Abstract
Blood or biopsies are often used to characterize metabolites that are modulated by exercising muscle. However, blood has inputs derived from multiple tissues, biopsies cannot discriminate between secreted and intracellular metabolites, and their invasive nature is challenging for frequent collections in sensitive populations (e.g., children and pregnant women). Thus, minimally invasive approaches to interstitial fluid (IF) metabolomics would be valuable. A catheter was designed to collect IF from the gastrocnemius muscle of acutely anesthetized adult male rats at rest or immediately following 20 min of exercise (~60% of maximal O2 uptake). Nontargeted, gas chromatography-time-of-flight mass spectrometry analysis was used to detect 299 metabolites, including nonannotated metabolites, sugars, fatty acids, amino acids, and purine metabolites and derivatives. Just 43% of all detected metabolites were common to IF and blood plasma, and only 20% of exercise-modified metabolites were shared in both pools, highlighting that the blood does not fully reflect the metabolic outcomes in muscle. Notable exercise patterns included increased IF amino acids (except leucine and isoleucine), increased α-ketoglutarate and citrate (which may reflect tricarboxylic acid cataplerosis or shifts in nonmitochondrial pathways), and higher concentration of the signaling lipid oleamide. A preliminary study of human muscle IF was conducted using a 20-kDa microdialysis catheter placed in the vastus lateralis of five healthy adults at rest and during exercise (65% of estimated maximal heart rate). Approximately 70% of commonly detected metabolites discriminating rest vs. exercise in rats were also changed in exercising humans. Interstitium metabolomics may aid in the identification of molecules that signal muscle work (e.g., exertion and fatigue) and muscle health.
Keywords: beta-oxidation, interstitium, metabolome
INTRODUCTION
Muscle metabolism is impacted by acute changes in metabolite flux during periods of exertion and by chronic changes coincident with long-term metabolic health. As an example of the latter, insulin resistance has been associated with less efficient/incomplete skeletal muscle long-chain fatty acid (LCFA) combustion and impaired metabolic flexibility (a shift from fat oxidation toward carbohydrate metabolism in response to an increased presence of insulin) (19, 20, 26, 42). The concept that fat metabolism is altered by insulin resistance is supported by cross-limb respiratory quotient measurements (20, 21, 26), reduced whole body oxidation of plasma-derived LCFAs during exercise (3, 28), and, potentially, more limited entry of LCFA carbon into the tricarboxylic acid (TCA) cycle in muscle of fasted subjects with type 2 diabetes mellitus (4). Increased fitness and physical activity are directly linked to improved muscle insulin sensitivity and overall cardiometabolic health, yet the specific molecular signals and mechanisms involved are not fully elaborated.
During acute exercise, fuel “crossover” occurs as muscle work increases in terms of maximal or peak O2 uptake, the relative carbohydrate contribution to ATP needs (percentage of energy requirements met by carbohydrates) increases, and the relative role of fatty acids wanes (6). An acute submaximal exercise bout led to significant increases (from rest) in plasma indexes of incomplete fatty acid (medium-chain acylcarnitines) oxidation (FAO) (46), in line with other reports (16, 24). Other studies have examined broader exercise-associated metabolite patterns through application of metabolomics in blood (8, 9, 16, 25, 29, 31–36) or in a limited number of muscle biopsy studies (7, 12). These reports provide insight into the large muscle- and body-wide metabolic shifts that occur when muscle work is engaged. However, blood analyses have limitations in terms of identifying muscle-specific metabolites. Biopsy is invasive, and metabolomics application to these samples cannot discern intracellular and extracellular metabolites.
Approaches that monitor metabolite profiles in the muscle bed itself during or following exercise would be advantageous to explore muscle metabolism more proximally and accurately. This would open the door to clarification of temporal or diurnal patterns and metabolic shifts with changes in metabolic health. Such methods may also help characterize metabolite patterns that associate with, or serve as signals for, muscle fatigue and exertion during physical activity. Regarding the latter, we recently discovered that palmitoylcarnitine activates a subset of muscle-innervating neurons (46). Minimally invasive catheters for collection of interstitial fluid (IF) also have value in applications for which heavily invasive methods are not feasible, vis-à-vis in pediatric populations, the elderly, and pregnant women. To this end, a small-bore perfluoroalkoxy (PFA) catheter was developed to collect small volumes of muscle IF in a manner that does not require dialysis. In a proof-of-principle study, IF metabolomics analyses were conducted using samples collected adult male rats at rest and after an exercise bout. Since it is not clear how well blood plasma metabolite patterns reflect muscle metabolism, condition- and rat-matched IF and plasma metabolomics patterns were compared. Although there is at least one example of metabolomics analysis of muscle IF (15), to our knowledge, no experiments that used this approach in an acute exercise paradigm have been reported.
RESEARCH DESIGN AND METHODS
Animal Studies, Catheter Design, and Sample Collection
All animal studies were conducted with approval of the University of Utah Institutional Animal Care and Use Committee. Ten adult male Sprague-Dawley rats (Charles River) were fed standard laboratory rodent chow (catalog no. 2920X, Envigo) before testing. Animals were housed on a 12:12-h light-dark cycle (lights on 0800–2000) at 20°C. Half of the rats were relegated to rest or exercise at the initial screening and then switched 1 wk later to the other treatment; this yielded a total of 10 samples per condition for plasma and 10 and 9 samples for IF in the rest and exercise conditions, respectively (1 IF sample for exercise was insufficient for analysis). At the time of initial testing, the average body weight was 232 g. Experiments were carried out on 4 workdays every week, with up to three rats used on each day. All rats, regardless of initial testing paradigm, were twice acclimated to treadmill running before the initial experimental day: at −4 days and −1 day, rats were subjected to two 5-min sessions at 10 m/min. On the experimental day, food was removed at 0800, ∼5–6 h before exercise (or rest), which started at 1300, 1330, and 1400, respectively, for each rat. Exercise was carried out on a level treadmill (model 800, IITC Life Science, Woodland Hills, CA) at 18 m/min for 20 min. The workload was expected to elicit ∼60% of maximal O2 uptake (14). Blood and IF samples were collected as follows. Immediately after treadmill exercise, rats were anesthetized with 50 mg/kg ketamine-5 mg/kg xylazine ip and placed on a 37°C circulating water pad with the animal’s tail hanging off the edge of the surgical table. After the tail was briefly heated in 42°C water for 30 s, either the left or right tail vein that lies lateral to the tail artery was located and sterilized with 10% iodine-povidone solution. Approximately 250 µl of blood were withdrawn with a butterfly catheter connected to a 1-ml syringe at ~20 µl/s. Immediately after the blood draw, the needle was cut away from the catheter, and the blood sample was carefully expelled into a clear 500-µl Eppendorf tube and centrifuged at 5,000 rpm (2,040 g; model 5415C, Eppendorf) for 10 min. After centrifugation, plasma was eluted in two ~50-µl aliquots into 200-µl tubes, with care taken not to disturb the red and white blood cell layers, and the tubes containing the plasma were placed into a mixture of dry ice pellets and methanol for freezing. The 500-µl Eppendorf tube, 1-ml syringes, and butterfly catheters were pretreated with 0.1 M EDTA as an anticoagulant (10 µl in the 500-µl tube).
IF samples were collected during centrifugation of the blood samples. Rats were laid on their stomach on the heating pad. Hair was shaved, skin was sterilized using 10% iodine-povidone solution, and a ~3-mm skin incision was made near the Achilles tendon in the hindleg to allow insertion of the catheter into the center of the gastrocnemius muscle, ~10 mm rostrocaudally. Care was taken to ensure that placement of the tip was parallel with muscle fibers, thus minimizing damage to tissue during insertion. The intravenous (IV) catheter was then withdrawn, with only the tip of the IF catheter left in the muscle. Accumulated IF was collected through the holes in the insertion tip by means of a syringe attached to the opposite end of the catheter, which created an area of low pressure within the lumen. Over a period of ≤5 min, ~2–5 µl of IF was collected from the gastrocnemius muscle. The IF was immediately transferred to a 200-µl tube and briefly centrifuged, and the tube was frozen in a mixture of dry ice pellets and methanol. Unlike blood collection, no apparatus was treated with EDTA. Thus, collections of both blood and IF samples were completed within 10 min after exercise ended. After the collection, the skin incision was closed with sterile cyan methacrylate glue, and anesthetic (150 µl of 1:1 2% lidocaine-0.5% bupivacaine) was injected into the muscle. Rats were allowed to recover from anesthesia on the heating pad and then returned to the housing cage. All samples were stored at −80°C for future analysis.
