Abstract
As the use of nanoparticles (NPs) is increasing, the potential toxicity and behavior of NPs in living systems need to be better understood. Our goal was to evaluate the developmental toxicity and bio-distribution of two different sizes of fluorescently-labeled SiO2 NPs, 25 and 115 nm, with neutral surface charge or with different surface functionalization, rendering them positively or negatively charged, in order to predict the effect of NPs in humans. We performed a zebrafish embryo toxicity test (ZFET) by exposing the embryos to SiO2 NPs starting from six hours post fertilization (hpf). Survival rate, hatching time, and gross morphological changes were assessed at 12, 24, 36, 48, 60, and 72 hpf. We evaluated the effect of NPs on angiogenesis by counting the number of sub-intestinal vessels between the second and seventh intersegmental vessels and gene expression analysis of vascular endothelial growth factor (VEGF) and VEGF receptors at 72 hpf. SiO2 NPs did not show any adverse effects on survival rate, hatching time, gross morphology, or physiological angiogenesis. We found that SiO2 NPs were trapped by the chorion up until to the hatching stage. After chemical removal of the chorion (dechorionation), positively surface-charged SiO2 NPs (25 nm) significantly reduced the survival rate of the fish compared to the control group. These results indicate that zebrafish chorion acts as a physical barrier against SiO2 NPs, and removing the chorions in ZFET might be necessary for evaluation of toxicity of NPs.
Keywords: silica nanoparticles, surface functionalization, zebrafish, embryo acute toxicity test, vascularization, bio-distribution, dechorionation
1. Introduction
Nanotechnologies are emerging technologies that have been gaining popularity in the last few decades and are still being developed. These technologies foresee nanoparticles (NPs) for many applications in the fields of medicine, microelectronics, catalysis, cosmetics, drug delivery, and imaging [1,2]. However, human exposure to nanomaterials is a concern since there might be a negative impact on health, not only in occupational settings where NPs are produced and used, but also in the general population and environment considering the life cycle of NPs from manufacturing to recycling and final disposal [3,4]. Due to their small size, which is in the size range of various cellular structures, NPs are capable of interacting with these structures, entering the cells, and/or translocating from the site of exposure toward the circulation and secondary organs [5,6]. NPs can impair cellular functions and be cytotoxic [7,8]. For that reason, it is of crucial importance to understand the key aspects of interactions of NPs with living systems and to properly compare benefits and potential hazard coming from use of nanomaterials.
Silicon dioxide (SiO2) NPs have numerous applications, such as additives to drugs, cosmetics, printer toners, varnishes, and food, due to their anti-agglomerations properties [9]. Their characteristics have led to the applications in cancer therapy, DNA delivery, and drug delivery [10,11,12]. The wide use of SiO2 NPs and their presence in general and in the occupational environment prioritize the study on their health and environmental effects and the analysis of their potential toxicity and underlying mechanisms, in order to offer essential information on in vivo behavior of SiO2 NPs as well as induced toxic responses.
Rodents are widely used to test the potential developmental toxicity of chemical substances, including NPs. The procedures for animal testing and research are highly regulated and the necessity of numerous animals is ethically discussed. Although reliable data for extrapolating toxicant effects to humans are obtained through rodent studies, these experiments are expensive, time consuming, and more restricted by ethics and law. Since genes, receptors, and molecular processes are highly conserved across animal phyla, zebrafish (Danio rerio) has been employed as an experimental tool in toxicology for testing chemicals and NPs as an alternative to rodents [13]. In addition, because of their transparent body wall at their younger age, it is easy to visualize and evaluate internal organ toxicities by using transgenic zebrafish with tissue-specific enhanced green fluorescent protein (EGFP) expression. Using vascular-EGFP strains, Tg (fli1:egfp)/nacre zebrafish, we previously investigated the effects of metal oxide NPs on angiogenesis [14].
In the present study, we investigated the effects of fluorescently-labeled SiO2 NPs of two different sizes: 25 and 115 nm, with a neutrally charged, pristine surface or with different surface functionalization, rendering them positively or negatively charged using the zebrafish embryo toxicity test method (ZFET). We also evaluated the effects of surface charge and size of SiO2 NPs on bio-distribution and vascularization in the embryos of vascular-EGFP zebrafish using fluorescent-labelled NPs.
2. Results and Discussion
2.1. Characterization of NPs in Suspension
In the present study, the in vivo effects of SiO2 NPs with three different surface charges were studied. We used rhodamine-labeled SiO2NPs 25 and 115 nm in diameter with hydroxyl function on the surface (pristine, neutral surface charge; N) or functionalized with amino (positive surface charge; +q) or carboxyl groups (negative surface charge; −q). The intensity-weighted hydrodynamic average diameters of dispersed NPs were determined by the dynamic light scattering (DLS) technology. Table 1 shows the mean hydrodynamic diameters and polydispersity index (PdI) of SiO2 NPs in embryo culture medium. The hydrodynamic average diameters of amino-, carboxyl-, and hydroxyl-modified 25 nm SiO2 NPs were 171.3 ± 8.58, 166.8 ± 2.05, and 132.2 ± 6.99 nm, respectively (Table 1), indicating that NPs were aggregated or agglomerated in the medium. Although DLS data provided the mean hydrodynamic diameters of >100 nm, the presence of nano-sized particles was confirmed in the medium (Table 1). The average hydrodynamic diameters of 115-nm SiO2 NPs were 228.9 ± 5.06, 152.7 ± 1.19, and 127.2 ± 1.10 nm for amino-, carboxyl-, and hydroxyl-modified, NPs respectively (Table 1). Hydrodynamic sizes of all NPs dispersed in the embryo medium did not change after 24 h.
Table 1.
