Abstract
Despite the advancement of transgenic and gene knockout animal models in the prostate cancer research, there is still a need for utilizing xenograft models. Xenografts can be grown in multiple sites/organs within immunocompromised animals such as mice and rats. Although prostate xenografts have been derived from many species, human cells and tissues are the most commonly used due to their potential clinical significance. Xenograft models that progress from one state or stage to another are commonly used to address important scientific questions including malignant transformation, metastatic spread, and castration resistance. Utilization of xenografts are commonly being used to assess the biology and genetics of prostate cancer, as well as, for therapeutic benefit.
In addition to models for the study of prostate cancer, xenografts are also utilized as a tool in precision medicine where patient derived xenografts (PDX) can be grown in multiple animals and assessed for therapeutic efficacy. The popularity of such xenograft models and PDXs have led to availability of these resources through public and commercial institutions. In this review, we describe both traditional and emerging models of prostate cancer and their potential uses. Further development of current models and introduction of new models will likely provide new insights and better understanding of prostatic carcinogenesis and progression.
Introduction
Prostate cancer is the second leading diagnosed cancer in men in the United States, trailing only lung cancer. Over 220,000 men are diagnosed per year with 27,000 deaths resulting from metastatic prostate cancer (SEER 2014). Prostate cancer is a relatively slowly progressing disease that occurs spontaneously in the aging dog and human as the ratio of testosterone to estradiol decreases. Recreating this hormone environment has led to prostatic carcinogenesis in mice, dogs, and rats (Ittmann et al., 2013; Leroy and Northrup, 2009; Ricke et al., 2008; Shirai et al., 2000; Wang et al., 2005). While these models have been immensely useful in understanding the role of specific factors involved in prostate cancer progression, they are limited by long tumor latency and low incidence of tumor development. As a localized disease, prostate cancer presents relatively low risks to the patients, with 5-year survival rates close to 100%. However, patients that have undergone androgen deprivation therapy (ADT) to treat high-grade prostate cancer will progress to castration resistant prostate cancer (CRPC) in 10-20% of cases, whereby the 5-year survival rate drops to 29% (Kirby et al., 2011). The events that lead to CRPC are not well understood, but invariably result from the ability of the prostate cells to grow in hormone-depleted environment. This can result from multiple mechanisms including “hypersensitivity” to androgens from androgen receptor (AR) upregulation, promiscuity of AR, ligand-independent activation of AR, or complete bypassing of the receptor through other receptors such as growth factor signaling (Arnold and Isaacs, 2002; Choong et al., 1996; Culig et al., 1994; Watson et al., 2015; Zhao et al., 2000). To date, few models allow for in-depth examination of the processes that lead to prostate carcinogenesis and the development of CRPC using human cells.
Traditional cell line and xenograft models as well as newer patient derived xenograft (PDX) models will described in this review.
Steroid Receptors and Prostate Cancer Progression
Prostate cancers are most often diagnosed in aging men, where the sex hormone milieu is typically marked by an increasing ratio of 17β-estradiol (E2) to testosterone (T) (Vermeulen et al., 2002). Androgens and estrogens have previously been implicated in prostatic carcinogenesis in the rat (Noble, 1977a, b). These hormones have also been shown to induce carcinogenesis in wild-type mice, and administration of testosterone in mouse retinoblastoma protein (pRb)-deficient prostatic epithelium promoted cancer progression (Ricke et al., 2008; Wang et al., 2000). It has since been established that sex hormones are critical in the progression of human prostate cancer; we and others have shown that the ratio of T to E2 is a key component (Ricke et al., 2006; Wang et al., 2001). Recapitulation of this aspect of prostate cancer carcinogenesis is useful in understanding the roles of stromal-epithelial interaction and hormone action.
Treatment of prostate epithelial cells with estrogen unopposed by androgens can induce squamous metaplasia of prostatic epithelium. (Bainborough, 1952; Cunha et al., 2001; Ricke et al., 2006; Risbridger et al., 2001). Estrogens work through estrogen receptors (ER), which are ligand activated transcription factors. There are at least two forms of the ER, α and β, that have been detected in the prostate. ERα and β are found in both the stroma and the epithelium (Ratliff, 2005; Ricke et al., 2008). As prostate cancer progresses, epithelial ERβ expression decreases; suggesting a protective role of ERβ (Christoforou et al., 2014; Ricke et al., 2008; Royuela et al., 2001). Using genome wide ER-knockout mice, we have observed that ERα is the main driver of prostate carcinogenesis (Ricke et al., 2008). The tissue specific role of ERα has yet to be resolved. However, given the critical function of stromal AR in prostatic carcinogenesis and organogenesis, ERα may play a similar role.
Androgens target the prostate and are regulators of growth, development, function, and maintenance. These effects are mediated through the androgen receptor (AR), present in both the stroma and the epithelium of the prostate (Heinlein and Chang, 2004; Nicholson et al., 2013a). Using tissue recombinants, we have shown that stromal AR is essential for prostate organogenesis and prostate cancer progression and metastasis while epithelial AR is not (Cunha et al., 2003; Cunha and Lung, 1979; Ricke et al., 2012). It has been suggested that AR functions as both a tumor suppressor and a proliferator, representing contradictory roles. To this point, restoring AR function in AR-negative PC3 cells results in decreased invasion and metastasis. In mice, eliminating AR in the prostate epithelium caused increased apoptosis of luminal cells; yet also resulted in increased epithelial basal cells (Litvinov et al., 2006a; Niu et al., 2008). Adult mice that lack epithelial AR develop prostates that display less differentiated but hyperproliferative tissue when compared to wild-type littermates (Simanainen et al., 2007; Wu et al., 2007). However, when AR is eliminated from stromal WPMY cells in vitro, a decrease in proliferation was observed, suggesting a stromal-mediated paracrine role of AR. Taken together, it is clear that the cooperation of ARs and ERs in both stroma and epithelium are important factors in prostate carcinogenesis. Models that address these aspects of the disease are necessary to elucidate the cross-talk between these critical receptors.
Several “progression” models have been created using the LNCaP cell line as well as other traditional prostate cancer cell lines (Sobel and Sadar, 2005a, b). Traditionally, most of these models use a late stage cell line representation of prostate cancer as a starting point and are often focused on one aspect such as tumor growth, metastasis, or androgen independence. There are a limited number of models that have utilized non-tumorigenic human prostatic epithelium as the starting point to assess prostate cancer (PRCA) progression. No single model exists that allows one to examine the progression of normal to metastatic cancer, androgen dependence to independence, genetic and epigenetic changes, as well as gene fusion events such as TMPRSS-ERG (transmembrane protease serine 2- ETS-related gene fusion). Such a model may allow for the better interrogation of events that lead to prostate carcinogenesis and disease progression.
