Abstract
Intervertebral disc degeneration is strongly associated with chronic low back pain, a leading cause of disability worldwide. Current back pain treatment approaches (both surgical and conservative) are limited to addressing symptoms, not necessarily the root cause. Not surprisingly therefore, long‐term efficacy of most approaches is poor. Cell‐based disc regeneration strategies have shown promise in preclinical studies, and represent a relatively low‐risk, low‐cost, and durable therapeutic approach suitable for a potentially large patient population, thus making them attractive from both clinical and commercial standpoints. Despite such promise, no such therapies have been broadly adopted clinically. In this perspective we highlight primary obstacles and provide recommendations to help accelerate successful clinical translation of cell‐based disc regeneration therapies. The key areas addressed include: (a) Optimizing cell sources and delivery techniques; (b) Minimizing potential risks to patients; (c) Selecting physiologically and clinically relevant efficacy metrics; (d) Maximizing commercial potential; and (e) Recognizing the importance of multidisciplinary collaborations and engaging with clinicians from inception through to clinical trials.
Keywords: biological therapies, biomaterials, preclinical models, stem cells
1. INTRODUCTION
Lower back pain is a leading cause of disability worldwide and the third most expensive health condition in the United States, with estimated health care spending in excess of $80 billion annually.1, 2 It is a significant epidemiological and socioeconomic problem affecting quality of life, and is the most common, non‐cancer reason for opioid prescription in the United States.3 Intervertebral disc degeneration is a progressive, cell‐mediated cascade of molecular, structural and biomechanical changes, which is strongly implicated as a cause of “discogenic” back pain.4 The incidence of disc degeneration is linked to aging, trauma, genetics, lifestyle and the presence of co‐morbidities.5, 6, 7, 8, 9 The intervertebral discs are the pliant, fibrocartilaginous joints required for transferring compressive loads and supporting complex mobility of the spine.10 Each disc is comprised of a central, proteoglycan‐rich, gelatinous nucleus pulposus (NP), a peripheral, fibrocartilaginous annulus fibrosus (AF), and superiorly and inferior, hyaline cartilage end plates that interface with the vertebral bodies.10 The earliest degenerative changes typically manifest in the NP, where reductions in proteoglycan content and hydration compromise biomechanical function, leading to progressive degeneration of the entire intervertebral joint.11, 12 Self‐repair and regeneration of the NP is limited by a low cell density and a limited nutrient supply.13, 14 Conventional treatments for disc degeneration are focused solely on alleviation of symptoms and often have limited long‐term efficacy.15, 16, 17 This is exacerbated by the lack of an accepted clinical standard for discogenic pain, where clinicians are often unable to identify a specific nociceptive cause.18, 19 Because of this, the rationale for choosing surgery for these patients is controversial. Spinal fusion does not restore natural biomechanical function and has been shown to induce adjacent segment pathology (ASP) caused by increased mechanical stress in adjacent segments.20 More specifically, in a study comparing 725 lumbar fusion cases to 725 randomly selected controls with chronic low back pain diagnoses, focusing on the outcome return to work (RTW) revealed only 26% of patients had RTW 2 years after fusion surgery, while 67% of nonsurgical controls had RTW within 2 years from the date of injury.21 In addition, of the patients receiving spinal fusion, 36% had complications and the reoperation rate was 27%, with 66% of the reoperations occurring within 2 years of the index surgery. Of the 36% of complications, failed and/or implant malposition represented 4.7% of cases, while late spinal complications such as disc space infection, pseudarthrosis, postlaminectomy syndrome, adjacent disc degeneration, stenosis, spondylolisthesis, and adjacent vertebral fracture represented 25.2% of cases, with the balance relating to early major systemic, neurologic and wound complications.
There is therefore a tremendous unmet need for new treatment options for patients with disc degeneration and associated lower back pain. To address this need, there has been significant recent interest in developing injectable cell‐based therapies for the treatment of disc degeneration with the specific aim to stimulate tissue repair.22 Such therapies represent a potentially low risk and low cost long term solution for a very large patient population, making them attractive from both clinical and commercial standpoints.
The pathway to bringing new cell therapies to patients involves extensive in vitro testing, pre‐clinical validation in small and large animal models, and human clinical trials to demonstrate safety, efficacy, and commercial viability. Successful translation involves multidisciplinary engagement and interactions between clinicians, scientists, engineers and industry. Clinicians are particularly important as they can provide access to primary human cells, advise on patient selection criteria and outcome metrics, assist with the design and conduct of clinical trials, support risk/value discussions with regulatory and reimbursement agencies, and advocate the treatment to patients and health systems.
Even after demonstrating success in animal studies, cell‐based therapies are at risk of stalling in the “valley of death”, which comprises multiple significant barriers that include scale‐up, manufacturing, regulatory, business and market obstacles. To effectively move cell therapies out of the lab and into the clinic, the following must be considered and addressed: (a) Optimizing cell sources and delivery techniques; (b) Minimizing potential risks to patients; (c) Selecting physiologically and clinically relevant efficacy metrics; (d) Working with industry and maximizing commercial potential; and (e) Recognizing the importance of forming multidisciplinary collaborations and engaging with clinicians from inception through to clinical trials. The focus of this article is to highlight and comprehensively review each of these critical areas, and in doing so provide a set of recommendations for successfully advancing cell‐based disc therapies from the lab to the clinic for first‐in‐human studies.
2. CURRENT COMMERCIAL APPROACHES FOR CELL‐BASED DISC REPAIR AND REGENERATION
A small number of companies have successfully entered the clinic to evaluate the efficacy of cell‐based disc regeneration treatments in humans. These treatments have employed a variety of cell types including those obtained from both homologous (from the disc) and non‐homologous (eg, from knee cartilage, adipose tissue) sources, and have utilized both autologous and allogeneic approaches. Autologous Disc Cell Transplantation (ADCT) by Co.Don AG (Berlin, Germany) has been in clinical use for the treatment of degenerate discs for a number of years,23 with recent investigations demonstrating that re‐implantation of culture‐expanded NP cells can retard degenerative changes in patients with herniated discs24; however, there are limitations associated with ADCT delivery and efficacy, including cell leakage following injection into the disc,25 diminished tissue forming capacity of culture expanded NP cells derived from degenerated tissue26 and the paucity of NP cells that can be isolated. The challenge of cell leakage is not unique to ADCT per se, and is a function of the delivery and surgical techniques employed. Any cell therapy relying on intradiscal needle delivery in a saline solution will likely suffer similar failings unless it is combined with some form of biomaterial (eg, hydrogel, microcarriers, microcapsules), which can enhance cellular retention in the disc space.
Two companies have disclosed results from initial clinical trials and have subsequently progressed to performing additional clinical trials. ISTO Biologics (St. Louis, Missouri) reported promising results from a Phase I study evaluating allogeneic juvenile chondrocytes mixed with a fibrin carrier,27 known as NuQu; however the Phase II study was recently terminated for undisclosed reasons (clinicaltrials.gov study identifier NCT01771471). Mesoblast Ltd. (Melbourne, Australia) also showed promising results using immunoselected adipose‐derived mesenchymal stem cells (MSCs) injected into the discs of patients, including finding that the treatment resulted in pain relief and improved water content in treated discs compared to controls.28, 29 When injected into degenerate ovine discs, these cells were shown to elicit regenerative effects.30 A larger, multi‐national Phase III study is ongoing to better assess the potential efficacy and safety of this treatment (clinicaltrials.gov study identifier NCT02412735). Biorestorative (Melville, New York) has evaluated BRTX‐100, an autologous stem cell therapy, in a preliminary human clinical study and has investigational new drug (IND) approval to start a trial in the United States (no clinicaltrials.gov information available).
A number of other companies are currently executing first‐in‐human clinical trials. Utilizing cells modified from adult disc tissue, DiscGenics Inc. (Salt Lake City, Utah) is conducting a clinical trial to evaluate safety and efficacy of the product candidate IDCT (injectable discogenic cell therapy) compared to controls over 2 years (clinicaltrials.gov study identifier NCT03347708). IDCT contains a mixture of specialized therapeutic progenitor cells engineered from donated adult disc tissue, or “discogenic” cells and a viscous carrier material. Promising animal data suggests a potential therapeutic benefit of IDCT, including disc height improvement and normalization of disc architecture, and a safe profile.31 Aesculap Biologics (Breinigsville, Pennsylvania) is currently testing NOVOCART DISC in a Phase II trial in the European Union. This treatment is a combination of autologous disc chondrocytes and a self‐polymerizing hydrogel (clinicaltrials.gov study identifier NCT01640457) that is already being used clinically for articular cartilage repair in the knee. Preliminary results from the Phase I trial of this therapy indicate the risks of adverse events occurring are comparable to elective disc surgery.32
In summary, commercial efforts to date have applied an extensive variety of cell‐based approaches for treating disc degeneration. While some of these have shown encouraging preclinical and clinical findings with respect to both safety and efficacy, none have achieved widespread clinical adoption.
