Abstract
Background:
Recent rat studies indicate that alcohol withdrawal can trigger a negative emotional state including anxiety- and depression-like behaviors and hyperalgesia, as well as elevated glutamatergic transmission and activity in lateral habenula neurons. TRPV1, a vanilloid receptor expressed in the habenula, is involved in pain, alcohol dependence, and glutamatergic transmission. We, therefore, hypothesized that TRPV1 contributes to the changes in both the behavioral phenotypes and the habenula activity in alcohol-withdrawn rats.
Methods:
Adult male Long-Evans rats (n=110 and 280 for electrophysiology and behaviors, respectively), randomly assigned into the alcohol and water (Naïve) groups, were trained to consume either alcohol or water-only using an intermittent-access procedure. Slice electrophysiology was used to measure spontaneous excitatory postsynaptic currents (sEPSCs) and firing of lateral habenula neurons. The primary outcome was the change in alcohol-related behaviors and lateral habenula activity induced by pharmacological manipulation of TRPV1 activity.
Results:
The basal frequency of sEPSCs and firing of lateral habenula neurons in alcohol-withdrawn rats was significantly increased. The TRPV1 antagonist capsazepine (10 µM) induced a stronger inhibition on sEPSCs (mean ± SD; by 26.1±27.9% [n=11] vs. 6.7±18.6% [n=17], P=0.027) and firing (by 23.4±17.6% [n=9] vs. 11.9±16.3% [n=12], P=0.025) in Withdrawn rats than Naive rats. By contrast, the TRPV1 agonist capsaicin (3 μM) produced a weaker potentiation in Withdrawn than Naïve rats (sEPSCs: by 203.6±124.7% [n=20] vs. 415.2±424.3% [n=15], P <0.001; firing: 38.1±14.7% [n=11] vs. 73.9±41.9% [n=11], P<0.001). Conversely, capsaicin’s actions in Naïve but not in Withdrawn rats were significantly attenuated by the pretreatment of TRPV1 endogenous agonist N-Oleoyldopamine. In Withdrawn rats, intra-habenula infusion of TRPV1 antagonists attenuated hyperalgesia, and anxiety-like behaviors, decreased alcohol consumption upon resuming drinking and elicited a conditioned place preference.
Conclusions:
Enhanced TRPV1 function may contribute to increased glutamatergic transmission and activity of lateral habenula neurons, resulting in the aberrant behaviors during ethanol withdrawal.
Introduction
Repeated cycles of excessive alcohol drinking and withdrawal induce a negative affective state and aberrant behaviors, including pain and anxiety 1. These psychiatric ailments act as negative reinforcers, promoting relapse drinking and the development of alcohol use disorders (AUDs)2.
Accumulating evidence indicates that the lateral habenula (LHb), an epithalamic structure in the brain, plays a crucial role in the aversive behaviors induced by many abused drugs, including alcohol3–9. Recently we demonstrated increased nociception10,11, anxiety- and depression-like behaviors12,13 in alcohol-withdrawn rats. These aberrant behaviors are concomitant with increased activity of, and glutamatergic transmissions to, LHb neurons12,13.
The TRP channel, vanilloid type 1 or TRPV1, is the receptor for capsaicin14, and a nonselective cation channel that has been implicated in pain and behaviors associated with drugs of abuse such as anxiety15. TRPV1 is in several brain regions including the habenula16,17. Given that TRPV1 has been suggested to be able to facilitate glutamate transmission18 and is a target of alcohol19, we hypothesized that LHb TRPV1 contributes to the hyper-glutamatergic and hyperactivity of LHb neurons as well as the aberrant behaviors in rats withdrawn from chronic alcohol exposure.
In this study, we test this hypothesis by assessing the role of TRPV1 in the electrophysiological activity of LHb neurons in epithalamic slices. We also measured the effect of pharmacological manipulations of TRPV1 in the LHb on pain, anxiety-, and depression-like behaviors and relapse-like drinking behaviors in rats withdrawn from chronic intermittent ethanol drinking.
Materials and methods
Animals
All experiments were performed in adult male Long Evans rats (n=110 for electrophysiology and n=280 for behaviors, 250–300g in the start of the study). All procedures were performed by the National Institutes of Health guidance and approved by the Animal Care and Utilization Committee of Rutgers, the State University of New Jersey, New Jersey Medical School. Rats were maintained on a 12:12-h light: dark regiment (light on 20:00 with ad libitum food and water unless indicated otherwise, all experiments were carried out during the dark cycle. These rats were randomly divided into the alcohol- and water-drinking groups and were numbered and randomly assigned to experimental groups thereafter. The primary outcome was the change in alcohol-related behaviors and LHb activity induced by pharmacological manipulation of LHb TRPV1 activity.
Ethanol Administration
Rats were trained to drink either 20% ethanol or water only under the intermittent access to two-bottle free choice (IA2BC) paradigm for 2–3 months20,21. A detailed description of the IA2BC paradigm is provided in the Supplementary Digital Content, Materials, and Methods. All subsequent experiments started at 10:00 in each experimental day and were conducted by experimenters blinded to group assignment.
Brain Slice Preparation and Electrophysiology
Animals were sacrificed under anesthesia using ketamine/xylazine (80 mg/10 mg/kg. i.p). Coronal epithalamic slices (250 μm thick) were cut at 10:00 on experiment day, from rats that were at 24 hours withdrawal from the last alcohol-drinking session in the IA2BC procedure for eight weeks (EtOH-WD), and from age-matched water drinking (Naïve) counterparts, in artificial cerebrospinal fluid (aCSF) containing the following (in mM): 126 NaCl, 2.5 KCl, 1.25 NaH2PO4, 1 MgCl2, 2 CaCl2, 25 NaHCO3, 0.3 L-ascorbate, and 11 glucose, saturated with carbogen (95% O2/5% CO2), as described22,23.
Electrophysiological events were recorded in warm (33℃) carbogenated aCSF (1.5–2.0 ml/min) from 11:00–18:00 h. Patch pipettes (6–8 MΩ) for voltage-clamp recordings contained (in mM): 140 Cs-methanesulfonate, 5 KCl, 2 MgCl2, 10 HEPES, 2 MgATP, 0.2 NaGTP, pH 7.2. Spontaneous firing was recorded by the loose-patch cell-attached technique. Spontaneous excitatory postsynaptic currents (sEPSCs) were recorded at a holding potential of −70 mV in the presence of gabazine (20 μM) and SCH50911 (10 μM) to block GABA(A) and GABA(B) receptors, respectively.
Cannula Implantation
Stereotaxic surgery was performed on rats after a stable baseline drinking level was reached, under anesthesia using ketamine/xylazine (80 mg/10 mg/kg. i.p), as described 20. A bilateral guide cannula (C235G-3.0, 23 gauge; Plastics One, Roanoke, VA) was aimed 1 mm above the LHb (in mm: AP: −3.85; ML: ±0.75; DV: −4.2 from the skull’s surface)12. One week after recovery from surgery, animals returned to drinking with the IA2BC paradigm. Age-matched naïve rats also received surgery and drank only water. To conclude the study, histological verification was completed as described20. Supplementary Digital Content, Figure 1 indicates the placement of cannula tips from animals that received surgery; rats with injection sites outside of the LHb (n=4) were not used in the data analysis.
Intra-LHb Microinjection
Microinjection was performed as described 13. Injectors were left in place for an additional 60s to allow for diffusion. Behavioral experiments proceeded as noted in the following methods.
Drugs
Common salts, 6, 7-Dinitroquinoxaline-2, 3-dione (DNQX), (2R)-amino-5-phosphonovaleric acid (AP5), capsazepine, N-Oleoyldopamine (OLDA), 2E-N-(2,3-Dihydro-1,4-benzodioxin-6-yl)-3-[4-(1,1-dimethylethyl)phenyl]-2-Propenamide (AMG9810), and capsaicin were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Thermal Pain Test
Thermal nociceptive thresholds were determined by measuring the paw-withdrawal latency (PWL) using the Hargreaves method10,24. The PWL was defined as the length of time between the start of the light beam and the lift of the hind paw. To avoid tissue damage, a 20-second cut-off time was used. A comparatively lower PWL was interpreted as hyperalgesia, whereas a higher PWL was interpreted as analgesia. To better compare the effects between the baselines of Naïve and EtOH-WD rats, the PWL data were transformed to represent the percent of maximum possible effect, or %MPE, using the following formula:
Conditioned Place Paradigm (CPP)
A separate group of Naïve and EtOH-WD rats was trained for conditioned place preference or aversion (CPP/CPA) to intra-LHb aCSF, DNQX, capsazepine or capsaicin to detect the presence of spontaneous pain25. We established an unbiased procedure26, consisting of three distinct phases: pre-conditioning, conditioning, and post-conditioning. Each phase was conducted during the 24-hour withdrawal time.
CPP Apparatus
We utilized a standard rat place preference apparatus (MED-CPP2–013C, Med Associates INC. Georgia, VT), which consisted of two chambers (30L×20W×20H, in cm) separated by a guillotine door. Chambers differed by tactile floor cues (mesh vs. rod) and wall colors (white vs. black). Photo beam detectors identified the activity and position of each rat (MED-PC Software, Med Associates INC.).