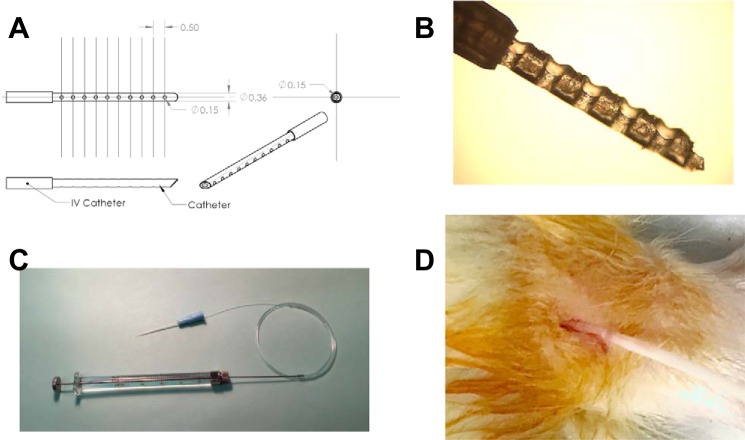
The IF extraction catheter comprises a segment of PFA tubing (IDEX Health and Science, Oak Harbor, WA) that can vary in length based on the muscle tissue into which it is placed. For the rat studies described here, a catheter with a 360-µm outer-diameter (OD) tube and a 150-µm inner-diameter (ID) lumen was made (Fig. 1). The tube contains 150-µm through holes placed orthogonally to the lumen axis and centered around the lumen. The holes were fabricated using a CO2 laser system (VLS3.60, Universal Laser Systems), spaced 500 µm apart, and located within 10 mm of the tip of the tubing, which was placed within the muscle tissue. Under a microscope, a razor blade was used to cut the insertion tip of the tubing at a 45° angle. The 360-µm-long tubing segment opposite the insertion tip was glued using UV cure adhesive (Loctite 3106), inserted into a larger segment of Tygon tubing [1.524 mm (0.060 inch) OD, 0.508 mm (0.020 inch) ID; Cole-Parmer 0642002], and cured in place with UV light. This process ensures that the glue does not occlude the tubing while providing an airtight seal. Each of the two tubing segments was 10 cm long. The two segments of tubing were attached, such that there was little or no dead space between the outer wall of the PFA tubing and the inner wall of the Tygon tubing, and their lumens were made continuous and without obstruction. Before the experiment, the tip of the IF catheter was led through an IV catheter (800 µm OD, 490 µm ID; catalog no. 2862, Deseret, Sandy, UT), with the opposite end of the catheter attached to a 50-µl Hamilton syringe (Fig. 1).
Fig. 1.
Intramuscular catheter used to collect interstitial fluid from the gastrocnemius muscle of adult male rats. A: schematic of catheter outer dimensions, inner dimensions, length, and through holes. B: magnified photograph of catheter tip. C: photograph of entire catheter-syringe apparatus. D: photograph of catheter inserted into gastrocnemius muscle of an anesthetized rat.
Feasibility Studies in Human Participants
Studies on human subjects were approved by the Florida State University Institutional Review Board, in accordance with the Declaration of Helsinki guidelines. Five healthy adults (3 female and 2 male, body mass index >18 to <35 kg/m2, 21–46 yr of age) were included. Participants were free of cardiovascular disease, hypertension, metabolic disease, acute or chronic infections, bleeding disorders, rheumatoid arthritis, or serious orthopedic problems that would impair their ability to exercise. Participants provided informed consent before participation.
Microdialysis.
Participants were studied late-morning through afternoon, with sample collection initiated ~5 h after a nonstandardized breakfast in three subjects (2 subjects had voluntarily skipped breakfast); water was provided ad libitum throughout the 5 h of sample collection. Microdialysis probes with a 20-kDa membrane cutoff (CMA 20 Elite, CMA Microdialysis, Solna, Sweden) were inserted into the vastus lateralis muscle under sterile technique and ethyl chloride spray local anesthesia, as previously described (22). Probes were perfused using microinfusion pumps (CMA 107, CMA/Microdialysis) at a flow rate of 2.0 µl/min with 0.9% saline containing 10 mmol/l ethanol. After insertion, probes were perfused for ≥60 min during an equilibration period without sample collection. Then three 20-min dialysate samples (40 µl each) were collected in the resting state, with the participant in a seated/reclined position. A 30-min (60-µl) sample was subsequently collected during upright lower-body cycle ergometer (Monark Exercise, Vansbro, Sweden) exercise, performed at an intensity eliciting a heart rate of 65% of the participant’s estimated maximum heart rate, as measured using a commercially available heart rate monitor (Polar Electro, Bethpage, NY). Dialysate samples were collected in capped 150-µl polyethylene collection vials, which were stored at −80°C for subsequent analysis.
Metabolomics Analysis
Plasma and IF samples used for nontargeted metabolomics of primary metabolism were delivered on dry ice to the West Coast Metabolomics Center at the University of California Davis Genome Center. For plasma, in-depth details of the analytical methods are described elsewhere (13). Briefly, samples were thawed, extracted, and derivatized by silylation-methyloximation before analysis by gas chromatography-quadrupole time-of-flight mass spectrometry (GC-TOF-MS). Results were processed by the Fiehnlab metabolomics BinBase database and matched against spectra from the Fiehnlab mass spectral library of 1,200 authentic metabolite spectra using retention index and mass spectrum information or the National Institute of Science and Technology (NIST 05) commercial library [thus yielding named (annotated) metabolites]. Metabolite data are provided as peak heights of quantifier ions normalized to the sum intensities of all annotated metabolites. Ions lacking structural identification (nonannotated metabolites) were assigned BinBase identification numbers (37) and included in all statistical analyses. Mass spectra, retention indexes, quantification ions, and presence in a complement of >1,500 studies can be interrogated for all nonannotated metabolites in a new publically available BinVestigate tool (23). Metabolomics data are provided as Supplemental Table S1 (Supplemental Material for this article is available online at the Journal website). IF was processed as follows. Extraction solvent composed of 3:3:2 acetonitrile-isopropanol-Milli-Q H2O was degassed by sonication and then bubbled with nitrogen gas for 5 min. Solvents were stored at −20°C and then kept on ice throughout the extraction (samples were also kept on ice). For each freshly thawed sample, 20 µl of 3:3:2 solvent were added, and the sample was lightly vortexed and then transferred to a 1.5-ml microcentrifuge tube. Each sample was subjected to this protocol twice to effectively transfer the entire sample to the tube. An additional 460 µl of 3:3:2 solvent were added to the new tubes and transferred sample, and the samples were vortexed for 20 s. Samples were shaken for 5 min at 4°C and centrifuged at 14,000 g for 2 min, and total supernatant was transferred to a clean 1.5-ml microcentrifuge tube. Samples were dried in a vacuum concentrator (Centrivap, Labconco) to dryness to prepare for derivatization. Methoxyamine hydrochloride (Meox) was dissolved in pyridine to a concentration of 40 mg/ml. After addition of 10 µl of Meox to each sample, the samples were shaken for 1.5 h at 30°C; then 41 µl of N-methyl-N-(trimethylsilyl) trifluoroacetamide were added. Samples were shaken for 30 min at 37°C and then centrifuged at 14,000 g for 2 min before transfer in deactivated, high-recovery crimp-capped autosampler vials for subsequent analysis by GC-TOF-MS with a 0.5-µl injection. Because the general extraction and derivatization conditions for plasma and IF are the same, they enable detection of the same metabolite classes. A blank (water flushed through an unused IF catheter) was also analyzed to ensure that metabolites reported here were not due to artifacts from the sampling device. Each rat sample was processed in its entirety; for human samples, 30 µl were processed for metabolomics analysis. For the resting condition in humans, 10 µl from each of the three resting period collections (see above) were pooled for analysis of 30 µl (to obtain a representative resting sample). Metabolite concentrations are semiquantitative, since they reflect the quantifier ion peak heights and not absolute concentrations in molar terms.
