Abstract
Introduction:
Transcatheter aortic valve replacement (TAVR) has emerged as an effective minimally-invasive alternative to surgical valve replacement in medium- to high-risk, elderly patients with calcific aortic valve disease and severe aortic stenosis. The rapid growth of the TAVR devices market has led to a high variety of designs, each aiming to address persistent complications associated with TAVR valves that may hamper the anticipated expansion of TAVR utility.
Areas Covered:
Here we outline the challenges and the technical demands that TAVR devices need to address for achieving the desired expansion, and review design aspects of selected, latest generation, TAVR valves of both clinically-used and investigational devices. We further review in detail some of the up-to-date modeling and testing approaches for TAVR, both computationally and experimentally, and additionally discuss those as complementary approaches to the ISO 5840–3 standard. A comprehensive survey of the prior and up-to-date literature was conducted to cover the most pertaining issues and challenges that TAVR technology faces.
Expert Commentary:
The expansion of TAVR over SAVR and to new indications seems more promising than ever. With new challenges to come, new TAV design approaches, and materials used, are expected to emerge, and novel testing/modeling methods to be developed.
Keywords: TAVI, Aortic Stenosis, Calcific aortic valve disease, Prosthetic Heart Valve, Valve Hydrodynamics, Thrombogenicity, Medical Device, ISO 5840
1. Calcific aortic valve disease
Calcific Aortic Valve Disease (CAVD) afflicts approximately 0.9% of the Unites States population with 2.8% of people over 75 years of age having moderate to severe aortic stenosis (AS), which is a narrowing of the aortic valve opening, ultimately leading to heart failure if untreated [1]. With CAVD progression into severe AS, valve narrowing limits blood flow into the aorta reducing forward flow in the systemic circulation and coronary perfusion. Further, stiffened valve leaflets (cusps) may also prevent their closure during diastole, leading to aortic insufficiency. The 2-year survival rate after onset of symptomatic AS is 50% and declines to 20% at 5 years with a rapid decline in survival thereafter [2]. The disease is characterized by the formation of tissue similar to bone [3] on the leaflets of the aortic valve. Valve calcification initiates with non-interacting nodules, which grow and coalesce until blood flow to the body is reduced [4]. Clinical symptoms include angina, syncope, and heart failure, thus early intervention is essential to limit morbidity and mortality [2]. However, patients usually do not have symptoms until the disease has progressed to an advanced stage [5].
2. The era of TAVR
Traditionally, replacement of the diseased valve with a prosthetic valve has been the most effective treatment for AS [6]. TAVR has become an established technology that provides an alternative to open-heart Surgical Aortic Valve Replacement (SAVR) [7]. It has emerged as an effective therapy for the unmet clinical need of inoperable patients with severe AS, often as their only life-saving treatment. In this type of minimally invasive intervention, a stent with a mounted bioprosthetic valve is delivered through the arterial tree, commonly in a transfemoral approach, and deployed through the stenotic native valve [8]. TAVR has the potential to replace traditional SAVR procedures in the future if its durability would be improved to a level similar to that of surgical bioprosthetic valves. The concept of TAVR devices began in 1999 with the start-up company PVT (NJ, USA) in collaboration with ARAN R&D (Caesarea, Israel), which completed the first-in-human feasibility studies in 2002 [9]. PVT was later acquired by Edwards Lifesciences Corp. (Irvine, CA, USA) in 2004. At the same year Medtronic (Minneapolis, MN, USA) began evaluating the feasibility of their own valves. With many successful feasibility trials in Europe (REVIVE, PARTNER Europe, TRAVERSE) both Edwards and Medtronic gained the Conformité Européenne (CE) mark in 2007 [9]. Currently, the only FDA-approved TAVR devices are the Edwards SAPIEN family balloon-expandable valves, and the Medtronic CoreValve family self-expandable valves, with the latest generation being the SAPIEN 3 and the Evolut Pro, respectively. In 2011, TAVR was indicated for use in inoperable patients (defined as a surgical risk with high risk patients avoiding surgery and deemed inoperable) with severe AS, thus fulfilling a previously unmet clinical need. In 2012, its indicated use was expanded to operable high-risk patients. In 2017, TAVR was granted commercial approval by the FDA for Intermediate-risk patients (with risk profiles that are generally suitable for surgical approaches). Following favorable results for TAVR in intermediate-risk patients [8], randomized trials are currently ongoing in low-risk (low risk profile for surgical operation) patients (PARTNER 3, Medtronic low-risk trial, and NOTION 2), comparing TAVR with SAVR [10]. Currently, approximately 180,000 patients can be considered potential TAVR candidates in the European Union and in Northern-America annually. This number might increase up to 270,000 if indications for TAVR expand to low-risk patients [11].
3. Review scope
The early and ongoing success of TAVR devices has led to a fast expansion of new TAVR valves and concepts that are rapidly introduced to the market. In this paper, we will discuss (i) the mechanical and biological requirements of a TAVR device, (ii) different TAVR design aspects using examples from existing devices, (iii) review in detail existing and complementary testing methods that are available today or that may be utilized in the future to address specific TAVR issues, both computational and experimental, and refer to the current corresponding ISO guidance.
4. TAVR performance guidance
The International Organization for Standardization (ISO) developed a set of guideline procedures for heart valves implanted with transcatheter techniques (ISO 5840–3). In terms of hydrodynamic performance, TAVR valves effective orifice area (EOA, a measure of the valve performance during systole) is expected to be higher than that of equivalent-size SAVR valves (ISO 5840–2), but at the same time are allowed a higher total regurgitant flow percentage (TAVR <20–25% of the stroke volume, SAVR <10–15%) to account for additional leak flows. However, the fact that a TAVR valve needs to be crimped and then deployed over a diseased and heavily calcified aortic valve makes it technically far more demanding to develop a successful TAVR device. Each new TAVR valve design must be thoroughly tested, both in bench testing and pre-clinical tests before reaching the first-in-man stage. We highlight several requirements and specific challenges that a TAVR valve needs to meet: (a) hemocompatibility, (b) hydrodynamics, (c) non-thrombogenicity, (d) high durability, (e) low calcification susceptibility, and (f) crimping & deployment stability (Figure 1). Aspect (f) makes the engineering of TAVR devices even more challenging compared to the surgical valve designs. TAVR designs can be generally grouped into balloon-expandable and self-expandable valves. Each concept has its own engineering challenges, both pre- and post-procedural.
Figure 1:
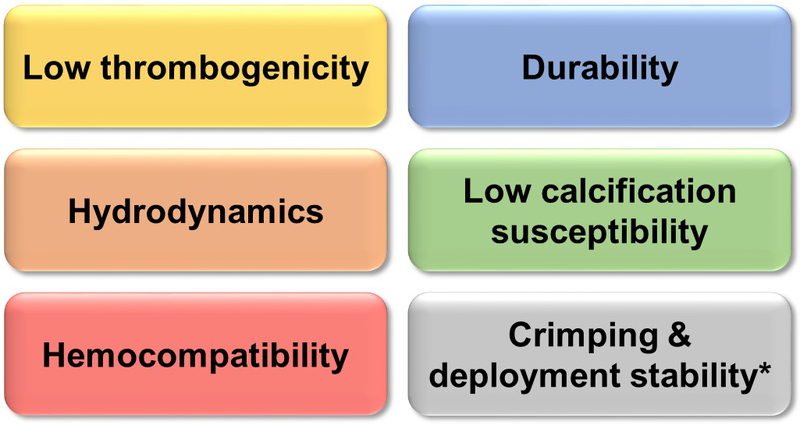
Highlights of a TAVR device key requirements. * The only requirement that is not shared with SAVR devices.
5. Common complications of TAVR
While improved greatly since the first-generation devices were introduced to the market, several persistent complications still exist, and are becoming of more concern with the expansion of TAVR to younger and lower-risk patients. Paravalvular leak (PVL), structural valve deterioration (SVD), permanent pacemaker implantation (PPI), valve thrombosis and strokes are the most common complications [12].
Roughly, 25% of all post-TAVR patients suffer from mild-to-more severe paravalvular aortic regurgitation (AR) [13]. Although in recent years the reported incidence of AR is decreasing, moderate-to-severe AR can still be expected at a rate of approximately 5% following TAVR, 10 times more frequently than after SAVR [10].
SVD is defined by permanent intrinsic changes of the valve (calcification, pannus, and leaflet failure) leading to degeneration and/or dysfunction [14]. The risk of SVD is heavily influenced by valve design and patient age at the time of implantation, with inverse association between age and prevalence of SVD [15]. Medium term follow-up with TAVR experience is so far reassuring in terms of SVD. However, the experience with bioprosthetic SAVR valves indicates that at least 10 years of follow-up are essential to establish the long-term durability of TAVR valves [14].
Latest-generation TAVR devices have been associated with higher incidence of cardiac conduction abnormalities (CCAs). Balloon aortic valvuloplasty is routinely employed before TAVR, helping facilitate easier deployment and minimize the likelihood of coronary occlusion but increases the risk of conduction abnormalities [16, 17] and may require concurrent PPI. Cases of left bundle branch block (LBBB) and total atrioventricular (AV) block have been repeatedly reported [16, 17, 18, 19]. Because of the proximity of the AV-node, mechanical stress and injury exerted by the deployed stent on the aortic annulus may damage the bundle of His fibers and those forming the left branch [19].
6. Extended and off-label uses for TAVR
For high-risk surgical patients or those deemed unable to undergo surgery, TAVR may be used off-label as an alternative to surgery or medical therapy.
A recent indication for TAVR devices is the replacement of failed bioprosthetic valves in a procedure that is called Valve-in-Valve (ViV). ViV, which can be used both for failing bioprosthetic SAVR [20] or TAVR valves [21, 22], allows the patients to avoid open heart surgery (if former TAVR valve recipients) or a repeated surgery (if former SAVR valve recipients). The failing bioprosthetic valve or TAVR valve is treated like the native stenotic valve and a TAVR valve is deployed into the annulus of the failed valve. The ViV procedure, however, increases the narrowing of the valve geometric orifice area and reduces the hydrodynamic performance, since the transcatheter valve is deployed within the orifice of the failed bioprosthetic or TAVR valves [12]. Therefore, the risk-benefit of ViV (versus SAVR) is dependent on the available geometric orifice area of each and every patient, and hence the option of ViV should not be planned to compensate for a lower-durability valve in the first procedure.
TAVR in bicuspid aortic valve (BAV) patients leads to a unique off-label use due to the radical variation in patients valve orifice geometry. Although BAV occurs in about 1% of the population [23], up to 75% of the BAV population will develop AS pathologies early on [24]. BAV patients have elliptical valve openings and major variations in calcific patterns (Raphe 0,1,2 with/without calcific commissures) that make transcatheter aortic valve (TAV) deployment more challenging than the intended use in AS patients (in terms of the TAV conforming to the native pathological BAV anatomy).
7. Search for alternative leaflet material: the polymer opportunity
To date, pericardium xenografts are the only leaflet material for flexible prosthetic aortic valve to gain FDA and CE approval. However, many of the persistent limitations associated with bioprosthetic valves (both SAVR and TAVR) are inherently related to the tissue material (i.e. calcific degeneration, crimping and deployment damage, durability). Other shortcoming of bioprosthetic valves is related to cost-effectiveness of the manufacturing process: (i) producing the harvested tissue from which leaflets can be processed with enough reliability and reproducibility involves an extremely high rejection rate (approximately 98%), (ii) the valves have to be sutured by hand, which limits automation and makes it non-pertinent for mass production. This has led to a constant search for alternative leaflet materials. Polymers provide better design freedom to overcome many of the aforementioned limitations as they offer the possibility to specifically design and optimize a TAVR valve from the bottom up, and can be potentially produced at high reproducibility and lower costs. However, all the attempts to date to develop a viable polymeric aortic valve have failed [25]. Many of these valves had promising early results, yet none had everything needed to gain FDA or CE approval (Figure 1). In recent years, new polymer technologies have emerged and subsequently novel polymeric aortic valve have been developed, showing very promising early in-vitro results [26]. Examples for such devices include the PolyNova xSIBS TAVR valve (PolyNova Cardiovascular Inc., Stony Brook, NY, USA), Triskele urethane (POSS-PCU) TAVR valve (UCL TAV™, University College London, London, UK), Foldax SAVR valve (Foldax Inc., Salt Lake City, UT, USA), SAT TAVI heparinized polyurethane valve (SAT, Cape Town, South Africa), and the Endurance Valve HA-LLDPE (Ohio State University, OH, USA). While some have been initially developed for surgical applications, the future of polymeric valves is likely in transcatheter applications where tissue leaflets are more vulnerable. Specific design aspects of these valves will be discussed in more details below.
8. TAVR design aspects
Figure 2 depicts various current TAVR devices with varying design approaches for addressing some of the aforementioned complications/limitations. In general, the various TAVR valves stent types are categorized as: balloon-expandable valves (e.g. SAPIEN 3), self-expandable valves (e.g. Evolut Pro), and other (mechanically expanded, e.g. Boston Scientific Lotus Edge Valve) (Table 1). Among those valves, currently only the SAPIEN 3 balloon-expandable and the Evolut Pro self-expandable are FDA-approved valves. The SAPIEN 3, Evolut Pro, ACURATE Neo, Allegra, Engager, JenaValve, Portico, Lotus Edge and the CENTERA have CE mark.
Figure 2:

Selected TAVR valves based on varying design approaches, both commercially-available and under-investigation devices.
Table 1:
Selected TAVR valves with varying design approaches.
| Valve | Approval for use | Stent/frame type | Crimped profile (Fr) |
Leaflets material |
Leaflets position |
Retreivable/ Repositioable |
|---|---|---|---|---|---|---|
| Evolut Pro (Medtronic) |
• FDA approved • CE mark |
Self-expandable (Nitinol) |
14 | Porcine pericardium | Supra-annular | Partially/Yes |
| ACURATE Neo (Boston Scientific) |
• CE mark | Self-expandable (Nitinol) |
18 | Porcine pericardium | Supra-annular | No/No |
| Allegra (NVT) |
• CE mark | Self-expandable (Nitinol) |
18 | Bovine pericardium | Supra-annular | - |
| Engager (Medtronic) |
• CE mark | Self-expandable (Nitinol) |
30 | Bovine pericardium | Supra-annular | Partially/ Yes |
| JenaValve (JenaValve Technology) |
• CE mark* | Self-expandable (Nitinol) |
32 | Porcine pericardium | Supra-annular | Partially/Yes |
| Portico (St. Jude Medical) |
• CE mark | Self-expandable (Nitinol) |
18 | Bovine pericardium | Intra-annular | Fully/Yes |
| SAPIEN 3 (Edwards Lifesciences) |
• FDA approved • CE mark |
Balloon-expandable (Cobalt-chromium) |
14/16 | Bovine pericardium | Intra-annular | No/No |
| Lotus Edge (Boston Scientific) |
• CE mark | Mechanical-expandable (Braided Nitinol) |
18/20 | Bovine pericardium | Intra-annular | Fully/Yes |
| CENTERA (Edwards Lifesciences) |
• CE mark | Self-expandable (Nitinol) |
14 | Bovine pericardium | Intra-annular | Partially/Yes |
| Direct Flow (Direct Flow Medical) |
• CE mark • No longer available |
Inflation of balloon rings by a polymer | 18 | Bovine pericardium | Intra-annular | Fully/No |
| Colibri (Colibri Heart Valve LLC) |
• Investigational | Balloon-expendable (Stainless steel) |
9 | ‘Dry’ porcine pericardium | Intra-annular | No/No |
| Meridian Valve (HLT Medical) |
• Investigational | Self-expandable (Braided Nitinol) |
18 | Porcine pericardium | Intra-annular | Fully/Yes |
| J-valve (JC Medical) |
• Investigational | Self-expandable (Nitinol) |
27 | Porcine pericardium | Intra-annular | - |
| FoldaValve (Folda LLC) |
• Investigational | Self-expandable (Nitinol) |
14 | Bovine pericardium | Intra-annular | Fully/Yes |
| Triskele (UCL TAV) |
• Investigational | Self-expandable (Nitinol) |
20 | Urethane (POSS-PCU) polymer | Intra-annular | Fully/Yes |
| SAT TAVI (Strait Access Technologies) |
• Investigational | Balloon-expandable (−) | - | Heparinized Polyurethane or bovine pericardium | Supra-annular | No/No |
| PolyNova Valve (PolyNova Cardiovascular Inc.) |
• Investigational | Self-expandable (Nitinol) |
16 | xSIBS polymer | Intra-annular | Partially/Yes |
| Endurance Valve (Ohio state University) |
• Investigational | Balloon-expandable (Cobalt-chromium) |
18 | HA-LLDPE polymer | Intra-annular | No/No |
Transapical JenaValve device (shown in Figure 2).
