Abstract
Background
Behaviour problems are prevalent among children born very preterm (≤ 32 weeks gestation), and have been associated with morphine exposure. Morphine accumulation in the brain is determined by genetic variations related to morphine biotransformation. The objective of the study was to investigate whether morphine-biotransformation genotypes contribute to individual differences in long-term effects of morphine on behaviour at 18 months corrected age (CA).
Methods
198 children born very preterm (24–32 weeks gestation) were followed from birth and seen at 18 months CA. Relationships between child behavior (Internalizing, Externalizing on the Child Behavior Checklist), morphine exposure, neonatal clinical variables, and morphine biotransformation gene variants in ABCB1, UGT1A9, UGT 2B7*2, ABCC2, ABCC3, SLCO1B1, CYP3A4, COMT were examined.
Findings
Neonatal clinical predictors and genotypes accounted for 39% of the overall variance in behaviour. In children with the minor allele of UGT1A9 rs17863783 (marker of UGT1A6*4, UDP-glucuronosyltransferase), greater morphine exposure (p = ·0011) was associated with more Internalizing behaviour. More Externalizing behaviour was predicted by greater morphine exposure in children with the COMT rs4680 Met/Met genotype (p = ·0006).
Interpretation
Genetic variations that affect relative accumulation of morphine in the brain, together with neonatal clinical factors, are differentially related to anxiety and depressive symptoms (internalizing) and to acting out (externalizing) behaviours at 18 months CA in children born very preterm.
Fund
NIH/NICHD HD039783 (REG); CIHR MOP86489 (REG), MOP68898 (SPM), MOP79262 (SPM, REG).
Keywords: Preterm, Pain, Morphine, Behaviour, Genetics, Polymorphism
Research in context.
Evidence before this study
Morphine is used in neonatal intensive care in many countries for infants requiring mechanical ventilation. Preclinical studies suggest morphine might associate with long-term changes in behaviour. However, the mechanism is largely unknown.
Added value of this study
In children born very preterm (≤ 32 weeks), UDP-glucuronosyltransferase UGT1A9 rs17863783 (synonymous variant of UGT1A6) and catechol-O-methyltransferase COMT rs4680 genotypes moderated the association between neonatal morphine exposure and behaviour at 18 months corrected age.
Implication of all the available evidence
Our findings highlight the importance of individual genetic differences in morphine biotransformation biomarkers in order to minimize long-term adverse effects of morphine exposure on behaviour in children born very preterm.
Alt-text: Unlabelled Box
1. Introduction
Morphine is used to manage distress in very preterm neonates undergoing mechanical ventilation in the neonatal intensive care unit (NICU). However there are concerns over possible long-term adverse effects on neurodevelopmental outcomes in this population due to their physiological immaturity and the difficulty of pain assessment. Morphine is the most common opioid analgesic used to treat neonatal pain associated with ventilation in the NICU [1,2], and is often used to lower the oxygen needs in ventilated neonates. Morphine is metabolized in the liver into 2 active compounds, morphine-3-glucuronide (M3G) and morphine-6-glucuronide (M6G) by UDP-glucuronosyltransferase. The former is an opioid antagonist, and the latter is a potent analgesic. Adverse effects of morphine exposure include increased hypotension risk [3], respiratory depression with prolonged need for ventilation and increased time to reach full feeds [4,5]. Morphine may have a specific effect on pulmonary mechanics or central (brainstem) respiration, possibly due to some as yet undefined direct toxicity such as histamine release and/or bronchospasm [6]. There is evidence in basic animal research suggesting morphine exposure induces long term changes in behaviour and brain function [7], and spatial recognition memory [8]. Therefore, it is important to understand the mechanism of morphine to improve the pain management for infants in the NICU and to improve the safety of morphine usage during ventilation.
Children born very preterm (≤ 32 weeks gestation) are exposed to repeated pain and stress of invasive clinical procedures during this critical period of rapid brain development. Despite the improved survival rate, children born very prematurely have greater risk for difficulty in attention, learning and memory, as well as behavioural and emotional problems [9]. Moreover, children born very preterm have increased rates of depressive and anxiety symptoms (internalizing behaviour) [[10], [11], [12], [13], [14], [15], [16]]. Our group have previously reported that greater morphine exposure in very preterm neonates was associated with more child internalizing behaviour at school age [17] and with reduced cerebellar growth from early in life to term equivalent age [18]. Neonatal pain/stress exposure was also associated with more behaviour problems at 7 age years in children born very preterm [19]. However, given similar exposure to neonatal pain/stress and morphine, not all children born very preterm will develop behaviour problems. One explanation is that variations in genes involved in morphine biotransformation may contribute to inter-individual variations in morphine response and susceptibility to adverse effects. Therefore, genetic predispositions and the interactions with environment may play an important role in identifying increased risk of behaviour problems in children born very preterm.
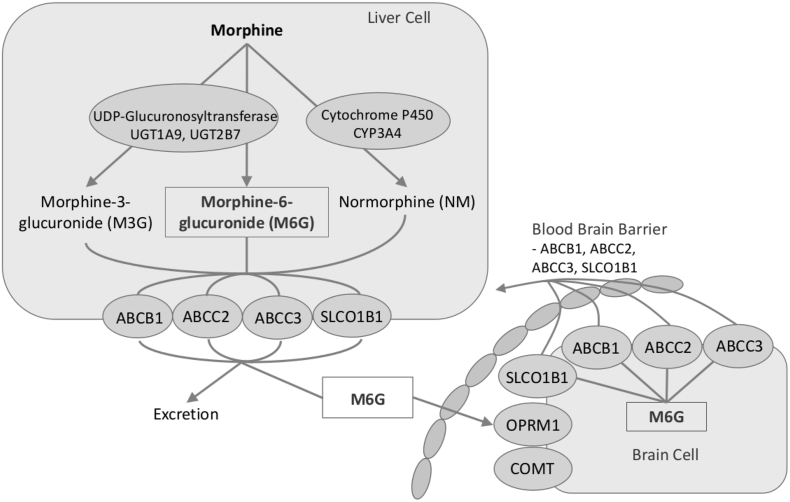
An opioid panel was created to better classify genetic variation using single nucleotide polymorphisms (SNP) in morphine's biotransformation pathway. Candidate gene variants that may influence responses to opioid therapy and pain perception were targeted based on previous evidence from (e.g. [20,21]), including multidrug resistance transporter (ABCB1), opioid receptor mu 1 (OPRM1), UDP glucuronosyltransferase 2B7 (UGT2B7) and 1A6 (UGT1A6), multidrug resistance-associated protein 2(ABCC2), multidrug resistance-associated protein 3 (ABCC3), solute carrier organic anion transporter family member 1B1 (SLCO1B1), cytochrome P450 (CYP3A4), and catechol-O-Methyltransferase (COMT). UGT1A9 rs17863783 is a synonymous variant (Val209Val) in UDP glucuronosyltransferase 1A6 (UGT1A6) and was included in this study in place of UGT1A6. A brief outline of their role is summarized (Fig. 1).
