Abstract
Bisphenol A (BPA) is a high-production chemical used in a variety of applications worldwide. While BPA has been documented as an endocrine disrupting chemical (EDC) having adverse health-related outcomes in multiple studies, risk assessment for BPA has lagged due to reliance on guideline toxicology studies over academic ones with endpoints considered more sensitive and appropriate. To address current controversies on BPA safety, the United States National Institute of Environmental Health Sciences (NIEHS), the National Toxicology Program (NTP) and the Food and Drug Administration (FDA) established the Consortium Linking Academic and Regulatory Insights on BPA Toxicity (CLARITY-BPA) using the NCTR-Sprague-Dawley rats. The goal of CLARITY-BPA is to perform a traditional regulatory toxicology study (Core study) in conjunction with multiple behavioural, molecular and cellular studies by academic laboratories focused on previously identified BPA-sensitive organ systems (Academic studies). Combined analysis of the data from both study types will be undertaken by the NTP with the aim of resolving uncertainties on BPA toxicity. To date, the Core study has been completed and a draft report released. Most of the academic studies have also been finalized and published in peer reviewed journals. In light of this important milestone, the PPTOX-VI meeting held in the Faroe Islands, 27–30 May 2018 devoted a plenary session to CLARITY-BPA with presentations by multiple investigators with the purpose of highlighting key outcomes. This MiniReview synthesizes the results of three academic studies presented at this plenary session, evaluates recently published findings by other CLARITY-BPA academic studies to provide an early combined overview of this emerging data, and places this in the context of the Core study findings. This coordinated effort revealed a plethora of significant BPA effects across multiple organ systems and BPA doses with non-monotonic responses across the dose-range utilized. Remarkably consistent across most studies, including the Core study, are low-dose effects (2.5, 25 and 250 μg BPA/kg body weight). Collectively, the findings highlighted herein corroborate a significant body of evidence that documents adverse effects of BPA at doses relevant to human exposures and emphasizes the need for updated risk assessment analysis.
Keywords: Bisphenol A, CLARITY-BPA, endocrine disruptor, EDC, estrogen
INTRODUCTION:
Despite a wealth of data showing that Bisphenol A (BPA) is an endocrine-disrupting chemical (EDC), there remains controversy as to whether exposure poses a risk to human health. The incongruence between the results from academic laboratories and the results of traditional regulatory toxicity studies has contributed to ongoing debate regarding the human health risks posed by BPA. Recognizing this controversy, an inter-agency collaboration was initiated between the National Institutes of Environmental Health Sciences (NIEHS), the National Toxicology Program and the US Food and Drug Administration (FDA). Together, they established the “Consortium Linking Academic and Regulatory Insights on BPA Toxicity” (CLARITY-BPA). The goal of CLARITY-BPA is to combine a traditional regulatory toxicology study, which would evaluate validated guideline endpoints, with detailed behavioural, cellular and molecular research, designed and performed by academic researchers, to ultimately resolve uncertainties on BPA toxicity [1]. This MiniReview highlights papers presented at a dedicated session of the PPTOX-VI meeting that aimed to highlight emerging data from academic laboratories that participated in CLARITY-BPA.
BPA is a high-production chemical with >5 million tons produced annually worldwide, for use in epoxy resins, polycarbonate plastics and other consumer goods. Its production and use continues to increase and contributes to widespread environmental contamination of waters, sediments, soil, air, wildlife and humans [2]. Because of frequent contact with consumer products manufactured with BPA, there are widespread human exposures. Most humans have detectable levels of BPA and BPA metabolites in their urine [3], indicating chronic exposure. Although controversial, estimates of daily intake range from 0.01 to > 5 μg/kg BW/day for adults and 0.01 to 13 μg/kg BW/day for children in westernized countries with higher exposures in Asia [2, 4–6]. BPA crosses the placental barrier [7], and detection of BPA and its metabolites in cord blood, foetal tissue, placenta and amniotic fluid indicates that foetal exposures occur [8–10]. Because foetuses and newborns have limited ability to transform BPA to its metabolites, exposures and potential risks may be greater than for adults [11].
BPA is an EDC that interferes with multiple hormone actions [12, 13]. Specifically, BPA acts as an agonist of membrane and nuclear estrogen receptors (ERs), and an antagonist of thyroid hormone receptor and androgen receptor signalling [14]. There is also some evidence that it can disrupt other signalling pathways including neuropeptide and GABA signalling [15].
More than 100 human epidemiology studies have examined associations between BPA exposures and human diseases [16]. Although these studies individually have limitations, they collectively suggest that current levels of exposure are associated with adverse health outcomes. Hundreds of controlled laboratory animal studies have also identified adverse health outcomes in mice, rats, zebrafish, non-human primates and other model species after exposures to BPA (reviewed in [17–19]). Endpoints as diverse as changes to the structure of the brain, behavioural and cognitive decrements, disruptions to metabolism and obesity, altered reproductive function (male and female), shifted age at pubertal onset, cardiac remodelling, and pre-cancerous and cancerous lesions in the mammary gland and prostate, have been reported, often following exposures below the US Environmental Protection Agency (EPA)’s reference dose of 50 μg/kg/day [20]. This extensive literature has demonstrated that there are vulnerable windows of exposure specific to each organ/endpoint, that many deficits induced by BPA become apparent only later in life, that effects can be sex-specific, and that BPA can sensitize animals to environmental insults encountered after the original period of BPA exposure.
Despite the extensive peer-reviewed literature, regulatory action for BPA has relied on the results of guideline studies, i.e. studies conducted according to internationally agreed upon protocols that include validated endpoints such as body weight, organ weight and histopathological evaluation of tissues. These studies are typically used by risk assessors because of their compliance with Good Laboratory Practices (GLP), a series of record-keeping methodologies that were implemented in response to research misconduct by chemical-testing laboratories [21]. To date, guideline studies have failed to identify effects of BPA at low doses [22–25]; instead, these studies have been used to identify a no-observed-adverse-effect-level (NOAEL) of 5 mg/kg/day, based on changes in body weight observed in rat dams exposed to higher doses [22].
For evaluation of EDCs, both academic and industry-affiliated scientists have noted that the endpoints included in guideline studies are not comprehensive [26, 27]; concerns have also been raised that guideline endpoints may not appropriately ‘map’ to human diseases of concern and may not be sensitive enough to determine ‘safe’ doses for human exposures (e.g., reference doses) [28]. To address these concerns, CLARITY-BPA included two parallel components: 1) A core GLP-compliant guideline study using 1 and 2-year chronic or developmental BPA exposures conducted at the FDA National Center for Toxicological Research (NCTR) laboratories, and 2) Hypothesis-driven studies evaluating non-guideline endpoints previously identified as sensitive to BPA exposure completed in academic laboratories by 14 NIEHS-funded research teams. By design, the academic studies allowed for comprehensive evaluations across a range of tissues and BPA doses to augment the classical guideline endpoints [29]. Importantly, all animals were dosed and handled at the GLP-compliant FDA-NCTR laboratories using a common protocol and study design.
In brief, NCTR Sprague-Dawley rats were gavaged daily with 0.3% aqueous carboxymethylcellulose (CMC) vehicle, one of five BPA doses (2.5 to 25,000 μg/kg BW/day), or ethinyl estradiol (0.05 or 0.5 μg/kg BW/day, a positive control for estrogenicity). Two exposure periods were examined: “continuous exposure” from gestation day 6 (GD6) to the time of sacrifice, and a “stop-dose” with exposure from GD6 to postnatal day (PND) 21 to examine the effects of developmental exposure. Tissues were collected at necropsy at PND 1, 21, 90, 180 and at 1 year by NCTR staff, coded and shipped to the academic laboratories for analysis. Although the pathologists that evaluated the guideline endpoints at the NCTR were unblinded to the control groups, all academic laboratory experiments were conducted in a fully blinded manner with identity of all control and exposure groups unknown throughout data collection. After study completion in the independent laboratories, the primary data were submitted to the Chemical Effects in Biological Systems Database (CEBS) maintained by the NIEHS in a read-only format so they could not be altered. After data were archived and quality review was performed by the Decoding Team, the decoded information was shared with the investigators to permit statistical analysis and publication of findings.
The CLARITY-BPA Consortium had multiple strengths but also some limitations (highlighted in Table 1) that should be taken under consideration when evaluating the utility of the studies. Ultimately, any final conclusions regarding the safety and potential health effects of BPA are expected to be derived from the combined analysis of the Core and Academic study components [29, 30]. Completion of this study will also shed light on the prior concerns raised about the ability of guideline studies to detect low-dose effects, and the comparative sensitivity and utility of guideline and non-guideline endpoints [13, 31].
Table 1:
CLARITY-BPA strengths and weaknesses in study design and implementation
| STRENGTHS: |
|
| LIMITATIONS: |
|
Remarkably, although these studies were conducted on varied tissues and endpoints, significant effects at the lowest BPA doses (2.5, 25 and 250 μg/kg/day) were consistently identified, with variable and, at times absent effects at the higher BPA doses (Table 2). We discuss these findings in the context of other CLARITY-BPA published studies and a similarly designed Danish guideline study examining BPA toxicity.
