Abstract
Background
Kidney transplantation is the optimal treatment for end‐stage kidney disease. Retrieval, transport and transplant of kidney grafts causes ischaemia reperfusion injury. The current accepted standard is static cold storage (SCS) whereby the kidney is stored on ice after removal from the donor and then removed from the ice box at the time of implantation. However, technology is now available to perfuse or "pump" the kidney during the transport phase or at the recipient centre. This can be done at a variety of temperatures and using different perfusates. The effectiveness of treatment is manifest clinically as delayed graft function (DGF), whereby the kidney fails to produce urine immediately after transplant.
Objectives
To compare hypothermic machine perfusion (HMP) and (sub)normothermic machine perfusion (NMP) with standard SCS.
Search methods
We searched the Cochrane Kidney and Transplant Register of Studies to 18 October 2018 through contact with the Information Specialist using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria
All randomised controlled trials (RCTs) and quasi‐RCTs comparing HMP/NMP versus SCS for deceased donor kidney transplantation were eligible for inclusion. All donor types were included (donor after circulatory (DCD) and brainstem death (DBD), standard and extended/expanded criteria donors). Both paired and unpaired studies were eligible for inclusion.
Data collection and analysis
The results of the literature search were screened and a standard data extraction form was used to collect data. Both of these steps were performed by two independent authors. Dichotomous outcome results were expressed as risk ratio (RR) with 95% confidence intervals (CI). Continuous scales of measurement were expressed as a mean difference (MD). Random effects models were used for data analysis. The primary outcome was incidence of DGF. Secondary outcomes included: one‐year graft survival, incidence of primary non‐function (PNF), DGF duration, long term graft survival, economic implications, graft function, patient survival and incidence of acute rejection.
Main results
No studies reported on NMP, however one ongoing study was identified.
Sixteen studies (2266 participants) comparing HMP with SCS were included; 15 studies could be meta‐analysed. Fourteen studies reported on requirement for dialysis in the first week post‐transplant (DGF incidence); there is high‐certainty evidence that HMP reduces the risk of DGF when compared to SCS (RR 0.77; 95% CI 0.67 to 0.90; P = 0.0006). HMP reduces the risk of DGF in kidneys from DCD donors (7 studies, 772 participants: RR 0.75; 95% CI 0.64 to 0.87; P = 0.0002; high certainty evidence), as well as kidneys from DBD donors (4 studies, 971 participants: RR 0.78, 95% CI 0.65 to 0.93; P = 0.006; high certainty evidence). The number of perfusions required to prevent one episode of DGF (number needed to treat, NNT) was 7.26 and 13.60 in DCD and DBD kidneys respectively. Studies performed in the last decade all used the LifePort machine and confirmed that HMP reduces the incidence of DGF in the modern era (5 studies, 1355 participants: RR 0.77, 95% CI 0.66 to 0.91; P = 0.002; high certainty evidence). Reports of economic analysis suggest that HMP can lead to cost savings in both the North American and European settings.
Two studies reported HMP also improves graft survival however we were not able to meta‐analyse these results. A reduction in incidence of PNF could not be demonstrated. The effect of HMP on our other outcomes (incidence of acute rejection, patient survival, hospital stay, long‐term graft function, duration of DGF) remains uncertain.
Authors' conclusions
HMP is superior to SCS in deceased donor kidney transplantation. This is true for both DBD and DCD kidneys, and remains true in the modern era (studies performed in the last decade). As kidneys from DCD donors have a higher overall DGF rate, fewer perfusions are needed to prevent one episode of DGF (7.26 versus 13.60 in DBD kidneys).
Further studies looking solely at the impact of HMP on DGF incidence are not required. Follow‐up reports detailing long‐term graft survival from participants of the studies already included in this review would be an efficient way to generate further long‐term graft survival data.
Economic analysis, based on the results of this review, would help cement HMP as the standard preservation method in deceased donor kidney transplantation.
RCTs investigating (sub)NMP are required.
Keywords: Humans, Kidney, Tissue Donors, Delayed Graft Function, Graft Rejection, Graft Rejection/epidemiology, Graft Survival, Incidence, Kidney Transplantation, Kidney Transplantation/mortality, Organ Preservation, Organ Preservation/instrumentation, Organ Preservation/methods, Perfusion, Perfusion/instrumentation, Perfusion/methods, Randomized Controlled Trials as Topic, Refrigeration, Refrigeration/instrumentation, Refrigeration/methods, Time Factors
The use of machines to preserve kidneys from deceased donors prior to transplantation
What is the issue?
Kidney transplantation is the best treatment for patients with end‐stage kidney disease. However, there are not enough donated organs to go around. In addition, whilst a donated kidney is outside of the body it is starved of oxygen, the halting of circulation allows small clots to form, which damages the organ. This damage remains a major barrier to transplantation as it renders many organs unusable and is associated with decreased survival of the kidneys which are transplanted. Traditionally kidneys were kept in ice (termed static cold storage). Machines which drive cold (hypothermic machine perfusion) or warm (normothermic machine perfusion) solutions through donated kidneys aim to decrease the damage done during transport and therefore improve the outcomes for these kidneys.
What did we do?
We performed a rigorous search for studies which compared hypothermic machine perfusion, normothermic machine perfusion and standard static cold storage. Data from included studies could then be combined to allow further analysis. Our primary outcome was rate of delayed graft function (DGF) (the number of patients who needed extra dialysis support in the week following transplant). Our main secondary outcome of interest was one‐year kidney survival (the number of transplanted kidneys functioning at one year).
What did we find?
Sixteen studies (2266 participants) comparing hypothermic machine perfusion with static cold storage were included. The use of hypothermic machine perfusion instead of standard static cold storage reduces the risk of DGF by approximately 23%. Two reports performed economic analysis, in the USA and European settings, and both estimated cost savings with the use of hypothermic machine perfusion. Two studies reported hypothermic machine perfusion prolongs the length of time that donated kidneys survive in the recipient, however we were unable to perform an analysis to confirm this. The effect of HMP on other outcomes (incidence of acute rejection, patient survival, hospital stay, long‐term kidney function, duration of DGF) remains uncertain.
No completed studies investigating normothermic machine perfusion were identified, but one ongoing study was identified.
Conclusions
Compared with standard static cold storage, hypothermic machine perfusion reduces the rate of DGF in kidneys from deceased donors, and likely results in increased survival of the transplanted kidney and overall cost savings. Studies looking at normothermic machine perfusion are required to assess if this results in superior outcomes.
Summary of findings
Summary of findings for the main comparison.
Hypothermic machine perfusion versus static cold storage for deceased donor kidney transplantation
| Hypothermic machine perfusion versus static cold storage for deceased donor kidney transplantation | ||||||
| Patient or population: deceased donor kidney transplantation Intervention: hypothermic machine perfusion Comparison: static cold storage | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with static cold storage | Risk with hypothermic machine perfusion | |||||
| DGF | 409 per 1,000 | 315 per 1,000 (274 to 368) | RR 0.77 (0.67 to 0.90) | 2138 (14) | ⊕⊕⊕⊕ HIGH | ‐ |
| DGF: DCD group | 501 per 1,000 | 376 per 1,000 (321 to 436) | RR 0.75 (0.64 to 0.87) | 772 (7) | ⊕⊕⊕⊕ HIGH | ‐ |
| DGF: DBD group | 342 per 1,000 | 266 per 1,000 (222 to 318) | RR 0.78 (0.65 to 0.93) | 971 (4) | ⊕⊕⊕⊕ HIGH | ‐ |
| DGF: modern era studies | 372 per 1,000 | 286 per 1,000 (245 to 338) | RR 0.77 (0.66 to 0.91) | 1355 (5) | ⊕⊕⊕⊕ HIGH | ‐ |
| DGF: pre‐2008 studies | 474 per 1,000 | 370 per 1,000 (289 to 470) | RR 0.78 (0.61 to 0.99) | 783 (9) | ⊕⊕⊕⊕ HIGH | ‐ |
| One year graft survival | See comments | See comments | ‐ | ‐ | ‐ | Meta‐analysis was not possible. There is strong evidence of improved graft survival with HMP |
| PNF | 65 per 1,000 | 57 per 1,000 (38 to 86) | RR 0.88 (0.58 to 1.33) | 1387 (7) | ⊕⊕⊕⊕ HIGH | ‐ |
| Duration of DGF | The mean duration of DGF was 11.8 days | Mean duration of DGF was 1.23 fewer days (5.87 fewer to 3.4 more) | ‐ | 220 (4) | ⊕⊝⊝⊝ VERY LOW 1 | ‐ |
| One year patient survival | 965 per 1,000 | 955 per 1,000 (917 to 994) | RR 0.99 (0.95 to 1.03) | 920 (3) | ⊕⊕⊝⊝ LOW 2 | ‐ |
| Treated acute rejection in the first year | 244 per 1,000 | 161 per 1,000 (90 to 285) | RR 0.66 (0.37 to 1.17) | 248 (2) | ⊕⊕⊝⊝ LOW 3 | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DBD: donor after brainstem death; DCD: donor after circulatory death; DGF: delayed graft function; MD: mean difference; PNF: primary non‐function; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded three levels: not all studies reporting DGF duration could be included in the meta‐analysis and high level of heterogeneity between studies
2 Downgraded two levels: not all studies reporting patient survival duration could be included in the meta‐analysis, and none of the studies were powered to allow analysis of patient survival
3 Downgraded two levels: reporting at different time points prevented inclusion of several studies into meta‐analysis. In addition different studies used different definitions for acute rejection, some being dependent on biopsies and some on clinical judgement.
Background
Description of the condition
End‐stage kidney disease (ESKD) is defined as an irreversible decline in kidney function that is severe enough to be fatal without renal replacement therapy (RRT). ESKD is a major debilitating condition with a drastic effect on patients' quality of life, as well as being associated with significant morbidity and death. It is a condition with growing worldwide prevalence, affecting an estimated 3.2 million people (Fresenius 2013). The maintenance treatment for such patients is regular RRT. The impact of dialysis on the ability of patients to lead normal lives is significant, requiring frequent hospital visits, as well as severely restricting travel. It is now widely accepted that kidney transplantation offers a survival advantage over all forms of RRT (Wolfe 1999). In addition, there is also an economic benefit of transplantation when compared with the high cost of dialysis. It has been estimated that kidney transplantation costs GBP £241,000 less than dialysis over a 10‐year period for a single patient (NHSBT 2009).
An estimated 77,818 kidney transplants were carried out in 2012 (GODT 2012). Potential recipients can be transplanted with a kidney graft (simply termed 'graft' for the remainder of the review) from a living or deceased donor. Deceased donors may be certified dead on the basis of brainstem death (donation after brainstem death; DBD) criteria or circulatory death (donation after circulatory death; DCD). However, kidneys from deceased donors have a higher incidence of delayed graft function (DGF) and primary non‐function (PNF) due to the trauma of brainstem death or circulatory arrest, as well as reperfusion injury when compared with live donor kidneys.
The growing disparity between supply and demand has led to increasing use of DCD organs and marginal organs from donors outside traditional transplantation protocols. A well‐accepted definition of extended/expanded criteria donor (ECD) is age over 60 years or over 50 years with a history of hypertension, kidney impairment or cause of death secondary to stroke (Port 2002). Most studies have shown that transplantation of organs from either DCD or ECD are associated with inferior short‐ and long‐term outcomes (Glyda 2012; Hwang 2014; Metzger 2003; Pascual 2008). This has focused attention on organ preservation techniques and ways to recondition organs in the donor and ex vivo prior to transplantation to potentially improve outcomes for recipients. However, the significant increase in cost of machine perfusion (MP) means that its widespread use depends on the demonstration of superiority, over the relatively inexpensive static cold storage (SCS). Although it is also important to note that at least some of the additional cost may be offset by reduced hospitalisation, complications, or both.
The use of MP brings with it further questions, such as what is the optimum perfusion temperature, preservation solution; pulsatile versus non‐pulsatile flow; and oxygenated versus non‐oxygenated perfusate. The main focus of this review will be to compare (sub)normothermic and hypothermic MP (HMP) versus SCS.
Description of the intervention
From the early days of organ transplantation, hypothermia was an effective means of preserving the organ in the absence of oxygenated circulation. Belzer 1968 successfully preserved human kidneys using HMP; although the machine was large, bulky and difficult to transport. Shortly thereafter, an electrolyte solution was developed that enabled preservation of a kidney for 24 hours in a container surrounded with ice, now termed SCS (Collins 1969). Subsequently, various other preservation solutions have superseded Euro‐Collins, most notably University of Wisconsin (UW), histidine‐tryptophan‐ketoglutarate (HTK), and Marshall's hyperosmolar citrate. The preservation solution used has an effect on the incidence of DGF, which may affect long‐term outcomes. In a meta‐analysis both UW and HTK were found to have similar DGF incidence, when compared with older preservation solutions like Euro‐Collins (O'Callaghan 2012, Table 3).
Table 1.
Comparison of preservation solution composition
| Solution name | Energy substrate | N+ | K+ | M2+ | Ca2+ | pH | Buffer | Osm | Impermeant |
| Euro‐Collins | Glucose | 10 | 108 | 0 | 0 | 7.4 | Bicarbonate/phosphate | 340 | Glucose |
| UW | Adenosine | 30 | 125 | 5 | ‐ | 7.4 | Phosphate | 325 | Lactobionate /raffinose |
| HTK | Ketoglutarate | 15 | 10 | 4 | 0.015 | 7.02 to 7.2 | Histidine | 310 | Mannitol |
| Belzer's | Adenine | 100 | 25 | 5 | 0.5 | 7.4 | HEPES | 320 | Gluconate /ribose |
| PBS140 | ‐ | 92 | 0 | 0 | 0 | 7.2 | Phosphate | 310 | Sucrose |
| Celsion® | Glutamate | 100 | 15 | 13 | 0.25 | 7.3 | Histidine | 320 | Lactobionate /mannitol |
| Marshall's hyperosmolar citrate | Citrate | 28 | 26 | 41 | ‐ | 7.1 | Citrate | 486 | Mannitol |
HEPES ‐ N‐2‐hydroxyethylpiperazine‐N‐2‐ethane sulfonic acid; HTK ‐ histidine‐tryptophan‐ketoglutarate; Osm ‐ osmolality; THAM ‐ trometamol; tris‐hydroxymethyl aminomethane; UW ‐ University of Wisconsin
As organ preservation solutions have evolved so have extracorporeal MP technologies. There are now several commercially available HMP devices which are broadly similar with minor variations in perfusion temperature (4oC to 10oC), flow (pulsatile versus non‐pulsatile) and provision of oxygenation (oxygenated versus non‐oxygenated). The most popular machines currently available are the LifePort® (Organ Recovery Systems; Itasca, Illinois), the KidneyAssist® (OrganAssist; Gronigen, Netherlands) and the Waters RM3® system (Rochester, Minnesota). The Gambro MP devices (Gambro, Lund, Sweden) were previously available alternatives. Once the kidney has been removed from the donor, the kidney is cannulated and connected to a disposable circuit designed specifically for the device. The donor kidney is then continuously perfused typically at temperatures between 6oC and 12oC within the battery‐operated device, whilst the kidney is transported to a suitable recipient.
The older Waters and Gambro pumps rely on continuous flow of cold perfusate to sustain hypothermia. This risks graft loss in the unlikely event of pump failure. In contrast, the newer LifePort perfusion device is able to revert to SCS in the event of pump failure, mitigating this risk.
More recently Professor Nicholson in Leicester, United Kingdom, has pioneered a technique of normothermic machine perfusion (NMP) using modified cardiopulmonary bypass equipment. This preservation technique is static and can be used to complement either SCS or HMP; as the kidney still has to be transferred to the recipient centre (Nicholson 2013). In the future commercially available transportable kidney normothermic perfusion machines may become available as there is now for the liver (OrganOx® metra™ device). Whilst NMP uses a perfusion temperature of 35oC to 37oC, further studies may employ (sub)NMP; defined as 20oC to 34oC.
