Abstract
Current treatment recommendations for resectable pancreatic cancer support upfront resection and adjuvant therapy. Randomized controlled trials offering comparison with the emerging neoadjuvant approach are lacking. This review aims to compare both treatment strategies for resectable pancreatic cancer. PubMed, MEDLINE, Embase, Cochrane Database and Cochrane Databases were searched for studies comparing neoadjuvant and surgery-first with adjuvant therapy for resectable pancreatic cancer. A Bayesian network meta-analysis was conducted using the Markov chain Monte Carlo method. Cochrane Collaboration’s risk of bias, ROBINS-I and GRADE tools were used to assess quality and risk of bias of included trials. 9 studies compared neoadjuvant therapy and surgery-first with adjuvant therapy (n = 22,285). Aggregate rate (AR) of R0 resection for neoadjuvant therapy was 0.8008 (0.3636–0.9144) versus 0.7515 (0.2026–0.8611) odds ratio (O.R.) 1.27 (95% CI 0.60–1.96). 1-year survival AR for neoadjuvant therapy was 0.7969 (0.6061–0.9500) versus 0.7481 (0.4848–0.8500) O.R. 1.38 (95% CI 0.69–2.96). 2-year survival AR for neoadjuvant therapy was 0.5178 (0.3000–0.5970) versus 0.5131 (0.2727–0.5346) O.R. 1.26 (95% CI 0.94–1.74). 5-year AR survival for neoadjuvant therapy was 0.2069 (0.0323–0.3300) versus 0.1783 (0.0606–0.2300) O.R. 1.19 (95% CI 0.65–1.73). In conclusion neoadjuvant therapy may offer benefit over surgery-first and adjuvant therapy. However, further randomized controlled trials are needed.
Introduction
Pancreatic cancer (PC) is the fourth and fifth most common cause of cancer deaths in the USA and Europe respectively1,2. Despite advances in surgical technique and adjuvant treatment, survival rates remain poor1,2. Early complete surgical resection is the only potentially curative treatment for PC and adjuvant therapy has been proven to prolong survival leading to surgery first with adjuvant therapy (SFadj) becoming the standard of care for resectable pancreatic cancer (RPC)3. However in reality most patients develop early recurrence, nullifying the potential benefits of high-risk surgery4 with up to 50% of patients failing to receive adjuvant therapy due to: post-operative complications, early metastases, reduced performance status and comorbidities5. This has resulted in the advent of neoadjuvant therapy (NAT) with the postulated benefits of: identifying aggressive tumour hence avoiding futile surgery, elimination of micrometastesis, increased feasibility of R0 resection and completion of multimodal treatment6,7.
NAT for RPC is an area of prime controversy and ongoing debate with a lack of large prospective randomised controlled trials (RCTs) offering direct comparison with SFadj approach8. Ambiguity surrounding the existing body of research has led critics to highlight the limitations of drawing optimistic conclusions from small studies that are underpowered and caution against loosing the window of resectability6,7. Although not their sole focus, meta-analysis by both Xu et al.9 and Andriulli et al.10 report only marginal benefit of NAT in terms of overall and disease-free survival in RPC7, whilst other studies report superiority of NAT approach for RPC11–14. Previous Markov decision analysis studies have reported slight benefit with NAT11,13,15. Often comparison studies include borderline resectable and locally advanced PC in NAT arm hence they do not offer a true like-for-like comparison.
In the clinical setting the role of NAT has widely been accepted for the management of locally advanced and borderline resectable cases of PC to increase the likelihood of achieving resection, particularly R0 resection6–8,13. However, ambiguities in the existing body of research concerning the management of RPC with either SFadj or NAT approach creates a dilemma in clinical decision-making. It has been established that optimal survival outcomes for PC are not obtained by resection alone, but require the delivery of additional treatment whether delivered as neoadjuvant or adjuvant therapy3,9–15. Both SFadj and NAT treatment approaches carry the risk of failing to achieve multimodal treatment delivery. The currently recommended standard of care for RPC, SFadj3, carries the risk of failing to receive adjuvant therapy despite having undergone surgery with its associated risks of morbidity and mortality4,5. NAT approach also carries the risk of disease that was initially resectable at presentation progressing to become unresectable which makes its role in the management of RPC controversial6,7. The question therefore arises as to whether NAT represents a less superior treatment approach to SFadj for RPC, or if NAT has the advantage of identifying aggressive tumour types, that would have resulted in early disease reoccurrence precluding adjuvant therapy, being identified prior to patients undergoing high-risk, costly yet futile surgery6,7. The aim of this review is to compare SFadj and NAT approach to the management of RPC on an intention-to-treat basis. Treatment outcomes include: R0 resection rates and 1, 2, 3, 4 and 5-year survival.
