Abstract
Background:
Lifetime risk of heart failure has been estimated to range from 20% to 46% in diverse sex and race groups. However, lifetime risk estimates for the two HF phenotypes, HF with preserved ejection fraction (HFpEF) and HF with reduced ejection fraction (HFrEF), is not known.
Methods:
Participant-level data from 2 large prospective cohort studies, the Cardiovascular Health Study (CHS) and the Multiethnic Study of Atherosclerosis (MESA), were pooled excluding individuals with prevalent HF at baseline. Remaining lifetime risk estimates for HFpEF (Ejection Fraction ≥ 45%) and HFrEF (Ejection Fraction < 45%) were determined at different index ages using a modified Kaplan-Meier method with mortality and the other HF subtype as competing risks.
Results:
We included 12,417 participants older than 45 years (22.2% blacks, 44.8% men) who were followed for median duration of 11.6 years with 2,178 overall incident HF events with 561 HFrEF events and 726 HFpEF events. At index age of 45 years, the lifetime risk for any HF through age 90 was higher in men than women (27.4% vs. 23.8%). Among HF subtypes, the lifetime risk for HFrEF was higher in men than women (10.6% vs. 5.8%). In contrast, the lifetime risk for HFpEF was similar in men and women. In race-stratified analyses, lifetime risk for overall HF was higher in non-blacks than blacks (25.9% vs. 22.4%). Among HF subtypes, the lifetime risk for HFpEF was higher in non-blacks than blacks (11.2% vs. 7.7%) while that for HFrEF was similar across the two groups. Among participants with antecedent myocardial infarction (MI) prior to HF diagnosis, the remaining lifetime risks for HFpEF and HFrEF were 2.5-fold and 4-fold higher, respectively, as compared with those without antecedent MI.
Conclusion:
Lifetime risks for HFpEF and HFrEF vary by sex, race, and history of antecedent MI. These insights into the distribution of HF risk and its subtypes could inform development of targeted strategies to improve population level HF prevention and control.
Keywords: Heart failure with preserved ejection fraction, Heart failure with reduced ejection fraction, Lifetime risk
Introduction
Heart Failure (HF) is a major public health problem with increasing prevalence, significant mortality and morbidity burden, and high cost of care. 1, 2 While the incidence of HF has declined modestly over time, its prevalence continues to increase, owing to the aging population, rising burden of HF risk factors such as diabetes, obesity, and physical inactivity, as well as lower myocardial infarction (MI) case-fatality rates.3, 4 This highlights the need for better strategies for estimation of HF risk and novel approaches to its prevention.
Lifetime risk estimates have emerged as an important concept in the field of cardiovascular disease epidemiology and prevention that provide a comprehensive assessment of the population-level disease burden while accounting for other competing risks such as mortality. 5 Previous investigations have reported overall HF lifetime risk estimates from 20% to 46%. 6–8 However, the lifetime risk estimates for the two HF phenotypes, HF with preserved ejection fraction (HFpEF) and HF with reduced ejection fraction (HFrEF) have not been studied. This is particularly important since the two HF subtypes differ considerably in the demographic distribution, underlying pathophysiological mechanisms, and available preventive and therapeutic options. 9–12 Furthermore, recent studies have also identified distinct risk factors that may differentially predict risk for HFpEF and HFrEF, highlighting the need for more targeted, subtype-specific approaches to prevention. 1, 13, 14 Better understanding of the lifetime risk estimates for HFpEF and HFrEF would provide insight into the population-level burden of the two HF subtypes that could have implications for resources allocated to preventive strategies.
Accordingly, in this study, we sought to define the lifetime risks of HFrEF and HFpEF at selected ages stratified by race, sex, and history of antecedent MI by pooling data from two large prospective cohort studies: the Cardiovascular Health Study (CHS) and the Multiethnic Study of Atherosclerosis (MESA).
Methods
Study population
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. We pooled participant-level data from the CHS and MESA study, two large prospective cohort studies. The study design, participant characteristics, and HF outcomes for these two well-established observational cohort studies have been previously published. 15, 16
Briefly, CHS is an ongoing prospective, observational cohort study that includes 5,888 community-dwelling older adults recruited from a random sample of Medicare-eligible older adults in two phases between 1989–1990 and 1992–1993 across 4 communities in the United States.16 MESA is an ongoing, prospective, community-based observational study of 6,814 middle age to older participants, from 6 distinct communities in the U.S. We included all participants from the two cohorts who were older than 45 years of age without prevalent HF at baseline.15 CHS and MESA studies were each approved by appropriate institutional review boards and informed consent was obtained from all the study participants. For the present analysis, de-identified participant information was obtained from each of the two cohort studies after the study protocol was approved by the Institutional Review Board at University of Texas Southwestern Medical Center, Dallas, Texas and the coordinating centers for both of the cohorts.
Measurement of baseline covariates
In both cohorts, participants underwent baseline examinations that included both self-reported and measured baseline risk factors, the details of which have been previously reported for each cohort.15, 16 Age, sex, race at baseline was self-reported while other clinical risk factors such as blood pressure and body mass index were measured using standard protocols in both cohorts. Other clinical characteristics such as history of diabetes and coronary artery disease at baseline was self-reported and confirmed based on use of medications at initial visit. Baseline characteristics reported differently across the two cohorts were harmonized into standardized categories for individual-level pooling. The main participant characteristics of interest for the lifetime risk estimation were: age (years), sex (men vs. women), ethnicity (non-blacks vs. blacks), prevalent MI at baseline (yes vs. no), and incident MI on follow-up antecedent to HF (yes vs. no).
