Abstract
Introduction
The extent of working memory (WM) and executive function (EF) impairment in mild cognitive impairment (MCI) is not well-characterized.
Methods
We compared 48 patients with MCI, 124 noncognitively impaired elderly healthy controls, and 57 patients with Alzheimer's disease (AD) on multiple WM/EF measures, frontal lobe integrity indexes, and functioning.
Results
Patients with MCI demonstrated worse performance on nearly all WM/EF tests. This profile of impairment was refined in a factor analysis that identified three primary WM/EF constructs: WM storage; speed and controlled visual search; and manipulation of information and problem solving. EF impairments were associated with reductions in prefrontal cortical thickness. WM/EF accounted for over 50% of the variance in functional competence.
Discussion
In MCI, WM/EF impairments are far from rare, based on specific compromises to frontal cortex circuitry, and are associated with loss of everyday functioning. WM/EF impairments, even at this potentially prodromal stage of AD, have clinically deleterious consequences.
Keywords: Mild cognitive impairment (MCI), Working memory, Executive function, Functional competence, Frontal cortex
1. Background
Mild cognitive impairment (MCI) is considered to be a symptomatic prodromal phase of Alzheimer's disease (AD) [1], [2]. Diagnostic criteria for MCI include objective evidence for cognitive impairment typically involving memory and subjective memory complaints, in the context of preserved functional abilities [3], [4]. However, MCI is a heterogeneous and complex clinical entity [5]. Some form of working memory (WM) and executive function (EF) impairment may be evident in MCI, not only in the nonamnestic form of the disorder, but also in the most common amnestic presentation [6], [7], [8]. These cognitive functions generally refer to “high-level” processes that coordinate cognitive, emotional, and behavioral functions to optimize performance in the pursuit of goals [9], [10]. Processes subserving these functions include planning and problem solving, set shifting, information updating, simultaneous storage and manipulation of information, and inhibition of prepotent responses, as well as more basic short-term maintenance of information [11], [12], [13], [14].
Based on behavioral data, simple WM maintenance functions are relatively better preserved with age than various control and information manipulation processes [15], including planning and inhibition, although it remains possible that executive processes may compensate to some degree for subtle declines in the former [16]. These executive processes are more severely compromised in AD both at late and early stages [17], [18], [19], [20], as well as “late” MCI [21], [22]. In addition, it has been proposed that episodic memory deficits contribute to the observed WM/EF deficits [23].
Importantly, while the typological differentiation of MCI allows for WM/EF impairment (as does AD), to date only limited numbers and types of WM/EF tests have been used to characterize these processes [24], [25], [26], [27], [28]. Moreover, characterization of WM/EF impairments has been limited by the use of composite scores including measures of general cognitive ability (memory, executive function, language, and visuospatial) that might obscure specific WM/EF impairments [29]. Recent research using cluster analytic techniques has demonstrated that individuals with MCI, a so-called “dysexecutive” MCI subtype was more severely impaired than the classical amnestic subtype and associated with greater longitudinal decline [25]. However, the restricted range of WM/EF tests in ADNI used in this study (i.e., confined to trail making part B) may not thoroughly capture the larger range of WM/EF processes.
The present study aimed to investigate the breadth and depth of WM/EF impairments in elderly healthy control individuals (EHCs), individuals with MCI, and individuals with AD. We hypothesized that WM/EF would be impaired in MCI and in AD. In this adequately powered study conducted with a reasonable range of WM/EF tests, our goals were to investigate 1) how pervasive, i.e., frequent, are WM/EF impairments in MCI (and AD); 2) how severe are these impairments; 3) the subdomains of WM/EF impairment; 4) relationships between WM/EF and everyday functional competence; and 5) brain morphometric predictors of WM/EF performance. This is the first study to comprehensively examine WM/EF in an MCI sample using these approaches.
2. Methods
2.1. Subjects
A total of 48 individuals with MCI defined following Petersen's criteria [30], and a sample of 57 “probable” AD diagnosed according to National Institutes of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association criteria [31] were identified for inclusion in these analyses from the Litwin-Zucker Alzheimer's Disease Center by diagnostic consensus conferences (comprising neurologists, neuropsychologists, and psychiatrists). Note that while all subjects in our MCI sample were amnestic, i.e., had episodic memory impairments <1.5 SDs below the mean, as were our AD subjects, cognitive impairments were not necessarily restricted to memory. A control sample of 124 noncognitively impaired EHC volunteers were included for comparison. Details about inclusion/exclusion criteria are elsewhere [32] and in the Supplement. All subjects provided written informed consent after receiving a complete description of the study.
2.2. Clinical stage
The global level of dementia of the sample was measured by the Clinical Dementia Rating (CDR) [33] and the Mini-Mental State Examination (MMSE) [34]. We used CDR sum of “boxes” to derive its global score. Patients with MCI obtained a score of CDR = 0.5 (questionable dementia) and patients with AD had scores of 1 or 2 (mild or moderate). EHCs had scores of 0. The MMSE is a 30-point cognitive screening test. All patients with MCI had scores greater than 23, all patients with AD had scores of 16 to 23, and all EHCs had scores greater than 23.
2.3. Neuropsychological assessment
We used a large test battery designed to assess different domains of WM/EF. The tests used to assess them are listed, and detailed descriptions are included in the Supplement: digit span, letter-number span, trail making test A and B (TMT-A and TMT-B), N Back (Zero- and One-back), Tower test, Stroop test. Memory tests used were selective reminding and logical memory.
2.4. Functional assessment
The University of California, San Diego, Performance-Based Skills Assessment (UPSA) is a performance-based measure of functional abilities that includes measures of simulated real-world activities, for example, planning a trip to the beach, remembering a phone number, and writing a check. We used the brief version of the task that includes the communication and comprehension/planning domains [35].