Methods
The protocol for this review was published in the PROSPERO online database of systematic reviews (CRD42018108673). This review followed the PRISMA checklist16.
A search was undertaken using MEDLINE, Embase, PubMed and Cochrane database. For each of the four searches, the entire database was included since 2000 up to and including 31st August 2018, with no further date restrictions or limits applied. Full search strategies and are detailed in supplementary material (Supplementary Methods1).
Search Strategy
After removal of duplicates, manual screening was carried out based on the title and abstract of articles identified in the database searches. Articles of probable or possible relevance to this review based on the title and abstract were reviewed in full. Following screening, reference lists and citations of all included papers were manually searched to identify any additional articles. This process was repeated until no new articles were identified.
Inclusion Criteria and Outcomes
RCTs and Prospective phase II and III trials offering comparison of NAT and SFadj approach for RPC, published in English language since 2000, involving chemo/radiotherapy-naive human subjects over 18 years of age with preoperatively staged RPC, or that reported outcomes for RPC separately, were included. As this produced only 2 studies prospective and retrospective cohort studies comparing NAT and SFadj, with the same inclusion criteria, were also included. RCTs comparing SFadj and surgery alone, with similar inclusion criteria, were also included for sensitivity analysis. Included trials had to report: protocol design, treatment regimes, number per arm, median age and co-morbidities of subjects, pre-treatment stage of pancreatic cancer, outcome from post NAT re-staging, surgical outcomes including resection rates, R0 resection rates and survival time. Case series and case reports were excluded, as were studies from identical patient cohorts. Studies that included borderline resectable, locally advanced and stage IV pancreatic cancer where results were inseparable, trials involving intra-operative radiotherapy and trials including disease other than pancreatic cancer were excluded.
Data collection
Search design and data extraction were performed by the lead reviewer and with second author performing independent quality assurance. Discrepancies were resolved by discussion between the reviewers. The following data was extracted from each study: study details (country, year, design, number of participants, mean age, sex, co-morbidity profile and presenting disease stage of participants in each arm), details of treatment protocols, treatment outcomes (rates of tumour resection, R0 resection rates, overall survival and disease free survival and 1, 2, 3, 4 and 5 year survival rates) and risk of bias data.
The Cochrane Collaboration’s risk of bias tool17 and ROBINS-I tool (Risk Of Bias In Non-randomized Studies - of Interventions)18. Grading of Recommendations Assessment Development and Evaluation (GRADE) tool was used to provide additional assessment of quality of evidence and rate certainty in estimates from network meta-analysis19,20.
Statistical analysis
This study was conducted on an intention-to-treat basis. Patients who dropped out, or who failed to receive multimodal treatment within, either SFadj or NAT pathways in the included studies were included in the overall and disease free survival analysis. The number of patients in the NAT pathway who presented with RPC but failed to undergo resection, and the number of patients who underwent surgery but failed to receive adjuvant therapy, were analysed using weighted pooled estimates of proportions calculated using Freeman-Tukey arcsine square root transformation under random effects model to account for heterogeneity.
For each outcome of interest, NetMetXl was used to draw a weighted network for all treatments assessed for the specific outcomes that accounted for the study population size of each included study21–23. This ensured that larger studies carried a greater weight within the network. A Bayesian network meta-analysis was conducted using the Markov chain Monte Carlo method in WinBUGS 1.4.3 (MRC Biostatistics Unit, Cambridge, and Imperial College School of Medicine, London, UK). To account for the inherent heterogeneity as a result of the different chemotherapy regimes, variations in multimodal treatment completion rates and differences in reported survival outcomes, analysis was run using a random effects, in addition to a fixed effects, model using vague priors as outlined in National Institute of Clinical Excellence Evidence Synthesis Series21,24,25. Pairwise comparisons between interventions were also summarized to provide ranking of impact of intervention on outcome based on the surface under the cumulative ranking (SUCRA) and summarized in rankograms21,25.
