Abstract
The Food and Drug Administration–approved clinical dose (1.5 mg/mL) of bone morphogenetic protein-2 (BMP2) has been reported to induce significant adverse effects, including cyst-like adipose-infiltrated abnormal bone formation. These undesirable complications occur because of increased adipogenesis, at the expense of osteogenesis, through BMP2-mediated increases in the master regulatory gene for adipogenesis, peroxisome proliferator-activated receptor-γ (PPARγ). Inhibiting PPARγ during osteogenesis has been suggested to drive the differentiation of bone marrow stromal/stem cells toward an osteogenic, rather than an adipogenic, lineage. We demonstrate that knocking down PPARγ while concurrently administering BMP2 can reduce adipogenesis, but we found that it also impairs BMP2-induced osteogenesis and leads to bone nonunion in a mouse femoral segmental defect model. In addition, in vitro studies using the mouse bone marrow stromal cell line M2-10B4 and mouse primary bone marrow stromal cells confirmed that PPARγ knockdown inhibits BMP2-induced adipogenesis; attenuates BMP2-induced cell proliferation, migration, invasion, and osteogenesis; and escalates BMP2-induced cell apoptosis. More important, BMP receptor 2 and 1B expression was also significantly inhibited by the combined BMP2 and PPARγ knockdown treatment. These findings indicate that PPARγ is critical for BMP2-mediated osteogenesis during bone repair. Thus, uncoupling BMP2-mediated osteogenesis and adipogenesis using PPARγ inhibition to combat BMP2's adverse effects may not be feasible.
The current gold standard for repairing critical-sized bone defects, which are addressed in >2.2 million surgical cases at costs exceeding $23.9 billion each year, is an autologous bone graft procedure.1, 2 However, these procedures are both invasive and risk donor site morbidity. In addition to these drawbacks, some patients are not eligible to receive this procedure because of inadequate availability or poor quality of the donor bone. Thus, there is a need for a novel treatment that enhances bone growth, but also does not require the use of substandard bone material or compromise the health of the donor site.
Currently, bone morphogenetic protein-2 (BMP2) is the most potent osteoinductive growth factor used in clinical practice. It has been found to induce bone regeneration in critical-sized bone defects made in multiple preclinical animal models.3, 4, 5, 6, 7 Collectively, BMP2 has been approved by the Food and Drug Administration with limited indications for use in anterior lumbar interbody fusions since 2002, open tibia shaft fractures with an intramedullary nail fixation since 2004, autogenous bone grafts for sinus augmentations since 2007, and localized alveolar ridge augmentations for defects associated with extraction sockets since 2007.8 Notably, BMP2 appears to have a species-specific dosing response. In other words, the concentration of BMP2 required for osteogenesis increases with phylogenetic complexity.9, 10, 11 The BMP2 concentration required for consistent bone formation in nonhuman primates ranges from 0.75 to 2.0 mg/mL, but smaller concentrations, ranging from 0.02 to 0.4 mg/mL, are required for rodents.11, 12, 13 On the basis of data obtained from nonhuman primate studies, the minimum effective human BMP2 concentration used in pilot and pivotal trials was initially chosen to be 1.5 mg/mL (with the total dose administered ranging between 4.2 and 12 mg), which is currently the maximum concentration approved for clinical use as a replacement for autogenous bone grafts in anterior lumbar interbody fusion procedures performed with an LT-CAGE Lumbar Tapered Fusion Device (Medtronic Spinal and Biologics, Memphis, TN).8, 11, 12, 14
Unfortunately, the Food and Drug Administration–approved clinical dose of BMP2 has been reported to induce significant adverse effects.15, 16, 17, 18 These include the formation of structurally abnormal bone, due to increased adipogenesis at the expense of osteogenesis, through BMP2-mediated increases in the master regulatory gene for adipogenesis, peroxisome proliferator-activated receptor-γ (PPARγ).19 Concomitantly, these adverse effects of BMP2 have been reproduced and verified in preclinical rodent and large animal models from our previous research and the research of other investigative groups.20, 21 Of particular importance, it was reported that high BMP2 concentrations (150, 300, and 600 μg/mL) are able to induce bone union in a rat femoral segmental defect model, but form cyst-like bony shells that are filled with adipose tissue.19 This finding suggests that large doses of BMP2 may induce excessive adipogenesis during the healing process, which produces newly formed bone of poor quality. As such, the dual effect of BMP2 on the induction of osteogenic and adipogenic signaling must be closely regulated to promote strong and healthy bone growth.
PPARγ is a critical signaling molecule involved in the promotion of adipocyte differentiation from mesenchymal stem cell progenitors.22 In addition, it has been investigated as an anti-inflammatory molecule.23 PPARγ has been assumed to have negative impacts on osteoblast differentiation due to the reciprocal relationship between osteoblastic and adipogenic differentiation.24, 25 Indeed, the intramedullary delivery of PPARγ lentiviral shRNA interference (PPARγ shRNA), also known as gene silencing in which gene expression is knocked down in a stable manner, results in increased trabecular bone formation with a significant decrease in lipid accumulation in rat femoral bone marrow.26 PPARγ suppression appears to enhance osteogenesis through the promotion of osteoblast formation and differentiation from bone marrow progenitors.27, 28, 29 These studies indicate that the inhibition of PPARγ may be an effective method to tip the balance of bone marrow stromal/stem cell (BMSC) differentiation toward an osteogenic, rather than an adipogenic, lineage. Previous reports have revealed that BMP2, at a regular dose of 50 to 100 ng/mL, can significantly up-regulate osteoblast transcription factors [runt related transcription factor (Runx) 2, distal-less homeobox (Dlx) 5, Osterix, Msh homeobox (Msx) 2, and AJ18] and an adipocyte transcription factor (PPARγ), which induces C26 mesenchymal progenitor differentiation into mature osteoblasts and adipocytes in basal medium.30 Moreover, 300 ng/mL BMP2 has the capacity to induce C3H10T1/2 cell differentiation into adipocytes in basal medium.11 With large doses of BMP2 seemingly able to activate adipocyte differentiation, inhibiting PPARγ during BMP2 administration was expected to increase osteogenic differentiation and new bone formation without the undesirable induction of adipogenesis.
In this study, we determined whether PPARγ inhibition with BMP2 administration could reduce excessive adipogenesis and improve the quality of regenerating bone in a critical-sized mouse femoral bone defect model. Reducing PPARγ expression may assist in unveiling the complex interactions and cross talk between key factors that regulate osteogenesis and adipogenesis in vivo. The possible underlying interactions between PPARγ and BMP2 were also explored to further determine osteogenic and adipogenic regulation.
Materials and Methods
Reagents and Antibodies
Human BMP2 (rhBMP2; Infuse Bone Graft) was purchased from Medtronic (Minneapolis, MN). The primary antibodies used in the immunohistochemical staining were as follows: anti-PPARγ (sc-7273; Santa Cruz Biotechnology, Dallas, TX), anti-osteocalcin (OCN; sc-18322; Santa Cruz Biotechnology), anti-Runx2 (sc-101145; Santa Cruz Biotechnology), anti–β-catenin (610153; BD Biosciences, East Rutherford, NJ), anti-CD31 (102503; Biolegend, San Diego, CA), anti–vascular endothelial growth factor (sc-57496; Santa Cruz Biotechnology), and anti-BMP receptor 2 (sc-393304; Santa Cruz Biotechnology). All other reagents for immunohistochemical staining were purchased from Dako (Agilent Technologies, Carpinteria, CA) unless otherwise specified. The primary antibodies used in the flow cytometric assay were mouse monoclonal anti–BMP receptor 2 (BMPR2; Ab130206; Abcam, London, UK) with a goat anti-mouse IgG (Ab150119, AF-647; Abcam) secondary antibody, anti-CD44 (560568, allophycocyanin-Cy7; BD Biosciences), anti–CD-105 (562759, phosphatidylethanolamine; BD Biosciences), and antibodies contained in the FITC Annexin V/Dead Cell Apoptosis Kit (V13242; Invitrogen, Paisley, UK).
Viral Production, Purification, and Efficiency Estimation
Lentiviral vectors with integrated PPARγ shRNA were used for long-term gene silencing of PPARγ. Scrambled shRNA or green fluorescent protein shRNA was integrated into lentiviral vectors and used as negative control. The lentiviral vectors encoding PPARγ shRNA, scramble shRNA, or green fluorescent protein shRNA were generated by cotransfection of 293T cells with the PPARγ shRNA plasmid vector (SC-29455-SH; Santa Cruz Biotechnology), scramble shRNA plasmid vector (1864 Addgene; Santa Cruz Biotechnology), or FG12 vector (14884 Addgene; Santa Cruz Biotechnology) with the pCMV-dR8.2-vprX helper plasmid and pCMV-VSVG envelope plasmid.26 The viral vectors were collected from transfected cell cultures, cell debris was removed by 0.22-mm filtration, and the remaining solution was concentrated by ultracentrifuge at 4°C, 32,310 × g for 60 minutes using Beckman SW32 rotors (Beckman Coulter, Inc., Brea, CA).26 The pellets containing the lentiviral vectors were resuspended with Dulbecco's modified Eagle's medium (Gibco, Gaithersburg, MD) to a concentration of 5 × 107 tissue culture infective dosage/mL. The lentiviral vector titer was estimated by measuring the gag p24 protein; 1 pg of the p24 reading was ascribed as the 10 tissue culture infective dosage for freshly isolated lentiviral vectors.26 Real-time PCR and DAPI/green fluorescent protein histologic quantification were used to analyze the efficiency of the shRNA lentiviral activity.31
Implant Material
Cylindrical poly(lactic-co-glycolic acid) (PLGA) scaffold implants were prepared as previously described.11, 19, 32 Briefly, the scaffold was fabricated from an 85:15 d, l-PLGA (inherent viscosity, 0.61 dL/g; Absorbable Polymers, Pelham, AL)/chloroform solution, briefly mixed with 200- to 300-μm diameter sucrose, and compressed into a polytetrafluoroethylene-ethylene mold to form a cylindrical shape (2.5-mm length × 1-mm diameter).11 The scaffolds were freeze dried overnight and were rinsed three times by immersion in distilled water to dissolve the sucrose to obtain a porous structure (94% porosity). All scaffolds were disinfected by immersion in 70% ethanol for 30 minutes, and then rinsed three times with distilled water.11
Next, the scaffolds were coated with hydroxyapatite to increase the biomechanical strength and efficiently load BMP2 and enhance the overall osteoconductivity, as the scaffolds were naturally hydrophobic after fabrication.11 Briefly, two sterile supersaturated solutions with ion concentrations five times greater than those in human plasma [simulated body fluid (SBF) 1 and 2] were sequentially applied to the scaffolds.11 The preparation and ionic concentrations of SBF 1 and SBF 2 were similar to the methods and final concentrations used in our previous studies.19, 33 To improve wetting and coating uniformity, dried PLGA scaffolds were subjected to glow discharge argon plasma etching (Harrick Scientific, Ossining, NY) immediately before the coating process. Etched scaffolds were then incubated in SBF 1 for 12 hours, and then immersed into SBF 2 for another 12 hours inside of a water-jacketed incubator at 37°C. Coated PLGA scaffolds were rinsed gently with sterile distilled water to wash away excess ions, and then dried in a laminar flow hood. Apatite-coated PLGA scaffolds did not experience a reduction in porosity, and maintained a static pore interconnectivity and pore size ranging between 200 and 300 μm. Shortly before implantation, scaffolds were impregnated with phosphate-buffered saline (PBS), BMP2, or BMP2 in combination with a control lentivirus or PPARγ shRNA lentivirus.11 The BMP2 dose selected for this study has been previously established and consistently induces adipocytic bone cysts in a femoral segmental defect (FSD) model.19 The BMP2 was diluted to the appropriate concentration in PBS, uniformly added to the scaffolds in a dropwise manner for 20 minutes, and lyophilized in a freeze drier overnight at −20°C.11
FSD Animal Model
The 3-month–old male Axin2+/− transgenic mice were used as the FSD model for potential Wnt signaling applications.34, 35 Axin2+/− mice are similar to wild-type mice in terms of their behavioral phenotype and bone formation capacity; they have been considered analogous to wild-type control mice in previous reports.35, 36 Animals were obtained from The Jackson Laboratory (Sacramento, CA) and housed on a 12:12-hour light-dark cycle with ad libitum access to laboratory rodent chow and water. Genotypes were determined as previously described.37 All surgical procedures were conducted in accordance with the Animal Care Guideline approved by the University of California, Los Angeles Chancellor's Animal Research Committee. Twenty mice were randomized into four experimental groups (n = 5 per group), as shown in Table 1.
