Abstract
The trafficking of G protein coupled-receptors (GPCRs) is one of the most exciting areas in cell biology due to recent advances demonstrating that GPCR signaling is spatially encoded. GPCRs, acting in a diverse array of physiological systems, can have differential signaling consequences depending on their subcellular localization. At the plasma membrane, GPCR organization could fine-tune the initial stages of receptor signaling by determining the magnitude of signaling and the type of effectors to which receptors can couple. This organization is mediated by the lipid composition of the plasma membrane, receptor-receptor interactions, and receptor interactions with intracellular scaffolding proteins. GPCR organization is subsequently changed by ligand binding and the regulated endocytosis of these receptors. Activated GPCRs can modulate the dynamics of their own endocytosis through changing clathrin-coated pit dynamics, and through the scaffolding adaptor protein β-arrestin. This endocytic regulation has signaling consequences, predominantly through modulation of the MAPK cascade. This review explores what is known about receptor sorting at the plasma membrane, protein partners that control receptor endocytosis, and the ways in which receptor sorting at the plasma membrane regulates downstream trafficking and signaling.
Keywords: Traffic, Intracellular Transport, arrestins, microdomains, clathrin-coated pits, scaffolds, MAPK, PDZ, oligomers, dimers
Graphical Abstract

1. Introduction
The organization and trafficking of G protein-coupled receptors (GPCRs) at the cell membrane are major regulators of receptor signaling. Since many GPCRs primarily respond to extracellular ligands, a receptor’s ability to respond to signals depends on its physical presence at the cell membrane.1 The spatial organization of receptors at the cell membrane is tightly controlled, as reported by an increasing number of studies. Receptor organization is likely dictated by the biochemical properties of the receptors themselves,2 lipid composition of the membrane,3 and the presence of a host of scaffolding proteins,4 although the mechanisms are still being elucidated. Once receptors bind a ligand, receptors rapidly reorganize to specific domains within the plasma membrane, which could help coordinate spatially restricted signaling. 5,6 GPCRs are further regulated at the cell surface by agonist-mediated endocytosis.7 After activation, receptors are sorted to endocytic domains through binding to the adaptor protein β-arrestin.8,9 Many GPCRs continue to signal during endocytosis, making use of β-arrestin as a signaling scaffold.10,11 Some GPCRs appear to regulate their own endocytic rate through modulation of clathrin-coated pit maturation.12–14 This regulation, which can differ between ligands acting at the same GPCR, is an additional method by which GPCR signaling can be spatially encoded.
This review explores our currently emerging understanding of how receptors are organized on the membrane, and how this organization could regulate downstream trafficking and signaling. We focus specifically on basal receptor localization and agonist-dependent redistribution, as well as the mechanics of GPCR modulation of receptor-mediated endocytosis of mammalian GPCRs. We also highlight currently open questions in the field relating to how GPCR localization and trafficking at the plasma membrane has physiological significance. Although the principles discussed focus on GPCR signaling specifically, they are applicable to many other signaling receptors or transmembrane proteins whose functions depend on spatial localization.
2. Basal receptor localization and agonist-dependent redistribution
GPCR organization in the plasma membrane is driven through receptor-receptor, receptor-lipid, and receptor-protein interactions that restrict and regulate receptor movement within the plasma membrane (Figure 1). Most GPCRs begin their signaling lives at the plasma membrane, although some receptors are basally localized to intracellular sites such as the ER or the trans-Golgi network.15–17 Once delivered to the plasma membrane, the three types of receptor interactions described below help GPCRs localize to specialized membrane domains and to specialized structures such as the neuronal postsynaptic density,18 primary cilia,19 and the outer segment of photoreceptor cells.20
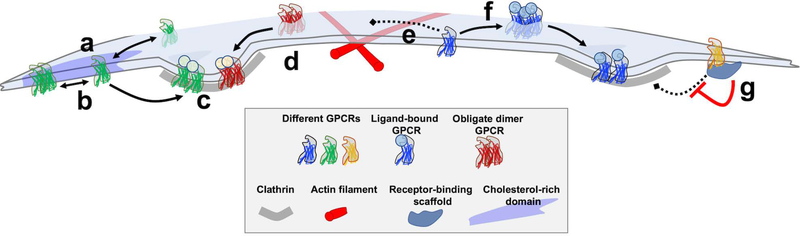
Figure 1:
GPCR organization at the plasma membrane is dynamic and regulated. a) Before agonist addition, many GPCRs exist at the plasma membrane as monomers. b) Through receptor-receptor interactions, some receptors dynamically exchange between monomeric and oligomeric states, with the degree of time a receptor spends in each of these states varying between different types of receptors. Receptors are enriched at cholesterol rich regions (darker blue) through receptor-lipid interactions, although they can diffuse between these domains and the surrounding membrane. c) After agonist addition, receptors cluster in clathrin coated pits (CCPs), regardless of their oligomeric state, but the rate at which a given receptor is sorted into CCPs can be variable. The gray bars denote the clathrin coat. d) Some receptors are obligate homodimers or heterodimers or higher order oligomers, existing always in these states. e) Receptor diffusion in the plasma membrane is restricted by actin and microtubule ‘fences’ (red rods) which confine receptors. f) Receptors can also cluster tightly together into domains that could mediate signaling after agonist addition prior to localizing to CCPs. g) Scaffolding proteins associate with and restrict the localization of certain GPCRs. Receptors bound to scaffolding proteins may be protected from endocytosis.
2.1. Receptor-Receptor Interactions
Despite a great deal of controversy over the past several decades, there is mounting evidence for the existence of semi-stable oligomeric GPCR complexes, as well as receptor-receptor interactions that drive receptor signaling and localization.21,22 Some of these interactions are stable and long-lasting,23,24 while some are transient and weak.25 These receptor-receptor interactions can produce homodimers of the same receptor,26 or heterodimers of two different receptors.27 Homodimerization is evolutionarily conserved. The yeast α-factor receptor (Ste2p) shows a significant tendency to dimerize,28,29 and dimerization of functional receptors might be required for receptor signaling 30. Dimeric receptor complexes can even couple to a single G protein or arrestin molecule, as demonstrated for the light-activated GPCR rhodopsin.31,32 Heteromers such as the μ/δ-opioid receptor dimer might couple to different effectors and induce functional effects distinct from their monomers.33–36 Receptors can oligomerize at multiple steps throughout the biosynthetic trafficking of GPCRs, with the ɣ-aminobutyric acid receptor type B (GABABR) requiring dimerization for ER export 37,38 whereas Ste2p dimerizes only at the plasma membrane.39
Receptor oligomerization regulates the diffusion of the receptors within the plasma membrane. The GABAB receptor, an obligate dimer,23,24 diffuses slowly within the plasma membrane and primarily exists as dimers and tetramers.40 The β1-adrenergic receptor (B1AR) exists predominantly as a monomer at the plasma membrane, and the closely related β2-adrenergic receptor splits its time roughly equally between monomeric and dimeric states.40 Receptor oligomerization is highly dynamic at physiological concentrations of receptor, as demonstrated by recent studies with the neurotensin receptor NTSR141 and rhodopsin42. Changes in diffusion rate may serve to change receptor-effector coupling, and GPCR-G protein complexes appear to diffuse much more rapidly than GPCRs alone.43
2.2. Receptor-Lipid Interactions
Receptor-lipid interactions can regulate receptor distribution at the plasma membrane, and potentially affect receptor signaling. Fluorescence correlation spectroscopy of the μ- and κ-opioid receptors (μOR, κOR) showed that these receptors are enriched in cholesterol-rich domains and tend to be excluded from ganglioside-rich plasma membrane domains.3 Some GPCRs contain a cholesterol binding site,44 and cholesterol has been a necessary additive to GPCR crystallization studies.45,46 Further evidence suggests that cholesterol is permissive of GPCR GEF activity at G proteins.47–49 Despite evidence showing a biochemical role of cholesterol in GPCR activity, the exact function of GPCRs residing at cholesterol-enriched membrane sites remains unknown. Since heterotrimeric G proteins can be differentially lipidated via palmitoylation, myristoylation, farnesolyation, and geranylgeranylation,50 it is possible that GPCR localization to different lipid microdomains could dictate coupling to different G proteins. Indeed, B2AR, which is predominantly coupled to Gαs,51 can couple to Gαi when restricted to lipid rafts.52 Receptor-lipid interactions could therefore sort GPCRs to membrane domains where receptors are best situated to signal through different G proteins or even interact with specific effectors or modifying enzymes, although this remains to be tested.
