Abstract
Genome sequencing data have recently demonstrated that eukaryote evolution has been remarkably influenced by the acquisition of a large number of genes by horizontal gene transfer (HGT) across different kingdoms. However, in depth-studies on the physiological traits conferred by these accidental DNA acquisitions are largely lacking. Here we elucidate the functional role of Sl gasmin, a gene of a symbiotic virus of a parasitic wasp that has been transferred to an ancestor of the moth species Spodoptera littoralis and domesticated. This gene is highly expressed in circulating immune cells (haemocytes) of larval stages, where its transcription is rapidly boosted by injection of microorganisms into the body cavity. RNAi silencing of Sl gasmin generates a phenotype characterized by a precocious suppression of phagocytic activity by haemocytes, which is rescued when these immune cells are incubated in plasma samples of control larvae, containing high levels of the encoded protein. Proteomic analysis demonstrates that the protein Sl gasmin is released by haemocytes into the haemolymph, where it opsonizes the invading bacteria to promote their phagocytosis, both in vitro and in vivo. Our results show that important physiological traits do not necessarily originate from evolution of pre-existing genes, but can be acquired by HGT events, through unique pathways of symbiotic evolution. These findings indicate that insects can paradoxically acquire selective advantages with the help of their natural enemies.
Author summary
Parasitic wasps are important insect biocontrol agents. These insects are beneficial for the ecological service they provide, which largely contributes to the control of natural populations of their hosts. Paradoxically, they can be beneficial also for non-host individuals attacked by mistake, if these survive after parasitization. In nature, this can happen to lepidopteran larvae when attacked by a wasp harbouring a symbiotic virus that can mediate horizontal gene transfer. Indeed, this virus, injected along with the egg in the body of the host, can get integrated in the genome of the parasitized larva, carrying along exogenous genes. Because the non-host regularly suppresses the parasitoid egg and/or juveniles, any surviving individual with a stable insertion of new genes in the germ line will represent an evolutionary novelty, with expanded functional capacities, if the resulting gene domestication event confers new physiological traits. The immune function here discovered demonstrates that symbiotic associations can drive unique evolutionary pathways, maximizing the fitness of interacting organisms, which evolve as a complex unit with a shared gene pool.
Introduction
Horizontal gene transfer (HGT) is a mechanism of accidental acquisition of genetic material by means other than reproduction, which in some evolutionary lineages, such as prokaryotes, is considered the major driving force in genome evolution [1]. In theory, all genes may undergo HGT, however current evidence on prokaryotes indicates that housekeeping genes, modulating cellular functions, are significantly more itinerant than regulatory genes [2, 3].
In eukaryotes, HGT occurrence in unicellular organisms has been frequently reported, while, in contrast, it has been considered rare in multicellular organisms, until the advent of high-throughput sequencing technologies, which have allowed the discovery of a considerable number of HGT events in these organisms. Indeed, although less common than in prokaryotes, HGT is far from being of marginal importance in genome evolution of multicellular eukaryotes [4–7)]. The HGT occurrence is frequent both in vertebrates and invertebrates, with bacteria and protists being the major gene donors, as they have established an extensive variety of symbiotic associations with higher organisms, which favours intimate contact and exchange of genetic material [6, 8]. This complements the major role played by transposable elements in shaping genome evolution [5–7, 9].
The study of HGT in eukaryotes has increasingly shed light on its frequency and on the functional categories of the genes involved [3, 5, 7, 9, 10]. However, only a few reports have addressed whether the transferred genes are neutrally included in the genome or functionally integrated into the biological pathways of the recipient organism (i.e., domestication).
Cases of HGT from microorganisms and plants, potentially conferring a functional benefit, have been described in rotifers, nematodes and arthropods [11]. Genome and transcriptome analyses of plant-parasitic nematodes indicate that success in host colonization and exploitation could have been favoured by genes acquired from bacteria through HGT [12–16]. These genes encode plant cell wall-degrading or -modifying enzymes [11–17], as well as enzymes involved in vitamin synthesis [16], or invertases expressed in the nematode digestive system, and likely involved in the metabolism of plant sucrose [13]. Similarly, there is increasing evidence to suggest that HGT plays an active role in modeling insect genomes, providing novel enzymes for digestion and metabolism. Indeed, in coffee berry borer (Hypothenemus hampei) [18] and mustard leaf (Phaedon cochleariae) [19] beetles, genes acquired from gut bacteria may confer the capacity to hydrolyze host plant polysaccharides; similarly, genes of bacterial and fungal origin may mediate carotenoid biosynthesis in the pea aphid [20] and metabolism of sugar and amino acids in some phytophagous species of Lepidoptera [10]. Moreover, genes conferring the putative capacity to detoxify plant defense chemicals have been acquired by HGT, both in insects and mites [21].
