Abstract
Intervertebral disc degeneration (IDD) is widely considered to be one of the main causes of lower back pain, which is a chronic progressive disease closely related to inflammation, nucleus pulposus (NP) cell apoptosis and extracellular matrix (ECM) degradation. Berberine (BBR) is an alkaloid compound with an anti-inflammatory effect and has been reported to exert therapeutic action in several inflammatory diseases, including osteoarthritis. Therefore, it was hypothesized that BBR may have a therapeutic effect on IDD through inhibition of the inflammatory response. The aim of the present study was to evaluate the influence of BBR on IDD in interleukin (IL)-1β-treated human NP cells in vitro. The results showed that BBR attenuated the upregulation of ECM-catabolic factors [matrix metalloproteinase (MMP)-3, MMP-13, a disintegrin and metalloproteinase with thrombospondin motif (ADAMTS)-4 and ADAMTS-5], and the downregulation of ECM-anabolic factors (type II collagen and aggrecan) following stimulation of the human NP cells with IL-1β. Treatment with BBR also protected human NP cells from IL-1β-induced apoptosis, as determined by western blotting and flow cytometry. Mechanistically, the IL-1β-stimulated degradation of IκBα, and the phosphorylation and translocation of nuclear factor (NF)-κB p65 were found to be attenuated by BBR, indicating that NF-κB pathway activation was suppressed by BBR in the IL-1β-treated human NP cells. The results of the experiments revealed a therapeutic potential of BBR for the prevention or treatment of IDD.
Keywords: intervertebral disc degeneration, berberine, inflammation, nuclear factor-κB, extracellular matrix degradation, apoptosis
Introduction
Lower back pain (LBP) is a common musculoskeletal disorder that severely affects the quality of life of human beings and imposes a substantial economic burden on society (1). Intervertebral disc (IVD) degeneration (IDD) has been shown to be the major contributor to LBP (2). IVD, composed of a central nucleus pulposus (NP) surrounded by annulus fibrosus and cartilaginous endplates, is an avascular organ. In healthy IVD, the hydrated NP, which is mainly composed of NP cells and is rich in the extracellular matrix (ECM), including type II collagen and aggrecan, is important in the physiological function of the disc in distributing the mechanical load acting on the spine, and in multi-axial flexibility (3,4). During the progression of IDD, the increased levels of pro-inflammatory cytokines, elevated cell apoptosis, increased production of ECM-catabolic proteinases, including matrix metalloproteinases (MMPs) and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS), and decreased synthesis of type II collagen and aggrecan have been observed within the NP tissue (5-7). These cellular and molecular changes lead to disruption of the physiological structure and function of IVD and to spinal instability, which are the main triggers of LBP (8).
The excessive production of proinflammatory molecules secreted by NP cells, including tumor necrosis factor (TNF)-α, interleukin (IL)-1α, IL-1β, IL-2 and IL-6, has been demonstrated to serve critical roles in the initiation and development of IDD (5,9). Among the above-mentioned inflammatory cytokines, IL-1β is widely considered to be the predominant cytokine that is expressed at high levels in degenerative IVD tissues and cells and has been shown to be involved in several pathological processes in NP cells, including inflammatory responses, oxidative stress, apoptosis, and an imbalance of ECM synthesis and degradation (10,11). Therefore, inhibiting the effect of IL-1β on NP cells may postpone the progression of IDD.
Berberine (BBR) is an isoquinoline alkaloid that is derived from several medicinal herbs, including Rhizoma Coptidis, Cortex Phellodendri, and Mahonia bealei, which have long been used in traditional Chinese medicine. BBR has been reported to possess multiple pharmacological effects, including anti-oxidative, anti-inflammatory and anti-apoptotic effects (12-14). Previous studies have found that BBR has therapeutic effects on musculoskeletal disorders, including rheumatoid arthritis and osteoarthritis, owing to its anti-inflammatory properties (15,16). Zhao et al showed that BBR treatment can protect articular cartilage from degeneration via activating the Akt-p70S6K-S6 signaling pathway in IL-1β-stimulated articular chondrocytes and in a rat osteoarthritis model (17). Hu et al reported that BBR decreases glycosaminoglycan release and nitric oxide production in IL-1β-stimulated chondrocytes (16). In addition, the administration of BBR was found by Zhou et al to prevent nitric oxide-induced chondrocyte apoptosis and cartilage degeneration in a rat model of osteoarthritis (18). As the morphology and avascular supply of NP cells are similar to those of chondrocytes, and BBR has been reported to inhibit the effects of oxidative stress in rat NP cells (19), it was hypothesized that BBR may prevent the development of IDD by protecting NP cells from IL-1β-induced degenerative effects. Therefore, the purpose of the present study was to investigate the influence of BBR on IL-1β-induced apoptosis and ECM degradation in human NP cells and to elucidate the underlying molecular mechanism.