2.5. MRI acquisition and extraction of brain morphometry measures
Scans were performed in a GE 3T MRI scanner using an 8-channel phased-array head coil. High resolution structural T1-weighted spoiled gradient-echo recalled (SPGR) images in the coronal plane were acquired with TR = 7.8 ms, TE = 3 ms, TI = 450 ms, flip angle = 20°, 24 cm field of view, 256 × 256 matrix for pixel dimensions of 0.9375 mm by 0.9375 mm, and 136 slices of 1.5 mm thickness. Images were parceled and segmented using the FreeSurfer software version 5.3 [36], [37]. See Supplement for a more detailed explanation about this procedure. Jack et al recently found in large ADNI and Mayo Clinic samples that using these exact methods (fully automated FreeSurfer v. 5.3. thickness method) afforded greater sensitivity and reliability than voxel-based morphometry techniques [38].
We used the following FreeSurfer-derived variables in three different regression models. The first model included volumetric measures of cortical gray matter volume, subcortical gray matter, and hippocampus, adjusted by age, sex, education, and estimated intracranial volume. The second model included thickness measures of regions of interest (ROI) within the frontal lobe: lateral orbitofrontal, medial orbitofrontal, pars opercularis, pars orbitalis, pars triangularis, rostral anterior cingulate, caudal anterior cingulate, rostral middle frontal, superior frontal, frontal pole, plus a general measure of cortical thickness of the whole brain; these measures were adjusted by age, sex, and education. The third model included subcortical global white matter volume, global white matter hypointensities volume, and volumes of white matter ROIs within the frontal lobe: we used the same ROIs as listed previously for subjacent white matter volumes; these measures were adjusted by age, sex, education, and estimated intracranial volume. Left and right ROIs were averaged.
2.6. Data analysis
Comparisons on sociodemographic, clinical status, cognitive variables, and MRI measures between groups were performed with chi square and F tests.
The statistical analysis approach was structured following the next steps: 1) Frequency of WM/EF impairment was assessed using chi square tests across diagnostic groups (EHC, MCI, and AD) by contrasting differences in number of subjects showing impairment on a given test. The cutoff for impairment was derived using z scores with mean and standard deviation of the EHC group for each test. A z score lower than -1 was considered impaired. Multiple comparisons were adjusted by Bonferroni correction (alpha of 0.05/12 tests, P = .004); this corrected level of statistical significance was applied for all the analysis described below. 2) To examine the breadth of WM/EF impairments within each individual we contrasted subjects in each group with 0-3 tests impaired and 4 or more tests impaired by chi square. 3) Depth (i.e., severity) of WM/EF impairments were assessed using general linear models (GLM) fitted to quantify differences between groups on each dependent variable (WM/EF tests) adjusted for post hoc comparisons using Bonferroni correction as indicated previously; effect sizes (ESs) were also computed using a correction approach [39]. 4) To ascertain subdomains of WM and EF impairment we used a factor analytic approach using data from all subjects. All cognitive measures were subjected to an exploratory factor analysis using maximum absolute correlation as prior communality estimates. Factors were extracted using the principal component method followed by a VARIMAX (orthogonal) rotation. 5) The relationship between WM/EF with memory and everyday function were assessed through Spearman correlation coefficients adjusting alpha level for significance by Bonferroni correction; in these correlations, the factors derived from factorial analysis plus UPSA functional scale score were used. Finally, 6) stepwise regression models, based on maximizing R2, were fitted to analyze the contribution of the variation in cognitive/functional factors that might be attributed to brain morphometry in the whole sample. Brain morphometry variables were fitted as independent predictors (See Section 2.5 for list of predictors) into a series of separate models using each one of the cognitive/functional factors as dependent variables.
To manage missing data only for cognitive variables, a within-group multiple imputation was performed in SAS 9.4 (PROC MI) software for missing data [40] (see Supplementary Data).
3. Results
The sociodemographic and clinical status characteristics for the three groups are presented in Table 1. The groups did not differ in gender ratios. There were differences in the number of years of education between EHC and AD, and MCI and AD (patients with AD having less years of education), but not between EHC and MCI. As expected, both MCI and AD groups showed higher scores on CDR (sum of boxes) compared with the EHC group and the AD group had a higher score compared with the MCI group. The groups also differed significantly on MMSE score; post hoc contrasts indicated that all groups differed from one another. As age and education showed differences between groups, GLM analyses were adjusted by these two variables; in addition, gender was also treated as a covariate in the analyses.
Table 1.
Sociodemographic and clinical status characteristics for EHC, MCI, and AD
| Variables | Groups |
Statistics |
Post hoc tests |
||||||
|---|---|---|---|---|---|---|---|---|---|
| EHCN = 124 | MCI N = 48 | AD N = 57 | Test | df | P value | EHC vs. MCI | EHC vs. AD | MCI vs. AD | |
| Age Mean (SD) | 73.17 (8.60) | 76.68 (10.27) | 76.58 (10.31) | F = 3.84 | 2, 226 | .02 | P = .07 | P = .06 | P = 1.00 |
| Gender M/F | 49/75 | 27/21 | 27/30 | χ2 = 4.09 | 2 | .13 | |||
| Education Mean (SD) | 16.33 (2.69) | 15.94 (2.86) | 14.10 (3.34) | F = 11.63 | 2, 222 | <.0001 | P = .70 | P < .0001 | P = .004 |
| CDR-SB Mean (SD) | 0.77 (0.98) | 2.64 (1.07) | 4.48 (1.88) | F = 28.05 | 2, 67 | <.0001 | P = .003 | P < .0001 | P < .0001 |
| MMSE Mean (SD) | 28.49 (1.40) | 25.96 (2.03) | 21.21 (4.28) | F = 159.93 | 2, 226 | <.0001 | P < .0001 | P < .0001 | P < .0001 |
Abbreviations: EHC, elderly healthy controls; MCI, mild cognitive impairment; AD, Alzheimer's disease; df, degrees of freedom; SD, standard deviation; M, male; F, female; CDR-SB, Clinical Dementia Rating–Sum of Boxes.