Table 1.
Experimental Groups for FSD Surgery
| Experimental group at 8 weeks | Animals, n | Scaffold volume, μL | Total dose |
|
|---|---|---|---|---|
| BMP2, μg (600 μg/mL) | shRNA lentivirus, pfu (1 × 107 pfu/mL) | |||
| PBS (control) | 5 | 3.5 | 0 | 0 |
| BMP2 | 5 | 3.5 | 2.1 | 0 |
| BMP2 + shControl | 5 | 3.5 | 2.1 | 35,000 |
| BMP2 + shPPARγ | 5 | 3.5 | 2.1 | 35,000 |
FSD, femoral segmental defect; pfu, plaque-forming unit.
The mouse FSD surgical procedure was performed on the basis of the RI system (RISystem, Davos, Switzerland) surgical technique guide (Figure 1, A and B). Using an aseptic surgical technique, a 15- to 20-mm longitudinal incision was made along the right femur from the greater trochanter of the thigh to the knee. The vastus lateralis muscle and biceps femoris muscle were split, the tensor fasciae latae muscle was elevated to the distal metaphysis of the femur while preserving the sciatic nerve, and the femoral shaft was exposed. Fixation of a six-hole polyether ether ketone plate (1 mm in length, 1.1 mm in width, 1 mm in height) (RISystem) was placed on the anterolateral surface of the femur and fixed with four locking screws (RISystem), which were drilled through the plate and both cortices of the femur. A 2.5-mm bone defect was generated using the Gigly saw (RISystem) with a 2.5-mm saw guide (RISystem) and sufficient irrigation. The volume of the defect was approximately 3.5 μL. An implant preloaded with one of the four different treatments was placed into the defect, and the overlying muscle, fascia, and skin were closed with a 6-0 Vicryl absorbable suture (Ethicon, Somerville, NJ). Faxitron radiographic X-ray imaging was performed immediately after surgery to evaluate potential complications that may occur after the fixation, and was repeated each subsequent week to evaluate the healing process.
Figure 1.
Large doses of BMP2 induce cystic bone formation in mouse femoral segmental defects (FSDs) 8 weeks after surgery. A: Schematic of the FSD surgical procedure. B: Intraoperative image of the FSD procedure with polyether ether ketone plate and cylindrical poly(lactic-co-glycolic acid) scaffold placement. C: Microcomputed tomography (microCT) and biomechanical evaluation. From top to bottom, two-dimensional (2D) radiography, sagittal sectioning, axial sectioning, density distribution [color indicates bone mineral density value, ranging from 0.217 (yellow) to 1.282 (blue) g/cm3], and three-dimensional (3D) reconstructed microCT images. The treatment groups were PBS, BMP2, BMP2 + shControl, and BMP2 + shPPARγ. Green boxed areas indicate the region of interest selected for 3D microCT reconstruction. Red boxed areas indicate the region of interest selected for microCT quantification in D. D: MicroCT quantification of bone volume/tissue volume (BV/TV) and trabecular number (Tb.N). Data are expressed as means ± SD. ∗∗∗P < 0.005 versus the PBS group; †P < 0.05, †††P < 0.005 versus the BMP2 + shControl group. Scale bar = 5 mm (C).
Three-Dimensional Microcomputed Tomography Analysis
Animals were euthanized 8 weeks after surgery by slow carbon dioxide (20% to 30%/minute) asphyxiation. A subsequent thoracotomy was performed as a confirmatory method of euthanasia. Femurs were harvested, paraformaldehyde fixed, and scanned by high-resolution microcomputed tomography (microCT; SkyScan 1176 microCT; Bruker, Kontich, Belgium) at an image resolution of 27.4 mm (55-kV and 181-mÅ radiation source, using a 0.5-mm aluminum filter).11 Three-dimensional reconstructions of the images were obtained using NRecon software version 1.7.0.4 (Bruker).
For femoral analyses, approximately 3 mm (0.5 mm more than the original surgical defect) centered on the defect area was used as the region of interest. The region of interest was identified and standardized on the basis of the position of the screws. A threshold of 20 was used for bone analysis. Two-dimensional X-ray images were obtained from axial planes made through the centers of the defect areas.11 A volume of interest of 500 slices (approximately 10 mm) containing the whole femur was selected for sagittal sectioning and three-dimensional reconstruction.11 Sagittally sectioned images were generated through the mediolateral center of the femur, which has been previously described.11 A volume of interest of 50 slices (approximately 1 mm), centered on the middle of the defect, was reconstructed for axial section images.11 All quantitative and structural morphometric data use nomenclature was described by the Nomenclature Committee of the American Society for Bone and Mineral Research.11, 38 The bone volume fraction (bone volume/tissue volume), bone mineral density, and trabecular number of the regions of interest were calculated at a volume of 150 slices (approximately 3 mm) with CTAn software version 1.16.4.1 (Bruker).
Histologic, Immunohistochemical, and Histomorphometric Analysis
Eight weeks after surgery, femurs were harvested, fixed in paraformaldehyde, decalcified in 19% EDTA, paraffin embedded, and divided into sections (5 μm thick). Three slides from each animal were selected for histomorphometric analysis, and all experimental animals were evaluated. Hematoxylin and eosin, Masson's trichrome, and immunohistochemical staining were performed as previously described.11 All primary antibodies were diluted to 1:100. Appropriate secondary antibodies were used at a dilution of 1:200.11 Photomicrographs were acquired using an Olympus BX51 microscope (×40, ×200, and ×400 magnification lens, UPLanFL; Olympus, Center Valley, PA), SZX12 microscopes (×10 magnification lens, DF PLAPO 1.2 pf; Olympus, Center Valley, PA), and a MicroFire digital microscope camera with Image Frame software version 2.1 (Optronics, Goleta, CA).11 Approximately four random consecutive ×400 magnification images of the tissue near the defect sites were acquired for analyses by three blinded examiners (C.W., J.T., and E.A.B.) using ImageJ software version 1.48 (NIH, Bethesda, MD; http://imagej.nih.gov/ij), and repeated in triplicate.31 All nomenclature, symbols, and units are used with regard to the established American Society for Bone and Mineral Research guidelines.11, 39
Cell Culture
Cells from the M2-10B4 cell line (M2 cells) (ATCC, Manassas, VA), a clone derived from BMSCs of a (C57BL/6J × C3H/HeJ) F1 mouse,40 were used and maintained in growth medium [RPMI 1640 medium (Invitrogen, Carlsbad, CA) with the addition of 10% heat-inactivated fetal bovine serum, 1 mmol/L sodium pyruvate, and 100 U/mL penicillin/streptomycin (Gibco)]. Osteogenic differentiation medium consisted of RPMI 1640 medium, 10% fetal bovine serum, 50 mg/mL ascorbic acid, and 3 mmol/L b-glycerophosphate.11 Growth medium was only used for cellular proliferation before treatments were applied, as previously reported.11, 41, 42
Primary mouse BMSCs were obtained from mice humeri and femurs.43 Bone samples were dissected, and the marrow cells were harvested by flushing the bones with Dulbecco's modified Eagle's medium using a 27-gauge syringe needle inserted into one end of the bone. The bone marrow cells were then filtered through a 70-mm nylon mesh filter (BD Falcon; BD Biosciences).26 Cells were plated into 6-well plastic cell culture plates at a density of 2.5 × 106 cells per well26 in Complete MethoCult Expansion Medium (STEMCELL Technologies, Vancouver, BC, Canada), which contained basal medium, 10× supplement, and MesenPure. MesenCult Expansion Medium (STEMCELL Technologies) has been optimized for the derivation and expansion of mouse BMSCs in vitro. Cells were incubated in a 37°C humidified chamber containing 95% air and 5% CO2. Cells from passage 2 and passage 4 were used for further experiments. Mouse osteogenic Stimulatory Supplements (STEMCELL Technologies) were used in conjunction with MesenCult Basal Medium (STEMCELL Technologies).
Knocking Down PPARγ Activity Using Lentiviral Vectors
Knockdown PPARγ in M2 cells and primary BMSCs was performed as previously described.26, 44 All work with lentiviral vectors was performed under biosafety level 2 conditions.44 Puromycin (sc-108071) was purchased from Santa Cruz Biotechnology. Briefly, cells were plated at 30% to 50% confluence and transfected with appropriate dilutions of lentiviral particles (multiplicity of infection = 10) for 24 hours. A puromycin titration (kill curve) was performed to optimize the concentration of puromycin. Cells were then cultured in growth medium containing 0.5 μg/mL puromycin to obtain stable transfected cell lines.
Real-Time PCR
Total RNA extraction and SYBR Green real time-PCR were performed as previously described.11, 45 SYBR Green real-time PCR primer sequences used for M2 cell studies are provided in Table 2. The total RNA present was isolated using the TRIzol Reagent protocol (Invitrogen, Waltham, MA) and DNaseI (Promega, Madison, WI), followed by isopropyl alcohol precipitation. cDNA was synthesized from 1000 ng of the total RNA using a SuperScript III Reverse-Transcriptase kit (Invitrogen, Waltham, MA). Then, quantitative PCR was performed using SYBR Select Master Mix (Applied Biosystems, Foster City, CA) following the supplier's protocol. Glyceraldehyde-3-phosphate dehydrogenase was used as the internal control for normalization. Real-time PCR was performed on days 3, 6, and 9. All fold-change values generated from three independent experiments are presented.