Materials and methods
Patient tissue samples
Between March and October 2017, human lumbar NP tissues were collected from 10 patients (six women and four men; mean age, 24.7 years; age range, 15-42 years) with idiopathic scoliosis who underwent deformity correction surgery with the approval of the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China). Written informed consent was obtained from all participants involved in the study. The degrees of degeneration of the discs of all participants were assessed using the modified Pfirrmann grading system (20) and were classified as grade II.
Human NP cell culture and treatment
Human NP cells were isolated using a method reported previously by Kang et al (21), and were then cultured in DMEM/F12 (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 15% of fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and 1% of a penicillin-streptomycin solution at 37°C in a humidified atmosphere containing 5% CO2. The cells were passaged twice for use in the following experiments. The human NP cells were seeded in a six-well plate at a density of 105 cells/well. On reaching 80-90% confluence, the NP cells were incubated with 25 µM BBR for 2 h prior to IL-1β (10 ng/ml) treatment for 24 h at 37°C. The NP cells were harvested for subsequent experiments. All experiments were conducted in triplicate.
Cell viability analysis
Cell viability was evaluated using a Cell Counting Kit-8 (CCK-8, Dojindo Molecular Technologies, Inc., Kumamoto, Japan). Briefly, the human NP cells were seeded in a 96-well plate (5×103 cells per well) and cultured as described above, followed by treatment with various concentrations of BBR (5, 10, 15, 20 or 25 µM) or IL-1β (10 ng/ml) for 24 h at 37°C. Subsequently, 10 µl of the CCK-8 dye was added into each well, followed by incubation at 37°C for 2 h. The optical density was measured at 450 nm on a microplate reader (Leica Microsystems GmbH, Wetzlar, Germany).
RNA extraction and reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
Total RNA was isolated from the human NP cells with TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The mRNA expression levels of type II collagen, aggrecan, MMP-3, MMP-13, ADAMTS-4 and ADAMTS-5 were quantified by RT-qPCR analysis on a 7500 Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.) under the cycling conditions recommended by the manufacturer. qPCR was performed using a SYBR Prime Script™ RT-qPCR kit (Takara Biotechnology Co., Ltd., Dalian, China). The reaction conditions were as follows: 95°C for 10 min, followed by 40 cycles at 95°C for 30 sec and 60°C for 30 sec. The gene expression levels were normalized to that of β-actin. Relative expression levels were analyzed using the 2−ΔΔCq method (22). The primer sequences used for RT-qPCR analysis were as follows: Type II collagen, forward 5′-AGA ACT GGT GGA GCA GCA AGA-3′ and reverse 5′-AGC AGG CGT AGG AAG GTC AT-3′; aggrecan, forward 5′-TGA GCG GCA GCA CTT TGA C-3′ and reverse 5′-TGA GTA CAG GAG GCT TGA GG-3′; MMP-3, forward 5′-TTC CTT GGA TTG GAG GTG AC-3′ and reverse 5′-AGC CTG GAG AAT GTG AGT GG-3′; MMP-13, forward 5′-CCC AAC CCT AAA CAT CCA A-3′ and reverse 5′-AAA CAG CTC CGC ATC AAC C-3′; ADAMTS-4, forward 5′-ACC CAA GCA TCC GCA ATC-3′ and reverse 5′-TGC CCA CAT CAG CCA TAC-3′; ADAMTS-5, forward 5′-GAC AGT TCA AAG CCA AAG ACC-3′ and reverse 5′-TTT CCT TCG TGG CAG AGT-3′; β-actin, forward 5′-AGC GAG CAT CCC CCA AAG TT-3′ and reverse 5′-GGG CAC GAA GGC TCA TCA TT-3′.
Western blotting
The western blotting procedure was performed to analyze protein levels. The treated human NP cells were lysed with RIPA lysis buffer, and protein concentration was measured using the BCA Protein Assay kit (Beyotime Institute of Technology, Haimen, China). The nuclear and cytoplasmic proteins were isolated with the Nuclear/Cytosolic Fractionation kit (BioVision, Inc., Mountain View, CA, USA). The concentration was measured using a BCA protein assay. A total of 40 µg protein per lane was separated by sodium dodecyl sulphate polyacrylamide gel electrophoresis on a 12% gel and transferred onto a polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA, USA). Following blocking with 5% non-fat milk in TBST, the membranes were incubated with primary antibodies against type II collagen (sc-7764; 142 kDa; Santa Cruz Biotechnology, Inc., Dallas, TX, USA; 1:8,000), aggrecan (ab3778; 50-60 kDa; Abcam, Cambridge, UK; 1:100), MMP-3 (14351; 60 kDa; Cell Signaling Technology, Inc., Danvers, MA, USA; 1:1,000), MMP-13 (ab39012; 54 kDa; Abcam; 1:4,000), ADAMTS-4 (ab185722; 90 kDa; Abcam; 1:1,000), ADAMTS-5 (ab41037; 73 kDa; Abcam; 1:200), B-cell lymphoma 1 (Bcl-2; ab32124; 26 kDa; Abcam; 1:1,000), Bcl-2-associated X protein (Bax; ab32503; 20 kDa; Abcam; 1:1,000), cleaved caspase3 (9664; 17 kDa; Cell Signaling Technology, Inc.; 1:1,000), nuclear factor (NF)-κB p65 (ab16502; 60 kDa; Abcam; 1:500), phosphorylated p65 (p-p65; ab86299; 60 kDa; Abcam; 1:1,000), inhibitor of κBα (IκBα; (9242; 39 kDa; Cell Signaling Technology, Inc.; 1:1,000), β-actin (ab8227; 42 kDa; Abcam; 1:2,000) and lamin B1 (ab16048; 66 kDa; Abcam; 1:1,000) overnight at 4°C, followed by incubation with the respective secondary antibodies (BA1054; Boster Biological Technology, Pleasanton, CA, USA, 1:5,000) at room temperature for 1 h. The protein bands were detected using an enhanced chemiluminescence system and analyzed quantitatively using BandScan software (version 4.30; BioMarin Pharmaceutical Inc., UK). β-actin and lamin B1 served as loading controls.
