Abstract
Purpose.
We assessed sex- and age-dependent differences in a cross-sectional analysis of cardiac autonomic nervous system (ANS) regulation during sleep in adolescents.
Methods.
Nocturnal heart rate (HR) and heart rate variability (HRV) metrics, reflecting ANS functioning, were analyzed across the night and within undisturbed rapid-eye movement (REM) and non-REM (NREM) sleep in 149 healthy adolescents (12–22y; 67 female) from the National Consortium on Alcohol and Neurodevelopment in Adolescence (NCANDA).
Results.
Nocturnal HR was slower in older, more pubertally-advanced boys than younger boys. In girls, HR did not vary according to age or maturity, although overall HRV and vagal modulation declined with age. While younger boys and girls had similar HR, the male-female HR difference increased by ~2.4bpm every year (p<.01). Boys and girls showed expected increases in total HRV across the night but this within-night “recovery” was blunted in girls compared to boys (p<.05). Also, the NREM-REM difference in HR was greater in girls (p<.01). Models exploring a role of covariates (sleep, mood, reproductive hormones, activity) in influencing HR and HRV showed few significant effects, apart from sedentary activity (higher in older girls), which partially mediated the sex × age interaction in HR.
Conclusions.
Sex-related differences in cardiac ANS function emerge during adolescence. The extent to which sex-age divergences in ANS function are adaptive or reflect underlying sex-specific vulnerability for the development of psychopathology and other health conditions in adolescence needs to be determined.
Keywords: Heart rate variability, saliva hormones, physical activity, health, polysomnographic sleep
INTRODUCTION
Adolescence is a period of dramatic development across physiological systems including the autonomic nervous system (ANS). Abnormal ANS function is implicated in the etiology of several physical and mental conditions (1) that may emerge in adolescence (2). Thus, effective functioning of the ANS is fundamental for maintaining an individuals’ mental and physical health. Understanding normal maturation of the ANS, and how behavioral and biological changes in adolescence affect its development, is crucial for timely identification of deviating trajectories in ANS function.
Cardiac ANS function is non-invasively assessed by analyzing heart rate (HR) and its beat-to-beat fluctuations, i.e. heart rate variability (HRV) (3). High frequency (HF) HRV is a well-accepted measure of vagal activity assessed in time (root mean square of differences between adjacent inter-beat intervals, RMSSD) and frequency (HF power within 0.15–0.40 Hz power spectrum) domains, reflecting HR variation with respiration (3). High resting HR, reduced total HRV (reflected by frequency-domain total power [TP] within the 0.03–0.40 Hz power spectrum and time-domain standard deviation of inter-beat intervals [SDNN]), and reduced HF HRV are all associated with cardiovascular (CV) risk and mortality (1, 3). Lower HRV is also associated with mood and anxiety disorders, and is a risk factor for psychopathology in adults and adolescents (1, 3, 4).
Recording HRV during sleep allows the investigation of changes in ANS function across the night, reflecting ANS recovery (5), and assessment of changes between non-rapid eye movement (NREM) and REM sleep, reflecting sleep stage-dependent ANS reactivity. NREM sleep is characterized by reduced HR and vagal dominance compared to REM sleep, in which CV and ANS activity is comparable to wakefulness (6). Sleep as a period of analysis for HRV has the advantage of being relatively free from external wake-related disruptive events that may affect HRV (7), allowing a stable measure of basal ANS tone (8).
HRV decreases with age but the effect of aging on HRV is not linear during the life span (9, 10). There is extensive literature about cardiac ANS control in healthy adults but less is known about ANS control within adolescence (10). HR progressively slows across childhood and adolescence (11) and HRV peaks in late childhood (between 15–18 years), and then declines with advancing age (10–12). Age-related ANS maturation is evident during sleep (13–15) and is hypothesized to reflect enhanced efficiency of neural integrative mechanisms (11). It remains unclear whether there are sex differences in ANS development (10). Few studies have focused on adolescence and findings are mixed, indicating higher HR in girls than boys (9, 16, 17), age-dependent sex differences in HR (18), or no sex difference (19). Similarly, some studies report lower HRV in girls (9, 20, 21), age-dependent sex differences in HRV (22), or no sex differences in HRV (11, 19). Sex differences in HR and HRV measures are evident in adults (23), with women having a faster HR, lower total HRV, and greater vagal activity than men but when these differences emerge is not clear. This state of relative vagal dominance in adult women (despite a higher resting HR) could protect them against CV disease (23).
Hormonal factors (along with anatomical and genetic factors) are implicated in sex differences in ANS and CV function (24). Estrogen affects electrophysiological properties of the myocardium (25) and sex steroids increase cardiovagal baroreflex sensitivity (26, 27). Sex differences in the hormonal milieu during puberty and differences in pubertal timing (starts later in boys), therefore, may impact the ANS over-and-above age in adolescents. Sex and age differences in ANS functioning could also be mediated by differences in activity or anthropometric factors, which change across adolescence, such as sleep (28), body composition and lifestyle factors (10). For example, sleep duration (29), fitness and/or physical activity (30) are associated with ANS activity in healthy children and adolescents, and obesity in children is associated with reduced vagal functioning (10). Substance use is also related to a poor ANS profile in adolescents (31).
