Abstract
Research on the cognitive neuroscience of aging has identified myriad neurocognitive processes that are affected by the aging process, with a focus on identifying neural correlates of cognitive function in aging. The present study aimed to test whether inter-network connectivity among 6 cognitive networks is sensitive to age-related changes in neural efficiency and cognitive functioning. A factor analytic connectivity approach was used to model network interactions during 11 cognitive tasks grouped into 4 primary cognitive domains: vocabulary, perceptual speed, fluid reasoning, and episodic memory. Results showed that both age and task domain were related to inter-network connectivity, and that some of the connections among the networks were associated with performance on the in-scanner tasks. These findings demonstrate that inter-network connectivity among several cognitive networks is not only affected by aging and task demands, but also shows a relationship with task performance. As such, future studies examining inter-network connectivity in aging should consider multiple networks, and multiple task conditions, in order to better measure dynamic patterns of network flexibility over the course of cognitive aging.
Studies of the cognitive neuroscience of aging have consistently found deleterious effects of aging on cognitive status and neural integrity and efficiency (C. L. Grady, 2008; Hedden & Gabrieli, 2004). Over the course of adulthood, there exists a gradual decline in memory function, executive function, working memory, and attentional resources, and a concomitant progression of neural degeneration, resulting in thinner cortex, white matter loss, and patterns of hyper- and hypo-recruitment of brain regions. As such, a primary focus of research in this field has centered on identifying neural correlates of cognitive function in older adulthood. Recent studies have combined multiple behavioral and imaging modalities in order to investigate cognitive decline in aging, and have found myriad neural metrics that may predict cognitive function in the context of aging (Hedden et al., 2016).
Recently neuroimaging research on cognitive aging has begun to utilize functional connectivity analyses to measure network-scale differences in neural recruitment between age groups. Of particular note is the emphasis placed on a neural network known as the default mode network (DMN), which is thought to be engaged primarily during rest and mind-wandering thought (Dosenbach et al., 2007; F. Esposito et al., 2006; Greicius, Supekar, Menon, & Dougherty, 2009; Laird et al., 2009). While this network has traditionally been studied during resting state functional magnetic resonance imaging (fMRI) scans, recent studies have examined how this network is engaged during a cognitive task, and have found that it “decouples” from task-related networks during performance of a task, such that correlated activity between a task-relevant network and the task-irrelevant DMN drops considerably in the presence of a task (Fox et al., 2005; C. L. Grady et al., 2010; Prakash, Heo, Voss, Patterson, & Kramer, 2012; Sala-Llonch et al., 2012). This network-based approach to fMRI analysis has gained traction within special populations, such as older adults with and without neurodegenerative disease, as it allows examination of complex large-scale networks known to be structurally affected by brain aging and dementia (Fujiyama et al., 2016; Hirsiger et al., 2016; Jones et al., 2015; Liu et al., 2015; Suckling et al., 2015). Importantly, unlike younger adults, older adults do not show the same degree of anti-correlation between the DMN and task-specific networks, and this lack of anti-correlation between networks negatively correlates with performance on the task (Miller et al., 2008; Prakash et al., 2012). These findings suggest that this relatively greater connectivity between these two networks may underlie age-related differences in task performance.
While this type of connectivity research has primarily focused on the DMN and executive function-related task networks (Damoiseaux, 2017; C. L. Grady et al., 2010; Prakash et al., 2012; Uddin, Kelly, Biswal, Castellanos, & Milham, 2009), more recent studies have broadened their study of inter-network connectivity to examine network interactions across multiple cognitive networks. Specifically, interactions between the DMN and the Dorsal Attention Network (DAN) have shown to be modulated by age and cognitive state (Amer, Anderson, Campbell, Hasher, & Grady, 2016; Damoiseaux, 2017; Dixon et al., 2017; R. Esposito et al., 2018; Spreng, Stevens, Viviano, & Schacter, 2016), and this interaction may be further coordinated by the frontoparietal control (FP) network (Avelar-Pereira, Bäckman, Wåhlin, Nyberg, & Salami, 2017; C. Grady, Sarraf, Saverino, & Campbell, 2016; Spreng, Sepulcre, Turner, Stevens, & Schacter, 2013). Additionally, another study found the salience network to be critically involved in coordinating the DMN and central executive networks, and found that this pattern was significantly disrupted in individuals with Mild Cognitive Impairment (MCI) (Chand, Wu, Hajjar, & Qiu, 2017).
In this vein, several studies have attempted to expand this study of internetwork connectivity to the whole brain by examining system segregation or internetwork connectivity across many neural networks. Most of these studies have utilized resting state fMRI data to show that older adults overall show greater between-network connections than younger adults (Chan, Park, Savalia, Petersen, & Wig, 2014; Ferreira et al., 2016; Geerligs, Maurits, Renken, & Lorist, 2014; Geerligs, Renken, Saliasi, Maurits, & Lorist, 2015; King et al., 2017), and generally show less segregated/modular network organization (Geerligs et al., 2015; Song et al., 2014). Further, some of these studies found a relationship between internetwork connectivity or segregation and performance on cognitive tasks (Chan et al., 2014; Geerligs et al., 2014; King et al., 2017), suggesting that this internetwork connectivity may underlie some of the variability in cognitive performance in the context of aging. However, while these larger-scale network studies provide critical information about whole-brain network connectivity across the adult lifespan, only one of the studies mentioned above examined functional connectivity during performance of a cognitive task (Geerligs et al., 2014). While some of the other studies make extensions to cognitive performance outside of the scanner, or relate resting state connectivity data to BOLD activation during cognitive tasks (Chan, Alhazmi, Park, Savalia, & Wig, 2017), none of the other studies examine internetwork connectivity across the whole brain during a cognitive challenge.
Thus, in order to bridge the findings from studies examining connectivity among a few networks during a cognitive task and those from studies examining internetwork connectivity across the whole brain at rest, the present study examined between-network connectivity across 6 cognitive networks (DMN, DAN, FP, cingulo-opercular, salience, and memory) during 11 cognitive tasks corresponding to 4 primary cognitive domains (vocabulary, perceptual speed, fluid reasoning, and episodic memory). Based on prior studies showing reductions in network decoupling during cognitive task performance, and generally greater connectivity between networks in aging, the hypotheses in the present study were as follows: (1) between-network connectivity patterns among these predefined cognitive networks will be affected by both task type and participant age, and (2) connectivity between specific pairs of cognitive networks will account for significant variability in task performance. Since task-relevance of networks will be in some ways determined by task domain, we expect patterns of inter-network connectivity to differ by domain. For example, while the DMN and memory networks may be irrelevant for FLUID and SPEED tasks, they may be relevant for VOCAB and MEM tasks, suggesting that patterns of interactions with these networks will likely be affected by task domain. On the other hand, networks such as the fronto-parietal network and cingulo-opercular network may be more broadly implicated in executive control, and thus might be more generally relevant during all cognitive tasks. Further, the salience network has been implicated as one that might coordinate correlated activity between task-relevant and task-irrelevant networks, and thus its task-relevance may not be as likely to differ by task type.
Regarding hypothesis 1, we expect that connectivity between task-relevant and task-irrelevant networks will be weaker than connectivity from task-relevant networks to other task-relevant networks. Since past studies on inter-network connectivity during a cognitive task have primarily used executive function tasks, these studies have found that task-relevant networks (i.e., FP, DAN) tend to show negative correlations with task-irrelevant networks (frequently the DMN) during a task, and that aging disrupts this pattern (Amer et al., 2016; Avelar-Pereira et al., 2017; Damoiseaux, 2017; Dixon et al., 2017; R. Esposito et al., 2018; Fox et al., 2005; C. Grady et al., 2016; C. L. Grady et al., 2010; Prakash et al., 2012; Sala-Llonch et al., 2012; Spreng et al., 2013; Spreng et al., 2016). As such, the task-specific hypotheses of the present study are as follows: (1) during FLUID and SPEED tasks, connections between task-relevant (FP, DAN, CO) and task-irrelevant (DMN) will be reduced and connections among task-relevant networks will be increased; and (2) during VOCAB and MEM tasks, there will be fewer differences in connections between networks, since the DMN and memory networks (considered task-irrelevant in FLUID/SPEED tasks) may in these tasks be task-relevant and implicated in mnemonic processing.
Methods
Sample.
The sample for the present study came from participants who completed the baseline visit for the Reference Ability Neural Network (RANN) study (N = 426) (Stern et al., 2014). All participants were native English speakers, right-handed, free of MRI contraindications, and read at a fourth grade reading level or above. Screening was performed prior to enrollment in order to ensure that no participants had any psychological or medical conditions that could affect cognitive function, and that older adults did not meet criteria for dementia or MCI at baseline. Age was trichotomized to enable testing of moderation by age, resulting in 3 age groups: young adults (YA; age 20–39, n = 118), middle-aged adults (MA; age 40–60, n = 131), and older adults (OA; age 61+, n = 177).