8.1. Paravalvular leak (PVL) –
The most common design approach in the latest valves’ designs for minimizing PVL is the incorporation of an outer skirt at the lower part (ventricular side) of the stent (Evolut Pro, ACURATE Neo, SAPIEN 3, Lotus Edge, Colibri, FoldaValve, SAT valve, Endurance valve). In the SAPIEN 3 for example, the outer PET fabric skirt is divided into pockets intended to fill with retrograde clotting blood and seal the gaps between the valve and the tissue, conceivably offering a certain degree of protection against PVL. Another approach for minimizing PVL, which is commonly adopted in the self-expandable devices, is expanding the ventricular side of the stent (flared annulus design) for better attaching the stent to the aortic root on the ventricular side (Evolut Pro, ACURATE Neo, Engager, CENTERA, Triskele, SAT TAVI). A unique stent design of the Lotus Edge, which is characterized by a thinner and denser mesh with an adaptive axially collapsing polycarbonate-urethane polymer sealing skirt along the annulus edge. It aims at better conforming to the shape of the native aortic root for minimizing the potential of PVL gaps forming post-deployment. Direct Flow valve (Direct Flow Medical Inc., Santa Rosa, CA, USA) is a non-metallic valve made of bovine pericardium and a conformable expandable cuff. After placement of the valve in position across the annulus, the cuff is inflated with liquid plastic polymer that hardens while keeping the cuff in place through a double ring. This design aims to achieve an atraumatic seal [27].
8.2. Durability –
Durability is now becoming a major concern of TAVR with its increasing expansion to younger and lower risk patients. Tissue leaflets have become the material of choice for PHV manufacturers, either for surgical of transcatheter valve applications, as these have proven record for passing the minimum ISO requirement of 200M cycles in the accelerated wear tests. In terms of tissue leaflet design for improving durability, the material allows only limited design freedom. Very little information is disclosed by the valve manufacturers who mostly protect it as proprietary information.
The Meridian Valve (HLT Medical, Maple Grove, MN, USA) leaflets are porcine pericardial tissue mounted on a wire form that flexes throughout the cardiac cycle. This design is intended to support leaflet motion and reduce tissue stress, which in turn may enhance the valve’s durability. The stent of the Allegra valve was designed such that when the valve is fully open, the leaflet’s free edges do not touch the stent, with the aim of preventing wear of the leaflets, and improving valve durability. An integrative approach that combines various TAVR valve challenges demonstrates the design flexibility using alternative materials such as polymers. The PolyNova polymeric TAVR valve was designed using the Device Thrombogenic Emulation (DTE) methodology for optimizing hydrodynamic and durability performances, as well as minimizing the valve thrombogenicity [28]. It yielded a leaflet design where flexural stresses were minimized by varying its thickness [28], and the nominal conformation of the leaflets (‘zero-stress’ position) was adjusted to be semi-open [29]. Currently, several polymeric investigational TAVR valves already met and surpassed the ISO 5840–3 durability standard (e.g., SAT TAVI, PolyNova).
Other than mechanical failure per se, durability of TAVs can also be affected by crimping and deployment –induced damage, calcific degeneration, and thrombosis. These will be discussed separately in the following sections.
8.3. Crimping damage –
In recent years there is a growing evidence that bioprosthetic valves are subjected to irreversible mechanical damage during valve crimping [30, 31] and deployment [32, 33]. With the trend growing to crimp TAVR devices into lower profile delivery catheters, the collagen fibers are prone to fragmentation in the tissue leaflets [34], applying significant tissue damage both on the leaflets surface and through its depth [31]. The damage to the leaflets is increased with lower crimping sizes [31], and with longer crimping durations [34]. The concern of crimping duration restricts most TAVR devices today to be crimped onto the delivery catheter and immediately deployed at the procedure site (cath-lab) using trained personnel, with limited time between crimping to deployment (e.g. < 20 minute with the SAPIEN 3 at 14 Fr crimping size). The Colibri valve (Colibri Heart Valve LLC, Broomfield, CO, USA) incorporates ‘dry’ porcine pericardium that allows for crimping and loading of the valve onto the delivery system at factory setting [35]. In addition, Colibri reports that their latest generation valve can be crimped up to 9 Fr (3 mm), which is the lowest profile reported to date. By factory-crimping the device into such a small profile the device can be used in many patients with challenging vascular access. However, the concern of crimping damage and its effect on long-term durability yet needs to be explored. The FoldaValve (Folda LLC, Rancho Santa Margarita, CA, USA) addressed the issue of stent-crimping damage by incorporating a unique crimping process that ends with the leaflets folded outside of the crimped stent, thus by avoiding leaflet damage by the stent struts, less pressure is effectively applied on them. This mechanism can furthermore provide further room for crimping the valves into a smaller French size without risking the leaflet damage [36]. Alternative approach for overcoming the limitation of crimping damage is to use materials that are less prone to damage as compared to the current harvested tissue derived materials for the leaflets. This could be achieved by engineering a leaflet material with yield stress that is far from the stresses that are expected during crimping. The PolyNova valve, for example, incorporates xSIBS polymer for the leaflets, which has shown in-vitro resistance to crimping-damage following 8 days of crimping.
8.4. Calcific degeneration
Aside from fatigue and structural damage associated with durability, formation of new calcific masses in the TAV leaflets can lead to valve failure from increased stresses or induced stenosis. The calcific masses can form from active processes where the immune responses of the body cause new calcific masses to grow, or passive processes where calcium ions can accumulate into defects in the tissue [37]. Glutaraldehyde is mainly used to chemically treat the two kinds of tissues (bovine and porcine pericardium) that are used for TAVs [38]. Although glutaraldehyde decreases immune response to the tissue leaflets, it does not completely eliminate the active calcification process [37]. Calcific degeneration is considered a major limitation of tissue valves and was traditionally addressed by valve manufacturers by chemically modifying the tissue leaflets, to make them more resistant to calcific deposition. While calcific degeneration may be associated with the valve design and leaflet kinematics, this kind of modification is less a design approach, but more of a chemical treatment of the implanted tissues. Choosing a different material for the leaflets, e.g. polymers, might also overcome this limitation, by engineering their chemistry to tackle both the passive and active calcific deposition. However, this will have to be carefully verified both in-vitro and in-vivo.
8.5. Thrombogenicity –
Thrombosis in heart valves is governed by the interaction between the blood flow (hemodynamics) and foreign material (hemocompatibility). Flow induced stresses are governed by the valve design and kinematics that dictate the resultant flow patterns formed. Those, combined with the foreign materials hemocompatibility, determine the thrombogenic potential of the valve. The flow-induced thrombogenicity could be initiated by several mechanisms: (i) PVL, which involves a confined retrograde jet flow into the left ventricle during diastole, exposes platelets to elevated shear stresses that may activate them, potentially leading to either thrombus or thromboemboli formation [39]. (ii) Stagnation regions in the valve neo-sinuses, with low washout, that may lead to thrombus formation on the leaflets [40, 41, 42]. With thrombus growth, it may limit the leaflets kinematics [43] and may also affect the valve durability [15]. (iii) Highly disturbed flow regime through the valve orifice at systole may lead to platelet activation and thromboemboli formation. This is common with mechanical surgical valves, and less problematic with bioprosthetic or polymeric SAVR valves. However, specifically for TAVR valves, because the valve is deployed over a diseased valve there is an inherent degree of narrowing that further accelerates the systolic blood flow through the valve and exacerbates the flow induced stresses.
In terms of minimizing the valve thrombogenicity using a dedicated design, the DTE methodology that was described above and was incorporated into the design of the PolyNova polymeric TAVR valve, allows to iteratively minimize flow disturbances such as recirculation regions and regions of strong shear flows [44, 45]. The polymeric SAT TAVI leaflets design focused on leaflets smoothness for further reducing thrombogenicity.
8.6. Stent design approaches –
Stent designs have evolved immensely since the conception of TAVR to address all of the design aspects and make a better product. Many valves have iterated on the original designs of the balloon-expandable SAPIEN valve or the self-expandable CoreValve to address the major complications, while other companies have created radically different designs to address them. For example, the newest generation SAPIEN 3 valve has benefited from wider-angle stent cells as well as a denser cell structure in the annulus region of the frame, allowing better coronary access in the supra-annular frame and better sealing around the annulus [46]. The Evolut Pro was designed with a different shape (versus the original CoreValve) over the annulus region for better sealing and increased radial force [47]. The CENTERA Valve (Edwards Lifesciences) is a new self-expandable design that incorporates the benefits of the SAPIEN low-profile frame with the ability to recapture and redeploy the valve [48]. The Lotus Edge (Boston Scientific) holds the claim for one of the only fully recapturable and redeployable valves as well as having a braided frame for increased sealing [49]. The Meridian valve, like the Lotus Edge, also utilizes braided nitinol stent that is fully recapturable and redeployable, yet with a completely different design. Some designs, like the Engager (Medtronic) [50], J-valve (JC Medical) [51] and JenaValve (JenaValve Technology) [52], have created stents that lock onto the native leaflets or engage with the native sinus cusps to anchor the stent. Other designs, like the FoldaValve, crimp the stent without contacting the tissue leaflets with the stent. Then, during deployment the leaflets are pulled back into the stent frame and take shape [36]. The Endurance valve stent was designed such that it requires only 9 polypropylene sutures, all of which are non-load bearing and function only to keep the leaflets in place during crimping and expansion. Generally, the progression of stent designs has been working towards smaller profile designs for decreased crimping profiles, recapturing/redeployment, and better anchoring. Design aspects of ‘Valve migration’ and ‘Valve orifice circularity’, which are also related to the stent design, are discussed below.
8.7. Valve migration –
Valve migration may result from improper anchoring of the TAVR valve. The risk is mostly of migration into the left ventricle as migration force is maximal during diastole. The majority of TAVR valves use radial force against the aortic root and the calcified leaflets to hold the valve in place (see Figure 2). The deployed valve is intentionally oversized within the native diseased calcified aortic root. Other self-expandable devices, combine radial force with anchoring struts that hold against the native leaflets, providing axial compression, and prevent the valve from migrating into the left ventricle during diastole (e.g. ACURATE Neo, Engager, J-Valve [53], JenaValve, SAT TAVI [54]); The SAT TAVI, for example, is designed for Rheumatic Heart Disease (RHD) patients, where the transcatheter valve is deployed into a compliant aortic root and valve that are non-calcified, and therefore radial force-based anchoring is not enough for preventing valve migration [55]. Moving the fixation to the native leaflets offers an increased ability to treat aortic regurgitation patients without AS and calcific masses to anchor against [55]. The Allegra valve (NVT, Germany), like some other supra-annular self-expandable TAVs comprise of a distal stent region that aims at stabilizing the valve against the ascending aorta. Unlike the other TAVs, the Allegra valve is not dependent on the diameter of the ascending aorta. Moreover, the radial force of the stent is increased in the basal part that provides implant anchoring. The JenaValve, which is implanted in a trans-apical approach, is completely supra-annular (e.g. does not penetrate into the left ventricle outflow tract) and relies only upon anchoring onto the calcified native leaflets. The Triskele has a stent design that mainly applies counteractive axial forces other than radial forces. This design also aims for reducing the perturbation of the AV-node and the Left Bundle Branch (LBB) [56]. The Direct Flow valve uniquely holds on both sides of the native aortic root using inflatable rings. In addition to providing axial securement to the anatomy, this approach also provides seal against PVL. The ACRUATE Neo (Boston Scientific) stent has aortic-facing arches in the upper crown, not to lock on the native leaflets, but rather to push the native leaflets away from the TAV leaflets and coronary ostia.
8.8. Valve orifice circularity –
Self-expandable TAVR valves are more prone to stent deformations after the deployment, and various designs address this issue by adopting supra-annular positions for the prosthetic leaflets with either a flaring or a certain degree of freedom from the stent confinement (e.g. Evolut Pro, ACURATE Neo, Allegra, Engager, JenaValve). Such device positioning allows the valve to assume a circular cross section where the leaflets extend, thus less affected by the deployed stent shape. The Meridian valve adopts a different approach: the wire form, independent of the outer support structure, is intended to allow the leaflets to maintain a circular geometry, preserving the flow area even in an eccentric diseased annulus.
9. Testing methods for TAVR valves
9.1. By the ISO
This ISO standard is designed to outline benchmark in-vitro testing for the Transcatheter Heart Valve (THV) devices to provide evidence of efficacy and safety for CE and FDA approvals before proceeding to clinical evaluations. The tests are used to assess physical, chemical, biological and mechanical properties of THVs and of their materials and components. ISO 5840 also defines operational conditions and performance requirements for THV substitutes. All TAVR valve manufacturers must follow a long list of bench tests that are defined by ISO 5840–3. FDA approval is dependent on the results of these tests and therefore it is critical to design the THV according to the pass/fail criteria as defined in the ISO. These tests include, among others: (i) Material property assessment, including biocompatibility (ISO 10993–1) and mechanical property testing (at various stages of manufacture), (ii) Device hydrodynamic performance, (iii) Structural performance, which includes device durability, structural component fatigue and component corrosion, and (iv) additional implant design evaluation like resistance to device migration, device MRI safety, radial resistive force, recoil (for balloon-expandable stents), etc. ISO 5840–3 also outlines the required evaluation criteria for the delivery systems, yet this is beyond the scope of this review.
While ISO 5840–3 defines the testing methods and minimum performance requirements for a THV, new testing approaches are being developed, mostly on the academic side, with the aim of optimizing both device assessment to be more realistic and optimizing the procedure and thus the clinical outcomes. These include both experimental as well as advanced computational simulations that complement each other. In addition, many of these methods are used to investigate new clinical indications for TAVR (e.g. ViV, or off-label TAVR use in BAV patients) that are excluded from ISO 5840, and whose long-term clinical outcome data is still sparse. Table 2 summarizes numerous publications to date related to TAVR testing, categorized according to testing aspects and parameters/cases of evaluation, both experimental and computational. Using this table, the readers could refer to what evaluation approaches for TAVR devices have been developed and utilized, and choose the most pertinent method, or develop their own, according to their needs. Description of selected testing methods/models, both computational and experimental, follows.