To further minimise the impact of heterogeneity of different chemotherapy combinations, treatment completion rates and reported survival analysis on the overall analysis, convergence was assessed using the Brooks-Gelman-Rubin method and by checking whether the Monte Carlo error is less than 5% of the standard deviation of the effect estimates and between-study variance21. The Markov chain Monte Carlo (MCMC) Bayesian network meta-analysis was fitted with three chains as a means of checking MCMC convergence21. The Brooks-Gelman-Rubin method compares within-chain and between-chain variances to calculate the potential scale reduction factor with a value close to one indicating when approximate convergence is reached21,26.
Inconsistency assessment, the conflict between direct and indirect evidence, is crucial to any network meta-analysis27. In accordance with the NICE decision-support documents28 inconsistency was measured by comparing deviance residuals and deviance information criteria (DIC) statistic in fitted consistency and inconsistency models21,27. Posterior mean deviance of the individual data points in the inconsistency model were plotted against their posterior mean deviance in the consistency model to identify any loops in the treatment network where inconsistency is present21.
A sensitivity network meta-analysis was carried out that also included RCTs that compared SFadj and surgery only.
Results
Eligible studies
A total of 14224 studies were identified through search of electronic databases (Medline/PubMed: 148; Embase: 14032; Cochrane Database: 1; Cochrane Trial Registry: 43). After removal of duplicates and studies that were not relevant on review of title and abstract, 452 studies underwent full text review (Supplementary Fig. 1a). 9 studies were identified that offered comparison between NAT and SFadj for treatment of RPC29–37. As only 2 of these studies were phase II trials29,30, one of which was randomized29 all studies were therefore included in the network meta-analysis. 4 studies were prospective31–34 and 3 studies were retrospective35–37 (Supplementary Table 1a; Supplementary Fig. 2).
6 studies (n = 371) reported the number of cases of RPC who received NAT and progressed to surgery29–31,33,34,37 giving a pooled proportion of 76.08% (95% CI: 60.826–88.509). Two studies reported response to NAT29,31. One study reported responses for resectable cases29 (complete response: 0; partial response: 4/31; stable disease 8/31; disease progress 12/31; 7 unrecorded). The study by Ielop et al.31 did not report this outcome separately for resectable only cases but included borderline cases also in reporting the outcomes of response to NAT (complete response: 5/45; partial response: 13/45; stable disease 5/45). 6 studies (n = 17596) reported the number of patients in the SFadj pathway who received adjuvant therapy31–33,35–37 giving a pooled proportion of 63.01% (95% CI: 59.452–66.489).
For sensitivity analysis, RCTs offering comparison between surgery and adjuvant therapy versus surgery alone were also included in a separate network meta-analysis. Electronic database search identified 25332 studies (Medline/PubMed: 3165; Embase: 21810; Cochrane Database: 1; Cochrane Trial Registry: 356). 15 studies were randomized controlled trials, 5 of which offered comparison between adjuvant therapy and surgery alone and were included in the sensitivity analysis (Supplementary Fig. 1b; Supplementary Table 1b; Supplementary Fig. 2b)38–42.
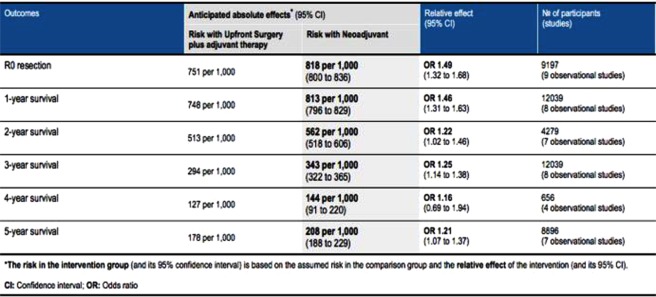
A summary of overall findings for each outcome measure is provided in Fig. 1.
Figure 1.
Summary of results of Bayesian network meta-analysis comparing upfront surgery and adjuvant therapy with neoadjuvant therapy for the management of resectable pancreatic cancer.