3. PATIENT SELECTION: EVEN THE BEST THERAPY WON'T SUCCEED IF APPLIED TO THE WRONG DISC
For effective clinical translation it will be important to stratify patient cohorts, for example using sophisticated magnetic resonance imaging techniques33, 34, 35, 36, 37 and quantification of circulating biomarkers38 to select suitable candidates for treatment. The opportunity for cell‐based therapy may be most appropriate at the early stage of the degenerative cascade, prior to the onset of advanced disc degeneration coupled with endplate calcification and extensive annular degeneration.39 As a first step, patient selection for cell‐based therapies should therefore be limited to single‐level, moderate severity disc degeneration, which is reflected by Pfirrmann Grade III or IV on MRI. Patients presenting with a Pfirrmann grade of II or V may represent a patient population either too mild or too advanced in the disease process to show measurable improvement.40
Currently, patient selection for intradiscal therapies includes chronic back pain (>4 VAS) and disability (>40 ODI) that is refractory to conservative care (longer than 6 months). Potential patients are excluded if there is evidence of clinically‐relevant vertebral/spinal abnormalities that include spondylolisthesis, spondylolysis, scoliosis, fracture, severe kyphosis, or disc herniation. Herniation patients may be suitable candidates for treatment if intradiscal therapies are applied in combination with AF repair. Within appropriate individuals, the offending level(s) is typically identified by imaging (MRI) plus other clinical criteria that may include invasive techniques such as provocative discography. While current imaging techniques can provide exquisite anatomic detail, standard MRI sequences do poorly at identifying specific pain generators.41 Recently, a group of international experts concluded that there is no accepted clinical standard for discogenic pain.18, 19, 42, 43 Equally important, treatment outcomes are currently judged by subjective and qualitative patient reports, which can be influenced by significant placebo effects.44 Therefore, to support clinical application and evaluation of intradiscal cellular therapies, there is a critical need for new techniques that localize pain generators and quantify therapeutic effects on treated levels. New, advanced imaging tools for this purpose are currently in development and have been summarized in several recent reviews.45, 46 Examples include MRI techniques such as T1ρ and T2 relaxation times, and chemical exchange saturation transfer (CEST), which, unlike more widely used subjective grading disc grading schemes, are quantitative surrogates for disc composition.
4. OPTIMIZING CELL SOURCES AND DELIVERY TECHNIQUES
4.1. The challenges of the degenerate disc microenvironment
The rationale for and benefits of delivery of cells to the NP are 2‐fold: firstly, transplanted cells may stimulate endogenous NP cells to produce neo‐matrix through paracrine effects; and secondly transplanted cells may adopt an NP cell‐like phenotype and directly reconstitute native tissue.47, 48 A central challenge, however, that limits the potential of cell‐based regeneration is the harsh local cellular microenvironment within the degenerate disc, which is characterized by low oxygen and nutrient supply, increased acidity, altered osmolarity, as well as elevated levels of pro‐inflammatory cytokines.49, 50, 51, 52, 53, 54 Therefore, there is a clear need to identify robust cell populations to enhance the likelihood of survival post‐injection, and characterize how such cells will function in the typical degenerate microenvironment to determine if they can contribute effectively to functional disc repair. The precise cell number for intradiscal delivery needs to be carefully considered, and to‐date the exact number of cells required for functional regeneration is unclear. This will most likely depend on the cell‐type being employed as well as the metabolic activity and percentage survival post intradiscal delivery. A recent review of clinical trials by Sakai and Schol reported cell numbers ranging from 1 to 40 million cells being used in investigations.55 It is postulated that the delivery of large numbers of cells may exacerbate the degenerative microenvironment due to competing nutrient demands, which may in turn result in ineffective clinical outcomes.56 Given the varying stages of degeneration and microenvironments that exist in vivo, it will be important to tailor or design treatments for individuals. One way to achieve this could be to condition stem cells prior to implantation by acclimatizing them to patient specific in vivo microenvironmental milieu, which may include inflammatory cytokines, acidity, oxygen or nutrient deprivation.57, 58, 59 These treatments must be assessed using appropriate in vitro and ex vivo culture conditions which mimic those of the degenerate niche to assess the likely behavior and survival of transplanted cells prior to progressing to in vivo testing.49
4.2. Primary cells, stem cells or gene therapy?
From a surgical and practical perspective, given the unique structure and location of the disc, the difficulty in obtaining autologous primary NP tissue and cells for therapeutic use from either herniated discs or adjacent intact levels is clear. Delivery of primary NP cells to the disc appears safe and has shown some potential for efficacy in early clinical trials.60 Additional limitations of primary cells include the diminished tissue forming capacity of culture expanded NP cells derived from degenerated tissue26 and the altered catabolic phenotype of such cells, together with the paucity of NP cells that can be isolated. Furthermore, the isolation of tissue from adjacent healthy disc levels may increase the risk of initiating degeneration at the harvest site.61 This has motivated many researchers into identifying and characterizing alternative cell sources for disc regeneration, which is a key step towards translating therapies into clinical practice.62 Primary, differentiated cells from other skeletal sites with reduced risk for donor site morbidity, such as articular and nasal cartilage, have been investigated in vitro and in animal models for NP regeneration.63, 64, 65 While these cell types do exhibit some phenotypic differences to native NP cells, their relative ease of isolation, differentiated state and higher propensity to deposit extracellular matrix may make them attractive alternatives in the future and warrant further consideration and exploration. The likelihood that these alternative cell types can adopt a true discogenic phenotype is remote, and whether the extracellular matrix constituents they deposit are suitable to provide biomechanical functionality and longevity to restore disc function remains to be fully established.
Stem cell therapies have received considerable attention due to their versatility, translatability and potential for long term native tissue regeneration. Some successful patient outcomes have been demonstrated in clinical studies using this approach.28, 29, 55, 66, 67, 68 The bone marrow has been the prime site of MSC isolation for disc regeneration applications, inclusive of in vitro, preclinical and clinical studies.61, 69 Progenitor cells derived from other tissue sources such as adipose tissue have also been shown to possess significant potential for differentiation and tissue forming capabilities.70, 71 Adipose derived stem cells (ADSCs) may provide a better alternative and candidate for cell therapy and disc regeneration, due to their abundance and ease of isolation, and may also have a lower inherent capacity than bone marrow derived MSCs to undergo endochondral ossification.72 Harvesting of autologous ADSCs can be performed in outpatient clinics with typical yields of up to 25 000 adherent ADSCs per gram of tissue.73 One of the potential challenges in utilizing stem cells is that they are undifferentiated. The precise mechanism of action for regeneration is unknown; whether injected stem cells have the capacity to differentiate towards a discogenic phenotype or stimulate resident native cells has not been clearly demonstrated in preclinical studies. Priming stem cells using growth factors or other environmental cues such as altered oxygen or glucose availability to direct or maintain a specific phenotype may enhance their regenerative potential,58, 59, 74, 75, 76 but may also add complexity to the regulatory approval process.
Notochordal cell‐based approaches for disc regeneration have received considerable interest in the past several years. Notochordal cells are the progenitors of adult NP cells, and their loss in humans during postnatal growth is thought to contribute to the onset of degeneration later in life.10 As notochordal cells themselves cannot be readily obtained for direct clinical application, most studies have focused on leveraging the therapeutic potential of notochordal cell‐secreted factors.77
Gene therapy approaches may hold significant promise for disc regeneration, for example through silencing of catabolic or activating anabolic pathways. Gene therapy has advantages over direct delivery of proteins or small molecules, among them the possibility of sustained efficacy through long‐term, regulated, endogenous synthesis of growth factors or anti‐inflammatory factors.78 However, the need to establish a safety profile and possible off target effects will most likely lead to a longer and more complex regulatory process before realizing a commercially available product and clinical translation.
4.3. Effective cell delivery and retention
With respect to delivery of cell‐based therapies, the primary mode of choice has been injection through the AF. A key translational advantage of this approach is that, with image‐guided assistance, it can be performed percutaneously. However, there are associated challenges that have the potential to compromise both safety and efficacy, including the potential for needles to induce permanent AF damage,79 cell leakage from the delivery site and diminished cell viability due to shear forces as cells are forced through small diameter needles.80, 81, 82 For example, injecting a cell suspension into the lumbar discs of rabbits resulted in a 90% loss of the injected cells within the first 30 minutes.83 Biomaterials as delivery vehicles, and in particular hydrogels, have been utilized to overcome retention issues and provide additional support for cell survival and phenotype retention.84, 85, 86, 87, 88, 89 Injectable biomaterial systems utilizing alginates, collagens, hyaluronic acid, fibrin and a variety of other substances have all shown promise for improving cell delivery for disc repair90, 91, 92; however, for clinical translation, the biomaterial itself will require regulatory approval. Using biomaterials already approved and in clinical use for other indications may accelerate the approval process. That being said, because the disc is avascular, carrier degradation products may accumulate to levels beyond those found in other target tissues, justifying disc‐specific toxicity testing. Selection of needle size for trans‐annular delivery should be a careful trade‐off between the minimum size necessary to prevent shear‐induced cell death and allow injection of viscous hydrogels, and the maximum size necessary to prevent permanent AF damage and/or cell leakage. In a recent study using a preclinical large frame goat model, a 22G needle was found not to induce discernable degenerative changes on MRI or histology after 12 weeks.93 In addition, the volume to be delivered needs to be optimized, as excessive volumes may result in over pressurization, increasing the likelihood of cell leakage upon needle withdrawal. Further, the spatial distribution of delivered cell populations may also impact cell differentiation or activity and subsequent regeneration outcomes, as injected materials will tend to preferentially concentrate within pre‐existing fissures that are common in degenerated discs. For hydrogel‐delivered cell populations, migration of cells through the NP and integration of the injected material with native tissue should be considered. An alternative delivery route that has been investigated, and which does not cause disruption to the AF, is through the pedicles/endplates (transpedicular approach);94, 95 however, whether such an approach will have detrimental effects to the integrity of the endplate in the long term is yet to be established.