For the present analyses, the following additional inclusion criteria were established: completion of all 11 in-scanner tasks (N=338; YA n=96, MA n=110, OA n=132), and less than 30% motion artifact data removal (scrubbing; Parkes, Fulcher, Yucel, & Fornito, 2018; Power, Barnes, Snyder, Schlaggar, & Petersen, 2012) within each of the four domain timeseries (N=312; YA n=96, MA n=103, OA n=113). Further, one additional inclusion criteria concerned appropriate fit of the planned models, and is detailed further alongside the analysis methodology described below. As a result, the balanced sample utilized in the analyses below was comprised of 142 (YA n=45, MA n=49, OA n=48) healthy adults between the ages of 20 and 80 (mean = 50.75, SD = 17.335) who met all inclusion criteria.
In-Scanner Cognitive Tasks.
The cognitive variables included in this study are comprised of their performance on tasks completed during the fMRI scan. The in-scanner tasks were designed to measure performance within each of the four reference abilities: vocabulary (VOCAB: synonyms and antonyms), perceptual speed (SPEED: digit symbol, letter comparison, pattern comparison), fluid reasoning (FLUID: paper folding, matrix reasoning, and letter sets), and episodic memory (MEMORY: logical memory, word order, and paired associates) (for further information on tasks please see Razlighi, Habeck, Barulli, & Stern, 2017; Stern et al., 2014). In the vocabulary domain, the synonyms and antonyms tasks required participants to select a synonym/antonym (respectively) for a selected word from among four different options displayed on the same screen (15 trials per task). In the speed domain, the digit symbol task required participants to examine a digit-symbol code table and determine whether a digit-symbol pair on the sample screen was correct (90 trials), and the letter and pattern comparison tasks required participants to view a pair of strings of letters or figures comprised of varying numbers of lines (respectively) presented simultaneously and indicate whether or not they were identical (60 trials per task). In the fluid domain, the paper folding task required participants to select from five images one that represented the pattern of holes that would result from a set of folds in a piece of paper through which a hole is punched, the matrix reasoning task required participants to recognize a pattern from a series of pictures and identify the last missing piece of the pattern from among eight options, and the letter sets task required participants to identify which of a series of five sets of letters violated a rule expressed by the other four sets (7–18 self-paced trials for each FLUID task). In the memory domain, the logical memory task required participants to read a story one or two sentences at a time and then answer detailed four-choice multiple choice questions about the story (2 stories; 10 questions per story), the word order task required participants to view a series of twelve words then later indicate which of four words immediately followed a probe word (10 probe trials), and the paired associates task required participants to view six pairs of words then indicate which of four options was originally paired with the probe word (2 lists of pairs; 6 probe trials per list). One task, the Picture Naming task from the VOCAB reference ability, was not included in the present analyses due to excessive in-scanner motion from participants speaking their responses aloud during the scanned task. Performance on VOCAB, FLUID, and MEMORY tasks is measured by number of correct responses, while performance on SPEED tasks is measured by average correct reaction time. In order to appropriately compare performance across these four domains, behavioral data were z-scored such that each domain score represents standardized deviation from the mean domain score of the entire set of subjects – positive values represent behavioral performance (accuracy or reaction time) values above the normal mean, while negative values represent behavioral performance values below the normal mean. As such, for VOCAB, FLUID, and MEMORY tasks, positive z-scores reflect performance that is better (higher accuracy) than the mean, while for SPEED tasks, negative z-scores reflect performance that is better (faster) than the mean.
fMRI Scan Parameters.
The present study collected fMRI scans during the in-scanner tasks mentioned above. All participants completed these scans on a 3.0T Philips Achieva Magnet over the course of two 2-hour MR imaging sessions. T1-weighted images of the whole brain were acquired for each subject with a Magnetization Prepared Rapid Gradient Echo (MPRAGE) sequence with the following parameters: TE/TR: 3/6.5 ms; Field of view: 256 mm; Flip angle: 8°; In-plane resolution: 256×256 voxels; Slice thickness/gap: 1/0 mm; Slices: 180. fMRI blood oxygen level-dependent (BOLD) scans were collected during each of the 11 in-scanner tasks mentioned above with the following parameters: TE/TR: 20/2000 ms; Field of view: 240mm; Flip angle: 72°; In-plane resolution: 112×112 voxels; Slice thickness/gap: 3/0 mm; Slices: 41.
fMRI Data Processing.
Images were preprocessed using an in-house developed native space method (Razlighi et al., 2014). Briefly, slice-timing correction was performed using the FSL slicetimer tool. We then used mcflirt (motion correction tool in the FSL package; Jenkinson, Beckmann, Behrens, Woolrich, & Smith, 2012) to register all volumes to a reference image (Jenkinson, Bannister, Brady, & Smith, 2002). The reference image was generated by registering (6 df, 256 bins mutual information, and Sinc interpolation) all volumes to the middle volume and averaging them. We then used the method described in Power et al. (2012) to calculate frame-wise displacement (FWD) from the six motion parameters and root mean square difference (RMSD) of the BOLD percentage signal in the consecutive volumes for each subject. To be conservative, we lowered the threshold of our RMSD to 0.3% (it was originally suggested to be 0.5%.). RMSD was computed on the motion-corrected volumes before temporal filtering. The contaminated volumes were detected by the criteria FWD > 0.5 mm or RMSD > 0.3%. Identified contaminated volumes were replaced with new volumes generated by linear interpolation of adjacent volumes. Volume replacement was done before band-pass filtering (Carp, 2013). The motion-corrected signals were passed through a band-pass filter with the cut-off frequencies of 0.01 and 0.09 Hz. We used flsmaths–bptf to do the filtering in this study (Jenkinson et al. 2012). Finally, we residualized the motion-corrected, scrubbed, and temporally filtered volumes by regressing out the FWD, RMSD, left and right hemisphere white matter, and lateral ventricular signals (Birn, Diamond, Smith, & Bandettini, 2006).
T1 image segmentation were done using FreeSurfer (Dale, Fischl, & Sereno, 1999; Fischl et al., 2002; Fischl et al., 2004) and visually checked for any inaccuracies. In the event that we observed any inaccuracy in the FreeSurfer segmentation, we made corrections using the FreeSurfer provided guidelines for troubleshooting data. The coordinates of the 264 putative functional nodes derived from a brain-wide graph described by Power and colleagues (2011) were then transferred to each subject’s T1 space via non-linear registration of the subject’s structural scan to the MNI template using the ANTS software package. A spherical mask with 10 mm radius, centered at each transferred coordinate was generated and intersected with the FreeSurfer gray-matter mask to obtained the ROI mask for the 264 functional nodes. An intermodal, intra-subject, rigid-body registration of fMRI reference image and T1 scan was performed with FLIRT with 6 degrees of freedom, normalized mutual information as the cost function (Jenkinson & Smith, 2001), and used to transfer all ROI masks from T1 space to fMRI space. These transferred ROI masks were used to average all the voxels within each mask to obtain a single fMRI time-series for each node.
Functional connectivity timeseries data were then extracted from each of the 264 coordinate-based ROIs within each participant’s preprocessed fMRI task scans. Six networks were pre-selected for analysis based on their role in cognitive processes identified in previous studies, and as outlined by the Power et al. (2011) coordinate system: fronto-parietal network (FP; 25 ROIs), cingulo-opercular network (CO; 14 ROIs), DAN (11 ROIs), memory network (MEM; 5 ROIs), salience network (SAL; 18 ROIs), and DMN (58 ROIs). Timeseries data were then concatenated by domain, yielding 4 sets of timeseries data that were modeled separately as blocked designs: VOCAB (concatenated synonyms and antonyms data; 388 volumes, SPEED (concatenated digit symbol, letter comparison, and pattern comparison data; 595 volumes), FLUID (concatenated paper folding, matrix reasoning, and letter sets data; 1290 volumes), and MEM (concatenated logical memory, word order, and paired associates data; 517 volumes).
Analytic Methodology.
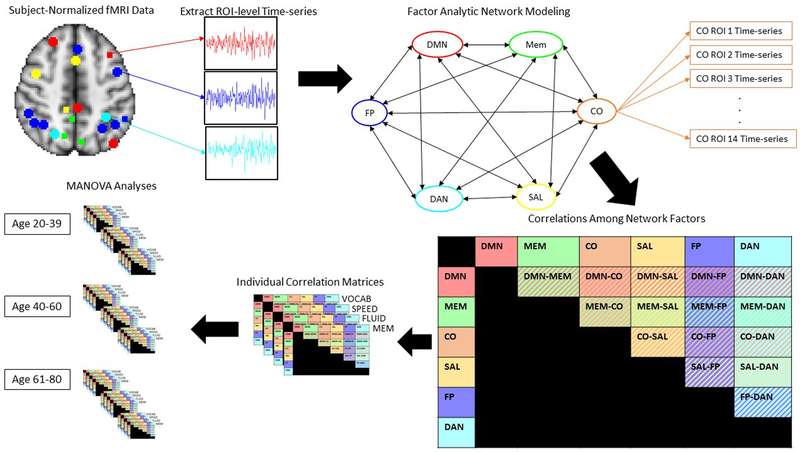
In order to model network-level timeseries interactions, a factor analytic functional connectivity methodology was utilized. Data were analyzed in R using a structural equation modeling approach, Model-Implied Instrumental Variable estimtation (Bollen, 1996, 2001), appropriate for high-dimensional data (MIIVsem; https://cran.r-project.org/package=MIIVsem, Fisher, Bollen, Gates, & Rönkkö, 2017). This approach is designed for robust estimation of high-dimensional structural equation models and has recently been extended to handle individual-level multivariate timeseries data (Fisher, Bollen, & Gates, In Press). Furthermore, this approach allows for the estimation of variance and covariance parameters of network timeseries along with bootstrap standard errors. Each individual’s ROI-level timeseries data from the 131 ROIs reflecting the networks selected above was loaded onto the corresponding predefined network latent factors, resulting in a 6-factor model estimated at the subject level, where the primary outcome measure was correlations between the 6 network factors (see Figure 1). This approach is not only a robust technique for modeling repeated measures at the participant level, but also represents a more flexible method for modeling network-level timeseries data in that it does not impose group-level assumptions on the contributions of each ROI with respect to network function. Thus, ROIs are allowed to freely load on the latent network factors, allowing participants to differ in the respective contributions of each ROI in the predefined networks, and reducing the effect of any outliers on network timeseries estimation. This analysis was performed separately for each cognitive domain of interest: VOCAB, SPEED, FLUID, and MEM, resulting in 4 correlation matrices of size 6×6 for each participant. Individual-level correlation coefficients between latent network factors for each of the 4 domains were then z-transformed and exported to SPSS for MANOVA and correlational analyses. The additional inclusion criterion referenced above was valid estimation of the pre-specified factor structure: in order to be included in the analyses, each participant had to have valid data for the estimation of the factor structure and correlations among factors. While variability in network architecture that deviates from the pre-specified criteria may be critical to examine, it was beyond the scope of the present analyses, and thus only participants whose network structure fit the structure that was pre-specified based on empirically defined network structures were included. Thus, after the data were exported to SPSS, all participants who had errors related to computation of latent factor variance appear during factor structure estimation were removed in order to ensure that only participants with valid estimation of the proposed factor structure were included in the analyses.