3.1. Frequency of WM/EF impairment in MCI and AD groups
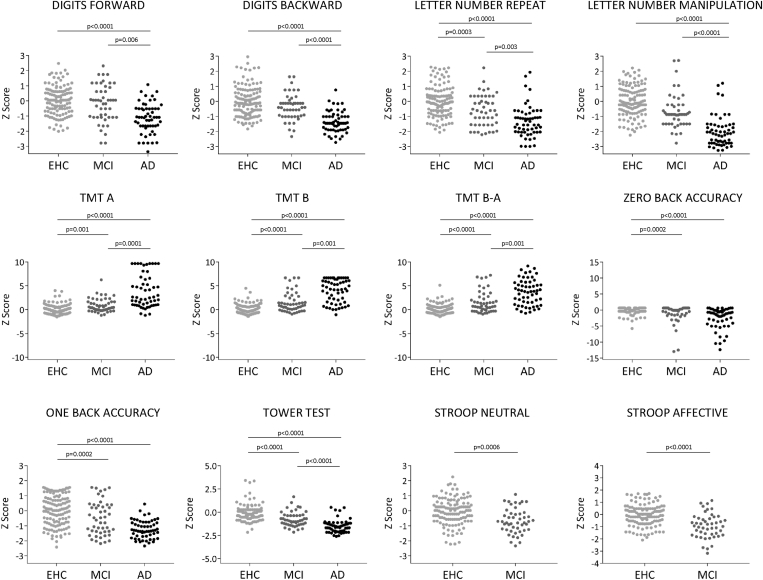
First, the proportion of impaired subjects on a given test was compared across the groups by chi square. For all tests, patients with MCI were more frequently impaired than EHCs and significantly so for all tests except digit span. In addition, the proportion of impaired MCI and EHC subjects was significantly smaller than the proportion in AD. These results are shown in Fig. 1 and Supplementary Table 1 that includes chi square values.
Fig. 1.
Frequency of WM/EF impairment in MCI and AD groups. Distribution of z scores between groups for each of the tests. Each dot in the graphs represents a subject. P values have been obtained through χ2 tests comparing the proportion of subjects below and above −1 z score between groups. Bonferroni adjustment for level of significance was set at P = .004. Note that there was a trend to significance in the comparison of EHC and MCI on digits backward (P = .009). Abbreviations: EHC, elderly healthy controls; MCI, mild cognitive impairment; AD, Alzheimer's disease; P, P value.
Second and as can be observed in Fig. 2, the modal MCI subject demonstrated impairments on average in five tests of twelve, whereas the modal AD subject demonstrated impairments in seven or nine tests (of ten). The modal EHC subjects did not demonstrate any impaired performances. Chi square analyses indicated that this difference in distribution was highly significant.
Fig. 2.
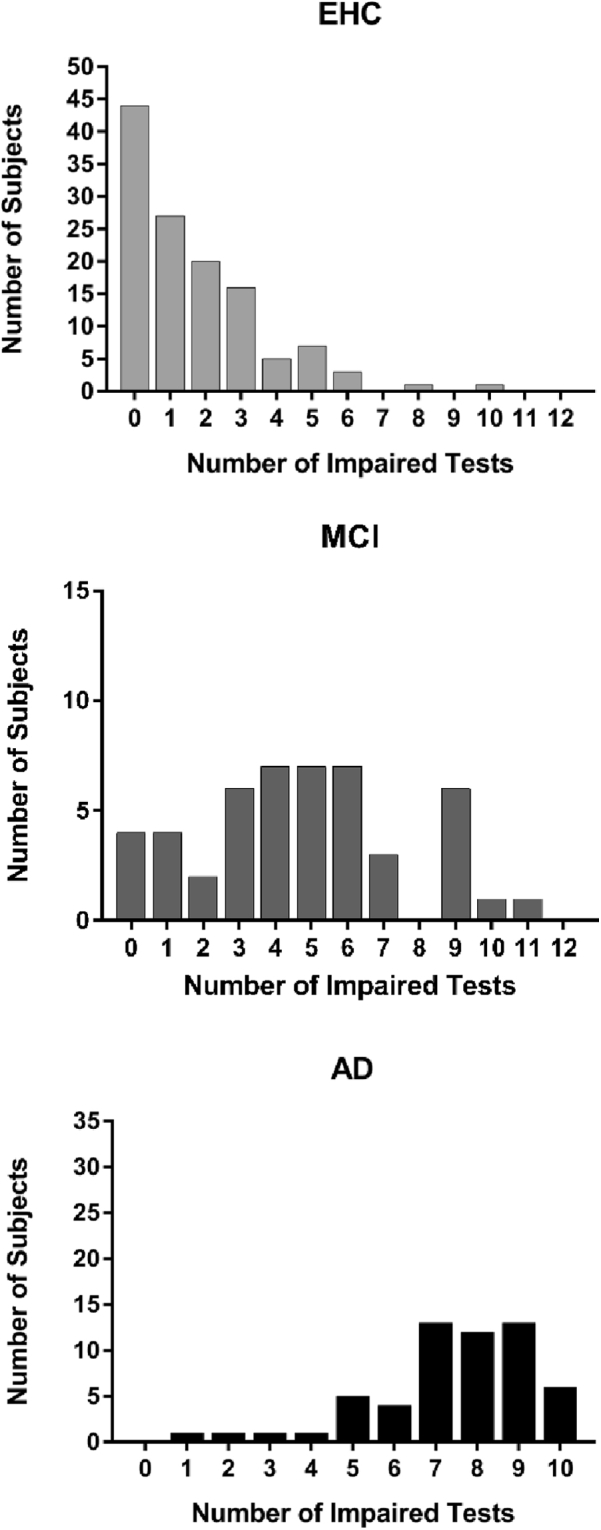
Frequency of impairments within subjects (breadth of impairment). Bar graphs represent number of subjects that have between 0 and 12 tests impaired (0 to 10 the AD group due to lack of data in Stroop neutral and affective tests). A between-groups comparison, taking into account the number of subjects with 3 or less tests impaired and number of subjects with more than 3 tests impaired, revealed significantly differences between all groups: 14% EHC, 67% MCI, and 95% had more than 3 tests impaired (EHC vs. MCI χ2 = 47.64, P < .0001; EHC vs. AD χ2 = 107.54, P < .0001; MCI vs. AD χ2 = 13.85, P = .0002). Abbreviations: EHC, elderly healthy controls; MCI, mild cognitive impairment; AD, Alzheimer's disease.