Flow cytometry
Following treatment with the different agents, the human NP cells were harvested, washed with cold PBS, and stained using the Annexin V-FITC Apoptosis Detection kit (Beyotime Institute of Biotechnology, Haimen, China). The fluorescence of the cells was determined immediately by flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA). The apoptotic rate was calculated as the sum of the percentages of early (Annexin V+/PI−) and late (Annexin V+/PI+) apoptotic cells.
Statistical analysis
The results are expressed as the mean ± standard deviation. Statistical analyses were performed using SPSS v.18.0 software (SPSS, Inc., Chicago, IL, USA). Differences between groups were evaluated by one-way analysis of variance followed by the Tukey test. P<0.05 was considered to indicate a statistically significant difference.
Results
Cell viability of human NP cells following treatment with BBR
The present study evaluated the effect of BBR on the viability of human NP cells at various concentrations (5, 10, 15, 20 or 25 µM) for 24 h using the CCK-8 assay. The results indicated that BBR was not significantly toxic to human NP cells at concentrations ranging between 5 and 25 µM (Fig. 1A). In addition, BBR was administered to IL-1β-treated human NP cells at various concentrations (15, 20 or 25 µM). Pretreatment with BBR had a significant protective effect against IL-1β-induced cell apoptosis, particularly at 25 µM (Fig. 1B). Therefore, 25 µM BBR was selected for subsequent experiments.
Figure 1.
Protective effect of BBR on human NP cell viability. (A) Influence of the indicated concentrations of BBR on the viability of NP cells at 24 h, as measured by a CCK-8 assay. (B) CCK-8 results of BBR-pretreated NP cells stimulated by IL-1β. Data are presented as the mean ± standard deviation. *P<0.05, vs. control group; #P<0.05, vs. IL-1β group. NP, nucleus pulposus; BBR, berberine; CCK-8, Cell Counting Kit-8; IL-1β, interleukin-1β.
Inhibitory effects of BBR on IL-1β-induced ECM degradation by human NP cells
To determine whether BBR affects IL-1β-induced ECM degradation by human NP cells, the present study assessed the mRNA and protein expression levels of the main components of the NP ECM, including type II collagen and aggrecan, and major NP ECM catabolic proteinases MMP-3, MMP-13, ADAMTS-4 and ADAMTS-5 by RT-qPCR and western blot analyses, respectively. The results revealed that IL-1β treatment markedly decreased the mRNA expression levels of type II collagen and aggrecan, whereas pretreatment with BBR attenuated the downregulation induced by IL-1β (Fig. 2A and B). It was also found that the mRNA expression levels of MMP-3, MMP-13, ADAMTS-4 and ADAMTS-5 in human NP cells were significantly increased following stimulation with IL-1β (Fig. 2C-F). However, BBR pretreatment resulted in a statistically significant attenuation of IL-1β-induced upregulation of these ECM catabolic proteinases (Fig. 2C-F). The same results were observed for protein expression levels (Fig. 2G-M)
Figure 2.
Effects of BBR on IL-1β-induced extracellular matrix degradation by human NP cells. Following treatment with BBR (25 µM) for 2 h, NP cells were stimulated with IL-1β (10 ng/ml) for 24 h and harvested for RT-qPCR and western blot analyses. mRNA expression of (A) type II collagen, (B) aggrecan, (C) MMP-3, (D) MMP-13, (E) ADAMTS-4 and (F) ADAMTS-5, as analyzed by RT-qPCR analysis. (G) Western blot analysis of the protein levels of (H) type II collagen, (I) aggrecan, (J) MMP-3, (K) MMP-13, (L) ADAMTS-4 and (M) ADAMTS-5. β-actin served as an internal control. Data are presented as the mean ± standard deviation. *P<0.05, vs. control group; #P<0.05, vs. IL-1β group. NP, nucleus pulposus; BBR, berberine; IL-1β, interleukin-1β; MMP, matrix metalloproteinase; ADAMTS, a disintegrin and metalloproteinase with thrombospondin motifs; RT-qPCR, reverse transcription-quantitative polymerase chain reaction.