Table 2:
Significant BPA and EE effects observed across multiple experiments, target organs and doses in CLARITY-BPA
|
TARGET ORGANS |
BPA DOSE (μg/kg BW/day) |
Ethinyl Estradiol (μg/kg BW/day) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| TREATMENT GROUPS | ENDPOINTS ASSESSED | 2.5 | 25 | 250 | 2,500 | 25,000 | 0.05 EE | 0.5 EE | |
| BRAIN | |||||||||
| SD1 | Stop-dose; PND 28 examined | AVPV size increase; females | <0.01 | <0.05 | ne | <0.001 | ne | ne | O |
| SD2 | AVPV size increase; males | O | <0.001 | ne | <0.001 | O | ne | O | |
| SD3 | MePD volume; males | O | O | ne | <0.01 | O | ne | O | |
| G1 | G6 to DOB: PND 1 examined | Hypothalamic ERα increase; females only | <0.01 | O | <0.01 | O | <0.01 | O | O |
| G2 | Hypothalamic ERß increase; females only | <0.01 | O | <0.05 | O | <0.05 | O | O | |
| G3 | Hippocampus ERß increase; males only | O | O | O | O | <0.01 | <0.01 | O | |
| G4 | Amygdala ERß increase; males | O | <0.01 | O | O | O | O | O | |
| G5 | Amygdala ERß increase; females | O | O | <0.05 | O | O | O | O | |
| G6 | Amygdala oxytocin receptor increase; males | O | <0.01 | <0.05 | O | O | O | O | |
| G7 | Amygdala oxytocin receptor increase; females | <0.05 | <0.01 | <0.05 | O | <0.05 | O | <0.01 | |
| G8 | Amygdala vasopressin increase; males | O | <0.01 | <0.05 | O | O | O | <0.05 | |
| G9 | Amygdala genes for GABA/glutamate signaling; females | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| HEART | |||||||||
| SD1 | Stop-dose | Heart weight decrease; females at 6 months | <0.02 | O | O | O | O | O | O |
| SD2 | Heart weight/body weight decrease; females at 6 months | <0.06 | O | O | O | O | O | O | |
| SD3 | LV wall thickness decrease; females at 3 months | <0.05 | O | O | O | O | O | <0.01 | |
| SD4 | Cardiomyopathy, increase severity; females at PND 21 | <0.05 | O | <0.05 | O | <0.02 | <0.0005 | O | |
| CD1 | Continuous-dose | LV wall thickness increase; females at 6 months | O | O | O | O | O | <0.05 | O |
| CD2 | Heart weight/body weight increase; females at 6 months | O | <0.05 | O | O | O | O | <0.001 | |
| PROSTATE | |||||||||
| SD+1 | Stop-dose: T+E at PND 90 | Lateral high-grade prostate intraepithelial neoplasia (HGPIN) at 1 year | <0.01 | na | <0.05 | na | <0.05 | ne | <0.05 |
| SD+2 | Dorsolateral ductal adenocarcinoma, multiplicity increase at 1 year | <0.01 | O | O | O | O | ne | O | |
| CD1 | Continuous-dose: 6 months | Stem cell # increase | <0.02 | O | O | ne | ne | ne | <0.02 |
| CD2 | Progenitor cell proliferation increased | O | <0.01 | <0.02 | ne | ne | ne | <0.01 | |
| CD3 | Stem cell lineage commitment shift to basal cells | O | <0.001 | <0.01 | ne | ne | ne | <0.001 | |
| OVARY | |||||||||
| SD1 | Stop-dose: PND 21 effects | Primordial follicle # decrease | O | O | <0.05 | O | O | O | O |
| SD2 | Primary follicle # decrease | <0.05 | O | <0.05 | O | O | O | O | |
| SD3 | Preantral follicle # decrease | <0.05 | O | O | O | O | O | O | |
| SD4 | Healthy follicle # decrease | <0.05 | O | <0.05 | O | O | O | O | |
| CD1 | Continuous-dose: 1 year | Serum estradiol | O | P=0.08 | O | <0.05 | <0.05 | <0.05 | <0.05 |
| IMMUNE | |||||||||
| CD1 | Continuous-dose: Spleenic effects | Myoid cDC cell increase; males at 3 months | <0.05 | O | O | O | O | O | O |
| CD2 | cDC cell decrease; males at 6 months | <0.01 | O | O | O | <0.01 | O | <0.001 | |
| CD3 | B-cell decrease; females at 6 months | O | <0.05 | O | O | O | O | O | |
| CD4 | APC cell decrease; males at 1 year | <0.05 | <0.05 | O | O | O | O | O | |
| CD5 | Macrophage decrease; males at 1 year | O | O | O | <0.05 | O | O | O | |
| CD6 | CD8 T cells and NKT cell increase; males at 1 year | O | O | O | O | <0.05 | O | O | |
| CD+1 | Continuous-dose: Spleenic response to activating stimuli ex vivo | Proliferation increase (to PWM); females at 6 months | O | <0.01 | O | <0.0001 | O | <0.001 | O |
| CD+2 | Proliferation decrease (to PWM); males at 6 months | <0.05 | O | <0.05 | <0.01 | O | O | <0.001 | |
| CD+3 | Proliferation increase (to PWM); males at 1 year | <0.01 | <0.05 | O | <0.01 | <0.01 | <0.01 | <0.001 | |
| CD+4 | Proliferation increase (to LPS); females at 1 year | O | O | O | O | <0.05 | O | O | |
| CD+5 | Proliferation increase (to LPS); males at 1 year | <0.05 | O | O | <0.05 | <0.01 | O | <0.0001 | |
| CD+6 | Proliferation decrease (to anti-CD3); females at 1 year | <0.05 | O | O | O | O | <0.01 | O | |
| CORE STUDY | |||||||||
| SD1 | Stop-dose | Mammary adenocarcinoma incidence increase; females at 2 years | <0.02 | O | O | O | O | O | O |
| SD2 | Ovarian follicular cysts; 1 year | O | O | O | O | <0.001 | O | <0.001 | |
| SD3 | Aberrant estrous cycle, early onset | O | O | O | <0.03 | O | O | O | |
| SD4 | Liver infiltration with mononuclear cells; females at 1 year | <0.05 | O | O | O | <0.05 | O | O | |
| SD5 | Kidney tubule cyst increase; females at 2 years | <0.01 | O | O | O | O | O | O | |
| SD6 | Serum protein and total bile acids decrease; males at 1 year | O | <0.05 | O | O | O | O | O | |
| SD7 | Platelets decrease; females at 1 year | O | O | O | O | <0.05 | <0.002 | O | |
| SD8 | Pancreas pigmentation increase; males at 1 year | O | O | X | O | O | O | O | |
| SD9 | Adrenal hyperplasia; males at 2 years | O | O | O | X | O | O | O | |
| SD10 | Pituitary cysts; males at 2 years | O | O | O | O | <0.05 | O | O | |
| SD11 | Pituitary gland hyperplasia; males at 2 years | O | O | O | O | <0.05 | <0.05 | O | |
| SD12 | Bone marrow hypercellularity; males at 2 years | O | O | X | O | X | O | O | |
| SD13 | Spleen lymphoid hyperplasia; males at 2 years | O | O | X | O | O | O | O | |
| SD14 | Thyroid cysts; females at 2 years | O | O | <0.05 | O | <0.05 | O | O | |
| SD15 | Epididymis, exfoliated germ cells increase; 2 years | O | O | O | <0.05 | O | O | O | |
| CD1 | Continuous-dose | Mammary gland alveoli dilation; females at 2 years | X | O | O | O | O | O | O |
| CD2 | Uterine apoptosis increase | O | O | O | O | <0.05 | O | <0.001 | |
| CD3 | Vaginal epithelial hyperplasia increase; females at 2 years | O | <0.05 | O | <0.05 | <0.05 | O | O | |
| CD4 | Vaginal epithelial hyperplasia increase; females at 1 year | O | O | O | O | O | O | <0.001 | |
| CD5 | Liver weight decrease; males at 1 year | <0.05 | O | O | O | O | O | O | |
| CD6 | Liver angiectasis incidence increase; males at 2 years | X | O | O | O | O | O | O | |
| CD7 | Liver mononuclear infiltration; males at 1 year | <0.05 | O | <0.05 | <0.05 | <0.05 | O | O | |
| CD8 | Liver mononuclear infiltration; males at 2 years | O | O | O | O | O | <0.05 | O | |
| CD9 | Liver fatty acid increase; males at 1 year | O | <0.05 | O | O | O | O | <0.05 | |
| CD10 | Kidney tubule cysts; females at 1 year | <0.02 | O | O | O | O | <0.05 | O | |
| CD11 | Kidney tubule cysts; males at 1 year | O | O | X | X | O | X | O | |
| CD12 | Kidney epithelial hyperplasia, males at 2 years | O | <0.01 | O | O | O | O | O | |
| CD13 | Hemoglobin increase; females at 1 year | O | <0.01 | O | O | O | O | O | |
| CD14 | Hemoglobin increase; males at 1 year | O | O | O | O | <0.05 | <0.05 | O | |
| CD15 | Body weight increase; females at 2 years | O | O | <0.05 | O | O | O | O | |
| CD16 | Pituitary hyperplasia; males at 2 years | O | <0.05 | O | O | <0.05 | O | <0.05 | |
| CD17 | Parathyroid gland hyperplasia; males at 2 years | O | <0.01 | O | O | O | O | <0.05 | |
| CD18 | Thyroid hyperplasia; males at 2 years | O | O | O | <0.01 | O | <0.01 | O | |
| CD19 | Eosinophil decrease; males and females at 1 year | O | O | <0.05 | O | O | O | <0.05 | |
| CD20 | Epididymis, exfoliated germ cells & lymphocytic infiltration increase; 1 year | O | O | O | O | <0.05 | O | <0.05 | |
| CD21 | Fat pad, retroperitoneal; females at 1 year | <0.05 | O | O | O | O | O | O | |
| CD22 | Prostate DLP chronic lymphocytic inflammation increase; 1 year | <0.05 | O | O | O | O | O | O | |
| CD23 | Prostate DLP chronic intraluminal inflammation increase; 2 years | <0.05 | O | O | O | O | O | O | |
Significant effects observed for each organ system were sequentially numbered (column at left): (SD) Stop-dose; (CD) Continuous-dose; (G) Gestation exposure only; (+) indicates secondary exposures in vivo or in vitro as described in the text. (na) not available; (ne) not examined; (O) no significant effect; (X) significant effect noted on Core Study Overview Table; data not available.
NEUROBIOLOGY and BEHAVIOR ENDPOINTS
Over a half century of work has established that perinatal estrogen, aromatized from testicular androgens, is essential for masculinization of the rodent brain [32, 33], and that gonadal hormones also contribute to human neural and behavioural sex differences [34, 35]. Manipulation of estrogen signalling during these critical periods of sexual differentiation can produce profound effects on brain architecture and behaviour. For example, female rodents exposed to estrogens during a tight, well-defined perinatal critical period spanning just before and after birth subsequently display male-like copulatory behaviour, while male rodents in which ER signalling is blocked fail to display male-typical mating behaviours and can instead display lordosis under the right conditions [33]. The location and density of estrogen and other hormonal receptors are critically important for the coordination of sexual differentiation throughout the brain and can radically change across neonatal life [36–38]. Studies using ER knockout mice have revealed a particularly critical role for ERα [39, 40], with ERβ and other hormones, including the neuropeptides oxytocin and vasopressin, playing a supporting role and facilitating social recognition and other behaviours fundamental to partner preference, courtship, social anxiety and copulation [41, 42]. Morphological sex differences also emerge in response to perinatal estrogen, most notably a larger SDN-POA in males and a larger AVPV in females. Thus, behaviour, ER expression, and subregion volume are all reliable endpoints of developmental endocrine disruption via interference with estrogen signalling.
Work by Patisaul and others using multiple rodent models has shown that developmental exposure to BPA can alter the structure and sexual differentiation of many brain regions including the AVPV, amygdala (Fig. 1) and medial preoptic area, and result in altered non-reproductive behaviors, particularly elevated anxiety [43–47]. Most significantly, Patisaul and colleagues have repeatedly shown, in multiple rat strains, that developmental BPA exposure can alter the expression of ERs in brain regions, such as the amygdala and hypothalamus, that coordinate reproductive and other sexually dimorphic behaviours [38, 48]. Two of these studies were done in the same animal strain as CLARITY-BPA, with rats treated at the NCTR-FDA labs under nearly identical conditions, with a similar dose range, as a prelude to the CLARITY-BPA studies at that facility.
Figure 1:
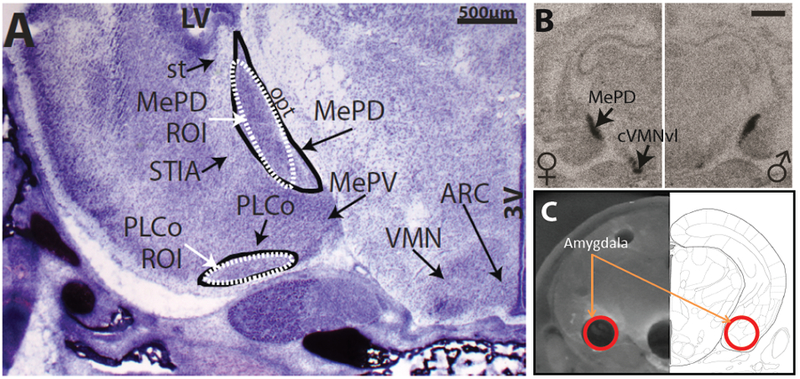
(A) The neonatal rat amygdala is a complex structure containing multiple subnuclei readily discernable with a NISSL stain. Two regions of particular interest (MePD and PLCo) are depicted (circled in black) along with the areas selected within those subregions for quantification by in situ hybridization (white ovals labelled ROI). (B) In situ hybridization allows for mRNA quantification on a subregional, and even individual cell, scale. In the representative autoradiograph, ERβ mRNA is clearly labelled in the MePD and neighboring VMN in both sexes. The two Patisaul studies done in collaboration with NCTR prior to CLARITY used this approach to quantify ERs in multiple brain regions including the hypothalamus and amygdala. (C) For the CLARITY study, regions including the amygdala were isolated by micropunch and the whole transcriptome sequenced. With this approach, anatomical resolution is reduced in exchange for the capacity to sequence many more genes. In all three studies, ER expression was found to be affected by BPA at doses as low as 2.5 μg/kg bw. Scale bar in B = 1000μm; 3V = third ventricle; ARC = arcuate nucleus; LV = lateral ventricle; MePD = medial postdorsal portion of the amygdala; opt = optic track; PLco = posterolateral cortical amygdaloid nucleus; ROI = region of interest; st = stria terminalis; VMN = ventromedial nucleus; cVMNvl = caudal portion of the ventrolateral ventromedial nucleus.