2.3. Receptor-Protein Interactions
GPCR interactions with cytoskeletal and signaling scaffolds regulate receptor organization at the plasma membrane. There are a multitude of known GPCR interacting proteins,4,53 but only a subset of these have been shown to participate directly in basal GPCR organization. GPCR localization to neuronal synapses has received particular attention. The localization of the metabotropic glutamate receptor 5 (mGluR5) is dependent on the scaffolding protein Homer.54 Another metabotropic glutamate receptor, mGluR7, is restricted to synapses through its interaction with the protein PICK1.55 This interaction is dependent on PICK1’s PSD95/Dlg/ZO-1 (PDZ) homology domain, which binds a PDZ ligand at the distal C-terminus of mGluR7. PSD95, another PDZ domain-containing protein, binds to the β1-adrenergic receptor (B1AR) and appears to increase surface expression of the GPCR.56 Many GPCRs feature PDZ ligands,57 and as these receptors are explored further in their native context in polarized cells it is likely many similar scaffolds to those described above will be discovered.
GPCR diffusion at the plasma membrane also appears to be regulated by the cortical cytoskeleton. A ‘fence and picket’ model of membrane organization previously proposed suggests that the diffusion of transmembrane protein ‘pickets’ is limited within differently sized membrane compartments that are demarcated by actin ‘fences’.58 Single molecule studies of μOR59 showed that receptors diffused in distinct membrane ‘compartments’, with straight line barriers that were assumed to be actin filaments. These early findings were recently reaffirmed when single molecule analysis of B2AR and the α2a-adrenergic receptor (A2AR) showed that these receptors avoid actin during their diffusion in the plasma membrane and that their diffusion is restricted to actin-bounded compartments.2
2.4. Agonist-Dependent Redistribution
Agonist binding and activation cause substantial reorganization of GPCRs from the basal state. Receptor reorganization includes receptor clustering, as well changes in receptor diffusion kinetics. For example, the μ opioid receptor (μOR) clusters upon activation with the endogenous-like ligand DAMGO, but not after treatment with the exogenous ligand morphine.6 DAMGO-dependent receptor clustering correlates with downstream signaling through the mitogen activated protein kinase (MAPK) cascade. Because of this MAPK activation, these receptor clusters have been suggested to be a specialized signaling domain. MAPK signaling from these putative signaling domains requires cholesterol at the plasma membrane, though whether receptor clustering independent of signaling requires cholesterol is not clear. Changes in receptor organization after agonist binding has been explored also at a single-molecule level. The Gαs-coupled B2AR and the Gαi-coupled A2AR do not change their diffusive behavior upon agonist activation, but their respective G proteins become more mobile.2 In this study, only a small fraction of both B2AR and A2AR molecules were shown to rapidly sort into clathrin-coated pits after agonist addition. In contrast to B2AR and A2AR, the Gαi/o-coupled metabotropic glutamate receptor mGluR3 (a class C GPCR with a much larger extracellular domain compared to the class A B2AR and A2AR) significantly slows its diffusion when bound to an agonist, and sees considerable redistribution into clathrin-coated pits after agonist treatment.43 The variability between GPCRs suggests that although there may be commonalities to how receptors behave immediately following agonist treatment, receptor redistribution patterns are worth investigating at the level of specific receptor types.
3. Agonist-mediated receptor endocytosis
The role and regulation of endocytosis - a well-known consequence of receptor activation - is currently being redefined in the field. Activated receptors are phosphorylated by G protein-receptor kinases, after which they recruit the adapter protein β-arrestin.1,60 β-arrestin binding sorts receptors into clathrin-coated pits – specialized endocytic domains on the plasma membrane.8,61,62 The traditional view of GPCR endocytosis (excellently reviewed previously63) was that it primarily served to desensitize receptors after agonist activation by removing them from the cell surface. Recent work has highlighted several novel aspects of GPCR endocytic trafficking: 1) GPCRs segregate to specialized endocytic domains, 2) GPCRs regulate the maturation of these endocytic domains, 2) this regulation has signaling consequences that differ both between receptors and between ligands acting at the same receptor. These recent advances, discussed below, are summarized in Figure 2.
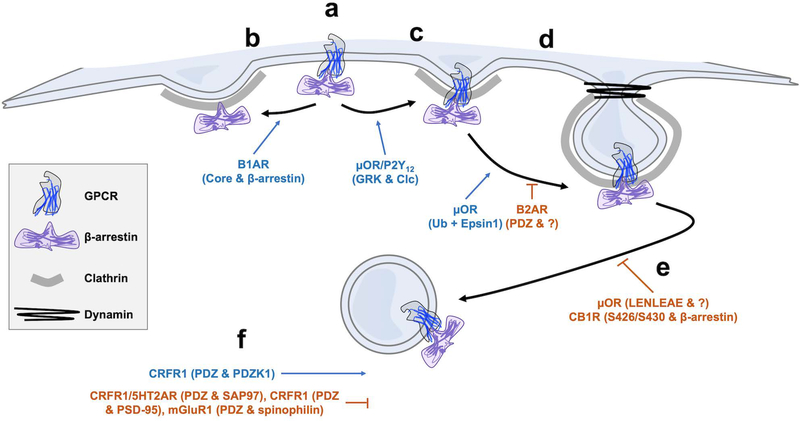
Figure 2:
GPCRs modulate endocytosis at distinct phases of the endocytic process. a) After ligand binding to a given receptor, β-arrestin is recruited to the receptor at the plasma membrane. b) In the case of B1AR, an interaction between β-arrestin and the B1AR core region causes β-arrestin to sort to clathrin-coated pits (CCPs) independent of the receptor. Other GPCRs sort with arrestin to CCPs. c) P2Y12 and μOR regulate clathrin light chain (CLC) phosphorylation through the activation of GPCR related kinases (GRKs) which is permissive of endocytosis continuing. d) After receptors are sorted into nascent CCPs, μOR is ‘proofread’ by Epsin1 to ensure that it is ubiquitinated before CCP maturation continues. At about the same phase, the PDZ ligand of B2AR delays recruitment of the GTPase dynamin through an unknown protein partner. e) After dynamin recruitment, μOR can delay dynamin-dependent scission through an unknown protein interacting with its C-terminal LENLEAE motif. CB1R, through an arrestin interaction mediated by two serines on its C-terminal tail, can also delay CCP lifetimes. f) Through as yet unknown mechanisms, GPCR interactions with PDZ domain containing proteins can globally upregulate (e.g. CRFR1 & PDZK1) or downregulate (e.g. mGluR1 & spinophilin) receptor internalization.