Study Measures: Outcomes of Interest
Primary outcomes of interest in this study were the incidence of overall HF and its subtypes, HFrEF and HFpEF. Both cohorts utilized an expert committee of physicians to adjudicate clinical HF outcomes on follow-up.17–20 In the MESA cohort, following the baseline examination, participants were followed up every 9 to 12 months via telephone to obtain information about interval hospitalizations and new cardiovascular diagnosis. Self-reported diagnosis was verified and confirmed by review of medical records for all hospitalizations or outpatient diagnosis. For this, medical records were abstracted by trained personnel and transmitted to the coordinating center for independent review and event adjudication by two physicians (cardiologists or cardiovascular epidemiologists). Any disagreement between the adjudicators was resolved by the morbidity and mortality classification committee. To establish incident HF, a participant was required to have HF symptoms and/or signs such as shortness of breath or edema along with a physician’s diagnosis of HF with appropriate medical therapy (probable HF), or an objective feature of the disease such as pulmonary edema on chest x-ray or echocardiographic evidence of LV systolic or diastolic dysfunction (definite HF). A new HF diagnosis was then classified as either HFpEF (LVEF ≥45%) or HFrEF (LVEF <45%) using available data on LVEF from review of medical records. In the CHS cohort, incident HF events were identified either by review of the medical records, CMS claims data, or participant survey answers at study visits. Events were then confirmed by an expert adjudication panel, which required the following for confirmation: the presence of a physician’s diagnosis with symptoms (shortness of breath, orthopnea, fatigue, paroxysmal nocturnal dyspnea) and/or signs (edema, rales, tachycardia) of HF, supporting radiological findings of HF (for example. pulmonary edema on chest x-ray), or any appropriate medical therapy for HF. These events were then classified as either HFpEF (LVEF ≥ 45%) or HFrEF (LVEF <45%) using data from cardiac imaging performed within 30 days of the event.19, 20
Statistical Analysis
Lifetime risk was determined using a life-table analysis via the Practical Incidence Estimator macro (SAS), which allows for variable follow up duration, and combines information on participants at each age attained during follow up.21 This approach is consistent with the previously described methods for estimation of lifetime risk for cardiovascular diseases.5, 7 Briefly, a modified Kaplan-Meier method was employed, using death free of HF as a competing risk to avoid overestimation of lifetime risk. A participant’s lifetime risk using this method, therefore, is simply the cumulative incidence of developing HF over their subsequent lifetime follow-up, which was set at age 90 years in this study. Each participant was followed from age of entry until first HF event, death, 90 years age, or censoring in the primary study cohort. Remaining lifetime risk estimates for HFpEF and HFrEF were calculated for index ages of 45, 55, 65, and 75 in the overall population as well as across sex (men vs. women) and race (blacks vs. non-blacks) based subgroups. We also determined the remaining lifetime risks for HFpEF and HFrEF in individuals with vs. without antecedent MI. For this we performed additional subgroup analyses to estimate the lifetime risk for HFpEF and HFrEF in participants with vs. without interval MI during follow-up. Individuals with a MI event during follow up prior to or concurrent with incident HF event were classified as having antecedent MI. Individuals without a MI event on follow-up or those with incident MI after HF incidence were classified as without antecedent MI.8 For each HF subtype outcome, mortality and incidence of the other HF subtype were treated as competing events. Finally, the differential impact of competing risk adjustment on HFpEF and HFrEF risks were evaluated by estimating lifetime risk with and without adjustment for competing risk of mortality and other HF subtype. Individuals with HF and missing ejection fraction were excluded for the HF subtype analysis.
Several sensitivity analyses were performed to test the robustness of our study findings. First, owing to significant missingness in EF among all HF outcomes, additional sensitivity analysis was performed to determine the impact of HF with missing EF on the observed race- and sex- based differences in HFpEF and HFrEF risk. For this, lifetime risk of HFpEF and HFrEF events at index age of 45 using were estimated using an extreme case scenario approach, first assuming all HF with missing EF events to be HFpEF events and then assuming all HF with missing EF events to be HFrEF events. Second, a sensitivity analysis was also performed to determine the lifetime risk of HF with missing EF at index age of 45 across different race- and sex-based subgroups. Third, a sensitivity analysis was performed using alternative EF cutoffs to define HFpEF (EF > 50%) and HFrEF (EF < 40%) to determine if the race- and sex- based differences in lifetime risk of HFpEF and HFrEF are sensitive to EF cut offs. Finally, additional subgroup analyses were performed to evaluate the impact of baseline diabetes status on lifetime risk of HFpEF and HFrEF. All statistical analyses were performed using SAS statistical software (version 9.1, SAS Institute, Cary, North Carolina)
Results
Overall, 12,417 participants were included in this analysis (44.8% men, 22.2% blacks). Baseline characteristics of the study participants can be found in Table 1. The age distribution of study participants across the two pooled cohorts are shown in supplemental figure 1. Overall, the MESA cohort included participants with a larger age distribution with all the younger participants (< 65 years of age) in the pooled cohort contributed by MESA. After the index age of 45 years, 2,178 incident HF events (726 incident HFpEF and 561 incident HFrEF events, rest with HF and missing EF) were observed over a median follow-up of 11.6 years.