The first of these studies [49] was conducted in collaboration with Sherry Ferguson at NCTR and used two doses of BPA (2.5 and 25 μg/kg BW) and two doses of EE (5 and 10 μg/kg BW) as well as a vehicle (CMC) and naïve control (which was removed from the cage but not gavaged). Dams were exposed prenatally from GD6 through the day of birth. The postnatal day (PND) 1 offspring were decapitated and the frozen heads sent to the Patisaul laboratory for quantification of ERα and ERβ expression in the hypothalamus and amygdala via in situ hybridization (Fig. 1). Effects were region-, dose- and sex-specific with some known sex differences in ER expression eliminated at the lowest BPA dose. BPA- and EE-related effects were directionally similar and exposure resulted in ER up-regulation in most cases. Notably, ER levels were markedly lower, particularly in the amygdala, in the vehicle controls compared to the naïve animals suggesting a suppressive effect of gavage on ER expression. This observation is significant, because gavage is traditionally the dosing method of choice for guideline toxicity studies. Additionally, exposure-related increases in ER expression generally returned expression levels to a range typical of the naïve animals. Thus, it was concluded that increased expression levels in the exposed animals likely reflected the interaction of BPA exposure and stress.
The second study [50] used animals obtained from a 90-day subchronic study conducted by NCTR as a prelude to CLARITY-BPA [51, 52]. For this project, only females were examined but vehicle controls of both sexes were included to 1) ensure known sex differences could be reliably detected and 2) establish the degree to which BPA and EE could “masculinize” the female brain. Four doses of BPA (2.5, 25, 260, 2700 μg/kg bw),and two EE doses were used, (0.5, 5 μg/kg BW) were used. Two exposure windows were used: GD6 through PND21 and GD6 through PND90. Offspring brains were collected on PND21 or PND90 and sent to the Patisaul laboratory for analysis of ER expression in the preoptic area via in situ hybridization. As in the PND1 study, effects were region- and sex-specific. In this case, BPA exposure generally decreased ER expression, which is opposite of what was observed on PND 1. This is not surprising given the dramatic age-dependent differences in baseline ER expression levels in the rodent brain [36–38]. Similar to the PND 1 study, ERβ appeared to be more sensitive. Interestingly, effects were not always monotonic, with some effects observable at the lowest two, but not the highest two, doses. BPA and EE were concordant in direction but not necessarily dose, with some cases where low-dose effects of BPA were observed but not low-dose EE.
The CLARITY-BPA studies in the Patisaul laboratory used two different groups of animals. One, collected on PND1, had five doses of BPA (2.5, 25, 250, 2,500 and 25,000 μg/kg BW) and two doses of EE (0.05 and 0.5 μg/kg BW). Exposure was exclusively prenatal. The second group, used for behavioural analyses at NCTR by the Patisaul laboratory and the laboratory of Cheryl Rosenfeld. In collaboration with Sherry Ferguson, CLARITY-BPA rats were transferred to a separate building at weaning for assessment of anxiety, exploratory behaviour and spatial navigation. In this second group, exposure spanned GD6 to PND21 and included only a subset of the dose groups (vehicle, 2.5, 25 and 2,500 μg/kg BW BPA, 0.5 μg/kg BW EE). One group of animals was tested as juveniles and another was tested in adulthood. Despite numerous prior studies showing robust and reproducible effects of developmental BPA exposure on anxiety and exploratory behaviors, effects in this case were subtle and sporadic [53, 54]. Some sex differences were either not detected or the opposite of expected in the vehicle controls leading to the conclusion that some behavioural sex differences may be uniquely different in this strain compared to other Sprague-Dawley strains [53].
The brains from the juvenile animals were then collected and subjected to morphometric analysis to look for evidence of abrogated volumetric sex differences [55]. All expected sex differences were detected in the vehicle controls and there was no instance where exposure eliminated those differences. Interestingly, all doses of BPA enlarged the female AVPV and a similar enlargement was observed in males at the 25 and 2,500 μg/kg BW dose levels. Because endogenous estrogen, via action on ERα, acts to reduce the volume of the AVPV [32, 33], the effect of BPA observed here is consistent with anti-estrogenic activity. An effect of BPA on MePD volume was also detected, but only in the right MePD in males exposed to the 2,500 μg BPA/kg BW/day exposure group. Because the left and right MePD have numerous structural and functional differences, an effect on only one side is biologically plausible, although the functional significance is not entirely clear.
The PND1 brains sent to the Patisaul laboratory were analysed by a combination of transcriptomics and qRT-PCR with the hypothesis that exposure would alter ER expression levels and other targets in the ER signalling cascade. Although the prior two studies with NCTR rats used in situ hybridization, here the three regions of interest (hypothalamus, hippocampus and amygdala) were isolated by micropunch (Fig. 1) and analysed by targeted and untargeted transcriptomics to obtain a richer picture of the suite of genes impacted by exposure. Overall, the greatest number of differentially expressed genes were found in the male hypothalamus and female amygdala [56, 57]. Heightened ERα and ERβ expression was observed in the hypothalamus of both sexes at 2.5, 25 and 2,500 μg BPA/kg bw. In the hippocampus, the only evidence of ER disruption was heightened ERβ expression in males at the 25,000 μg/kg bw dose. Similarly, only ERβ was altered in the amygdala with expression levels non-monotonically heightened in both sexes.
The CLARITY transcriptome data are consistent with the data obtained in the first study the Patisaul laboratory did in conjunction with NCTR, which also found heightened ER expression in BPA-exposed animals. Because the CLARITY-BPA study used micropunched tissue containing the entire region of interest, it lacks the resolution obtainable via in situ hybridization and thus could not recapitulate the subregional differences observed in that first study (Fig. 2). Nevertheless, it successfully reproduced evidence that prenatal BPA exposure disrupts neonatal ER expression in the hypothalamus and amygdala. Other genes altered by BPA included oxytocin and GABA vesicular transporter (Slc32a1) in the hypothalamus, oxytocin in the hippocampus, and oxytocin and vasopressin receptors in the amygdala. Nearly identical outcomes were found in adult hippocampus and hypothalamus by the Rosenfeld laboratory working on CLARITY-BPA animals dosed at 2,500 μg/kg BW from GD 6 through PND 21 [58]. Numerous genes involved in glutamate signalling were also found to be disrupted in the amygdala. Collectively, these data are consistent with prior work by Patisaul and others showing that BPA impacts estrogen, oxytocin and vasopressin pathways throughout the brain [15, 45, 48, 59–61].
Figure 2:
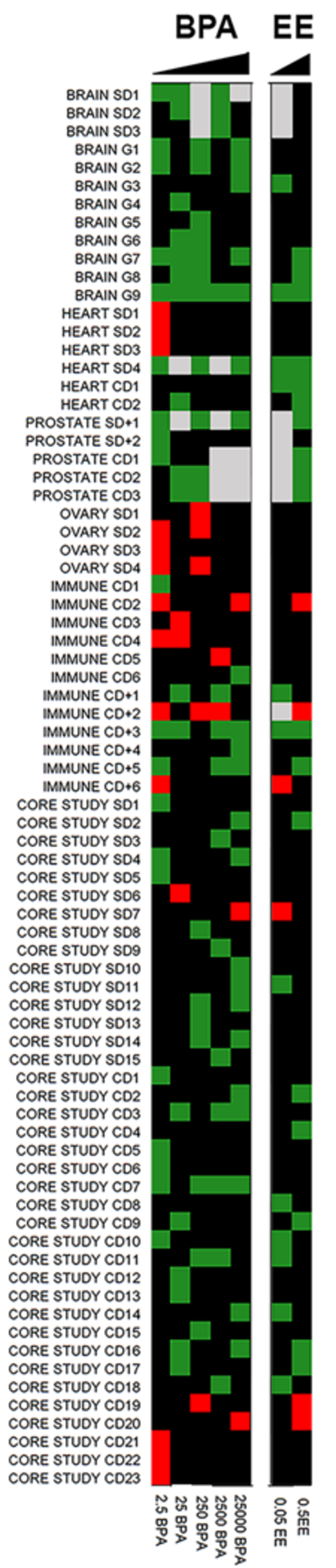
Significant BPA effects from CLARITY-BPA Academic and Core studies available to date are visually represented as a heat map over 5 BPA doses (25,000, 2,500, 250, 25 and 2.5 μg/kg BW/day) and 2 EE doses (0.5 and 0.05 μg/kg BW/day).. The specific endpoints for various organ systems are numbered at left with corresponding details provided in Table 2. Green indicates increased effects, red indicates decreased effects and black represents no significant difference relative to vehicle controls for each group. Areas of grey represent doses or treatments that were not examined.
In summary, the CLARITY-BPA studies, and the two collaborative studies with NCTR that proceeded them, confirm that developmental exposure to BPA, at doses as low as 2.5 μg/kg BW, can alter ER expression in the brain. Directionality differs with age with heightened expression generally observed in neonates and suppressed expression observed in adolescents. Disruption of brain ER is one of the most consistently observed outcomes of developmental BPA exposure. Additionally, the CLARITY-BPA studies from the Patisaul and Rosenfeld laboratories provide further confirmatory evidence that developmental BPA exposure alters oxytocin- and vasopressin-related signalling pathways, and the volume of the AVPV.
CARDIAC ENDPOINTS
The heart of both males and females express estrogen receptors [62]. Estrogens (i.e. 17β-estradiol) normally act to regulate heart function and vascular tone through direct actions on cardiac and vascular tissues, and by regulating autonomic nervous system activity. The impacts of endogenous estrogens and BPA are sex-specifically regulated and differ in males and females [63–67]. Prior work by the Belcher laboratory demonstrated that sub-nanomolar concentrations of BPA and 17β-estradiol could sex-specifically alter rapid estrogen-signalling in cultured adult rodent cardiomyocytes [68]. Those effects of BPA were found only in females and mediated through an ERα and ERβ−dependent mechanism that disrupted Ca2+ handling and modified excitation–contraction coupling. In both rats and wildtype and ER knockout mice, those estrogen-like effects of BPA were shown to sex specifically increase arrhythmia frequencies in response to β-adrenergic stress in females [65].