CPP Test Procedure
Phase I: Pre-conditioning (Day 1)
Animals were allowed free access to the apparatus for 15 min. Rats with a strong initial preference (>75%) for one chamber were excluded from further analysis.
Phase II: Conditioning (Day 3)
During conditioning, one drug session and one aCSF session were performed, separated by 6 hours. During this phase, the guillotine door was closed, and animals were confined in one chamber for 15 min immediately following intra-LHb drug infusion.
Phase III: Post-conditioning (Day 5)
For post-conditioning, the guillotine door was opened, and the time spent in each chamber was recorded during the 15-minute test period. The preference score was defined as the difference in time spent in the drug-paired chamber on the post-conditioning day versus the pre-conditioning day. Positive or negative scores indicated preference or aversion, respectively.
Elevated plus maze test (EPM)
We utilized the EPM to assess anxiety-like behaviors, as described12,27. During the 5-minute test, time spent in the open arms, number of entries into the open arms and total distance traveled were recorded using Smart 3.0 (Pan lab Harvard Apparatus, Barcelona, Spain). EPM testing took place 15 min after drug infusion. Each animal was exposed to the EPM one-time only.
Marble burying test (MBT)
We also utilized the MBT to assess anxiety-like behaviors, as described12. Following intra-LHb injection, animals were placed into the test cage containing 20 marbles. The numbers of marbles buried in the bedding (to 2/3 their depth) after 30 minutes were counted.
Forced swimming test (FST)
We used a modified FST to measure depressive-like behaviors, in a transparent plastic tube (diameter = 24.5 cm, height = 51 cm), filled to 30 cm with water at 23–25 °C. Each rat was placed in the tube of water for 15 min on the first session of the test and for 5 min on the test session 24 h later. Immobility was defined as the rat floating in the water without struggling and only making movements necessary to keep its head above water.
Statistical Analysis
All values in the text and figures indicate the mean ± S.D. The sample sizes we used were based on previous publications7,12 and no statistical power calculation was conducted prior to the study. All statistical calculations were carried out using Sigmaplot 14.0. (SYSTAT software, CA). Baseline electrophysiological data were recorded for 5–10 minutes, before drug superfusion, and during the washout. Since the basal EPSCs/firing frequency and amplitude varies in each cell, changes induced by each drug were calculated by the percent change. Data recorded during the initial control period were averaged and normalized to 100%. Prior to analysis, the distributions of the variables under study were examined using histograms. The use of parametric statistical analysis was deemed appropriate. The data of basal sEPSCs or firings were analyzed using student’s unpaired two tailed t-test between groups. The effects induced by drugs on sEPSCs/firing were analyzed using a two-way ANOVA with “group” (Naïve vs. EtOH-WD) as between-group factors and “dose” (Capsaicin: 0.01–30 µM; capsazepine or AMG9810: 0.1–100 μM; OLDA: 0.2–10 μM) or “pretreatment” (aCSF vs. 10μM N-Oleoyldopamine) as within-subject factor followed by the Tukey multiple comparisons test, as detailed in the figure legends. A Kolmogorov-Smirnov test was used to evaluate statistical significance for cumulative data. Dose-response data were fitted to the logistic equation: y=100xa/(xa+xoa), where y is the percentage change, x is the concentration of capsaicin, a is the slope parameter, and xo is the capsaicin concentration which induces a half-maximal change.
All the animals survived at the end of the behavior experiment and there were no missing data except 4 rats with injection sites outside of the LHb. Data from the behaviors were subject to a two-way ANOVA with “group” (Naïve vs. EtOH-WD) as between-group factors and treatment as within-subject factor. Changes in pain threshold and ethanol-drinking data were subjected to a two-way repeated measured ANOVA to extract significant main effect. Conditioned place test and hyperalgesia data were analyzed using a Pearson correlation. Statistical significance was declared at P < 0.05. Data were analyzed by experimenters who were blinded to the treatment history.
Results
Elevation of TRPV1 function mediates hyper-glutamatergic transmission and hyperactivity of LHb neurons during ethanol withdrawal
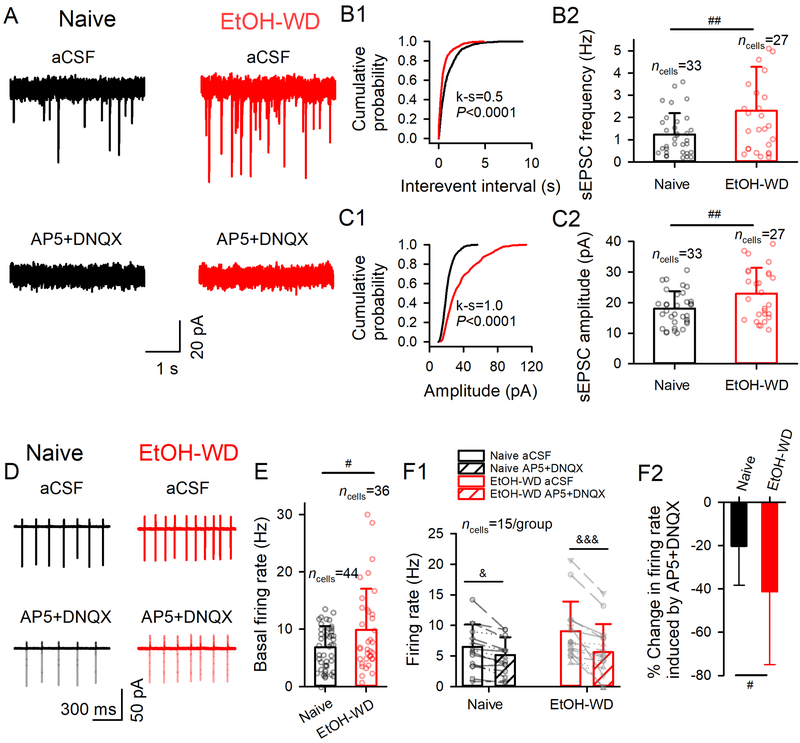
Consistent with a recent report28, the frequency and amplitude of sEPSCs in LHb neurons were significantly enhanced in slices from ethanol-withdrawn (EtOH-WD) rats compared to Naïve rats (frequency: 2.3±2.0 vs. 1.2±1.0 Hz, t(58) = 2.71, P = 0.009, fig. 1A, B1–2; amplitude: 22.9±8.4 vs. 18.1±8.7 pA, t(58) = 2.64, P = 0.011; fig. C1–2). The sEPSCs were eliminated by the glutamate antagonists (2R)-amino-5-phosphonovaleric acid (AP5, 50 μM) plus 6, 7-Dinitroquinoxaline-2, 3-dione (DNQX, 20 μM), indicating that these are events mediated by glutamate receptors. The spontaneous action potential firing frequency of LHb neurons was also markedly higher in slices from EtOH-WD rats (9.9±7.2 vs. 6.8±3.7 Hz, t(78) = 2.44, P = 0.017; fig. 1D, E). Moreover, bath application of AP5 (50 μM) plus DNQX (20 μM) significantly decreased the basal firing rate (main effect of Treatment F1,28 = 44.61, P < 0.001, two-way RM ANOVA; fig. 1F1), and the inhibitions differed between groups (main effect of Group × Treatment interaction: F1,28 = 8.57, P = 0.007), with a greater reduction in EtOH-WD rats (from 9.1±4.8 to 5.6±4.6 Hz, P < 0.001) than naïve rats (from 6.5±3.7 to 5.1±2.9 Hz, P = 0.013). Since the basal sEPSC/firing rates varied among LHb neurons of both groups of rats, we calculated the percent change before and during drug application for each cell in the following electrophysiological experiments. As expected, glutamate antagonists induced a significantly stronger inhibition of spontaneous firing in EtOH-WD than in Naïve rats (41.3±33.7% vs. 20.3±18.0%, t(28) = 2.13, P = 0.042; fig. 1F2), indicating that the hyper-glutamatergic state contributes to LHb hyperactivity during ethanol withdrawal.
Fig. 1. Enhanced glutamate transmission and activity of LHb neurons in ethanol-withdrawn rats.
(A) sEPSCs recorded from a slice of a Naïve rat (black) and a rat at 24hr withdrawal from chronic drinking in the IA2BC paradigm for two months (EtOH-WD, red). The events were eliminated by the glutamate receptor antagonists (50 μM AP5 and 20 µM DNQX). Cumulative probability plots of inter-event intervals (B1) and amplitudes of sEPSCs (C1). Summary data of frequency (B2) and amplitude (C2) of sEPSCs. (D) Spontaneous firing of LHb neurons recorded in the cell-attached mode in the absence and presence of AP5 plus DNQX in Naïve and EtOH-WD rats. (E) Firing rate of LHb neurons from Naïve and EtOH-WD rats. (F1) Firing rate of LHb neurons of Naïve and EtOH-WD rats before and after the application of AP5+DNQX. (F2) Inhibition of firing by AP5+DNQX was significantly greater in EtOH-WD rats than in Naïve rats. Data are expressed as mean ± SD. #P < 0.05, ##P < 0.01 EtOH-WD compared with the naïve group, student unpaired t-test (two-tailed). &P < 0.05, &&&P < 0.001, aCSF compared with AP5+DNQX, two-way repeated-measure ANOVA followed by Tukey post hoc comparison. Cell numbers in each figure are indicated.