5. MINIMIZING POTENTIAL RISKS TO PATIENTS
Demonstrating safety is critical for disc therapies, as the consequences of an adverse event are potentially severe. The disc is one of the most highly loaded tissues in the body, which creates the significant potential that injected biomaterials will migrate in response to long term, cyclic loading. Material that is expelled from the disc space may impinge on nerves and result in neurological complications or even paralysis. Therefore, a fundamental safety requirement of a disc therapy, particularly one that involves a structural component such as a hydrogel or scaffold, is that it structurally integrates, and remains completely and permanently contained within the disc space upon delivery. Retention should be verified in the presence of physiological loading; ex vivo, this can be accomplished through extensive cyclic and multiaxial loading. Long term in vivo retention can be assessed via incorporation of radiopaque contrast agents or nanoparticles at the time of delivery, facilitating non‐invasive and three dimensional longitudinal assessments.85
Ensuring that a therapy does not impart local or systemic toxicity is also a critical safety consideration, and in vivo biocompatibility studies are generally a requirement of the FDA (food and drug administration) and other regulatory bodies for new biological therapies. Toxicity could potentially result directly from the implanted material, or over time from its degradation by‐products. For example, biomaterials that break down into acidic by‐products in the confined space of the disc could contribute to eliciting a catabolic, local, cellular response.96 Toxicity and biocompatibility can be assessed in vitro through cellular co‐culture studies and tissue or organ culture studies, and in vivo via implantation subcutaneously as well as in the disc space itself. Outcome measures from in vivo studies may include assessing the viability of endogenous cells and recruitment of neutrophils, macrophages, mast cells and fibroblasts, and the extent of fibrous encapsulation at the site of implantation, which can provide indications of an inflammatory response. Monitoring for signs of systemic toxicity (acute and chronic) is also essential, through assessment of changes in behavior, weight and appetite, reflexes and sensitization, in addition to blood chemistry, hematology and anatomic pathology.
Prior to clinical testing, stem cell therapies must be carefully vetted in vitro and through preclinical studies. Genetic instability of undifferentiated, multi‐ or pluripotent cells poses a safety risk as it increases the chances of tumorigenesis and cancer.97 Pluripotent stem cells such as embryonic and induced pluripotent stem cells exhibit intrinsic genetic instability that can result in teratoma formation.97 Emerging non‐integrating techniques for cellular reprogramming, for example using synthetic mRNA or chemical methods, may mitigate these risks without compromising induction efficiency.98, 99 Adult, multipotent stem cells, while not exhibiting the same tumorigenic risk as pluripotent stem cells, may also exhibit genetic instability through chromosomal aberrations that manifest progressively with increasing numbers of expansion passages.97 Minimizing the length of culture and degree of cellular manipulation prior to implantation minimizes the propensity for these in vitro genetic alterations to occur. Assessing the heterogeneity of stem cell populations in culture is also important to reduce tumorigenic and immunogenic risk.
Gene therapy, which employs cellular reprogramming, poses unique safety challenges that may limit or complicate translatability.100 These risks, which include the potential for insertional mutagenesis and cancer, and antigenicity with associated adverse immunological responses, result in additional regulatory hurdles, which may be harder to overcome for therapies targeting non‐life‐threatening conditions such as disc degeneration. Risks associated with gene therapy may be mitigated through careful selection of gene delivery systems (eg, viral vs non‐viral), which minimize risk without comprising efficacy.101
The method and frequency of administration will also impact a therapy's safety profile. Therapies that can be delivered using minimally invasive procedures, such as image‐guided percutaneous injection, and do not require general anesthesia, will pose less risk to patients than therapies that require an open surgical approach. Examples of such therapies might include injection of stem cells, hydrogels or drugs directed at the NP. Other strategies, such as implantation of cell‐seeded engineered constructs to repair or replace degenerate disc tissue, may necessitate an open surgical approach, however the required surgical procedures should carry no greater risk than those in current clinical use. Similarly, therapies that require a single administration to achieve long term efficacy will have a more attractive safety profile than those that require multiple administrations.
6. SELECTING PHYSIOLOGICALLY AND CLINICALLY RELEVANT EFFICACY METRICS
6.1. Defining and understanding the clinical problem
Optimization of disease‐modifying activity should be informed by knowledge of the patient, their clinical needs, and underlying pain and disability mechanisms. The most important objective of a treatment for painful disc degeneration is long term (>6 months) alleviation of symptoms. Current treatment strategies, whether they be conservative or surgical, may be effective in providing short term relief from pain, but their long‐term efficacy is more problematic, in part because they do not seek to restore disc structure or mechanical function. Given the nature of disc degeneration and its cascading effect on adjacent tissues and overall spine mechanical properties, it is often difficult to specify the fundamental treatment target. Disruption of normal disc biomechanical properties is associated with accelerated degeneration of other structures such as facet joints, and osteophyte formation due to adaptive bony changes or calcification of ligaments.102, 103 Back pain patients commonly have associated leg pain and symptoms which are frequently secondary to nerve compression, due to stenosis caused by loss of disc height and/or bulging or herniation. Consequently, stabilization (if not restoration) of disc structure is a key goal.
Back pain can also arise from irritation of peripheral disc tissues. For example, pro‐inflammatory crosstalk between the disc and vertebra is thought to underlie bone marrow edema evidenced as Modic changes (MC) on clinical MRI.104 Cellular expression of cytokines (eg, IL‐1, −6, and −10, and RANKL)104, 105 with MC is linked to endplate erosions which strongly associate with back pain.106, 107 These observations indicate that inflammation suppression is another important therapeutic goal.
For cell therapies to be clinically adopted, they need to demonstrate benefits beyond current treatment options. The rationale for choosing between surgical and nonsurgical care for chronic low back pain patients is not well‐defined. While the benefit of surgery for “mechanical” back pain (such as instability and sciatica) is supported by significant outcome literature, the appropriate intervention for disc‐related pain is less clear. Fusion surgery may alleviate pain by restoring intervertebral height and decompressing nerves, but immobilizes the intervertebral joint.15, 17 Metal/polymer‐based total disc arthroplasties preserve a limited degree of joint mobility, but are subject to wear and potential failure requiring revision surgery, and have not seen widespread adoption.108 Reported success of surgical care for back pain ranges from 41% to 57%,109 and with 5% to 16% early complication and reoperation rates.110 Discectomy may alleviate symptoms by removing herniated or bulging disc material and decompressing nerves, but the damaged disc is not repaired and may continue to degenerate, and many patients continue to experience pain and require ongoing treatment.111 Non‐surgical approaches such as steroid injections and physical therapy are temporary treatments that seek to mask symptoms that might otherwise resolve over time. Recurrent treatment for unresolving symptoms is more problematic as permanent nerve damage can occur. Non‐surgical, like surgical, treatment does not alter the long term progression of degeneration. Restoration of disc biomechanical function is therefore key to effective long‐term alleviation of painful symptoms.
The primary attraction of biological, cell‐based disc regeneration therapies is that they have the potential to alleviate symptoms and stabilize disc structure as well as biomechanical function by reconstituting native tissue structures. When delivered in combination with a structural biomaterial such as a hydrogel or composite scaffold, cell‐based therapies can provide immediate normalization of disc structure and mechanical function, while bioactive elements such as cells or growth factors work in vivo to suppress inflammation and progressively replace the biomaterial with native tissue. The three most important objectives to demonstrate efficacy of biological disc regeneration therapies are therefore alleviation of pain, stabilization or restoration of structure, and normalization of biomechanical function. Outcome measures for in vitro, in vivo preclinical, and clinical evaluation of such therapies should be selected with these goals in mind.
6.2. Appropriate selection and implementation of model systems to demonstrate efficacy
Pre‐clinical demonstration of efficacy and safety using in vitro and in vivo model systems is an iterative process (Figure 1), and typically begins with simple two‐dimensional (2D) or three‐dimensional (3D) cell culture models that have limited biological complexity but are cost effective and high throughput. Experiments may then progress to more complex 3D culture models or organ culture systems that incorporate more elements of the in vivo cellular environment. Smaller animal models such as mice, rats or rabbits may then be used to establish preliminary in vivo efficacy, and immunocompromised small animal models enable preliminary in vivo evaluation of human cells. Preclinical evaluations may then be conducted using larger animals such as pigs, sheep, goats or dogs. The reason for such an iterative approach to demonstrating therapeutic efficacy reflects the need to balance experimental control, cost and throughput on the one hand during proof‐of‐concept, with the need to incorporate biological complexity and demonstrate long term efficacy on the other, to progress towards clinical translation. At a minimum, a broad understanding of the biological mechanisms underlying cell‐based regeneration strategies is required to achieve efficacy, both for hypothesis generation at the study outset and to direct troubleshooting and optimization.
Figure 1.