Table 2:
Up to date testing methods for TAVR valves according to testing categories, both in-vitro and in-silico, and the corresponding ISO guidance. CCA – Cardiac conduction abnormalities; CFD – computational fluid dynamics; FEA – finite element analysis; FSI – fluid-structure interaction; PVL – Paravalvular leak; ViV – valve-in-valve.
| Aspects | In-vitro | In-silico | Comments |
|---|---|---|---|
| Crimping | Leaflet Damage [31, 33, 99, 100, 101], Radial Force [127, 128] ISO-5840-3 Sec 7.2.5.7, Calcification [34] | Stent deformation analysis [73, 74] and validation of radial force [129]* | |
| Stent deployment Balloon-expandable |
AR rupture [59], ViV [57, 130, 131], Positioning [33, 57, 58], Coronary Flow [57], Patient Specific [66, 67, 68, 69, 70, 71, 72, 110], Pullout Force [58] ISO-5840-3 Sec 7.2.5.1, Paravalvular Gaps [69, 70, 72], Structural Performance ISO-5840-3 Sec 7.2.4, Recoil ISO-5840-3 Sec 7.2.5.5 | Stent positioning [74, 81, 82], Leaflets stress analysis [79, 80, 132], Stent deformation [83, 133], Paravalvular gaps [84, 133Ŧ, 134], AR rupture [84], AR material calibration [135]Ŧ, ViV [82] | FEA (in-silico) |
| Stent deployment Self-expandable |
ViV [60, 61, 136], Positioning [56, 58, 60, 61, 62, 63, 64, 65], Patient Specific [66, 67], Pullout Force [58, 64, 68] ISO-5840-3-3 Sec 7.2.5.1, Structural Performance ISO-5840-3 Sec 7.2.4 | Stent positioning [78], Stent deformation [76Ŧ, 94*, 137, 138], Paravalvular gaps [76Ŧ, 78, 139], CCA [17, 139] Ŧ, AR material model calibration [140] | FEA (in-silico) |
| Leaflet mechanics | Eccentric [111], Oversizing [112], High-resolution strain analysis [113] | Eccentric deployment [75, 141, 142], Material model calibration [86, 143*, 144*], ViV [85] | FEA (in-silico) |
| Durability | Durability [37, 103, 131] ISO-5840-3 Sec 7.2.4.1, | [85, 145] | |
| Calcification susceptibility | Accelerated [114, 115] | ||
| Hydrodynamics | Baseline [29, 56, 103, 107, 108, 131] ISO-5840-3 Sec 7.2.3, Patient-specific [29, 71, 104], ViV [58, 60, 64, 65, 105, 106, 136], Sinus Stasis [61, 63, 65, 102, 104, 105, 107], Eccentric [62, 111], Leaflet Dynamics [112, 146], patient-specific PVL [69, 72] | Patient-specific [147, 148, 149], Patient-specific PVL [90] Ŧ, Eccentric deployment [89]*, Leaflets dynamics [93, 94, 146]* | CFD/FSI (in-silico) |
| Thrombogenicity | Crimping/Balloon Damage [100], Blood Stagnation [40, 117], Biocompatibility [116, 150] ISO-5840-3-3 Sec 7.2.2.2, ViV [117], PVL [39], Flow-induced platelet activation [28, 29] | Blood stagnation [42, 151, 152]*, Leaflets durability [85] |
Validated in-vitro,
Validated in-vivo
9.2. Computational models
Computational models have been developed to assist clinicians in pre-procedural planning and in understanding the underlying biomechanics of the clinical complications in cardiovascular disease, such as CAVD. In other cases, computational models have also been developed for optimizing TAVR device design and performance during R&D stages. Several studies addressed various aspects of TAVR procedure via Finite Element Analysis (FEA) [33, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72], from crimping to deployment, in order to evaluate performance of TAVR valves in specific settings. Among the advantages of computational models: (i) once established, it allows analysis of the results in extremely high spatial and temporal resolution, (ii) it allows analysis of more parameters than in an equivalent experimental setup, without interfering with the measurements, (iii) it allows investigation of more complex models and various scenarios while simultaneously considering more factors, and (iv) isolation of any of the input parameters and performing sensitivity analyses are relatively easy and cost-effective- as compared to equivalent costly experiments. Due to these reasons computational simulations serve as an excellent tool to complement experimental testing approaches. Below are highlights surveying recent modeling efforts relevant and/or specific to TAVR.
9.2.1. Valve Crimping
Crimping has been shown to have an impact on bioprosthetic leaflets [30, 31] and a FEA can serve to conduct stress analyses and assess the damage risk [73], which in turn may affect the valve durability. The inclusion of the prosthetic leaflets appears to have a negligible effect on the stent longitudinal and axial deformation during the crimping [74], thus allowing to simplify crimping models in following analyses for reduced computational cost. Stresses experienced in prosthetic leaflets were computationally compared between Edwards SAPIEN bioprosthetic valve and PolyNova polymeric valve. The peak values normalized to the ultimate tensile stresses were 9.1% and 24.7% for the PolyNova and SAPIEN valves, respectively, indicating that the polymeric leaflets should potentially sustain less damage during crimping to a similar catheter profile [73].
9.2.2. Stent deployment
Stent deployment can be categorized according to the type of TAVR valves, namely, balloon-expandable and self-expandable valves. Unlike balloon-expandable TAVR valve, the self-expandable TAVR valve’s prosthetic leaflets tend to show more often eccentric post-deployment configuration along with the stent- its effect on the leaflets’ stress distribution was studied via FEA [75]. FEAs of self-expandable TAVR valves are mostly of the Medtronic CoreValve family. Similar to balloon-expandable valves studies, these aim in assisting pre-procedural planning to minimize potential clinical complications. Specifically, FE simulations can predict the presence of paravalvular gaps based on the distance between the frame and luminal side of the aortic root, and can be further validated with clinical data. Bosman et al. (2016) and Schultz et al. (2016) compared the results of stent deployment with post-TAVR CT-based reconstruction and the calculation of paravalvular gaps with post-op angiographic and echocardiographic aortic regurgitation grade [76, 77]. The deployment strategy was also modeled in terms of valve implantation depth and release angle. The stent cross-section eccentricity was measured for each configuration and results show their direct impact on the final stent deformation [78].
As mentioned above, stent deployment models are often developed with the exclusion of prosthetic leaflets for simplicity reasons. In a comparative analysis Bailey et al. (2015) showed that the inclusion of the prosthetic leaflets in a complete model of a balloon-expandable TAVR led to a discrepancy of 0.236% of the expanded frame diameter after being deployed in a patient-specific aortic root [79]. On the other hand, the impact of stent deployment on deformations in the prosthetic leaflets was studied through more complex models. In these studies, the TAVR valve was deployed in different circumferential orientations relative to the native valve and it was found that in the specific analyzed cases it is preferable to deploy the device with the prosthetic leaflets aligned with the native leaflets, as the stresses in the valve increased when the distance between the prosthetic commissures decreased. This potentially could represent a sufficient increase in stress to induce variation in device lifespan, which would therefore have a direct impact on valve durability This consideration remains valid on a patient-to-patient basis, since TAVR valve final deformation is dependent on the individual anatomy and calcifications distribution [80].
9.2.3. Valve positioning
Positioning the valve prior to the deployment is one of the procedural parameters which has been shown to affect the stent landing zone and anchorage onto the native tissue. Several patient-specific studies with different levels of accuracy were published to investigate the stent deformation in different scenarios [74, 81, 82]. Bianchi et al. (2016) quantified the stent anchorage to the calcified native leaflets in three procedural scenarios (midway, distal and proximal). Observed contact area resulted in a sudden drop in the recoil phase of the stent for the proximal configuration, leading to stent dislodgment into the LV, as occurred in the clinical setting (Figure 3) [74]. Calcification distribution has been shown to represent the main predictor of TAVR performance, as shown by Sturla et al. (2016), in which different calcification patterns were implemented based on ex-vivo measurements on three explanted human aortic valve leaflets [83]. FE models are capable of assessing complications such as risk of aortic root rupture based on stress developed in the sinus during balloon-expansion. Material failure criteria were included to pinpoint the location of the soft tissue failure upon stent expansion [84], based on a unreferenced maximum stress threshold of σ=2.5 MPa.
Figure 3:
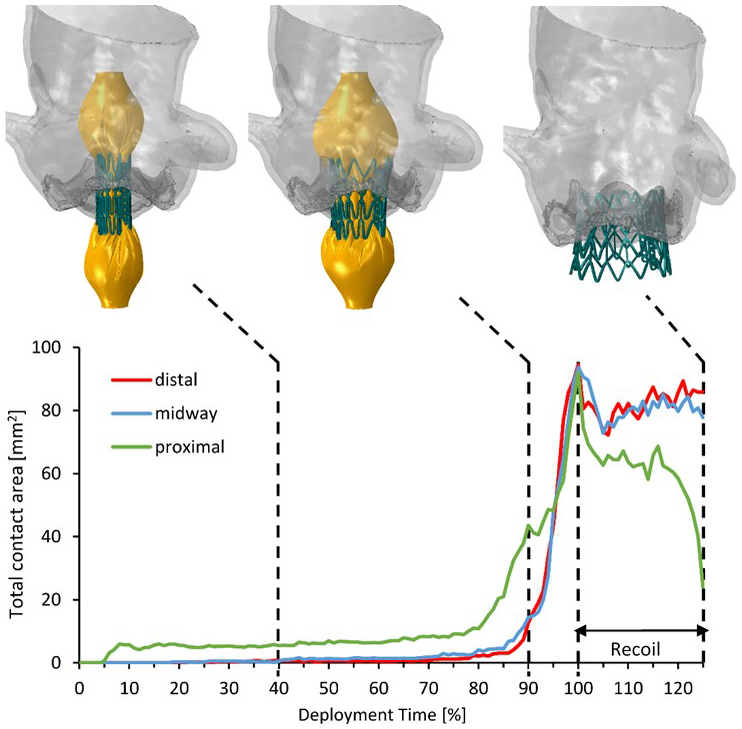
Total contact area calculated in the deployment and recoil phases for the distal, midway and proximal configurations. The model in the midway configuration is shown in three instances: 40% and 90% of the deployment time, and at the end of the recoil. Adapted by permission from John Wiley and Sons, Artificial Organs (Bianchi M. et al. Effect of Balloon-Expandable Transcatheter Aortic Valve Replacement Positioning: A Patient-Specific Numerical Model)©2016.
9.2.4. Leaflet mechanics and durability
TAV leaflet fatigue damage was studied via FEA under cyclic pressurization in different levels of under-expansion (1, 2, 3 mm reduction in diameter) (Figure 4) [85]. In the under-expanded valves, the TAV leaflets exhibited severe pin-wheeling (diastolic deformation of coapted leaflets with inherent twisting pattern) during valve closure, which drastically increased leaflet stresses, and resulted in accelerated fatigue damage of the leaflets. This study ascertains that an under-expansion >9% will significantly impact the durability, even though clinically a range of 10–15% under-expansion is generally considered acceptable.
Figure 4:
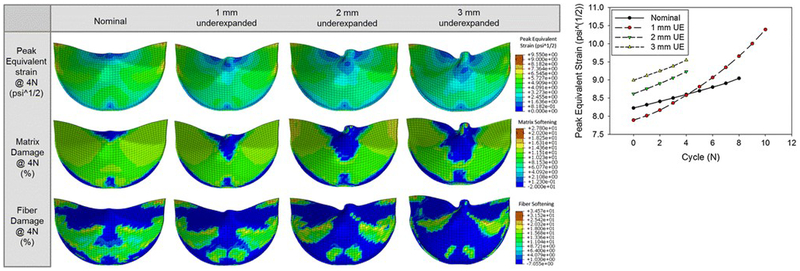
Left- Contour plots of the peak equivalent strain and the matrix and fiber damage at the 4th cycle fatigued state for each case. Right- The peak equivalent strain observed in the leaflets for each case and cycle. Reprinted by permission from Springer Nature, Annals of Biomedical Engineering (Martin C. et al. Transcatheter Valve Underexpansion Limits Leaflet Durability: Implications for Valve-in-Valve Procedures)©2016.
The mechanical response of the materials used to fabricate TAVR valve leaflets is often analyzed computationally. These were compared and tested using FEA under static pressure-only loading conditions to examine the effects of tissue thickness and anisotropy on the valve deformation and stress distribution [86]. Results showed that bovine pericardium leaflets experience lower maximum principal stress than porcine leaflets. Similar aspects can be analyzed for TAVR ViV utility, adding on the complexity of the models, thereby requiring simplification assumptions [82].
9.2.5. Effect of TAVR on cardiac conduction abnormalities
FEA was employed to assess contact pressures in patient-specific TAVR deployment models, in the region of the aortic root that encompasses the AV conduction system, which was determined by identifying the membranous septum (MS). The analysis was conducted for a large set of patients (n=112) with and without emergent CCAs, such as new LBBB or high-degree AV block. In patients with these CCAs, the results showed a significant increase in contact pressure and contact pressure index [17].
To date, the beating action of the heart was not investigated in TAVR deployment simulations. The Living Heart Human Model (LHHM) is an electro-mechanically coupled heart simulator provided by Dassault Systèmes SIMULIA (Johnston, RI, USA) inclusive of the entire heart and the electrophysiology and fibrous architecture of the myocardium. The mechanical behavior of the model was validated against clinical data to assure physiological and realistic responses [87]. This model can be employed to investigate the interaction of the heart wall motion on TAVR valves performance and its effect on risk of CCAs through quantifying dynamic post-deployment stresses and strains in the proximity of the AV-node, MS, and LBB (Figure 5) [88].
Figure 5:
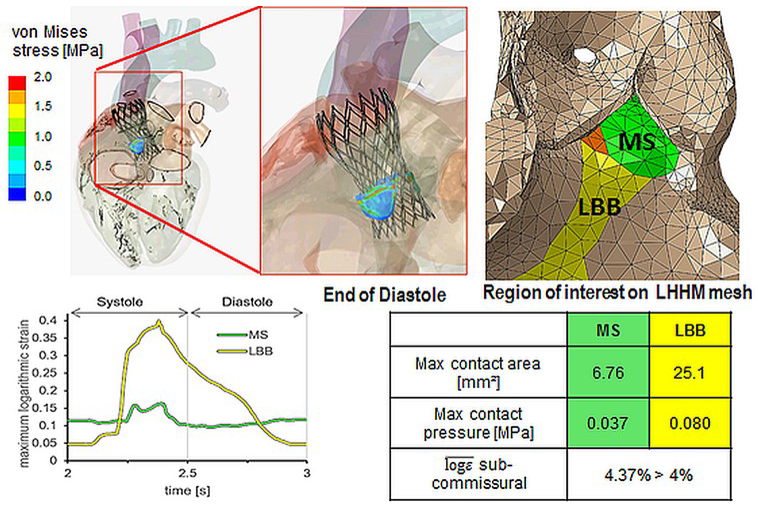
Post TAVR deployment stresses and strains in SIMULIA beating heart model in close proximity of the AV node (animation freeze frame- Top) - for assessing a mechanical threshold predictive of CCAs (bottom). LHHM – living heart human model; VBB – left bundle branch; MS – membranous septum. (Adapted from Ghosh R.P. et al. P6317Simulation of transcatheter aortic valve performance in a beating heart. European Heart Journal 2018;39(Suppl_1): ehy566.P6317-ehy566.P6317. By permission of Oxford University Press).
9.2.6. Hydrodynamics
Computational Fluids Dynamics (CFD) has been used to study and characterize hydrodynamics in TAVR patient-specific models, although to a lower extent given the complex interacting geometries that come into play. Sirois et al. (2018) investigated the effect of elliptical deployment and under-expansion on the transvalvular flow of isolated valves. Increases in transvalvular pressure gradients indicated that under-expanded deployment has a much greater negative effect on the TAV hemodynamics compared with elliptical deployment [89].
CFD was employed to study post-deployment AR in a large set of patients for which predicted values were compared and in agreement with angiographic and echocardiographic data [90]. Fluid-structure Interaction (FSI) analysis is capable of simulating leaflets mechanical response to blood flow and vice versa by solving the fluid and structural mechanics governing equations simultaneously [91]. It is commonly employed to study the prosthetic valves hemodynamics, and examine leaflets kinematics and maximum stress magnitudes [44, 92, 93, 94, 95, 96]. Wu et al. (2015) were the first to develop an FSI model to study a self-expandable TAVR valve deployed in a patient-specific aortic root geometry [94]. This model, however, did not include the calcified native leaflets, which could affect the flow analysis. Ghosh et al. (2017) implemented an ALE-FSI model to compare the PolyNova TAVR and SAVR valve’s hydrodynamics together with leaflets mechanical stresses during the cardiac cycle [96]. This SAVR valve was also studied using an “operator-split” FSI approach, which was used to evaluate the valve thrombogenicity [44].
9.2.7. Thrombogenicity
Valve thrombosis was addressed and quantified through blood residence time of randomly distributed platelets in close proximity of the leaflets [42], and compared for intra- and supra- annular positioning cases of a TAV model. The thrombogenicity of a TAVR valve can be characterized by utilizing the concept of stress accumulation (SA) that was extensively used in studies investigating other cardiovascular devices [97, 98]. In these flow analyses, the blood is modeled as two-phase Newtonian fluid with viscosity of 0.0035 kg/m-s and density of 1,081 kg/m3 with platelets assumed as neutrally-buoyant 3 µm solid spherical particles. This two-phase flow model computes the particle-fluid interactions (drag, lift and Basset forces, and turbulent fluctuations). The shear loading histories of the platelet trajectories flowing through the TAVR valve can be computed by incorporating the cumulative linear product of the instantaneous shear stress and exposure time along each platelet trajectories. The overall SA reached by individual platelets along their trajectories is collapsed into a probability density function (PDF) to statistically represent the distribution of the SA of all trajectories for each TAVR valve configuration analyzed (its ‘thrombogenic footprint’)[44]. This allows for direct comparison of different configurations on two ranges of the PDF—the main mode and the tail region. The main mode of PDF represents the mean SA value for most of the trajectories flowing through the device (bulk flow), and the tail region represents the distribution of the trajectories at the higher and riskier SA range, which translates into a higher risk of activating platelets, i.e., increase the device thrombogenicity.