Statistical Analysis
R (version 3.1.2, http://www.r-project.org/) was used for all statistical analyses and figures. Group differences between exercise and rest in rats were compared using Wilcoxon’s signed-rank test, a nonparametric test to compare paired samples. For rat studies, multiple-comparison adjustments were made using the Benjamini-Hochberg method, with statistical significance set at an adjusted P < 0.05. The main difference between the univariate Wilcoxon signed-rank test (for paired samples) and the multivariate partial least squares-discriminant analysis (PLS-DA; see below) is that the latter can deal with autocorrelation or multicollinearity, which is expected in metabolomics data. Therefore, many metabolites that were not significant by the classical univariate method after adjustment for multiple comparisons were significant by the multivariate method.
Data preprocessing for multivariate analysis for rat studies.
All metabolite data were first checked for missing values and outliers. Samples were detected as outliers if they were far outside the 95% confidence ellipse (Hotelling’s T2) in a multivariate principal component (PC) analysis plot. We compute the Mahalanobis distance of each sample from the center of the k-dimensional PC space (k being the number of PCs that explains ≥70% of the variation in the data). Under the null hypothesis, all samples arise from the same multivariate normal distribution. Therefore, the distance from the center of a k-dimensional PC space should follow a χ2 distribution with k degrees of freedom. Thus P values can be computed for the Mahalanobis distances. In this study, no such outlier was detected in PC analysis plots. One sample was missing for IF, and thus no metabolomics data were available for the analysis, as noted. No metabolites were filtered out, inasmuch as none had missing data. For standardization across the wide range of absolute concentration data of the metabolite variables in the models, the data were centered around zero and scaled to unit variance before multivariate analysis.
PLS-DA for rat studies.
PLS-DA, a supervised method of pattern recognition, was used to identify metabolites that were significantly associated with group differences (exercise vs. rest). The optimum number of latent components in each model was selected based on minimization of the cross-validated prediction error. The model parameters R2 (goodness of fit) and Q2 (goodness of prediction), calculated by sevenfold internal cross-validation, were used to assess the quality of the models. In all comparisons, the optimum models were further validated by repeated permutations (n = 100) of the sample identifiers, and a reference distribution of all R2 and Q2 values from the permuted PLS-DA models was vetted against the actual model. For each model, the major latent variables were represented in a scores scatterplot, and the significant predictors were identified based on their variable importance on projection (VIP) scores. PLS-DA models for IF and plasma were built in two steps. In step 1, the optimum models were built following the above-described protocol. The top metabolites that contributed to the PLS-DA models with VIP scores ≥1 were then selected to build further-refined models to identify a smaller subset of major contributors. For clarity, we present the graphs from refined models in results but provide all data, including initial model VIP scores (see Supplemental Tables S1a and S1b). For IF, an optimum two-component refined PLS-DA model captured ~53.7% of the variation in the predictor variables that correlated with ~86.9% (R2) of variation in the rest and exercise samples, with a predictive estimate (Q2) of 62.2%. For plasma, the refined model captured ~33.1% variation in the predictor variables that correlated with 94.5% variation in the rest and exercise samples, with Q2 = 79.5%.
The metabolites that were significant by univariate or multivariate analysis were classified according to the main classes of the metabolite databases at the National Institutes of Health Metabolomics Data Repository (http://www.metabolomicsworkbench.org) and graphically presented as heat maps of log2 fold changes (exercise vs. rest) for each rat.
RESULTS
Metabolomics of Rat Skeletal Muscle IF
A total of 299 metabolites were detected in IF from rested and 20-min-exercised rats (Supplemental Table S1). Generally speaking, there was substantial within-group variance for most metabolites for IF, making detection of significant exercise vs. rest differences by univariate statistics (paired Mann-Whitney U-test) challenging. To identify IF metabolites that best discriminate the resting vs. exercised metabolic phenotypes, a refined PLS-DA model was generated using metabolites with a VIP score ≥1 from the initial model that contained all 299 detected metabolites. The refined model using 139 metabolites (plus phenylethylamine, which was significantly different by univariate paired Mann-Whitney U-test) successfully discriminated samples derived from rest or exercise conditions, as evident from the scores plot, where groups separated primarily along the latent variable 1 axis (Fig. 2A; each symbol represents the PLS-DA score for a single rat). Interstitial metabolites reflective of exercise status that were included in the refined PLS-DA model and have known identities to allow for grouping into biochemical classes are discussed below.
Fig. 2.
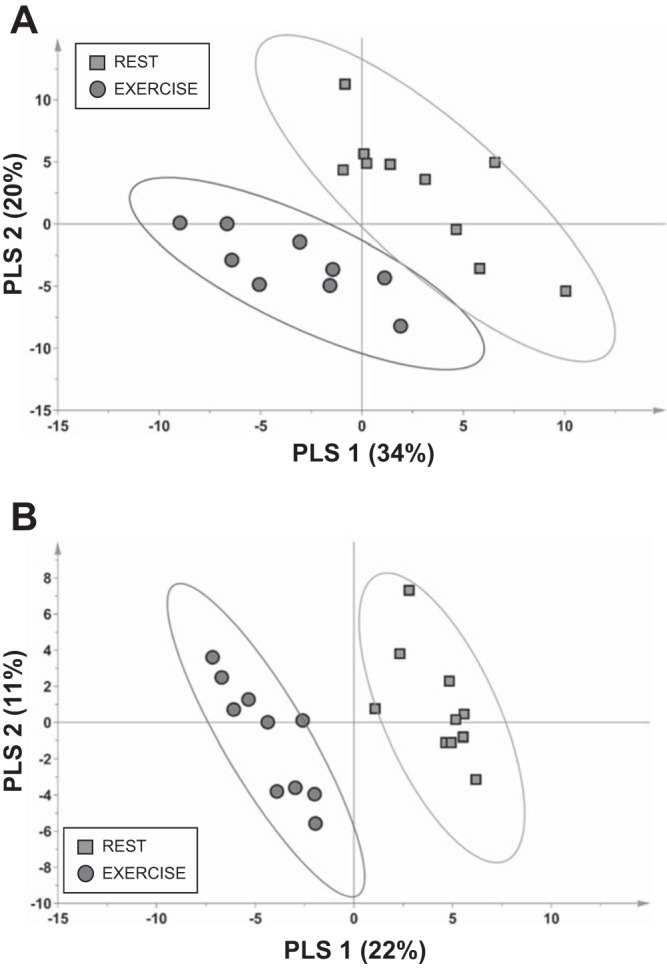
Plots of partial least squares (PLS)-discriminant analysis scores highlight that variances in concentrations of select metabolites in muscle interstitial fluid (A) and blood plasma (B) can readily discriminate the rested state from the immediate postexercise state in adult male rats. Each score from individual rats is shown. Confidence regions of group clusters are presented as 95% confidence ellipses based on Hotelling’s T2 statistic. Exercise samples were collected in anesthetized animals ~10 min after cessation of the acute exercise bout.
Amino acids and derivatives; nitrogenous metabolites.
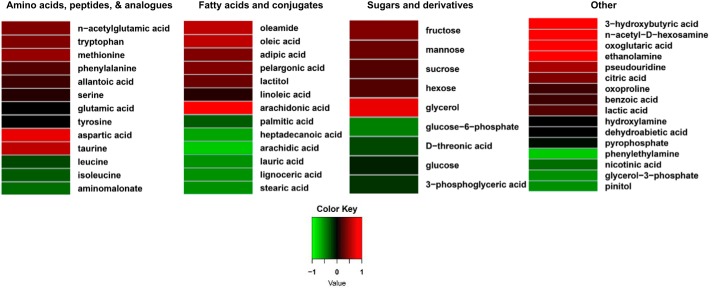
IF concentration of most metabolites in this class increased following the 20-min exercise bout (Fig. 3 and Supplemental Table S2). The most robust of these (e.g., with >25% increases) were aspartic acid, tryptophan, allantoic acid (a purine derivative), methionine, and N-acetylglutamate (a product of acetyl-CoA and glutamate via N-acetylglutamate synthase). Phenylalanine was increased 21%. Concentrations of several metabolites (e.g., isoleucine, leucine, and aminomalonate) were reduced (Fig. 3 and Supplemental Table S2) and several others (e.g., serine, tyrosine, and glutamic acid) displayed small increases following exercise. Urea concentration in IF was ~28% lower postexercise. The concentration of taurine, a cysteine metabolite, was doubled in IF following exercise.