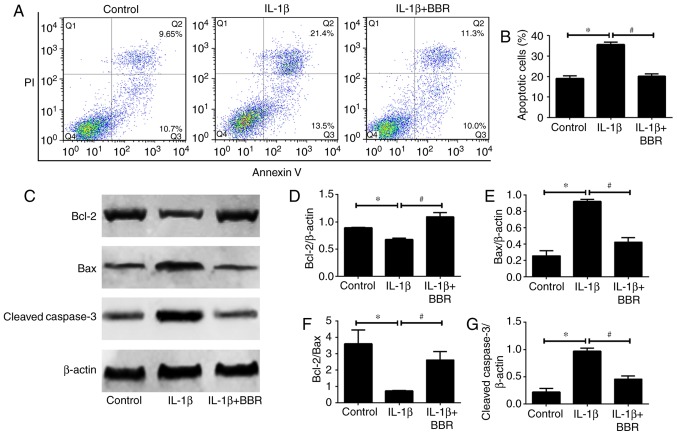
BBR protects human NP cells from IL-1β-induced cell apoptosis
As NP cell apoptosis is important in the progression of IDD, the present study investigated the influence of BBR on IL-1β-induced human NP cell apoptosis. Flow cytometric analysis revealed that IL-1β increased the rate of apoptosis compared with that in the untreated control (Fig. 3A and B). When the human NP cells were cotreated with BBR and IL-1β, the results showed a significant decrease in the rate of apoptosis (Fig. 3A and B). Furthermore, the expression levels of apoptosis-related proteins (Bcl-2, Bax and cleaved caspase3) were assessed. The protein levels of Bax (pro-apoptotic) and cleaved caspase3 (pro-apoptotic) were increased and the protein level of Bcl-2 (anti-apoptotic) was decreased in the IL-1β-treated human NP cells (Fig. 3C-G). These trends were reversed by BBR pretreatment (Fig. 3C-G).
Figure 3.
Impact of BBR on IL-1β-induced human NP cells apoptosis. Following treatment with BBR (25 µM) for 2 h, NP cells were stimulated with IL-1β (10 ng/ml) for 24 h and harvested for flow cytometry and western blotting. (A) Flow cytometry was used to determine the (B) rates of apoptosis of human NP cells. (C) Western blot analysis and quantification of protein levels of (D) Bcl-2, (E) Bax, (F) Bcl-2/Bax and (G) cleaved caspase 3. β-actin served as an internal control. Data are presented as the mean ± standard deviation. *P<0.05, vs. control group; #P<0.05, vs. IL-1β group. NP, nucleus pulposus; BBR, berberine; IL-1β, interleukin-1β; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein.
Suppressive effects of BBR on the IL-1β-induced activation of NF-κB in human NP cells
The NF-κB pathway has been found to be aberrantly activated in IDD and has been demonstrated to be an important mediator of the IL-1β-induced degenerative effects in human NP cells. Therefore, to further examine the underlying mechanism of BBR-induced anti-degenerative effects, the present study determined whether BBR regulates the NF-κB pathway in IL-1β-treated human NP cells. The extent of NF-κB p65 phosphorylation and the intracellular distribution of p65 were first evaluated by western blotting. Compared with the untreated group, a higher ratio p-p65/p65 and nuclear translocation of p65 from the cytoplasm were observed following IL-1β stimulation (Fig. 4A-C), indicating activation of the NF-κB signaling pathway in the IL-1β-treated human NP cells. However, pretreatment with BBR markedly suppressed this IL-1β-induced phosphorylation and translocation of p65 (Fig. 4A-C). IκBα is an inhibitor protein for p65, the phosphorylation of IκBα and its subsequent degradation induce the translocation of p65 to the nucleus. Therefore, the level of cytoplasmic IκBα was analyzed by western blotting in human NP cells. IL-1β treatment induced the degradation of cytoplasmic IκBα, whereas this effect was attenuated by BBR pretreatment (Fig. 4D).
Figure 4.
Effects of BBR on IL-1β-induced NF-κB pathway activity in human NP cells. (A) Total protein levels of NF-κB p65 and its phosphorylated form were analyzed by western blotting. Protein levels of p65 in the (B) cytoplasm and (C) nucleus were determined by western blotting. (D) Protein levels of IκBα in the cytoplasm were evaluated by western blotting. β-actin or lamin B1 was used as an internal control. Data are presented as the mean ± standard deviation. *P<0.05, vs. control group; #P<0.05, vs. IL-1β group. NP, nucleus pulposus; BBR, berberine; IL-1β, interleukin-1β; NF-κB, nuclear factor-κB; IκBα, inhibitor of κBα; p-, phosphorylated.