The current cross-sectional study aims to investigate sex- and age-dependent differences in HRV measures in a large sample of healthy adolescents (12–22y) participating in the National Consortium on Alcohol and Neurodevelopment in Adolescence (NCANDA). HRV measures were obtained during undisturbed NREM and REM sleep, and across hours of the night. We hypothesized that older age and advanced pubertal development would be associated with a slower HR and that girls would have a faster HR than boys overall. We also investigated the influence of sleep, reproductive hormones, body mass index, depressive symptoms, physical and sedentary activity, and alcohol exposure, on HRV.
METHODS
Participants
149 healthy adolescents participated in a sleep component of the multi-site longitudinal NCANDA study at SRI International (N=119) and University of Pittsburgh (N=30). Details about NCANDA methodology (32) and the sleep project (28) are described elsewhere. Sample characteristics are from data-release version: NCANDA_DATA_00010_V2. The study was approved by Institutional Review Boards at both sites. Adult participants consented to participate and minors provided written assent along with consent from a parent/legal guardian.
Participants had an in-person visit that included weight and height measurements (body mass index was calculated and converted into a body-mass-for-age percentile according to U.S. reference standards) and validated questionnaires, including: Pubertal Development Scale (PDS) (33), a self-report measure of pubertal stage; National Youth physical activity and nutrition survey (NYPANS), to assess self-reported physical and sedentary activity, Pittsburgh Sleep Quality Index (PSQI), to assess perceived sleep quality, and Center for Epidemiologic Studies Depression Scale (CESD) to assess depression symptoms. Twenty-six adolescents exceeded baseline alcohol and drug use criteria for no-to-low exposure (see 32). Participant characteristics are reported in Table 1. Participants were free from severe medical conditions, current/past DSM-IV Axis-I disorders, and sleep disorders, assessed by an in-lab clinical polysomnographic (PSG) evaluation. None of the participants was using medication known to affect sleep (e.g. hypnotics) or the CV system (e.g. antihypertensives).
Table 1.
Demographic characteristics and self-report measures of pubertal development, physical and sedentary activity, sleep quality and depressive symptoms.
| Females Mean (SD) |
±95%CI | Males Mean (SD) |
±95%CI | |
|---|---|---|---|---|
| Sample, No. | 67 | - | 82 | - |
| Age, y | 15.7 (2.4) | 15.2–16.3 | 15.4 (2.1) | 14.9–15.9 |
| Pubertal development, PDS scores a | 3.4 (0.7) | 3.2–3.5 | 2.8 (0.7) | 2.7–2.9 |
| Girls post-menarche, % | 82.0 | - | - | - |
| Ethnicity | ||||
| ▪ Caucasian, % | 68.7 | - | 72.0 | - |
| ▪ Asian, % | 16.4 | - | 20.7 | - |
| ▪ African-American, % | 14.9 | - | 7.3 | - |
| Exceeds baseline drinking criteria, % b | 22.4 | - | 13.4 | - |
| Current smokers (>1 cigarette per week), % | 0 | - | 0 | - |
|
Body mass index ▪ Absolute, Kg.m−2 ▪ Percentile - Obese (≥95th percentile), % - Overweight (85th to <95th percentile), % - Healthy Weight (5th to <85th percentile), % - Underweight (<5th percentile), % |
21.9 (5.2) 56.3 (28.9) 10.4 10.4 73.1 6.0 |
20.7–23.2 49.2–63.3 - - - - |
21.8 (4.5) 59.6 (26.7) 9.8 14.6 73.2 2.4 |
20.8–22.8 53.8–65.5 - - - - |
| Daily Activity c | ||||
| ▪ N days in past 7 days of at least 60 min of physical activity | 3.3 (1.9) | 2.9–3.8 | 4.1 (1.9) | 3.6–4.5 |
| ▪ N hours of sedentary screen-time activity (e.g., watching TV, computer games) on an average school day | 3.0 (1.5) | 2.7–3.4 | 3.0 (1.3) | 2.6–3.3 |
| Sleep quality, PSQI total score d | 4.3 (2.4) | 3.7–4.9 | 3.7 (2.2) | 3.3–4.2 |
| ▪ Exceeded cut-off for poor sleep quality (PSQI ≥5), % | 34.8 | - | 28.4 | - |
| Depressive symptoms, CESD total score e | 7.0 (6.6) | 5.3–8.7 | 5.7 (3.8) | 4.8–6.6 |
| ▪ Exceeded subthreshold depressive symptoms (CESD ≥16), % | 13.3 | - | 1.4 | - |
Pubertal Development Scale (PDS), missing in one female and four males;
defined in Brown and colleagues [32]. Exceeds group reported median [25th, 75th percentile] of 5.5 [2.75, 17.75] days in the past year on which they consumed alcohol and a median of 1 [1.0, 3.0] binge drinking episodes (>4 drinks for girls, >5 drinks for boys) in the past year. Compared with no/low users (medians of 0 for both these measures);
determined by the National Youth physical activity and nutrition survey (NYPANS),missing in three females and 10 males;
Pittsburgh Sleep Quality Index (PSQI), missing in one female and one male;
Center for Epidemiologic Studies Depression Scale (CESD), missing in seven females and 8 males.