How the intervention might work
Hypothermia slows the metabolism of cells. In general, for every 10oC drop, the metabolism rate halves (Wilson 2006). SCS works by removing blood and clots from the kidney graft and replacing this with an acellular preservation solution in a hypothermic environment. Pulsatile preservation up‐regulates nitric oxide production by vascular endothelium (Gallinat 2013), as well as clearing the microcirculation of debris and toxic metabolites. Proponents hypothesise that the ultimate result of MP is a reduced intra‐renal resistance at the time of in vivo reperfusion and better earlier transplant function.
NMP or EVNP (ex vivo normothermic perfusion; as it is also known) technology is in its infancy and the exact beneficial mechanism of action debated. In brief, whilst the recipient is undergoing anaesthesia and preliminary surgery, the kidney is prepared and connected to a modified cardiopulmonary bypass circuit using a red‐cell based perfusate (Nicholson 2013). The perfusate lacks mediators of reperfusion injury like leukocytes, complement, platelets but includes vasodilators and heparin. Experimental work has shown that this combination improves early transplant function in a porcine model (Bagul 2008).
Why it is important to do this review
Ischaemia reperfusion injury in a kidney transplant manifests as DGF with PNF if the injury is severe. In a recent review looking at ECD/DCD kidneys one year graft survival was only 73% and PNF rate of 12.5% in one subset that had been transplanted (Kosmoliaptsis 2015). In addition, DGF leads to an increased requirement for RRT, prolonged hospitalisation and often more investigations ‐ incurring significant extra financial costs. In our own institution these extra peritransplant financial costs for DCD recipients with DGF have been estimated to be GBP £4500 per patient (Wilson 2014). In cases of PNF the recipient requires a second operation to remove the kidney and returns to dialysis with an immune system sensitised and difficult to match for repeat transplantation.
DGF is most commonly defined as the requirement for dialysis within the first week after implantation (Mallon 2013), although common measures of kidney function estimation may be used such as estimated glomerular filtration rate (eGFR; Cockcroft‐Gault or MDRD).
SCS is a simple method of storage, and is relatively cheap compared to MP. Robust evidence for the benefits of MP are required to justify these increased initial costs. This review will critically appraise and summate the current randomised controlled trial (RCT) literature to analyse the potential benefit of novel preservation technologies in kidney transplantation, both in terms of patient centred outcomes and the financial implications at a societal level.
Objectives
To compare hypothermic HMP and (sub)NMP with standard SCS.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) looking at normothermic and HMP versus SCS for kidney transplantation from deceased donors were eligible for inclusion. For a study to be included, one group must have been randomised to cold storage with a commercially available preservation solution (Table 3) and one to a MP technique.
Types of participants
Inclusion criteria
All RCTs and quasi‐RCTs comparing HMP or NMP with SCS for donor human kidneys were eligible for inclusion. We anticipated that some studies would be limited to ECD or DCD kidneys, whereas others would not be selective. We further anticipated that some studies would randomise one kidney in a pair to one modality and the other kidney to the control group: in other unpaired studies both organs from the same donor will have been randomised. We included both paired and unpaired studies.
We also included studies where a recipient received dual kidney transplants as long as the same modality (HMP, NMP or SCS) was used for both grafts.
Exclusion criteria
We planned to exclude studies where the kidney graft was used as part of a composite or multi‐visceral transplantation, although no such studies were identified. The meta‐analysis was restricted to human studies, as although there are good animal transplantation models available, we did not anticipate that these would examine the inter‐relationship of immunosuppression, ischaemia‐reperfusion injury and preservation modality over the required length of follow‐up to provide data with direct clinical applicability.
Types of interventions
We searched for studies comparing the following interventions.
HMP versus SCS
HMP versus (sub)NMP
(Sub)NMP versus SCS.
This initial version of the review will only include comparison of HMP and SCS, as (sub)NMP RCTs are yet to be completed.
Types of outcome measures
Primary outcomes
Incidence of DGF (defined as requirement for postoperative dialysis)
Secondary outcomes
One‐year graft survival
Duration of DGF
Episodes of biopsy‐proven rejection
Incidence of PNF
Patient survival
Presence of fibrosis on biopsy
Economic implications
Quality of life
Hospital stay
Early hospital costs
Number of allograft ultrasound scans
Number of allograft biopsies
Incidence of acute rejection
Kidney function at three, six, nine and 12 months (serum creatinine (SCr) and glomerular filtration rate (GFR))
Two, three, and five‐year graft survival.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Register of Studies up to 18 October 2018 through contact with the Information Specialist using search terms relevant to this review. The Register contains studies identified from the following sources.
Monthly searches of the Cochrane Central Register of Controlled Trials CENTRAL
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney and transplant conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of search strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about Cochrane Kidney and Transplant.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of review articles, relevant studies and clinical practice guidelines.
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies that may have been relevant to the review. Titles and abstracts were screened independently by two authors, who discarded studies that were not applicable, however studies and reviews which were thought to include relevant data or information on studies were retained initially. Two authors independently assessed retrieved abstracts and, if necessary, the full text of these studies to determine which studies satisfied the inclusion criteria.
Data extraction and management
Data extraction was carried out independently by two authors using standard data extraction forms. Where more than one publication of one study existed, reports were grouped together and the publication with the most complete data was used in the analyses. Where relevant outcomes were only published in earlier versions these data were used.
Assessment of risk of bias in included studies
The following items were independently assessed using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study (detection bias)?
Participants and personnel
Outcome assessors
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For dichotomous outcomes (e.g. incidence of DGF or PNF) results were expressed as risk ratio (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment (e.g. duration of DGF), the mean difference (MD) was used.
Missing standard deviations were imputed from data presented in the published data (P values). Imputation of standard deviations from other studies (Furukawa 2006), as described in our protocol, was not appropriate . We recognise that all imputation techniques involve making assumptions about unknown statistics, and we avoided their use where possible.
As specified in our protocol we attempted to analyse graft survival and patient survival by extracting time‐to‐event data from publications and entering the O‐E and V statistics into RevMan and then performing analysis with a log rank approach. Unfortunately there was insufficient reporting of time‐to‐event data to allow this.
Unit of analysis issues
We did not anticipate any challenges with non‐standard designs such as cross‐over or cluster RCTs. In the future, there may be studies in which a graft is initially transported with either SCS or HMP and then subjected to NMP prior to implantation. These will be analysed by the "dominant" preservation type.
Dealing with missing data
Any further information required from the original author was requested by written correspondence (e.g. emailing corresponding author). Evaluation of important numerical data such as screened, randomised patients as well as intention‐to‐treat, as‐treated and per‐protocol population was carefully performed. Attrition rates, for example drop‐outs, losses to follow‐up and withdrawals were investigated. Issues of missing data and imputation methods (for example, last‐observation‐carried‐forward) were critically appraised (Higgins 2011).
Assessment of heterogeneity
Statistical heterogeneity was explored and potential sources identified (including subgroup analysis as described below). Heterogeneity was analysed using a Chi2 test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I2 test (Higgins 2003). I2 values of 25%, 50% and 75% were taken to indicate low, medium and high levels of heterogeneity.
Assessment of reporting biases
Where possible, funnel plots were used to assess for the potential existence of small study bias (Higgins 2011).
Data synthesis
Data was pooled using the random‐effects model, but the fixed‐effects model was also used to ensure robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was used to explore possible sources of heterogeneity (e.g. inclusion of ECD or DCD kidneys). Potential subgroup analyses included:
DCD versus DBD criteria donors kidneys
ECD versus standard criteria kidneys
HMP at the time of donation versus HMP at the recipient centre
Long preservation times (≥ 24 hours) versus short (< 24 hours)
Era of study (those performed in the 'modern era' with newer MP devices such as the LifePort device versus studies performed previously; 'pre‐2008').
Sensitivity analysis
We performed sensitivity analyses in order to explore the influence of the following factors on effect size.
Repeating the analysis taking account of risk of bias, as specified
Repeating the analysis excluding any very long or large studies to establish how much they dominate the results.
'Summary of findings' tables
The main results of the review are presented in a 'Summary of findings' table. This table presents key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2011a). The 'Summary of findings' table also includes an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008; GRADE 2011). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2011b). The following outcomes are presented in the 'Summary of findings' table.
Incidence of DGF (defined as requirement for postoperative dialysis)
One‐year graft survival
Incidence of PNF
Duration DGF
One‐year patient survival
Incidence of acute rejection.
Results
Description of studies
The following section contains broad descriptions of the studies considered in this review. For further details on each individual study please see the characteristics of studies tables; Characteristics of included studies, Characteristics of excluded studies.
No studies reported on NMP, however one ongoing study was identified. The rest of this review therefore deals entirely with HMP.
Results of the search
A PRISMA flow chart for the studies included in this review can be found in Figure 1.
Figure 1.
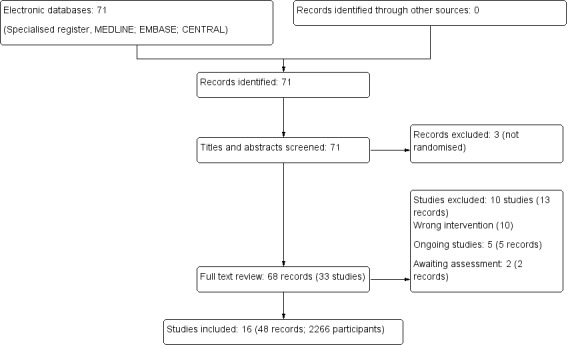
Study flow diagram
After searching the Register we identified 71 records. After duplicates were removed and titles and abstracts screened we retrieved 68 full‐text articles for further assessment. Of these, 16 studies (48 records) were included and 10 studies (13 records) were excluded. Five ongoing studies were identified (Hosgood 2017; ISRCTN35082773; ISRCTN63852508; NCT02525510; NCT02621281). Two studies were completed prior to publication, however no results are as yet available (ISRCTN50082383; NCT01170910). These seven studies will be assessed in a future update of this review
Included studies
In total 2266 recipients of cadaveric kidney transplants from 16 different studies (Alijani 1985; Amaduzzi 2011; Chen 2014c; Halloran 1985; Heil 1987; Kwiatkowski 1996; Matsuno 1994; Merion 1990; Moers 2009; Mozes 1985; PPART 2010; Tedesco‐Silva 2017; van der Vliet 2001; Veller 1994; Wang 2017; Zhong 2017) included in this review; full details for each study can be found in the Characteristics of included studies table.
Insufficient information was provided in the abstract by Amaduzzi 2011. Attempts to gain further information by contacting the corresponding author failed and therefore results of this study could not be included in the meta‐analyses.
Of the 16 studies, four were performed in the USA (Alijani 1985; Heil 1987; Merion 1990; Mozes 1985), five in Europe (Amaduzzi 2011; Kwiatkowski 1996; Moers 2009; PPART 2010; van der Vliet 2001), three in China (Chen 2014c; Wang 2017; Zhong 2017), one in Japan (Matsuno 1994), one in Canada (Halloran 1985), one in South Africa (Veller 1994), and one in Brazil (Tedesco‐Silva 2017).
All but one of the included studies used a paired design, with one kidney from each donor being preserved with MP and one preserved with SCS. Halloran 1985 was the only study to not use a paired design, instead randomising a donor to have both kidneys preserved with MP or SCS.
The type of MP device used varied between studies. The most commonly used device was the Waters Mox‐100 pulsatile, which was used by seven studies (Alijani 1985; Halloran 1985; Heil 1987; Kwiatkowski 1996; Merion 1990; Mozes 1985; Veller 1994). The LifePort Pulsatile Perfusion machine was used in six studies (Chen 2014c; Moers 2009; PPART 2010; Tedesco‐Silva 2017; Wang 2017; Zhong 2017), the Gambro Pulsatile Perfusion machine was used in van der Vliet 2001, and the APS‐02 (Nikiso) machine was used in Matsuno 1994. Amaduzzi 2011 did not report the type of MP device used.
Excluded studies
Full details for individual studies can be found in the Characteristics of excluded studies table.
Three studies compared different cold storage solutions (Alijani 1987; Baatard 1993; Wamser 1990); two compared different MP solutions (Guarrera 2004; Guarrera 2004a); one compared reflush solutions (Lodge 1993); two compared different MP additives (Polyak 1998; Polyak 2002); and two compared different MP techniques (Tisone 1999; Wszola 2013).
Risk of bias in included studies
The following section contains an overview of some of the common biases present in the included studies. For further details on each individual study please see Characteristics of included studies; a summary of the risk of bias information for each study can be found in Figure 2, and a summary of the of the total risk of bias in different domains can be found in Figure 3.
Figure 2.
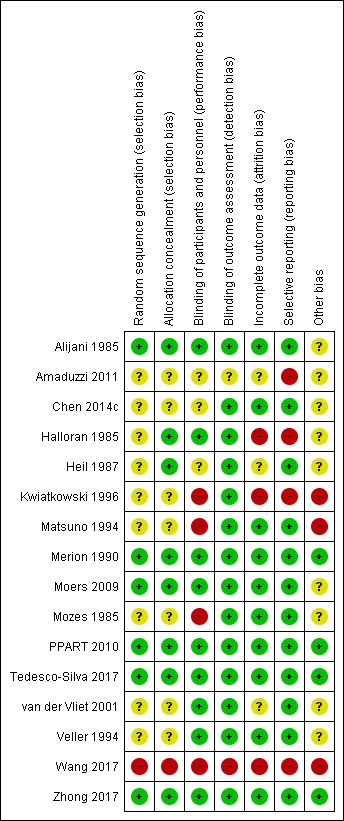
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Figure 3.
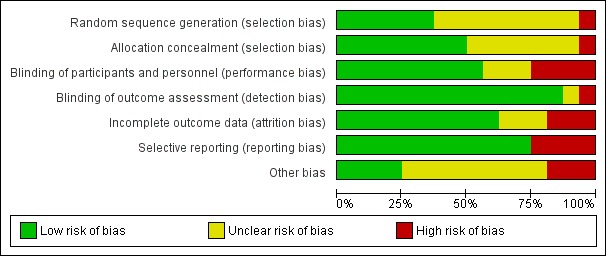
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Four of the studies (Heil 1987; Moers 2009; PPART 2010; Tedesco‐Silva 2017) described appropriate methods of randomisation and allocation concealment, resulting in a low risk of bias. One study (Zhong 2017) did not describe randomisation or allocation in detail, however all consecutive donors were assessed for inclusion, with valid reasons given for any exclusions. Therefore, the risk of bias remained low. Two studies (Alijani 1985; Merion 1990) described quasi‐RCTs, with a paired alternating design; each donor had one kidney preserved with SCS and the other with MP, alternating between left and right. However, as these studies used a paired design with consecutive donors, and good explanations for any exclusions, our assessment was that the risk of selection bias remained low.
In seven studies (Amaduzzi 2011; Chen 2014c; Kwiatkowski 1996; Matsuno 1994; Mozes 1985; van der Vliet 2001; Veller 1994) there was insufficient information on randomisation and allocation techniques to make a judgement of the risk of bias. However, as these studies are all paired RCT, it is unlikely that selection bias will be present unless the decision to include a donor in the study was made after visualisation of the organs by the organ retrieval team.
For the single study which did not use a paired design (Halloran 1985), the potential for selection bias is potentially higher. No information was given regarding how patients were randomised, however inclusion and randomisation of each donor happened prior to procurement. This lowers the risk of selection bias, as donors could not be selected based on features only apparent at surgery.
Wang 2017 gave no information on how kidneys were randomised, and the authors were allowed to swap kidneys between groups. As no intention‐to‐treat analysis was performed it was considered to be at high risk of allocation bias. On the advice of referees during the peer review process this study was included.
