Abstract
Alzheimer disease (AD) is a progressive neurodegenerative disorder associated with aging and characterized pathologically by the presence of senile plaques, neurofibrillary tangles, and neurite and synapse loss. Amyloid beta-peptide (1–42) [Aβ(1–42)], a major component of senile plaques, is neurotoxic and induces oxidative stress in vitro and in vivo. Redox proteomics has been used to identify proteins oxidatively modified by Aβ(1–42) in vitro and in vivo. In this review, we discuss these proteins in the context of those identified to be oxidatively modified in animal models of AD, and human studies including Familial AD (FAD), Preclinical AD (PCAD), Mild Cognitive Impairment (MCI), Early AD (EAD), Late AD (LAD), Down syndrome (DS) and DS with AD (DS/AD). These redox proteomics studies indicate that Aβ(1–42)-mediated oxidative stress occurs early in AD pathogenesis and results in altered antioxidant and cellular detoxification defenses, decreased energy yielding metabolism and mitochondrial dysfunction, excitotoxicity, loss of synaptic plasticity and cell structure, neuroinflammation, impaired protein folding and degradation, and altered signal transduction. Improved access to biomarker imaging and the identification of lifestyle interventions or treatments to reduce Aβ production could be beneficial in preventing or delaying the progression of AD.
Keywords: Redox proteomics, Alzheimer disease, amyloid β-peptide, oxidative stress
Graphical Abstract
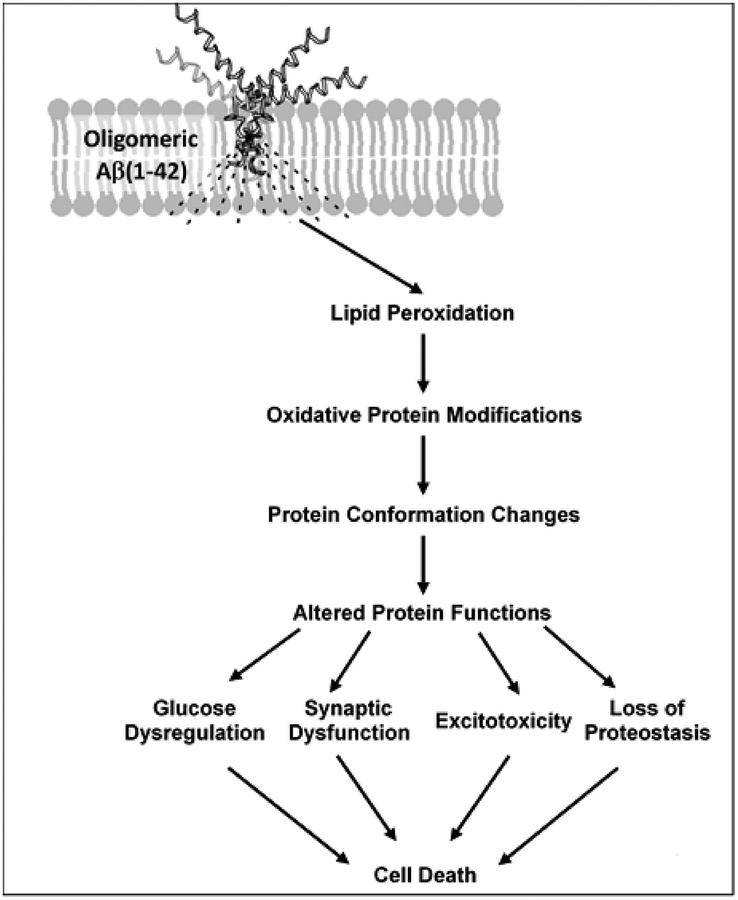
In this review we discuss how redox proteomics provided insights into how Aβ(1–42)-mediated cell death in Alzheimer disease (AD) and its earlier stage, amnestic mild cognitive impairment (MCI), and in in vitro and in vivo models thereof, is initiated by lipid peroxidation. Oligomeric Aβ(1–42) embeds in the lipid bilayer inducing lipid peroxidation, leading to reactive 4-hydroxy-2-nonenal (HNE). HNE covalently binds Cys, His, and Lys residues on proteins by Michael addition, changing the proteins’ conformation and decreasing their function. Key processes in brain that are damaged and dysfunctional as a result of Aβ(1–42)-mediated HNE modification identified by redox proteomics are glucose metabolism, synaptic plasticity, synaptic glutamate clearance, and proteostasis, ultimately leading to neuronal death.
Alzheimer Disease (AD) is a neurodegenerative disorder characterized by changes in brain chemistry associated with cognitive decline and aging. AD is the most common cause of dementia (Wilson et al. 2012; Barker et al. 2002) and is currently the 6th leading cause of death in the United States (US). It is estimated that there are over 5.7 million Americans currently living with AD and of those 5.5 million are age 65 or older; however, with the aging baby boomer population, it is projected that the number of Americans age 65 or older living with AD will increase to 7.1 million by 2025 and to 13.8 million by 2050. Additionally, due to medical advances, it is also expected that the population of Americans living beyond age 85 will increase subsequently increasing the population most at risk of developing AD. Given that no definitive cause, treatment, preventative, or cure exists for AD, the US is facing a growing public health crisis as national costs associated with AD are projected to increase from $277 billion to $1.1 trillion by 2050 (Alzheimer’s Association 2018).
One percent or fewer of AD cases are familial, due to inherited genetic mutations (Bekris et al. 2010), meaning that the overwhelming majority of cases are sporadic and likely due to a concert of multiple factors. The greatest risk factors for developing AD include age, family history, and apolipoprotein E (ApoE) genotype. Three allelic forms of ApoE exist: ε2, ε3, and ε4. ε3 is the most common form followed by ε4, and finally ε2 (Mahley and Rall 2000). ε4 is associated with an increased risk of developing AD with heterozygotes having a three times greater risk and homozygotes having an eight to twelve times greater risk than those homozygous in ε3 (Loy et al. 2014; Holtzman et al. 2012). While the ApoE ε4 is associated with an increased risk of developing AD, it does not guarantee that one will develop AD (Chouraki et al. 2014). Those with the ε2 form have a decreased risk compared to those with the ε3 form (Loy et al. 2014; Holtzman et al. 2012). Collectively, age, family history, and genetics are factors beyond our control; however, there are also a number of risk factors that can be influenced by lifestyle modifications over which we do have control. Obesity (Rönnemaa et al. 2011; Loef et al. 2013; Anstey et al. 2011; Gottesman et al. 2017a), diabetes (Wu et al. 2008; Gudala et al. 2013; Vagelatos et al. 2013; Reitz et al. 2011), impaired glucose metabolism (Rönnemaa et al. 2011; Crane et al. 2013), hyperlipidemia (Solomon et al. 2009; Meng et al. 2014), and hypertension (Launer et al. 2000; Ninomiya et al. 2011; Debette et al. 2011; Livingston et al. 2017; Gottesman et al. 2017b) are all associated with increased risk of developing AD. Increased physical activity (Willis et al. 2012; Tan et al. 2017; Wiley et al. 2016; Stephen et al. 2017; Blondell et al. 2014; Koscak 2017) and consuming a heart-healthy diet are associated with a decreased risk of developing AD (Barberger-Gateau et al. 2007; Hardman et al. 2016; Lourida et al. 2013; Morris et al. 2015a; Morris et al. 2015b; Féart et al. 2010; Solfrizzi et al. 2011). Recent studies indicate that brain changes associated with AD may begin 20 or more years before the onset of cognitive symptoms (Villemagne et al. 2013; Reiman et al. 2012; Jack et al. 2009; Bateman et al. 2012) suggesting that lifestyle interventions that decrease midlife obesity, diabetes, hypertension, and high cholesterol could potentially reduce the prevalence, or delay the onset, of Alzheimer’s disease. Delay of onset by 5 years is estimated to reduce costs by 33 (Alzheimer’s Association 2015) to 39 percent (Zissimopoulos et al. 2014).
Extraneuronal deposition of amyloid beta-peptide (Aβ), measured by positron emission tomography (PET), precedes (Aizenstein et al. 2008; Mintun et al. 2006; Rowe et al. 2010) and positively correlates with cognitive decline and cerebral atrophy (Villemagne et al. 2013). Aβ is a 40- to 42-amino acid peptide derived from amyloid precursor protein (APP) by the concerted proteolytic action of β-secretase at the amino-terminus and γ-secretase at the carboxy-terminus. Aβ(1–42), which comprises the majority of Aβ found in senile plaques in AD brain (Selkoe 2001), is the more toxic of these species (Butterfield 1997; Butterfield et al. 2002; Copani et al. 1991; Walsh and Teplow 2012) with several studies indicating that, rather than fibrils, small soluble oligomers are the toxic form of Aβ (Drake et al. 2003; Lambert et al. 2001; Oda et al. 1995; Walsh et al. 1999). Genetic mutations that lead to familial AD (FAD) involve the genes encoding presenilin-1 or presenilin-2, which comprise the catalytic element of γ-secretase, or APP and all subsequently increase the production of Aβ(1–42) (Selkoe 2001; Goate and Hardy 2012; Jarrett et al. 1993; Scheuner et al. 1996). Down Syndrome (DS) lends further genetic evidence as to the importance of APP and the consequential role of Aβ in AD pathogenesis. DS patients have three copies of chromosome 21 which contains the gene encoding APP resulting in increased production of Aβ (Beyreuther et al. 1993) which initially accumulates intracellularly, but then decreases intracellularly as extracellular Aβ deposits form (Gyure et al. 2001; Mori et al. 2002). If DS patients live long enough, they develop brain pathology and cognitive symptoms indistinguishable from AD (Wisniewski et al. 1985; Lott and Head 2005; Lott and Dierssen 2010).
Progression of AD can be viewed as a continuous spectrum consisting of four stages: preclinical AD (PCAD), mild cognitive impairment (MCI), early AD (EAD), and late-stage AD (LAD). IN PCAD, the patient is fully functional, but brain pathology changes – which can be divided into three stages – have begun to develop unbeknownst to the patient (Dubois et al. 2016; Vos et al. 2013; Pietrzak et al. 2015; Small et al. 2001; Sperling et al. 2011; Vlassenko et al. 2012). Stage 1 is classified by the presence of abnormal Aβ biomarkers, stage 2 is classified by the presence of abnormal Aβ biomarkers and neuronal injury, and stage 3 is an extension of stage 2 in which subtle cognitive changes are present (Sperling et al. 2011). MCI represents the transition between PCAD and EAD in which cognitive decline is noticeable but does not interfere with activities of daily living. MCI is further subdivided into amnestic MCI (aMCI) and non-amnestic MCI with aMCI having a higher conversion rate to AD (Kelley and Petersen 2007; Petersen 2000, 2004). EAD and LAD are marked by noticeable and progressive cognitive decline and behavioral changes that significantly impact the ability of the patient to carry out everyday functions. Imaging studies document progressive neuropathology and neurodegeneration as the disease advances from EAD into LAD (Drayer et al. 1985; Farrow et al. 2007; Jack et al. 1999, 2018; Rowe et al. 2010; Villemagne et al. 2013).
Elevated oxidative stress is implicated in the pathogenesis of AD (Butterfield et al. 2013; Swomley and Butterfield 2015) and is also detected in DS brain (Cenini et al. 2012; Jovanovic et al. 1998; Perluigi and Butterfield 2011, 2012). Aβ(1–42) induces oxidative stress in vitro and in vivo (Hensley et al. 1994; Butterfield et al. 1999; Butterfield and Boyd-Kimball 2004, 2018; Sultana et al. 2009) and, in AD brain. There are many hypotheses regarding the mechanism by which Aβ(1–42)-mediated oxidative stress plays a causative role in the progression of AD (Cheignon et al. 2018); however, our laboratory proposed that oligomers of Aβ(1–42) insert into the bilayer initiating lipid peroxidation (Butterfield and Lauderback 2002; Butterfield et al. 2001; Mark et al. 1997; Markesbery 1997; Mattson 1997) and the increased protein, lipid, DNA, and RNA oxidation observed in AD brain (Butterfield and Lauderback 2002; Butterfield and Stadtman 1997; Smith et al. 1997; Hensley et al.1995; Markesbery and Lovell 1998; Zhang et al. 1994; Mecocci et al. 1994; Gabbita et al. 1998; Shan and Lin 2006; Ding et al. 2006; Butterfield and Boyd-Kimball, 2018). Redox proteomics studies have allowed insight into the specific proteins susceptible to oxidation. Biomarkers of oxidative modification include protein carbonyls and the lipid peroxidation by-product, 4-hydroxy-2-trans-nonenal (HNE), and a marker of nitration, 3-nitrotyrosine (3-NT), which have been the foci of most redox proteomics analyses relevant to AD to date (Subramaniam et al. 1997; Butterfield and Perluigi 2017; Swomley et al. 2014; Sultana and Butterfield 2009, 2011; Sultana et al. 2009). Such irreversible oxidative protein modifications induced by Aβ(1–42) initiate conformation changes resulting in altered or lost function with consequences toward disease progression (Butterfield et al. 2007; Butterfield and Boyd-Kimball 2018). Reversible oxidative modifications, including S-nitrosylation (Nakamura et al. 2013) and cysteine oxidation (Gu and Robinson 2016), may also provide insights into pathogenesis; however, the focus of this review is irreversible oxidative modifications associated with Aβ(1–42) indexed by protein carbonyls, HNE and 3-NT as we summarize our current knowledge of Aβ(1–42)-induced protein oxidation in vitro and in vivo as evidenced by redox proteomics. Here, we first highlight the proteins identified by redox proteomics as oxidatively modified in these models, relate these same proteins that also are oxidized in aMCI and AD brain, and then discuss the implications of such modifications in the context of our current knowledge of AD.
In vitro Aβ(1–42)-induced protein oxidation
Currently, redox proteomics studies of the in vitro effects of Aβ(1–42) have focused solely on protein carbonylation (Table 1). In cortical synaptosomes incubated with Aβ(1–42), β-actin, glial fibrillary acid protein (GFAP), and dihydropyrimidinase-related protein-2 (DRP-2 also called collapsin response mediated protein-2, CRMP-2) were found to be significantly carbonylated (Boyd-Kimball et al. 2005a); however, in primary neuronal culture incubated with Aβ(1–42) 14-3-3ζ, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), pyruvate kinase (PK), and malate dehydrogenase (MDH) were found to be increasing carbonylated (Boyd-Kimball et al. 2005b; Sultana et al. 2006d). Proteins identified as oxidized by Aβ(1–42) in vitro are consistent with proteins identified to be oxidized in AD and aMCI brain (Castegna et al. 2002a,b, 2003; Aksenov et al. 2001; Sultana et al. 2006a,c, 2007; Butterfield et al. 2006a; Reed et al. 2008).
Table 1:
Aβ(1–42)-Induced Carbonylated Proteins in vitro
| Function | Protein | Model | References |
|---|---|---|---|
| Energy Metabolism | Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | primary neuronal culture | Boyd-Kimball et al. 2005b; Sultana et al. 2006d |
| Malate dehydrogenase (MDH) | primary neuronal culture | Sultana et al. 2006d | |
| Pyruvate kinase (PK) | primary neuronal culture | Sultana et al. 2006d | |
| Synaptic plasticity, vesicle-mediated transport, and cell structure | Dihydropyrimidinase-related protein 2 (DRP-2) | cortical synaptosomes | Boyd-Kimball et al. 2005a |
| β-actin | cortical synaptosomes | Boyd-Kimball et al. 2005a | |
| Neuroinflammation | Glial fibrillary acidic protein (GFAP) | cortical synaptosomes | Boyd-Kimball et al. 2005a |
| Signal transduction | 14-3-3ζ | primary neuronal culture | Boyd-Kimball et al. 2005b; Sultana et al. 2006d |
In vivo Aβ(1–42)-induced protein oxidation: Models of Alzheimer’s Disease
Various models of the in vivo effects of Aβ(1–42) have been studied using redox proteomics (Table 2). One such model includes the intracerebral ventricular (ICV) injection of Aβ(1–42) into the nucleus basalis magnocellularis (NBM) of 3-month-old male Wistar rats. Seven days post-injection, 14-3-3ζ and chaperonin 60 (HSP60) were found to be significantly carbonylated in the NBM. Notably, this modeled also showed that the oxidative damage induced by Aβ(1–42) could spread beyond the site of injection resulting in the significant carbonylation of α- and β-tubulin and glutamine synthetase (GS) in the cortex, and 14-3-3ζ, β-synuclein, pyruvate dehydrogenase (PDH), GAPDH, and phosphoglycerate mutase 1 (PGM1) in the hippocampus (Boyd-Kimball et al. 2005c).
Table 2:
Aβ(1–42)-Induced Carbonylated Proteins in vivo
| Function | Protein | Model | Tissue | References |
|---|---|---|---|---|
| Antioxidants and Cellular Detoxification Defenses | Glutathione S-Transferase | Canineb | -- | Opii et al. 2008 |
| C. elegansb | -- | Boyd-Kimball et al. 2006 | ||
| Adenosine kinase | C. elegansb | -- | Boyd-Kimball et al. 2006 | |
| Arginine kinase | C. elegansb | -- | Boyd-Kimball et al. 2006 | |
| Glutamate dehydrogenase | Canineb | -- | Opii et al. 2008 | |
| Lipid binding protein | C. elegansb | -- | Boyd-Kimball et al. 2006 | |
| Energy Metabolism | acyl-CoA DH (medium chain) | C. elegansb | -- | Boyd-Kimball et al. 2006 |
| acyl-CoA DH (short-chain) | C. elegansb | -- | Boyd-Kimball et al. 2006 | |
| α-enolase | Canineb | -- | Opii et al. 2008 | |
| Glyceraldehyde-3-phosphate dehydrogenase | Canineb | -- | Opii et al. 2008 | |
| Wistar Rata | HP | Boyd-Kimball et al. 2005c | ||
| Malate dehydrogenase | C. elegansb | -- | Boyd-Kimball et al. 2006 | |
| Phosphoglycerate mutase 1 | Wistar Rata | HP | Boyd-Kimball et al. 2005c | |
| Pyruvate dehydrogenase (PDH) (lipoamide) | Wistar Rata | HP | Boyd-Kimball et al. 2005c | |
| Transketolase | C. elegansb | -- | Boyd-Kimball et al. 2006 | |
| Excitotoxicity | Glutamine Synthetase | Wistar Rata | Cortex | Boyd-Kimball et al. 2005c |
| Synaptic plasticity, vesicle-mediated transport, and cell structure | actin | C. elegansb | -- | Boyd-Kimball et al. 2006 |
| β-synuclein | Wistar Rata | HP | Boyd-Kimball et al. 2005c | |
| Fascin actin bundling protein | Canineb | -- | Opii et al. 2008 | |
| Myosin regulatory light chain | C. elegans | -- | Boyd-Kimball et al. 2006 | |
| Neurofilament triplet L | Canineb | -- | Opii et al. 2008 | |
| α-/β-tubulin | Wistar Rata | Cortex | Boyd-Kimball et al. 2005c | |
| Protein Synthesis | Elongation factor 1 γ | C. elegansb | -- | Boyd-Kimball et al. 2006 |
| Protein Folding and Degradation | HSP60 | Wistar Rata | NBM | Boyd-Kimball et al. 2005c |
| Proteasome α-subunit | C. elegansb | -- | Boyd-Kimball et al. 2006 | |
| Proteasome β-subunit | C. elegansb | -- | Boyd-Kimball et al. 2006 | |
| Signal Transduction | 14-3-3ζ | Wistar Rata | HP, NBM | Boyd-Kimball et al. 2005c |
Note: HP, hippocampus; NBM, nucleus basalis magnocellaris
ICV injection of Aβ(1–42) into the NBM with analysis 7 days post injection;
expresses Aβ(1–42) with human amino acid sequence.
C. elegans expressing human Aβ(1–42) were previously reported to cause elevated protein carbonyls correlated with aggregated Aβ(1–42) (Yatin et al. 1999). Subsequent studies used a mRNA surveillance system to degrade foreign mRNA at permissive (regular) growth temperatures. The non-permissive elevated growth temperature caused the surveillance system to be inactive, resulting in expression of Aβ(1–42) for any given time chosen by the experimenter. Return to permissive temperature stopped Aβ(1–42) expression. Expression of Aβ(1–42) for 24 h led to elevated oxidative damage (protein carbonyls) in the worm that was correlated with paralysis, since a muscle wall promoter was used to express Aβ(1–42), and there was absence of Aβ fibril formation, consistent with the notion that oligomers of Aβ(1–42) inserted into the muscle cell membranes led to oxidative stress (Drake et al. 2003). Redox proteomics analysis of this C. elegans strain identified both short and medium chain acyl-CoA dehydrogenase (acyl CoA DH), elongation factor 1 γ (Ef-1 γ), MDH, arginine kinase (AK), RACK1 ortholog, myosin regulatory light chain (MRLC), actin, adenosine kinase (ADK), lipid binding protein (LBP), transketolase (TK), proteasome α- and β-subunit, and glutathione-S-transferase (GST) to be significantly carbonylated (Boyd-Kimball et al. 2006).
The canine model of the aged beagle deposits Aβ(1–42) identical to the human amino acid sequence as a function of age (Miligram et al. 2004; Head 2013). Long-term administration of an antioxidant-rich diet and behavioral enrichment resulted in the significantly decreased carbonylation of glutamate dehydrogenase (GDH), GAPDH, α-enolase, neurofilament triplet L, GST P, and fascin actin bundling protein compared to age-matched control (Opii et al. 2008). These findings support a role for lifestyle intervention as a therapeutic approach to prevent or delay Aβ(1–42) induced oxidative stress.
Redox proteomics has also been utilized to analyze several transgenic or knock-in rodent models of AD including the APP/PS-1 double human mutant knock-in mouse (the APP/PS-1 mouse), the triple transgenic AD mouse (3×Tg-AD), the senescence accelerated mouse P8 (SAMP8), and the J20 mouse (Table 3). The APP/PS1 double human knock-in mouse is the APPNLh/NLh × PS-1P264L/P264L strain which expresses normal levels of human APP, but significantly increased levels of Aβ(1–42) prior to two months of age. Aβ deposition occurs by 6 months of age and increases linearly as a function of age (Flood et al. 2002) similar to Aβ solubility and deposition exhibited in human brain during the progression of AD (Murphy et al. 2007). This age-dependent increase in Aβ(1–42) production correlates with cognitive impairment (Bruce-Keller et al. 2011; Webster et al. 2013), oxidative stress (Abdul et al. 2008; Huang et al. 2010), decreased manganese superoxide dismutase (MnSOD or SOD2) activity and mitochondrial respiration (Anantharaman et al. 2006; Sompol et al. 2008), and loss of phospholipid asymmetry (Bader Lange et al. 2010). Redox proteomics was used to analyze the oxidative modification of proteins, as indexed by protein carbonyls, in the brain of APPNLh/NLh × PS-1P264L/P264L mice as a function of age compared to age-matched wild-type control. Proteins identified to be significantly oxidized can be broadly classified as structural, regulatory, synaptic, and involved in glucose metabolism. The structural protein β-actin was found to be significantly oxidized at 1 month and 15 months of age while the synaptic protein β-synuclein was found to be significantly oxidized at 12 months of age. The regulatory proteins identified were 14-3-3ζ/δ/γ and peptidylprolyl cis-trans isomerase-1 (Pin1). 14-3-3ζ/δ/γ was found to be significantly oxidized at 1, 9, 12, and 15 months, while Pin-1 was found to be significantly oxidized at 9, 12 and 15 months of age. α-enolase, pyruvate dehydrogenase E1 (PDH), and ATP synthase α-subunit – all proteins involved in generating cellular energy – were determined to be significantly oxidized. α-enolase was found to be significantly oxidized at 6, 9, 12, and 15 months and ATP synthase was found to be significantly oxidized at 12 and 15 months while PDH was found to be significantly oxidized only at 6 months of age (Sultana et al. 2011a).