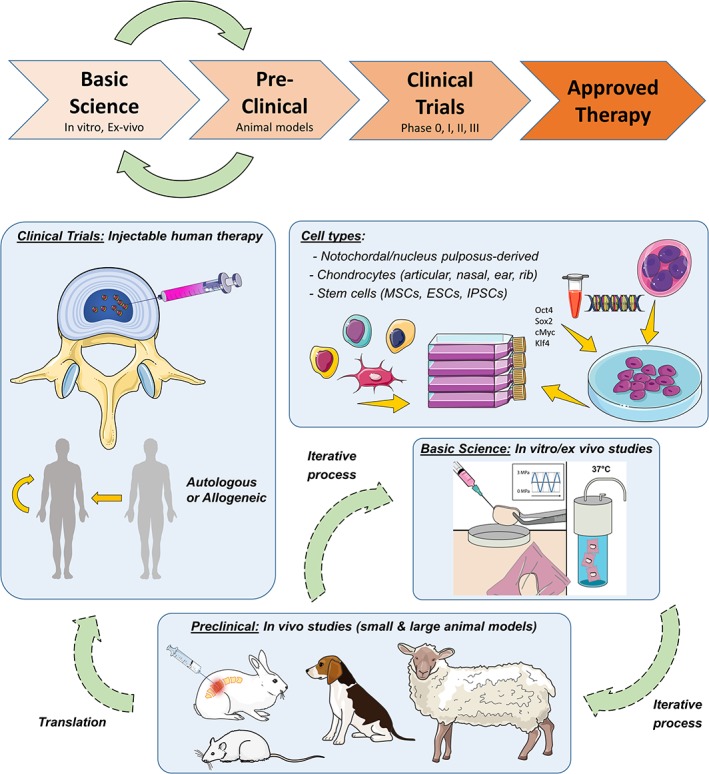
Requisite steps for demonstrating the efficacy of cell‐based disc regeneration therapies. Model systems should be applied iteratively, balancing experimental control, cost and throughput in the early stages with biological complexity and clinical relevance at more advanced stages, in order to maximize the chances of success in the clinic
In order to maximize the physiological relevance of experimental data, it is important that model systems effectively recapitulate the in vivo cellular microenvironment of the degenerate human disc. If therapeutic efficacy is evaluated only under idealized experimental conditions, the chances of failure upon preclinical or clinical translation are high. While this is true to an extent for therapies targeting any number of conditions (ie, not just disc degeneration), the disc microenvironment poses unique challenges—biochemical, biophysical and biomechanical—to therapeutic cell survival and function. With respect to the biochemical environment, as the disc has little or no direct blood supply, the cells must survive and function with access to very limited oxygen and nutrition. As discs degenerate, even in the early stages, this biochemical environment is further characterized by increasing local catabolic cytokine expression112 and acidity.113 Therapeutic cell types such as MSCs are more sensitive to microenvironmental stressors such as oxygen, nutrient deprivation and inflammatory stimuli compared to endogenous cells.114, 115, 116
For in vitro cell culture models, mimicking this in vivo disc microenvironment can be accomplished by culturing cells in low oxygen, and reduced glucose and serum, to simulate nutrient deprivation.115, 116, 117 The degenerate environment can be further simulated by including catabolic mediators such as inflammatory cytokines and by increasing acidity.118, 119, 120 The physical environment can be mimicked through culturing in soft 3D scaffolds such as hydrogels. Mechanical stress can be simulated using bioreactors that apply dynamic compressive loads and/or hydrostatic pressure.121, 122, 123, 124 Organ culture systems that employ living, degenerate discs from human donors may be the ultimate platform to effectively mimic the biological complexity of the in vivo cellular microenvironment, particularly when combined with a bioreactor that applies physiological loading.125
Ensuring accurate recapitulation of the human disc cellular microenvironment is equally important when moving to in vivo systems. One of the greatest challenges facing in vivo models of disc therapeutics is the need to replicate the size, and in particular the height of the human disc, necessary to accurately recapitulate physiological nutritional demands. Large animals obviously more closely approximate the nutritional environment compared to rodents, however, even in livestock, lumbar disc size is still significantly smaller than in humans (eg, lumbar disc height in sheep is ~4 mm compared to ~11 mm in humans).126
Therapeutic cell types such as MSCs may exhibit different phenotypic characteristics as a function of donor, age, species and anatomical site, and these characteristics may be closely linked to regenerative potential.127, 128, 129, 130 While the development phase may necessitate the use of non‐human cells that are accessible and compatible with in vitro and in vivo models, the results obtained with these cells may not accurately reflect the performance of human cells that will be used clinically. It is therefore important to validate findings using cells from human donors, wherever possible controlling for variables such as age, sex, co‐morbidities and anatomical site. Human cells may be obtained both from commercial sources, as well as from clinical collaborators undertaking spinal procedures. The latter of these sources is particularly attractive where it permits recruitment of donors who would also be prospective recipients of disc therapeutics. For example, a surgeon performing a procedure such as a spinal fusion may be able to provide not only surgical waste tissue as a source of endogenous disc cells, but also bone marrow from the adjacent vertebrae without subjecting the patient to any additional surgical procedures or risks. As already discussed, organ culture using degenerate human discs obtained from cadavers represents an avenue for evaluating therapeutic efficacy not only using human cells, but also in a physiologically accurate and degenerative microenvironment. The importance of cell source also extends to the preclinical development phase. For example, a therapy that demonstrates efficacy in vitro using cells of a particular age and species, may not demonstrate similar efficacy in a large animal model of a different age and species. In vitro studies should therefore seek to validate a therapeutic approach using cells from the full range of sources that will be required at each phase of development.
6.3. Pain and disability outcome measures
As alleviation of pain is the primary goal of disc therapies, including pain as an outcome measure in laboratory experiments is desirable. Selection of physiologically‐relevant pain outcome measures in the preclinical setting is complicated, as in the clinical setting pain is subjective and its direct source may be ill‐defined.131, 132 Pain outcome measures can be classified in different ways. They may include surrogates such as nerve ingrowth and regression,133, 134 local and systemic levels of secreted neurogenic and inflammatory factors,135, 136 as well as imaging to demonstrate nerve compression.45 Direct outcome measures of pain may include assessments of overall behavior and mobility, and pain sensitivity assays.137 Small animal models are particularly attractive with respect to assessing pain, as they are compatible with most if not all of these outcome measure types. For example, if a therapy is being evaluated in a mouse or rat, it is possible and practical to assess mobility, pain sensitivity, and serum and radiological biomarkers in vivo, and tissue level expression of neurogenic markers post mortem. Using in vitro models, pain outcomes are limited to surrogates such as expression of neurogenic factors and nerve ingrowth. Large animal models are in theory compatible with many of the same outcome measures as small animal models, but practical considerations make implementation of functional and pain sensitivity assays more challenging.
6.4. Structural and biomechanical outcome measures
Including appropriate outcome measures to assess restoration of disc structure and mechanical function is equally important, as these are the key indicators of the likelihood for a therapy to provide long term alleviation of symptoms. Like pain, the ability to assess these parameters directly or indirectly is dependent on the type of model system. With respect to structure, the most clinically relevant outcome measure is stabilization or restoration of disc height as demonstrated through imaging (MRI, plain radiographs or computed tomography), and is possible for in vivo models as well as in vitro whole‐disc organ culture models.93, 138, 139 Importantly, these non‐invasive imaging metrics can be applied longitudinally and thus used to confirm both acute regenerative effects and sustained, long term efficacy. With respect to biomechanical properties, in vitro model systems that incorporate three‐dimensional constructs or whole‐disc organ culture facilitate direct assessment of mechanical function.89, 140 For in vivo models, mechanical properties can be assessed ex vivo on intact spinal motion segments or isolated tissue samples.85 In the case of motion segments, multi‐axis loads can be applied to similar physiological deformations, including those likely to lead to failure.141 Direct, in vivo evaluation of disc mechanical properties is possible using emerging technologies such as magnetic resonance elastography.142, 143 Where direct evaluation is impossible, assessments of extracellular matrix composition and organization through biochemical and histological assays, or imaging (eg, MRI or contrast‐enhanced CT), represent important surrogates for mechanical function.33, 34, 35, 36, 37
7. MAXIMIZING COMMERCIAL POTENTIAL
Given the vast socio‐economic costs associated with low back pain and the high costs of current treatment approaches, it would appear, superficially, to be straight forward to establish the commercial case for a new disc therapy, particularly one for which clinical safety and efficacy can be established. However, bringing a biological disc therapy to market is a long, complex and expensive process, and one that to date has been met with little success. Basic scientists, including those working in the translational space, are often focused foremost on probing basic mechanistic questions and rapidly disseminating findings. They may be less concerned about addressing questions of commercial viability, despite this being an essential criterion for widespread clinical adoption. A new biological therapy may “check” key boxes with respect to safety and efficacy in vitro, in animal models, and even in clinical trials, but fail at the final hurdle if it is not perceived as commercially viable. Some of the factors impacting potential commercial viability can be addressed early on, including clearly identifying the prospective target patient population size, protecting intellectual property, and assessing the competitive landscape. Other factors should be considered and continuously reviewed as the concept evolves, including complexity, cost of components and preparation time, in addition to the ease and method of administration. Early and frequent discussions with regulators (eg, FDA) are key to defining the studies required to translate such products into the clinic, and to ensure that manufacturing approaches will meet the standards required for human testing.
One predictor of the potential commercial viability of a new therapy is the size of the prospective target patient population. Further, the overall manufacturing costs associated with producing the product, in contrast to the reimbursement/pricing potential of the treatment, must be favorable. In considering the clinical motivation for developing a specific biological disc therapy, the overall socioeconomic burden is often cited, as is the current lack of effective treatment options. In establishing and justifying the clinical need for a specific, new therapeutic approach, it is necessary to move beyond general patient populations (ie, “degenerative disc disease”) and stratify subgroups of patients that will be suitable candidates for that therapeutic approach based on the specific disease features experienced. For example, injectable disc therapies may be tailored to target a patient subgroup that exhibits a specific set of symptoms or a narrow window of degeneration severity that includes an intact annulus and relatively healthy endplates. The need for a therapy targeting symptomatic low back pain in patients with mild to moderate severity disc degeneration (eg, minimally invasive cell therapy) could be justified by citing the number of patients in this subgroup that are not candidates for surgery and are unresponsive to conservative treatment approaches. Alternatively, the need for a new therapy targeting patients with severe disc degeneration (eg, tissue engineered total disc replacements) could be justified by citing the number of patients that exhibit poor outcomes from current surgical approaches such as fusion or total disc arthroplasty.
The second important consideration to ensure future commercial viability is early protection of relevant intellectual property. At most academic institutions, protection of intellectual property begins by submitting an invention disclosure to the relevant university office, which will then assess the concept or technology for commercialization potential. Such disclosures are a requirement for any potentially patentable invention, and institutions may stipulate that this should be done in advance of any public disclosure (eg, publication of manuscripts or conference presentations). Without such protection before public disclosure, the ownership is lost, and no patent may be filed, significantly decreasing the chances of the product ever being translated to the clinic. Following disclosure, where an invention is identified as having commercialization potential, the institution should be equipped to guide the investigator through the intellectual property protection process, including filing of patents. Trade secrets represent an alternative approach to patenting an idea, though not one necessarily supported by academic institutions. When a patent is published and becomes public, smart competitors have the opportunity to circumvent it by implementing minor alterations or improvements to the overall concept. In the case of trade secrets, key properties of the product that are critical to its function never enter the public domain.