All but eight participants had a clinical/adaptation night a few nights before the PSG recording night. Girls who were post-menarche were studied irrespective of menstrual cycle phase, however, only 5 girls (of 116 sampled) had saliva progesterone levels > 40 pg.ml−1, reflecting probable recordings in the luteal phase. Each night, a breath alcohol test (S75 Pro, BACtrack Breathalyzers) and urine drug test (10 Panel iCup drug test, Instant Technologies, Inc.) confirmed the absence of recent alcohol or drug use.
Sleep Assessment
PSG assessment was performed using Compumedics Grael® HD-PSG systems (Compumedics, Abbotsford, Victoria, Australia) and standard sleep metrics were calculated (see 28): time in bed (TIB, min), total sleep time (TST, min), wake after sleep onset (WASO, min), sleep efficiency (SE, %). Electroencephalographic (EEG) delta power (μV2.Hz−1, 0.3-<4Hz) at a frontal site (F3) in NREM sleep was also calculated (28).
Assessment of Autonomic Nervous System Functioning
Electrocardiograph (ECG) data were collected at 256 Hz using electrodes in a modified lead II Einthoven configuration. R-waves were automatically detected and manually checked, and normal-to-normal inter-beat intervals (IBIs, ms) calculated.
Frequency domain HRV analysis was performed on artifact-free, two-min bins of undisturbed NREM (N2+N3) and REM sleep selected throughout the night according to modified rules described in (34). Two-min bins had to be preceded by four epochs of the same sleep stage. HR, narrow absolute HF (HFna), total power (TP, 0.03–0.4 Hz), and peak frequency in the HF range (HFpf, Hz; measure of respiratory rate) were calculated separately for REM and NREM sleep (see 34). Differences in these variables between NREM and REM sleep were also calculated (ΔHR, ΔTP, ΔHFpf, and ΔHFna).
Time domain HRV analysis was performed on consecutive 5-min windows irrespective of awakenings/arousals or sleep stage changes across the night. Standard deviation of IBIs (SDNN), and RMSSD, were calculated and averaged across the first 7h of the night. Within-night slopes for SDNN and RMSSD were calculated from linear regression equations, representing the change in these variables as a function of time of night, reflecting both sleep and circadian influences (positive numbers reflect within-night increases, negative values reflect within-night decreases).
Reproductive Hormone Assessment
Passive-drool saliva samples were collected from 116 participants at the SRI site after waking. Samples were frozen and batch-analyzed (ZRT laboratories, Beaverton, OR) using liquid chromatography–tandem mass spectrometry. Testosterone, estradiol, and Dehydroepiandrosterone (DHEA) were analyzed in all participants. Progesterone was analyzed in girls. Limits of detection for testosterone, estradiol, DHEA, and progesterone were, respectively, 3.2pg.ml−1, 0.24pg.ml−1, 17.1pg.ml−1, 5.1pg.ml−1. The intra-assay coefficients of variation range from 3.4 to 8.5 % over the following concentrations: testosterone (9.8–83.5pg.ml−1); estradiol (0.7–2.5pg.ml−1); DHEA (35.6–567pg.ml−1); progesterone (22.2–93.2pg.ml−1).
Statistical analysis
Multivariate regression models were used to investigate age-, sex-, and age-by-sex dependent differences in HRV measures, including NREM-REM differentials and within-night slopes. Age was centered at the mean in all models. Alternative models used pubertal development scores instead of age. Models that included site, ethnicity and BMI percentile showed similar results and since none of these factors was significant, they were dropped from final models. Where there was a significant interaction, Pearson’s correlations were run separately in males and females to characterize the relationships in each sex.
Additional regression models based on frequency-domain HRV measures in NREM sleep were used to assess the influence on ANS measures of reproductive hormones (log-transformed to normalize the distribution), physical activity, sedentary activity, exceeding alcohol use criteria (yes/no), sleep (TST, WASO, EEG delta power), and depressive symptoms. Separate models were run with each factor along with sex, age, and sex-by-age interaction terms. Cases with missing values were dropped from the model. If a covariate was significant, follow-up mediation analysis using “binary mediation” in Stata was conducted to determine if that factor mediated the sex-by-age interaction effect, which was set as the independent variable, with sex and age as covariates, and the factors listed above as mediators. The mediation effect was tested using bootstrapping.
F-, p- and R2-values are provided for each significant model; t- and p-values, coefficients and their standard error (B [SE]) are provided for significant predictors. Analyses were performed using Stata/SE 14.1 for Windows.