Table 3:
Oxidatively Modified Proteins Identified in AD mouse models
| Function | Protein | Transgenic Mouse Model | Age (months) | Oxidative Modification | References |
|---|---|---|---|---|---|
| Cellular Redox Homeostasis and Detoxification Defense | Peroxiredoxin 2 (Prx2) | SAMP8a | 12 | C↓ | Poon et al. 2005 |
| Energy Metabolism | Aconitase | 3×Tg-AD | 3 | C↑ | Shen et al. 2015 |
| Aldolase | SAMP8a | 12 | C↓ | Poon et al. 2005 | |
| ATP Synthase | APP/PS1 KI | 12,15 | C↑ | Sultana et al. 2011a | |
| Creatine Kinase (CK) | SAMP8 | 12 | C↑ | Poon et al. 2004 | |
| α-enolase | APP/PS1 KI | 6,9,12,15 | C↑ | Sultana et al. 2011a | |
| 3×Tg-AD | 3 | C↑ | Shen et al. 2015 | ||
| SAMP8b | 12 | N↑ | Fiorini et al. 2013 | ||
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | 3×Tg-AD | 3 | C↑ | Shen et al. 2015 | |
| Isocitrate dehydrogenase (IDH) | SAMP8b | 12 | N↑ | Fiorini et al. 2013 | |
| Lactate dehydrogenase (LDH) | SAMP8b | 12 | N↑ | Fiorini et al. 2013 | |
| SAMP8 | 12 | C↑ | Poon et al. 2004 | ||
| Malate dehydrogenase (MDH) | 3×Tg-AD | 3 | C↑ | Shen et al. 2015 | |
| Phosphoglycerate kinase (PGK) | 3×Tg-AD | 3 | C↑ | Shen et al. 2015 | |
| Pyruvate dehydrogenase (PDH) (E1) | APP/PS1 KI | 6 | C↑ | Sultana et al. 2011a | |
| Synaptic plasticity, vesicle-mediated transport and cell structure | β-synuclein | APP/PS1 KI | 12 | C↑ | Sultana et al. 2011a |
| Dihydropyrimidinase-related protein 1 (DRP-1) | SAMP8b | 12 | N↑ | Fiorini et al. 2013 | |
| Dihydropyrimidinase-related protein 2 (DRP-2) | SAMP8 | 12 | C↑ | Poon et al. 2004 | |
| SAMP8b | 12 | N↓ | Fiorini et al. 2013 | ||
| Dynamin-1 | SAMP8b | 12 | N↑ | Fiorini et al. 2013 | |
| Vesicle-fusing ATPase | SAMP8b | 12 | N↑ | Fiorini et al. 2013 | |
| Septin 11 | SAMP8b | 12 | N↑ | Fiorini et al. 2013 | |
| Neuropolypeptide h3 (PEBP1) | J20 | 9 | N↓ | Robinson et al. 2011 | |
| β-Actin | APP/PS1 KI | 1,15 | C↑ | Sultana et al. 2011a | |
| Actin related protein 2 | SAMP8b | 12 | N↑ | Fiorini et al. 2013 | |
| Coronin1A | SAMP8a | 12 | C↓ | Poon et al. 2005 | |
| Neurofilament light polypeptide | 3×Tg-AD | 3 | C↑ | Shen et al. 2015 | |
| α-Spectrin 2 | SAMP8 | 12 | C↑ | Poon et al. 2004 | |
| Tubulin β−2A chain | 3×Tg-AD | 3 | C↑ | Shen et al. 2015 | |
| Neuroinflammation | GFAP | SAMP8b | 12 | N↑ | Fiorini et al. 2013 |
| Protein Folding and Degradation | HSP90AB1 | 3×Tg-AD | 3 | C↑ | Shen et al. 2015 |
| Protein disulfide-isomerase A3 | 3×Tg-AD | 3 | C↑ | Shen et al. 2015 | |
| T-complex protein 1 subunit ε | 3×Tg-AD | 3 | C↑ | Shen et al. 2015 | |
| Signal Transduction | 14-3-3ζ/δ/γ | APP/PS1 KI | 1,9,12,15 | C↑ | Sultana et al. 2011a |
| 14-3-3ζ/δ | 3×Tg-AD | 3 | C↑ | Shen et al. 2015 | |
| Pin1 | J20 | 9 | N↓ | Robinson et al. 2011 | |
| APP/PS1 KI | 9,12,15 | C↑ | Sultana et al. 2011a |
Note: C, carbonylation; N, nitration;
oxidative modification was significantly increased;
oxidative modification was significantly decreased;
Antisense Oligonucleotide directed against Aβ region of APP gene;
Antisense Oligonucleotide directed against PS-1 gene;
The 3×Tg-AD mouse model develops age related progressive neuropathology including senile plaques, neurofibrillary tangles, and synaptic dysfunction (Oddo et al. 2003a). Increased intracellular Aβ is detected by 3 months of age with cognitive impairment observed at 4 months of age (Billings et al. 2005) and extracellular deposition occurs by 6 months of age (Oddo et al. 2003a,b). Redox proteomics analysis of carbonylated proteins in the hippocampus of 3 month-old 3×Tg-AD mice identified 14-3-3ζ/γ, mitochondrial aconitase, GS, GAPDH, α-enolase, phosphoglycerate kinase 1 (PGK1), heat shock protein 90-β, mitochondrial MDH, neurofilament light polypeptide, protein disulfide-isomerase A3, T-complex protein 1 subunit ε, and tubulin β−2A chain to be significantly oxidized compared to age-matched non-transgenic controls (Shen et al. 2015).
SAMP8 mice are a model for gerontological and age-related dementia research that exhibit learning and memory deficits with age (Flood and Morley 1993, 1998). These changes correlate with increased oxidative stress (Butterfield et al. 1997; Farr et al. 2003; Poon et al., 2005; Butterfield and Poon 2005) starting as early as 4 months. SAMP8 mice exhibit an age-related increase in APP (Morley et al. 2000; Nomura et al. 1996) and hippocampal Aβ peptide (Kumar et al. 2000; Takeda 2009) depositing as granules in the hippocampus by 6 months of age (Takemura et al. 1993; Del Valle et al. 2010;) with plaque formation late in life at 20 months (Morley et al. 2000). Cognitive deficits occur prior to plaque formation supporting the role for small, soluble oligomers of Aβ inducing oxidative damage resulting in learning and memory impairments. Consequently, the SAMP8 mouse is considered a model of oxidative stress and AD (Morley et al. 2012). Redox proteomics analysis of the brain of 12 month old SAMP8 mice identified lactate dehydrogenase 2 (LDH-2), creatine kinase (CK), DRP-2 (CRMP-2), and α-spectrin 2 to be significantly oxidized as indexed by protein carbonyls compared to 4 month old SAMP8 mice (Poon et al. 2004a). Antisense oligonucleotide (AO) directed against the Aβ region of APP gene in aged SAMP8 mice significantly decreased APP protein levels, reversed learning and memory deficits (Kumar et al. 2000) and significantly decreased brain Aβ levels and oxidative stress biomarkers compared to age-matched SAMP8 mice that received a random AO (Poon et al. 2004b). In this model, carbonylation of aldolase 3 (Aldo 3), coronin 1a (Coro 1a), and peroxiredoxin 2 (Prx2) was found to be significantly decreased in the 12-month-old SAMP8 mice that received the AO directed against the Aβ region of APP gene (Poon et al. 2005). Another study determined that the age-related increase in APP and Aβ may be due to an age-related increase in PS-1 protein level in the hippocampus of SAMP8 mice (Kumar et al. 2009). AO directed against the PS-1 gene in 12 month old SAMP8 mice resulted in significantly decreased PS-1 protein levels, oxidative stress parameters, and reversed learning and memory deficits in the hippocampus. In this model, nitration of LDH, DRP-1 (CRMP-1), dynamin-1 (Dyn-1), GFAP, vesicle-fusing ATPase (NSF), α-enolase, isocitrate dehydrogenase (IDH), actin-related protein 2 (Actr2), and septin-11 (SEP11) was significantly decreased in SAMP8 mice receiving AO directed against the PS-1 gene while nitration of DRP-2 (CRMP-2) and peptidase α (DPL2) was significantly increased (Fiorini et al. 2013).
The J20 mouse model expresses human APP with the Swedish (670/671KM->NL) and Indiana (717V->F) FAD mutations generating increased Aβ(1–42) by 2 months of age with plaques forming by 5 months of age (Mucke et al. 2000). This increase in soluble Aβ(1–42) correlates with a decrease in presynaptic terminals (Mucke et al. 2000) and an increase in oxidative stress (Butterfield et al. 2010) and learning and memory deficits (Mucke et al. 2000; Galvan et al. 2006; Harris et al. 2010) that again supports the role of soluble Aβ(1–42)-induced oxidative stress in synaptic toxicity in AD. Redox proteomics analysis has shown decreased nitration of phosphatidylethanolamine-binding protein 1 (PEBP-1) and Pin1 in brain of 9-month-old J20 mice compared to the age-matched non-transgenic control (Robinson et al. 2011).
Collectively, redox proteomics analyses of in vivo models of Aβ(1–42)-induced oxidative stress can be categorized as involved in cellular redox homeostasis and detoxification defense, glucose metabolism, synaptic plasticity, vesicle-mediated transport and cell structure, neuroinflammation, protein folding and degradation, and signal transduction and are consistent with reports of such functional proteins being oxidatively modified and usually dysfunctional in aMCI and AD brains (Sultana et al. 2006a,c, 2007; Reed et al. 2008; Aluise et al. 2011; Castegna et al. 2002a,b, 2003; Aksenov et al. 2001; Butterfield et al. 2006a).
In vivo Aβ(1–42)-induced protein oxidation: PCAD, MCI, AD, and DS Human Studies
Redox proteomics analyses were conducted to identify oxidatively modified proteins in brain representing all stages of the AD continuum and DS (Table 4). In PCAD inferior parietal lobule (IPL), a significant increase in insoluble monomeric Aβ(1–42) is observed compared to control, but no difference in oligomeric Aβ or oxidative stress biomarkers were detected (Aluise et al. 2010). MCI IPL, however, exhibits increased protein carbonyls compared to PCAD IPL. Redox proteomics analysis showed α-enolase and heat shock protein 90 (HSP90) to be significantly carbonylated in MCI IPL compared to PCAD IPL (Aluise et al. 2011). Carbonic anhydrase II (CA II), heat shock protein 70 (HSP70), mitogen-activated protein kinase I (MAPKI), and syntaxin binding protein I (SBP1) are also significantly carbonylated in MCI IPL compared to age-matched control (Sultana et al. 2010). In MCI hippocampus, α-enolase, GS, pyruvate kinase M2 (PKM2), and Pin1 were identified to be significantly carbonylated compared to age-matched control (Butterfield et al. 2006a). HNE-bound protein modifications have also been assessed in MCI hippocampus and IPL. In the hippocampus, α-enolase, LDH, PGK, HSP70, carbonyl reductase, and neuropolypeptide h3 (PEBP1) had significantly increased levels of protein-bound HNE while β-actin, PK, initiation factor α (eIF-α), and elongation factor Tu (EF-Tu) were identified in MCI IPL. ATP synthase was found to have increased levels of protein-bound HNE in both MCI hippocampus and IPL (Reed et al. 2008). Protein nitration in MCI IPL and hippocampus has also been assessed by redox proteomics. α-enolase was found to be significantly nitrated in both MCI IPL and hippocampus while glucose related protein precursor, aldolase, GST mu (GSTM3), multidrug resistant protein (MRP3), and 14-3-3γ were significantly nitrated in MCI IPL and peroxiredoxin 6 (Prx6), DRP-2 (CRMP-2), fascin 1, and HSP70 isoform A8 were significantly nitrated in MCI hippocampus (Sultana et al. 2007).
Table 4:
Oxidatively Modified Proteins Identified in aMCI, EAD, LAD, DS, and DS/AD Brain
| Protein | Disease State | Brain Region | Oxidative Modification | References |
|---|---|---|---|---|
| Cellular Redox Homeostasis and Detoxification Defense | ||||
| Carbonyl reductase | MCIa | HP | H | Reed et al. 2008 |
| Glutamate dehydrogenase (GDH) | DSa | FC | H | Di Domenico et al. 2014 |
| DS/ADa,b | FC | H | Di Domenico et al. 2014 | |
| Glutathione S-Transferase (GST) Mu | MCIa | IPL | N | Sultana et al. 2007 |
| Multidrug resistance protein 3 (MRP3) | MCIa | IPL | N | Sultana et al. 2007 |
| Peroxiredoxin 2 (Prx2) | DSa | FC | H | Di Domenico et al. 2014 |
| Peroxiredoxin 6 (Prx6) | MCIa | HP | N | Sultana et al. 2007 |
| LADa | HP | H | Perluigi et al. 2009 | |
| Cu,Zn Superoxide dismutase (SOD) | DS/ADa | FC | H | Di Domenico et al. 2014 |
| Mn Superoxide dismutase (SOD) | EADa | IPL | H | Reed et al. 2009 |
| LADa | IPL | H | Perluigi et al. 2009 | |
| Energy Metabolism | ||||
| Aconitase | LADa | HP | H | Perluigi et al. 2009 |
| DS/ADb | FC | H | Di Domenico et al. 2014 | |
| Aldolase | MCIa | IPL | N | Sultana et al. 2007 |
| EADa | IPL | C | Sultana et al. 2010 | |
| LADa | HP | H | Perluigi et al. 2009 | |
| DSa | FC | H | Di Domenico et al. 2014 | |
| ATP Synthase | MCIa | HP | H | Reed et al. 2008 |
| MCIa | IPL | H | Reed et al. 2008 | |
| EADa | IPL | H | Reed et al. 2009 | |
| LADa | HP | N | Sultana et al. 2006a | |
| LADa | IPL | H | Perluigi et al. 2009 | |
| Creatine Kinase BB (CK) | LADa | IPL | C | Castegna et al. 2002a |
| Cyt b-c1 RIeske Protein | DSa | FC | H | Di Domenico et al. 2014 |
| DS/ADa | FC | H | Di Domenico et al. 2014 | |
| α-enolase | MCIa | HP | C | Butterfield et al. 2006a |
| MCIa | HP | N | Sultana et al. 2007 | |
| MCIa | HP | H | Reed et al. 2008 | |
| MCIa | IPL | N | Sultana et al. 2007 | |
| MCIa | IPL | C | Aluise et al. 2011 | |
| EADa | IPL | H | Reed et al. 2009 | |
| LADa | HP | C | Sultana et al. 2006c | |
| LADa | HP | N | Sultana et al. 2006a | |
| LADa | HP | H | Perluigi et al. 2009 | |
| LADa | IPL | C | Categna et al. 2002b | |
| LADa | IPL | N | Categna et al. 2003 | |
| DS/ADa | FC | H | Di Domenico et al. 2014 | |
| Glucose Regulated Protein Precursor | MCIa | IPL | N | Sultana et al. 2007 |
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | LADa | HP | N | Sultana et al. 2006a |
| DS/ADa | FC | H | Di Domenico et al. 2014 | |
| Lactate dehydrogenase (LDH) | MCIa | HP | H | Reed et al. 2008 |
| Malate dehydrogenase (MDH) | DS/ADa | FC | H | Di Domenico et al. 2014 |
| EADa | IPL | H | Reed et al. 2009 | |
| Phosphoglycerate kinase (PGK) | MCIa | HP | H | Reed et al. 2008 |
| Phosphoglycerate mutase 1 (PGM1) | EADa | IPL | C | Sultana et al. 2010 |
| LADa | HP | C | Sultana et al. 2006c | |
| Pyruvate kinase (PK) | MCIa | IPL | H | Reed et al. 2008 |
| DSa | FC | H | Di Domenico et al. 2014 | |
| MCIa | HP | C | Butterfield et al. 2006a | |
| Succinyl CoA-3-ketoacid-CoA transferase-1 (SCOT-1) | DSa | FC | C | Di Domentico et al. 2013 |
| Triose phosphate isomerase (TPI) | EADa | IPL | H | Reed et al. 2009 |
| LADa | HP | C | Sultana et al. 2006c | |
| LADa | IPL | N | Castegna et al. 2003 | |
| Excitotoxicity | ||||
| Glutamine Synthetase (GS) | MCIa | HP | C | Butterfield et al. 2006a |
| LADa | IPL | C | Castegna et al. 2002a | |
| LADa | IPL | H | Perluigi et al. 2009 | |
| Synaptic plasticity, vesicle-mediated transport and cell structure | ||||
| Carbonic anhydrase II (CA II) | MCIa | IPL | C | Sultana et al. 2010 |
| LADa | HP | C | Sultana et al. 2006c | |
| LADa | HP | N | Sultana et al. 2006a | |
| Dihydropyrimidinase-related protein 1 (DRP-1) | DS/ADa,b | FC | H | Di Domenico et al. 2014 |
| Dihydropyrimidinase-related protein 2 (DRP-2) | MCIa | HP | N | Sultana et al. 2007 |
| EADa | IPL | H | Reed et al. 2009 | |
| LADa | HP | C | Sultana et al. 2006c | |
| LADa | IPL | C | Castegna et al. 2002b | |
| LADa | IPL | H | Perluigi et al. 2009 | |
| DSa | FC | H | Di Domenico et al. 2014 | |
| DS/ADa,b | FC | H | Di Domenico et al. 2014 | |
| Fascin 1 | MCIa | HP | N | Sultana et al. 2007 |
| Neuropolypeptide h3 (PEBP1) | MCIa | HP | H | Reed et al. 2008 |
| LADa | IPL | N | Castegna et al. 2003 | |
| Syntaxin binding protein I (SBP1) | MCIa | IPL | C | Sultana et al. 2010 |
| DS/ADa,b | FC | H | Di Domenico et al. 2014 | |
| γ-synaptosomal protein like soluble N-ethylmaleimide-sensitive factor (γ-SNAP) | LADa | HP | C | Sultana et al. 2006c |
| Voltage-dependent anion-channel protein-1 (VDAC-1) | LADa | HP | N | Sultana et al. 2006a |
| β-actin | MCIa | IPL | H | Reed et al. 2008 |
| α-internexin | DSa | FC | H | Di Domenico et al. 2014 |
| Myelin binding protein (MBP) | DSa | FC | H | Di Domenico et al. 2014 |
| DS/ADb | FC | H | Di Domenico et al. 2014 | |
| Neurofilament medium polypeptide (NMP) | DS/ADa | FC | H | Di Domenico et al. 2014 |
| α-tubulin | LADa | HP | H | Perluigi et al. 2009 |
| Neuroinflammation | ||||
| Glial fibrillary acidic protein (GFAP) | EADa | IPL | C | Sultana et al. 2010 |
| DSa | FC | H | Di Domenico et al. 2014 | |
| DS/ADa | FC | H | Di Domenico et al. 2014 | |
| Protein Synthesis | ||||
| Elongation factor-Tu (EF-Tu) | MCIa | IPL | H | Reed et al. 2008 |
| Initiation factor α (eIF-α) | MCIa | IPL | H | Reed et al. 2008 |
| Protein Folding and Degradation | ||||
| Cathepsin D (CatD) | DSa | FC | C | Di Domentico et al. 2013 |
| 78kDa glucose-regulated protein (GRP78) | DSa | FC | C | Di Domentico et al. 2013 |
| DS/ADa,b | FC | H | Di Domenico et al. 2014 | |
| Heat shock cognate 71 (HSC71) | LADa | IPL | C | Castegna et al. 2002b |
| DS/ADa | FC | H | Di Domenico et al. 2014 | |
| Heat shock protein (HSP70) | MCIa | HP | H | Reed et al. 2008 |
| MCIa | HP | N | Sultana et al. 2007 | |
| MCIa | IPL | C | Sultana et al. 2010 | |
| Heat shock protein (HSP90) | MCIc | IPL | C | Aluise et al. 2011 |
| DS/ADb | FC | H | Di Domenico et al. 2014 | |
| T-complex protein 1 subunit β (TCPB) | DS/ADa | FC | H | Di Domenico et al. 2014 |
| Ubiquitin carboxy-terminal hydrolase L-1 (UCH-L1) | LADa | FC | C | Choi et al. 2004 |
| LADa | HP | C | Sultana et al. 2006c | |
| LADa | IPL | C | Castegna et al. 2002a | |
| DSa | FC | C | Di Domentico et al. 2013 | |
| DSa | FC | H | Di Domentico et al. 2014 | |
| Vo-ATPase | DSa | FC | C | Di Domentico et al. 2013 |
| Signal Transduction | ||||
| 14-3-3γ | MCIa | IPL | N | Sultana et al. 2007 |
| Mitogen-activated protein kinase I (MAPKI) | MCIa | IPL | C | Sultana et al. 2010 |
| Peptidylprolyl cis-trans isomerase-1 (Pin1) | MCIa | HP | C | Butterfield et al. 2006a |
| LADa | HP | C | Sultana et al. 2006b; Sultana et al. 2006c | |
Note: FC, frontal cortex; HP, hippocampus; IPL, inferior parietal lobule; C, carbonylation; N, nitration; H, HNE bound
compared to age-matched control
compared to DS brain
compared to PCAD
In EAD IPL, phosphoglycerate mutase 1 (PGM1), GFAP, and fructose bisphosphate aldolase C (FBA-C) are significantly carbonylated (Sultana et al. 2010), while MnSOD, α-enolase, ATP synthase, DRP-2 (CRMP-2), MDH, and triose phosphate isomerase (TPI) have significant increases in protein-bound HNE compared to age-matched control (Reed et al. 2009). Assessment of HNE-bound proteins in LAD have identified increased levels modifying MnSOD, ATP synthase, DRP-2 (CRMP-2), and GS in IPL and aldolase, aconitase, α-enolase, Prx6, and α-tubulin in hippocampus compared to respective age-matched controls (Perluigi et al. 2009). Also in LAD brain, ubiquitin carboxy-terminal hydrolase L-1 (UCH-L1), DRP-2 (CRMP-2) and α-enolase were found to be significantly carbonylated in both IPL and hippocampus compared to respective age-matched controls (Castegna et al. 2002a,b; Sultana et al. 2006c). Creatine kinase BB (CK BB), GS, and heat shock cognate 71 (HSC71) also were significantly carbonylated in IPL (Castegna et al. 2002a,b), and Pin1, PGM1, CA II, TP1, and γ-synaptosomal protein like soluble N-ethylmaleimide-sensitive factor (γ-SNAP) were significantly carbonylated in hippocampus (Sultana et al. 2006c). CAII, GAPDH, ATP synthase, and voltage-dependent anion-channel protein-1 (VDAC1) are significantly nitrated in LAD hippocampus, and TPI and neuropeptide h3 are increasingly nitrated in LAD IPL. α-enolase was found to be significantly nitrated in LAD IPL and hippocampus (Castegna et al. 2003; Sultana et al. 2006a). Frontal cortex of two FAD patients each with a mutation in PS1 have been analyzed using redox proteomics. Because of the limited sample size only proteins with elevated carbonylation compared to age-matched controls were reported. These proteins included γ-enolase, dimethylarginine dimethylaminohydrolase 1 (DMDMAH-1), UCH-L1, and actin (Butterfield et al. 2006b), consistent with the notion that glucose metabolism, nitric oxide metabolism, synaptic remodeling, and protein quality control are detrimentally affected in inherited AD.