Characterization of nanoparticles (NPs).
| NPs | Hydrodynamic Size (nm) | PdI | Intensity of Particles of less than 100 nm (%) | Volume of Particles of less than 100 nm (%) |
|---|---|---|---|---|
| 25 nm +q | 171.3 ± 8.58 | 0.394 ± 0.076 | 18.7 ± 1.9 | 33.9 ± 4.4 |
| 25 nm −q | 166.8 ± 2.05 | 0.113 ± 0.013 | 4.63 ± 0.75 | 10.5 ± 1.6 |
| 25 nm N | 132.2 ± 6.99 | 0.294 ± 0.060 | 28.5 ± 0.26 | 45.4 ± 4.8 |
| 115 nm +q | 228.9 ± 5.06 | 0.326 ± 0.021 | – | – |
| 115 nm −q | 152.7 ± 1.19 | 0.140 ± 0.006 | – | – |
| 115 nm N | 127.2 ± 1.10 | 0.081 ± 0.015 | – | – |
Values are mean ± SD (standard deviation) of four independent experiments. PdI: polydispersity index.
SiO2 NPs were aggregated or agglomerated in the medium (Table 1). The aggregation state of the NPs influenced the final toxicological outcome. Note that we performed the series of experiments using NPs dispersed in Danieau’s solution supplemented with albumin (0.012 mg/mL) in order to obtain a better dispersion of NPs. However, this experimental set-up (presence of albumin in Danieau’s solution) was toxic for the embryos on its own without any presence of NPs. Moreover, the dispersibility of the NPs was not much improved. Therefore, we decided to continue the treatment of the embryos using the media that are the most suitable for their normal growth and development.
2.2. Effects of SiO2 NPs on the Development of Zebrafish Embryo
The zebrafish embryo toxicity test (ZFET) has emerged as an alternative in vivo approach to assessing developmental toxicity [15], with some variations including use of tissue-specific EGFP fish lines alone [16] or in combination with behavior assays [17,18]. There are several variations in ZFET protocols for assessment of targeted toxicity though OECD-defined guidelines for ZFET in 2013 [19].
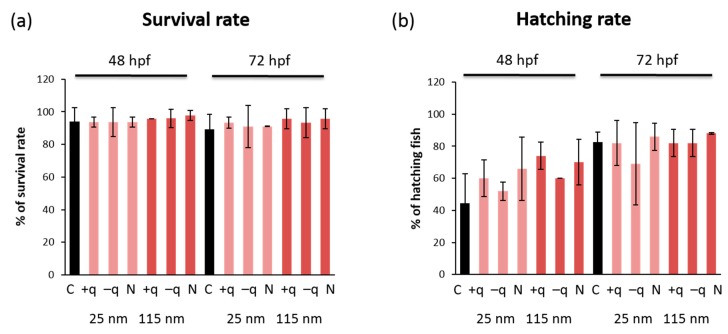
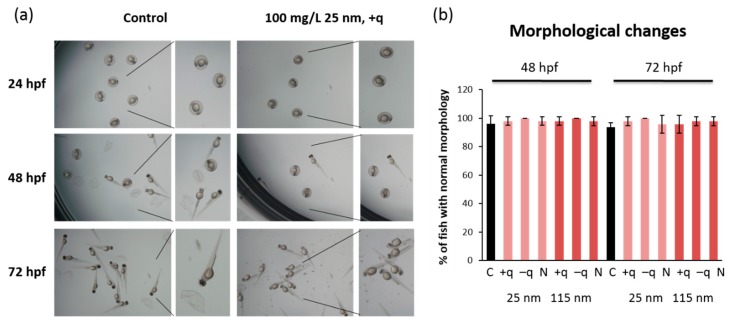
To evaluate the developmental toxicity of SiO2 NPs, the zebrafish embryos were exposed to SiO2 NPs with different surface charges of two different sizes, 25 and 115 nm, starting from 6 hpf. Survival rate, hatching rate, and gross morphological changes were examined at each time point of 12, 24, 36, 48, 60, and 72 hpf after exposure to SiO2 NPs at 3.125, 6.25, 12.5, 25, 50, and 100 mg/L (Figures S1 and S2). The survival rate (Figure 1a) and hatching rate (Figure 1b) at 48 and 72 hpf were not affected by exposure to all types of SiO2 NPs at the highest concentration (100 mg/L). Correspondingly, there were no obvious morphological changes at 72 hpf in zebrafish exposed to all types of SiO2 NPs at the highest concentration (100 mg/L) (Figure 2a,b). These results are partially in accordance with the previous study that showed no toxicity in the early life stage of zebrafish exposed to core-shell silica NPs [20]. Although Li et al. [21] previously reported that exposure to smaller SiO2 NPs, whose diameter was less than 50 nm, at 300–1000 mg/L caused Parkinson’s-like behavior in adult zebrafish. No locomotor disturbance was observed at 72 hpf in zebrafish exposed to 100 mg/L for all types of SiO2 NPs in the present study.
Figure 1.
The effect of exposure to SiO2 NPs on the development of zebrafish embryos. (a) Survival rate and (b) hatching rate after exposure to 100 mg/L of 25- or 115-nm SiO2 NPs with different surface charges at 48 and 72 hpf. Data are represented as mean ± SD (standard deviation).
Figure 2.
The effects of SiO2 NPs on gross morphological features of zebrafish embryos. (a) Representative images of embryos exposed to positively charged SiO2 NPs (25 nm) at 100 mg/L and (b) gross morphological changes after the exposure to 100 mg/L of 25 or 115 nm SiO2 NPs at 48 and 72 hpf. Data are represented as mean ± SD.
