Abstract
Stress exposure during development can impact both the expression of individual traits and associations between traits, but whether stress results in stronger or weaker associations between traits is unclear. In this study, we examined within- and among-trait associations for morphological and physiological traits in zebra finches (Taeniopygia guttata) exposed to corticosterone (CORT) during the nestling and fledging stages as well as in control birds. Birds exposed to CORT exhibited stronger within-trait correlations over time and stronger associations among traits. We found preliminary evidence that birds that died before the median age of death had stronger within- and among-trait correlations independent of treatment, and among CORT-treated birds, smaller birds were more likely to survive beyond the median age than larger birds. These findings suggest that stress hormone exposure in early life can result in reduced developmental flexibility, with potential fitness ramifications, and that these costs may be greater for larger offspring. Furthermore, our results provide experimental evidence for pleiotropic effects of hormones during development through altered patterns of phenotypic correlation.
Keywords: corticosterone, development, mortality, morphometrics, phenotypic programming, zebra finch
Graphical Abstract:

Introduction
Stress during the early developmental period can have a disproportionately large impact on an organism’s phenotype (Matthews 2002; Monaghan 2008; Spencer 2017). These early life challenges can have transient or permanent impacts on phenotypic expression depending on the type, duration, and magnitude of the stressors, the developmental stage at which the stress occurs (e.g., Matthews 2002; Monaghan and Haussmann 2015), and the traits examined. There is a large body of work documenting short- and long-term effects of early life stress on trait expression across taxa and across trait types (e.g., behavior [Nowicki et al. 2002; Pechtel and Pizzagalli 2011; Merrill et al. 2016, 2017], morphology [Denver et al. 1998; Schmidt et al. 2014], and physiology [Merrill and Grindstaff 2015; Monaghan and Haussmann 2015]), indicating that most organisms exhibit some degree of phenotypic plasticity in response to early life challenges.
Despite broad interest in developmental programming, most studies of early life stress measure the mean trait expression of only one or a few traits at a single time point. This includes work examining the impacts of early life conditions on trait canalization and developmental stability (e.g., Van Dongen 2006). Canalization results in decreased variance in trait expression across the population, whereas developmental stability limits variation of trait expression within individuals (Debat and David 2001; Lazic et al. 2016). Fluctuating asymmetry is one widely used measure of developmental stability (Klingenberg et al. 2002; Klingenberg 2015) and has been used as a tool to assess levels of developmental stress (Klingenberg 2003; Lazicetal. 2013). However, early life conditions can influence not only trait expression, including trait symmetry, but also the strength of associations within traits over time (e.g., repeatability and predictive developmental trajectories), as well as associations between traits (Careau et al. 2014a, 2014b). There are two primary areas in which ecologists have investigated the effects of early life stress on trait associations: work examining the emergence of trade-offs under resource-limiting or otherwise stressful conditions and work on behavioral syndromes. Studies of trade-offs between traits are generally conducted on traits that compete for resources and often report a pattern of increased strength of correlation (inverse correlations, in these cases) under stressful conditions (e.g., Blount et al. 2003; O’Hagan et al. 2015). For work on associations between behavior and physiology or on the repeatability of behavioral or physiological traits, the results have been mixed. Some studies report increased strength of correlations between traits in stressful environments (e.g., Killen et al. 2012; reviewed in Killen et al. 2013; Careau et al. 2014b), whereas others report weaker correlations between traits in stressful environments (e.g., reviewed in Killen et al. 2013; Careau et al. 2014a). These disparate patterns could be due to trait-specific differences in stress-induced variance or differences in the severity (Killen et al. 2013) or timing (sensu Lindström 1999) of the stressor.
Stressors occur in many forms (e.g., nutritional, thermal, social, immunological), but among vertebrates, they share a common regulatory effector: glucocorticoid (GC) hormones. Glucocorticoid levels such as corticosterone (the dominant GC in birds, reptiles, amphibians, and rodents) and cortisol (the dominant GC in nonrodent mammals and fish) are mediated via the hypothalamo-pituitary-adrenal/interrenal (HPA) axis, which releases GCs in response to perceived stressors. Increases in corticosterone or cortisol (both hereafter referred to as CORT) initiate a cascade of physiological, metabolic, and behavioral processes meant to help organisms cope with challenges (Wingfield et al. 1998; Wingfield and Sapolsky 2003). Sustained production of and exposure to CORT during adulthood, however, has deleterious effects on organisms, including loss of energy reserves, reduced immune defenses, and increased oxidative burden (Breuner et al. 2008; Bonier et al. 2009; Haussmann and Marchetto 2010; Crespi et al. 2013). These are generally ephemeral changes in somatic or physiological condition, and once the stressor is removed, organisms are typically able to return to the prior somatic and physiological state. Exposure to CORT or stress during development, conversely, can lead to permanent phenotypic programming (Lindström 1999; Monaghan 2008; Schmidt et al. 2014; Merrill et al. 2017). Whether these altered phenotypes are adaptive or maladaptive depends on the ecological context (sensu Gluckman et al. 2005; Monaghan 2008) and how stressed individuals perform relative to the normal, non-stressed phenotype. If organisms experience chronic stress during development, then adaptive developmental programming should allow individuals to modify life-history strategies to maximize lifetime reproductive success under poor environmental conditions (Gluckman et al. 2005; Monaghan 2008). It is also possible that the plastic responses to stress represent the “best of a bad job” (sensu Denver et al. 1998) and are adaptive in the sense that the organism would have otherwise died but they have reduced fitness compared to the unstressed phenotype, regardless of prevailing environmental conditions (Denver and Crespi 2006). Although many studies have demonstrated that developmental CORT exposure impacts trait expression, we have little understanding of the effects of early life CORT exposure on individual developmental trajectories for size-linked morphological traits or the effects of stress on the associations within and between physiological systems across development.
To broaden our understanding of the role that early life stress has on phenotypic programming and the capacity for stress to impact organism-wide developmental trajectories, we examined how chronic exposure to corticosterone during the nestling and fledging periods impacted the strength of associations within and among morphological and physiological traits in zebra finches (Taeniopygia guttata). We measured the expression of three morphological and four physiological traits during the nestling and fledging stages and at additional time points during the immature and adult periods (between days 60 and 108 posthatch; Zann 1996). In related work, we found short-term effects of developmental CORT exposure on CORT levels but no long-term impact on HPA-axis function or innate immune defenses or on acute-phase protein production or antibody production following subsequent immune challenge (Grindstaff and Merrill 2017). Here we examined the associations among these measures to determine whether early life stress impacted the strength of correlations across physiological traits. We also examined within-trait correlations over time for both morphological and physiological measures to determine whether early life stress impacted within-trait correlations. These tests differ from many measures of repeatability because traits were assessed across development and under differing contexts (e.g., prior to and following immune challenge). Assuch, these analyses provide an estimation of how strongly predictive the first measurement is of the subsequent measure. Finally, we recorded age at death to determine whether CORT treatment impacted survival and whether there were differences in trait associations for birds that died before the median age of death compared to those that survived beyond the median point.