Genes possibly implicated in controlling arthropod immunity or their interactions with other organisms also undergo HGT, as reported for Drosophila [5]. Several parasitic chalcidoid wasp species have acquired chitinase genes from fungi, which are expressed in the venom gland to produce virulence factors injected into the host at the oviposition [22]. The recurrent and independent transfer of bacterial genes encoding antibacterial toxins to distinct eukaryotic lineages suggests that these genes can enhance the fitness by adding new immune defense barriers [23]. Only recently it has been demonstrated that these domesticated sequences express active antibacterial effectors in the recipient organisms [24]. Indeed, in the tick Ixodes scapularis, this allows the control of the proliferation of the Lyme disease agent, Borrelia burgdorferi, in order to keep its abundance at optimal levels for an effective vectoring activity. However, an active role of these genes in the modulation of the immune response against infection is still elusive.
Polydnaviruses (PDV) have recently been identified as a new source of sequences contributing to insect genome evolution by HGT [6, 25]. PDV are viral symbionts of braconid (Bracovirus-BV) and ichneumonid (Ichnovirus-IV) parasitic wasps attacking lepidopteran larvae, which are injected during the oviposition [26, 27]. They express virulence factors in parasitized hosts triggering immunosuppression and a number of physiological alterations to allow progeny survival and development [26–29]. The PDV are integrated into the wasp’s genome as provirus, while free virions are produced only in the calyx region of the wasp’s ovary. These contain multiple DNA circles, some of which, upon infection of host tissues, become integrated into its genome, starting the expression of virulence factors without undergoing replication [27, 28]. This latter integration property has been proposed as the main route of entrance of genetic material from the BV associated with the parasitic wasp Cotesia congregata (CcBV) into the genome of non-permissive hosts, which survive and can transmit CcBV sequences integrated in the germ line [25]. The viral origin of one of these insertions in the moth species Spodoptera exigua, named gasmin, is unequivocally corroborated by the presence of a bracoviral regulatory sequence [25]. A recent work has tried to analyse the microevolutionary forces driving the domestication of gasmin [30]. This was done by baculovirus-mediated expression of gasmin in the larvae of a population lacking a functional gasmin gene (i.e., the European population of S. exigua that bears a truncated non-functional gasmin gene). The reduced mortality of larvae infected by a baculovirus expressing gasmin was attributed to a putative protecting role exerted by this protein against viral infection and/or replication [30]. However, this presumed antiviral defense barrier was surprisingly associated with an enhanced susceptibility of S. exigua larvae to bacterial infection [30]. Collectively, the functional evidence provided was largely indirect and strongly influenced by baculovirus infection, which can have effects difficult to tease apart from those induced by gasmin. Then, the microevolutionary scenario cannot be unequivocally interpreted, since a clear conclusion on the effective physiological role of this viral protein and on the adaptive advantage conferred by the domestication of its coding gene is still lacking.
In order to fill this gap, here we report a detailed molecular and functional characterization of a gasmin homologue, identified in S. littoralis, using a gene silencing approach instead of a baculovirus mediated expression in a gasmin-free environment, in order to get rid of all potential experimental artifacts that have apparently influenced previous studies. Our work demonstrates the important role of gasmin in the modulation of the cellular immune response. This sheds new light on the importance that HGT can have in the evolution of metazoan genomes, and on how innate defense barriers in insects can be paradoxically shaped by parasitic wasps and their associated viral symbionts, which are potent pathogens of the wasp’s host.
Results
Spodoptera littoralis gasmin gene (Sl gasmin) and its expression profile
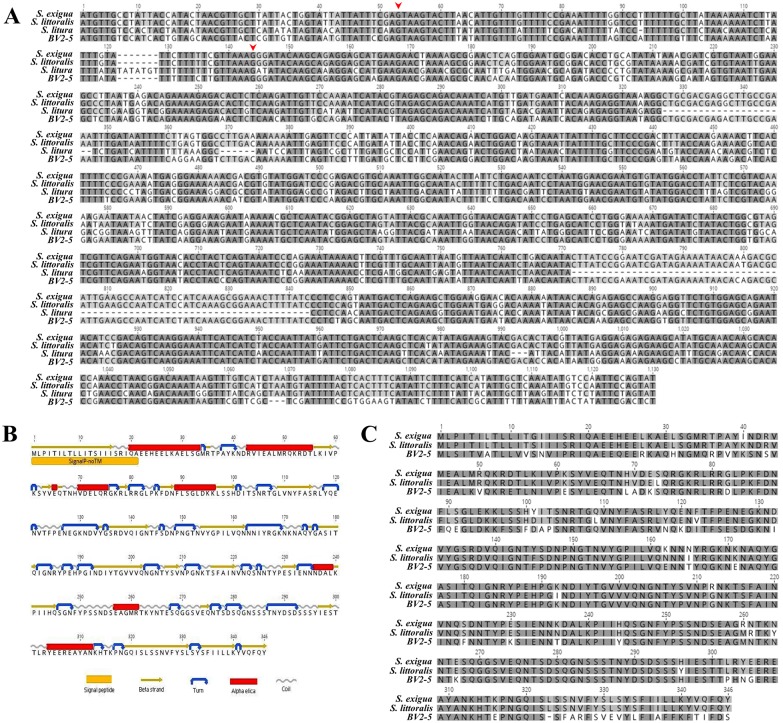
The genomic sequence of Sl gasmin, including the intron (1126 bp), shows very high identity with S. exigua gasmin (KP406767), S. litura gasmin (obtained through data mining; MTZO01009970.1) and with viral BV2-5 (AJ632326) (98, 72 and 86% respectively) (Fig 1A). The coding DNA sequence (CDS) encodes a predicted protein of 346 amino acids (aa) (with a putative signal peptide of 21 aa) (Fig 1B and 1C), that shows 95% and 76% sequence identity with S. exigua gasmin and with the homologue viral protein encoded by CcBV (CcBV 25.3; CAG17487), respectively (Fig 1B and 1C).