R0 Resection Rates
The network offering pairwise comparison of rates of R0 resection between NAT and SFadj included 8 studies and 9197 participant (NAT: n = 2626; SFadj: n = 6571). The aggregate rate of R0 resection for NAT was 0.8008 (0.3636–0.9144) compared to 0.7515 (0.2826–0.8611) for SFadj. Both fixed effects (O.R: 1.49; 95% CI: 1.32–1.68) and random effects (O.R: 1.27; 95% CI: 0.60–1.96) models favoured NAT. NAT was found to have superior positive impact on outcome of R0 resection (SUCRA: 0.8124 versus 0.1876).
1-year Survival Rates
Pairwise comparison for 1-year survival of NAT versus SFadj was based on 8 studies and 12011 participants (NAT: n = 2708; SFadj: n = 9303). Aggregate rate of 1-year survival was higher in NAT at 0.7969 (0.6061–0.9500) versus 0.7481 (0.4848–0.8500). Both fixed effects (O.R. 1.46 95% CI: 1.31–1.63) and random effects (O.R. 1.38 95% CI: 0.69–2.96) models favoured NAT. NAT also has a stronger positive impact on the outcome of 1-year survival (SUCRA: 0.84 v 0.16).
For sensitivity analysis a network also including RCTs of SFadj versus surgery only was constructed based on a total of 10 studies and 12483 patients (NAT: n = 2708; SFadj: n = 9540; Surgery only: n = 235). 8 studies compared NAT and SFadj (n = 12011) and 2 studies compared SFadj and surgery only (n = 472). NAT was found to be superior in both fixed and random effects models (Supplementary Fig. 3). Aggregate rate of 1-year survival was highest in NAT (0.7957; range 0.6205–0.9500) followed by SFadj (0.7478; range 0.4848–0.8500) then surgery only (0.7314; range 0.7250–0.7500). Again NAT was found to have strongest positive impact on outcome of 1-year survival (SUCRA 0.7836; Supplementary Fig. 3c).
2-year Survival Rates
Network pairwise comparison of NAT and SFadj for 2-year survival was based on 7 studies (n = 4251; NAT n = 903; SFadj: 3348). Aggregate rate of 2-year survival was 0.5178 (0.3000–0.5970) versus 0.5131 (0.2727–0.5346) in favour of NAT. Both fixed effects (O.R. 1.22; 95% CI: 1.02–1.46) and random effects model (O.R. 1.26; 95% CI: 0.94–1.74) favoured NAT with SUCRA 0.95 for NAT.
Inclusion of SFadj versus Surgery only RCTs in a network based on 9 studies (n = 4723; NAT: n = 903; SFadj: n = 3585; Surgery only: n = 235) also demonstrated superiority of NAT for 2-year survival in both fixed and random effects model (Supplementary Fig. 4). Aggregate of 2-year survival was 0.5217 (0.3000–0.5970) for NAT compared to 0.5107 (0.2727–0.5346) for SFadj and 0.4149 (0.4000–0.4200) for surgery only.
3-year Survival Rates
Pairwise comparison of NAT versus SFadj was based on a network comprising 8 studies (n = 12011; NAT: n = 2708; SFadj: n = 9303) and demonstrated superiority of NAT with aggregate rate of 0.3367 (0.1212–0.3900) to 0.2943 (0.1800–0.4700). Again both fixed effect (O.R. 1.25 95% CI 1.14–1.38) and random effects (O.R. 1.19 9% CI 0.86–1.51) models favored NAT with SUCRA 0.9 demonstrating stronger positive effect with NAT on outcomes of 3-year survival.
Inclusion of SFadj versus Surgery only RCTs in a network produced comparisons based on 9 studies (n = 12365; NAT: 2708; SFadj: n = 9482; Surgery only: n = 175). NAT was superior in both fixed and random effects models with aggregate rate 0.3400 (0.2000–0.4194) compared to 0.2951 (0.1800–0.4700) for SFadj and 0.2050 (0.2050–0.2050) for surgery only (Supplementary Fig. 5).
4-year Survival Rates
Only pairwise comparison of NAT and SFadj could be offered, as SFadj versus surgery only RCTS did not report 4-year survival rates. This network was based on 4 studies (n = 656). NAT was superior with aggregate rate 0.1416 (0.0303–0.2500) compared to 0.1269 (0.0606–0.2000). Fixed effects (O.R. 1.16 95% 0.69–1.94) and random effects model (O.R 1.03 95% CI 0.27–3.13) favored NAT.