RESULTS
ANS functioning during sleep
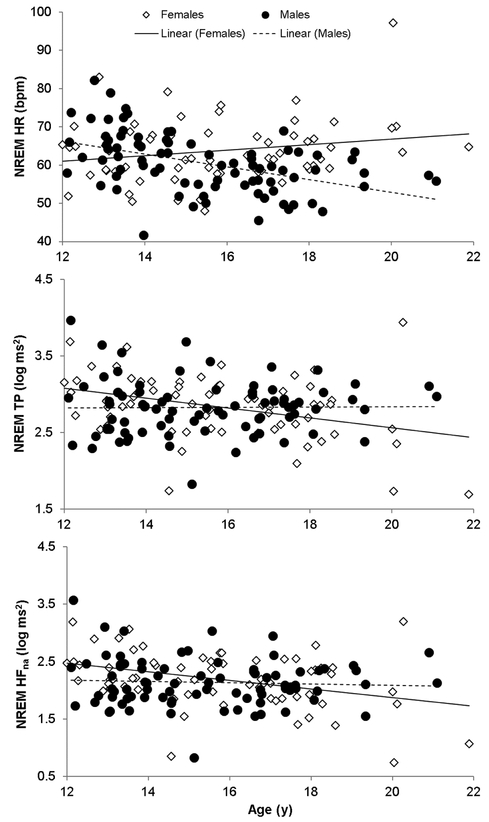
Models were significant for NREM HR (F3,145=9.84, p<.001, R2=.17), TP (F3,145=3.65, p=.014, R2=.07) and HFna (F3,145=3.97, p=.009, R2=.08). There was a sex × age interaction effect (B=1.19[.27], t=4.42, p<.001) for NREM HR, which was negatively related to age in males (r=−.48, p<.001), but unrelated to age in females (Figure 1). Consequently, NREM HR was similar in younger boys and girls, but progressively diverged between sexes with increasing age (the female-male difference in HR increased by, approximately, 2.4 bpm every year). There were sex × age interaction effects for TP (B=−.03[.01], t=−2.42, p=.017) and HFna (B=−.03[.02], t=−2.00, p=.048), which were both negatively related with age in females (TP: r=−0.36, p=.003; HFna: r=−0.37, p=.002) but unrelated to age in males (Figure 1).
Figure 1.
Relationships between age and heart rate (HR) and heart rate variability total power (TP) and narrow absolute high frequency (HFna) power in 82 male and 67 female adolescents, showing divergent patterns according to sex.
HFpf (reflecting respiration rate) was similar in boys (.26±.03 Hz) and girls (.26±.03 Hz) and showed no variation with age. The model that replaced age with PDS score was significant for HR (F3,145=6.79, R2=0.13, p<.001), with a significant interaction term (B =3.0[.92], t=3.27, p=.001). In boys, higher PDS scores (more mature) were associated with a lower HR (r=−.42, p<.001) whereas PDS scores and HR were unrelated in girls (r=.12, p=0.343). Models with PDS were not significant for TP or HFna.
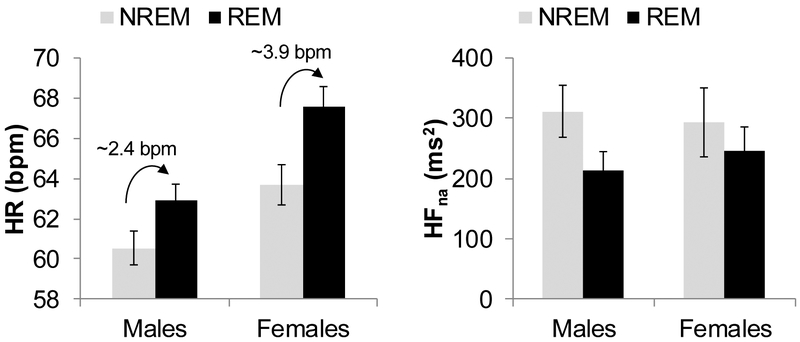
The model for the NREM-REM differential in HR was significant (F3, 145=2.83, p=.041, R2=.05), with a main effect of sex (B=.77[.27], t=2.84, p=.005): girls showed a greater NREM-REM difference in HR than did boys (Figure 2). Models for TP, HFna, and HFpf were not significant.
Figure 2.
Heart rate (HR) and heart rate variability narrow absolute high frequency power (HFna) in arousal-free rapid-eye-movement (REM) and non-REM (NREM) sleep in 82 male and 67 female adolescents. NREM-REM differences for HR are significantly greater in females (~3.9 bpm) compared to males (~2.4 bpm). Vertical bars represent standard errors.
Time-domain HRV analysis across the night produced similar sex-by-age interaction effects for HRV indices. The regression models were significant for SDNN (F3, 145=5.51, p=.001, R2=.10), and RMSSD (F3, 145=3.61, p=.015, R2=.07). Significant sex × age interaction effects for SDNN (B=−.02 [.01], t=−3.15, p=.002) and RMSSD (B=−.02 [.01], t=−2.66, p=.009) indicated that SDNN and RMSSD were negatively related with age in female (SDNN: r=−.33, p=.007; RMSSD: r=−.33, p=.006) but not male adolescents. Models that replaced age with PDS score for time domain HRV measures were not significant.
The regression model for within-night slope of SDNN was also significant (F3, 145=4.85, p=.003, R2=.09). While both male and female adolescents showed increases in SDNN across the night, the slope of the increase was greater in boys (sex effect: B=−.04[.02], t=−3.10, p=.002).