Fig 1. Sequence analysis.
DNA sequence alignment of gasmin homologues in 3 Spodoptera species and in CcBV (BV2-5) (the red arrowheads delimit the intronic region) (A), predicted secondary structure (B) and sequence conservation of Sl gasmin (C). Secondary structure prediction of Sl gasmin was carried out with the EMBOSS: Garnier algorithm; the Inter ProScan tool identified a potential signal-peptide. Bioinformatics analyses were performed using Geneious v6.1.6 (Biomatters, available from www.geneious.com) (A and C). DNA and protein alignments were performed using the Clustal W algorithm; black and grey shadings indicate identity and high conservation of amino acids, respectively.
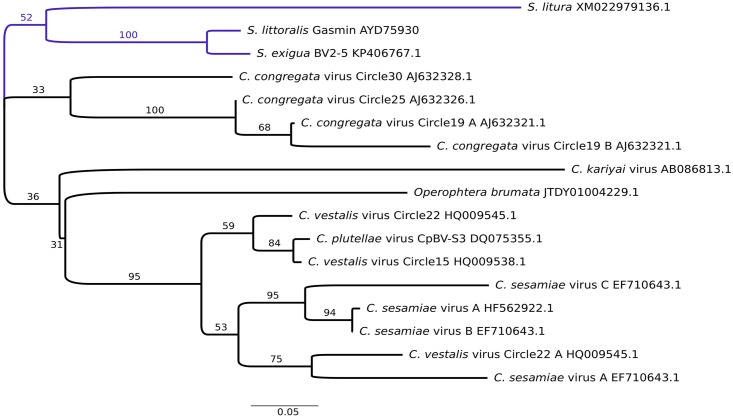
To learn more about the origin of the Sl gasmin, we retrieved all sequences showing high similarity, available in NCBI, and constructed a similarity tree using the predicted amino acid sequences (Fig 2). Phylogenetic analyses strongly support the monophyly of the S. exigua and S. littoralis protein sequences (BS = 100), and indicate that S. litura represents a sister to these sequences. Other relationships, including the placement of a homologue identified in the winter moth (Operophtera brumata), are poorly supported and available sampling does not permit the confident assignment of likely donors for any of the lepidopteran genes. While all of the lepidopteran proteins (with the exception of the S. exigua and S. littoralis sequences) are highly divergent from one-another, examination of the aligned protein sequences reveals that most indels are concentrated in regions that also show indels and high divergence among viral sequences, possibly consistent either with rapid evolution under altered selective constraints in various lepidopteran groups, or with multiple acquisitions and independent losses in insects. Further sequence sampling would be required to conclusively resolve this issue.
Fig 2. Molecular phylogenetic tree of gasmin-like proteins.
The evolutionary history was inferred for aligned sequences by the Maximum Likelihood method, under the JTT amino acid substitution matrix. The sequence identity of Spodoptera littoralis gasmin with the retrieved protein sequences was higher than 60% in all cases, except for S. litura (57%) and Cotesia kariyai bracovirus (47%). Query cover was above 94%, except for C. vestalis virus circle 22 (79%) and C. kariyai bracovirus (63%). The numbers above the branches denote the bootstrap proportions.
To investigate the existence of selective pressure on S. littoralis and S. exigua sequences we used FEL (Fixed Effects Likelihood), a program which uses a maximum-likelihood approach to infer nonsynoymous (dN) and synonymous (dS) substitution rates on a per-site basis for a given coding alignment and corresponding phylogeny. As expected, negative purifying selection has been observed at 26 sites with P values≤0.05 (S1 File).
The expression profile of Sl gasmin in different tissues of S. littoralis larvae was analyzed by qRT-PCR. The haemocytes (i.e., the circulating immune cells) were by far the most active site of transcription (One-Way ANOVA: F(3,24) = 49.07, n = 7, P<0.0001, df = 27) (Fig 3), suggesting a key-role of this gene in the immune response.
Fig 3. Sl gasmin transcript level in different tissues of Spodoptera littoralis larvae.