Figure 1.
Factor Analytic connectivity analysis pipeline.
In order to explore the effects of specific connection (within-subjects: i.e., FP-DMN), cognitive domain (within-subjects: VOCAB, SPEED, FLUID, and MEM), age (between-subjects: YA, MA, and OA), and their interactions on inter-network correlations, multivariate analyses of variance were conducted. Follow-up Spearman correlational analyses were then conducted to test for any relationship between inter-network connectivity and task performance. Due to the exploratory nature of this analysis, a Spearman correlational approach was utilized over a Pearson correlational approach in order to test for robust monotonic relationships between connectivity and task performance without making any assumptions about the linearity of this relationship (i.e., Geerligs et al., 2014; Geerligs et al., 2015). In the first set of correlational analyses, Spearman correlational tests were conducted across the entire age range. Next, similar to previous studies assessing the relationship between functional connectivity and cognitive/behavioral outcomes (Chan et al., 2014; Ferreira et al., 2016; Geerligs et al., 2015), partial Spearman correlational tests were conducted with respect to age in order to remove any pure effect of age on both task performance and functional connectivity. Finally, Spearman correlational tests were conducted within each of the three age groups in order to examine whether these relationships remained stable across the three age groups.
Results
Participants.
Demographic characteristics of participants in the present study are summarized in Table 1. In order to test whether this sub-sample of the data set was in any way biased, independent sample t-tests were performed to ensure no group differences between excluded and included participants on Age (p = 0.091), Education (p = 0.311), Gender (p = 0.820), Race (p = 0.168), Ethnicity (p = 0.716), NART IQ (p = 0.070), and SPEED (p = 0.124) and MEM (p = 0.265) task performance; however, included participants tended to have higher scores on VOCAB (p = 0.040) and FLUID (p = 0.008) tasks. Further, chi-square tests were run for each inclusion/exclusion step in order to ensure that exclusion of participants was not biased by age group. Chi-square tests for each exclusion step showed that at enrollment there were more OAs than MAs or YAs (p=0.001), more OAs were excluded for not having balanced fMRI task data (p=0.002) and greater than 30% scrubbing (p<.001), however there was no bias in number of participants excluded for improper estimation of factor structure (p=0.383). Thus, more OAs were excluded for not completing all 11 in-scanner tasks and for having more data scrubbed, however participant drop-out was similar for proper estimation of factor structure.
Table 1.
Demographic characteristics of the sample by age group: Young Adults (YA; age 20–39), Middle Adulthood (MA; age 40–60), and Older Adults (OA; age 61–80).
| YA | MA | OA | Overall | Test Statistic | |
|---|---|---|---|---|---|
| (p-value) | |||||
| N | 45 | 49 | 48 | 142 | n/a |
| Age (SD) | 29.73 (5.127) | 50.69 (5.864) | 70.50 (4.524) | 50.75 (17.335) | F2,139=711.735 (<.001)1 |
| Education (SD) | 16.16 (1.731) | 15.71 (1.947) | 16.90 (2.769) | 16.25 (2.242) | F2,139=3.554 (0.031)2 |
| NART IQ (SD) | 114.81 (8.279) | 115.93 (8.301) | 121.66 (6.222) | 117.54 (8.165) | F2,134=10.598 (<.001)3 |
| % Female | 61.4 | 49.0 | 57.5 | 55.7 | χ22=1.527 (0.466) |
| VOCAB | −0.252 (0.846) | 0.146 (0.122) | 0.466 (0.792) | 0.126 (0.875) | F2,138=8.582 (<.001)4 |
| SPEED | −0.660 (0.690) | −0.043 (0.712) | 0.371 (0.748) | −0.085 (0.825) | F2,134=22.713 (<.001)1 |
| FLUID | 0.480 (0.740) | 0.093 (0.748) | −0.053 (0.740) | 0.166 (0.770) | F2,136=6.198 (0.003)5 |
| MEM | 0.406 (0.667) | 0.053 (0.594) | −0.239 (0.631) | 0.068 (0.678) | F2,135=11.813 (<.001)5 |
All three groups significantly differ (p<.05; Tukey HSD)
OA>MA (p<.05; Tukey HSD)
OA>MA and YA (p<.05; Tukey HSD)
OA>YA
YA>MA and OA (p<.05; Tukey HSD)
Note: NART=National Adult Reading Test. Bolded test statistics indicate those that are significant, and bolded group-level means highlight values that significantly differ by group.
Further, in order to examine this effect of age on scrubbing across all 338 participants with balanced fMRI task data, a 3 (age group: YA, MA, OA) × 4 (task domain: VOCAB, SPEED, FLUID, MEM) MANOVA was used to probe the effects of age and domain on percent scrubbing. Results showed that there was a main effect of age (F2,335=14.081, p<.001) and a main effect of domain (F3,1005=18.595, p<.001) but no interaction between domain and age (F6,1005=1.646, p=0.131). The main effect of age showed that older adults (M=9.530%) had more data scrubbed than both younger (M=3.741%; p<.001) and middle-aged (M=5.906%; p=0.003) participants. Further, more older (n=19) and middle-aged (n=7) adults were excluded for having greater than 30% scrubbing on any given task domain than younger adults (n=0). The main effect of domain showed that FLUID (M=8.420%) tasks had more data scrubbed than VOCAB (M=5.762%, p<.001), SPEED (M=5.990%, p<.001), or MEM (M=6.655%, p<.001) tasks. However, of note is that FLUID tasks were the longest task scans of the two scan sessions, so this greater proportional amount of motion during these tasks could be partly due to longer acquisition times.
MANOVA Results.
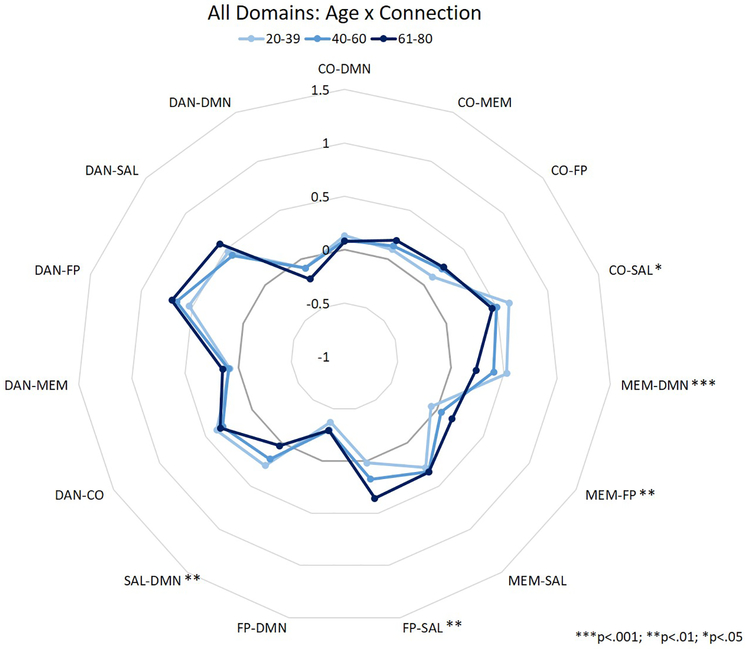
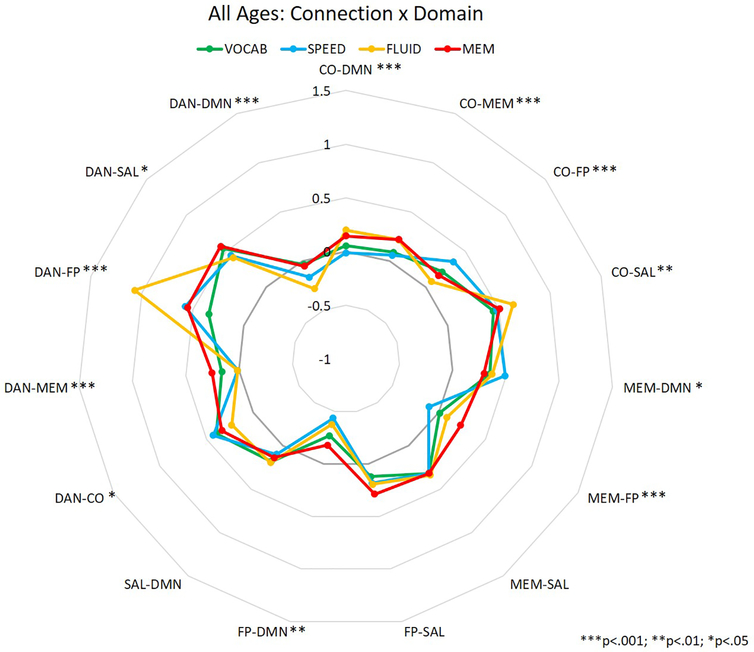
Inter-network correlation values were analyzed in a MANOVA to assess the independent and interactive effects of participant age (between-participants), task domain (within-participants), and network pairing (within-participants) on inter-network correlations during performance of the in-scanner tasks. Thus, the primary interactions of interest were between age and network pairing, domain and network pairing, and the three-way interaction, as these would suggest that between-network correlations are affected by participant age, task domain, or both. Means for each connection for the aforementioned two-way interactions are depicted in figures 2 (age × connection) and 3 (domain × connection), and represent summaries of the data used for the MANOVA analyses reported below. The values depicted are the mean correlation between two specific networks for each age group (Figure 2) or task domain (Figure 3).