We predicted that prolonged exposure to CORT during the nestling and fledging stages would result in weaker associations within and among morphological and physiological traits as a result of developmental dysregulation and the decoupling of physiological processes (sensu Killen et al. 2013; Careau et al. 2014a). We also predicted that CORT-treated birds would die at an earlier age (sensu Saino et al. 2005; Haussmann et al. 2012; Grace et al. 2017) and that birds that died earlier would have weaker associations within and among traits. To our knowledge, this is the first study to examine the effects of early life stress on associations within and among morphometric and physiological traits, as well as the link between trait association strength and longevity.
Material and Methods
Research Animals and Housing
For details on housing and physiological measures, see Grindstaff and Merrill (2017). In brief, parents of birds used for this study were housed in breeding pairs in wire cages. Off-spring were moved to larger, single-sex cages with 3–4 individuals on day 50 posthatch. Lighting was maintained on a 13.5L∶10.5D photoperiod, and temperatures were held constant. All birds had ad lib access to water and food. Research was approved by the Oklahoma State University Institutional Animal Care and Use Committee and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Research Council 2010).
Corticosterone Treatment
Young were assigned to either the CORT treatment (N = 74) or the control group (N = 77) during the nestling period. Within broods, we attempted to balance the number of young assigned to the CORT and control groups. From days 12–15 posthatch, birds in the CORT group received a twice-daily oral dose of 0.124 mg/mL of CORT in 25 μL of peanut oil. Between days 16 and 28 posthatch, the dosage increased to 0.163 mg/mL of CORT in 25 μL of peanut oil administered twice daily to account for increased mass (Spencer et al. 2009). Control birds were given an equal volume of peanut oil throughout the treatment period (Spencer et al. 2009).
Immune Challenges
Once birds reached 60 days posthatch, one-half of the birds in each of the two nestling treatment groups (CORT and control) were challenged with lipopolysaccharide (LPS; N = 61) and the other birds received a control injection (N = 57). Again, we attempted to balance treatment groups within families. LPS-challenged birds received a subcutaneous abdominal injection of 1.0 mg LPS/kg body weight (Sigma-Aldrich L7261, St. Louis) in 50 μL of sterilized phosphate-buffered saline (PBS; Sigma-Aldrich P5368, St. Louis), and control birds were injected with 50 μL of PBS. The injections (LPS and control) were repeated on day 100 posthatch.
Morphometric Assessment
On days 0 and 5 posthatch, birds were weighed using a digital scale (0.01 g). Beginning on day 10 posthatch, birds were weighed, tarsus length was measured using digital calipers (0.01 mm), and wing length was measured using a wing rule (0.5 mm). Birds were measured again on days 17, 28, and 60.
Blood Sample Collection for Measurement of CORT Levels
To confirm that CORT treatment resulted in elevated circulating CORT levels, we collected blood samples (∼50 μL from the brachial vein) from all birds on day 14 posthatch, 10 min after dosing the bird with either CORT or peanut oil, as appropriate (Khan and Robert 2013). As previously reported (Grindstaff and Merrill 2017), we did find an increase in circulating CORT concentrations in experimental nestlings compared to controls (CORT-treated birds: 58.3±2.6 ng/mL; control birds: 14.7±2.5 ng/mL). The magnitude of the difference between the CORT levels measured in control and CORT-treated birds closely reflects the magnitude of increase in CORT levels in response to a 30-min restraint stress in our population (L. Merrill and J. L. Grindstaff, unpublished data), and we refer to this sample as day 14 acute CORT. We also collected 2 blood samples (50 μL each) from each bird on day 60 posthatch; the first (baseline) was collected immediately prior to the primary injection of LPS or PBS, and the second was collected 1 h after injection (postinjection).Blood was centrifuged for 7 min at 1,845×g, and plasma was separated from the red blood cells and stored at −80°C until analysis. For all assays, samples were evenly balanced by treatments and sex across plates.
Quantification of CORT Levels
CORT concentrations were quantified using an enzyme immunoassay kit from Enzo Life Sciences (ADI-901–097, Plymouth Meeting, MA) that had been previously validated for use with zebra finches (Wada et al. 2007; Merrill and Grindstaff 2015). Samples were diluted 1∶40 in 1% steroid displacement reagent and assay buffer. Plates were read using a Biotek ELx808 microplate reader at 405 nm. The average intra- and interassay coefficients of variation were 6.9% and 22.4%, respectively.
Antibody Response to LPS Injection
On days 68 and 108 posthatch, blood samples (∼50 mL) were collected to quantify anti-LPS antibody levels in both birds injected with LPS and control birds. Antibody titers were quantified using enzyme-linked immunosorbent assays following previously described methods (Grindstaff et al. 2005; Grindstaff 2008; Merrill and Grindstaff 2014). The average intra- and interplate coefficients of variation were 7.68% and 13.64%, respectively.
Haptoglobin
Blood samples were collected from all birds on day 100 posthatch, immediately prior to injection with either PBS or LPS, and 12 h postinjection, to quantify production of haptoglobin in response to the injection (Hegemann et al. 2013). Haptoglobin concentrations were quantified using a commercial kit (Tri-Delta Diagnostics, TP-801) following the kit instructions. The average intra- and interplate coefficients of variation were 7.08% and 6.32%, respectively.
Bacterial Killing Ability
We quantified the birds’ plasma bacterial killing ability (BKA) from samples collected on day 36 posthatch following methods in Matson et al. (2006), Millet et al. (2007), and Morrison et al. (2009). In brief, 5 μL of plasma were added to a combination of CO2-independent media (Gibco, Invitrogen) + 4 mM L-glutamine (90 μL) and the E. coli (ATCC 8739) bacterial broth (10 μL), incubated at 40°C for 20 min, and then pipetted onto agar plates in 50 μL aliquots in duplicate. The solution was evenly distributed on the plates and then incubated overnight at 37°C. The following day, the bacterial colonies were counted and killing ability was calculated by subtracting the mean colony numbers for a bird’s two plates from the control mean and then dividing that by the control mean. Intra-assay coefficient of variation mean was 10% for day 36 BKA.
Statistical Analyses
To assess the impact of early life CORT treatment on mean measures of morphological traits (mass, tarsus, and wing length), we used general linear mixed models for these traits on days 17, 28, and 60 posthatch. We also ran models for day 10 posthatch to ensure that there were no differences in the treatment groups prior to CORT manipulation. To examine whether CORT treatment impacted longevity, we ran a survival analysis (SAS 9.4, Proc PHREG) in which we used age at death in days as the dependent variable and birds that were still alive as of this writing were censored. For all analyses, we included CORT treatment (CORT, control) as a fixed effect; maternal identity as a random effect; and hatch order, hatch date, sex, and clutch number (i.e., which clutch in the female’s life a given nestling originated from) as covariates in initial models for each dependent variable. Covariates that were significant (P < .05) were retained in the final model. To determine whether any treatment-related differences in morphometric traits were due to the selective loss of smaller or larger chicks from the treatment groups, we ran general linear models for mass, tarsus length, and wing length at day 10 as described above, but with death prior to or after the median date, CORT treatment, and the interaction of the two as fixed effects. We used median age rather than mean because 31 birds were still alive at the time of writing. In all models, we used maximum likelihood, and the denominator degrees of freedom were approximated using the containment method (Littell et al. 2006); for the generalized linear mixed models, we used the logit link function.