2.3. Effect on Angiogenesis in Zebrafish Embryos
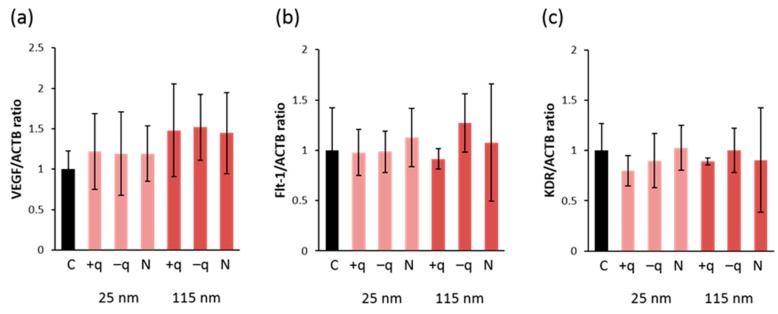
To evaluate the potential toxicity on angiogenesis, the number of sub-intestinal vessels running transversally between planes including the second and seventh intersegmental vessels was counted in 72 hpf embryos. The result showed that there were no significant differences in the number of transversely-running sub-intestinal vessels in zebrafish exposed to all types of SiO2 NPs at the concentration of 100 mg/L. Previously, we demonstrated that exposure to CuO NPs reduced the number of transversely-running sub-intestinal vessels in the same TG zebrafish at 5 dpf and down-regulated the expression of VEGF and VEGF receptors in endothelial cells sorted by the Fluorescence Activated Cell Sorter (FACS) [14]. Brundo et al. [22] showed that Au NPs exposure affected the expression of biomarkers, such as metallothionein, in zebrafish embryos even with normal survival rate and phenotype. Therefore, we analyzed the gene expression of biomarkers involved in angiogenesis (vegfa and its receptors: flt-1 and kdr). We found that there were no significant changes in their expression in zebrafish embryos exposed to all types of NPs (Figure 3a–c). These results indicate that SiO2 NPs have no effects on angiogenesis in all size and types of surface-charges in regular ZFET.
Figure 3.
Gene expression of VEGFa and VEGF receptors in zebrafish. Expression levels of (a) VEGFa and VEGF receptors: (b) Flt-1 and (c) KDR in embryos exposed to 100 mg/L of 25 or 115 nm SiO2 NPs with different surface-charge at 48 and 72 hpf. The mRNA levels were normalized by the amount of β-action mRNA. Data are represented as mean ± SD.
The SD values of the gene expression of VEGF and its receptors are relatively high in the present study. However, these variations are possible because another study [23] that investigated the expression of VEGF in zebrafish embryos similarly reported high SD values in their data. Total RNA was extracted from the whole body of zebrafish and was subjected to quantitative PCR in both studies. This might explain how the variations in gene expression of VEGF and its receptors were high. If the expression of VEGF and its receptors had been analyzed using total RNA isolated from the sorted green fluorescent protein (GFP)-positive endothelial cells, the variations might have been minimal. Further studies are needed to evaluate SiO2 NPs effect on angiogenesis and the gene expression associated with angiogenesis.
2.4. Protective Effect of Chorion Against SiO2 NPs
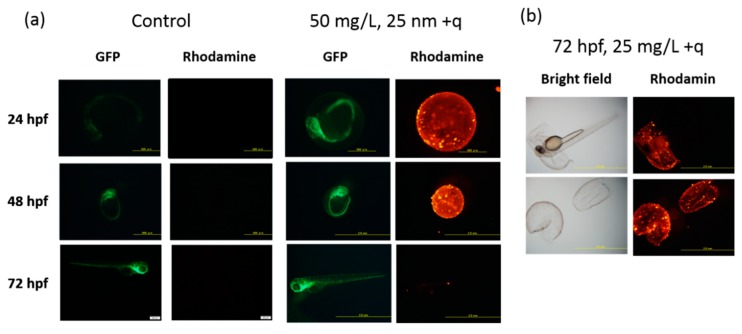
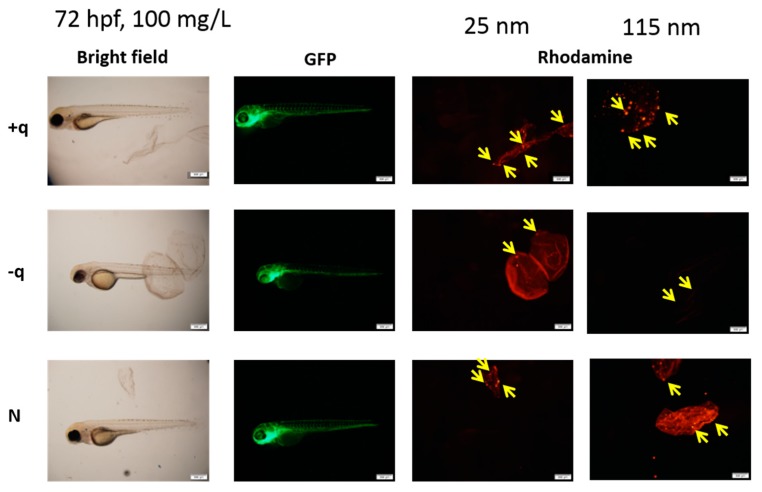
Contrary to the present ZFET results, several reports showed that SiO2 NPs exert acute toxicity in vitro (immortalized mammalian cell lines) and in vivo (mouse and rat) [24,25]. Physico-chemical properties, such as size, surface area, and surface properties including charges, were found to play a key role in the toxicity of SiO2 NPs [26]. To better understand the non-toxic effect of SiO2 NPs in ZFET, we examined the localization of NPs using rhodamine-labelled SiO2 NPs in the zebrafish embryos. As shown in Figure 4, all types of SiO2 NPs accumulated on the surface of chorion before hatching. Even after hatching, the NPs were not detected inside the fish bodies (Figure 5).
Figure 4.