As discussed previously, redox proteomics analyses of DS brain can provide insights into the in vivo effects of Aβ(1–42). In DS frontal cortex, GDH, Prx2, complex III Rieske protein (cyt b-c1), fructose bisphosphate aldolase-A, FBA-C, DRP-2, GFAP, UCH-L1, α-internexin, and myelin binding protein (MBP) have significantly elevated levels of protein-bound HNE (Di Domenico et al. 2014), while succinyl CoA-3-ketoacid-CoA transferase 1 (SCOT-1), cathepsin D (CatD), 78kDa glucose-regulated protein (GRP78), UCH L-1, and Vo-type proton ATPase (Vo-ATPase) were significantly carbonylated (Di Domenico et al. 2013) compared to age-matched control. Protein-bound HNE levels of Cu,ZnSOD, cyt b-c1, α-enolase, GAPDH, MDH, GFAP, HSC71, T-complex protein 1 subunit β (TCPB), and neurofilament medium polypeptide (NMP) were significantly increased in frontal cortex from DS with AD (DS/AD) compared to age-matched control while GDH, DRP-1, DRP-2, SBP1, GRP78, aconitase, HSP90B1 and MBP were significantly increased in DS/AD frontal cortex compared to DS (Di Domenico et al. 2014).
Redox proteomics analyses of MCI, AD, and DS brain provides insight into the proteins of the proteome that are particularly sensitive to oxidative modification. Notably, GS is oxidatively modified in MCI (Butterfield et al. 2006a) and LAD brain (Castegna et al. 2002a; Perluigi et al. 2009), and ATP synthase, α-enolase, and DRP-2 (CRMP-2) are oxidatively modified in MCI (Butterfield et al. 2006a; Sultana et al. 2007; Reed et al. 2008; Aluise et al. 2011), EAD (Reed et al. 2009) and LAD (Castegna et al. 2002b, 2003; Sultana et al. 2006a,c; Perluigi et al. 2009). α-enolase also was oxidatively modified in DS/AD brain, while DRP-2 was also oxidized in both DS and DS/AD brain (Di Domenico et al. 2014). Likewise, redox proteomics analyses provide a snapshot of the oxidative modification of the proteome at a given point in time. Interestingly, HSP 70 and HSP90 are only oxidized in MCI brain (Sultana et al. 2007, 2010; Reed et al. 2008; Aluise et al. 2011), suggesting that oxidative modification of these molecular chaperones and maintenance of proper protein folding may be affected early in the progression of AD. Similarly, PK which catalyzes the final step in aerobic glycolysis is oxidatively modified only in MCI (Butterfield et al. 2006a; Reed et al. 2008) and DS brain (Di Domenico et al. 2014). Aβ(1–42) induces oxidative modification of DRP-2 (Boyd-Kimball et al. 2005a) and PK (Sultana et al. 2006d) in vitro and GS in vivo (Boyd-Kimball et al. 2005c) strongly support the idea that Aβ(1–42) plays a significant role in the oxidative modification of these proteins in AD or DS brain.
Serum, CSF, and Peripheral Lymphocyte Mitochondria
Adaptation of redox proteomics methods to analyze plasma, serum, and cerebrospinal fluid (CSF) (Di Domenico et al. 2016a) and circulating biomarkers of protein oxidation in Alzheimer’s disease have been previously reviewed (Aluise et al. 2008; Di Domenico et al. 2011; Lista et al. 2013). In cerebrospinal fluid, decreased Aβ(1–42) and increased tau phosphorylated at threonine 181 (p-tau181) correlate with disease progression from MCI to AD (Mattsson et al. 2009; Di Domenico et al. 2016b). Just like changes in brain pathology, CSF levels of Aβ(1–42) and tau begin to change about two decades prior to the onset of symptoms, with changes in Aβ(1–42) preceding changes in tau (Wildsmith et al. 2014; Bateman et al. 2012; Buchhave et al. 2012). Redox proteomics analysis of CSF identified α−1β-glycoprotein, vitamin D-binding protein (DBP), ApoE, prostaglandin-H2 D-isomerase (PGDS), and α−1-anti-trypsin (A1AT) to be significantly carbonylated in both aMCI and AD compared to age-matched control. Gelsolin was the only protein identified to be significantly carbonylated in CSF of AD compared to aMCI (Di Domenico et al. 2016b). λ chain precursor has also been identified as significantly carbonylated in AD CSF compared to control (Korolainen et al. 2007). Redox proteomics analysis of plasma has identified A1AT, fibrinogen γ-chain precursor protein, transferrin, and hemopexin to be significantly carbonylated in AD compared to age-matched control (Choi et al. 2002; Yu et al. 2003). More recently, increased carbonylation of α2-macroglobulin has been identified in AD plasma compared to control, while increased carbonylation of haptoglobin has also been identified in AD plasma and aMCI plasma compared to control. Increased carbonylation of haptoglobin was also detected between AD and aMCI plasma (Cocciolo et al. 2012). Moreover, plasma levels of heme oxygenase −1/biliverdin reductase-A, that normally produce the antioxidant bilirubin, are found at different levels in plasma in AD (Di Domenico et al. 2012). More redox proteomics studies of serum and CSF are necessary to expand our understanding of the progression of AD and potentially identify additional biomarkers.
Peripheral lymphocyte mitochondria showed elevated oxidative stress in both aMCI and AD that correlated with decreased levels of small molecule antioxidants, with elevated levels of Aβ(1–42), and with decreased performance on tests of cognition (Sultana et al. 2011b, 2013b).
Redox Proteomics and Aβ(1–42)-mediated oxidative stress in the progression of AD
Aβ(1–42) induces oxidative stress by forming small soluble oligomers that insert into the lipid bilayer and initiate lipid peroxidation, forming free radicals that further propagate lipid peroxidation events by abstracting allylic hydrogen atoms from acyl chains of lipids in the lipid bilayer (Figure 1). This process leads to a chain reaction eventually resulting in lipid peroxidation products, such as HNE that further contribute to protein oxidation by reacting with proteins via Schiff base chemistry or with Cys, Lys, and His residues of proteins by Michael Addition (Butterfield et al. 2001, 2007, 2013; Butterfield and Lauderback 2002; Halliwell 2006; Di Domenico et al. 2017; Luczaj et al. 2017) (Figure 2). Such protein modifications result in conformational changes and often loss of function (Table 5) leading to necrotic or apoptotic cell death (Subramaniam et al. 1997; Butterfield and Stadtman 1997).
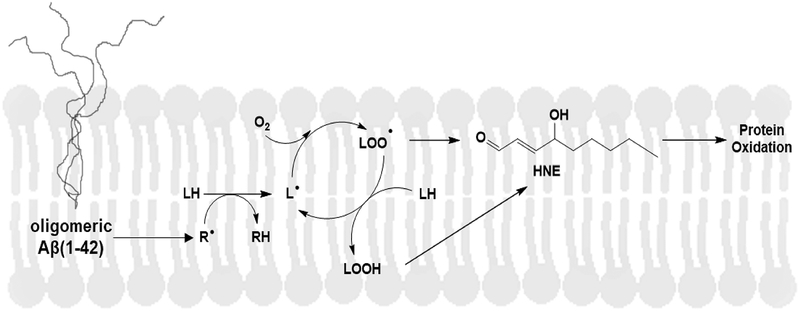
Figure 1:
Oligomeric Aβ(1–42)-induces lipid peroxidation. Oligomeric Aβ(1–42) embedded in the lipid bilayer initiates free radical formation (R.) mediated by Met 35 which leads to abstraction of a labile, allylic hydrogen atom from unsaturated fatty acids forming lipid radicals (L.) that rapidly combine with oxygen to form lipid peroxyl radicals (LOO.). Lipid peroxyl radicals propagate lipid peroxidation by abstracting allylic hydrogen atoms from other unsaturated fatty acids in the membrane forming lipid hydroperoxides (LOOH) and lipid radicals (L.), thereby propagating the chain reaction. The reactive lipid peroxidation product, 4-hydroxy-2-trans-nonenal (HNE) can be formed from lipid hydroperoxides of arachidonic acid, linoleic acid, and linolenic acid. This process of lipid peroxidation, coupled to HNE formation and subsequent reaction with and dysfunction of cellular proteins, amplifies greatly the effects of a small amount of free radical formation on Aβ(1–42).
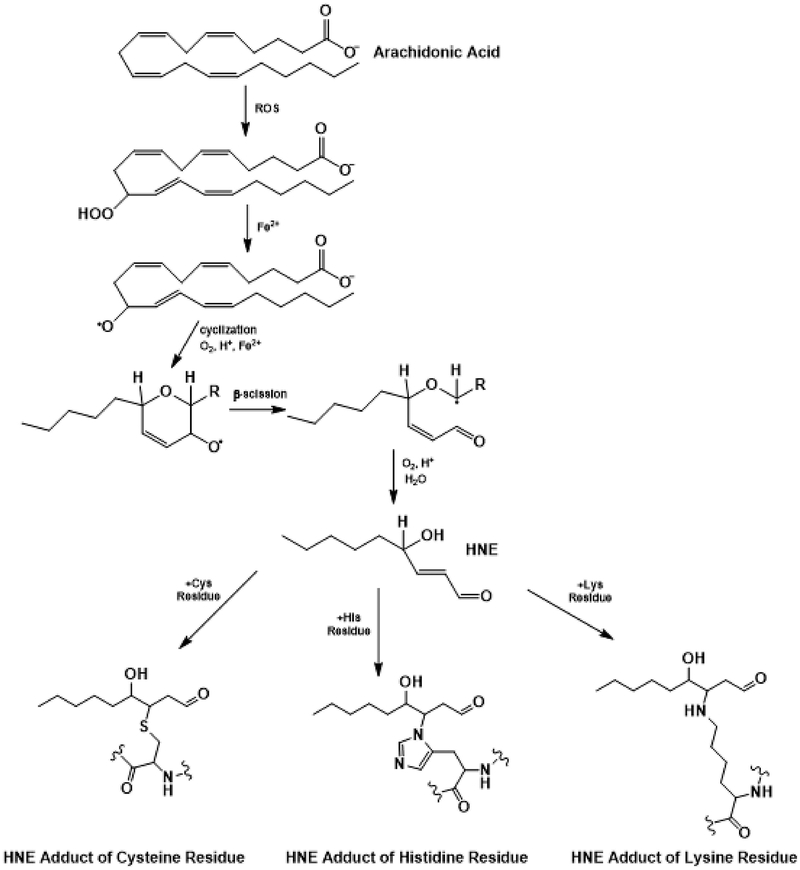
Figure 2:
HNE is formed from arachidonic acid and leads to oxidative protein modifications by reacting with Cys, His, and Lys residues via Michael addition.
Table 5:
Summary of Oxidatively Modified Proteins Identified by Redox Proteomics and Enzymatic Activity in aMCI, AD, and DS Brain
| Protein | Disease State | Brain Region | Oxidative Modification | Enzyme Activity | References |
|---|---|---|---|---|---|
| Cellular Redox Homeostasis and Detoxification Defense | |||||
| Mn Superoxide dismutase (SOD) | EADa | IPL | H | ↓ | Reed et al. 2009 |
| Energy Metabolism | |||||
| Aconitase | LADa | HP | H | ↓ | Perluigi et al. 2009 |
| ATP Synthase | MCIa | HP | H | ↓ | Reed et al. 2008 |
| MCIa | IPL | H | ↓ | Reed et al. 2008 | |
| LADa | IPL | H | ↓ | Perluigi et al. 2009 | |
| α-enolase | MCIa | HP | C | ↓ | Butterfield et al. 2006a |
| EADa | IPL | H | ↓ | Reed et al. 2009 | |
| Lactate dehydrogenase (LDH) | MCIa | HP | H | ↓ | Reed et al. 2008 |
| Malate dehydrogenase (MDH) | EADa | IPL | H | ↑ | Reed et al. 2009 |
| Pyruvate kinase (PK) | MCIa | IPL | H | ↓ | Reed et al. 2008 |
| MCIa | HP | C | ↓ | Butterfield et al. 2006a | |
| Synaptic plasticity, vesicle-mediated transport and cell structure | |||||
| Carbonic anhydrase II (CA II) | LADa | HP | C | ↓ | Sultana et al. 2006c |
| Protein Folding and Degradation | |||||
| Ubiquitin carboxy-terminal hydrolase L-1 (UCH-L1) | LADa | HP | C | ↓ | Sultana et al. 2006c |
| DSa | FC | C | ↓ | Di Domenico et al. 2013 | |
| Signal Transduction | |||||
| Peptidylprolyl cis-trans isomerase-1 (Pin1) | MCIa | HP | C | ↓ | Butterfield et al. 2006a |
| LADa | HP | C | ↓ | Sultana et al. 2006b | |
Note: FC, frontal cortex; HP, hippocampus; IPL, inferior parietal lobule; C, carbonylation; N, nitration; H, HNE bound
compared to age-matched control
Imaging studies indicate that increase in brain Aβ(1–42) may begin up to 20 years prior to the onset of cognitive symptoms (Villemagne et al. 2013; Reiman et al. 2012; Jack et al. 2009; Bateman et al. 2012). Early detection of Aβ(1–42) pathology may allow a timeframe for interventions that may prevent or delay the onset of cognitive symptoms; however, a greater understanding of the brain chemistry changes resulting from the increase in Aβ(1–42) is necessary to elucidate the progression of AD. Redox proteomics is a high-throughput method that has provided insight into the proteins that are particularly susceptible to oxidative damage as AD neuropathology accumulates from PCAD through LAD (Swomley and Butterfield 2015; Sultana et al. 2013a; Sultana and Butterfield 2013; Reed et al. 2010; Butterfield and Sultana 2007). Here we will focus our discussion to proteins found to be oxidatively modified in two or more of the models previously described (Figure 3). These proteins that have been identified to be oxidatively modified can be broadly classified into the following categories: antioxidants and cellular detoxification defenses, energy yielding metabolism and mitochondrial dysfunction, excitotoxicity, synaptic plasticity and cell structure, neuroinflammation, protein folding and degradation, and signal transduction.
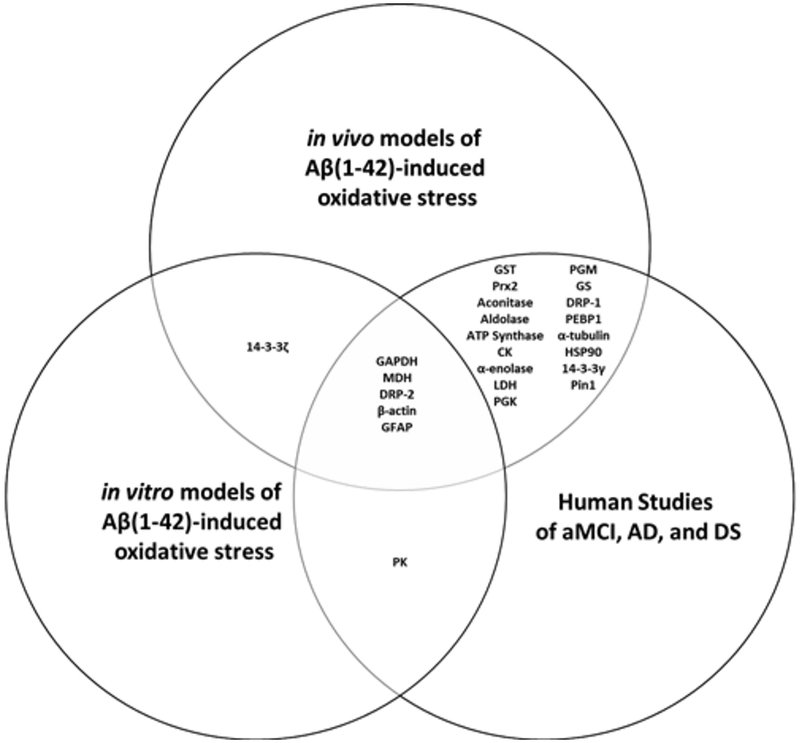
Figure 3:
Venn diagram of proteins commonly oxidized in vitro and in vivo by Aβ(1–42) and in human studies of aMCI, AD, and DS brain.
Abbreviations: CK creatine kinase, DRP-1 dihyropyrimidinase-related protein-1 or collapsin response mediated protein-1 (CRMP-1), DRP-2 dihyropyrimidinase-related protein-2 or collapsin response mediated protein-2 (CRMP-2), GAPDH glyceraldehyde-3-phosphate dehydrogenase, GFAP glial fibrillary acidic protein, GS glutamine synthase, GST glutathione S-transferase, HSP90 heat shock protein 90, MDH malate dehydrogenase, LDH lactate dehydrogenase, PEBP1 neuropolypeptide h3 or hippocampal cholinergic neurostimulating peptide precursor protein (HCNPpp), PGK phosphoglycerate kinase, PGM phosphoglycerate mutase, Pin1 peptidyl cis-trans isomerase-1, PK, pyruvate kinase, Prx2 peroxiredoxin 2.
Antioxidants and cellular detoxification defenses
GSTs catalyze the conjugation of HNE to the cysteinyl residue of glutathione (GSH) forming GS-HNE adducts to be cleared from the cell by the multidrug resistant protein, MRP-1 (Sharma et al. 2004; Balogh and Atkins 2011). GST is carbonylated in vivo in C. elegans expressing Aβ(1–42) (Boyd-Kimball et al. 2006) and in the canine model of aging (Opii et al. 2008). Additionally, GST is excessively nitrated in MCI IPL (Sultana et al. 2007) and has significantly elevated levels of protein-bound HNE in AD hippocampus resulting in significantly decreased activity (Sultana and Butterfield 2004; Lovell et al. 1998). Decreased GST activity significantly impacts the activity of this detoxification process preventing the clearance of HNE, allowing for increased accumulation of HNE (Singhal et al. 2015; Luczaj et al. 2017; Siems and Grune 2003) and the subsequent HNE-modification of other proteins.
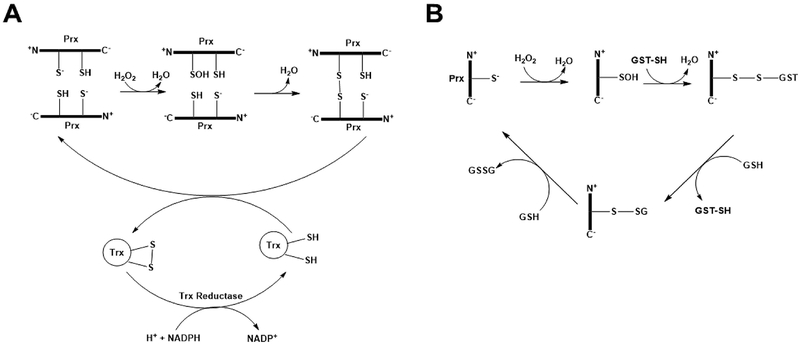
Peroxiredoxins (Prxs) are a family of thiol-dependent peroxidases that rapidly reduce H2O2 (Wood et al. 2003). Prxs are subcategorized into 2-Cys-Prx or 1-Cys-Prx enzymes. 2-Cys-Prx isoforms function as dimers each containing a conserved Cys residue in the amino-terminal region that reacts with peroxides and is oxidized to sulfenic acid. The sulfenic acid moiety then oxidizes an additional conserved Cys residue in the carboxy-terminal region of the other subunit to form an intrasubunit disulfide bond that is resolved by thioredoxin in an NADPH-dependent reduction. 1-Cys-Prx isoforms react with peroxides in the same manner as 2-Cys-Prx, but function catalytically as a monomer and lack the sacrificial carboxy-terminal Cys residue (McBean et al. 2015). Oxidized 1-Cys-Prx isoforms form a disulfide linkage with GST that is resolved by successive reaction with GSH (Rhee and Kil 2017) (Figure 4). Prx2 is a 2-Cys-Prx that is expressed primarily in the cytosol of neurons (Hattori and Oikawa 2007) and is oxidized in DS brain (Di Domenico et al. 2014). Oxidation of Prx2 was significantly decreased in brain of SAMP8 mice treated with an antisense oligonucleotide (AO) directed against the Aβ region of APP indicating that oxidative modification of Prx2 is Aβ(1–42) dependent (Poon et al. 2005). Prx6 is a 1-Cys-Prx isoform that is expressed in the cytosol and nucleus of astrocytes and oligodendrocytes (Hattori and Oikawa 2007) and is oxidatively modified in MCI (Sultana et al. 2007) and LAD brain (Perluigi et al. 2009). Expression of Prx2 is increased in AD and DS brain (Kim et al. 2001; Krapfenbauer et al. 2003), but this may be a compensatory mechanism. Oxidative modification of Prx2 and Prx6 not only contribute to impairment of the antioxidant response in the central nervous system in AD, but also may impact the signaling properties of Prxs and their ability repair peroxidized cell membranes (Fisher 2017) and to prevent, or delay, apoptotic cell death (Hampton and O’Connor 2016). In addition to their antioxidant function, Prxs also exhibit thiol redox-regulated chaperone activity (Kumsta and Jakob 2009; Conway and Lee 2015). Irreversible oxidative modifications of Prxs including carbonylation, nitration, and protein-bound HNE may inhibit the thiol redox-regulated activation of Prx chaperone activity contributing to increased protein misfolding and aggregation in AD brain (Kundra et al. 2017).
Figure 4:
Mechanisms of reduction of H2O2 by 2-Cys-Prx and 1-Cys-Prx by peroxiredoxin are shown in A and B, respectively. The reader is referred to the text for further details.