For all analyses in which we examined patterns of co-variation, we used only measures for which we had at least 15 individuals in each CORT treatment so as not to draw inferences from small sample sizes. To assess within-trait associations, we examined the three morphological traits (mass, tarsus length, and wing length) at days 10 (2 days prior to the start of CORT treatment) and 60 posthatch (end of the measurement period) and four physiological traits at different time points (see table 1 for details). The day 14 CORT sample represented a temporal intermediate between baseline and stress-induced CORT, but rather than including associations between day 14 CORT and both day 60 CORT baseline and postinjection (which exhibited nearly identical pairwise-association absolute differences between CORT and control birds), we selected day 60 CORT baseline. For among-trait associations, we examined the three morphological traits on day 60 posthatch and nine physiological traits (table 2). We used morphological measurements from day 60 because that was the final day we recorded all morphological traits, and at that point, birds should be at, or close to, their final adult size. These measurements thus reflect permanent programming effects of CORT treatment. We selected physiological trait groupings first based on the closest temporal associations that met the minimum sample size standard. Second, when the measurement of one trait was approximately equidistant to another trait measured at two time points (e.g., day 36 BKA and day 14 acute and day 60 baseline CORT), we included both associations. Third, we limited the pairings to those that made the most biological sense to include. For instance, we examined day 36 BKA and day 100 baseline haptoglobin but did not include day 36 BKA and 12 h postinjection haptoglobin because that haptoglobin measure is a relatively transient-induced response, whereas the other two are baseline levels and should reflect the same physiological state. We examined both day 68 and day 108 antibody concentrations because day 68 should reflect a primary antibody response, and the concentrations on day 108 should reflect a secondary response. Finally, we included correlations between both haptoglobin measures and both antibody measures because the induced haptoglobin levels are in response to LPS challenge and therefore are likely to be linked to the anti-LPS antibody responses. Associations were selected based on these criteria prior to analyses, and all data are deposited in the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.vd158h6 (Merrill and Grindstaff 2018).
Table 1:
Within-trait correlations by treatment
| Parameters |
|||||
|---|---|---|---|---|---|
| First time point | Second time point | Treatment | N | r | P |
| D10 mass | D60 mass | Control | 60 | .35 | .007 |
| CORT | 57 | .58 | <.001 | ||
| D10 tarsus | D60 tarsus | Control | 60 | .31 | .016 |
| CORT | 57 | .46 | <.001 | ||
| D10 wing | D60 wing | Control | 60 | .15 | .249 |
| CORT | 57 | .22 | .108 | ||
| D14 acute CORT | D60 baseline CORT | Control | 50 | .32 | .028 |
| CORT | 49 | .41 | .005 | ||
| D60 baseline CORT | D60 stress CORT | Control | 50 | .17 | .246 |
| CORT | 49 | .20 | .177 | ||
| D68 anti-LPS Ab | D108 anti-LPS Ab | Control | 23 | .32 | .122 |
| CORT | 20 | .62 | .004 | ||
| D100 baseline Hp | D100 12-h Hp | Control | 41 | .37 | .022 |
| CORT | 42 | .55 | <.001 | ||
Note: Within-trait correlation coefficients for morphometric and physiological traits in zebra finches (Taeniopygia guttata) exposed to corticosterone (CORT) or control birds. D = day posthatch on which the measure was taken; anti-LPS Ab = concentration of antilipopolysaccharide antibodies; Hp = haptoglobin. Day 60 stress CORT is the sample collected 1 h postinjection. Boldface indicates the treatment group with stronger correlations.
Table 2:
Among-trait correlations by treatment
| Parameters |
|||||
|---|---|---|---|---|---|
| Variable 1 | Variable 2 | Treatment | N | r | P |
| D60 mass | D60 tarsus | Control | 60 | .52 | <.001 |
| CORT | 57 | .63 | <.001 | ||
| D60 tarsus | D60 wing | Control | 60 | .43 | .001 |
| CORT | 57 | .35 | .007 | ||
| D60 mass | D60 wing | Control | 60 | .39 | .002 |
| CORT | 57 | .47 | <.001 | ||
| D14 acute CORT | D36 BKA | Control | 54 | −.16 | .260 |
| CORT | 51 | −.32 | .023 | ||
| D36 BKA | D60 baseline CORT | Control | 50 | .00 | .984 |
| CORT | 49 | −.32 | .025 | ||
| D36 BKA | Hp baseline | Control | 42 | −.17 | .290 |
| CORT | 43 | −.26 | .093 | ||
| D36 BKA | D68 LPS Ab | Control | 31 | .18 | .340 |
| CORT | 26 | .33 | .098 | ||
| D36 BKA | D108 LPS Ab | Control | 23 | .21 | .343 |
| CORT | 20 | .30 | .192 | ||
| D60 baseline CORT | Hp baseline | Control | 34 | −.09 | .632 |
| CORT | 38 | −.04 | .801 | ||
| D60 baseline CORT | D68 LPS Ab | Control | 25 | .10 | .623 |
| CORT | 22 | .12 | .590 | ||
| D60 baseline CORT | D108 LPS Ab | Control | 20 | −.29 | .209 |
| CORT | 17 | .13 | .623 | ||
| Hp baseline | D68 LPS Ab | Control | 29 | −.03 | .867 |
| CORT | 25 | −.44 | .028 | ||
| Hp baseline | D108 LPS Ab | Control | 22 | .20 | .381 |
| CORT | 19 | −.52 | .022 | ||
| Hp 12-h | D68 LPS Ab | Control | 28 | .03 | .879 |
| CORT | 25 | −.58 | .002 | ||
| Hp 12-h | D108 LPS Ab | Control | 22 | .02 | .944 |
| CORT | 19 | −.62 | .005 | ||
Note: Among-trait correlation coefficients in zebra finches (Taeniopygia guttata) exposed to corticosterone (CORT) or control birds. BKA = bacterial killing ability. See table 1 for description of other parameters. Boldface indicates the treatment group with stronger correlations.
We ran pairwise correlations on z-transformed values for each within- and among-trait comparison and used the absolute value of the resulting correlation coefficient to assess the strength of trait associations. To ensure that LPS exposure was not consistently impacting our assessment of the effects of CORT treatment, we ran correlations for traits that were assessed post LPS-challenge (day 60 CORT postinjection, 12 h haptoglobin, and days 68 and 108 anti-LPS anti-bodies) broken down by CORT (yes/no) and LPS (yes/no), and examined the resulting r values (see appendix, available online). To determine whether CORT treatment impacted within- or among-trait covariance, we ran paired two-tailed t-tests of the absolute r values in which the association (e.g., day 10 wing length×day 10 mass) was the nominal variable, and the r value for the CORT-treated birds was matched with the r value for the control birds. We ran similar analyses for birds that died before the median age at death and those that survived to that age or longer.