3.1. Segregation of GPCRs in endocytic domains
Understanding GPCR regulation of endocytosis requires a broad understanding of the steps of clathrin-mediated endocytosis (CME) that GPCR cargo may be able to regulate. Endocytic cargo, including GPCRs, are sorted into nascent clathrin-coated pits (CCPs) through interaction with adaptor proteins possessing both cargo- and clathrin-binding domains. Although adaptor protein 2 (AP2) is the canonical adaptor for a host of CME cargo, there are a variety of endocytic sorting signals and cognate adaptors that bind them.64 CCP maturation to an internalized vesicle is a highly regulated process.65,66 A maturing CCP proceeds through multiple ‘checkpoints’ before undergoing eventual dynamin-dependent scission.67,68 Adaptor proteins play a prominent role in implementing these checkpoints during CCP maturation.69,70
The diversity in both the number of cargoes internalized via CME as well as the adaptor proteins used for this internalization suggests that multiple routes for biochemically distinct CME pathways may exist. Different cargo sorting signals for CME are differentially saturable, indicating a variety of mechanisms for cargo association with clathrin.71 Overexpression of distinct cargoes – the transferrin receptor (TfR), the epidermal growth factor receptor (EGFR), and the low-density lipoprotein receptor (LDLR) – can saturate each cargo’s respective endocytosis, but do not interfere with the endocytosis of the other cargos.72,73 A straightforward explanation is that different cargoes recruit distinct adapters and endocytic accessory proteins to nascent CCPs. Consistent with this, the adaptor AP2 is required for the internalization of TfR but not EGFR.74 But the number of adaptors identified are far fewer than the number of potential cargoes. Alternatively, cargo could change the lipid environment in which CCPs form. For example, EGFR-positive CCPs form preferentially from cholesterol and sphingolipid rich membrane rafts, a phenomenon which does not appear to be conserved by other endocytic cargo.75
GPCRs can sort into specific subsets of CCPs. Activated B2AR and μOR are present only in a subset of the CCPs through which TfR endocytoses, both in fixed cell analyses and when visualizing endocytosis at the resolution of single scission events, although the extent of overlap between these cargo in the same CCPs has been variable across studies.12,76–78 Further, not all GPCRs sort to the same subset of pits. The purinergic receptors P2Y1 and P2Y12 localize to different CCP subsets, with P2Y12 internalizing in the same CCPs as B2AR while P2Y1 internalizes by a distinct clathrin-dependent pathway.79 GPCRs and β-arrestin clusters that form at the cell membrane in response to agonist colocalize with preexisting clathrin clusters,9 suggesting that GPCRs can cluster in a subset of extant CCPs as opposed to exclusively nucleating specialized new CCPs. GPCRs also show different biochemical requirements for endocytosis. Both P2Y12 and μOR require phosphorylated clathrin light chain for efficient clathrin-mediated endocytosis, whereas TfR does not.80,81 B2AR endocytosis is blocked at 16°C while TfR endocytosis is not.77 Segregation of different cargo into specific subsets of CCPs could allow individual control over clustering and endocytosis of different receptors.
3.2. Receptor Regulation of Endocytosis
Regardless of the degree to which CCPs specialize based on cargo, it is clear that GPCRs can regulate CCP dynamics 12,13,78. Although CCPs containing the δ opioid receptor (δOR) last ~40 seconds at the plasma membrane before undergoing dynamin-dependent scission, CCPs containing a chimeric δOR with B1AR’s C-terminal PDZ ligand last 3 times as long. CCPs containing B2AR, which has its own PDZ ligand, also last longer than CCPs containing δOR. PDZ ligands extend the duration of clathrin-coated pits (‘CCP lifetimes’) by delaying dynamin recruitment at the CCPs in which these receptors reside.12 However, work with μOR has shown that this is not the only mechanism by which GPCRs can regulate CCP lifetimes. μOR promotes long CCP lifetimes by delaying scission after dynamin recruitment.78 Despite differing mechanisms, both of these studies pinpointed receptor control of CCP lifetimes as being mediated through amino acid motifs in the C-termini of the identified receptors (PDZ ligands ‘DSLL’ or ‘ESKV’ for B2AR and db1, a unique ‘LENLEAE’ motif for μOR). How these sequences regulate CCP dynamics is not known. The dynamin-dependent scission at the end of vesicle maturation could be a point at which CME can be regulated. The protein kinase Src phosphorylates dynamin2 and the actin nucleating factor cortactin to be permissive of TfR endocytosis.82 The kinase GSK3B also regulates dynamin activity through inactivating phosphorylation of dynamin1.83 Interestingly, these kinases could be regulated by GPCRs themselves, providing a potential feedback mechanism for precise control of endocytic dynamics at the level of individual CCPs.
Different ligands acting at the same receptor have distinct effects on CCP lifetimes, pointing to the physiological relevance of CCP regulation. This was first shown with the cannabinoid receptor 1 (CB1R), where the exogenous ligand WIN 55,212–2 caused shorter CCP lifetimes than the endogenous ligand 2-arachidonoylglycerol (2-AG).13 Subsequent work with μOR revealed that the clinically relevant ligand morphine produced significantly shorter endocytic lifetimes compared to endomrophin-2, one of the receptor’s endogenous agonists.14 For both CB1R and μOR, longer receptor cluster lifetimes correlated with increased MAPK activation, suggesting that CCPs sustain a signaling complex that links receptors to MAPK, as discussed below.
GPCRs regulate endocytosis through their downstream signaling and interactions with scaffolding proteins. In the case of P2Y12 and μOR it is possible that activation of GPCR regulated kinases (GRKs) downstream of these receptors might regulate endocytosis in general by changing the phosphorylation state of clathrin light chain, but it remains unclear whether this phosphorylation plays a role in changing CCP lifetimes.80 When investigating protein partners that might mediate GPCR control of lifetimes, the PDZ ligands of the β-adrenoreceptors provide tantalizing targets given their requirement for lifetime extension, but no PDZ-domain containing partner has been identified that regulates CCP lifetimes for these receptors. However, a host of other GPCRs have been shown to have PDZ-dependent regulation of their endocytosis, although not specifically though regulating CCP lifetimes. The PDZ ligands of the serotonin 2A receptor (5HT2AR) and the corticotropin releasing factor receptor 1 (CRFR1) both bind the PDZ-domain containing protein synapse associated protein 97 (SAP97), and overexpression of SAP97 slowed global endocytic rate for both of these receptors.84,85 For CRFR1 alone, expression of the protein PDZK1 increased the receptor’s endocytic rate,86 while PSD-95 expression decreased the receptor’s endocytic rate87 in a manner consistent with the stabilizing effect PSD-95 has on B1AR.56 Another GPCR, the metabotropic glutamate receptor 1 (mGluR1), interacts with the PDZ-domain containing protein spinophilin and has subsequently decreased endocytosis. The parathyroid hormone receptor (PTHR) interacts with the Na/H-exchanger regulatory factor 1 (NHERF1) through a PDZ-domain dependent interaction, and NHERF1 inhibits internalization of PTHR following agonist treatment.88 These are only a handful of examples of PDZ-domain containing proteins known to regulate GPCR internalization. For all of these PDZ interactions, it has not yet been investigated whether these effects on endocytosis are mediated through extension of CCP lifetimes or through blocking receptors from sorting into CCPs in the first place, although published results with B2AR’s extended CCP lifetimes make this a tantalizing question.