Fig. 1.
Genes involved in the morphine metabolism.
Morphine is glucuronidated to morphine-3-glucuronide (M3G) and morphine-6-glucuronide (M6G) by UGT2B7 in the liver and UGT1A9 in the liver. Cytochrome P450 CYP3A4 is involved in the biotransformation of morphine to normorphine (NM). Transporters (ABCB1, ABCC2, ABCC3 and SLCO1B1) are also included in this figure as they influence clearance of morphine and their metabolites. The transporters present at the blood-brain barrier as well as metabolic enzymes (COMT) and receptor (OPRM1) also play an important role in the pharmacokinetics of morphine.
Greater enzymatic activity of UGT2B7, UGT1A9 and CYP3A4 is associated with higher levels of morphine metabolites. Greater density of OPRM1 is associated with greater morphine analgesic effect, while greater density of ABCB1, ABCC2, ABCC3 and SLCO1B1 was associated with less analgesic effect. COMT is associated with pain sensitivity and may be associated with OPRM1 density.
In order to further our understanding of the possible mechanisms that may lead to long-term behaviour problems related to neonatal morphine exposure in children born very preterm, the aim of this study was to explore the roles of SNPs in the morphine biotransformation pathway and their interactions with morphine exposure (adjusted for confounding clinical factors) to predict behaviour problems in children born very preterm at 18 months corrected age.
2. Method
2.1. Participants
Participants were included from a convenience sample of infants combined from two longitudinal studies of long-term effects of neonatal pain/stress on brain, stress and neurodevelopment of children born very preterm (24–32 weeks gestation) (e.g. [22,23]), admitted to the level III NICU at BC Women's Hospital in Vancouver Canada. The first cohort was recruited 2000–2004 (N = 58) and the second cohort 2006–2012 (N = 133). Total of N = 191 children born very preterm at 18 months corrected age (CA) were included in this study (105 boys/86 girls; mean age 19·3 months CCA [s.d. = 1·1 months] range 17·9–23·8 months). 12.5% of the 2000–2004 and 7.8% of the 2006–2012 cohort was lost to follow-up. Parents completed questionnaires while children were undergoing psychometric testing.
Exclusion criteria were no genetic syndromes, or major cognitive, sensory, motor impairments, or diagnosis of autism spectrum disorder. Patients with major cognitive, sensory or motor impairment were excluded because the etiology of their poorer outcomes would be very different and we will be unable to conclude whether the poorer outcomes were due to disability or due to early exposure to pain-related stress. This study was approved by the Clinical Research Ethics Board of the University of British Columbia and the Research Ethics Board of the B.C. Children's & Women's Hospitals, and conforms to the conventions set out in the Declaration of Helsinki. Written informed consent was obtained from parents.
2.2. Neonatal data
Daily prospective medical and nursing chart reviews were carried out by an experienced neonatal research nurse. The data collection included but was not limited to, GA, birth weight, illness severity on day 1 (Score for Neonatal Acute Physiology [SNAP]- II [24]), days of mechanical ventilation, presence of culture proven infection, number of surgeries, and cumulative dose of morphine (Table 2). We quantified neonatal pain/stress as the number of skin-breaking procedures (e.g., heel lance, peripheral intravenous or central line insertion, chest-tube insertion, and nasogastric tube insertion) during the stay in the NICU, as previously reported (e.g. [23,25]). Each attempt at a procedure was counted as one skin-break; all nursing staff in our NICU have been trained to precisely record each attempt. Cumulative morphine exposure was calculated as the average daily dose (i.e. intravenous dose plus intravenous-equivalent oral dose) adjusted for daily body weight, multiplied by the number of days morphine was administered.
Table 2.
CPCA component loadings of CBCL behavior problems predicted by G1 G2 independently and interactively.
|
G1 G2 Independently |
G1 × G2 interactively |
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
PC1ind |
PC1 G1×G2 |
PC2 G1×G2 |
|||||||
| Variables | Loadings | p valuea | BHFDRb | Loadings | p valuea | BHFDRb | Loadings | p valuea | BHFDRb |
| Internalizing pro0062lems | 0·8826 | <0·0001 | 0·0002 | 0·7254 | <0·0001 | <0·0001 | 0·4687 | 0·0014 | 0·0054 |
| Externalizing problems | 0·1060 | 0·6823 | 0·7798 | 0·3288 | 0·0004 | 0·0018 | 0·8658 | <0·0001 | <0·0001 |
| Affective problems | 0·7605 | 0·0209 | 0·0577 | 0·7279 | 0·0022 | 0·0054 | 0·4103 | 0·0117 | 0·0187 |
| Anxiety problems | 0·8496 | 0·0216 | 0·0577 | 0·8789 | 0·0027 | 0·0054 | 0·0696 | 0·6931 | 0·6931 |
| Pervasive dev problems | 0·7329 | 0·0430 | 0·0860 | 0·6535 | 0·0193 | 0·0258 | 0·4589 | 0·0703 | 0·0937 |
| Sleep problems | 0·5811 | 0·0901 | 0·1442 | 0·6868 | 0·0055 | 0·0088 | 0·3242 | 0·2218 | 0·2535 |
| Attention deficit/hyperactivity problems | −0·0802 | 0·7987 | 0·7987 | 0·3021 | 0·1695 | 0·1937 | 0·7438 | 0·0021 | 0·0043 |
| Opposition defiant problems | 0·3979 | 0·2235 | 0·2980 | 0·2083 | 0·2077 | 0·2077 | 0·8358 | 0·0004 | 0·0015 |
Bolded and italicized for p < ·0063 after adjusted for Bonferroni for the number of outcomes.
Bolded for p < ·05 after adjusted for 5% Benjamini Hochberg FDR (BHFDR).
Morphine was the opioid used for routine management of mechanical ventilation in the preterm population at this site. The morphine starting dose was according to the hospital pharmacy protocol based on the infant's weight on that day. Morphine dosing was then adjusted according to ongoing clinical assessment of pain and illness severity by the nursing and medical staff. Other opioids were only given in the context of surgery, thus rarely used in the study site. Number of surgeries was included as a neonatal clinical variable in data analyses.
2.3. Measures/assessments
Parents completed the Child Behaviour Checklist (CBCL) for children ages 1·5 to 5 years, [26] the most widely used questionnaire for identifying problem behaviours in children. The six DSM –oriented scales (Affective Problems, Anxiety Problems, Pervasive Developmental Problems, Sleep Problems, Attention Deficit/Hyperactivity Problems, and Oppositional Defiant Problem) and two higher order-factors of Internalizing and Externalizing Problems were used in the study. Raw scores were converted to age-standardized scores (T scores with mean = 50 and SD = 10) based on the normative samples of children for age separately by sex [26]. The CBCL has an alpha coefficient of 0·97 [26].