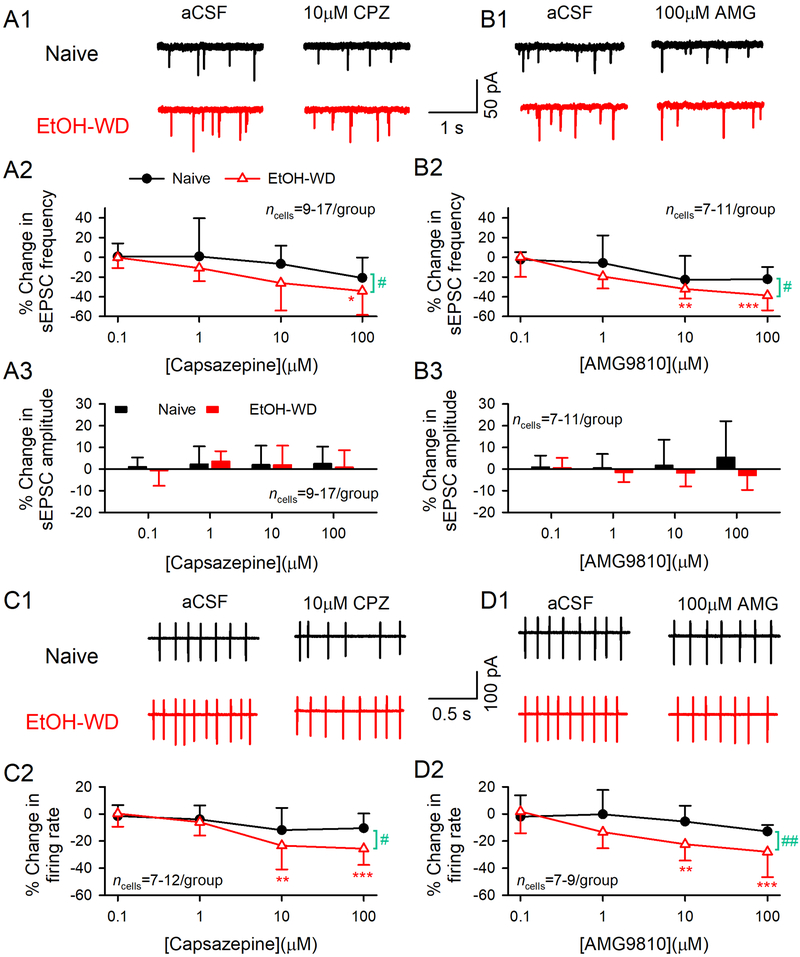
To determine whether TRPV1 plays a role in the hyper-glutamatergic and hyperactivity state of LHb neurons of EtOH-WD rats, we examined the effects of capsazepine, a TRPV1 antagonist, on sEPSCs and firings in LHb neurons. Bath application of capsazepine (CPZ, 0.1–100 μM) dose-dependently inhibited the sEPSC frequency (main effect of Dose: F3, 76 = 5.59, P = 0.002; fig. 2A1–2) and firing rate (F3, 67 = 9.62, P < 0.0001; fig. 2C1–2) in slices from EtOH-WD rats, but not Naïve rats. Thus, 10μM capsazepine induced significantly greater reduction on glutamate transmission (by 26.1 ± 27.9% in EtOH-WD rat vs. 6.7 ± 18.6% in naïve rats, main effect of Group: F1,76 = 5.06, P = 0.027) and neuronal activity (by 23.4 ± 17.6% in EtOH-WD rats vs. 11.9 ± 16.3% in naïve rats, main effect of Group: F1,67 = 5.28, P = 0.025) during ethanol withdrawal. No group × dose interaction was found (sEPSCs: F3,76 = 0.62, P = 0.606; Firing: F3,67=1.90, P = 0.139). Since capsazepine could also inhibit other ion channels and receptors29–31, we repeated the experiments with AMG9810 (AMG, 0.1–100 μM), a more selective TRPV1 antagonist. AMG9810 also induced a much stronger inhibition in EtOH-WD than in Naïve rats on sEPSC frequency (Group: F1,61 = 4.88, P = 0.031; Dose: F3,61 = 10.83, P < 0.0001; fig. 2B1–2) and firing rate (Group: F1,55 = 7.96, P = 0.007; Dose: F3,55 = 5.81, P = 0.002; fig. 2D1–2). No significant difference was detected between the group × dose interaction (sEPSCs: F3, 61 = 0.97, P = 0.413, Firing: F3, 55 = 1.67, P = 0.183).
Fig.2. TRPV1 antagonist capsazepine and AMG9810 induce a stronger inhibition of glutamate transmission and firing in LHb neurons from EtOH-WD rats.
Representative traces of sEPSCs in the absence and presence of capsazepine (CPZ, A1) and AMG9810 (AMG, B1) in Naïve (●) and EtOH-WD (△) rats. Capsazepine and AMG9810 (0.1–100 μM) induced changes in the frequency (CPZ: A2, AMG: B2) and amplitude (CPZ: A3, AMG: B3) of sEPSCs. Example traces of firing in the absence and presence of capsazepine (C1) and AMG9810 (D1). Summary graph of inhibition induced by capsazepine (C2) and AMG9810 (D2). Capsazepine and AMG9810 produced a significantly greater inhibition on sEPSC frequency and firing rate in EtOH-WD rats. *P < 0.05, **P < 0.01, ***P < 0.001 relative to 0.1 μM CPZ/AMG. #P < 0.05, ##P < 0.01 Naïve in comparison with EtOH-WD rats. Data were analyzed with two-way ANOVA and Tukey post hoc comparison.
Furthermore, neither capsazepine (F3,76 = 0.41, P = 0.743; fig. 2A3) nor AMG9810 (F3,61 = 0.14, P = 0.939; fig.2B3) at all the doses tested significantly altered the sEPSC amplitude. These data indicated that LHb TRPV1 channels are tonically activated under physiological and pathological conditions. More importantly, LHb TRPV1 basal activity is elevated in EtOH-WD rats.
Capsaicin-induced enhancement of glutamatergic transmission and activity of LHb neurons is weaker in ethanol-withdrawn rats
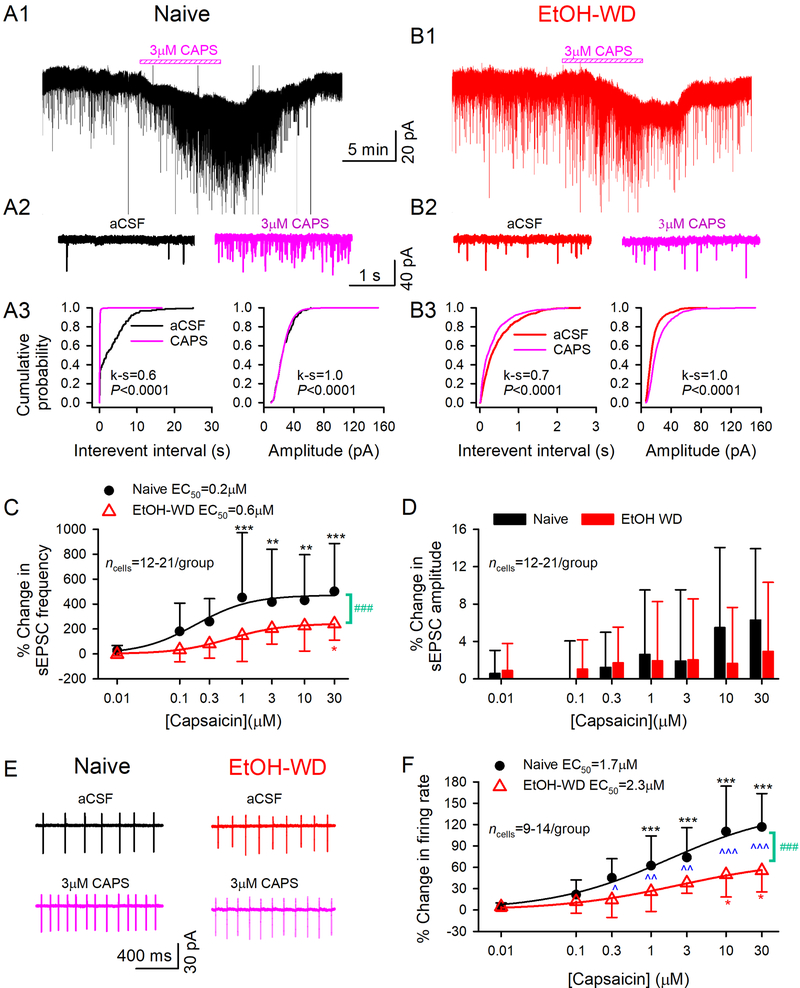
To further assess the effects of TRPV1 on synaptic plasticity during ethanol withdrawal, we examined the effect of the agonist capsaicin on the electrophysiological properties of LHb neurons. Bath perfusion of capsaicin (CAPS, 0.01–30μM) concentration-dependently increased the frequency of sEPSCs in LHb neurons in both Naïve (fig. 3A) and EtOH-WD (fig. 3B) rats (main effect of Dose: F6,208 = 7.95, P < 0.001; fig. 3C), with EC50s of 0.2 and 0.6 μM, respectively. We also detected a marked increase in cumulative probability of frequencies of sEPSCs, suggesting an increase in presynaptic glutamate release by capsaicin application (fig. 3A3, B3 left panels). Importantly, capsaicin’s action was significantly greater in Naïve than in EtOH-WD rats (203.6 ± 124.7% in EtOH-WD rats vs. 415.2 ± 424.3% in naïve rats induced by 3 μM capsaicin, main effect of Group: F1, 208 = 28.20, P < 0.001; fig. 3C). Though capsaicin-induced increases in sEPSC frequency were accompanied by a higher incidence of larger sEPSCs (k-s test, fig. 3A3, B3 right panels), the mean amplitude did not significantly change in all the doses tested in either Naïve or EtOH-WD rats (main effect of Group: F1, 208 = 1.12, P = 0.291; Dose: F6, 208 = 1.95, P = 0.075; fig. 3D). No significant difference was detected between the group × dose interaction (frequency: F6, 208 = 0.88, P = 0.508, amplitude: F6, 208 = 0.82, P = 0.558).