Discussion
LBP affects 80% of the global population, and IDD is widely recognized as the main cause of LBP (2). The prevention or reversal of IDD is a potential treatment of LBP; however, the pathological basis and mechanisms underlying IDD remain to be fully elucidated. Current evidence suggests that the excessive inflammatory response, an increase in the proportion of apoptotic NP cells, and the imbalance between anabolism and catabolism of NP ECM are closely associated with the development of IDD (7,8,23); therefore, targeting these pathological processes is a novel strategy for the treatment of IDD. Medical treatments of LBP caused by IDD mainly include conservative approaches and, rarely, surgical procedures. Conservative approaches, including non-steroidal anti-inflammatory drugs, are prescribed to inhibit inflammation and to relieve the symptom of back pain temporarily; however, the widespread and long-term use of non-steroidal anti-inflammatory drugs results in considerable adverse effects (24). Surgical procedures, including spine fusion and discectomy (25), which cannot preserve the function of IVD, imposes a financial burden on the patient and is not an optimal choice. Therefore, investigating novel drug treatments that can promote endogenous repair of degenerative IVD and prevent IDD development is crucial, particularly for early-stage IDD. In previous years, plant-derived compounds have attracted the attention of those investigating IDD treatment owing to their anti-inflammatory effects and few adverse effects (10,26). BBR, an isoquinoline alkaloid extracted from Coptidis Rhizoma and Cortex Phellodendri, has been shown to have potent therapeutic potential against inflammatory diseases (12,27). The present study provides the first evidence, to the best of our knowledge, that BBR has pharmacological inhibitory effects on ECM degradation and apoptosis in IL-1β-stimulated human NP cells. The results also showed that BBR inhibits the IL-1β-induced activation of the NF-κB pathway in human NP cells (Fig. 5).
Figure 5.
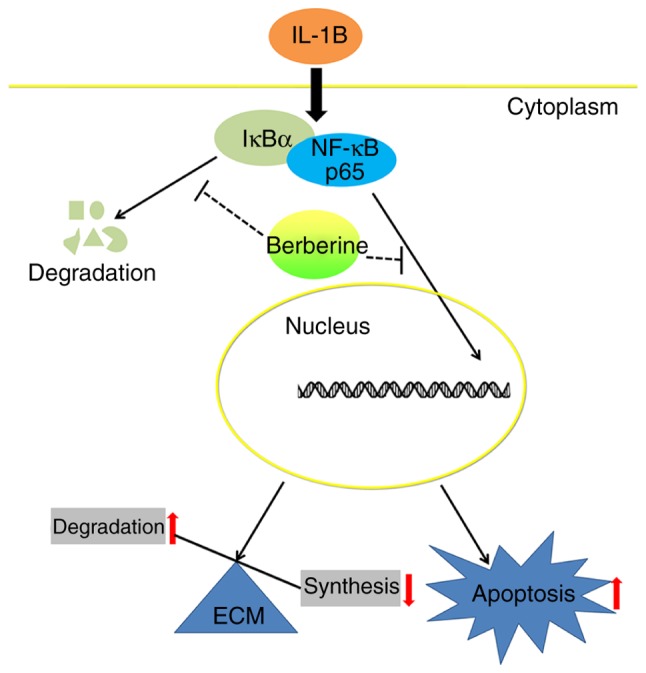
Schematic model of the signaling mechanisms underlying the inhibitory effect of BBR on ECM catabolism and apoptosis in IL-1β-stimulated human NP cells. NP, nucleus pulposus; BBR, berberine; IL-1β, interleukin-1β; NF-κB, nuclear factor-κB; IκBα, inhibitor of κBα; ECM, extracellular matrix.
IL-1β is a major risk factor of IDD, with potent proinflammatory activity involving stimulation of the production of multiple proinflammatory mediators (28). It has been demonstrated that IL-1β is involved in multiple pathological processes related to the development of IDD (5,10,29). The progressive degradation of the NP ECM, which mainly consists of type II collagen and aggrecan (performing a vital function in the maintenance of the physiological function of IVD), is the most prominent feature of IDD. MMPs and ADAMTSs are the major ECM-degrading enzymes in IDD, and their functions in IDD have been investigated extensively (30). MMP-3 and MMP-13 have been identified as the main collagenases that lead to degradation of type II collagen in NP, whereas ADAMTS-4 and ADAMTS-5 are the primary aggrecanases due to their potent and specific activity in cleaving aggrecan. The expression of all these enzymes, which are often regarded as the catabolic markers of IDD, has been found to be upregulated in degenerative NP (31). The inhibition of MMPs and ADAMTSs has a therapeutic effect on IDD in vitro and in vivo. There is substantial evidence that, in NP cells, IL-1β can promote the production of ECM-degrading enzymes that degrade type II collagen and aggrecan in NP ECM (5,10). In the present study, the results showed that IL-1β treatment resulted in the upregulation of MMP-3, MMP-13, ADAMTS-4 and ADAMTS-5 and the downregulation of type II collagen and aggrecan in human NP cells, as expected, and that BBR reversed these changes induced by IL-1β. These results suggest that BBR has the potential to control the progression of IDD by restoring the balance between ECM anabolism and catabolism in human NP cells.