Following up on those in vitro findings, in vivo studies using a continuous life-long dietary exposure protocol of BPA or control 17α-ethinyl estradiol exposures were performed in CD-1 mice [64, 69–72]. BPA exposure spanned five orders of magnitude from ~4 μg/kg BW/day to ~50,000 μg/kg BW/day with a 10-fold dose interval. Three doses of 17α-ethinyl estradiol resulting in exposures of ~0.02, 0.2 and 0.15 μg/kg BW/day were also included. These studies demonstrated significant, and most often, sex-specific effects of BPA on a variety of reproductive, metabolic, immune and cardiovascular related endpoints [64, 69–72]. In the heart, only minor exposure-related increases in heart weight, left ventricular (LV) wall thickness, and modest changes in collagen content in the extracellular matrix of hearts were found in males exposed to 430 and 4400 μg BPA/kg BW/day. In contrast, modest decreases in LV wall thickness and a remarkable decrease in collagen were observed in hearts of females at the lowest BPA dose (5 μg/kg BW/day). In females, BPA exposure resulted in an extraordinary increase in sensitivity of the heart to isoproterenol-induced ischaemic damage and hypertrophy that was likely due to alterations in the collagen extracellular matrix and modified cardiac fat metabolism. This conclusion was supported by cardiac transcriptome analysis that revealed BPA exposure caused sex-specific changes in expression of components of the extracellular matrix and in dysregulation of fatty acid metabolism and glycolytic metabolism. These alterations were consistent with the observed histological changes in the collagen extracellular matrix and pathological remodelling observed in BPA-exposed hearts [64].
The published finding analysing cardiac effects in CLARITY-BPA NCTR-Sprague Dawley rat hearts [73], found limited evidence of BPA or EE alterations on gross cardiac endpoints related to hypertrophy. While alterations in LV wall thickness were not observed at any dose in either sex, a significant decrease in heart weight and heart weight normalized to body weight was observed in females exposed to 2.5 μg/kg BW, a finding consistent with previous findings in mice [64].. Exposure-related changes in collagen accumulation in males were limited to the highest EE dose group with increased collagen accumulation in PND21 males. In female hearts, decreased collagen content was observed in rats treated with 25,000 μg/kg BW BPA and 0.5 μg/kg BW EE at PND90 and 6 months, respectively.
The lack of overt morphology phenotypes in the CLARITY-BPA or EE exposed hearts was not surprising since pathology associated with the majority of cardiac insults typically becomes evident only after adverse cardiovascular events such as cardiac ischaemia or myocardial infarction [64, 66]. For experimental studies with rodents, it is well-accepted that an intervention resulting in increased β-adrenergic stress, ischaemic injury or genetic manipulations are necessary to reveal cardiac fibrosis, hypertrophy or phenotypes indicative of overt cardiac pathology [74]. Such manipulations were not possible in the CLARITY-BPA study and limit any interpretations resulting from negative data. Additionally, compared to control mice, the hearts of control NCTR-Sprague Dawley rats had relatively higher levels of collagen due to known species-specific differences in the proportions of myocytes and fibroblasts present in murine and rat hearts [75].
Progressive cardiac myopathy (PCM) is a common background lesion in some rat strains that is suspected to arise from localized microvascular dysfunction. The common occurrence of PCM lesions in Sprague-Dawley rats has presented challenges for analysing cardiotoxicity in regulatory toxicology studies of chemicals and pharmaceuticals [76–80]. Previous studies had not analysed PCM lesions in hearts of young animals. In the CLARITY-BPA study, a remarkable abundance of early PCM lesions was detected in the hearts of most control and exposed animals analysed at PND21 [73]. Consistent with PCM found in adults, the lesion incidence and severity was greater in control males than in females. In BPA- or EE-treated females at PND21, cardiomyopathy incidence was increased compared to control females and a significant increase in severity was found for 2.5, 250 or 25,000 μg BPA/kg BW/day and both EE groups. In a male exposed to 250 μg BPA/kg BW/day and a female from each of the two lowest BPA dose groups (2.5 and 25 μg/kg BW/day), a diffuse degeneration phenotype involving much of the myocardium was also observed [73]. This high level of cardiac pathology across much of the myocardium is an accepted indication of exposure-related cardiotoxicity [76].
At PND90 and 6 months, cardiomyopathy in both males and females was observed in 100% of control samples from both the stop dose and the continuous dose arms of the CLARITY-BPA study [73]. At PND90, the diffuse degeneration phenotype was again observed in males and females from the continuous (males: BPA 25, 250, 25,000; EE 0.5 μg/kg/day; females: BPA 2.5, 25; EE 0.05, 0.5 μg/kg/day) and stop dose (males: BPA 250, 25,000; EE 0.05, 0.5 μg/kg/day; Females 250 μg/kg/day) exposure groups.
The increases in PCM observed in females at PND21 and the notable increase in myocardial degeneration at PND90 suggests that BPA and EE impact cardiovascular health resulting in an early onset of vascular dysfunction and progression of cardiomyopathy. These findings are clear indicators of exposure-related cardiotoxicity in the 2.5 μg/kg/day BPA group resulting from increases in adverse vascular events, findings that support a NOAEL of <2.5 μg/kg/day for effects in the heart.
It is also notable that the largest observed morphometric effects in the CLARITY-BPA study animals analysed were due to treatment duration (“stop-dose” versus “continuous dose”) which altered body weight and cardiac collagen accumulation in control animals, an effect arising from experimental differences between the two different study designs [73]. At PND90, mean body weight of stop dose control males was 9.5% greater than in the continuously exposed control group; at 6 months, mean body weight of stop dose control males was 9.1% greater than continuously exposed controls. Those observed differences in body weights between continuously dosed males and males dosed only until weaning at PND21 indicate that there were effects of post-weaning dosing procedures and/or the CMC vehicle. The sex-specific decreased weight of males dosed daily with vehicle by gavage is consistent with previous studies showing that prolonged postnatal stress in males decreases weight gain over time, and that female SD rats are resistant to these effects of stress [81–83].
PROSTATE ENDPOINTS
It is well established that the developing prostate gland has heightened sensitivity to estrogenic exposures which can reprogram the organ to elevated disease risk in adulthood [84–86]. In this context, previous work from the Prins, Ho and Walker laboratories determined that early-life, low-dose BPA exposure reprogrammed the rat prostate epigenome and increased its susceptibility to hormonal carcinogenesis with ageing, a finding that was subsequently confirmed in a humanised prostate model [87–94].This is germane to human disease since elevated estrogen levels rise in ageing men [95] and together with androgens, induce prostate cancer in the rat and human epithelium [96, 97]. As part of the CLARITY-BPA consortium, the Prins laboratory had two main goals: 1) re-assess whether developmental BPA exposures across multiple doses could alter susceptibility to estrogen-driven carcinogenesis with ageing, and 2) determine whether chronic low-dose BPA exposures might target prostate epithelial stem and progenitor cells [98]
For goal 1, NCTR Sprague-Dawley rats were gavaged daily with vehicle, EE (0.5 μg/kg BW), 2.5, 25, 250, 2500 or 25,000 μg BPA/kg BW from GD6 to one year (continuous dose), from GD6 to PND21 (stop-dose), or from GD6 to PND21 (stop dose) with testosterone plus estradiol-17β (T+E) implants given at PND90 to drive carcinogenesis with ageing. The T+E treatment resulted in normal circulating testosterone levels and a 2-fold estradiol-17β elevation to model rising estrogen levels in ageing men. Prostates were collected at one-year necropsy, coded and analysed for histopathology by Maarten Bosland, DVM, PhD who was blinded to treatments and controls. As previously reported by the Prins laboratory [87, 94], developmental or continuous exposure to BPA alone at any dose did not produce prostate pathology that differed from vehicle controls. However, in response to T+E, rats given developmental EE, 2.5, 250 or 25000 μg BPA/kg BW showed significant increases in lateral prostate prostatic intraepithelial neoplasia (PIN) severity compared to vehicle controls at one year, shifting from low-grade PIN in controls to high-grade PIN in rats developmentally exposed to BPA with the highest PIN score observed at the 2.5 μg BPA/kg BW dose. PIN could not be evaluated in the rats given 25 or 2500 μg BPA/kg BW due to limited sample size. Of note, in humans, high-grade PIN is a precursor lesion of prostate cancer, whereas low-grade PIN is not considered clinically relevant. Importantly, there was a four-fold increase in dorsolateral ductal adenocarcinoma multiplicity in rats developmentally exposed to 2.5 μg BPA/kg BW plus adult T+E. Together, these CLARITY-BPA findings confirm previous reports that low-dose BPA exposures augment prostate cancer risk.
To address goal 2, NCTR Sprague-Dawley rats were gavaged daily with vehicle, ethinyl estradiol (EE; 0.5 μg/kg BW), 2.5, 25 or 250 μg BPA/kg BW from GD6 to 6 months whereupon prostates were removed and shipped on ice overnight to the Prins laboratory for stem cell isolation and culture [98]. Dorsolateral lobe epithelial stem cells were isolated by direct prostasphere 3-D culture and passaged 3 times in the absence of hormone or BPA to enhance stem cell purification. Dose-specific responses to BPA exposures were observed for stem and progenitor cells. In vivo exposure to EE and 2.5 μg BPA doubled the total prostasphere number, reflecting increased stem cell numbers in the adult prostates. Prostasphere size, a marker of progenitor cell proliferation in the cultured spheroids, was increased steeply by EE and 25 μg BPA/kg BW and to a lesser degree by 250 μg BPA/kg BW compared to vehicle-treated rats. To assess the effects of BPA on prostate stem cell lineage commitment, progenitor lineage markers were quantified by q-RT-PCR in the prostasphere cells. Tightly paralleling prostasphere size effects, exposure to EE and 25 μg/kg BW BPA significantly increased CK5, Sox2 and HoxB13 expression while EE, 25 and 250 μg/kg BW BPA suppressed CK8, Trop2 and Tbx3 mRNA. This indicates that chronic BPA exposures permanently modify the lineage commitment of the prostate stem cell progeny, increasing basal progenitors and suppressing luminal progenitor cells. Together, these results show that chronic low-dose BPA exposure alters adult prostate stem cell homeostasis in a dose-dependent manner, increasing stem cell numbers at the lowest dose and elevating progenitor cell proliferation while also shifting lineage commitment to favour basal progenitor cells at a 10- and 100-fold higher dose. That the 0.5 μg EE/kg BW exposure resulted in similar stem and progenitor cell effects indicates that they are mediated through ER pathways. The dose-specific responses observed over a 100-fold BPA dose range is likely due to differential engagement of ER populations and membrane versus nuclear signalling pathways. These findings are particularly relevant in light of reports that cancer risk is strongly correlated with the number of normal stem cell divisions across multiple human tissues, including the prostate [99]. Furthermore, since tumour initiating cells for human prostate cancer are largely localized to the basal cell compartment [100], it is possible that increased basal cell numbers in the prostate may increase the opportunity for tumour initiation by secondary hormone exposures as occurs in our animal model.
In summary, the CLARITY-BPA findings show the most significant effects on the prostate occur at the lowest doses examined, with peak effects observed at the 2.5 μg BPA/kg BW dose. Together, our results confirm that low-dose developmental BPA exposure increases prostate cancer susceptibility to ageing-associated increases circulating estrogens which may be underpinned, in part, by reprogramed stem cells.
OVARIAN ENDPOINTS
Prior studies have demonstrated that low-dose BPA exposures during perinatal development affect three types of endpoints in the ovary: 1) exposures at the onset of meiosis increase the likelihood that a female will ovulate chromosomally abnormal eggs in adulthood; 2) exposures during follicle formation induce an increase in multi-oocyte follicles and reduce the total pool of oocytes; and 3) exposures throughout gestation affect the adult ovary including the induction of polycystic ovaries. These effects have been observed in several mammalian species including rat, mouse, sheep and monkey [101–108] as well as in nematodes [109] and in human foetal ovaries exposed in vitro [110].