We confirmed normality of residuals and homogeneity of variance, and all analyses were run in SAS 9.4 and JMP 10.0 (SAS Institute, Cary, NC). Antibody titer and haptoglobin data were log +1 transformed to achieve normality prior to analysis. Two outliers due to methodological error were removed from analyses, although inclusion of these values did not qualitatively or quantitatively impact results. Variation in sample sizes for each analysis (see tables 1, 2, and 4) reflects changes in the number of individuals due to mortality over time, plasma limitations, and removal of the two outliers.
Table 4:
Within- and among-trait correlations by longevity
| Parameters |
|||||
|---|---|---|---|---|---|
| Variable 1 | Variable 2 | Median death | N | r | P |
| D0 mass | D5 mass | After | 78 | .39 | <.001 |
| Before | 70 | .65 | <.001 | ||
| D0 mass | D10 mass | After | 78 | .29 | <.001 |
| Before | 71 | .46 | <.001 | ||
| D5 mass | D10 mass | After | 78 | .72 | <.001 |
| Before | 69 | .77 | <.001 | ||
| D10 mass | D10 tarsus | After | 78 | .83 | <.001 |
| Before | 71 | .83 | <.001 | ||
| D10 tarsus | D10 wing | After | 78 | .86 | <.001 |
| Before | 71 | .93 | <.001 | ||
| D10 mass | D10 wing | After | 78 | .76 | <.001 |
| Before | 71 | .78 | <.001 | ||
Note: Within- and among-trait correlation coefficients in zebra finches (Taeniopygia guttata) that died prior to the median age at death (before) and those that survived to that age or beyond (after). See table 1 for a description of parameters. Boldface indicates the group that died before or after the median in which within- and among-trait correlations were stronger.
Results
Effects of CORT Treatment on Mean Expression of Morphological Traits and Survival
There were no differences in morphological measures between treatment groups prior to CORT treatment as assessed from day 10 measurements (P > 75). On day 17 posthatch, CORT-treated birds weighed significantly less than control birds but did not differ in tarsus or wing length (table 3). On the final day of CORT dosing (day 28 posthatch), CORT-treated birds weighed significantly less and had marginally shorter wings than control birds, but tarsus length did not differ (table 3). We also found a sex difference in day 28 wing length, in which females had shorter wings than males, although this did not persist beyond day 28 (table 3). On day 60 posthatch, CORT-treated birds had significantly shorter wings, but mass and tarsus length did not differ (table 3). We found no effect of CORT treatment on longevity (χ2 = 0.79, P = .37), but there was a sex difference in which females died earlier than males (χ2 = 13.61, P < .001; female age at death = 1,005.5 days; male age at death = 1,363.7 days). We also found significant interaction effects between CORT treatment and age at death category for day 10 morphometric traits (mass: F1, 100 = 5.39, P = .02; tarsus length: F1, 100 = 4.79, P = .03; wing length: F1, 199 = 5.54, P = .02), in which control birds that died before the median age of death were similar in size at day 10 compared to those that survived to at least the median age (mass: F1, 42 = 1.64, P = .21; tarsus length: F1, 42 = .92, P = .34; wing length: F1, 40 =0.04, P = .85), whereas CORT-treated birds that died before the median age of death were larger at day 10 than those that survived to at least the median age (mass: F1, 39 = 3.24, P = .08; tarsus length: F1, 39 = 4.87, P = .03; wing length: F1, 38 = 4.63, P = .04).
Table 3:
Effects of CORT on size-related parameters
| Mass |
Tarsus |
Wing |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day | Parameter | Est. | SE | P | Est. | SE | P | Est. | SE | P |
| 17 | CORT trt | .32 | .14 | .02 | .05 | .08 | .5 | .42 | .49 | .39 |
| 28 | CORT trt | .59 | .15 | <.001 | .09 | .07 | .24 | .41 | .21 | .06 |
| Sex | … | … | … | … | … | … | .52 | .23 | .03 | |
| 60 | CORT trt | .21 | .19 | .27 | .06 | .08 | .48 | .72 | .28 | .01 |
Note: Model outputs from general linear mixed models examining the effect of corticosterone treatment (CORT trt) on morphometric traits in zebra finches (Taeniopygia guttata). Birds were exposed to CORT between days 12 and 28, and mass, tarsus length, and wing length were measured on days 17, 28, and 60. Boldface indicates values that were significant in the final models.
Within- and Among-Trait Correlations
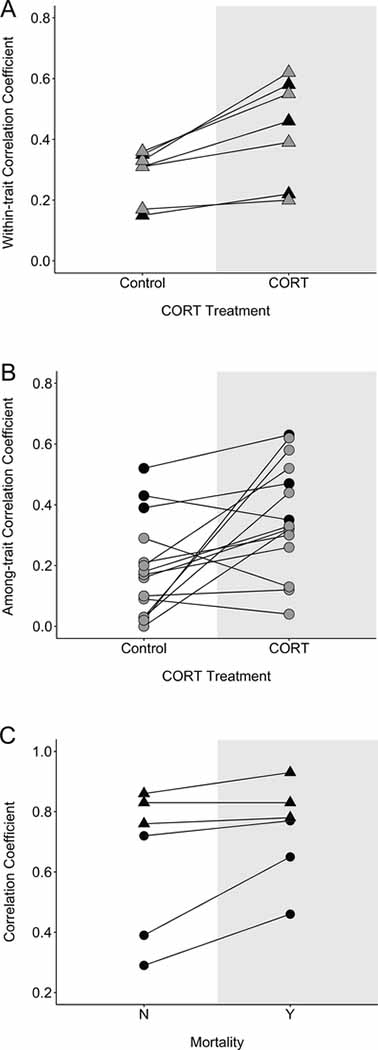
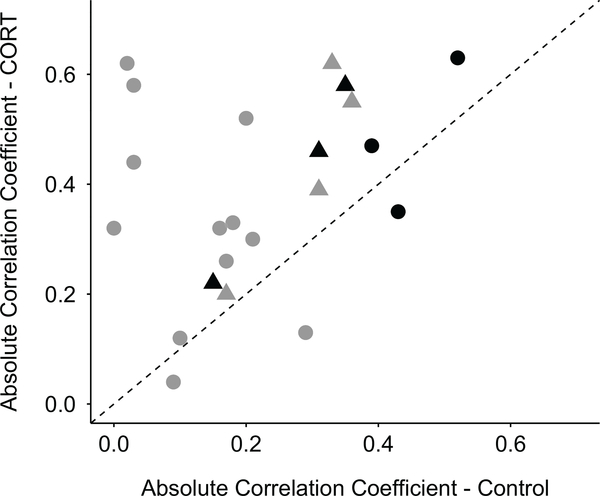
CORT treatment resulted in consistently and significantly stronger within-trait associations for all traits assessed (table 1; figs. 1, 2, A1; figs. A1–A5 are available online; paired t-test: t ratio = 4.17, df = 6, P = .006). Among-trait correlations were consistently stronger as well for CORT-treated birds (table 2; figs. 1, 2, A2; paired t-test: t ratio = 3.02, df = 14, P = .009). Among-trait correlations in morphological traits were stronger for CORT-treated birds for two of the three associations (table 2; figs. 1, 2, A2). Correlations between physiological traits averaged higher for CORT-treated birds than for controls (table 2; fig. 1), and all associations except two (day 60 baseline CORT and both day 100 baseline haptoglobin and day 108 anti-LPS antibody) were stronger for CORT-treated birds (table 2; figs. 2, A2).