Table 1:
Baseline characteristics of the study participants
| Variable | Age Group 1 (45 – 55 years) | Age Group 2 (>55 to 65 years) | Age Group 3 (>65 to 75 years) | Age Group 4 > 75 years | ||||
|---|---|---|---|---|---|---|---|---|
| Men n = 906 | Women n = 1034 | Men n = 881 | Women n = 1000 | Men n = 2487 | Women n = 3279 | Men n = 1293 | Women n = 1530 | |
| BMI (kg/m2) | 28.2 (4.4) | 29.2 (6.9) | 28.2 (4.7) | 29.3 (6.2) | 27.2 (4.0) | 27.6 (5.6) | 26.0 (3.8) | 26.4 (4.9) |
| Blacks (%) | 26.3 | 28.7 | 25.5 | 29.3 | 19.7 | 20.6 | 16.2 | 21.1 |
| Whites (%) | 36.9 | 37.4 | 38.6 | 37.1 | 67.6 | 69.1 | 71.7 | 67.1 |
| Hispanics (%) | 24.7 | 23.0 | 23.8 | 21.9 | 7.7 | 6.4 | 7.3 | 6.9 |
| Other races (%) | 12.1 | 10.8 | 12.0 | 11.7 | 5.1 | 3.9 | 4.8 | 4.9 |
| DM (%) | 8.8 | 6.8 | 15.0 | 11.6 | 15.1 | 11.2 | 13.6 | 11.7 |
| HTN (%) | 24.1 | 25.2 | 39.5 | 44.1 | 46.5 | 47.4 | 47.6 | 59.6 |
| Former Smoker (%) | 34.6 | 25.2 | 42.6 | 29.6 | 55.8 | 33.0 | 53.8 | 26.2 |
| Current Smoker (%) | 20.5 | 17.3 | 17.8 | 13.2 | 12.0 | 13.1 | 6.7 | 5.8 |
| Current Alcohol use (%) | 39.7 | 21.5 | 41.7 | 21.3 | 39.0 | 20.4 | 34.0 | 16.1 |
| Antecedent MI (%) | 3.0 | 0.9 | 4.2 | 2.3 | 16.3 | 12.1 | 15.9 | 12.6 |
Continuous data presented as mean (SD)
BMI: Body mass index; DM: Diabetes mellitus; HTN: Hypertension; MI: Myocardial Infarction
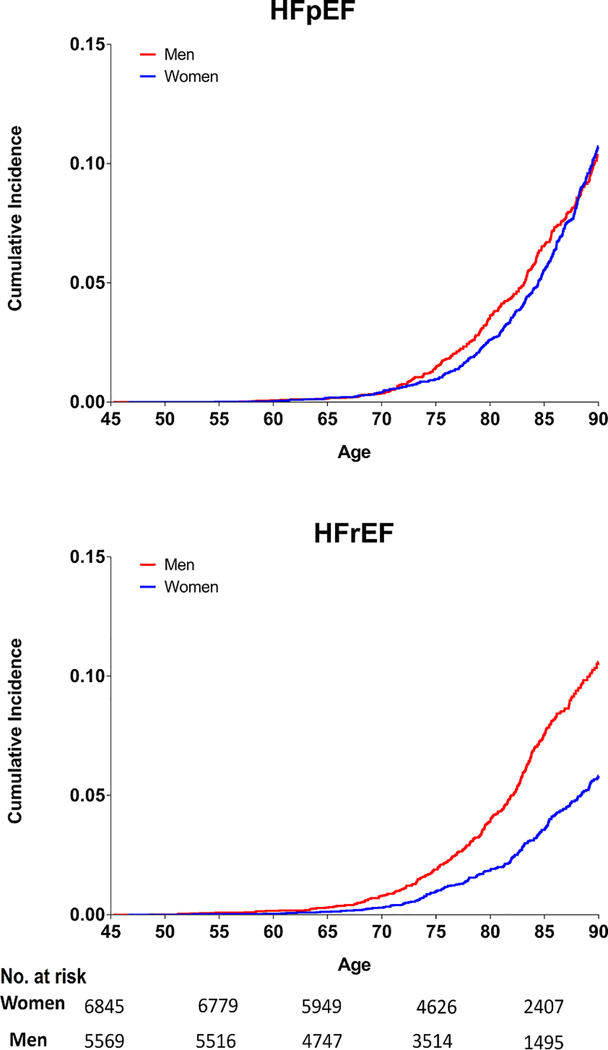
In sex-stratified analyses, the remaining lifetime risk of overall HF was higher in men than women, regardless of the index age (Table 2). Among the two HF subtypes, lifetime risk of HFpEF at index age 45 through 90 was similar in men and women (Figure 1, Table 2). In contrast, for HFrEF, the lifetime risk estimates were up to 1.8-fold higher in men as compared with women (Figure 1, Table 2). The lifetime risks for HFpEF and HFrEF were similar in men while women had substantially higher lifetime risk for HFpEF than HFrEF. The lifetime risk of HFpEF and HFrEF were not substantially lower at higher index ages in men and women (Table 2). Similar sex-based differences in lifetime risk of HFpEF and HFrEF were also observed in cohort-specific sensitivity analysis of MESA and CHS participants separately (data not shown).
Table 2:
Lifetime risk for different heart failure phenotypes stratified by sex at selected index ages
| Index Age (y) | Lifetime Risk (%) (95% Confidence Interval) | |||||
|---|---|---|---|---|---|---|
| Overall HF | HFpEF | HFrEF | ||||
| Women | Men | Women | Men | Women | Men | |
| 45 | 23.8 (22.4 – 25.1) | 27.4 (25.9 – 29.0) | 10.7 (9.6 – 11.8) | 10.4 (9.1 – 11.6) | 5.8 (5.03 – 6.6) | 10.6 (9.4 – 11.8) |
| 55 | 23.7 (22.4 – 25.0) | 27.4 (25.9 – 29.0) | 10.7 (9.6 – 11.8) | 10.4 (9.2 – 11.6) | 5.8 (5.0 – 6.6) | 10.5 (9.3 – 11.8) |
| 65 | 23.6 (22.3 – 25.0) | 27.3 (25.8 – 28.9) | 10.7 (9.5 – 11.8) | 10.4 (9.1 – 11.6) | 5.8 (5.0 – 6.6) | 10.5 (9.2 – 11.7) |
| 75 | 22.5 (21.1 – 23.8) | 25.9 (24.3 – 27. 6) | 10.3 (9.2 – 11.5) | 9.7 (8.4 – 11.0) | 5.2 (4.4 – 6.0) | 9.5 (8.2 – 10.8) |
Figure 1:
Sex-stratified remaining lifetime risk of HFpEF and HFrEF in study participants at index age 45 through 90. Number of participants at risk for HFpEF & HFrEF in each subgroup at different index ages (45, 55, 65,75, 85) are shown below the x-axis.