The relative cost and complexity of a therapeutic strategy, with respect to production, preparation and administration will also impact commercial viability. A complex and costly in vitro approach may be necessary during early stages of development; however, investigators should consider how this can be reduced as the development process advances towards preclinical and clinical translation. This process of refinement might include minimizing (or eliminating) the number of growth factors, cell types or biomaterial components, substituting growth factors with cheaper compounds, and reducing the time needed for in vitro manipulation prior to administration, which may include cell expansion or conditioning.
For first or second generation therapeutic products, investigators should also be cognizant and take steps at an early stage to avoid developing “complex” approaches, which will most likely face significant regulatory hurdles and barriers. For example, this might include considering using autologous vs allogeneic cells, or using appropriately conditioned and sourced adult stem cells vs pluripotent cells that require viral reprogramming. Therapies that require in vitro manipulation using animal serum face tough regulatory hurdles and alternative culture conditions should be considered. If the approach includes gene therapy, then gene delivery systems with the best safety profile should be selected. The number of regulatory hurdles could be further reduced by incorporating or substituting compounds that have an established safety record and are already FDA approved for other indications.
The final important consideration with respect to ensuring commercial viability is to undertake a comprehensive survey of the current competitive landscape. This requires identifying competing therapeutics, including those in clinical use and those undergoing clinical or preclinical trials, that target a similar cohort of patients, and reviewing where those competing products are with respect to the development pipeline. If a competing product has not already come to market, it should be estimated when that is likely to happen. Even if the number of competing products is relatively small, factors such as relative risk, cost and potential efficacy compared to the therapy being proposed should be assessed.
8. THE IMPORTANCE OF MULTIDISCIPLINARY COLLABORATION AND ENGAGING WITH CLINICIANS
Closely linked to commercial viability and prospective market uptake is the willingness of clinicians to embrace the newly developed therapeutic approach. There may be resistance within a particular market sector to switch away from traditional treatment approaches, for example, for philosophical, financial or logistical reasons. To ensure a new disc therapy will successfully and effectively translate from the lab to the clinic, basic scientists must therefore work to ensure that the new product will ultimately be enthusiastically embraced by those clinicians required to administer it. To achieve this, it is essential to engage clinicians early and continuously from inception through to translation, and these clinical collaborators may well already be passionate about identifying novel treatment strategies for their low back pain patients. Not only will the participation of clinicians (as the end users) aid market acceptance, it will also add significant value to the overall scientific endeavor. In the early stages, clinicians will be able to draw on their extensive, firsthand experience to help identify prospective target patient populations, and advise on existing treatment modalities and their limitations. As development progresses through in vitro evaluations, clinical collaborators can provide access to primary human cells, for example from surgical waste tissue, that can be used to maximize the physiological relevance of results. During preclinical evaluation in animal models, clinical collaborators can work to optimize application of the treatment, surgically or otherwise, advise on selection of clinically relevant outcome measures and evaluate success benchmarks. As a therapy approaches clinical translation, clinical collaborators can disseminate and promote preclinical findings to colleagues at clinical scientific meetings, assist with the design and conduct of clinical trials, including recruitment of trial participants, navigate the regulatory landscape, and advocate the treatment to patients and health systems. It is thus crucial that clinical collaborators fully understand the character of the product and underlying science.
Engagement with clinicians can also be enhanced through dissemination of research findings not only at major basic science‐intensive meetings such as the Orthopedic Research Society (ORS), but also at meetings that have stronger clinical participation, such as those of the International Society for the Study of the Lumbar Spine (ISSLS), the Cervical Spine Research Society (CSRS), the North American Spine Society (NASS), the American Academies of Orthopedic (AAOS) and Neurological (AANS) surgeons. Clinicians are frequent and active participants in these meetings, and constitute leadership positions, and are thus the ideal advocacy vehicles in these fora. Equally, it is pivotal to promote and encourage multidisciplinary interactions at both scientific and clinically focused meetings to accelerate the field towards clinical translation and ensure we maximize our efforts in treating degenerative disc disease.
9. SUMMARY
Despite an overwhelming clinical need, cell‐based therapies for disc degeneration and low back pain have to date failed to achieve widespread clinical adoption. Over the last decade, tremendous advances have been made towards understanding the underlying pathophysiology, defining and optimizing therapeutic cell types, and refining and applying physiologically relevant model systems for their evaluation. The goal now must be to more effectively translate laboratory findings into the clinic to achieve improved patient outcomes. To this end, the primary intention of this perspective from members of the Orthopedic Research Society Spine Section is to provide a consensus with respect to the central challenges that have limited effective clinical translation of cell‐based therapies. Motivating this perspective was a uniform and sincere belief among Spine Section members that cell‐based therapies, if effectively designed and implemented in conjunction with appropriate diagnostic tools, patient selection criteria, and with the support of industry partners have the potential for substantial clinical impact. Recommendations for researchers to effectively address these challenges are provided, falling broadly under the key themes of “Safety, Efficacy, Commercial Viability and Engaging Clinicians” (summarized in Table 1). The authors urge researchers to consider and leverage these recommendations to enhance the translational relevance and clinical potential of their research endeavors and activities.
Table 1.
Key recommendations for successful clinical translation of injectable cell‐based disc therapies
| Safety | Efficacy | Commercial viability | Engaging clinicians |
|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CONFLICT OF INTEREST
Drs Smith, Buckley, Sakai, Mauck and Malhotra have no conflicts of interest to declare. Dr Silverman is an employee of DiscGenics, Inc. Dr Le Maitre is a named inventor on a patent for an injectable hydrogel system (GB2493933). Dr Lotz is a founder of Relivient Medsystems and Nocimed.
Author contributions
All authors contributed to the writing of the manuscript. Drs Smith and Buckley designed the outline and revised the manuscript, and all authors read and approved the final version prior to submission.
ACKNOWLEDGEMENTS
Dr Smith received funding support from the National Institutes of Health (R21AR070959 and R01AR071975), the Department of Veteran's Affairs (I01RX001321), and the Catherine D. Sharp Foundation. Dr Buckley was supported by Science Foundation Ireland Career Development Award (15/CDA/3476). Dr Le Maitre was supported by funding from the Arthritis Research UK and Medical Research Council (UK) (MR/P026796/1). Dr Lotz was supported by funding from the National Institutes of Health (R01AR063705). The authors would like to acknowledge and thank Servier Medical Art (www.servier.com) for their image bank used to produce Figure 1.
Smith LJ, Silverman L, Sakai D, et al. Advancing cell therapies for intervertebral disc regeneration from the lab to the clinic: Recommendations of the ORS spine section. JOR Spine. 2018;1:e1036. 10.1002/jsp2.1036
Funding information Arthritis Research UK; Catherine D. Sharpe Foundation; Medical Research Council (UK), Grant/Award Number: MR/P026796/1; National Institute of Arthritis and Musculoskeletal and Skin Diseases, Grant/Award Numbers: R01AR063705, R01AR071975, R21AR070959; Science Foundation Ireland, Grant/Award Number: 15/CDA/3476; U.S. Department of Veterans Affairs, Grant/Award Number: I01RX001321
Contributor Information
Lachlan J. Smith, Email: lachlans@pennmedicine.upenn.edu.
Conor T. Buckley, Email: conor.buckley@tcd.ie.