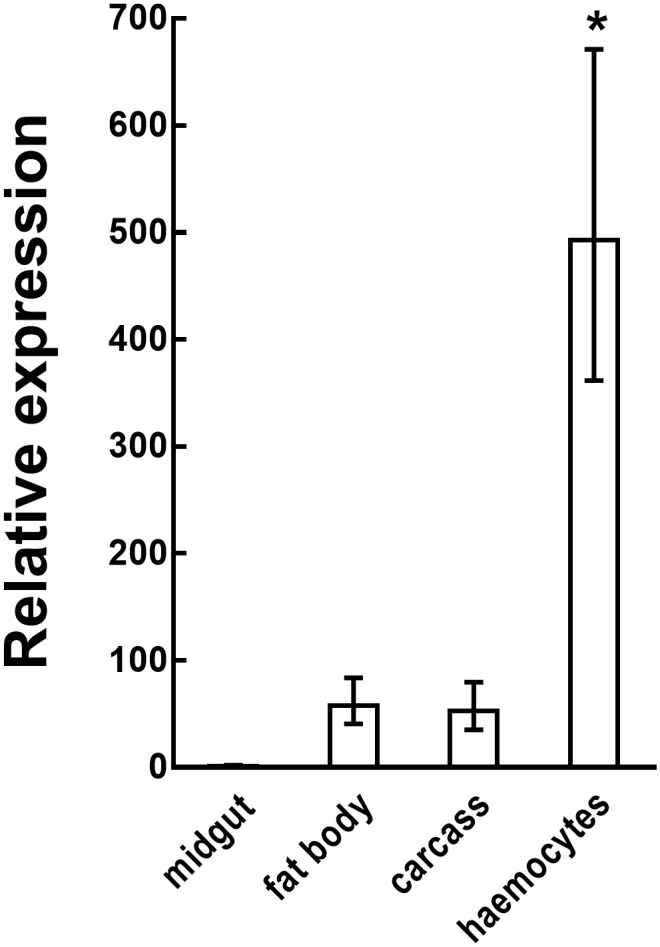
Sl gasmin relative expression, determined by qRT-PCR, was significantly higher in the haemocytes compared with the other tissues analyzed. S. littoralis β-actin was used as reporter gene. The values reported are the mean ± S.E. (*P<0.001, One-Way ANOVA followed by Bonferroni’s test, n = 7).
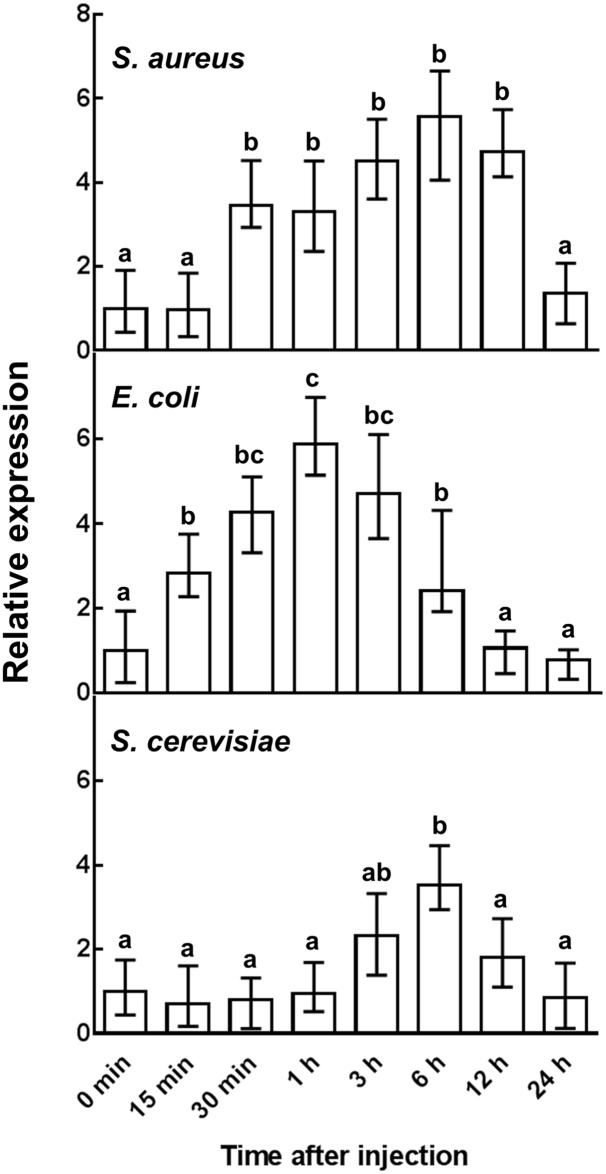
Because this gene is of viral origin, and its viral homologue is expressed in parasitized hosts, we formulated the hypothesis that it confers a selective advantage both to the wasp bearing the donor viral symbiont and to the recipient moth species. Indeed, because the protection of wasp’s juveniles against host immune responses is mediated by broad immunosuppressive strategies, it is reasonable to assume that a reinforcement of the antimicrobial barriers, by preventing secondary infections of the host, is also beneficial for the wasp progeny. To corroborate this hypothesis, Sl gasmin transcription was assessed in response to microbial challenge. The injection of both Gram-positive (Staphylococcus aureus) and Gram-negative (Escherichia coli) bacteria in S. littoralis larvae, as well as of the yeast Saccharomyces cerevisiae, significantly enhanced the transcription of Sl gasmin in the haemocytes (One-Way ANOVA S. aureus: F(7,88) = 150.68, P<0.0001, n = 12, df = 95; E. coli: F(7,88) = 169.61, P<0.0001, n = 12, df = 95; S. cervisiae: F(7,88) = 68.072, P<0.0001, n = 12, df = 95), with slightly different temporal profiles (Fig 4). The expression level of the target gene increased very rapidly following bacteria injection, while S. cerevisiae challenge triggered a comparatively slower response soon after injection, followed by a gradual decrease to the basal level 12 h post-injection (Fig 4).