3.3. Signaling Consequences of Receptor-Regulated Endocytosis
The signaling effects of GPCR-mediated extension of lifetimes are primarily mediated through β-arrestins. For CB1R, a receptor mutant that binds β-arrestin1 more strongly than the wild-type receptor also increases CCP lifetimes of the exogenous ligand WIN 55,212–2.89 These extended lifetime CCPs produce stronger MAPK activation downstream of β-arrestin as measured by phosphorylation of the MAP kinases ERK 1 & 2.13 Extended lifetimes downstream of endogenous agonists at μOR also serve to extend the duration of the receptor/arrestin interaction and to increase ERK1/2 activation.14 Recent work has shown that following activation of B1AR, β-arrestin can translocate to CCPs even in the absence of GPCR translocation. These arrestin-positive CCPs subsequently have significantly prolonged lifetimes and this results in increased ERK1/2 phosphorylation downstream of B1AR agonists.90 This discovery was extended by showing that GPCRs can act as β-arrestin activators without necessitating a stable interaction between the receptor and β-arrestin.91,92 This implicates GPCRs as not just cargo, but also regulators of protein trafficking themselves. All of the above findings fit with an emerging model whereby GPCRs affect multiple modes of β-arrestin function through interactions at functionally distinct sites.93,94 Notably, the dependence of ERK1/2 activation on lengthened endocytic lifetimes has so far been demonstrated only with receptors whose activation of MAPK is dependent on β-arrestin.
Several receptors that rely on PDZ domain-containing proteins to regulate their endocytosis show positive coupling between endocytosis and ERK1/2 activation. Studies with CRFR1 and 5HT2AR show that the PDZ-domain containing protein SAP97 slows the endocytic rate of these receptors while increasing their ligand-dependent ERK1/2 activation.84,85 However, the effects of PDZ-domain containing protein on receptor trafficking and signaling are not always so stereotyped. For example, PDZK1 overexpression increases CRFR1 ERK1/2 activation while having no effect on CRFR1 endocytosis, but this same overexpression increases 5HT2AR ERK1/2 activation while slowing 5HT2AR endocytosis.86 The uncoupling of ERK1/2 activation to endocytic lifetimes suggests that, at least for some receptors prolonged endocytic rate may serve a different role.
There is a dearth of evidence directly connecting endocytic lifetimes to specific protein components in the MAPK cascade. Studies exploring PDZ-dependent modulation of endocytosis and ERK1/2 have not demonstrated a direct interaction between any PDZ domain-containing scaffolds and components of the MAPK cascade.4,84–87,95,96 For some GPCRs, β-arrestin is required for connecting endocytic lifetimes to ERK1/2 activation. But the specific molecular mechanism through which β-arrestin controls ERK1/2 activation is not clear.13,14,90 Differences in endocytic lifetimes may also contribute to further downstream spatial encoding of GPCR signaling. The recent explosion in the study of GPCR endosomal signaling (recently reviewed97) opens up the exciting possibility that changes in receptor duration on the plasma membrane might affect trafficking at post-endocytic stages of GPCR trafficking.
The changes on the receptors that drive control of endocytic lifetimes are not clear. One potential mechanism through which endocytic lifetimes might regulate downstream trafficking is through regulating the phosphorylation state of GPCRs. GPCR phosphorylation changes in response to agonist, and receptor phosphorylation is a known regulator of GPCR trafficking and signaling. GPCRs are phosphorylated by many kinases including GRKs98–101 and PKA101–103. Receptor phosphorylation begins at the plasma membrane independent of endocytosis104, and at least some phosphorylation is present on receptors throughout post-endocytic trafficking.105,106 Receptor phosphorylation is important for post-endocytic trafficking107,108 and for endosomal receptor signaling109. Modulating GPCR ubiquitination is another potential target of endocytic lifetime regulation. Ubiquitination has been implicated in GPCR trafficking and signaling (reviewed by Trejo and colleagues in this same issue). The endocytosis of yeast GPCRs Ste2p and Ste3p depends on ubiquitination, after which they might recruit alpha arrestins or other unique adapters. Ubiquitination plays a prime role in the p38 signaling downstream of the protease activated receptor 1110. Ubiquitination is required for effective β-arrestin recruitment at the interleukin-8 chemokine receptor (CXCR2)111, and for regulating the dynamics of internalization of the μOR.112 The latter mechanism is mediated through a ubiquitin binding motif in the endocytic accessory protein Epsin1, suggesting that cells proofread receptor modification states before allowing the receptor to internalize.
In summary, GPCRs are not passive components in endocytic trafficking. Rather, they can control the dynamics of endocytic components. This control might modulate downstream signaling pathways including the MAPK pathway. It is possible that the endocytic lifetimes indirectly regulate the phosphorylation or ubiquitination states of receptors themselves, which in turn could regulate interactions of receptors with components of the endocytic pathway, although there is little evidence to support this model at present. The interplay between signaling and endocytic control is an area with tremendous potential that still needs to be understood better.
4. Concluding Remarks and Future Directions
In the past decade, we have learnt much about GPCR organization at the plasma membrane, but much still remains to be learned both with regards to the nature of basal organization, the mechanism of endocytic regulation, and the physiological effects of both. For example, while targets such as PDZ proteins and β-arrestins have been confirmed, we do not understand how these interactions modify CME. Further, ERK1/2 is the main signaling output that has been measured downstream of CCP regulation, but the physiological consequences of ERK1/2 activation remain unknown. Nevertheless, current work has uncovered the potential physiological and translational impact of spatial organization of signaling receptors and has underlined the importance of studying receptor trafficking. Another interesting aspect that still needs to be addressed is the contribution of GPCR reorganization and endocytic control on how different ligands acting at the same receptor can bias downstream signaling to different effector-driven pathways – a phenomenon termed biased agonism or functional selectivity.113 Although the correlation of endocytic lifetimes and arrestin-mediated activation of MAPK suggest a mechanism through which different agonists may produce bias, the exploration of how membrane organization relates to bias is still in its infancy. Learning how receptor partitioning into lipid domains regulates signaling, or how receptor oligomerization is regulated, may also be key to understanding the pleiotropy of signaling and mechanisms of bias.
The next frontier is to validate the importance of the mechanistic findings discussed to receptor physiology in vivo. Model cells, where receptors can be heterologously expressed, receptors and effectors specifically mutated or modified, and signaling outputs isolated, have been indispensable in understanding the fundamental principles of receptor organization and trafficking. Nevertheless, as we continue to use these models to tease out mechanistic details, a concomitant step is to move the study of receptor localization and function into physiologically relevant systems expressing endogenous receptors. Some of the receptor-lipid, receptor-receptor, and receptor-protein interactions have been demonstrated directly in primary cells of interest. At present, the degree of endocytic specialization in primary cells and the role of endocytic regulation is still not well understood. Newer advances in imaging and profiling receptor location and signaling and in inducible stem cells, as well as using animal models with cell-specific expression and gene-editing tools, provide exciting avenues for addressing the role of spatial organization in receptor physiology in vivo. As we continue to validate findings in specific physiological systems, we anticipate that this will open a new druggable proteome, allowing pharmaceutical targeting of trafficking factors to regulate the endogenous signaling of GPCRs that are important in physiology and disease.
Synopsis:
G protein-coupled receptors (GPCRs) have long been known to signal from the plasma membrane via multiple signaling pathways. GPCR organization at the plasma membrane plays a critical role in regulating the signaling consequences of receptor activation by regulating the interactions of receptors with specific effector proteins. GPCR organization can be regulated by dynamic association with proteins and membrane microdomains. Furthermore, GPCRs also regulate their own endocytic rate in a ligand- and receptor-dependent manner, with consequences for receptor signaling. This review explores our current understanding of basal organization of GPCRs in the plasma membrane and the functional consequences of this organization on GPCR endocytosis and signaling.