Fig. 3.
Heat maps depicting log fold concentration changes in muscle interstitial fluid metabolites in adult male rats following an acute exercise bout relative to the rested state. Only metabolites that were contributing factors in the interstitial fluid partial least squares-discriminant analysis model (see Fig. 2A) and were annotated are shown. Mean concentration values from each condition were used to generate heat map results (from 9 rats). Metabolites were classified according to the main classes of the metabolite databases at the National Institutes of Health Metabolomics Data Repository (http://www.metabolomicsworkbench.org).
Fatty acids and derivatives.
In the period immediately following acute exercise, IF concentrations of unsaturated LCFAs, including oleic (C18:1), arachidonic (20:4), and linoleic (18:2) acids, were increased at least ~50% relative to the rested state (Fig. 3 and Supplemental Table S2). In distinct contrast, IF concentrations of saturated LCFAs, including palmitic (C16:0), heptadecanoic (C17:0), stearic (C18:0), arachidic (C20:0), and lignoceric (C24:0) acids, were reduced postexercise (Fig. 3 and Supplemental Table S2). The medium-chain fatty acids pelargonic (C9:0) and lauric (C12:0) acids displayed disparate patterns of a modest 15% increase and 22% decrease, respectively. IF concentration of the ketone body 3-hydroxybutyric acid (also known as β-hydroxybutyrate) was substantially increased following exercise.
Monosaccharides, sugars, and derivatives.
IF concentrations of a variety of monosaccharides, including fructose (which was at very low abundance during exercise and at rest), mannose, and a hexose (“hexose” is mostly glucose, but the peak represents the nonmethoximated product that is not separated from other hexoses), were increased postexercise compared with the rested state (Fig. 3 and Supplemental Table S2). The IF concentration of glucose-6-phosphate was ~30% lower following exercise, whereas that of lactate increased ~36% (Fig. 3 and Supplemental Table S2). Although IF glucose was a discriminating variable in the refined PLS-DA model, glucose concentration difference with exercise was minimal. The sugar acids isothreonic (or d-threonic) acid and 3-phosphoglycerate were ~33% increased and 46% decreased, respectively.
Oleamide, other metabolites, and unknowns.
Exercise elicited substantial changes in several metabolites from various classes (Fig. 3 and Supplemental Table S2). Particularly striking was the endocannabinoid-signaling lipid amide metabolite oleamide, for which IF levels rose more than threefold compared with the rested state. Other IF metabolites that increased substantially with exercise were N-acetyl-d-hexosamine and ethanolamine (Fig. 3 and Supplemental Table S2). Citric acid and α-ketoglutarate, which can be derived from TCA cycle metabolism, among other sources (e.g., amino acid metabolism for α-ketoglutarate), were increased ~26% and ~59%, respectively. The metabolites glycerol-α-phosphate, pinitol, and nicotinic acid were ~29–44% lower in postexercise IF (Fig. 3 and Supplemental Table S2).
Metabolomics of Rat Plasma
A total of 353 metabolites were detected in plasma from rested and 20-min-exercised rats (Supplemental Table S3). To identify plasma metabolites that best discriminate the resting vs. exercised metabolic phenotype, a refined PLS-DA model was generated using plasma metabolites with a VIP score >1 from the initial model that included all 353 plasma metabolites. The refined model using 108 metabolites could successfully discriminate samples derived from rest or exercise, as evident from the scores plot, where groups separated primarily along the latent variable 1 axis (Fig. 2B). Plasma metabolites reflective of exercise status that were included in the refined PLS-DA model and have known identities to allow for grouping into biochemical classes are discussed below.
Amino acids and derivatives; nitrogenous metabolites.
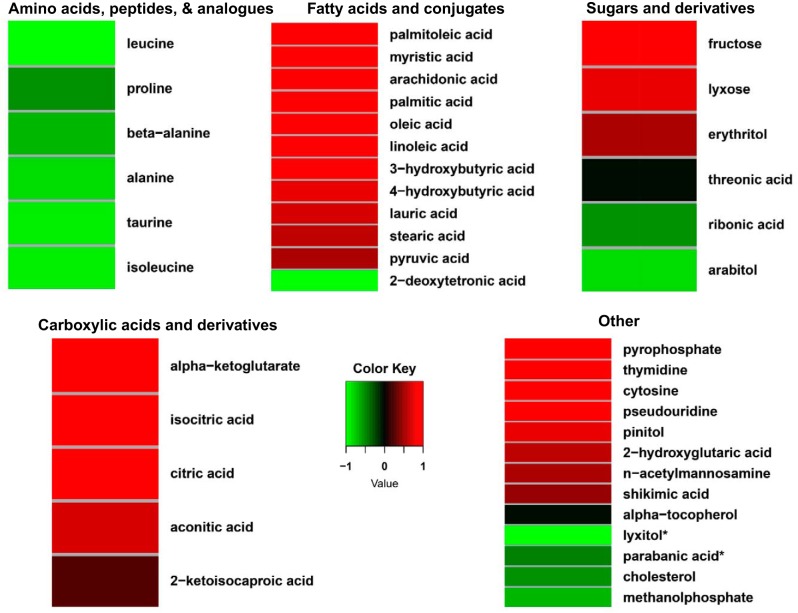
Plasma amino acids displayed a consistent exercise-associated decrease in concentration following a 20-min exercise bout (Fig. 4 and Supplemental Table S3). Plasma urea concentration was modestly reduced by ~10% following exercise. The concentration of plasma taurine, a cysteine metabolite, was 50% lower with exercise than in the rested state, in distinct contrast to the directional change in IF.
Fig. 4.
Heat maps depicting log fold concentration changes in blood plasma metabolites in adult male rats following an acute exercise bout relative to the rested state. Only metabolites that were contributing factors in the interstitial fluid partial least squares-discriminant analysis model (see Fig. 2B) and that were annotated are shown. Mean concentration values from each condition were used to generate heat map results (from 10 rats). Metabolites were classified according to the main classes of the metabolite databases at the National Institutes of Health Metabolomics Data Repository (http://www.metabolomicsworkbench.org). *Not classified in the database.
Fatty acids and derivatives.
Plasma concentrations of unsaturated LCFAs, including oleic, arachidonic, palmitoleic, and linoleic acids (Fig. 4 and Supplemental Table S3), were ~50–200% higher in the period immediately following an acute exercise bout than in the rested state. Saturated LCFAs, including palmitic and stearic acids, were also increased, although more modestly, in the postexercise period. The plasma concentration of the ketone body 3-hydroxybutyrate was increased nearly threefold following exercise.
Monosaccharides, sugars, and derivatives.
There were few sugars that were discriminating variables in plasma for comparison of rest with the postexercise period. Plasma concentrations of the low-abundance sugars fructose and lyxose (an aldopentose) were higher (~40–50%), as were concentrations of the sugar acid isothreonic acid (~26%) and the sugar alcohol erythritol (~30%), following exercise (Fig. 4 and Supplemental Table S3). The sugar alcohols arabitol and lyxitol were lower (~20%) and the sugar acid ribonic acid was reduced ~23% in plasma following exercise.
Other metabolites and unknowns.
Exercise elicited substantial changes in several metabolites from various classes (Fig. 4 and Supplemental Table S3). Plasma concentrations of citric acid, isocitric and aconitic acids, and α-ketoglutarate, which can be derived from TCA cycle metabolism (among other sources), were increased ~23–54% postexercise compared with the resting state (Fig. 4 and Supplemental Table S3). The pyrimidine-associated metabolites cytosine and thymidine were also increased in plasma postexercise. Plasma concentrations of the plant-derived xenometabolites shikimic acid and pinitol were more than twofold and ~34% higher, respectively, postexercise. The plasma concentration of a potential oxidation radical marker, parabanic acid, was lower (~29%), as was that of methanol phosphate (~63%).