Table 2.
Mouse Primer Sequences for Real-Time PCR
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Gapdh | 5′-TGCACCACCAACTGCTTAGC-3′ | 5′-CCACCACCCTGTTGCTGTAG-3′ |
| Bmp2 | 5′-GTGTGGTCCACCGCATCAC-3′ | 5′-GGCAGACATTGTATCTCTAGG-3′ |
| Pparγ | 5′-GGAAAGACAACGGACAAATCA-3′ | 5′-TACGGATCGAAACTGGCAA-3′ |
| Alp | 5′-TGCCACTGTGAGAAGACCTG-3′ | 5′-TGCACAGGAAGTGAGTCTGG-3′ |
| Runx2 | 5′-CCGCACGACAACCGCACCAT-3′ | 5′-CGCTCCGGCCCACAAATCTC-3′ |
| Ocn | 5′-GCAATAAGGTAGTGAACAGACTCC-3′ | 5′-AGCAGGGTTAAGCTCACACTG-3′ |
| Bmpr2 | 5′-GAAACGATAATCATTGCTTTGGC-3′ | 5′-CCCTGTTTCCGGTCTCCTGT-3′ |
| Bmpr1a | 5′-TCATGTTCAAGGGCAGAATCTAGA-3′ | 5′-GGCAAGGTATCCTCTGGTGCTA-3′ |
| Bmpr1b | 5′-CCTCGGCCCAAGATCCTAC-3′ | 5′-CCTAGACATCCAGAGGTGACA-3′ |
Cell Proliferation Analysis
Cell proliferation was measured at 24, 48, and 72 hours by the Vybrant MTT Cell Proliferation Assay Kit (Life Technologies, Camarillo, CA), as previously reported.46 Briefly, 2 × 103 cells/cm2 of M2 cells, PPARγ shRNA lentivirus transfected stable (shPPARγ) M2 cells, and control shRNA lentivirus transfected stable (shControl) M2 cells were seeded into 96-well plates with 200 μL fresh growth medium (5% serum) 6 hours before treatment with or without 300 ng/mL BMP. At each time point, 10 μL of 12 mmol/L MTT stock solution reagent (Sigma-Aldrich, St. Louis, MO) was added into chambers and incubated for 4 hours at 37°C. The absorbance was read using a plate reader (model 680; Bio-Rad, Hercules, CA) at 570 nm.
Wound-Healing Assay
A monolayer scratch assay44 was used to compare the migratory ability of M2 cells, shPPARγ M2 cells, and shControl M2 cells, with the presence or absence of 300 ng/mL BMP2. Cells were cultured on 6-well plates to confluence, and monolayers were wounded with a sterile 200-μL pipette tip.44 The cultures were then washed with PBS to remove detached cells, and treated with either PBS or 300 ng/mL BMP2.44 Images were captured using phase-contrast microscopy at 0, 12, and 21 hours after treatment.44 Migration was quantified by measuring the average wound gap between the wound edges before and after the treatment, and calculated as follows: Cell Migration (%) = [(Gap0h − Gap12h)/Gap0h] × 100.47
Cell Invasion Assay
Cell invasion assays were performed in 8-μm pore Boyden chambers (Transwell; Corning, Lowell, MA).48, 49 Briefly, M2 cells, shPPARγ M2 cells, and shControl M2 cells were seeded in 100-mm dishes and cultured to 80% to 90% confluence. After reaching 80% to 90% confluence, the cells were starved overnight for 16 hours. Then, the cells were trypsinized, resuspended in 0.5% serum medium, and plated onto the top of each chamber insert.44 The bottom chamber was filled with 0.5% serum medium. BMP2 (300 ng/mL) was added to the insert and bottom chamber. After 21 hours, the inserts were removed and washed; cells that migrated to the bottom of the inserts were fixed with methanol, whereas the nonmigrated cells staying on the upper surface of the filter were removed carefully with a cotton swab. Migrated cells were stained with DAPI for 5 minutes, and then counted in five randomly selected fields under an inverted microscope (Olympus, Tokyo, Japan).44
Mineralization Assay
M2, shPPARγ M2, and shControl M2 cells were seeded at 2 × 105 cells per well in 6-well plates for 24 hours to full confluence, and then cultured with osteogenic differentiation medium with or without 300 ng/mL BMP2. These experiments were repeated with bisphenol A diglycidyl ether (BADGE), an antagonist of PPARγ, instead of PPARγ shRNA transfection; M2 cells were treated with PBS, 300 ng/mL BMP2, or 50 μmol/L BADGE. The medium was replenished every 3 days, and the experiments were concluded after 21 days. Alizarin Red staining was then performed as previously described.50 Monolayers were rinsed with PBS and fixed with 3 mL absolute ethanol per well for 30 minutes at room temperature. Alizarin Red Stain Solution (CM-0058; Lifeline Cell Technology, Frederick, MD) was added and incubated for 15 minutes. The absorbance was read at 405 nm with a plate reader.
Flow Cytometric Assay
To analyze BMPR2-positive cells, primary BMSCs were isolated using previously described methods from Axin2+/− mice 3 days after intramedullary injection43, 51 with the same treatment dosages and animal conditions applied in the FSD surgery groups. The cells were washed with PBS and diluted to a final concentration of 1 × 106 cell/mL. The cell suspension (100 μL) was incubated for 20 minutes with BMPR2, CD44, CD105, CD31, and CD45 antibodies in the dark at room temperature and analyzed by flow cytometry, according to the manufacturer's instructions, using a BD FACS caliber flow cytometry system (BD Biosciences). CD44/CD105 were used as stem cell markers, whereas CD31/CD45 were used as hematopoietic cell markers. All primary antibodies were used at a dilution of 1:100. The secondary antibody was used at a dilution of 1:1000. The data were analyzed with FACS Diva software version 4.0 (BD Biosciences).
To analyze M2 cell apoptosis, M2 cells were treated with PBS, 300 ng/mL BMP2, BMP2 + shPPARγ (using a stable transfected M2 cell line that had a 90% knockdown of PPARγ, which was selected from PPARγ shRNA transfected colonies), or BMP2 + shControl (using a stable M2 cell line selected from control shRNA transfected colonies) for 3 days, and subjected to a flow cytometry test using the FITC Annexin V/Dead Cell Apoptosis Kit protocol.
Statistical Analysis
Generally, data are presented as the means ± SD. Statistical significance quantification was performed with GraphPad Prism software version 5 (GraphPad, San Diego, CA). The P values were generated using a two-way analysis of variance with the Bonferroni correction or multiple t-tests. P < 0.05 was considered statistically significant.
Results
A Large Dose of BMP2 Induces Cystic Bone Formation and Bony Union in a Mouse Segmental Femoral Defect Model
Using a mouse FSD model (Figure 1, A and B), the cystic bone formation induced by large doses of BMP2 was successfully recapitulated, as portrayed by the microCT and histologic assessments (Figures 1C and 2A). Briefly, cylindrical PLGA scaffolds impregnated with different treatments were implanted into 2.5-mm mouse femur defect sites during surgery.
Figure 2.
Histologic evaluation of BMP2 + shPPARγ cotreated femoral segmental defect femurs reveals decreased osteogenesis and adipogenesis 8 weeks after surgery. A: Hematoxylin and eosin (H&E) staining reveals bone union and the cystic bone formation (a large extension of bone that extends beyond the original defect margins surrounded by thin cortical bone) induced by large doses of BMP2. BMP2 + shPPARγ cotreated samples experience bone nonunion, with newly formed bone confined to the defect sites, defect margins that remodeled to form square caps, and unabsorbed poly(lactic-co-glycolic acid) scaffolds that were located between the two caps. Insets show regions at lower magnification. B: Masson's trichrome staining reveals fibrous tissue (light blue) in the control samples, abundant adipocytes (large white droplets) in the BMP2 samples, and osteoid matrix (dark blue) ossifying into mature trabecular bone (red) in the BMP2 + shPPARγ cotreated samples. C: Osteocalcin (OCN) immunohistochemical (IHC) analysis. Arrows indicate positive OCN staining. D: PPARγ IHC analysis. E: Histomorphometric analyses are based on H&E staining. Bone volume/tissue volume (BV/TV) and trabecular number (Tb.N) are shown. F and G: Quantification of OCN (F) and PPARγ (G) IHC analysis. All fold-changes are reported relative to the PBS control group. Data are expressed as means ± SD. ∗∗∗P < 0.005 versus the PBS group; †††P < 0.005 versus the BMP2 + shControl group. Scale bars: 5 mm (A); 1 mm (B); 0.1 mm (C and D). Def, boundaries and extent of the defect area.
All parameters related to bone formation gradually increased over the post-surgical 8-week period. The PBS group exhibited minimal new bone formation in the defect area, and the defect margins remodeled to form rounded caps that are characteristic of bone nonunion. This insufficient remodeling left obvious bone defects and unresolved PLGA scaffolds within the original defect sites. In contrast, the defects in the group treated with a large dose of BMP2 showed bony union with markedly increased new bone formation, although bridging of the defects was variable among samples and large cysts were present between the regenerated bone (Figure 1C). Moreover, the new bone formed after BMP2 treatment was not limited to the original defect margins, but instead showed remarkable overgrowth with a 105.8% higher bone volume/tissue volume and a 125.4% higher trabecular number compared with the PBS group (P < 0.005) (Figure 1D). The cystic bone formed had a thin, continuous cortical shell that expanded beyond the original defect edges externally, sparse and thin trabecular formations internally, and massive fat infiltrations in the bone marrow cavity that were confirmed by histology (Figure 2, A and B). The β-catenin expression did not appear to be significantly different among the various treatment groups, as determined by immunohistochemical staining (Supplemental Figure S1).
PPARγ Knockdown Suppresses BMP2-Induced Cystic Bone Formation and Bone Union in Vivo
MicroCT evaluation of complete FSD reconstructions (Figure 1C) revealed that cystic bone formations and bone union occurred in the BMP2 and BMP2 + shControl groups. Unexpectedly, most of the samples in the BMP2 + shPPARγ group showed bone nonunion, with regenerated bone that was confined to the defect area and defect margins that remodeled to form square caps. MicroCT-based quantification of new bone regeneration revealed that the BMP2 + shPPARγ group had a 48.4% lower bone volume/tissue volume (P < 0.01) and a 51.8% lower trabecular number (P < 0.05) compared with the BMP2 + shControl group (Figure 1D). No statistically significant differences were detected between the BMP2 and BMP2 + shControl groups.