Energy Yielding Metabolism and Mitochondrial Dysfunction
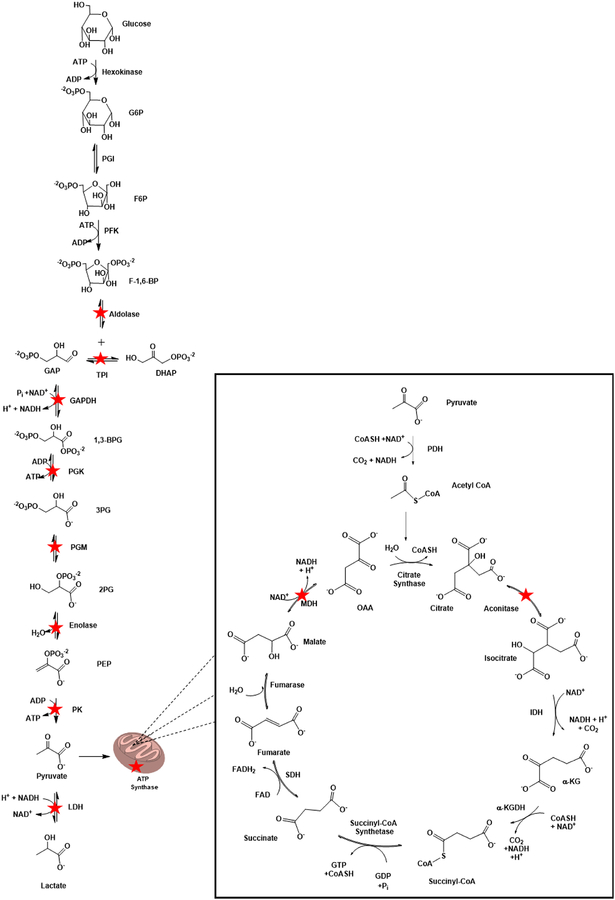
Glycolytic enzymes including aldolase, TPI, GAPDH, PGK, PGM, enolase, and PK are significantly oxidatively modified in AD brain (Sultana et al. 2006a,c, 2007, 2010; Perluigi et al. 2009; Reed et al. 2008, 2009; Castegna et al. 2003; Butterfield et al. 2006a). Of these, GAPDH was found to be oxidatively modified in primary neuronal culture treated with Aβ(1–42) (Boyd-Kimball et al. 2005b; Sultana et al. 2006d), in hippocampus of rats following ICV injection of Aβ(1–42) (Boyd-Kimball et al. 2006), in aged canine (Opii et al. 2008), in 3×Tg-AD mice (Shen et al. 2015), in LAD (Sultana et al. 2006a), and DS/AD brain (Di Domenico et al. 2014), while oxidation of PK was observed in MCI and DS and was induced by Aβ(1–42) in primary neuronal culture (Sultana et al. 2006d). α-enolase is extensively oxidized in multiple AD mouse models (Sultana et al. 2011a; Shen et al. 2015; Fiorini et al. 2013) and in MCI (Butterfield et al. 2006a; Sultana et al. 2007; Reed et al. 2008; Aluise et al. 2011), EAD (Reed et al. 2009), LAD (Castegna et al. 2002b, 2003; Sultana et al. 2006a,c; Perluigi et al. 2009), and DS/AD brain (Di Domenico et al. 2014). Decreased glucose catabolism in the progression of AD has been well documented with the use of PET imaging studies (Yamaguchi et al. 1997; Marcus et al. 1997; Kato et al. 2016). The brain preferentially utilizes glucose (Sokoloff et al. 1977), but can also utilize ketone bodies in times of need (Hertz and Rothman 2016), to meet its cellular energy needs; however, beyond the production of ATP, glucose is also needed as a precursor for neurotransmitter biosynthesis, and plays a role in regulation of cell death and autophagy (Hertz and Chen 2017; Mergenthaler et al. 2013). Oxidative modification of glycolytic enzymes could result in glucose hypometabolism in AD.
Mitochondrial dysfunction also plays a role in AD pathogenesis (Reddy and Reddy 2011; Tu et al. 2014). In an APP/PS1 transgenic mouse model of AD, greater mitochondrial dysfunction is evident in the hippocampus and cortex that corresponded with increased Aβ mitochondrial levels and cognitive deficits (Dragicevic et al. 2010). In female 3×Tg-AD mice, mitochondrial dysfunction corresponded with the onset of oxidative stress, but precedes the development of plaque and tangle neuropathology (Yao et al. 2009), suggesting the mitochondrial dysfunction is an early event in AD pathogenesis. MDH, an important enzyme in the tricarboxylic acid (TCA) cycle, has been identified by redox proteomics as oxidized by Aβ(1–42) in vitro (Sultana et al. 2006d) and in vivo (Boyd-Kimball et al. 2006) and in 3×Tg-AD mice (Shen et al. 2015), EAD (Reed et al. 2009), and DS/AD brain (Di Domenico et al. 2014). Beyond the TCA cycle, MDH is important in the malate-aspartate shuttle that regenerates cytosolic NAD+ for GAPDH and glycolytic activity. Aconitase, another TCA cycle enzyme, is oxidized in 3×Tg-AD mice (Shen et al. 2015) and in LAD (Perluigi et al. 2009) and DS/AD brain (Di Domenico et al. 2014). ATP synthase (complex V) is also oxidized in APP/PS1 double human mutant knock- in mice (Sultana et al. 2011a) and in MCI (Reed et al. 2008), EAD (Reed et al. 2009), and LAD brain (Sultana et al. 2006a; Perluigi et al. 2009). Oxidation and altered function of mitochondrial enzymes critical for maintaining cellular energy needs could contribute to synaptic dysfunction, neurite loss and cell death evident in AD brain (Figure 5). See further discussion of glucose dysmetabolism in the progression of AD below in the Conclusion and Future Directions section.
Figure 5:
A summary of the cytosolic and mitochondrial enzymes critical for energy yielding metabolism and mitochondrial function are shown. Enzymes that are oxidatively modified by HNE in the progression of AD are starred for emphasis. Note that a stoichiometry of two exists for all reactions shown following the production of GAP.
Abbreviations: G6P: Gluocose-6-Phosphate; PGI: Phosphoglucose isomerase; F6P: Fructose 6 Phosphate; PFK: Phosphofructokinase; F-1,6-BP: Fructose-1,6-Bisphosphatase; TPI: Triose Phosphate Isomerase; GAP: Glyceraldehyde-3-Phosphate; DHAP: Dihydroxyacetone Phosphate; GAPDH: Glyceraldehyde-3-Phosphate Dehydrogenase; 1,3-BPG: 1,3-Bisphosphoglycerate; PGK: Phosphoglycerate Kinase; 3PG: 3-Phosphoglycerate; PGM: Phosphoglycerate Mutase; 2PG: 2-Phosphoglycerate; PEP: Phosphoenolpyruvate; PK: Pyruvate Kinase; LDH: Lactate Dehydrogenase; PDH: Pyruvate Dehydrogenase; OAA: Oxaloacetate; IDH: Isocitrate Dehydrogenase; α-KG: α-Ketoglutarate; α-KGDH: α-Ketoglutarate Dehydrogenase; SDH: Succinate Dehydrogenase; MDH: Malate Dehydrogenase.
Excitotoxicity
Glutamate is an excitatory neurotransmitter that is cleared from the synapse by the glial glutamate transporter (GLT-1), also known as the excitatory amino acid transporter-2 (EAAT2), where it is converted to glutamine by glutamine synthetase (GS). GLT-1 activity is decreased in AD brain (Masliah et al. 1996), which is likely due to the elevated levels of HNE bound to GLT-1 in AD brain (Lauderback et al. 2001). Redox proteomics has identified GS as oxidatively modified in vivo in C. elegans expressing Aβ(1–42) (Boyd-Kimball et al. 2006), and in MCI (Sultana et al. 2010) and LAD brain (Castegna et al. 2002a; Perluigi et al. 2009). GS activity is significantly decreased in AD brain (Smith et al. 1991; Hensley et al. 1995) and is likely explained by the increased protein-bound HNE and carbonylation present in AD brain (Perluigi et al. 2009; Castegna et al. 2002a). Taken together, oxidation of GLT-1 and GS could result in decreased clearance of synaptic glutamate inducing excitotoxicity and neuronal death.
Synaptic plasticity and cell structure
Dihydropyrimidinase-related protein-2 (DRP-2), also known as collapsin response mediator protein-2 (CRMP-2), was first characterized as functioning in neurite outgrowth by guiding the axonal growth cone (Goshima et al. 1995; Inagaki et al. 2001). More recently, DRP-2 has been identified as a microtubule associated protein that functions as an adaptor in several additional processes including establishing neuronal polarity, kinesin-dependent transport, neurotransmitter release, and Ca2+ channel regulation (Hensley et al. 2011; Hensley and Kursula 2016). DRP-2 is oxidatively modified by Aβ(1–42) in vitro (Boyd-Kimball et al. 2005a) and in vivo in SAMP8 mice (Poon et al. 2004; Fiorini et al. 2013) and in MCI (Sultana et al. 2007), EAD (Reed et al. 2009), LAD (Categna et al. 2002b; Sultana et al. 2006c; Perluigi et al. 2009), DS, and DS/AD brain (Di Domenico et al. 2014). Cytoskeletal proteins β-actin and α-tubulin also are susceptible to oxidative modification by Aβ(1–42). β-Actin is oxidized in vitro and in vivo by Aβ(1–42)-related processes (Boyd-Kimball et al. 2005a, 2006), in APP/PS1 double human mutant knock-in mice (Sultana et al. 2011a), and in MCI and FAD brain (Reed et al. 2008; Butterfield et al. 2006b), while α-tubulin is oxidized in vivo by Aβ(1–42) (Boyd-Kimball et al. 2005c), in 3×Tg-AD (Shen et al. 2015), and in LAD brain (Perluigi et al. 2009). DRP-2 has been shown to mediate axonal growth by interacting with and stabilizing tubulin (Rogemond et al. 2008; Chae et al. 2009) and indirectly stabilizing actin microfilaments (Hensley and Kursula 2016). Oxidative modification and conformation changes of DRP-2, α-tubulin, and β-actin likely impacts this interaction in addition to vesicle-mediated transport and thereby contribute to the loss of cognition in AD.
The cholinergic system is significantly disrupted in AD brain (Ferreira-Vieira et al. 2016). Cholinergic neurons are lost in the progression of AD (Whitehouse et al. 1981; Whitehouse et al. 1982), and choline acetyl transferase (ChAT), which catalyzes the synthesis of acetylcholine (ACh), exhibits decreased activity in AD brain (Wilcock et al. 1982). Levels of HNE-bound ChAT are significantly increased by Aβ(1–42) in vitro (Butterfield and Lauderback 2002) suggesting that Aβ(1–42)-induced oxidative modification may contribute to the decreased ChAT activity observed in AD brain. Redox proteomics has identified neuropolypeptide h3 to be oxidatively modified in vivo in the J20 mouse (Robinson et al. 2011), and in MCI (Reed et al. 2008) and LAD brain (Castegna et al. 2003). Neuropolypeptide h3 is also known as phosphatidylethanolamine binding protein-1 (PEBP-1), which is a pleiotropic protein that binds ATP in addition to phosphatidylethanolamine (Sato et al. 2017). PEBP-1 is also known as hippocampal cholinergic neurostimulating peptide precursor protein (HCNPpp) and Raf kinase inhibitor protein (RKIP). Reduced HCNPpp gene expression has been shown in AD hippocampus (Maki et al. 2002) and HCNPpp has been shown to co-localize with DRP-2 at presynaptic terminals in hippocampal neurons (Kato et al. 2012). HCNP, an 11 amino acid peptide, is cleaved from within the amino-terminus of HCNPpp and increases ACh synthesis by increasing ChAT levels (Ojika et al. 2000). Oxidative modification of HCNPpp may further disrupt presynaptic function in AD brain by impacting its interaction with DRP-2 and may potentially alter its recognition and cleavage to yield HCNP further impacting cholinergic neurotransmission.
Neuroinflammation
GFAP is the main constituent of the intermediate filament system in adult astrocytes and is considered a marker of reactive gliosis (Pekny and Pekna 2004). Reactive astrocytes can provide some neuroprotective and repair functions in mild to moderate astrogliosis; however, extreme astrogliosis can result in glial scar formation that is reported to inhibit axon regeneration (Sofroniew 2009; Silver and Miller 2004). Reactive astrogliosis is evident in MCI, suggesting it occurs early in the progression of AD (Carter et al. 2012). Reactive astrocytes are co-localized with senile plaques (Nagele et al. 2003) and produce proinflammatory signals (Li et al. 2011) that enhance Aβ(1–42) production in astrocytes (Blasko et al. 2000; Frost and Li 2017) and impair microglial phagocytosis (Koenigsknecht-Talboo and Landreth 2005). Aβ(1–42) has been shown to play a role in initiating inflammation in the CNS (Minter et al. 2016). GFAP is oxidized in vitro (Boyd-Kimball et al. 2005a) by Aβ(1–42), and in vivo in SAMP8 mice brain (Fiorini et al. 2013) and EAD (Sultana et al. 2010), DS, and DS/AD brain (Di Domenico et al. 2014). Oxidation of GFAP could indicate damage to astrocytes consistent with astrocytic dysfunction (Acosta et al. 2017; Garwood et al. 2017) and neuroinflammation (Latta et al. 2015) evidenced in AD brain and exacerbate glutamate excitotoxicity as previously described.
Protein folding and degradation
Protein homeostasis, or proteostasis, is the maintenance of the proteome such that all proteins are in the correct conformation, location, and concentration for their function. Structure dictates function; consequently, chaperones are required to prevent misfolding while sorting and trafficking functions are required to target proteins to the appropriate cellular location, and, in addition, the concentration of each protein is controlled by regulating protein synthesis and degradation (Klaips et al. 2018). The Unfolded Protein Response (UPR) is a protective mechanism in which the endoplasmic reticulum (ER) responds to increased levels of unfolded or misfolded proteins in the ER lumen. UPR works in concert with endoplasmic reticulum associated degradation (ERAD) that directs unfolded proteins for destruction by the ubiquitin proteasome system (UPS) (Penke et al. 2018). Failure of the UPR to keep up this screening mechanism leads to the buildup of misfolded proteins in the ER which accumulate in mitochondria-associated ER membranes and cause mitochondrial dysfunction (Volgyi et al. 2015; Schon and Area-Gomez 2013) resulting in aberrant APP processing, decreased energy production, and triggering apoptotic cell death consistent with AD (Area-Gomez and Schon 2016; Hiramatsu et al. 2015; Safra et al. 2013). Several molecular chaperones have been identified by redox proteomics to be oxidatively modified AD brain and models thereof. HSP60 is increasingly carbonylated by Aβ(1–42) in vivo (Boyd-Kimball et al. 2006), while HSC71 exhibited increased carbonylation in LAD brain (Castegna et al. 2002b) and increased protein-bound HNE in DS/AD brain (Di Domenico et al. 2014). HSP70 is oxidized and nitrated in MCI brain (Reed et al. 2008; Sultana et al. 2007, 2010), and HSP90 is oxidatively modified in 3×Tg-AD mice (Shen et al.2015) and in MCI (Aluise et al. 2011) and DS/AD brain (Di Domenico et al. 2014). Oxidation of HSPs may prevent appropriate interactions with target proteins, resulting in increased protein misfolding and impaired repair mechanisms consistent with the increased protein aggregation evident in AD brain (Ashraf et al. 2014; Ross and Poirier 2004). Additionally, molecular chaperones including HSC71, HSP70, and HSP90 have been found in the synaptic compartment and play an important role in maintaining synaptic proteostasis (Gorenberg and Chandra 2017). Oxidation and altered function of these chaperones could further contribute to loss of synaptic plasticity and decreased cognition.
Proteasomal activity is decreased in AD brain (Keller et al. 2000) with a reduction in trypsin and chymotrypsin-like activities being reported (Lopez-Salon et al. 2003; Mishto et al. 2006). A similar decrease in proteasomal activity also exists in DS brain (Di Domenico et al. 2013). Inhibition of proteasomal activity impairs synaptic plasticity in animals (Lopez-Salon et al. 2001; Fonseca et al. 2006), which may indicate that decreased proteasomal activity in AD brain may play a role in loss of synaptic plasticity. Oligomeric Aβ(1–42) inhibits proteasomal activity in vitro, and proteasomal function decreases in 3×Tg-AD mice at ages consistent with oligomeric Aβ(1–42) production suggesting that Aβ(1–42) may also inhibit proteasomal function in vivo (Tseng et al. 2008). Redox proteomics analysis of C. elegans expressing Aβ(1–42) identified both α- and β-proteasomal subunits to be oxidatively modified (Boyd-Kimball et al. 2006) suggesting a likely mechanism by which proteasome function is decreased in AD brain.
Aβ(1–42) induces inhibition of long-term potentiation (LTP), a form of synaptic plasticity associated with learning and memory, in hippocampal slices from wild type mice (Cullen et al. 1997; Walsh et al. 2002) and an APP/PS1 transgenic mouse model that overexpresses Aβ(1–42) also shows loss of LTP as early as 3 months of age (Trinchese et al. 2004). UCH-L1 ameliorates Aβ(1–42)-induced LTP loss in vitro and in vivo. This APP/PS1 transgenic mouse model of AD also shows no difference in the protein level of UCH-L1 in the hippocampus, but rather a shift in UCH-L1 from soluble to insoluble with a significant decrease in UCH-L1 specific activity in the insoluble fraction compared to the soluble fraction (Gong et al. 2006). UCH-L1 is an abundant protein localized to neurons that is a deubiquitinating enzyme (Wilkinson et al. 1989; Nijman et al. 2005), but also has been reported to stabilize monoubiquitinylated proteins in vivo (Osaka et al. 2003) and is itself regulated by ubiquitinylation (Setsuie and Wada 2007; Bishop et al. 2016). In dimeric form, UCH-L1 has ATP-independent ligase activity (Liu et al. 2002). Redox proteomics identified that brain-resident UCH-L1 is oxidatively modified in FAD (Butterfield et al. 2006b), LAD (Castegna et al. 2002a; Choi et al. 2004; Sultana et al. 2006c), and DS (Di Domenico et al. 2013, 2014). Additionally, UCH-L1 activity is significantly decreased in AD brain compared to age-matched control, suggesting that the increased carbonylation of the protein impairs its function (Sultana et al. 2006c). Likewise, oxidative modification of UCH-L1 by HNE significantly decreases its hydrolase activity (Nishikawa et al. 2003) while significantly increasing its insolubility and protein aggregation, likely due to structural alterations (Kabuta et al. 2008). Taken together, these studies suggest that oxidative modification of UCH-L1 causes conformational change resulting in loss of deubiquitinylation activity, resulting in increased protein aggregation and impaired UPS function and loss of synaptic plasticity consistent with what is known in AD.
Signal transduction
14-3-3 proteins are highly expressed in neuronal cytosol but are also detected in other cellular compartments. 14-3-3 proteins consist of 7 isoforms that have been found to interact with up to 200 different proteins to regulate metabolism, cell cycle, differentiation, autophagy, and apoptosis (Umahara et al. 2012; Steinacker et al. 2011; Heverin et al. 2012). 14-3-3 proteins function by inducing conformational change in a target, by using their hydrophobic domain to occlude structural features of a target, or by scaffolding (Heverin et al. 2012). Decreased expression of 14-3-3ζ induces an ER stress response and exacerbates excitotoxicity in vitro (Murphy et al. 2008) and overexpression of 14-3-3ζ ameliorates these alterations in vivo (Brennan et al. 2013). 14-3-3ζ also is reported to regulate neurite outgrowth (Ramser et al. 2010), differentially regulate protein kinase C (Acs et al. 1995) and may act to sequester misfolded proteins (Kaneko and Hachiya 2006). 14-3-3 proteins co-localize with neurofibrillary tangles (NFTs) in AD brain (Layfield et al. 1996) and 14-3-3ζ co-immunoprecipitates with microtubule associated tau (Hashiguchi et al. 2000). 14-3-3ζ facilitates glycogen synthase kinase 3β (GSK3β)-dependent phosphorylation of tau in culture (Agarwal-Mawal et al. 2003; Yuan et al. 2003) suggesting a role for 14-3-3ζ in formation of neurofibrillary tangles (Sluchanko and Gusev 2011; Li and Paudel 2016). 14-3-3ζ was identified to be oxidatively modified by Aβ(1–42) in vitro (Boyd-Kimball et al. 2005b; Sultana et al. 2006) and in vivo (Boyd-Kimball et al. 2005c) and in APP/PS1 double human mutant knock-in (Sultana et al. 2011a) and 3×Tg-AD mice (Shen et al. 2015). It should be noted that oxidized 14-3-3ζ was detected at an early age in both transgenic mouse models. Thus, oxidative modification of 14-3-3ζ occurred prior to Aβ deposition in the APP/PS1 double human mutant knock-in mice and prior to both Aβ deposition and tau pathology in the 3×Tg-AD mice. 14-3-3γ protein levels are increased in AD brain (Fountoulakis et al. 1999) and 14-3-3γ is oxidatively modified in MCI brain (Sultana et al. 2007) indicating that oxidative modification of 14-3-3 occurs early in the pathogenesis of AD and could have impacts on axonal guidance and transport, protein aggregation, autophagy, and apoptosis, all functions affected in AD.
Pin1 is a peptidyl prolyl cis/trans isomerase that induces a conformational change in phosphoproteins possessing phosphorylated Ser/Thr-Pro motifs (Lu et al. 1996; Lu et al. 1999a; Zhou et al. 1999; Liou et al. 2011). Notably, Pin1 activity is decreased by phosphorylation post-translational modification (Lu and Zhou 2007). In the CNS, Pin1 is expressed primarily in neurons and is mainly localized to the nuclei in normal brain; however, in AD brain, the Pin1 is colocalized in the cytoplasm with neurofibrillary tangles and purifies with paired helical filaments of hyperphosphorylated tau from AD brain (Lu et al. 1999b; Ramakrishnan et al. 2003). p-Thr231 tau has been shown to promote further phosphorylation of tau (Lin et al. 2007) leading to hyperphosphorylation (Hamdane et al. 2003). p-Thr231 tau is a target for Pin1 binding, and conformational change of the peptidyl proline bond in tau from cis to trans facilitates phosphoprotein phosphatase A2 (PPA2) mediated dephosphorylation of tau (Lu et al. 1999b; Ranganathan et al. 1997; Zhou et al. 2000). Pin1 knockout mice develop tau hyperphosphorylation and filament formation with age (Liou et al. 2003) and, when Pin1 knockout mice were crossed with Tg2576 mice that overexpress APP with the Swedish mutations K670N and M671L, knockout of Pin1 was found to increase amyloidogenic processing of APP resulting in increased intracellular Aβ(1–42) accumulation (Pastorino et al. 2006). Redox proteomics has identified Pin1 to be oxidatively modified in J20 (Robinson et al. 2011) and APP/PS1 double human mutant knock-in mice (Sultana et al. 2011a) and in MCI (Butterfield et al. 2006a) and LAD brain (Sultana et al. 2006b,c). Pin1 activity is significantly deceased in both MCI (Butterfield et al. 2006a) and LAD brain (Sultana et al. 2006b). Further study has shown that Cys113 of Pin1 is oxidatively modified in vivo in AD brain and in AD mouse models. In vitro oxidation of Cys113 results in loss of function, but not substrate recognition indicating that oxidized Pin1 can bind and trap substrates but be unable to complete the catalytic cycle. Mutation of this residue to alanine resulted in loss of catalytic function confirming that Cys113 is critical for Pin1 activity (Chen et al. 2015). Taken together, as Pin1 activity is proposed to promote nonamyloidogenic processing of APP, decreased Pin1 activity could shift APP processing further toward the amyloidogenic pathway resulting in increased production of Aβ(1–42) (Pastorino et al. 2006). Likewise, as Pin1 plays a role in regulating the phosphorylation state of tau, impaired Pin1 activity could lead to tau hyperphosphorylation and neurofibrillary tangles present in AD brain (Wang et al. 2007; Zhou et al. 2000). Impaired Pin1 activity in AD brain could also affect cell cycle regulation (Lin et al. 2015), UPS protein degradation (Liou et al. 2011) and synaptic plasticity (Xu et al. 2017).