Acknowledgements
ZYW would like to thank Jennifer Kunselman and Lili Trifilio for essential feedback and comments. M.A.P. was supported by NIH GM117425 and NSF 1517776.
Footnotes
Conflict of interest: All authors on this work agree to its content and hereby declare no competing commercial interests relating to this submitted work.
References:
- 1.Hausdorff WP, Caron MG, Lefkowitz RJ. Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J. 1990;4(11):2881–2889. [PubMed] [Google Scholar]
- 2.Sungkaworn T, Jobin M-L, Burnecki K, Weron A, Lohse MJ, Calebiro D. Single-molecule imaging reveals receptor-G protein interactions at cell surface hot spots. Nature Publishing Group. 2017;550(7677):543–547. doi: 10.1038/nature24264. [DOI] [PubMed] [Google Scholar]
- 3.Rogacki MK, Golfetto O, Tobin SJ, et al. Dynamic lateral organization of opioid receptors (kappa, muwt and muN40D ) in the plasma membrane at the nanoscale level. Traffic. May 2018. doi: 10.1111/tra.12582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunn HA, Ferguson SSG. PDZ Protein Regulation of G Protein-Coupled Receptor Trafficking and Signaling Pathways. Molecular Pharmacology. 2015;88(4):624–639. doi: 10.1124/mol.115.098509. [DOI] [PubMed] [Google Scholar]
- 5.Zastrow von M, Kobilka BK. Antagonist-dependent and -independent steps in the mechanism of adrenergic receptor internalization. Journal of Biological Chemistry. 1994;269(28):18448–18452. [PubMed] [Google Scholar]
- 6.Halls ML, Yeatman HR, Nowell CJ, et al. Plasma membrane localization of the μ-opioid receptor controls spatiotemporal signaling. Sci Signal. 2016;9(414):ra16–ra16. doi: 10.1126/scisignal.aac9177. [DOI] [PubMed] [Google Scholar]
- 7.Wolfe BL, Trejo J. Clathrin-dependent mechanisms of G protein-coupled receptor endocytosis. Traffic. 2007;8(5):462–470. doi: 10.1111/j.1600-0854.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- 8.Goodman OB, Krupnick JG, Santini F, et al. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996;383(6599):447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 9.Santini F, Gaidarov I, Keen JH. G protein–coupled receptor/arrestin3 modulation of the endocytic machinery. The Journal of Cell Biology. 2002;156(4):665–676. doi: 10.1083/jcb.200110132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.ph.69.013107.100021. [DOI] [PubMed] [Google Scholar]
- 11.Peterson YK, Luttrell LM. The Diverse Roles of Arrestin Scaffolds in G Protein-Coupled Receptor Signaling. Michel MC, ed. Pharmacological Reviews. 2017;69(3):256–297. doi: 10.1124/pr.116.013367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puthenveedu MA, Zastrow von M. Cargo Regulates Clathrin-Coated Pit Dynamics. Cell. 2006;127(1):113–124. doi: 10.1016/j.cell.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 13.Flores-Otero J, Ahn KH, Delgado-Peraza F, Mackie K, Kendall DA, Yudowski GA. Ligand-specific endocytic dwell times control functional selectivity of the cannabinoid receptor 1. Nat Comms. 2014;5:4589. doi: 10.1038/ncomms5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinberg ZY, Zajac AS, Phan T, Shiwarski DJ, Puthenveedu MA. Sequence-Specific Regulation of Endocytic Lifetimes Modulates Arrestin-Mediated Signaling at the μ Opioid Receptor. Molecular Pharmacology. 2017;91(4):416–427. doi: 10.1124/mol.116.106633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grimsey NL, Graham ES, Dragunow M, Glass M. Cannabinoid Receptor 1 trafficking and the role of the intracellular pool: implications for therapeutics. Biochemical Pharmacology. 2010;80(7):1050–1062. doi: 10.1016/j.bcp.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Shiwarski DJ, Darr M, Telmer CA, Bruchez MP, Puthenveedu MA. PI3K class II α regulates δ-opioid receptor export from the trans-Golgi network. Mostov KE, ed. Molecular Biology of the Cell. 2017;28(16):2202–2219. doi: 10.1091/mbc.E17-01-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rozenfeld R, Devi LA. Regulation of CB1 cannabinoid receptor trafficking by the adaptor protein AP-3. FASEB J. 2008;22(7):2311–2322. doi: 10.1096/fj.07-102731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Møller TC, Wirth VF, Roberts NI, et al. PDZ domain-mediated interactions of G protein-coupled receptors with postsynaptic density protein 95: quantitative characterization of interactions. PLoS ONE. 2013;8(5):e63352. doi: 10.1371/journal.pone.0063352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schou KB, Pedersen LB, Christensen ST. Ins and outs of GPCR signaling in primary cilia. EMBO reports. 2015;16(9):1099–1113. doi: 10.15252/embr.201540530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pearring JN, Salinas RY, Baker SA, Arshavsky VY. Protein sorting, targeting and trafficking in photoreceptor cells. Prog Retin Eye Res. 2013;36:24–51. doi: 10.1016/j.preteyeres.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Devi LA. Heterodimerization of G-protein-coupled receptors: pharmacology, signaling and trafficking. Trends in Pharmacological Sciences. 2001;22(10):532–537. [DOI] [PubMed] [Google Scholar]
- 22.Ferré S, Casadó V, Devi LA, et al. G protein-coupled receptor oligomerization revisited: functional and pharmacological perspectives. Pharmacological Reviews. 2014;66(2):413–434. doi: 10.1124/pr.113.008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White JH, Wise A, Main MJ, et al. Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature. 1998;396(6712):679–682. doi: 10.1038/25354. [DOI] [PubMed] [Google Scholar]
- 24.Jones KA, Borowsky B, Tamm JA, et al. GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature. 1998;396(6712):674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- 25.Gavalas A, Lan TH, Liu Q, Corrêa IR, Javitch JA, Lambert NA. Segregation of Family A G Protein-Coupled Receptor Protomers in the Plasma Membrane. Molecular Pharmacology. 2013;84(3):346–352. doi: 10.1124/mol.113.086868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hébert TE, Bouvier M. Structural and functional aspects of G protein-coupled receptor oligomerization. Biochem Cell Biol. 1998;76(1):1–11. [DOI] [PubMed] [Google Scholar]
- 27.Jordan BA, Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399(6737):697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Overton MC, Blumer KJ. G-protein-coupled receptors function as oligomers in vivo. Current Biology. 2000;10(6):341–344. [DOI] [PubMed] [Google Scholar]
- 29.Gehret AU, Bajaj A, Naider F, Dumont ME. Oligomerization of the yeast alpha-factor receptor: implications for dominant negative effects of mutant receptors. Journal of Biological Chemistry. 2006;281(30):20698–20714. doi: 10.1074/jbc.M513642200. [DOI] [PubMed] [Google Scholar]