Fig.3. TRPV1 agonist capsaicin induces a stronger potentiation of glutamate transmission and firing in LHb neurons from ethanol naive rats.
Representative traces showing enhancement of sEPSCs induced by 3 µM capsaicin in LHb neurons from a Naive (A1) or an EtOH-WD (B1) rat. (A2-B2) Exemplar current traces were acquired in A1 and B1, before and during capsaicin application. Cumulative probability plots show higher incidence of events with shorter inter-event interval, and amplitude before and after capsaicin application in the LHb neurons of Naive (A3) and EtOH-WD (B3) rats (k-s test). Capsaicin elicited a concentration-dependent increase in sEPSC frequency (C), which was significantly greater in Naïve than in EtOH-WD neurons. The smooth curve is the best fit to the data by the logistic equation. Capsaicin did not significantly alter the mean sEPSC amplitude (D). (E) A representative example of increased spontaneous firing rate induced by 3 µM capsaicin in a Naïve or an EtOH-WD neuron. (F) Capsaicin caused a concentration-dependent increase in firing rate, which was significantly greater in the Naïve than the EtOH-WD neurons. *P < 0.05, **P < 0.01, ***P < 0.001 relative to 0.01 μM capsaicin; ###P < 0.001, Naïve in comparison with EtOH-WD rats. ^P < 0.05, ^^P < 0.01, ^^^P < 0.001, Naïve in comparison with EtOH-WD rats underwent the same dose of capsaicin. Data were analyzed with two-way ANOVA and Tukey post hoc comparison.
Capsaicin also concentration-dependently increased LHb neuronal activity (main effect of Dose: F6, 142 = 19.78, P < 0.0001), with EC50s of 1.7 and 2.3 μM, respectively in Naïve and EtOH-WD groups. Additionally, capsaicin’s action was greater in Naïve than in EtOH-WD rats (Group: F1, 142 = 42.0, P < 0.001; Group × Dose interaction: F6, 142 = 2.86, P = 0.012; 38.1 ± 14.7% in EtOH-WD rats vs. 73.9 ± 41.9% in naïve rats induced by 3μM capsaicin, post hoc P = 0.009; fig. 3E, F). These data indicate that the LHb neurons of EtOH-WD rats are less sensitive to capsaicin.
Capsaicin-induced enhancement of glutamatergic transmission and activity of LHb neurons is weaker in the presence of the TRPV1 endogenous agonist N-Oleoyldopamine
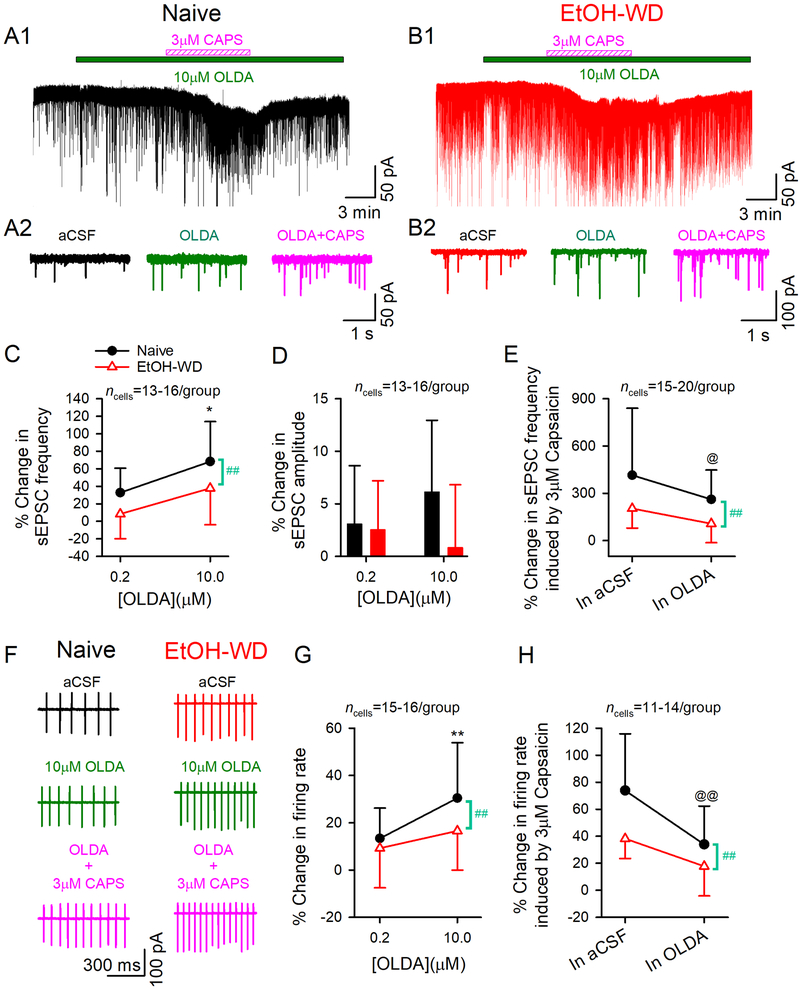
To test whether the decreased capsaicin response in EtOH-WD rats is due to an elevated endogenous TRPV1 activity, we pretreated slices with TRPV1 endogenous agonist N-Oleoyldopamine (OLDA, 0.2 or 10 µM) 32 for 6–8 min before the co-application of capsaicin (3μM) (fig. 4A, B). High (10 μM), but not low (0.2 μM) concentration of OLDA significantly enhanced sEPSC frequency in Naïve but not in EtOH-WD rats (main effect of Dose: F1,54 = 11.01, P = 0.002; Group: F1,54 = 7.83, P = 0.007; fig. 4A, B, C). No difference was found for group × dose interaction (F1,54 = 0.09, P = 0.762).
Fig.4. Pretreatment with endogenous TRPV1 agonist N-oleoyldopamine attenuates capsaicin-induced enhancement in glutamate transmission and firing in LHb neurons.
Representative traces showing enhancement of sEPSCs induced by N-oleoyldopamine (OLDA,10 μM) followed by capsaicin (3 μM) in a Naive (A1) and an EtOH-WD (B1) neuron. (A2-B2) Exemplar current traces were acquired in A1 and B1, before and during OLDA application, and OLDA/capsaicin co-application. Application of a high concentration OLDA (10 μM) enhanced sEPSC frequency (C) in LHb neurons of naïve but not in EtOH-WD rats. (D) OLDA did not alter sEPSC amplitude. (E) In the presence of 10 μM OLDA, capsaicin (3μM) produced a weaker increase on sEPSC frequency in naïve rats. (F) Representative traces showing enhancement of firing induced by OLDA alone or OLDA/capsaicin co-application in neurons of Naïve and EtOH-WD rats. (G) OLDA (10 μM) activated the LHb neurons of naïve rats but not that of EtOH-WD rats. (H) With the pretreatment of OLDA (10 μM), capsaicin (3 μM) produced a weaker increase in firing in naïve rats. *P < 0.05, **P < 0.01 relative to respective 0.2μM OLDA. ##P < 0.01, Naïve in comparison with EtOH-WD rats. @P < 0.05, @@P < 0.01, capsaicin-induced change within OLDA comparison to without OLDA (aCSF).
Notably, capsaicin (3 μM)-induced enhancement on sEPSC frequency was significantly decreased with Oleoyldopamine (10 μM) pretreatment compared with Oleoyldopamine -free solution in Naïve rats (main effect of Pretreatment: F1, 61 = 6.58, P = 0.013), but not in EtOH-WD rats (main effect of Group: F1, 61 = 8.0, P = 0.006; Pretreatment × Group interaction: F1, 61 = 0.66, P = 0.419; fig. 4A, B, E). These chemicals did not change the mean sEPSC amplitude (Oleoyldopamine alone or Oleoyldopamine plus capsaicin; fig. 4A, B, D). Furthermore, Oleoyldopamine dose-dependently accelerated the firing in Naïve rats (F1, 60 = 7.47, P = 0.008; fig. 4F, G) but not in EtOH-WD rats, thus producing a stronger excitation in Naïve rats (main effect of Group: F1, 60 = 4.06, P = 0.049; Group × Dose interaction: F1, 60 = 1.18, P = 0.281). In the presence of Oleoyldopamine (10 μM), capsaicin (3 μM)–induced increase in firing was significantly attenuated in Naïve rats but not in EtOH-WD rats (main effect of Pretreatment: F1, 46 = 14.13, P < 0.001; Group: F1, 46 = 10.43, P = 0.002; Group × Pretreatment interaction: F1, 46 = 1.48, P = 0.231; fig. 4H). These results support the view that an elevated endogenous TRPV1 activity may contribute to the blunted response to capsaicin in EtOH-WD rats.