Concurrence of Muscle IF and Blood Plasma Metabolite Patterns in Rats
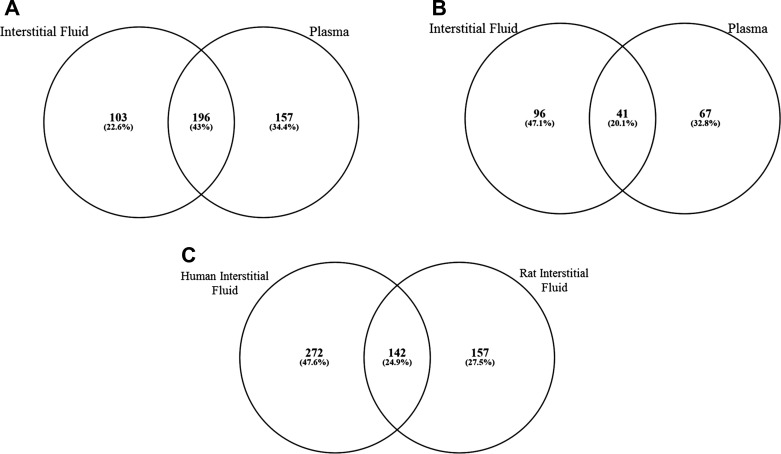
Of the 456 distinct molecules detectable by GC-TOF-MS in the samples (in IF and plasma), ~43% were detected in both IF and plasma (Fig. 5A and Supplemental Table S4a). There were 103 metabolites detected in IF but not plasma, and 157 were detected in plasma but not IF. Differences in a total of 204 metabolites contributed to PLS-DA models that discriminated rest vs. exercise in either IF or plasma. Of these, only 41 were included in both IF and plasma in rest vs. exercise models (Fig. 5B and Supplemental Table S4b). This represents just 20% overlap, indicating that the muscle IF and plasma pools respond differently to exercise with respect to metabolite patterns. The chemical class information for annotated metabolites in each pool is provided in Supplemental Table S4 tabs; there was no obvious pool-specific segregation by metabolite class.
Fig. 5.
Concurrence between adult male rat muscle interstitial fluid and rat blood plasma metabolites that were detected using the gas chromatography-time-of-flight analytical platform (A), rat metabolites included in partial least squares-discriminant analysis models that discriminated resting from exercised states (B), and detected human interstitial fluid metabolites (C). Numbers within each section of the Venn diagram represent the number of metabolites, and percentages refer to the fraction of all detected metabolites within that section. Comparator lists may be found in Supplemental Tables S4 (rat comparisons) and S6 (rat vs. human comparison).
Metabolomics of Human Skeletal Muscle IF
The muscle bed microdialysis method allowed for collection of IF during exercise. The small sample size and focus of the experiment as a feasibility study preclude application of formal statistical analysis, yet the results provide remarkable first-ever insight into the global metabolite pool in human IF. A total of 414 metabolites were detected in human muscle IF (Supplemental Table S5). Of these, 142 were also detected in male rat IF (Fig. 5C and Supplemental Table S4c). The chemical class information for annotated metabolites comparing rat and human IF is provided in Supplemental Table S4 tabs; there was no obvious pool-specific segregation by metabolite class. This preliminary study of exercise-associated human IF metabolomics indicated that a substantial number of metabolites displayed a mean change in IF concentration of ≥|25%| compared with the overnight-fasted, resting condition (Supplemental Table S5). We acknowledge that there were many differences in terms of experimental conditions and also catheter type when the studies in humans were compared with studies in rats. Despite these important caveats, it is notable that, of the rat metabolites that contributed to the discrimination of rest vs. exercise (Fig. 2), 78 were also detected in human IF and, of these, ∼70% changed in the same direction as seen in rats in response to exercise. While we acknowledge the preliminary nature of our findings, the results are consistent with the hypothesis that many acute exercise-associated metabolic shifts in skeletal muscle are shared between rats and humans.
DISCUSSION
This study determined, for the first time, how an acute exercise bout influences large-scale metabolite profiles in the IF of the skeletal muscle bed in a rodent model and, in preliminary studies, also measured IF metabolite shifts during a moderate-exercise test in healthy human subjects. To our knowledge, only one previous study has applied metabolomics analysis to human muscle IF, in the resting and recovery period of fibromyalgia patients long after exercise stopped (at 60–80 min after cessation of exercise), following a 1 h 40 min repetitive arm-work test and subsequent Trier social stress test (15). The current proof-of-principle experiments further establish that IF metabolomics analysis is a minimally invasive method that allows for identification of hundreds of exercise-responsive metabolites in this pool. Furthermore, this study indicates that the IF pool is clearly distinct from the blood plasma pool with respect to metabolite profiles. Considering the importance of muscle metabolism to whole body fuel utilization and insulin action and the impact of physical activity and sedentary behavior on metabolic health, application of muscle IF metabolomics should prove to be a valuable tool for understanding the molecular underpinnings associated with these outcomes.
Particularly interesting was the lack of concurrence of rat IF and plasma metabolite patterns. This was true for both the rested and exercise conditions, indicating that measurement of plasma alone will not fully discern muscle metabolism and metabolic flux. This general concept is in line with studies that have applied nontargeted metabolomics to compare muscle biopsy and plasma in nonexercising young and elderly subjects (12) and experiments in mice that considered patterns of select acylcarnitines in specific muscle groups vs. plasma in the fed-to-fasted transition (38). Flux rates into and out of each pool for many metabolites could lead to disparate accumulation in IF vs. blood, and muscle-derived metabolites might be enriched in the IF pool. Should metabolites prominent in the much smaller IF pool make their way to the large-volume blood pool, a dilution effect coupled to blood flow transiting the metabolites away from the tissue bed would lead to a lower concentration in the peripheral blood than IF. More research is needed to define metabolite flux rates and net directional flow into and out of the muscle IF to understand drivers of the IF metabolite profile at rest and during muscular exertion.
Several exercise-associated muscle IF metabolite patterns are notable. First, increased IF concentrations of α-ketoglutarate (~59% higher) and citric acid (~26% higher) differentiated the exercised vs. rested states in rats. In our preliminary study in humans, mean IF concentrations of citric acid (citrate) and malic acid (malate) were increased (62% and 31%, respectively) during exercise. The etiology of these patterns cannot be ascertained from the current experimental design, but it is plausible that exercise triggers efflux of mitochondrial TCA intermediates through cataplerosis, coincident with higher FAO: in experiments using mouse muscle mitochondria (39) or isolated perfused rat hearts (44), increasing FAO promoted cataplerotic export of α-ketoglutarate, citrate, and/or malate. It has been suggested that regulation of the mitochondrial TCA transporter plays a role in FAO-induced cataplerosis (44). If exercise-associated FAO activates cataplerosis (and/or reduces net anaplerosis), the TCA cycle intermediate pool could drop; if this does occur, this may help explain why exercise acutely raises concentrations of blood markers of incomplete β-oxidation (16, 24, 46).