- 30.Choudhary P, Loewen MC. Quantification of mutation-derived bias for alternate mating functionalities of the Saccharomyces cerevisiae Ste2p pheromone receptor. J Biochem. 2016;159(1):49–58. doi: 10.1093/jb/mvv072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sommer ME, Hofmann KP, Heck M. Arrestin-rhodopsin binding stoichiometry in isolated rod outer segment membranes depends on the percentage of activated receptors. J Biol Chem. 2011;286(9):7359–7369. doi: 10.1074/jbc.M110.204941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jastrzebska B, Ringler P, Palczewski K, Engel A. The rhodopsin-transducin complex houses two distinct rhodopsin molecules. J Struct Biol. 2013;182(2):164–172. doi: 10.1016/j.jsb.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.George SR, Fan T, Xie Z, et al. Oligomerization of mu- and delta-opioid receptors. Generation of novel functional properties. Journal of Biological Chemistry. 2000;275(34):26128–26135. doi: 10.1074/jbc.M000345200. [DOI] [PubMed] [Google Scholar]
- 34.Hasbi A, Nguyen T, Fan T, et al. Trafficking of preassembled opioid mu-delta heterooligomer-Gz signaling complexes to the plasma membrane: coregulation by agonists. Biochemistry. 2007;46(45):12997–13009. doi: 10.1021/bi701436w. [DOI] [PubMed] [Google Scholar]
- 35.Rozenfeld R, Devi LA. Receptor heterodimerization leads to a switch in signaling: beta-arrestin2-mediated ERK activation by mu-delta opioid receptor heterodimers. FASEB J. 2007;21(10):2455–2465. doi: 10.1096/fj.06-7793com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfeiffer M, Kirscht S, Stumm R, et al. Heterodimerization of substance P and mu-opioid receptors regulates receptor trafficking and resensitization. Journal of Biological Chemistry. 2003;278(51):51630–51637. doi: 10.1074/jbc.M307095200. [DOI] [PubMed] [Google Scholar]
- 37.Margeta-Mitrovic M, Jan YN, Jan LY. A trafficking checkpoint controls GABA(B) receptor heterodimerization. Neuron. 2000;27(1):97–106. [DOI] [PubMed] [Google Scholar]
- 38.Doly S, Shirvani H, Gäta G, et al. GABAB receptor cell-surface export is controlled by an endoplasmic reticulum gatekeeper. Mol Psychiatry. 2016;21(4):480–490. doi: 10.1038/mp.2015.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cevheroğlu O, Kumaş G, Hauser M, Becker JM, Son ÇD. The yeast Ste2p G protein-coupled receptor dimerizes on the cell plasma membrane. Biochim Biophys Acta Biomembr. 2017;1859(5):698–711. doi: 10.1016/j.bbamem.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 40.Calebiro D, Rieken F, Wagner J, et al. Single-molecule analysis of fluorescently labeled G-protein-coupled receptors reveals complexes with distinct dynamics and organization. Proceedings of the National Academy of Sciences. 2013;110(2):743–748. doi: 10.1073/pnas.1205798110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dijkman PM, Castell OK, Goddard AD, et al. Dynamic tuneable G protein-coupled receptor monomer-dimer populations. Nat Comms. 2018;9(1):1710. doi: 10.1038/s41467-018-03727-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mishra AK, Gragg M, Stoneman MR, et al. Quaternary structures of opsin in live cells revealed by FRET spectrometry. Biochem J. 2016;473(21):3819–3836. doi: 10.1042/BCJ20160422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yanagawa M, Hiroshima M, Togashi Y, et al. Single-molecule diffusion-based estimation of ligand effects on G protein-coupled receptors. Sci Signal. 2018;11(548):eaao1917. doi: 10.1126/scisignal.aao1917. [DOI] [PubMed] [Google Scholar]
- 44.Hanson MA, Cherezov V, Griffith MT, et al. A specific cholesterol binding site is established by the 2.8 A structure of the human beta2-adrenergic receptor. Structure. 2008;16(6):897–905. doi: 10.1016/j.str.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cherezov V, Rosenbaum DM, Hanson MA, et al. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 2007;318(5854):1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rasmussen SGF, DeVree BT, Zou Y, et al. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature. 2011;477(7366):549–555. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dawaliby R, Trubbia C, Delporte C, et al. Allosteric regulation of G protein–coupled receptor activity by phospholipids. Nat Chem Biol. 2015;12(1):35–39. doi: 10.1038/nchembio.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pucadyil TJ, Chattopadhyay A. Cholesterol modulates ligand binding and G-protein coupling to serotonin(1A) receptors from bovine hippocampus. Biochim Biophys Acta. 2004;1663(1–2):188–200. doi: 10.1016/j.bbamem.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 49.Oates J, Faust B, Attrill H, Harding P, Orwick M, Watts A. The role of cholesterol on the activity and stability of neurotensin receptor 1. Biochim Biophys Acta. 2012;1818(9):2228–2233. doi: 10.1016/j.bbamem.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 50.Vögler O, Barceló JM, Ribas C, Escribá PV. Membrane interactions of G proteins and other related proteins. Biochim Biophys Acta. 2008;1778(7–8):1640–1652. doi: 10.1016/j.bbamem.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 51.Cerione RA, Codina J, Benovic JL, Lefkowitz RJ, Birnbaumer L, Caron MG. The mammalian beta 2-adrenergic receptor: reconstitution of functional interactions between pure receptor and pure stimulatory nucleotide binding protein of the adenylate cyclase system. Biochemistry. 1984;23(20):4519–4525. doi: 10.1021/bi00315a003. [DOI] [PubMed] [Google Scholar]
- 52.Xiang Y, Rybin VO, Steinberg SF, Kobilka B. Caveolar localization dictates physiologic signaling of beta 2-adrenoceptors in neonatal cardiac myocytes. Journal of Biological Chemistry. 2002;277(37):34280–34286. doi: 10.1074/jbc.M201644200. [DOI] [PubMed] [Google Scholar]
- 53.Ritter SL, Hall RA. Fine-tuning of GPCR activity by receptor-interacting proteins. Nat Rev Mol Cell Biol. 2009;10(12):819–830. doi: 10.1038/nrm2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ango F, Pin JP, Tu JC, et al. Dendritic and axonal targeting of type 5 metabotropic glutamate receptor is regulated by homer1 proteins and neuronal excitation. J Neurosci. 2000;20(23):8710–8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boudin H, Craig AM. Molecular determinants for PICK1 synaptic aggregation and mGluR7a receptor coclustering: role of the PDZ, coiled-coil, and acidic domains. Journal of Biological Chemistry. 2001;276(32):30270–30276. doi: 10.1074/jbc.M102991200. [DOI] [PubMed] [Google Scholar]
- 56.Hu LA, Tang Y, Miller WE, et al. beta 1-adrenergic receptor association with PSD-95. Inhibition of receptor internalization and facilitation of beta 1-adrenergic receptor interaction with N-methyl-D-aspartate receptors. Journal of Biological Chemistry. 2000;275(49):38659–38666. doi: 10.1074/jbc.M005938200. [DOI] [PubMed] [Google Scholar]
- 57.Romero G, Zastrow von M, Friedman PA. Role of PDZ proteins in regulating trafficking, signaling, and function of GPCRs: means, motif, and opportunity. Adv Pharmacol. 2011;62:279–314. doi: 10.1016/B978-0-12-385952-5.00003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ritchie K, Iino R, Fujiwara T, Murase K, Kusumi A. The fence and picket structure of the plasma membrane of live cells as revealed by single molecule techniques (Review). Molecular Membrane Biology. 2009;20(1):13–18. doi: 10.1080/0968768021000055698. [DOI] [PubMed] [Google Scholar]