Fig 4. Relative expression level of Sl gasmin after microbial challenge.
The transcription level of Sl gasmin, determined by qRT-PCR, was significantly up-regulated in Spodoptera littoralis larvae by injection of the Gram-positive bacterium Staphylococcus aureus, the Gram-negative bacterium Escherichia coli, and the yeast Saccharomyces cerevisiae. S. littoralis β-actin was used as reporter gene. Data points are the mean ± S.E. of 3 biological replicates. Different letters above each bar indicate significant differences (P<0.05, One-Way ANOVA followed by Bonferroni’s test).
Functional analysis of Sl gasmin by RNAi
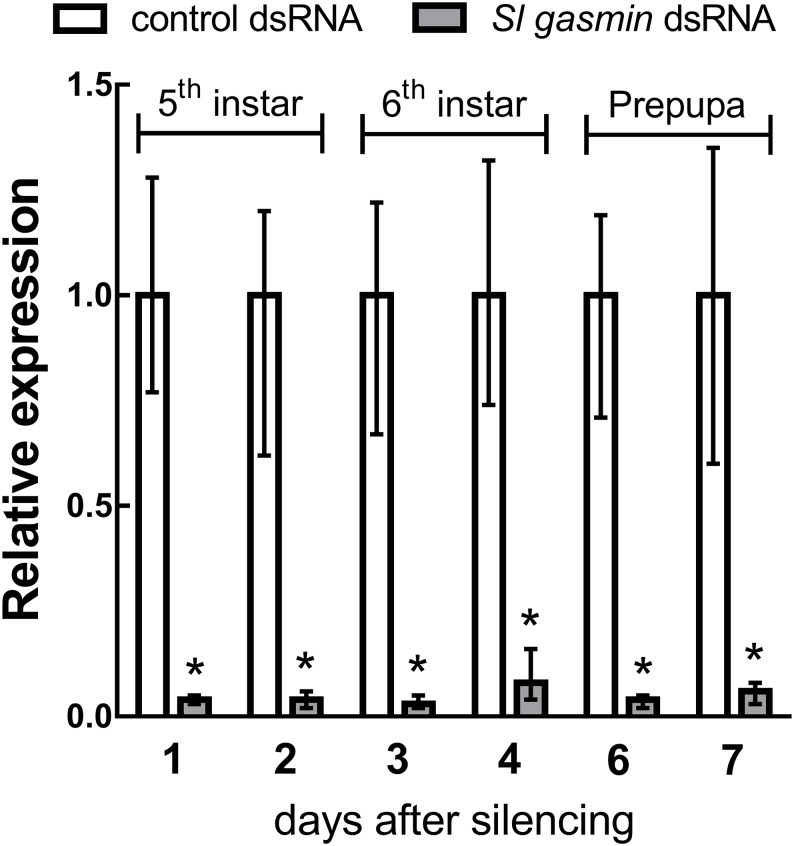
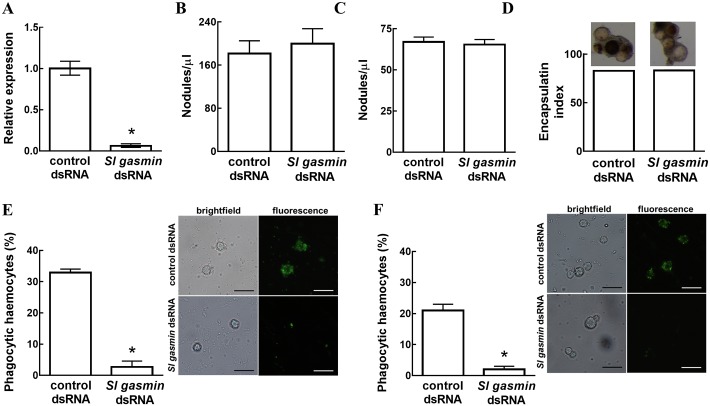
The very high transcription level in the haemocytes and its enhancement following microbial challenge strongly suggested the important role for Sl gasmin in the immune response mounted by S. littoralis larvae. To characterize Sl gasmin at the functional level, we pursued a loss-of-function strategy, through RNAi-mediated silencing, which proved to be very efficient in S. littoralis [31, 32], like in many other lepidopteran species [33–39], in spite of several cases of refractiveness to RNAi reported for species/strains belonging to this order [39]. Oral treatment of experimental larvae with Sl gasmin dsRNA was effective in silencing the target gene from the first day of the 5th instar until the prepupal stage (Student’s t test. Day 1: t = 26.838, df = 14, P<0.0001; day 2: t = 11.362, df = 14, P<0.0001; day 3: t = 15.408, df = 14, P<0.0001; day 4: t = 18.456, df = 14, P<0.0001; day 6: t = 22.903, df = 14, P<0.0001; day 7: t = 15.258, df = 14, P<0.0001) (Fig 5).