Figure 2.
Between-network correlation coefficients for the interaction between age and connection. Asterisks represent significance of age difference at each connection.
Figure 3.
Between-network correlation coefficients for the interaction between task domain and connection. Asterisks represent significance of domain difference at each connection.
A 3 (age group: YA, MA, and OA) × 4 (domain: VOCAB, SPEED, FLUID, and MEM) × 15 (inter-network connection) MANOVA revealed a significant main effect of domain (F3,417 = 8.577, p<.001), a main effect of connection (F14,1946 = 84.563, p<.001), an interaction between connection and age group (F28,1946 = 4.024, p<.001), and an interaction between domain and connection (F42,5838 = 12.309, p<.001). The main effect of age (F2,139 = 0.116, p = 0.891), and the interactions between domain and age group (F6,417 = 0.626, p = 0.710), and domain, connection, and age group (F84,5754 = 1.185, p = 0.120) were not significant. Post-hoc analyses of the interactions between connection and age (see Table 2; Figure 2), and connection and domain (see Table 3; Figure 3) showed a significant effect of age and domain on inter-network connection strength across many inter-network connections. In the interaction between age and connection, OAs showed greater correlations between MEM-FP and FP-SAL than YAs, while YAs showed greater correlations between CO-SAL, MEM-DMN, and SAL-DMN than OAs (see Table 2; Figure 2). In the interaction between domain and connection, only 2 connections did not show a significant effect of domain on the correlations between networks: MEM-SAL and SAL-DMN. The 13 remaining connections showed some alteration in correlation strength depending upon task domain – for example, the DAN-FP connection showed significantly greater connectivity during tasks of fluid reasoning than tasks from any other domains (and greater connectivity during memory tasks than vocabulary tasks; see Table 3; Figure 3).
Table 2.
MANOVA results for post-hoc analyses of the interaction between age group (Young Adult or YA; Middle Adulthood or MA, and Older Adult or OA) and connection (df between=2, df within = 139 for all statistics reported). Contrasts are represented in columns 4–6 by the mean difference in z-transformed correlation coefficients between groups (p-value in parentheses).
| Connection | F-value | p-value | YA vs. MA | YA vs. OA | MA vs. OA |
|---|---|---|---|---|---|
| CO-DMN | .202 | .817 | n/a | n/a | n/a |
| CO-MEM | 2.635 | .075 | n/a | n/a | n/a |
| CO-FP | 1.685 | .189 | n/a | n/a | n/a |
| CO-SAL | 3.119 | .047 | 0.131 (0.277) | 0.212 (0.038) | 0.081 (0.597) |
| MEM-DMN | 9.671 | <.001 | 0.124 (0.251) | 0.337 (<.001) | 0.214 (0.016) |
| MEM-FP | 7.351 | .001 | −0.044 (0.764) | −0.228 (0.001) | −0.184 (0.010) |
| MEM-SAL | .737 | .481 | n/a | n/a | n/a |
| FP-SAL | 5.742 | .004 | −0.133 (0.312) | −0.309 (0.003) | −0.176 (0.126) |
| FP-DMN | .472 | .625 | n/a | n/a | n/a |
| SAL-DMN | 5.914 | .003 | 0.119 (0.344) | 0.292 (0.002) | 0.173 (0.101) |
| DAN-CO | .458 | .634 | n/a | n/a | n/a |
| DAN-MEM | .798 | .452 | n/a | n/a | n/a |
| DAN-FP | 1.767 | .175 | n/a | n/a | n/a |
| DAN-SAL | 2.720 | .069 | n/a | n/a | n/a |
| DAN-DMN | 1.201 | .304 | n/a | n/a | n/a |
Note: CO=Cingulo-Opercular Network, DMN=Default Mode Network, MEM=Memory Network, FP=Fronto-Parietal Network, SAL=Salience Network, DAN=Dorsal Attention Network. Bolded values reflect mean differences that are statistically significant.
Table 3.
MANOVA results for post-hoc analyses of the interaction between cognitive task domain and connection (df between=3, df within = 129 for all statistics reported). Contrasts are represented in columns 4–9 by the mean difference in z-transformed correlation coefficients between groups (p-value in parentheses).
| Connection | F-value | p-value | Vocab vs. Speed | Vocab vs. Fluid | Vocab vs. Memory | Speed vs. Fluid | Speed vs. Memory | Fluid vs. Memory |
|---|---|---|---|---|---|---|---|---|
| CO-DMN | 8.319 | <.001 | 0.060 (>.999) | −0.149 (0.016) | −0.127 (0.060) | −0.208 (<.001) | −0.187 (0.001) | 0.022 (>.999) |
| CO-MEM | 12.995 | <.001 | 0.039 (>.999) | −0.169 (0.001) | −0.184 (<.001) | −0.209 (<.001) | −0.223 (<.001) | −0.014 (>.999) |
| CO-FP | 7.602 | <.001 | −0.095 (0.318) | 0.129 (0.051) | 0.064 (>.999) | 0.224 (<.001) | 0.159 (0.008) | −0.066 (>.999) |
| CO-SAL | 4.702 | .003 | 0.049 (>.999) | −0.185 (0.043) | −0.105 (0.750) | −0.234 (0.004) | −0.154 (0.150) | 0.080 (>.999) |
| MEM-DMN | 3.548 | .014 | −0.172 (0.060) | −0.041 (>.999) | 0.028 (>.999) | 0.131 (0.294) | 0.200 (0.016) | 0.069 (>.999) |
| MEM-FP | 12.535 | <.001 | 0.107 (0.230) | −0.033 (>.999) | −0.204 (0.001) | −0.140 (0.041) | −0.311 (<.001) | −0.171 (0.006) |
| MEM-SAL | .211 | .889 | n/a | n/a | n/a | n/a | n/a | n/a |
| FP-SAL | 2.153 | .093 | n/a | n/a | n/a | n/a | n/a | n/a |
| FP-DMN | 4.410 | .004 | 0.086 (>.999) | 0.075 (>.999) | −0.117 (0.384) | −0.012 (>.999) | −0.203 (0.008) | −0.191 (0.015) |
| SAL-DMN | .357 | .784 | n/a | n/a | n/a | n/a | n/a | n/a |
| DAN-CO | 4.048 | .007 | 0.009 (>.999) | 0.165 (0.012) | 0.060 (>.999) | 0.156 (0.021) | 0.050 (>.999) | −0.106 (0.281) |
| DAN-MEM | 11.854 | <.001 | 0.118 (0.106) | 0.190 (0.001) | −0.080 (0.623) | 0.072 (0.874) | −0.198 (<.001) | −0.270 (<.001) |
| DAN-FP | 32.339 | <.001 | −0.221 (0.049) | −0.777 (<.001) | −0.189 (0.141) | −0.556 (<.001) | −0.032 (>.999) | 0.588 (<.001) |
| DAN-SAL | 5.086 | .002 | 0.174 (0.066) | 0.212 (0.012) | 0.014 (>.999) | 0.038 (>.999) | −0.159 (0.117) | −0.198 (0.023) |
| DAN-DMN | 7.496 | <.001 | 0.019 (>.999) | 0.200 (0.003) | −0.052 (>.999) | 0.181 (0.009) | −0.071 (>.999) | −0.252 (<.001) |
Note: CO=Cingulo-Opercular Network, DMN=Default Mode Network, MEM=Memory Network, FP=Fronto-Parietal Network, SAL=Salience Network, DAN=Dorsal Attention Network. Bolded values reflect mean differences that are statistically significant.