- 59.Suzuki K, Ritchie K, Kajikawa E, Fujiwara T, Kusumi A. Rapid Hop Diffusion of a G-Protein-Coupled Receptor in the Plasma Membrane as Revealed by Single-Molecule Techniques. Biophysical Journal. 2005;88(5):3659–3680. doi: 10.1529/biophysj.104.048538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lohse MJ, Benovic JL, Codina J, Caron MG, Lefkowitz RJ. beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science. 1990;248(4962):1547–1550. [DOI] [PubMed] [Google Scholar]
- 61.Grady EF, Gamp PD, Jones E, et al. Endocytosis and recycling of neurokinin 1 receptors in enteric neurons. Neuroscience. 1996;75(4):1239–1254. [DOI] [PubMed] [Google Scholar]
- 62.Ferguson SS, Downey WE, Colapietro AM, Barak LS, Menard L, Caron MG. Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science. 1996;271(5247):363–366. [DOI] [PubMed] [Google Scholar]
- 63.Hanyaloglu AC, Zastrow MV. Regulation of GPCRs by Endocytic Membrane Trafficking and Its Potential Implications. Annu Rev Pharmacol Toxicol. 2008;48(1):537–568. doi: 10.1146/annurev.pharmtox.48.113006.094830. [DOI] [PubMed] [Google Scholar]
- 64.Traub LM. Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat Rev Mol Cell Biol. 2009;10(9):583–596. doi: 10.1038/nrm2751. [DOI] [PubMed] [Google Scholar]
- 65.Kaksonen M, Roux A. Mechanisms of clathrin-mediated endocytosis. Nature Publishing Group. 2018;19(5):313–326. doi: 10.1038/nrm.2017.132. [DOI] [PubMed] [Google Scholar]
- 66.Taylor MJ, Perrais D, Merrifield CJ. A High Precision Survey of the Molecular Dynamics of Mammalian Clathrin-Mediated Endocytosis. Schmid SL, ed. PLoS Biol. 2011;9(3):e1000604. doi: 10.1371/journal.pbio.1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mettlen M, Stoeber M, Loerke D, Antonescu CN, Danuser G, Schmid SL. Endocytic accessory proteins are functionally distinguished by their differential effects on the maturation of clathrin-coated pits. Molecular Biology of the Cell. 2009;20(14):3251–3260. doi: 10.1091/mbc.E09-03-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Loerke D, Mettlen M, Yarar D, et al. Cargo and dynamin regulate clathrin-coated pit maturation. Hughson F, ed. PLoS Biol. 2009;7(3):e57. doi: 10.1371/journal.pbio.1000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kadlecova Z, Spielman SJ, Loerke D, Mohanakrishnan A, Reed DK, Schmid SL. Regulation of clathrin-mediated endocytosis by hierarchical allosteric activation of AP2. The Journal of Cell Biology. 2017;216(1):167–179. doi: 10.1083/jcb.201608071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hong SH, Cortesio CL, Drubin DG. Machine-Learning-Based Analysis in Genome-Edited Cells Reveals the Efficiency of Clathrin-Mediated Endocytosis. CellReports. 2015;12(12):2121–2130. doi: 10.1016/j.celrep.2015.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marks MS, Woodruff L, Ohno H, Bonifacino JS. Protein targeting by tyrosine- and di-leucine-based signals: evidence for distinct saturable components. The Journal of Cell Biology. 1996;135(2):341–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Warren RA, Green FA, Stenberg PE, Enns CA. Distinct saturable pathways for the endocytosis of different tyrosine motifs. Journal of Biological Chemistry. 1998;273(27):17056–17063. [DOI] [PubMed] [Google Scholar]
- 73.Warren RA, Green FA, Enns CA. Saturation of the endocytic pathway for the transferrin receptor does not affect the endocytosis of the epidermal growth factor receptor. Journal of Biological Chemistry. 1997;272(4):2116–2121. [DOI] [PubMed] [Google Scholar]
- 74.Motley A, Bright NA, Seaman MNJ, Robinson MS. Clathrin-mediated endocytosis in AP-2-depleted cells. The Journal of Cell Biology. 2003;162(5):909–918. doi: 10.1083/jcb.200305145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Puri C, Tosoni D, Comai R, et al. Relationships between EGFR signaling-competent and endocytosis-competent membrane microdomains. Molecular Biology of the Cell. 2005;16(6):2704–2718. doi: 10.1091/mbc.E04-07-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lampe M, Pierre F, Al-Sabah S, Krasel C, Merrifield CJ. Dual single-scission event analysis of constitutive transferrin receptor (TfR) endocytosis and ligand-triggered β2-adrenergic receptor (β2AR) or Mu-opioid receptor (MOR) endocytosis. Molecular Biology of the Cell. 2014;25(19):3070–3080. doi: 10.1091/mbc.E14-06-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cao TT, Mays RW, Zastrow von M. Regulated endocytosis of G-protein-coupled receptors by a biochemically and functionally distinct subpopulation of clathrin-coated pits. Journal of Biological Chemistry. 1998;273(38):24592–24602. [DOI] [PubMed] [Google Scholar]
- 78.Soohoo AL, Puthenveedu MA. Divergent modes for cargo-mediated control of clathrin-coated pit dynamics. Molecular Biology of the Cell. 2013;24(11):1725–1734. doi: 10.1091/mbc.E12-07-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mundell SJ, Luo J, Benovic JL, Conley PB, Poole AW. Distinct Clathrin-Coated Pits Sort Different G Protein-Coupled Receptor Cargo. Traffic. 2006;7(10):1420–1431. doi: 10.1111/j.1600-0854.2006.00469.x. [DOI] [PubMed] [Google Scholar]
- 80.Smythe FFMFACMCGSRWGHEKSME, Foley M, Cooke A, et al. Endocytosis of G Protein-Coupled Receptors Is Regulated by Clathrin Light Chain Phosphorylation. Current Biology. 2012;22(15):1361–1370. doi: 10.1016/j.cub.2012.05.034. [DOI] [PubMed] [Google Scholar]
- 81.Maib H, Ferreira F, Vassilopoulos S, Smythe E. Cargo regulates clathrin-coated pit invagination via clathrin light chain phosphorylation. The Journal of Cell Biology. 2018;112:jcb.201805005. doi: 10.1083/jcb.201805005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cao H, Chen J, Krueger EW, McNiven MA. SRC-mediated phosphorylation of dynamin and cortactin regulates the “constitutive” endocytosis of transferrin. Molecular and Cellular Biology. 2010;30(3):781–792. doi: 10.1128/MCB.00330-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reis CR, Chen P-H, Srinivasan S, Aguet F, Mettlen M, Schmid SL. Crosstalk between Akt/GSK3β signaling and dynamin-1 regulates clathrin-mediated endocytosis. EMBO J. 2015;34(16):2132–2146. doi: 10.15252/embj.201591518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dunn HA, Walther C, Godin CM, Hall RA, Ferguson SSG. Role of SAP97 protein in the regulation of corticotropin-releasing factor receptor 1 endocytosis and extracellular signal-regulated kinase 1/2 signaling. J Biol Chem. 2013;288(21):15023–15034. doi: 10.1074/jbc.M113.473660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dunn HA, Walther C, Yuan GY, Caetano FA, Godin CM, Ferguson SSG. Role of SAP97 in the Regulation of 5-HT2AR Endocytosis and Signaling. Molecular Pharmacology. 2014;86(3):275–283. doi: 10.1124/mol.114.093476. [DOI] [PubMed] [Google Scholar]
- 86.Walther C, Caetano FA, Dunn HA, Ferguson SSG. PDZK1/NHERF3 differentially regulates corticotropin-releasing factor receptor 1 and serotonin 2A receptor signaling and endocytosis. Cell Signal. 2015;27(3):519–531. doi: 10.1016/j.cellsig.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 87.Dunn HA, Chahal HS, Caetano FA, et al. PSD-95 regulates CRFR1 localization, trafficking and β-arrestin2 recruitment. Cell Signal. 2016;28(5):531–540. doi: 10.1016/j.cellsig.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 88.Wang B, Bisello A, Yang Y, Romero GG, Friedman PA. NHERF1 regulates parathyroid hormone receptor membrane retention without affecting recycling. Journal of Biological Chemistry. 2007;282(50):36214–36222. doi: 10.1074/jbc.M707263200. [DOI] [PubMed] [Google Scholar]