Figure 1:
Mean trait associations by corticosterone (CORT) treatment (A, B) and mortality (C). Triangles and circles represent the mean absolute value of the z-transformed correlation coefficient for a given trait, and the line connects the same trait for CORT and control birds (A, B) and for birds that died prior to the median age of survival (Y) versus those that survived to at least the median age of death (N; C). Black triangles are within morphological trait associations (A, C), gray triangles are within physiological trait associations (A), black circles are among morphological trait associations (B, C), and gray circles are among physiological trait associations (B).
Figure 2:
Absolute z-transformed correlation coefficient values for corresponding trait associations. Triangles are within-trait associations, circles are among-trait associations, black shapes are morphological traits, and gray shapes are physiological traits. The line represents a 1∶1 relationship, and values above the line reflect associations that were stronger for birds treated with corticosterone (CORT) during development.
Approximately 25% of the nestlings died over the course of the 108-day sampling period, and while this was roughly split between treatment groups (∼20% for CORT-treated birds, ∼26% for control birds), it limited our ability to examine associations within and among traits for those that died prior to the median age of death compared to those that survived at least that long. Due to the sample size disparity, and because we were also interested in assessing traits prior to the onset of CORT treatment to determine whether associations within and among traits could expose underlying differences in critical processes linked to survival, we examined withintrait associations between mass on days 0, 5, and 10 posthatch and among-trait correlations for mass, tarsus length, and wing length on day 10 posthatch, on the basis of whether the bird survived to the median age. Birds that died before the median age of death had stronger within-trait correlations for all three associations, but the strength of the correlations was not significantly different (paired t-test: t ratio = 1.44, df = 2, P = .29). Among-trait correlations were also stronger in birds that died earlier for two of the three associations, although again this difference was not significant (paired t-test: t ratio = 2.63, df = 2, P = .12; table 4; figs. 1, 2, A3). We ran a z-test comparing the number of within- and among-trait associations that were stronger for birds that died before the median age at death (six of six associations), and it was significantly different from random (P < .001).
We found no evidence for a consistent effect of LPS challenge, or an interaction effect between CORT and LPS on associations among or within traits (figs. A4, A5).
Discussion
Early life stress has been shown to impact mean trait expression across a wide range of taxa and trait types (Lindström 1999; Monaghan 2008; Schmidt et al. 2014; Merrill et al. 2017). The influence of developmental stress on within- and among-trait associations, however, has received less attention. Here we demonstrated that prolonged exposure to corticosterone during the nestling and fledging stages in zebra finches negatively impacted mean morphological trait expression to differing degrees during and after CORT exposure but also that CORT treatment significantly impacted the strength of both within- and among-trait associations for a suite of morphological and physiological parameters. Moreover, we found an indication that, independent of treatment, birds that died prior to the median age of death were more likely to have stronger within- and among-trait correlations prior to the onset of the experimental treatment than birds that survived to the median age or longer. Together, these findings provide compelling evidence that early life stress impacts more than the expression of specific traits; it appears to alter an individual’s phenotypic flexibility.
CORT exposure during the nestling and fledging stages resulted in birds that weighed less on days 17 and 28 posthatch and had shorter wings on day 60 posthatch compared to control birds. These findings are similar to those of prior studies documenting short- and long-term negative effects of CORT exposure on growth (Spencer and Verhulst 2007; Mueller et al. 2009; Crino et al. 2014), but interestingly, we found no effect on tarsus length. The lack of tarsus length difference between treatments suggests that CORT exposure did not negatively impact skeletal development in these birds (Senar and Pascual 1997). The differences in mass and wing length indicate that prolonged CORT exposure resulted in a temporary reduction in body mass (perhaps due to increased energy expenditures associated with elevated CORT; Lin et al. 2006; Schmidt et al. 2012) and a more protracted or permanent reduction in wing development. Much of the measured wing length is feather, and there is evidence that elevated CORT inhibits feather growth (Romero et al. 2005). Among CORT-treated birds, larger individuals at day 10 were more likely to die before the median age of death. This elevated mortality for large CORT-treated birds could be a product of CORT inducing higher metabolic rates (Lin et al. 2006; Schmidt et al. 2012) and thus increased energy requirements that larger birds were unable to maintain. Regardless of the cause, size-linked mortality is unlikely to be responsible for the differences in morphological traits between CORT and control birds because only one CORT-treated bird had died by day 17, two by day 28, and 11 by day 60. CORT treatment did not impact survival, but we did find a significant difference between the sexes, in which females died at a younger age than males. Female-biased mortality has been widely reported in zebra finches (e.g., Martins 2004 and references therein), but sex did not appear to impact the other analyses.
In addition to variable differences in mass and wing length related to CORT treatment, we found consistent differences in within-trait associations for both morphological and physiological traits, in which CORT-treated birds exhibited stronger correlations. For the morphological traits, this consisted of associations between traits on days 10 and 60 posthatch. Day 10 was 2 days prior to experimental manipulation, and the tighter associations for CORT-treated birds indicate that exposure to CORT resulted in a more rigid developmental trajectory in which size at day 10 more strongly predicted size at day 60 than it did for control birds. The same pattern for physiological traits suggests that CORT exposure during development resulted in a more limited range of trait expression across systems.
We also found that among-trait correlations were significantly stronger for CORT-treated birds than controls. This pattern was especially strong for associations among traits that were weakly or negatively correlated in control birds but in which CORT-treated birds exhibited strongly inverse associations (table 2). This was most pronounced in associations between haptoglobin and anti-LPS antibodies (Ab), suggesting that early life exposure to CORT resulted in a trade-off between the two immunological proteins. Haptoglobin is an acute-phase protein that typically increases in circulation within a few hours of immune challenge and, by binding free heme, helps to protect the organism from oxidative damage and prevents invading bacteria from being able to use the iron in heme (Exton 1997). Anti-LPS Ab production typically takes a few days to peak, but both haptoglobin and antibody production are dependent on protein and, in particular, the amino acid lysine (Iseri and Klasing 2014). The inverse relationship in CORT-treated birds may reflect protein or lysine limitation driving patterns of investment in one mode of defense or the other. Conversely, it may be that this trade-off is not directly due to nutritional limitations but reflects a developmental shift in immunological investment. It is not uncommon for organisms to trade off between different arms of the immune system (e.g., Sheldon and Verhulst 1996; Lochmiller and Deerenberg 2000; Lee 2006), and our data may reflect differential investment in aspects of immunity driven by early life challenge. We also examined whether LPS challenge influenced within- and among-trait associations but found no consistent effects (figs. A4, A5), indicating that early life CORT treatment appears to be more important in driving these associations than LPS exposure later in life.