3.2. Depth of WM/EF impairment in MCI and AD
The MCI group demonstrated medium to large ES compared with the EHC group (Table 2 and Supplementary Fig. 1) in Tower test (ES = 0.88), TMT-B (ES = 0.85), TMT B-A (ES = 0.78), letter-number manipulation (ES = 0.71), and One-back accuracy (ES = 0.55). Stroop neutral inhibition and affective inhibition also showed a significant impairment in patients with MCI compared with EHCs (ES of 0.54 and 0.84, respectively). Compared to patients with AD, patients with MCI showed better performance on all executive functions (ES ranging from 0.74 for One-back accuracy to 1.39 for TMT-A), with the exception of zero-back accuracy on which MCI and AD were equally impaired as compared with EHC. Patients with AD showed significant impairment compared with EHC in all measures (ES ranging from 0.87 to 2.20). In keeping with these results, GLMs and least squares means (Table 2) demonstrated significant group differences for all measures; post hoc EHC-MCI differences (Bonferroni corrected) were present for TMT-B, TMT B-A, Tower test, letter-number manipulation, and One-back accuracy. See Supplementary Fig. 1 for all visual comparisons among the three diagnostic groups.
Table 2.
GLMs and least squares means between group performance on WM/EF tests
| Variables | EHC (N = 124) Mean (SE) | MCI (N = 48) Mean (SE) | AD (N = 57) Mean (SE) | GLM | P | Post hoc (ES) |
|---|---|---|---|---|---|---|
| Digits forward Raw correct score |
8.77 (0.16) | 8.61 (0.26) | 7.24 (0.24) | F5, 219 = 15.55 | <.0001 | EHC vs. MCI P = .84 (ES = 0.09) EHC vs. AD P < .0001 (ES = 0.94) MCI vs. AD P = .0005 (ES = 0.75) |
| Digits backward Raw correct score |
7.18 (0.19) | 6.50 (0.30) | 4.34 (0.28) | F5, 219 = 19.02 | <.0001 | EHC vs. MCI P = .14 (ES = 0.32) EHC vs. AD P < .0001 (ES = 1.33) MCI vs. AD P < .0001 (ES = 1.02) |
| Letter-number repetition Raw correct score |
7.11 (0.19) | 6.08 (0.30) | 4.55 (0.29) | F5, 219 = 15.24 | <.0001 | EHC vs. MCI P = .01 (ES = 0.49) EHC vs. AD P < .0001 (ES = 1.19) MCI vs. AD P = .0009 (ES = 0.71) |
| Letter-number manipulation Raw correct score |
5.32 (0.14) | 4.23 (0.22) | 2.45 (0.21) | F5, 219 = 39.82 | <.0001 | EHC vs. MCI P = .0002 (ES = 0.70) EHC vs. AD P < .0001 (ES = 1.82) MCI vs. AD P < .0001 (ES = 1.13) |
| TMT-A seconds |
34.92 (2.95) | 44.53 (4.60) | 90.01 (4.35) | F5, 219 = 27.18 | <.0001 | EHC vs. MCI P = .19 (ES = 0.29) EHC vs. AD P < .0001 (ES = 1.67) MCI vs. AD P < .0001 (ES = 1.39) |
| TMT-B seconds |
83.73 (5.00) | 130.90 (7.80) | 206.69 (7.37) | F5, 219 = 49.04 | <.0001 | EHC vs. MCI P < .0001 (ES = 0.85) EHC vs. AD P < .0001 (ES = 2.20) MCI vs. AD P < .0001 (ES = 1.37) |
| TMT B-A seconds |
49.41 (4.40) | 87.26 (6.87) | 140.82 (6.48) | F5, 219 = 36.56 | <.0001 | EHC vs. MCI P < .0001 (ES = 0.78) EHC vs. AD P < .0001 (ES = 1.85) MCI vs. AD P < .0001 (ES = 1.10) |
| Zero-back accuracy | 97.58 (0.89) | 92.50 (1.39) | 88.91 (1.32) | F5, 219 = 8.70 | <.0001 | EHC vs. MCI P = .007 (ES = 0.52) EHC vs. AD P < .0001 (ES = 0.87) MCI vs. AD P = .15 (ES = 0.36) |
| One-back accuracy | 61.86 (2.09) | 49.35 (3.27) | 32.18 (3.08) | F5, 219 = 19.53 | <.0001 | EHC vs. MCI P = .004 (ES = 0.55) EHC vs. AD P < .0001 (ES = 1.27) MCI vs. AD P = .0005 (ES = 0.74) |
| Tower test | 17.45 (0.51) | 12.48 (0.80) | 8.24 (0.76) | F5, 219 = 24.62 | <.0001 | EHC vs. MCI P < .0001 (ES = 0.88) EHC vs. AD P < .0001 (ES = 1.61) MCI vs. AD P = .0005 (ES = 0.74) |
| Stroop neutral T score |
37.34 (0.60) | 33.73 (0.97) | - | F5, 162 = 23.97 | <.0001 | ES = 0.54 |
| Stroop affective T score |
39.23 (0.65) | 33.14 (1.04) | - | F5, 162 = 23.97 | <.0001 | ES = 0.84 |
3.3. Subdomains of WM/EF impairment
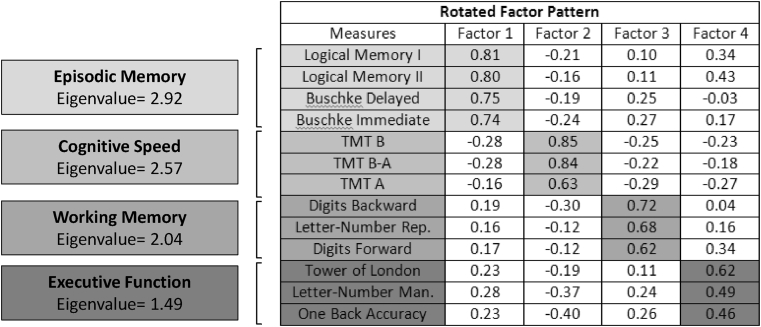
The factor analysis scree test revealed four interpretable factors (Fig. 3). Tests showing a factor loading of 0.45 (or greater) were considered to load on a given factor. All episodic memory measures (logical memory immediate and delayed and Buschke immediate and delayed) loaded on the first factor (labeled as episodic memory) with an eigenvalue of 2.92. Measures derived from TMT test loaded on another factor (cognitive speed) with an eigenvalue of 2.57. Digits (forward and backward) and letter-number repetition loaded on the third factor (labeled working memory maintenance) with an eigenvalue of 2.04. Finally, the Tower test, letter-number manipulation, and one-back accuracy loaded on the fourth factor (labeled executive function) with an eigenvalue of 1.49. A separate factor analysis was performed with only EHC and MCI samples to allow inclusion of Stroop inhibition task measures, resulting in identification of an additional factor (cognitive control) wherein these two measures loaded with an eigenvalue of 1.92. We used these results to derive factor scores based on test z scores only (i.e., loading weights were not used) to be used in analyses below. GLMs for between-group analyses of factor scores are shown in Supplementary Table 2. All EHC-MCI post hoc contrasts were highly significant with the exception of the working memory maintenance factor.