- 89.Delgado-Peraza F, Ahn KH, Nogueras-Ortiz C, et al. Mechanisms of Biased β-Arrestin-Mediated Signaling Downstream from the Cannabinoid 1 Receptor. Molecular Pharmacology. 2016;89(6):618–629. doi: 10.1124/mol.115.103176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Eichel K, Jullie D, Zastrow von M. β-Arrestin drives MAP kinase signalling from clathrin-coated structures after GPCR dissociation. Nat Cell Biol. 2016;18(3):303–310. doi: 10.1038/ncb3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Eichel K, Jullié D, Barsi-Rhyne B, et al. Catalytic activation of β-arrestin by GPCRs. Nature. 2018;459:356. doi: 10.1038/s41586-018-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Latorraca NR, Wang JK, Bauer B, et al. Molecular mechanism of GPCR-mediated arrestin activation. Nature. 2018;35:308. doi: 10.1038/s41586-018-0077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cahill TJ III, Thomsen ARB, Tarrasch JT, et al. Distinct conformations of GPCR–β-arrestin complexes mediate desensitization, signaling, and endocytosis. Proceedings of the National Academy of Sciences. 2017;114(10):2562–2567. doi: 10.1073/pnas.1701529114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kumari P, Srivastava A, Ghosh E, et al. Core engagement with β-arrestin is dispensable for agonist-induced vasopressin receptor endocytosis and ERK activation. York J, ed. Molecular Biology of the Cell. 2017;28(8):1003–1010. doi: 10.1091/mbc.E16-12-0818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hammad MM, Dunn HA, Ferguson SSG. MAGI Proteins Regulate the Trafficking and Signaling of Corticotropin-Releasing Factor Receptor 1 via a Compensatory Mechanism. Journal of Molecular Signaling. 2016;11(1):219–16. doi: 10.5334/1750-2187-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hammad MM, Dunn HA, Ferguson SSG. MAGI proteins can differentially regulate the signaling pathways of 5-HT2AR by enhancing receptor trafficking and PLC recruitment. Cell Signal. 2018;47:109–121. doi: 10.1016/j.cellsig.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 97.Eichel K, Zastrow von M. Subcellular Organization of GPCR Signaling. Trends in Pharmacological Sciences. 2018;39(2):200–208. doi: 10.1016/j.tips.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pitcher JA, Touhara K, Payne ES, Lefkowitz RJ. Pleckstrin homology domain-mediated membrane association and activation of the beta-adrenergic receptor kinase requires coordinate interaction with G beta gamma subunits and lipid. Journal of Biological Chemistry. 1995;270(20):11707–11710. [DOI] [PubMed] [Google Scholar]
- 99.Pitcher JA, Inglese J, Higgins JB, et al. Role of beta gamma subunits of G proteins in targeting the beta-adrenergic receptor kinase to membrane-bound receptors. Science. 1992;257(5074):1264–1267. [DOI] [PubMed] [Google Scholar]
- 100.Benovic JL, Strasser RH, Caron MG, Lefkowitz RJ. Beta-adrenergic receptor kinase: identification of a novel protein kinase that phosphorylates the agonist-occupied form of the receptor. Proceedings of the National Academy of Sciences. 1986;83(9):2797–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fredericks ZL, Pitcher JA, Lefkowitz RJ. Identification of the G protein-coupled receptor kinase phosphorylation sites in the human beta2-adrenergic receptor. Journal of Biological Chemistry. 1996;271(23):13796–13803. [DOI] [PubMed] [Google Scholar]
- 102.Hausdorff WP, Bouvier M, O’Dowd BF, Irons GP, Caron MG, Lefkowitz RJ. Phosphorylation sites on two domains of the beta 2-adrenergic receptor are involved in distinct pathways of receptor desensitization. Journal of Biological Chemistry. 1989;264(21):12657–12665. [PubMed] [Google Scholar]
- 103.Benovic JL, Pike LJ, Cerione RA, et al. Phosphorylation of the mammalian beta-adrenergic receptor by cyclic AMP-dependent protein kinase. Regulation of the rate of receptor phosphorylation and dephosphorylation by agonist occupancy and effects on coupling of the receptor to the stimulatory guanine nucleotide regulatory protein. Journal of Biological Chemistry. 1985;260(11):7094–7101. [PubMed] [Google Scholar]
- 104.Iyer V, Tran TM, Foster E, Dai W, Clark RB, Knoll BJ. Differential phosphorylation and dephosphorylation of beta2-adrenoceptor sites Ser262 and Ser355,356. Br J Pharmacol. 2006;147(3):249–259. doi: 10.1038/sj.bjp.0706551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tran TM, Friedman J, Baameur F, Knoll BJ, Moore RH, Clark RB. Characterization of beta2-Adrenergic Receptor Dephosphorylation: Comparison with the Rate of Resensitization. Molecular Pharmacology. 2006;71(1):47–60. doi: 10.1124/mol.106.028456. [DOI] [PubMed] [Google Scholar]
- 106.Trester-Zedlitz M, Burlingame A, Kobilka B, Zastrow von M. Mass spectrometric analysis of agonist effects on posttranslational modifications of the beta-2 adrenoceptor in mammalian cells. Biochemistry. 2005;44(16):6133–6143. doi: 10.1021/bi0475469. [DOI] [PubMed] [Google Scholar]
- 107.Puthenveedu MA, Lauffer B, Temkin P, et al. Sequence-dependent sorting of recycling proteins by actin-stabilized endosomal microdomains. Cell. 2010;143(5):761–773. doi: 10.1016/j.cell.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bowman SL, Soohoo AL, Shiwarski DJ, Schulz S, Pradhan AA, Puthenveedu MA. Cell-Autonomous Regulation of Mu-Opioid Receptor Recycling by Substance P. CellReports. 2015;10(11):1925–1936. doi: 10.1016/j.celrep.2015.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bowman SL, Shiwarski DJ, Puthenveedu MA. Distinct G protein-coupled receptor recycling pathways allow spatial control of downstream G protein signaling. The Journal of Cell Biology. 2016;214(7):797–806. doi: 10.1083/jcb.201512068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Grimsey NJ, Aguilar B, Smith TH, et al. Ubiquitin plays an atypical role in GPCR-induced p38 MAP kinase activation on endosomes. The Journal of Cell Biology. 2015;210(7):1117–1131. doi: 10.1083/jcb.201504007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Leclair HM, Dubois SM, Azzi S, Dwyer J, Bidère N, Gavard J. Control of CXCR2 activity through its ubiquitination on K327 residue. BMC Cell Biol. 2014;15(1):38. doi: 10.1186/s12860-014-0038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Henry AG, Hislop JN, Grove J, Thorn K, Marsh M, Zastrow von M. Regulation of Endocytic Clathrin Dynamics by Cargo Ubiquitination. Developmental Cell. 2012;23(3):519–532. doi: 10.1016/j.devcel.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Luttrell LM, Maudsley S, Bohn LM. Fulfilling the Promise of “Biased” G Protein-Coupled Receptor Agonism. Molecular Pharmacology. 2015;88(3):579–588. doi: 10.1124/mol.115.099630. [DOI] [PMC free article] [PubMed] [Google Scholar]