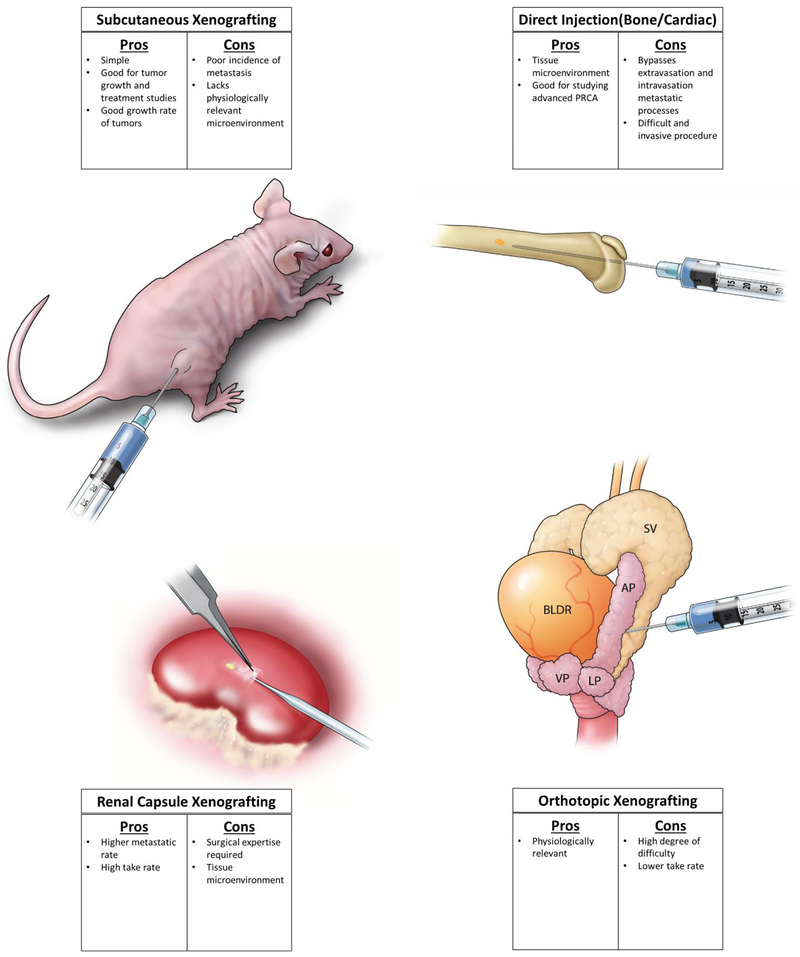
Over the years, there have been many efforts to develop xenograft and implantation methods to create a model that accurately represent prostate cancer and prostate cancer progression. Subcutaneous xenografting was developed when researchers began implanting prostatic tissues from patients into athymic nude mice, creating one of the first patient derived xenograft lines, PC-82 (Hoehn et al., 1980). An advantage of subcutaneous xenografting is that it is an efficient way to monitor growth of tumors. A major disadvantage is that they often fail to produce metastases in mice. In an attempt to mimic the prostate cancer at the site of implantation, orthotopic xenografting was introduced (Stephenson et al., 1992). This model has the advantage of cells encountering an environment similar to the organ in which they originated, possibly offering a more clinically and biologically relevant model. Orthotopic implantation of cell lines has been successful in generating new cell lines that are metastatic. This method was used to generate PC-3 and LNCaP lineages, which were discovered to metastasize, specifically to bones (Pettaway et al., 1996). More recently, subrenal capsule xenografting has been used as a way to xenograft cells that could be recovered at a very high rate. The high degree of vascularity that is present at this site allows for implanted material to grow more successfully than other methods (Nicholson et al., 2013b; Wang et al., 2005). Additionally, implanted cells have also metastasized from this site to distant lymph nodes and tissues. Unlike orthotopic xenografting, this method lacks the advantage of having a prostatic environment. This can, in part, be overcome by introducing different populations of stromal cells through tissue recombination technology. This method, although more difficult and requires surgical expertise, does have improved take rates compared to other sites. Finally, several methods have been employed to specifically study metastasis of prostate cancer cells including cardiac injection, tail vein injection, and direct injection into bone or other tissues. These methods allow researchers to study the behavior of cells at specific sites outside the prostate, but bypasses the steps of extravasation and intravasation of cancer cells. In this review, we will discuss current and historical xenograft models of prostate cancer carcinogenesis in which the addition or subtraction of hormones was used to induce a more advanced phenotype as well as some of the newer patient derived xenograft models.
Current “progression” models
For decades, the most common cell lines used in prostate cancer research have been LNCaP, DU-145, and PC3. LNCaP has been the most used cell line in prostate cancer research with over 7,000 manuscripts indexed on PubMed. Following LNCaP, PC3 has been the second most used cell line with 4,313 references. Through prolonged passaging in mice and subsequent selection of increasingly malignant phenotypes, an expansive list of derivative lines has been established. These derivative lines vary in androgen sensitivity and ability to grow and metastasize. However, it is important to note that researchers began these experiments using cell lines that were taken from patients with advanced disease, metastasis, or were undergoing ADT.
Xenograft Models using Benign Cell Lines
RWPE-1 + MNU
One of the few non-tumorigenic human prostate epithelial cell lines is RWPE-1. These cells were harvested from the peripheral zone of a 54-year-old white male donor whose prostate histology appeared normal. RWPE-1 cells were isolated from plated acini that were then immortalized with HPV 18. RWPE-2 cells were then created by transforming them with insertion of a Ki-ras oncogene. Both cell lines reportedly express AR/PSA mRNA and protein and display a dose-dependent increase in growth following treatment with androgens (Bello et al., 1997; Rhim et al., 1994). When RWPE-1 is injected into nude mice with or without Matrigel, malignant tumors do not form. In contrast, RWPE-2 cells readily form malignant tumors in vivo and are moderately invasive when compared to DU-145. In another attempt to create a malignant cell line out of RWPE-1, cells were treated with N-methyl-N-nitrosurea (MNU), a chemical carcinogen, and injected subcutaneously into adult male nude mice (Rivette et al., 2005). After 10 weeks, tumors were harvested, cultured, re-injected and grown for another 10 weeks. Following this second round of xenotransplantation injection, isolated cells were then plated in soft agar and colonies were selected to re-inject for another round. The resulting cell lines were established: WPE1-NA22, WPE1-NB14, WPE1-NB11 and WPE1-NB26 (cells were isolated in order of increasing malignancy). As a final step, metastatic WPE1-NB26 cells were injected and tumors were harvested giving rise to the most malignant cell lines, WPE1-NB26-64 and WPE1-NB26-65. Collectively, this cell series mimics progression characteristics from benign, to low, to highly malignant stages (Rivette et al., 2005). Growth and MMP expression increases significantly when comparing parental to tumorigenic lines. When NB26 cells are injected intravenously into nude mice, 2/5 mice had evidence of lung metastases. However, the relevance of a DNA alkylating agent, i.e. MNU, that results in the accumulation of mutations in DNA and prostate cancer is yet to be determined (Golding et al., 1997). Therefore, while this model is valuable to the study of different stages of prostate cancer, the method of induced carcinogenesis may not represent the human disease process.
NHPrEI and BHPrEI Tissue Recombination Xenograft Model
To avoid issues with viral transformation, a series of spontaneously immortalized benign human prostate epithelial cell lines called NHPrE1 and BHPrE1 were generated (Jiang et al., 2010). Although these cell lines lack AR expression in vitro, tissue recombination with inductive urogenital sinus mesenchyme (UGM) induced luminal epithelial differentiation with AR expression. NHPrE1 cells were characterized as a “progenitor” line due to their high expression of CD133/CD44/OCT4/PTEN and the ability of as few as 10 cells to regenerate fully secretory glandular structures when recombined with inductive UGM. The BHPrEI cells were characterized as an “intermediate” cell line due to enhanced expression of p63/p53/p21/RB and the necessity to xenograft a minimum of 200,000 cells with inductive UGM to regenerate fully differentiated glandular structures. Genomic analysis detailed the small number of rearrangements and amplifications likely responsible for the immortalization. These cell lines represent a major advancement in our ability to accurately model transformation-independent effects of specific genes on cancer initiation. Future studies will assess the role of hormones and stroma, as well as oncogenes and tumor suppressor genes in carcinogenesis.
BPH1 Cancer Progression Model
Most models that will be discussed in this review use either subcutaneous or orthotopic injection to generate sublines. Subcutaneous injection generally does not produce reliable rates of metastasis, and orthotopic xenografting is technically more difficult and has a low limit of material that can be injected. Subrenal capsule xenografting of prostate tissue was introduced in the 1970’s and allowed for a high rate of tumor recovery in a relatively simple procedure. The high degree of vascularity results in rapid tumor growth in most cases and allows for metastasis to distant tissues (Ricke et al., 2006; Wang et al., 2005). Sex steroids have long been known to be important to both growth and maintenance of the prostate (Huggins, 1943; Huggins and Hodges, 1972; Nelles et al., 2011; Wibowo et al., 2011). Addition or removal of sex steroids have resulted in the generation of dozens of sublines from established xenografts/cell lines, and we know that steroids play a pivotal role in the carcinogenesis of prostate cancer (Bosland, 2000; Wilding, 1992). The BPH1 cell line is another example of the limited supply of benign prostate cell lines. This cell line was isolated from a transurethral resection of 68-year-old white male exhibiting urinary symptoms (Hayward et al., 1995; Hayward et al., 2001). Cells were immortalized with SV40 large T-antigen and one clone was used to derive the BPH1 cell line. The status of the androgen receptor in BPH1 cells has been controversial, but literature favors evidence for an absence of both AR and PSA protein. Whereas other benign cell lines used chemicals, oncogenes, or loss of tumor suppressors to induce malignant transformation, use of “natural” inducers such as hormones and stromal-microenvironment may be more biologically relevant. Several studies have used BPH1 cells that were transformed by the microenvironment, which included both hormonal and stromal components. This recapitulated the conditions similar to the aging male prostate environment. When BPH1 cells were recombined with rodent urogenital sinus mesenchyme (rUGM) and xenografted, they form solid branching epithelial cords that could become canalized (Wang et al., 2001). However, when these UGM+BPH1 recombinants are grown in hosts that are treated with testosterone (T) and 17β-estradiol (E2), they form invasive carcinomas. The resultant tumors shrink following host castration, and tumors were capable of being transplanted and growing in a new host for at least 6 generations, which was the longest time point assessed. In the absence of stroma but in the presence of hormones, BPH1 cells survive and proliferate at the implantation site but do not form cancers. The hormonal milieu that was required to induce carcinogenesis in BPH1 cells was then further elucidated in 2006. Varying doses of T:E2 treatments in hosts were employed to determine if the amount or ratio of sex steroids was an important factor in the process of carcinogenesis. It was found that only groups with T:E2 ratios of 25:2.5 or 100:10 conferred carcinogenesis. In groups that had low T:E2 ratios of 0:2.5 or 2.5:2.5, benign squamous metaplasia, a non-malignant growth formed (Ricke et al., 2006). To form cancer, the concentration of T had to be at least 10× higher than E2 and at least 25 mg of T had to be present at the start of treatment. This experiment linked the importance of a decreasing ratio of T:E2 to the process of carcinogenesis. After 4 months of treatment, the rate of carcinogenesis and malignant transformation in BPH1 tissue recombinants was 87.5%. Rate of metastasis was also monitored in this study. After 2 months of treatment with the optimal ratio of hormones, 2/25 mice were found to have lung or liver metastases. In addition, 2/3 treated from another group had renal lymph node metastases. Once the cells had undergone malignant transformation using T+E2, they were able to grow in mice in the absence of both hormones and co-injected stromal cells. This is the first model to demonstrate that non-tumorigenic human epithelial cells could develop into tumorigenic and metastatic cells using stroma and naturally occurring hormones to promote malignancy. Since the publication in 2006, we have been able to isolate various stages of progression using this method of transformation. An advantage of this model is that changes in malignancy can be compared back to the parental line. Using the benign/non-tumorigenic cell lines, one can focus on aspects of prevention of progression, while at the same time studying aspects of treatment using metastatic cell lines.