Blinding
Nine studies were considered to be at low risk of performance bias; Moers 2009 performed adequate blinding at the time of organ offer; PPART 2010 did not perform blinding, but did perform randomisation for which kidney was transplanted first; the remaining seven studies (Alijani 1985; Halloran 1985; Merion 1990; Tedesco‐Silva 2017; van der Vliet 2001; Veller 1994; Zhong 2017) did not perform blinding but showed no significant difference in CIT, suggesting that the organs preserved by different methods were treated similarly.
Four studies (Kwiatkowski 1996; Matsuno 1994; Mozes 1985; Wang 2017) were considered to be at high risk of performance bias. In three of these studies (Kwiatkowski 1996; Matsuno 1994; Mozes 1985) the CIT was significantly longer in the MP group. Kwiatkowski 1996 describes routinely transplanting the SCS kidney prior to the MP kidney. As increased CIT is known to be detrimental, this generates a performance bias which may lessen the predicted positive effects of MP. Wang 2017 perform no blinding and no reporting of CIT. Therefore, one group may have routinely been transplanted first, adding bias to the results. In addition, it is not stated whether assessors of acute rejection were blinded.
In three studies (Amaduzzi 2011; Chen 2014c; Heil 1987) there was insufficient information on blinding and CIT to make a judgement of the risk of performance bias.
Although none of the studies performed blinding of the outcome assessors, we deemed this to be an unlikely source of bias, given the outcome measures chosen by most studies. For this reason, 14 studies were deemed to be at low risk of detection bias. Wang 2017 did not report blinding of outcome assessors however it's outcomes (non‐standard definition of DGF and acute rejection) put it at higher risk of detection bias.
Incomplete outcome data
Ten studies (Alijani 1985; Chen 2014c; Matsuno 1994; Merion 1990; Moers 2009; Mozes 1985; PPART 2010; Tedesco‐Silva 2017; Veller 1994; Zhong 2017) provided either full follow‐up data for all included patients, or valid reasons for any exclusions, and were therefore considered to be at low risk of attrition bias.
Three studies (Halloran 1985; Kwiatkowski 1996; Wang 2017) were considered to be at high risk of attrition bias. In the Halloran 1985 study, 13 patients which were originally randomised to MP, instead received SCS; no follow‐up information was provided for these patients so intention to treat analysis could not be performed. In Kwiatkowski 1996, data on DGF was missing for six patients with no explanation. In addition, the 10‐year graft survival data gave only percentages with no absolute numbers to indicate level of follow‐up. Wang 2017 had "time‐zero biopsies" as an outcome, but no data was given for the groups as a whole; only H+E stains and electron microscopy from a single pair of kidneys are presented.
In three studies (Amaduzzi 2011; Heil 1987; van der Vliet 2001) there was insufficient information to make a judgement of the risk of attrition bias.
Selective reporting
The majority of the studies (Alijani 1985; Chen 2014c; Heil 1987; Matsuno 1994; Merion 1990; Moers 2009; Mozes 1985; PPART 2010; Tedesco‐Silva 2017; van der Vliet 2001; Veller 1994; Zhong 2017) reported all expected outcomes in a complete and unambiguous fashion.
The remaining three studies (Halloran 1985; Kwiatkowski 1996; Wang 2017) were considered to be at high risk of reporting bias. Halloran 1985 used a complex and unusual definition for DGF, for unclear reasons. However, data included in our analysis was taken directly from the number of dialyses in week one table; so their reporting anomaly has not directly impacted on this meta‐analysis. Amaduzzi 2011 and Kwiatkowski 1996 did not report all relevant data, and most of the data which was reported was either incomplete or reported ambiguously with percentages rather than absolute values. Wang 2017 used a non‐standard definition of DGF and failed to provide data on the number of participants requiring dialysis in the first week post‐transplant. There were also issues with selective reporting of the outcome 'time‐zero biopsies' described in further detail in the characteristics of included studies table.
Other potential sources of bias
Several manuscripts had very short methods sections making full assessment of further biases difficult. Matsuno 1994 did not state the duration of the study, whether they were consecutive cases, or how inclusion/randomisation took place and was therefore considered to be at high risk of bias. Kwiatkowski 1996 was considered to be at high risk of bias‐ as the CIT was different between the groups.
Another potential source of bias is the lack of intention‐to‐treat analysis. Only one of the studies (PPART 2010) described using intention to treat analysis. In two studies (Alijani 1985; Halloran 1985) a change in perfusion technique led to exclusion from the study. In Moers 2009 a "switch in preservation methods changed the initial randomization". In Wang 2017 several kidneys were swapped between groups and intention to treat analysis not performed. In Zhong 2017 no grafts were swapped between groups therefore intention to treat analysis was not performed. For the remaining 10 studies (Amaduzzi 2011; Chen 2014c; Heil 1987; Kwiatkowski 1996; Matsuno 1994; Merion 1990; Mozes 1985; Tedesco‐Silva 2017; van der Vliet 2001; Veller 1994) there was insufficient information to assess whether intention‐to‐treat analysis had been performed.
One potential source of bias in all studies was the lack of blinding‐ the surgical team performing the transplant were aware of treatment allocation. This information may have changed the decision threshold to dialyse in the early post‐operative period, depending on physician and surgeon pre‐conceptions.
Effects of interventions
See: Table 1
Hypothermic machine perfusion versus static cold storage
See Table 1
The results for Amaduzzi 2011 could not be included in any of the meta‐analyses.
Delayed graft function
All studies reported DGF as their primary outcome. Other than Wang 2017, all studies used the definition stated in our methods section, or provided data on how many patients required dialysis in the first week post transplant. This meant that 2138 participants could be included in the meta‐analysis. The use of HMP reduces the risk of DGF (Analysis 1.1 (14 studies, 2138 participants): RR 0.77, 95% CI 0.67 to 0.90; P = 0.0006; I2 = 33%; high certainty evidence). This equates to 10.35 HMPs required to prevent one case of DGF. The level of heterogeneity between studies as measured by I2 test was low. A funnel plot including data on DGF incidence can be found in Figure 4. This plot is symmetrical and does not suggest the presence of publication bias.
Analysis 1.1.
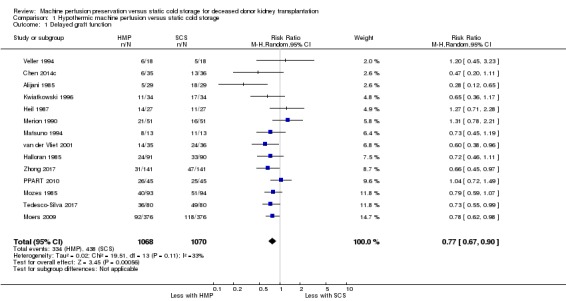
Comparison 1 Hypothermic machine perfusion versus static cold storage, Outcome 1 Delayed graft function.
Figure 4.
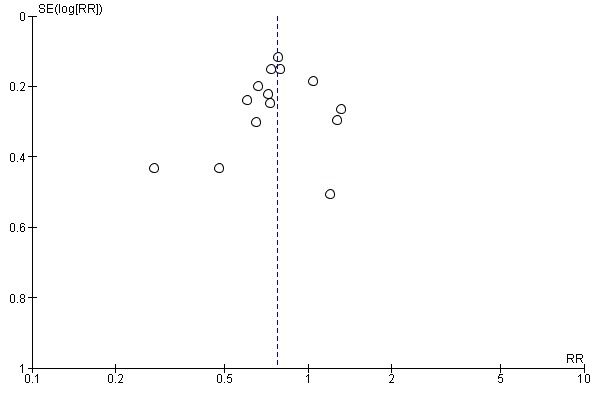
Funnel plot of comparison: 1 Hypothermic machine perfusion versus static cold storage, outcome: 1.1 Delayed graft function.
Sensitivity analysis was performed. Moers 2009 was the largest study, and contributed 752 of 2138 participants. When this study was excluded from the meta‐analysis, the risk of DGF (RR 0.77, 95% CI 0.65 to 0.91; P = 0.003) and the level of heterogeneity (I2 = 39%; P = 0.08) were not affected. Four studies were assessed to have high risk of bias in at least one category (Halloran 1985; Kwiatkowski 1996; Matsuno 1994; Mozes 1985 (Figure 2). Removing all four of these studies from the meta‐analysis, the risk of DGF (RR 0.79, 95% CI 0.64 to 0.97; P = 0.03) remained similar, but a medium level of heterogeneity was found (I2 = 52%; P = 0.03).
Wang 2017 did not report on the number of patients requiring dialysis in the first week post‐transplant (the definition of DGF used in this review) and therefore could not be included in our meta‐analysis. Using their non‐standard definition of DGF they reported a significant improvement in DGF incidence with HMP. Sensitivity analysis was performed, adding the Wang 2017 data (using their non‐standard definition of DGF) to the rest of the studies; the relative risk of DGF (RR 0.77, 95% CI 0.66 to 0.89; P = 0.0003) remained similar, and the level of heterogeneity remained low (I2 = 32%; P = 0.11).
Amaduzzi 2011 reported "No statistically significant difference was found between graft preserved by machine perfusion and cold storage in terms of DGF rate (37,8% vs 30%, respectively p>0.05)."
To ensure robustness of the model, the main analysis (Analysis 1.1) was repeated using a fixed effects model; HMP continued to demonstrate a significant relative risk reduction when compared to SCS (RR 0.76, 95% CI 0.68 to 0.86; P < 0.0001), and heterogeneity remained low (I2 = 33%; P = 0.11).
Subgroup analyses were performed to look for differing treatment effects in various groups. Initial subgroup analysis compared DBD with DCD donors. Six studies looked at DCD (Chen 2014c; Kwiatkowski 1996; Matsuno 1994; PPART 2010; van der Vliet 2001; Zhong 2017), three studies looked at DBD (Mozes 1985; Tedesco‐Silva 2017; Veller 1994), four studies did not specify the donor type (Alijani 1985; Halloran 1985; Heil 1987; Merion 1990), and one study (Moers 2009) reported both DCD and DBD data separately. HMP reduces DGF in the DCD group (Analysis 1.2.1 (7 studies, 772 participants): RR 0.75, 95% CI 0.64 to 0.87; P = 0.0002; I2 = 1%; high certainty evidence), as well as in the DBD group (Analysis 1.2.2 ( 4 studies, 971 participants): RR 0.78, 95% CI 0.65 to 0.93; P = 0.006; I2 = 0%; high certainty evidence). The number of perfusions required to prevent one episode of DGF was 7.26 and 13.60 in DCD and DBD grafts respectively. The level of heterogeneity in both the DCD and DBD subgroups was low. There was no evidence for a differing treatment effect in DBD and DCD donors (P = 0.72). Of note, due to the publication date of the four studies which did not specify donor type (Alijani 1985; Halloran 1985; Heil 1987; Merion 1990) these likely represent DBD donors. A separate analysis was performed to assess the robustness of the subgroup findings including these studies in the DBD subgroup; similar results were found with HMP leading to a relative risk reduction (RR 0.82, 95% CI 0.66 to 1.02; P = 0.07) and there remained no evidence of differing treatment effects in DCD and DBD grafts (P = 0.51).
Analysis 1.2.
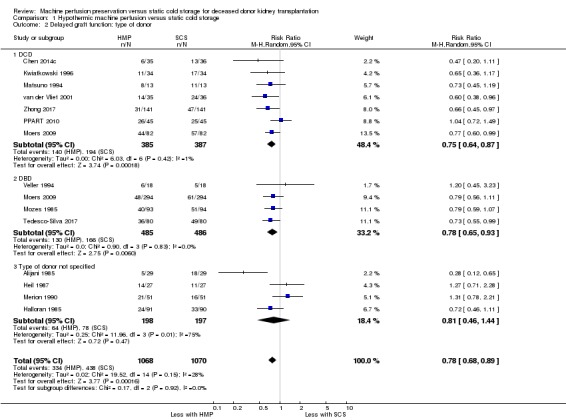
Comparison 1 Hypothermic machine perfusion versus static cold storage, Outcome 2 Delayed graft function: type of donor.
Subgroup analysis was performed looking at the era of study. Five studies reporting DGF in a standard fashion were performed in the last decade (Chen 2014c; Moers 2009; PPART 2010; Tedesco‐Silva 2017; Zhong 2017). All five of these 'modern era' studies used the LifePort perfusion machine. Studies performed over a decade ago ('pre 2008') used older perfusion machines (Waters Mox‐100, Gambro pulsatile perfusion machine, or Nikiso machine). HMP reduced the risk of DGF when compared with SCS in studies performed in the 'modern era'; (Analysis 1.3.1 (5 studies, 1355 participants): RR 0.77, 95% CI 0.66 to 0.91; P = 0.002; I2 = 15%; high certainty evidence). Studies published 'pre‐2008' also demonstrated a reduction in the risk of DGF with HMP (Analysis 1.3.2 (9 studies, 783 participants): RR 0.78, 95% CI 0.61 to 0.99; P = 0.04; I2 = 46%; high certainty evidence). There was no evidence for a differing treatment effect in studies performed in the 'modern era' vs studies performed 'pre‐2008' (P = 0.97).
Analysis 1.3.
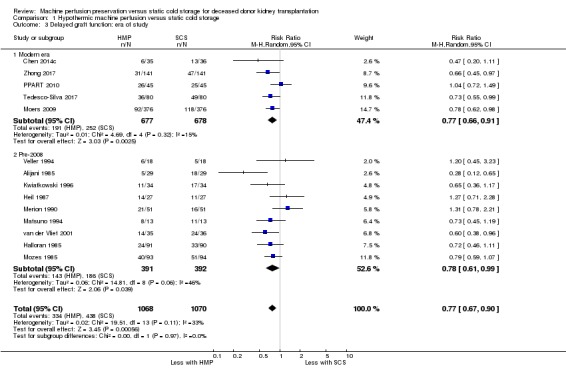
Comparison 1 Hypothermic machine perfusion versus static cold storage, Outcome 3 Delayed graft function: era of study.
Subgroup analysis based on short (< 24 hours) or long (≥ 24 hours) mean cold ischaemic times (CIT) was also performed. Six studies reported short CIT (Matsuno 1994; Merion 1990; Moers 2009; PPART 2010; Veller 1994; Zhong 2017), six studies reported long CIT (Alijani 1985; Halloran 1985; Kwiatkowski 1996; Mozes 1985; Tedesco‐Silva 2017; van der Vliet 2001), and two studies did not report CIT (Chen 2014c; Heil 1987). There was a reduction in the risk of DGF with HMP using a long CIT (Analysis 1.4.2 (6 studies, 725 participants): RR 0.69, 95% CI 0.57 to 0.83; P < 0.0001; I2 = 16%). In the six studies reporting a short CIT (1288 participants), There was little or no reduction in the risk of DGF with HMP and short CIT (Analysis 1.4.1 (6 studies, 1288 participants): RR 0.86, 95% CI 0.70 to 1.04; P = 0.11; I2= 28%). When the two studies which did not report CIT were removed from the analysis, a test for differing treatment effects provided no evidence that the effect of HMP was different between subgroups with short versus long CIT (P = 0.11). The most highly powered study (Moers 2009) reported short mean CIT (15 hours) and found a significant reduction in DGF incidence with HMP (adjusted odds ratio, 0.57; P = 0.01).
Analysis 1.4.
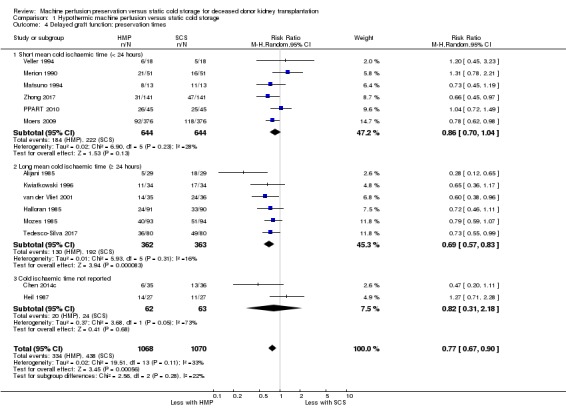
Comparison 1 Hypothermic machine perfusion versus static cold storage, Outcome 4 Delayed graft function: preservation times.