The bone quality of the FSD samples was analyzed histologically 8 weeks after surgery (Figure 2). In the center of the defects, the PBS group defects showed nonunion with no newly formed trabecular bone. BMP2 treatment induced bone union and the PLGA scaffolds were absorbed, but the regenerated bone contained a copious amount of adipose tissue that was integrated within sparse trabecular bone. In addition, the regenerated bone was surrounded by a thin cortical bone cyst that extended almost 1.5 mm beyond the defect edges. In contrast, the BMP2 + shPPARγ cotreatment group experienced bone nonunion, unresolved PLGA scaffolds, less bone formation, and less adipose infiltration than the BMP2 group (Figure 2A). Histomorphometric analyses on hematoxylin and eosin sections revealed a significantly decreased bone volume/tissue volume ratio and trabecular number in BMP2 + shPPARγ treated samples compared with the samples from the BMP2 or BMP2 + shControl groups (Figure 2E). Masson's trichrome staining revealed fibrous tissue formation in the PBS samples, prolific adipocytes in the BMP2 samples, and osteoid matrices ossifying to mature bone in the BMP2 + shPPARγ sections. In the BMP2 and BMP2 + shControl groups, the cut femoral bone ends had bony trabeculations protruding into the defect margins. Samples from both groups had vast reactive tissue zones with increased cellularity that extended far beyond the surgical bone defect edges into the adjacent bone and muscle (Figure 2B).
Markers for osteogenic and adipogenic differentiation were further evaluated by histology. Immunostaining for OCN (Figure 2, C and F), a marker of bone matrix formation and osteoblastic differentiation, and PPARγ (Figure 2, D and G) confirmed that defects from the BMP2 + shPPARγ group exhibited decreased OCN and PPARγ expression compared with the BMP2 + shControl group. In contrast, the BMP2 + shPPARγ samples induced significantly lower OCN signal intensity, especially around the mineralized parts of the new bone matrices, substantially smaller reactive tissue zones with decreased amounts of trabecular bone that were mostly confined to the surgical defect areas, and nominal tissue reactivity in the adjacent muscle tissue.
PPARγ Knockdown Inhibits BMP2-Induced Osteogenesis
To determine whether PPARγ knockdown with BMP2 cotreatment corresponded with a decrease in osteogenesis, the expression of early (Runx2), intermediate [alkaline phosphatase (ALP)], and final osteogenic differentiation (OCN and Alizarin Red–positive nodules) markers was analyzed in vitro.11 Meanwhile, immunostaining for Runx2 (Figure 3, A and D) confirmed that the BMP2 + shPPARγ group exhibited decreased Runx2 expression compared with the BMP2 + shControl group. To reduce discrepancies of individual lentiviral transfection efficiency, stable cell lines with PPARγ shRNA or control shRNA were first generated and selected (Supplemental Figure S2). A stable M2 cell line with a 90% knockdown of PPARγ was used for the subsequent experiments, and was cultured under osteogenic conditions. The matrix mineralization was quantified by measuring the nodular calcium produced during 3 weeks of osteogenic differentiation after Alizarin Red staining.
Figure 3.
PPARγ deletion leads to decreased osteogenesis. A: Immunohistochemical (IHC) staining of an early osteoblastic marker (Runx2). B: Real-time PCR analysis of runt related transcription factor (Runx) 2, alkaline phosphatase (ALP), osteocalcin (OCN), and PPARγ expression in M2 cell line cells treated with PBS, 300 ng/mL BMP2, BMP2 + shPPARγ (using a stable transfected M2 cell line that had a 90% knockdown of PPARγ, which was selected from PPARγ shRNA transfected colonies), or BMP2 + shControl (using a stable M2 cell line selected from control shRNA transfected colonies) for 3, 6, and 9 days. All fold-changes are reported relative to the PBS control group. C: Alizarin Red staining to determine mineralization in M2 cells treated with PBS, 300 ng/mL BMP2, BMP2 + shPPARγ, or BMP2 + shControl for 21 days. Insets show representative images of Alizarin Red staining of a whole well at lower magnification. D: Quantification of Runx2 IHC analysis. E: Quantification of Alizarin Red staining intensity. F: Real-time PCR analysis of Runx2 and PPARγ expression in mouse primary bone marrow stromal cells treated with PBS, BMP2, BMP2 + shPPARγ, or BMP2 + shControl for 3 days. All fold-changes are reported relative to the PBS control group. Data are expressed as means ± SD. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.005 versus the PBS group; †P < 0.05, ††P < 0.01, and †††P < 0.005 versus the BMP2 + shControl group. Scale bars: 0.1 mm (A); 0.2 mm (C).
As expected, the BMP2 and BMP2 + shControl groups showed increased RNA expression of early (Runx2), intermediate (ALP), and final osteogenic differentiation (OCN and Alizarin Red–positive nodules) markers when compared with the PBS control group (Figure 3, B, C, and E). However, when knocking down PPARγ in the BMP2 cotreatment group, the expression of all of the osteogenic markers was significantly decreased relative to the BMP2 or BMP2 + shControl groups (P < 0.01). These findings were consistent with the 8-week microCT and histologic results showing bone nonunion in the in vivo experiments. Repeating these experiments in mouse primary BMSCs demonstrated that the BMP2 + shPPARγ group had significantly decreased Runx2 and PPARγ expression compared with the BMP2 + shControl group (Figure 3F). Last, repeating these experiments with BADGE, a chemical antagonist of PPARγ, showed the same trend observed in the PPARγ shRNA experiments (Supplemental Figure S3). BADGE and BMP2 cotreatment significantly decreased mineralization compared with BMP2 treatment alone.
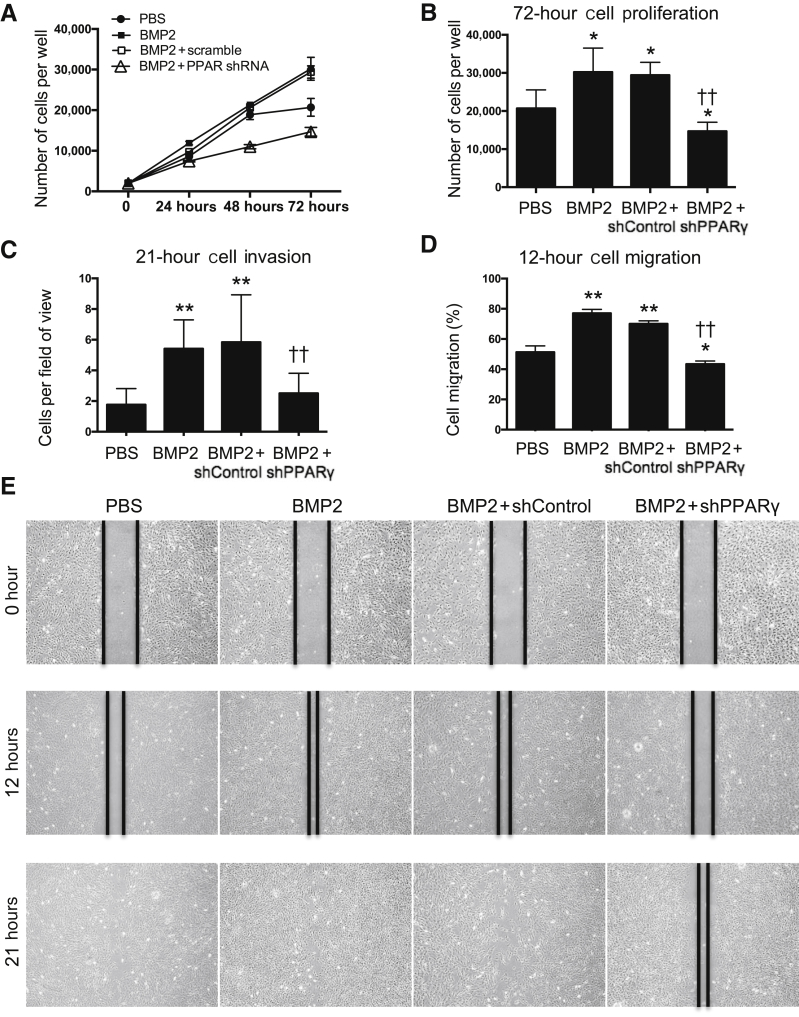
PPARγ Knockdown Significantly Alters BMP2-Induced Cell Proliferation, Migration, and Invasion
With the unexpected findings from the mouse FSD in vivo model and in vitro mineralization experiments, cellular function was investigated by examining cellular proliferation, migration, and invasion using selected transfected M2-10B4 cells.
Cell proliferation can be drastically altered in response to various stimuli, which leads to various osteogenic differentiation outcomes. As expected, BMP2 is a strong inducer of M2 cell proliferation. Knocking down PPARγ significantly decreased the BMP2-induced cell proliferation (P < 0.01) (Figure 4A). At the 72-hour time point, the average number of cells per well increased from the initially seeded 2000 to 30,205 in the BMP2 group. In contrast, proliferation in the BMP2 + shPPARγ group appeared to be diminished by 50.01% when compared with the BMP2 + shControl group. The average number of cells per well after 72 hours only reached 14,680 (P < 0.01) (Figure 4B).
Figure 4.
PPARγ repression results in reduced bone marrow stromal cell proliferation, migration, and differentiation that are typically induced by BMP2. A: MTT assay of M2 cells treated with PBS, 300 ng/mL BMP2, BMP2 + shPPARγ (using a stable transfected M2 cell line that had a 90% knockdown of PPARγ, which was selected from PPARγ shRNA transfected colonies), or BMP2 + shControl (using a stable M2 cell line selected from control shRNA transfected colonies). B: Quantification of viable cells after 72 hours. C: Transwell invasion assay of M2 cells conducted after 21 hours. D: M2 cell wound-healing assay after 12 hours. E: Migration was quantified by measuring the average wound gap between the wound edges before and after treatment. The black lines were drawn along the edges of the wounds at indicated time points. The percentage of cells that migrated at 12 hours was calculated as follows: Cell Migration (%) = [(Gap0h – Gap12h)/Gap0h] × 100. Data are expressed as means ± SD. ∗P < 0.05, ∗∗P < 0.01 versus the PBS group; ††P < 0.01 versus the BMP2 + shControl group. Original magnification, ×4 (E).
An in vitro wound-healing model was used to assess cell migration by generating scrape wounds in confluent cell cultures. As expected, BMP2 significantly increased cell migration in comparison to the PBS group. shControl cells, with the addition of BMP2 treatment, began to migrate into the denuded area 6 hours after being scratched, and wound closure was nearly complete (>90%) after 21 hours. In contrast, shPPARγ cells did not migrate as much as the noninfected M2 cells in the presence of BMP2 (P < 0.01), as indicated by a smaller amount of cells in the denuded area when observed 12 and 21 hours after scratching (Figure 4, D and E). These observations indicate that knocking down PPARγ decreased cell migration that was normally induced by BMP2.