Factors influencing ANS function
Table 2 shows reproductive hormone levels in adolescents with saliva samples. Testosterone (r=.57, p<.001) and Estradiol (r=.35, p=.014) were positively related to age in boys, and DHEA levels were positively related to age in boys (r=.32, p=.009) and girls (r=.49, p=.001). In boys only, Testosterone correlated with NREM HR (r=−.34, p=.008), and HFna (r=−.28, p=.028). When Testosterone was added to the main regression models, it was no longer a significant factor for NREM HR, although, it was a marginally significant factor in the model predicting NREM HFna (F4, 106=5.06, p<.001, R2=.16; B=−.03[.16], t=−2.97, p=.049).
Table 2.
Average (SD) morning reproductive hormone levels in saliva in 53 female and 63 male adolescents.
| Females Mean (SD) |
±95%CI | Males Mean (SD) |
±95%CI | |
|---|---|---|---|---|
| Testosterone, pg.ml−1 | 8.7 (4.6) | 7.5–10.0 | 80.3 (43.5) | 69.4–91.3 |
| Undetectable (<3 pg.ml−1), No. | 2 | - | 2 | - |
| Estradiol, pg.ml−1 | 1.0 (0.9) | 0.8–1.3 | 0.4 (0.3) | 0.3–0.5 |
| Undetectable ( <0.3 pg.ml-)1, No. | 5 | - | 14 | - |
| DHEA, pg.ml−1 | 179.2 (115.8) | 147.0–211.5 | 133.8 (77.6) | 114.4–153.2 |
Dehydroepiandrosterone (DHEA).
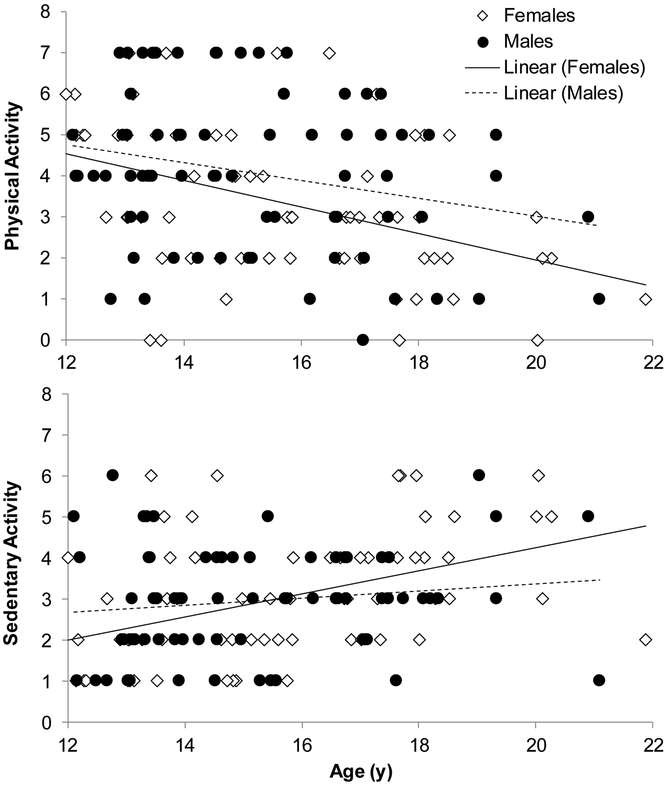
Physical activity correlated with age in boys (r=−.24, p=.04) and girls (r=−.42, p=.001), with older adolescents reporting fewer days per week of >60 min physical activity. Sedentary activity correlated positively with age in girls (r=.44, p<.001) but not in boys. Physical activity was not a significant factor in any models. Sedentary activity was a significant factor in the model predicting NREM HR (F4,131=8.70, p<.001, R2=.21; B=1.35[0.47], p=.004), with the model explaining 5% more variance than the basic model. Sedentary activity correlated with NREM HR in girls (r=.44, p<.001) but not in boys (Figure 3). Sedentary activity mediated 10.6% of the total effect of the sex-by-age interaction term on NREM HR (p=.050).
Figure 3.
Age-related differences in physical activity (number of days of at least 60 min of physical activity over the past week) and sedentary activity (number of hours of sedentary activity like time spent watching TV during a normal school day) across adolescence.
None of the other factors investigated was a significant factor in the regression models.
DISCUSSION
Age-dependent differences in HR and HRV were evident during sleep across the narrow age range of adolescence, with sex differences emerging in older adolescents, supporting the need for consideration of sex-specific analysis of HRV (23, 35). Boys showed an age-dependent reduction in HR across the adolescent period whereas girls did not and consequently, older girls had a faster HR than did boys, an effect that was also apparent when replacing age with pubertal status. Further, while we did not find a main effect of sex in total HRV or vagal-related HRV measures, girls but not boys showed an age-dependent reduction in total HRV and vagal activity. Most previous studies have not considered sex-by-age interaction effects during adolescence although overall sex differences have been reported. Some have reported higher HR and a lower HRV in female compared with male adolescents (20, 22). Although they did not report an age-by-sex interaction for HR, Hedger-Archbold and colleagues (17) found that average HR decreased by more than 10 bpm in males, whereas it decreased by less than 1 bpm in females from age 12 to age 16–17, consistent with our findings.