Conclusion and Future Directions
Redox proteomics has been invaluable in identifying proteins that are particularly sensitive to oxidative modification pinpointing molecular targets in cellular processes or signaling pathways that are damaged and may be dysfunctional. In PCAD brain total Aβ(1–42) is significantly increased, but no significant difference in oxidative stress biomarkers were detected compared to control brain (Aluise et al. 2010); However, in MCI only small differences in AD neuropathology are observed compared to PCAD, but protein carbonyls are significantly increased in MCI brain compared to PCAD brain (Aluise et al. 2011). Some protein expression changes exist in PCAD brain compared to control brain (Aluise et al. 2010), but in our hands no oxidatively modified proteins were detected until MCI (Aluise et al. 2011) providing further evidence that a window of opportunity for treatment or lifestyle interventions may exist if AD can be detected in its earliest stages. Redox proteomics has the potential to screen for oxidized proteins in peripheral tissues; however, more studies of peripheral tissues along the progression of PCAD to LAD are needed to identify possible peripheral biomarkers that correlate with the changes observed in brain. Such studies have the potential to lead to the development of tools that could aid in diagnosis and staging the of disease beyond our current methods.
Overproduction and accumulation of Aβ(1–42) is known to induce oxidative stress and is key to the neuropathogenesis of AD. Redox proteomics has provided insight into the molecular targets susceptible to Aβ(1–42)-induced oxidative modification. In this review, we surveyed the proteins identified by redox proteomics to be oxidized by Aβ(1–42) in vitro and in vivo and discussed these in the context of oxidatively modified proteins identified by redox proteomics analyses of animal models of AD, human FAD, PCAD, MCI, EAD, LAD, DS and DS/AD brain. These commonalities indicate that Aβ(1–42)-induced oxidative modifications involve proteins that largely contribute to decreased energy yielding metabolism, synaptic dysfunction and excitotoxicity, and loss of proteostasis due to impaired molecular chaperones and degradation contributing to protein misfolding and aggregation, altered APP processing yielding increased production of Aβ(1–42) and, ultimately, resulting in cell death (Figure 6). Taken together, these redox proteomics studies indicate that Aβ(1–42)-mediated oxidative stress occurs early in AD progression. Consequently, improved access to biomarker imaging and the identification of treatments to reduce Aβ production or the oxidative damage induced by Aβ could be beneficial in preventing or delaying the progression of AD.
Figure 6:
Aβ(1–42)-mediated cell death is initiated by lipid peroxidation. Oligomeric Aβ(1–42)-embeds in the lipid bilayer inducing lipid peroxidation and propagating free radical reactions that oxidatively modify proteins resulting in conformational change and altered function impairing glucose metabolism, synaptic plasticity, synaptic glutamate clearance, and proteostasis, ultimately leading to cell death.
Indeed, a recent study demonstrates the value of imaging modalities in determining the order of pathological alterations in the progression of AD (Gordon et al. 2018). Consistent with our findings using redox proteomics and our overall Aβ(1–42)-centered hypothesis for oxidative stress, glucose dysmetabolism, and neuronal death in AD (Butterfield 2014; Butterfield and Boyd-Kimball 2018; Butterfield and Lauderback 2002; Butterfield et al. 2001, 2002, 2007, 2013), Gordon et al. (2018) demonstrated using PET imaging on thousands of individuals who are known to have dominantly inherited AD over a period of 22 years prior to expected onset of symptoms to 3 years following symptoms (the DIAN Network) that Aβ(1–42) deposition was the first pathological alteration observed, followed later by glucose dysmetabolism. In the current review, Aβ(1–42) oligomers were shown to oxidatively modify glycolytic and TCA enzymes and portions of ATP synthase, thereby decreasing glucose metabolism resulting in lower levels of ATP. This, in turn, leads to loss of neuronal membrane potential, opening voltage-gated Ca2+ channels with consequent influx of detrimental Ca2+, and eventual neuronal death. Gordon et al. (2018) showed by MRI that the third temperol alteration in AD pathology was thinning of the hippocampus and frontal cortex, consistent with neuronal death, in agreement with our redox proteomics findings and overall hypothesis.
Redox proteomics has identified potential promising therapeutic targets and pathways for eventually slowing if not halting progression of this devastating dementing disorder that is AD. Continued use of redox proteomics in the stages of AD likely will provide additional insights and new targets and pathways that may be beneficial in treating this disease.
ACKNOWLEDGEMENTS
This work was supported in part by grants from the National Institute on Aging. We thank the faculty of the Sanders-Brown Center on Aging for providing well-characterized specimens for the studies from our laboratory mentioned in this review.
Abbreviations:
- 3-NT
3-nitrotyrosine
- 3×Tg-AD
triple transgenic AD mouse
- A1AT
α−1-anti-trypsin
- Actr2
actin-related protein 2
- Acyl CoA DH
acyl-CoA dehydrogenase
- ADK
adenosine kinase
- AK
arginine kinase
- Aldo 3
aldolase 3
- aMCI
Amnestic Mild Cognitive Impairment
- AO
antisense oligonucleotide
- ApoE
Apolipoprotein E
- APP
Amyloid Precursor Protein
- Aβ
Amyloid beta-peptide
- CA II
carbonic anhydrase II
- CatD
cathepsin D
- ChAT
choline acetyltransferase
- CK BB
creatine kinase BB
- CK
creatine kinase
- Coro 1a
coronin 1a
- CRMP-1
collapsing response mediated protein-1
- CRMP-2
collapsing response mediated protein-2
- cyt b-c1
complex III Rieske protein
- DBP
vitamin D-binding protein
- DMDMAH-1
dimethylarginine dimethylaminohydrolase 1
- DPL2
peptidase α
- DRP-1
dihydropyrimidinase-related protein-1
- DRP-2
dihydropyrimidinase-related protein-2
- DS
Down syndrome
- DS/AD
Down syndrome with Alzheimer’s disease
- Dyn-1
dynamin-1
- EAAT2
excitatory amino acid transporter-2
- EAD
Early Alzheimer’s disease
- EF-1γ
elongation factor 1 γ
- EF-Tu
elongation factor Tu
- eIF-α
initiation factor α
- ERAD
endoplasmic reticulum associated degradation
- FAD
Familial Alzheimer’s disease
- FBA-C
fructose bisphosphate aldolase C
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GDH
glutamate dehydrogenase
- GFAP
glial fibrillary acid protein
- GLT-1
glial glutamate transporter
- GRP78
78kDa glucose-regulated protein
- GS
glutamine synthetase
- GSH
glutathione
- GST
glutathione-S-transferase
- GSTM3
GST mu
- HCNP
hippocampal cholinergic neurostimulating peptide
- HCNPpp
hippocampal cholinergic neurostimulating peptide precursor protein
- HNE
4-hydroxy-2-trans-nonenal
- HSC71
heat shock cognate 71
- HSP60
chaperonin 60
- HSP70
heat shock protein 70
- HSP90
heat shock protein 90
- ICV
intracerebral ventricular
- IPL
inferior parietal lobule
- LAD
Late Alzheimer’s disease
- LBP
lipid binding protein
- LDH-2
lactate dehydrogenase 2
- MAPKI
mitogen-activated protein kinase I
- MBP
myelin binding protein
- MCI
Mild Cognitive Impairment
- MDH
malate dehydrogenase
- MnSOD or SOD2
manganese superoxide dismutase
- MRLC
myosin regulatory light chain
- MRP1
multidrug resistant protein 1
- MRP
multidrug resistant protein
- NBM
nucleus basalis magnocellularis
- NMP
neurofilament medium polypeptide
- NSF
vesicle-fusing ATPase
- PCAD
Preclinical Alzheimer’s disease
- PDH
pyruvate dehydrogenase
- PEBP-1
phosphatidylethanolamine-binding protein 1 or neuropolypeptide h3
- PET
Positron Emission Tomography
- PGDS
prostaglandin-H2 D-isomerase
- PGK1
phosphoglycerate kinase 1
- PGM1
phosphoglycerate mutase 1
- Pin1
peptidylprolyl cis-trans isomerase-1
- PK
pyruvate kinase
- PKM2
pyruvate kinase M2
- Prx2
peroxiredoxin 2
- Prx6
peroxiredoxin 6
- Prxs
peroxiredoxins
- PS-1
presenilin 1
- SAMP8
senescence accelerated mouse
- SBP1
syntaxin binding protein I
- SCOT-1
succinyl CoA-3-ketoacid-CoA transferase 1
- SEP11
septin-11
- TCA
tricarboxylic acid cycle
- TCPB
T-complex protein 1 subunit β
- TK
transketolase
- UCH-L1
ubiquitin carboxy-terminal hydrolase L-1
- UPR
Unfolded Protein Response
- UPS
ubiquitin proteasome system
- VDAC1
voltage-dependent anion-channel protein-1
- Vo-ATPase
Vo-type proton ATPase
- γ-SNAP
soluble N-ethylmaleimide-sensitive factor
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
References
- Acs P, Szallasi Z, Kazanietz MG and Blumberg PM (1995) Differential activation of PKC isozymes by 14-3-3 zeta protein. Biochem. Biophys. Rev. Commun 216, 103–109. [DOI] [PubMed] [Google Scholar]
- Abdul HM, Sultana R, St. Clair DK, Markesbery WR and Butterfield DA (2008) Oxidative damage in brain from human mutant APP/PS-1 double knock-in mice as a function of age. Free Radic. Biol. Med 45, 1420–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta C, Anderson HD and Anderson CM (2017) Astrocyte dysfunction in Alzheimer disease. J. Neurosci. Res 95, 2430–2447. [DOI] [PubMed] [Google Scholar]
- Agarwal-Mawal A, Qureshi HY, Cafferty PW, Yuan Z, Han D, Lin R and Paudel HK (2003) 14-3-3 connects glycogen synthase kinase-3 beta to tau within a brain microtubule-associated tau phosphorylation complex. J. Biol. Chem 278, 12722–12728. [DOI] [PubMed] [Google Scholar]
- Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Ziolko SK, James JA, Snitz BE, Houck PR, Bi W, Cohen AD, Lopresti BJ, DeKosky ST, Halligan EM and Klunk WE (2008) Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch. Neurol 65, 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksenov MY, Aksenova MV, Butterfield DA, Geddes JW and Markesbery WR (2001) Protein Oxidation in the Alzheimer’s Disease Brain: Analysis of Protein Carbonyls by Immunocytochemistry and Two-Dimensional Western Blotting. Neuroscience 103, 373–383. [DOI] [PubMed] [Google Scholar]
- Aluise CD, Sowell RA and Butterfield DA (2008) Peptides and proteins in plasma and cerebrospinal fluid as biomarkers for the prediction, diagnosis, and monitoring of therapeutic efficacy of Alzheimer’s disease. Biochim. Biophys. Acta 1782, 549–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aluise CD, Robinson RAS, Beckett TL, Murphy MP, Cai J, Pierce WM, Markesbery MR and Butterfield DA (2010) Preclinical Alzheimer disease: Brain oxidative stress, Aβ peptide and proteomics. Neurobiol. Dis 39, 221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aluise CD, Robinson RAS, Cai J, Pierce WM, Markesbery WR and Butterfield DA (2011) Redox proteomics analysis of brains from subjects with amnestic mild cognitive impairment compared to brains from subjects with preclinical Alzheimer’s disease: Insights into memory loss in MCI. J. Alzheimers Dis 23, 257–269. [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Association. (2015) Changing the trajectory of Alzheimer’s disease: how a treatment by 2025 saves lives and dollars Alzheimer’s Association, Chicago. [Google Scholar]
- Alzheimer’s Association (2018) Alzheimer’s Disease Facts and Figures. Alzheimers Dement 14, 367–429. [Google Scholar]
- Anantharaman M, Tangpong J, Keller JN, Murphy MP, Markesbery WR, Kiningham KK and St. Clair DK (2006) β-amyloid mediated nitration of manganese superoxide dismutase: implication for oxidative stress in a APPNLh/NLh × PS-1P264L/P264L double knock-in mouse model of Alzheimer’s disease. Am. J. Pathol 168, 1608–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstey KJ, Cherbuin N, Budge M and Young J (2011) Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes. Rev 12, e426–e437. [DOI] [PubMed] [Google Scholar]
- Area-Gomez E, and Schon EA (2016) Mitochondria-associated ER membrane and Alzheimer disease. Curr. Opin. Genet. Dev 38, 90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf GM, Greig NH, Khan TA, Hassan I, Tabrez S, Shakil S, Sheikh IA, Zaidi SK, Akram M, Jabir NR, Firoz CK, Naeem A, Alhazza IM, Damanhouri GA and Kamal MA (2014) Protein misfolding and aggregation in Alzheimer’s disease and type 2 diabetes mellitus. CNS Neurol. Disord. Drug Targets 13, 1280–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader Lange ML, St. Clair D, Markesbery WR, Studzinski CM, Murphy MP and Butterfield DA (2010) Age-related loss of phospholipid asymmetry in APPNLh/APPNLh × PS-1P264L/PS-1P264L human double mutant knock-in mice: Relevance to Alzheimer’s disease. Neurobiol. Dis 38, 104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balogh LM and Atkins WM (2011) Interactions of glutathione transferase with 4-hydroxy-2-nonenal. Drug Metab. Rev 43, 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargerger-Gateau P, Raffaitin C, Letenneur L, Tzourio C, Dartigues JF and Alpérovitch A (2007) Dietary patterns and risk of dementia: the Three-City cohort study. Neurology 69, 1921–1930. [DOI] [PubMed] [Google Scholar]
- Barker WW, Luis CA, Kashuba A, Harwood DG, Loewenstein D, Waters C, Jimison P, Shepherd E, Sevush S, Graff-Radford N, Newland D, Todd M, Miller B, Gold M, Heilman K, Doty L, Goodman I, Robinson B, Pearl G, Dickson D and Duara R (2002) Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis. Assoc. Disord 16, 203–212. [DOI] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TLS, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, Holtzman DM, Santacruz A, Buckles V, Oliver A, Moulder K, Aisen PS, Ghetti B, Klunk WE, McDade E, Martins RN, Masters CL, Mayeux R, Ringman JM, Rossor MN, Schofield PR, Sperling RA, Salloway S and Morris JC (2012) Clinical and biomarker changes in dominantly inherited Alzheimer’s Disease. New Engl. J. Med 367, 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekris LM, Yu CE, Bird RD and Tsuang DW (2010) Genetics of Alzheimer disease. J. Geriatr. Psychiatry Neurol 23, 213–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyreuther K, Pollwein P, Multhaup G, Mönning U, König G, Dyrks T, Schubert W and Masters CL (1993) Regulation and expression of the Alzheimer’s beta/A4 amyloid protein precursor in health, disease, and Down’s syndrome. Ann. N. Y. Acad. Sci 695, 91–102. [DOI] [PubMed] [Google Scholar]
- Billings LM, Oddo S, Green KN, McGaugh JL and LaFerla FM (2005) Intraneuronal Ab causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron, 45, 675–688. [DOI] [PubMed] [Google Scholar]
- Bishop P, Rocca D and Henley JM (2016) Ubiquitin C-terminal hydrolase L1 (UCH-L1): structure, distribution and roles in brain function and dysfunction. Biochem. J 473, 2453–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasko I, Veerhuis R, Stampfer-Kountchev M, Saurwein-Teissl M, Eikelenboom P and Grubeck-Loebenstein B (2000) Costimulatory effects of interferon-gamma and interleukin-1beta or tumor necrosis factor alpha on the synthesis of Abeta1–40 and Abeta1–42 by human astrocytes. Neurobiol. Dis 7, 682–689. [DOI] [PubMed] [Google Scholar]
- Blondell SJ, Hammersley-Mather R and Veerman JL (2014) Does physical activity prevent cognitive decline and dementia?: A systematic review and meta-analysis of longitudinal studies. BMC Public Health 14, 510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd-Kimball D, Castegna A, Sultana R, Poon HF, Petroze R, Lynn BC, Klein JB and Butterfield DA (2005a) Proteomic identification of proteins oxidized by Aβ(1–42) in synaptosomes: Implications for Alzheimer’s disease. Brain Res 1044, 206–215. [DOI] [PubMed] [Google Scholar]
- Boyd-Kimball D, Sultana R, Poon HF, Mohmmad-Abdul H, Lynn BC, Klein JB, and Butterfield DA (2005b) γ-Glutamylcysteine Ethyl Ester Protection of Proteins from Aβ(1–42)-Mediated Oxidative Stress in Neuronal Cell Culture: A Proteomics Approach. J. Neurosci. Res 79, 707–713. [DOI] [PubMed] [Google Scholar]
- Boyd-Kimball D, Sultana R, Poon HF, Lynn BC, Casamenti F, Pepeu G, Klein JB and Butterfield DA (2005c) Proteomic identification of proteins specifically oxidized by intracerebral injection of amyloid β-peptide (1–42) into rat brain: implications for Alzheimer’s Disease. Neuroscience 132, 313–324. [DOI] [PubMed] [Google Scholar]
- Boyd-Kimball D, Poon HF, Lynn BC, Cai J, Pierce WM Jr., Klein JB, Ferguson J, Link CD and Butterfield DA (2006) Proteomic identification of proteins specifically oxidized in Caenorhabditis elegans expressing human Aβ(1–42): Implications for Alzheimer’s Disease. Neurol. Aging 27, 1239–1249. [DOI] [PubMed] [Google Scholar]
- Brennan GP, Jimenez-Mateos EM, McKiernan RC, Engel T, Tzivion G and Henshall DC (2013) Transgenic overexpression of 14-3-3 zeta protects hippocampus against endoplasmic reticulum stress and status epilepticus in vivo. PLoS ONE 8, e54491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Gupta S, Knight AG, Beckett TL, McMullen JM, Davis PR, Murphy MP, Van Eldik LJ, St. Clair D and Keller JN (2011) Cognitive impairment in humanized APP × PS-1 mice is linked to Aβ1–42 and NOX activation. Neurobiol. Dis 44, 317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchhave P, Minthon L, Zetterberg H, Walin AK, Blennow K and Hansson O (2012) Cerebrospinal fluid levels of β-amyloid 1–42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch Gen. Psychiatry 69, 98–106. [DOI] [PubMed] [Google Scholar]
- Butterfield DA (1997) beta-amyloid-associated free radical oxidative stress and neurotoxicity: implications for Alzheimer’s disease. Chem. Res. Toxicol 10, 495–506. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Howard BJ, Yatin S, Allen KL and Carney JM (1997) Free radical oxidation of brain proteins in accelerated senescence and its modulation by N-tert-butyl-alpha-phenylnitrone. Proc. Natl. Acad. Sci. USA 94, 674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA and Stadtman ER (1997) Protein oxidation processes in aging brain. Adv. Cell. Aging Gerontol 2, 161–191. [Google Scholar]
- Butterfield DA, Yatin SM and Link CD (1999) In vitro and in vivo protein oxidation induced by Alzheimer’s disease amyloid beta-peptide (1–42). Ann. N. Y. Acad. Sci 893, 265–268. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Drake J, Pocernich C and Castegna A (2001) Evidence of oxidative damage in Alzheimer’s disease brain: central role for amyloid beta-peptide. Trends Mol. Med 7, 548–554. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Castegna A, Lauderback CM and Drake J (2002) Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer’s disease brain contributes to neuronal death. Neurobiol. Aging 23, 655–664. [DOI] [PubMed] [Google Scholar]
- Butterfield DA and Lauderback CM (2002) Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: potential causes and consequences involving amyloid β-peptide-associated free radical oxidative stress. Free Radic. Biol. Med 32, 1050–1060. [DOI] [PubMed] [Google Scholar]
- Butterfield DA and Boyd-Kimball D (2004) Amyloid beta-peptide(1–42) contributes to the oxidative stress and neurodegeneration found in Alzheimer’s disease brain. Brain Pathol 14, 426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA and Poon HF (2005) The senescence-accelerated prone mouse (SAMP8): A model of age-related cognitive decline with relevance to alterations of the gene expression and protein abnormalities in Alzheimer’s disease. Exp. Gerontol 40, 774–783. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Poon HF, St. Clair D, Keller JN, Pierce WM, Klein JB and Markesbery WR (2006a) Redox Proteomics identification of oxidatively modified hippocampal proteins in mild cognitive impairment: Insights into the development of Alzheimer’s disease. Neurobiol. Dis 22, 223–232. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Gnjec A, Poon HF, Castegna A, Pierce WM, Klein JB and Martins R (2006b) Redox proteomics identification of oxidatively modified brain proteins in inherited Alzheimer’s disease: An initial assessment. J. Alzheimers Dis 10, 391–397. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Reed R, Newman SF and Sultana R (2007) Roles of amyloid beta-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer’s disease and mild cognitive impairment. Free Rad. Biol. Med 43, 658–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA and Sultana R (2007) Redox proteomics identification of oxidatively modified brain proteins in Alzheimer’s disease and mild cognitive impairment: insights into the progression of this dementing disorder. J. Alzheimers Dis 12, 61–72. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Galvan V, Bader Lange M, Tang H, Sowell RA, Spilman P, Fombonne J, Gorostiza O, Zhange J, Sultana R and Bredesen DE (2010) In vivo oxidative stress in brain of Alzheimer’s disease transgenic mice: requirement for methionine 35 in amyloid β-peptide of APP. Free Radic. Biol. Med 48, 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Swomley AM and Sultana R (2013) Amyloid β-peptide (1–42)-induced oxidative stress in Alzheimer disease: Importance in disease pathogenesis and progression. Antioxid. Redox Signal 19, 823–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA (2014) The 2013 SFRBM Discovery Award: Selected discoveries from the Butterfield Laboratory of oxidative stress and its sequela in brain in cognitive disorders exemplified by Alzheimer disease and chemotherapy induced cognitive impairment. Free Radic. Biol. Med 74, 157–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA and Perluigi M (2017) Redox proteomics: a key tool for new insights into protein modification with relevance to disease. Antioxid. Redox Signal 26, 277–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA and Boyd-Kimball D (2018) Oxidative stress, amyloid-β peptide, and altered key molecular pathways in the pathogenesis and progression of Alzheimer’s disease. J. Alzheimer Dis 62, 1345–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter SF, Scholl M, Almkvist O, Wall A, Engler H, Langstrom B and Nordberg A (2012) Evidence for astrocytosis in prodromal Alzheimer disease provided by 11C-deuterium-L-deprenyl: a multitracer PET paradigm combining 11C-Pittsburgh compound B and 18F-FDG. J. Nucl. Med 53, 37–46. [DOI] [PubMed] [Google Scholar]