2.4. Genotyping
Scientific literature and publicly available databases were screened and functional polymorphisms in candidate genes (ABCB1, ABCC2, ABCC3, COMT, CYP3A4, OPRM1, and SLCO1B1) were selected for genotyping. DNA was extracted from maternal saliva samples using a QiaSymphony™ DNA Purification Instrument (Qiagen, USA) according to the manufacturer's protocol.
Variants in ABCB1 (rs2032582, rs1045642, rs1128503), OPRM1 (rs563649, rs1799971), UGT2B72*2 (rs7662029), UGT1A9 (rs17863783), ABCC2 (rs2273697, rs17222723, rs8187710), ABCC3 (rs2277624, rs11568591), SLCO1B1 (rs4149056), CYP3A4 (rs2242480), COMT (rs4680) were genotyped using TaqMan® genotyping assays according to the manufacturer's recommendations for ABCB1 (C_7586662_10, C_11711720C_30 + C_11711720D_40, C_7586657_20), OPRM1 (C_809947_10, C_8950074_1), UGT2B7*2 (C_30720663_20), UGT1A6 (C_25972736_20), ABCC2 (C_22272980_20, C_25591743_30, C_22272567_30), ABCC3 (C_15885015_40, C_31810858_20), SLCO1B1 (C_30633906_10), CYP3A4 (C_26201900_30), and COMT (C_25746809_50) (Thermo-Fisher, USA).
2.5. Statistical analysis
Constrained Principal component analysis (CPCA) with interaction terms was used to examine sex, morphine exposure (adjusted for neonatal clinical confounders: GA, SNAP-II, infection, number of invasive procedures, number of surgeries), genotypes of genes involved in the morphine metabolic pathway (ABCB1 (rs2032582, rs1045642, rs1128503), OPRM1 (rs563649, rs1799971), UGT2B7*2 (rs7662029), UGT1A9 (rs17863783), ABCC2 (rs2273697, rs17222723, rs8187710), ABCC3 (rs2277624, rs11568591), SLCO1B1 (rs4149056), CYP3A4 (rs2242480), COMT (rs4680)), and their interactions in relation to behaviours measured by the CBCL (Internalizing, Externalizing), Affective, Anxiety, Pervasive Developmental, Sleep, Attention Deficit/Hyperactivity, and Oppositional Defiant problems). CPCA is a 2-step process, referred to as the external and internal analysis.
The external analysis consists of a multivariate multiple regression of the dependent measures on the independent measures, producing predicted and residual scores for each dependent measure. In the present study, the matrix of predicted scores (Z) consisted of CBCL outcomes that were regressed onto neonatal clinical variables (G1) and genotypes (G2), then to the interactions between clinical variables and genotypes (G1 xG2). For G2, the homozygous and heterozygous of minor alleles of rs2032582_ABCB1, rs2273697_ABCC2, rs8187710_ABCC2, rs2277624_ABCC3, rs11568591_ABCC3, rs4149056_SLCO1B1, and rs2242480_CYP3A4 were combined and coded as 1 while the major alleles were coded as 0 because there were <5 homozygous minor alleles. For the rest, homozygous of major alleles was coded as 0, heterozygous was 1, and homozygous of minor alleles was 2. Therefore the major alleles were the reference categories in all subsequent analyses. Missing genotypes (0.1%) were imputed by KNN (K-Nearest-Neighbor) methods. The internal analysis consists of principal component analyses on each of the aforementioned matrices (outcomes, independent main effects only, and interaction terms). The resulting component solutions (overall, predicted, and residual solutions) were examined to determine which CBCL behaviours were explained by the neonatal clinical variables, genotypes and their interactions. CPCA results were bootstrapped 1000 times to compute confidence interval and p-values. Multiple comparisons were adjusted by 5% False Discovery Rate (FDR) (Benjamini & Hochberg, 1995; Storey & Tibshirani, 2003) and Bonferroni method. Details of CPCA have been described previously [27,28]. Computations for CPCA were done using MATLAB v 8.5.0 (R2015a) (The MathWorks, 2010, Natick, Massachusetts). Because of the extensive number of interaction terms in the CPCA, Generalized Linear Modeling (GZLM) was then performed using SPSS (version 25) to confirm the results.
3. Results
3.1. Subject characteristics
N = 191 children born very preterm, with complete neonatal clinical data and the CBCL were included in the study. Only 0·1% of genotype data was missing. CBCL behavioural problems subscales at 18 months and neonatal characteristics were summarized in Table 1. We could not examine the role of sedatives since only eight infants received a sedative (midazolam).
Table 1.
Participant characteristics.
| N = 191 | Mean (SD) | Range |
|---|---|---|
| Male (number, %) | 105 (55) | |
| Gestational age at birth (weeks) | 28·2 (2·5) | 24·0–32·3 |
| Birth weight (g) | 1125 (395) | 459–2350 |
| Skin-breaks | 130 (85) | 20–446 |
| Cumulative morphine dose (mg/kg) | 4·3 (10·0) | 0–58·3 |
| Cumulative midazolam dose (mg/kg) | 4·7 (12·5) | 0–77·0 |
| Days of mechanical ventilation | 32 (23) | 0–83 |
| Illness severity day I (SNAP-II) | 14·0 (13·0) | 0–56 |
| Number of surgeries | 0·5 (1·0) | 0–4 |
| Postnatal infection (yes/no, %) | 100 (52) |
3.2. Constrained principal component analysis (CPCA)
The external analysis of CPCA showed that the predictors (sex, GA, neonatal infection, number of invasive procedures, number of surgeries, illness severity on day 1, morphine exposure, ABCB1, OPRM1, UGT1A9, ABCC2, ABCC3, SLCO1B1, CYP3A4, and COMT genotypes) accounted for 38·5% of the overall variance in CBCL behaviour problems. One component was extracted from the predicted solutions by neonatal clinical variables (G1) and genotypes (G2) independently (PC1ind), which corresponded to 5·2% of the overall variance. Interactions between the neonatal variables (G1) and genotypes (G2) accounted for 33·3% of variance of CBCL behaviour problems. Two main components, which corresponded to 17·3% (PC1 G1×G2) and 16·0% (PC2 G1×G2) respectively, were extracted from the predicted solutions by the interactions.