Representative fluorescence microscopy images of zebrafish embryos. (a) Images of embryos exposed to 0 (control) or 50 mg/L of positively charged SiO2 NPs (25 nm) at 24, 48, and 72 hpf and (b) embryos exposed to 25 mg/L of positively charged SiO2 NPs (25 nm) at 72 hpf.
Figure 5.
Representative fluorescence microscopy images of embryos exposed to 100 mg/L of 25 or 115 nm SiO2 NPs with different surface charges at 72 hpf.
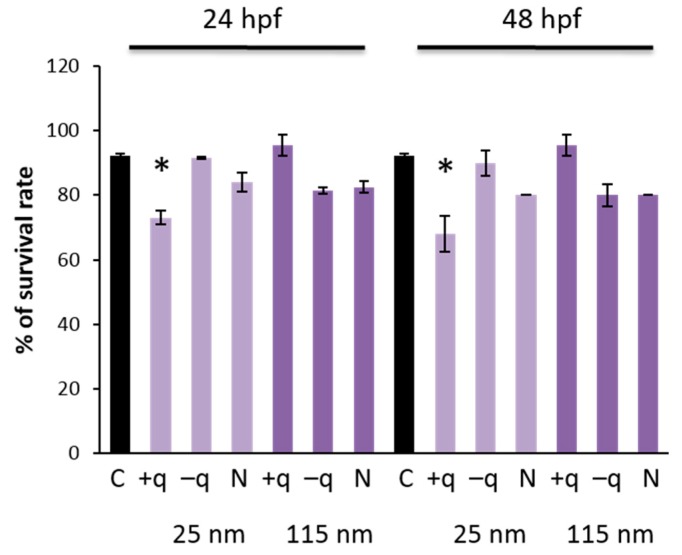
To evaluate the protective effect of chorion against SiO2 NPs, we exposed dechorionated (chorion removed) embryos to SiO2 NPs. After chemical removal of the chorion at 24 hpf, survival rate was not affected and gross morphological changes were not observed in the embryos exposed to 100 mg/L of SiO2 NPs at 48 and 72 hpf. Accordingly, the chorion was carefully removed at 6 hpf and the embryos were exposed to different surface-charged SiO2 NPs. As a result, exposure to positively surface-charged SiO2 NPs (25 nm) significantly (p < 0.05) reduced the survival rate of zebrafish at 24 and 48 hpf (Figure 6). It has been shown that positively charged NPs showed a higher cell uptake rate owing to its electrostatic interaction with cells [27,28]. Since the cell membrane is negatively charged in general, reduction in the survival rate of zebrafish by positive surface-charged NPs might be due to the higher uptake of NPs into cells, resulting from interaction of positively charged NPs and negatively charged cell membrane. However, further studies are needed to test this hypothesis.
Figure 6.
Survival rate at 24 and 48 hpf in zebrafish embryos exposed to 100 mg/L of 25 or 115 nm SiO2 NPs with different surface charges after chemical removal of chorion at 6 hpf. Data are represented as mean ± SD. * p < 0.05 compared with the control (ANOVA followed by Dunnett’s multiple comparison test).
In rodents, several kinds of metal-bearing NPs exhibit reproductive [29] and neurodevelopmental toxicities [30], probably due to oxidative stress, inflammation, or DNA damage [31]. Apoptosis induced by DNA damage and/or oxidative stress might be a mechanism underlying the developmental toxicity of SiO2 NPs in zebrafish embryos, as shown in other studies on NP toxicity [32,33,34]. In addition, our results demonstrated that all types of SiO2 NPs, regardless of the size or charges, were completely trapped by chorion, and subsequently blocked from exhibiting the toxicity to zebrafish embryos (Figure 5). Chorion might adsorb NPs as a natural barrier, as suggested by other studies [35,36]. The chorion possesses canals whose pore size is approximately 0.6–0.7 µm [37], which is larger than the size of the NPs in the present study, suggesting that the barrier property of chorion against NP transport is explained by adsorption of NPs by the chorion.
In the preset study, we focused on the effects of SiO2 NPs on the development of zebrafish and the effects of SiO2 NPs on angiogenesis. Because aorta and large vessels are formed by 48 hpf and small vessels in the abdomen, such as sub-intestinal vessels, are formed by around 72 hpf in zebrafish [38], we exposed SiO2 NPs at 6 hpf and observed the effect of SiO2 NPs on the formation of blood vessel at 72 hpf. The evaluation of the toxicity of SiO2 NPs by the exposure to SiO2 NPs after the chorions disappear at 48 hpf is also important. Further investigation is needed to determine the effects of SiO2 NPs after the absence of chorion. The effects of NPs on young zebrafish (after 8 dpf) should also be evaluated because the larvae become independent and start oral feeding after the nutrients contained in the yolk sac are exhausted. This topic can be considered as an essential theme for further investigation.
3. Materials and Methods
3.1. Preparation and Characterization of NPs Suspensions
Rhodamine-labeled silica NPs, 25 and 115 nm in diameter, with hydroxyl groups on the surface (neutral surface charge; N) or functionalized with amino modified (positive surface charge; +q) or carboxyl modified (negative surface charge; –q) were purchased from HiQ-Nano, Arnesano, Italy. All NPs were suspended in water at 25 mg/L and then dispersed with 0.3× Danieau’s solution (17.4 mM NaCl, 0.21 mM KCl, 0.18 mM Ca(NO3)2, 0.12 mM MgSO4, and 1.5 mM HEPES buffer, pH 7.6). The particle size distribution was determined with a nano-zetasizer (Zetasizer Nano S; Malvern Instruments, Worcestershire, UK), which uses DLS technique [39]. Hydrodynamic sizes of all NPs dispersed in the embryo medium at the highest concentration used for the treatment were analyzed from the time of exposure up to 24 h in order to check the stability of the NP solutions during the contact with embryos. The fluorescence intensities of three types of silica NPs were measured at different concentrations using an ARVO™MX 1420 Multilabel Counter (Perkin Elmer, Waltham, MA, USA). The successive dilutions of the medium at particle concentrations of 100, 50, 25, 12.5, 6.25, and 3.125 mg/L were completed immediately prior to exposure.