5-year Survival Rates
Network pairwise comparison of 5-year survival for NAT and SFadj was based on 7 studies (n = 8896; NAT: n = 2558; SFadj: n = 6338). Aggregate rate for NAT was 0.2069 (0.0323–0.3300) compared to 0.1783 (0.0606–0.2300). Fixed effects (O.R 2.21 95% CI: 1.07–1.37) and random effects (vague prior) (O.R. 1.19 95% 0.65–1.73) favored NAT with SUCRA 0.82 for NAT association with 5-year survival.
Inclusion of SFadj versus surgery only RCTs was based on 11 studies (n = 9675; NAT n = 2558; SFadj n = 6730; Surgery only n = 387). NAT was superior across fixed effects and random effects models with aggregate rate 0.2069 (0.0323–0.3300) followed by 0.1814 (0.0606–0.2640) for SFadj and 0.1418 (0.1040–0.2200) for surgery only (Supplementary Fig. 6).
Convergence, Inconsistency and Assessment of Strength of Recommendations
Convergence was achieved across all models and no issues were identified with inconsistency. In 2-year survival analysis and 5-year survival analysis there was a marginal preference towards fixed effects model as determined by the DIC statistic.
Overall this analysis marginally favors NAT for treatment of RPC across outcomes of R0 resection, 1, 2, 3, 4 and 5-year survival. This is based on the best available studies and did not alter on sensitivity analysis. However, issues pertaining to quality and level of bias of available studies are an issue that weakens the strength and level of certainty of any such recommendations (Supplementary Fig. 7).
Discussion
SFadj is a well established treatment pathway for RPC3. NAT is supported by current guidelines for borderline resectable and locally advanced PC but its role in the management of RPC remains controversial8,13. Postulated benefits of NAT include: identifying aggressive tumour types hence avoiding futile surgery, elimination of micrometastesis, increased R0 resection rate and increased rate of completion of multimodal treatment considering that up to 50% of patients treated in SFadj pathway fail to receive adjuvant therapy5–7. However, controversy in the role of NAT for RPC arises from the potential of loosing the window of resectability6,7. In the absence of conclusive results from large multi-centered RCTs, this study, the first of its kind, utilizes existing studies comparing NAT and SFadj for the treatment of RPC in a Bayesian network meta-analysis to offer an important interim source of information to inform the ongoing debate regarding the best treatment for RPC.
In terms of survival time, from direct and indirect comparisons our analysis found that NAT was marginally superior to SFadj across 1, 2, 3, 4 and 5-year survival. These findings are corroborated by previous attempts to synthesize existing evidence comparing SFadj and NAT for RPC. Meta-analysis by both Xu et al.9 and Andriulli et al.10 reported marginal benefit of NAT for RPC in terms of OS and DFS for resectable cases. However, neither of these reports focused solely on NAT and therefore omitted significant studies from their meta-analysis7. Sharma et al.11 and de Gus et al.13 synthesized published data in a Markov decision-analysis model to compared NAT and SFadj for the treatment of RPC and also reported marginal benefit of NAT. More recently Versteijne et al.14 reported more significant survival benefit with NAT in their meta-analysis but the reported weighted mean overall survival time included borderline resectable cases therefore captured the effect of conversion to resectability affecting overall survival time in NAT pathway. The reported weighted mean overall survival time for resectable only cases was lower although still superior to SFadj14.
The second key outcome explored through direct and indirect comparison was the rate of R0 resection, which is known to impact survival time43. Once again NAT was found to be superior to SFadj which is in keeping with the hypothesis that NAT results in higher rates of R0 resection6,7,44. However, definitions of R0 resection can vary between studies, which could potentially impact reported outcomes14. In this study convergence was achieved across all models comparing this outcome and no issues with inconsistency were identified in our analysis.
A key clinical concern when selecting a treatment pathway for RPC is the delivery of multimodal treatment: resection in the NAT pathway and receipt of adjuvant therapy in the SFadj pathway. Our analysis of pooled proportions found that 63% of patients in the SFadj pathway received adjuvant therapy, and 76% in the NAT pathway underwent resection. These findings are in keeping with the results of a recent meta-analysis of pooled proportions that reported 68.6% of patients in SFadj received adjuvant therapy and 76.8% of resectable cases in NAT pathways underwent resection14.