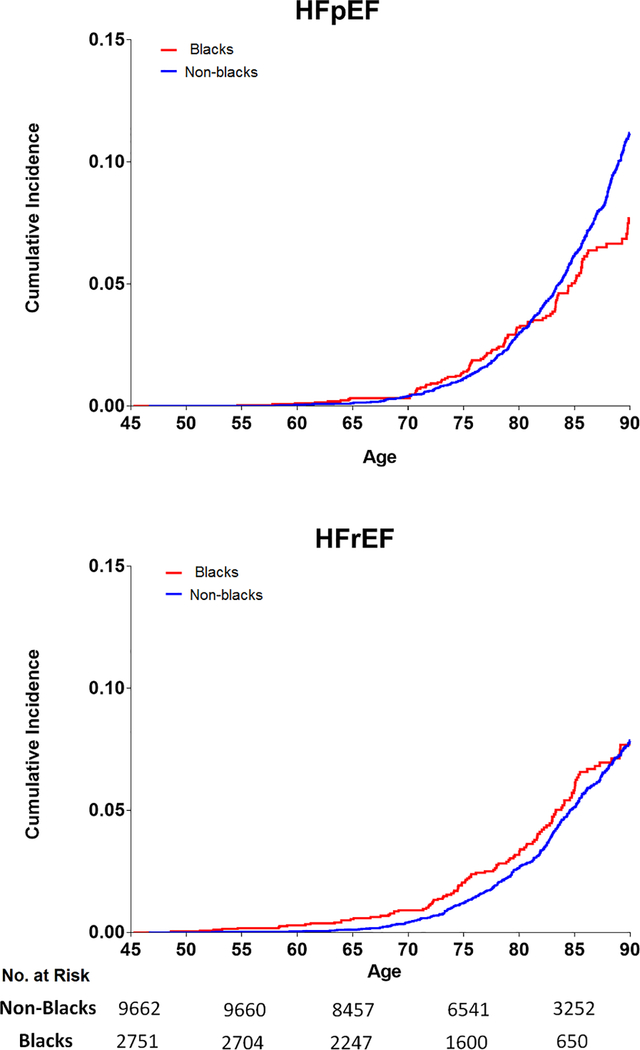
In race-stratified analyses, the remaining lifetime risk of overall HF was higher in non-blacks than blacks, regardless of the index age (Table 3). For the two HF subtypes, the remaining lifetime risk of HFrEF at age 45 through 90 was similar in non-blacks vs. blacks (Figure 2, Table 3). In contrast, for HFpEF, the lifetime risk estimates were ~1.5 fold higher in non-blacks as compared with blacks (Figure 2, Table 3). Blacks had similar lifetime risk of HFpEF and HFrEF while non-blacks had higher risk of HFpEF than HFrEF over the remaining lifespan. The lifetime risk of HFpEF & HFrEF was similar at higher index ages in both blacks and non-blacks (Table 3).
Table 3:
Lifetime risk of different heart failure phenotypes across race/ethnicity-based subgroups
| Age (y) | Lifetime Risk (%) (95% Confidence Interval) | |||||
|---|---|---|---|---|---|---|
| Overall HF | HFpEF | HFrEF | ||||
| Non-black | Black | Non-black | Black | Non-black | Black | |
| 45 | 25.9 (24.8 – 27.0) | 22.4 (20.1 – 24.6) | 11.2 (10.2 – 12.1) | 7.7 (6.0 – 9.4) | 7.9 (7.1 – 8.7) | 7.7 (6.1 – 9.2) |
| 55 | 25.9 (24.8 – 27.0) | 22.3 (20.1 – 24.6) | 11.2 (10.3 – 12.1) | 7.7 (6.1 – 9.4) | 7.9 (7.1 – 8.6) | 7.6 (6.0 – 9.1) |
| 65 | 25.9 (24.7 – 27.0) | 22.0 (19.7 – 24.3) | 11.2 (10.2 – 12.1) | 7.6 (5.9 – 9.3) | 7.8 (7.0 – 8.6) | 7.3 (5.8 – 8.9) |
| 75 | 24.6 (23.5 – 25.8) | 20.2 (17.8 – 22.6) | 10.7 (9.7 – 11.7) | 7.0 (5.2 – 8.7) | 7.1 (6.3 – 7.9) | 6.2 (4.7 – 7.8) |
Figure 2:
Race-stratified (blacks vs. non-blacks) remaining lifetime risk of HFpEF and HFrEF in study participants at index age 45 through 90. Number of participants at risk for HFpEF & HFrEF in each subgroup at different index ages (45, 55, 65,75, 85) are shown below the x-axis.