Fig. 3.
Subdomains of WM/EF impairment. Rotated factor pattern of all cognitive measures extracted by VARIMAX rotation. Four factors were retained: episodic memory, cognitive speed, working memory, and executive function. Tests with a factor loading of 0.45 (or greater) were considered to load on a given factor.
3.4. WM/EF relationships with functional competency performance
In the MCI group (Supplementary Table 3), functional competence on the UPSA was significantly correlated with working memory (ρ = 0.38) and cognitive control (ρ = 0.47) factors. In the EHC group executive function was a significant correlate of functional competence (ρ = 0.33). In AD relationships between WM/EF and functioning were more widespread.
With respect to episodic memory correlations with WM/EF in the EHC and MCI groups, these were consistently small and not significant, suggesting that WM/EF is not driven by memory failures. In AD, WM/EF impairments were correlated with memory suggestive of a “disease blurring” factor due to more global impairments.
We next sought to determine the amount of variance in the UPSA that could be predicted by all three WM/EF factors in a multivariate regression model in the whole group of participants. In this model, the executive, cognitive speed, and working memory factors were entered in prespecified order. The full model was highly significant (F6,218 = 46.82, P < .0001). Moreover, all WM/EF entered significantly. The R2 was 0.42 (P < .0001) for the executive factor; 0.09 (P < .0001) for cognitive speed; and 0.01 (P = .02) for working memory. Demographics (age, sex, and education) were forced to enter and their combined R2 = 0.04.
3.5. Association between WM/EF and brain morphometry
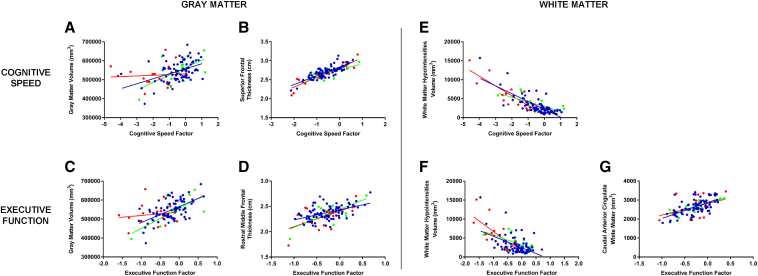
Significant associations in the regression models between WM/EF domains and FreeSurfer morphometric measures are displayed in Fig. 4.
Fig. 4.
Association between WM/EF and brain morphometry. Scatterplots showing regression lines from significant brain morphometric predictors of cognitive factors derived from factor analysis. Volume variables are in the form of mm3, thickness variables are in the form of cm, and cognitive factors are in the form of predicted z scores (from significant regression models). Blue dots and line represent EHC group; green dots and line represent MCI; red dots and line represent AD. Values on cognitive speed (seconds to complete TMT-A and TMT-B tasks) have been inverted to represent higher positive score with better performance. (A) Scatterplot of global gray matter volume as a significant predictor of cognitive speed (t = 5.13, P < .0001). (B) Scatterplot of superior frontal thickness as a significant predictor of cognitive speed (t = 3.04, P = .003). (C) Scatterplot of gray matter volume as a significant predictor of executive function (t = 4.10, P < .0001). (D) Scatterplot of rostral middle frontal thickness as a significant predictor of executive function (t = 3.04, P < .003). (E) Scatterplot of white matter hypointensities as a significant predictor of cognitive speed (t = −4.54, P < .0001). (F) Scatterplot of white matter hypointensities as a significant predictor of executive function (t = −4.10, P = .005). (G) Scatterplot of caudal anterior cingulate white matter volume as a significant predictor of executive function (t = 2.54, P = .01).
3.5.1. Gray matter
Cognitive speed was predicted by cortical gray matter volume with an R2 of 0.18 (F5, 108 = 8.03, P < .0001). In the cortical thickness model, cognitive speed was also predicted by superior frontal thickness (F4, 109 = 4.77, P = .001; R2 = 0.07), as well as medial temporal lobe measures (the latter data not shown). Executive function was predicted by cortical gray matter volume (F5, 108 = 6.48, P < .0001; R2 = 0.12). In the cortical thickness model, executive function was predicted by rostral middle frontal thickness (F5, 108 = 4.37, P = .001; R2 = 0.03); pars triangularis thickness served as a “suppressor” variable as when it was included it strengthened (i.e., “unsuppressed”) the relationship between middle frontal cortical thickness and the executive factor. Finally, episodic memory was predicted by hippocampal volume (R2 = 0.10), plus middle temporal and parahippocampus thickness (R2 = 0.03 and R2 = 0.07, respectively).
3.5.2. White matter
Cognitive speed was predicted by white matter hypointensity volume with an R2 of 0.14 (F5, 108 = 6.78, P < .0001). Executive function was predicted by white matter hypointensity volume (R2 = 0.08) and caudal anterior cingulate white matter volume (R2 = 0.05) in a significant model (F6, 107 = 5.41, P < .0001).