3.2. Zebrafish
Zebrafish were maintained in the facility at Mie University according to the standard operational guidelines. The nacre/fli1:egfp zebrafish with transparency, which facilitates in vivo monitoring of vascularization, were obtained by cross-breeding nacre mutants [40] and fli1:egfp transgenic zebrafish [41]. The zebrafish were acclimated to experimental conditions (28 ± 0.5 °C; light:dark/14:10 h; daily water change), as described previously [42]. All animal procedures were conducted according to the Japanese Animal Welfare Regulation Act and Management of Animals (Ministry of Environment of Japan) and complied with international guidelines. Ethical approval from the local Institutional Animal Care and Use Committee was not sought, since this law does not mandate the protection of fish.
3.3. Zebrafish Embryo Toxicity Test (ZFET)
Zebrafish embryo toxicity test (ZFET) for SiO2 NPs was performed according to the Organisation for Economic Co-operation and Development (OECD) guideline [19]. The day before egg collection, a single female and two male nacre/fli1:egfp zebrafish were placed in mating tanks. The next morning, mating was initiated by light stimuli and the fertilized eggs were collected. These eggs were incubated in 0.3× Danieau’s solution in a 6-well plate at the concentration of 20 eggs/well in 3 mL medium at 28 °C. The good quality eggs that were beginning to develop a yolk sac and animal pore were chosen. At 6 h post fertilization (hpf), the embryos were exposed by immersion in 0.3× Danieau’s solution or the same solution containing SiO2 NPs up to 72 hpf. Embryo medium was changed every 24 h with a new solution of NPs. Fluorescent image acquisition was performed using a Leica MZ16F stereoscopic microscope (Leica Microsystems, Wetzlar, Germany) equipped with DP71 digital camera (Olympus, Tokyo, Japan).
3.4. Evaluation of Survival Rate, Hatching Rate, and Gross Morphological Changes
Each of the 20 embryos were incubated in a 6-well plate with 3 mL of embryo medium. Then, the embryos were exposed to 3.125, 6.25, 12.5, 25, and 50 mg/L or 6.25, 12.5, 25, 50, and 100 mg/L of rhodamine-labeled silica NPs 25 or 115 nm in diameter from 6 to 72 hpf. Survival rate, hatching time, and gross morphological changes were examined at each time point: 12, 24, 36, 48, 60, and 72 hpf. The number of dead embryos/fish, the number of non-hatched or hatched embryos, and the number of deformed embryos/fish were recorded. Using MZ16F stereotypic microscope (Leica Microsystems, Wetzlar, Germany), the embryos were first observed using bright field and then fluorescence, and the photos were captured at the indicated time points.
3.5. Evaluation of NPs Localization
The embryos were anesthetized using 100 ppm 2-phenoxyethanol (ethylene glycol monophenylether) in 0.3× Danieau’s solution in the wells and mounted on the slides. The embryos were incubated in the embryo medium for at least 15–20 min for rinsing to remove NPs before anesthesia. To check the localization of the rhodamine-labeled SiO2 NPs in the embryos, live imaging of embryos after exposure was performed using MZ16F stereotypic microscope (Leica Microsystems) with a DP71 digital camera (Olympus, Tokyo, Japan) at 24, 48, and 72 hpf.
3.6. Evaluation of Development of Subintestinal Vessels and Measurement of Gene Expression of Vascular Endothelial Growth Factor (VEGF) and VEGF Receptors
After 72 hpf, zebrafish were transferred to 96-well microplates and observed for gross morphological changes under a fluorescent stereomicroscope. The number of transversely-running subintestinal vessels (perpendicular to the anterior-posterior axis) between the transverse planes containing the 2nd and 7th intersegmental vessels, was counted. Total RNA was extracted from pooled embryos using the RNAqueous-MicroTotal RNA Isolation kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. The concentration of total RNA was quantified by spectrophotometry (ND-1000; NanoDrop Technologies, Wilmington, DE, USA). The first-strand cDNA was synthesized from 200 ng of total RNA using the SuperScript III cDNA Synthesis Kit (Thermo Fisher Scientific) with oligo dT primers (Thermo Fisher Scientific). cDNA (n = 5 in each group) was subjected to quantitative polymerase chain reaction (PCR) analysis with FastStart Universal Probe Master Mix (Roche, Basel, Switzerland) and primers for VEGFa and VEGF receptors (VEGFR1; Flt-1: Fms-like tyrosine kinase and VEGFR2; KDR: kinase insert domain receptor) using an ABI 7300 Real-Time PCR system (Thermo Fisher Scientific), as described previously [14].The gene expression level was normalized to that of β-actin in the same cDNA.
3.7. Evaluation of Survival Rate and Gross Morphological Changes after Dechorionation of Zebrafish Embryos
To evaluate the protective effect of chorion against SiO2 NPs, we exposed SiO2 NPs to dechorionated (chorion removed) embryos. For chorion removal (dechorionation), 6 or 24 hpf embryos were immersed in 1.5 mg/mL of pronase (Roche Molecular Systems, Pleasanton, CA, USA) and the chorion was carefully removed according to the standard protocol [43]. After chemical removal of chorion at 6 hpf, the embryos were exposed to 100 mg/L of rhodamine-labeled SiO2 NPs at the same time (6 hpf). Survival rate and gross morphological changes were examined at 24 and 48 hpf. After chemical removal of chorion at 24 hpf, the embryos were exposed to the same dose of SiO2 NPs from 6 hpf, and survival rate and gross morphological changes were examined at 48 and 72 hpf.