Differences among studies in age ranges, use of age as a continuous or categorical variable, period of analysis (ranging from minutes to 24h, and from wake to sleep), contribute to the conflicting findings. In a recent meta-analysis of studies including those in adolescents, Koenig and Thayer (23) found that females have a higher resting HR and lower total HRV but greater HF power, reflecting greater relative cardiac vagal dominance, compared to males. HRV measures are negatively correlated with age in adults (36) so vagal HRV measures that we found were relatively stable across adolescent years in boys may show a marked decreasing pattern in the adult years, in men.
Relationships between HR and HRV variables with age were not necessarily aligned: boys showed an age-dependent difference in HR but not in HRV measures and girls showed an age-dependent difference in HRV measures but not in HR. Possibly, the age-related decline in HR in boys is not attributed to a shift in increased vagal activity and rather reflects a reduction in sympathetic modulation of the sinus node in adolescent boys, as suggested elsewhere (22). Future work of specific sympathetic indicators is required to investigate this possibility. Changes in arterial baroreflex sensitivity (10, 11), cardiac volume relative to body size (10, 22), neural control of the heart (23), or behavioral and hormonal factors (10) could also contribute to age- and sex-dependent differences in HRV indices across adolescence.
Neither BMI nor self-reported physical activity influenced HRV in this group of mostly healthy-weight adolescents. However, sedentary activity partly mediated the age-by-sex interaction effect for HR, with higher sedentary activity related to a higher HR in girls only. A previous study of sleep-HRV measures in male and female adolescents (17) reported that physical activity explained much of the difference in sleeping HR between sexes. While we did not find an effect of physical activity, our results show that sedentary activity influences sleeping HR, particularly in girls, and could be worth investigating as a modifiable behavior to lower HR and reduce CV risk.
We investigated relationships between salivary reproductive hormones and HRV measures and found mostly null effects. In univariate analysis, testosterone was associated with higher HR and lower HFna in boys, but not in girls, and was marginally significant when added to the main model for HFna. There is limited work about the role of reproductive hormones in influencing ANS function, and none in adolescents, to our knowledge. Longitudinal investigations are needed to better understand whether reproductive hormones play a role in influencing ANS development during this period.
Sleep did not affect sex-by-age interaction effects for HRV measures, implying that sex differences in sleep that we previously reported (higher WASO in older boys) (28) did not mediate sex-age differences in HRV. However, we found novel sex differences in HRV measures within sleep. HRV changed across the night to a similar extent in older and younger adolescents but there was a greater increase in HRV (reflected by SDNN) across the night in boys than in girls. Reasons for this sex difference and relevance for understanding ANS–sleep relationships are unclear. Possibly, the stronger SDNN recovery across the night in boys reflects a sex difference in circadian regulation of the ANS. Heart rate and HRV measures are under sleep and circadian control (6) and evidence indicates that the circadian profile of these measures changes across the age span and differs between sexes (36). One study in adolescents reported a delay of about 1 h in the circadian curve of HRV HF in boys compared to girls, but no sex differences were evident for SDNN acrophase (37).
Girls had a stronger increase in HR in REM relative to NREM sleep compared with boys (see Figure 2), implying that they may have a more reactive cardiac response to sleep-dependent state changes. REM sleep is associated with increased sympathetic dominance and reduced vagal functioning, which drives the increase in HR. Interestingly, we previously found a blunted HR increase in association with K-complexes (single instance slow waves during sleep), suggesting lower ANS reactivity, in girls compared to boys (38). A more blunted response to K-complexes yet a magnified response to REM sleep in girls is intriguing and shows the complexity of the interaction between autonomic and central nervous systems during sleep. Further studies are needed to investigate sex differences in other aspects of ANS reactivity such as stress responses, relevant for understanding psychopathology, including disorders with a female preponderance such as insomnia (39) and depression.
Here, we used sleep as a prolonged, relatively stable period to analyze HRV (7), which allowed measurement of basal ANS functioning. Our HRV data were unlikely to be confounded by respiration (3) since we did not find sex or age differences in HFpf (indirectly reflecting respiration rate). Also, the use of “narrow bands” in calculating HRV frequency domain metrics (see (34)) reduces the likelihood of breathing influences on the calculation of HRV measures. While we did not conduct recordings in a specific menstrual cycle phase in girls, we confirmed that only 5 (of 116) girls had detectable progesterone levels consistent with the luteal phase.
A limitation of the current study is the lower age boundary of 12 years such that few participants, particularly girls, were in the early pubertal stages, and we may have missed some differences in ANS development in relation to puberty onset. Critically, adolescence is a dynamic period and future longitudinal analyses are required to confirm cross-sectional findings reported here. Longitudinal analysis would be particularly helpful in identifying mediating factors in sex- and age-dependent ANS divergences such as different timing in pubertal development. Further, longitudinal investigations of the onset of psychopathology will help define age- and sex-specific trajectories of risk using HRV as a biomarker.