Development of hyperalgesia during withdrawal from the IA2BC drinking paradigm
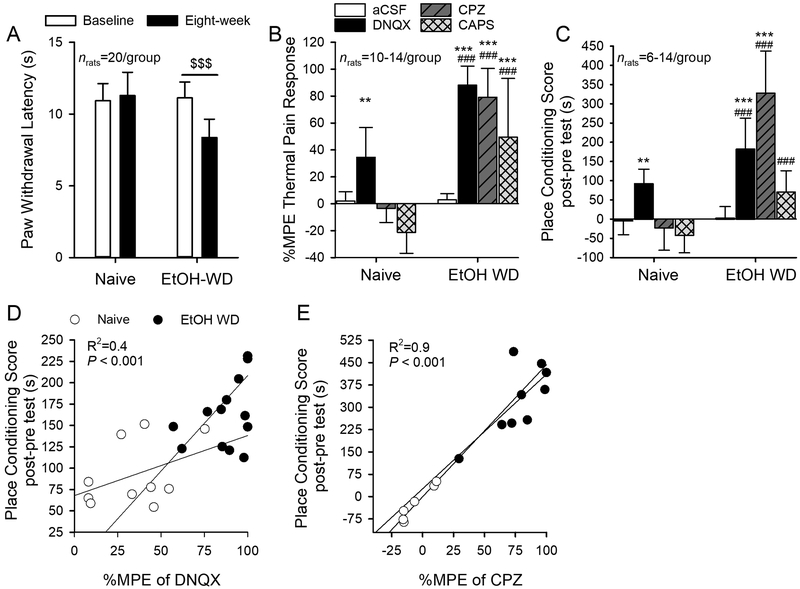
All rats survived after the experiments and all data were analyzed as intended, thus there were no missing values for our behavior experiments. We next examined whether changes in LHb TRPV1 in EtOH-WD rats contributes to the changes in nociception. To characterize the changes in nociception, we measured the paw withdrawal latency (PWL) to thermal stimuli at 24-hour withdrawal from the last drinking session after the 24- session (eight-week) drinking period. There was a significant interaction between the group (Naïve vs. EtOH-WD) and time (baseline vs. eight-week) (Two-way RM ANOVA: F 1, 272 = 52.58, P < 0.0001; fig.5A). The PWL was significantly reduced after 24 drinking sessions in EtOH-WD rats (Post hoc P < 0.001, eight-week vs. baseline), suggesting that chronic intermittent ethanol consumption and withdrawal induce hyperalgesia.
Fig. 5. Capsazepine mitigates hyperalgesia and spontaneous pain in ethanol-withdrawn rats.
(A) The paw withdrawal latency (PWL) to thermal stimuli was significantly reduced in EtOH-WD rats at 24hr withdrawal, in comparison to the Naïve counterparts. $$$P < 0.001 relative to respective baseline. Two-way RM ANOVA followed by Tukey post hoc comparison. (B) Intra-LHb injection of capsazepine (CPZ) significantly increased the nociceptive response in EtOH-WD rats. The change in nociceptive response is expressed as percent maximum peak effect (%MPE). Intra-LHb DNQX significantly increased PWL in both Naïve and EtOH-WD rats. (C) In the place conditioning paradigm, intra-LHb CPZ significantly increased the place conditioning score in EtOH-WD rats; intra-LHb DNQX significantly increased the CPP score in both Naïve and EtOH-WD rats. **P <0.01, ***P < 0.001 relative to respective aCSF treatment; ###P < 0.001 Naïve vs. EtOH-WD rats. The place conditioning score was significantly correlated with %MPE with intra-LHb DNQX (D), and CPZ (E) in EtOH-WD rats.
Inhibition of LHb TRPV1 reduces hyperalgesia in ethanol-withdrawn rats
To investigate the role of LHb TRPV1 on pain during ethanol withdrawal, we examined the PWL 10 min after intra-LHb injection of capsazepine (CPZ, 60 nmol) or capsaicin (CAPS, 6 nmol). To better compare the drug’s effects between the groups, we converted the PWL to the percentage of maximum possible effect (%MPE, see Method section).
Intra-LHb capsazepine or capsaicin produced a significant increase in the %MPE of the PWL in the EtOH-WD, but not the Naive rats, confirmed by two-way ANOVA (main effect of Group: F1,74 = 134.12, P < 0.0001; Treatment: F3,74 = 36.14, P < 0.0001; Group × Treatment interaction: F3,74 = 15.72, P < 0.0001; fig. 5B) followed by Tukey post hoc test (both P < 0.001, Naïve vs. EtOH-WD; both P < 0.001 CPZ/CAPS vs. aCSF). These results suggest that both positive and negative modulations of LHb TRPV1 function can reduce hyperalgesia of EtOH-WD rats. Additionally, intra-LHb AMG9810 significantly decreased the PWL in EtOH-WD rats (Group × Treatment interaction: F1,36 = 87.75, P < 0.001; see Supplementary Digital Content, fig 2A).
Our electrophysiological data indicate that activation of TRPV1 can enhance AMPAR-mediated glutamatergic transmission, we, therefore, investigated the role of AMPARs in the LHb in the pain response. Consistent with our recent report13, intra-LHb injection of AMPAR antagonist DNQX (10 μM, 200 nl/side) induced a significant increase in the %MPE to the thermal stimuli in both EtOH-WD (P < 0.001, DNQX vs. aCSF) and Naïve (P = 0.003) rats, and was significantly greater in the EtOH-WD rats (P < 0.001, Naïve vs. EtOH-WD; fig. 5B). These data indicate that LHb AMPARs play a critical role in nociception, and enhanced glutamate signaling could be responsible for the hyperalgesia during ethanol withdrawal.
Correlation between place conditioning and hyperalgesia
Next, we examined the effect of pharmacological inhibition of LHb TRPV1 channels on the spontaneous pain using a conditioned place paradigm. In terms of the place conditioning score, there was a main effect of group (F1,67 = 85.50, P < 0.0001, fig. 5C), a main effect of treatment (F3,67 = 28.15, P < 0.001), and a significant interaction between group and treatment (F3,67 = 21.79, P < 0.001). EtOH-WD (Post hoc P < 0.001, CPZ vs. aCSF), but not Naïve rats (P = 0.941), spent significantly longer time in the chamber paired with an intra-LHb infusion of capsazepine. Thus, capsazepine induced a conditioned place preference (CPP) in EtOH-WD rats (P < 0.001 vs. Naïve). Additionally, intra-LHb DNQX elicited a strong CPP in both EtOH-WD (P < 0.001 vs. aCSF) and Naïve (P = 0.007) rats and was significantly stronger in the EtOH-WD group (P = 0.001 vs. Naive).
To determine if spontaneous and evoked pain are correlated, we plotted the place conditioning score against the %MPE obtained from the same group of rats (fig. 5D-E). The data indicated a strong, positive correlation between the CPP score and the %MPE of DNQX (fig. 5D) or capsazepine (fig. 5E) in EtOH-WD rats. In Naïve rats, there was a strong, positive correlation between the CPP score and the %MPE of DNQX (fig. 5D), suggesting that inhibition of LHb AMPARs can have an analgesic and rewarding effect. Overall, these results suggest that LHb TRPV1 contributes to both evoked and spontaneous pain in EtOH-WD rats.
LHb TRPV1 channels contribute to anxiety-like behaviors
Recently, we have shown that LHb inhibition can alleviate the elevated anxiety in EtOH-WD rats 8 In this study, we examined the effect of pharmacological manipulation of LHb TRPV1 channels on the anxiety levels of rats using the elevated plus maze (EPM)28. As expected, we observed elevated anxiety levels in EtOH-WD rats reflected by the reduction in time spent in the open arms (main effect of Group × Treatment interaction: F2,34 = 14.72, P < 0.001; P = 0.004 for post hoc comparisons, Naïve vs. EtOH-WD; fig 6A,B) and in open arm entries (Group × Treatment interaction: F2,34 = 8.85; P < 0.001; P < 0.001 for post hoc comparisons; fig 6C) in the EPM. In the EtOH-WD group, intra-LHb capsazepine significantly increased the time spent (main effect of Treatment: F2,34 = 9.43, P <0.001; P < 0.001 vs. aCSF for post hoc comparisons; fig. 6B) and the number of entries (F2,34 = 7.45, P = 0.002; P = 0.002 for post hoc comparisons; fig. 6C) to the open arms, indicating that antagonism of LHb TRPV1 reduces anxiety-like behaviors. Importantly, capsazepine did not alter the total distance traveled in the EPM (main effect of Treatment: F2,27 = 1.43, P = 0.257, fig. 6D), suggesting locomotor activity was not altered. These results were also consistent with pharmacological manipulation of the LHb with alternate antagonist AMG9810 (see Supplementary Digital Content, fig 2B-D).
Fig. 6. Activation of LHb TRPV1 channels contributes to anxiety- and depression-like behaviors.