Patient Derived Xenografts (PDXs)
Perhaps the largest limitation of most models in use, is the inability to address the aspect of tumor heterogeneity. Most cell lines are inherently homogenous and increase in homogeneity over time due to the most proliferative cells taking over the culture.
Prostate cancer, on the other hand, is a heterogeneous disease. Spatial sampling and sequencing of prostate tumors has shown significant heterogeneity in multifocal tumors arising in the same patient (Boutros et al., 2015). In addition, the typical resurgence of prostate cancer that can follow long-term androgen deprivation therapy points to sub-clones of cells that could be present and resistant to therapy in early stages of disease. Androgen receptor mutations are rarely seen in non-treated primary tumors, and almost exclusively arise in therapeutically treated and metastatic prostate cancer (Taplin and Balk, 2004; Tilley et al., 1996). This heterogeneity and variable nature of prostate cancer is a facet that is largely missed in most cell line and xenograft models. Patient derived xenografts (PDX) are one way to increase growth of heterogeneous cancer within and between patients. Tumor samples can be harvested within a patient and directly transplanted subcutaneously, orthotopically, or under the renal capsule of immunocompromised animals. These xenografts maintain their stromal components, architecture, and heterogeneity that are present in the primary tumor isolate. This method is especially useful for studying tumor response to various pharmacologic and therapeutic treatment strategies. In particular it could be invaluable in developing strategies that are specific to individual patients, improving precision medicine strategies.
CWR22 and 22Rv1 Xenograft Model
The CWR xenograft models were established as a serially-transplantable xenograft harvested from transurethral resections of the prostate (TURP) of several patients (Nagabhushan et al., 1996; Pretlow et al., 1993; Wainstein et al., 1994). All patients that contributed to the generation of CWR lines (CWR22, 21, 31, and 91) had advanced stage D prostate cancer, as well as, bone metastases. CWR22 cells were not injected in suspension during the initial experiments, but were instead injected as minced tissue combined with Matrigel. Initial experiments injected the cells at various sites in the mouse, with subcutaneous, bone, and testiclar sites growing best. It is of interest to note that of the 4 animals injected in the prostate, none formed tumors. Tumors also failed to grow in all female mice. One of the advantages of these xenografts is after castration of injected animals, the tumor initially regresses and PSA decreases, much like the human disease. After prolonged androgen deprivation, tumors relapse and re-establish in 25-50% of animals, then designated as CWR22R. Relapsed tumors grow at a significantly slower rate than CWR22 tumors. This was one of the first models that could successfully recapitulate relapse and regrowth in vivo as well as in soft agar. Later a purified cell line was established from a relapsed CWR22 tumor xenograft, designated 22Rv1 (Sramkoski et al., 1999). Initial experiments using CWR22 cells from xenografts ended with cultures being overgrown with mouse cells. When establishing the 22Rv1 cell line, researchers used flow cytometry, targeting CD44 to select for a pure, human cell line. This cell line is reportedly similar to the parental CWR22 xenografts, expresses PSA in vivo and has similar xenograft morphology and growth (Sramkoski et al., 1999). Following establishment and use of the CWR22 model, other researchers began to examine and characterize it, attempting to define its progression from hormone dependent to hormone independent. In 2002, a novel AR mutation that conferred a loss of the ligand binding domain was identified in both CWR22R and 22Rv1. This mutation contains an in-frame tandem duplication of exon 3 which originated in the original CWR22R xenograft (Tepper et al., 2002). The progression of CWR22 tumors to the relapsed 22Rv1 tumors continues to be a valuable resource for studying the progression of PRCA to CRPC.
In all cases that have been described in this section, researchers used the manipulation of hormones to induce changes in prostate cancer cells and to the subsequent derivations. These techniques were used to derive cell lines representing singular aspects of prostate cancer progression. Regardless, these models represent aspects of prostate cancer progression that will add to the understanding of PRCA.
LuCaP Xenograft Models
The LuCaP series of 21 patient derived xenografts was derived over the span of 15 years from 1991-2005 when 261 prostate cancer samples were collected from 156 patients (Corey et al., 2003; Ellis et al., 1996; Nguyen et al., 2017; True et al., 2002). These samples were implanted subcutaneously into nude mice. While the overall take rate was low (10%), researchers were still able to create a diverse set of transplantable PDX’s. Of the 21 PDX’s that were further characterized, 4 were taken from the primary graft site while 17 were taken from a spectrum of metastatic sites. Within this series there are 4 PDX’s that are AR-negative (LuCaP 49, 93, 145.1, 145.2), and all were designated as neuroendocrine prostate cancers (NEPC) based on coinciding synaptophysin (SYP) expression. Researchers also tested for ERG, PTEN, and SYP by IHC; as well as response to either castration or docetaxel. The responses and markers varied across the 21 samples. Almost all of the harvested samples retained the host histological features and protein expression patterns.
More recently, researchers have further characterized this series of xenografts and have shown that it covers a wide breadth of disease variability and heterogeneity that is in prostate cancer from patient to patient. The most common genomic alterations in xenografts were AR amplification (8/21), PTEN loss (12/21), RBI loss (16/21), and TMPRSS2-ERG rearrangement (10/21), which are consistent with human disease phenotypes. Most of these phenotypes were seen in the host patient with the exception of a five AR amplifications that occurred following transplantation (Linja et al., 2001; Lotan et al., 2017; Macoska et al., 1992; Visakorpi et al., 1995). In addition to AR amplification, 4/21 of the xenografts exhibited AR mutations in the AR ligand domain, a characteristic often seen in mCRPC (Tilley et al., 1996). AR transcript levels and splice variants were also analyzed as a part of characterization and varied across the 21 samples. ARV7, a splice variant most commonly seen in mCRPC, was significantly upregulated in PDX’s that originated in castration-resistant patients (Guo et al., 2009). Up to 150 passages of xenografts were analyzed for retention of the characteristics described here and it was found that xenografts continued to display host tumor features (Nguyen et al., 2017).
Finally, researchers analyzed the LuCaP xenograft series for use in testing responses to ADT and docetaxel treatment. The ability to grow patient tumors in mice and test various treatment strategies are techniques that will prove indispensable in the future. Cumulatively, the LuCaP series displayed variable responses to ADT, ranging from complete regression to striking progression. This outcome mimicked what is seen in human patients with most responding to ADT, while some have little to no response. In some instances, mice displayed signs of tumor heterogeneity contributing to response. Mice that harbored the same PDX in multiple sites displayed differing responses between lesions. After prolonged treatment under castrate conditions, most PDXs adapt and regrow. The time of regression ranged from 2-25 weeks, and neuroendocrine PDXs showed no response to ADT. Interestingly, AR mRNA and signaling had no correlation with response to ADT. Docetaxel, which is the most common chemotherapy used in men with mCRPC, again showed varied responses across the xenograft series (Petrylak et al., 2004). Interestingly, the LuCaP 35 PDX was the only xenograft that displayed an initial negative response to docetaxel treatment that coincided with significant decreases in host body weight. Because significant host body weight loss was not seen in any of the other mice treated with docetaxel, this points to a unique interaction between the tumor and docetaxel. Unfortunately, like most prostate cancer models, the LuCaP PDXs do not spontaneously metastasize to bone even when implanted orthotopically. However, nine of the sublines did show osteoblastic lesions when injected directly into the bone, a condition that mimics that of the human.