BMP2 has been recognized as a strong chemotactic factor that has significant impacts on recruiting stem cells to sites of bone regeneration.52, 53, 54 Treatment with BMP2 alone showed shControl cells had significantly increased cell migration (169%) relative to M2 cells in the PBS group (Figure 4C). The shPPARγ cells treated with BMP2 demonstrated a significantly decreased cell migration (57%) compared with that of the BMP2 + shControl cells (P < 0.005). Taken together, these findings support the claim that knocking down PPARγ decreased cell migration, whereas BMP2 appeared to promote cell migration in M2-10B4 cells.
Overall, significant decreases in cell proliferation, migration, and invasion were observed in cells with reduced expressions of PPARγ that were treated with a large dose of BMP2. These findings distinctly contrast with those showing significant increases in these functional parameters in M2 cells treated with BMP2 alone.
PPARγ Knockdown Escalates BMP2-Induced Cell Apoptosis
PPARγ knockdown attenuated BMP2-induced cell proliferation, migration, and invasion. In addition to assessing these parameters, flow cytometry was performed to determine cell apoptosis and cell survival after BMP2 treatment with and without PPARγ knockdown. Cell apoptosis appeared to be significantly higher in the BMP2 + shPPARγ group (18.4%) than in the PBS (1.6%) and BMP2 (3.2%) groups (P < 0.005) (Figure 5, A and B).
Figure 5.
PPARγ knockdown escalates BMP2-induced cell apoptosis. A: Flow cytometry analysis of M2 cell apoptosis. M2 cells treated with PBS, 300 ng/mL BMP2, or BMP2 + shPPARγ (using a stable transfected M2 cell line that had a 90% knockdown of PPARγ, which was selected from PPARγ shRNA transfected colonies), or BMP2 + shControl (using a stable M2 cell line selected from control shRNA transfected colonies) for 3 days and subjected to flow cytometry testing. B: Quantification of apoptotic cells. All fold-changes are reported relative to the PBS group. Data are expressed as means ± SD. ∗∗∗P < 0.005 versus the PBS group; †††P < 0.005 versus the BMP2 + shControl group. FITC, fluorescein isothiocyanate; PI-A, propidium iodide–active; Q, quadrant.
BMP Receptor 2 and 1B Expression Is Significantly Inhibited by the Combinatorial BMP2 and PPARγ shRNA Treatment
The in vitro findings described above indicate that the cellular responses to BMP2 stimulation were significantly altered with PPARγ inhibition. To further decipher the underlying mechanism of these perturbations, a BMP2 cell surface receptor was examined for its expression among cultured M2 cells and primary bone marrow stromal cells, in addition to its distribution in the tissue of mouse FSD samples. By using immunohistochemical staining inside the bone marrow cavity in vivo, a decreased expression and staining intensity of BMPR2 was observed in bone marrow cells treated with BMP2 + shPPARγ in comparison to those treated with BMP2 alone (P < 0.05) (Figure 6, A and B). Following the same trend, real-time PCR analysis revealed that the RNA expression of BMPR2 was significantly decreased after knocking down PPARγ and cotreating with BMP2 in both the stable M2 cell line and mouse primary BMSCs (Figure 6, C and D). Flow cytometry confirmed that the population of BMPR2-positive primary BMSCs was significantly decreased in the BMP2 + shPPARγ group relative to that in the BMP2 + shControl group (P < 0.005) (Figure 6, E and F).
Figure 6.
PPARγ inhibition leads to reduced BMP receptor 2 (BMPR2) expression. A: Immunohistochemical (IHC) staining of BMPR2. B: Quantification of IHC analysis of BMPR2. C: Real-time PCR analysis of BMPR2 mRNA expression in M2 cells treated with PBS, 300 ng/mL BMP2, BMP2 + shPPARγ (using a stable transfected M2 cell line that had a 90% knockdown of PPARγ, which was selected from PPARγ shRNA transfected colonies), or BMP2 + shControl (using a stable M2 cell line selected from control shRNA transfected colonies) for 3, 6, and 9 days. D: Real-time PCR analysis of BMPR2 mRNA expression in mouse primary bone marrow stromal cells (BMSCs) treated with PBS, BMP2, BMP2 + shPPARγ, or BMP2 + shControl for 3 days. E: Flow cytometry analysis of BMPR2 expression on mouse primary BMSCs. PBS, 600 μg/mL BMP2, BMP2 + PPARγ shRNA (1 × 107 plaque-forming units/mL; multiplicity of infection = 10), or BMP2 + shControl treatments were injected into Axin2+/− mouse femur cavities. BMSCs were isolated and subjected to flow cytometry test on day 3 after injection. F: Quantification of flow cytometry analysis of BMPR2-expressing mouse primary BMSCs among different treatment groups. All fold-changes are reported relative to the PBS group. Data are expressed as means ± SD. n = 6 per group (E). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.005 versus the PBS group; †P < 0.05, ††P < 0.01 versus the BMP2 + shControl group. Scale bar = 0.1 mm (A). P2, negative area.
In addition to these experiments, real-time PCR was performed to explore endogenous BMP2, BMPR1A, and BMPR1B mRNA changes. In general, the endogenous BMP2 and BMPR1B mRNA changes followed the similar pattern to that of BMPR2, which were significantly increased in the BMP2 group and significantly down-regulated in the BMP2 + PPARγ inhibition group after 3 days of treatment (Supplemental Figure S4, A and C). The BMPR1A mRNAs in the BMP2-treated groups were all increased compared with the PBS group (P < 0.05), but the significant changes by PPARγ inhibition were only seen at day 3 (P < 0.05), and this difference was not statistically significant at days 6 and 9 (Supplemental Figure S4B). Notably, the BMPR1B was consistently down-regulated to a level much lower than the PBS control when BMP2 was applied with PPARγ inhibition, particularly at days 6 and 9 after treatment. This may be a partial explanation of the reduced mineralization in vitro observed with BMP2 + shPPARγ treatment.
Discussion
After its original Food and Drug Administration approval, BMP2 has been widely used in orthopedic clinics for augmenting lumbar spinal fusion and oral maxillary bone defects. The clinical efficacy of these applications has been favorable, but with some concerns regarding its adverse effects in certain cases and circumstances. In particular, BMP2's off-label use in cervical spinal fusion and long bone fractures has been found to induce structurally abnormal bone formation.15, 16, 18 These concerns must be addressed urgently with practical resolutions. Fortunately, BMP2 has been extensively studied as a bone growth factor in various experimental settings in vitro and in vivo. A high BMP2 concentration (150, 300, and 600 μg/mL) can fuse critical-sized bone defects in a rat femoral segmental defect model, but forms cyst-like bony shells filled with adipose tissue.19 Using a rat spinal fusion model, it was found that, although bone bridging was observed with BMP2 treatment, it was accompanied by inner radiolucency that suggests cyst-like bone formation.55 A large dose of BMP2 also induces cyst-like bone formations in a sheep spinal fusion model20 and mandible defect model.21 Additional studies have shown newly formed bone of poor quality and low mineral density with an interspersed lipid deposition after BMP2 implantation, leading to cyst-like bone formation.56, 57 BMP2-induced cyst-like bone formation was often reported to be attributed to the collagen sponge carrier.19 The advantages of collagen carrier included wound-healing properties, semipermeability, and early wound stabilization,58 but the disadvantages, such as poor mechanical properties, lead to its squeezable nature,59 fast-release kinetics, and retention of <5% of BMP2 after 14 days of implantation.60, 61 In this study, a hydroxyapatite-coated PLGA scaffold was used, which had better mechanical properties, slower release kinetics, and, most important, high stability and low toxicity for the delivery of RNA interference and BMP2 protein simultaneously.62 However, the high dose of BMP2 loaded into the PLGA scaffold may have exceeded the slow release capacity of the PLGA scaffold, thus eliciting an adverse effect similar to a squeezable collagen sponge—namely, a more rapid burst release of high BMP2 concentration to local target tissues. Interestingly, it was demonstrated that NEL-like molecule-1 combined with BMP2 optimizes osteogenesis in a rat femoral segmental defect model by minimizing the formation of BMP2-induced adipose-filled cyst-like bone formations.11 Thus, despite the robust pro-osteogenic effects that BMP2 induces on BMSCs, the proadipogenic effects appear to decrease the overall quality of the newly formed bone. BMSC fate is hypothesized to be regulated by an antagonistic balance between Runx2 and PPARγ.63
PPARγ is the master regulator of adipogenesis, and is both sufficient and necessary for adipogenesis; no other factor that can induce normal adipogenesis in the absence of PPARγ has been identified.64 Because of the reciprocal relationship between osteoblastic and adipogenic differentiation, reports indicate that PPARγ inhibits osteogenesis by stimulating adipogenesis, and reducing the activity of PPARγ increases osteogenesis by inhibiting adipogenesis. PPARγ promotes adipogenesis on activation, which suppresses osteogenesis by down-regulating Runx2 expression, inhibiting the DNA binding ability of Runx2, and transactivating the osteocalcin promoter.65, 66 PPARγ shRNA delivery (used for long-term PPARγ silencing) significantly reduced PPARγ expression and lipid droplet accumulation in mouse BMSCs, and increased pro-osteoblastogenic and antiosteoclastic effects after intramedullary injection into femoral bone marrow, which promoted an improved trabecular bone architecture.26 PPARγ inhibition may also lead to an increase in mineralization of the extracellular matrix, as well as an increased activity of alkaline phosphatase.67 Theoretically, it seemed that suppressing the expression of PPARγ might be an effective strategy in combating BMP2 adverse effects by shifting the BMSC fate toward an osteoblast lineage to generate better-quality bone. Herein, the expression of PPARγ was inhibited to explore a possible strategy for reducing the abnormally robust adipogenesis induced by large doses of BMP2.
Unexpectedly, the results did not seem to confirm this hypothesis. Knocking down PPARγ significantly decreased BMP2-induced adipogenesis, but more important, it led to grossly apparent bone nonunion when compared with BMP2 treatment alone. PPARγ knockdown attenuated BMP2-induced cell proliferation, migration, and invasion, and escalated cell apoptosis. BMP2 alone induces osteoblast apoptosis at a mature stage.68 Conversely, PPARγ alone can prevent apoptosis by regulating the antiapoptotic effects of the Bcl-2 family proteins; however, the protective effect may have been hindered when PPARγ was knocked down with shRNA.69 Bcl-2 was shown to be expressed solely in differentiated bone marrow mesenchymal cells,70 which may suggest that the apoptotic cells found in our study were at a differentiated stage. Future studies of cell lineage tracing during the bone-healing process might provide better understanding and elucidate the protective role of PPARγ knockdown versus the apoptotic effects of combinatorial treatment of PPARγ knockdown and BMP2. Furthermore, these results are different from those gathered when inhibiting PPARγ alone, which suggests that PPARγ is crucial in BMP2-mediated bone regeneration. Some researchers have reported that PPARγ suppression inhibits adipogenesis, but does not promote human mesenchymal stem cell osteogenesis.71 On the other hand, although PPARγ has been recognized as an inducer of adipogenesis, it also acts as an anti-inflammatory molecule. PPARγ delivered by chitosan gold nanoparticles onto titanium surfaces inhibited implant-induced inflammation and induced bone mineralization of MC-3T3E1 osteoblast-like cells, with enhanced expression of osteogenic molecules like BMP2/7, from up-regulating the antioxidant molecule heme oxygenase-1.72 Meanwhile, PPARγ has also been found to play an important role in angiogenesis.73, 74 These effects may all contribute to the bone-healing process. Herein, we focused on exploring possible changes in BMSC function because BMSCs are among BMP2's main target cells that contribute to osteogenesis.