REFERENCES
- 1. Dieleman JL, Baral R, Birger M, et al. US spending on personal health care and public health, 1996‐2013. JAMA. 2016;316:2627‐2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Global Burden of Disease Study 2013 Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990‐2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743‐800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ringwalt C, Gugelmann H, Garrettson M, et al. Differential prescribing of opioid analgesics according to physician specialty for Medicaid patients with chronic noncancer pain diagnoses. Pain Res Manag. 2014;19:179‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Freemont AJ. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology. 2009;48:5‐10. [DOI] [PubMed] [Google Scholar]
- 5. Ashley JW, Enomoto‐Iwamoto M, Smith LJ, et al. Intervertebral disc development and disease‐related genetic polymorphisms. Genes Dis. 2016;3:171‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vo NV, Hartman RA, Patil PR, et al. Molecular mechanisms of biological aging in intervertebral discs. J Orthop Res. 2016;34:1289‐1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dario AB, Ferreira ML, Refshauge KM, Lima TS, Ordoñana JR, Ferreira PH. The relationship between obesity, low back pain, and lumbar disc degeneration when genetics and the environment are considered: a systematic review of twin studies. Spine J. 2015;15:1106‐1117. [DOI] [PubMed] [Google Scholar]
- 8. Vieira LA, dos Santos AA, Peluso C, Barbosa CP, Bianco B, Rodrigues LMR. Influence of lifestyle characteristics and VDR polymorphisms as risk factors for intervertebral disc degeneration: a case‐control study. Eur J Med Res. 2018;23:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Teraguchi M, Yoshimura N, Hashizume H, et al. Progression, incidence, and risk factors for intervertebral disc degeneration in a longitudinal population‐based cohort: the Wakayama Spine Study. Osteoarthritis Cartilage. 2017;25:1122‐1131. [DOI] [PubMed] [Google Scholar]
- 10. Smith LJ, Nerurkar NL, Choi KS, Harfe BD, Elliott DM. Degeneration and regeneration of the intervertebral disc: lessons from development. Dis Model Mech. 2011;4:31‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Adams MA, McNally DS, Dolan P. 'Stress' distributions inside intervertebral discs. The effects of age and degeneration. J Bone Joint Surg Br. 1996;78:965‐972. [DOI] [PubMed] [Google Scholar]
- 12. Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine. 2004;29:2691‐2699. [DOI] [PubMed] [Google Scholar]
- 13. Maroudas A, Stockwell RA, Nachemson A, Urban J. Factors involved in the nutrition of the human lumbar intervertebral disc: cellularity and diffusion of glucose in vitro. J Anat. 1975;120:113‐130. [PMC free article] [PubMed] [Google Scholar]
- 14. Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine. 2004;29:2700‐2709. [DOI] [PubMed] [Google Scholar]
- 15. Eck JC, Sharan A, Ghogawala Z, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 7: lumbar fusion for intractable low‐back pain without stenosis or spondylolisthesis. J Neurosurg Spine. 2014;21:42‐47. [DOI] [PubMed] [Google Scholar]
- 16. Raj PP. Intervertebral disc: anatomy‐physiology‐pathophysiology‐treatment. Pain Pract. 2008;8:18‐44. [DOI] [PubMed] [Google Scholar]
- 17. Zhang C, Berven SH, Fortin M, Weber MH. Adjacent segment degeneration versus disease after lumbar spine fusion for degenerative pathology: a systematic review with meta‐analysis of the literature. Clin Spine Surg. 2016;29:21‐29. [DOI] [PubMed] [Google Scholar]
- 18. Foster NE, Anema JR, Cherkin D, et al. Prevention and treatment of low back pain: evidence, challenges, and promising directions. Lancet. 2018;391:2368‐2383. [DOI] [PubMed] [Google Scholar]
- 19. Hartvigsen J, Hancock MJ, Kongsted A, et al. What low back pain is and why we need to pay attention. Lancet. 2018;391:2356‐2367. [DOI] [PubMed] [Google Scholar]
- 20. Lee JC, Choi SW. Adjacent segment pathology after lumbar spinal fusion. Asian Spine J. 2015;9:807‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nguyen TH, Randolph DC, Talmage J, Succop P, Travis R. Long‐term outcomes of lumbar fusion among workers' compensation subjects: a historical cohort study. Spine. 2011;36:320‐331. [DOI] [PubMed] [Google Scholar]
- 22. Tong W, Lu Z, Qin L, et al. Cell therapy for the degenerating intervertebral disc. Transl Res. 2017;181:49‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hohaus C, Ganey TM, Minkus Y, Meisel HJ. Cell transplantation in lumbar spine disc degeneration disease. Eur Spine J. 2008;17:S492‐S503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meisel HJ, Siodla V, Ganey T, Minkus Y, Hutton WC, Alasevic OJ. Clinical experience in cell‐based therapeutics: disc chondrocyte transplantation. A treatment for degenerated or damaged intervertebral disc. Biomol Eng. 2007;24:5‐21. [DOI] [PubMed] [Google Scholar]
- 25. Hegewald AA, Endres M, Abbushi A, et al. Adequacy of herniated disc tissue as a cell source for nucleus pulposus regeneration. J Neurosurg Spine. 2011;14:273‐280. [DOI] [PubMed] [Google Scholar]
- 26. Masuda K. Biological repair of the degenerated intervertebral disc by the injection of growth factors. Eur Spine J. 2008;17(suppl 4):441‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Coric D, Pettine K, Sumich A, Boltes MO. Prospective study of disc repair with allogeneic chondrocytes presented at the 2012 Joint Spine Section Meeting. J Neurosurg‐Spine. 2013;18:85‐95. [DOI] [PubMed] [Google Scholar]
- 28. Yoshikawa T, Ueda Y, Miyazaki K, Koizumi M, Takakura Y. Disc regeneration therapy using marrow mesenchymal cell transplantation: a report of two case studies. Spine (Phila Pa 1976). 2010;35:E475‐E480. [DOI] [PubMed] [Google Scholar]
- 29. Orozco L, Soler R, Morera C, Alberca M, Sánchez A, García‐Sancho J. Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation. 2011;92:822‐828. [DOI] [PubMed] [Google Scholar]
- 30. Ghosh P, Moore R, Vernon‐Roberts B, et al. Immunoselected STRO‐3+ mesenchymal precursor cells and restoration of the extracellular matrix of degenerate intervertebral discs. J Neurosurg Spine. 2012;16:479‐488. [DOI] [PubMed] [Google Scholar]
- 31. Hiraishi S, Schol J, Sakai D, et al. Discogenic cell transplantation directly from a cryopreserved state in an induced intervertebral disc degeneration canine model. JOR Spine. 2018;1:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tschugg A, Diepers M, Simone S, et al. A prospective randomized multicenter phase I/II clinical trial to evaluate safety and efficacy of NOVOCART disk plus autologous disk chondrocyte transplantation in the treatment of nucleotomized and degenerative lumbar disks to avoid secondary disease: safety results of Phase I‐a short report. Neurosurg Rev. 2017;40:155‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mwale F, Demers CN, Michalek AJ, et al. Evaluation of quantitative magnetic resonance imaging, biochemical and mechanical properties of trypsin‐treated intervertebral discs under physiological compression loading. J Magn Reson Imaging. 2008;27:563‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Antoniou J, Epure LM, Michalek AJ, Grant MP, Iatridis JC, Mwale F. Analysis of quantitative magnetic resonance imaging and biomechanical parameters on human discs with different grades of degeneration. J Magn Reson Imaging. 2013;38:1402‐1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mulligan KR, Ferland CE, Gawri R, Borthakur A, Haglund L, Ouellet JA. Axial T1rho MRI as a diagnostic imaging modality to quantify proteoglycan concentration in degenerative disc disease. Eur Spine J. 2015;24:2395‐2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu Q, Tawackoli W, Pelled G, et al. Detection of low back pain using pH level‐dependent imaging of the intervertebral disc using the ratio of R1rho dispersion and ‐OH chemical exchange saturation transfer (RROC). Magn Reson Med. 2015;73:1196‐1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gullbrand SE, Ashinsky BG, Martin JT, et al. Correlations between quantitative T2 and T1rho MRI, mechanical properties and biochemical composition in a rabbit lumbar intervertebral disc degeneration model. J Orthop Res. 2016;34:1382‐1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weber KT, Alipui DO, Sison CP, et al. Serum levels of the proinflammatory cytokine interleukin‐6 vary based on diagnoses in individuals with lumbar intervertebral disc diseases. Arthritis Res Ther. 2016;18:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sakai D. Future perspectives of cell‐based therapy for intervertebral disc disease. Eur Spine J. 2008;17(suppl 4):452‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Coric D, Pettine K, Sumich A, Margaret O, Boltes RN. Prospective study of disc repair with allogeneic chondrocytes. In: Vaccaro AR editor. Chart showing the intradiscal surgical treatment options. J Neurosurg Spine. 2013;18:85‐95. [DOI] [PubMed] [Google Scholar]
- 41. Sheehan NJ. Magnetic resonance imaging for low back pain: indications and limitations. Ann Rheum Dis. 2010;69:7‐11. [DOI] [PubMed] [Google Scholar]
- 42. Buchbinder R, van Tulder M, Oberg B, et al. Low back pain: a call for action. Lancet. 2018;391:2384‐2388. [DOI] [PubMed] [Google Scholar]
- 43. Clark S, Horton R. Low back pain: a major global challenge. Lancet. 2018;391:2302‐2302. [DOI] [PubMed] [Google Scholar]
- 44. Harden RN, Saracoglu M, Connolly S, et al. "Managing" the placebo effect: the single‐blind placebo lead‐in response in two pain models. Pain Med. 2016;17:2305‐2310. [DOI] [PubMed] [Google Scholar]
- 45. Lotz JC, Haughton V, Boden SD, et al. New treatments and imaging strategies in degenerative disease of the intervertebral disks. Radiology. 2012;264:6‐19. [DOI] [PubMed] [Google Scholar]
- 46. Samartzis D, Borthakur A, Belfer I, et al. Novel diagnostic and prognostic methods for disc degeneration and low back pain. Spine J. 2015;15:1919‐1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Richardson SM, Walker RV, Parker S, et al. Intervertebral disc cell‐mediated mesenchymal stem cell differentiation. Stem Cells. 2006;24:707‐716. [DOI] [PubMed] [Google Scholar]