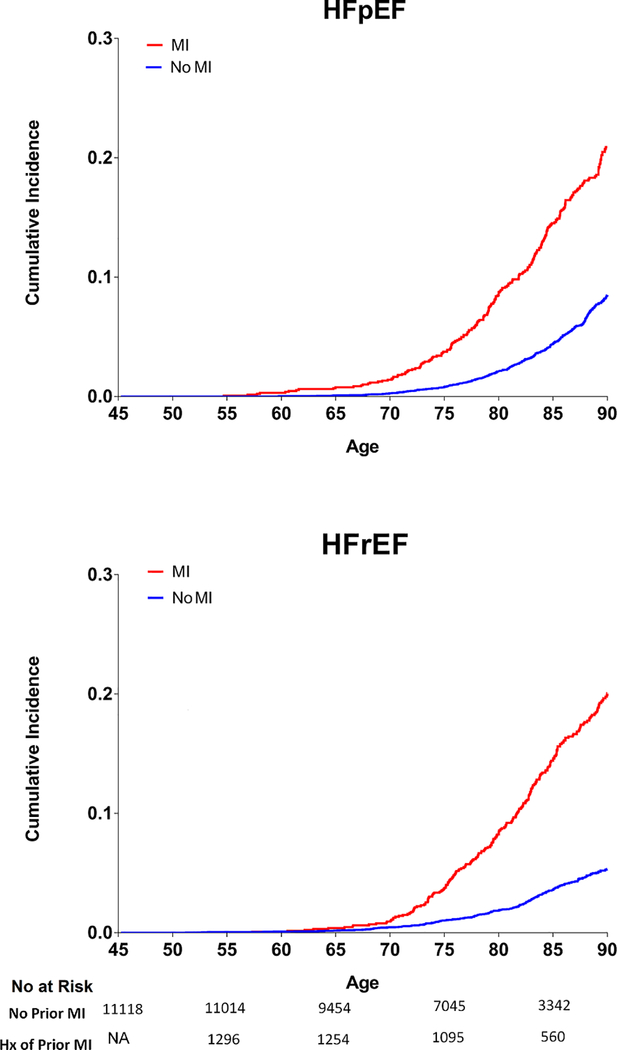
The remaining lifetime risk of overall HF was 2.5-fold higher in participants with antecedent MI compared with those without antecedent MI, at each index age (Table 4). For the two HF subtypes, the lifetime risks of both HFpEF and HFrEF at index age 45 through 90 were significantly higher (2.5 to 4-fold higher respectively) in participants with antecedent MI compared to those without antecedent MI (Figure 3, Table 4). Participants with antecedent MI had similar lifetime risk of HFpEF and HFrEF while those without antecedent MI had higher risk of HFpEF than HFrEF (Table 4). Similar patterns of results were observed across increasing index ages in those with and without antecedent MIs.
Table 4:
Lifetime risk of different heart failure phenotypes among patients with vs. without antecedent myocardial infarction prior to heart failure incidence
| Index Age (y) | Lifetime Risk (%) (95% Confidence Interval) | |||||
|---|---|---|---|---|---|---|
| Overall HF | HFpEF | HFrEF | ||||
| MI | No MI | MI | No MI | MI | No MI | |
| 45 | 53.7 (50.9 – 56.5) | 20.0 (19.0 – 21.0) | 20.9 (18.4 – 23.4) | 8.5 (7.6 – 9.3) | 20.0 (17.6 – 22.5) | 5.3 (4.7 – 6.0) |
| 55 | 53.7 (50.8 – 56.5) | 20.0 (18.9– 21.0) | 20.8 (18.4 – 23.3) | 8.5 (7.7 – 9.4) | 20.1 (17.6 – 22.5) | 5.3 (4.6 – 5.9) |
| 65 | 53.3 (50.5 – 56.2) | 19.9 (18.9 – 20.9) | 20.4 (17.9 – 22.9) | 8.5 (7.7 – 9.4) | 20.0 (17.5 – 22.4) | 5.2 (4.6 – 5.9) |
| 75 | 50.7 (47.7 – 53.8) | 18.8 (17.8 – 19.9) | 19.1 (16.5 – 21.7) | 8.2 (7.3 – 9.1) | 18.2 (15.7 – 20.7) | 4.6 (3.9 – 5.2) |
Figure 3:
Remaining lifetime risk of HFpEF and HFrEF in study participants at index age 45 through 90 among participants with vs. without antecedent MI. Number of participants at risk for HFpEF & HFrEF in each subgroup at different index ages (45, 55, 65,75, 85) are shown below the x-axis.
The impact of competing risks on lifetime risk for HFpEF and HFrEF were assessed by estimating the cumulative risk of HFpEF and HFrEF in unadjusted models without accounting for the competing risk for death and the other HF subtype. The unadjusted cumulative risk of HFpEF and HFrEF at age 45 through 90 was slightly higher than the adjusted lifetime risk estimates (Table 5). Furthermore, competing risk adjustment was associated with similar attenuation in the lifetime risk of both HF subtypes.