Our results advance the understanding of normal ANS development across adolescence in boys and girls. Such information can aid the identification of pathological deviating ANS patterns, which may differ for boys and girls, as well as factors contributing to healthy ANS development, which could inform early life interventions to mitigate the development of mental and physical health conditions later in life. While poor ANS functioning is associated with many physical and mental disorders, it may also serve as a key focus for intervention because ANS function, and HRV specifically, can be improved with treatment.
Acknowledgments.
We would like to thank Devin Prouty for his study co-ordination, and Justin Greco, Lena Kardos, Rebecca Carr, David Sugarbaker, David Dresser, Stephanie Claudatos, Sarah Inkelis, and Sarah Gratzmiller for their effort in the data collection process. The content is solely the responsibility of the authors and does not necessarily represent the official views the National Institutes of Health.
Sources of Funding. This study was supported by the National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA); grants: AA021690 (DBC), AA021696 (IMC+FCB), AA021695 (AP and KMP) and AA021692 (SAB and SFT).
ABBREVIATION LIST
Heart rate variability measures
- IBIs
inter-beat intervals
- HF
high frequency
- HRV
heart rate variability
- LF
low frequency
- RMSSD
root mean square of differences between adjacent inter-beat intervals
- SDNN
standard deviation of inter-beat intervals
- TP
total power
Sleep-related measures
- NREM
non-rapid-eye movement
- PSG
polysomnography
- REM
rapid-eye movement
- SE
sleep efficiency
- TIB
time spent in bed
- TST
total sleep time
- WASO
wake after the sleep onset
Footnotes
Conflict of Interest. None.
REFERENCE LIST
- [1].Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol 2010;141:122–131. [DOI] [PubMed] [Google Scholar]
- [2].Jones P Adult mental health disorders and their age at onset. Br J Psychiatry Suppl 2013;54:s5–s1. [DOI] [PubMed] [Google Scholar]
- [3].Camm AJ, Malik M, Bigger J, et al. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996;93:1043–1065. [PubMed] [Google Scholar]
- [4].Koenig J, Kemp A, Beauchaine T, et al. Depression and resting state heart rate variability in children and adolescents-A systematic review and meta-analysis. Clin Psychol Rev 2016;46:136–150. [DOI] [PubMed] [Google Scholar]
- [5].de Zambotti M, Sugarbaker D, Trinder J, et al. Acute stress alters autonomic modulation during sleep in women approaching menopause. Psychoneuroendocrinology 2016;66:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Trinder J, Waloszek J, Woods M, et al. Sleep and cardiovascular regulation. Pflugers Arch 2012;463:161–168. [DOI] [PubMed] [Google Scholar]
- [7].Brandenberger G, Buchheit M, Ehrhart J, et al. Is slow wave sleep an appropriate recording condition for heart rate variability analysis? Auton Neurosci 2005;121:81–86. [DOI] [PubMed] [Google Scholar]
- [8].Orr WC, Elsenbruch S, Harnish MJ. Autonomic regulation of cardiac function during sleep in patients with irritable bowel syndrome. Am J Gastroenterol 2000;95:2865–2871. [DOI] [PubMed] [Google Scholar]
- [9].Umetani K, Singer D, McCraty R, et al. Twenty-four hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. J Am Coll Cardiol 1998;31:593–601. [DOI] [PubMed] [Google Scholar]
- [10].Eyre E, Duncan M, Birch S, et al. The influence of age and weight status on cardiac autonomic control in healthy children: A review. Auton Neurosci 2014;186:8–21. [DOI] [PubMed] [Google Scholar]
- [11].Lenard Z, Studinger P, Mersich B, et al. Maturation of cardiovagal autonomic function from childhood to young adult age. Circulation 2004;110:2307–2312. [DOI] [PubMed] [Google Scholar]
- [12].Kazuma N, Otsuka K, Wakamatsu K, et al. Heart rate variability in normotensive healthy children with aging. Clin Exp Hypertens 2002;24:83–89. [DOI] [PubMed] [Google Scholar]
- [13].Massin M, Maeyns K, Withofs N, et al. Circadian rhythm of heart rate and heart rate variability. Arch Dis Child 2000;83:179–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Michels N, Clays E, De Buyzere M, et al. Children’s Sleep and Autonomic Function: Low Sleep Quality Has an Impact on Heart Rate Variability. Sleep 2013;36:1939–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Archbold K, Johnson N, Goodwin J, et al. Normative heart rate parameters during sleep for children aged 6 to 11 years. J Clin Sleep Med 2010;6:47–50. [PMC free article] [PubMed] [Google Scholar]
- [16].Scholle S, Wiater A, Scholle H. Normative values of polysomnographic parameters in childhood and adolescence: cardiorespiratory parameters. Sleep Med 2011;12:988–996. [DOI] [PubMed] [Google Scholar]