A strength of this study is that only studies of RPC, identified through comprehensive literature search, were included to offer a true like-for-like comparison based on currently available evidence. Analysis of NAT versus SFadj were based on direct comparisons to strengthen certainty of findings with indirect comparisons drawn from inclusion of SFadj versus surgery only in sensitivity analysis which did not alter network findings. However, this study also shares the limitations of the existing body of evidence pertaining to treatment of RPC: heterogeneity and small underpowered sample size10. Although random effects modeling was employed to counter heterogeneity, overall there is a lack of RCTs comparing NAT and SFadj for RPC7,10,11,13. Only one of the two phase II trials were randomized29 with the remaining studies being either prospective or retrospective studies which raises serous concerns about bias and reduced certainty in the recommendations drawn from the network meta-analysis. However, unlike the majority of existing network meta-analysis45–47, this study went beyond only assessing bias of included trials to utilise GRADE approach to rate the certainty in estimates from our network meta-analysis20,48–51. Hence this study not only furthers the ongoing current debate regarding best treatment for RPC by offering an important interim analysis, but adds a further dimension by highlighting limitations of the body of evidence on which this analysis is based.
To conclude our Bayesian network meta-analysis shows that NAT for treatment of RPC is no worse than traditional SFadj approach and may even hold benefit across outcomes of: R0 resection, 1, 2, 3, 4 and 5-year survival. This finding in the context of limitations of existing studies means that conclusive superiority of one approach over another for RPC cannot be determined without a degree of uncertainty. This highlights three important directions for future research: 1) rigorous head-to-head comparison of NAT and SFadj for treatment of RPC 2) cost-effectiveness analysis of NAT versus SFadj and 3) exploring methods of predictive statistical modeling to identify patients who are more likely to receive and benefit from differing treatment modalities within competing pathways. By moving research in this direction it is hoped that we can find a path from ambiguity to delivering personalized medicine with associated benefits for patients and resource utilization.
Supplementary information
Acknowledgements
Professor Colin McKay and the West of Scotland Pancreatic Cancer Unit for their support.
Author Contributions
Dr. Bradley was the lead researcher and undertook study design, data collection and analysis and writing and re-drafting of the manuscript. Dr. Van Der Meer acted as second reviewer, provided study supervision and was involved in editing and re-drafting the manuscript.
Data Availability
The datasets analysed during the current study are publicly available and sources are cited in supplementary material.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-40951-6.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 3.Neoptolemos JP, et al. Adjuvant chemoradio- therapy and chemotherapy in resectable pancreatic cancer: a randomized controlled trial. Lancet. 2001;358:1576–85. doi: 10.1016/S0140-6736(01)06651-X. [DOI] [PubMed] [Google Scholar]
- 4.Winter JM, et al. Survival after resection of pancreatic adenocarcinoma: results from a single institution over three decades. Ann Surg Oncol. 2012;19:169. doi: 10.1245/s10434-011-1900-3. [DOI] [PubMed] [Google Scholar]
- 5.Bilimoria KY, et al. Multimodality therapy for pancreatic cancer in the U.S.: utilization, outcomes, and the effect of hospital volume. Cancer. 2007;110:1227–34. doi: 10.1002/cncr.22916. [DOI] [PubMed] [Google Scholar]
- 6.Asare EA, et al. Neoadjuvant treatment sequencing adds value to the care of patients with operable pancreatic cancer. Journal of Surgical Oncology. 2016;114(3):291–295. doi: 10.1002/jso.24316. [DOI] [PubMed] [Google Scholar]
- 7.Lee J, et al. Clinical impact of neoadjuvant treatment in resectable pancreatic cancer: a systematic review and meta-analysis protocol. BMJ. 2016;6(3):1–9. doi: 10.1136/bmjopen-2015-010491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tempero MA, Malafa MP, Behrman SW. Pancreatic adenocarcinoma, version 2.2014: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2014;12:1083–93. doi: 10.6004/jnccn.2014.0106. [DOI] [PubMed] [Google Scholar]