Overall, the newly characterized series of LuCaP PDXs represents a series of models that will be very valuable in assessing prostate cancer progression, CRPC, and testing new treatment drugs and strategies. Unlike in vitro models and xenografts created from cell lines, this xenograft model has the ability to retain host heterogeneity, molecular signatures, and stromal compartments, all important in progression and treatment in the clinic. Further characterization and use of this model may illuminate novel aspects of the role of the microenvironment, the stroma, progression, and drug interactions.
Commercially Available PDXs
Recently, the development of PDXs from the Living Tumor Laboratory, a public repository of growing PDXs from both surgery and the core biopsies that are extensively characterized has been established (Lin et al., 2014a; Lin et al., 2014b). These PDX models are xenografts of patient tissue harvested from both surgery and core biopsy. Using the subrenal xenografting method (SRC) robust growth was observed from 7 of 18 patient samples, while 9 of the tumors were viable despite remaining quiescent for >2 years. All transplanted xenografts maintained histopathologic features of the matched patient tumor. Transplanted tissue had also shown key chromosomal alterations that are most often observed in patients: loss of TP53, NKX3.1, RBI, and PTEN (Lin et al., 2014a; Rubin and De Marzo, 2004). Chromosomal comparisons between the patient’s tumor and xenografts was performed on 5 samples and showed high conservation of both gene expression levels and gross genomic structure.
Following transplantation of the 5 different biopsy foci from a single patient, it was observed that each xenograft had a different rate of growth and metastatic capability; highlighting the importance of tumor heterogeneity and how it is maintained using this model. Of the 14 patients who donated tissue to the experiment, two had been diagnosed with NEPC. NEPC represents a rare yet growing population of CRPC (Beltran et al., 2016). All other patients had been diagnosed with adenocarcinoma, the most common form of prostate cancer. When mice harboring xenografts were treated with bicalutamide or castration, all adenocarcinomas initially regressed, while the NEPCs did not respond to castration or bicalutamide treatment. In addition, after several months in castrate conditions, two of the adenocarcinoma xenografts had developed resistance to bicalutamide treatment and demonstrated growth in castrate conditions.
Most strikingly, one of the xenografted adenocarcinoma lines developed into NEPC following androgen deprivation. While the patient tumor initially expressed AR and PSA, following several months of growth in a castrated mouse, the xenograft was entirely AR and PSA negative and expressed several neuroendrocrine markers including synaptophysin (SYP) and chromogranin A (CHGA). This represents a model of transformation from adenocarcinoma to NEPC. Of the 9 grafts that remained quiescent in mice, only 2 came from patients who later had PSA recurrence (Beltran et al., 2011). However, of the 7 grafts that grew readily in mice, all 7 patients had PSA recurrence. Latency of regrowth also correlated with overall time to PSA recurrence; the faster a tumor established in mice, the quicker that patient progressed to recurrence. Much like the LuCaP series, these models provide means for study of treatment of heterogeneous tumors and development of personalized medicine. The investigators have yet to report the ability of xenografts to metastasize to bone, another critical component of prostate cancer progression. These xenografts can be passaged in mice over several years and are available for study from the Living Tumor Laboratory (livingtumorlab.com).
Even with the continuing development of PDXs, a number of disadvantages to the model remain. Perhaps most notably is the low rate of establishment, making it necessary to obtain a high number of samples. This can make it impossible for some labs because it is often difficult to obtain sufficient primary patient tissue to be used for xenografting. Furthermore, their inability to be grown in vitro as well as their high costs are other reasons for limited application. Nonetheless, they represent unique model systems to study prostate cancer. In addition, there are other commercial repositories including Jackson Labs and Charles River that offer PDXs for purchase. However, prostate cancer is not currently well represented in these consortiums, with only 6 prostate cancer PDXs being offered by Jackson Labs. The advantages of PDXs offered to researchers outweigh many of the negatives, and hence will likely lead to more companies and non-profit institutions offering these valuable resources in the future.
Xenograft Models using Malignant Cell Lines
LNCaP and Derivatives
Perhaps the most widely used cell line in prostate cancer research has been the LNCaP cell line. This cell line was isolated from a needle biopsy of a lymph node that had a metastatic lesion (Horoszewicz et al., 1980). LNCaP has a doubling time that is significantly shorter than the other commonly used lines; however it is capable of anchorage independent growth (Horoszewicz et al., 1983). When LNCaP was initially isolated, it was capable of forming tumors in 50% of mice when injected subcutaneously. However, since this original experiment, LNCaPs now require injection with collagen or Matrigel to form subcutaneous tumors. Tumors grow at the same rate in both male and female mice, but form earlier in male mice (Horoszewicz et al., 1983). What has made this cell line particularly attractive to researchers is that it expresses both AR and ER. While it does express AR, the receptor contains the T877A mutation, which confers promiscuous binding activity of the receptor (Veldscholte et al., 1990). This makes some studies on inhibitors and steroids difficult to interpret. Because LNCaP contains AR and is hormone sensitive, the derivation of sublines has been focused on creating lines that are androgen insensitive. Thus, the list of derivations is much more expansive than any other cell line. The technique of re-passaging cell lines through the prostate orthotopically and subsequently isolating cells from the prostate and lymph node generated the LNCaP-Pro and LNCaP-LN series. As the researchers continued to inject, isolate, culture, and then re-inject; the tumorigenicity and metastatic rate increased. LNCaP-Pro3 had the highest rate of tumorigenicity, forming tumors in 8/8 mice injected and forming metastatic lesions in 6/8 mice. All LNCaP-LN lines retained the same degree of metastatic ability, metastasizing in 60-70% of mice. However, the highest rate of tumorigenicity was seen in the LNCaP-LN3 line, forming tumors in 19/19 mice injected. The LNCaP-LN3 line was also unaffected by orchiectomy 48 hours following injection and when grown in vitro without androgen, PSA produced was 10-fold higher than that of the parental line. All other variants were unable to form tumors following orchiectomy (Pettaway et al., 1996).
Perhaps the best-known LNCaP sublines are the LNCaP-C4 variants. The first three cell lines that began this series of derivations were the M, C4, and C5 cell lines. These were created when researchers injected mice with mixtures of LNCaP cells with the human bone stromal cell line, MS (Wu et al., 1994). After 4 weeks, the mouse hosts were castrated and after another 4 weeks, the LNCaP-C4 cell line was isolated. Another set of animals had cells isolated from tumors following 5 weeks of castration, and became the LNCaP-C5 line. As a control, the LNCaP-M cell line was established from intact hosts following 12 weeks of growth. The C4 cell line was then co-injected with MS cells back into mice that were again castrated and the C4-2 cell line was isolated (Thalmann et al., 1994). All 4 cell lines express AR and PSA mRNA with M, C4-2, and C5 expressing significantly higher levels. The C4-2 line is the only line that can form tumors in castrated mice consistently in the absence of MS cells and served as another jumping off point for the next set of derivations (Thalmann et al., 1994; Wu et al., 1998).
The focus of the next study was to create cell lines that had increased metastatic ability, specifically to bone. This led to the creation of the LNCaP-C4-2B progression series (Table 1). This study was designed much like the previous study and started with the injection of C4-2 cells subcutaneously and orthotopically in both intact and castrated mice. In both intact and castrated mice, cells that were injected orthotopically formed tumors 100% of the time (Thalmann et al., 2000). However, out of the 66 subcutaneous injections in intact mice, only one tumor formed, while tumors did not form in castrated mice. It is interesting to note that although there was no tumor formation following subcutaneous injection into castrated mice, these mice had the highest incidence of paraplegia and osseous metastases (50%). Intact mice had lower incidences of paraplegia when compared to castrated mice. Four LNCaP-C4-B variants were created as well as a control cell line from the primary tumor and a lymph node, C4-2-Pr and C4-2-Ln respectively. All cell lines exhibited increased invasion when compared to parental LNCaP and had shown an increased production of PSA.