Table 5:
Lifetime risk of heart failure with preserved ejection fraction and heart failure with reduced ejection fraction at index age 45 with vs. without adjustment for competing risk
| Lifetime Risk (%) (95% Confidence Interval) | ||||
|---|---|---|---|---|
| HFpEF | HFrEF | |||
| Competing risk Adjusted | Without Competing risk adjustment | Competing risk Adjusted | Without Competing risk adjustment | |
| Overall Cohort | 10.6 (9.8 – 11.4) | 12.2 (11.2 – 13.1) | 7.9 (7.2 – 8.6) | 9.0 (8.2 – 9.8) |
| Men | 10.4 (9.1 – 11.6) | 12.6 (11.0 – 14.2) | 10.6 (9.4 – 11.8) | 12.6 (11.1 – 14.1) |
| Women | 10.7 (9.6 – 11.8) | 11.9 (10.6 – 13.1) | 5.8 (5.03 – 6.6) | 6.5 (5.6 – 7.4) |
| Blacks | 7.7 (6.0 – 9.4) | 9.6 (7.4 – 11.7) | 7.7 (6.1 – 9.2) | 9.0 (7.1 – 10.9) |
| Non-Blacks | 11.2 (10.2 – 12.1) | 12.7 (11.6 – 13.8) | 7.9 (7.1 – 8.7) | 8.9 (8.0 – 9.8) |
Several sensitivity analyses were performed to test the robustness of our study findings. The impact of HF with missing EF events on the observed lifetime risk estimates for HFpEF and HFrEF was also determined using two additional approaches. First, the sex- and race- based differences in lifetime risk estimates of HF with missing EF events at different index ages was calculated and noted to be in between that for HFpEF and HFrEF (Supplemental Table 1). Second, the sex- and race-based differences in lifetime risk of HFpEF and HFrEF in the extreme case scenario (first, assuming all HF with missing EF events as HFpEF in one extreme scenario and then as HFrEF) analysis, was consistent with that observed in the primary analysis (Supplemental Table 2). In a sensitivity analysis using alternative ejection fraction cut offs for HFrEF (< 40%) and HFpEF (>50%), the sex- and race-based differences in lifetime risk of HF subtypes were similar to that observed in the primary analysis (Supplemental Table 3). Additional sensitivity analyses were also performed to evaluate the lifetime risk of HFpEF and HFrEF among study participants with or without prevalent diabetes at baseline. The remaining lifetime risk of HFpEF at age 45 through 90 was similar among those with vs. without diabetes at baseline. In contrast, the remaining lifetime risk for HFrEF at index age 45 through 90 was 1.6-fold higher in those with vs. without DM at baseline (Supplemental Table 4).
Discussion
In this individual participant-level pooled analysis from 2 large prospective cohort studies, we observed that, over the lifetime, compared to women, men have a higher lifetime risk of overall HF and HFrEF with a similar lifetime risk of HFpEF. Second, compared with blacks, non-blacks have a similar lifetime risk of developing HFrEF but a higher risk of HFpEF. Finally, the lifetime risks of HFpEF and HFrEF are similar and substantially higher in those with vs. without antecedent MI. To our knowledge, this is the first study to describe the lifetime risk estimates of the two HF subtypes, HFpEF and HFrEF.
The study may have public health implications. HF is a growing public health problem with increasing morbidity and rising health care costs attributed to HF care.1, 2 Among the HF subtypes, the burden of HFpEF is increasing in the community more so than HFrEF and novel approaches to its prevention are urgently needed. 4, 22, 23 Our study findings provide insights into the lifetime risk of HF subtypes across relevant population subgroups. These data may guide health policy makers and public health investigators to predict the relative population burden of the two distinct HF subtypes. It will also help identify the high-risk patient population for HFrEF and HFpEF and allow for appropriate disease-specific resource allocation and targeting of preventive strategies. For example, women and non-blacks may be a higher risk subgroup for HFpEF than HFrEF and may benefit from more aggressive implementation of HFpEF prevention strategies. To this end, previous studies have demonstrated that aggressive hypertension control may lower the risk of incident HFpEF.24 Similarly, recent studies have also identified lifestyle risk factors such as obesity and physical inactivity as potentially modifiable targets for HFpEF prevention.25 Futures studies are needed to determine if intensive lifestyle interventions may lower HFpEF incidence in these higher risk populations.
Consistent with the previously reported observations of higher short-term risk of HFrEF in men, we observed higher lifetime risk of HFrEF in men than women.13, 14, 26 This may be related to higher burden and earlier onset of coronary heart disease in men vs. women. It is noteworthy that lifetime risk of HFpEF in men was also high and comparable to that in women. This contrasts with the prevailing notion that HFpEF is predominantly a disease of women based on observations from cross sectional studies with higher proportion of women vs. men in hospitalized HFpEF patients. 27–29 Findings from the present study highlight the significantly high lifetime risk of HFpEF in men similar to that observed in women and argue for increased focus on HFpEF prevention in men. Consistent with our findings, competing risk adjusted analysis evaluating the predictors of HFpEF and HFrEF in community based cohorts such as PREVEND and the Framingham Heart Study, female sex was not associated with increased risk of HFpEF as compared with males over 8–10 year follow up.14, 26
Among racial/ethnic groups, we observed that the lifetime risk of HFpEF was lower in blacks than non-blacks. In contrast, the lifetime risk of HFrEF was not different between the two groups. Previous studies have demonstrated lower lifetime risk of overall HF in blacks as compared with non-blacks.7, 30 The findings from our study suggest that this may be largely related to the lower lifetime risk for HFpEF in blacks vs. non-blacks with similar risk of HFrEF in the two subgroups. The lower lifetime risk of HFpEF in blacks observed in our study is consistent with lower short-term risk of HFpEF reported in the non-black participants in other cohorts. 19, 30, 31 The observed lower lifetime risk of HFpEF in blacks may be related to higher competing risk of death among African Americans before developing HFpEF.7 As observed in our study, competing risk attenuates the lifetime risk of HFpEF more than that of HFrEF, largely because HFpEF development tends to occur at an older age than HFrEF. Furthermore, the lower incidence of non-fatal MI and higher incidence of fatal MI in blacks vs. non-blacks as observed in recent studies may also contribute to lower lifetime risk of HF blacks.32 It is noteworthy that a previous study from the ARIC community surveillance observed that blacks had higher rates of first HF hospitalization than whites as well as a greater proportion of HFrEF vs. HFpEF events.33 However, in contrast to the findings from our study, these observations were based on cross-sectional assessment of HF hospitalizations rates across different race-groups and do not reflect the cumulative lifetime risk of incident HF (outpatient or inpatient) or account for the competing risk of death or other HF subtype. Furthermore, more than half of participants with a first HF hospitalization event reported prior history of HF diagnosis in ARIC cohort and thus, the reported HF hospitalizations do not reflect true HF incidence rates. In contrast, the incident HF events in the present study represent first HF diagnosis in the inpatient or outpatient setting. These differences in risk assessment strategies and outcome definition may explain the observed differences in HF risk.