Task Performance Correlations.
In order to assess any relationships between task performance and inter-network connectivity during the task, Spearman correlational analyses were conducted. Full correlation tables are presented for each of the three correlational analyses presented here (see Tables 4–6), however only results that survive multiple comparisons correction using the Benjamini-Hochberg procedure across all correlations analyzed (60 per analysis – 15 connections × 4 domains) are discussed here. In the first set of results, Spearman correlational tests were conducted across the entire age range. Results showed that FLUID task performance was negatively correlated with DAN-MEM connectivity, such that better performance on FLUID tasks was associated with less correlated BOLD signal between DAN and MEM networks (see Table 4; r139=−0.312, p<.001). In the second set of results, partial Spearman correlational tests were conducted with respect to age in order to remove any pure effect of age on both task performance and functional connectivity. After removing the effect of age from these relationships, the correlation between FLUID performance and DAN-MEM connectivity remained significant (see Table 5; r139=−0.285, p=0.001). In the third set of results, Spearman correlational tests were conducted within each age group in order to examine whether these relationships remained stable across the three age groups. Results from this analysis showed that across the three age groups, the only correlation that survived FDR correction was a negative relationship between VOCAB task performance and FP-DMN connectivity in YA (see Table 6; r45=−0.483, p=0.001).
Table 4.
Correlation table for Spearman correlation coefficient (p-values in parentheses; significant correlations bolded) for the relationship between task performance in each domain (VOCAB=Vocabulary, SPEED=Perceptual Speed, FLUID=Fluid Reasoning, MEM=Episodic Memory) and each internetwork connection.
| VOCAB | SPEED | FLUID | MEM | |
|---|---|---|---|---|
| CO-DMN | 0.033 (0.701) | 0.022 (0.798) | −0.096 (0.263) | −0.004 (0.962) |
| CO-MEM | 0.078 (0.359) | −0.047 (0.587) | −0.070 (0.410) | 0.024 (0.781) |
| CO-FP | −0.021 (0.804) | 0.086 (0.319) | −0.096 (0.259) | 0.064 (0.459) |
| CO-SAL | 0.017 (0.841) | −0.152 (0.076) | 0.073 (0.395) | −0.018 (0.835) |
| MEM-DMN | 0.048 (0.571) | 0.070 (0.419) | 0.229 (0.007) | 0.212 (0.013) |
| MEM-FP | −0.014 (0.869) | 0.063 (0.465) | −0.171 (0.044) | −0.165 (0.053) |
| MEM-SAL | 0.172 (0.041) | 0.090 (0.293) | −0.004 (0.963) | 0.068 (0.429) |
| FP-SAL | −0.139 (0.099) | 0.085 (0.323) | −0.220 (0.009) | −0.040 (0.644) |
| FP-DMN | −0.151 (0.074) | 0.076 (0.379) | −0.189 (0.026) | 0.056 (0.516) |
| SAL-DMN | 0.227 (0.007) | −0.014 (0.868) | 0.156 (0.067) | 0.055 (0.523) |
| DAttn-CO | 0.120 (0.156) | −0.039 (0.654) | −0.001 (0.993) | 0.019 (0.822) |
| DAttn-MEM | 0.120 (0.158) | −0.093 (0.280) | −0.312 (<.001)* | −0.115 (0.178) |
| DAttn-FP | 0.104 (0.218) | 0.086 (0.319) | 0.201 (0.018) | −0.050 (0.560) |
| DAttn-SAL | −0.075 (0.376) | 0.042 (0.629) | −0.219 (0.010) | 0.077 (0.370) |
| DAttn-DMN | 0.062 (0.468) | −0.015 (0.862) | −0.177 (0.037) | 0.114 (0.182) |
Note: CO=Cingulo-Opercular Network, DMN=Default Mode Network, MEM=Memory Network, FP=Fronto-Parietal Network, SAL=Salience Network, DAN=Dorsal Attention Network. Bolded correlation coefficients represent those that are significant at p<.05; correlation coefficients with an asterisk represent those that survive multiple comparison correction.
Table 6.
Correlation table for Spearman correlation coefficient (p-values in parentheses; significant correlations bolded) for the relationship between task performance in each domain (VOCAB=Vocabulary, SPEED=Perceptual Speed, FLUID=Fluid Reasoning, MEM=Episodic Memory) and each internetwork connection by participant age group (Young Adult or YA; Middle Adulthood or MA, and Older Adult or OA).
| VOCAB | SPEED | FLUID | MEM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| YA | MA | OA | YA | MA | OA | YA | MA | OA | YA | MA | OA | |
| CO-DMN | 0.269 (0.074) | −0.029 (0.843) | −0.168 (0.259) | 0.128 (0.426) | −0.039 (0.789) | −0.072 (0.629) | −0.254 (0.096) | −0.189 (0.198) | 0.026 (0.863) | −0.231 (0.132) | −0.128 (0.388) | 0.184 (0.221) |
| CO-MEM | 0.047 (0.758) | 0.002 (0.991) | −0.086 (0.566) | −0.006 (0.972) | −0.369 (0.009) | 0.063 (0.673) | 0.005 (0.975) | −0.075 (0.611) | −0.033 (0.825) | 0.044 (0.775) | 0.349 (0.015) | −0.092 (0.544) |
| CO-FP | −0.193 (0.205) | −0.116 (0.429) | 0.261 (0.077) | −0.085 (0.596) | 0.171 (0.239) | −0.009 (0.953) | −0.113 (0.466) | 0.030 (0.839) | −0.028 (0.851) | −0.124 (0.422) | 0.298 (0.040) | 0.081 (0.594) |
| CO-SAL | 0.014 (0.928) | −0.093 (0.524) | 0.176 (0.235) | 0.119 (0.457) | −0.215 (0.137) | −0.071 (0.633) | 0.017 (0.914) | −0.135 (0.359) | 0.132 (0.376) | −0.275 (0.070) | 0.073 (0.620) | −0.042 (0.782) |
| MEM-DMN | 0.258 (0.087) | 0.222 (0.124) | −0.219 (0.140) | 0.393 (0.011) | 0.080 (0.586) | 0.108 (0.470) | 0.170 (0.270) | 0.305 (0.035) | −0.098 (0.511) | 0.149 (0.335) | 0.181 (0.219) | 0.031 (0.837) |
| MEM-FP | −0.229 (0.130) | −0.165 (0.256) | 0.139 (0.351) | 0.001 (0.994) | −0.117 (0.422) | −0.069 (0.643) | −0.231 (0.132) | −0.297 (0.041) | 0.225 (0.129) | −0.094 (0.542) | 0.091 (0.541) | −0.113 (0.454) |
| MEM-SAL | 0.236 (0.119) | 0.213 (0.141) | 0.014 (0.924) | 0.190 (0.235) | −0.065 (0.659) | 0.099 (0.509) | −0.046 (0.769) | 0.008 (0.957) | 0.158 (0.290) | −0.107 (0.491) | 0.191 (0.194) | 0.245 (0.101) |
| FP-SAL | −0.218 (0.150) | −0.205 (0.157) | −0.147 (0.325) | 0.165 (0.302) | −0.096 (0.510) | −0.167 (0.263) | −0.029 (0.851) | −0.311 (0.031) | −0.153 (0.305) | −0.121 (0.433) | 0.105 (0.479) | 0.130 (0.388) |
| FP-DMN | −0.483 (0.001)* | −0.333 (0.019) | 0.348 (0.017) | 0.091 (0.571) | −0.209 (0.150) | 0.150 (0.314) | −0.374 (0.012) | −0.327 (0.023) | 0.242 (0.101) | −0.107 (0.489) | 0.086 (0.563) | 0.021 (0.890) |
| SAL-DMN | 0.448 (0.002) | 0.342 (0.016) | 0.050 (0.74) | 0.150 (0.349) | −0.029 (0.843) | 0.194 (0.191) | 0.015 (0.923) | 0.283 (0.051) | −0.085 (0.572) | −0.159 (0.304) | 0.068 (0.645) | −0.081 (0.591) |
| DAttn-CO | −0.019 (0.901) | 0.158 (0.280) | 0.180 (0.226) | −0.281 (0.075) | 0.169 (0.246) | 0.042 (0.777) | −0.074 (0.634) | −0.115 (0.435) | 0.213 (0.150) | −0.390 (0.009) | 0.273 (0.061) | −0.056 (0.711) |
| DAttn-MEM | 0.251 (0.096) | −0.011 (0.939) | 0.091 (0.542) | 0.152 (0.344) | −0.073 (0.619) | −0.287 (0.050) | −0.273 (0.073) | −0.399 (0.005) | −0.114 (0.444) | −0.232 (0.130) | 0.082 (0.579) | 0.016 (0.914) |
| DAttn-FP | −0.013 (0.931) | −0.031 (0.834) | 0.007 (0.961) | −0.165 (0.302) | 0.026 (0.857) | 0.014 (0.928) | 0.279 (0.066) | 0.429 (0.002) | −0.030 (0.839) | −0.033 (0.832) | 0.062 (0.676) | −0.011 (0.940) |
| DAttn-SAL | −0.239 (0.114) | 0.042 (0.772) | −0.082 (0.584) | −0.030 (0.852) | 0.070 (0.632) | 0.035 (0.816) | −0.047 (0.763) | −0.361 (0.012) | −0.145 (0.330) | −0.016 (0.917) | 0.295 (0.042) | 0.165 (0.274) |
| DAttn-DMN | 0.195 (0.200) | −0.040 (0.785) | 0.013 (0.932) | 0.370 (0.017) | −0.135 (0.356) | 0.037 (0.804) | −0.340 (0.024) | −0.349 (0.015) | 0.164 (0.270) | −0.086 (0.577) | −0.014 (0.927) | 0.153 (0.312) |
Note: CO=Cingulo-Opercular Network, DMN=Default Mode Network, MEM=Memory Network, FP=Fronto-Parietal Network, SAL=Salience Network, DAN=Dorsal Attention Network. Bolded correlation coefficients represent those that are significant at p<.05; correlation coefficients with an asterisk represent those that survive multiple comparison correction.