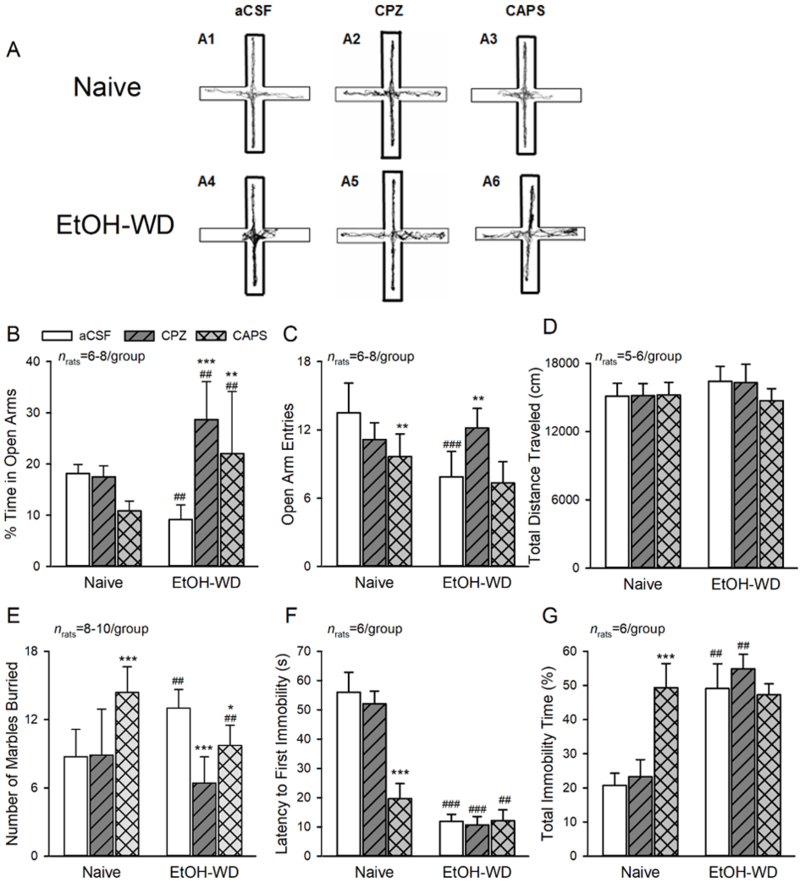
(A-D) Elevated Plus Maze (EPM) Data. (A1, A4) Representative traces show EtOH-WD rats spend less time in open arms compared to Naive rats. In Naïve rats, intra-LHb either capsazepine (CPZ, A2) or capsaicin (CAPS, A3), did not alter the time in (B), and the entries (C) to the open arms. In EtOH-WD rats, CPZ (A5) or CAPS (A6) significantly increased open arm time (B). (D) The total distance traveled in the EPM was not changed. (E) In the marble burying test, EtOH-WD rats buried significantly more marbles than Naïve rats. In Naïve rats, CAPS increased the marbles buried; in EtOH-WD rats, CPZ or CAPS significantly decreased the marbles buried. (F, G), In the forced swimming test, EtOH-WD rats had a significantly shorter latency to first immobility (F) and longer total immobility time (G) compared to naïve rats. CPZ did not alter the latency or total immobility time, while CAPS significantly reduced the latency and increase the immobility time in Naïve rats. *P<0.05, **P<0.01, ***P<0.001 relative to respective aCSF. ##P < 0.01, ###P < 0.001, Naïve in comparison with EtOH-WD rats. Data were analyzed with two-way ANOVA and Tukey post hoc comparison.
There was a significant effect of interaction between group and drug treatment in the marble burying test (MBT) (F2,42 =14.14, P < 0.001; fig. 6E). Naïve rats receiving aCSF infusion buried significant less marbles, compared to EtOH-WD rats, receiving aCSF infusion (Post hoc P = 0.001), further confirmed enhanced anxiety levels in EtOH-WD rats. Moreover, EtOH-WD animals receiving intra-LHb capsazepine (P < 0.001 vs. aCSF) or capsaicin (P = 0.03 vs. aCSF) buried significantly less marbles, while Naïve rats treated with intra-LHb capsaicin buried significantly more marbles (P < 0.001 vs. aCSF). Together, these results suggest that LHb TRPV1 contributes to the anxiety-like behaviors in rats and that LHb TRPV1 function is altered after repeated cycles of ethanol drinking and withdrawal.
LHb TRPV1 involves in depression-like behaviors
The latency of first immobility time (F2,30 = 63.01, P < 0.0001; fig. 6F) and total immobility time (F2,30 = 37.17, P < 0.0001; fig. 6G) of the forced swimming test (FST) were tested using a two-way ANOVA and showed a significant main effect of group × treatment interaction. EtOH-WD rats had a significantly shorter latency to first immobility (Post hoc P < 0.001) and a longer immobility time (P < 0.001) than Naïve rats (fig. 6F, G), indicating EtOH-WD rats displayed depression-like behaviors. In parallel, intra-LHb infusion of capsaicin (CAPS) substantially shortened the latency to first immobility, and prolonged the total immobility time, thus producing depression-like behaviors in Naïve rats (both P < 0.001 vs. aCSF), without changing either the latency to first immobility (P = 0.996) or the total immobility time (P = 0.817) in EtOH-WD rats. Conversely, intra-LHb infusion of capsazepine (CPZ) had no effect on both factors in ether Naïve or EtOH-WD rats. These results suggest that activation of LHb TRPV1 is sufficient to induce depression-like behaviors, but LHb TRPV1 is not involved in the depression-like behaviors during ethanol withdrawal.
Inhibition of LHb TRPV1 channels decreases relapse-like ethanol consumption
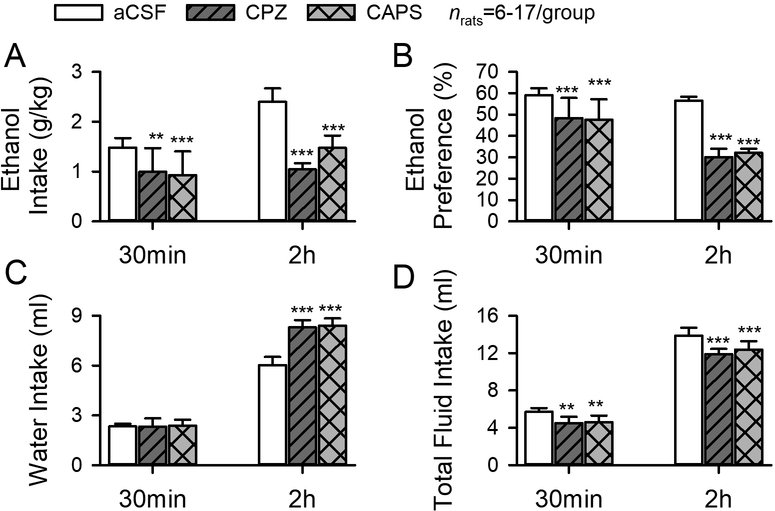
Since the aversive responses during withdrawal may contribute to relapse drinking, we next investigated the impact of LHb TRPV1 channels on ethanol consumption when the rats resumed drinking after 24 hours of withdrawal from the last ethanol session. Intra-LHb infusion of capsazepine or capsaicin significantly decreased ethanol intake, which was paralleled by a significant decrease in preference for ethanol after 30-min and 2-hour access to ethanol (main effect of Treatment: F2,53 = 59.16, P < 0.001; fig. 7A), and a significant increase in water intake at 2 hour (F2,53 = 61.45, P < 0.001; fig. 7C), thus reducing ethanol preference (F2,53 = 102.67, P < 0.001; fig. 7B). The total fluid intake also slightly but significantly reduced (F2,53 =32.36, P < 0.001; fig. 7D).
Fig.7. Intra-LHb capsazepine and capsaicin significantly decreases ethanol intake (A), preference (B), and total fluid intake (D), but increases water intake (C).
**P < 0.01, ***P < 0.001 relative to respective aCSF treatment, two-way ANOVA followed by Tukey post hoc test.
Discussion
While TRPV1 has a well-established role in the pain response, studies are now uncovering its role in psychiatric disorders including drug addiction. This study investigated the role of LHb TRPV1 channels in the aberrant behaviors occurring during ethanol withdrawal. We observed that the frequency of spontaneous firing and sEPSCs of LHb neurons was increased in slices from animals withdrawn from repeated ethanol administration. Importantly, the frequency of these events was reduced by TRPV1 antagonists capsazepine and AMG9810. The reduction was stronger in ethanol-withdrawn than naïve animals. Furthermore, aversive behaviors such as increased nociception sensitivity and anxiety-like behaviors in withdrawn rats were alleviated by intra-LHb capsazepine. This treatment also produced a significant place preference, and reduced ethanol consumption and preference. These results suggest that enhanced TRPV1 function may contribute to these aversive behaviors during withdrawal.
We have previously reported increased frequency of sEPSCs and firing of LHb neurons28 as well as phosphorylated AMPAR expression in the LHb of ethanol-withdrawn rats13, and inhibition of LHb AMPARs reduced anxiety-like behaviors, as well as alcohol intake13. Here, we reported that inhibition of LHb AMPARs elicited both an analgesic effect and place preference suggesting that LHb AMPARs contribute to nociception and reward.