Although initially planned in the protocol, subgroup analyses separating standard versus ECD, and HMP during transport versus HMP at the recipient centre, were not completed. This was due to insufficient reporting of these subgroups across the included studies. We feel this does not limit the review, as the original reason for considering these analyses was to investigate sources of significant heterogeneity, and heterogeneity was found to be low as described above.
The highly powered Moers 2009 performed subgroup analysis to compare SCD with ECD. Incidence of DGF was found to be lower with HMP versus SCS in both the SCD (n = 484, adjusted OR 0.59, 95% CI 0.35 to 1.02) and ECD (n = 188, adjusted OR 0.51, 95% CI 0.24 to 1.09) subgroups. There was no evidence for different treatment effect in these two subgroups (P = 0.75).
Overall, there is high certainty evidence that HMP reduces the risk of DGF.
One‐year graft survival
Eight studies reported one‐year graft survival data (Chen 2014c; Halloran 1985; Moers 2009; PPART 2010; Tedesco‐Silva 2017; van der Vliet 2001; Veller 1994; Zhong 2017) (see Table 4). Many of the studies did not provide information on how the graft survival percentages were calculated. Often only a percentage is given with no indication of statistical significance or the number of people who were followed up to one year. There is not enough information in the studies to analyse the data in a time‐to‐event fashion, as recommended by Cochrane and as specified in our protocol. Unfortunately, it was not possible to meta‐analyse the data; many studies do not provide raw data and studies use different definitions of graft survival (some censoring for death, others not, some providing raw data, others giving the output of a Cox regression model which adjusts for other factors).
Table 2.
Summary of studies reporting one‐year graft survival
| Study ID | Number of participants | One year graft survival results | Information |
| Chen 2014c | 72 | SCS: 91.7% HMP: 97.2% (P = 0.307) |
No information on how percentages were calculated. Therefore, likely not time‐to‐event analysis, and unknown whether graft survival was censored for death. Insufficient information to assess how many patients were followed‐up for a full year |
| Halloran 1985 | 181 | SCS 69.5% HMP 74.9% (“not significant”) |
Survival % is from cox regression time‐to‐event analysis. No P value or further information was provided which may allow inclusion in a meta‐analysis. Death counted as graft failure. Most patients were not followed up for a full year but no further information was given on this |
| Moers 2009 | 672 in graft survival analysis | SCS 90% HMP 94% (P = 0.04) Cox HR 0.52 (P = 0.03) |
Used log‐rank and cox proportional hazards model. Graft survival censored for death (in those dying with a functioning graft). Graft survival rates are a result of this time‐to‐event death censored analysis |
| PPART 2010 | 90 | SCS 44/45 (97.8%) MP 42/45 (93.3%) (P = 0.3) |
They give actual numbers for numbers of grafts which failed by 1 year. Death was not counted as graft failure. Time‐to‐event analysis not performed |
| Tedesco‐Silva 2017 | 160 | SCS 72/78 (92.3%) HMP 72/80 (90%) (P = 1.000) |
They give actual numbers for numbers of grafts which failed by 1 year. Death was not counted as graft failure. Time‐to‐event analysis not performed |
| van der Vliet 2001 | 76 | SCS 84.2% HMP 76.3% |
No information on how percentages were calculated. Therefore, likely not time‐to‐event analysis, and unknown whether graft survival was censored for death. Insufficient information to assess how many patients were followed‐up for a full year. No P value was reported |
| Veller 1994 | 36 | SCS 82% HMP 83% |
No information on how percentages were calculated. Therefore, likely not time‐to‐event analysis, and unknown whether graft survival was censored for death. Insufficient information to assess how many patients were followed‐up for a full year. No P value was reported |
| Zhong 2017 | 282 | SCS 93% HMP 98% (P = 0.026) |
Graft survival was analysed using a log‐rank test. Graft survival was censored for death (in those dying with a functioning graft). Graft survival estimates are based on time‐to‐event analysis and raw data for number of graft losses was not given. Hazard ratios were not reported |
HMP ‐ hypothermic machine perfusion; SCS ‐ static cold storage
The two most powerful studies (Moers 2009 and Zhong 2017) both reported statistically significant benefits in one‐year graft survival with HMP versus SCS. Moers 2009, which included mostly DBD kidneys, used appropriate time‐to‐event analysis and reported a statistically significant improvement with HMP (90% SCS versus 94% HMP, log‐rank P = 0.04; Cox HR for one‐year graft loss, 0.52; P = 0.03). Zhong 2017, which included only DCD kidneys, used log‐rank test analysis and also reported a statistically significant improvement with HMP (93% SCS versus 98% HMP; P = 0.026). As described in Table 4, the remaining studies report non‐significant differences in one‐year graft survival (Chen 2014c; Halloran 1985; PPART 2010; Tedesco‐Silva 2017), or do not provide P values (van der Vliet 2001; Veller 1994).
Whilst Mozes 1985 does not report one‐year graft survival directly, there is a table reporting graft loss. Using this table, one‐year graft survival estimates of 60.7% and 65.4% in SCS and HMP groups respectively were calculated. However, mathematical inconsistencies were identified in the table, so these calculations are likely inaccurate.
Heil 1987 only provided graft survival data on those kidneys which experienced DGF. They reported that kidneys which experienced DGF had one‐year graft survival rates of 74% and 89% for SCS and HMP respectively (P < 0.05). However, they did not state how many of the 25 kidneys which experienced DGF were followed up for the full year.
Primary non‐function
Seven studies (Halloran 1985; Matsuno 1994; Moers 2009; Mozes 1985; PPART 2010; Tedesco‐Silva 2017; van der Vliet 2001) reported on PNF. There was no evidence that the use of HMP affected the risk of developing PNF when compared to SCS (Analysis 1.5 (7 studies, 1387 participants): RR 0.88, 95% CI 0.58 to 1.33; P = 0.55; I2 = 0%; high certainty evidence). A funnel plot showed no signs of asymmetry (Figure 5). The results remained unchanged when studies assessed to have a high risk of bias in at least one area (Halloran 1985; Matsuno 1994; Mozes 1985) were removed from the analysis (RR 1.05, 95% CI 0.37 to 3.02; P = 0.92).
Figure 5.
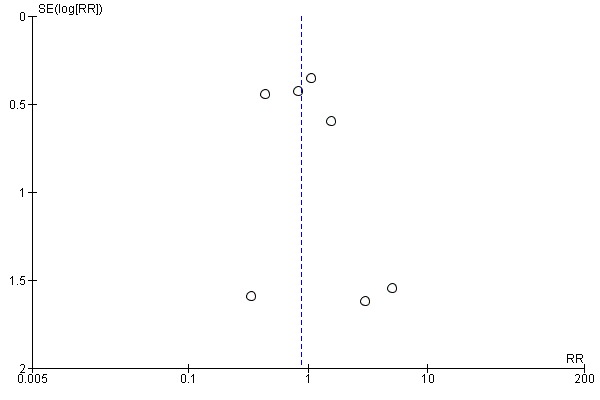
Funnel plot of comparison: 1 Hypothermic machine perfusion versus static cold storage, outcome: 1.5 Primary non‐function.
Duration of delayed graft function
Six studies (Heil 1987; Matsuno 1994; Moers 2009; Mozes 1985; PPART 2010; Tedesco‐Silva 2017) reported on duration of DGF. Only four of these studies could be included in the meta‐analysis; three included mean and SD data (Heil 1987; Mozes 1985; Tedesco‐Silva 2017), and Matsuno 1994 provided means and a P value which allowed imputation of a conservative SD estimate. It is uncertain whether HMP reduces the duration of DGF (Analysis 1.6 (4 studies, 220 participants): MD ‐1.23 days, 95% CI ‐5.87 to 3.40; P = 0.60; very low certainty evidence). The level of heterogeneity was high (I2 = 88%), with two studies reporting statistically significant reductions in DGF duration with HMP (Heil 1987; Matsuno 1994) and one study reporting statistically significant increases in DGF duration with HMP (Mozes 1985). It is important to note that Analysis 1.6 does not display all of the available evidence on DGF duration, only that evidence presented in a way to allow meta‐analysis.
Analysis 1.6.
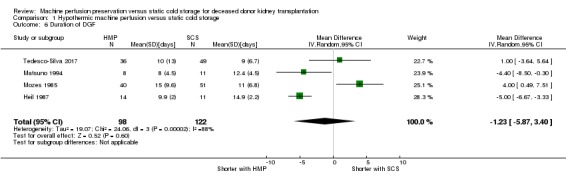
Comparison 1 Hypothermic machine perfusion versus static cold storage, Outcome 6 Duration of DGF.
Means and SD could not be imputed for Moers 2009 or PPART 2010 which only reported median, range and a P value for the duration of DGF. Whilst this prevented their inclusion in the meta‐analysis, they do provide further evidence. PPART 2010 reported no significant difference in duration of DGF; 7 days (range 1 to 33) for SCS versus 5 days (range 1 to 92) for HMP (P = 0.40). However, the European study by Moers 2009 did report a significant reduction in DGF duration with HMP; 13 days (range 1 to 41) for SCS versus 10 days (range 1 to 48) for HMP (P = 0.04).
Overall, three studies report a significant reduction in DGF (Heil 1987; Matsuno 1994; Moers 2009) duration with HMP, one reports an increase in DGF duration (Mozes 1985), and two were inconclusive (PPART 2010; Tedesco‐Silva 2017).
Long‐term graft survival
Three studies (Kwiatkowski 1996; Moers 2009; Zhong 2017) provided data on longer term transplant survival. Moers 2009 continued to follow up all 672 participants from their main analysis as well as an additional 80 participants from their extended DCD dataset for three years. Overall, three‐year graft survival was significantly improved by HMP versus SCS (91% versus 87%; adjusted hazard ratio for transplant failure, 0.60; P = 0.04). This benefit was most pronounced in the subgroup of grafts from ECD (86% versus 76%; adjusted hazard ratio, 0.38; P = 0.01). Interestingly, the authors state that a significant three‐year graft survival benefit was not seen in the DCD subgroup, but no further data or explanation was given. This could simply represent a lack of power due to the smaller number of patients in this subgroup (164 participants).
Zhong 2017 followed all 282 included participants for three years. The log‐rank test was used to analyse three‐year graft survival, censoring for death (in those who died with a functioning graft). In their large cohort of DCD recipients, the three‐year graft survival rate in the HMP group was significantly higher than that in the SCS group (93% versus 82%; P = 0.036).
Kwiatkowski 1996 provided the longest follow up data, reporting 10‐year graft survival. The group which received HMP had improved 10‐year graft survival when compared to the SCS group (68.2% versus 43.0%), however this was not statistically significant (P = 0.08). This may be a result of the low power of the study, with 37 patients in each arm. The study did report a significant difference in the frequency of "return to dialysis" between the groups (50% SCS versus 25% HMP; P = 0.02), however this does not appear to be a pre‐specified outcome and may suffer selective reporting bias.
Patient survival
Four studies reported on one‐year patient survival (Halloran 1985; Moers 2009; PPART 2010; Tedesco‐Silva 2017). Halloran 1985 reported survival data calculated from Cox regression. As this was the only study to provide time‐to‐event data, meta‐analysis using this could not be applied. Three studies (Moers 2009; PPART 2010; Tedesco‐Silva 2017) reported the number of patients who had died at one year. These studies could be combined. There no evidence that HMP has an effect on one‐year patient survival (Analysis 1.7 (3 studies, 920 participants): RR 0.99, 95% CI 0.95 to 1.03; P = 0.58; I2 = 20%; low certainty evidence). Halloran 1985 also reported a non‐significant impact on one‐year patient survival (88.8% SCS versus 94.9% HMP; P = “not significant”).
Analysis 1.7.
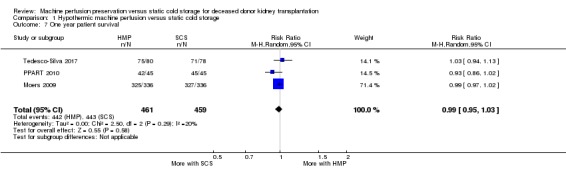
Comparison 1 Hypothermic machine perfusion versus static cold storage, Outcome 7 One year patient survival.
Whilst Mozes 1985 does not report one‐year patient survival directly, there is a table reporting patient survival. Using this table, one‐year patient survival estimates of 89.0% and 89.7% in SCS and HMP groups respectively were calculated. However, mathematical inconsistencies were identified in the table, so these calculations are likely inaccurate.
Two studies (Kwiatkowski 1996; Moers 2009) provided longer follow‐up of patients. Moers 2009 simply states that there were no significant differences in three‐year patient survival between HMP and SCS groups (n = 752). Kwiatkowski 1996 reports that there were no significant differences in 10‐year patient survival between HMP and SCS groups (86.5% versus 83.7%, n = 72; P = ns).
Economic implications
Two reports performed economic evaluation. Both of these performed their analysis based on the results of Moers 2009. Both reports confirm cost savings with HMP, one in the US and one in the European setting. Groen 2012 reported estimated mean total costs of $8668 with HMP versus $11,294 with SCS in the European setting. Garfield 2009 performed US projections and reported that HMP improved mean costs when compared to SCS in both standard criteria donors ($92,561 versus $104,118) and ECD ($106,012 versus $114,530). One of the main reasons for the cost savings, were lower dialysis costs in the HMP group due to decreased incidence of DGF.
Quality of life
No studies reported quality of life.
Hospital stay
Four studies (Chen 2014c; Moers 2009; Tedesco‐Silva 2017; Wang 2017) reported on length of hospital stay. Reporting was insufficient to allow meta‐analysis. Chen 2014c reported a significantly shorter hospital stay with HMP compared to SCS (16.8 days versus 21.4 days; P = 0.046), but did not state whether these values were means or medians, and did not provide standard deviations or inter‐quartile ranges. Wang 2017 reported a significantly shorter mean hospital stay with HMP compared with SCS (12.3 ± 4.4 versus 19.4 ± 7.2 respectively; P = 0001). Moers 2009 found no significant difference in the median length of hospital stay between groups (18 days SCS versus 19 days HMP; P = 0.78). Tedesco‐Silva 2017 also found no significant difference in hospital stay between groups (15.6 ± 11.7 days SCS versus 13.5 ± 10.5 HMP; P = 0.629).
Graft function
Five studies reported on graft function (Moers 2009; PPART 2010; Tedesco‐Silva 2017; van der Vliet 2001; Zhong 2017). The following measures were reported: SCr, creatinine clearance, estimated glomerular filtration rate (eGFR), area under the curve of creatinine for the first two weeks post‐transplant, creatinine reduction ratio post‐transplant, and urine output. These were reported at time points ranging from seven days to one year. As studies reported different outcomes at different time points meta‐analysis could not be completed.
Moers 2009 reported creatinine clearance at 14 days, and area under the creatinine curve during the first 14 days. Median creatinine clearance at 14 days was not significantly different in the HMP group compared with the SCS group (42 HMP versus 40 SCS; P = 0.25). By performing daily SCr measurements, Moers 2009 was able to calculate area under the curve for the first 14 days post‐transplant, with a lower value equating to better graft function. HMP significantly decreased the median area under the curve compared to SCS (1456 HMP versus 1787 SCS; P = 0.01). This difference is to be expected given the decreased incidence of DGF with HMP which was reported by the same study.
Zhong 2017 reported SCr and urine output in the first week post‐transplant. Data was collected on both of these outcomes every day for the first seven days post‐transplant. "Analysis for repeated measurement data" was used to compare SCS with HMP. They reported that HMP led to a significant decrease in median SCr (F = 5.165; P = 0.024), and a significant increase in median urine output (F = 3.962; P = 0.047), in the first seven days post‐transplant.