As part of the CLARITY-BPA consortium, Flaws and colleagues examined the effects of exposure to EE (0.05 or 0.5 μg/kg BW), or BPA (2.5, 25, 250, 2500 or 25,000 μg BPA/kg BW) from GD6 to one year (continuous), or from GD6 to PND21 (stop-dose), on ovarian morphology and sex steroid hormone production [111]. Ovarian endpoints including follicle health, follicle number, and follicle stage were evaluated at PND1, PND21, PND90, 6 months and 12 months of age. Effects of BPA were only observed at PND21, prior to puberty; BPA significantly decreased the number of primordial follicles (250 μg/kg/day), number of primary follicles (2.5 and 250 μg/kg/day), and number of total healthy follicles (2.5 and 250 μg/kg/day). These same outcomes were not disrupted by EE, the positive control. In fact, the lower dose of EE (0.05 μg/kg/day) did not affect any ovarian endpoints at any age.
Another important role for the ovary is sex steroid production. Prior work from the Flaws laboratory and others has demonstrated that BPA can disrupt ovarian steroidogenesis [112]. An in vitro study found that exposure to 10 and 100 μg/mL concentrations of BPA disrupted expression of steroidogenesis genes in mouse antral follicles, ultimately decreasing production of progesterone, androstenedione, testosterone and estradiol [113].
Within the CLARITY-BPA study, Flaws and colleagues also examined steroidogenesis in females from both the stop-dose and continuous-dose groups at PND21, PND90, 6 months and 1 year of age [111]. Continuous exposure to EE (0.5 μg/kg BW) significantly decreased serum progesterone levels at PND21, PND90 and 6 months of age, whereas the lower dose of EE and all doses of BPA had no effect on this hormone. Continuous exposure to either dose of EE, or the two highest doses of BPA (2500 and 25000 μg/kg BW), significantly decreased serum estradiol levels. Similar declines were also observed in the three lower BPA groups, but these did not reach statistical significance, likely due to small sample sizes that diminished statistical power.
In summary, these results suggest that the ovary is an important target of BPA, with effects that occur both at the level of reproductive function (e.g., health and number of follicles) and endocrine function (e.g., production of sex steroids). Although the CLARITY-BPA study has limitations due to small sample sizes for some measures, the effects observed in this study are consistent with prior studies showing harm after low-dose exposures [112].
IMMUNE ENDPOINTS
Prior studies examining the effects of BPA on immune cells have been conducted both in vitro and in vivo, and epidemiological evidence suggests associations between BPA and immune cell mediated diseases including asthma [114]. In vitro studies suggested that BPA could alter expression of cytokines and chemokines from a range of different immune cell types, alter mast cell degranulation, and enhance antigen presentation. In rodents, significant variability in responses was observed across studies; these studies examined different exposure periods, doses, routes of exposure and health outcomes. Some studies revealed effects of BPA on inflammatory cytokines in the spleen without effects in the lung [115], whereas others observed lung inflammation and airway hyperactivity [116, 117]. Effects of BPA on innate immune responses were also observed [118]. Prior to CLARITY, an expert panel concluded that there was credible evidence that BPA could induce abnormal immune responses after relatively low levels of exposure (5 μg/kg/day) during development [119].
Within the CLARITY-BPA consortium, the Kaminski laboratory examined immune cell populations residing in the spleen and thymus [120]. Only animals that were continuously exposed to BPA throughout life were utilized in two published studies resulting from this work. At PND21, the composition of the spleen and thymus were not affected by BPA exposure, and neither the lymphoid nor the myeloid populations were altered in their composition within the spleen. At PND90, there were similarly no effects of BPA, with the exception of the spleen-associated myeloid populations, which showed increases in the number of cDC cells in males exposed to 2.5 μg/kg/day. By 6 months of age, more disruptions to immune cell populations became evident: there was an increase in total spleen cellularity and a decrease in B-cell populations in females exposed to 25 μg/kg/day; males treated with either 2.5 or 2500 μg/kg/day also displayed decreases in the number of mature cDC cells at this age. By 12 months of age, low-dose effects of BPA were observed only in males, with decreases in the number of APC cells (after treatment with 2.5 or 25 μg/kg/day) and decreases in the number of macrophages (after treatment with 2500 μg/kg/day) in the spleen.
The second study from the Kaminski group examined the response of the immune system to stimuli administered to cells ex vivo from animals exposed continuously to BPA [121]. Spleen cell proliferation was not affected by BPA treatment alone, but after induction by an activating agent, PWM, heightened responses were seen in females at 6 months of age (exposed to 2.5 or 2500 μg BPA/kg/day) whereas diminished responses were seen in males of the same age (exposed to 2.5, 250 or 2500 μg BPA/kg/day). Spleen cell proliferation was also affected by BPA exposure at 12 months of age, where males from the 2.5, 25, 2500 and 25,000 μg/kg/day groups had heightened responses to the PWM activating agent; females were not affected by PWM, but did respond to another activating agent (Anti-CD3/28), with diminished responses that were significant in the 2.5 μg/kg/day group. Finally, the study authors isolated specific kinds of immune cells including T-cells and Natural Killer Cells. Sex-specific effects of low-dose BPA were observed, although the timing of evaluation (6 versus 12 months of age) also influenced these outcomes.
Overall, the CLARITY-BPA study authors downplay the significance of their results, noting that a large number of individual outcomes were examined (530 in the first study, 630 in the second study) with few that were deemed statistically significant. Here, we have merged many of these individual outcomes to instead see the overall effect (e.g., 40 individual measurements were taken to evaluate the myeloid cell lineage: 5 different cells types, 2 sexes, at 4 different ages; we combined these to a single measure: disruption of the myeloid cell lineage). When viewed in this way, a clear pattern emerges for several parameters: low doses of BPA alter how immune cells respond to stimulation, with effects that are different in males and females.
SPERM ENDPOINTS
Because sperm are relatively easy to access, prior studies have conducted in vitro investigations into the effects of BPA on a range of outcomes including proliferation and survival, motility, velocity, ATP content and gene expression, among others. These studies have found effects of BPA at low concentrations (10−12 – 10−9 M) [122–124]. In rodents, developmental exposures to BPA have been shown to disrupt a number of male reproductive functions including alterations to sperm count, motility and density [125].
One report from the CLARITY-BPA study from the Boekelheide laboratory found no effect of BPA on sperm endpoints in males exposed continuously and evaluated at PND90 [126]. Outcomes that were evaluated included testis weight, epididymal weight, sperm production (estimated from testicular homogenization-resistant spermatid heads), disruptions to spermiation (retained spermatid heads in the seminiferous tubules), and apoptotic germ cells. BPA also did not disrupt sperm mRNA levels or DNA methylation. Unfortunately, this CLARITY-BPA study did not include any positive control groups treated with EE, making it difficult to evaluate the sensitivity of the measurements or the outcomes. Since only the continuous BPA exposures were assessed and not the stop-dose group, we cannot directly compare these findings to an independent CLARITY-like study where exposure to 25 μg BPA/kg BW/day from GD7 to PND22 decreased epididymal sperm counts Furthermore, as some estrogenic effects on male reproduction are best observed beyond young adulthood [127], it would have been useful to evaluate the rats at the one year necropsy to determine whether BPA exposures affected sperm endpoints throughout adulthood.
RESULTS OF THE DRAFT CORE STUDY:
Here, we will focus on the effects of BPA that were observed at the three lowest doses in the CLARITY core study [128]; additional effects were observed at higher doses as well, but the seriousness of the adverse outcomes disrupted at these three lowest doses is telling (see Table 2). At the lowest dose, 2.5 μg/kg/day, females that were exposed only during early development displayed increased incidence of mammary adenocarcinoma and abnormal infiltration of the liver with mononuclear cells at 1 year of age and an increased incidence of renal tubule cysts at 2 years; males displayed an increased incidence of pancreas pigmentation at 2 years of age. In animals that were continuously exposed, there was an increased incidence of cysts in the kidney renal tubule in females at 1 year of age; increased chronic inflammation of the dorsal and lateral prostate and decreased liver weight in males at 1 year of age; increased kidney neuropathy in females at 2 years of age; and increased mammary alveolar dilation, and increased incidence of liver angiectasis in males at 2 years of age.
In animals exposed to 25 μg/kg/day during development only, males displayed decreased serum protein levels and total bile acids at 1 year of age. In animals that were continuously exposed to this dose, females had increased hemoglobin concentrations at 1 year of age; increased vaginal epithelial hyperplasia at 2 years of age; and at 2 years of age, males had increased hyperplasia of the transitional epithelium of the kidney and increased hyperplasia of the parathyroid gland.
Finally, exposure to 250 μg/kg/day during development increased thyroid ultimobranchal cysts in females at 2 years of age; increased spleen pigmentation in males at 1 year of age; and increased pituitary gland cysts, bone marrow hypercellularity, and lymphoid hyperplasia in the spleen of males at 2 years of age. Continuous exposure to this dose decreased eosinophil number and increased serum alkaline phosphatase levels in females at 1 year of age; decreased the percentage of blood cells that were eosinophils and increased infiltration of the liver with mononuclear cells in males at 1 year of age; increased the body weight of females starting at 96 weeks of age; and increased the incidence of cysts in the kidney renal tubule of males at 2 years of age.
In analysing the ~36 significant changes as a function of BPA exposures in the Core Study, 50% were found in only low-dose BPA exposure groups, 30% in only the 2 high-dose groups and 20% found with both low-and-high-dose exposures (Table 2). Although the authors of the draft core report dismiss these findings because they were seen in only one arm of the study (developmental exposure versus continuous exposure), or because they were only seen at the lower doses (e.g., indicative of non-monotonic responses), the number and severity of many of these endpoints with the majority occurring at low-dose exposures raises serious concern about the safety of BPA in the low dose range.
ANOTHER CLARITY-LIKE STUDY:
A previous guideline study of BPA was conducted at the National Food Institute of Denmark [129]. Wistar rats were gavaged with one of four BPA doses (25, 250, 5000 or 50,000 μg/kg/day) from GD 7 through lactational day 22; this period is similar to the stop-dose group in the CLARITY-BPA study, except that the postnatal treatment continued to be given to the mother rather than the pup. Like CLARITY, the Danish study also included additional non-guideline endpoints including evaluations of behaviours (sugar preference, spatial learning ability) [130], male reproductive outcomes (sperm count) [130], and mammary growth parameters (male epithelial outgrowths, female mammary intraductal hyperplasias) [131].
Remarkably, the authors found that male offspring in the lowest dose group (25 μg/kg/day) had significantly fewer sperm and larger mammary gland epithelial trees compared to controls. Female offspring from this dose group had masculinized neurobehaviours (in spatial learning and preference for sugar) and increased body weights in later life (9–13 months of age). Females exposed to 250 μg/kg/day also had increased mammary intraductal hyperplasias. These effects were observed without seeing changes to ‘core’ outcomes like organ weight. The adverse effects seen in the Danish study are mostly consistent with what was observed in CLARITY-BPA, with the exception of sperm outcomes, increasing confidence in these findings. Considering their results, the Danish study authors concluded that the European reference dose of 4 μg/kg/day is not sufficiently protective [130]. (The US reference dose is even higher: 50 μg/kg/day.)