Before this study, the role of TRPV1 in the LHb was unknown. To understand LHb TRPV1 function, we first utilized the competitive antagonist capsazepine 33. Capsazepine significantly reduced sEPSC frequency, suggesting a presynaptic effect18, although a postsynaptic effect may also be involved. This idea is consistent with previous reports suggesting that activation of TRPV1 increases the influxes of Na+ and Ca2+ that facilitate depolarization and release of neurotransmitters34 including glutamate18,35,36. Notably, capsazepine might also inhibit the TRPM8 channel29,30, voltage-gated calcium channels31, and nicotinic acetylcholine receptors. However, the effects of capsazepine we reported here may act through TRPV1, since the more selective TRPV1 antagonist AMG9810 elicited a similar effect.
Remarkably, in LHb neurons of ethanol-withdrawn rats compared with Naïve rats, spontaneous firing was faster, and capsazepine’s inhibition was stronger, suggesting that enhanced TRPV1 function may contribute to LHb hyperactivity. Also, capsazepine suppressed spontaneous firing and sEPSCs, suggesting that LHb TRPV1 is tonically activated37,38 (but see18). The selective TRPV1 agonist, capsaicin enhanced sEPSCs and firing of LHb neurons, but this enhancement was much weaker in ethanol-withdrawn animals, suggesting a higher level of TRPV1 function during ethanol withdrawal. This view is supported the data showing that capsaicin-induced enhancement was reduced in the presence of the endogenous agonist N-Oleoyldopamine in the LHb neurons of Naïve but not withdrawn rats. However, the smaller effect of capsaicin in ethanol-withdrawn animals conflicts with studies in other states which show that when TRPV1 function is enhanced (i.e., inflammatory hyperalgesia) capsaicin responses are increased. The mechanism underlying this confliction is currently unknown. This difference may suggest withdrawal from chronic intermittent alcohol administration induces a specific change in LHb TRPV1.
Procedures based on classical and operant conditioning principles in rodents have been established and validated to study nociception and evaluate the effects of analgesics. They are an important addition to the traditional stimulus-evoked pain measurements39–41. The CPP procedure has been used to study ongoing or spontaneous pain25, centered around the concept that the attenuation of ongoing pain is rewarding. Therefore, we investigated the effect of capsazepine with this procedure. Indeed, capsazepine elicited a significant place preference in ethanol-withdrawn, but not in Naïve rats, suggesting capsazepine attenuated aversiveness of ethanol withdrawal. The AMPAR antagonist DNQX elicited a strong place preference in both Naive and withdrawn animals, suggesting that inhibition of LHb AMPARs is rewarding. The CPP data are consistent with the data of the paw withdrawal latency to thermal stimuli in both groups of rats. Together, these results suggest that inhibition of LHb-TRPV1 could reduce aversiveness induced by withdrawal, which would make LHb TRPV1 a potential therapeutic target for those suffering from alcohol use disorders.
Anxiety is an early withdrawal symptom that develops after cessation of ethanol consumption in alcoholics41,42. While some people may use alcohol to relieve anxiety, the negative effects of this type of self-medication quickly outweigh any of the short-term positive relief one may experience41–44. Therefore, from a medical standpoint, it is best to treat both alcohol abuse and anxiety simultaneously as addressing only alcohol abuse will allow anxiety to reoccur, leading to relapse drinking as a means of coping with the issue. In our study, inhibition of LHb TRPV1 channels with capsazepine or AMG9810, significantly reduced anxiety-like behaviors measured in the elevated plus maze or marble-burying test, but not depression-like behaviors measured in the forced swimming test. Importantly, inhibition of LHb TRPV1 significantly reduced relapse-like drinking. In summary, the data suggest that targeting TRPV1 channels in the LHb could reduce both pain and anxiety-like behaviors resulted from ethanol withdrawal and potentially curb relapse to alcohol.
To our knowledge, there is no information regarding what percent of the LHb neurons are TRPV1 positive. However, our electrophysiological data showed that the more than 80% of LHb neurons in naïve rats (88/101 neurons on sEPSCs, and 63/76 neurons on firing) responded to TRPV1 agents suggesting that TRPV1 is expressed in the majority of the LHb neurons. About 95% of LHb neurons are glutamatergic 45. LHb neurons receive glutamatergic projections from the basal ganglia and prefrontal cortex, and project to midbrain monoaminergic nuclei. Destinations include the dopaminergic ventral tegmental area and substantia nigra, GABAergic rostromedial tegmental nucleus, serotonergic raphe and encephalin-producing periaqueductal grey9,46. This study showed that the activation of TRPV1 increased glutamatergic transmission and activity of LHb neurons. Future studies are needed to investigate the sources of these glutamatergic inputs and the projections of the LHb neurons activated by TRPV1.
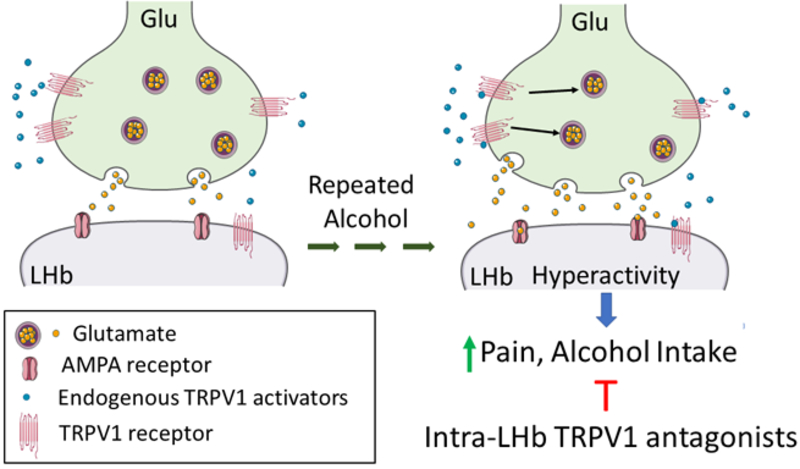
In conclusion, we observed that LHb TRPV1 function increased during ethanol withdrawal which may contribute to increased glutamate transmission and activity of LHb neurons, as well as hyperalgesia and anxiety-like behaviors. Inhibition of LHb TRPV1 alleviated these aberrant behaviors (fig. 8). Thus, LHb TRPV1 channels could be a novel therapeutic target against alcohol use disorders.
Fig. 8. Cartoon demonstrating that repeated alcohol and withdrawal can increase endogenous TRPV1 activity which may increase glutamate (Glu) release and hyperactivity of lateral habenula (LHb) neurons and hyperalgesia and alcohol intake.
Supplementary Material
Acknowledgments:
Tibor Rohacs, M.D., Ph.D. Professor, Department of Pharmacology, Physiology & Neuroscience Rutgers - New Jersey Medical School, 185 S. Orange Avenue, Newark, NJ 07103, For reviewing and offering constructive feedback on the manuscript.
Priscilla White, M.D., Department of Anesthesiology, Rutgers - New Jersey Medical School, 185 S. Orange Avenue, Newark, NJ 07103, For editing the manuscript.
Jing Li, M.D., Ph.D., Research Associate, Department of Anesthesiology, Physiology, and Pharmacology, Rutgers - New Jersey Medical School, 185 S. Orange Avenue, Newark, NJ 07103, For comments on the manuscript.
Seungwoo Kang, Ph.D., Postdoctoral fellow, Department of Anesthesiology, Physiology, and Pharmacology, Rutgers - New Jersey Medical School, 185 S. Orange Avenue, Newark, NJ 07103, For comments on the manuscript.
Ying Li, Ph.D., Research Associate, Department of Pharmacology, Physiology, and Neuroscience, Rutgers - New Jersey Medical School, 185 S. Orange Avenue, Newark, NJ 07103, For comments on the manuscript.
Funding Statement: This work was supported by NIH grants AA021657 and AA022292.
Footnotes
Clinical trial number and registry URL: Not applicable.
Prior Presentations: 1) Society for Neuroscience, November 11–15 2017 at the Walter E. Washington Convention Center in Washington, DC; 2) Research Society for Alcoholism June 25–29 2016, New Orleans, Louisiana.
Conflicts of Interest: The authors declare no conflicts of interest, financial or otherwise.