9.3. Experimental methods
As outlined above, computational models may provide information and insights into THV performance that are difficult or not possible to achieve from experiments. However, numerical simulation results are affected greatly by the input parameters, and the numerical setting, and therefore special attention should be given in choosing the input and output parameters. Validation should be performed whenever possible; however, due to the high complexity of the more advanced models, this is not always feasible. It is therefore crucial to understand the limitations and interpretation of the generated simulations data so that experiments and simulations would complement each other. Most importantly, when both in-vitro and in-silico methods are considered, it is very beneficial to consider means of validation/comparison already at the design phase of the valve and its experimental setup. With PHVs evolution toward transcatheter applications, researchers have started developing in-vitro models that extend beyond the ISO guidelines in terms of complexity and scope as to focus on specific aspects of CAVD complications in order to innovate TAVR devices designs and optimize their clinical performance and safety. Below we highlight some of these methods.
9.3.1. Crimping damage of the THV leaflets is not addressed directly in ISO 5840–3; however, all tests with the THV device should be performed on valves after crimping and deployment according to their intended use. This implicitly includes the effects of crimping and deployment in other tests, such as hydrodynamics and durability. However, the ISO covers minimum performance requirements for current TAVR intended use but not for future indications. Crimping-associated damage in the leaflet may affect long-term durability of the THV device in-situ, either before or beyond the 5-years use indicated by the ISO. In fact, this is a major concern that impede the expansion of TAVR to younger and lower risk patients [15]. Crimping-associated damage to bioprosthetic leaflets have been demonstrated in many in-vitro studies [30, 31, 33, 99, 100, 101]. Damage was examined with imaging techniques such as surface characterization with scanning electron microscopy (SEM) and subsurface with second-harmonic generation microscopy of the collagen fibers. Damage can also be assessed and quantified with indexing images, hydraulic conductance [4], or mechanical testing [6, 41].
9.3.2. Hydrodynamic performance assessment of THVs is the process of characterizing the flow and pressure behavior of the deployed valve. Pulse Duplicators (PDs) are traditionally used to measure the ISO 5840–3 Sec 7.2.3 outlined parameters. Standard FDA ISO compliant PDs generally consist of a compliant left ventricle model, a functional mitral valve and a simplified aortic valve annulus mounted in a simplified rigid aortic root structure (Figure 6). The ventricle is usually actuated with a linear piston pump, allowing direct control of the stroke volume and cardiac cycle. ISO 5840–3 specifies minimum device performance requirements based on the valve diameter and at a single cardiac output (CO 5 L/min at 70 BPM, MAP 100 mmHg, systolic period 35%), with additional standardized performance testing at multiple COs. ISO 5840–3 additionally describes testing the THV in circular and elliptical annulus mounts that attempt to cover the range of labeled/intended implant dimensions and to assess oversizing characteristics. The hydrodynamics guidelines in ISO 5840–3 are primarily concerned with specifying and calculating an effective orifice area (EOA), which is a modified measure of the Bernoulli equation for estimating an opening area of the valve in peak systolic flow, pressure gradient across the valve in systole, and the regurgitant volume, that is comprised of the closing and leak volumes.
Figure 6:
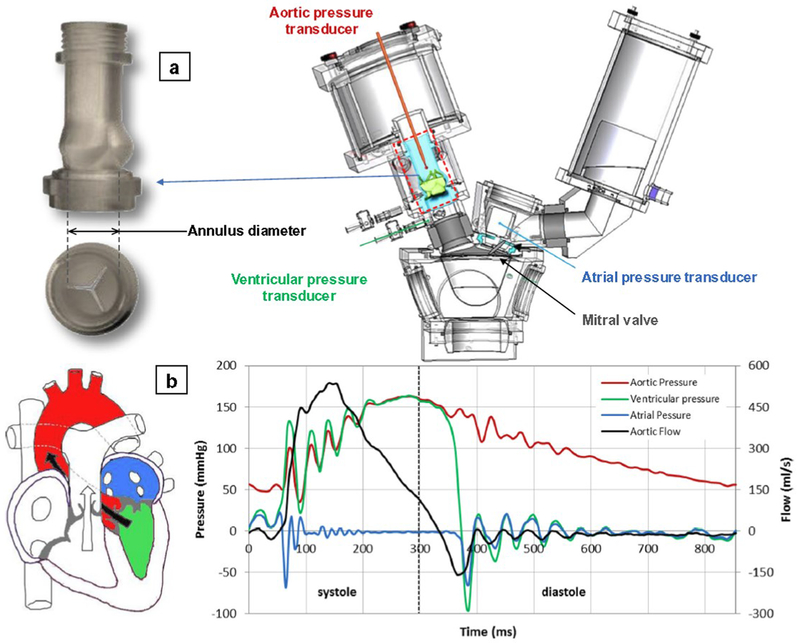
Example of a Pulse Duplicator (PD) for baseline hydrodynamic testing mitral and aortic valves (Vivitro PD, VivitroLabs, Victoria, BC). (a) mock native aortic root model for deployment of the TAVR device; (b) a typical diagram of transvalvular pressures/flows over a cardiac cycle at CO of 5 l/min. Reprinted by permission from Springer Nature, J Cardiovascular and Translational Research (Rahmani B. et al. In Vitro Hydrodynamic Assessment of a New Transcatheter Heart Valve Concept (the TRISKELE))©2017.
ISO 5840–3 N.4.3.5 outlines the need for characterizing the THV flow field which has been extensively developed in literature with digital particle image velocimetry (DPIV) [40, 61, 62, 63, 64, 65, 102, 103, 104, 105, 106, 107, 108]. DPIV allows quantitative analysis of the flow field velocity, shear and particle residence times, which are critical for predicting risks of thrombosis (Figure 7). Gunning et al. (2014) used DPIV to extensively characterize the developed flow field from a severe eccentric deployed TAVR [62]. The results show increased shear stresses and increased turbulence which may influence hemolysis, which cannot be characterized by traditional hydrodynamics. Studies of sinus stasis times, or particle residence times in the aortic sinus are often performed with DPIV experiments [61, 63, 65, 102, 104, 105, 107]. These studies commonly focus on the positioning of the TAVR valve, at supra-annular and sub-annular alignments, and its influence of the hydrodynamics. Midha et al. (2017) used DPIV to characterize the residence times in the neosinus of a TAVR valve and compared to clinical 4DCT data to show that supra-annular positioning of the THV can directly influence residence times and thrombosis formation [40].
Figure 7:
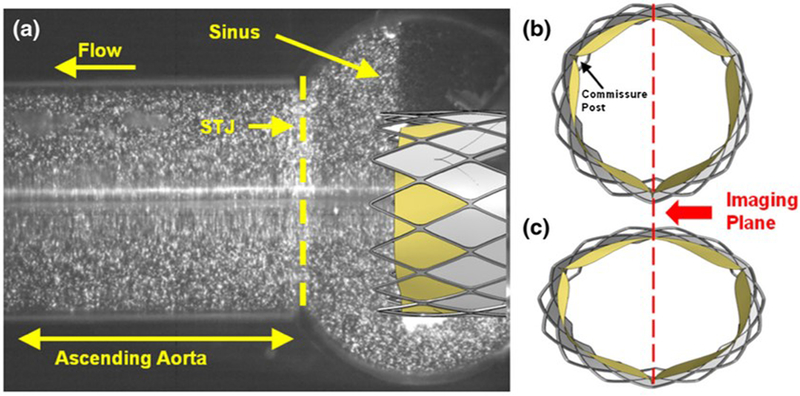
PIV setup for eccentric THV deployment. (a) Raw image of the particle laden fluid flow from the imaging plane. Idealized schematic of (b) circular and (c) eccentric deployed TAVRs at peak systole with imaging plane coincident with the lower commissure post and line of coaptation of the lower leaflets. Reprinted by permission from Springer Nature, Annals of Biomedical Engineering (Gunning et al. An in vitro evaluation of the impact of eccentric deployment on transcatheter aortic valve hemodynamics)©2014.
DPIV techniques are based on optical clarity of the model, and therefore THVs are mostly limited to analysis of the flow downstream of where the TAV stent extends. Analysis of the flow regimes within the valve region itself could be possible to a certain extent, depending on the specific valve being tested, yet requires improvisation for replacing the metallic stent with a transparent frame substitute [40]. As an example, TAVR supra-annular designs such as the Medtronic CoreValve family may be unsuitable for DPIV analysis.
9.3.3. Patient-specific hydrodynamics
With advancements in 3D-printing technology, recent in-vitro models are recreating patient-specific geometries for TAVR hydrodynamics analysis and deployment characterization [29, 66, 67, 68, 69, 70, 71, 72, 104, 109, 110]. Most importantly, development of such models sets the ground for testing TAVs under more realistic scenarios, leading to more realistic hydrodynamic predictions, which are often inferior to what is evaluated under the simplified mock anatomy of traditional PDs [71]. In that, the hydrodynamic test conditions might be referred to as ‘baseline hydrodynamics’, while the testing under diseased CAVD anatomies complements it by adding the anatomical variability as a factor. Such novel setups open the door for experimental tests that were until recently available only via complex computational simulations. They additionally provide a new mean for validation of the latter. New 3D printed elastomers allow the user to tune the mechanical properties of the material and directly print the patient aortic root, native leaflets and calcification patterns. Qian et al. (2017) used 3D printed aortic roots to quantify the anchoring strains on the annulus/root structure showing a bulge post deployment of the TAV caused by the calcifications [69]. These bulge areas corresponded to the locations of large PVL in the patient based on clinical transesophageal echocardiography [69]. Tanaka et al. (2018) took the 3D printed aortic structure to the next experimental level by mounting the patient specific roots/valves, and patient specific silicone aorta into a pulsatile flow system. The resulting PVL from the deployed TAV was directly measured with ultrasound, and location and magnitude of PVL were compared to the patient’s clinical data [72]. The work of Rotman et al. (2018) focused on casting patient specific replicas of calcific aortic valves that have similar performance characteristics as diseased aortic valves (Figure 8). Integrated into a dedicated pulsatile flow system of the complete upper body circulation (Replicator, Vascular Simulations LLC, Stony Brook, NY, USA), these valves allow to study the whole TAVR procedure in a severe AS patient-specific anatomy, as well as the deployment characterization in such a way that an improved hydrodynamics can be achieved before and after the procedure [71]. Such a platform might also be ideal for medical centers for TAVR training in challenging anatomies, or where there is a consideration of what size or type of TAVR device would provide the best outcome.
Figure 8:
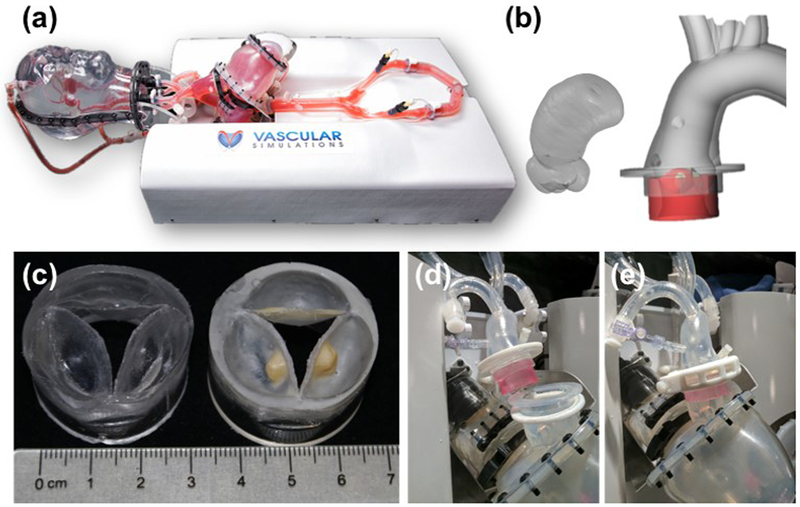
Novel platform for hydrodynamic testing in a diseased patient-specific CAVD anatomy. (a) – Vascular Simulations (Stony Brook, NY, USA) upper body arterial Replicator. (b) left – reconstruction of ascending aorta and aortic root based on a CT scan of a patient, based on which aortic root models, shown on the right, were developed. (c) – Corresponding aortic valve models, with (right) or without (left) calcific deposits, (d-e) - the modular valve model (red colored for visibility) fitted into the aorta and left ventricle. Reprinted by permission from Springer Nature, Cardiovascular Engineering and Technology (Rotman O.M. et al. Realistic Vascular Replicator for TAVR Procedures)©2018.
9.3.4. Leaflet mechanics
Gunning et al. (2015) used a marker array on the TAV leaflet surface to characterize the strain of the leaflet during the cardiac cycle, using high speed imaging. This study showed that eccentric deployments of the TAV induced increased strains that may lead to reduced durability [111]. Maleki et al. (2015) used high speed imaging to track the leaflet edges and estimate bending stresses. Results showed that oversizing the valves, up to 20%, had little impact on the hydrodynamics but increased the stresses [112]. Heide-Jorgensen et al. (2016) demonstrated a novel method for optical high spatiotemporal strain analysis for TAV (Figure 9). This method enabled high-resolution imaging of all three TAV leaflets simultaneously. A coating technique for applying a stochastic pattern on the leaflets of the TAV was developed, replacing the marker array of previous studies. It should be noted that implementing any of these techniques requires imaging with dual high-speed cameras setup [113].
Figure 9:
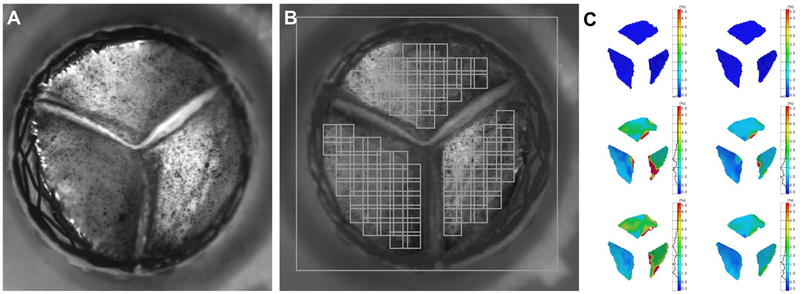
Method for optical high spatiotemporal strain analysis for transcatheter aortic valves in-vitro. A – A closed TAVR valve surrounded by the nitinol stent seen from the aortic point of view. The surface of the each leaflet is covered with a fine pattern of ink speckles with a particle size less than 1 μm. The ink is applied directly to the surface of the leaflets by an airbrush. B - Post processing of the experimental data of from the TAVR valve seen by one of two high-speed cameras. The applied facet field is marked by the squares, which each contains a unique signature based on the gray level intensity of the pixels inside it. Using stereophotogrammetry, the facet field is transformed into a three-dimensional surface. To reduce computational time areas with no interest is masked out and no facets were applied. Since the facets need to be visual for both cameras computation of the 3D structure along edges, such as the coaptation lines, is not possible. C - Visualization of the strain distribution of the TAVR valve leaflets on the surface created from digital image correlation. The left column depicts the von Mises strains at varying time points, and the right column depicts the major principal strains. Reprinted from Heide-Jorgensen et al. A Novel Method for Optical High Spatiotemporal Strain Analysis for Transcatheter Aortic Valves In Vitro. Journal of Biomechanical Engineering. 2016 Mar;138(3):4032501. With permission by ASME.
9.3.5. Durability
ISO 5840–3 7.2.4.1 and Annex O describe the guidelines for in-vitro durability testing of THVs to anticipate device lifetimes in-situ. These guidelines require that the THV remains intact and functional over 200 million in-vitro cycles (equivalent to 5 years in-vivo operation), which requires periodic hydrodynamic performance evaluations. This test is traditionally performed in accelerated wear testers that induce rapid cycling of the PHVs, commonly at 600–1,200 bpm, depending on the valve being tested and its capacity to fully open and close at high frequencies. Currently, there is no computational model that could reliably replace the accelerated wear testing. Completion of the durability testing usually takes 3–6 months. This is highly time-consuming for valve manufacturers, especially if design changes in the TAV are required. Of note, this test does not capture the main failure mode of bioprosthetic valves in-vivo, the structural valve degeneration, which is usually caused by calcification of the tissue material [37].