- 9.Xu CP, et al. Effect of chemoradiotherapy and neoadjuvant chemoradiotherapy in resectable pancreatic cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2014;140:549–59. doi: 10.1007/s00432-013-1572-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andriulli A, et al. Neoadjuvant/preoperative gemcitabine for patients with localized pancreatic cancer: a meta-analaysis of prospective studies. Ann Surg Oncol. 2012;19:1644–62. doi: 10.1245/s10434-011-2110-8. [DOI] [PubMed] [Google Scholar]
- 11.Sharma G, et al. Efficacy of neoadjuvant versus adjuvant versus adjuvant therapy for resectable pancreatic adenocarcinoma: a decision analysis. Ann Surg Oncol. 2015;22:1229–37. doi: 10.1245/s10434-015-4711-0. [DOI] [PubMed] [Google Scholar]
- 12.de Felice F, et al. Neoadjuvant strategy as initial treatment in resectable pancreatic cancer: concrete evidence of benefit. Anticancer Res. 2014;34:4673–6. [PubMed] [Google Scholar]
- 13.de Gus SW, et al. Neoadjuvant therapy versus upfront surgical strategies in resectable pancreatic cancer: a markov decision analysis. Eur J Surg. 2016;42(10):1552–60. doi: 10.1016/j.ejso.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 14.Versteijne E, et al. Meta‐analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. The British Journal of Surgery. 2018;105(8):946–958. doi: 10.1002/bjs.10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.VanHouten JP, White RR, Jackson GP. A decision model of therapy for potentially resectable pancreatic cancer. The Journal of Surgical Research. 2012;174(2):222–230. doi: 10.1016/j.jss.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. 2009;3:123–130. [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JPT, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sterne JAC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomized studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shunemann, H. et al. GRADEpro GDT. https://gradepro.org/product/#about [accessed 6th September 2018].
- 20.Brignardello-Petersen R, et al. Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. Journal of Clinical epidemiology. 2018;93:36–44. doi: 10.1016/j.jclinepi.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Brown S, et al. A Microsoft-Excel-based tool for running and critically appraising network meta-analyses—an overview and application of NetMetaXL. Systematic Reviews. 2014;3:110. doi: 10.1186/2046-4053-3-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown, S. et al. NetMetaXL. http://www.netmetaxl.com/index.html [accessed 6th Spetember 2018]
- 23.Chaimani A, Higgins JPT, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One. 2013;8:e76654. doi: 10.1371/journal.pone.0076654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision-making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making. 2013;33:607–617. doi: 10.1177/0272989X12458724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salanti G, Ades A, Ioannidis J. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163–171. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 26.Brooks S, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat. 1998;7:434–455. [Google Scholar]
- 27.Dias S, et al. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making. 2013;33:641–656. doi: 10.1177/0272989X12455847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spiegelhalter DJ, Best NG, Carlin BP, Van der Linde A. Bayesian measures of model complexity and fit. Journal of the Royal Statistical Society. 2002;64(4):583–616. doi: 10.1111/1467-9868.00353. [DOI] [Google Scholar]
- 29.Golcher H, et al. Neoadjuvant chemoradiation therapy with gemcitabine/cisplatin and surgery versus immediate surgery in resectable pancreatic cancer: results of the first prospective randomized phase II trial. Strahlentherapie Und Onkologie. 2015;191:7–16. doi: 10.1007/s00066-014-0737-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vento P, et al. Impact of preoperative chemoradiotherapy on survival in patients with resectable pancreatic cancer. World Journal of Gastroenterology: WJG. 2007;13(21):2945–2951. doi: 10.3748/wjg.v13.i21.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ielpo B, et al. Preoperative treatment with gemcitabine plus nab-paclitaxel is a safe and effective chemotherapy for pancreatic adenocarcinoma. Eur J Surg Oncol. 2016;42:1394–1400. doi: 10.1016/j.ejso.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Roland CL, et al. Neoadjuvant therapy is associated with a reduced lymph node ratio in patients with potentially resectable pancreatic cancer. Annals of surgical oncology. 2015;22(4):1168–1175. doi: 10.1245/s10434-014-4192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tzeng CW, et al. Treatment sequencing for resectable pancreatic cancer: influence of early metastases and surgical complications on multimodality therapy completion and survival. J Gastrointest Surg. 2014;18:16–24. doi: 10.1007/s11605-013-2412-1. [DOI] [PubMed] [Google Scholar]