Table 5.
Correlation table for Spearman correlation coefficient (p-values in parentheses; significant correlations bolded) for the relationship between task performance in each domain (VOCAB=Vocabulary, SPEED=Perceptual Speed, FLUID=Fluid Reasoning, MEM=Episodic Memory) and each internetwork connection after controlling for participant age.
| VOCAB | SPEED | FLUID | MEM | |
|---|---|---|---|---|
| CO-DMN | 0.009 (0.919) | −0.022 (0.802) | −0.100 (0.250) | −0.056 (0.521) |
| CO-MEM | 0.007 (0.934) | −0.131 (0.132) | 0.004 (0.962) | 0.080 (0.361) |
| CO-FP | 0.004 (0.961) | 0.045 (0.609) | −0.033 (0.709) | 0.110 (0.206) |
| CO-SAL | 0.078 (0.374) | −0.113 (0.194) | −0.003 (0.975) | −0.094 (0.282) |
| MEM-DMN | 0.075 (0.388) | 0.175 (0.044) | 0.177 (0.042) | 0.102 (0.242) |
| MEM-FP | −0.054 (0.540) | −0.080 (0.361) | −0.183 (0.035) | −0.094 (0.280) |
| MEM-SAL | 0.127 (0.146) | 0.079 (0.365) | 0.069 (0.431) | 0.094 (0.282) |
| FP-SAL | −0.164 (0.059) | −0.081 (0.352) | −0.163 (0.060) | 0.038 (0.666) |
| FP-DMN | −0.148 (0.088) | −0.036 (0.684) | −0.162 (0.062) | 0.003 (0.975) |
| SAL-DMN | 0.251 (0.004) | 0.114 (0.191) | 0.124 (0.153) | −0.056 (0.523) |
| DAttn-CO | 0.131 (0.132) | 0.050 (0.568) | −0.025 (0.777) | −0.024 (0.786) |
| DAttn-MEM | 0.102 (0.244) | −0.069 (0.431) | −0.285 (0.001)* | −0.064 (0.465) |
| DAttn-FP | −0.031 (0.726) | 0.007 (0.937) | 0.251 (0.004) | −0.036 (0.680) |
| DAttn-SAL | −0.086 (0.327) | 0.056 (0.520) | −0.211 (0.015) | 0.155 (0.075) |
| DAttn-DMN | 0.062 (0.477) | 0.052 (0.555) | −0.156 (0.073) | 0.032 (0.712) |
Note: CO=Cingulo-Opercular Network, DMN=Default Mode Network, MEM=Memory Network, FP=Fronto-Parietal Network, SAL=Salience Network, DAN=Dorsal Attention Network. Bolded correlation coefficients represent those that are significant at p<.05; correlation coefficients with an asterisk represent those that survive multiple comparison correction.
Discussion
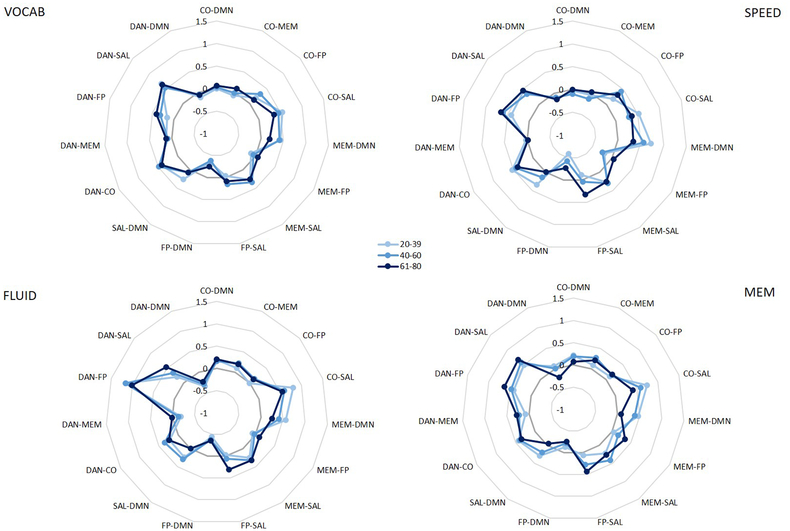
Results from the present study show that patterns of inter-network connectivity during task performance are modulated by age and task domain. Specifically, age may particularly affect connectivity directed from the memory and salience networks, such that older adults show greater connectivity between the memory network and a task-relevant network (FP), altered connectivity directed from the salience network to task-relevant networks (CO and FP), and reduced connectivity from memory and salience networks to the DMN. However, the present set of results did not replicate findings from previous studies showing reduced anti-correlation between the DMN and FP in older adults (Miller et al., 2008; Prakash et al., 2012) – in the interaction between age and connection, there was no effect of age on connectivity between these two networks. That being said, when examining means for the connection by domain interaction within each age group, some interesting trends emerged which may suggest that the effect of age on FP-DMN connectivity only exists during specific types of tasks (specifically, speed tasks). While this 3-way interaction was not significant, its p-value of 0.120 in the MANOVA analyses could suggest that we were simply underpowered to detect this complex effect (see Figure 4). Thus while we failed to replicate that result here, it could be due to our inclusion of tasks tapping into multiple cognitive domains, rather than focusing on connectivity during one specific cognitive task. Specifically, if only the SPEED domain is modeled, there is a significant difference between older and younger adults in FP-DMN connectivity; however, given the scope of analyses in the present study we were unable to detect this small of an effect (see Figure 4; SPEED). As such, while this connection may be affected by aging in working memory, executive function, or processing speed tasks, it may not be a critically age-sensitive connection across all cognitive domains.
Figure 4.
Between-network correlation coefficients for the nonsignificant interaction between age and connection within each domain.
Further, the present study also did not provide evidence for a generally more connected brain in the context of aging. Specifically, the main effect of age was not significant, suggesting that within this sample and during these tasks, older adults do not show overall greater connectivity across these networks relative to younger adults. Several past studies examining functional connectivity in the context of aging found that, generally speaking, older adults show more between-network connections than younger adults (Chan et al., 2014; Geerligs et al., 2014; King et al., 2017), a more positively-connected brain graph (Ferreira et al., 2016), and reduced segregation/modularity of brain systems (Geerligs et al., 2015; Song et al., 2014). However, all but one (Geerligs et al., 2014) of these studies utilized resting state fMRI data to probe such effects, and one of the studies similarly found patterns of inter-network connectivity that either increased or decreased with age (Geerligs et al., 2015). Thus, the present study may not lie in contrast to these findings, but may instead represent a novel approach for modeling and investigating the effect of age on inter-network connectivity.
We also found that connection strength between networks differed by the type of task being performed during the scan. From a broader perspective, these connectivity patterns appear to cluster such that certain tasks have more similar connectivity patterns between networks (i.e., vocab and speed, vocab and memory, and fluid and memory), while other tasks have largely dissimilar connectivity patterns between networks (i.e., speed and memory, speed and fluid, and vocab and fluid). This finding suggests that cognitive networks do modulate their connections to other networks based on cognitive task domain. While past studies on resting state connectivity suggest its utility in revealing age-related differences in network function, structure, and organization (Damoiseaux, 2017; Damoiseaux et al., 2008; Dennis & Thompson, 2014; R. Esposito et al., 2018; Hedden et al., 2016; Spreng et al., 2016), they may not fully represent how connectivity might change in the presence of a cognitive challenge. Though changes in network structure or activation were outside of the scope of the present analyses, our results show how predefined, cognitively relevant networks may alter their relationships to each other in response to the demands of the task.
We also found that two inter-network connections showed a direct correlation with behavior: greater connectivity between the dorsal attention network and the memory network was associated with poorer FLUID performance across the whole sample (and after controlling for participant age), and greater connectivity between the frontoparietal network and DMN was associated with poorer performance on VOCAB tasks. While implicating different networks and tasks, these results somewhat mirror findings from previous studies showing differential connectivity patterns between older and younger adults that can be linked to differences in task performance (Miller et al., 2008; Prakash et al., 2012). Further, one interesting trend evident in the correlational analyses conducted separately within each age group is the difference in the relationship between FP-DMN and VOCAB task performance based on age group. While the negative relationship between FP-DMN connectivity and VOCAB performance was only significant for the younger adults in the sample after correcting for multiple comparisons, the fact that this relationship is positively related to VOCAB performance in older adults is a novel and unexpected finding (see Table 6). While previous studies found that FP-DMN connectivity was consistently associated with poorer performance on executive function tasks (Damoiseaux, 2017; C. L. Grady et al., 2010; Prakash et al., 2012; Uddin et al., 2009), this study found a similar relationship with VOCAB task performance in younger adults (and marginally so in middle adulthood), but found the opposite relationship with task performance in older adults. One potential effect that might influence this relationship is the superior performance on VOCAB tasks by older adults – it could be that these tasks are easier for older adults than they are for younger adults, and thus this relationship between FP-DMN connectivity and task performance (frequently found to be a negative relationship in executive function tasks) simply reflects processing difficulty during the task. However, this finding of a positive relationship between FP-DMN connectivity and task performance in older adults was an unexpected finding in this study, and thus interpretation of its meaning is purely speculative. By linking these connectivity patterns to task performance, this study adds to existing literature in identifying brain-based metrics that are directly linked to cognitive outcomes.