9.3.6. Calcification susceptibility
An equivalent type of testing complementary to the accelerated wear testing has been developed to assess in-vitro the calcification susceptibility of a PHV. This test utilizes the accelerated wear testers that are used for the traditional durability testing and uses a pro-calcific compound as the working fluid (instead of saline) [114, 115]. It is not clear how accurately such protocols characterize PHV calcification susceptibility; yet, if performed in comparison to reference valves with known clinical history of calcification tendency, it can provide a valuable tool. It should be noted that the only available alternative is to perform chronic testing in a large animal model, which is (i) very expensive, (ii) ethically debatable, (iii) includes biological variability and thus requires large sample size, and ultimately (iv) there is no large animal CAVD model- resulting in a TAVR valve that is implanted in a healthy animal aortic root, and as such does not reflect implantation in a CAVD human patient.
9.3.7. Thrombogenicity
In-vitro models of thrombus formation and thrombogentic potential have been adapted to THV devices with some studies observing platelet aggregation on the surface of the bioprosthetic leaflet via SEM and histophathology [116]. Platelet aggregation and thrombus formation studies usually consist of flowing platelets or blood over the tissue samples at steady flows with matching physiological shear rates. Bourget et al (2017) combined observations of crimping and balloon dilation damage with blood cell uptake, showing increased rates of uptake with increased tissue damage [100]. A more unique study performed by Richardt et al (2018) used a clotting milk model to highlight regions of potential for thrombus deposition due to flow stasis [117].
Other Bench-top thrombogenicity protocols include testing under pulsatile flow conditions. Jesty and Bluestein (1999) developed a modified prothrombinase assay for measuring platelet activity state (PAS) [118] in order to quantify cardiovascular devices thrombogenicity [119], i.e., the tendency of the device to induce platelet activation by the flow patterns through it. This protocol uses fresh human gel-filtered platelets suspended in a platelet buffer together with other coagulation factors that are critical for the formation of thrombin. Using acetylation of prothrombin, the thrombin generated in the PAS assay is defective and cannot participate further in the coagulation cascade; therefore it does not activate other platelets and does not form fibrin fibers (Figure 10a) [118, 120]. This hemophilia-like in-vitro scenario creates a 1:1 correspondence between the agonist for platelets activation (e.g., the flow induced stresses) and thrombin concentrations measured, which is termed the PAS. Combining this protocol in a pulsatile flow loop with a cardiovascular device one can quantify the platelet activity rate of the device (the slope of PAS) as a function of the recirculation time that can be used to compare the thrombogenic potential of various designs (Figure 10b). This protocol has been used extensively to characterize and optimize the thromboresistance of cardiovascular devices such as ventricular assist devices and PHVs- including polymeric valves [6, 45, 120, 121, 122, 123, 124, 125, 126]. Recently, it was used to evaluate the thrombogenicity of a polymeric TAVR valve in a complementary hemodynamic testing approach that included also baseline hydrodynamics and patient-specific hydrodynamic testing [29].
Figure 10:
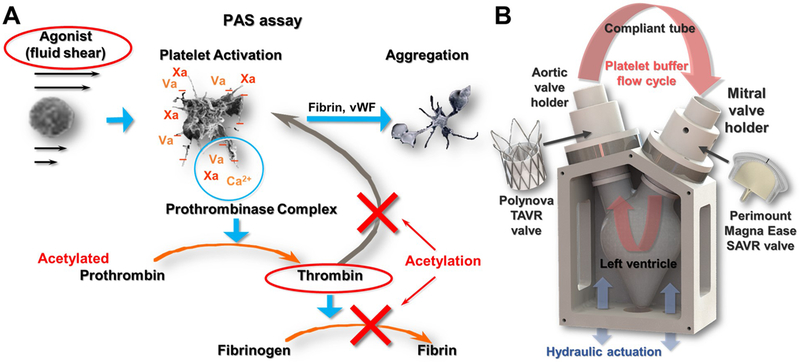
(A) Platelet Activity State (PAS) thrombogenicity methodology concept, in which the agonist for platelet activation (here fluid shear) is correlated 1:1 with thrombin formation (Adapted from Bluestein D. et al. Research Approaches for Studying Flow Induced Thromboembolic Complications in Blood Recirculating Devices. Expert Review of Medical Devices 2004;1(1):65-80. With permission by Taylor & Francis Ltd). (B) Implementation of the PAS assay with TAVR valve testing, using a mock left ventricle model and pulsating flow of gel-filtered human platelets in a closed flow loop.
10. Expert commentary
TAVR as an effective alternative to surgical valve replacement in high-risk, CAVD patients with severe AS represents a disruptive technology that has challenged the hegemony of the surgical approach for valve replacement that dominated the field for decades. However, the promise of TAVR as a longer-term solution that could also offer an effective alternative to SAVR for lower risk patients, could be at risk. This may impede TAVR initial phenomenal success as a life-saving solution for inoperable patients. The accumulating clinical evidence of various limitations and adverse events that were described in our review, include failed delivery due to tortuous aortic geometry and severe valvular calcification, valve migration, PVL, embolization with increased stroke risk, and CCAs following TAVR. With longer follow-up clinical studies new serious complications are being increasingly recognized, such as thrombosis with persistent pro-thrombotic risk, valve leaflet thickening, and valve deterioration. Current TAVR technology is based on tissue leaflets adapted to, but not specifically designed and optimized for TAVR. Those may sustain damage during crimping and deployment on nodular valve calcifications, resulting in limited durability and impaired functionality. Clearly, studying the potential pitfalls and the underlying mechanisms that may lead to various TAVR failure modes is a key for designing future generation TAVR devices that will overcome these limitations. A comprehensive integrated approach is required in order to truly facilitate their expansion into younger, lower risk patients, and for additional emerging indications.
New generation TAVR devices will have to effectively address the aforementioned clinical complications before their anticipated expansion to lower risk patients could be fully realized. In addition, off-label opportunities for TAVR are quickly emerging and will challenge existing and newer generation TAVs even more. This will likely lead to dedicated TAV designs for new indications, perhaps with dedicated patient-specific designs as the ultimate goal. Clinical complications that require better design solutions include better addressing the persistent issue of PVL and the need of PPI because of TAVR-induced CCAs. Other complications that will need to be addressed in terms of device design are thrombogenicity and durability, as was demonstrated with the DTE methodology [28, 121]. The clinical follow-up of the valves durability has been difficult with the elderly/frail cohort that is associated with the early use of TAVR, although certain failure modes were already observed and documented [14, 37]. As clinical trials expand to lower risk and younger patients, durability is becoming a critical aspect and longer follow up clinical studies will be required.
Extended and off-label uses of TAVR devices have been conducted in medical centers world-wide. The report by Hira et al. (2018) summarizes the clinical outcomes of many of those [153]. Such uses include the ViV procedure discussed earlier, where research ambiguity exists in terms of matching valve oversizing parameters and positioning within the surgical valve. BAV patients leads to a unique off-label use for TAVR. BAV patients have elliptical valve openings and major variations in calcific patterns that could possibly leading to asymmetrical flow patterns. Clinical results show numerous complication rates among the early generation TAVR devices with BAV treatments, but a significant improvement with newer generation devices, that appear to match complication rates similar to those observed in the tricuspid cohort [23, 154, 155, 156, 157, 158, 159, 160]. Many authors highlight the fact that severe PVL and highly elliptical stent deployments are still prevalent in BAV patients with newer generation devices, which leads the authors to question the device durability. This leaves the door open for possible new devices with dedicated designs targeted to achieve better valve performance for the BAV patient population.
Polymeric PHVs have been under development for decades, but no polymeric aortic valve received CE mark or FDA approval yet. However, disruptive newer polymer valve technologies that have recently appeared around the globe (e.g. PolyNova xSIBS valve, the Triskele urethane valve, SAT TAVI heparinized-polyurethane valve, and the HA-LLDPE Endurance Valve) significantly differ from the old-generation polymeric PHVs, currently demonstrating the great potential for finally becoming a viable substitute for bioprosthetic heart valves. In addition, the potential advantages that polymers offer such as the ability to manufacture at mass production with complete reproducibility, the higher engineering freedom, and the ability to cope with persistent complications that are traditionally associated with bioprosthetic leaflets (e.g. calcific degeneration and durability), strongly indicate that polymeric TAVR valves may transform the field and help fulfill its early promise.
Finally, there is a clear ongoing trend for more sophisticated experimental and computational methods that emerge around the globe. These include, among others, patient-specific models with pulse duplicators that were not available until recently, and now allow evaluation of TAV devices under more realistic scenarios of AS anatomy. Integrating between such experimental and computational methods provides a powerful tool for valve manufacturers, for optimizing the design and performance of their devices with much less dependency on costly animal studies, and for adapting their device to new indications of use.
11. Five-year view
The rapid growing of the TAVR market and the rate of new TAVR devices emerging every year are likely to continue in the following five years [161]. With TAVR already expanding to lower risk patients, the inherent complications associated with tissue-based TAVR valves, such as calcific degeneration and durability, are a growing concern. This will likely be addressed by additional unique design approaches, yet also by search of alternative leaflets materials. Several novel polymer technologies have been introduced in the recent years, with very promising early results. We envision that a first prosthetic polymeric aortic valve may move forward toward gaining regulatory approval within this period. The crimping race for lower profile valves is expected to continue and cross slightly below the current lowest 14 Fr record. However, considering the inherent crimping-damage issues related with tissue-leaflets, we predict that the next significant crimping record will be gained only with alternative leaflet materials. Finally, we anticipate that advanced modeling approaches and experimental testing methods (e.g. advanced CFD and FSI, patient-specific simulations, pulse duplicators designed for TAVR experiments) will get more traction in the regulatory process, offering critical complementary information on the TAV performance.
Key Issues.
With TAVR expanding into younger and lower risk patients, TAVR valve durability and improved hemodynamics become a critical issue.
The variability of TAVR devices designs that exist today is remarkable, demonstrating the wide range of design approaches to address common pitfalls and complications of TAVR.
New extended and off-label uses for TAVR (e.g. ViV and BAV) are stretching the limits of current devices, and might require dedicated TAV designs for improving clinical outcome in these unique pathologies.
Persistent complications of TAVs related to the tissue leaflets accelerates the need for investigating alternative materials.
New-generation polymeric valves demonstrate potential to substitute tissue TAVs with ability to improve clinical performance and durability, introduce dedicated designs for currently off-label uses, and enhance manufacturing efficacy and reproducibility.
Cutting-edge computational and experimental testing methods that complement the ISO standards are essential for a deeper examination of current TAVs pitfalls, for improving future generation valves, and expansion to new indications.
Acknowledgments
Funding
This paper was funded by an NIH-NIBIB Quantum award Phase II-C (1U01EB012487–0, DB) and NIH-NHLBI STTR Phase I award (R41-HL134418, DB), the Center for Biotechnology: a New York State Center for Advanced Technology, New York State Department of Economic Development; and New York State Accelerate Long Island support.
Abbreviations
- AR
Aortic regurgitation
- AS
Aortic stenosis
- AV
Atrioventricular
- CAVD
Calcific aortic valve disease
- CCA
Cardiac conduction abnormalities
- CFD
Computational fluid dynamics
- CO
Cardiac output
- DTE
Device thrombogenic Emulation
- DPIV
Digital particle image velocimetry
- EOA
Effective orifice area
- FEA
Finite element analysis
- FSI
Fluid-structure interaction
- ISO
International organization for standardization
- LBB
Left bundle branch
- LBBB
Left bundle branch block
- PAS
Platelet activity state
- PD
Pulse duplicator
- PHV
Prosthetic heart valve
- PVL
Paravalvular leak
- PPI
Permanent pacemaker implantation
- RHD
Rheumatic heart disease
- SAVR
Surgical aortic valve replacement
- SVD
Structural valve deterioration
- TAV
Transcatheter aortic valve
- TAVR
Transcatheter aortic valve replacement
- THV
Transcatheter heart valve
- ViV
Valve-in-valve
Footnotes
Declaration of interest
OM Rotman is a consultant for Polynova Cardiovascular Inc. D Bluestein has stock ownership in Polynova Cardiovascular Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
References of Interest*
Heide Jorgensen et al. (2016) [113] – In-vitro high resolution leaflet strain analysis
Midha et al. (2017) [40] – DPIV analysis in TAV neosinuses with implication on thrombosis
Bianchi et al. (2016) [74] – High-resolution 3D modeling of patient-specific aortic root
Rocatello et al. (2018) [17] – Computational patient-specific model for assessing cardiac conduction abnormalities post TAVR
References of Considerable Interest**
Rotman et al. (2018) [71] – In-vitro patient-specific TAVR testing
Financial & Competing Interests Disclosure
Alavi et al. (2014) [31] – Crimping damage assessment in tissue leaflets
Schultz et al. (2016) [76] – Clinically-validated computational model of patient-specific TAVR deployment
Wu et al. (2015) [94] – First FSI model to assess TAVR valve performance
- 1.Franzoni I, Latib A, Maisano F, et al. Comparison of incidence and predictors of left bundle branch block after transcatheter aortic valve implantation using the CoreValve versus the Edwards valve. The American journal of cardiology. 2013. August 15;112(4):554–9. doi: 10.1016/j.amjcard.2013.04.026. PubMed PMID: 23726173; eng. [DOI] [PubMed] [Google Scholar]
- 2.Kurtz CE, Otto CM. Aortic stenosis: clinical aspects of diagnosis and management, with 10 illustrative case reports from a 25-year experience. Medicine. 2010. November;89(6):349–79. doi: 10.1097/MD.0b013e3181fe5648. PubMed PMID: 21057260; eng. [DOI] [PubMed] [Google Scholar]