- 34.Fujii T, et al. Clinical benefits of neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreatic head: an observational study using inverse probability of treatment weighting. J Gastroenterol. 2017;52:81–93. doi: 10.1007/s00535-016-1217-x. [DOI] [PubMed] [Google Scholar]
- 35.de Gus SWL, et al. Neoadjuvant therapy affects margins and margins affects all: perioperative and survival outcomes in resected pancreatic adenocarcinoma. HPB. 2017;20:573–581. doi: 10.1016/j.hpb.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Mokdad AA, et al. Neoadjuvant therapy followed by resection versus upfront resection for resectable pancreatic cancer: a propensity score matches analysis. Journal of Clinical Oncology. 2017;35(5):515–522. doi: 10.1200/JCO.2016.68.5081. [DOI] [PubMed] [Google Scholar]
- 37.Papalezova KT, et al. Does preoperative therapy optimize outcomes in patients with resectable pancreatic cancer? Journal of Surgical Oncology. 2012;106:111–118. doi: 10.1002/jso.23044. [DOI] [PubMed] [Google Scholar]
- 38.Ueno H, et al. A randomised phase III trial comparing gemcitabine with surgery-only in patients with resected pancreatic cancer: Japanese study group of adjuvant therapy for pancreatic cancer. Br J Cancer. 2009;101:908–15. doi: 10.1038/sj.bjc.6605256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oettle H, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473–81. doi: 10.1001/jama.2013.279201. [DOI] [PubMed] [Google Scholar]
- 40.Kosuge T, et al. A multicentre randomized controlled trial to evaluate the effect of adjuvant cisplatin and 5-fluorouracil therapy after curative resection in cases of pancreatic cancer. Jpn J Clin Oncol. 2006;36:159–165. doi: 10.1093/jjco/hyi234. [DOI] [PubMed] [Google Scholar]
- 41.Smeenk HG, et al. Long-term survival and metastatic pattern of pancreatic and periampullary cancer after adjuvant chemoradiation or observation: long-term results of EORTC trial 40891. Ann Surg. 2007;246:734–40. doi: 10.1097/SLA.0b013e318156eef3. [DOI] [PubMed] [Google Scholar]
- 42.Morak MJM, et al. Adjuvant intra-arterial chemotherapy and radiotherapy versus surgery alone in resectable pancreatic and periampullary cancer: a prospective randomized controlled trial. Ann Surg. 2008;248:1031–1041. doi: 10.1097/SLA.0b013e318190c53e. [DOI] [PubMed] [Google Scholar]
- 43.Howard TJ, et al. A margin‐negative R0 resection accomplished with minimal postoperative complications is the surgeon’s contribution to long‐term survival in pancreatic cancer. J Gastrointest Surg. 2006;10:1338–1345. doi: 10.1016/j.gassur.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 44.Chua TC, Saxena A. Preoperative chemoradiation followed by surgical resection for resectable pancreatic cancer: a review of current results. Surg Oncol. 2011;20:161–168. doi: 10.1016/j.suronc.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 45.Bafeta A, et al. Analysis of the systematic reviews process in reports of network meta-analyses: methodological systematic review. BMJ. 2013;347:f3675. doi: 10.1136/bmj.f3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hutton B, et al. The quality of reporting methods and results in network meta-analyses: an overview of reviews and suggestions for improvement. PLoS One. 2014;9:e92508. doi: 10.1371/journal.pone.0092508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zarin W, et al. Characteristics and knowledge synthesis approach for 456 network meta-analyses: a scoping review. BMC Med. 2017;15:3. doi: 10.1186/s12916-016-0764-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faltinsen, E. G. et al. Network meta-analysis: the highest level of medical evidence? BMJ Evidence-Based Medicine. Published Online First, 10.1136/bmjebm-2017-110887 (14 March 2018). [DOI] [PubMed]
- 49.Guyatt GH, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Puhan MA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349:g5630. doi: 10.1136/bmj.g5630. [DOI] [PubMed] [Google Scholar]
- 51.Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JPT. Evaluating the quality of evidence from a network meta-analysis. PLoS One. 2014;9(7):e99682. doi: 10.1371/journal.pone.0099682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analysed during the current study are publicly available and sources are cited in supplementary material.