Table 1:
Summary of C4-2 variants generated from LNCaP
| Cell Line | Host | Injection Site | Derived From | Tumorigenicity S.C. | Paraplegia |
|---|---|---|---|---|---|
| C4-2B-2 | Intact | Sub C | Bone Met. | 4/4 | 1/4 |
| C4-2B-3 | Castrated | Sub C | Bone Met. | 7/8 | 3/8 |
| C4-2B-4 | Castrated | Orthotopic | Bone Met. | 4/4 | 1/4 |
| C4-2B-5 | Castrated | Sub C | Bone Met | 4/4 | 1/4 |
Two LNCaP variants were created by long-term culture in the absence of androgen (Culig et al., 1999). The LNCaP-abl and LNCaP-AI both exhibit similar levels of androgen receptor as parental LNCaP, but are able to grow in an androgen-depleted environment. The LNCaP-abl line was sub-cultured for 87 passages and responded positively to androgens up until passage 75. Following passage 75, the cells started to be inhibited by androgens. Researchers also noted that bicalutamide switched from being an antagonist in parental lines, to an agonist in LNCaP-abl (Culig et al., 1999). LNCaP-abl cells grew well in intact mice without testosterone supplementation and were unable to grow in mice with testosterone supplementation. LNCaP-abl xenografts grew best when implanted in castrated mice that also received bicalutamide treatment, reaching an average size of 140mm2. When injected into the prostates of mice, LNCaP-abl cells can metastasize to both lymph node and bone sites. The LNCaP-AI was derived in a similar experiment of passaging cells for an extended period in the absence of androgens. The parental line used in this experiment was LNCaP-FGC cells, which are a faster growing cell line than the initially established LNCaP line. Faster growth is the only difference between LNCaP-FGC and LNCaP. LNCaP-AI cells were cultured for up to 6 months in the absence of androgen. At this point 99% of the cells had died and the remaining cells were used to establish the cell line. Unlike the LNCaP-abl cell line, LNCaP-AI still exhibited a growth effect following stimulation with androgen. The LNCaP-abl cells are also more resistant to apoptosis induced by TPA, likely through increased expression of the anti-apoptotic gene BCL2 (Lu et al., 1999). These cells also exhibit increased expression of p21 and decreased expression of p16. Several other variants have been established using androgen deprivation and prolonged cell culture. These are known as the LNCaP-C series and the LNCaP-104 series, which will not be covered in this review (Kokontis et al., 1998). A more recent LNCaP line was created by culturing LNCaP cells in the presence of IL-6 for a prolonged period of time. These cells exhibited better growth in vivo likely through the reduction of pRb (Steiner et al., 2003). Together, the multitude of LNCaP variants have allowed researchers to examine many different mechanisms of gained hormone independence and prostate cancer progression.
PC3 and Derivatives
The PC3 cell line was derived from a vertebral metastasis of a 62-year-old white male (Kaighn et al., 1979). This cell line is similar to DU-145 in that it is androgen insensitive and lacks AR and PSA expression. It is important to note that re-expression of AR in PC3 results in decreased motility, migration, and invasion (Huo et al., 2015; Litvinov et al., 2006b; Niu et al., 2008). PC3 cells express high levels of EGFR and TGF-α, while they are deficient in PTEN. An interesting observation of this cell line is the presence of the transferrin receptor, which allows for growth stimulation via bone marrow-derived transferrin (Keer et al., 1990). This is one of the fastest growing cell lines in vivo, but has recently begun to represent features of neuroendocrine carcinoma (Tai et al., 2011). The PC3 line expresses the neuroendocrine markers CD44, CgA, and NSE. The study of neuroendocrine prostate carcinomas (NEPC) is a rapidly expanding field. While it is represented in a small subset of patients, NEPC is untreatable and survival times for those patients are drastically shorter than the rest of the population and therefore must be studied further (Parimi et al., 2014). Regardless of this, PC3 has been invaluable in understanding tumors that no longer respond to conventional therapy. Because this cell line is already androgen independent, creation of sublines has been focused on variants that are more metastatic or metastasize to certain tissues.
The PC3-M line was derived from grossly visible lesions on the liver of a nude mouse that received intrasplenic injection of PC3 cells 6 weeks prior. These cells were designated PC3-M (Kaighn et al., 1979; Kozlowski et al., 1984). From the PC3-M cell lines, several more cell lines were created by passaging or injecting PC3-M cells into the prostates of nude mice. With each round of injection, the most metastatic cells were selected, cultured and then re-injected back into the prostate (Pettaway et al., 1996). With this technique, they created the PC-3M-Pro4 and PC-3M-LN4, harvested from the prostate and lymph node respectively. Both cell lines grow significantly faster than their parental PC3/PC-3M lines. The LN4 variant has the highest incidence of metastasis and tumorigenicity. Another 4 sublines were created by injecting cells into the tail vein of nude mice after 3 rounds of selection through Boyden chambers (Table 2). The most invasive cells after 3 rounds of Boyden chamber selection were then injected into the tail vein of nude mice. Once the cells had metastasized to soft tissues, they were harvested and plated. This process was repeated 5 times and 4 cell lines were isolated based on where they preferentially metastasized (Wang and Stearns, 1991). A caveat of this series of derivations is that specificity of the metastatic site decreased as passage and cell number increase.
Table 2:
Summary of PC-3M variants.
| PC3 Variant | Preferential Site of Metastasis | Secondary Sites of Metastasis |
|---|---|---|
| PC3-ML | Lumbar vertebrae | Lung, Liver, Brain, Colon |
| PC3-MR | Rib Cartilage | Lung |
| PC3-MC | Mandible | Brain, Colon |
| PC3-MK | Right knee bone | Colon |
Summary
The models that have been described in this review have immensely furthered our understanding of the prostate cancer carcinogenesis and progression. Cell line models and their derivatives have provided researchers with the ability to quickly and efficiently study aspects of the disease in vitro and in vivo. However, these models have been limited by the inability to recapitulate aspects of cancer progression, initiation, and heterogeneity, yet model at least one important feature. In addition, many of these cell lines were founded in men that were not receiving the same treatments that are given today (e.g. enzalutamide). Over time, cell line models have come closer to emulating the human disease through the use of tissue recombinants containing a stromal component and the use of hormones treatments. Increasingly, researchers are turning to PDXs to study aspects of heterogeneity, treatment, and progression. While PDXs are time consuming and generally have low take rates, they retain characteristics such as biomarkers, heterogeneity, histological features, and androgen response of the host tumor. Take rates of PDXs have been significantly improved through the use of subrenal capsule xenografting which provides high vascularization and lymphatic flow which provides a supply of nutrients, hormones, oxygen, and growth factors to the xenografts (Ott and Knox, 1976). The ability to establish a xenograft has been correlated with the aggressiveness of the host tumor. Early stage prostate tumors are generally slower growing and do not establish as well. Even when using tissues harvested from metastatic lesions graft take rates are around 25-30% (Alsop et al., 2016).
More recently, PDX consortiums are being established by public and commercial institutions that offer mice bearing PDXs from patients for collaboration or purchase. This may allow more labs with limited resources to acquire and use PDXs. Use of a wide breadth of models, including those that are described here will be necessary for ultimately deciphering this disease, understanding therapeutic mechanisms, and increasing precision medicine. We must also focus on the development of new models, new therapies, and the improvement of those models already established.
Supplementary Material
Figure 1. Xenografting Sites:
Depiction of the most common sites of xenografting that are used in prostate.
Figure 2: Tissue recombination using human prostatic epithelia (BPH1) and mouse stroma (UGM) grown in hormone treated mice leads to malignant transformation and metastasis.
BPH1 and UGM cells were implanted under the kidney capsules of mice, and were allowed to grow for 2-4 months while receiving sustained doses of T + E2, effectively mimicking the environment seen in men as they age.
Acknowledgements
This work was supported by the NIH U54 DK104310 and NIH Training Grant 4T32CA009135-39. Thanks to Ms. Sally Griffith-Oh for assistance in generating figures.