Limitations and Future Directions.
One limitation of the present study is the number of participants whose data were not usable in the context of the present analyses. While the balanced design, stringent scrubbing criteria, and appropriateness of factor structure fit allowed for a robust set of data for analysis, they also resulted in significant data loss. That being said, one advantage of this method is that errors are produced if the predefined network structure is not an appropriate fit for the data. While past studies have conducted analyses by parcellating correlation matrices based on predefined network organization, there is no validation as to whether these ROI network memberships are appropriate for each individual included in the analyses. The present study, therefore, excluded roughly 54% of all valid data (sample size after scrubbing exclusion criteria=312; sample size of final analyses=142), however the data that were included are data that we know fit the predefined network parcellation scheme. Had the analysis included these individuals (as it would have if we had examined connectivity using a standard correlational/graph theoretical approach), it would have included participants whose connectivity patterns may not have been appropriately modeled based on the predefined networks, thus potentially weakening the results. However, excluding these individuals also considerably reduced the power of the current study, which in turn might limit the generalizability of the findings presented here. While this may somewhat limit the strength of the conclusions of the present study, the within-subjects design of the analyses somewhat assuages concerns about the strength of the effect of task domain on the observed connectivity patterns.
One additional aspect of the study that may have limited the strength of the results is the fact that an externally-derived network parcellation scheme was utilized for defining network membership of ROIs in the present analyses (Power et al., 2011). This parcellation scheme was based on network organization at rest, however the authors cross-validated its networks with task activation-based data in order to determine cognitive relevance of networks, and to validate spatial distributions. As such, one strength of utilizing this parcellation system is that it was not derived based on the present data, and thus the present results are not simply an artefact of double-dipping (Kriegeskorte, Simmons, Bellgowan, & Baker, 2009). However, it is also possible that this network parcellation scheme may not represent an ideal fit for task-based connectivity data since it was derived during rest in an external sample, or that it may not be appropriate for participants in the present study as evidenced by the number of subjects excluded for not exhibiting good model fit. Given that the present study examined connectivity across a wide range of ages (20–80) and across 4 cognitive domains (VOCAB, SPEED, FLUID, and MEM), using an external network parcellation in the present analyses was crucial to avoid using a network parcellation that was biased by participant age or cognitive domain. While it would have been possible to derive separate network parcellations for each cognitive domain in younger adults, then utilize these across all participants, this would have resulted in differential network structure for each cognitive domain (and, by definition, poorer fit for older adults), which would have made network-based comparisons across the 4 domains inappropriate. Thus, while the results may have been slightly weakened by using this external network parcellation, it was necessary in order to appropriately test the hypotheses of the present study.
Further, a difference was found in VOCAB and FLUID task performance between those participants who were included and those who were excluded from the present analyses. While task performance was not included as inclusion/exclusion criteria for these analyses, the subsample of individuals who met eligibility for these analyses tended to do better on these tasks. However, given that these included and excluded participants did not differ based on age, education, and NART IQ, this difference may not be representative of a systematic bias in the subsample of included participants.
Another limitation is the difference in NART IQ between older and younger/middle-aged participants. While sampling was conducted in an unbiased, randomized way, this unequal distribution emerged, such that older adults tended to have higher verbal IQ than younger adults. This is consistent with previous studies reporting preserved or improved vocabulary/semantic memory in the context of healthy aging (Verhaeghen, 2003), which may be reflective of accrued verbal experience over adulthood, a cohort effect, or participation bias in that older adults with better cognitive functioning or more educational experience may be more likely to want to participate in cognitive aging studies, or some combination of the two. While these sources of bias are impossible to disentangle in the present study, this may be a general limitation of most cross-sectional studies of cognitive aging.
Conclusions.
Results from the present study suggest that in the context of aging, inter-network connectivity among a set of cognitive networks may be modulated by both age and cognitive process being employed. Further, some of the differences in connectivity patterns based on age may also represent inefficient patterns of network recruitment, resulting in poorer behavioral performance as a result of this network inefficiency.
Acknowledgements:
The authors thank Dr. Kathleen Gates at the University of North Carolina at Chapel Hill for her assistance with the MIIVsem analyses. This research was supported by a grant from the National Institute on Aging (R01 AG038465, PI Dr. Stern).
Contributor Information
Eleanna Varangis, Cognitive Neuroscience Division, Department of Neurology, College of Physicians and Surgeons, Columbia University.
Qolamreza Razlighi, Cognitive Neuroscience Division, Department of Neurology, College of Physicians and Surgeons, Columbia University.
Christian G. Habeck, Cognitive Neuroscience Division, Department of Neurology, College of Physicians and Surgeons, Columbia University
Zachary Fisher, Department of Psychology and Neuroscience, University of North Carolina at Chapel Hill.
Yaakov Stern, Cognitive Neuroscience Division, Department of Neurology, College of Physicians and Surgeons, Columbia University, 630 W 168th Street, P&S Box 16, New York, NY 10032, (212) 342-1350.
References
- Amer T, Anderson JAE, Campbell KL, Hasher L, & Grady CL (2016). Age differences in the neural correlates of distraction regulation: A network interaction approach. NeuroImage, 139, 231–239. doi: 10.1016/j.neuroimage.2016.06.036 [DOI] [PubMed] [Google Scholar]
- Avelar-Pereira B, Bäckman L, Wåhlin A, Nyberg L, & Salami A (2017). Age-Related Differences in Dynamic Interactions Among Default Mode, Frontoparietal Control, and Dorsal Attention Networks during Resting-State and Interference Resolution. Front Aging Neurosci, 9. doi: 10.3389/fnagi.2017.00152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith MA, & Bandettini PA (2006). Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. NeuroImage, 31(4), 1536–1548. doi: 10.1016/j.neuroimage.2006.02.048 [DOI] [PubMed] [Google Scholar]
- Bollen K (1996). An alternative two stage least squares (2SLS) estimator for latent variable equations. Psychometrika, 61(1), 109–121. doi: 10.1007/BF02296961 [DOI] [Google Scholar]
- Bollen K (2001). Two-stage least squares and latent variable models: Simultaneous estimation and robustness to misspecifications In Cudeck R, Toit S, & Sörbom D (Eds.), Structural equation modeling: Present and Future, A Festschrift in Honor of Karl Jöreskog (pp. 119–138). Lincoln, IL: Scientific Software. [Google Scholar]
- Carp J (2013). Optimizing the order of operations for movement scrubbing: Comment on Power et al. NeuroImage, 76, 436–438. doi: 10.1016/j.neuroimage.2011.12.061 [DOI] [PubMed] [Google Scholar]
- Chan MY, Alhazmi FH, Park DC, Savalia NK, & Wig GS (2017). Resting-State Network Topology Differentiates Task Signals across the Adult Life Span. J Neurosci, 37(10), 2734–2745. doi: 10.1523/jneurosci.2406-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MY, Park DC, Savalia NK, Petersen SE, & Wig GS (2014). Decreased segregation of brain systems across the healthy adult lifespan. Proc Natl Acad Sci U S A, 111(46), E4997–5006. doi: 10.1073/pnas.1415122111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chand GB, Wu J, Hajjar I, & Qiu D (2017). Interactions of the Salience Network and Its Subsystems with the Default-Mode and the Central-Executive Networks in Normal Aging and Mild Cognitive Impairment. Brain Connect, 7(7), 401–412. doi: 10.1089/brain.2017.0509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, & Sereno MI (1999). Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage, 9(2), 179–194. doi: 10.1006/nimg.1998.0395 [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS (2017). Effects of aging on functional and structural brain connectivity. NeuroImage, 160, 32–40. doi: 10.1016/j.neuroimage.2017.01.077 [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, … Rombouts SA (2008). Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex, 18(8), 1856–1864. doi: 10.1093/cercor/bhm207 [DOI] [PubMed] [Google Scholar]
- Dennis EL, & Thompson PM (2014). Functional brain connectivity using fMRI in aging and Alzheimer’s disease. Neuropsychol Rev, 24(1), 49–62. doi: 10.1007/s11065-014-9249-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon ML, Andrews-Hanna JR, Spreng RN, Irving ZC, Mills C, Girn M, & Christoff K (2017). Interactions between the default network and dorsal attention network vary across default subsystems, time, and cognitive states. NeuroImage, 147, 632–649. doi: 10.1016/j.neuroimage.2016.12.073 [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, … Petersen SE (2007). Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A, 104(26), 11073–11078. doi: 10.1073/pnas.0704320104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito F, Bertolino A, Scarabino T, Latorre V, Blasi G, Popolizio T, … Di Salle F (2006). Independent component model of the default-mode brain function: Assessing the impact of active thinking. Brain Res Bull, 70(4–6), 263–269. doi: 10.1016/j.brainresbull.2006.06.012 [DOI] [PubMed] [Google Scholar]
- Esposito R, Cieri F, Chiacchiaretta P, Cera N, Lauriola M, Di Giannantonio M, … Ferretti A (2018). Modifications in resting state functional anticorrelation between default mode network and dorsal attention network: comparison among young adults, healthy elders and mild cognitive impairment patients. Brain Imaging Behav, 12(1), 127–141. doi: 10.1007/s11682-017-9686-y [DOI] [PubMed] [Google Scholar]
- Ferreira LK, Regina AC, Kovacevic N, Martin Mda G, Santos PP, Carneiro Cde G, … Busatto GF (2016). Aging Effects on Whole-Brain Functional Connectivity in Adults Free of Cognitive and Psychiatric Disorders. Cereb Cortex, 26(9), 3851–3865. doi: 10.1093/cercor/bhv190 [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, … Dale AM (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, … Dale AM (2004). Automatically parcellating the human cerebral cortex. Cereb Cortex, 14(1), 11–22. [DOI] [PubMed] [Google Scholar]
- Fisher Z, Bollen K, & Gates KM (In Press). Dynamic factor models with model implied instrumental variable estimation. Multivariate Behavioral Research. doi: 10.1080/00273171.2018.1519406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher Z, Bollen K, Gates K, & Rönkkö M (2017). MIIVsem: Model Implied Instrumental Variable (MIIV) estimation of structural equation models (Version 0.5.0): R package.