- 3.Mohler ER 3rd, Gannon F, Reynolds C, et al. Bone formation and inflammation in cardiac valves. Circulation. 2001. March 20;103(11):1522–8. doi: 10.1161/01.cir.103.11.1522. PubMed PMID: 11257079. [DOI] [PubMed] [Google Scholar]
- 4.Weinberg EJ, Schoen FJ, Mofrad MR. A computational model of aging and calcification in the aortic heart valve. PloS one. 2009;4(6):e5960. doi: 10.1371/journal.pone.0005960. PubMed PMID: 19536285; PubMed Central PMCID: PMC2693668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenhek R, Binder T, Porenta G, et al. Predictors of outcome in severe, asymptomatic aortic stenosis. The New England journal of medicine. 2000. August 31;343(9):611–7. doi: 10.1056/NEJM200008313430903. PubMed PMID: 10965007. [DOI] [PubMed] [Google Scholar]
- 6.Sheriff J, Girdhar G, Chiu WC, et al. Comparative efficacy of in vitro and in vivo metabolized aspirin in the DeBakey ventricular assist device. Journal of thrombosis and thrombolysis. 2014. May;37(4):499–506. doi: 10.1007/s11239-013-0997-6. PubMed PMID: 24043375; PubMed Central PMCID: PMC4160029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao C, Ang SC, Indraratna P, et al. Systematic review and meta-analysis of transcatheter aortic valve implantation versus surgical aortic valve replacement for severe aortic stenosis. Annals of cardiothoracic surgery. 2013. January;2(1):10–23. doi: 10.3978/j.issn.2225-319X.2012.11.09. PubMed PMID: 23977554; PubMed Central PMCID: PMC3741825. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones BM, Krishnaswamy A, Tuzcu EM, et al. Matching patients with the ever-expanding range of TAVI devices. Nature reviews Cardiology. 2017. October;14(10):615–626. doi: 10.1038/nrcardio.2017.82. PubMed PMID: 28682324; eng. [DOI] [PubMed] [Google Scholar]
- 9.Cribier A The development of transcatheter aortic valve replacement (TAVR). Global Cardiol Sci Pract 2016; 2016(4):e201632. 12/30 11/30/received 12/12/accepted. doi: 10.21542/gcsp.2016.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durko AP, Osnabrugge RL, Kappetein AP. Long-term outlook for transcatheter aortic valve replacement. Trends Cardiovasc Med 2018. April;28(3):174–183. doi: 10.1016/j.tcm.2017.08.004. PubMed PMID: 28838702. [DOI] [PubMed] [Google Scholar]
- 11.Durko AP, Osnabrugge RL, Van Mieghem NM, et al. Annual number of candidates for transcatheter aortic valve implantation per country: current estimates and future projections. Eur Heart J. 2018. March 12. doi: 10.1093/eurheartj/ehy107. PubMed PMID: 29546396. [DOI] [PubMed] [Google Scholar]
- 12.Dasi LP, Hatoum H, Kheradvar A, et al. On the Mechanics of Transcatheter Aortic Valve Replacement. Ann Biomed Eng 2017. February;45(2):310–331. doi: 10.1007/s10439-016-1759-3. PubMed PMID: 27873034; PubMed Central PMCID: PMCPMC5300937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Généreux P, Head SJ, Hahn R, et al. Paravalvular Leak After Transcatheter Aortic Valve Replacement: The New Achilles’ Heel? A Comprehensive Review of the Literature. Journal of the American College of Cardiology. 2013. 2013/March/19/;61(11):1125–1136. doi: 10.1016/j.jacc.2012.08.1039. [DOI] [PubMed] [Google Scholar]
- 14.Cahill TJ, Chen M, Hayashida K, et al. Transcatheter aortic valve implantation: current status and future perspectives. Eur Heart J. 2018. April 27. doi: 10.1093/eurheartj/ehy244. PubMed PMID: 29718148. [DOI] [PubMed] [Google Scholar]
- 15.Arsalan M, Walther T. Durability of prostheses for transcatheter aortic valve implantation. Nature reviews Cardiology. 2016. June;13(6):360–7. doi: 10.1038/nrcardio.2016.43. PubMed PMID: 27053461. [DOI] [PubMed] [Google Scholar]
- 16.Campelo-Parada F, Nombela-Franco L, Urena M, et al. Timing of Onset and Outcome of New Conduction Abnormalities Following Transcatheter Aortic Valve Implantation: Role of Balloon Aortic Valvuloplasty. Revista espanola de cardiologia (English ed). 2017. May 26. doi: 10.1016/j.rec.2017.04.010. PubMed PMID: 28566243. [DOI] [PubMed] [Google Scholar]
- 17.Rocatello G, El Faquir N, De Santis G, et al. Patient-Specific Computer Simulation to Elucidate the Role of Contact Pressure in the Development of New Conduction Abnormalities After Catheter-Based Implantation of a Self-Expanding Aortic Valve. Circulation: Cardiovascular Interventions. 2018;11(2). doi: 10.1161/circinterventions.117.005344. [DOI] [PubMed] [Google Scholar]
- 18.Ben-Dor I, Pichard AD, Satler LF, et al. Complications and Outcome of Balloon Aortic Valvuloplasty in High-Risk or Inoperable Patients. JACC: Cardiovascular Interventions. 2010;3(11):1150–1156. doi: 10.1016/j.jcin.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 19.Siontis GCM, Jüni P, Pilgrim T, et al. Predictors of Permanent Pacemaker Implantation in Patients With Severe Aortic Stenosis Undergoing TAVRA Meta-Analysis. Journal of the American College of Cardiology. 2014;64(2):129–140. doi: 10.1016/j.jacc.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 20.Gallo M, Dvir D, Demertzis S, et al. Transcatheter valve-in-valve implantation for degenerated bioprosthetic aortic and mitral valves. Expert Rev Med Devices. 2016. August;13(8):749–58. doi: 10.1080/17434440.2016.1207521. PubMed PMID: 27359372. [DOI] [PubMed] [Google Scholar]
- 21.Witkowski A, Jastrzebski J, Dabrowski M, et al. Second transcatheter aortic valve implantation for treatment of suboptimal function of previously implanted prosthesis: review of the literature. J Interv Cardiol 2014. June;27(3):300–7. doi: 10.1111/joic.12120. PubMed PMID: 24731263. [DOI] [PubMed] [Google Scholar]
- 22.Saji M, Tobaru T, Higuchi R, et al. Repeat transcatheter aortic valve replacement using a 23 mm Evolut R in a small patient with a failed 20 mm SAPIEN XT. Cardiovascular intervention and therapeutics. 2018. March 26. doi: 10.1007/s12928-018-0519-8. PubMed PMID: 29582354; eng. [DOI] [PubMed] [Google Scholar]
- 23.Perlman GY, Blanke P, Dvir D, et al. Bicuspid Aortic Valve Stenosis: Favorable Early Outcomes With a Next-Generation Transcatheter Heart Valve in a Multicenter Study. JACC Cardiovasc Interv 2016. April 25;9(8):817–824. doi: 10.1016/j.jcin.2016.01.002. PubMed PMID: 27101906. [DOI] [PubMed] [Google Scholar]
- 24.Hamm CW, Arsalan M, Mack MJ. The future of transcatheter aortic valve implantation. Eur Heart J. 2016. March 7;37(10):803–10. doi: 10.1093/eurheartj/ehv574. PubMed PMID: 26578195. [DOI] [PubMed] [Google Scholar]
- 25.Kheradvar A, Groves EM, Dasi LP, et al. Emerging trends in heart valve engineering: Part I. Solutions for future. Ann Biomed Eng 2015. April;43(4):833–43. doi: 10.1007/s10439-014-1209-z. PubMed PMID: 25488074. [DOI] [PubMed] [Google Scholar]
- 26.Bezuidenhout D, Williams DF, Zilla P. Polymeric heart valves for surgical implantation, catheter-based technologies and heart assist devices. Biomaterials. 2015. January;36:6–25. doi: 10.1016/j.biomaterials.2014.09.013. PubMed PMID: 25443788. [DOI] [PubMed] [Google Scholar]
- 27.Lefevre T, Colombo A, Tchetche D, et al. Prospective Multicenter Evaluation of the Direct Flow Medical Transcatheter Aortic Valve System: 12-Month Outcomes of the Evaluation of the Direct Flow Medical Percutaneous Aortic Valve 18F System for the Treatment of Patients With Severe Aortic Stenosis (DISCOVER) Study. JACC Cardiovasc Interv 2016. January 11;9(1):68–75. doi: 10.1016/j.jcin.2015.09.027. PubMed PMID: 26762913. [DOI] [PubMed] [Google Scholar]
- 28.Claiborne TE, Sheriff J, Kuetting M, et al. In vitro evaluation of a novel hemodynamically optimized trileaflet polymeric prosthetic heart valve. Journal of biomechanical engineering. 2013. February;135(2):021021. doi: 10.1115/1.4023235. PubMed PMID: 23445066; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rotman OM, Kovarovic B, Chiu WC, et al. Novel Polymeric Valve for Transcatheter Aortic Valve Replacement Applications: In Vitro Hemodynamic Study. Ann Biomed Eng 2018. September 7. doi: 10.1007/s10439-018-02119-7. PubMed PMID: 30194551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khoffi F, Heim F. Mechanical degradation of biological heart valve tissue induced by low diameter crimping: an early assessment. J Mech Behav Biomed Mater. 2015. April;44:71–5. doi: 10.1016/j.jmbbm.2015.01.005. PubMed PMID: 25621851. [DOI] [PubMed] [Google Scholar]
- 31.Alavi SH, Groves EM, Kheradvar A. The effects of transcatheter valve crimping on pericardial leaflets. Ann Thorac Surg 2014. April;97(4):1260–6. doi: 10.1016/j.athoracsur.2013.11.009. PubMed PMID: 24444873. [DOI] [PubMed] [Google Scholar]
- 32.Convelbo C, El Hafci H, Petite H, et al. Traumatic leaflet injury: comparison of porcine leaflet self-expandable and bovine leaflet balloon-expandable prostheses. Eur J Cardiothorac Surg 2018. May 1;53(5):1062–1067. doi: 10.1093/ejcts/ezx451. PubMed PMID: 29272377. [DOI] [PubMed] [Google Scholar]
- 33.de Buhr W, Pfeifer S, Slotta-Huspenina J, et al. Impairment of pericardial leaflet structure from balloon-expanded valved stents. J Thorac Cardiovasc Surg 2012. June;143(6):1417–21. doi: 10.1016/j.jtcvs.2011.11.001. PubMed PMID: 22244562. [DOI] [PubMed] [Google Scholar]
- 34.Kiefer P, Gruenwald F, Kempfert J, et al. Crimping may affect the durability of transcatheter valves: an experimental analysis. Ann Thorac Surg 2011. July;92(1):155–60. doi: 10.1016/j.athoracsur.2011.03.020. PubMed PMID: 21718842. [DOI] [PubMed] [Google Scholar]
- 35.Fish RD, Paniagua D, Urena P, et al. The Colibri heart valve: theory and practice in the achievement of a low-profile, pre-mounted, pre-packaged TAVI valve. EuroIntervention. 2013. September 10;9 Suppl:S111–4. doi: 10.4244/EIJV9SSA23. PubMed PMID: 24025948. [DOI] [PubMed] [Google Scholar]
- 36.Kheradvar A, Groves EM, Tseng E. Proof of concept of FOLDAVALVE, a novel 14 Fr totally repositionable and retrievable transcatheter aortic valve. EuroIntervention. 2015. September;11(5):591–6. doi: 10.4244/EIJY15M03_04. PubMed PMID: 25772904. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez-Gabella T, Voisine P, Puri R, et al. Aortic Bioprosthetic Valve Durability: Incidence, Mechanisms, Predictors, and Management of Surgical and Transcatheter Valve Degeneration. J Am Coll Cardiol 2017. August 22;70(8):1013–1028. doi: 10.1016/j.jacc.2017.07.715. PubMed PMID: 28818190. [DOI] [PubMed] [Google Scholar]
- 38.Caballero A, Sulejmani F, Martin C, et al. Evaluation of transcatheter heart valve biomaterials: Biomechanical characterization of bovine and porcine pericardium. Journal of the Mechanical Behavior of Biomedical Materials. 2017. 2017/November/01/;75:486–494. doi: 10.1016/j.jmbbm.2017.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scotten LN, Siegel R. Thrombogenic potential of transcatheter aortic valve implantation with trivial paravalvular leakage. Ann Transl Med 2014. May;2(5):43. doi: 10.3978/j.issn.2305-5839.2014.05.04. PubMed PMID: 25333018; PubMed Central PMCID: PMCPMC4200687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Midha PA, Raghav V, Sharma R, et al. The Fluid Mechanics of Transcatheter Heart Valve Leaflet Thrombosis in the Neosinus. Circulation. 2017. October 24;136(17):1598–1609. doi: 10.1161/CIRCULATIONAHA.117.029479. PubMed PMID: 28724752. [DOI] [PubMed] [Google Scholar]
- 41.Marwan M, Mekkhala N, Goller M, et al. Leaflet thrombosis following transcatheter aortic valve implantation. J Cardiovasc Comput Tomogr 2018. Jan-Feb;12(1):8–13. doi: 10.1016/j.jcct.2017.11.002. PubMed PMID: 29195844. [DOI] [PubMed] [Google Scholar]
- 42.Vahidkhah K, Barakat M, Abbasi M, et al. Valve thrombosis following transcatheter aortic valve replacement: significance of blood stasis on the leaflets. Eur J Cardiothorac Surg 2017. May 1;51(5):927–935. doi: 10.1093/ejcts/ezw407. PubMed PMID: 28100471. [DOI] [PubMed] [Google Scholar]
- 43.Nakatani S Subclinical leaflet thrombosis after transcatheter aortic valve implantation. Heart. 2017. December;103(24):1942–1946. doi: 10.1136/heartjnl-2017-311818. PubMed PMID: 28780575. [DOI] [PubMed] [Google Scholar]
- 44.Piatti F, Sturla F, Marom G, et al. Hemodynamic and thrombogenic analysis of a trileaflet polymeric valve using a fluid-structure interaction approach. Journal of biomechanics. 2015. August 21. doi: 10.1016/j.jbiomech.2015.08.009. PubMed PMID: 26329461; Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Claiborne TE, Xenos M, Sheriff J, et al. Toward optimization of a novel trileaflet polymeric prosthetic heart valve via device thrombogenicity emulation. ASAIO journal. 2013. May-Jun;59(3):275–83. doi: 10.1097/MAT.0b013e31828e4d80. PubMed PMID: 23644615; PubMed Central PMCID: PMC3648888. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rao RS, Maniar H, Zajarias A. Sapien Valve: Past, Present, and Future. Cardiac Interventions Today. 2015. Mar-Apr 2015. [Google Scholar]
- 47.Hafiz AM, Pinto D. CoreValve Evolut R in Current TAVR Practice. Cardiac Interventions Today. 2016. Mar-Apr 2016. [Google Scholar]
- 48.Ribeiro HB, Urena M, Kuck KH, et al. Edwards CENTERA valve. EuroIntervention. 2012. September;8 Suppl Q:Q79–82. doi: 10.4244/EIJV8SQA14. PubMed PMID: 22995117. [DOI] [PubMed] [Google Scholar]
- 49.Meredith IT, Hood KL, Haratani N, et al. Boston Scientific Lotus valve. EuroIntervention. 2012. September;8 Suppl Q:Q70–4. doi: 10.4244/EIJV8SQA12. PubMed PMID: 22995115. [DOI] [PubMed] [Google Scholar]
- 50.Sündermann SH, Holzhey D, Bleiziffer S, et al. Medtronic Engager™ bioprosthesis for transapical transcatheter aortic valve implantation. EuroIntervention. 2013;9(S):S97–S100. doi: 10.4244/EIJV9SSA19. [DOI] [PubMed] [Google Scholar]
- 51.Ye J, Lee AJ, Blanke P, et al. The first transapical transcatheter aortic valve-in-valve implantation using the J-valve system into a failed biophysio aortic prosthesis in a patient with high risk of coronary obstruction. Catheter Cardiovasc Interv 2018. March 30. doi: 10.1002/ccd.27604. PubMed PMID: 29602249. [DOI] [PubMed] [Google Scholar]
- 52.Schäfer U, Schirmer J, Schofer N, et al. First-in-human implantation of a novel transfemoral self-expanding transcatheter heart valve to treat pure aortic regurgitation. EuroIntervention. 2017;13(11):1297–1300. doi: 10.4244/EIJ-D-17-00502. [DOI] [PubMed] [Google Scholar]
- 53.Cheng J, Chen M, Zhu D, et al. Successful trans-apical aortic valve implantation for a high risk patient with aortic stenosis using a new second-generation TAVI device - J-Valve system. J Cardiothorac Surg 2015. January 17;10:5. doi: 10.1186/s13019-015-0207-z. PubMed PMID: 25595419; PubMed Central PMCID: PMCPMC4299398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scherman J, Bezuidenhout D, Ofoegbu C, et al. Tavi for low to middle income countries. European Heart Journal. 2017;38(16):1182–1184. doi: 10.1093/eurheartj/ehx169. [DOI] [Google Scholar]
- 55.Spina R, Anthony C, Muller DW, et al. Transcatheter Aortic Valve Replacement for Native Aortic Valve Regurgitation. Interventional cardiology (London, England). 2015. March;10(1):49–54. doi: 10.15420/icr.2015.10.1.49. PubMed PMID: 29588674; PubMed Central PMCID: PMCPMC5808468. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rahmani B, Tzamtzis S, Sheridan R, et al. In Vitro Hydrodynamic Assessment of a New Transcatheter Heart Valve Concept (the TRISKELE). J Cardiovasc Transl Res 2017. April;10(2):104–115. doi: 10.1007/s12265-016-9722-0. PubMed PMID: 28028692; PubMed Central PMCID: PMCPMC5437138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Azadani AN, Jaussaud N, Ge L, et al. Valve-in-valve hemodynamics of 20-mm transcatheter aortic valves in small bioprostheses. Ann Thorac Surg 2011. August;92(2):548–55. doi: 10.1016/j.athoracsur.2011.04.009. PubMed PMID: 21704287; eng. [DOI] [PubMed] [Google Scholar]