PPART 2010 reported on the creatinine reduction ratio between day one and day two, and the creatinine reduction ratio between immediately pre‐transplant and day five. They also reported eGFR at day seven, three months, and one year. There were no significant differences in any of these values between the HMP and SCS groups, in keeping with similar DGF rates in each group.
Tedesco‐Silva 2017 provide data for mean SCr and eGFR (± SD) at time points of 7, 14, 21, 28, and 365 days. They reported that "mean serum creatinine was significantly lower in the HMP group compared with the SCS at both 14 days (3.0 ± 2.2 HMP versus 4.1 ± 3.2 mg/dL; P = 0.005) and 21 days (2.3 ± 1.8 HMP versus 3.0 ± 2.6 mg/dL; P = 0.021)". Although these results are significant, they did not perform statistical corrections for multiple comparisons, and found no evidence for differences in SCr at any of the other three time points, or significant differences in eGFR at any of the five time points.
van der Vliet 2001 reported mean SCr (± SD) at 3 months post‐transplant; there was no significant difference between HMP and SCS groups (174 ± 25 HMP versus 162 ± 11 µmol/L SCS group; P = 0.68).
Overall there is no evidence that long‐term graft function is affected. The significant improvements seen in short‐term graft function are analogous to the significant improvements seen in DGF incidence.
Episodes of acute rejection or fibrosis on biopsy
Five studies (Kwiatkowski 1996; Moers 2009; PPART 2010; Tedesco‐Silva 2017; Wang 2017) reported acute rejection. As they reported on acute rejection over different time periods meta‐analysis including all studies was not possible. Only PPART 2010 and Tedesco‐Silva 2017 had a shared time point; acute rejection within one year. HMP may make little or no difference to acute rejection at one year (Analysis 1.8 (2 studies, 248 participants): RR 0.66, 95% CI 0.37 to 1.17; P = 0.15; I2 = 13%; low certainty evidence).
Analysis 1.8.
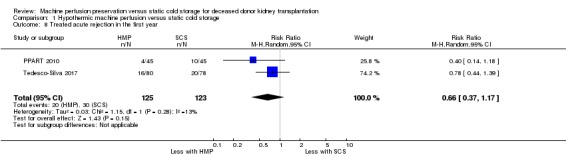
Comparison 1 Hypothermic machine perfusion versus static cold storage, Outcome 8 Treated acute rejection in the first year.
In addition to the one year data, PPART 2010 also reported a lower incidence of biopsy‐proven acute rejection in the HMP group within the first three months (n = 90; 22% SCS versus 7% HMP; P = 0.06), although this is not significant. Tedesco‐Silva 2017 reports incidence of treated acute rejection within the first month (n = 160; 16.3% SCS versus 8.8%; P = 0.151); again the lower incidence of acute rejection in the HMP group is not significant. Moers 2009 reported on incidence of biopsy‐proven acute rejection at 14 days, and found similar rates between the groups (n = 672; 13.7% SCS versus 13.1% HMP; P = 0.91).
Kwiatkowski 1996 reported incidence of treated acute rejection during the full duration of follow‐up (median 22 months, range 7 to 37 months). They found that incidence of acute rejection was lower with HMP (n = 74; 51% SCS versus 35% HMP) but this was not statistically significant. Kwiatkowski 1996 did not state whether the follow‐up duration was similar between groups, therefore the validity of these results are questionable.
Wang 2017 reported on incidence of acute rejection, however it is not stated whether this is biopsy‐proven rejection or clinical rejection. It is also not stated over what time period acute rejection data was collected over. They report acute rejection in 1/24 kidneys undergoing HMP and 2/24 kidneys undergoing SCS (P = 0.551),
Fibrosis on biopsy was not reported by any studies.
Number of allograft ultrasound scans
No studies reported the number of ultrasound scans.
Number of allograft biopsies
No studies reported the number of allograft biopsies.
Normothermic machine perfusion versus hypothermic machine perfusion or static cold storage
To date no RCT has been published which includes a NMP arm. Our search identified one ongoing RCT comparing NMP with SCS which could be included in future updates of this review (Hosgood 2017).
Discussion
Summary of main results
Overall, 16 studies (2266 participants) were included. These studies all compared HMP with standard SCS. None of the included studies investigated (sub)NMP, however one ongoing normothermic study was identified (Hosgood 2017).
The use of HMP reduced the rate of DGF compared to SCS (RR 0.77, 95% CI 0.67 to 0.90; P = 0.0006, high certainty evidence). This result was also observed for both DCD (7 studies, 772 participants: RR 0.75, 95% CI 0.64‐0.87; P = 0.0002), and DBD subgroups (7 studies, 971 participants: RR 0.78, 95% CI 0.65‐0.93; P = 0.006). There was no evidence for differing treatment effect between these groups (P = 0.72). That said, as the overall incidence of DGF is higher in the DCD subgroup, HMP prevents more episodes of DGF in DCD grafts in absolute terms. Therefore, the number of HMP required to prevent one episode of DGF (number needed to treat; NNT) is lower in DCD grafts; 7.26 and 13.60 in DCD and DBD grafts respectively. There was no evidence that the beneficial effect of HMP varies depending on duration of CIT (Analysis 1.4). Studies published in the last decade ('modern era') all used the LifePort HMP device. Clearly these studies are especially relevant for practice today. In these 'modern era' studies, HMP with LifePort decreased the incidence of DGF compared with SCS (5 studies, 1355 participants: RR 0.77, 95% CI 0.66 to 0.91; P = 0.002; high certainty evidence).
Economic analysis based on results from the large Moers 2009 study suggest that HMP is cost effective in both the European and US setting. The main reason for cost savings in the HMP group was the lower incidence of DGF. The reported risk of DGF with HMP reported by Moers 2009 (RR 0.77) was similar to the overall risk from our meta‐analysis (RR 0.78). Therefore, the cost savings reported based on results from Moers 2009 are generalisable to our meta‐analysis as a whole; we feel it is almost certain that HMP results in cost savings.
Although graft survival was reported in some form by 10 studies, it was insufficiently accurately reported to allow meta‐analysis. A summary of all studies reporting on overall one‐year graft survival is provided in Table 4. The EuroTransplant study (Moers 2009) reported a significant graft survival benefit of HMP compared with SCS, at both one year (90% SCS versus 94% HMP, log‐rank P = 0.04; Cox HR for one‐year graft loss, 0.52; P = 0.03) and three years (87% SCS versus 91% HMP; adjusted hazard ratio for transplant failure, 0.60; P = 0.04). It is important to note that this study included predominantly DBD kidneys. Zhong 2017 used log‐rank test analysis and also reported a statistically significant survival benefit of HMP compared with SCS, at both one (93% SCS versus 98% HMP; P = 0.026) and three years (82% SCS versus 93% HMP; P = 0.036) in their cohort of DCD kidney recipients. Both of these studies were well designed, and well powered. Together they provide strong evidence that HMP improves graft survival in kidneys from both DBD and DCD donors. Other studies reporting graft survival were less well powered and did not report significant differences in transplant survival.
Overall, we feel that transplant centres should consider the use of HMP in all kidney transplants on the basis of the benefits listed above (reduced incidence of DGF, cost savings, and improved graft survival), which have all been demonstrated/confirmed by studies in the modern era (those performed in the last decade). This is especially important in DCD kidneys, where the number of perfusions needed to prevent one episode of DGF is far lower (7.26 versus 13.60 in DBD kidneys).
Four studies reported on patient survival and none of these found significant differences between HMP and SCS. It is likely that any effect on patient survival is small, and beyond the detection size of these samples.
Based on high quality evidence from 7 studies, there was no evidence that HMP has an impact on incidence of PNF (RR 0.88, 95% CI 0.58 to 1.33; P = 0.55). There are two explanations for this. PNF is an inevitable event which HMP has no effect. Alternatively, this is a type 2 statistical error, as the incidence of PNF is low and it may be difficult to demonstrate significant differences in PNF incidence.
There is some good evidence that HMP reduces the duration of DGF, with three studies finding reductions in DGF duration (including the highly powered Moers 2009). However, one study contradicts this (Mozes 1985) and two found no significant differences. Not all studies provided mean and SD data, and these could not be imputed due to evidence of positive skew, therefore meta‐analysis was not possible. The contradictory evidence from these studies may be due to hospital and physician differences in criteria for dialysis. Further studies looking at duration of DGF would likely change the estimate therefore this evidence is very low certainty.
Five studies reported on transplant function. This was reported in various ways (based on SCr or urine output), at various time points, preventing meta‐analysis. Three studies reported significant improvements in graft function in the short term with HMP (Moers 2009; Tedesco‐Silva 2017; Zhong 2017). This is in keeping with the lower incidence of DGF in the HMP group reported by these studies. The three studies (PPART 2010; Tedesco‐Silva 2017; van der Vliet 2001) which looked at graft function at time points greater than one month, found no significant differences in long‐term graft function, although the level of certainty is low.
Five studies reported on acute rejection. All of these reported a lower incidence of acute rejection with the use of HMP, however this result was not significant in any studies. Reporting at various time points prevented meta‐analysis of all five studies. Only two studies could be included in meta‐analysis; HMP may make little or no difference to acute rejection at one year (RR 0.66, 95% CI 0.37 to 1.17; P = 0.15).
Four studies reported length of hospital stay. Two reported no significant differences between HMP and SCS (Moers 2009; Tedesco‐Silva 2017). Two small studies (Chen 2014c; Wang 2017) reported a significant reduction in hospital stay with HMP.
Other secondary outcomes (quality of life, number of ultrasound scans, number of biopsies) were not reported by any studies.
Overall completeness and applicability of evidence
The 16 studies included in this review were from a range of different locations (USA, Europe, China, Japan, Canada, South Africa and Brazil). Some studies reported on the use of HMP in DCD kidneys and some on DBD kidneys. Studies with both short and long mean CIT were also well represented. Many of the studies were reported in the last decade. Overall, this makes the results of this review generalisable and therefore applicable to many different transplant settings.
DGF data was available from all studies, although reporting by Wang 2017 was incompatible with the standard definition of DGF used in this review. Our main secondary outcome, one‐year graft survival, was only reported by seven studies, and insufficient reporting prevented meta‐analysis. With the exception of one‐year graft survival and PNF, our secondary outcomes were reported by a minority of studies, and differences in reporting often prevented meta‐analysis. The authors of several studies were contacted to try and gather additional unreported data. Unfortunately, none of the corresponding authors responded to inquiries. In one instance this prohibited the inclusion of the results of this study in our meta‐analyses (Amaduzzi 2011).
Moers 2009 was a large study and contributed to a lot of the outcomes included in this review. It could therefore be argued that this study dominates our results. However, the evidence from this study was gathered from 60 different hospitals throughout Europe, and included DBD, DCD, SCD and ECD, so results from this study are generalisable to the current European setting.
We have reported numbers of perfusions needed to prevent one episode of DGF in our results. This number depends on the incidence of DGF, so these figures may not be applicable to transplant centres which have particularly high or low rates of DGF.
Quality of the evidence
A summary of identified biases can be found in the Risk of bias in included studies section above. Where bias could be assessed, studies were generally well designed leading to a low risk of bias. Wang 2017 was considered to be at high risk of bias. In some studies there was a tendency to leave the HMP kidney for longer. The resulting increase in CIT introduces bias, and may lead to an underestimate of the positive effect of HMP in these studies. Many of the older studies (especially those published before 2000) had very short manuscripts making risk of bias difficult to assess.
The primary outcome of all included studies was incidence of DGF. This outcome is measured in the first week post‐transplant, whilst the patient is still in hospital. This means that participants were very unlikely to be lost to follow‐up and there was virtually no missing data for the outcome of DGF. In addition, this outcome tended to be reported in a standardised fashion, with studies simply reporting raw data for the numbers of patients with DGF in each group, allowing inclusion of all studies except Wang 2017 in meta‐analysis. This resulted in high certainty evidence for this outcome.
None of our other outcomes were reported by all studies. In addition, other outcomes tended to be reported in different ways by different studies, which often prevented inclusion of all studies into meta‐analysis. This meant that the quality of evidence for outcomes other than incidence of DGF was lower (see Table 1 for more information).
Potential biases in the review process
We attempted to limit biases at every stage in our review. The search for studies was performed in a systematic fashion using the Cochrane Kidney and Transplant Specialised Register. Two independent authors screened the identified studies prior to inclusion in the review. A standardised data extraction form was used to collect data from included studies. This was done independently by two authors, and any discrepancies were resolved. Subgroup analysis was only performed if pre‐specified in our protocol, to limit bias from multiple comparisons. There is however always a possibility that we failed to identify some relevant studies.
Agreements and disagreements with other studies or reviews
O'Callaghan 2013 was the previous large meta‐analysis comparing HMP with SCS. They also used incidence of DGF as their primary outcome. They included seven RCTs in their meta‐analysis, all of which are also included in our review. O'Callaghan 2013 reported a significant decrease in DGF with HMP, reporting a RR of 0.81 (similar to our meta‐analysis). As our review was able to include more studies we were able to demonstrate a significant reduction in DGF in both DCD and DBD subgroups, which O'Callaghan 2013 did not demonstrate. As in our review, O'Callaghan 2013 was not able to perform meta‐analysis of graft survival data. In contrast to O'Callaghan 2013, there are now sufficient studies to provide strong evidence that HMP leads to improved graft survival compared to SCS.
Authors' conclusions
There is high certainty evidence that HMP reduces the incidence of DGF when compared to SCS, in both DBD and DCD kidneys. The number of perfusions required to prevent one episode of DGF was 7.26 and 13.60 in DCD and DBD kidneys respectively, demonstrating that HMP is especially beneficial in DCD grafts. Previous economic analysis suggests that this alone makes HMP a cost‐effective intervention. HMP may also decrease the duration of DGF when it develops.
There is strong evidence that HMP has a positive impact on transplant survival in both the short and long term, in both DBD and DCD grafts. This is to be expected given previous research has shown the DGF is associated with higher rates of kidney loss (Yarlagadda 2009).
Overall, there is high certainty evidence for the benefits of HMP in terms of incidence of DGF, the cost savings that this has been shown to produce, and the improved transplant survival (all of which have been demonstrated/confirmed by studies published in the last decade). This is especially important in DCD kidneys, where the number of perfusions needed to prevent one episode of DGF is far lower (7.26 versus 13.60 in DBD kidneys).
Further studies comparing HMP with SCS and reporting only DGF incidence are not required. Any new studies should include data on duration of DGF and incidence of acute rejection. Data should also be included to allow meta‐analysis of transplant survival in a time‐to‐event fashion.
Follow‐up reports detailing long‐term graft survival from participants of the studies already included in this review would be an efficient way to generate long‐term graft survival data. This is vital in assessing the long‐term benefits of HMP.
Economic analysis based on the results of this review would help cement HMP as the standard preservation method in deceased donor kidney transplantation.
RCTs investigating (sub)NMP are required.
As described above, current studies fail to provide evidence of a benefit in terms of PNF. Research investigating the use of perfusion parametrics such as flow, pressure and resistance, to perform viability assessment may be useful in preventing incidences of PNF.
Acknowledgements
The author team would like thank the referees and to acknowledge the ongoing help and assistance of the Cochrane Kidney and Transplant group. The research was funded in part by the National Institute for Health Research Blood and Transplant Research Unit (NIHR BTRU) in Organ Donation and Transplantation at the University of Cambridge in collaboration with Newcastle University and in partnership with NHS Blood and Transplant (NHSBT). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, the Department of Health or NHSBT.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE (OVID) |
|
| EMBASE (OVID) |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1.