We also found a substantially higher risk of HFpEF among individuals with antecedent MI. This is consistent with the association between history of ischemic heart disease and higher risk of HFpEF over 8–10 year follow-up that has been reported previously. In the Framingham cohort, history of CAD was strongly associated with risk of both HFpEF and HFrEF.14 Similarly, in a recent pooled analysis using data from 4 large cohorts, prior MI was associated with higher risk of both HFpEF & HFrEF.13 We observed that the risk of HFpEF and HFrEF were comparable among individuals with antecedent MI. Among individuals without an MI, the lifetime risk of HFpEF was higher than HFrEF. Taken together, these findings suggest that MI increases the risk of both HFpEF and HFrEF but more so for HFrEF, and therefore, prevention of atherosclerotic disease burden may be a useful strategy to lower the population burden of both HF subtypes.
The primary strengths of our study include the large sample size, availability of clinically adjudicated data on HFpEF and HFrEF events in the included study cohorts, use of similar adjudication criteria for clinical HF, and use of same EF cutoff for HFpEF and HFrEF in the two study cohorts. Several important limitations to our study are also noteworthy. First, the two pooled cohorts had different entry criteria and index ages which may have affected lifetime risk estimates in the pooled study population. Similarly, the risk factor burden was also different across the two cohorts, which may have influenced the lifetime risk estimates. Second, ejection fraction data were missing in a significant proportion of HF events (~40%) and contributed to an underestimation of the lifetime risk estimates. However, despite the underestimation of overall lifetime risk burden, it is unlikely that the comparison of lifetime risk across different sex-, race-, and MI history based subgroups would be affected by the missing EF events as demonstrated in the extreme case scenario sensitivity analysis (Supplemental table 2). Third, we do not have data on objective biomarkers such as NT-ProBNP levels at the time of incident HF diagnosis or use of guideline recommended HFrEF therapies after HF diagnosis. Future studies using other databases with available medication use data from index hospitalization are needed to better understand the use of these evidence-based therapies across different groups.
In conclusion, findings from our study suggest that the lifetime risks for HFpEF and HFrEF vary by sex, race, and history of antecedent MI. These findings may be informative to understand the population burden of the two HF subtypes and help formulate disease-specific preventive strategies in the at-risk population.
Supplementary Material
Clinical Perspective.
What is new?
The lifetime risk estimates for the two phenotypes of heart failure (HF), HF with preserved ejection fraction (HFpEF) and HF with reduced ejection fraction(HFrEF) have not been reported previously.
Compared with women, men have a higher lifetime risk of HFrEF with a similar lifetime risk of HFpEF.
Compared with blacks, non-blacks have a similar lifetime risk of developing HFrEF but a higher risk of HFpEF
Lifetime risks of HFpEF and HFrEF are similar and substantially higher in those with vs. without antecedent MI
What are the clinical implications?
These findings provide insights into the long-term burden of HFpEF & HFrEF across different population subgroups
These findings may help health policy makers in appropriate resource allocation for targeting of HFpEF and HFrEF-specific preventive strategies in the at-risk population.
Acknowledgement:
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
The authors also thank the investigators, staff, participants of the CHS study for their contribution. A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org
Funding Sources: Dr. Berry receives funding from (1) the Dedman Family Scholar in Clinical Care endowment at University of Texas Southwestern Medical Center, and (2) 14SFRN20740000 from the American Heart Association prevention network. This project was done in collaboration with the American Heart Association Strategically Focused Research Network centers for prevention at UTSW, Dallas and Northwestern University, Chicago.
MESA: This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from National Center for Advancing Translational Sciences.
CHS: This research was supported by contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Footnotes
Conflicts of Interest & Disclosures: None
References:
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Statistics C and Stroke Statistics S. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ and Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–52. [DOI] [PubMed] [Google Scholar]
- 3.Dunlay SM, Roger VL and Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;14(10):591–602. [DOI] [PubMed] [Google Scholar]
- 4.Gerber Y, Weston SA, Redfield MM, Chamberlain AM, Manemann SM, Jiang R, Killian JM and Roger VL. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med. 2015;175:996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, Greenland P, Van Horn L, Tracy RP and Lloyd-Jones DM. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;366:321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bleumink GS, Knetsch AM, Sturkenboom MC, Straus SM, Hofman A, Deckers JW, Witteman JC and Stricker BH. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure The Rotterdam Study. Eur Heart J. 2004;25:1614–9. [DOI] [PubMed] [Google Scholar]
- 7.Huffman MD, Berry JD, Ning H, Dyer AR, Garside DB, Cai X, Daviglus ML and Lloyd-Jones DM. Lifetime risk for heart failure among white and black Americans: cardiovascular lifetime risk pooling project. J Am Coll Cardiol. 2013;61:1510–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D’Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D and Framingham Heart S. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–72. [DOI] [PubMed] [Google Scholar]
- 9.Dhingra A, Garg A, Kaur S, Chopra S, Batra JS, Pandey A, Chaanine AH and Agarwal SK. Epidemiology of heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2014;11:354–65. [DOI] [PubMed] [Google Scholar]