- 48. Miyamoto T, Muneta T, Tabuchi T, et al. Intradiscal transplantation of synovial mesenchymal stem cells prevents intervertebral disc degeneration through suppression of matrix metalloproteinase‐related genes in nucleus pulposus cells in rabbits. Arthritis Res Ther. 2010;12:R206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thorpe A, Bach FC, Tryfonidou MA, et al. Leaping the hurdles in developing regenerative treatments for the intervertebral disc from preclinical to clinical. JOR Spine. 2018;1:e1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wuertz K, Godburn K, Neidlinger‐Wilke C, Urban J, Iatridis JC. Behavior of mesenchymal stem cells in the chemical microenvironment of the intervertebral disc. Spine. 2008;33:1843‐1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin‐1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7:R732‐R745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Le Maitre CL, Hoyland JA, Freemont AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL‐1beta and TNFalpha expression profile. Arthritis Res Ther. 2007;9:R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Holm S, Maroudas A, Urban JP, Selstam G, Nachemson A. Nutrition of the intervertebral disc: solute transport and metabolism. Connect Tissue Res. 1981;8:101‐119. [DOI] [PubMed] [Google Scholar]
- 54. Urban JPG, Mcmullin JF. Swelling pressure of the lumbar intervertebral disks – influence of age, spinal level, composition, and degeneration. Spine. 1988;13:179‐187. [DOI] [PubMed] [Google Scholar]
- 55. Sakai D, Schol J. Cell therapy for intervertebral disc repair: clinical perspective. J Orthop Translat. 2017;9:8‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bendtsen M, Bunger C, Colombier P, et al. Biological challenges for regeneration of the degenerated disc using cellular therapies. Acta Orthop. 2016;87:39‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Redondo‐Castro E, Cunningham C, Miller J, et al. Interleukin‐1 primes human mesenchymal stem cells towards an anti‐inflammatory and pro‐trophic phenotype in vitro. Stem Cell Res Ther. 2017;8:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pei M. Environmental preconditioning rejuvenates adult stem cells' proliferation and chondrogenic potential. Biomaterials. 2017;117:10‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Naqvi SM, Gansau J, Buckley CT. Priming and cryopreservation of microencapsulated marrow stromal cells as a strategy for intervertebral disc regeneration. Biomed Mater. 2018;13:034106. [DOI] [PubMed] [Google Scholar]
- 60. Mochida J, Sakai D, Nakamura Y, et al. Intervertebral disc repair with activated nucleus pulposus cell transplantation: a three‐year, prospective clinical study of its safety. Eur Cell Mater. 2015;29:202‐212. [DOI] [PubMed] [Google Scholar]
- 61. Oehme D, Goldschlager T, Ghosh P, et al. Cell‐based therapies used to treat lumbar degenerative disc disease: a systematic review of animal studies and human clinical trials. Stem Cells Int. 2015;946031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vedicherla S, Buckley CT. Cell‐based therapies for intervertebral disc and cartilage regeneration – current concepts, parallels, and perspectives. J Orthop Res. 2017;35:8‐22. [DOI] [PubMed] [Google Scholar]
- 63. Acosta F Jr, Metz L, Liu J, et al. Juvenile chondrocytes are superior to undifferentiated mesenchymal stem cells for porcine intervertebral disc repair. Spine J. 2008;50S:8. [Google Scholar]
- 64. Vedicherla S, Buckley CT. In vitro extracellular matrix accumulation of nasal and articular chondrocytes for intervertebral disc repair. Tissue Cell. 2017;49:503‐513. [DOI] [PubMed] [Google Scholar]
- 65. Tsaryk R, Silva‐Correia J, Oliveira JM, et al. Biological performance of cell‐encapsulated methacrylated gellan gum‐based hydrogels for nucleus pulposus regeneration. J Tissue Eng Regen Med. 2017;11:637‐648. [DOI] [PubMed] [Google Scholar]
- 66. Pettine KA, Murphy MB, Suzuki RK, Sand TT. Percutaneous injection of autologous bone marrow concentrate cells significantly reduces lumbar discogenic pain through 12 months. Stem Cells. 2015;33:146‐156. [DOI] [PubMed] [Google Scholar]
- 67. Noriega DC, Ardura F, Hernandez‐Ramajo R, et al. Intervertebral disc repair by allogeneic mesenchymal bone marrow cells: a randomized controlled trial. Transplantation. 2017;101:1945‐1951. [DOI] [PubMed] [Google Scholar]
- 68. Pennicooke B, Moriguchi Y, Hussain I, et al. Biological treatment approaches for degenerative disc disease: a review of clinical trials and future directions. Cureus. 2016;8:e892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Richardson SM, Kalamegam G, Pushparaj PN, et al. Mesenchymal stem cells in regenerative medicine: focus on articular cartilage and intervertebral disc regeneration. Methods. 2016;99:69‐80. [DOI] [PubMed] [Google Scholar]
- 70. Guilak F, Estes BT, Diekman BO, Moutos FT, Gimble JM. 2010 Nicolas Andry Award: multipotent adult stem cells from adipose tissue for musculoskeletal tissue engineering. Clin Orthop Relat Res. 2010;468:2530‐2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jurgens WJ, Oedayrajsingh‐Varma MJ, Helder MN, et al. Effect of tissue‐harvesting site on yield of stem cells derived from adipose tissue: implications for cell‐based therapies. Cell Tissue Res. 2008;332:415‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Diekman BO, Rowland CR, Lennon DP, Caplan AI, Guilak F. Chondrogenesis of adult stem cells from adipose tissue and bone marrow: induction by growth factors and cartilage‐derived matrix. Tissue Eng Part A. 2010;16:523‐533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yang X, Li X. Nucleus pulposus tissue engineering: a brief review. Eur Spine J. 2009;18:1564‐1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hudson KD, Bonassar LJ. Hypoxic expansion of human mesenchymal stem cells enhances three‐dimensional maturation of tissue‐engineered intervertebral discs. Tissue Eng Part A. 2017;23:293‐300. [DOI] [PubMed] [Google Scholar]
- 75. Adesida AB, Mulet‐Sierra A, Jomha NM. Hypoxia mediated isolation and expansion enhances the chondrogenic capacity of bone marrow mesenchymal stromal cells. Stem Cell Res Ther. 2012;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Clarke LE, McConnell JC, Sherratt MJ, et al. Growth differentiation factor 6 and transforming growth factor‐beta differentially mediate mesenchymal stem cell differentiation, composition, and micromechanical properties of nucleus pulposus constructs. Arthritis Res Ther. 2014;16:R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Purmessur D, Schek RM, Abbott RD, Ballif BA, Godburn KE, Iatridis JC. Notochordal conditioned media from tissue increases proteoglycan accumulation and promotes a healthy nucleus pulposus phenotype in human mesenchymal stem cells. Arthritis Res Ther. 2011;13:R81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Evans CH, Huard J. Gene therapy approaches to regenerating the musculoskeletal system. Nat Rev Rheumatol. 2015;11:234‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Michalek AJ, Funabashi KL, Iatridis JC. Needle puncture injury of the rat intervertebral disc affects torsional and compressive biomechanics differently. Eur Spine J. 2010;19:2110‐2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Vadala G, Sowa G, Hubert M, et al. Mesenchymal stem cells injection in degenerated intervertebral disc: cell leakage may induce osteophyte formation. J Tissue Eng Regen Med. 2012;6:348‐355. [DOI] [PubMed] [Google Scholar]
- 81. Li YY, Diao HJ, Chik TK, et al. Delivering mesenchymal stem cells in collagen microsphere carriers to rabbit degenerative disc: reduced risk of osteophyte formation. Tissue Eng Part A. 2014;20:1379‐1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Moyer HR, Kinney RC, Singh KA, Williams JK, Schwartz Z, Boyan BD. Alginate microencapsulation technology for the percutaneous delivery of adipose‐derived stem cells. Ann Plast Surg. 2010;65:497‐503. [DOI] [PubMed] [Google Scholar]
- 83. Bertram H, Kroeber M, Wang H, et al. Matrix‐assisted cell transfer for intervertebral disc cell therapy. Biochem Biophys Res Commun. 2005;331:1185‐1192. [DOI] [PubMed] [Google Scholar]
- 84. Thorpe AA, Dougill G, Vickers L, et al. Thermally triggered hydrogel injection into bovine intervertebral disc tissue explants induces differentiation of mesenchymal stem cells and restores mechanical function. Acta Biomater. 2017;54:212‐226. [DOI] [PubMed] [Google Scholar]
- 85. Gullbrand SE, Schaer TP, Agarwal P, et al. Translation of an injectable triple‐interpenetrating‐network hydrogel for intervertebral disc regeneration in a goat model. Acta Biomater. 2017;60:201‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhou XP, Wang JK, Fang WJ, et al. Genipin cross‐linked type II collagen/chondroitin sulfate composite hydrogel‐like cell delivery system induces differentiation of adipose‐derived stem cells and regenerates degenerated nucleus pulposus. Acta Biomater. 2018;71:496‐509. [DOI] [PubMed] [Google Scholar]
- 87. Zeng Y, Chen C, Liu W, et al. Injectable microcryogels reinforced alginate encapsulation of mesenchymal stromal cells for leak‐proof delivery and alleviation of canine disc degeneration. Biomaterials. 2015;59:53‐65. [DOI] [PubMed] [Google Scholar]
- 88. Frith JE, Cameron AR, Menzies DJ, et al. An injectable hydrogel incorporating mesenchymal precursor cells and pentosan polysulphate for intervertebral disc regeneration. Biomaterials. 2013;34:9430‐9440. [DOI] [PubMed] [Google Scholar]
- 89. Smith LJ, Gorth DJ, Showalter BL, et al. In vitro characterization of a stem cell‐seeded triple interpenetrating network hydrogel for functional regeneration of the nucleus pulposus. Tissue Eng. 2014;20:1841‐1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Blanquer SBG, Grijpma DW, Poot AA. Delivery systems for the treatment of degenerated intervertebral discs. Adv Drug Deliv Rev. 2015;84:172‐187. [DOI] [PubMed] [Google Scholar]
- 91. Malafaya PB, Silva GA, Reis RL. Natural–origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv Drug Deliv Rev. 2007;59:207‐233. [DOI] [PubMed] [Google Scholar]
- 92. Pereira DR, Silva‐Correia J, Oliveira JM, Reis RL. Hydrogels in acellular and cellular strategies for intervertebral disc regeneration. J Tissue Eng Regen Med. 2013;7:85‐98. [DOI] [PubMed] [Google Scholar]
- 93. Gullbrand SE, Malhotra NR, Schaer TP, et al. A large animal model that recapitulates the spectrum of human intervertebral disc degeneration. Osteoarthritis Cartilage. 2017;25:146‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Vadala G, De Strobel F, Bernardini M, et al. The transpedicular approach for the study of intervertebral disc regeneration strategies: in vivo characterization. Eur Spine J. 2013;22(suppl 6):S972‐S978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Vadala G, Russo F, Pattappa G, et al. The transpedicular approach as an alternative route for intervertebral disc regeneration. Spine (Phila Pa 1976). 2013;38:E319‐E324. [DOI] [PubMed] [Google Scholar]