Component loadings, their bootstrapped p values and FDR adjusted p values for CBCL behaviours, predicted by clinical variables (G1) and genotype (G2) independently and interactively (G1 × G2) are summarized in Table 2. All CPCA components (G1 and G2 independently and G1 × G2) were positively loaded on all CBCL behaviours, which indicated that greater component loadings were associated with greater CBCL behaviour problems. For behaviour problems explained by the neonatal variables (G1) and genotypes (G2) independently, one component was extracted (PC1ind) and it was significantly loaded on CBCL Internalizing (p < .0001). Two components (PC1 G1×G2 and PC2 G1×G2) from the interactions between neonatal variables and genotypes were extracted to predict CBCL behaviour problems. The dominant loadings on the PC1 G1×G2 were distributed mainly on CBCL Internalizing (p = 5·8 × 10−7), Externalizing (p = 0·0004), Affective (p = ·0014), Anxiety (p = ·0031), and Sleep problems (p = ·0022). Meanwhile, PC2 G1×G2 were dominantly loaded on Externalizing (p = 2·2 × 10−16), Internalizing (p = 0·0014), Attention Deficit/Hyperactivity (p = ·0021) and Oppositional Defiant behaviours (p = ·0004).
Predictor loadings for clinical variables (G1) and genotypes (G2) independently are summarized In Appendix Table 1. Positive loadings were associated with greater CBCL Internalizing. Component 1 (PC1ind) reflected neonatal infection, invasive procedures, rs1128503_ABCB1, rs563649_OPRM1, rs17863783_UGT1A9, rs2273697_ABCC2, rs2277624_ABCC3, rs11568591_ABCC3, and rs2242480_CYP3A4 genotypes. Greater neonatal invasive procedures and illness severity on day 1 were associated with greater CBCL Internalizing (p = 3·74 × 10−7 and p = ·004 respectively). Less ABCB1 activity, which may lead to greater morphine biotransformation, was associated with less CBCL Internalizing (p = ·0003). Also, less OPRM1 production was associated with less CBCL Internalizing (p = 0·0002). Minor alleles of multidrug transporter ABCC3 genotypes (rs2277624 and rs11568591) were also associated with less CBCL Internalizing (p = 0·001 and 0·00002 respectively). Finally, the minor allele of UGT2B7*2 was associated with less CBCL Internalizing (p = 1·8 × 10−6).
All predictor loadings for clinical variables and genotypes interactively (G1 × G2) are provided in Appendix Table 2. The results were then split into 2 tables for the ease of interpretation: Significant interaction component 1 (PC1 G1×G2) (Table 3a), and Significant interaction component 2 (PC2 G1×G2) (Table 3b). In Tables 3a, PC1 G1×G2, which was associated dominantly with CBCL Internalizing behaviour, was mainly explained by the interactions between UGT1A9 genotypes and neonatal variables. In Children with UGT1A9 minor allele, greater morphine exposure and greater number of surgeries were also associated with greater Internalizing (p = .0003 and p = .0001 respectively). Results were not significant for UGT1A9 major allele. Using the predictor loading of UGT1A9 × morphine dose (0·233) and component loading of CBCL Internalizing (0·725), every 9·3 mg/kg dose of morphine in children with a minor allele of UGT1A9 was associated with 7·8 increase in the CBCL Internalizing T score at 18 months. In children with ABCB1 rs2032582 any T allele, greater number of invasive procedures was associated with greater Internalizing as well (p = ·0003). There was no significant association between UGT1A9 genotypes and morphine exposure (r = ·023, p = ·751).
Table 3a.
Significant interaction terms for component 1 (PC1 G1×G2) Internalizing Problems.
|
G1 |
G2 |
PC1 G1×G2 |
|||
|---|---|---|---|---|---|
| Variables | Variables | loadings | p valuesa | FDRb | |
| Morphine dose | × | rs17863783_UGT1A9 | 0·233 | 0·0011 | 0·0288 |
| Invasive procedures | × | rs1045642_ABCB1 | 0·265 | 0·0003 | 0·0146 |
| Number of surgeries | × | rs17863783_UGT1A9 | 0·272 | 0·0003 | 0·0146 |
Bolded and italicized for p < ·00048after adjusted for Bonferroni for the number of outcomes.
Bolded for p < ·05 after adjusted for 5% Benjamini Hochberg FDR (BHFDR).
Table 3b.
Significant interaction terms for component 2 (PC2 G1xG2) Externalizing Problems.
|
G1 |
G2 |
PC2 G1xG2 |
|||
|---|---|---|---|---|---|
| Variables | Variables | Loadings | p valuesa | FDRb | |
| Morphine dose | × | rs4680_COMT | 0·2522 | 0·0006 | 0·0210 |
| Neonatal infection | × | rs1045642_ABCB1 | −0·2465 | 0·0005 | 0·0210 |
Bolded and italicized for p < ·00048after adjusted for Bonferroni for the number of outcomes.
Bolded for p < ·05 after adjusted for 5% Benjamini Hochberg FDR (BHFDR).
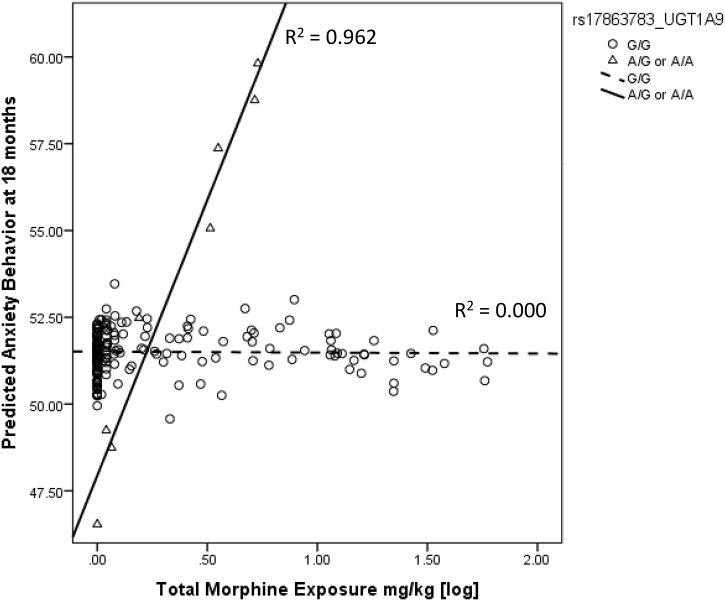
After using Constrained Principal Analysis (CPCA) to identify the significant interaction between UGT1A9 and morphine exposure, to confirm our finding, a separate Generalized Linear Model (GZLM) was used to evaluate the relationship between morphine exposure and CBCL Anxiety, moderated by UGT1A9 genotypes. Morphine exposure, UTG1A9 genotype, and their interaction were predictors, with GA, infection, number of invasive procedures, number of surgeries, illness severity on day 1 as covariates. CBCL Anxiety at 18 months was significantly predicted by the interaction between UGT1A9 genotype and morphine exposure. In children with a UGT1A9 minor allele, greater morphine exposure was associated with greater anxiety behaviour (R2 = 0.962, p = .001) (Fig. 2).
Fig. 2.
Interaction between UGT1A9 rs17863783 genotype and morphine exposure predicts behaviour problem at 18 months.