Fig 5. Sl gasmin silencing in Spodoptera littoralis larvae.
After oral administration of control or Sl gamin dsRNA during 4th instar, the expression of Sl gamin, determined by qRT-PCR was significantly down-regulated until pupation. S. littoralis β-actin was used as reporter gene. The values reported are the mean ± S.E. (*P<0.0001, Student’s t test).
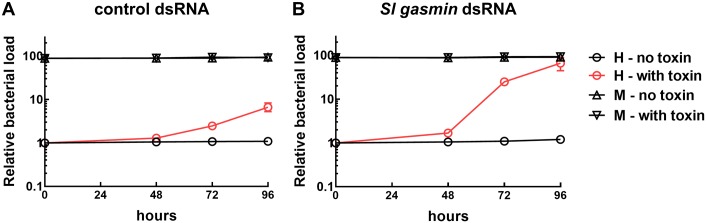
The relevance of the immune role played by Sl gasmin in vivo was assessed by scoring the impact of the silencing of its coding gene on the host septicaemia induced by Bacillus thuringiensis toxin Cry1Ca, using the experimental approach previously described to study the Bt killing mechanism [32]. Cry toxins produced by this entomopathogenic bacterium are active upon ingestion. When in the midgut, they interact with brush border membranes of epithelial cells and cause the osmotic lysis of these latter. The resulting tissue lesions allow the entrance of gut microflora into the insect haemocoel, causing septicaemia and insect death [32]. Then, we hypothesized that the reduced immunocompetence associated with Sl gasmin silencing should result in the enhancement of Cry1Ca killing activity, as a consequence of uncontrolled bacterial proliferation. Indeed, this was the case. Sl gasmin dsRNA treatment significantly enhanced the mortality of S. littoralis larvae treated with Cry1Ca toxin (Table 1), and this enhancement was associated with a significant increase of bacterial load in the haemolymph (Fig 6, see S1 Table for statistics). These data clearly support the key-role played by this gene in the modulation of the antimicrobial immune response in vivo, and nicely corroborate the proposed key-role of septicaemia in the Bt killing mechanism [32].
Table 1. Enhancement of Cry1Ca toxicity by host immunosuppression.
| Treatmenta | LC50b | TIc | |
|---|---|---|---|
| Control dsRNA | Sl gasmin dsRNA | ||
| Cry1Ca | 6.8 (4.5–10.0) | 2.7 (1.6–4.3) | 2.5 (1.2–5.2) |
aToxicity of Cry1Ca toxin was assessed on Spodoptera littoralis larvae after RNAi-mediated silencing of the immune gene Sl gasmin (Sl gasmin dsRNA), and in immune competent controls (control dsRNA).
bConcentration (μg Cry1Ca/cm2 diet) killing 50% of experimental larvae, with 90% fiducial limits reported in parentheses.
cThe toxicity increase (TI) is calculated as the ratio between LC50 values scored in control dsRNA control larvae, and in larvae treated with Sl gasmin dsRNA; 95% fiducial limits are reported in parentheses.
Fig 6. Relative quantification of bacterial load by qRT-PCR.
Relative change over time of the bacterial load in Spodoptera littoralis larvae, exposed to control dsRNA (A) or Sl gasmin dsRNA (B) and fed with artificial diet on which a solution of Bacillus thuringiensis Cry1Ca toxin (2.7 μg/cm2) was layered. The bacterial load in the haemolymph (H, red lines with empty circles) resulted significantly influenced by toxin treatment, both in control and silenced larvae, with these latter showing a much higher bacterial load increase over time (see S1 Table for statistics), whereas no significant changes were observed in the midgut (M).
Thereafter, we performed specific experiments to understand which component of the immune response was controlled by Sl gasmin. We focused on the three main cellular responses against foreign invaders that are mediated by haemocytes: nodulation, encapsulation and phagocytosis [40–41]. On the occasion of each experiment performed to assess the impact of Sl gasmin silencing on immune response, we always checked the level of gene silencing on day 1 of 5th instar larvae (t = 37.873, P<0.0001, df = 46, n = 24) (Fig 7A).