BMPs activate target cells primarily through canonical BMP signaling, which initiates when BMPs associate with type 1 and type 2 BMP receptors. These receptors are transmembrane serine/threonine kinases, and combine to generate a multimeric receptor ligand complex. Absence of the BMPR2 results in the down-regulation of Runx2 expression, whereas the up-regulation of the BMPR2 leads to an increase in the expression of Runx2.75 Up-regulated Runx2 indicates a potential increase in osteogenesis. Consistent with our results, decreased expression of BMPR2 and BMPR1B in BMSCs may result from reducing PPARγ expression, which would, in turn, down-regulate the BMP2/SMAD signaling and impair bone regeneration. This observation suggests that PPARγ, which has been traditionally recognized as an adipogenic transcription factor, may also be essential in BMP2/SMAD signaling that mediates differences in BMSC osteoblastogenesis. Herein, although the focus was on the BMP2 canonical pathway, it is possible that the involvement and cross talk with the BMP2 noncanonical–Smad-independent signaling (ie, p38 mitogen-activated protein kinase)76 and/or Wnt pathways may assist in regulating BMSC differentiation during bone formation and regeneration. In addition, the exact mechanisms that knock down PPARγ could suppress BMP2-induced proliferation, migration, and invasion, and increase in BMP2-induced apoptosis remains to be further explored in conjunction with the reduction in BMPR2 and BMPR1B presence.
In conclusion, bone healing is a highly complex, temporally coordinated process that includes inflammation, cellular ingress and proliferation, angiogenesis, BMSC differentiation, and bone remodeling phases. Knocking down PPARγ may disrupt these phases at any point, and appears to attenuate BMP2-induced bone generation. The dosing and timing of the combinatorial use of BMP2 and PPARγ inhibition may also influence the delicate balance of osteogenesis and adipogenesis induced by BMP2 therapy used in clinical settings.
Acknowledgments
We thank the University of California, Los Angeles Translational Pathology Core Laboratory and California NanoSystems Institute for expertise; and Emma Copeland and Samantha Rustia for excellent technical assistance.
Footnotes
Supported by California Institute for Regenerative Medicine Early Translational II Research award TR2-01821 (C.S.); NIH/National Institute of Dental and Craniofacial Research grant R21 DE0177711 (C.S. and K.T.); NIH/NIAMS grants R01 AR061399-02 (C.S.), R01 AR061399-01A1 (C.S.), R01 AR066782-01 (K.T.), R01 AR068835-01A1 (C.S), and RO1 DE01607 (C.S.); University of California Discovery grant 07-10677 (C.S.); the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at the University of California, Los Angeles Innovation award (C.S.); and National Natural Science Foundation of China grant 51272286 (S.G.).
X.Z. and K.T. contributed equally as senior authors.
Disclosures: None declared.
Supplemental material for this article can be found at https://doi.org/10.1016/j.ajpath.2018.11.019.
Contributor Information
Shu Guo, Email: sguo@cmu.edu.cn.
Chia Soo, Email: bsoo@ucla.edu.
Supplemental Data
Immunohistochemistry staining for β-catenin (A) and quantification (B). No significant difference is found among the groups. Data are expressed as means ± SD. Scale bar = 0.1 mm (A).
Real-time PCR analysis of PPARγ expression in stable M2 cell lines and transient transfected primary bone marrow stromal cells. Single colonies of stable cell lines were isolated using 0.5 μg/mL puromycin. A: Stable M2 single colonies transfected with PPARγ shRNA. shPPARγ C4, with 90% knockdown of PPARγ, was used in the in vitro experiments. B: Stable M2 single colonies transfected with control shRNA. shControl A3, with similar level of PPARγ expression as the PBS group, was used in the in vitro experiments. All fold-changes are reported relative to the PBS group. Data are expressed as means ± SD. ∗∗P < 0.01, ∗∗∗P < 0.005 versus the PBS group.
Inhibiting PPARγ with bisphenol A diglycidyl ether (BADGE), an antagonist of PPARγ, leads to significantly decreased mineralization compared with BMP2 treatment alone. A: Alizarin Red staining of mineralization in M2 cells treated with PBS, 300 ng/mL BMP2, or BMP2 + 50 μmol/L BADGE for 21 days. Insets: Representative Alizarin Red–staining images of a whole well at lower magnification. B: Quantification of Alizarin Red staining intensity. All fold-changes are reported relative to the PBS group. Data are expressed as means ± SD. ∗∗P < 0.01 versus the PBS group; ††P < 0.01 versus the BMP2 + shControl group. Scale bar = 0.2 mm (A).
Real-time PCR analysis of endogenous BMP2, BMPR1A, and BMPR1B mRNA expression changes. Endogenous BMP2 (A), BMPR1A (B), and BMPR1B (C) mRNA expression in M2 cells treated with PBS, 300 ng/mL BMP2, BMP2 + shPPARγ (using a stable transfected M2 cell line that had a 90% knockdown of PPARγ, which was selected from PPARγ shRNA transfected colonies), or BMP2 + shControl (using a stable M2 cell line selected from control shRNA transfected colonies) for 3, 6, and 9 days. All fold-changes are reported relative to the PBS group. Data are expressed as means ± SD. ∗P < 0.05, ∗∗P < 0.01 versus the PBS group; †P < 0.05, ††P < 0.01 versus the BMP2 + shControl group.
References
- 1.Giannoudis P.V., Dinopoulos H., Tsiridis E. Bone substitutes: an update. Injury. 2005;36 Suppl 3:S20–S27. doi: 10.1016/j.injury.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 2.Yelin E.H., Trupin L.S., Sebesta D.S. Transitions in employment, morbidity, and disability among persons ages 51-61 with musculoskeletal and non-musculoskeletal conditions in the US, 1992-1994. Arthritis Rheum. 1999;42:769–779. doi: 10.1002/1529-0131(199904)42:4<769::AID-ANR22>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 3.Alt V., Borgman B., Eicher A., Heiss C., Kanakaris N.K., Giannoudis P.V., Song F. Effects of recombinant human bone morphogenetic protein-2 (rhBMP-2) in grade III open tibia fractures treated with unreamed nails: a clinical and health-economic analysis. Injury. 2015;46:2267–2272. doi: 10.1016/j.injury.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Garrison K.R., Donell S., Ryder J., Shemilt I., Mugford M., Harvey I., Song F. Clinical effectiveness and cost-effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: a systematic review. Health Technol Assess. 2007;11:1–150. doi: 10.3310/hta11300. iii-iv. [DOI] [PubMed] [Google Scholar]
- 5.Mendoza M.C., Sonn K.A., Kannan A.S., Bellary S.S., Mitchell S.M., Singh G., Park C., Yun C., Stock S.R., Hsu E.L., Hsu W.K. The effect of vancomycin powder on bone healing in a rat spinal rhBMP-2 model. J Neurosurg Spine. 2016;25:147–153. doi: 10.3171/2015.11.SPINE15536. [DOI] [PubMed] [Google Scholar]
- 6.Starman J.S., Bosse M.J., Cates C.A., Norton H.J. Recombinant human bone morphogenetic protein-2 use in the off-label treatment of nonunions and acute fractures: a retrospective review. J Trauma Acute Care Surg. 2012;72:676–681. doi: 10.1097/TA.0b013e318232cf5a. [DOI] [PubMed] [Google Scholar]
- 7.Tinsley B.A., Dukas A., Pensak M.J., Adams D.J., Tang A.H., Ominsky M.S., Ke H.Z., Lieberman J.R. Systemic administration of sclerostin antibody enhances bone morphogenetic protein-induced femoral defect repair in a rat model. J Bone Joint Surg Am. 2015;97:1852–1859. doi: 10.2106/JBJS.O.00171. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Food and Drug Administration . 2007. InFUSE Bone Graft/LT- CAGE Lumbar Tapered Fusion Device: Summary of Safety and Effectiveness Data.https://www.accessdata.fda.gov/cdrh_docs/pdf/P000058b.pdf.https://www.accessdata.fda.gov/cdrh_docs/pdf/P000058b.pdf Available at. (accessed February 3, 2019) [Google Scholar]
- 9.Walker D.H., Wright N.M. Bone morphogenetic proteins and spinal fusion. Neurosurg Focus. 2002;13:e3. doi: 10.3171/foc.2002.13.6.4. [DOI] [PubMed] [Google Scholar]
- 10.Rengachary S.S. Bone morphogenetic proteins: basic concepts. Neurosurg Focus. 2002;13:e2. doi: 10.3171/foc.2002.13.6.3. [DOI] [PubMed] [Google Scholar]
- 11.Shen J., James A.W., Zhang X., Pang S., Zara J.N., Asatrian G., Chiang M., Lee M., Khadarian K., Nguyen A., Lee K.S., Siu R.K., Tetradis S., Ting K., Soo C. Novel Wnt regulator NEL-like molecule-1 antagonizes adipogenesis and augments osteogenesis induced by bone morphogenetic protein 2. Am J Pathol. 2016;186:419–434. doi: 10.1016/j.ajpath.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boden S.D., Kang J., Sandhu H., Heller J.G. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine. 2002;27:2662–2673. doi: 10.1097/00007632-200212010-00005. [DOI] [PubMed] [Google Scholar]
- 13.Choi J.W., Jeong W.S., Yang S.J., Park E.J., Oh T.S., Koh K.S. Appropriate and effective dosage of BMP-2 for the ideal regeneration of calvarial bone defects in beagles. Plast Reconstr Surg. 2016;138:64e–72e. doi: 10.1097/PRS.0000000000002290. [DOI] [PubMed] [Google Scholar]
- 14.McKay B., Sandhu H.S. Use of recombinant human bone morphogenetic protein-2 in spinal fusion applications. Spine. 2002;27:S66–S85. doi: 10.1097/00007632-200208151-00014. [DOI] [PubMed] [Google Scholar]
- 15.Vaidya R., Weir R., Sethi A., Meisterling S., Hakeos W., Wybo C.D. Interbody fusion with allograft and rhBMP-2 leads to consistent fusion but early subsidence. J Bone Joint Surg Br. 2007;89:342–345. doi: 10.1302/0301-620X.89B3.18270. [DOI] [PubMed] [Google Scholar]
- 16.Shields L.B.E., Raque G.H., Glassman S.D., Campbell M., Vitaz T., Harpring J., Shields C.B. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine. 2006;31:542–547. doi: 10.1097/01.brs.0000201424.27509.72. [DOI] [PubMed] [Google Scholar]