Hypothermic machine perfusion versus static cold storage
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Delayed graft function | 14 | 2138 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.67, 0.90] |
| 2 Delayed graft function: type of donor | 14 | 2138 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.68, 0.89] |
| 2.1 DCD | 7 | 772 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.64, 0.87] |
| 2.2 DBD | 4 | 971 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.65, 0.93] |
| 2.3 Type of donor not specified | 4 | 395 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.46, 1.44] |
| 3 Delayed graft function: era of study | 14 | 2138 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.67, 0.90] |
| 3.1 Modern era | 5 | 1355 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.66, 0.91] |
| 3.2 Pre‐2008 | 9 | 783 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.61, 0.99] |
| 4 Delayed graft function: preservation times | 14 | 2138 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.67, 0.90] |
| 4.1 Short mean cold ischaemic time (< 24 hours) | 6 | 1288 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.70, 1.04] |
| 4.2 Long mean cold ischaemic time (≥ 24 hours) | 6 | 725 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.57, 0.83] |
| 4.3 Cold ischaemic time not reported | 2 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.31, 2.18] |
| 5 Primary non‐function | 7 | 1387 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.58, 1.33] |
| 6 Duration of DGF | 4 | 220 | Mean Difference (IV, Random, 95% CI) | ‐1.23 [‐5.87, 3.40] |
| 7 One year patient survival | 3 | 920 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.95, 1.03] |
| 8 Treated acute rejection in the first year | 2 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.37, 1.17] |
Analysis 1.5.
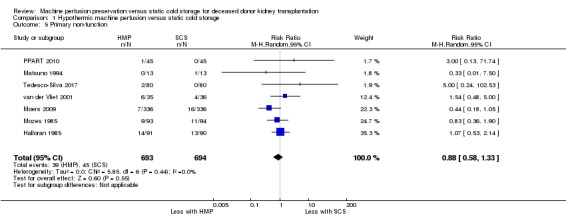
Comparison 1 Hypothermic machine perfusion versus static cold storage, Outcome 5 Primary non‐function.
Differences between protocol and review
In the protocol we included studies looking at (sub)NMP. As anticipated, no such studies could be included in this version of the review. However, an ongoing study has been identified which is looking at NMP and could be included in future versions of this review.
Although mentioned in the protocol, subgroup analyses separating standard versus ECD, and HMP during transport versus HMP at the recipient centre, were not completed. This was due to insufficient reporting of these subgroups across the included studies. We feel this does not limit the review, as the original reason for considering these analyses was to investigate sources of significant heterogeneity, and heterogeneity was found to be low for our primary outcome (Analysis 1.1). In the initial protocol we did not include subgroup analysis based on the 'era of study'. However, this subgroup analysis was suggested by a peer reviewer and our sign off editor, and was therefore added.
In the protocol we stated that transplant survival and patient survival would be analysed as time‐to‐event data, with O‐E and V statistics entered into RevMan. However, due to insufficient reporting, transplant survival could not be entered into a meta‐analysis, and patient survival could only be analysed as number of patients alive at one year (dichotomous).
It is widely accepted that the key measure of success of a preservation technique is its ability to reduce the incidence of DGF. This is evidenced by the fact that all included studies used DGF as their primary outcome. Therefore, DGF incidence was chosen as the primary outcome, and one‐year graft survival was changed to a secondary outcome for the final version of this review (despite one‐year graft survival being listed as a primary outcome in the original protocol).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods |
|
|
| Participants |
|
|
| Interventions |
Machine perfusion
Static cold storage
Mean CIT
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Although it is quasi‐randomised, it is 38 consecutive donors and the reasons for exclusions are clear and appropriate. The fact that it is 38 consecutive donors means that selection bias is likely not a large source of bias |
| Allocation concealment (selection bias) | Low risk | See random sequence generation above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but CIT was not significantly longer in either group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding, but outcome measurements are unlikely to be affected by the lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data reported for all kidneys, and reason for exclusion of donors and discarding of kidneys was explained clearly |
| Selective reporting (reporting bias) | Low risk | Appropriate outcome clearly reported |
| Other bias | Unclear risk | Short methods section, as expected given the date of the study. Intention to treat analysis not performed, but this only affected one kidney pair |
| Methods |
|
|
| Participants |
|
|
| Interventions | Machine perfusion
Cold static storage
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Not all outcomes listed were reported; data presented could not be meta‐analysed |
| Other bias | Unclear risk | Insufficient information to permit judgement |
| Methods |
|
|
| Participants |
|
|
| Interventions | Machine perfusion
Static cold storage
Mean CIT
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding not discussed, but outcome measurements are unlikely to be affected by the lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were followed up |
| Selective reporting (reporting bias) | Low risk | Study outcomes were appropriate, and concisely reported |
| Other bias | Unclear risk | As this is only an abstract, thorough analysis of biases is impossible |
| Methods |
|
|
| Participants |
|
|
| Interventions | Machine perfusion
Static cold storage
Mean CIT
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Patients were randomised before procurement, immediately after consent for organ donation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but CIT was not statistically significantly different between groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding, but outcome measurements are unlikely to be affected by the lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 13 kidneys randomised to MP and were then changed to SCS. These patients were not reported and were excluded from the study; intention to treat analysis was not employed |
| Selective reporting (reporting bias) | High risk | They used a complex and unusual definition for DGF, for unclear reasons. However, data included in our analysis will be taken directly from the number of dialyses in week one table, so this bias will not impact on the meta analysis |
| Other bias | Unclear risk | A relatively short methods section |
| Methods |
|
|
| Participants |
|
|
| Interventions | Machine perfusion
Static cold storage
Mean CIT
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used to decide which kidney would receive MP |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information on blinding, or on differences in CIT between groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information, but outcome measurements are unlikely to be affected by the lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Low risk | Appropriate outcomes, adequately reported |
| Other bias | Unclear risk | Extremely short manuscript making thorough analysis of biases impossible |
| Methods |
|
|
| Participants |
|
|
| Interventions | Machine perfusion
Static cold storage
Mean CIT
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Very few details given, only that the kidneys were paired and randomised |
| Allocation concealment (selection bias) | Unclear risk | Very few details given, only that the kidneys were paired and randomised |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding and the kidneys were transplanted such that the SCS kidney was routinely transplanted before the MP kidney |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding, but outcome measurements are unlikely to be affected by the lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No reason given as to why there was no DGF information on 6 patients. In terms of 10‐year graft survival, this was only given as a percentage with no indication to how many patients were followed up |
| Selective reporting (reporting bias) | High risk | A lot of data was not reported, and most of the data reported was incomplete or reported ambiguously |
| Other bias | High risk | A relatively short manuscript. CIT was significantly different between the groups |
| Methods |
|
|
| Participants |
|
|
| Interventions | Machine perfusion
Static cold storage
Mean CIT
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study does not specify how, or at what time, the randomisation takes place. It only actually mentions that the study is randomised in the concluding paragraph |
| Allocation concealment (selection bias) | Unclear risk | The study does not specify how, or at what time, the randomisation takes place. It only actually mentions that the study is randomised in the concluding paragraph |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding and there was a significant difference in CIT, suggesting that the groups were treated differently. Prolonged CIT is likely to affect the primary outcome of DGF |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding, but outcome measurements are unlikely to be affected by the lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data available for all included donors |
| Selective reporting (reporting bias) | Low risk | The outcomes reported were appropriate and expected |
| Other bias | High risk | The study does not state the duration of the study, whether they are consecutive cases, or how inclusion/randomisation took place |
| Methods |
|
|
| Participants |
|
|
| Interventions | Machine perfusion
Static cold storage
Mean CIT
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Although an alternating design was used, all consecutive donors between two time periods were considered for inclusion. 51 of the 60 donors were included, and clear reasons were given for exclusion of the nine donors. This and the fact that the study used a paired design, means that the selection bias is unlikely to have altered the results of this study |
| Allocation concealment (selection bias) | Low risk | See random sequence generation above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding but this is unlikely to affect the outcome, especially as there was no difference in CIT between the groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding, but outcome measurements are unlikely to be affected by the lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow up data for all included patients and clear explanations for the nine excluded patients |
| Selective reporting (reporting bias) | Low risk | Appropriate outcomes, well reported |
| Other bias | Low risk | A thorough descriptive methods section |
| Methods |
|
|
| Participants |
|
|
| Interventions | Machine perfusion
Static cold storage
Mean CIT
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation lists for each study region |
| Allocation concealment (selection bias) | Low risk | Allocation happened prior to procurement, whilst the donor was still in the ICU |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | They performed blinding at the time of organ offer, so a centre could not turn down a kidney on the basis of storage method |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding, but outcome measurements are unlikely to be affected by the lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 patient out of 672 was lost to follow‐up. The rest were followed up to at least one year |
| Selective reporting (reporting bias) | Low risk | All outcomes reported were present in the original study protocol except for PNF |
| Other bias | Unclear risk | Intention to treat analysis likely not completed |
| Methods |
|
|
| Participants |
|
|
| Interventions | Machine perfusion
Static cold storage
Mean CIT
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Recipient centres were informed of the preservation method used and left MP kidneys for longer; more kidneys had a CIT > 36 hours in the MP group (P = 0.01) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding, but outcome measurements are unlikely to be affected by the lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The 5 kidneys not used were as a result of "recipient unavailability" which is unlikely to be a source of bias. Thorough outcome data was reported for the remaining 187 patients |
| Selective reporting (reporting bias) | Low risk | Appropriate outcome measures, well reported |
| Other bias | Unclear risk | Limited methods section, as expected due to the date of the study |
| Methods |
|
|
| Participants |
|
|
| Interventions | Machine perfusion
Static cold storage
Mean CIT
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random sequences were used |
| Allocation concealment (selection bias) | Low risk | Central allocation at duty office of NHS blood and transplant |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but there was a randomisation scheme to dictate which kidney would be transplanted first, therefore the MP kidney was not always left to suffer longer CIT as is the case in some other studies |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding, but outcome measurements are unlikely to be affected by the lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Full follow‐up data for all patients, using an intention to treat model |
| Selective reporting (reporting bias) | Low risk | Outcomes suitable, with adequate reporting |
| Other bias | Low risk | All kidneys were transplanted, the indices gained from the MP process were not used to decide whether a kidney was transplanted |
| Methods |
|
|
| Participants |
|
|
| Interventions | Machine perfusion
Static cold storage
Mean CIT
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using a web based program (www.randomization.com) |
| Allocation concealment (selection bias) | Low risk | Once a random sequence was generated using a web based program allocations were placed in opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding but this is unlikely to affect the outcome, especially as there was no difference in CIT between the groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding, but outcome measurements are unlikely to be affected by the lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear reasons given for excluded pairs of kidneys. Primary outcome data reported for all 160 included participants. Only 2/160 were lost to follow up and were therefore not included in the graft/patient survival analysis |
| Selective reporting (reporting bias) | Low risk | Outcomes suitable, with adequate reporting |
| Other bias | Low risk | Kidneys were assessed to ensure that both kidneys were suitable for HMP/SCS before randomisation. This removes the potential bias associated with excluding kidneys only if a kidney with unusual vascular anatomy is randomised to HMP |
| Methods |
|
|
| Participants |
|
|
| Interventions | Machine perfusion
Static cold storage
Mean CIT
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Paired design with one kidney randomly assigned to each group. No information on how or at what stage the randomisation was done |
| Allocation concealment (selection bias) | Unclear risk | Too little information was given to allow assessment of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but the difference in CIT between the arms was not statistically significantly different |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding, but outcome measurements are unlikely to be affected by the lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5/76 lost to follow‐up, with no information on DGF. No explanation given for the patients lost to follow up, but similar numbers lost from each group |
| Selective reporting (reporting bias) | Low risk | Appropriate outcome measures, with each reported in the results section |
| Other bias | Unclear risk | Very concise materials and methods section so difficult to assess level of bias |
| Methods |
|
|
| Participants |
|
|
| Interventions | Machine perfusion
Static cold storage
Mean CIT
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It does not state how or at what stage the decision was made as to which kidney would receive MP and which would receive SCS. Moreover it is unclear whether the study was randomised or quasi‐randomised. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but comparable CIT between the groups, and the study does describe randomisation of kidneys in terms of allocation to recipients |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding, but outcome measurements are unlikely to be affected by the lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data is available for all included participants |
| Selective reporting (reporting bias) | Low risk | Suitable outcome measures with results reported for each |
| Other bias | Unclear risk | Relatively short manuscript lacking details of methods of randomisation |
| Methods |
|
|
| Participants |
|
|
| Interventions | Machine perfusion
Static cold storage
Mean CIT
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | There was no information on how kidneys were randomised. Furthermore, surgeons could swap kidneys from the HMP to the SCS if they felt the aortic patch was too small or if the anatomy of the renal arteries was non‐standard. No intention to treat analysis was applied. No indication was given as to how many kidneys were swapped between groups |
| Allocation concealment (selection bias) | High risk | Allocation concealment was not possible with the study design, kidneys were moved between groups without intention to treat analysis |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding and no reporting of CIT. Therefore one group may have routinely been transplanted first, adding bias to the results |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of DGF data. No information is given as to whether the assessors of "acute rejection" or "time zero biopsies" were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | For the outcome "time‐zero biopsies" no data is given for the groups as a whole. Only H+E stains and electron microscopy from a single pair of kidneys are presented |