Fig 7. Cellular immune responses by Spodoptera littoralis larvae as affected by RNAi mediated silencing of the gene Sl gasmin.
The oral administration of Sl gasmin dsRNA induced a significant transcriptional down-regulation of the target gene in the haemocytes of S. littoralis (A). S. littoralis β-actin was used as reporter gene. Gene silencing did not influence nodulation of the Gram-negative bacterium Escherichia coli (B) and of the yeast Saccharomyces cerevisiae (C). Chromatography beads injected into S. littoralis larvae orally treated with Sl gasmin dsRNA were regularly encapsulated and melanized as in controls (D). Conversely, RNAi mediated silencing of Sl gasmin significantly reduced the phagocytic capacity of haemocytes against Gram-negative (E. coli) (E) and Gram-positive (Staphyloccus aureus) (F) bacteria. The values reported are the mean ± S.E. (*P<0.001, Student’s t test). Bars: 15 μm.
Gene silencing did not interfere with the nodulation response (i.e., multicellular aggregation of haemocytes that entrap a large number of microorganisms) induced against either bacteria (Fig 7B) or yeast (Fig 7C) (E. coli: t = 0.501, P = 0.6208, df = 26, n = 14; S. cervisiae: t = 0.386, P = 0.7028, df = 26, n = 14). Similarly, encapsulation (i.e., formation of a multilayered capsule of haemocytes around the non-self object) and melanization of chromatographic beads injected into experimental larvae were not affected by gene silencing (t = 0.674, P = 0.5056, df = 22 n = 14) (Fig 7D).
In contrast, phagocytosis of bacteria was strongly inhibited in experimental larvae treated with Sl gasmin dsRNA, as their haemocytes were almost completely unable to internalize either Gram-negative (E. coli) (t = 13.610, P<0.0001, df = 19, n = 11) (Fig 7E) or Gram-positive (S. aureus) bacteria (t = 10.725, P<0.0001, df = 20, n = 11), within 10 min or 30 min of incubation, respectively (Fig 7F). We focused on such a short time in order to detect any precocious enhancement of one of the immune barriers most rapidly activated by foreign invaders [41–43].
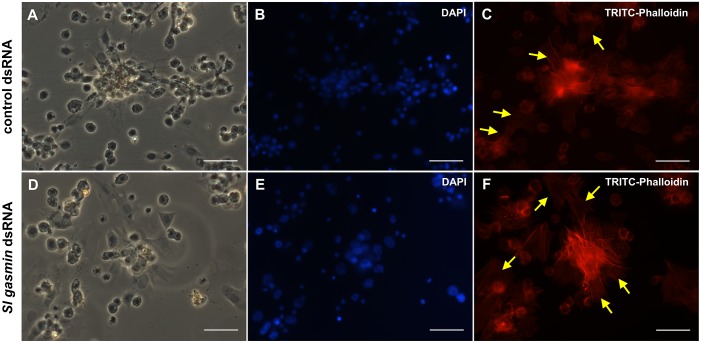
To check if the disruption of phagocytosis was due to a negative effect of gene silencing on cytoskeleton architecture and dynamics, the haemocyte spreading/adhesion and the distribution/polymerization of actin were investigated, as readouts of their immunocompetence. Haemocytes extracted from both control and silenced larvae showed identical levels of aggregation and adhesion on glass surfaces (Fig 8A and 8D), large nuclei (Fig 8B and 8E), and a similar network of polymerized actin, underlying plasma membrane protrusions, such as lamellipodia (sheet-like protruding two-dimensional lobes of the plasma membrane at the leading edge of the cell) and filopodia (protruding one-dimensional microspikes of the cell membrane) extensions (Fig 8C and 8F). These findings demonstrated that the adhesion properties of haemocytes, which strongly depend upon proper actin cytoskeletal dynamics, were completely unaffected by gene silencing.
Fig 8. In vitro behaviour and actin cytoskeleton of Spodoptera littoralis haemocytes.
Bright-field images (A, D), DAPI (nuclear DNA) (B, E) and TRITC-Phalloidin (F-actin) staining (C, F) of haemocytes extracted from S. littoralis larvae, orally treated with control (GFP dsRNA) or Sl gasmin dsRNA. Different types of haemocytes from experimental and control larvae adhered and spread on glass slides singly or in clusters (A, D), showing similar actin networks of polymerized actin; lamellipodia are denoted with yellow arrows (C, F). Bars: 30 μm.