CONCLUSIONS:
Many datasets within the CLARITY-BPA study have documented effects of BPA in the low-dose range, often at the lowest dose provided, i.e. 2.5 μg/kg BW/day and frequently in the stop-dose cohorts, specifically suggesting heightened sensitivity to developmental exposures. For carcinoma risk, heightened mammary cancer incidence and prostate cancer multiplicity were uniquely seen in the cohort developmentally exposed to 2.5 μg/kg BW/day, which is cause for great concern. These low-dose effects were observed not only in the Academic Studies as highlighted herein, but in the Core Study itself; significant effects on mammary cancer, prostatic inflammation, kidney cysts and increased body weight within the Core Study are especially notable. It is also noteworthy that many of these CLARITY outcomes have been observed across multiple previous studies, including similar low-dose effects on neurobehaviours that have been observed both in CLARITY and in the Danish study, effects on sexually dimorphic brain regions that have been observed in CLARITY and across numerous prior academic studies, and enhanced prostatic carcinogenic susceptibility observed in CLARITY and previous academic laboratories.
There are numerous outcomes, across different organ systems that were disrupted in animals exposed to 2.5, 25 and 250 μg BPA/kg/day. If these effects at the lowest doses were observed less frequently or limited to single system, their importance could be questioned. However, when results from the Core study and Academic studies published thus far are combined, we identify a total of 75 endpoints with significant BPA effects noted relative to controls (Table 2). Of those, 51% appear only in low-dose groups (at or below 250 μg/kg BW/day), 21% in high-dose only rats (2,500 and 25,000 μg/kg BW/day) and 27% across both low and high-dose exposure groups. Thus, the large majority of identified effects are observed in low-dose exposed animals. This is best visualized using a heat-map approach (Fig. 2) where BPA effects are found across a range of doses with a preponderance in the three low-dose groups.These results are particularly noteworthy in the context of several limitations of the study design (summarized in Table 1).
As discussed in the Introduction, BPA effects are known to be mediated through multiple cellular receptors and signalling pathways [14, 15]. As BPA engagement of these multiple receptors/pathways occurs at widely different doses (low-dose for membrane receptor pathways; high-dose activation/antagonism for nuclear receptors), one would expect to observe non-linear BPA effects across increasing doses [132], as is seen in a considerable number of endpoints in the CLARITY-BPA studies. Further, expression of the different receptors varies widely across organs and cell types which can lead to dose-selective responses in different organs within the same animals. This argues strongly against dismissing significant BPA effects observed at single doses or in non-monotonic patterns and, in fact, supports the patterns observed thus far as scientifically sound.
Collectively, the CLARITY results highlighted herein contribute to and strongly corroborate a significant body of evidence that documents adverse effects of BPA at low doses. Additional studies are anticipated from the academic participants over the next year. As academic investigators who have long studied BPA, we propose that the cumulative results of the CLARITY study provide solid documentation of BPA effects in the low-dose range, below the current NOAEL, and conclude that the TDI dose is not reasonably protective of public health.
Acknowledgments
This study is part of the National Institute of Environmental Health Sciences (NIEHS) CLARITY-BPA Consortium supported by NIEHS grant U01 ES020886 (GSP), U011ES020929 (HBP), R03ES023098 (SMB). LNV was supported by NIEHS Award Number K22ES025811 and was not involved in the CLARITY-BPA Consortium. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank Dr. Rebecca Smith and Lynn Birch at the University of Illinois with assistance in graphics.
Footnotes
Presented, in part, at the PPTOX-VI meeting in Torshavn, Faroe Islands, 28–30 May 2018
REFERENCES
- 1.Birnbaum LS, Bucher JR, Collman GW, Zeldin DC, Johnson AF, Schug TT, et al. Consortium-based science: The NIEHS’s multipronged, collaborative approach to assessing the health effects of Bisphenol A. Environ Health Perspect. 2012;120:1640–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corrales J, Kristofco LA, Steele WB, Yates BS, Breed CS, Williams ES, et al. Global Assessment of Bisphenol A in the Environment: Review and Analysis of Its Occurrence and Bioaccumulation. Dose-Response. 2015;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Envir Hlth Prospect. 2008;116:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geens T, Aerts D, Berthot C, Bourguignon JP, Goeyens L, Lecomte P, et al. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem Toxicol. 2012;50:3725–40. [DOI] [PubMed] [Google Scholar]
- 5.Covaci A, Den Hond E, Geens T, Govarts E, Koppen G, Frederiksen H, et al. Urinary BPA measurements in children and mothers from six European member states: Overall results and determinants of exposure. Environmental research. 2015;141:77–85. [DOI] [PubMed] [Google Scholar]
- 6.Vandenberg LN, Hunt PA, Myers JP, Vom Saal FS. Human exposures to bisphenol A: mismatches between data and assumptions. Rev Environ Health. 2013;28:37–58. [DOI] [PubMed] [Google Scholar]
- 7.Balakrishnan B, Henare K, Thorstensen EB, Ponnampalam AP, Mitchell MD. Transfer of bisphenol A across the human placenta. Am J Obsetr Gynecol. 2010;202:e1–7. [DOI] [PubMed] [Google Scholar]
- 8.Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect. 2010;118:1055–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerona RR, Woodruff TJ, Dickenson CA, Pan J, Schwartz JM, Sen S, et al. Bisphenol-A (BPA), BPA glucuronide, and BPA sulfate in midgestation umbilical cord serum in a northern and central California population. Environmental science & technology. 2013;47:12477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Padmanabhan V, Siefert K, Ransom S, Johnson T, Pinkerton J, Anderson L, et al. Maternal bisphenol-A levels at delivery: a looming problem? J Perinatology. 2008;28:258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nahar MS, Liao C, Kannan K, Dolinoy DC. Fetal liver bisphenol A concentrations and biotransformation gene expression reveal variable exposure and altered capacity for metabolism in humans. J Biochem Mol Toxicol. 2013;27:116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reif DM, Martin MT, Tan SW, Houck KA, Judson RS, Richard AM, et al. Endocrine profiling and prioritization of environmental chemicals using ToxCast data. Environ Health Perspect. 2010;118:1714–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zoeller RT, Brown TR, Doan LL, Gore AC, Skakkebaek NE, Soto AM, et al. Endocrine-disrupting chemicals and public health protection: a statement of principles from the Endocrine Society. Endocrinology. 2012;153:4097–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Acconcia F, Pallottini V, Marino M. Molecular Mechanisms of Action of BPA. Dose-Response. 2015;13:1559325815610582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patisaul HB. Endocrine Disruption of Vasopressin Systems and Related Behaviors. Frontiers in endocrinology. 2017;8:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rochester JR. Bisphenol A and human health: A review of the literature. Reprod Toxicol. 2013;42C:132–55. [DOI] [PubMed] [Google Scholar]
- 17.Chapin RE, Adams J, Boekelheide K, Gray LE Jr., Hayward SW, Lees PS, et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res B Dev Reprod Toxicol. 2008;83:157–395. [DOI] [PubMed] [Google Scholar]
- 18.vom Saal FS, Akingbemi BT, Belcher SM, Birnbaum LS, Crain DA, Eriksen M, et al. Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod Toxicol. 2007;24:131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocrine reviews. 2015;36:E1–E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vandenberg LN, Ehrlich S, Belcher SM, Ben-Jonathan N, Dolinoy DC, Hugo ER, et al. Low dose effects of Bisphenol A: An integrated review of in vitro, laboratory animal and epidemiology studies. Endocrine Disruptors. 2013;1:e25078. [Google Scholar]
- 21.Myers JP, vom Saal FS, Akingbemi BT, Arizono K, Belcher S, Colborn T, et al. Why public health agencies cannot depend upon ‘Good Laboratory Practices’ as a criterion for selecting data: the case of bisphenol-A. Environ Health Perspect. 2009;117:309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tyl RW, Myers CB, Marr MC, Thomas BF, Keimowitz AR, Brine DR, et al. Three-generation reproductive toxicity study of dietary bisphenol A in CD Sprague-Dawley rats. Toxicol Sci. 2002;68:121–46. [DOI] [PubMed] [Google Scholar]
- 23.Tyl RW, Myers CB, Marr MC, Sloan CS, Castillo NP, Veselica MM, et al. Two-generation reproductive toxicity study of dietary bisphenol A in CD-1 (Swiss) mice. Toxicol Sci. 2008;104:362–84. [DOI] [PubMed] [Google Scholar]
- 24.Stump DG, Beck MJ, Radovsky A, Garman RH, Freshwater LL, Sheets LP, et al. Developmental neurotoxicity study of dietary bisphenol A in Sprague-Dawley rats. Toxicol Sci. 2010;115:167–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delclos KB, Camacho L, Lewis SM, Vanlandingham MM, Latendresse JR, Olson GR, et al. Toxicity evaluation of bisphenol A administered by gavage to Sprague Dawley rats from gestation day 6 through postnatal day 90. Toxicol Sci. 2014;139:174–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tyl RW. Basic exploratory research versus guideline-compliant studies used for hazard evaluation and risk assessment: bisphenol A as a case study. Environ Health Perspect. 2009;117:1644–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myers JP, Zoeller RT, vom Saal FS. A clash of old and new scientific concepts in toxicity, with important implications for public health. Environ Health Perspect. 2009;117:1652–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maffini MV, Vandenberg LN. Closing the gap: Improving additives safety evaluation to reflect human health concerns. Envirin Risk Assess Remediation. 2017;1:26–33. [Google Scholar]
- 29.Schug TT, Heindel JJ, Camacho L, Delclos KB, Howard P, Johnson AF, et al. A new approach to synergize academic and guideline-compliant research: the CLARITY-BPA research program. Reprod Toxicol. 2013;40:35–40. [DOI] [PubMed] [Google Scholar]
- 30.Heindel JJ, Newbold RR, Bucher JR, Camacho L, Delclos KB, Lewis SM, et al. NIEHS/FDA CLARITY-BPA research program update. Reprod Toxicol. 2015;58:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vandenberg LN. Low-dose effects of hormones and endocrine disruptors. Vitamin Horm. 2014;94:129–65. [DOI] [PubMed] [Google Scholar]
- 32.McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88:91–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–36. [DOI] [PubMed] [Google Scholar]
- 34.Wallen K Hormonal influences on sexually differentiated behavior in nonhuman primates. Frontiers in neuroendocrinology. 2005;26:7–26. [DOI] [PubMed] [Google Scholar]
- 35.McCarthy MM. Sex differences in the developing brain as a source of inherent risk. Dialogues in clinical neuroscience. 2016;18:361–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao J, Patisaul HB. Sexually dimorphic expression of hypothalamic estrogen receptors alpha and beta and Kiss1 in neonatal male and female rats. J Comp Neurol. 2011;519:2954–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao J, Patisaul HB. Sex specific expression of estrogen receptors alpha and beta and kiss1 in the postnatal rat amygdala. J Comp Neurol. 2013;521:465–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao J, Joyner L, Mickens JA, Leyrer SM, Patisaul HB. Sex-specific Esr2 mRNA expression in the rat hypothalamus and amygdala is altered by neonatal bisphenol A exposure. Reproduction. 2014;147:537–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rissman EF, Wersinger SR, Fugger HN, Foster TC. Sex with knockout models: behavioral studies of estrogen receptor alpha. Brain research. 1999;835:80–90. [DOI] [PubMed] [Google Scholar]