Abbreviations:
- PDX
patient derived xenograft
- UGM
urogenital mesenchyme
- AR
androgen receptor
- ER
estrogen receptor
- NEPC
neuroendocrine prostate cancer
- TURP
transurethral resection of the prostate
- TMPRSS2-ERG
transmembrane protease serine 2, ETS-related gene fusion
- CRPC
castration resistant prostate cancer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alsop K, Thorne H, Sandhu S, Hamilton A, Mintoff C, Christie E, Spruyt O, Williams S, McNally O, Mileshkin L, Ananda S, Hallo J, Loi S, Scott C, Savas P, Devereux L, O’Brien P, Gunawardena S, Hampson C, Strachan K, Jaravaza RD, Francis V, Young G, Ranson D, Samaranayake R, Stevens D, Boyle S, Fedele C, Topp M, Ho G, Teo ZL, Taylor RA, Papargiris MM, Lawrence MG, Wang H, Risbridger GP, Haynes NM, Medon M, Johnstone RW, Vidacs E, Arnau GM, Vergara IA, Papenfuss AT, McArthur G, Waring P, Carvosso S, Angel C, Gyorki D, Solomon B, Mitchell G, Shanley S, Francis PA, Dawson SJ, Haffenden A, Tidball E, Volchek M, Pyman J, Madadin M, Leditschke J, Cordner S, Shackleton M, Bowtell DD, 2016. A community-based model of rapid autopsy in end-stage cancer patients. Nature biotechnology 34, 1010–1014. [DOI] [PubMed] [Google Scholar]
- Arnold JT, Isaacs JT, 2002. Mechanisms involved in the progression of androgen-independent prostate cancers: it is not only the cancer cell’s fault. Endocrine-related cancer 9, 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainborough AR, 1952. Squamous metaplasia of prostate following estrogen therapy. J Urol 68, 329–336. [DOI] [PubMed] [Google Scholar]
- Bello D, Webber MM, Kleinman HK, Wartinger DD, Rhim JS, 1997. Androgen responsive adult human prostatic epithelial cell lines immortalized by human papillomavirus 18. Carcinogenesis 18, 1215–1223. [DOI] [PubMed] [Google Scholar]
- Beltran H, Prandi D, Mosquera JM, Benelli M, Puca L, Cyrta J, Marotz C, Giannopoulou E, Chakravarthi BVSK, Varambally S, Tomlins SA, Nanus DM, Tagawa ST, Van Allen EM, Elemento O, Sboner A, Garraway LA, Rubin MA, Demichelis F, 2016. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Med 22, 298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran H, Rickman DS, Park K, Chae SS, Sboner A, MacDonald TY, Wang Y, Sheikh KL, Terry S, Tagawa ST, Dhir R, Nelson JB, de la Taille A, Allory Y, Gerstein MB, Perner S, Pienta KJ, Chinnaiyan AM, Wang Y, Collins CC, Gleave ME, Demichelis F, Nanus DM, Rubin MA, 2011. Molecular Characterization of Neuroendocrine Prostate Cancer and Identification of New Drug Targets. Cancer discovery 1, 487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosland MC, 2000. The role of steroid hormones in prostate carcinogenesis. Journal of the National Cancer Institute. Monographs, 39–66. [DOI] [PubMed] [Google Scholar]
- Boutros PC, Fraser M, Harding NJ, de Borja R, Trudel D, Lalonde E, Meng A, Hennings-Yeomans PH, McPherson A, Sabelnykova VY, Zia A, Fox NS, Livingstone J, Shiah Y-J, Wang J, Beck TA, Have CL, Chong T, Sam M, Johns J, Timms L, Buchner N, Wong A, Watson JD, Simmons TT, P’ng C, Zafarana G, Nguyen F, Luo X, Chu KC, Prokopec SD, Sykes J, Dal Pra A, Berlin A, Brown A, Chan-Seng-Yue MA, Yousif F, Denroche RE, Chong LC, Chen GM, Jung E, Fung C, Starmans MHW, Chen H, Govind SK, Hawley J, D’Costa A, Pintilie M, Waggott D, Hach F, Lambin P, Muthuswamy LB, Cooper C, Eeles R, Neal D, Tetu B, Sahinalp C, Stein LD, Fleshner N, Shah SP, Collins CC, Hudson TJ, McPherson JD, van der Kwast T, Bristow RG, 2015. Spatial genomic heterogeneity within localized, multifocal prostate cancer. Nat Genet 47, 736–745. [DOI] [PubMed] [Google Scholar]
- Choong CS, Sturm MJ, Strophair JA, McCulloch RK, Tilley WD, Leedman PJ, Hurley DM, 1996. Partial androgen insensitivity caused by an androgen receptor mutation at amino acid 907 (Gly-->Arg) that results in decreased ligand binding affinity and reduced androgen receptor messenger ribonucleic acid levels. The Journal of clinical endocrinology and metabolism 81, 236–243. [DOI] [PubMed] [Google Scholar]
- Christoforou P, Christopoulos PF, Koutsilieris M, 2014. The Role of Estrogen Receptor β in Prostate Cancer. Molecular Medicine 20, 427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey E, Quinn JE, Buhler KR, Nelson PS, Macoska JA, True LD, Vessella RL, 2003. LuCaP 35: a new model of prostate cancer progression to androgen independence. The Prostate 55, 239–246. [DOI] [PubMed] [Google Scholar]
- Culig Z, Hobisch A, Cronauer MV, Radmayr C, Trapman J, Hittmair A, Bartsch G, Klocker H, 1994. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer research 54, 5474–5478. [PubMed] [Google Scholar]
- Culig Z, Hoffmann J, Erdel M, Eder IE, Hobisch A, Hittmair A, Bartsch G, Utermann G, Schneider MR, Parczyk K, Klocker H, 1999. Switch from antagonist to agonist of the androgen receptor bicalutamide is associated with prostate tumour progression in a new model system. British journal of cancer 81, 242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Hayward SW, Wang YZ, Ricke WA, 2003. Role of the stromal microenvironment in carcinogenesis of the prostate. Int J Cancer 107, 1–10. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Lung B, 1979. The importance of stroma in morphogenesis and functional activity of urogenital epithelium. In vitro 15, 50–71. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Wang YZ, Hayward SW, Risbridger GP, 2001. Estrogenic effects on prostatic differentiation and carcinogenesis. Reprod Fertil Dev 13, 285–296. [DOI] [PubMed] [Google Scholar]
- Ellis WJ, Vessella RL, Buhler KR, Bladou F, True LD, Bigler SA, Curtis D, Lange PH, 1996. Characterization of a novel androgen-sensitive, prostate-specific antigen-producing prostatic carcinoma xenograft: LuCaP 23. Clinical cancer research : an official journal of the American Association for Cancer Research 2, 1039–1048. [PubMed] [Google Scholar]
- Golding BT, Bleasdale C, McGinnis J, Müller S, Rees HT, Rees NH, Farmer PB, Watson WP, 1997. The mechanism of decomposition of N-methyl-N-nitrosourea (MNU) in water and a study of its reactions with 2’-deoxyguanosine, 2’-deoxyguanosine 5’-monophosphate and d(GTGCAC). Tetrahedron 53, 4063–4082. [Google Scholar]
- Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, Chen H, Kong X, Melamed J, Tepper CG, Kung HJ, Brodie AM, Edwards J, Qiu Y, 2009. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer research 69, 2305–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward SW, Dahiya R, Cunha GR, Bartek J, Deshpande N, Narayan P, 1995. Establishment and characterization of an immortalized but non-transformed human prostate epithelial cell line: BPH-1. In vitro cellular & developmental biology. Animal 31, 14–24. [DOI] [PubMed] [Google Scholar]
- Hayward SW, Wang Y, Cao M, Hom YK, Zhang B, Grossfeld GD, Sudilovsky D, Cunha GR, 2001. Malignant transformation in a nontumorigenic human prostatic epithelial cell line. Cancer research 61, 8135–8142. [PubMed] [Google Scholar]
- Heinlein CA, Chang C, 2004. Androgen receptor in prostate cancer. Endocr Rev 25, 276–308. [DOI] [PubMed] [Google Scholar]
- Hoehn W, Schroeder FH, Reimann JF, Joebsis AC, Hermanek P, 1980. Human prostatic adenocarcinoma: some characteristics of a serially transplantable line in nude mice (PC 82). The Prostate 1, 95–104. [DOI] [PubMed] [Google Scholar]
- Horoszewicz JS, Leong SS, Chu TM, Wajsman ZL, Friedman M, Papsidero L, Kim U, Chai LS, Kakati S, Arya SK, Sandberg AA, 1980. The LNCaP cell line--a new model for studies on human prostatic carcinoma. Progress in clinical and biological research 37, 115–132. [PubMed] [Google Scholar]
- Horoszewicz JS, Leong SS, Kawinski E, Karr JP, Rosenthal H, Chu TM, Mirand EA, Murphy GP, 1983. LNCaP model of human prostatic carcinoma. Cancer research 43, 1809–1818. [PubMed] [Google Scholar]
- Huggins C, 1943. ENDOCRINE CONTROL OF PROSTATIC CANCER. Science (New York, N.Y.) 97, 541–544. [DOI] [PubMed] [Google Scholar]
- Huggins C, Hodges CV, 1972. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA: a cancer journal for clinicians 22, 232–240. [DOI] [PubMed] [Google Scholar]
- Huo C, Kao YH, Chuu CP, 2015. Androgen receptor inhibits epithelial-mesenchymal transition, migration, and invasion of PC-3 prostate cancer cells. Cancer letters 369, 103–111. [DOI] [PubMed] [Google Scholar]