Second, exercise-associated differences in nitrogen-containing IF metabolites, amino acids and derivatives, contributed to the discrimination of rest vs. exercise in rats. Several amino acids (aspartate, tryptophan, methionine, phenylalanine, serine, tyrosine, and glutamate) were increased in IF with exercise. In the preliminary study of exercised human subjects, IF concentrations were also increased for ≥12 amino acids. It may be speculated that exercise induces net flux of amino acids from the blood pool into the muscle, which is not exactly matched to muscle uptake and catabolism. Because of the lack of flux measurements and the significant animal-to-animal and person-to-person variability in the responses of some amino acids, it is not possible to make definite conclusions regarding the main drivers of exercise-associated IF amino acid patterns. Based on blood acylcarnitine patterns, we previously hypothesized (46) that, in the initial acute phase of a moderate-exercise bout in humans, branched-chain amino acid flux through the branched-chain ketoacid dehydrogenase complex is reduced due to inhibition of the enzyme (and perhaps other dehydrogenases involved in amino acid catabolism) by increased FAO and the attendant increase in the NADH-to-NAD+ ratio (1, 10). In the preliminary experiment in humans, mean IF concentration of the amino acid derivative 2-hydroxybutanoic acid (2-HB; also known as 2-hydroxybutyrate or α-hydroxybutryate) was increased ~50% in samples collected coincident with exercise. 2-HB accumulation is suggested to reflect, in part, dampened flux of the 2-HB precursor 2-ketobutyrate through branched-chain ketoacid dehydrogenase complex, coupled to lactate dehydrogenase activity (which is thought to convert 2-ketobutyrate to 2-HB under conditions of an increased NADH-to-NAD+ ratio as seen during active FAO) (1). Taurine was another nitrogen-containing metabolite significantly increased in rat IF with exercise (~2-fold compared with rest). Since skeletal muscle mRNA expression of cysteine dioxygenase 1 and cysteine sulfinic acid decarboxylase, the first two enzymes in the cysteine metabolic pathway to taurine, is trivial in mice and humans [http://biogps.org (45)], the increased taurine concentration in IF during exercise likely originates in other tissues (e.g., liver and adipose). In support of this idea, higher plasma taurine helped differentiate exercised from rested rats in the current study. Interestingly, taurine was not detected in human IF with exercise, which may point to a species-specific effect in cysteine/taurine metabolism in this context.
Third, muscle IF metabolomics results support the idea that acute exercise, with coincident accumulation of AMP, triggers purine catabolism and accumulation of derivatives. In rats, increased IF allantoic acid (also known as allantoin) was a feature of the model differentiating exercise from rest. Allantoic acid production increased following metabolic stress in muscle cell cultures (27) and with exercise in human muscle biopsies and blood (18), conditions in which high-energy phosphates are consumed and AMP pools rise. Allantoin is derived from uric acid as part of the pathway AMP → IMP → inosine → hypoxanthine → xanthine → uric acid → allantoic acid. The concept that exercise activates flux through this pathway in muscle is further bolstered by the preliminary results of IF metabolites in humans: mean concentrations of inosine, hypoxanthine, and uric acid were substantially increased during the exercise bout.
Fourth, patterns of carbohydrates and lipids in rat IF were consistent with a major and expected impact of exercise on metabolism of these fuels. At the intensity of exercise in both the rat and human studies, IF concentration of lactic acid rose, as anticipated. In both rats and humans, the levels of ketone body 3-hydroxybutanoic acid (also known as 3-hydroxybutyric acid or β-hydroxybutyrate) were higher in IF, consistent with enhanced liver ketogenesis at the workload and duration of exercise used in our studies. Indeed, a higher blood β-hydroxybutyrate concentration was a defining variable for exercise in the rats (blood metabolites were not available from the human pilot study). Exercise elicited an increase in IF glycerol concentration in rats and humans, but plasma glycerol was not a factor in the multivariate models defining rest vs. exercise in rats. It is intriguing to consider that this pattern reflected hydrolysis of localized, resident triglyceride stores in muscle, which can be significant based on isotope flux studies (43). There was an exercise-associated increase in unsaturated fatty acid concentrations in IF, yet, unexpectedly, concentrations of virtually all saturated fatty acids detected in IF were lower after exercise. The mechanisms that explain such disparate patterns of unsaturated and saturated fatty acids in rat IF are elusive, especially in light of a generally shared exercise-associated increase in all blood plasma fatty acids. The exercise-associated fatty acid patterns in IF were quite different in the human preliminary study: saturated and unsaturated fatty acids were lower during exercise than at rest. We suspect that rat and human fatty acid pattern differences are explained, in part, by the timing and conditions of sample collection, which differed between studies (~10 min after exercise cessation under anesthesia in rats vs. coincident with exercise in humans). During moderate exercise in humans, the decrease in blood plasma fatty acid concentrations is concurrent with enhanced body-wide and muscle FAO (46); such events may have contributed to the exercise-associated reduction in fatty acid concentrations in IF in the human pilot study. Blood fatty acid concentrations rise rapidly to even exceed resting values in humans, coincident with exercise recovery (46); if this did occur in our rat model, this phenomenon may have influenced IF fatty acid profiles, since they were measured after a brief period of recovery.
Fifth, it was interesting to observe a nearly threefold increase in IF concentration of the endocannabinoid-signaling lipid molecule oleamide following exercise in rats. This metabolite was not detected in the blood plasma pool under our conditions, suggesting localized production of oleamide around the muscle bed itself. Myocyte or muscle tissue production of oleamide has not, to our knowledge, been described previously, and muscle expresses negligible transcript encoding the key oleamide synthesis pathway enzyme peptidylglycine α-amidating monooxygenase [http://biogps.org (45)]. Yet, in their biochemistry studies, Driscoll and colleagues (11, 30) showed that cytochrome c can also synthesize oleamide, and even the oleamide precursor oleoylglycine, from oleoyl-CoA substrate, in a substrate concentration-dependent manner. Future experiments are required to validate the cellular origins of oleamide. In light of the established role of oleamide as a modulator of serotonin action on neuronal 5-hydroxytryptamine receptors (2, 5, 17, 40, 41), it is exciting to consider if exercise-induced oleamide in muscle serves to signal exertion, fatigue, or other information to muscle-innervating somatosensory neurons. This metabolite was not detected in human IF in our pilot study: whether this reflects a species-specific biology or is due to different sampling conditions remains to be determined.
Strengths, Limitations, and Future Directions
The proof-of-principle studies outlined here established that use of muscle IF metabolomics to monitor acute exercise-associated shifts in metabolism is feasible in both rodents and humans. The results for rat IF indicate that, to fully capture the nuances of muscle-associated metabolism, it is necessary to move beyond blood metabolite profiling and into a more direct interrogation of the metabolome of the muscle bed itself. The IF catheter approach has particular utility for this purpose when characterizing temporal, within-subject alterations in muscle metabolism, especially in sensitive populations, such as children, the elderly, and pregnant women, for which repeated muscle biopsy is not feasible.
Limitations.
First, the human feasibility experiments included a small sample size and, hence, large interindividual differences in IF responses to exercise, possibly due to a wide range of body mass index, age, and examination of men and women; future studies will control for these important variables and others, such as collection time and lead-in diet. Second, for the rat studies, anesthesia, the slight delay in IF collection postexercise, and the catheter insertion process with potential acute inflammation/stress may have contributed to variability. Third, the interpretations are limited to select metabolites, and extraction of more sample material and/or application of additional analytical platforms would provide a more complete metabolomics profile for very-low-abundance metabolites and across multiple chemical classes. Fourth, we acknowledge that the use of two different catheter systems and molecular weight cutoffs, plus different experimental paradigms, limits our ability to compare metabolomics patterns across species; a more direct test of species-specific patterns awaits experiments directed at this question. Fifth, without metabolite flux measurements, the origins of concentration differences between conditions or pools could not be confirmed (e.g., tissue origins and production/utilization). Finally, as with all multivariate statistical models, there is always the potential for overfitting, and there is a need to follow up on metabolite leads in future validation studies.
Despite several limitations, the present studies revealed novel exercise-associated metabolic patterns and stark contrasts between IF and blood pools, forming the foundation for future experiments to address origins of these outcomes. Considering the challenges of repeated muscle biopsy and the inability to discern intracellular from extracellular metabolites from tissue samples, catheterization of the muscle interstitium is a promising, minimally invasive tool for examination of temporal and conditional changes in muscle metabolism through application of metabolomics. Of many future areas of interest that can apply this technique, exploration into the origins of exercise-associated shifts in xenometabolite concentrations in IF and plasma is intriguing. Here, shikimic acid and pinitol were identified in rats as exercise-modified metabolites, and previously we reported that acute exercise increases plasma cis-3,4-methylene-heptanoylcarnitine (a xeno-lipid derivative) in humans (46). Other studies could explore sex-specific muscle metabolomics or IF metabolism throughout the lifespan in response to exercise. In summary, cataloging and tracking muscle IF metabolites have promise to identify new pathways and potential signaling molecules reflective of muscle health, metabolism, exertion, and fatigue.