- Castegna A, Aksenov M, Aksenova M, Thongboonkerd V, Klein JB, Pierce WM, Booze R, Markesbery WR and Butterfield DA (2002a) Proteomic Identification of Oxidatively Modified Proteins in Alzheimer’s Disease Brain. Part I: Creatine Kinase BB, Glutamine Synthase, and Ubiquitin Carboxy-Terminal Hydrolase L-1. Free Radical Biol. & Med 33, 562–571. [DOI] [PubMed] [Google Scholar]
- Castegna A, Aksenov M, Aksenova M, Thongboonkerd V, Klein JB, Pierce WM, Booze R, Markesbery WR and Butterfield DA (2002b) Proteomic Identification of Oxidatively Modified Proteins in Alzheimer’s Disease Brain. Part II: Dihydropyrimidinase-Related Protein 2, α-Enolase, and Heat Shock Cognate 71, J. Neurochem 82, 1524–1532. [DOI] [PubMed] [Google Scholar]
- Castegna A, Thongboonkerd V, Klein JB, Lynn B, Markesbery WR and Butterfield DA (2003) Proteomic Identification of Nitrated Proteins in Alzheimer’s Disease Brain. J. Neurochem 85, 1394–1401. [DOI] [PubMed] [Google Scholar]
- Cenini G, Dowling AL, Beckett TL, Barone E, Mancuso C, Murphy MP, Levine H 3rd, Lott IT, Schmitt FA, Butterfield DA and Head E (2012) Association between frontal cortex oxidative damage and beta-amyloid as a function of age in Down syndrome. Biochim. Biophys. Acta 1822, 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae YC, Lee S, Heo K, Ha SH, Jung Y, Kim JH, Ihara Y, Suh PG and Ryu SH (2009) Collapsin response mediator protein-2 regulates neurite formation by modulating tubulin GTPase activity. Cell Signal 21, 1818–1826. [DOI] [PubMed] [Google Scholar]
- Cheignon C, Tomas M, Bonnefont-Rousselot D, Faller P, Hureau C, and Collin F (2018) Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol 14, 450–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-H, Li W, Sultana R, You M-H, Kondo A, Shahpasand K, Kim BM, Luo M-L, Nechama M, Lin Y-M, Yao Y, Lee TH, Zhou XZ, Swomley AM and Butterfield DA (2015) Pin1 cysteine-113 oxidation inhibits its catalytic activity and cellular function in Alzheimer’s disease. Neurobiol. Dis 76, 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Malakowsky CA, Talent JM, Conrad CC and Gracy RW (2002) Identification of oxidized plasma proteins in Alzheimer’s disease. Biochem. Biophys. Res. Commun 293, 1566–1570. [DOI] [PubMed] [Google Scholar]
- Choi J, Levey AI, Weintraub ST, Rees HD, Gearing M, Chin LS and Li L (2004) Oxidative modifications and down-regulation of ubiquitin carboxyl-terminal hydrolase L1 associated with idiopathic Parkinson’s and Alzheimer’s diseases. J. Biol. Chem 279, 13256–13264. [DOI] [PubMed] [Google Scholar]
- Chouraki V and Seshadri S (2014) Genetics of Alzheimer’s disease. Adv. Genet 87, 245–294. [DOI] [PubMed] [Google Scholar]
- Cocciolo A, Di Domenico F, Coccia R, Fiorini A, Cai J, Pierce WM, Mecocci P, Butterfield DA and Perluigi M (2012) Decreased expression and increased oxidation of plasma haptoglobin in Alzheimer’s disease: Insights from redox proteomics. Free Radic. Biol. Med 53, 1868–1876. [DOI] [PubMed] [Google Scholar]
- Conway ME and Lee C (2015) The redox switch that regulates molecular chaperones. BioMol Concepts 6, 269–284. [DOI] [PubMed] [Google Scholar]
- Copani A, Koh JY and Cotman CW (1991) Beta-amyloid increases neuronal susceptibility to injury by glucose deprivation. Neuroreport 2, 763–765. [DOI] [PubMed] [Google Scholar]
- Crane PK, Walker R, Hubbard RA, Li G, Nathan DM, Zheng H, Haneuse S, Craft S, Montine TJ, Kahn SE, McCormick W, McCurry SM, Bowen JD and Larson EB (2013) Glucose levels and risk of dementia. N. Engl. J. Med 369, 540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen WK, Suh YH, Anwyl R and Rowan MJ (1997) Block of LTP in rat hippocampus in vivo by beta-amyloid precursor protein fragments. Neuroreport 8, 3213–3217. [DOI] [PubMed] [Google Scholar]
- Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, Wolf PA and DeCarli C (2011) Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology 77, 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Valle J, Duran-Vilaregut J, Manich G, Casadesús G, Smith MA, Camins A, Pallàs M, Pelegri C and Vilaplana J (2010) Early amyloid accumulation in the hippocampus of SAMP8 mice. J. Alzheimers Dis 19, 1303–1315. [DOI] [PubMed] [Google Scholar]
- Di Domenico F, Coccia R, Butterfield DA and Perluigi M (2011) Circulating biomarkers of protein oxidation for Alzheimer disease: Expectations within limits. Biochim. Biophys. Acta 1814, 1785–1795. [DOI] [PubMed] [Google Scholar]
- Di Domenico F, Barone E, Mancuso C, Perluigi M, Cocciolo A, Mecocci P, Butterfield DA and Coccia R (2012) HO-1/BVR-A system analysis in plasma from probable Alzheimer’s disease and mild cognitive impairment subjects: A potential biochemical marker for the prediction of the disease. J. Alzheimers Dis 32, 277–289. [DOI] [PubMed] [Google Scholar]
- Di Domenico F, Coccia R, Cocciolo A, Murphy MP, Cenini G, Head E, Butterfield DA, Giorgi A, Schininà ME, Mancuso C, Cini C and Perluigi M (2013) Impairment of proteostasis network in Down syndrome prior to the development of Alzheimer’s disease neuropathology: Redox proteomics analysis of human brain. Biochim. Biophys. Acta 1832, 1249–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Domenico F, Pupo G, Tramutola A, Giorgi A, Schininà ME, Coccia R, Head E, Butterfield DA and Perluigi M (2014) Redox proteomics analysis of HNE-modified proteins in Down syndrome brain: clues for understanding the development of Alzheimer’s disease. Free Radic. Biol. Med 71, 270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Domenico F, Perluigi M and Butterfield DA (2016a) Redox proteomics in human biofluids: Sample preparation, separation and immunochemical tagging for analysis of protein oxidation. Methods Mol. Biol 1303, 391–403. [DOI] [PubMed] [Google Scholar]
- Di Domenico F, Pupo G, Giraldo E, Badìa M-C, Monllor P Lloret A, Schininà ME, Giorgi A, Cini C, Tramutola A, Butterfield DA, Viña J and Perluigi M (2016b) Oxidative signature of cerebrospinal fluid from mild cognitive impairment and Alzheimer disease patients. Free Radic. Biol. Med 91, 1–9. [DOI] [PubMed] [Google Scholar]
- Di Domenico F, Tramutola A and Butterfield DA (2017) Role of 4-hydroxy-2-nonenal (HNE) in the pathogenesis of Alzheimer disease and other selected age-related neurodegenerative disorders. Free Radic. Biol. Med 111, 253–261. [DOI] [PubMed] [Google Scholar]
- Ding Q, Markesbery WR, Cecarini V and Keller JN (2006) Decreased RNA, and increased RNA oxidation, in ribosomes from early Alzheimer’s disease. Neurochem. Res 31, 705–710. [DOI] [PubMed] [Google Scholar]
- Dragicevic N, Mamcarz M, Zhu Y, Buzzeo R, Tan J, Arendash GW, and Bradshaw PC (2010) Mitochondrial amyloid-beta levels are associated with the extent of mitochondrial dysfunction in different brain regions and the degree of cognitive impairment in Alzheimer’s transgenic mice. J. Alzheimers Dis 20, S535–550. [DOI] [PubMed] [Google Scholar]
- Drake J, Link CD and Butterfield DA (2003) Oxidative stress precedes fibrillar deposition of Alzheimer’s disease amyloid beta-peptide (1–42) in a transgenic Caenorhabditis elegans model. Neurobiol. Aging 24, 415–420. [DOI] [PubMed] [Google Scholar]
- Drayer BP, Heyman A, Wilkinson W, Barrett L and Weinberg T (1985) Early-onset Alzheimer’s disease: an analysis of CT findings. Ann. Neurol 17, 407–410. [DOI] [PubMed] [Google Scholar]
- Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, Bakardjian H, Benali H, Bertram L, Blennow K, Broich K, Cavedo E, Crutch S, Dartigues J-F, Duyckaerts C, Epelbaum S, Frisoni GB, Gauthier S, Genthon R, Gouw AA, Habert M-O, Holtzman DM, Kivipelto M, Lista S, Molinuevo J-L, O’Bryant SE, Rabinovivi GS, Rowe C, Salloway S, Schneider LS, Sperling R, Teichmann M, Carrillo MC, Cummings J and Jack CR Jr. from the Proceedings of the Meeting of the International Working Group (IWG) and the American Alzheimer’s Association on “The Preclinical State of AD”; July 23, 2015; Washington, DC, USA. (2016) Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimers Dement 12, 292–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr SA, Poon HF, Dogrukol-Ak D, Drake J, Banks WA, Eyerman E, Butterfield DA and Morley JE (2003) The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J. Neurochem 84, 1173–1183. [DOI] [PubMed] [Google Scholar]
- Farrow TF, Thiyagesh SN, Wilkinson ID, Parks RW, Ingram L and Woodruff PW (2007) Fronto-temporal-lobe atrophy in early-stage Alzheimer’s disease identified using an improved detection methodology. Psychiatry Res 155, 11–19. [DOI] [PubMed] [Google Scholar]
- Féart C, Samieri C and Barberger-Gateau P (2010) Mediterranean diet and cognitive function in older adults. Curr. Opin. Clin. Nutr. Metab. Care 13, 14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira-Vieira TH, Guimaraes M, Silva FR and Ribeiro FM (2016) Alzheimer’s disease: Targeting the cholinergic system. Curr. Neuropharmacol 14, 101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorini A, Sultana R, Forster S, Perluigi M, Cenini G, Cini C, Cai J, Klein JB, Farr SA, Niehoff ML, Morley JE, Kumar VB and Butterfield DA (2013) Antisense directed against PS-1 gene decreases brain oxidative markers in aged senescence accelerated mice (SAMP8) and reverses learning and memory impairment: A proteomics study. Free Radic. Biol. Med 65, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher AB (2017) Peroxiredoxin 6 in the repair of peroxidized cell membranes and cell signaling. Arch Biochem. Biophys 617, 68–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood DG, Reaume AG, Dorfman KS, Lin Y-G, Lang DM, Trusko SP, Savage MJ, Annaert WG, De Strooper B, Siman R and Scott RW (2002) FAD mutant PS-1 gene-targeted mice: Increased Aβ42 and Aβ deposition without APP overproduction. Neurobiol. Aging 23, 335–348. [DOI] [PubMed] [Google Scholar]
- Flood JF and Morley JE (1993) Age-related changes in footshock avoidance acquisition and retention in senescence accelerated mouse (SAM). Neurobiol Aging 14, 153–157. [DOI] [PubMed] [Google Scholar]
- Flood JF and Morley JE (1998) Learning and memory in the SAMP8 mouse. Neurosci. Biobehav. Rev 22, 1–20. [DOI] [PubMed] [Google Scholar]
- Fonseca R, Vabulas RM, Hartl FU Bonhoeffer T and Nägerl UV (2006) A balance of protein synthesis and proteasome-dependent degradation determines the maintenance of LTP. Neuron 52, 239–245. [DOI] [PubMed] [Google Scholar]
- Fountoulakis M, Cairns N and Lubec G (1999) Increased levels of 14-3-3 gamma and epsilon proteins in brain of patients with Alzheimer’s disease and Down syndrome. J. Neural. Transm. Suppl 57, 323–335. [DOI] [PubMed] [Google Scholar]
- Frost GR and Li Y-M (2017) The role of astrocytes in amyloid production and Alzheimer’s disease. Open Biol 7, 170228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbita SP, Lovell MA and Markesbery WR (1998) Increased nuclear DNA oxidation in the brain in Alzheimer’s disease. J. Neurochem 71, 2034–2040. [DOI] [PubMed] [Google Scholar]
- Galvan V, Gotostiza OF, Banwait S, Ataie M, Logvinova AV, Sitaraman S, Carlson E, Sagi SA, Chevallier N, Jin K, Greenberg DA and Bredesen DE (2006) Reversal of Alzheimer’s-like pathology and behavior in human APP transgenic mice by mutation of Asp 664. Proc. Natl. Acad. Sci. USA 103, 7130–7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garwood CJ, Ratcliffe LE, Simpson JE, Heath PR, Ince PG and Wharton SB (2017) Review: Astrocytes in Alzheimer’s disease and other age-associated dementias: a supporting player with a central role. Neuropathol. Appl. Neurobiol 43, 281–298. [DOI] [PubMed] [Google Scholar]
- Goate A and Hardy J (2012) Twenty years of Alzheimer’s disease-causing mutations. J. Neurochem 120, Suppl 1, 3–8. [DOI] [PubMed] [Google Scholar]
- Gong B, Cao Z, Zheng P, Vitolo OV, Liu S, Staniszewski A, Moolman D, Zhang H, Shelanski M and Aranci O (2006) Ubiquitin hydrolase Uch-L1 rescues beta-amyloid-induced decreases in synaptic function and contextual memory. Cell 126, 775–788. [DOI] [PubMed] [Google Scholar]
- Gordon BA, Blazey TM, Su Y, Hari-Raj A, Dincer A, Flores S, Christensen J, McDade E, Wang G, Xiong C, Cairns NJ, Hassenstab J, Marcus DS, Fagan AM, Jack CR Jr., Hornbeck RC, Paumier KL, Ances BM, Berman SB, Brickman AM, Cash DM, Chhatwal JP, Correia S, Forster S, Fox NC, Graff-Radford NR, la Fougere C, Levin J, Masters CL, Rossor MN, Salloway S, Saykin AJ, Schofield PR, Thompson PM, Weiner MM, Holtzman DM, Raichle ME, Morris JC, Bateman RJ and Benzinger TL (2018) Spatial patterns of neuroimaging biomarker change in individuals from families with autosomal dominant Alzheimer’s disease: a longitudinal study. Lancet Neurol 17, 241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorenberg EL and Chandra SS (2017) The role of co-chaperones in synaptic proteostasis and neurodegenerative disease. Front. Neurosci 11, 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima Y, Nakamura F, Strittmatter P and Strittmatter SM (1995) Collapsin-induced growth cone collapse mediated by an intracellular protein related to UNC-33. Nature 376, 509–514. [DOI] [PubMed] [Google Scholar]
- Gottesman RF, Schneider AL, Zhou Y, Coresh J, Green E, Gupta N, Knopman DS, Mintz A, Rahmim A, Sharrett AR, Wagenknecht LE, Wong DF and Mosley TH (2017a) Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA 317, 1443–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman RF, Albert MS, Alonso A, Coker LH, Coresh J, Davis SM, Deal JA, McKhann GM, Mosley TH, Sharrett AR, Schneider ALC, Windham BG, Wruck LM and Knopman DS (2017b) Associations between midlife vascular risk factors and 25-year incident dementia in atherosclerosis risk in communities (ARIC) cohort. JAMA Neurol 74, 1246–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L and Robinson RAS (2016) Proteomic approaches to quantify cysteine reversible modifications in aging and neurodegenerative diseases. Proteomics Clin. Appl 10, 1159–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudala K, Bansal D, Schifano F and Bhansali A (2013) Diabetes mellitus and risk of dementia: A meta-analysis of prospective observational studies. J. Diabetes Investig 4, 640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyure KA, Durham R, Stewart WF, Smialek JE and Troncoso JC (2001) Intraneuronal abeta-amyloid precedes development of amyloid plaques in Down syndrome. Arch. Pathol. Lab. Med 125, 489–492. [DOI] [PubMed] [Google Scholar]
- Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? J. Neurochem 97, 1634–1658. [DOI] [PubMed] [Google Scholar]
- Hamdane M, Sambo AV, Delobel P, Bégard S, Violleau A, Delacourte A, Bertrand P, Benaviades J and Buée L (2003) Mitotic-like tau phosphorylation by p25-Cdk5 kinase complex. J. Biol. Chem 278, 34026–34034. [DOI] [PubMed] [Google Scholar]
- Hampton MB and O’Connor KM (2016) Peroxiredoxins and the regulation of cell death. Mol. Cell 39, 72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardman RJ, Kennedy G, Macpherson H, Scholey AB and Pipingas A (2016) Adherence to a Mediterranean-Style Diet and Effects on Cognition in Adults: A Qualitative Evaluation and Systematic Review of Longitudinal and Prospective Trials. Front. Nutr 3, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Devidze N, Halabisky B, Lo I, Thwin MT, Yu G-Q, Bredesen DE, Masliah E and Mucke L (2010) Many neuronal and behavioral impairments in transgenic mouse models of Alzheimer’s disease are independent of caspase cleavage of the amyloid precursor protein. J. Neurosci 30, 372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiguchi M, Sobue K and Paudel HK (2000) 14-3-3zeta is an effector to tau protein phosphorylation. J. Biol. Chem 275, 25247–25254. [DOI] [PubMed] [Google Scholar]
- Hattori F and Oikawa S (2007) Peroxiredoxins in the central nervous system. Subcell. Biochem 44, 357–374. [DOI] [PubMed] [Google Scholar]
- Head E (2013) A canine model of human aging and Alzheimer’s disease. Biochim. Biophys. Acta 1832, 1384–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley K, Carney J, Mattson M, Aksenova M, Harris M, Wu JF, Floyd R and Butterfield DA (1994) A model for β-amyloid aggregation and neurotoxicity based on free radical generating capacity of the peptide: Insights into Alzheimer disease. Proc. Nat. Acad. Sci. USA 91, 3270–3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley K, Hall N, Subramaniam R, Cole R, Harris M, Aksenov M, Aksenova A, Gabbita SP, Wu JF, Carney JM, Lovell M, Markesbery WR and Butterfield DA (1995) Brian regional correspondence between Alzheimer’s disease histopathology and biomarkers of protein oxidation. J. Neurochem 65, 2146–2156. [DOI] [PubMed] [Google Scholar]
- Hensley K, Venkova K, Christov A, Gunning W and Park J (2011) Collapsin response mediator protein-2: An emerging pathologic feature and therapeutic target for neurodisease indications. Mol. Neurobiol 43, 180–191. [DOI] [PubMed] [Google Scholar]
- Hensley K and Kursula P (2016) Collapsin response mediator protein-2 (CRMP2) is a plausible etiological factor and potential therapeutic target in Alzheimer’s disease: Comparison and contrast with microtubule-associated protein tau. J. Alzheimers Dis 53, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L and Rothman DL (2016) Glucose, lactate, β-hydroxybutyrate, acetate, GABA, and succinate as substrates for synthesis of glutamate and GABA in glutamine-glutamate/GABA cycle. Adv. Neurobiol 13, 9–42. [DOI] [PubMed] [Google Scholar]
- Hertz L and Chen Y (2017) Integration between glycolysis and glutamate-glutamine cycle flux may explain preferential glycolytic increase during brain activation, requiring glutamate. Front. Integr. Neurosci 11, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heverin M, Brennan GP, Koehler CJ, Treumann A and Henshall DC (2012) Proteomic analysis of 14-3-3 zeta binding proteins in the mouse hippocampus. Int. J. Physiol. Pathophysiol. Pharmacol 4, 74–83 [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu N, Chiang W-C, Kurt TD, Sigurdson CJ and Lin JH (2015) Multiple mechanisms of unfolded protein response-induced cell death. Am. J. Pathol 185, 1800–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Herz J and Bu G (2012) Apolipoprotein E and apolipoprotein E receptors: Normal biology and roles in Alzheimer disease. Cold Spring Harb. Perspect. Med 2, a006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Aluise CD, Joshi G, Sultana R, St. Clair DK, Markesbery WR and Butterfield DA (2010) Potential in vivo amelioration by N-acetyl-L-cysteine of oxidative stress in brain in human double mutant APP/PS-1 knock-in mice: toward therapeutic modulation of mild cognitive impairment. J. Neurosci. Res 88, 2618–2629. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Chihara K, Arimura N, Menager C, Kawano Y, Matsuo N, Nishimura T, Amano M and Kaibuchi K (2001) CRMP-2 induces axons in cultured hippocampal neurons. Nat. Neurosci 4, 781–782. [DOI] [PubMed] [Google Scholar]
- Jr. Jack C. R., Petersen RC, Xu YC, O’Brien PC, Smith GE, Ivnik RJ, Boeve BF, Waring SC, Tangalos EG and Kokmen E (1999) Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology 52, 1397–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jr. Jack C. R., Lowe VJ, Weigand SD, Wiste HJ, Senjem ML, Knopman DS, Shiung MM, Gunter JL, Boeve BF, Kemp BJ, Weiner M, Petersen RC and the Alzheimer’s Disease Neuroimaging Initiative. (2009) Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: implications for sequence of pathological events in Alzheimer’s disease. Brain 132, 1355–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jr. Jack C. R., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, Elliott C, Masliah E, Ryan L and Silverberg N (2018) NIA-AA Reseearch Framework: Toward a biological definition of Alzheimer’s disease 14, 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett JT, Berger EP and Lansbury PT Jr. (1993) The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer’s disease. Biochemistry 32, 4693–4697. [DOI] [PubMed] [Google Scholar]
- Jovanovic SV, Clements D and MacLeod K (1998) Biomarkers of oxidative stress are significantly elevated in Down syndrome. Free Radic. Biol. Med 25, 1044–1048. [DOI] [PubMed] [Google Scholar]
- Kabuta T, Setsuie R, Mitsui T, Kinugawa A, Sakurai M, Aoki S, Uchida K and Wada K (2008) Aberrant molecular properties shared by familial Parkinson’s disease-associated mutant UCH-L1 and carbonyl-modified UCH-L1. Hum. Mol. Genet 17, 1482–1496. [DOI] [PubMed] [Google Scholar]
- Kaneko K and Hachiya NS (2006) The alternative role of 14-3-3 zeta as a sweeper of misfolded proteins in disease conditions. Med. Hypotheses 67, 169–171. [DOI] [PubMed] [Google Scholar]
- Kato D, Mitake S, Mizuno M, Kanamori T, Suzuki T, Ojika K and Matsukawa N (2012) Co-localization of hippocampal cholinergic neurostimulating peptide precursor with collapsing response mediator protein-2 at presynaptic terminals in hippocampus. Neurosci. Lett 517, 92–97. [DOI] [PubMed] [Google Scholar]