3.8. Statistical Analysis
All parameters were expressed as mean ± standard deviation (SD). Statistical analyses were performed using one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc test. A p value less than 0.05 was considered statistically significant.
4. Conclusions
The toxicity of SiO2 NPs has been studied for many years and is linked with chronic bronchitis, emphysema, and silicosis in mammals [44,45,46]. For evaluation of developmental toxicity of SiO2 NPs, the zebrafish embryo is a powerful tool as an alternative model of rodents and as a suitable model for rapid growth and live imaging of the internal organs. However, OECD-approved ZFET protocol typically employs zebrafish embryos with intact chorions, which can obstruct NPs uptake independently from the size and surface charges. Our results suggest that chorion removal (dechorionation) in 6 hpf embryos might be necessary to determine the toxicological impact of NPs.
Acknowledgments
The authors thank Michiko Ariyoshi and Saori Ichikawa for breeding zebrafish and Junya Kuroyanagi and Kiyora Izuoka for experimental support.
Abbreviations
| ANOVA | one-way analysis of variance |
| DLS | dynamic light scattering |
| EGFP | enhanced green fluorescent protein |
| Flt-1 | Fms-like tyrosine kinase |
| hpf | hours post fertilization |
| KDR | kinase insert domain receptor |
| NPs | nanoparticles |
| PdI | polydispersity index |
| SD | standard deviation |
| SiO2 | silicon dioxide |
| VEGF | vascular endothelial growth factor |
| ZFET | zebrafish embryo acute toxicity test |
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1422-0067/20/4/882/s1.
Author Contributions
S.V. contributed to study design. S.V. and Y.S. performed the experiments, analyzed the data, and wrote the manuscript. S.I. designed and supervised the project and contributed to data interpretation and manuscript revision. M.K. and W.W. performed the experiments. T.T., S.B., and L.T. designed and supervised the project. G.I. reviewed the manuscript and provided comments. All authors read and approved the final manuscript.
Funding
This work was supported by a grant (Z9013040) of the Japan Society for the Promotion of Science (JSPS) postdoctoral fellowships for foreign researchers, JSPS KAKENHI Grant Number JP17K08590, and a grant (#LS059) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) in Japan, and a grant from Casio Science Promotion Foundation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Pan J., Wan D., Gong J. PEGylated liposome coated QDs/mesoporous silica core-shell nanoparticles for molecular imaging. Chem. Commun. (Camb.) 2011;47:3442–3444. doi: 10.1039/c0cc05520d. [DOI] [PubMed] [Google Scholar]
- 2.Yetisen A.K., Qu H., Manbachi A., Butt H., Dokmeci M.R., Hinestroza J.P., Skorobogatiy M., Khademhosseini A., Yun S.H. Nanotechnology in textiles. ACS Nano. 2016;10:3042–3068. doi: 10.1021/acsnano.5b08176. [DOI] [PubMed] [Google Scholar]
- 3.Fröhlich E., Roblegg E. Oral uptake of nanoparticles: Human relevance and the role of in vitro systems. Arch. Toxicol. 2016;90:2297–2314. doi: 10.1007/s00204-016-1765-0. [DOI] [PubMed] [Google Scholar]
- 4.Pietroiusti A., Bergamaschi E., Campagna M., Campagnolo L., de Palma G., Iavicoli S., Leso V., Magrini A., Miragoli M., Pedata P., et al. The unrecognized occupational relevance of the interaction between engineered nanomaterials and the gastro-intestinal tract: A consensus paper from a multidisciplinary working group. Part Fibre Toxicol. 2017;14:47. doi: 10.1186/s12989-017-0226-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oberdörster G., Maynard A., Donaldson K., Castranova V., Fitzpatrick J., Ausman K., Carter J., Karn B., Kreyling W., Lai D., et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: Elements of a screening strategy. Part Fibre Toxicol. 2005;2:8. doi: 10.1186/1743-8977-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Y.W., Cambre M., Lee H.J. The Toxicity of nanoparticles depends on multiple molecular and physicochemical mechanisms. Int. J. Mol. Sci. 2017;18:2702. doi: 10.3390/ijms18122702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tada-Oikawa S., Ichihara G., Fukatsu H., Shimanuki Y., Tanaka N., Watanabe E., Suzuki Y., Murakami M., Izuoka K., Chang J., et al. Titanium dioxide particle type and concentration influence the inflammatory response in caco-2 cells. Int. J. Mol. Sci. 2016;17:576. doi: 10.3390/ijms17040576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ude V.C., Brown D.M., Viale L., Kanase N., Stone V., Johnston H.J. Impact of copper oxide nanomaterials on differentiated and undifferentiated Caco-2 intestinal epithelial cells; assessment of cytotoxicity, barrier integrity, cytokine production and nanomaterial penetration. Part Fibre Toxicol. 2017;14:31. doi: 10.1186/s12989-017-0211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Y., Quan G., Wu Q., Zhang X., Niu B., Wu B., Huang Y., Pan X., Wu C. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm. Sin. B. 2018;8:165–177. doi: 10.1016/j.apsb.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kempen P.J., Greasley S., Parker K.A., Campbell J.L., Chang H.Y., Jones J.R., Sinclair R., Gambhir S.S., Jokerst J.V. Theranostic mesoporous silica nanoparticles biodegrade after pro-survival drug delivery and ultrasound/magnetic resonance imaging of stem cells. Theranostics. 2015;5:631–642. doi: 10.7150/thno.11389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durfee P.N., Lin Y.S., Dunphy D.R., Muñiz A.J., Butler K.S., Humphrey K.R., Lokke A.J., Agola J.O., Chou S.S., Chen I.M., et al. Mesoporous silica nanoparticle-supported lipid bilayers (protocells) for active targeting and delivery to individual leukemia cells. ACS Nano. 2016;10:8325–8345. doi: 10.1021/acsnano.6b02819. [DOI] [PubMed] [Google Scholar]