NP cells are important for maintaining the structural integrity of IVD by generating the molecular components of ECM. Recently, an apoptosis-associated decrease in the NP cell number was proposed as an important mechanism underlying the development of IDD (32,33). Therefore, treatment of IDD not only depends on the NP ECM homeostasis but also on inhibiting the apoptosis of NP cells. Several studies have demonstrated that IL-1β is an important regulator of the apoptosis of NP cells (34,35). The majority of rabbit NP cells were shown to undergo apoptosis following IL-1β stimulation and exhibit morphological changes related to apoptosis, whereas a combined treatment with insulin-like growth factor 1 and IL-1β significantly reduced IL-1β-mediated apoptosis (35). Shen et al (34) reported that IL-1β induces the mitochondrial pathway in NP cells by increasing the expression ratio Bax/Bcl-2 and by releasing cytochrome c from the mitochondria to the cytoplasm, subsequently activating downstream caspases 9 and 3 to complete the apoptotic process. In addition, Chen et al (19) found that BBR may mitigate oxidative-stress-induced apoptosis through the mitochondrial pathway. The results of flow cytometric analysis in the present study revealed that BBR effectively prevented IL-1β-induced apoptosis. The data also indicated that BBR attenuated the downregulation of Bcl-2 and the upregulation of Bax and cleaved caspase 3 at the protein level in IL-1β-treated human NP cells. Taken together, these results suggest that BBR protects human NP cells from IL-1β-induced apoptosis.
Various intracellular signaling pathways are activated in response to inflammatory stimulation associated with IDD, thereby mediating the increase in the production of a downstream effector that is closely involved in the progression of IDD (36). As one of the most critical intracellular signaling proteins, NF-κB can regulate the expression of genes associated with ECM degradation and cell apoptosis in IL-1β-treated human NP cells (21,37). Inhibiting the activation of NF-κB is regarded as a potential therapeutic strategy against IDD. Under normal conditions, NF-κB is located in the cytoplasm bound to an inhibitory protein, IκB, which prevents NF-κB from entering the nucleus. Upon stimulation by IL-1β, the IκB protein is phosphorylated and degraded, resulting in the translocation of NF-κB from the cytoplasm to the nucleus. Subsequently, NF-κB facilitates gene transcription by binding to specific sequences in the promoter region of NF-κB-responsive genes, which upregulate the production of catabolic enzymes, inflammatory mediators and cyto-kines (5,10). To further elucidate the molecular mechanism underlying the inhibitory effect of BBR on ECM degradation and apoptosis in IL-1β-treated NP cells, the present study assessed the influence of BBR on the IL-1β-induced activation of NF-κB in human NP cells. The results revealed that BBR significantly inhibited the IL-1β-induced upregulation of the phosphorylation of NF-κB p65 and its nuclear translocation in human NP cells. In addition, the IL-1β-induced decrease in the level of cytoplasmic IκBα was reversed by BBR, indicating that treatment with BBR inhibited the degradation of IκBα, thereby maintaining NF-κB in an inactive state. Taken together, these results suggest that the therapeutic action of BBR on IDD may be mediated by suppression of the NF-κB signaling pathway.
However, as human NP cells cultured in vitro and human NP cells in vivo may respond differently to BBR treatment, whether BBR is effective as a IDD treatment in vivo remains to be fully elucidated. Therefore, animal experiments that can truly mimic IDD pathogenesis are urgently required to confirm the protective effect of BBR against IDD and to advance current understanding of the molecular mechanisms underlying this effect.
In conclusion, the results of the present study suggest that BBR exerts potent anti-ECM catabolic and anti-apoptotic actions by inhibiting the IL-1β-induced activation of NF-κB in human NP cells, indicating that BBR may be validated as an effective therapeutic agent for IDD in the future.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
JW and ZY designed the study. LL wrote the manuscript. LL, JH, and QW conducted the experiments. LL, JH, QW, YA, and WC collected the data. JW, ZY, and LL analyzed the data. JW and ZY reviewed and revised the manuscript. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Martin BI, Deyo RA, Mirza SK, Turner JA, Comstock BA, Hollingworth W, Sullivan SD. Expenditures and health status among adults with back and neck problems. JAMA. 2008;299:656–664. doi: 10.1001/jama.299.6.656. [DOI] [PubMed] [Google Scholar]