GRANTS
This research was supported by intramural US Department of Agriculture-Agricultural Research Service Project 6026-51000-010-05S and National Institute of Diabetes and Digestive and Kidney Diseases Grants R01 DK-078328 (to S.H. Adams) and U24 DK-097154 and U2C ES-030158 (to O. Fiehn) and a National Institutes of Health Research Enhancement Award (R15) to R. C. Hickner.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.Z., A.R.L., and S.H.A. conceived and designed research; J.Z., R.C.H., C.J.L., B.K.G., and O.F. performed experiments; S.B., O.F., and S.H.A. analyzed data.; J.Z., S.B., R.C.H., A.R.L., and S.H.A. interpreted results of experiments; J.Z., S.B., C.J.L., B.K.G., and S.H.A. prepared figures; J.Z., S.B., R.C.H., O.F., and S.H.A. drafted manuscript; J.Z., S.B., R.C.H., A.R.L., C.J.L., B.K.G., O.F., and S.H.A. edited and revised manuscript; J.Z., S.B., R.C.H., A.R.L., C.J.L., B.K.G., O.F., and S.H.A. approved final version of manuscript.
Supplemental Data
ACKNOWLEDGMENTS
The authors thank Soeren Hoehne (University of Utah) for drawing the schematic in Fig. 1A and Holly Clarke (Florida State University) and Heidi Kluess (Auburn University) for assisting with human clinical studies.
REFERENCES
- 1.Adams SH. Emerging perspectives on essential amino acid metabolism in obesity and the insulin-resistant state. Adv Nutr 2: 445–456, 2011. doi: 10.3945/an.111.000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alberts GL, Chio CL, Im WB. Allosteric modulation of the human 5-HT7A receptor by lipidic amphipathic compounds. Mol Pharmacol 60: 1349–1355, 2001. doi: 10.1124/mol.60.6.1349. [DOI] [PubMed] [Google Scholar]
- 3.Blaak EE, van Aggel-Leijssen DP, Wagenmakers AJ, Saris WH, van Baak MA. Impaired oxidation of plasma-derived fatty acids in type 2 diabetic subjects during moderate-intensity exercise. Diabetes 49: 2102–2107, 2000. doi: 10.2337/diabetes.49.12.2102. [DOI] [PubMed] [Google Scholar]
- 4.Blaak EE, Wagenmakers AJ. The fate of [U-13C]palmitate extracted by skeletal muscle in subjects with type 2 diabetes and control subjects. Diabetes 51: 784–789, 2002. doi: 10.2337/diabetes.51.3.784. [DOI] [PubMed] [Google Scholar]
- 5.Boger DL, Patterson JE, Jin Q. Structural requirements for 5-HT2A and 5-HT1A serotonin receptor potentiation by the biologically active lipid oleamide. Proc Natl Acad Sci USA 95: 4102–4107, 1998. doi: 10.1073/pnas.95.8.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooks GA, Mercier J. Balance of carbohydrate and lipid utilization during exercise: the “crossover” concept. J Appl Physiol (1985) 76: 2253–2261, 1994. doi: 10.1152/jappl.1994.76.6.2253. [DOI] [PubMed] [Google Scholar]
- 7.Bruno C, Patin F, Bocca C, Nadal-Desbarats L, Bonnier F, Reynier P, Emond P, Vourc’h P, Joseph-Delafont K, Corcia P, Andres CR, Blasco H. The combination of four analytical methods to explore skeletal muscle metabolomics: better coverage of metabolic pathways or a marketing argument? J Pharm Biomed Anal 148: 273–279, 2018. doi: 10.1016/j.jpba.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Chorell E, Moritz T, Branth S, Antti H, Svensson MB. Predictive metabolomics evaluation of nutrition-modulated metabolic stress responses in human blood serum during the early recovery phase of strenuous physical exercise. J Proteome Res 8: 2966–2977, 2009. doi: 10.1021/pr900081q. [DOI] [PubMed] [Google Scholar]
- 9.Chorell E, Svensson MB, Moritz T, Antti H. Physical fitness level is reflected by alterations in the human plasma metabolome. Mol Biosyst 8: 1187–1196, 2012. doi: 10.1039/c2mb05428k. [DOI] [PubMed] [Google Scholar]
- 10.Corkey BE, Martin-Requero A, Walajtys-Rode E, Williams RJ, Williamson JR. Regulation of the branched chain α-ketoacid pathway in liver. J Biol Chem 257: 9668–9676, 1982. [PubMed] [Google Scholar]
- 11.Driscoll WJ, Chaturvedi S, Mueller GP. Oleamide synthesizing activity from rat kidney: identification as cytochrome c. J Biol Chem 282: 22353–22363, 2007. doi: 10.1074/jbc.M610070200. [DOI] [PubMed] [Google Scholar]
- 12.Fazelzadeh P, Hangelbroek RW, Tieland M, de Groot LC, Verdijk LB, van Loon LJ, Smilde AK, Alves RD, Vervoort J, Müller M, van Duynhoven JP, Boekschoten MV. The muscle metabolome differs between healthy and frail older adults. J Proteome Res 15: 499–509, 2016. doi: 10.1021/acs.jproteome.5b00840. [DOI] [PubMed] [Google Scholar]
- 13.Fiehn O, Garvey WT, Newman JW, Lok KH, Hoppel CL, Adams SH. Plasma metabolomic profiles reflective of glucose homeostasis in non-diabetic and type 2 diabetic obese African-American women. PLoS One 5: e15234, 2010. doi: 10.1371/journal.pone.0015234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gleeson TT, Baldwin KM. Cardiovascular response to treadmill exercise in untrained rats. J Appl Physiol 50: 1206–1211, 1981. doi: 10.1152/jappl.1981.50.6.1206. [DOI] [PubMed] [Google Scholar]
- 15.Hadrévi J, Ghafouri B, Sjörs A, Antti H, Larsson B, Crenshaw AG, Gerdle B, Hellström F. Comparative metabolomics of muscle interstitium fluid in human trapezius myalgia: an in vivo microdialysis study. Eur J Appl Physiol 113: 2977–2989, 2013. doi: 10.1007/s00421-013-2716-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen JS, Zhao X, Irmler M, Liu X, Hoene M, Scheler M, Li Y, Beckers J, Hrabĕ de Angelis M, Häring HU, Pedersen BK, Lehmann R, Xu G, Plomgaard P, Weigert C. Type 2 diabetes alters metabolic and transcriptional signatures of glucose and amino acid metabolism during exercise and recovery. Diabetologia 58: 1845–1854, 2015. doi: 10.1007/s00125-015-3584-x. [DOI] [PubMed] [Google Scholar]
- 17.Hedlund PB, Carson MJ, Sutcliffe JG, Thomas EA. Allosteric regulation by oleamide of the binding properties of 5-hydroxytryptamine7 receptors. Biochem Pharmacol 58: 1807–1813, 1999. doi: 10.1016/S0006-2952(99)00274-9. [DOI] [PubMed] [Google Scholar]
- 18.Hellsten Y, Tullson PC, Richter EA, Bangsbo J. Oxidation of urate in human skeletal muscle during exercise. Free Radic Biol Med 22: 169–174, 1997. doi: 10.1016/S0891-5849(96)00286-9. [DOI] [PubMed] [Google Scholar]
- 19.Kelley DE. Skeletal muscle fat oxidation: timing and flexibility are everything. J Clin Invest 115: 1699–1702, 2005. doi: 10.1172/JCI25758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol Endocrinol Metab 277: E1130–E1141, 1999. doi: 10.1152/ajpendo.1999.277.6.E1130. [DOI] [PubMed] [Google Scholar]