- Kato T, Inui Y, Nakamura A and Ito K (2016) Brain fluordeoxyglucose (FDG) PET in dementia. Ageing Res. Rev 30, 73–84. [DOI] [PubMed] [Google Scholar]
- Keller JN, Hanni KB and Markesbery WR (2000) Impaired proteasome function in Alzheimer’s disease. J. Neurochem 75, 436–439. [DOI] [PubMed] [Google Scholar]
- Kelley BJ and Petersen RC (2007) Alzheimer’s disease and mild cognitive impairment. Neurol. Clin 25, 577–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Fountoulakis M, Cairns N and Lubec G (2001) Protein levels of human peroxiredoxin subtypes in brains of patients with Alzheimer’s disease and Down syndrome. J. Neural. Transm Suppl. 61, 223–235. [DOI] [PubMed] [Google Scholar]
- Klaips CL, Jayaraj GG and Hartl FU (2018) Pathways of cellular proteostasis in aging and disease. J. Cell. Biol 217, 51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsknecht-Talboo J and Landreth GE (2005) Microglial phagocytosis induced by fibrillar beta-amyloid and IgGs are differentially regulated by proinflammatory cytokines. J. Neurosci 25, 8240–8249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korolainen MA, Nyman TA, Nyyssönen P, Hartikainen ES and Pirttilä T (2007) Multiplexed proteomic analysis of oxidation and concentrations of cerebrospinal fluid proteins in Alzheimer’s disease. Clinical Chem 53, 657–665. [DOI] [PubMed] [Google Scholar]
- Koščak TB (2017) Physical activity improves cognition: possible explanations. Biogerontology 18, 477–483. [DOI] [PubMed] [Google Scholar]
- Krapfenbauer K, Engidawork E, Cairns N, Fountoulakis M and Lubec G (2003) Aberrant expression of peroxiredoxin subtypes in neurodegenerative disorders. Brain Res 967, 152–160. [DOI] [PubMed] [Google Scholar]
- Kumar VB, Farr SA, Flood JF, Kamlesh V, Franko M, Banks WA, and Morley JE (2000) Site-directed antisense oligonucleotide decreases the expression of amyloid precursor protein and reverses deficits in learning and memory in aged SAMP8 mice. Peptides 21, 1769–1775. [DOI] [PubMed] [Google Scholar]
- Kumar VB, Franko M, Banks WA, Kasinadhuni P, Farr SA Vyas K, Choudhuri V and Morley JE (2009) Increase in presenilin 1 (PS1) levels in senescence-accelerated mice (SAMP8) may indirectly impair memory by affecting amyloid precursor protein (APP) processing. J. Exp. Biol 212, 494–498. [DOI] [PubMed] [Google Scholar]
- Kumsta C and Jakob U (2009) Redox-Regulated Chaperones. Biochemistry 48, 4666–4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundra R, Ciryam P, Morimoto RI, Dobson CM and Vendruscolo M (2017) Protein homeostasis of a metastable subproteome associated with Alzheimer’s disease. Proc. Natl. Acad. Sci. U. S. A 114, E5703–E5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JC, Mann DM, Harris JM, Chartier-Harlin MC, Cumming A, Coates J, Lemmon H, St. Clair D, Iwatsubo T and Lendon C (2001) The −48 C/T polymorphism in the presenilin 1 promoter is associated with increased Abeta load in brain. J. Med. Genet 38, 353–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latta CH, Brothers HM and Wilcock DM (2015) Neuroinflammation in Alzheimer’s disease; a source of heterogeneity and target for personalized therapy. Neuroscience 302, 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauderback CM, Hackett JM, Huang FF, Keller JN, Szweda LI, Markesbery WR and Butterfield DA (2001) The glial glutamate transporter, GLT-1, is oxidatively modified by 4-hydroxy-2-nonenal in the Alzheimer’s disease brain: Role of Aβ1–42. J. Neurochem 78, 413–416. [DOI] [PubMed] [Google Scholar]
- Launer LJ, Ross GW, Petrovitch H, Massaki K, Foley D, White LR and Havlik RJ (2000) Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol. Aging 21, 49–55. [DOI] [PubMed] [Google Scholar]
- Layfield R, Fergusson J, Aitken A, Lowe J, Landon M and Mayer RJ (1996) Neurofibrillary tangles of Alzheimer’s disease brains contain 14-3-3 proteins. Neurosci. Lett 209, 57–60. [DOI] [PubMed] [Google Scholar]
- Li C, Zhao R, Gao K, Wei Z, Yin MY, Lau LT, Chui D and Yu AC (2011) Astrocytes: implications for neuroinflammatory pathogenesis of Alzheimer’s disease. Curr. Alzheimer Ra 8, 67–80. [DOI] [PubMed] [Google Scholar]
- Li T and Paudel HK (2016) 14-3-3ζ mediates tau aggregation in human neuroblastoma M17 cells. PLoS ONE 11, e0160635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C-H, Li H-Y, Lee Y-C, Calkins MJ, Lee K-H, Yang C-N and Lu P-J (2015) Landscape of Pin1 in the cell cycle. Exp. Biol. Med 240, 403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YT, Cheng JT, Liang LC, Ko CY, Lo YK and Lu PJ (2007) The binding and phosphorylation of Thr231 is critical for Tau’s hyperphosphorylation and functional regulation by glycogen synthase kinase 3 beta. J. Neurochem 103, 802–813. [DOI] [PubMed] [Google Scholar]
- Liou YC, Sun A, Ryo A, Zhou XZ, Huang HK, Uchida T, Bronson R, Bing G, Li X, Hunter T and Lu KP (2003) Role of prolyl isomerase Pin1 in protecting against age-dependent neurodegeneration. Nature 424, 556–561. [DOI] [PubMed] [Google Scholar]
- Liou YC, Zhou XZ and Lu KP (2011) Prolyl isomerase Pin1 as a molecular switch to determine the fate of phosphoproteins. Trends Biochem. Sci 36, 501–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lista S, Faltraco F, Prvulovic D and Hampel H (2013) Blood and plasma-based proteomic biomarker research in Alzheimer’s disease. Prog. Neurobiol 101–102, 1–17. [DOI] [PubMed] [Google Scholar]
- Liu Y, Fallon L, Lashuel HA, Liu Z and Lansbury PT Jr. (2002) The UCH-L1 gene encodes two opposing enzymatic activities that affect alpha-synuclein degradation and Parkinson’s disease susceptibility. Cell 111, 209–218. [DOI] [PubMed] [Google Scholar]
- Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, Ballard C, Banerjee S, Burns A, Cohen-Mansfield J, Cooper C, Fox N, Gitlin LN, Howard R, Kales HC, Larson EB, Ritchie K, Rockwood K, Sampson EL Samus Q, Schneider LS, Selbæk G, Teri L, and Mukadam N (2017) Dementia prevention, intervention, and care. Lancet 390, 2673–2734. [DOI] [PubMed] [Google Scholar]
- Loef M and Walach H (2013) Midlife obesity and dementia: meta-analysis and adjusted forecast of dementia prevalence in the United States and China. Obesity (Silver Spring) 21, E51–E55. [DOI] [PubMed] [Google Scholar]
- Lopez-Salon M, Alonso M, Vianna MR, Viola H, Mello e Souza T, Izquierdo I, Pasquini JM and Medina JH (2001) The ubiquitin-proteasome cascade is required for mammalian long-term formation. Eur. J. Neurosci 14, 1820–1826. [DOI] [PubMed] [Google Scholar]
- Lopez-Salon M, Pasquini L, Besio Moreno M, Paswuini JM and Soto E (2003) Relationship between beta-amyloid degradation and the 26S proteasome in neural cells. Exp. Neurol 180, 131–143. [DOI] [PubMed] [Google Scholar]
- Lott IT and Head E (2005) Alzheimer’s disease and Down syndrome: factors in pathogenesis. Neurbiol. Aging 26, 383–389. [DOI] [PubMed] [Google Scholar]
- Lott IT and Dierssen M (2010) Cognitive deficits and associated neurological complications in individuals with Down’s syndrome. Lancet Neurol 9, 623–633. [DOI] [PubMed] [Google Scholar]
- Lourida I, Soni M, Thompson-Coon J, Purandare N, Lang IA, Ukoumunne OC and Llewellyn DJ (2013) Mediterranean diet, cognitive function, and dementia: a systematic review. Epidemiology 24, 479–489. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Xie C and Markesbery WR (1998) Decreased glutathione transferase activity in brain and ventricular fluid in Alzheimer’s disease. Neurology 51, 1562–1566. [DOI] [PubMed] [Google Scholar]
- Loy CT, Schofield PR, Turner AM and Kwok JBJ (2014) Genetics of dementia. Lancet 383, 828–840. [DOI] [PubMed] [Google Scholar]
- Lu KP, Hanes SD and Hunter T (1996) A human peptidyl-prolyl isomerase essential for regulation of mitosis. Nature 380, 544–547. [DOI] [PubMed] [Google Scholar]
- Lu KP and Zhou XZ (2007) The prolyl isomerase PIN1: a pivotal new twist in phosphorylation signaling and disease. Nat. Rev. Mol. Cell. Biol 8, 904–916. [DOI] [PubMed] [Google Scholar]
- Lu PJ, Zhou XZ, Shen M and Lu KP (1999a) Function of WW domains as phosphoserine- or phosphothreonine-binding modules. Science 283, 1325–1328. [DOI] [PubMed] [Google Scholar]
- Lu PJ, Wulf G, Zhou XZ, Davies P and Lu KP (1999b) The prolyl isomerase Pin1 restores the function of Alzheimer-associated phosphorylated tau protein. Nature 399, 784–788. [DOI] [PubMed] [Google Scholar]
- Luczaj W, Gegotek A and Skrzydlewska E (2017) Antioxidants and HNE in redox homeostasis. Free Radic. Biol. Med 111, 87–101. [DOI] [PubMed] [Google Scholar]
- Mahley RW and Rall SC Jr. (2000) Apolipoprotein E: Far more than a lipid transport protein. Annu. Rev. Genomics. Hum. Genet 1, 507–537. [DOI] [PubMed] [Google Scholar]
- Maki M, Matsukawa N, Yuasa H, Otsuka Y, Yamamoto T, Akatsu H, Okamoto T, Ueda R and Ojika K (2002) Decreased expression of hippocampal cholinergic neurostimulating peptide precursor protein mRNA in the hippocampus in Alzheimer’s disease. J. Neuropathol. Exp. Neurol 61, 176–185. [DOI] [PubMed] [Google Scholar]
- Marcus DL and Freedman ML (1997) Decreased brain glucose metabolism in microvessels from patients with Alzheimer’s disease. Ann. N. Y. Acad. Sci 826, 248–253. [DOI] [PubMed] [Google Scholar]
- Mark RJ, Lovell MA, Markesbery WR, Uchida K and Mattson MP (1997) A role for 4-hydroxynonenal, an aldehydic product of lipid peroxidation, in disruption of ion homeostasis and neuronal death induced by amyloid beta-peptide. J. Neurochem 68, 255–264. [DOI] [PubMed] [Google Scholar]
- Markesbery WR (1997) Oxidative stress hypothesis in Alzheimer’s disease. Free Radic. Biol. Med 23, 134–147. [DOI] [PubMed] [Google Scholar]
- Markesbery WR and Lovell MA (1998) Four-hydroxynonenal, a product of lipid peroxidation, is increased in the brain in Alzheimer’s disease. Neurobiol. Aging 19, 33–36. [DOI] [PubMed] [Google Scholar]
- Masliah E, Alford M, DeTeresa R, Mallory M and Hansen L (1996) Deficient glutamate transport is associated with neurodegeneration in Alzheimer’s disease. Ann. Neurol 40, 759–766. [DOI] [PubMed] [Google Scholar]
- Mattson MP (1997) Cellular actions of beta-amyloid precursor protein and its soluble and fibrillogenic derivatives. Physiol. Rev 77, 1081–1132. [DOI] [PubMed] [Google Scholar]
- Mattsson N, Zetterberg H, Hansson O, Andreasen N, Parnetti L, Jonsson M, Herukka SK, van der Flier WM, Blankenstein MA, Ewers M, Rich K, Kaiser E, Verbeek M, Tsolaki M, Mulugeta E, Rosén E, Aarsland D, Visser PJ, Schröder J, Marcusson J, de Leon M, Hampel H, Scheltens P, Pirttilä T, Wallin A, Jönhagen ME, Minthon L, Winblad B and Blennow K (2009) CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. J. Am. Med. Assoc 302, 385–393. [DOI] [PubMed] [Google Scholar]
- McBean GJ, Aslan M, Griffiths HR and Torrão RC (2015) Thiol redox homeostasis in neurodegenerative disease. Redox Biology 5, 186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecocci P, MacGarvey U and Beal MF (1994) Oxidative damage to mitochondrial DNA is increased in Alzheimer’s disease. Ann. Neurol 36, 747–751. [DOI] [PubMed] [Google Scholar]
- Meng XF, Yu JT, Wang HF, Tan MS, Wang C, Tan CC and Tan L (2014) Midlife vascular risk factors and the risk of Alzheimer’s disease: a systematic review and meta-analysis. J. Alzheimers Dis 42, 1295–1310. [DOI] [PubMed] [Google Scholar]
- Mergenthaler P, Lindauer U, Dienel GA and Meisel A (2013) Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci 36, 587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgram NW, Head E, Zicker SC, Ikeda-Douglas C, Murphey H, Muggenberg MA, Siwak CT, Tapp RD, Lowry SR and Cotman CW (2004) Long-term treatment with antioxidants and a program of behavioral enrichment reduces age-dependent impairment in discrimination and reversal learning in beagle dogs. Exp. Gerontol 39, 753–765. [DOI] [PubMed] [Google Scholar]
- Minter MR, Taylor JM and Crack PJ (2016) The contribution of neuroinflammation to amyloid toxicity in Alzheimer’s disease. J. Neurochem 136, 457–474. [DOI] [PubMed] [Google Scholar]
- Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, Klunk WE, Mathis CA, DeKosky ST and Morris JC (2006) [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology 67, 446–452. [DOI] [PubMed] [Google Scholar]
- Mishto M, Bellavista E, Santoro A, Stolzing A, Ligorio C, Nacmias B, Spazzafumo L, Chiappelli M, Licasto F, Sorbi S, Pession A, Ohm T, Grune T, Franceschi C (2006) Immunoproteasome and LMP2 polymorphism in aged and Alzheimer’s disease brains. Neurobiol. Aging 27, 54–66. [DOI] [PubMed] [Google Scholar]
- Mori C, Spooner ET, Wisniewsk KE, Wisniewski TM, Yamaguch H, Saido TC, Tolan DR, Selkoe DJ and Lemere CA (2002) Intraneuronal Abeta42 accumulation in Down syndrome brain. Amyloid 9, 88–102. [PubMed] [Google Scholar]
- Morley JE, Armbrecht HJ, Farr SA and Kumar VB (2012) The senescence accelerated mouse (SAMP8) as a model for oxidative stress and Alzheimer’s disease. Biochim. Biophys. Acta 1822, 650–656. [DOI] [PubMed] [Google Scholar]
- Morley JE, Kumar VB, Bernardo AE, Farr SA, Uezu K, Tumosa N and Flood JF (2000) β-amyloid precursor protein in SAMP8 mice affects learning and memory. Peptides 21, 1761–1767. [DOI] [PubMed] [Google Scholar]
- Morris MC, Tangney CC, Wang Y, Sacks FM, Barnes LL, Bennett DA, and Aggarwal NT (2015a) MIND diet slows cognitive decline with aging. Alzheimers Dement 11, 1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA and Aggarwal NT (2015b) MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement 11, 1007–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu G-Q, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K and McConlogue L (2000) High-level neuronal expression of Aβ1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J. Neurosci 20, 4050–4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MP, Beckett TL, Ding Q, Patel E, Markesbery WR, St. Clair DK, LeVine H III. and Keller JN (2007) Aβ solubility and deposition during AD progression and in APP × PS-1 knock-in mice. Neurobiol. Dis 27, 301–311. [DOI] [PubMed] [Google Scholar]
- Murphy N, Bonner HP, Ward MW, Murphy BM, Prehn JH and Henshall DC (2008) Depletion of 14-3-3 zeta elicits endoplasmic reticulum stress and cell death and increases vulnerability to kainate-induced injury in mouse hippocampal culture. J. Neurchem 106, 978–988. [DOI] [PubMed] [Google Scholar]
- Nagele RG, D’Andrea MR, Lee H, Venkataraman V and Wang HY (2003) Astrocytes accumulate A beta 42 and give rise to astrocytic amyloid plaques in Alzheimer disease brain. Brain Res 971, 179–209. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Tu S, Akhtar MW, Sunico CR, Okamoto S and Lipton SA (2013) Aberrant protein S-nitrosylation in neurodegenerative diseases. Neuron 78, 596–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijman SM, Luna-Vargas MP, Velds A, Brummelkamp TR, Dirac AM, Sixma TK and Bernards R (2005) A genomic and functional inventory of deubiquitinating enzymes. Cell 123, 773–786. [DOI] [PubMed] [Google Scholar]
- Ninomiya T, Ohara T, Hirakawa Y, Yoshida D, Hata J, Kanba S, Iwaki T and Kiyohara Y (2011) Midlife and late-life blood pressure and dementia in Japanese elderly: the Hisayama study. Hypertension 58, 22–28. [DOI] [PubMed] [Google Scholar]
- Nisikawa K, Li H, Kawamura R, Osaka H, Wang YL, Hara Y, Hirokawa T, Manago Y, Amano T, Noda M, Aoki S and Wada K (2003) Alterations of structure and hydrolase activity of parkinsonism-associated human ubiquitin carboxyl-terminal hydrolase L1 variants. Biochem. Biophys. Res. Commun 304, 176–183. [DOI] [PubMed] [Google Scholar]
- Nomura Y, Yamanaka Y, Kitamura Y, Arima T, Ohnuki T, Oomura Y, Sasaki K, Nagashima K and Ihara Y (1996) Senescence-accelerated mouse. Neurochemical studies on aging. Ann. N. Y. Acad. Sci 786, 410–418. [DOI] [PubMed] [Google Scholar]
- Oda T, Wals P, Osterburg HH, Johnson SA, Pasinetti GM, Morgan TE, Rozovsky I, Stine WB, Snyder SW, Holzman TF, Krafft GA and Finch CE (1995) Clusterin (apoJ) alters the aggregation of amyloid β-peptide (Aβ1–42) and forms slowly sedimenting Aβ complexes that cause oxidative stress. Exp. Neurol 136, 22–31. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y and LaFerla FM (2003a) Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron 39, 409–421. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Kitazawa M, Tseng BP and LaFerla FM (2003b) Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol. Aging 24, 1063–1070. [DOI] [PubMed] [Google Scholar]
- Ojika K, Mitake S, Tohdoh N, Appel SH, Otsuka Y, Katada E and Matsukawa N (2000) Hippocampal cholinergic neurostimulating peptides (HCNP). Prog. Neurobiol 60, 37–83. [DOI] [PubMed] [Google Scholar]
- Opii WO, Joshi G, Head E, Milgram NW, Muggenburg BA, Klein JB, Pierce WM, Cotman CW and Butterfield DA (2008) Proteomic identification of brain proteins in the canine model of human aging following a long-term treatment with antioxidants and a program of behavioral enrichment: Relevance to Alzheimer’s disease. Neurobiol. Aging 29, 51–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaka H, Wang YL, Takada K, Takizawa D, Setsuie R, Li H, Sato Y, Nishikawa K, Sun YJ, Sakurai M, Harada T, Hara Y, Kimura I, Chiba S, Namikawa K, Kiyama H, Noda M, Aoki S and Wada K (2003) Ubiquitin carboxy-terminal hydrolase L1 binds to and stabilizes monubiquitin in neuron. Hum. Mol. Genet 12, 1945–1958. [DOI] [PubMed] [Google Scholar]
- Pastorino L, Sun A, Lu P-J, Zhou XZ, Balastik M, Finn G, Wulf G, Lim J, Li S-H, Li X, Xia W, Nicholson LK and Lu KP (2006) The prolyl isomerase Pin1 regulates amyloid precursor protein processing and amyloid-β production. Nature 440, 528–534. [DOI] [PubMed] [Google Scholar]
- Pekny M and Pekna M (2004) Astrocyte intermediate filaments in CNS pathologies and regeneration. J. Pathol 204, 428–437. [DOI] [PubMed] [Google Scholar]
- Penke B, Bogár F, Crul T, Sántha M, Tóth ME and Vígh L (2018) Heat shock proteins and autophagy pathways in neuroprotection: from molecular bases to pharmacological interventions. Int. J. Mol. Sci 19, 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perluigi M, Sultana R, Cenini G, Di Domenico F, Memo M, Pierce WM, Coccia R and Butterfield DA (2009) Redox proteomics identification of 4-hydroxynonenal-modified brain proteins in Alzheimer’s disease: Role of lipid peroxidation in Alzheimer’s disease pathogenesis. Proteomics Clin. Appl 3, 682–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perluigi M and Butterfield DA (2011) The identification of protein biomarkers for oxidative stress in Down syndrome. Expert Rev. Proteomics 8, 427–429. [DOI] [PubMed] [Google Scholar]
- Perluigi M and Butterfield DA (2012) Oxidative stress and Down syndrome: A route toward Alzheimer-like dementia. Curr. Gerontol. Geriatr. Res 2012, 724904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC (2000) Mild cognitive impairment: transition between aging and Alzheimer’s disease. Neurologia 15, 93–101. [PubMed] [Google Scholar]
- Petersen RC (2004) Mild cognitive impairment as a diagnostic entity. J. Intern. Med 256, 183–194. [DOI] [PubMed] [Google Scholar]
- Pietrzak RH, Lim YY, Ames D, Harrington K, Restrepo C, Martins RN, Rembach A, Laws SM, Masters CL, Villemagne VL, Rowe CC, Maruff P and Australian Imaging, Biomarkers and Lifestyle Flagship Study of ageing. (2015) Trajectories of memory decline in preclinical Alzheimer’s disease: results from the Australian Imaging, Biomarkers and Lifestyle Flagship Study of ageing. Neurobiol. Aging 36, 1231–1238. [DOI] [PubMed] [Google Scholar]
- Poon HF, Castegna A, Farr SA, Thongboonkerd V, Lynn BC, Banks WA, Morley JE, Klein JB and Butterfield DA (2004a) Quantitative proteomics analysis of specific protein expression and oxidative modification in aged senescence-accelerated-prone 8 mice brain. Neuroscience 126, 915–926. [DOI] [PubMed] [Google Scholar]
- Poon HF, Joshi G, Sultana R, Farr SA, Banks WA, Morley JE, Calabrese V, and Butterfield DA (2004b) Antisense directed at the Aβ region of APP decreases brain oxidative markers in aged senescence accelerated mice. Brain Res 1018, 86–96. [DOI] [PubMed] [Google Scholar]
- Poon HF, Farr SA, Banks WA, Pierce WM, Klein JB, Morley JE and Butterfield DA (2005) Proteomic identification of less oxidized brain proteins in aged senescence-accelerated mice following administration of antisense oligonucleotide directed at the Aβ region of amyloid precursor protein. Molecular Brain Res 138, 8–16. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan P, Dickson DW and Davies P (2003) Pin1 colocalization with phosphorylated tau in Alzheimer’s disease and other tauopathies. Neurobiol. Dis 14, 251–264. [DOI] [PubMed] [Google Scholar]
- Ramser EM, Wolters G, Dityateva G, Dityatev A, Schachner M and Tilling T (2010) The 14-3-3ζ protein binds to the cell adhesion molecule L1, promotes L1 phosphorylation by CKII and influences L1-dependent neurite outgrowth. PLoS ONE 5, e13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan R, Lu KP, Hunter T and Noel JP (1997) Structural and functional analysis of the mitotic rotamase Pin1 suggests substrate recognition is phosphorylation dependent. Cell 89, 875–886. [DOI] [PubMed] [Google Scholar]