Glucocorticoids have been widely recognized as important mediators of behavioral and physiological processes (Wing-field et al. 1998; Sapolsky et al. 2000; French et al. 2007; Crespi et al. 2013), but this study represents the first demonstration of alterations in relationships within- and among-traits as a result of early life exposure to CORT. CORT receptors are found in most tissue and cell types (Ballard et al. 1974; Lattin et al. 2012), meaning that variation in CORT levels can result in pleiotropic effects. How, and to what extent, exposure to elevated levels of CORT during development acts to influence the permanent expression of specific traits (e.g., production of haptoglobin)—or whether effects are broader but harder to detect (e.g., across all immunological tissues)—remains unclear. The window during which an organism is most susceptible to the programming effects of stress likely varies by species and potentially even within species. Thus the timing of the stressor likely is important in determining the magnitude of the effects (sensu Lindström 1999). Killen et al. (2013) also suggested that the magnitude of the stressor determines the impact on trait associations and that mild-to-moderate stressors result in stronger correlations, whereas severe stressors mask or attenuate relationships between physiological and behavioral traits. We predicted that CORT treatment would mimic exposure to a severe stressor and, consequently, that we would find weaker associations within and among morphological and physiological traits, but it is possible that our protocol instead mimicked exposure to mild or moderate stressors. The regulatory mechanisms responsible for impacting trait covariance are unknown at this stage, but future work examining the effects of stressors of different severity on mean trait expression, trait covariance, and patterns of gene expression for those traits could help uncover some of the mechanisms.
Our results provide experimental evidence that the hormonal environment early in life can have pleiotropic effects on the phenotype. Although the potential for hormones to exert pleiotropic effects is widely recognized (e.g., Ketterson and Nolan 1999; Williams 2012), very few empirical studies have provided quantitative evidence of this process. However, Cox et al. (2015, 2016) also compared phenotypic correlations between treatment and control groups similar to what we have done here and found that associations among sexually dimorphic and testosterone-mediated traits were stronger in female brown anole lizards (Anolis sagrei) when individuals with experimentally elevated testosterone levels were included in the analyses than when only control females with low testosterone levels were examined. In addition to comparing pairwise associations, the phenotypic covariance matrices (P) can be compared between control and hormone-exposed individuals (with sufficiently large sample sizes for all traits) to statistically evaluate the potential for hormones to alter multivariate selection gradients and correlational selection. Any changes to phenotypic covariances resulting from early life stress, however, should not be heritable (unless variation in the reaction norms has a heritable component), thus the evolutionary response to selection should not be altered (McGlothlin and Ketterson 2008). This complex dynamic warrants further investigation, and we encourage other researchers to consider utilizing quantitative genetic analyses to more explicitly assess the potential for hormone exposure to impact multivariate selection (McGlothlin and Ketterson 2008; Cox et al. 2016, 2017).
It has been proposed that stress induces adaptive plasticity to help organisms cope with challenging conditions (Denver et al. 1998; Denver and Crespi 2006; Monaghan 2008). Plasticity can occur in different forms: it can be expressed on a trait-by-trait basis, in which early life experience impacts the mean expression of specific traits; it can occur at the whole-organism level, in which the expression of all traits are impacted; and plasticity can influence trait covariance. Our work has shown that early life exposure to CORT exerts generally weak and variable effects on long-term morphological and physiological trait expression (this study; Grindstaff and Merrill 2017) but consistent effects on associations within and among traits. This is striking given that we examined associations at the within- and among-trait levels for both morphological and physiological traits. This pattern of stronger correlations for CORT-treated birds indicates that the plastic response to stress exposure (altered correlation strength) results in reduced flexibility. If flexibility, defined in this case as trait covariance, is constrained by early life stress, then this alteration can have profound impacts on the organism and could potentially limit the capacity of an organism to respond to changing environmental conditions. Indeed, we found preliminary evidence that the strength of trait covariance is linked to rates of mortality. There was no difference in mortality between CORT-treated and control birds, but when we examined correlations within and among traits, we found that birds that died before the median age at death had stronger correlations than birds that survived to at least the median age at death. Trait covariance may predict mortality as a result of reduced flexibility, constraining developmental trajectories to such an extent that birds unable to maintain the constrained trajectory die earlier. Our data showing that birds that were larger at day 10 were more likely to die earlier within the CORT treatment but not among control birds provides some tentative evidence in support of this idea. These questions require further investigation, but we believe that they provide a strong impetus for examining within and among-trait correlations in the context of early life stress.
Supplementary Material
Acknowledgments
We would like to thank Kent Andersson, Sara Dawson, Katerina Faust, Lisa Hughes, Madeleine Naylor, Sarah Oppenborn, Alecia Rains, and Matthew Waselik for their help with data collection and aviary maintenance. Thanks also to Tara Stewart Merrill for feedback on the manuscript and figure design assistance and to Joel McGlothlin and Daniel Bolnick for helpful input on the analyses and interpretation of the results. Funding for this research was provided by the National Institutes of Health (grant 1R15HD066378-01). The authors declare no conflict of interests.