- 21.Kelley DE, Simoneau JA. Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitus. J Clin Invest 94: 2349–2356, 1994. doi: 10.1172/JCI117600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.La Favor JD, Dubis GS, Yan H, White JD, Nelson MA, Anderson EJ, Hickner RC. Microvascular endothelial dysfunction in sedentary, obese humans is mediated by NADPH oxidase: influence of exercise training. Arterioscler Thromb Vasc Biol 36: 2412–2420, 2016. doi: 10.1161/ATVBAHA.116.308339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai Z, Tsugawa H, Wohlgemuth G, Mehta S, Mueller M, Zheng Y, Ogiwara A, Meissen J, Showalter M, Takeuchi K, Kind T, Beal P, Arita M, Fiehn O. Identifying metabolites by integrating metabolome databases with mass spectrometry cheminformatics. Nat Methods 15: 53–56, 2018. doi: 10.1038/nmeth.4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehmann R, Zhao X, Weigert C, Simon P, Fehrenbach E, Fritsche J, Machann J, Schick F, Wang J, Hoene M, Schleicher ED, Häring HU, Xu G, Niess AM. Medium chain acylcarnitines dominate the metabolite pattern in humans under moderate intensity exercise and support lipid oxidation. PLoS One 5: e11519, 2010. doi: 10.1371/journal.pone.0011519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis GD, Farrell L, Wood MJ, Martinovic M, Arany Z, Rowe GC, Souza A, Cheng S, McCabe EL, Yang E, Shi X, Deo R, Roth FP, Asnani A, Rhee EP, Systrom DM, Semigran MJ, Vasan RS, Carr SA, Wang TJ, Sabatine MS, Clish CB, Gerszten RE. Metabolic signatures of exercise in human plasma. Sci Transl Med 2: 33ra37, 2010. doi: 10.1126/scitranslmed.3001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mandarino LJ, Consoli A, Jain A, Kelley DE. Interaction of carbohydrate and fat fuels in human skeletal muscle: impact of obesity and NIDDM. Am J Physiol Endocrinol Metab 270: E463–E470, 1996. doi: 10.1152/ajpendo.1996.270.3.E463. [DOI] [PubMed] [Google Scholar]
- 27.Matsuki N, Inaba M, Ono K. Catabolism of cytoplasmic and intramitochondrial adenine nucleotides in C2C12 skeletal myotube under chemical hypoxia. J Vet Med Sci 64: 341–347, 2002. doi: 10.1292/jvms.64.341. [DOI] [PubMed] [Google Scholar]
- 28.Mensink M, Blaak EE, van Baak MA, Wagenmakers AJ, Saris WH. Plasma free fatty acid uptake and oxidation are already diminished in subjects at high risk for developing type 2 diabetes. Diabetes 50: 2548–2554, 2001. doi: 10.2337/diabetes.50.11.2548. [DOI] [PubMed] [Google Scholar]
- 29.Mueller-Hennessen M, Sigl J, Fuhrmann JC, Witt H, Reszka R, Schmitz O, Kastler J, Fischer JJ, Müller OJ, Giannitsis E, Weis T, Frey N, Katus HA. Metabolic profiles in heart failure due to non-ischemic cardiomyopathy at rest and under exercise. ESC Heart Fail 4: 178–189, 2017. doi: 10.1002/ehf2.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mueller GP, Driscoll WJ. In vitro synthesis of oleoylglycine by cytochrome c points to a novel pathway for the production of lipid signaling molecules. J Biol Chem 282: 22364–22369, 2007. doi: 10.1074/jbc.M701801200. [DOI] [PubMed] [Google Scholar]
- 31.Nieman DC, Gillitt ND, Sha W, Meaney MP, John C, Pappan KL, Kinchen JM. Metabolomics-based analysis of banana and pear ingestion on exercise performance and recovery. J Proteome Res 14: 5367–5377, 2015. doi: 10.1021/acs.jproteome.5b00909. [DOI] [PubMed] [Google Scholar]
- 32.Nieman DC, Scherr J, Luo B, Meaney MP, Dréau D, Sha W, Dew DA, Henson DA, Pappan KL. Influence of pistachios on performance and exercise-induced inflammation, oxidative stress, immune dysfunction, and metabolite shifts in cyclists: a randomized, crossover trial. PLoS One 9: e113725, 2014. doi: 10.1371/journal.pone.0113725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nieman DC, Sha W, Pappan KL. IL-6 linkage to exercise-induced shifts in lipid-related metabolites: a metabolomics-based analysis. J Proteome Res 16: 970–977, 2017. doi: 10.1021/acs.jproteome.6b00892. [DOI] [PubMed] [Google Scholar]
- 34.Nieman DC, Shanely RA, Gillitt ND, Pappan KL, Lila MA. Serum metabolic signatures induced by a three-day intensified exercise period persist after 14 h of recovery in runners. J Proteome Res 12: 4577–4584, 2013. doi: 10.1021/pr400717j. [DOI] [PubMed] [Google Scholar]
- 35.Nieman DC, Shanely RA, Luo B, Meaney MP, Dew DA, Pappan KL. Metabolomics approach to assessing plasma 13- and 9-hydroxy-octadecadienoic acid and linoleic acid metabolite responses to 75-km cycling. Am J Physiol Regul Integr Comp Physiol 307: R68–R74, 2014. doi: 10.1152/ajpregu.00092.2014. [DOI] [PubMed] [Google Scholar]
- 36.Peake JM, Tan SJ, Markworth JF, Broadbent JA, Skinner TL, Cameron-Smith D. Metabolic and hormonal responses to isoenergetic high-intensity interval exercise and continuous moderate-intensity exercise. Am J Physiol Endocrinol Metab 307: E539–E552, 2014. doi: 10.1152/ajpendo.00276.2014. [DOI] [PubMed] [Google Scholar]
- 37.Scholz M, Fiehn O. SetupX—a public study design database for metabolomic projects. Pac Symp Biocomput 12: 169–180, 2007. [PubMed] [Google Scholar]
- 38.Schooneman MG, Achterkamp N, Argmann CA, Soeters MR, Houten SM. Plasma acylcarnitines inadequately reflect tissue acylcarnitine metabolism. Biochim Biophys Acta 1841: 987–994, 2014. doi: 10.1016/j.bbalip.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Seifert EL, Fiehn O, Bezaire V, Bickel DR, Wohlgemuth G, Adams SH, Harper ME. Long-chain fatty acid combustion rate is associated with unique metabolite profiles in skeletal muscle mitochondria. PLoS One 5: e9834, 2010. doi: 10.1371/journal.pone.0009834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas EA, Carson MJ, Neal MJ, Sutcliffe JG. Unique allosteric regulation of 5-hydroxytryptamine receptor-mediated signal transduction by oleamide. Proc Natl Acad Sci USA 94: 14115–14119, 1997. doi: 10.1073/pnas.94.25.14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas EA, Carson MJ, Sutcliffe JG. Oleamide-induced modulation of 5-hydroxytryptamine receptor-mediated signaling. Ann NY Acad Sci 861: 183–189, 1998. doi: 10.1111/j.1749-6632.1998.tb10190.x. [DOI] [PubMed] [Google Scholar]
- 42.Ukropcova B, McNeil M, Sereda O, de Jonge L, Xie H, Bray GA, Smith SR. Dynamic changes in fat oxidation in human primary myocytes mirror metabolic characteristics of the donor. J Clin Invest 115: 1934–1941, 2005. doi: 10.1172/JCI24332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Loon LJ. Intramyocellular triacylglycerol as a substrate source during exercise. Proc Nutr Soc 63: 301–307, 2004. doi: 10.1079/PNS2004347. [DOI] [PubMed] [Google Scholar]
- 44.Vincent G, Comte B, Poirier M, Rosiers CD. Citrate release by perfused rat hearts: a window on mitochondrial cataplerosis. Am J Physiol Endocrinol Metab 278: E846–E856, 2000. doi: 10.1152/ajpendo.2000.278.5.E846. [DOI] [PubMed] [Google Scholar]
- 45.Wu C, Orozco C, Boyer J, Leglise M, Goodale J, Batalov S, Hodge CL, Haase J, Janes J, Huss JW III, Su AI. BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol 10: R130, 2009. doi: 10.1186/gb-2009-10-11-r130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang J, Light AR, Hoppel CL, Campbell C, Chandler CJ, Burnett DJ, Souza EC, Casazza GA, Hughen RW, Keim NL, Newman JW, Hunter GR, Fernandez JR, Garvey WT, Harper ME, Fiehn O, Adams SH. Acylcarnitines as markers of exercise-associated fuel partitioning, xenometabolism, and potential signals to muscle afferent neurons. Exp Physiol 102: 48–69, 2017. doi: 10.1113/EP086019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.