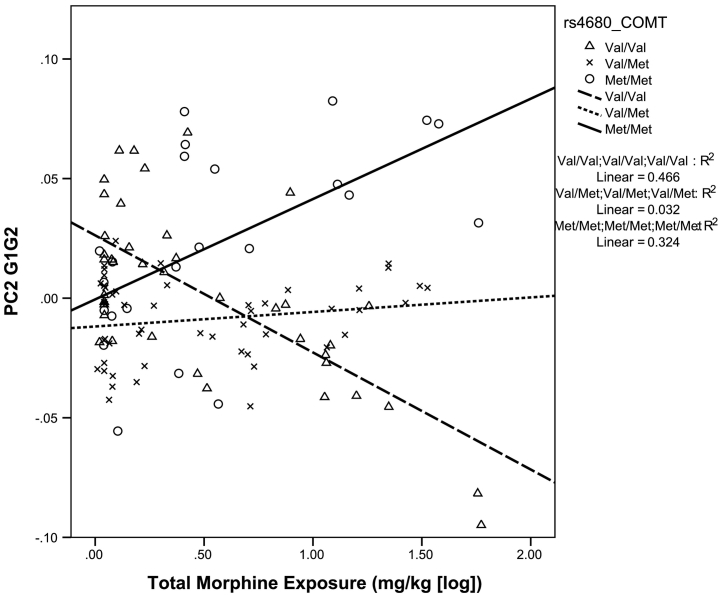
Component 2 of the interaction matrix (PC2 G1×G2), which was associated dominantly with CBCL Externalizing, was mainly explained by interaction between COMT genotype and morphine exposure (p = ·0006). In children with COMT Met/Met genotype, greater morphine exposure was associated with greater PC2 G1×G2 (Externalizing Problems). However, in children with COMT Val/Val genotype, less PC2 G1×G2 was associated with greater morphine exposure (Fig. 3). There is no significant correlation between UGT1A9 genotypes and morphine exposure (r = ·060, p = ·4121). PC2 G1×G2 was also explained by the interactions between the number of invasive procedures, neonatal infection and genotypes of multi-drugs transporters (ABCB1, ABCC3) (Table 3b). Sex was not significant in interaction terms or main effects, therefore we re-ran the Constrained Principal Component Analysis (CPCA) model without sex as a variable. In the CPCA model without sex as a variable, all loadings and p-values were identical and the results remained unchanged.
Fig. 3.
Interaction between COMT rs4680 genotype and morphine exposure predict behaviour problem at 18 months.
4. Discussion
This is the first study to our knowledge that investigate the associations between neonatal morphine exposure, SNPs in morphine biotransformation, and later behavioural outcomes. In an exploratory study, we examined the role of single nucleotide polymorphisms in a morphine biotransformation panel (ABCB1, ABCC2, ABCC3, OPRM1, UGT2B7, UTG1A9, SLCO1B1, CYP3A4, and COMT) and their interactions with neonatal clinical factors, in relation to behaviour problems in children born very preterm at 18 months corrected age. We found that anxiety and depressive (internalizing) behaviours at 18 months were significantly related to interactions between neonatal morphine exposure and UGT1A9 genotype; and externalizing behaviour was significantly related to the interaction between neonatal morphine exposure and COMT genotype.
In this study, the relationship between neonatal morphine exposure and internalizing behaviour at 18 months was moderated by UGT1A9 genotype such that in children with the minor allele (rs17863783 T allele) of UGT1A9, higher morphine dose was associated with more internalizing behaviour. In our dataset, each increase of 9.3 mg/kg in the total exposure of morphine in children with a minor allele of UGT1A9 was associated with 7.8 increase in the CBCL Internalizing T score at 18 months after adjusting for other clinical confounders. One standard deviation in a CBCL T score is 10, therefore the higher dosing of morphine is associated with a clinically important impact on child behaviour (more than ¾ of a standard deviation), for children with this minor allele. UGT1A9 rs17863783 is a synonymous variant (Val209Val) in UDP glucuronosyltransferase 1A6 (UGT1A6), and is highly expressed in the liver, kidney and bladder, and moderately expressed in the brain [21,29]. UGT1A6 is known to glucuronidate several different substrates including morphine, and this variant “tags” a specific haplotype of UGT1A6 (UGT1A6*4) that has been shown to have altered enzyme activity [29]. However, this glucuronidate effect might be substrate specific. The minor allele of rs17863783 has been associated with increased mRNA expression and protein levels of UGT1A6 in-vitro and in human liver tissue samples [30]. Therefore children with a minor allele of rs17863783 may have greater UGT1A6 enzyme activity and lower morphine metabolites levels. However, no study linking this SNP with morphine metabolites levels is available and further studies are required. Carleton and colleagues recently identified this variant as highly associated with a serious adverse drug reaction to a commonly used chemotherapeutic agent [21]. The results of our present study further suggest the important role of UDP-glucuronosyltransferase as an early stage of morphine biotransformation to convert morphine to either morphine-3-glucuronide or morphine-6-glucuronide. One possible explanation of greater internalizing behaviour may be due to the accumulation of M3G and M6G from the increased UDP-glucuronosyltransferase levels [31]. M3G has been shown to dose-dependently evoke neuro-excitatory behaviours in rats [32] and may lead to neurotoxicity [33] [34].
Both UGT1A6 and UGT2B7 are involved in xenobiotic biotransformation, and UGT2B7 isoform is the most important in morphine biotransformation [20,21]. Therefore, it was unexpected to find an association of UGT1A6 with morphine exposure and long-term behaviour outcomes instead of UGT2B7. One possible explanation may be due to the location of gene expression in the brain. Although in fetal brain, both UGT1A6 and UGT2B7 are expressed at very low levels, King et al. 1999 found that UGT2B7 expression was lower than UGT1A6 in the fetal brain and not expressed in brain cortex [35]. Also, UGT1A6 in brain has a much higher activity for serotonin than UGT2B7 [35], and thus may have a greater role in long term behaviour change.