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, & Raichle ME (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A, 102(27), 9673–9678. doi: 10.1073/pnas.0504136102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiyama H, Van Soom J, Rens G, Gooijers J, Leunissen I, Levin O, & Swinnen SP (2016). Age-Related Changes in Frontal Network Structural and Functional Connectivity in Relation to Bimanual Movement Control. J Neurosci, 36(6), 1808–1822. doi: 10.1523/JNEUROSCI.3355-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerligs L, Maurits NM, Renken RJ, & Lorist MM (2014). Reduced specificity of functional connectivity in the aging brain during task performance. Hum Brain Mapp, 35(1), 319–330. doi: 10.1002/hbm.22175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerligs L, Renken RJ, Saliasi E, Maurits NM, & Lorist MM (2015). A Brain-Wide Study of Age-Related Changes in Functional Connectivity. Cereb Cortex, 25(7), 1987–1999. doi: 10.1093/cercor/bhu012 [DOI] [PubMed] [Google Scholar]
- Grady C, Sarraf S, Saverino C, & Campbell K (2016). Age differences in the functional interactions among the default, frontoparietal control, and dorsal attention networks. Neurobiol Aging, 41, 159–172. doi: 10.1016/j.neurobiolaging.2016.02.020 [DOI] [PubMed] [Google Scholar]
- Grady CL (2008). Cognitive neuroscience of aging. Ann N Y Acad Sci, 1124, 127–144. doi: 10.1196/annals.1440.009 [DOI] [PubMed] [Google Scholar]
- Grady CL, Protzner AB, Kovacevic N, Strother SC, Afshin-Pour B, Wojtowicz M, … McIntosh AR (2010). A multivariate analysis of age-related differences in default mode and task-positive networks across multiple cognitive domains. Cereb Cortex, 20(6), 1432–1447. doi: 10.1093/cercor/bhp207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, & Dougherty RF (2009). Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex, 19(1), 72–78. doi: 10.1093/cercor/bhn059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, & Gabrieli JD (2004). Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci, 5(2), 87–96. doi: 10.1038/nrn1323 [DOI] [PubMed] [Google Scholar]
- Hedden T, Schultz AP, Rieckmann A, Mormino EC, Johnson KA, Sperling RA, & Buckner RL (2016). Multiple Brain Markers are Linked to Age-Related Variation in Cognition Cereb Cortex (Vol. 26, pp. 1388–1400). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsiger S, Koppelmans V, Mérillat S, Liem F, Erdeniz B, Seidler RD, & Jäncke L (2016). Structural and functional connectivity in healthy aging: Associations for cognition and motor behavior. Hum Brain Mapp, 37(3), 855–867. doi: 10.1002/hbm.23067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, & Smith S (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17(2), 825–841. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, & Smith SM (2012). FSL. NeuroImage, 62(2), 782–790. doi: 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- Jenkinson M, & Smith S (2001). A global optimisation method for robust affine registration of brain images. Med Image Anal, 5(2), 143–156. [DOI] [PubMed] [Google Scholar]
- Jones DT, Knopman DS, Gunter JL, Graff-Radford J, Vemuri P, Boeve BF, … Initiative A. s. D. N. (2015). Cascading network failure across the Alzheimer’s disease spectrum. Brain. doi: 10.1093/brain/awv338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BR, van Ruitenbeek P, Leunissen I, Cuypers K, Heise KF, Santos Monteiro T, … Swinnen SP (2017). Age-Related Declines in Motor Performance are Associated With Decreased Segregation of Large-Scale Resting State Brain Networks. Cereb Cortex, 1–13. doi: 10.1093/cercor/bhx297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PSF, & Baker CI (2009). Circular analysis in systems neuroscience – The dangers of double dipping. Nat Neurosci, 12(5), 535–540. doi: 10.1038/nn.2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, & Fox PT (2009). Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. J Neurosci, 29(46), 14496–14505. doi: 10.1523/JNEUROSCI.4004-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhang X, Yu C, Duan Y, Zhuo J, Cui Y, … Liu Y (2015). Impaired Parahippocampus Connectivity in Mild Cognitive Impairment and Alzheimer’s Disease. J Alzheimers Dis. doi: 10.3233/JAD-150727 [DOI] [PubMed] [Google Scholar]
- Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, … Sperling RA (2008). Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci U S A, 105(6), 2181–2186. doi: 10.1073/pnas.0706818105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes L, Fulcher B, Yucel M, & Fornito A (2018). An evaluation of the efficacy, reliability, and sensitivity of motion correction strategies for resting-state functional MRI. NeuroImage, 171, 415–436. doi: 10.1016/j.neuroimage.2017.12.073 [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, & Petersen SE (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59(3), 2142–2154. doi: 10.1016/j.neuroimage.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, … Petersen SE (2011). Functional network organization of the human brain. Neuron, 72(4), 665–678. doi: 10.1016/j.neuron.2011.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash RS, Heo S, Voss MW, Patterson B, & Kramer AF (2012). Age-related differences in cortical recruitment and suppression: implications for cognitive performance. Behav Brain Res, 230(1), 192–200. doi: 10.1016/j.bbr.2012.01.058 [DOI] [PubMed] [Google Scholar]
- Razlighi QR, Habeck C, Barulli D, & Stern Y (2017). Cognitive neuroscience neuroimaging repository for the adult lifespan. NeuroImage, 144(Pt B), 294–298. doi: 10.1016/j.neuroimage.2015.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razlighi QR, Habeck C, Steffener J, Gazes Y, Zahodne LB, MacKay-Brandt A, & Stern Y (2014). Unilateral disruptions in the default network with aging in native space. Brain Behav, 4(2), 143–157. doi: 10.1002/brb3.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala-Llonch R, Pena-Gomez C, Arenaza-Urquijo EM, Vidal-Pineiro D, Bargallo N, Junque C, & Bartres-Faz D (2012). Brain connectivity during resting state and subsequent working memory task predicts behavioural performance. Cortex, 48(9), 1187–1196. doi: 10.1016/j.cortex.2011.07.006 [DOI] [PubMed] [Google Scholar]
- Song J, Birn RM, Boly M, Meier TB, Nair VA, Meyerand ME, & Prabhakaran V (2014). Age-related reorganizational changes in modularity and functional connectivity of human brain networks. Brain Connect, 4(9), 662–676. doi: 10.1089/brain.2014.0286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Sepulcre J, Turner GR, Stevens WD, & Schacter DL (2013). Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. Journal of cognitive neuroscience(25), 74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Viviano JD, & Schacter DL (2016). Attenuated anticorrelation between the default and dorsal attention networks with aging: evidence from task and rest. Neurobiol Aging, 45, 149–160. doi: 10.1016/j.neurobiolaging.2016.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Habeck C, Steffener J, Barulli D, Gazes Y, Razlighi Q, … Salthouse T (2014). The Reference Ability Neural Network Study: motivation, design, and initial feasibility analyses. NeuroImage, 103, 139–151. doi: 10.1016/j.neuroimage.2014.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suckling J, Simas T, Chattopadhyay S, Tait R, Su L, Williams G, … O’Brien JT (2015). A Winding Road: Alzheimer’s Disease Increases Circuitous Functional Connectivity Pathways. Front Comput Neurosci, 9, 140. doi: 10.3389/fncom.2015.00140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AM, Biswal BB, Castellanos FX, & Milham MP (2009). Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum Brain Mapp, 30(2), 625–637. doi: 10.1002/hbm.20531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaeghen P (2003). Aging and vocabulary scores: a meta-analysis. Psychol Aging, 18(2), 332–339. [DOI] [PubMed] [Google Scholar]