- 2.Luoma K, Riihimaki H, Luukkonen R, Raininko R, Viikari-Juntura E, Lamminen A. Low back pain in relation to lumbar disc degeneration. Spine (Phila Pa 1976) 2000;25:487–492. doi: 10.1097/00007632-200002150-00016. [DOI] [PubMed] [Google Scholar]
- 3.Pattappa G, Li Z, Peroglio M, Wismer N, Alini M, Grad S. Diversity of intervertebral disc cells: Phenotype and function. J Anat. 2012;221:480–496. doi: 10.1111/j.1469-7580.2012.01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakai D, Grad S. Advancing the cellular and molecular therapy for intervertebral disc disease. Adv Drug Deliv Rev. 2015;84:159–171. doi: 10.1016/j.addr.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Yang W, Yu XH, Wang C, He WS, Zhang SJ, Yan YG, Zhang J, Xiang YX, Wang WJ. Interleukin-1β in intervertebral disk degeneration. Clin Chim Acta. 2015;450:262–272. doi: 10.1016/j.cca.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 6.Wu B, Meng C, Wang H, Jia C, Zhao Y. Changes of proteoglycan and collagen II of the adjacent intervertebral disc in the cervical instability models. Biomed Pharmacother. 2016;84:754–758. doi: 10.1016/j.biopha.2016.09.077. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Li K, Han X, Mao C, Zhang K, Zhao T, Zhao J. The imbalance between TIMP3 and matrix-degrading enzymes plays an important role in intervertebral disc degeneration. Biochem Biophys Res Commun. 2016;469:507–514. doi: 10.1016/j.bbrc.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 8.Freemont AJ. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology (Oxford) 2009;48:5–10. doi: 10.1093/rheumatology/ken396. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto J, Maeno K, Takada T, Kakutani K, Yurube T, Zhang Z, Hirata H, Kurakawa T, Sakai D, Mochida J, et al. Fas ligand plays an important role for the production of pro-inflammatory cytokines in intervertebral disc nucleus pulposus cells. J Orthop Res. 2013;31:608–615. doi: 10.1002/jor.22274. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Xuan J, Gu YT, Shi KS, Xie JJ, Chen JX, Zheng ZM, Chen Y, Chen XB, Wu YS, et al. Celastrol reduces IL-1β induced matrix catabolism, oxidative stress and inflammation in human nucleus pulposus cells and attenuates rat intervertebral disc degeneration in vivo. Biomed Pharmacother. 2017;91:208–219. doi: 10.1016/j.biopha.2017.04.093. [DOI] [PubMed] [Google Scholar]
- 11.Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat Rev Rheumatol. 2014;10:44–56. doi: 10.1038/nrrheum.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, Chen Z, Yang S, Wang Y, Huang Z, Gao J, Tu S, Rao Z. Berberine ameliorates collagen-induced arthritis in rats associated with anti-inflammatory and anti-angiogenic effects. Inflammation. 2014;37:1789–1798. doi: 10.1007/s10753-014-9909-y. [DOI] [PubMed] [Google Scholar]
- 13.Li Z, Geng YN, Jiang JD, Kong WJ. Antioxidant and anti-inflammatory activities of berberine in the treatment of diabetes mellitus. Evid Based Complement Alternat Med. 2014;2014;289264 doi: 10.1155/2014/289264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang Y, Huang M, Jiang X, Liu Q, Chang X, Guo Y. The neuroprotective effects of Berberine against amyloid β-protein-induced apoptosis in primary cultured hippocampal neurons via mitochondria-related caspase pathway. Neurosci Lett. 2017;655:46–53. doi: 10.1016/j.neulet.2017.06.048. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, He X, Zhang CF, Guo CR, Wang CZ, Yuan CS. Anti-arthritic effect of berberine on adjuvant-induced rheumatoid arthritis in rats. Biomed Pharmacother. 2017;89:887–893. doi: 10.1016/j.biopha.2017.02.099. [DOI] [PubMed] [Google Scholar]
- 16.Hu PF, Chen WP, Tang JL, Bao JP, Wu LD. Protective effects of berberine in an experimental rat osteoarthritis model. Phytother Res. 2011;25:878–885. doi: 10.1002/ptr.3359. [DOI] [PubMed] [Google Scholar]
- 17.Zhao H, Zhang T, Xia C, Shi L, Wang S, Zheng X, Hu T, Zhang B. Berberine ameliorates cartilage degeneration in interleukin-1β-stimulated rat chondrocytes and in a rat model of osteoarthritis via Akt signalling. J Cell Mol Med. 2014;18:283–292. doi: 10.1111/jcmm.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Y, Liu SQ, Yu L, He B, Wu SH, Zhao Q, Xia SQ, Mei HJ. Berberine prevents nitric oxide-induced rat chondrocyte apoptosis and cartilage degeneration in a rat osteoarthritis model via AMPK and p38 MAPK signaling. Apoptosis. 2015;20:1187–1199. doi: 10.1007/s10495-015-1152-y. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Zheng Z, Wang J, Tang C, Khor S, Chen J, Chen X, Zhang Z, Tang Q, Wang C, et al. Berberine suppresses apoptosis and extracellular matrix (ECM) degradation in nucleus pulposus cells and ameliorates disc degeneration in a rodent model. Int J Biol Sci. 2018;14:682–692. doi: 10.7150/ijbs.24081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2001;26:1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 21.Kang L, Hu J, Weng Y, Jia J, Zhang Y. Sirtuin 6 prevents matrix degradation through inhibition of the NF-κB pathway in intervertebral disc degeneration. Exp Cell Res. 2017;352:322–332. doi: 10.1016/j.yexcr.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Purmessur D, Walter BA, Roughley PJ, Laudier DM, Hecht AC, Iatridis J. A role for TNFα in intervertebral disc degeneration: A non-recoverable catabolic shift. Biochem Biophys Res Commun. 2013;433:151–156. doi: 10.1016/j.bbrc.2013.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roelofs PD, Deyo RA, Koes BW, Scholten RJ, van Tulder MW. Nonsteroidal anti-inflammatory drugs for low back pain: An updated Cochrane review. Spine (Phila Pa 1976) 2008;33:1766–1774. doi: 10.1097/BRS.0b013e31817e69d3. [DOI] [PubMed] [Google Scholar]
- 25.Phillips FM, Reuben J, Wetzel FT. Intervertebral disc degeneration adjacent to a lumbar fusion. An experimental rabbit model. J Bone Joint Surg Br. 2002;84:289–294. doi: 10.1302/0301-620X.84B2.11937. [DOI] [PubMed] [Google Scholar]
- 26.Zhang B, Xu L, Zhuo N, Shen J. Resveratrol protects against mitochondrial dysfunction through autophagy activation in human nucleus pulposus cells. Biochem Biophys Res Commun. 2017;493:373–381. doi: 10.1016/j.bbrc.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 27.Chandirasegaran G, Elanchezhiyan C, Ghosh K, Sethupathy S. Berberine chloride ameliorates oxidative stress, inflammation and apoptosis in the pancreas of Streptozotocin induced diabetic rats. Biomed Pharmacother. 2017;95:175–185. doi: 10.1016/j.biopha.2017.08.040. [DOI] [PubMed] [Google Scholar]
- 28.Jimbo K, Park JS, Yokosuka K, Sato K, Nagata K. Positive feedback loop of interleukin-1beta upregulating production of inflammatory mediators in human intervertebral disc cells in vitro. J Neurosurg Spine. 2005;2:589–595. doi: 10.3171/spi.2005.2.5.0589. [DOI] [PubMed] [Google Scholar]
- 29.Johnson ZI, Schoepflin ZR, Choi H, Shapiro IM, Risbud MV. Disc in flames: Roles of TNF-α and IL-1β in intervertebral disc degeneration. Eur Cells Mater. 2015;30:104–116. doi: 10.22203/eCM.v030a08. discussion 116–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang WJ, Yu XH, Wang C, Yang W, He WS, Zhang SJ, Yan YG, Zhang J. MMPs and ADAMTSs in intervertebral disc degeneration. Clin Chim Acta. 2015;448:238–246. doi: 10.1016/j.cca.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 31.Kang L, Yang C, Yin H, Zhao K, Liu W, Hua W, Wang K, Song Y, Tu J, Li S, et al. MicroRNA-15b silencing inhibits IL-1β-induced extracellular matrix degradation by targeting SMAD3 in human nucleus pulposus cells. Biotechnol Lett. 2017;39:623–632. doi: 10.1007/s10529-016-2280-3. [DOI] [PubMed] [Google Scholar]
- 32.Han Y, Li X, Yan M, Yang M, Wang S, Pan J, Li L, Tan J. Oxidative damage induces apoptosis and promotes calcification in disc cartilage endplate cell through ROS/MAPK/NF-κB pathway: Implications for disc degeneration. Biochem Biophys Res Commun. 2017 Mar 23; doi: 10.1016/j.bbrc.2017.03.111. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 33.Cheng X, Zhang L, Zhang K, Zhang G, Hu Y, Sun X, Zhao C, Li H, Li YM, Zhao J. Circular RNA VMA21 protects against intervertebral disc degeneration through targeting miR-200c and X linked inhibitor-of-apoptosis protein. Ann Rheum Dis. 2018;77:770–779. doi: 10.1136/annrheumdis-2017-212056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen J, Xu S, Zhou H, Liu H, Jiang W, Hao J, Hu Z. IL-1β induces apoptosis and autophagy via mitochondria pathway in human degenerative nucleus pulposus cells. Sci Rep. 2017;7:41067. doi: 10.1038/srep41067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang CC, Zhou JS, Hu JG, Wang X, Zhou XS, Sun BA, Shao C, Lin Q. Effects of IGF-1 on IL-1β-induced apoptosis in rabbit nucleus pulposus cells in vitro. Mol Med Rep. 2013;7:441–444. doi: 10.3892/mmr.2012.1238. [DOI] [PubMed] [Google Scholar]
- 36.Wuertz K, Vo N, Kletsas D, Boos N. Inflammatory and catabolic signalling in intervertebral discs: The roles of NF-κB and MAP kinases. Eur Cell Mater. 2012;23:103–119. doi: 10.22203/ecm.v023a08. discussion 119–120. [DOI] [PubMed] [Google Scholar]
- 37.Li Z, Wang X, Pan H, Yang H, Li X, Zhang K, Wang H, Zheng Z, Liu H, Wang J. Resistin promotes CCL4 expression through toll-like receptor-4 and activation of the p38-MAPK and NF-κB signaling pathways: Implications for intervertebral disc degeneration. Osteoarthritis Cartilage. 2017;25:341–350. doi: 10.1016/j.joca.2016.10.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.