- 12.Guisasola E., Asín L., Beola L., de la Fuente J.M., Baeza A., Vallet-Regí M. Beyond traditional hyperthermia: In vivo cancer treatment with magnetic-responsive mesoporous silica nanocarriers. ACS Appl. Mater. Interfaces. 2018;10:12518–12525. doi: 10.1021/acsami.8b02398. [DOI] [PubMed] [Google Scholar]
- 13.Hill A.J., Teraoka H., Heideman W., Peterson R.E. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 2005;86:6–19. doi: 10.1093/toxsci/kfi110. [DOI] [PubMed] [Google Scholar]
- 14.Chang J., Ichihara G., Shimada Y., Tada-Oikawa S., Kuroyanagi J., Zhang B., Suzuki Y., Sehsah R., Kato M., Tanaka T., et al. Copper oxide nanoparticles reduce vasculogenesis in transgenic zebrafish through down-regulation of vascular endothelial growth factor expression and induction of apoptosis. J. Nanosci. Nanotechnol. 2015;15:2140–2147. doi: 10.1166/jnn.2015.9762. [DOI] [PubMed] [Google Scholar]
- 15.Inoue A., Nishimura Y., Matsumoto N., Umemoto N., Shimada Y., Maruyama T., Kayasuga K., Morihara M., Katagi J., Shiroya T., et al. Comparative study of the zebrafish embryonic toxicity test and mouse embryonic stem cell test to screen developmental toxicity of human pharmaceutical drugs. Fundament. Toxicol. Sci. 2016;3:79–87. doi: 10.2131/fts.3.79. [DOI] [Google Scholar]
- 16.Delov V., Muth-Köhne E., Schäfers C., Fenske M. Transgenic fluorescent zebrafish Tg(fli1:EGFP)y¹ for the identification of vasotoxicity within the zFET. Aquat. Toxicol. 2014;150:189–200. doi: 10.1016/j.aquatox.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Basnet R.M., Guarienti M., Memo M. Zebrafish Embryo as an in vivo model for behavioral and pharmacological characterization of methylxanthine drugs. Int. J. Mol. Sci. 2017;18:596. doi: 10.3390/ijms18030596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishimura Y., Murakami S., Ashikawa Y., Sasagawa S., Umemoto N., Shimada Y., Tanaka T. Zebrafish as a systems toxicology model for developmental neurotoxicity testing. Congenit. Anom. (Kyoto) 2015;55:1–16. doi: 10.1111/cga.12079. [DOI] [PubMed] [Google Scholar]
- 19.Busquet F., Strecker R., Rawlings J.M., Belanger S.E., Braunbeck T., Carr G.J., Cenijn P., Fochtman P., Gourmelon A., Hübler N., et al. OECD validation study to assess intra- and inter-laboratory reproducibility of the zebrafish embryo toxicity test for acute aquatic toxicity testing. Regul. Toxicol. Pharmacol. 2014;69:496–511. doi: 10.1016/j.yrtph.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 20.Fent K., Weisbrod C.J., Wirth-Heller A., Pieles U. Assessment of uptake and toxicity of fluorescent silica nanoparticles in zebrafish (Danio rerio) early life stages. Aquat. Toxicol. 2010;100:218–228. doi: 10.1016/j.aquatox.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 21.Li X., Liu B., Li X.L., Li Y.X., Sun M.Z., Chen D.Y., Zhao X., Feng X.Z. SiO2 nanoparticles change colour preference and cause Parkinson’s-like behaviour in zebrafish. Sci. Rep. 2014;4:3810. doi: 10.1038/srep03810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brundo M.V., Pecoraro R., Marino F., Salvaggio A., Tibullo D., Saccone S., Bramanti V., Buccheri M.A., Impellizzeri G., Scuderi V., et al. Toxicity evaluation of new engineered nanomaterials in zebrafish. Front. Physiol. 2016;7:130. doi: 10.3389/fphys.2016.00130. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Hogan B.M., Schulte-Merker S. How to Plumb a Pisces: Understanding Vascular Development and Disease Using Zebrafish Embryos. Dev. Cell. 2017;42:567–583. doi: 10.1016/j.devcel.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 24.Petrache Voicu S.N., Dinu D., Sima C., Hermenean A., Ardelean A., Codrici E., Stan M.S., Zărnescu O., Dinischiotu A. Silica nanoparticles induce oxidative stress and autophagy but not apoptosis in the MRC-5 cell line. Int. J. Mol. Sci. 2015;16:29398–29416. doi: 10.3390/ijms161226171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Großgarten M., Holzlechner M., Vennemann A., Balbekova A., Wieland K., Sperling M., Lendl B., Marchetti-Deschmann M., Karst U., Wiemann M. Phosphonate coating of SiO2 nanoparticles abrogates inflammatory effects and local changes of the lipid composition in the rat lung: A complementary bioimaging study. Part Fibre. Toxicol. 2018;15:31. doi: 10.1186/s12989-018-0267-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Napierska D., Thomassen L.C., Lison D., Martens J.A., Hoet P.H. The nanosilica hazard: Another variable entity. Part Fibre. Toxicol. 2010;7:39. doi: 10.1186/1743-8977-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung T.H., Wu S.H., Yao M., Lu C.W., Lin Y.S., Hung Y., Mou C.Y., Chen Y.C., Huang D.M. The effect of surfacecharge on the uptake and biological function of mesoporoussilicananoparticles in 3T3-L1 cells and human mesenchymal stem cells. Biomaterials. 