The relationship between morphine exposure and externalizing behaviour was moderated by COMT rs4680 genotype so that in Met/Met children, greater morphine exposure was associated with greater externalizing behaviour. In our dataset, every 9.3 mg/kg dose of morphine in Met/Met children was associated with 4.5 increase in the CBCL Externalizing T score (close to half a standard deviation) at 18 months after adjusting for other clinical confounders. In children with COMT Val/Val, greater morphine exposure was associated with less externalizing behaviour. Specifically, in children with the Val/Val genotype, each 9.4 mg/kg dose of morphine was associated with a 4.5 decrease in the Externalizing T score after adjusting for other clinical confounders. The COMT rs4680 Met allele has previously been associated with greater pain sensitivity [36,37], and was also associated with higher pain scores after painful procedures in adults [38]. COMT genotype was also found to be associated with morphine pharmacodynamics [39]; adult cancer patients with COMT 158Met/Met genotype were shown to require lower morphine doses for the cancer neurotrophic pain than the Val/Val genotype [40]. Moreover, infants with Val/Val may have diminished opioid-induced pain relief [41,42] and thus may require higher morphine dos. Matsuoka et al. (2012) propose that may be due to increased density of mu-opioid receptors in Met/Met individuals [43,44]. We found that in COMT Val/Val children, greater morphine exposure was associated with less externalizing behaviour. One recent study of preterm neonates exposed to low dose morphine (median 0.8 mg/kg; range 0.5–1.2 mg/kg, as compared to mean 3.6 mg/kg and range 0–58.8 mg/kg in the present study) has suggested that morphine-treated children showed fewer problems with executive functions by parent report [45]. Improved executive function including better inhibition may thus result in less externalizing behaviour in early childhood [46]. Children with COMT Val/Val genotype who have lower morphine efficacy may thus be protected by morphine. However, our previous findings suggested that morphine at higher doses may be associated with adverse brain development [17,18,28]. Morphine dosing in the NICU is adjusted according to clinical response. Therefore it is likely that neonates with the COMT rs4680 Met allele would receive higher exposure to morphine. However, children with greater pain sensitivity and with higher morphine efficacy (COMT Met/Met) may not necessary have sufficiently effective biotransformation. Such increase in morphine dose in neonates with higher efficacy of morphine with moderate or low morphine biotransformation may lead to adverse drug reactions and poorer long-term outcomes. Our result suggests that the practice of increasing the morphine dose according to clinical response has to be more carefully assessed and documented. With the advancement of fast and high throughput genotyping technology, these results may support the advantage of personalized medicine where we will know that morphine is better for some neonates and another medications may be better for others.
Some children in this study had behaviour problems despite no or low morphine exposure, indicating that the particular genotype (for example the COMT Met/Met individual) may have greater susceptibility for anxiety and depression [47,48]. Another important point is that these morphine biotransformation-related genes are also involved in other pathways so that we only captured a part of their functions in this study.
We chose to use DSM-oriented subscales in addition to empirically based broad-band syndrome scales (internalizing and externalizing) based on previous studies showing that DSM-oriented subscales was able to better discriminate affective problem from anxiety problem in young girls, and provide good supplementary information [49]. Also, the use of DSM nosology may be easier to align with behavioural clinical diagnosis at older ages.
We did not find any significant sex-specific effects. It was possible that there may be sex dimorphic associations between clinical variables, genotypes and behavioural outcomes, however, our study did not have the power to explore all 3-ways interactions between sex, morphine exposure, and genotypes because of the low frequencies of some rare variants (e.g. UGT1A9 rs1786378, ABCC3 rs11568591). Another limitation is that we were unable to investigate possible gene-gene interactions (e.g. COMT and OPRM1 combined high-risk genotypes [42]; a far larger sample size would be necessary to evaluate these interactions. Moreover, we could not examine effects of additional exposure to other opioids such as fentanyl in the context of surgery, or to sedatives.
Our exploratory analysis highlighted a few biomarkers of morphine biotransformation and their interactions with clinical variables. However, gene expression levels in liver and brain, and the levels morphine metabolites cannot be directly measured in human subjects. Animal models are needed to further confirm the functions of the genes.
5. Conclusion
Our findings suggest that higher exposure to neonatal morphine is associated with more internalizing and externalizing behaviours at corrected age 18 months in children born very preterm, but that effects are moderated by genetic markers in the morphine metabolic pathway, especially the variants of UDP-glucuronosyltransferase and catechol-O-methyltransferase. We found complex relationships between different biomarkers in the morphine biotransformation pathway and neonatal clinical factors in relation to long-term behavioural outcomes. These findings highlight the importance of individual genetic differences in morphine biotransformation biomarkers in order to minimize long-term adverse effects of high morphine exposure on behaviour in children born very preterm.
The following are the supplementary data related to this article.
Predictor loadings for G1 G2 independently.
Predictor loadings for G1 G2 independently.
Predictor loadings for G1 × G2.
Funding sources
This work was supported by The Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health NIH/NICHD HD039783 (REG); and the Canadian Institutes of Health Research CIHRMOP86489 (REG), MOP68898 (SPM), MOP79262 (SPM, REG). We were not paid to write this article by a pharmaceutical company or other agency.
Declaration of interests
Dr. Ross, Dr. Miller, Dr. Grunau report grants from Canadian Institutes for Health Research, during the conduct of the study. Dr. Grunau and Dr. Chau reports grants from Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health NIH/NICHD, grants from Canadian Institutes of Health Research, during the conduct of the study. Mr. Chau, Dr. Carleton, Dr. Synnes have nothing to disclose.
Author contributions
CMY Chau conducted data collection, data analysis, and together with CJD Ross, interpreted the data and wrote the manuscript, contributing equally as the first authors. V Chau, SP Miller and AR Synnes participated in data collection and edited the manuscript. B Carleton and RE Grunau designed the study, contributed to interpretation and edited the manuscript, contributing equally as the senior authors. All authors provided feedback on drafts of this article, and read and approved the final manuscript.
Acknowledgements
We thank all the families who participated in the study and the staff in the Neonatal Follow-up Program at BC Women's Hospital.