| Selective reporting (reporting bias) | High risk | A non‐standard definition for DGF was used as the primary outcome of the study. The number of participants requiring dialysis in the first week post‐transplant (the standard definition for DGF) was not reported. No specific outcome was associated with the "time‐zero biopsies". In the results section some features of H+E stains, and electron microscopy were reported, leaving a high risk of selection bias. Furthermore, only H+E stains and electron microscopy from a single pair of kidneys are presented |
| Other bias | High risk | Although acute rejection is reported as an outcome, it is not stated whether this is biopsy proven rejection or clinical rejection. It is also not stated over what time period acute rejection data was collected over |
| Methods |
|
|
| Participants |
|
|
| Interventions | Machine perfusion
Static cold storage
Mean CIT
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | No specific information on random sequence generation, but all consecutive donors were assessed for inclusion, and valid reasons were given for any excluded donors |
| Allocation concealment (selection bias) | Low risk | No specific information on allocation concealment but all consecutive donors were assessed for inclusion, and valid reasons were given for any excluded donors |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Transplanting teams were blinded to the perfusion parameter readings. Transplant teams were not blinded to the storage method used but this is unlikely to affect the outcome, especially as there was no difference in CIT between the groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding, but outcome measurements are unlikely to be affected by the lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Full follow‐up was reported for all participants |
| Selective reporting (reporting bias) | Low risk | Outcomes suitable, with adequate reporting |
| Other bias | Low risk | No kidneys were swapped between groups. An independent scientific steering committee composed of clinicians and scientists was solely responsible for the design, conduct, data analysis, and manuscript preparation |
ATN ‐ acute tubular necrosis; CCD ‐ cardiocirculatory death; CIT ‐ cold ischaemic time; CrCl ‐ creatinine clearance; DBD ‐ donor after brainstem death; DCD ‐ donor after circulatory death; ECD ‐ extended/expanded criteria donor; ICU ‐ intensive care unit; MP ‐ machine perfusion; PNF ‐ primary non‐function; SCr ‐ serum creatinine; SCS ‐ static cold storage
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alijani 1987 | Wrong intervention: compared 2 cold storage solutions |
| Baatard 1993 | Wrong intervention: compared 2 cold storage solutions |
| Guarrera 2004 | Wrong intervention: compared 2 solutions for MP |
| Guarrera 2004a | Wrong intervention: compared 2 solutions for MP |
| Lodge 1993 | Wrong intervention: compared 2 types of reflush solutions |
| Polyak 1998 | Wrong intervention: compared the type of additive used in MP |
| Polyak 2002 | Wrong intervention: compared the type of additive used in MP |
| Tisone 1999 | Wrong intervention: compared gravity to high pressure perfusion |
| Wamser 1990 | Wrong intervention: compared 2 cold storage solutions |
| Wszola 2013 | Wrong intervention: compared 2 MP techniques |
MP ‐ machine perfusion
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Multicentre RCT; sequential study design ‐ maximum of 270 recipients |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | One kidney will be placed upon the LifePort pulsatile perfusion machine. The other will be placed in standard cold‐storage ice‐box. |
| Outcomes |
|
| Notes | The following is listed as an editorial note in the ISRCTN registry (http://www.isrctn.com/ISRCTN50082383): 18/12/2017: The overall study end date was updated from 01/12/2016 to 31/05/2017. |
| Methods | Multicenter, prospective, open, controlled and randomised trial comparing static incubation and pulsatile machine perfusion in expanded criteria donors |
| Participants | Inclusion criteria for donors (ECD)
Inclusion criteria for recipient
Exclusion criteria for recipient
|
| Interventions | Static incubation
Pulsatile perfusion
|
| Outcomes |
|
| Notes | Study marked as complete (www.clinicaltrials.gov/ct2/show/study/NCT01170910) but no results available despite attempted contact with the authors. |
DGF ‐ delayed graft function; ECD ‐ extended/expanded criteria donors; SCr ‐ serum creatinine
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Improving function of transplanted kidneys |
| Methods | UK‐based phase II multicentre RCT; not strictly paired |
| Participants | 400 patients receiving a kidney from a DCD donor (categories III and IV, controlled) in the UK setting |
| Interventions | On arrival at the transplant centre, kidneys will be randomised to receive either normothermic MP (n = 200) or remain in SCS (n = 200). Kidneys undergoing normothermic MP will be perfused with an oxygenated packed red cell solution at near body temperature for 60 min prior to transplantation |
| Outcomes |
|
| Starting date | 01/03/2015 |
| Contact information | Ms Sarah Hosgood Addenbrooke's Hospital Hills Road Cambridge CB2 0QQ United Kingdom |
| Notes | Estimated completion date 30/01/2021 |
| Trial name or title | A multi‐centre, randomised, controlled study of pre‐transplant machine perfusion of heart‐beating donor kidneys prior to renal transplantation |
| Methods | Multicentre RCT |
| Participants | 200 patients (aged 18 years and over, either gender) undergoing transplantation of a kidney from a heart‐beating cadaver donor |
| Interventions | MP of the kidney before transplantation versus SCS |
| Outcomes | To determine whether a brief period of MP reduces the incidence of DGF following kidney transplantation
|
| Starting date | Overall study start date: 28/06/2006 |
| Contact information | Mr Christopher Watson Department of Surgery Box 202 Addenbrooke's Hospital Cambridge CB2 2QQ United Kingdom |
| Notes | The following is listed as an editorial note in the ISRCTN registry (http://www.isrctn.com/ISRCTN35082773): 10/08/2017: No publications found in PubMed, verifying study status with principal investigator |
| Trial name or title | COPE‐POMP: ‘in house’ pre‐implantation oxygenated hypothermic machine perfusion reconditioning after cold storage versus cold storage alone in expanded criteria donor (ECD) kidneys from brain dead donors |
| Methods | Prospective parallel group RCT patient‐blinded controlled multicentre non‐paired superiority study |
| Participants | Kidneys donated after brain death from donors fulfilling the United Network for Organ Sharing (UNOS) ECD criteria. Participants are expected from Germany, Belgium, the Netherlands and UK |
| Interventions | ECD kidneys will be randomised to be preserved using either SCS alone or SCS followed by hypothermic oxygenated MP Group 1: the kidney will be retrieved and stored in cold storage solution until back‐table preparation and kidney transplantation are performed Group 2: the kidney will be placed in cold storage solution until arrival at the recipient's transplant centre. Following back‐table preparation the kidney will be placed on the Kidney Assist device to be perfused with cold oxygenated Belzer's Machine preservation solution until immediately before implantation |
| Outcomes |
|
| Starting date | 01/12/2013 |
| Contact information | Prof Andreas Paul Department of General Visceral and Transplant Surgery University Hospital Essen Hufelandstr. 55 Essen 45147 Germany |
| Notes | Study ongoing as of 15/12/2016, expected to finish 31/12/2018 |
| Trial name or title | A randomized trial of mild hypothermia and machine perfusion in deceased organ donors for protection against delayed graft function in kidney transplant recipients |
| Methods | Parallel assignment RCT to assess the effect of mild hypothermia in the deceased organ donor before organs are recovered, with or without subsequent hypothermic MP of the kidney after recovery and prior to transplantation |
| Participants | DBD donors > 18 years in the USA |
| Interventions | Enrolled donors will be divided into two populations based on local organ procurement organization criteria: pump eligible and not pump eligible. These categories will then be split to result in five arms:
|
| Outcomes |
|
| Starting date | 26/07/2017 |
| Contact information | Claus Niemann, MD; Claus.Niemann@ucsf.edu Darren Malinoski, MD; malinosk@ohsu.edu |
| Notes | Estimated completion date 26/07/2021 |
| Trial name or title | Clinical impact of hypothermic machine perfusion in renal transplant recipients (CIHMP) |
| Methods | Multicentre prospective, paired, RCT to compare HMP with SCS. Factors during the MP, such as the pressure, flow rate, and resistance index will also be investigated. This study aims to recruit 200 donors |
| Participants | DCD donors > 16 years in China |
| Interventions | SCS versus HMP with a kidney transporter machine |
| Outcomes |
|
| Starting date | December 2015 |
| Contact information | Chenguang Ding, PhD; doctor_ding@126.com Wujun Xue, PhD; xwujun@126.com |
| Notes | Estimated completion date January 2019 |
CRR ‐ creatinine reduction ratio; DCD ‐ donor after circulatory death; DGF ‐ delayed graft function; ECD ‐ extended/expanded criteria donors; eGFR ‐ estimated glomerular filtration rate; (H)MP ‐ (hypothermic) machine perfusion; PNF ‐ primary non‐function; RCT ‐ randomised controlled trial; SCS ‐ static cold storage
Contributions of authors
Draft the protocol: RF, JM, DT, CW
Study selection: ST, RF
Extract data from studies: ST, RF
Enter data into RevMan: ST, RF
Carry out the analysis: ST, RF
Interpret the analysis: ST, RF, MG
Draft the final review: ST, RF, JM, MG, DT, CW
Disagreement resolution: CW
Specialist input: DT
Update the review: ST, RF
Sources of support
Internal sources
No sources of support supplied
External sources
-
NIHR Blood and Transplant Research Unit, UK.
This study was supported by the National Institute for Health Research NIHR Blood and Transplant Research Unit in Organ Donation and Transplantation at the University of Cambridge, in collaboration with Newcastle University and in partnership with National Health Service Blood and Transplant (NHSBT). The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR, the Department of Health or NHSBT.
Declarations of interest
Samuel J Tingle: none known
Rodrigo S Figueiredo: none known
John AG Moir: none known
Michael Goodfellow: none known
David Talbot: essentially none, but I have received help in attending transplant meetings in the past. This has usually come from one of four companies (Astellas, Wyeth, Novartis and Roche)
Colin H Wilson: none known
New
References
References to studies included in this review
- Alijani MR, Cutler JA, DelValle CJ, Morres DN, Fawzy A, Pechan BW, et al. Single‐donor cold storage versus machine perfusion in cadaver kidney preservation. Transplantation 1985;40(6):659‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Amaduzzi A, Catena F, Montori G, Ravaioli M, Pinna A. Hypotermic machine perfusion (HMP) versus static cold storage (CS) in kidney allograft preservation. Prospective case‐control trial [abstract no: RO‐077]. Transplant International 2011;24(Suppl 2):151. [EMBASE: 70527605] [Google Scholar]
- Chen G, Ko D, Wang C, Yuan X, Qiu J, Han M, et al. Impact of machine perfusion on outcomes of kidney transplantation from donation after cardiac death: a prospective randomized controlled trial [abstract no: D2675]. Transplantation 2014;98(Suppl 1):267. [EMBASE: 71544357]24992357 [Google Scholar]
- Halloran P, Aprile M. A randomized prospective trial of cold storage versus pulsatile perfusion for cadaver kidney preservation. Transplantation 1987;43(6):827‐32. [MEDLINE: ] [PubMed] [Google Scholar]; Halloran P, Aprile M, Robinette M. A randomized prospective trial of cold storage versus pulsatile perfusion for cadaver kidney preservation. Transplantation Proceedings 1985;17(1 Part 2):1471‐3. [EMBASE: 1985080743] [PubMed] [Google Scholar]
- Heil JE, Canafax DM, Sutherland DE, Simmons RL, Dunning M, Najarian JS. A controlled comparison of kidney preservation by two methods: machine perfusion and cold storage. Transplantation Proceedings 1987;19(1 Pt 3):2046. [MEDLINE: ] [PubMed] [Google Scholar]
- Danielewicz R, Kwiatkowski A, Polak W, Kosieradzki M, Michalak G, Wegrowicz I, et al. An assessment of ischemic injury of the kidney for transplantation during machine pulsatile preservation. Transplantation Proceedings 1997;29(8):3580‐1. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Kosieradzki M, Danielewicz R, Kwiatkowski A, Polak W, Wegrowicz‐Rebandel I, Walaszewski J, et al. Rejection rate and incidence of acute tubular necrosis after pulsatile perfusion preservation. Transplantation Proceedings 1999;31(1‐2):278‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Kwiatkowski A, Danielewicz R, Polak W, Michalak G, Paczek L, Walaszewski J, et al. Storage by continuous hypothermic perfusion for kidney harvested from hemodynamically unstable donors. Transplantation Proceedings 1996;28(1):306‐7. [MEDLINE: ] [PubMed] [Google Scholar]; Kwiatkowski A, Wszola M, Kosieradzki M, Danielewicz R, Ostrowski K, Domagala P, et al. The early and long term function and survival of kidney allografts stored before transplantation by hypothermic pulsatile perfusion. A prospective randomized study. Annals of Transplantation 2009;14(1):14‐7. [MEDLINE: ] [PubMed] [Google Scholar]; Wszola M, Kwiatkowski A, Kosieradzki M, Danielewicz R, Ostrowski K, Domagala P, et al. Machine perfusion (MP) and cold storage (CS) of kidneys allografts ‐ prospective study of long‐term function [abstract no: P941]. Transplant International 2007;20(Suppl 2):322. [Google Scholar]
- Matsuno N, Sakurai E, Tamaki I, Uchiyama M, Kozaki K, Kozaki M. The effect of machine perfusion preservation versus cold storage on the function of kidneys from non‐heart‐beating donors. Transplantation 1994;57(2):293‐4. [MEDLINE: ] [PubMed] [Google Scholar]
- Merion RM, Oh HK, Port FK, Toledo‐Pereyra LH, Turcotte JG. A prospective controlled trial of cold‐storage versus machine‐perfusion preservation in cadaveric renal transplantation. Transplantation 1990;50(2):230‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gallinat A, Moers C, Treckmann J, Smits JM, Leuvenink HG, Lefering R, et al. Machine perfusion versus cold storage for the preservation of kidneys from donors >= 65 years allocated in the Eurotransplant Senior Programme. Nephrology Dialysis Transplantation 2012;27(12):4458‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Garfield SS, Poret AW, Evans RW. The cost‐effectiveness of organ preservation methods in renal transplantation: US projections based on the machine preservation trial. Transplantation Proceedings 2009;41(9):3531‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Groen H, Moers C, Smits JM, Treckmann J, Monbaliou D, Rahmel A, et al. Machine perfusion versus static cold storage in kidney transplant [abstract no: O10.4]. Annals of the Academy of Medicine of Singapore 2009;38(Suppl 6):S44. [CENTRAL: CN‐00747267] [Google Scholar]; Groen H, Moers C, Smits JM, Treckmann J, Monbaliu D, Rahmel A, et al. Cost‐effectiveness of hypothermic machine preservation versus static cold storage in renal transplantation. American Journal of Transplantation 2012;12(7):1824‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Groen H, Moers C, Smits JM, Treckmann J, Gelder F, Rahmel A, et al. Cost‐effectiveness of hypothermic machine perfusion versus static cold storage in kidney transplantation: first results of the prospective European RCT [abstract no: 265]. Transplantation 2008;86(2 Suppl):93. [CENTRAL: CN‐00747268] [Google Scholar]; Jochmans I, Moers C, Smits JM, Leuvenink HG, Treckmann J, Paul A, et al. Machine perfusion versus cold storage for the preservation of kidneys donated after cardiac death: a multicenter, randomized, controlled trial. Annals of Surgery 2010;252(5):756‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Kox J, Moers C, Monbaliu D, Strelniece A, Treckmann J, Jochmans I, et al. The benefits of hypothermic machine preservation and short cold ischemia times in deceased donor kidneys. Transplantation 2018;102(8):1344‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Moers C, Jochmans I, Treckmann J, Smits J, Homan van der Heide J, Squifflet JP, et al. Better graft survival with machine perfusion than cold storage after three years: follow‐up analysis of the European multicentre RCT in deceased‐donor kidney transplantation [abstract no: LB‐O‐003]. Transplant International 2011;24(Suppl 2):93‐4. [EMBASE: 70527404] [Google Scholar]; Moers C, Pirenne J, Paul A, Ploeg RJ, Machine Preservation Trial Study Group. Machine perfusion or cold storage in deceased‐donor kidney transplantation. New England Journal of Medicine 2012;366(8):770‐1. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Moers C, Smits JM, Maathuis MH, Treckmann J, Gelder F, Napieralski BP, et al. Machine perfusion or cold storage in deceased‐donor kidney transplantation. New England Journal of Medicine 2009;360(1):7‐19. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Moers C, Smits JM, Maathuis MJ, Treckmann J, Gelder F, Napieralski BP, et al. Transplantation after hypothermic machine perfusion versus static cold storage of deceased donor kidneys: a prospective randomized controlled trial [abstract no: 175]. American Journal of Transplantation 2008;8(Suppl 2):225. [CENTRAL: CN‐00671830] [Google Scholar]; Moers C, Varnav OC, Treckmann J, Monbaliu D, Rakhorst G, Paul A, et al. GST and HFABP values during machine perfusion of deceased donor kidneys are independent predictors of delayed graft function, but not of primary non‐function and graft survival [abstract no: O‐159]. Transplant International 2009;22(Suppl 2):42. [Google Scholar]; Moers C, Varnav OC, Heurn E, Jochmans I, Kirste GR, Rahmel A, et al. The value of machine perfusion perfusate biomarkers for predicting kidney transplant outcome. Transplantation 2010;90(9):966‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Nagelschmidt M, Minor T, Gallinat A, Moers C, Jochmans I, Pirenne J, et al. Lipid peroxidation products in machine perfusion of older donor kidneys. Journal of Surgical Research 2013;180(2):337‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Paul A, Moers C, Smits J, Maathuis H, Homan van der Heide JH, Heurn E, et al. Machine perfusion versus cold storage in transplantation of kidneys from older deceased donors: results of a prospective randomized multicenter trial [abstract no: 236]. Transplantation 2008;86(2 Suppl):83. [CENTRAL: CN‐00765605] [Google Scholar]; Paul A, Moers C, Smits JM, Maathuis MH, Gallinat A, Napieralski BP, et al. Machine perfusion vs cold storage in transplantation of kidneys from donors older than 65 years: results of a randomized multicenter trial [abstract no: 152]. American Journal of Transplantation 2009;9(Suppl 2):235. [CENTRAL: CN‐00765608] [Google Scholar]; Pirenne J, Smits J, Moers C, Maathuis M, Treckmann J, Napieralski B, et al. Machine perfusion versus cold storage preservation in non‐heart‐beating kidney donation and transplantation: results of a multicentre trial in Eurotransplant [abstract no: 146]. American Journal of Transplantation 2009;9(Suppl 2):233. [CENTRAL: CN‐00765607] [Google Scholar]; Pirenne J, Smits JM, Moers C, Maathuis MJ, Treckmann J, Napieralski BP, et al. Machine perfusion versus cold storage preservation in non‐heart‐beating kidney donation and transplantation: first results of a multicentre trial in Eurotransplant [abstract no: O‐158]. Transplant International 2009;22(Suppl 2):41. [Google Scholar]; Treckmann J, Minor T, Gallinat A, Moers C, Jochmanns I, Pirenne J, et al. Lipid peroxidation products and alpha glutathione‐S‐transferase in machine perfusate of older donor kidneys are associated with post‐transplant outcome [abstract no: 1349]. American Journal of Transplantation 2012;12(Suppl S3):424. [Google Scholar]; Treckmann J, Moers C, Smits JM, Gallinat A, Jochmans I, Squifflet JP, et al. Machine perfusion in clinical trials: "machine vs. solution effects". Transplant International 2012;25(5):e69‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Treckmann J, Moers C, Smits JM, Gallinat A, Maathuis MH, Kasterop‐Kutz M, et al. Machine perfusion versus cold storage for preservation of kidneys from expanded criteria donors after brain death. Transplant International 2011;24(6):548‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Gelder F, Moers C, Smits JM, Maathuis MHJ, Treckmann J, Napieralski BP, et al. Machine perfusion versus cold storage preservation in non‐heart‐beating kidney donation and transplantation: first results of multicentre trial in Eurotransplant [abstract no: 234]. Transplantation 2008;86(2 Suppl):82. [CENTRAL: CN‐00765606]18622282 [Google Scholar]
- Mozes MF, Finch WT, Reckard CR. Comparison of cold storage and machine perfusion in the preservation of cadaver kidneys: a prospective, randomized study. Transplantation Proceedings 1985;17(1 II):1474‐7. [EMBASE: 1985080744] [Google Scholar]
- Goldfarb DA. Re: Cold machine perfusion versus static cold storage of kidneys donated after cardiac death: A UK multicenter randomized controlled trial. Journal of Urology 2011;186(4):1392‐3. [EMBASE: 2011511848] [DOI] [PubMed] [Google Scholar]; Watson C, Wells A, Roberts R, Akoh J, Friend P, Akyol M. Machine preservation of kidneys donated after cardiac death does not reduce delayed graft function: the PPart Study [abstract no: 232]. Transplantation 2008;86(2 Suppl):81. [Google Scholar]; Watson C, Wells A, Roberts R, Blackwell J, Akoh J, Friend P, et al. Machine preservation of kidneys donated after cardiac death does not reduce delayed graft function [abstract no: 246]. American Journal of Transplantation 2008;8(Suppl 2):244. [Google Scholar]; Watson CJ, Wells AC, Roberts RJ, Akoh JA, Friend PJ, Akyol M, et al. Cold machine perfusion versus static cold storage of kidneys donated after cardiac death: a UK multicenter randomized controlled trial. American Journal of Transplantation 2010;10(9):1991‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Wells A, Roberts R, Blackwell J, Akoh J, Friend P, Akyol M, et al. Machine preservation of kidneys donated after cardiac death does not reduce delayed graft function: the PPART study [abstract no: O19]. British Transplantation Society (BTS).11th Annual Congress; 2008 Apr 16‐18; Glasgow, UK. 2008. [Google Scholar]
- Marinho Neto H, Aires V, Offerni J, Luconi W, Luconi P, Braga S, et al. The influence of static versus machine dynamic perfusion kidney graft preservation on delayed graft function incidence: a prospective, randomized study [abstract no: C179]. American Journal of Transplantation 2016;16(Suppl 3):658‐9. [EMBASE: 611699172] [Google Scholar]; Tedesco‐Silva H Jr, Mello Offerni JC, Ayres Carneiro V, Ivani de Paula M, Neto ED, Brambate Carvalhinho Lemos F, et al. Randomized trial of machine perfusion versus cold storage in recipients of deceased donor kidney transplants with high incidence of delayed graft function. Transplantation Direct 2017;3(5):e155. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vliet JA, Kievit JK, Hene RJ, Hilbrands LB, Kootstra G. Preservation of non‐heart‐beating donor kidneys: a clinical prospective randomised case‐control study of machine perfusion versus cold storage. Transplantation Proceedings 2001;33(1‐2):847. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Veller MG, Botha JR, Britz RS, Gecelter GR, Beale PG, Margolius LP, et al. Renal allograft preservation: a comparison of University of Wisconsin solution and of hypothermic continuous pulsatile perfusion. Clinical Transplantation 1994;8(2 (Pt 1)):97‐100. [MEDLINE: ] [PubMed] [Google Scholar]
- Wang W, Xie D, Hu X, Yin H, Liu H, Zhang X. Effect of hypothermic machine perfusion on the preservation of kidneys donated after cardiac death: a single‐center, randomized, controlled trial. Artificial Organs 2017;41(8):753‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Zhong Z, Lan J, Ye S, Liu Z, Fan L, Zhang Y, et al. Outcome improvement for hypothermic machine perfusion versus cold storage for kidneys from cardiac death donors. Artificial Organs 2017;41(7):647‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Zhong Z, Liu Z, Fu Z, Zhang Y, Ko D, Wang Y, et al. Hypothermic machine perfusion improves kidney viability through amelioration of vasospasm and edema of podocytes and renal tubular epithelial cells [abstract]. American Journal of Transplantation 2015;15(Suppl 3). [EMBASE: 71953566] [Google Scholar]
References to studies excluded from this review
- Alijani MR, Helfrich GB, Fawzy A, Cutler JA, Bates SB, Andrews PM. Clinical evaluation of a new kidney cold storage solution [abstract]. Kidney International 1987;31(1):453. [CENTRAL: CN‐00550449] [Google Scholar]
- Baatard R, Pradier F, Dantal J, Karam G, Cantarovich D, Hourmant M, et al. Prospective randomized comparison of University of Wisconsin and UW‐modified, lacking hydroxyethyl‐starch, cold‐storage solutions in kidney transplantation. Transplantation 1993;55(1):31‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Guarrera JV, Polyak M, O'Mar AB, Kapur S, Stubenbord WT, Kinkhabwala M. Pulsatile machine perfusion with Vasosol solution improves early graft function after cadaveric renal transplantation.[Retraction in Stubenbord WT, Kinkhabwala M, Kapur S. Transplantation. 2005 Jun 27;79(12):1774; PMID: 15973193]. Transplantation 2004;77(8):1264‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Guarrera JV, Polyak MM, Arrington B, Boykin J, Brown T, Jean‐Jacques MA, et al. Pushing the envelope in renal preservation; improved results with novel perfusate modifications for pulsatile machine perfusion of cadaver kidneys.[Erratum appears in Transplant Proc. 2004 Jul‐Aug;36(6):1853 Note: O'Mar Arrington, B [corrected to Arrington, B]]. Transplantation Proceedings 2004;36(5):1257‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lodge JP, Kashi SH, Lam FT, Lord A, Giles GR. The reflush effect‐‐a prospective analysis of late perfusion. Transplantation 1993;55(3):567‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Polyak MM, Arrington BO, Stubenbord WT, Kapur S, Kinkhabwala M. Prostaglandin E1 influences pulsatile preservation characteristics and early graft function in expanded criteria donor kidneys. Journal of Surgical Research 1999;85(1):17‐25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Polyak MM, Arrington BO, Stubenbord WT, Kinkhabwala M. Prostaglandin E1 improves pulsatile preservation characteristics and early graft function in expanded criteria donor kidneys. ASAIO Journal 1998;44(5):M610‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Polyak MM, Guarerra JV, Arrington BO, Kapur S, Stubenbord WT, Kinkhabwala M. Comparison of vasosol versus viaspan in the cold stored and machine preserved kidney [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00416475] [Google Scholar]
- Tisone G, Orlando G, Pisani F, Iaria G, Negrini S, Pollicita S, et al. Gravity perfusion versus high‐pressure perfusion in kidney transplantation: results from a prospective randomized study. Transplantation Proceedings 1999;31(8):3386‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wamser P. A new cold storage solution for kidney preservation. Comparing UW and Eurocollins solution. Wiener Klinische Wochenschrift 1990;102(6):177‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Wszola M, Domagala P, Diuwe P, Kwiatkowski A, Gorski L, Kieszek R, et al. One‐year transplantation results of kidneys preserved by machine perfusion ‐ Lifeport kidney transporter versus waters RM3 ‐ Prospective study [abstract no: O19‐0041]. Transplant International 2012;25(Suppl 1):15. [EMBASE: 70759806] [Google Scholar]; Wszola M, Domagala P, Diuwe P, Kwiatkowski A, Gorski L, Kieszek R, et al. One‐year transplantation results of kidneys preserved by machine perfusion. LifePort kidney transporter versus Waters RM3‐prospective study [abstract no: 648]. American Journal of Transplantation 2012;12(Suppl S3):221. [CENTRAL: CN‐01658615] [Google Scholar]; Wszola M, Kwiatkowski A, Diuwe P, Domagala P, Gorski L, Kieszek R, et al. One‐year results of a prospective, randomized trial comparing two machine perfusion devices used for kidney preservation. Transplant International 2013;26(11):1088‐96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Watson C. CArdiac Death kidney Machine Perfusion trial. www.isrctn.com/ISRCTN50082383 (first received 6 September 2011).
- NCT01170910. Pulsatile perfusion preservation in kidney transplantation from expanded criteria donors (IMPULSION). www.clinicaltrials.gov/ct2/show/NCT01170910 (first received 27 July 2010).
References to ongoing studies
- Hosgood SA, Saeb‐Parsy K, Wilson C, Callaghan C, Collett D, Nicholson ML. Protocol of a randomised controlled, open‐label trial of ex vivo normothermic perfusion versus static cold storage in donation after circulatory death renal transplantation. BMJ Open 2017;7(1):e012237. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson C. A multi‐centre, randomised, controlled study of pre‐transplant machine perfusion of heart‐beating donor kidneys prior to renal transplantation (HBDPump 2005). www.isrctn.com/ISRCTN35082773 (first received 10 May 2005).
- Paul A. COPE‐POMP: ‘in house’ pre‐implantation oxygenated hypothermic machine perfusion reconditioning after cold storage versus cold storage alone in expanded criteria donor (ECD) kidneys from brain dead donors. www.isrctn.com/ISRCTN63852508 (first received 28 February 2014).
- Malinoski D. Deceased organ donor interventions to protect kidney graft function. www.clinicaltrials.gov/ct2/show/NCT02525510 (first received 17 August 2015).
- Ding C. Clinical impact of hypothermic machine perfusion in renal transplant recipients (CIHMP). www.clinicaltrials.gov/ct2/show/NCT02621281 (first received 3 December 2015).
Additional references
- Bagul A, Hosgood SA, Kaushik M, Kay MD, Waller HL, Nicholson ML. Experimental renal preservation by normothermic resuscitation perfusion with autologous blood. British Journal of Surgery 2008;95(1):111‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Belzer FO, Ashby BS, Gulyassy PF, Powell M. Successful seventeen‐hour preservation and transplantation of human‐cadaver kidney. New England Journal of Medicine 1968;278(11):608‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Collins GM, Bravo‐Shugarman M, Terasaki PI. Kidney preservation for transportation. Initial perfusion and 30 hours' ice storage. Lancet 1969;2(7632):1219‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Fresenius Medical Care. ESRD patients in 2013: a global perspective. Fresenius, Bad Homburg, Germany. www.vision‐fmc.com/files/ESRD_Patients_in_2013.pdf (last accessed 20 April 2015).
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gallinat A, Fox M, Lüer B, Efferz P, Paul A, Minor T. Role of pulsatility in hypothermic reconditioning of porcine kidney grafts by machine perfusion after cold storage. Transplantation 2013;96(6):538‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Garfield SS, Poret AW, Evans RW. The cost‐effectiveness of organ preservation methods in renal transplantation: US projections based on the machine preservation trial. Transplantation Proceedings 2009;41(9):3531‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Glyda M, Wlodarczyk Z, Czaoiewski W. Results of renal transplantation from expanded criteria deceased donors ‐ a single‐center experience. Annals of Transplantation 2012;17(1):35‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Global Observatory on Donation, Transplantation (GODT). 2012 estimates. www.transplant‐observatory.org/Pages/home.aspx (accessed 14 April 2015).
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt G, Oxman A D, Akl E A, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64:383‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Groen H, Moers C, Smits JM, Treckmann J, Monbaliu D, Rahmel A, et al. Cost‐effectiveness of hypothermic machine preservation versus static cold storage in renal transplantation. American Journal of Transplantation 2012;12(7):1824‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Hwang JK, Park SC, Kwon KH, Choi BS, Kim JI, Yang CW, et al. Long‐term outcomes of kidney transplantation from expanded criteria deceased donors at a single center: comparison with standard criteria deceased donors. Transplantation Proceedings 2014;46(2):431‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kosmoliaptsis V, Salji M, Bardsley V, Chen Y, Thiru S, Griffiths MH, et al. Baseline donor chronic renal injury confers the same transplant survival disadvantage for DCD and DBD kidneys. American Journal of Transplantation 2015;15(3):754‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mallon DH, Summers DM, Bradley JA, Pettigrew GJ. Defining delayed graft function after renal transplantation: simplest is best. Transplantation 2013;96(10):885‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Metzger RA, Delmonico FL, Feng S, Port FK, Wynn JJ, Merion RM. Expanded criteria donors for kidney transplantation. American Journal of Transplantation 2003;3 Suppl 4:114‐25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- NHS Blood and Transplant. Cost effectiveness of transplantation. www.nhsbtmediaservices.blob.core.windows.net/organ‐donation‐assets/pdfs/Organ_Donation_Registry_Fact_Sheet_7_21337.pdf (accessed 7 January 2019).
- Nicholson ML, Hosgood SA. Renal transplantation after ex vivo normothermic perfusion: the first clinical study. American Journal of Transplantation 2013;13(5):1246‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- O’Callaghan JM, Knight SR, Morgan RD, Morris PJ. Preservation solutions for static cold storage of kidney allografts: a systematic review and meta‐analysis. American Journal of Transplantation 2012;12(4):896‐906. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- O'Callaghan JM, Morgan RD, Knight SR, Morris PJ. Systematic review and meta‐analysis of hypothermic machine perfusion versus static cold storage of kidney allografts on transplant outcomes. British Journal of Surgery 2013;100(8):991‐1001. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pascual J, Zamora J, Pirsch JD. A systematic review of kidney transplantation from expanded criteria donors. American Journal of Kidney Diseases 2008;52(3):553‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Port FK, Bragg‐Gresham JL, Metzger RA, Dykstra DM, Gillespie BW, Young EW, et al. Donor characteristics associated with reduced graft survival: an approach to expanding the pool of kidney donors. Transplantation 2002;74(9):1281‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Wilson CH, Brook NR, Talbot D. Preservation solutions for solid organ transplantation. Mini‐Reviews in Medicinal Chemistry 2006;6(10):1081‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wilson CH, Stamp S, Wyrley‐Birch H, Dosani T, Rix D, French J, et al. The early economic costs of delayed graft function in DCD kidney transplantation. 17th Annual Congress of the British Transplantation Society, Glasgow. 2014. [Google Scholar]
- Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. New England Journal of Medicine 1999;341(23):1725‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Yarlagadda SG, Coca SG, Formica RN Jr, Poggio ED, Parikh CR. Association between delayed graft function and allograft and patient survival: a systematic review and meta‐analysis. Nephrology Dialysis Transplantation 2008;24(3):1039‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Figueiredo RS, Moir JA, Talbot D, Wilson CH. Normothermic and hypothermic machine perfusion preservation versus static cold storage for deceased donor kidney transplantation. Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI: 10.1002/14651858.CD011671] [DOI] [PMC free article] [PubMed] [Google Scholar]