Literature Cited
- Ballard P, Baxter J, Higgins S, Rousseau G, and Tomkins G 1974. General presence of glucocorticoid receptors in mammalian tissues. Endocrinology 94:998–1002. [DOI] [PubMed] [Google Scholar]
- Blount JD, Metcalfe NB, Birkhead TR, and Surai PF 2003. Carotenoid modulation of immune function and sexual attractiveness in zebra finches. Science 300:125–127. [DOI] [PubMed] [Google Scholar]
- Bonier F, Moore IT, Martin PR, and Robertson RJ 2009. The relationship between fitness and baseline glucocorticoids in a passerine bird. General and Comparative Endocrinology 163:208–213. [DOI] [PubMed] [Google Scholar]
- Breuner CW, Patterson SH, and Hahn TP 2008. In search of relationships between the acute adrenocortical response and fitness. General and Comparative Endocrinology 157:288–295. [DOI] [PubMed] [Google Scholar]
- Careau V, Buttemer WA, and Buchanan KL 2014a. Developmental stress can uncouple relationships between physiology and behaviour. Biology Letters 10:20140834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careau V 2014b. Early-developmental stress, repeatability, and canalization in a suite of physiological and behavioral traits in female zebra finches. Integrative and Comparative Biology 54:539–554. [DOI] [PubMed] [Google Scholar]
- Cox CL, Hanninen AF, Reedy AM, and Cox RM 2015. Female anoles retain responsiveness to testosterone despite the evolution of androgen-mediated sexual dimorphism. Functional Ecology 29:758–767. [Google Scholar]
- Cox RM, Costello RA, Camber BE, and McGlothlin JW 2017. Multivariate genetic architecture of the Anolis dewlap reveals both shared and sex-specific features of a sexually dimorphic ornament. Journal of Evolutionary Biology 30:1262–1275. [DOI] [PubMed] [Google Scholar]
- Cox RM, McGlothlin JW, and Bonier F 2016. Hormones as mediators of phenotypic and genetic integration: an evolutionary genetics approach. Integrative and Comparative Biology 56:126–137. [DOI] [PubMed] [Google Scholar]
- Crespi EJ, Williams TD, Jessop TS, and Delehanty B 2013. Life history and the ecology of stress: how do glucocorticoid hormones influence life-history variation in animals? Functional Ecology 27:93–106. [Google Scholar]
- Crino OL, Prather CT, Driscoll SC, Good JM, and Breuner CW 2014. Developmental stress increases reproductive success in male zebra finches. Proceedings of the Royal Society B 281: 20141266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debat V, and David P 2001. Mapping phenotypes: canalization, plasticity and developmental stability. Trends in Ecology and Evolution 16:555–561. [Google Scholar]
- Denver RJ, and Crespi EJ 2006. Stress hormones and human developmental plasticity: lessons from tadpoles. Neoreviews 7:e183–e188. [Google Scholar]
- Denver RJ, Mirhadi N, and Phillips M 1998. Adaptive plasticity in amphibian metamorphosis: response of Scaphiopus hammondii tad-poles to habitat desiccation. Ecology 79:1859–1872. [Google Scholar]
- Exton MS 1997. Infection-induced anorexia: active host defence strategy. Appetite 29:369–383. [DOI] [PubMed] [Google Scholar]
- French SS, McLemore R, Vernon B, Johnston GIH, and Moore MC 2007. Corticosterone modulation of reproductive and immune systems trade-offs in female tree lizards: long-term corticosterone manipulations via injectable gelling material. Journal of Experimental Biology 210:2859–2865. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, and Spencer HG 2005. Predictive adaptive responses and human evolution. Trends in Ecology and Evolution 20:527–533. [DOI] [PubMed] [Google Scholar]
- Grace JK, Froud L, Meillere A, and Angelier F 2017. House sparrows mitigate growth effects of post-natal glucocorticoid exposure at the expense of longevity. General and Comparative Endocrinology 253:1–12. [DOI] [PubMed] [Google Scholar]
- Grindstaff JL 2008. Maternal antibodies reduce costs of an immune response during development. Journal of Experimental Biology 211: 654–660. [DOI] [PubMed] [Google Scholar]
- Grindstaff JL, Demas GE, and Ketterson ED 2005. Diet quality affects egg size and number but does not reduce maternal antibody transmission in Japanese quail Coturnix japonica. Journal of Animal Ecology 74:1051–1058. [Google Scholar]
- Grindstaff JL, and Merrill L 2017. Developmental corticosterone treatment does not program immune responses in zebra finches (Taeniopygia guttata). Journal of Experimental Zoology A 327:262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussmann MF, Longenecker AS, Marchetto NM, Juliano SA, and Bowden RM 2012. Embryonic exposure to corticoster one modifies the juvenile stress response, oxidative stress and telomere length. Proceedings of the Royal Society B 279:1447–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussmann MF, and Marchetto NM 2010. Telomeres: linking stress and survival, ecology and evolution. Current Zoology 56:714–727. [Google Scholar]
- Hegemann A, Matson KD, Versteegh MA, Villegas A, and Tieleman BI 2013. Immune response to an endotoxin challenge involves multiple immune parameters and is consistent among the annual-cycle stages of a free-living temperate zone bird. Journal of Experimental Biology 216:2573–2580. [DOI] [PubMed] [Google Scholar]
- Iseri VJ, and Klasing KC 2014. Changes in the amount of lysine in protective proteins and immune cells after a systemic response to dead Escherichia coli: implications for the nutritional costs of immunity. Integrative and Comparative Biology 54:922–930. [DOI] [PubMed] [Google Scholar]
- Ketterson ED, and Nolan V Jr. 1999. Adaptation, exaptation, and constraint: a hormonal perspective. American Naturalist 154(suppl.): S4–S25. [DOI] [PubMed] [Google Scholar]
- Khan N, and Robert K 2013. Does sex matter? differential responses to corticosterone administration in the zebra finch. Zoology 116:293–299. [DOI] [PubMed] [Google Scholar]
- Killen SS, Marras S, Metcalfe NB, McKenzie DJ, and Domenici P 2013. Environmental stressors alter relationships between physiology and behaviour. Trends in Ecology and Evolution 28:651–658. [DOI] [PubMed] [Google Scholar]
- Killen SS, Marras S, Ryan MR, Domenici P, and McKenzie DJ 2012. A relationship between metabolic rate and risk-taking behaviour is revealed during hypoxia in juvenile European sea bass. Functional Ecology 26:134–143. [Google Scholar]
- Klingenberg CP 2003. Fluctuating asymmetry and animal welfare: how far are we? and how far should we go? Veterinary Journal 166: 5–6. [DOI] [PubMed] [Google Scholar]
- Klingenberg CP 2015. Analyzing fluctuating asymmetry with geometric morphometrics: concepts, methods, and applications. Symmetry 7:843–934. [Google Scholar]
- Klingenberg CP, Barluenga M, and Meyer A 2002. Shape analysis of symmetric structures: quantifying variation among individuals and asymmetry. Evolution 56:1909–1920. [DOI] [PubMed] [Google Scholar]
- Lattin CR, Waldron-Francis K, Richardson JW, de Bruijn R, Bauer CM, Breuner CW, and Romero LM 2012. Pharmacological characterization of intracellular glucocorticoid receptors in nine tissues from house sparrow (Passer domesticus). General and Comparative Endocrinology 179:214–220. [DOI] [PubMed] [Google Scholar]
- Lazic MM, Carretero MA, Crnobrnja-Isailovic J, and Kaliontzopoulou A 2016. Postnatal dynamics of developmental stability and canalization of lizard head shape under different environmental conditions. Evolutionary Biology 43:368–379. [Google Scholar]
- Lazic MM, Kaliontzopoulou A, Carretero MA, and Crnobrnja-Isailovic J 2013. Lizards from urban areas are more asymmetric: using fluctuating asymmetry to evaluate environmental disturbance. PLoS ONE 8:e84190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KA 2006. Linking immune defenses and life history at the levels of the individual and the species. Integrative and Comparative Biology 46:1000–1015. [DOI] [PubMed] [Google Scholar]