- 17.Irie K., Alpaslan C., Takahashi K., Kondo Y., Izumi N., Sakakura Y., Tsuruga E., Nakajima T., Ejiri S., Ozawa H., Yajima T. Osteoclast differentiation in ectopic bone formation induced by recombinant human bone morphogenetic protein 2 (rhBMP-2) J Bone Miner Metab. 2003;21:363–369. doi: 10.1007/s00774-003-0430-x. [DOI] [PubMed] [Google Scholar]
- 18.Boraiah S., Paul O., Hawkes D., Wickham M., Lorich D.G. Complications of recombinant human BMP-2 for treating complex tibial plateau fractures: a preliminary report. Clin Orthop Relat Res. 2009;467:3257–3262. doi: 10.1007/s11999-009-1039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zara J.N., Siu R.K., Zhang X., Shen J., Ngo R., Lee M., Li W., Chiang M., Chung J., Kwak J., Wu B.M., Ting K., Soo C. High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue Eng Part A. 2011;17:1389–1399. doi: 10.1089/ten.tea.2010.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan H.C., Lee S., Ting K., Shen J., Wang C.C., Nguyen A., Berthiaume E.A., Zara J.N., Turner A.S., Seim H.B., Kwak J.H., Zhang X.L., Soo C. Cyst-like osteolytic formations in recombinant human bone morphogenetic protein-2 (rhBMP-2) augmented sheep spinal fusion. Am J Pathol. 2017;187:1485–1495. doi: 10.1016/j.ajpath.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan J., Pi-Anfruns J., Guo M., Im D.C.S., Cui Z.K., Kim S., Wu B.M., Aghaloo T.L., Lee M. Small molecule-mediated tribbles homolog 3 promotes bone formation induced by bone morphogenetic protein-2. Sci Rep. 2017;7:7518. doi: 10.1038/s41598-017-07932-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wahli W., Braissant O., Desvergne B. Peroxisome proliferator activated receptors: transcriptional regulators of adipogenesis, lipid metabolism and more. Chem Biol. 1995;2:261–266. doi: 10.1016/1074-5521(95)90045-4. [DOI] [PubMed] [Google Scholar]
- 23.Moraes L.A., Piqueras L., Bishop-Bailey D. Peroxisome proliferator-activated receptors and inflammation. Pharmacol Ther. 2006;110:371–385. doi: 10.1016/j.pharmthera.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Wahli W. PPAR gamma: ally and foe in bone metabolism. Cell Metab. 2008;7:188–190. doi: 10.1016/j.cmet.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Ge C., Cawthorn W.P., Li Y., Zhao G., Macdougald O.A., Franceschi R.T. Reciprocal control of osteogenic and adipogenic differentiation by ERK/MAP kinase phosphorylation of Runx2 and PPARgamma transcription factors. J Cell Physiol. 2016;231:587–596. doi: 10.1002/jcp.25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.James A.W., Shen J., Khadarian K., Pang S., Chung G., Goyal R., Asatrian G., Velasco O., Kim J., Zhang X., Ting K., Soo C. Lentiviral delivery of PPARgamma shRNA alters the balance of osteogenesis and adipogenesis, improving bone microarchitecture. Tissue Eng Part A. 2014;20:2699–2710. doi: 10.1089/ten.tea.2013.0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee M.J., Chen H.T., Ho M.L., Chen C.H., Chuang S.C., Huang S.C., Fu Y.C., Wang G.J., Kang L., Chang J.K. PPARgamma silencing enhances osteogenic differentiation of human adipose-derived mesenchymal stem cells. J Cell Mol Med. 2013;17:1188–1193. doi: 10.1111/jcmm.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akune T., Ohba S., Kamekura S., Yamaguchi M., Chung U.I., Kubota N., Terauchi Y., Harada Y., Azuma Y., Nakamura K., Kadowaki T., Kawaguchi H. PPAR gamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113:846–855. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takada I., Suzawa M., Matsumoto K., Kato S. Suppression of PPAR transactivation switches cell fate of bone marrow stem cells from adipocytes into osteoblasts. Ann NY Acad Sci. 2007;1116:182–195. doi: 10.1196/annals.1402.034. [DOI] [PubMed] [Google Scholar]
- 30.Kato S., Kawabata N., Suzuki N., Ohmura M., Takagi M. Bone morphogenetic protein-2 induces the differentiation of a mesenchymal progenitor cell line, ROB-C26, into mature osteoblasts and adipocytes. Life Sci. 2009;84:302–310. doi: 10.1016/j.lfs.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Xu Y., Hammerick K.E., James A.W., Carre A.L., Leucht P., Giaccia A.J., Longaker M.T. Inhibition of histone deacetylase activity in reduced oxygen environment enhances the osteogenesis of mouse adipose-derived stromal cells. Tissue Eng Part A. 2009;15:3697–3707. doi: 10.1089/ten.tea.2009.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chou Y.F., Huang W.B., Dunn J.C.Y., Miller T.A., Wu B.M. The effect of biomimetic apatite structure on osteoblast viability, proliferation, and gene expression. Biomaterials. 2005;26:285–295. doi: 10.1016/j.biomaterials.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 33.Li W., Zara J.N., Siu R.K., Lee M., Aghaloo T., Zhang X., Wu B.M., Gertzman A.A., Ting K., Soo C. Nell-1 enhances bone regeneration in a rat critical-sized femoral segmental defect model. Plast Reconstr Surg. 2011;127:580–587. doi: 10.1097/PRS.0b013e3181fed5ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu H.M., Jerchow B., Sheu T.J., Liu B., Costantini F., Puzas J.E., Birchmeier W., Hsu W. The role of Axin2 in calvarial morphogenesis and craniosynostosis. Development. 2005;132:1995–2005. doi: 10.1242/dev.01786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGee-Lawrence M.E., Carpio L.R., Bradley E.W., Dudakovic A., Lian J.B., van Wijnen A.J., Kakar S., Hsu W., Westendorf J.J. Runx2 is required for early stages of endochondral bone formation but delays final stages of bone repair in Axin2-deficient mice. Bone. 2014;66:277–286. doi: 10.1016/j.bone.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DasGupta R., Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- 37.McGee-Lawrence M.E., Li X.D., Bledsoe K.L., Wu H., Hawse J.R., Subramaniam M., Razidlo D.F., Stensgard B.A., Stein G.S., van Wijnen A.J., Lian J.B., Hsu W., Westendorf J.J. Runx2 protein represses Axin2 expression in osteoblasts and is required for craniosynostosis in Axin2-deficient mice. J Biol Chem. 2013;288:5291–5302. doi: 10.1074/jbc.M112.414995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bouxsein M.L., Boyd S.K., Christiansen B.A., Guldberg R.E., Jepsen K.J., Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 39.Parfitt A.M., Drezner M.K., Glorieux F.H., Kanis J.A., Malluche H., Meunier P.J., Ott S.M., Recker R.R. Bone histomorphometry: standardization of nomenclature, symbols, and units: report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 40.Sutherland H.J., Eaves C.J., Lansdorp P.M., Thacker J.D., Hogge D.E. Differential regulation of primitive human hematopoietic cells in long-term cultures maintained on genetically engineered murine stromal cells. Blood. 1991;78:666–672. [PubMed] [Google Scholar]
- 41.Kha H.T., Basseri B., Shouhed D., Richardson J., Tetradis S., Hahn T.J., Parhami F. Oxysterols regulate differentiation of mesenchymal stem cells: pro-bone and anti-fat. J Bone Miner Res. 2004;19:830–840. doi: 10.1359/JBMR.040115. [DOI] [PubMed] [Google Scholar]
- 42.Marion N.W., Mao J.J. Mesenchymal stem cells and tissue engineering. Methods Enzymol. 2006;420:339–361. doi: 10.1016/S0076-6879(06)20016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nadri S., Soleimani M., Hosseni R.H., Massumi M., Atashi A., Izadpanah R. An efficient method for isolation of murine bone marrow mesenchymal stem cells. Int J Dev Biol. 2007;51:723–729. doi: 10.1387/ijdb.072352ns. [DOI] [PubMed] [Google Scholar]
- 44.Sangani R., Pandya C.D., Bhattacharyya M.H., Periyasamy-Thandavan S., Chutkan N., Markand S., Hill W.D., Hamrick M., Isales C., Fulzele S. Knockdown of SVCT2 impairs in-vitro cell attachment, migration and wound healing in bone marrow stromal cells. Stem Cell Res. 2014;12:354–363. doi: 10.1016/j.scr.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Chen W., Zhang X., Siu R.K., Chen F., Shen J., Zara J.N., Culiat C.T., Tetradis S., Ting K., Soo C. Nfatc2 is a primary response gene of Nell-1 regulating chondrogenesis in ATDC5 cells. J Bone Miner Res. 2011;26:1230–1241. doi: 10.1002/jbmr.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calvier L., Chouvarine P., Legchenko E., Hoffmann N., Geldner J., Borchert P., Jonigk D., Mozes M.M., Hansmann G. PPAR gamma links BMP2 and TGF beta 1 pathways in vascular smooth muscle cells, regulating cell proliferation and glucose metabolism. Cell Metab. 2017;25:1118–1134. doi: 10.1016/j.cmet.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 47.Zheng Z., Lee K.S., Zhang X., Nguyen C., Hsu C., Wang J.Z., Rackohn T.M., Enjamuri D.R., Murphy M., Ting K., Soo C. Fibromodulin-deficiency alters temporospatial expression patterns of transforming growth factor-beta ligands and receptors during adult mouse skin wound healing. PLoS One. 2014;9:e90817. doi: 10.1371/journal.pone.0090817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang B., Luo Q., Chen Z., Sun J., Xu B., Ju Y., Song G. Cyclic mechanical stretching promotes migration but inhibits invasion of rat bone marrow stromal cells. Stem Cell Res. 2015;14:155–164. doi: 10.1016/j.scr.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 49.Zheng Z., Jian J., Velasco O., Hsu C.Y., Zhang K., Levin A., Murphy M., Zhang X., Ting K., Soo C. Fibromodulin enhances angiogenesis during cutaneous wound healing. Plast Reconstr Surg Glob Open. 2014;2:e275. doi: 10.1097/GOX.0000000000000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cowan C.M., Jiang X., Hsu T., Soo C., Zhang B., Wang J.Z., Kuroda S., Wu B., Zhang Z., Zhang X., Ting K. Synergistic effects of Nell-1 and BMP-2 on the osteogenic differentiation of myoblasts. J Bone Miner Res. 2007;22:918–930. doi: 10.1359/jbmr.070312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zilber S., Epstein N., Lee S.W., Larsen M., Ma T., Smith R.L., Biswal S., Goodman S.B. Mouse femoral intramedullary injection model: technique and microCT scan validation. J Biomed Mater Res B. 2008;84b:286–290. doi: 10.1002/jbm.b.30872. [DOI] [PubMed] [Google Scholar]