References
- 1.Hall R.W., Boyle E., Young T. Do ventilated neonates require pain management? Semin Perinatol. 2007 Oct;31(5):289–297. doi: 10.1053/j.semperi.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Carbajal R., Eriksson M., Courtois E., Boyle E., Avila-Alvarez A., Andersen R.D. Sedation and analgesia practices in neonatal intensive care units (EUROPAIN): results from a prospective cohort study. Lancet Respir Med. 2015 Oct;3(10):796–812. doi: 10.1016/S2213-2600(15)00331-8. [DOI] [PubMed] [Google Scholar]
- 3.Hall R.W., Kronsberg S.S., Barton B.A., Kaiser J.R., Anand K.J., NEOPAIN Trial Investigators Group Morphine, hypotension, and adverse outcomes among preterm neonates: who's to blame? Secondary results from the NEOPAIN trial. Pediatrics. 2005 May;115(5):1351–1359. doi: 10.1542/peds.2004-1398. [DOI] [PubMed] [Google Scholar]
- 4.Simons S.H., van Dijk M., van Lingen R.A., Roofthooft D., Duivenvoorden H.J., Jongeneel N. Routine morphine infusion in preterm newborns who received ventilatory support: a randomized controlled trial. JAMA. 2003 Nov 12;290(18):2419–2427. doi: 10.1001/jama.290.18.2419. [DOI] [PubMed] [Google Scholar]
- 5.Anand K.J., Hall R.W., Desai N., Shephard B., Bergqvist L.L., Young T.E. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet. 2004 May 22;363(9422):1673–1682. doi: 10.1016/S0140-6736(04)16251-X. [DOI] [PubMed] [Google Scholar]
- 6.Levene M. Morphine sedation in ventilated newborns: who are we treating? Pediatrics. 2005 Aug;116(2):492–493. doi: 10.1542/peds.2005-0441. [DOI] [PubMed] [Google Scholar]
- 7.Handelmann G.E., Dow-Edwards D. Modulation of brain development by morphine: effects on central motor systems and behavior. Peptides. 1985;6(Suppl. 2):29–34. doi: 10.1016/0196-9781(85)90131-7. [DOI] [PubMed] [Google Scholar]
- 8.Ma M.X., Chen Y.M., He J., Zeng T., Wang J.H. Effects of morphine and its withdrawal on Y-maze spatial recognition memory in mice. Neuroscience. 2007 Jul 29;147(4):1059–1065. doi: 10.1016/j.neuroscience.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 9.Gray R.F., Indurkhya A., McCormick M.C. Prevalence, stability, and predictors of clinically significant behavior problems in low birth weight children at 3, 5, and 8 years of age. Pediatrics. 2004 Sep;114(3):736–743. doi: 10.1542/peds.2003-1150-L. [DOI] [PubMed] [Google Scholar]
- 10.Aarnoudse-Moens C.S., Weisglas-Kuperus N., van Goudoever J.B., Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009 Aug;124(2):717–728. doi: 10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- 11.Bhutta A.T., Cleves M.A., Casey P.H., Cradock M.M., Anand K.J.S. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA. 2002;288(6):728–737. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- 12.Anderson P., Doyle L.W., Victorian Infant Collaborative Study Group Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. JAMA. 2003 Jun 25;289(24):3264–3272. doi: 10.1001/jama.289.24.3264. [DOI] [PubMed] [Google Scholar]
- 13.Grunau R.E., Whitfield M.F., Fay T.B. Psychosocial and academic characteristics of extremely low birth weight (< or =800 g) adolescents who are free of major impairment compared with term-born control subjects. Pediatrics. 2004 Dec;114(6):e725–e732. doi: 10.1542/peds.2004-0932. [DOI] [PubMed] [Google Scholar]
- 14.Loe I.M., Lee E.S., Luna B., Feldman H.M. Behavior problems of 9-16 year old preterm children: biological, sociodemographic, and intellectual contributions. Early Hum Dev. 2011 Apr;87(4):247–252. doi: 10.1016/j.earlhumdev.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt L.A., Miskovic V., Boyle M.H., Saigal S. Shyness and timidity in young adults who were born at extremely low birth weight. Pediatrics. 2008 Jul;122(1):e181–e187. doi: 10.1542/peds.2007-3747. [DOI] [PubMed] [Google Scholar]
- 16.van Baar A.L., Vermaas J., Knots E., de Kleine M.J., Soons P. Functioning at school age of moderately preterm children born at 32 to 36 weeks' gestational age. Pediatrics. 2009 Jul;124(1):251–257. doi: 10.1542/peds.2008-2315. [DOI] [PubMed] [Google Scholar]
- 17.Ranger M., Synnes A.R., Vinall J., Grunau R.E. Internalizing behaviours in school-age children born very preterm are predicted by neonatal pain and morphine exposure. Eur J Pain. 2014;18(6):844–852. doi: 10.1002/j.1532-2149.2013.00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zwicker J.G., Miller S.P., Grunau R.E., Chau V., Brant R., Studholme C. Smaller cerebellar growth and poorer neurodevelopmental outcomes in very preterm infants exposed to neonatal morphine. J Pediatr. 2016;172:81–87. doi: 10.1016/j.jpeds.2015.12.024. May. (e2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chau C.M.Y., Ranger M., Sulistyoningrum D., Devlin A.M., Oberlander T.F., Grunau R.E. Neonatal pain and COMT Val158Met genotype in relation to serotonin transporter (SLC6A4) promoter methylation in very preterm children at school age. Front Behav Neurosci. 2014:8(409). doi: 10.3389/fnbeh.2014.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lotsch J., Skarke C., Liefhold J., Geisslinger G. Genetic predictors of the clinical response to opioid analgesics: clinical utility and future perspectives. Clin Pharmacokinet. 2004;43(14):983–1013. doi: 10.2165/00003088-200443140-00003. [DOI] [PubMed] [Google Scholar]
- 21.Visscher H., Ross C.J., Rassekh S.R., Barhdadi A., Dube M.P., Al-Saloos H. Pharmacogenomic prediction of anthracycline-induced cardiotoxicity in children. J Clin Oncol. 2012 May 1;30(13):1422–1428. doi: 10.1200/JCO.2010.34.3467. [DOI] [PubMed] [Google Scholar]
- 22.Grunau R.E., Haley D.W., Whitfield M.F., Weinberg J., Yu W., Thiessen P. Altered basal cortisol levels at 3, 6, 8 and 18 months in infants born at extremely low gestational age. J Pediatr. 2007 Feb;150(2):151–156. doi: 10.1016/j.jpeds.2006.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grunau R.E., Whitfield M.F., Petrie-Thomas J., Synnes A.R., Cepeda I.L., Keidar A. Neonatal pain, parenting stress and interaction, in relation to cognitive and motor development at 8 and 18 months in preterm infants. Pain. 2009 May;143(1–2):138–146. doi: 10.1016/j.pain.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richardson D.K., Corcoran J.D., Escobar G.J., Lee S.K. SNAP-II and SNAPPE-II: Simplified newborn illness severity and mortality risk scores. J Pediatr. 2001;138(1):92–100. doi: 10.1067/mpd.2001.109608. 01// [DOI] [PubMed] [Google Scholar]
- 25.Brummelte S., Grunau R.E., Chau V., Poskitt K.J., Brant R., Vinall J. Procedural pain and brain development in premature newborns. Ann Neurol. 2012 Mar;71(3):385–396. doi: 10.1002/ana.22267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Achenbach T.M., Rescorla L.A. University of Vermont, Research Center for Children, Youth, & Families; Burlington, VT: 2000. Manual for ASEBA preschool forms & profiles. [Google Scholar]