- Reddy PH and Reddy TP (2011) Mitochondria as a therapeutic target for aging and neurodegenerative diseases. Curr. Alzheimer Res 8, 393–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed T, Perluigi M, Sultana R, Pierce WM, Klein JB, Turner DM, Coccia R, Markesbery WR and Butterfield DA (2008) Redox proteomic identification of 4-hydroxy-2-nonenal-modified brain proteins in amnestic mild cognitive impairment: Insight into the role of lipid peroxidation in the progression and pathogenesis of Alzheimer’s disease. Neurobiol. Dis 30, 107–120. [DOI] [PubMed] [Google Scholar]
- Reed TT, Pierce WM, Markesbery WR and Butterfield DA (2009) Proteomic identification of HNE-bound proteins in early Alzheimer’s disease: Insights into the role of lipid peroxidation in the progression of AD. Brain Res 1274, 66–76. [DOI] [PubMed] [Google Scholar]
- Reed TT, Sultana R and Butterfield DA (2010) Redox proteomics of oxidatively modified brain proteins in Mild Cognitive Impairment, in Neuroproteomics, (Alzate O ed), CRC Press/Taylor & Frances, Boca Raton. [PubMed] [Google Scholar]
- Reiman EM, Zuiroz YT, Fleisher AS, Chen K, Velez-Pardo C, Jimenez-Del-Rio M, Fagan AM, Shah AR, Alvarez S, Arbelaez A, Giraldo M, Acosta-Baena N, Sperling RA, Dickerson B, Stern CE, Tirado V, Munoz C, Reiman RA, Huentelman MJ, Alexander GE, Langbaum JBM, Kosik KS, Tariot PN and Lopera F (2012) Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer’s disease in the presenilin 1 E280A kindred: a case-control study. Lancet Neurol 11, 1048–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz C, Brayne C and Mayeux R (2011) Epidemiology of Alzheimer disease. Nat. Rev. Neurol 7, 137–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SG and Kil IS (2017) Multiple functions and regulations of mammalian peroxiredoxins. Annu. Rev. Biochem 86, 749–775. [DOI] [PubMed] [Google Scholar]
- Robinson RAS, Lange MB, Sultana R, Galvan V, Fombonne J, Gorostiza O, Zhang J, Warrie G, Cai J, Pierce WM, Bredesen DE and Butterfield DA (2011) Differential expression and redox proteomics analyses of an Alzheimer disease transgenic mouse model: effects of the amyloid-β peptide of amyloid precursor protein. Neuroscience 177, 207–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogemond V, Auger C, Giraudon P, Bechi M, Auvergnon N, Belin M-F, Honnorat J and Moradi-Ameli M (2008) Processing and nuclear localization of CRMP2 during brain development induce neurite outgrowth inhibition. J. Biol. Chem 283, 14751–14761. [DOI] [PubMed] [Google Scholar]
- Rönnemaa E, Zethelius B, Lannfelt L and Kilander L (2011) Vascular risk factors and dementia: 40-year follow-up of a population-based cohort. Dement. Geriatr, Cogn. Disord 31, 460–466. [DOI] [PubMed] [Google Scholar]
- Ross CA and Poirier MA (2004) Protein aggregation and neurodegenerative disease. Nat. Med 10, S10–S17. [DOI] [PubMed] [Google Scholar]
- Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, Fripp J, Tochon-Danguy H, Morandeau L, O’Keefe G, Price R, Raniga P, Robins P, Acosta O, Lenzo N, Szoeke C, Salvado O, Head R, Martins R, Masters CL, Ames D and Villemagne VL (2010) Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol. Aging 31, 1275–1283. [DOI] [PubMed] [Google Scholar]
- Safra M, Ben-Hamo S, Kenyon C and Henis-Korenblit S (2013) The ire-1 ER-stress-response pathway is required for normal secretory-protein metabolism in C. elegans. J. Cell. Sci 126, 4136–4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Ohi Y, Kato D, Mizuno M, Takase H, Kanamori T, Borlongan CV, Haji A and Matsukawa N (2017) Hippocampal cholinergic neurostimulating peptide as a possible modulating factor against glutamatergic neuronal disability by amyloid oligomers. Cell Tansplant 26, 1542–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, Larson E, Levy-Lahad E, Viitanen M, Peskind E, Poorkaj P, Schellenberg G, Tanzi R, Wasco W, Lannfelt L, Selkoe D and Younkin S Secreted amyloid-beta protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat. Med 2, 864–870. [DOI] [PubMed] [Google Scholar]
- Schon EA and Area-Gomez E (2013) Mitochondria-associated ER membranes in Alzheimer disease. Mol. Cell. Neurosci 55, 26–36. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ (2001) Alzheimer’s disease: genes, proteins, and therapy. Physiol. Rev 81, 741–766. [DOI] [PubMed] [Google Scholar]
- Setsuie R and Wada K (2007) The functions of UCH-L1 and its relation to neurodegenerative diseases. Neurochem. Intl 51, 105–111. [DOI] [PubMed] [Google Scholar]
- Shan X and Lin CL (2006) Quantification of oxidized RNAs in Alzheimer’s disease. Neurobiol. Aging 27, 657–662. [DOI] [PubMed] [Google Scholar]
- Sharma R, Yang Y, Sharma A, Awasthi A and Awasthi YC (2004) Antioxidant role of glutathione S-transferases: protection against oxidant toxicity and regulation of stress-mediated apoptosis. Antioxid. Redox. Signal 6, 289–300. [DOI] [PubMed] [Google Scholar]
- Shen L, Chen C, Yang A, Chen Y, Liu Q and Ni J (2015) Redox proteomics identification of specifically carbonylated proteins in the hippocampi of triple transgenic Alzheimer’s disease mice at its earliest pathological stage. J. Proteomics 123,101–113. [DOI] [PubMed] [Google Scholar]
- Siems W and Grune T (2003) Intracellular metabolism of 4-hydroxynonenal. Mol. Asp. Med 24, 167–175. [DOI] [PubMed] [Google Scholar]
- Silver J and Miller JH (2004) Regeneration beyond the glial scar. Nat. Rev. Neurosci 5, 146–156. [DOI] [PubMed] [Google Scholar]
- Singhal SS, Singh SP, Singhal P, Horne D, Singhal J and Awasthi S (2015) Antioxidant role of glutathione S-transferases: 4-hydroxynonenal, a key molecule in stress-mediated signaling. Toxicol. Appl. Pharmacol 289, 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluchanko N and Gusev NB (2011) Probable participation of 14-3-3 in tau protein oligomerization and aggregation. J. Alzheimer Dis 27, 467–476. [DOI] [PubMed] [Google Scholar]
- Small BJ, Fratiglioni L and Bäckman L (2001) Canaries in a coal mine: cognitive markers of preclinical Alzheimer’s disease. Arch Gen. Psychiatry 58, 859–860. [DOI] [PubMed] [Google Scholar]
- Smith CD, Carney JM, Starke-Reed PE, Oliver CN, Stadtman ER and Floyd RA (1991) Excess brain protein oxidation and enzyme dysfunction in normal and Alzheimer’s disease. Proc. Natl. Acad. Sci. U. S. A 87, 10540–10543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Richey PL, Harris LM, Sayre JS and Beckman G (1997) Widespread peroxynitrite-mediated damage in Alzheimer’s disease. J. Neurosci 17, 2653–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV (2009) Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 32, 638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O and Shinohara M (1977) The [14C]deoxyglucose method for measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J. Neurochem 28, 897–916. [DOI] [PubMed] [Google Scholar]
- Solfrizzi V, Panza F, Frisardi V, Seripa D, Logroscino G Imbimbo BP and Pilotto A (2011) Diet and Alzheimer’s disease risk factors or prevention: the current evidence. Expert. Rev. Neurother 11, 677–708. [DOI] [PubMed] [Google Scholar]
- Soloman A, Kivipelto M, Wolozin B, Zhou J and Whitmer RA (2009) Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement. Geriatr. Cogn. Disord 28, 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompol P, Ittarat W, Tangpong J, Chen Y, Doubinskaia I, Batinic-Haberle I, Abdul HM, Butterfield DA and St. Clair DK (2008) A neuronal model of Alzheimer’s disease: an insight into the mechanisms of oxidative stress-mediated mitochondrial injury. Neuroscience 153, 120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR Jr., Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrilo MC, Thies B, Morrison-Bogorad M, Wagster MV and Phelps CH (2011) Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association working groups on diagnostic guidelines for Alzheimer’s disease. Alzherimers Dement 7: 280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinacker P, Aitken A and Otto M (2011) 14-3-3 proteins in neurodegeneration. Semin. Cell Dev. Biol 22, 696–704. [DOI] [PubMed] [Google Scholar]
- Stephen R, Hongisto K, Solomon A and Lönnroos E (2017) Physical activity and Alzheimer’s disease: A Systematic Review. J. Gerontol. A. Biol. Med. Sci 72, 733–739. [DOI] [PubMed] [Google Scholar]
- Subramaniam R, Roediger F, Jordan B, Mattson MP, Keller JN, Waeg G and Butterfield DA (1997) The lipid peroxidation product, 4-hydroxy-2-trans-nonenal, alters the conformation of cortical synaptosomal membrane proteins. J. Neurochem 69, 1161–1169. [DOI] [PubMed] [Google Scholar]
- Sultana R and Butterfield DA (2004) Oxidatively modified GST and MRP1 in Alzheimer’s disease brain: Implications for accumulation of reactive lipid peroxidation products. Neurochem. Res 29, 2215–2220. [DOI] [PubMed] [Google Scholar]
- Sultana R, Poon HF, Cai J, Pierce WM, Merchant M, Klein JB, Markesbery WR and Butterfield DA (2006a) Identification of nitrated proteins in Alzheimer’s disease brain using a redox proteomics approach. Neurobiol. Dis 22, 76–87. [DOI] [PubMed] [Google Scholar]
- Sultana R, Boyd-Kimball D, Poon HF, Cai J, Pierce WM, Klein JB, Markesbery WR, Zhou XZ, Lu KP and Butterfield DA (2006b) Oxidative modification and down-regulation of Pin1 in Alzheimer’s disease hippocampus: A redox proteomics analysis. Neurobiol. Aging 27, 918–925. [DOI] [PubMed] [Google Scholar]
- Sultana R, Boyd-Kimball D, Poon HF, Cai J, Pierce WM, Klein JB, Merchant M, Markesbery WR and Butterfield DA (2006c) Redox Proteomics Identification of Oxidized Proteins in Alzheimer’s Disease Hippocampus and Cerebellum: An Approach to Understand Pathological and Biochemical Alterations in AD. Neurobiol. Aging 27, 1564–1576. [DOI] [PubMed] [Google Scholar]
- Sultana R, Newman SF, Mohmmad-Abdul H, Cai J, Pierce WM, Klein JB, Merchant M and Butterfield DA (2006d) Protective Effect of D609 Against Amyloid-Beta1–42-Induced Oxidative Modification of Neuronal Proteins: Redox Proteomics Study. J. Neurosci. Res 84, 409–417. [DOI] [PubMed] [Google Scholar]
- Sultana R, Reed T, Perluigi M, Coccia R, Pierce WM and Butterfield DA (2007) Proteomic identification of nitrated brain proteins in amnestic mild cognitive impairment: A regional study. J. Cell. Molec. Med 11, 839–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana R and Butterfield DA (2009) Proteomics identification of carbonylated and HNE-bound brain proteins in Alzheimer’s disease. Methods Mol. Biol 566, 123–135. [DOI] [PubMed] [Google Scholar]
- Sultana R, Perluigi M and Butterfield DA (2009) Oxidative modified proteins in Alzheimer’s disease (AD), mild cognitive impairment, and animal models of AD: role of Abeta in pathogenesis. Acta Neuropathol 118, 131–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana R, Perluigi M, Newman SF, Pierce WM, Cini C, Coccia R and Butterfield DA (2010) Redox proteomic analysis of carbonylated brain proteins in mild cognitive impairment and early Alzheimer’s disease. Antioxid. Redox Signal 12, 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana R and Butterfield DA (2011) Identification of the oxidative stress proteome in brain. Free Radic. Biol. Med 50, 487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana R, Robinson RAS, Di Domenico F, Abdul HM, St. Clair DK, Markesbery WR, Cai J, Pierce WM and Butterfield DA (2011a) Proteomics identification of specifically carbonylated brain proteins in APPNLh/APPNLh × PS-1P264L/PS-1P264L human double mutant knock-in mice model of Alzheimer disease as a function of age. J. Proteomics 74, 2430–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana R, Mecocci P, Mangialasche F, Cecchetti R and Butterfield DA (2011b) Increased protein and lipid oxidative damage in mitochondria isolated from lymphocytes from patients with Alzheimer disease: Insights into the role of oxidative stress in Alzheimer’s disease and initial investigations into a potential biomarker for this dementing disorder. J. Alzheimers Dis 24, 77–84. [DOI] [PubMed] [Google Scholar]
- Sultana R and Butterfield DA (2013) Oxidative modification of brain proteins in Alzheimer’s disease: perspective on future studies based on results of redox proteomics studies. J. Alzheimers Dis 33, S243–251. [DOI] [PubMed] [Google Scholar]
- Sultana R, Perluigi M and Butterfield DA (2013a) Lipid peroxidation triggers neurodegeneration: a redox proteomics view into the Alzheimer disease brain. Free Radic. Biol. Mol 62, 157–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana R, Baglioni M, Cecchetti R, Cai J, Klein JB, Bastiani P, Ruggiero C, Mecocci P and Butterfield DA (2013b) Lymphocyte mitochondria: toward identification of peripheral biomarkers in progression of Alzheimer disease. Free Radic. Biol. Med 65, 595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swomley AM, Förster S, Keeney JT, Triplett J, Zhang Z, Sultana R and Butterfield DA (2014) Abeta, oxidative stress in Alzheimer disease: Evidence based on proteomics studies. Biochim. Biophys. Acta 1842, 1248–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swomley AM and Butterfield DA (2015) Oxidative stress in Alzheimer disease and mild cognitive impairment: evidence from human data provided by redox proteomics. Arch Toxicol 89, 1669–1680. [DOI] [PubMed] [Google Scholar]
- Takeda T (2009) Senescence-accelerated mouse (SAM) with special reference to neurodegeration models, SAMP8 and SAMP10 mice. Neurochem. Res 34, 639–659. [DOI] [PubMed] [Google Scholar]
- Takemura M, Nakamura S, Akiguchi I, Ueno M, Oka N, Ishikawa S, Shimada A, Kimura J and Takeda T (1993) β/A4 protein-like immunoreactive granular structures in the brain of senescence-accelerated mice. Am. J. Pathol 142, 1887–1897. [PMC free article] [PubMed] [Google Scholar]
- Tan ZS, Spartano NL, Beiser AS, DeCarli C, Auerbach SH, Vasan RS and Seshadri S (2017) Physical activity, brain volume, and dementia risk: The Framingham Study. J. Gerontol. A Biol. Sci. Med. Sci 72, 789–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchese F, Liu S, Battaglia F, Walter S, Mathews PM and Arancio O (2004) Progressive age-related development of Alzheimer-like pathology in APP/PS1 mice. Ann. Neurol 55, 801–814. [DOI] [PubMed] [Google Scholar]
- Tseng BP, Green KN, Chan JL, Blurton-Jones M and LaFerla FM (2008) Abeta inhibits the proteasome and enhances amyloid and tau accumulation. Neurobiol. Aging 29, 1607–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu S, Okamoto S, Lipton SA and Xu H (2014) Oligomeric Ab-induced synaptic dysfunction in Alzheimer’s disease. Mol. Neurodegener 9, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umahara T, Uchihara T and Iwamoto T (2012) Structure-oriented review of 14-3-3 protein isoforms in geriatric neuroscience. Geriart. Gerontol. Int 12, 586–599. [DOI] [PubMed] [Google Scholar]
- Vagelatos NT and Eslick GD (2013) Type 2 diabetes as a risk factor for Alzheimer’s disease: the confounders, interactions, and neuropathology associated with this relationship. Epidemiol. Rev 35, 152–160. [DOI] [PubMed] [Google Scholar]
- Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvador O, Szoeke C, Macaulay SL, Martins R, Maruff P, Ames D, Rowe CC and Masters CL (2013) Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol 12, 357–367. [DOI] [PubMed] [Google Scholar]
- Vlassenko AG, Benzinger TL and Morris JC (2012) PET amyloid-beta imaging in preclinical Alzheimer’s disease. Biochim. Biophys. Acta 1822, 370–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgyi K, Juhasz G, Kovacs Z and Penke B (2015) Dysfunction of endoplasmic reticulum (ER) and mitochondria (MT) in Alzheimer’s disease: the role of the ER-MT Cross-Talk. Curr. Alzheimer Res 12, 655–672. [DOI] [PubMed] [Google Scholar]
- Vos SJ, Xiong C, Visser PJ, Jasielec MS, Hassenstab J, Grant EA, Cairns NJ, Morris JC, Holtzman DM and Fagan AM (2013) Preclinical Alzheimer’s disease and its outcome: a longitudinal cohort study. Lancet Neurol 12, 957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Hartley DM, Kusumoto Y, Fezoui Y, Condron MM, Lomakin A, Benedek GB, Selkoe DJ and Teplow DB (1999) Amyloid beta-protein fibrillogenesis: structure and biological activity of protofibrillar intermediates. J. Biol. Chem 274, 25945–25952. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ and Selkoe DJ (2002) Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416, 535–539. [DOI] [PubMed] [Google Scholar]
- Walsh DM and Teplow DB (2012) Alzheimer’s disease and the amyloid beta-protein. Prog. Mol. Biol. Transl. Sci 107, 101–124. [DOI] [PubMed] [Google Scholar]
- Wang S, Simon BP, Bennett DA, Schneider JA, Malter JS and Wang DS (2007) The significance of Pin1 in the development of Alzheimer’s disease. J. Alzheimers Dis 11, 13–23. [DOI] [PubMed] [Google Scholar]
- Webster SJ, Bachstetter AD and Van Eldik LJ (2013) Comprehensive behavioral characterization of an APP/PS-1 double knock-in mouse model of Alzheimer’s disease. Alzheimers Res. Ther 5, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse PJ, Price DL, Clark AW, Coyle JT and DeLong MR (1981) Alzheimer disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann. Neurol 10, 122–126. [DOI] [PubMed] [Google Scholar]
- Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT and Delon MR (1982) Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science 215, 1237–1239. [DOI] [PubMed] [Google Scholar]
- Wilcock GK, Esiri MM, Bowen DM and Smith CC (1982) Alzheimer’s disease. Correlation of cortical choline acetyltransferase activity with the severity of dementia and histological abnormalities. J. Neurol. Sci 57, 407–417. [DOI] [PubMed] [Google Scholar]
- Wildsmith KR, Schauer SP, Smith AM, Arnott D, Zhu Y, Haznedar J, Kaur S, Mathews WR and Honigberg LA (2014) Identification of longitudinally dynamic biomarkers in Alzheimer’s disease cerebrospinal fluid by targeted proteomics. Mol. Neurodegener 9, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson KD, Lee KM, Deshpande S, Duerksen-Hughes P, Boss JM and Pohl J (1989) The neuron-specific protein PGP 9.5 is a ubiquitin carboxyl-terminal hydrolase. Science 246, 670–673. [DOI] [PubMed] [Google Scholar]
- Willey JZ Gardener H, Caunca MR, Moon YP, Dong C, Cheung YK, Sacco RL, Elkind MS and Wright CB (2016) Leisure-time physical activity associates with cognitive decline: The Northern Manhattan Study. Neurology 86, 1897–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis BL, Gao A, Leonard D, DeFina LF and Berry JD (2012) Midlife fitness and the development of chronic conditions in later life. Arch. Intern. Med 172, 1333–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Segawa E, Boyle PA, Anagnos SE, Hizel LP and Bennett DA (2012) The natural history of cognitive decline in Alzheimer’s disease. Psychol. Aging 27, 1008–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski KE, Wisniewski HM and Wen GY (1985) Occurrence of neuropathological changes and dementia of Alzheimer’s disease in Down’s syndrome. Ann. Neurol 17, 278–282. [DOI] [PubMed] [Google Scholar]
- Wood ZA, Schroder E, Harris JR and Poole LB (2003) Structure, mechanism, and regulation of peroxiredoxins. Trends. Biochem. Sci 28, 32–40. [DOI] [PubMed] [Google Scholar]
- Wu W, Brickman AM, Luchsinger J, Ferrazzano P, Pichiule P, Yoshita M, DeCarli C, Barnes CA, Mayeux R, Vannucci SJ and Small SA (2008) The brain in the age of old: the hippocampal formation is targeted differentially by disease of late life. Ann. Neurol 64, 698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Ren Z, Chow FE, Tsai T, Liu T, Rizzolio F, Boffo S, Xu Y, Huang S, Lippa CF and Gong Y (2017) Pathological role of peptidyl-prolyl isomerase Pin1 in the disruption of synaptic plasticity in Alzheimer’s disease. Neural Plast 2017, 3270725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Meguro K, Itoh M, Hayasaka C, Shimada M, Yamazaki H and Yamadori A (1997) Decreased cortical glucose metabolism correlates with hippocampal atrophy in Alzheimer’s disease as shown by MRI and PET. J. Neurol. Neurosurg. Psychiatry 62, 596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT and Brinton RD (2009) Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. U. S. A 106, 14670–14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatin SM, Link CD and Butterfield DA (1999) In vitro and in vivo oxidative stress associated with Alzheimer’s amyloid β-peptide (1–42). Neurobiol. Aging 20, 325–330. [DOI] [PubMed] [Google Scholar]
- Yu HL, Chertkow HM, Bergman H and Schipper HM (2003) Aberrant profiles of native and oxidized glycoproteins in Alzheimer plasma. Proteomics 3, 2240–2248. [DOI] [PubMed] [Google Scholar]
- Yuan Z, Agarwal-Mawal A and Paudel HK (2004) 14-3-3 binds to and mediates phosphorylation of microtubule-associated tau protein by Ser9-phosphorylated glycogen synthase kinase 3beta in the brain. J. Biol. Chem 279, 26105–26114. [DOI] [PubMed] [Google Scholar]
- Zhang J, Dawson VL, Dawson TM and Snyder SH (1994) Nitric oxide activation of poly(ADP-ribose) synthetase in neurotoxicity. Science 263, 687–689. [DOI] [PubMed] [Google Scholar]
- Zhou XZ, Lu PJ, Wulf G and Lu KP (1999) Phosphorylation-dependent prolyl isomerization: a novel regulatory mechanism. Cell. Mol. Life Sci 56, 788–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XZ, Kops O, Werner A, Lu PJ, Shen M, Stoller G, Küllertz G, Stark M, Fischer G and Lu KP (2000) Pin1-dependent prolyl isomerization regulates dephosphorylation of Cdc25c and tau proteins. Mol. Cell 6, 873–883. [DOI] [PubMed] [Google Scholar]
- Zissimopoulos J, Crimmins E and St. Clair P (2014) The value of delaying Alzheimer’s disease onset. Forum Health Econ. Policy 18, 25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]