- [17].Hedger-Archbold K, Sorensen S, Goodwin J, et al. Average Heart Rates of Hispanic and Caucasian Adolescents during Sleep: Longitudinal Analysis from the TuCASA Cohort. J Clin Sleep Med 2014;10:991–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Allen M, Matthews K. Hemodynamic responses to laboratory stressors in children and adolescents: the influences of age, race, and gender. Psychophysiology 1997;34:329–339. [DOI] [PubMed] [Google Scholar]
- [19].Goto M, Nagashima M, Baba R, et al. Analysis of heart rate variability demonstrates effects of development on vagal modulation of heart rate in healthy children. J Pediatr 1997;130:725–729. [DOI] [PubMed] [Google Scholar]
- [20].Faulkner M, Hathaway D, Tolley B. Cardiovascular autonomic function in healthy adolescents. Heart Lung 2003;32:10–22. [DOI] [PubMed] [Google Scholar]
- [21].Cysarz D, Linhard M, Edelhäuser F, et al. Unexpected Course of Nonlinear Cardiac Interbeat Interval Dynamics during Childhood and Adolescence. PLoS One 2011;6:e19400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Silvetti M, Drago F, Ragonese P. Heart rate variability in healthy children and adolescents is partially related to age and gender. Int J Cardiol 2001;81:169–174. [DOI] [PubMed] [Google Scholar]
- [23].Koenig J, Thayer J. Sex differences in healthy human heart rate variability: A meta-analysis. Neurosci Biobehav Rev 2016;64:288–310. [DOI] [PubMed] [Google Scholar]
- [24].Mercuro G, Deidda M, Piras A, et al. Gender determinants of cardiovascular risk factors and diseases. J Cardiovasc Med 2010;11:207–220. [DOI] [PubMed] [Google Scholar]
- [25].Rosano G, Leonardo F, Dicandia C, et al. Acute electrophysiologic effect of estradiol 17beta in menopausal women. Am J Cardiol 2000;86:1385–1387. [DOI] [PubMed] [Google Scholar]
- [26].El-Mas M, Afify E, Mohy E- DM, et al. Testosterone facilitates the baroreceptor control of reflex bradycardia: role of cardiac sympathetic and parasympathetic components. J Cardiovasc Pharmacol 2001;38:754–763. [DOI] [PubMed] [Google Scholar]
- [27].el-Mas M, Abdel-Rahman A. Estrogen enhances baroreflex control of heart rate in conscious ovariectomized rats. Can J Physiol Pharmacol 1998;76:381–386. [PubMed] [Google Scholar]
- [28].Baker F, Willoughby A, de Zambotti M, et al. Age-Related Differences in Sleep Architecture and Electroencephalogram in Adolescents in the National Consortium on Alcohol and NeuroDevelopment in Adolescence Sample. Sleep 2016;39:1429–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jarrin D, McGrath J, Poirier P. Autonomic dysfunction: a possible pathophysiological pathway underlying the association between sleep and obesity in children at-risk for obesity. J Youth Adolesc 2015;44:285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Blom EH, Olsson EM, Serlachius E, et al. Heart rate variability is related to self-reported physical activity in a healthy adolescent population. Eur J Appl Physiol 2009;106:877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Evans B, Greaves-Lord K, Euser A, et al. Alcohol and tobacco use and heart rate reactivity to a psychosocial stressor in an adolescent population. Drug Alcohol Depend 2012;126:296–303. [DOI] [PubMed] [Google Scholar]
- [32].Brown SA, Brumback T, Tomlinson K, et al. The National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA): A Multisite Study of Adolescent Development and Substance Use. J Stud Alcohol Drugs 2015;76:895–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Petersen AC, Crockett L, Richards M, et al. A self-report measure of pubertal status: Reliability, validity, and initial norms. J Youth Adolesc 1988;17:117–133. [DOI] [PubMed] [Google Scholar]
- [34].Trinder J, Kleiman J, Carrington M, et al. Autonomic activity during human sleep as a function of time and sleep stage. Journal of sleep research 2001;10:253. [DOI] [PubMed] [Google Scholar]
- [35].Michels N, Clays E, De Buyzere M, et al. Determinants and reference values of short-term heart rate variability in children. Eur J Appl Physiol 2013;113:1477–1488. [DOI] [PubMed] [Google Scholar]
- [36].Bonnemeier H, Richardt G, Potratz J, et al. Circadian profile of cardiac autonomic nervous modulation in healthy subjects: differing effects of aging and gender on heart rate variability. J Cardiovasc Electrophysiol 2003;14:791–799. [DOI] [PubMed] [Google Scholar]
- [37].Rodríguez-Colón S, He F, Bixler EO, et al. The Circadian Pattern of Cardiac Autonomic Modulation and Obesity in Adolescents. Clin Auton Res 2014;24:265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].de Zambotti M, Willoughby A, Franzen P, et al. K-complexes: interaction between the central and autonomic nervous systems during sleep. Sleep 2016;39:1129–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].de Zambotti M, Goldstone A, Colrain IM, et al. Insomnia disorder in adolescence: diagnosis, impact, and treatment. Sleep Med Rev IN PRESS. [DOI] [PMC free article] [PubMed] [Google Scholar]