- Ittmann M, Huang J, Radaelli E, Martin P, Signoretti S, Sullivan R, Simons BW, Ward JM, Robinson BD, Chu GC, Loda M, Thomas G, Borowsky A, Cardiff RD, 2013. Animal Models of Human Prostate Cancer: The Consensus Report of the New York Meeting of the Mouse Models of Human Cancers Consortium Prostate Pathology Committee. Cancer research 73, 2718–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Strand DW, Fernandez S, He Y, Yi Y, Birbach A, Qiu Q, Schmid J, Tang DG, Hayward SW, 2010. Functional remodeling of benign human prostatic tissues in vivo by spontaneously immortalized progenitor and intermediate cells. Stem cells (Dayton, Ohio) 28, 344–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW, 1979. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Investigative urology 17, 16–23. [PubMed] [Google Scholar]
- Keer HN, Kozlowski JM, Tsai YC, Lee C, McEwan RN, Grayhack JT, 1990. Elevated transferrin receptor content in human prostate cancer cell lines assessed in vitro and in vivo. J Urol 143, 381–385. [DOI] [PubMed] [Google Scholar]
- Kirby M., Hirst C Crawford ED, 2011. Characterising the castration-resistant prostate cancer population: a systematic review. International Journal of Clinical Practice 65, 1180–1192. [DOI] [PubMed] [Google Scholar]
- Kokontis JM, Hay N, Liao S, 1998. Progression of LNCaP prostate tumor cells during androgen deprivation: hormone-independent growth, repression of proliferation by androgen, and role for p27Kip1 in androgen-induced cell cycle arrest. Molecular endocrinology (Baltimore, Md.) 12, 941–953. [DOI] [PubMed] [Google Scholar]
- Kozlowski JM, Fidler IJ, Campbell D, Xu ZL, Kaighn ME, Hart IR, 1984. Metastatic behavior of human tumor cell lines grown in the nude mouse. Cancer research 44, 3522–3529. [PubMed] [Google Scholar]
- Leroy BE, Northrup N, 2009. Prostate cancer in dogs: comparative and clinical aspects. Veterinary journal (London, England : 1997) 180, 149–162. [DOI] [PubMed] [Google Scholar]
- Lin D, Wyatt AW, Xue H, Wang Y, Dong X, Haegert A, Wu R, Brahmbhatt S, Mo F, Jong L, Bell RH, Anderson S, Hurtado-Coll A, Fazli L, Sharma M, Beltran H, Rubin M, Cox M, Gout PW, Morris J, Goldenberg L, Volik SV, Gleave ME, Collins CC, Wang Y, 2014a. High fidelity patient-derived xenografts for accelerating prostate cancer discovery and drug development. Cancer research 74, 1272–1283. [DOI] [PubMed] [Google Scholar]
- Lin D, Xue H, Wang Y, Wu R, Watahiki A, Dong X, Cheng H, Wyatt AW, Collins CC, Gout PW, Wang Y, 2014b. Next generation patient-derived prostate cancer xenograft models. Asian journal of andrology 16, 407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linja MJ, Savinainen KJ, Saramaki OR, Tammela TL, Vessella RL, Visakorpi T, 2001. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer research 61, 3550–3555. [PubMed] [Google Scholar]
- Litvinov IV, Antony L, Dalrymple SL, Becker R, Cheng L, Isaacs JT, 2006a. PC3, but not DU145, human prostate cancer cells retain the coregulators required for tumor suppressor ability of androgen receptor. The Prostate 66, 1329–1338. [DOI] [PubMed] [Google Scholar]
- Litvinov IV, Antony L, Dalrymple SL, Becker R, Cheng L, Isaacs JT, 2006b. PC3, but not DU145, human prostate cancer cells retain the coregulators required for tumor suppressor ability of androgen receptor. The Prostate 66, 1329–1338. [DOI] [PubMed] [Google Scholar]
- Lotan TL, Heumann A, Rico SD, Hicks J, Lecksell K, Koop C, Sauter G, Schlomm T, Simon R, 2017. PTEN loss detection in prostate cancer: comparison of PTEN immunohistochemistry and PTEN FISH in a large retrospective prostatectomy cohort. Oncotarget. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, Tsai SY, Tsai MJ, 1999. Molecular mechanisms of androgen-independent growth of human prostate cancer LNCaP-AI cells. Endocrinology 140, 5054–5059. [DOI] [PubMed] [Google Scholar]
- Macoska JA, Powell IJ, Sakr W, Lane MA, 1992. Loss of the 17p chromosomal region in a metastatic carcinoma of the prostate. J Urol 147, 1142–1146. [DOI] [PubMed] [Google Scholar]
- Nagabhushan M, Miller CM, Pretlow TP, Giaconia JM, Edgehouse NL, Schwartz S, Kung HJ, de Vere White RW, Gumerlock PH, Resnick MI, Amini SB, Pretlow TG, 1996. CWR22: the first human prostate cancer xenograft with strongly androgen-dependent and relapsed strains both in vivo and in soft agar. Cancer research 56, 3042–3046. [PubMed] [Google Scholar]
- Nelles JL, Hu WY, Prins GS, 2011. Estrogen action and prostate cancer. Expert review of endocrinology & metabolism 6, 437–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HM, Vessella RL, Morrissey C, Brown LG, Coleman IM, Higano CS, Mostaghel EA, Zhang X, True LD, Lam H-M, Roudier M, Lange PH, Nelson PS, Corey E, 2017. LuCaP Prostate Cancer Patient-Derived Xenografts Reflect the Molecular Heterogeneity of Advanced Disease an--d Serve as Models for Evaluating Cancer Therapeutics. The Prostate 77, 654–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson TM, Sehgal PD, Drew SA, Huang W, Ricke WA, 2013a. Sex steroid receptor expression and localization in benign prostatic hyperplasia varies with tissue compartment. Differentiation; research in biological diversity 85, 140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson TM, Uchtmann KS, Valdez CD, Theberge AB, Miralem T, Ricke WA, 2013b. Renal capsule xenografting and subcutaneous pellet implantation for the evaluation of prostate carcinogenesis and benign prostatic hyperplasia. Journal of visualized experiments : JoVE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Y, Altuwaijri S, Lai KP, Wu CT, Ricke WA, Messing EM, Yao J, Yeh S, Chang C, 2008. Androgen receptor is a tumor suppressor and proliferator in prostate cancer. Proceedings of the National Academy of Sciences of the United States of America 105, 12182–12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble RL, 1977a. The development of prostatic adenocarcinoma in Nb rats following prolonged sex hormone administration. Cancer research 37, 1929–1933. [PubMed] [Google Scholar]
- Noble RL, 1977b. Sex steroids as a cause of adenocarcinoma of the dorsal prostate in Nb rats, and their influence on the growth of transplants. Oncology 34, 138–141. [DOI] [PubMed] [Google Scholar]
- Ott CE, Knox FG, 1976. Tissue pressures and fluid dynamics in the kidney. Federation proceedings 35, 1872–1875. [PubMed] [Google Scholar]
- Parimi V, Goyal R, Poropatich K, Yang XJ, 2014. Neuroendocrine differentiation of prostate cancer: a review. American Journal of Clinical and Experimental Urology 2, 273–285. [PMC free article] [PubMed] [Google Scholar]
- Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr., Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, Benson MC, Small EJ, Raghavan D, Crawford ED, 2004. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. The New England journal of medicine 351, 1513–1520. [DOI] [PubMed] [Google Scholar]
- Pettaway CA, Pathak S, Greene G, Ramirez E, Wilson MR, Killion JJ, Fidler IJ, 1996. Selection of highly metastatic variants of different human prostatic carcinomas using orthotopic implantation in nude mice. Clinical cancer research : an official journal of the American Association for Cancer Research 2, 1627–1636. [PubMed] [Google Scholar]
- Pretlow TG, Wolman SR, Micale MA, Pelley RJ, Kursh ED, Resnick MI, Bodner DR, Jacobberger JW, Delmoro CM, Giaconia JM, Pretlow TP, 1993. Xenografts of Primary Human Prostatic Carcinoma. JNCI: Journal of the National Cancer Institute 85, 394–398. [DOI] [PubMed] [Google Scholar]
- Ratliff TL, 2005. Estrogen receptor alpha and imprinting of the neonatal mouse ventral prostate by estrogen. J Urol 174, 1149. [DOI] [PubMed] [Google Scholar]
- Rhim JS, Webber MM, Bello D, Lee MS, Arnstein P, Chen LS, Jay G, 1994. Stepwise immortalization and transformation of adult human prostate epithelial cells by a combination of HPV-18 and v-Ki-ras. Proceedings of the National Academy of Sciences of the United States of America 91, 11874–11878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricke EA, Williams K, Lee YF, Couto S, Wang Y, Hayward SW, Cunha GR, Ricke WA, 2012. Androgen hormone action in prostatic carcinogenesis: stromal androgen receptors mediate prostate cancer progression, malignant transformation and metastasis. Carcinogenesis 33, 1391–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricke WA, Ishii K, Ricke EA, Simko J, Wang Y, Hayward SW, Cunha GR, 2006. Steroid hormones stimulate human prostate cancer progression and metastasis. Int J Cancer 118, 2123–2131. [DOI] [PubMed] [Google Scholar]