4. Discussion
To the best of our knowledge, this is the most comprehensive study of WM/EF to date in MCI. It is also the largest non-ADNI study on WM/EF in MCI and it offers complementary findings that usefully extend and refine the heavily mined ADNI database. Tasks included 1) assays of simple WM storage that engages the phonological loop (digits forward and letter-number repetition); 2) more complex measures involving simultaneous storage and manipulation of information involving such processes as multiple sequencing of items, fully mentalized planning, and updating of information (digits backward, letter-number manipulation, N back task, Tower of London, and Stroop test); and 3) set shifting in the context of speed demands and organized visual search (TMT-A and TMT-B). Severity of impairment in the latter two types of task was in the medium and large range, respectively, in MCI by z score analyses and these differences were highly significant when contrasted with the EHC group. Moreover, the modal MCI subject demonstrated impairment on five measures, while the modal EHC demonstrated no impairments. This is one of the few studies to include such a variety of WM/EF tests within the same MCI sample.
The factor structure of the sample suggested three primary subdomains of WM/EF. Trail making tests (TMT A, B, and B-A) loaded on a single factor. As these tests are timed, when impaired in MCI and AD, they indicate slowed speed of processing. These tests also demand controlled visual search that in turn might modulate speed. What we have termed the executive subdomain comprised verbal and nonverbal tests that appear to reflect demands for processes that involve information manipulation of information maintained in storage, including multiple resequencing (of letters and numbers), updating (One-back), and mentalized planning and hypothesis testing (Tower test). A third factor involved short-term maintenance of information with reduced demand for manipulation. It may reflect the so-called phonological working memory loop and was found to be relatively preserved in MCI. The presence of another non-WM/EF factor comprised episodic memory tasks was not unexpected and provides a referent for the validity of our findings.
Another study examined executive function in MCI and identified two factors [41]: one consisted of tests that involved generating novel responses and problem solving and the other involved speed of processing and inhibition. In the ADNI database, Park and Manly [42] identified a speed/executive TMT factor and a digit span factors. Neither of these studies examined associations of factors to brain morphometry nor to functional competence, nor did they include the broad range of tests that we used.
Impressively, the WM/EF measures were generally robust predictors of a validated performance-based measure of functional competence (the UPSA) in MCI and AD. In MCI, both working memory and cognitive control factors correlated significantly with the UPSA, while in AD speed of processing/visual search, executive function, and episodic memory, all had significant correlations with the UPSA. In the whole sample, the three WM/EF subdomains accounted for nearly 60% of the variance in functional competency assessed by the UPSA. This suggests that WM/EF processes are engaged by real-world scenarios, and if they are compromised, real world function will likely demonstrate compromises.
With respect to the relationships between the identified WM/EF factors and brain morphometry, we found that global cortical thickness was a significant predictor for working memory maintenance, cognitive speed and visual search, and executive function. In keeping with what is known about dedicated circuitry, we found that superior prefrontal cortex, a region that includes the supplemental and frontal eye fields, was a predictor of speed, perhaps related to dynamic visual search. Indeed, it is now generally appreciated that directed efficient visual search drives TMT speed (or lack thereof), not psychomotor speed per se [43]. Superior frontal cortex is also thought to be an important node in the dorsal attention network and supports selection of visual information and shifting between visual stimuli [44].
Our finding that the middle frontal gyrus was associated with the executive factor is consistent with multiple models of executive control and problem-solving [45]. In these models, executive tasks that demand more than simple storage are supported by dorsal-lateral prefrontal cortex. To reinforce the validity of our approach, we found that episodic memory performance was predicted by hippocampal volume. The associations between WM/EF factors and cortical measures that we found may be placed in this context of prior findings in that more specific prefrontal morphometric reductions and more global gray matter reductions both play roles in impairment [46], [47]. We acknowledge that our sample does not have biological confirmation of AD pathology. However our design included a meticulous procedure to dissect clinical, cognitive, and functional profiles for each participant in consensus meetings with neurologists, neuropsychologists, and psychiatrists.
Our findings suggest that white matter abnormalities may also contribute to WM/EF impairments. In particular, white matter volume reductions and signal abnormalities have been associated with frontal executive function and processing speed compromises in aging and AD [48], [49]. Moreover, mixed brain pathologies are frequently present in MCI and AD cases and have usually been found to be additive to AD histopathology in contributing to the clinical phenotype [50]. We found that white matter volume reductions subjacent to the anterior cingulate were associated with reductions in executive function. These may reflect white matter shrinkage that interrupts connectivity or loss of projection fibers from that area of cortex. Perhaps more strikingly, we found that white matter hypointensity volume reductions were associated with attenuations in executive function and speed as have others [48], [51]. Such imaging findings are usually thought to be part of spectrum of small vessel disease and have been identified using other imaging techniques (i.e., FLAIR and T2) and at postmortem. In T1-weighted imaging, T1-weighted hypointensities tend to follow a periventricular and deep white matter distribution [52].
In summary, we found that WM/EF impairments are far from rare, that they are based on specific compromises to frontal cerebral circuitry, and that these WM/EF impairments are associated with loss of everyday functional abilities. The results that we report thus suggest that circuitry-based WM/EF impairments even at this potentially prodromal stage of AD have clinically deleterious consequences.
Research in context.
-
1.
Systematic review: The authors reviewed the literature using traditional sources (e.g., PubMed) and meeting abstracts and presentations. While working memory (WM) and executive function (EF) impairments have not been widely studied in mild cognitive impairment (MCI), there have been several recent studies that highlight the importance of frontal/executive functions in the presentation of the disorder. These relevant citations are appropriately cited.
-
2.
Interpretation: In MCI, WM/EF impairments are far from rare, are based on specific compromises to frontal cortex circuitry, and are associated with loss of everyday functional abilities. Thus, even at this potentially prodromal stage of AD, WM/EF impairments have clinically deleterious consequences.
-
3.
Future directions: The manuscript provides a new framework to incorporate WM and EF dysfunction into the clinical characterization of MCI. This work will help to better understand compromises in everyday functional competence and the underlying frontal lobe substrate.