- 58.Midha PA, Raghav V, Condado JF, et al. Valve Type, Size, and Deployment Location Affect Hemodynamics in an In Vitro Valve-in-Valve Model. JACC Cardiovasc Interv 2016. August 8;9(15):1618–28. doi: 10.1016/j.jcin.2016.05.030. PubMed PMID: 27491613. [DOI] [PubMed] [Google Scholar]
- 59.Aksoy O, Paixao AR, Marmagkiolis K, et al. Aortic annular rupture during TAVR: Mini review. Cardiovasc Revasc Med 2016. Apr-May;17(3):199–201. doi: 10.1016/j.carrev.2016.03.005. PubMed PMID: 27157295. [DOI] [PubMed] [Google Scholar]
- 60.Azadani AN, Reardon M, Simonato M, et al. Effect of transcatheter aortic valve size and position on valve-in-valve hemodynamics: An in vitro study. The Journal of Thoracic and Cardiovascular Surgery. 2017. doi: 10.1016/j.jtcvs.2016.12.057. [DOI] [PubMed] [Google Scholar]
- 61.Groves EM, Falahatpisheh A, Su JL, et al. The effects of positioning of transcatheter aortic valves on fluid dynamics of the aortic root. ASAIO journal. 2014. Sep-Oct;60(5):545–552. doi: 10.1097/MAT.0000000000000107. PubMed PMID: 25010918; PubMed Central PMCID: PMCPMC4334568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gunning PS, Saikrishnan N, McNamara LM, et al. An in vitro evaluation of the impact of eccentric deployment on transcatheter aortic valve hemodynamics. Ann Biomed Eng 2014. June;42(6):1195–206. doi: 10.1007/s10439-014-1008-6. PubMed PMID: 24719050. [DOI] [PubMed] [Google Scholar]
- 63.Kumar G, Raghav V, Lerakis S, et al. High Transcatheter Valve Replacement May Reduce Washout in the Aortic Sinuses: an In-Vitro Study. The Journal of heart valve disease. 2015. January;24(1):22–9. PubMed PMID: 26182616; eng. [PubMed] [Google Scholar]
- 64.Midha PA, Raghav V, Condado JF, et al. How Can We Help a Patient With a Small Failing Bioprosthesis?: An In Vitro Case Study. JACC Cardiovasc Interv 2015. December 28;8(15):2026–33. doi: 10.1016/j.jcin.2015.08.028. PubMed PMID: 26627992; eng. [DOI] [PubMed] [Google Scholar]
- 65.Midha PA, Raghav V, Okafor I, et al. The Effect of Valve-in-Valve Implantation Height on Sinus Flow. Annals of biomedical engineering. 2016:1–8. [DOI] [PubMed] [Google Scholar]
- 66.Kalejs M, von Segesser LK. Rapid prototyping of compliant human aortic roots for assessment of valved stents. Interact Cardiovasc Thorac Surg 2009. February;8(2):182–6. doi: 10.1510/icvts.2008.194134. PubMed PMID: 19036761. [DOI] [PubMed] [Google Scholar]
- 67.Maragiannis D, Jackson MS, Igo SR, et al. Replicating Patient-Specific Severe Aortic Valve Stenosis With Functional 3D Modeling. Circ Cardiovasc Imaging. 2015. October;8(10):e003626. doi: 10.1161/CIRCIMAGING.115.003626. PubMed PMID: 26450122. [DOI] [PubMed] [Google Scholar]
- 68.Mummert J, Sirois E, Sun W. Quantification of biomechanical interaction of transcatheter aortic valve stent deployed in porcine and ovine hearts. Ann Biomed Eng 2013. March;41(3):577–86. doi: 10.1007/s10439-012-0694-1. PubMed PMID: 23161165; PubMed Central PMCID: PMCPmc3594518. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qian Z, Wang K, Liu S, et al. Quantitative Prediction of Paravalvular Leak in Transcatheter Aortic Valve Replacement Based on Tissue-Mimicking 3D Printing. JACC Cardiovascular imaging. 2017. July;10(7):719–731. doi: 10.1016/j.jcmg.2017.04.005. PubMed PMID: 28683947; eng. [DOI] [PubMed] [Google Scholar]
- 70.Ripley B, Kelil T, Cheezum MK, et al. 3D printing based on cardiac CT assists anatomic visualization prior to transcatheter aortic valve replacement. J Cardiovasc Comput Tomogr 2016. Jan-Feb;10(1):28–36. doi: 10.1016/j.jcct.2015.12.004. PubMed PMID: 26732862; PubMed Central PMCID: PMCPMC5573584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rotman OM, Kovarovic B, Sadasivan C, et al. Realistic Vascular Replicator for TAVR Procedures. Cardiovascular engineering and technology. 2018. April 13. doi: 10.1007/s13239-018-0356-z. PubMed PMID: 29654509; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tanaka Y, Saito S, Sasuga S, et al. Quantitative assessment of paravalvular leakage after transcatheter aortic valve replacement using a patient-specific pulsatile flow model. Int J Cardiol 2018. May 1;258:313–320. doi: 10.1016/j.ijcard.2017.11.106. PubMed PMID: 29544953. [DOI] [PubMed] [Google Scholar]
- 73.Bianchi M, Ghosh RP, Marom G, et al. , editors. Simulation of Transcatheter Aortic Valve Replacement in patient-specific aortic roots: Effect of crimping and positioning on device performance. 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); 2015. 25–29 August. 2015. [DOI] [PubMed] [Google Scholar]
- 74.Bianchi M, Ghosh RP, Marom G, et al. Effect of Balloon-Expandable Transcatheter Aortic Valve Replacement Positioning: A Patient-Specific Numerical Model. Artificial Organs. 2016;40(12):E292–E304. doi: 10.1111/aor.12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gunning PS, Vaughan TJ, McNamara LM. Simulation of self expanding transcatheter aortic valve in a realistic aortic root: implications of deployment geometry on leaflet deformation. Ann Biomed Eng 2014. September;42(9):1989–2001. doi: 10.1007/s10439-014-1051-3. PubMed PMID: 24912765; eng. [DOI] [PubMed] [Google Scholar]
- 76.Schultz C, Rodriguez-Olivares R, Bosmans J, et al. Patient-specific image-based computer simulation for theprediction of valve morphology and calcium displacement after TAVI with the Medtronic CoreValve and the Edwards SAPIEN valve. EuroIntervention. 2016. January 22;11(9):1044–52. doi: 10.4244/eijv11i9a212. PubMed PMID: 26788707; eng. [DOI] [PubMed] [Google Scholar]
- 77.Bosmans B, Famaey N, Verhoelst E, et al. A validated methodology for patient specific computational modeling of self-expandable transcatheter aortic valve implantation. Journal of biomechanics. 2016. 2016/September/06/;49(13):2824–2830. doi: 10.1016/j.jbiomech.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 78.Morganti S, Brambilla N, Petronio AS, et al. Prediction of patient-specific post-operative outcomes of TAVI procedure: The impact of the positioning strategy on valve performance. Journal of biomechanics. 2016. August 16;49(12):2513–9. doi: 10.1016/j.jbiomech.2015.10.048. PubMed PMID: 26748728; eng. [DOI] [PubMed] [Google Scholar]
- 79.Bailey J, Curzen N, Bressloff NW. Assessing the impact of including leaflets in the simulation of TAVI deployment into a patient-specific aortic root. Computer methods in biomechanics and biomedical engineering. 2015:1–12. [DOI] [PubMed] [Google Scholar]
- 80.Bailey J, Curzen N, Bressloff NW. The Impact of Imperfect Frame Deployment and Rotational Orientation on Stress within the Prosthetic Leaflets During Transcatheter Aortic Valve Implantation. Journal of biomechanics. 2016. doi: 10.1016/j.jbiomech.2016.12.031. [DOI] [PubMed] [Google Scholar]
- 81.Auricchio F, Conti M, Morganti S, et al. Simulation of transcatheter aortic valve implantation: a patient-specific finite element approach. Comput Methods Biomech Biomed Engin 2014;17(12):1347–57. doi: 10.1080/10255842.2012.746676. PubMed PMID: 23402555. [DOI] [PubMed] [Google Scholar]
- 82.Capelli C, Bosi GM, Cerri E, et al. Patient-specific simulations of transcatheter aortic valve stent implantation. Medical & biological engineering & computing. 2012. February;50(2):183–92. doi: 10.1007/s11517-012-0864-1. PubMed PMID: 22286953; eng. [DOI] [PubMed] [Google Scholar]
- 83.Sturla F, Ronzoni M, Vitali M, et al. Impact of different aortic valve calcification patterns on the outcome of Transcatheter Aortic Valve Implantation: a finite element study. Journal of biomechanics. 2016. doi: 10.1016/j.jbiomech.2016.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Q, Kodali S, Primiano C, et al. Simulations of transcatheter aortic valve implantation: implications for aortic root rupture. Biomechanics and modeling in mechanobiology. 2014. April 16. doi: 10.1007/s10237-014-0583-7. PubMed PMID: 24736808; Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Martin C, Sun W. Transcatheter Valve Underexpansion Limits Leaflet Durability: Implications for Valve-in-Valve Procedures [journal article]. Annals of Biomedical Engineering. 2016:1–11. doi: 10.1007/s10439-016-1738-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li K, Sun W. Simulated Thin Pericardial Bioprosthetic Valve Leaflet Deformation Under Static Pressure-Only Loading Conditions: Implications for Percutaneous Valves [journal article]. Annals of Biomedical Engineering. 2010;38(8):2690–2701. doi: 10.1007/s10439-010-0009-3. [DOI] [PubMed] [Google Scholar]
- 87.Groves EM, Falahatpisheh A, Su JL, et al. The effects of positioning of transcatheter aortic valves on fluid dynamics of the aortic root. ASAIO Journal. 2014. Sep-Oct;60(5):545–552. doi: 10.1016/j.euromechsol.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ghosh R, Marom G, Bianchi M, et al. P6317Simulation of transcatheter aortic valve performance in a beating heart. European Heart Journal. 2018;39(suppl_1):ehy566.P6317–ehy566.P6317. doi: 10.1093/eurheartj/ehy566.P6317. [DOI] [Google Scholar]
- 89.Sirois E, Mao W, Li K, et al. Simulated Transcatheter Aortic Valve Flow: Implications of Elliptical Deployment and Under-Expansion at the Aortic Annulus. Artif Organs. 2018. April 2. doi: 10.1111/aor.13107. PubMed PMID: 29608034; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.de Jaegere P, De Santis G, Rodriguez-Olivares R, et al. Patient-Specific Computer Modeling to Predict Aortic Regurgitation After Transcatheter Aortic Valve Replacement. JACC: Cardiovascular Interventions. 2016;9(5):508–512. doi: 10.1016/j.jcin.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 91.Marom G Numerical Methods for Fluid–Structure Interaction Models of Aortic Valves. Archives of Computational Methods in Engineering. 2014:1–26. [Google Scholar]
- 92.Luraghi G, Wu W, De Gaetano F, et al. Evaluation of an aortic valve prosthesis: Fluid-structure interaction or structural simulation? Journal of biomechanics. 2017. doi: 10.1016/j.jbiomech.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kemp I, Dellimore K, Rodriguez R, et al. Experimental validation of the fluid–structure interaction simulation of a bioprosthetic aortic heart valve. Australas Phys Eng Sci Med 2013. 2013/September/01;36(3):363–373. doi: 10.1007/s13246-013-0213-1. English. [DOI] [PubMed] [Google Scholar]
- 94.Wu W, Pott D, Mazza B, et al. Fluid-Structure Interaction Model of a Percutaneous Aortic Valve: Comparison with an In Vitro Test and Feasibility Study in a Patient-Specific Case. Ann Biomed Eng 2015. August 21. doi: 10.1007/s10439-015-1429-x. PubMed PMID: 26294009. [DOI] [PubMed] [Google Scholar]
- 95.Kamensky D, Hsu M-C, Schillinger D, et al. An immersogeometric variational framework for fluid–structure interaction: Application to bioprosthetic heart valves. Computer methods in applied mechanics and engineering. 2015;284:1005–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ghosh R, Marom G, Rotman O, et al. Comparative Fluid-Structure Interaction Analysis of Polymeric Transcatheter and Surgical Aortic Valves’ Hemodynamics and Structural Mechanics. Journal of biomechanical engineering. 2018. June 25. doi: 10.1115/1.4040600. PubMed PMID: 30029207; eng. [DOI] [PubMed] [Google Scholar]
- 97.Chiu WC, Girdhar G, Xenos M, et al. Thromboresistance comparison of the HeartMate II ventricular assist device with the device thrombogenicity emulation- optimized HeartAssist 5 VAD. Journal of biomechanical engineering. 2014. February;136(2):021014. doi: 10.1115/1.4026254. PubMed PMID: 24337144; PubMed Central PMCID: PMCPmc4023653. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chiu W-C, Alemu Y, McLarty AJ, et al. Ventricular Assist Device Implantation Configurations Impact Overall Mechanical Circulatory Support System Thrombogenic Potential. ASAIO journal. 2017;63(3):285–292. doi: 10.1097/mat.0000000000000488. PubMed PMID: 00002480–201705000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Amahzoune B, Bruneval P, Allam B, et al. Traumatic leaflet injury during the use of percutaneous valves: a comparative study of balloon- and self-expandable valved stents. European Journal of Cardio-Thoracic Surgery. 2013;43(3):488–493. doi: 10.1093/ejcts/ezs359. [DOI] [PubMed] [Google Scholar]
- 100.Bourget JM, Zegdi R, Lin J, et al. Correlation between structural changes and acute thrombogenicity in transcatheter pericardium valves after crimping and balloon deployment. Morphologie. 2017. 2017/March/01/;101(332):19–32. doi: 10.1016/j.morpho.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 101.Convelbo C, Guetat P, Cambillau M, et al. Crimping and deployment of balloon-expandable valved stents are responsible for the increase in the hydraulic conductance of leaflets. Eur J Cardiothorac Surg 2013. December;44(6):1045–50. doi: 10.1093/ejcts/ezt175. PubMed PMID: 23554448; eng. [DOI] [PubMed] [Google Scholar]
- 102.Ducci A, Pirisi F, Tzamtzis S, et al. Transcatheter aortic valves produce unphysiological flows which may contribute to thromboembolic events: An in-vitro study. Journal of biomechanics. 2016. December 8;49(16):4080–4089. doi: 10.1016/j.jbiomech.2016.10.050. PubMed PMID: 27836502; PubMed Central PMCID: PMCPMC5179499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kheradvar A, Groves EM, Falahatpisheh A, et al. Emerging trends in heart valve engineering: Part IV. Computational Modeling and Experimental Studies. Ann Biomed Eng 2015. October;43(10):2314–33. doi: 10.1007/s10439-015-1394-4. PubMed PMID: 26224522. [DOI] [PubMed] [Google Scholar]
- 104.Hatoum H, Dollery J, Lilly SM, et al. Impact of patient-specific morphologies on sinus flow stasis in transcatheter aortic valve replacement: An in vitro study. J Thorac Cardiovasc Surg 2018. June 7. doi: 10.1016/j.jtcvs.2018.05.086. PubMed PMID: 29980299; PubMed Central PMCID: PMCPMC6056004. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hatoum H, Dollery J, Lilly SM, et al. Implantation Depth and Rotational Orientation Effect on Valve-in-Valve Hemodynamics and Sinus Flow. Ann Thorac Surg 2018. March 1. doi: 10.1016/j.athoracsur.2018.01.070. PubMed PMID: 29501642; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hatoum H, Dollery J, Lilly SM, et al. Effect of severe bioprosthetic valve tissue ingrowth and inflow calcification on valve-in-valve performance. Journal of biomechanics. 2018. June 6;74:171–179. doi: 10.1016/j.jbiomech.2018.04.039. PubMed PMID: 29753455; PubMed Central PMCID: PMCPMC5977402. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hatoum H, Dollery J, Lilly SM, et al. Sinus Hemodynamics Variation with Tilted Transcatheter Aortic Valve Deployments. Ann Biomed Eng 2018. August 27. doi: 10.1007/s10439-018-02120-0. PubMed PMID: 30151733; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hatoum H, Yousefi A, Lilly S, et al. An in vitro evaluation of turbulence after transcatheter aortic valve implantation. J Thorac Cardiovasc Surg 2018. June 2. doi: 10.1016/j.jtcvs.2018.05.042. PubMed PMID: 29961588; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vukicevic M, Mosadegh B, Min JK, et al. Cardiac 3D Printing and its Future Directions. JACC Cardiovascular imaging. 2017. February;10(2):171–184. doi: 10.1016/j.jcmg.2016.12.001. PubMed PMID: 28183437; PubMed Central PMCID: PMCPMC5664227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vukicevic M, Vekilov DP, Grande-Allen JK, et al. Patient-specific 3D Valve Modeling for Structural Intervention. Structural Heart. 2017;1(5–6):236–248. doi: 10.1080/24748706.2017.1377363. [DOI] [Google Scholar]