- 40.Ogawa S, Lubahn DB, Korach KS, Pfaff DW. Behavioral effects of estrogen receptor gene disruption in male mice. Neurobiology. 1997;94:1476–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choleris E, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW, Ogawa S. An estrogen-dependent four-gene micronet regulating social recognition: a study with oxytocin and estrogen receptor-alpha and -beta knockout mice. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:6192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choleris E, Devidze N, Kavaliers M, Pfaff DW. Steroidal/neuropeptide interactions in hypothalamus and amygdala related to social anxiety. Prog Brain Res. 2008;170:291–303. [DOI] [PubMed] [Google Scholar]
- 43.Wolstenholme JT, Rissman EF, Connelly JJ. The role of bisphenol A in shaping the brain, epigenome and behavior. Horm Behav. 2011;59:296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patisaul HB, Fortino AE, Polston EK. Neonatal genistein or bisphenol-A exposure alters sexual differentiation of the AVPV. Neurotoxicology and teratology. 2006;28:111–8. [DOI] [PubMed] [Google Scholar]
- 45.Patisaul HB, Sullivan AW, Radford ME, Walker DM, Adewale HB, Winnik B, et al. Anxiogenic effects of developmental bisphenol a exposure are associated with gene expression changes in the juvenile rat amygdala and mitigated by soy. PLoS ONE. 2012;7:e43890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCaffrey KA, Jones B, Mabrey N, Weiss B, Swan SH, Patisaul HB. Sex specific impact of perinatal bisphenol A (BPA) exposure over a range of orally administered doses on rat hypothalamic sexual differentiation. NeuroToxicology. 2013;36:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nesan D, Sewell LC, Kurrasch DM. Opening the black box of endocrine disruption of brain development: Lessons from the characterization of Bisphenol A. Hormones and behavior. 2018;101:50–8. [DOI] [PubMed] [Google Scholar]
- 48.Cao J, Mickens JA, McCaffrey KA, Leyrer SM, Patisaul HB. Neonatal Bisphenol A exposure alters sexually dimorphic gene expression in the postnatal rat hypothalamus. NeuroToxicology. 2012;33:23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cao J, Rebuli ME, Rogers J, Todd KL, Leyrer SM, Ferguson SA, et al. Prenatal bisphenol A exposure alters sex-specific estrogen receptor expression in the neonatal rat hypothalamus and amygdala. Toxicological sciences. 2013;133:157–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rebuli ME, Cao J, Sluzas E, Delclos KB, Camacho L, Lewis SM, et al. Investigation of the effects of subchronic low dose oral exposure to bisphenol A (BPA) and ethinyl estradiol (EE) on estrogen receptor expression in the juvenile and adult female rat hypothalamus. Toxicol Sci. 2014;140:190–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Churchwell MI, Camacho L, Vanlandingham MM, Twaddle NC, Sepehr E, Delclos KB, et al. Comparison of life-stage-dependent internal dosimetry for bisphenol a, ethinyl estradiol, a reference estrogen, and endogenous estradiol to test an estrogenic mode of action in sprague dawley rats. Toxicol Sci. 2014;139:4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Delclos KB, Camacho L, Lewis SM, Vanlandingham MM, Latendresse JR, Olson GR, et al. Toxicity evaluation of bisphenol A administered by gavage to Sprague Dawley rats from gestation day 6 through postnatal day 90. Toxicological sciences : an official journal of the Society of Toxicology. 2014;139:174–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rebuli ME, Camacho L, Adonay ME, Reif DM, Aylor DL, Patisaul HB. Impact of Low-Dose Oral Exposure to Bisphenol A (BPA) on Juvenile and Adult Rat Exploratory and Anxiety Behavior: A CLARITY-BPA Consortium Study. Toxicol Sci. 2015;148:341–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson SA, Javurek AB, Painter MS, Ellersieck MR, Welsh TH Jr., Camacho L, et al. Effects of developmental exposure to bisphenol A on spatial navigational learning and memory in rats: A CLARITY-BPA study. Horm Behav. 2016;80:139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arambula SE, Fuchs J, Cao J, Patisaul HB. Effects of perinatal bisphenol A exposure on the volume of sexually-dimorphic nuclei of juvenile rats: A CLARITY-BPA consortium study. Neurotoxicology. 2017;63:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arambula SE, Belcher SM, Planchart A, Turner SD, Patisaul HB. Impact of Low Dose Oral Exposure to Bisphenol A (BPA) on the Neonatal Rat Hypothalamic and Hippocampal Transcriptome: A CLARITY-BPA Consortium Study. Endocrinology. 2016;157:3856–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arambula SE, Jima D, Patisaul HB. Prenatal bisphenol A (BPA) exposure alters the transcriptome of the neonate rat amygdala in a sex-specific manner: a CLARITY-BPA consortium study. Neurotoxicology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheong A, Johnson SA, Howald EC, Ellersieck MR, Camacho L, Lewis SM, et al. Gene Expression and DNA Methylation Changes in the Hypothalamus and Hippocampus of Adult Rats Developmentally Exposed to Bisphenol A or Ethinyl Estradiol: A CLARITY-BPA Consortium Study. Epigenetics. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sullivan AW, Beach EC, Stetzik LA, Perry A, D’Addezio AS, Cushing BS, et al. A Novel Model for Neuroendocrine Toxicology: Neurobehavioral Effects of BPA Exposure in a Prosocial Species, the Prairie Vole (Microtus ochrogaster). Endocrinology. 2014;155:3867–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adewale HB, Todd KL, Mickens JA, Patisaul HB. The impact of neonatal bisphenol-A exposure on sexually dimorphic hypothalamic nuclei in the female rat. Neurotoxicology. 2011;32:38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wolstenholme JT, Edwards M, Shetty SR, Gatewood JD, Taylor JA, Rissman EF, et al. Gestational Exposure to Bisphenol A Produces Transgenerational Changes in Behaviors and Gene Expression. Endocrinology. 2012;153:3828–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grohe C, Kahlert S, Lobbert K, Vetter H. Expression of oestrogen receptor alpha and beta in rat heart: role of local oestrogen synthesis. J Endocrinol. 1998;156:R1–7. [DOI] [PubMed] [Google Scholar]
- 63.Belcher SM, Chen Y, Yan S, Wang HS. Rapid estrogen receptor-mediated mechanisms determine the sexually dimorphic sensitivity of ventricular myocytes to 17beta-estradiol and the environmental endocrine disruptor bisphenol A. Endocrinology. 2012;153:712–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Belcher SM, Gear RB, Kendig EL. Bisphenol A alters autonomic tone and extracellular matrix structure and induces sex-specific effects on cardiovascular function in male and female CD-1 mice. Endocrinology. 2015;156:882–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yan S, Chen Y, Dong M, Song W, Belcher SM, Wang HS. Bisphenol A and 17beta-estradiol promote arrhythmia in the female heart via alteration of calcium handling. PLoS ONE. 2011;6:e25455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patel BB, Kasneci A, Bolt AM, Di Lalla V, Di Iorio MR, Raad M, et al. Chronic Exposure to Bisphenol A Reduces Successful Cardiac Remodeling After an Experimental Myocardial Infarction in Male C57bl/6n Mice. Toxicological sciences : an official journal of the Society of Toxicology. 2015;146:101–15. [DOI] [PubMed] [Google Scholar]
- 67.Patel BB, Raad M, Sebag IA, Chalifour LE. Lifelong exposure to bisphenol a alters cardiac structure/function, protein expression, and DNA methylation in adult mice. Toxicol Sci. 2013;133:174–85. [DOI] [PubMed] [Google Scholar]
- 68.Belcher SM, Chen Y, Yan S, Wang HS. Rapid estrogen receptor-mediated mechanisms determine the sexually dimorphic sensitivity of ventricular myocytes to 17beta-estradiol and the environmental endocrine disruptor bisphenol a. Endocrinology. 2011;153:712–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gear RB, Belcher SM. Impacts of Bisphenol A and Ethinyl Estradiol on Male and Female CD-1 Mouse Spleen. Scientific reports. 2017;7:856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kendig EL, Buesing DR, Christie SM, Cookman CJ, Gear RB, Hugo ER, et al. Estrogen-like disruptive effects of dietary exposure to bisphenol A or 17alpha-ethinyl estradiol in CD1 mice. International journal of toxicology. 2012;31:537–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kendziorski JA, Belcher SM. Strain-specific induction of endometrial periglandular fibrosis in mice exposed during adulthood to the endocrine disrupting chemical bisphenol A. Reproductive toxicology. 2015;58:119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kendziorski JA, Kendig EL, Gear RB, Belcher SM. Strain specific induction of pyometra and differences in immune responsiveness in mice exposed to 17α-ethinyl estradiol or the endocrine disrupting chemical bisphenol A. Reproductive Toxicology. 2012;34:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gear R, Kendziorski JA, Belcher SM. Effects of bisphenol A on incidence and severity of cardiac lesions in the NCTR-Sprague-Dawley rat: A CLARITY-BPA study. Toxicol Lett. 2017;275:123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rai V, Sharma P, Agrawal S, Agrawal DK. Relevance of mouse models of cardiac fibrosis and hypertrophy in cardiac research. Molecular and Cellular Biochemistry. 2016:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am J Physiol Heart Circ Physiol. 2007;293:29. [DOI] [PubMed] [Google Scholar]
- 76.Jokinen MP, Lieuallen WG, Boyle MC, Johnson CL, Malarkey DE, Nyska A. Morphologic aspects of rodent cardiotoxicity in a retrospective evaluation of National Toxicology Program studies. Toxicologic pathology. 2011;39:850–60. [DOI] [PubMed] [Google Scholar]
- 77.Kemi M, Keenan KP, McCoy C, Hoe CM, Soper KA, Ballam GC, et al. The relative protective effects of moderate dietary restriction versus dietary modification on spontaneous cardiomyopathy in male Sprague-Dawley rats. Toxicologic pathology. 2000;28:285–96. [DOI] [PubMed] [Google Scholar]
- 78.Chanut F, Kimbrough C, Hailey R, Berridge B, Hughes-Earle A, Davies R, et al. Spontaneous cardiomyopathy in young Sprague-Dawley rats: evaluation of biological and environmental variability. Toxicologic pathology. 2013;41:1126–36. [DOI] [PubMed] [Google Scholar]
- 79.Rubin ZA, Areceo RJ, Bishop SP, Kerns WD, Mesfin GM, Sandusky GE, editors. Nonproliferative lesions of the heart and vasculature in rats Guides for Toxicologic Pathology; 2000; Washington D.C.: STP/ARP/AFIP. [Google Scholar]
- 80.Jokinen MP, Lieuallen WG, Johnson CL, Dunnick J, Nyska A. Characterization of spontaneous and chemically induced cardiac lesions in rodent model systems: the national toxicology program experience. Cardiovascular toxicology. 2005;5:227–44. [DOI] [PubMed] [Google Scholar]
- 81.Bassett JR, Cairncross KD. Morphological changes induced in rats following prolonged exposure to stress. Pharmacology Biochemistry and Behavior. 1975;3:411–20. [DOI] [PubMed] [Google Scholar]
- 82.Baker S, Rees S, Chebli M, LeMarec N, Godbout R, Huta V, et al. Effects of gestational stress: 2. Evaluation of male and female adult offspring. Brain research. 2009;1302:194–204. [DOI] [PubMed] [Google Scholar]
- 83.Baker S, Chebli M, Rees S, LeMarec N, Godbout R, Bielajew C. Effects of gestational stress: 1. Evaluation of maternal and juvenile offspring behavior. Brain research. 2008;1213:98–110. [DOI] [PubMed] [Google Scholar]