- 10.Pandey A, Darden D and Berry JD. Low Fitness in Midlife: A Novel Therapeutic Target for Heart Failure with Preserved Ejection Fraction Prevention. Prog Cardiovasc Dis. 2015;58:87–93. [DOI] [PubMed] [Google Scholar]
- 11.Shah SJ. Sedentary Lifestyle and the Risk for HFpEF: Are “Huff-Puff Health Clubs” the Answer? J Am Coll Cardiol. 2017;69:1143–1146. [DOI] [PubMed] [Google Scholar]
- 12.Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA and Paulus WJ. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation. 2016;134:73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho JE, Enserro D, Brouwers FP, Kizer JR, Shah SJ, Psaty BM, Bartz TM, Santhanakrishnan R, Lee DS, Chan C, Liu K, Blaha MJ, Hillege HL, van der Harst P, van Gilst WH, Kop WJ, Gansevoort RT, Vasan RS, Gardin JM, Levy D, Gottdiener JS, de Boer RA and Larson MG. Predicting Heart Failure With Preserved and Reduced Ejection Fraction: The International Collaboration on Heart Failure Subtypes. Circ Heart Fail. 2016;9(6). pii: e003116. doi: 10.1161/CIRCHEARTFAILURE.115.003116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho JE, Lyass A, Lee DS, Vasan RS, Kannel WB, Larson MG and Levy D. Predictors of new-onset heart failure: differences in preserved versus reduced ejection fraction. Circ Heart Fail. 2013;6:279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr., Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M and Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. [DOI] [PubMed] [Google Scholar]
- 16.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A and et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–76. [DOI] [PubMed] [Google Scholar]
- 17.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, Cruise RG and Theroux S. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–85. [DOI] [PubMed] [Google Scholar]
- 18.Seliger SL, de Lemos J, Neeland IJ, Christenson R, Gottdiener J, Drazner MH, Berry J, Sorkin J and deFilippi C. Older Adults, “Malignant” Left Ventricular Hypertrophy, and Associated Cardiac-Specific Biomarker Phenotypes to Identify the Differential Risk of New-Onset Reduced Versus Preserved Ejection Fraction Heart Failure: CHS (Cardiovascular Health Study). JACC Heart Fail. 2015;3:445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silverman MG, Patel B, Blankstein R, Lima JA, Blumenthal RS, Nasir K and Blaha MJ. Impact of Race, Ethnicity, and Multimodality Biomarkers on the Incidence of New-Onset Heart Failure With Preserved Ejection Fraction (from the Multi-Ethnic Study of Atherosclerosis). Am J Cardiol. 2016;117:1474–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Opdahl A, Ambale Venkatesh B, Fernandes VR, Wu CO, Nasir K, Choi EY, Almeida AL, Rosen B, Carvalho B, Edvardsen T, Bluemke DA and Lima JA. Resting heart rate as predictor for left ventricular dysfunction and heart failure: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2014;63:1182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beiser A, D’Agostino RB Sr., Seshadri S, Sullivan LM and Wolf PA. Computing estimates of incidence, including lifetime risk: Alzheimer’s disease in the Framingham Study. The Practical Incidence Estimators (PIE) macro. Stat Med. 2000;19:1495–522. [DOI] [PubMed] [Google Scholar]
- 22.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y and Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–9. [DOI] [PubMed] [Google Scholar]
- 23.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL and Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9. [DOI] [PubMed] [Google Scholar]
- 24.Davis BR, Kostis JB, Simpson LM, Black HR, Cushman WC, Einhorn PT, Farber MA, Ford CE, Levy D, Massie BM, Nawaz S and Group ACR. Heart failure with preserved and reduced left ventricular ejection fraction in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Circulation. 2008;118:2259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pandey A, Allen NB, Ayers C, Reis JP, Moreira HT, Sidney S, Rana JS, Jacobs DR Jr., Chow LS, de Lemos JA, Carnethon M and Berry JD. Fitness in Young Adulthood and Long-Term Cardiac Structure and Function: The CARDIA Study. JACC Heart Fail. 2017;5:347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brouwers FP, de Boer RA, van der Harst P, Voors AA, Gansevoort RT, Bakker SJ, Hillege HL, van Veldhuisen DJ and van Gilst WH. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur Heart J. 2013;34:1424–31. [DOI] [PubMed] [Google Scholar]
- 27.Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, O’Connor CM, Sun JL, Yancy CW, Young JB, Investigators O-H and Hospitals. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50:768–77. [DOI] [PubMed] [Google Scholar]
- 28.Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, Hernandez AF, Fonarow GC, Get With the Guidelines Scientific Advisory C and Investigators. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 2012;126:65–75. [DOI] [PubMed] [Google Scholar]
- 29.Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC, Committee ASA and Investigators. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47:76–84. [DOI] [PubMed] [Google Scholar]
- 30.Eaton CB, Pettinger M, Rossouw J, Martin LW, Foraker R, Quddus A, Liu S, Wampler NS, Hank Wu WC, Manson JE, Margolis K, Johnson KC, Allison M, Corbie-Smith G, Rosamond W, Breathett K and Klein L. Risk Factors for Incident Hospitalized Heart Failure With Preserved Versus Reduced Ejection Fraction in a Multiracial Cohort of Postmenopausal Women. Circ Heart Fail. 2016; 9(10). pii: e002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L, Liebelt JJ, Madan N, Shan J and Taub CC. Comparison of Predictors of Heart Failure With Preserved Versus Reduced Ejection Fraction in a Multiracial Cohort of Preclinical Left Ventricular Diastolic Dysfunction. Am J Cardiol. 2017;119:1815–1820. [DOI] [PubMed] [Google Scholar]
- 32.Colantonio LD, Gamboa CM, Richman JS, Levitan EB, Soliman EZ, Howard G and Safford MM. Black-White Differences in Incident Fatal, Nonfatal, and Total Coronary Heart Disease. Circulation. 2017;136:152–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang PP, Chambless LE, Shahar E, Bertoni AG, Russell SD, Ni H, He M, Mosley TH, Wagenknecht LE, Samdarshi TE, Wruck LM and Rosamond WD. Incidence and survival of hospitalized acute decompensated heart failure in four US communities (from the Atherosclerosis Risk in Communities Study). Am J Cardiol. 2014;113:504–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.