- 111.Gunning PS, Saikrishnan N, Yoganathan AP, et al. Total ellipse of the heart valve: the impact of eccentric stent distortion on the regional dynamic deformation of pericardial tissue leaflets of a transcatheter aortic valve replacement. J R Soc Interface. 2015. December 6;12(113):20150737. doi: 10.1098/rsif.2015.0737. PubMed PMID: 26674192; PubMed Central PMCID: PMCPMC4707849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maleki H, Shahriari S, Labrosse M, et al. Effect of Aortic Annulus Size and Prosthesis Oversizing on the Hemodynamics and Leaflet Bending Stress of Transcatheter Valves: An In Vitro Study. Can J Cardiol 2015. August;31(8):1041–6. doi: 10.1016/j.cjca.2015.03.026. PubMed PMID: 26211709. [DOI] [PubMed] [Google Scholar]
- 113.Heide-Jorgensen S, Kumaran Krishna S, Taborsky J, et al. A Novel Method for Optical High Spatiotemporal Strain Analysis for Transcatheter Aortic Valves In Vitro. Journal of biomechanical engineering. 2016. March;138(3):4032501. doi: 10.1115/1.4032501. PubMed PMID: 26784124. [DOI] [PubMed] [Google Scholar]
- 114.Boloori Zadeh P, Corbett SC, Nayeb-Hashemi H. In-vitro calcification study of polyurethane heart valves. Mater Sci Eng C Mater Biol Appl 2014. February 1;35:335–40. doi: 10.1016/j.msec.2013.11.015. PubMed PMID: 24411385. [DOI] [PubMed] [Google Scholar]
- 115.Golomb G, Wagner D. Development of a new in vitro model for studying implantable polyurethane calcification. Biomaterials. 1991. May;12(4):397–405. PubMed PMID: 1888809. [DOI] [PubMed] [Google Scholar]
- 116.Gauvin R, Marinov G, Mehri Y, et al. A comparative study of bovine and porcine pericardium to highlight their potential advantages to manufacture percutaneous cardiovascular implants. Journal of biomaterials applications. 2013. November;28(4):552–65. doi: 10.1177/0885328212465482. PubMed PMID: 23142967; eng. [DOI] [PubMed] [Google Scholar]
- 117.Richardt D, Haban-Rackebrandt SL, Stock S, et al. A matter of thrombosis: different thrombus-like formations in balloon-expandable transcatheter aortic valve prostheses. Eur J Cardiothorac Surg 2018. February 15. doi: 10.1093/ejcts/ezy033. PubMed PMID: 29462284. [DOI] [PubMed] [Google Scholar]
- 118.Jesty J, Bluestein D. Acetylated prothrombin as a substrate in the measurement of the procoagulant activity of platelets: elimination of the feedback activation of platelets by thrombin. Analytical biochemistry. 1999. July 15;272(1):64–70. doi: 10.1006/abio.1999.4148. PubMed PMID: 10405294; eng. [DOI] [PubMed] [Google Scholar]
- 119.Bluestein D, Yin W, Affeld K, et al. Flow-induced platelet activation in mechanical heart valves. The Journal of heart valve disease. 2004. May;13(3):501–8. PubMed PMID: 15222299; eng. [PubMed] [Google Scholar]
- 120.Bluestein D Research Approaches for Studying Flow Induced Thromboembolic Complications in Blood Recirculating Devices. Expert Rev Medical Devices. 2004;1(1):65–80. [DOI] [PubMed] [Google Scholar]
- 121.Girdhar G, Xenos M, Alemu Y, et al. Device Thrombogenicity Emulation: A Novel Method for Optimizing Mechanical Circulatory Support Device Thromboresistance. PloS one. 2012;7(3):e32463. doi: 10.1371/journal.pone.0032463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bluestein D, Einav S, Slepian MJ. Device thrombogenicity emulation: A novel methodology for optimizing the thromboresistance of cardiovascular devices. Journal of biomechanics. 2012. December 6. doi: S0021–9290(12)00685–9 [pii] 10.1016/j.jbiomech.2012.11.033. PubMed PMID: 23219278; Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Claiborne TE, Girdhar G, Gallocher-Lowe S, et al. Thrombogenic potential of Innovia polymer valves versus Carpentier-Edwards Perimount Magna aortic bioprosthetic valves. ASAIO journal. 2011. Jan-Feb;57(1):26–31. doi: 10.1097/MAT.0b013e3181fcbd86. PubMed PMID: 20930618; eng. [DOI] [PubMed] [Google Scholar]
- 124.Jesty J, Yin W, Perrotta P, et al. Platelet activation in a circulating flow loop: Combined effects of shear stress and exposure time. Platelets. 2003;14(3):143–149. doi: 10.1080/0953710031000092839. [DOI] [PubMed] [Google Scholar]
- 125.Sheriff J, Claiborne TE, Tran PL, et al. Physical Characterization and Platelet Interactions under Shear Flows of a Novel Thermoset Polyisobutylene-based Co-polymer. ACS Appl Mater Interfaces. 2015. October 7;7(39):22058–66. doi: 10.1021/acsami.5b07254. PubMed PMID: 26398588; PubMed Central PMCID: PMCPMC4608843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bluestein D Towards optimization of the thrombogenic potential of blood recirculating cardiovascular devices using modeling approaches. Expert Rev Med Devices. 2006. May;3(3):267–70. doi: 10.1586/17434440.3.3.267. PubMed PMID: 16681446; eng. [DOI] [PubMed] [Google Scholar]
- 127.Egron S, Fujita B, Gullon L, et al. Radial Force: An Underestimated Parameter in Oversizing Transcatheter Aortic Valve Replacement Prostheses: In Vitro Analysis with Five Commercialized Valves. ASAIO journal. 2017. September 5. doi: 10.1097/MAT.0000000000000659. PubMed PMID: 28885378. [DOI] [PubMed] [Google Scholar]
- 128.Mazilu D, Li M, Kocaturk O, et al. Self-Expanding Stent and Delivery System for Aortic Valve Replacement. Journal of Medical Devices. 2012;6(4):041006–041006-9. doi: 10.1115/1.4007750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhao T, Martinez A, Cao H. Experimental Validation of SAPIENTM Transcatheter Heart Valve Finite Element Analysis Models. Journal of Medical Devices. 2013;7(4):040914–040914. doi: 10.1115/1.4025981. [DOI] [Google Scholar]
- 130.Azadani AN, Jaussaud N, Matthews PB, et al. Valve-in-valve implantation using a novel supravalvular transcatheter aortic valve: proof of concept. Ann Thorac Surg 2009. December;88(6):1864–9. doi: 10.1016/j.athoracsur.2009.08.004. PubMed PMID: 19932250; eng. [DOI] [PubMed] [Google Scholar]
- 131.Walther T, Dehdashtian MM, Khanna R, et al. Trans-catheter valve-in-valve implantation: in vitro hydrodynamic performance of the SAPIEN+cloth trans-catheter heart valve in the Carpentier-Edwards Perimount valves. Eur J Cardiothorac Surg 2011. November;40(5):1120–6. doi: 10.1016/j.ejcts.2011.02.056. PubMed PMID: 21466959; eng. [DOI] [PubMed] [Google Scholar]
- 132.Xuan Y, Krishnan K, Ye J, et al. Stent and leaflet stresses in a 26-mm first-generation balloon-expandable transcatheter aortic valve. J Thorac Cardiovasc Surg 2017. May;153(5):1065–1073. doi: 10.1016/j.jtcvs.2016.12.016. PubMed PMID: 28108064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Morganti S, Conti M, Aiello M, et al. Simulation of transcatheter aortic valve implantation through patient-specific finite element analysis: two clinical cases. Journal of biomechanics. 2014. August 22;47(11):2547–55. doi: 10.1016/j.jbiomech.2014.06.007. PubMed PMID: 24998989; eng. [DOI] [PubMed] [Google Scholar]
- 134.Wang Q, Sirois E, Sun W. Patient-specific modeling of biomechanical interaction in transcatheter aortic valve deployment. Journal of biomechanics. 2012. July 26;45(11):1965–71. doi: 10.1016/j.jbiomech.2012.05.008. PubMed PMID: 22698832; PubMed Central PMCID: PMC3392407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bosi GM, Capelli C, Cheang MH, et al. Population-specific material properties of the implantation site for transcatheter aortic valve replacement finite element simulations. Journal of biomechanics. 2018 2018/February/20/. doi: 10.1016/j.jbiomech.2018.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Falahatpisheh A, Morisawa D, Toosky TT, et al. A calcified polymeric valve for valve-in-valve applications. Journal of biomechanics. 2017. January 4;50:77–82. doi: 10.1016/j.jbiomech.2016.11.027. PubMed PMID: 27887725. [DOI] [PubMed] [Google Scholar]
- 137.Russ C, Hopf R, Hirsch S, et al. Simulation of transcatheter aortic valve implantation under consideration of leaflet calcification. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference 2013;2013:711–4. doi: 10.1109/embc.2013.6609599. PubMed PMID: 24109786; eng. [DOI] [PubMed] [Google Scholar]
- 138.Gessat M, Hopf R, Pollok T, et al. Image-based mechanical analysis of stent deformation: concept and exemplary implementation for aortic valve stents. IEEE transactions on bio-medical engineering. 2014. January;61(1):4–15. doi: 10.1109/tbme.2013.2273496. PubMed PMID: 24626769; eng. [DOI] [PubMed] [Google Scholar]
- 139.Hopf R, Sündermann SH, Born S, et al. Postoperative analysis of the mechanical interaction between stent and host tissue in patients after transcatheter aortic valve implantation. Journal of biomechanics. 2017. 2017/February/28/;53:15–21. doi: 10.1016/j.jbiomech.2016.12.038. [DOI] [PubMed] [Google Scholar]
- 140.Finotello A, Morganti S, Auricchio F. Finite element analysis of TAVI: Impact of native aortic root computational modeling strategies on simulation outcomes. Medical Engineering & Physics. 2017. doi: 10.1016/j.medengphy.2017.06.045. [DOI] [PubMed] [Google Scholar]
- 141.Duraiswamy N, Weaver JD, Ekrami Y, et al. A Parametric Computational Study of the Impact of Non-circular Configurations on Bioprosthetic Heart Valve Leaflet Deformations and Stresses: Possible Implications for Transcatheter Heart Valves [journal article]. Cardiovascular engineering and technology. 2016;7(2):126–138. doi: 10.1007/s13239-016-0259-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gauvin R, Marinov G, Mehri Y. et al. A comparative study of bovine and porcine pericardium to highlight their potential advantages to manufacture percutaneous cardiovascular implants. J Biomater Appl 2013. November;28(4):552–565. doi: 10.1016/j.jbiomech.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 143.Richardt D, Haban-Rackebrandt SL, Stock S, et al. A matter of thrombosis: different thrombus-like formations in balloon expandable transcatheter aortic valve prostheses. Eur J Cardiothorac Surg 2018. February 15 DOI: 10.1093/ejcts/ezy033 [DOI] [PubMed] [Google Scholar]
- 144.Jesty J, Bluestein D. Acetylated prothrombin as a substrate in the measurement of the procoagulant activity of platelets: elimination of the feedback activation of platelets by thrombin. Anal Biochem 1999. July 15;272(1):64–70. doi: 10.1016/j.jmbbm.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 145.Bluestein D, Yin W, Affeld K, et al. Flow-induced platelet activation in mechanical heart valves. J Heart Valve Dis 2004. May;13(3):501–508. doi: 10.1016/j.jbiomech.2015.07.031. [DOI] [PubMed] [Google Scholar]
- 146.Gharaie SH, Mosadegh B, Morsi Y. In Vitro Validation of a Numerical Simulation of Leaflet Kinematics in a Polymeric Aortic Valve Under Physiological Conditions. Cardiovascular engineering and technology. 2018. March;9(1):42–52. doi: 10.1007/s13239-018-0340-7. PubMed PMID: 29322329. [DOI] [PubMed] [Google Scholar]
- 147.Sirois E, Wang Q, Sun W. Fluid Simulation of a Transcatheter Aortic Valve Deployment into a Patient-Specific Aortic Root. Cardiovascular engineering and technology. 2011. 2011/September/01;2(3):186–195. doi: 10.1007/s13239-011-0037-7. English. [DOI] [Google Scholar]
- 148.Tan FPP, Xu XY, Torii R, et al. Comparison of Aortic Flow Patterns Before and After Transcatheter Aortic Valve Implantation. Cardiovascular engineering and technology. 2012. 2012/March/01;3(1):123–135. doi: 10.1007/s13239-011-0073-3. English. [DOI] [Google Scholar]
- 149.Kopanidis A, Pantos I, Alexopoulos N, et al. Aortic Flow Patterns After Simulated Implantation of Transcatheter Aortic Valves. Hellenic journal of cardiology : HJC = Hellenike kardiologike epitheorese. 2015. Sep-Oct;56(5):418–28. PubMed PMID: 26429371; eng. [PubMed] [Google Scholar]
- 150.Reeve L, Baldrick P. Biocompatibility assessments for medical devices - evolving regulatory considerations. Expert Rev Med Devices. 2017. February;14(2):161–167. doi: 10.1080/17434440.2017.1280392. PubMed PMID: 28080154. [DOI] [PubMed] [Google Scholar]
- 151.Sheriff J, Claiborne TE, Tran PL, et al. Physical characterization and platelet interactions under shear flows of a novel thermoset polyisobutylene-based co-polymer. ACS Appl Mater Interfaces. 2015. October 7;7(39):22058–22066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vahidkhah K, Javani S, Abbasi M, et al. Blood Stasis on Transcatheter Valve Leaflets and Implications for Valve-in-Valve Leaflet Thrombosis. Ann Thorac Surg 2017. September;104(3):751–759. doi: 10.1016/j.athoracsur.2017.02.052. PubMed PMID: 28483152. [DOI] [PubMed] [Google Scholar]
- 153.Hira RS, Vemulapalli S, Li Z, et al. Trends and Outcomes of Off-label Use of Transcatheter Aortic Valve Replacement: Insights From the NCDR STS/ACC TVT Registry. JAMA Cardiol 2017. August 1;2(8):846–854. doi: 10.1001/jamacardio.2017.1685. PubMed PMID: 28636718; PubMed Central PMCID: PMCPMC5710589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Guyton RA, Padala M. Transcatheter Aortic Valve Replacement in Bicuspid Aortic Stenosis: Early Success But Concerning Red Flags. JACC Cardiovasc Interv 2016. April 25;9(8):825–827. doi: 10.1016/j.jcin.2016.02.042. PubMed PMID: 27101907. [DOI] [PubMed] [Google Scholar]
- 155.Hayashida K, Bouvier E, Lefevre T, et al. Transcatheter aortic valve implantation for patients with severe bicuspid aortic valve stenosis. Circulation Cardiovascular interventions. 2013. June;6(3):284–91. doi: 10.1161/circinterventions.112.000084. PubMed PMID: 23756698; eng. [DOI] [PubMed] [Google Scholar]
- 156.Mylotte D, Lefevre T, Sondergaard L, et al. Transcatheter aortic valve replacement in bicuspid aortic valve disease. J Am Coll Cardiol 2014. December 9;64(22):2330–9. doi: 10.1016/j.jacc.2014.09.039. PubMed PMID: 25465419. [DOI] [PubMed] [Google Scholar]
- 157.Patel SV, Sonani R, Singh V, et al. Outcomes of transcatheter aortic valve replacement for bicuspid aortic stenosis - a systematic review of existing literature. Expert Rev Pharmacoecon Outcomes Res 2017. December;17(6):579–585. doi: 10.1080/14737167.2017.1391692. PubMed PMID: 29017405. [DOI] [PubMed] [Google Scholar]
- 158.Wijesinghe N, Ye J, Rodes-Cabau J, et al. Transcatheter aortic valve implantation in patients with bicuspid aortic valve stenosis. JACC Cardiovasc Interv 2010. November;3(11):1122–5. doi: 10.1016/j.jcin.2010.08.016. PubMed PMID: 21087746; eng. [DOI] [PubMed] [Google Scholar]
- 159.Yoon SH, Bleiziffer S, De Backer O, et al. Outcomes in Transcatheter Aortic Valve Replacement for Bicuspid Versus Tricuspid Aortic Valve Stenosis. J Am Coll Cardiol 2017. May 30;69(21):2579–2589. doi: 10.1016/j.jacc.2017.03.017. PubMed PMID: 28330793. [DOI] [PubMed] [Google Scholar]
- 160.Yoon SH, Lefevre T, Ahn JM, et al. Transcatheter Aortic Valve Replacement With Early- and New-Generation Devices in Bicuspid Aortic Valve Stenosis. J Am Coll Cardiol 2016. September 13;68(11):1195–1205. doi: 10.1016/j.jacc.2016.06.041. PubMed PMID: 27609682. [DOI] [PubMed] [Google Scholar]
- 161.Transcatheter Aortic Valve Replacement/Implantation (TAVR/TAVI) Market Size, Share & Trends Analysis Report By Procedure (Transapical, Transfemoral, Transaortic), By Country, Vendor Landscape, And Segment Forecast, 2018–2025. 2018. p. 1–90. [Google Scholar]