- 84.Rajfer J, Coffey DS. Sex steroid imprinting of the immature prostate. Investigative Urology. 1978;16:186–90. [PubMed] [Google Scholar]
- 85.Zondek T, Mansfield MD, Attree SL, Zondek LH. Hormone levels in the fetal and neonatal prostate. Acta Endocrinologica. 1986;112:447–56. [DOI] [PubMed] [Google Scholar]
- 86.Prins GS, Birch L, Habermann H, Chang WY, Tebeau C, Putz O, et al. Influence of neonatal estrogens on rat prostate development. Reproductive Fertility Development. 2001;13:241–52. [DOI] [PubMed] [Google Scholar]
- 87.Ho SM, Tang WY, Belmonte de Frausto J, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer research. 2006;66:5624–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Prins GS, Ye SH, Birch L, Ho SM, Kannan K. Serum bisphenol A pharmacokinetics and prostate neoplastic responses following oral and subcutaneous exposures in neonatal Sprague-Dawley rats. Reprod Toxicol. 2011;31:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tang WY, Morey LM, Cheung YY, Birch L, Prins GS, Ho SM. Neonatal exposure to estradiol/bisphenol A alters promoter methylation and expression of Nsbp1 and Hpcal1 genes and transcriptional programs of Dnmt3a/b and Mbd2/4 in the rat prostate gland throughout life. Endocrinology. 2012;153:42–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Prins GS, Hu WY, Shi GB, Hu DP, Majumdar S, Li G, et al. Bisphenol A promotes human prostate stem-progenitor cell self-renewal and increases in vivo carcinogenesis in human prostate epithelium. Endocrinology. 2014;155:805–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wong RL, Wang Q, Trevino LS, Bosland MC, Chen J, Medvedovic M, et al. Identification of secretaglobin Scgb2a1 as a target for developmental reprogramming by BPA in the rat prostate. Epigenetics. 2015;10:127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Q, Trevino LS, Wong RL, Medvedovic M, Chen J, Ho SM, et al. Reprogramming of the Epigenome by MLL1 Links Early-Life Environmental Exposures to Prostate Cancer Risk. Molecular endocrinology. 2016;30:856–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cheong A, Zhang X, Cheung YY, Tang WY, Chen J, Ye SH, et al. DNA methylome changes by estradiol benzoate and bisphenol A links early-life environmental exposures to prostate cancer risk. Epigenetics. 2016;11:674–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Prins GS, Ye SH, Birch L, Zhang X, Cheong A, Lin H, et al. Prostate Cancer Risk and DNA Methylation Signatures in Aging Rats following Developmental BPA Exposure: A Dose-Response Analysis. Environ Health Perspect. 2017;125:077007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vermeulen A, Kaufman JM, Goemaere S, van Pottelberg I. Estradiol in elderly men. Aging Male. 2002;5:98–102. [PubMed] [Google Scholar]
- 96.Bosland MC, Ford H, Horton L. Induction at high incidence of ductal prostate adenocarcinomas in NBL/Cr and Sprague-Dawley Hsd:SD rats treated with a combination of testosterone and estradiol-17β or diethylstilbestrol. Carcinogenesis. 1995;16:1311–7. [DOI] [PubMed] [Google Scholar]
- 97.Hu WY, Shi GB, Lam HM, Hu DP, Ho SM, Madueke IC, et al. Estrogen-initiated transformation of prostate epithelium derived from normal human prostate stem-progenitor cells. Endocrinology. 2011;152:2150–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Prins GS, Hu WY, Xie L, Shi GB, Hu DP, Birch L, et al. Evaluation of Bisphenol A (BPA) exposures on prostate stem cell homeostasis and prostate cancer risk in the NCTR-Sprague-Dawley rat: An NIEHS/FDA CLARITY-BPA Consortium Study Environmental Health Perspectives 2018;in revision. [DOI] [PMC free article] [PubMed]
- 99.Tomasetti C, Li L, Vogelstein B. Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention. Science (New York, NY). 2017;355:1330–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Goldstein AS, Huang J, Guo C, Garraway IP, Witte ON. Identification of a cell of origin for human prostate cancer. Science (New York, NY). 2010;329:568–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hunt PA, Lawson C, Gieske M, Murdoch B, Smith H, Marre A, et al. Bisphenol A alters early oogenesis and follicle formation in the fetal ovary of the rhesus monkey. Proc Natl Acad Sci U S A. 2012;109:17525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hunt PA, Koehler KE, Susiarjo M, Hodges CA, Ilagan A, Voigt RC, et al. Bisphenol a exposure causes meiotic aneuploidy in the female mouse. Curr Biol. 2003;13:546–53. [DOI] [PubMed] [Google Scholar]
- 103.Susiarjo M, Hunt P. Bisphenol A exposure disrupts egg development in the mouse. Fertil Steril. 2008;89:e97. [DOI] [PubMed] [Google Scholar]
- 104.Susiarjo M, Hassold TJ, Freeman E, Hunt PA. Bisphenol A Exposure In Utero Disrupts Early Oogenesis in the Mouse. PLoS Genetics. 2007;3:e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rodriguez HA, Santambrosio N, Santamaria CG, Munoz-de-Toro M, Luque EH. Neonatal exposure to bisphenol A reduces the pool of primordial follicles in the rat ovary. Reprod Toxicol. 2010;30:550–7. [DOI] [PubMed] [Google Scholar]
- 106.Rivera OE, Varayoud J, Rodriguez HA, Munoz-de-Toro M, Luque EH. Neonatal exposure to bisphenol A or diethylstilbestrol alters the ovarian follicular dynamics in the lamb. Reprod Toxicol. 2011. [DOI] [PubMed] [Google Scholar]
- 107.Karavan JR, Pepling ME. Effects of estrogenic compounds on neonatal oocyte development. Reprod Toxicol. 2012;34:51–6. [DOI] [PubMed] [Google Scholar]
- 108.Zhang HQ, Zhang XF, Zhang LJ, Chao HH, Pan B, Feng YM, et al. Fetal exposure to bisphenol A affects the primordial follicle formation by inhibiting the meiotic progression of oocytes. Mol Biol Rep. 2012;39:5651–7. [DOI] [PubMed] [Google Scholar]
- 109.Allard P, Colaiacovo MP. Bisphenol A impairs the double-strand break repair machinery in the germline and causes chromosome abnormalities. Proc Natl Acad Sci U S A. 2010;107:20405–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brieno-Enriquez MA, Robles P, Camats-Tarruella N, Garcia-Cruz R, Roig I, Cabero L, et al. Human meiotic progression and recombination are affected by Bisphenol A exposure during in vitro human oocyte development. Hum Reprod. 2011;26:2807–18. [DOI] [PubMed] [Google Scholar]
- 111.Patel S, Brehm E, Gao L, Rattan S, Ziv-Gal A, Flaws JA. Bisphenol A Exposure, Ovarian Follicle Numbers, and Female Sex Steroid Hormone Levels: Results From a CLARITY-BPA Study. Endocrinology. 2017;158:1727–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Peretz J, Vrooman L, Ricke WA, Hunt PA, Ehrlich S, Hauser R, et al. Bisphenol a and reproductive health: update of experimental and human evidence, 2007–2013. Environ Health Perspect. 2014;122:775–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Peretz J, Flaws JA. Bisphenol A down-regulates rate-limiting Cyp11a1 to acutely inhibit steroidogenesis in cultured mouse antral follicles. Toxicol Appl Pharmacol. 2013;271:249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Robinson L, Miller R. The Impact of Bisphenol A and Phthalates on Allergy, Asthma, and Immune Function: a Review of Latest Findings. Current environmental health reports. 2015;2:379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.O’Brien E, Bergin IL, Dolinoy DC, Zaslona Z, Little RJ, Tao Y, et al. Perinatal bisphenol A exposure beginning before gestation enhances allergen sensitization, but not pulmonary inflammation, in adult mice. Journal of developmental origins of health and disease. 2014;5:121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Petzold S, Averbeck M, Simon JC, Lehmann I, Polte T. Lifetime-dependent effects of bisphenol A on asthma development in an experimental mouse model. PLoS One. 2014;9:e100468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nakajima Y, Goldblum RM, Midoro-Horiuti T. Fetal exposure to bisphenol A as a risk factor for the development of childhood asthma: an animal model study. Environ Health. 2012;11:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Roy A, Bauer SM, Lawrence BP. Developmental exposure to bisphenol A modulates innate but not adaptive immune responses to influenza A virus infection. PLoS ONE. 2012;7:e38448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hessel EV, Ezendam J, van Broekhuizen FA, Hakkert B, DeWitt J, Granum B, et al. Assessment of recent developmental immunotoxicity studies with bisphenol A in the context of the 2015 EFSA t-TDI. Reprod Toxicol. 2016;65:448–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li J, Bach A, Crawford RB, Phadnis-Moghe AS, Chen W, D’Ingillo S, et al. CLARITY-BPA: Effects of chronic Bisphenol A exposure on the immune system: Part 1 - Quantification of the relative number and proportion of leukocyte populations in the spleen and thymus. Toxicology. 2018;396–397:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li J, Bach A, Crawford RB, Phadnis-Moghe AS, Chen W, D’Ingillo S, et al. CLARITY-BPA: Effects of chronic bisphenol A exposure on the immune system: Part 2 - Characterization of lymphoproliferative and immune effector responses by splenic leukocytes. Toxicology. 2018;396–397: 54–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sheng ZG, Zhu BZ. Low concentrations of bisphenol A induce mouse spermatogonial cell proliferation by G protein-coupled receptor 30 and estrogen receptor-alpha. Environ Health Perspect. 2011;119:1775–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hulak M, Gazo I, Shaliutina A, Linhartova P. In vitro effects of bisphenol A on the quality parameters, oxidative stress, DNA integrity and adenosine triphosphate content in sterlet (Acipenser ruthenus) spermatozoa. Comp Biochem Physiol C Toxicol Pharmacol. 2013;158:64–71. [DOI] [PubMed] [Google Scholar]
- 124.Sheng ZG, Huang W, Liu YX, Zhu BZ. Bisphenol A at a low concentration boosts mouse spermatogonial cell proliferation by inducing the G protein-coupled receptor 30 expression. Toxicol Appl Pharmacol. 2013;267:88–94. [DOI] [PubMed] [Google Scholar]
- 125.Manfo FP, Jubendradass R, Nantia EA, Moundipa PF, Mathur PP. Adverse effects of bisphenol A on male reproductive function. Reviews of environmental contamination and toxicology. 2014;228:57–82. [DOI] [PubMed] [Google Scholar]
- 126.Dere E, Anderson LM, Huse SM, Spade DJ, McDonnell-Clark E, Madnick SJ, et al. Effects of continuous bisphenol A exposure from early gestation on 90day old rat testes function and sperm molecular profiles: A CLARITY-BPA consortium study. Toxicol Appl Pharmacol. 2018;347:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hess RA, Bunick D, Lee KH, Bahr J, Taylor JA, Korach KS, et al. A role for oestrogens in the male reproductive system. Nature. 1997;390:509–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.NTP. Draft NTP Research Report on the CLARITY-BPA Core Study: A Perintala and Chronic Extended-Dose-Range Study of Bisphenol A in Rats. National Toxicology Program Research Report. 2018;9:1–249. [PubMed] [Google Scholar]
- 129.Vandenberg LN, Prins GS. Clarity in the face of confusion: new studies tip the scales on bisphenol A (BPA). Andrology. 2016;4:561–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hass U, Christiansen S, Boberg J, Rasmussen MG, Mandrup K. Low-dose effect of developmental bisphenol A exposure on sperm count and behaviour in rats. 2016;4:594–607. [DOI] [PubMed] [Google Scholar]
- 131.Mandrup K, Boberg J, Isling LK, Christiansen S, Hass U. Low-dose effects of bisphenol A on mammary gland development in rats. Andrology. 2016;4:673–83. [DOI] [PubMed] [Google Scholar]
- 132.Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr., Lee DH, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012;33:378–455. [DOI] [PMC free article] [PubMed] [Google Scholar]