- Lin H, Sui SJ, Jiao HC, Buyse J, and Decuypere E 2006. Impaired development of broiler chickens by stress mimicked by corticosterone exposure. Comparative Biochemistry and Physiology A 143: 400–405. [DOI] [PubMed] [Google Scholar]
- Lindström J 1999. Early development and fitness in birds and mammals. Trends in Ecology and Evolution 14:343–348. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger K, and Schabenberger O 2006. SAS system for mixed models. SAS Institute, Cary, NC. [Google Scholar]
- Lochmiller RL, and Deerenberg C 2000. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos 88:87–98. [Google Scholar]
- Martins TLF 2004. Sex-specific growth rates in zebra finch nestlings: a possible mechanism for sex ratio adjustment. Behavioral Ecology 15:174–180. [Google Scholar]
- Matson KD, Tieleman BI, and Klasing KC 2006. Capture stress and the bactericidal competence of blood and plasma in five species of tropical birds. Physiological and Biochemical Zoology 79:556–564. [DOI] [PubMed] [Google Scholar]
- Matthews SG 2002. Early programming of the hypothalamopituitary-adrenal axis. Trends in Endocrinology and Metabolism 13: 373–380. [DOI] [PubMed] [Google Scholar]
- McGlothlin JW, and Ketterson ED 2008. Hormone-mediated suites as adaptations and evolutionary constraints. Philosophical Transactions of the Royal Society B 363:1611–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill L, and Grindstaff JL 2014. Maternal antibody transfer can lead to suppression of humoral immunity in developing zebra finches (Taeniopygia guttata). Physiological and Biochemical Zoology 87:740–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill L 2015. Pre and post-natal antigen exposure can program the stress axis of adult zebra finches: evidence for environment matching. Brain Behavior and Immunity 45:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill L 2018. Data from: Early-life stress strengthens trait covariance: a plastic response that results in reduced flexibility. American Naturalist, Dryad Digital Repository, http://doi:10.5061/dryad.vd158h6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill L, Naylor MF, Dalimonte M, McLaughlin S, Stewart TE, and Grindstaff JL 2017. Early-life immune activation increases song complexity and alters phenotypic associations between sexual ornaments. Functional Ecology 31:2263–2273, doi: 10.1111/1365-2435.12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill L, Naylor MF, and Grindstaff JL 2016. Imperfect past and present progressive: beak color reflects early-life and adult exposure to antigen. Behavioral Ecology 27:1320–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet S, Bennett J, Lee KA, Hau M, and Klasing KC 2007. Quantifying and comparing constitutive immunity across avian species. Developmental and Comparative Immunology 31:188–201. [DOI] [PubMed] [Google Scholar]
- Monaghan P 2008. Early growth conditions, phenotypic development and environmental change. Philosophical Transactions of the Royal Society B 363:1635–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan P, and Haussmann MF 2015. The positive and negative consequences of stressors during early life. Early Human Development 91:643–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison ES, Ardia DR, and Clotfelter ED 2009. Cross-fostering reveals sources of variation in innate immunity and hematocrit in nestling tree swallows Tachycineta bicolor. Journal of Avian Biology 40:573–578. [Google Scholar]
- Mueller C, Jenni-Eiermann S, and Jenni L 2009. Effects of a short period of elevated circulating corticosterone on postnatal growth in free-living Eurasian kestrels Falco tinnunculus. Journal of Experimental Biology 212:1405–1412. [DOI] [PubMed] [Google Scholar]
- National Research Council. 2010. Guide for the care and use of laboratory animals. 8th ed. National Academies Press, Washington, DC. [Google Scholar]
- Nowicki S, Searcy WA, and Peters S 2002. Brain development, song learning and mate choice in birds: a review and experimental test of the “nutritional stress hypothesis”. Journal of Comparative Physiology A 188:1003–1014. [DOI] [PubMed] [Google Scholar]
- O’Hagan D, Andrews CP, Bedford T, Bateson M, and Nettle D 2015. Early life disadvantage strengthens flight performance tradeoffs in European starlings, Sturnus vulgaris. Animal Behaviour 102: 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P, and Pizzagalli DA 2011. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology 214:55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero LM, Strochlic D, and Wingfield JC 2005. Corticosterone inhibits feather growth: potential mechanism explaining seasonal down regulation of corticosterone during molt. Comparative Biochemistry and Physiology A 142:65–73. [DOI] [PubMed] [Google Scholar]
- Saino N, Romano M, Ferrari RP, Martinelli R, and Moller AP 2005. Stressed mothers lay eggs with high corticosterone levels which produce low-quality offspring. Journal of Experimental Zoology A 303:998–1006. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, and Munck AU 2000. How do glucocorticoids influence stress responses? integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews 21:55–89. [DOI] [PubMed] [Google Scholar]
- Schmidt KL, MacDougall-Shackleton EA, Kubli SP, and MacDougall-Shackleton SA 2014. Developmental stress, condition, and birdsong: a case study in song sparrows. Integrative and Comparative Biology 54:568–577. [DOI] [PubMed] [Google Scholar]
- Schmidt KL, MacDougall-Shackleton EA, and MacDougall-Shackleton SA 2012. Developmental stress has sex-specific effects on nestling growth and adult metabolic rates but no effect on adult body size or body composition in song sparrows. Journal of Experimental Biology 215:3207–3217. [DOI] [PubMed] [Google Scholar]
- Senar JC, and Pascual J 1997. Keel and tarsus length may provide a good predictor of avian body size. Ardea 85:269–274. [Google Scholar]
- Sheldon BC, and Verhulst S 1996. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends in Ecology and Evolution 11:317–321. [DOI] [PubMed] [Google Scholar]
- Spencer KA 2017. Developmental stress and social phenotypes: integrating neuroendocrine, behavioural and evolutionary perspectives. Philosophical Transactions of the Royal Society B 372:20160242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KA, Evans NP, and Monaghan P 2009. Postnatal stress in birds: a novel model of glucocorticoid programming of the hypothalamic-pituitary-adrenal axis. Endocrinology 150:1931–1934. [DOI] [PubMed] [Google Scholar]
- Spencer KA, and Verhulst S 2007. Delayed behavioral effects of postnatal exposure to corticosterone in the zebra finch (Taeniopygia guttata). Hormones and Behavior 51:273–280. [DOI] [PubMed] [Google Scholar]
- Van Dongen S 2006. Fluctuating asymmetry and developmental instability in evolutionary biology: past, present and future. Journal of Evolutionary Biology 19:1727–1743. [DOI] [PubMed] [Google Scholar]
- Wada H, Hahn TP, and Breuner CW 2007. Development of stress reactivity in white-crowned sparrow nestlings: total corticosterone response increases with age, while free corticosterone response remains low. General and Comparative Endocrinology 150:405–413. [DOI] [PubMed] [Google Scholar]
- Williams TD 2012. Hormones, life-history, and phenotypic variation: opportunities in evolutionary avian endocrinology. General and Comparative Endocrinology 176:286–295. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Maney DL, Breuner CW, Jacobs JD, Lynn S, Ramenofsky M, and Richardson RD 1998. Ecological bases of hormone-behavior interactions: the “emergency life history stage”. American Zoologist 38:191–206. [Google Scholar]
- Wingfield JC, and Sapolsky RM 2003. Reproduction and resistance to stress: when and how. Journal of Neuroendocrinology 15:711–724. [DOI] [PubMed] [Google Scholar]
- Zann RA 1996. The zebra finch: a synthesis of field and laboratory studies. Oxford University Press, New York. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.