- Ricke WA, McPherson SJ, Bianco JJ, Cunha GR, Wang Y, Risbridger GP, 2008. Prostatic hormonal carcinogenesis is mediated by in situ estrogen production and estrogen receptor alpha signaling. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 22, 1512–1520. [DOI] [PubMed] [Google Scholar]
- Risbridger G, Wang H, Young P, Kurita T, Wang YZ, Lubahn D, Gustafsson JA, Cunha G, 2001. Evidence that epithelial and mesenchymal estrogen receptor-alpha mediates effects of estrogen on prostatic epithelium. Developmental biology 229, 432–442. [DOI] [PubMed] [Google Scholar]
- Rivette AS, Tokar EJ, Williams DE, Mackenzie CD, Ablin RJ, Webber MM, 2005. Selection of cell lines with enhanced invasive phenotype from xenografts of the human prostate cancer cell line WPE1-NB26. Journal of experimental therapeutics & oncology 5, 111–123. [PubMed] [Google Scholar]
- Royuela M, de Miguel MP, Bethencourt FR, Sanchez-Chapado M, Fraile B, Arenas MI, Paniagua R, 2001. Estrogen receptors alpha and beta in the normal, hyperplastic and carcinomatous human prostate. The Journal of endocrinology 168, 447–454. [DOI] [PubMed] [Google Scholar]
- Rubin MA, De Marzo AM, 2004. Molecular genetics of human prostate cancer. Mod Pathol 17, 380–388. [DOI] [PubMed] [Google Scholar]
- Shirai T, Takahashi S, Cui L, Futakuchi M, Kato K, Tamano S, Imaida K, 2000. Experimental prostate carcinogenesis - rodent models. Mutation research 462, 219–226. [DOI] [PubMed] [Google Scholar]
- Simanainen U, Allan CM, Lim P, McPherson S, Jimenez M, Zajac JD, Davey RA, Handelsman DJ, 2007. Disruption of prostate epithelial androgen receptor impedes prostate lobe-specific growth and function. Endocrinology 148, 2264–2272. [DOI] [PubMed] [Google Scholar]
- Sobel RE, Sadar MD, 2005a. Cell lines used in prostate cancer research: a compendium of old and new lines--part 1. J Urol 173, 342–359. [DOI] [PubMed] [Google Scholar]
- Sobel RE, Sadar MD, 2005b. Cell lines used in prostate cancer research: a compendium of old and new lines--part 2. J Urol 173, 360–372. [DOI] [PubMed] [Google Scholar]
- Sramkoski RM, Pretlow TG, Giaconia JM, Pretlow TP, Schwartz S, Sy MS, Marengo SR, Rhim JS, Zhang D, Jacobberger JW, 1999. A new human prostate carcinoma cell line, 22Rv1. In vitro cellular & developmental biology. Animal 35, 403–409. [DOI] [PubMed] [Google Scholar]
- Steiner H, Godoy-Tundidor S, Rogatsch H, Berger AP, Fuchs D, Comuzzi B, Bartsch G, Hobisch A, Culig Z, 2003. Accelerated in vivo growth of prostate tumors that up-regulate interleukin-6 is associated with reduced retinoblastoma protein expression and activation of the mitogen-activated protein kinase pathway. Am J Pathol 162, 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson RA, Dinney CP, Gohji K, Ordonez NG, Killion JJ, Fidler IJ, 1992. Metastatic model for human prostate cancer using orthotopic implantation in nude mice. Journal of the National Cancer Institute 84, 951–957. [DOI] [PubMed] [Google Scholar]
- Tai S, Sun Y, Squires JM, Zhang H, Oh WK, Liang CZ, Huang J, 2011. PC3 is a cell line characteristic of prostatic small cell carcinoma. The Prostate 71, 1668–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taplin M-E, Balk SP, 2004. Androgen receptor: A key molecule in the progression of prostate cancer to hormone independence. Journal of Cellular Biochemistry 91, 483–190. [DOI] [PubMed] [Google Scholar]
- Tepper CG, Boucher DL, Ryan PE, Ma AH, Xia L, Lee LF, Pretlow TG, Kung HJ, 2002. Characterization of a novel androgen receptor mutation in a relapsed CWR22 prostate cancer xenograft and cell line. Cancer research 62, 6606–6614. [PubMed] [Google Scholar]
- Thalmann GN, Anezinis PE, Chang SM, Zhau HE, Kim EE, Hopwood VL, Pathak S, von Eschenbach AC, Chung LW, 1994. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer research 54, 2577–2581. [PubMed] [Google Scholar]
- Thalmann GN, Sikes RA, Wu TT, Degeorges A, Chang SM, Ozen M, Pathak S, Chung LW, 2000. LNCaP progression model of human prostate cancer: androgen-independence and osseous metastasis. The Prostate 44, 91–103 Jul 101;144(102). [DOI] [PubMed] [Google Scholar]
- Tilley WD, Buchanan G, Hickey TE, Bentel JM, 1996. Mutations in the androgen receptor gene are associated with progression of human prostate cancer to androgen independence. Clinical cancer research : an official journal of the American Association for Cancer Research 2, 277–285. [PubMed] [Google Scholar]
- True LD, Buhler K, Quinn J, Williams E, Nelson PS, Clegg N, Macoska JA, Norwood T, Liu A, Ellis W, Lange P, Vessella R, 2002. A neuroendocrine/small cell prostate carcinoma xenograft-LuCaP 49. Am J Pathol 161, 705–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldscholte J., Ris-Stalpers C., Kuiper GG., Jenster G., Berrevoets C., Claassen E., van Rooij HC., Trapman J., Brinkmann AO., Mulder E., 1990. A mutation in the ligand binding domain of the androgen receptor of human LNCaP cells affects steroid binding characteristics and response to anti-androgens. Biochemical and biophysical research communications 173, 534–540. [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Kaufman JM, Goemaere S, van Pottelberg I, 2002. Estradiol in elderly men. The aging male : the official journal of the International Society for the Study of the Aging Male 5, 98–102. [PubMed] [Google Scholar]
- Visakorpi T, Hyytinen E, Koivisto P, Tanner M, Keinanen R, Palmberg C, Palotie A, Tammela T, Isola J, Kallioniemi OP, 1995. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet 9, 401–406. [DOI] [PubMed] [Google Scholar]
- Wainstein MA, He F, Robinson D, Kung HJ, Schwartz S, Giaconia JM, Edgehouse NL, Pretlow TP, Bodner DR, Kursh ED, et al. , 1994. CWR22: androgen-dependent xenograft model derived from a primary human prostatic carcinoma. Cancer research 54, 6049–6052. [PubMed] [Google Scholar]
- Wang M, Stearns ME, 1991. Isolation and characterization of PC-3 human prostatic tumor sublines which preferentially metastasize to select organs in S.C.I.D. mice. Differentiation; research in biological diversity 48, 115–125. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hayward SW, Donjacour AA, Young P, Jacks T, Sage J, Dahiya R, Cardiff RD, Day ML, Cunha GR, 2000. Sex hormone-induced carcinogenesis in Rb-deficient prostate tissue. Cancer research 60, 6008–6017. [PubMed] [Google Scholar]
- Wang Y, Revelo MP, Sudilovsky D, Cao M, Chen WG, Goetz L, Xue H, Sadar M, Shappell SB, Cunha GR, Hayward SW, 2005. Development and characterization of efficient xenograft models for benign and malignant human prostate tissue. The Prostate 64, 149–159. [DOI] [PubMed] [Google Scholar]
- Wang Y, Sudilovsky D, Zhang B, Haughney PC, Rosen MA, Wu DS, Cunha TJ, Dahiya R, Cunha GR, Hayward SW, 2001. A human prostatic epithelial model of hormonal carcinogenesis. Cancer research 61, 6064–6072. [PubMed] [Google Scholar]
- Watson PA, Arora VK, Sawyers CL, 2015. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nature reviews. Cancer 15, 701–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibowo E, Schellhammer P, Wassersug RJ, 2011. Role of estrogen in normal male function: clinical implications for patients with prostate cancer on androgen deprivation therapy. J Urol 185, 17–23. [DOI] [PubMed] [Google Scholar]
- Wilding G, 1992. The importance of steroid hormones in prostate cancer. Cancer surveys 14, 113–130. [PubMed] [Google Scholar]
- Wu CT, Altuwaijri S, Ricke WA, Huang SP, Yeh S, Zhang C, Niu Y, Tsai MY, Chang C, 2007. Increased prostate cell proliferation and loss of cell differentiation in mice lacking prostate epithelial androgen receptor. Proceedings of the National Academy of Sciences of the United States of America 104, 12679–12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HC, Hsieh JT, Gleave ME, Brown NM, Pathak S, Chung LW, 1994. Derivation of androgen-independent human LNCaP prostatic cancer cell sublines: role of bone stromal cells. Int J Cancer 57, 406–412. [DOI] [PubMed] [Google Scholar]
- Wu TT, Sikes RA, Cui Q, Thalmann GN, Kao C, Murphy CF, Yang H, Zhau HE, Balian G, Chung LW, 1998. Establishing human prostate cancer cell xenografts in bone: induction of osteoblastic reaction by prostate-specific antigen-producing tumors in athymic and SCID/bg mice using LNCaP and lineage-derived metastatic sublines. Int J Cancer 77, 887–894. [DOI] [PubMed] [Google Scholar]
- Zhao XY, Malloy PJ, Krishnan AV, Swami S, Navone NM, Peehl DM, Feldman D, 2000. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nat Med 6, 703–706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.