- 96. Gorth DJ, Mauck RL, Chiaro JA, et al. IL‐1ra delivered from poly(lactic‐co‐glycolic acid) microspheres attenuates IL‐1beta‐mediated degradation of nucleus pulposus in vitro. Arthritis Res Ther. 2012;14:R179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Goldring CEP, Duffy PA, Benvenisty N, et al. Assessing the safety of stem cell therapeutics. Cell Stem Cell. 2011;8:618‐628. [DOI] [PubMed] [Google Scholar]
- 98. Warren L, Manos PD, Ahfeldt T, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Singh VK, Kumar N, Kalsan M, Saini A, Chandra R. Mechanism of induction: induced pluripotent stem cells (iPSCs). J Stem Cells. 2015;10:43‐62. [PubMed] [Google Scholar]
- 100. Rothe M, Schambach A, Biasco L. Safety of gene therapy: new insights to a puzzling case. Curr Gene Ther. 2014;14:429‐436. [DOI] [PubMed] [Google Scholar]
- 101. Wu P, Chen H, Jin R, et al. Non‐viral gene delivery systems for tissue repair and regeneration. J Transl Med. 2018;16:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kumaresan S, Yoganandan N, Pintar FA, Maiman DJ, Goel VK. Contribution of disc degeneration to osteophyte formation in the cervical spine: a biomechanical investigation. J Orthop Res. 2001;19:977‐984. [DOI] [PubMed] [Google Scholar]
- 103. Paholpak P, Dedeogullari E, Lee C, et al. Do modic changes, disc degeneration, translation and angular motion affect facet osteoarthritis of the lumbar spine. Eur J Radiol. 2018;98:193‐199. [DOI] [PubMed] [Google Scholar]
- 104. Dudli S, Sing DC, Hu SS, et al. ISSLS PRIZE IN BASIC SCIENCE 2017: intervertebral disc/bone marrow cross‐talk with Modic changes. Eur Spine J. 2017;26:1362‐1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Torkki M, Majuri ML, Wolff H, et al. Osteoclast activators are elevated in intervertebral disks with Modic changes among patients operated for herniated nucleus pulposus. Eur Spine J. 2016;25:207‐216. [DOI] [PubMed] [Google Scholar]
- 106. Kerttula L, Luoma K, Vehmas T, Grönblad M, Kääpä E. Modic type I change may predict rapid progressive, deforming disc degeneration: a prospective 1‐year follow‐up study. Eur Spine J. 2012;21:1135‐1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Mok FPS, Samartzis D, Karppinen J, Fong DYT, Luk KDK, Cheung KMC. Modic changes of the lumbar spine: prevalence, risk factors, and association with disc degeneration and low back pain in a large‐scale population‐based cohort. Spine J. 2016;16:32‐41. [DOI] [PubMed] [Google Scholar]
- 108. Saifi C, Cazzulino A, Park C, et al. National trends for primary and revision lumbar disc arthroplasty throughout the United States. Global Spine J. 2018;8:172‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wei J, Song Y, Sun L, Lv C. Comparison of artificial total disc replacement versus fusion for lumbar degenerative disc disease: a meta‐analysis of randomized controlled trials. Int Orthop. 2013;37:1315‐1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ibrahim T, Tleyjeh IM, Gabbar O. Surgical versus non‐surgical treatment of chronic low back pain: a meta‐analysis of randomised trials. Int Orthop. 2008;32:107‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sherman J, Cauthen J, Schoenberg D, Burns M, Reaven NL, Griffith SL. Economic impact of improving outcomes of lumbar discectomy. Spine J. 2010;10:108‐116. [DOI] [PubMed] [Google Scholar]
- 112. Johnson ZI, Schoepflin ZR, Choi H, et al. Disc in flames: roles of TNF‐alpha and IL‐1beta in intervertebral disc degeneration. Eur Cell Mater. 2015;30:104‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Nachemson A. Intradiscal measurements of pH in patients with lumbar rhizopathies. Acta Orthop Scand. 1969;40:23‐42. [DOI] [PubMed] [Google Scholar]
- 114. Mohanraj B, Huang AH, Yeger‐McKeever MJ, et al. Chondrocyte and mesenchymal stem cell derived engineered cartilage exhibits differential sensitivity to pro‐inflammatory cytokines J Orthop Res; 2018. 10.1002/jor.24061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Farrell MJ, Shin JI, Smith LJ, et al. Functional consequences of glucose and oxygen deprivation on engineered mesenchymal stem cell‐based cartilage constructs. Osteoarthritis Cartilage. 2014;23:134‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Gorth DJ, Lothstein KE, Chiaro JA, et al. Hypoxic regulation of functional extracellular matrix elaboration by nucleus pulposus cells in long‐term agarose culture. J Orthop Res. 2015;33:747‐754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Naqvi SM, Buckley CT. Extracellular matrix production by nucleus pulposus and bone marrow stem cells in response to altered oxygen and glucose microenvironments. J Anat. 2015;227:757‐766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Naqvi SM, Buckley CT. Bone marrow stem cells in response to intervertebral disc‐like matrix acidity and oxygen concentration: implications for cell‐based regenerative therapy. Spine (Phila Pa 1976). 2016;41:743‐750. [DOI] [PubMed] [Google Scholar]
- 119. Wuertz K, Godburn K, Iatridis JC. MSC response to pH levels found in degenerating intervertebral discs. Biochem Biophys Res Commun. 2009;379:824‐829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wuertz K, Godburn K, Neidlinger‐Wilke C, et al. Behavior of mesenchymal stem cells in the chemical microenvironment of the intervertebral disc. Spine (Phila Pa). 2008;1976(33):1843‐1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Chan SC, Ferguson SJ, Wuertz K, et al. Biological response of the intervertebral disc to repetitive short‐term cyclic torsion. Spine (Phila Pa 1976). 2011;36:2021‐2030. [DOI] [PubMed] [Google Scholar]
- 122. Chan SCW, Walser J, Käppeli P, Shamsollahi MJ, Ferguson SJ, Gantenbein‐Ritter B. Region specific response of intervertebral disc cells to complex dynamic loading: an organ culture study using a dynamic torsion‐compression bioreactor. PLoS One. 2013;8:e72489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Walter BA, Korecki CL, Purmessur D, Roughley PJ, Michalek AJ, Iatridis JC. Complex loading affects intervertebral disc mechanics and biology. Osteoarthritis Cartilage. 2011;19:1011‐1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Shah BS, Chahine NO. Dynamic hydrostatic pressure regulates nucleus pulposus phenotypic expression and metabolism in a cell density‐dependent manner. J Biomech Eng. 2018;140:021003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Rosenzweig DH, Gawri R, Moir J, et al. Dynamic loading, matrix maintenance and cell injection therapy of human intervertebral discs cultured in a bioreactor. Eur Cell Mater. 2016;31:26‐39. [DOI] [PubMed] [Google Scholar]
- 126. O'Connell GD, Vresilovic EJ, Elliott DM. Comparison of animals used in disc research to human lumbar disc geometry. Spine (Phila Pa 1976). 2007;32:328‐333. [DOI] [PubMed] [Google Scholar]
- 127. Choudhery MS, Badowski M, Muise A, Pierce J, Harris DT. Donor age negatively impacts adipose tissue‐derived mesenchymal stem cell expansion and differentiation. J Transl Med. 2014;12:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kim M, Erickson IE, Huang AH, Garrity ST, Mauck RL, Steinberg DR. Donor variation and optimization of human mesenchymal stem cells chondrogenesis in hyaluronic acid. Tissue Eng Part A. 2018. 10.1089/ten.TEA.2017.0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Fellows CR, Matta C, Zakany R, et al. Adipose, bone marrow and synovial joint‐derived mesenchymal stem cells for cartilage repair. Front Genet. 2016;7:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Yim RL, Lee JT, Bow CH, et al. A systematic review of the safety and efficacy of mesenchymal stem cells for disc degeneration: insights and future directions for regenerative therapeutics. Stem Cells Dev. 2014;23:2553‐2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Malik KM, Cohen SP, Walega DR, Benzon HT. Diagnostic criteria and treatment of discogenic pain: a systematic review of recent clinical literature. Spine J. 2013;13:1675‐1689. [DOI] [PubMed] [Google Scholar]
- 132. Salzberg L. The physiology of low back pain. Prim Care. 2012;39:487‐498. [DOI] [PubMed] [Google Scholar]
- 133. Binch ALA, Cole AA, Breakwell LM, et al. Nerves are more abundant than blood vessels in the degenerate human intervertebral disc. Arthritis Res Ther. 2015;17:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Fagan AB, Sarvestani G, Moore RJ, Fraser RD, Vernon‐Roberts B, Blumbergs PC. Innervation of anulus tears: an experimental animal study. Spine. 2010;35:1200‐1205. [DOI] [PubMed] [Google Scholar]
- 135. Purmessur D, Freemont AJ, Hoyland JA. Expression and regulation of neurotrophins in the nondegenerate and degenerate human intervertebral disc. Arthritis Res Ther. 2008;10:R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Khan AN, Jacobsen HE, Khan J, et al. Inflammatory biomarkers of low back pain and disc degeneration: a review. Ann N Y Acad Sci. 2017;1410:68‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Shi C, Qiu S, Riester SM, et al. Animal models for studying the etiology and treatment of low back pain. J Orthop Res. 2018;36:1305‐1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Bach FC, Tellegen AR, Beukers M, et al. Biologic canine and human intervertebral disc repair by notochordal cell‐derived matrix: from bench towards bedside. Oncotarget. 2018;9:26507‐26526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Piazza M, Peck SH, Gullbrand SE, et al. Quantitative MRI correlates with histological grade in a percutaneous needle injury mouse model of disc degeneration. J Orthop Res. 2018. 10.1002/jor.24028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Walter BA, Illien‐Junger S, Nasser PR, et al. Development and validation of a bioreactor system for dynamic loading and mechanical characterization of whole human intervertebral discs in organ culture. J Biomech. 2014;47:2095‐2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Berger‐Roscher N, Casaroli G, Rasche V, Villa T, Galbusera F, Wilke HJ. Influence of complex loading conditions on intervertebral disc failure. Spine. 2017;42:E78‐E85. [DOI] [PubMed] [Google Scholar]
- 142. Walter BA, Mageswaran P, Mo X, et al. MR elastography‐derived stiffness: a biomarker for intervertebral disc degeneration. Radiology. 2017;285:167‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Cortes DH, Magland JF, Wright AC, Elliott DM. The shear modulus of the nucleus pulposus measured using magnetic resonance elastography: a potential biomarker for intervertebral disc degeneration. Magn Reson Med. 2014;72:211‐219. [DOI] [PMC free article] [PubMed] [Google Scholar]