References
- 1.Egli M, Koob GF, Edwards S: Alcohol dependence as a chronic pain disorder. Neurosci Biobehav Rev 2012; 36: 2179–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koob GF, Simon EJ: The Neurobiology of Addiction: Where We Have Been and Where We Are Going. J Drug Issues 2009; 39: 115–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jhou TC, Good CH, Rowley CS, Xu SP, Wang H, Burnham NW, Hoffman AF, Lupica CR, Ikemoto S: Cocaine drives aversive conditioning via delayed activation of dopamine-responsive habenular and midbrain pathways. J Neurosci 2013; 33: 7501–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haack AK, Sheth C, Schwager AL, Sinclair MS, Tandon S, Taha SA: Lesions of the lateral habenula increase voluntary ethanol consumption and operant self-administration, block yohimbine-induced reinstatement of ethanol seeking, and attenuate ethanol-induced conditioned taste aversion. PLoS One 2014; 9: e92701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Velasquez KM, Molfese DL, Salas R: The role of the habenula in drug addiction. Front Hum Neurosci 2014; 8: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glover EJ, McDougle MJ, Siegel GS, Jhou TC, Chandler LJ: Role for the Rostromedial Tegmental Nucleus in Signaling the Aversive Properties of Alcohol. Alcohol Clin Exp Res 2016; 40: 1651–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zuo W, Fu R, Hopf FW, Xie G, Krnjevic K, Li J, Ye JH: Ethanol drives aversive conditioning through dopamine 1 receptor and glutamate receptor-mediated activation of lateral habenula neurons. Addict Biol 2017; 22: 103–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah A, Zuo W, Kang S, Li J, Fu R, Zhang H, Bekker A, Ye JH: The lateral habenula and alcohol: Role of glutamate and M-type potassium channels. Pharmacol Biochem Behav 2017; 162: 94–102 [DOI] [PubMed] [Google Scholar]
- 9.Graziane NM, Neumann PA, Dong Y: A Focus on Reward Prediction and the Lateral Habenula: Functional Alterations and the Behavioral Outcomes Induced by Drugs of Abuse. Front Synaptic Neurosci 2018; 10: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu R, Gregor D, Peng Z, Li J, Bekker A, Ye J: Chronic intermittent voluntary alcohol drinking induces hyperalgesia in Sprague-Dawley rats. Int J Physiol Pathophysiol Pharmacol 2015; 7: 136–44 [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Fu C, Liu H, Fu R, Zuo W, Kang S, Chen P, Gregor D, Paulose R, Bekker A, Ye JH: Electroacupuncture Attenuates Hyperalgesia in Rats Withdrawn from Chronic Alcohol Drinking via Habenular Mu Opioid Receptors. Alcohol Clin Exp Res 2017; 41: 637–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang S, Li J, Zuo W, Fu R, Gregor D, Krnjevic K, Bekker A, Ye JH: Ethanol Withdrawal Drives Anxiety-Related Behaviors by Reducing M-type Potassium Channel Activity in the Lateral Habenula. Neuropsychopharmacology 2017; 42: 1813–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Kang S, Fu R, Wu L, Wu W, Liu H, Gregor D, Zuo W, Bekker A, Ye JH: Inhibition of AMPA receptor and CaMKII activity in the lateral habenula reduces depressive-like behavior and alcohol intake in rats. Neuropharmacology 2017; 126: 108–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szallasi A, Blumberg PM: Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev 1999; 51: 159–212 [PubMed] [Google Scholar]
- 15.Naziroglu M, Demirdas A: Psychiatric Disorders and TRP Channels: Focus on Psychotropic Drugs. Curr Neuropharmacol 2015; 13: 248–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mezey E, Toth ZE, Cortright DN, Arzubi MK, Krause JE, Elde R, Guo A, Blumberg PM, Szallasi A: Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc Natl Acad Sci U S A 2000; 97: 3655–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yee JR, Kenkel W, Caccaviello JC, Gamber K, Simmons P, Nedelman M, Kulkarni P, Ferris CF: Identifying the integrated neural networks involved in capsaicin-induced pain using fMRI in awake TRPV1 knockout and wild-type rats. Front Syst Neurosci 2015; 9: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Musella A, De Chiara V, Rossi S, Prosperetti C, Bernardi G, Maccarrone M, Centonze D: TRPV1 channels facilitate glutamate transmission in the striatum. Mol Cell Neurosci 2009; 40: 89–97 [DOI] [PubMed] [Google Scholar]
- 19.Blednov YA, Harris RA: Deletion of vanilloid receptor (TRPV1) in mice alters behavioral effects of ethanol. Neuropharmacology 2009; 56: 814–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Bian W, Dave V, Ye JH: Blockade of GABA(A) receptors in the paraventricular nucleus of the hypothalamus attenuates voluntary ethanol intake and activates the hypothalamic-pituitary-adrenocortical axis. Addict Biol 2011; 16: 600–14 [DOI] [PubMed] [Google Scholar]
- 21.Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE: Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res 2008; 32: 1816–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuo W, Chen L, Wang L, Ye JH: Cocaine facilitates glutamatergic transmission and activates lateral habenular neurons. Neuropharmacology 2013; 70: 180–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuo W, Wang L, Chen L, Krnjevic K, Fu R, Feng X, He W, Kang S, Shah A, Bekker A, Ye JH: Ethanol potentiates both GABAergic and glutamatergic signaling in the lateral habenula. Neuropharmacology 2017; 113: 178–187 [DOI] [PubMed] [Google Scholar]
- 24.Wang W, Petralia RS, Takamiya K, Xia J, Li YQ, Huganir RL, Tao YX, Yaster M: Preserved acute pain and impaired neuropathic pain in mice lacking protein interacting with C Kinase 1. Mol Pain 2011; 7: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navratilova E, Xie JY, Okun A, Qu C, Eyde N, Ci S, Ossipov MH, King T, Fields HL, Porreca F: Pain relief produces negative reinforcement through activation of mesolimbic reward-valuation circuitry. Proc Natl Acad Sci U S A 2012; 109: 20709–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cunningham CL, Ferree NK, Howard MA: Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology (Berl) 2003; 170: 409–22 [DOI] [PubMed] [Google Scholar]
- 27.Walf AA, Frye CA: The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc 2007; 2: 322–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Zuo W, Fu R, Xie G, Kaur A, Bekker A, Ye JH: High Frequency Electrical Stimulation of Lateral Habenula Reduces Voluntary Ethanol Consumption in Rats. Int J Neuropsychopharmacol 2016; 19: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weil A, Moore SE, Waite NJ, Randall A, Gunthorpe MJ: Conservation of functional and pharmacological properties in the distantly related temperature sensors TRVP1 and TRPM8. Mol Pharmacol 2005; 68: 518–27 [DOI] [PubMed] [Google Scholar]
- 30.Behrendt HJ, Germann T, Gillen C, Hatt H, Jostock R: Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay. Br J Pharmacol 2004; 141: 737–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Docherty RJ, Yeats JC, Piper AS: Capsazepine block of voltage-activated calcium channels in adult rat dorsal root ganglion neurones in culture. Br J Pharmacol 1997; 121: 1461–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spicarova D, Palecek J: The role of the TRPV1 endogenous agonist N-Oleoyldopamine in modulation of nociceptive signaling at the spinal cord level. J Neurophysiol 2009; 102: 234–43 [DOI] [PubMed] [Google Scholar]
- 33.Bevan S, Hothi S, Hughes G, James IF, Rang HP, Shah K, Walpole CS, Yeats JC: Capsazepine: a competitive antagonist of the sensory neurone excitant capsaicin. Br J Pharmacol 1992; 107: 544–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montell C: Physiology, phylogeny, and functions of the TRP superfamily of cation channels. Sci STKE 2001; 2001: re1. [DOI] [PubMed] [Google Scholar]
- 35.Medvedeva YV, Kim MS, Usachev YM: Mechanisms of prolonged presynaptic Ca2+ signaling and glutamate release induced by TRPV1 activation in rat sensory neurons. J Neurosci 2008; 28: 5295–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shoudai K, Peters JH, McDougall SJ, Fawley JA, Andresen MC: Thermally active TRPV1 tonically drives central spontaneous glutamate release. J Neurosci 2010; 30: 14470–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xing J, Li J: TRPV1 receptor mediates glutamatergic synaptic input to dorsolateral periaqueductal gray (dl-PAG) neurons. J Neurophysiol 2007; 97: 503–11 [DOI] [PubMed] [Google Scholar]
- 38.Li DP, Chen SR, Pan HL: VR1 receptor activation induces glutamate release and postsynaptic firing in the paraventricular nucleus. J Neurophysiol 2004; 92: 1807–16 [DOI] [PubMed] [Google Scholar]
- 39.Shippenberg TS, Stein C, Huber A, Millan MJ, Herz A: Motivational effects of opioids in an animal model of prolonged inflammatory pain: alteration in the effects of kappa- but not of mu-receptor agonists. Pain 1988; 35: 179–86 [DOI] [PubMed] [Google Scholar]
- 40.Sufka KJ: Conditioned place preference paradigm: a novel approach for analgesic drug assessment against chronic pain. Pain 1994; 58: 355–66 [DOI] [PubMed] [Google Scholar]
- 41.Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K: Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry 2004; 61: 807–16 [DOI] [PubMed] [Google Scholar]
- 42.Kushner MG, Sher KJ, Erickson DJ: Prospective analysis of the relation between DSM-III anxiety disorders and alcohol use disorders. Am J Psychiatry 1999; 156: 723–32 [DOI] [PubMed] [Google Scholar]
- 43.Santucci AC, Cortes C, Bettica A, Cortes F: Chronic ethanol consumption in rats produces residual increases in anxiety 4 months after withdrawal. Behav Brain Res 2008; 188: 24–31 [DOI] [PubMed] [Google Scholar]
- 44.Schuckit MA, Hesselbrock V: Alcohol dependence and anxiety disorders: what is the relationship? Am J Psychiatry 1994; 151: 1723–34 [DOI] [PubMed] [Google Scholar]
- 45.Aizawa H, Kobayashi M, Tanaka S, Fukai T, Okamoto H: Molecular characterization of the subnuclei in rat habenula. J Comp Neurol 2012; 520: 4051–66 [DOI] [PubMed] [Google Scholar]
- 46.Fu R, Mei Q, Zuo W, Li J, Gregor D, Bekker A, Ye J: Low-dose ethanol excites lateral habenula neurons projecting to VTA, RMTg, and raphe. Int J Physiol Pathophysiol Pharmacol 2017; 9: 217–230 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.