- 52.Myers T.J., Longobardi L., Willcockson H., Temple J.D., Tagliafierro L., Ye P., Li T.S., Esposito A., Moats-Staats B.M., Spagnoli A. BMP2 regulation of CXCL12 cellular, temporal, and spatial expression is essential during fracture repair. J Bone Miner Res. 2015;30:2014–2027. doi: 10.1002/jbmr.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Herberg S., Susin C., Pelaez M., Howie R.N., Moreno de Freitas R., Lee J., Cray J.J., Jr., Johnson M.H., Elsalanty M.E., Hamrick M.W., Isales C.M., Wikesjo U.M., Hill W.D. Low-dose bone morphogenetic protein-2/stromal cell-derived factor-1beta cotherapy induces bone regeneration in critical-size rat calvarial defects. Tissue Eng Part A. 2014;20:1444–1453. doi: 10.1089/ten.tea.2013.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Felthaus O., Gosau M., Ettl T., Prantl L., Morsczeck C. Migration of human dental follicle cells in vitro. J Periodontal Res. 2014;49:205–212. doi: 10.1111/jre.12096. [DOI] [PubMed] [Google Scholar]
- 55.Yuan W., James A.W., Asatrian G., Shen J., Zara J.N., Tian H.J., Siu R.K., Zhang X.L., Wang J.C., Dong J. NELL-1 based demineralized bone graft promotes rat spine fusion as compared to commercially available BMP-2 product. J Orthop Sci. 2013;18:646–657. doi: 10.1007/s00776-013-0390-5. [DOI] [PubMed] [Google Scholar]
- 56.James A.W., Zara J.N., Zhang X.L., Askarinam A., Goyal R., Chiang M., Yuan W., Chang L., Corselli M., Shen J., Pang S., Stoker D., Wu B., Ting K., Peault B., Soo C. Perivascular stem cells: a prospectively purified mesenchymal stem cell population for bone tissue engineering. Stem Cell Transl Med. 2012;1:510–519. doi: 10.5966/sctm.2012-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aghaloo T., Jiang X., Soo C., Zhang Z., Zhang X., Hu J., Pan H., Hsu T., Wu B., Ting K., Zhang X. A study of the role of nell-1 gene modified goat bone marrow stromal cells in promoting new bone formation. Mol Ther. 2007;15:1872–1880. doi: 10.1038/sj.mt.6300270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rho K.S., Jeong L., Lee G., Seo B.M., Park Y.J., Hong S.D., Roh S., Cho J.J., Park W.H., Min B.M. Electrospinning of collagen nanofibers: effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials. 2006;27:1452–1461. doi: 10.1016/j.biomaterials.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 59.Yarlagadda P.K., Chandrasekharan M., Shyan J.Y. Recent advances and current developments in tissue scaffolding. Biomed Mater Eng. 2005;15:159–177. [PubMed] [Google Scholar]
- 60.Uludag H., D'Augusta D., Palmer R., Timony G., Wozney J. Characterization of rhBMP-2 pharmacokinetics implanted with biomaterial carriers in the rat ectopic model. J Biomed Mater Res. 1999;46:193–202. doi: 10.1002/(sici)1097-4636(199908)46:2<193::aid-jbm8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 61.Haidar Z.S., Hamdy R.C., Tabrizian M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair, part A: current challenges in BMP delivery. Biotechnol Lett. 2009;31:1817–1824. doi: 10.1007/s10529-009-0099-x. [DOI] [PubMed] [Google Scholar]
- 62.Singha K., Namgung R., Kim W.J. Polymers in small-interfering RNA delivery. Nucleic Acid Ther. 2011;21:133–147. doi: 10.1089/nat.2011.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang X., Guo J., Zhou Y.S., Wu G. The roles of bone morphogenetic proteins and their signaling in the osteogenesis of adipose-derived stem cells. Tissue Eng Part B Rev. 2014;20:84–92. doi: 10.1089/ten.teb.2013.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kawai M., Rosen C.J. PPARgamma: a circadian transcription factor in adipogenesis and osteogenesis. Nat Rev Endocrinol. 2010;6:629–636. doi: 10.1038/nrendo.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pan G., Cao J., Yang N., Ding K., Fan C., Xiong W.C., Hamrick M., Isales C.M., Shi X.M. Role of glucocorticoid-induced leucine zipper (GILZ) in bone acquisition. J Biol Chem. 2014;289:19373–19382. doi: 10.1074/jbc.M113.535237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jeon M.J., Kim J.A., Kwon S.H., Kim S.W., Park K.S., Park S.W., Kim S.Y., Shin C.S. Activation of peroxisome proliferator-activated receptor-gamma inhibits the Runx2-mediated transcription of osteocalcin in osteoblasts. J Biol Chem. 2003;278:23270–23277. doi: 10.1074/jbc.M211610200. [DOI] [PubMed] [Google Scholar]
- 67.Graneli C., Karlsson C., Brisby H., Lindahl A., Thomsen P. The effects of PPAR-gamma inhibition on gene expression and the progression of induced osteogenic differentiation of human mesenchymal stem cells. Connect Tissue Res. 2014;55:262–274. doi: 10.3109/03008207.2014.910198. [DOI] [PubMed] [Google Scholar]
- 68.Hyzy S.L., Olivares-Navarrete R., Schwartz Z., Boyan B.D. BMP2 induces osteoblast apoptosis in a maturation state and noggin-dependent manner. J Cell Biochem. 2012;113:3236–3245. doi: 10.1002/jcb.24201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ren Y., Sun C., Sun Y., Tan H., Wu Y., Cui B., Wu Z. PPAR gamma protects cardiomyocytes against oxidative stress and apoptosis via Bcl-2 upregulation. Vascul Pharmacol. 2009;51:169–174. doi: 10.1016/j.vph.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 70.Oliver L., Hue E., Rossignol J., Bougras G., Hulin P., Naveilhan P., Heymann D., Lescaudron L., Vallette F.M. Distinct roles of Bcl-2 and Bcl-Xl in the apoptosis of human bone marrow mesenchymal stem cells during differentiation. PLoS One. 2011;6:e19820. doi: 10.1371/journal.pone.0019820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu W.H., Li F.G., Chen X.Y., Li J.T., Wu Y.H., Huang L.H., Wang Z., Li P., Wang T., Lahn B.T., Xiang A.P. PPARgamma suppression inhibits adipogenesis but does not promote osteogenesis of human mesenchymal stem cells. Int J Biochem Cell Biol. 2012;44:377–384. doi: 10.1016/j.biocel.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 72.Bhattarai G., Lee Y.H., Lee N.H., Park I.S., Lee M.H., Yi H.K. PPARgamma delivered by Ch-GNPs onto titanium surfaces inhibits implant-induced inflammation and induces bone mineralization of MC-3T3E1 osteoblast-like cells. Clin Oral Implants Res. 2013;24:1101–1109. doi: 10.1111/j.1600-0501.2012.02517.x. [DOI] [PubMed] [Google Scholar]
- 73.Kotlinowski J., Jozkowicz A. PPAR gamma and angiogenesis: endothelial cells perspective. J Diabetes Res. 2016;2016:8492353. doi: 10.1155/2016/8492353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hansmann G., de Jesus Perez V.A., Alastalo T.P., Alvira C.M., Guignabert C., Bekker J.M., Schellong S., Urashima T., Wang L., Morrell N.W., Rabinovitch M. An antiproliferative BMP-2/PPARgamma/apoE axis in human and murine SMCs and its role in pulmonary hypertension. J Clin Invest. 2008;118:1846–1857. doi: 10.1172/JCI32503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang K., Tang X.D., Guo W., Xu X.L., Ren T.T., Ren C.M., Wang S.D., Bao X., Zhang F., Sun K.K. BMPR2-pSMAD1/5 signaling pathway regulates RUNX2 expression and impacts the progression of dedifferentiated chondrosarcoma. Am J Cancer Res. 2016;6:1302–1316. [PMC free article] [PubMed] [Google Scholar]
- 76.Wu M., Chen G., Li Y.P. TGF-beta and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016;4:16009. doi: 10.1038/boneres.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Immunohistochemistry staining for β-catenin (A) and quantification (B). No significant difference is found among the groups. Data are expressed as means ± SD. Scale bar = 0.1 mm (A).
Real-time PCR analysis of PPARγ expression in stable M2 cell lines and transient transfected primary bone marrow stromal cells. Single colonies of stable cell lines were isolated using 0.5 μg/mL puromycin. A: Stable M2 single colonies transfected with PPARγ shRNA. shPPARγ C4, with 90% knockdown of PPARγ, was used in the in vitro experiments. B: Stable M2 single colonies transfected with control shRNA. shControl A3, with similar level of PPARγ expression as the PBS group, was used in the in vitro experiments. All fold-changes are reported relative to the PBS group. Data are expressed as means ± SD. ∗∗P < 0.01, ∗∗∗P < 0.005 versus the PBS group.
Inhibiting PPARγ with bisphenol A diglycidyl ether (BADGE), an antagonist of PPARγ, leads to significantly decreased mineralization compared with BMP2 treatment alone. A: Alizarin Red staining of mineralization in M2 cells treated with PBS, 300 ng/mL BMP2, or BMP2 + 50 μmol/L BADGE for 21 days. Insets: Representative Alizarin Red–staining images of a whole well at lower magnification. B: Quantification of Alizarin Red staining intensity. All fold-changes are reported relative to the PBS group. Data are expressed as means ± SD. ∗∗P < 0.01 versus the PBS group; ††P < 0.01 versus the BMP2 + shControl group. Scale bar = 0.2 mm (A).
Real-time PCR analysis of endogenous BMP2, BMPR1A, and BMPR1B mRNA expression changes. Endogenous BMP2 (A), BMPR1A (B), and BMPR1B (C) mRNA expression in M2 cells treated with PBS, 300 ng/mL BMP2, BMP2 + shPPARγ (using a stable transfected M2 cell line that had a 90% knockdown of PPARγ, which was selected from PPARγ shRNA transfected colonies), or BMP2 + shControl (using a stable M2 cell line selected from control shRNA transfected colonies) for 3, 6, and 9 days. All fold-changes are reported relative to the PBS group. Data are expressed as means ± SD. ∗P < 0.05, ∗∗P < 0.01 versus the PBS group; †P < 0.05, ††P < 0.01 versus the BMP2 + shControl group.