Acknowledgments
This work was supported by the Litwin-Zucker Alzheimer's Research Center and by grant RO1 AG038734 (Goldberg TE, PI) from the National Institutes of Health. Jesus J. Gomar is supported by a fellowship grant from the Alzheimer's Association (AACFD-16-438886). Dr. Goldberg receives royalties for the use of the Brief Assessment of Cognition in Schizophrenia (BACS) in clinical trials. The rest of the authors have nothing to disclose.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dadm.2018.12.010.
Supplementary data
References
- 1.Petersen R.C., Doody R., Kurz A., Mohs R.C., Morris J.C., Rabins P.V. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 2.Albert M.S., DeKosky S.T., Dickson D., Dubois B., Feldman H.H., Fox N.C. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen R.C., Morris J.C. Mild cognitive impairment as a clinical entity and treatment target. Arch Neurol. 2005;62:1160–1163. doi: 10.1001/archneur.62.7.1160. [DOI] [PubMed] [Google Scholar]
- 4.Winblad B., Palmer K., Kivipelto M., Jelic V., Fratiglioni L., Wahlund L.O. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 5.Libon D.J., Xie S.X., Eppig J., Wicas G., Lamar M., Lippa C. The heterogeneity of mild cognitive impairment: a neuropsychological analysis. J Int Neuropsychol Soc. 2010;16:84–93. doi: 10.1017/S1355617709990993. [DOI] [PubMed] [Google Scholar]
- 6.Albert M., Blacker D., Moss M.B., Tanzi R., McArdle J.J. Longitudinal change in cognitive performance among individuals with mild cognitive impairment. Neuropsychology. 2007;21:158–169. doi: 10.1037/0894-4105.21.2.158. [DOI] [PubMed] [Google Scholar]
- 7.Tabert M.H., Manly J.J., Liu X., Pelton G.H., Rosenblum S., Jacobs M. Neuropsychological prediction of conversion to Alzheimer disease in patients with mild cognitive impairment. Arch Gen Psychiatry. 2006;63:916–924. doi: 10.1001/archpsyc.63.8.916. [DOI] [PubMed] [Google Scholar]
- 8.Lafleche G., Albert M.S. Executive function deficits in mild Alzheimer's disease. Neuropsychology. 1995;9:313–320. [Google Scholar]
- 9.Alvarez J.A., Emory E. Executive function and the frontal lobes: A meta-analytic review. Neuropsychol Rev. 2006;16:17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- 10.Elliot R. Executive functions and their disorders. Br Med Bull. 2006;65:49–59. doi: 10.1093/bmb/65.1.49. [DOI] [PubMed] [Google Scholar]
- 11.Randolph C., Gold J.M., Goldberg T.E. The neuropsychology of schizophrenia. In: Heilman, Valenstein, editors. Clinical Neuropsychology. Third Edition. Oxford University Press; 1980. [Google Scholar]
- 12.Goldberg T.E., Weinberger D.R., Berman K.F., Pliskin N.H., Podd M.H. Further evidence for dementia of the prefrontal type in schizophrenia? A controlled study of teaching the Wisconsin Card Sorting Test. Arch Gen Psychiatry. 1987;44:1008–1014. doi: 10.1001/archpsyc.1987.01800230088014. [DOI] [PubMed] [Google Scholar]
- 13.Mattay V.S., Goldberg T.E., Sambataro F., Weinberger D.R. Neurobiology of cognitive aging: insights from imaging genetics. Biol Psychol. 2008;79:9–22. doi: 10.1016/j.biopsycho.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baddeley A., Hitch G.J. Working Memory. In: Bower, editor. The psychology of learning and motivation: Advances in research and theory. Academic Press; New York: 1974. pp. 47–89. [Google Scholar]
- 15.Reuter-Lorenz P.A., Sylvester C.Y.C. Cognitive Neuroscience of Aging: Linking cognitive and cerebral aging. Oxford University Press; 2009. The Cognitive Neuroscience of Working Memory and Aging. [Google Scholar]
- 16.Amieva H., Lafont S., Rouch-Leroyer I., Rainville C., Dartigues J.F., Orgogozo J.M. Evidencing inhibitory deficits in Alzheimer disease through interference effects and shifting disabilities in Stroop Test. Arch Clin Neuropsychol. 2004;19:791–803. doi: 10.1016/j.acn.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Binetti G., Magni E., Padovani A., Cappa S.F., Bianchetti A., Trabucchi M. Executive dysfunction in early Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1996;60:91–93. doi: 10.1136/jnnp.60.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambon R.M., Patterson K., Graham N., Dawson K., Hodges J. Homogeneity and heterogeneity in mild cognitive impairment and Alzheimer's disease: A cross-sectional and longitudinal study of 55 cases. Brain. 2003;126:2350–2362. doi: 10.1093/brain/awg236. [DOI] [PubMed] [Google Scholar]
- 19.Collette F., Van der Linden M., Salmon E. Executive dysfunction in Alzheimer's disease. Cortex. 1999;35:57–72. doi: 10.1016/s0010-9452(08)70785-8. [DOI] [PubMed] [Google Scholar]
- 20.Swanberg M., Tractenberg R.E., Mohs R., Thal L.J., Cummings J. Executive dysfunction in alzheimer disease. Arch Neurol. 2004;61:556–560. doi: 10.1001/archneur.61.4.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albert M.S., Moss M.B., Tanzi R., Jones K. Preclinical prediction of AD using neuropsychological tests. J Int Neuropsychological Soc. 2001;7:631–639. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- 22.Dickerson B.C., Sperling R.A., Hyman B.T., Albert M.S., Blacker D. Clinical prediction of Alzheimer disease dementia across the spectrum of mild cognitive impairment. Arch Gen Psychiatry. 2007;64:1443–1450. doi: 10.1001/archpsyc.64.12.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baudic S., Barba G.D., Thibaudet M.C., Smagghe A., Remy P., Traykov L. Executive function deficits in early Alzheimer's disease and their relations with episodic memory. Arch Clin Neuropsychol. 2006;21:15–21. doi: 10.1016/j.acn.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Saunders N.L., Summers M.J. Longitudinal deficits to attention, executive, and working memory in subtypes of mild cognitive impairment. Neuropsychology. 2011;25:237–248. doi: 10.1037/a0021134. [DOI] [PubMed] [Google Scholar]