2007;28:2959–2966. doi: 10.1016/j.biomaterials.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Jambhrunkar S., Qu Z., Popat A., Yang J., Noonan O., Acauan L., Ahmad Nor Y., Yu C., Karmakar S. Effect of surfacefunctionality of silicananoparticles on cellular uptake and cytotoxicity. Mol. Pharm. 2014;11:3642–3655. doi: 10.1021/mp500385n. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Q., Ding Y., He K., Li H., Gao F., Moehling T.J., Wu X., Duncan J., Niu Q. Exposure to alumina nanoparticles in female mice during pregnancy induces neurodevelopmental toxicity in the offspring. Front. Pharmacol. 2018;9:253. doi: 10.3389/fphar.2018.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Umezawa M., Onoda A., Takeda K. Developmental toxicity of nanoparticles on the brain. Yakugaku Zasshi. 2017;137:737–738. doi: 10.1248/yakushi.16-00214. [DOI] [PubMed] [Google Scholar]
- 31.Wang Z., Zhang T., Huang F. The reproductive and developmental toxicity of nanoparticles: A bibliometric analysis. Toxicol. Ind. Health. 2018;34:169–177. doi: 10.1177/0748233717744430. [DOI] [PubMed] [Google Scholar]
- 32.Zhao X., Ren X., Zhu R., Luo Z., Ren B. Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria-mediated apoptosis in zebrafish embryos. Aquat. Toxicol. 2016;180:56–70. doi: 10.1016/j.aquatox.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 33.Du J., Cai J., Wang S., You H. Oxidative stress and apotosis to zebrafish (Danio rerio) embryos exposed to perfluorooctane sulfonate (PFOS) and ZnO nanoparticles. Int. J. Occup. Med. Environ. Health. 2017;30:213–229. doi: 10.13075/ijomeh.1896.00669. [DOI] [PubMed] [Google Scholar]
- 34.Eryılmaz O., Ateş P.S., Ünal İ., Üstündağ Ü., Bay S., Alturfan A.A., Yiğitbaşı T., Emekli-Alturfan E., Akalın M. Evaluation of the interaction between proliferation, oxidant-antioxidant status, Wnt pathway, and apoptosis in zebrafish embryos exposed to silver nanoparticles used in textile industry. J. Biochem. Mol. Toxicol. 2018;32 doi: 10.1002/jbt.22015. [DOI] [PubMed] [Google Scholar]
- 35.Lee K.J., Nallathamby P.D., Browning L.M., Osgood C.J., Xu X.H. In vivo imaging of transport and biocompatibility of single silver nanoparticles in early development of zebrafish embryos. ACS Nano. 2007;1:133–143. doi: 10.1021/nn700048y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duan J., Yu Y., Shi H., Tian L., Guo C., Huang P., Zhou X., Peng S., Sun Z. Toxic effects of silica nanoparticles on zebrafish embryos and larvae. PLoS ONE. 2013;8:e74606. doi: 10.1371/journal.pone.0074606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim K.T., Tanguay R.L. The role of chorion on toxicity of silver nanoparticles in the embryonic zebrafish assay. Environ. Health Toxicol. 2014;29:e2014021. doi: 10.5620/eht.e2014021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Astin J.W., Haggerty M.J., Okuda K.S., Le Guen L., Misa J.P., Tromp A., Hogan B.M., Crosier K.E., Crosier P.S. Vegfd can compensate for loss of Vegfc in zebrafish facial lymphatic sprouting. Development. 2014;141:2680–2690. doi: 10.1242/dev.106591. [DOI] [PubMed] [Google Scholar]
- 39.Wu W., Ichihara G., Hashimoto N., Hasegawa Y., Hayashi Y., Tada-Oikawa S., Suzuki Y., Chang J., Kato M., D’Alessandro-Gabazza C.N., et al. Synergistic effect of bolus exposure to zinc oxide nanoparticles on bleomycin-induced secretion of pro-fibrotic cytokines without lasting fibrotic changes in murine lungs. Int. J. Mol. Sci. 2014;16:660–676. doi: 10.3390/ijms16010660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lister J.A., Robertson C.P., Lepage T., Johnson S.L., Raible D.W. Nacre encodes a zebrafish microphthalmia-related protein that regulates neural-crest-derived pigment cell fate. Development. 1999;126:3757–3767. doi: 10.1242/dev.126.17.3757. [DOI] [PubMed] [Google Scholar]
- 41.Lawson N.D., Weinstein B.M. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Bio. 2002;248:307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- 42.Shimada Y., Hirano M., Nishimura Y., Tanaka T. A high-throughput fluorescence-based assay system for appetite-regulating gene and drug screening. PLoS ONE. 2012;7:e52549. doi: 10.1371/journal.pone.0052549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio Rerio) 4th ed. University of Oregon Press; Eugene, OR, USA: 2000. [Google Scholar]
- 44.Ross M.H., Murray J. Occupational respiratory disease in mining. Occup. Med. (Lond.) 2004;54:304–310. doi: 10.1093/occmed/kqh073. [DOI] [PubMed] [Google Scholar]
- 45.Hnizdo E., Sullivan P.A., Bang K.M., Wagner G. Association between chronic obstructive pulmonary disease and employment by industry and occupation in the US population: A study of data from the Third National Health and Nutrition Examination Survey. Am. J. Epidemiol. 2002;156:738–746. doi: 10.1093/aje/kwf105. [DOI] [PubMed] [Google Scholar]
- 46.Hnizdo E., Vallyathan V. Chronic obstructive pulmonary disease due to occupational exposure to silica dust: A review of epidemiological and pathological evidence. Occup. Environ. Med. 2003;60:237–243. doi: 10.1136/oem.60.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.