- 27.Ranger M., Chau C.M., Garg A., Woodward T.S., Beg M.F., Bjornson B. Neonatal pain-related stress predicts cortical thickness at age 7 years in children born very preterm. PLoS One. 2013 Oct 18;8(10) doi: 10.1371/journal.pone.0076702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ranger M., Zwicker J.G., Chau C.M., Park M.T., Chakravarthy M.M., Poskitt K. Neonatal pain and infection relate to smaller cerebellum in very preterm children at school age. J Pediatr. 2015 Aug;167(2):292–298. doi: 10.1016/j.jpeds.2015.04.055. (e1) [DOI] [PubMed] [Google Scholar]
- 29.Nagar S., Zalatoris J.J., Blanchard R.L. Human UGT1A6 pharmacogenetics: identification of a novel SNP, characterization of allele frequencies and functional analysis of recombinant allozymes in human liver tissue and in cultured cells. Pharmacogenetics. 2004 Aug;14(8):487–499. doi: 10.1097/01.fpc.0000114771.78957.cb. [DOI] [PubMed] [Google Scholar]
- 30.Tang W., Fu Y.P., Figueroa J.D., Malats N., Garcia-Closas M., Chatterjee N. Mapping of the UGT1A locus identifies an uncommon coding variant that affects mRNA expression and protects from bladder cancer. Hum Mol Genet. 2012 Apr 15;21(8):1918–1930. doi: 10.1093/hmg/ddr619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Z.Z., Li L., Wang L., Yuan L.M., Xu M.C., Gu J.K. The regioselective glucuronidation of morphine by dimerized human UGT2B7, 1A1, 1A9 and their allelic variants. Acta Pharmacol Sin. 2017 Aug;38(8):1184–1194. doi: 10.1038/aps.2016.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartlett S.E., Cramond T., Smith M.T. The excitatory effects of morphine-3-glucuronide are attenuated by LY274614, a competitive NMDA receptor antagonist, and by midazolam, an agonist at the benzodiazepine site on the GABAA receptor complex. Life Sci. 1994;54(10):687–694. doi: 10.1016/0024-3205(94)00552-4. [DOI] [PubMed] [Google Scholar]
- 33.Mao J., Sung B., Ji R.R., Lim G. Neuronal apoptosis associated with morphine tolerance: evidence for an opioid-induced neurotoxic mechanism. J Neurosci. 2002 Sep 1;22(17):7650–7661. doi: 10.1523/JNEUROSCI.22-17-07650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mao J., Price D.D., Mayer D.J. Experimental mononeuropathy reduces the antinociceptive effects of morphine: implications for common intracellular mechanisms involved in morphine tolerance and neuropathic pain. Pain. 1995 Jun;61(3):353–364. doi: 10.1016/0304-3959(95)00022-K. [DOI] [PubMed] [Google Scholar]
- 35.King C.D., Rios G.R., Assouline J.A., Tephly T.R. Expression of UDP-glucuronosyltransferases (UGTs) 2B7 and 1A6 in the human brain and identification of 5-hydroxytryptamine as a substrate. Arch Biochem Biophys. 1999 May 1;365(1):156–162. doi: 10.1006/abbi.1999.1155. [DOI] [PubMed] [Google Scholar]
- 36.Diatchenko L., Slade G.D., Nackley A.G., Bhalang K., Sigurdsson A., Belfer I. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005 Jan 1;14(1):135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- 37.Nackley A.G., Shabalina S.A., Tchivileva I.E., Satterfield K., Korchynskyi O., Makarov S.S. Human catechol-O-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science. 2006 Dec. 22;314(5807):1930–1933. doi: 10.1126/science.1131262. [DOI] [PubMed] [Google Scholar]
- 38.Ahlers S.J., Elens L.L., van Gulik L., van Schaik R.H., van Dongen E.P., Bruins P. The Val158Met polymorphism of the COMT gene is associated with increased pain sensitivity in morphine-treated patients undergoing a painful procedure after cardiac surgery. Br J Clin Pharmacol. 2013 Jun;75(6):1506–1515. doi: 10.1111/bcp.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rakvag T.T., Ross J.R., Sato H., Skorpen F., Kaasa S., Klepstad P. Genetic variation in the catechol-O-methyltransferase (COMT) gene and morphine requirements in cancer patients with pain. Mol Pain. 2008 Dec. 18;4 doi: 10.1186/1744-8069-4-64. (64-8069-4-64) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsuoka H., Arao T., Makimura C., Takeda M., Kiyota H., Tsurutani J. Expression changes in arrestin beta 1 and genetic variation in catechol-O-methyltransferase are biomarkers for the response to morphine treatment in cancer patients. Oncol Rep. 2012 May;27(5):1393–1399. doi: 10.3892/or.2012.1660. [DOI] [PubMed] [Google Scholar]
- 41.Elens L., Norman E., Matic M., Rane A., Fellman V., van Schaik R.H. Genetic predisposition to poor opioid response in preterm infants: impact of KCNJ6 and COMT polymorphisms on pain relief after endotracheal intubation. Ther Drug Monit. 2016 Aug;38(4):525–533. doi: 10.1097/FTD.0000000000000301. [DOI] [PubMed] [Google Scholar]
- 42.Matic M., Simons S.H., van Lingen R.A., van Rosmalen J., Elens L., de Wildt S.N. Rescue morphine in mechanically ventilated newborns associated with combined OPRM1 and COMT genotype. Pharmacogenomics. 2014 Jul;15(10):1287–1295. doi: 10.2217/pgs.14.100. [DOI] [PubMed] [Google Scholar]
- 43.Zubieta J.K., Heitzeg M.M., Smith Y.R., Bueller J.A., Xu K., Xu Y. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003 Feb 21;299(5610):1240–1243. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]
- 44.Berthele A., Platzer S., Jochim B., Boecker H., Buettner A., Conrad B. COMT Val108/158Met genotype affects the mu-opioid receptor system in the human brain: evidence from ligand-binding, G-protein activation and preproenkephalin mRNA expression. Neuroimage. 2005 Oct 15;28(1):185–193. doi: 10.1016/j.neuroimage.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 45.de Graaf J., van Lingen R.A., Valkenburg A.J., Weisglas-Kuperus N., Groot Jebbink L., Wijnberg-Williams B. Does neonatal morphine use affect neuropsychological outcomes at 8 to 9 years of age? Pain. 2013 Mar;154(3):449–458. doi: 10.1016/j.pain.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 46.Utendale W.T., Hastings P.D. Developmental changes in the relations between inhibitory control and externalizing problems during early childhood. Infant Child Develop. 2011;20(2):181–193. [Google Scholar]
- 47.Olsson C.A., Byrnes G.B., Anney R.J., Collins V., Hemphill S.A., Williamson R. COMT Val(158)Met and 5HTTLPR functional loci interact to predict persistence of anxiety across adolescence: results from the Victorian Adolescent Health Cohort Study. Genes Brain Behav. 2007 Oct;6(7):647–652. doi: 10.1111/j.1601-183X.2007.00313.x. [DOI] [PubMed] [Google Scholar]
- 48.Aberg E., Fandino-Losada A., Sjoholm L.K., Forsell Y., Lavebratt C. The functional Val158Met polymorphism in catechol-O-methyltransferase (COMT) is associated with depression and motivation in men from a Swedish population-based study. J Affect Disord. 2011 Mar;129(1–3):158–166. doi: 10.1016/j.jad.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 49.Ebesutani C., Bernstein A., Nakamura B.J., Chorpita B.F., Higa-McMillan C.K., Weisz J.R. Concurrent validity of the child behavior checklist DSM-oriented scales: correspondence with DSM diagnoses and comparison to syndrome scales. J Psychopathol Behav Assess. 2010 Sep;32(3):373–384. doi: 10.1007/s10862-009-9174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Predictor loadings for G1 G2 independently.
Predictor loadings for G1 G2 independently.
Predictor loadings for G1 × G2.