- 25.Bondi M.W., Edmonds E.C., Jak A.J., Clark L.R., Delano-Wood L., McDonald C.R. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. J Alzheimers Dis. 2014;42:275–289. doi: 10.3233/JAD-140276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomar J.J., Bobes-Bascaran M.T., Conejero-Goldberg C., Davies P., Goldberg T.E., Alzheimer's Disease Neuroimaging I Utility of combinations of biomarkers, cognitive markers, and risk factors to predict conversion from mild cognitive impairment to Alzheimer disease in patients in the Alzheimer's disease neuroimaging initiative. Arch Gen Psychiatry. 2011;68:961–969. doi: 10.1001/archgenpsychiatry.2011.96. [DOI] [PubMed] [Google Scholar]
- 27.Manly J.J., Tang M.X., Schupf N., Stern Y., Vonsattel J.P., Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol. 2008;63:494–506. doi: 10.1002/ana.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belleville S., Chertkow H., Gauthier S. Working memory and control of attention in persons with Alzheimer's disease and mild cognitive impairment. Neuropsychology. 2007;21:458–469. doi: 10.1037/0894-4105.21.4.458. [DOI] [PubMed] [Google Scholar]
- 29.Huey E.D., Manly J.J., Tang M.X., Schupf N., Brickman A.M., Manoochehri M. Course and etiology of dysexecutive MCI in a community sample. Alzheimers Dement. 2013;9:632–639. doi: 10.1016/j.jalz.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petersen R.C. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 31.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 32.Goldberg T.E., Koppel J., Keehlisen L., Christen E., Dreses-Werringloer U., Conejero-Goldberg C. Performance-based measures of everyday function in mild cognitive impairment. Am J Psychiatry. 2010;167:845–853. doi: 10.1176/appi.ajp.2010.09050692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris J.C. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 34.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 35.Gomar J.J., Harvey P.D., Bobes-Bascaran M.T., Davies P., Goldberg T.E. Development and cross-validation of the UPSA short form for the performance-based functional assessment of patients with mild cognitive impairment and Alzheimer disease. Am J Geriatr Psychiatry. 2011;19:915–922. doi: 10.1097/JGP.0b013e3182011846. [DOI] [PubMed] [Google Scholar]
- 36.Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 37.Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 38.Schwarz C.G., Gunter J.L., Wiste H.J., Przybelski S.A., Weigand S.D., Ward C.P. A large-scale comparison of cortical thickness and volume methods for measuring Alzheimer's disease severity. Neuroimage Clin. 2016;11:802–812. doi: 10.1016/j.nicl.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hedges L.V., Olkin L., editors. Statistical Methods for Meta-Analysis. Academic Press, INC; London: 1985. [Google Scholar]
- 40.Yuan Y. Multiple Imputation Using SAS Software. J Stat Softw. 2011;45:1–25. doi: 10.18637/jss.v045.i01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brandt J., Aretouli E., Neijstrom E., Samek J., Manning K., Albert M.S. Selectivity of executive function deficits in mild cognitive impairment. Neuropsychology. 2009;23:607–618. doi: 10.1037/a0015851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park L.Q., Gross A.L., McLaren D.G., Pa J., Johnson J.K., Mitchell M., Alzheimer's Disease Neuroimaging Initiative Confirmatory factor analysis of the ADNI Neuropsychological Battery. Brain Imaging Behav. 2012;6:528–539. doi: 10.1007/s11682-012-9190-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kirchner C., Volker I., Bock O.L. Age related deficits on the trail making test part: separating specific deficits in visual search from generalized sensorimotor slowing. Int J Pschology Behav Sci. 2014;4:208–214. [Google Scholar]
- 44.Vossel S., Geng J.J., Fink G.R. Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist. 2014;20:150–159. doi: 10.1177/1073858413494269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Unterrainer J.M., Owen A.M. Planning and problem solving: from neuropsychology to functional neuroimaging. J Physiol Paris. 2006;99:308–317. doi: 10.1016/j.jphysparis.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 46.Edmonds E.C., Eppig J., Bondi M.W., Leyden K.M., Goodwin B., Delano-Wood L. Heterogeneous cortical atrophy patterns in MCI not captured by conventional diagnostic criteria. Neurology. 2016;87:2108–2116. doi: 10.1212/WNL.0000000000003326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dickerson B.C., Feczko E., Augustinack J.C., Pacheco J., Morris J.C., Fischl B. Differential effects of aging and Alzheimer's disease on medial temporal lobe cortical thickness and surface area. Neurobiol Aging. 2009;30:432–440. doi: 10.1016/j.neurobiolaging.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leritz E.C., Shepel J., Williams V.J., Lipsitz L.A., McGlinchey R.E., Milberg W.P. Associations between T1 white matter lesion volume and regional white matter microstructure in aging. Hum Brain Mapp. 2014;35:1085–1100. doi: 10.1002/hbm.22236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chong J.S.X., Liu S., Loke Y.M., Hilal S., Ikram M.K., Xu X. Influence of cerebrovascular disease on brain networks in prodromal and clinical Alzheimer's disease. Brain. 2017;140:3012–3022. doi: 10.1093/brain/awx224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schneider J.A., Wilson R.S., Bienias J.L., Evans D.A., Bennett D.A. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 2004;62:1148–1155. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]
- 51.Jacobs H.I., Leritz E.C., Williams V.J., Van Boxtel M.P., van der Elst W., Jolles J. Association between white matter microstructure, executive functions, and processing speed in older adults: the impact of vascular health. Hum Brain Mapp. 2013;34:77–95. doi: 10.1002/hbm.21412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wardlaw J.M., Valdes Hernandez M.C., Munoz-Maniega S. What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J Am Heart Assoc. 2015;4:001140. doi: 10.1161/JAHA.114.001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.