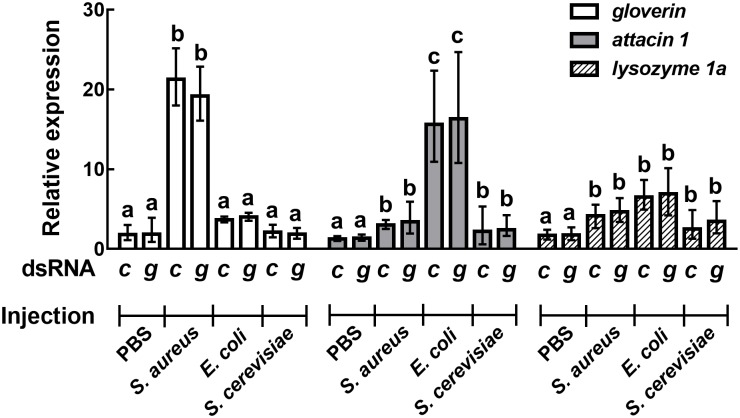
To assess whether the Sl gasmin silencing had any impact on S. littoralis humoral immune responses, we analyzed the transcription profiles of genes encoding humoral effectors secreted by haemocytes in the haemolymph, including antimicrobial peptides (AMP) and lysozyme [44, 45], following immune challenge with different microorganisms. No negative effects of Sl gasmin silencing on the ability of haemocytes to mount a humoral response were detected. Indeed an increase of transcript level of humoral effectors upon immune challenge was observed both in silenced and control larvae (Fig 9, see S1 Table for statistics).
Fig 9. Humoral immune response by Spodoptera littoralis larvae as affected by RNAi mediated silencing of the immune gene Sl gasmin.
Immune challenge with Gram-positive (Staphyloccus aureus), Gram-negative (Escherichia coli) bacteria or with the yeast Saccharomyces cerevisiae was performed on S. littoralis larvae after oral administration of control (c) or Sl gasmin (g) dsRNA. After 3 hours from the immune challenge, the transcript level of genes encoding the humoral effectors was significantly enhanced (attacin 1: F(3,126) = 68.13, P<0.0001; gloverin F(3,126) = 184.16, P<0.0001; lysozyme 1a F(3,126) = 33.53, P<0.0001), but was not influenced by gene silencing (see S1 Table for statistics). S. littoralis β-actin was used as reporter gene. The values reported are the mean ± S.E. Different letters denote significant differences between treatments, within each gene considered.
Collectively, these results indicate that Sl gasmin exerts a key-role in the modulation of phagocytosis by larval haemocytes.
Sl gasmin is an opsonizing factor
The presence of a signal peptide in Sl gasmin suggested that it is likely secreted by haemocytes and exerts its role in the haemolymph plasma. To investigate this hypothesis, the putative presence of Sl gasmin in the haemolymph plasma and its changes in response to gene silencing were assessed by liquid chromatography-tandem mass spectrometry in the multiple reaction mode (LC-MRM/MS). Specific peptides belonging to Sl gasmin sequence were selected (S2 Table) and monitored by MRM analysis of the tryptic digest of haemolymph samples, leading to the unambiguous identification of the protein.
The observed changes of Sl gasmin transcript level induced by gene silencing (One-Way ANOVA: F(4,40) = 401.6, P<0.0001, n = 9, df = 44) (Fig 10A) were perfectly mirrored by changes in the abundance of the protein in the haemolymph plasma (Kruskal-Wallis test: KW = 36.28, P<0.0001, n = 8) (Fig 10B). Moreover, the injection of E. coli into control larvae induced a steep increase of Sl gasmin transcription (Fig 10A) and of the encoded protein titer in the haemolymph plasma (Fig 10B).
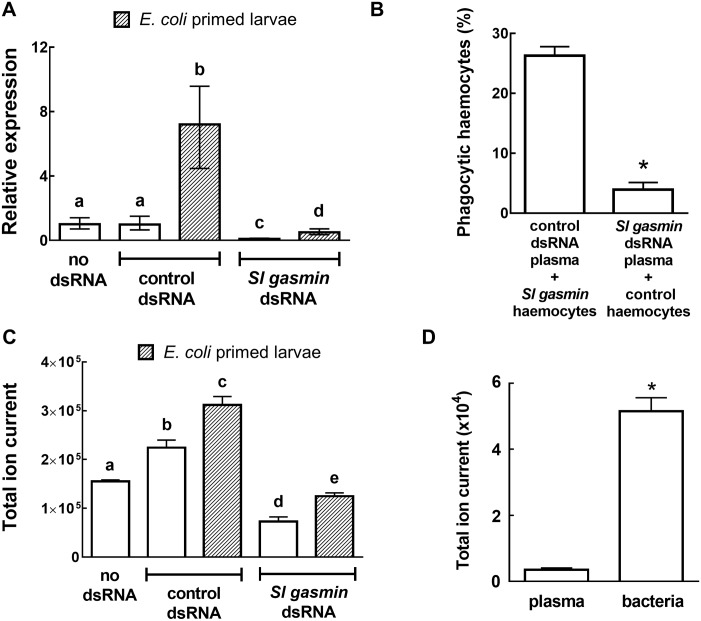
Fig 10. Phagocytosis by Spodoptera littoralis larvae as affected by the presence in the plasma of the opsonizing factor Sl gasmin.
RNAi mediated silencing of the gene Sl gasmin significantly reduced its transcription level, determined by qRT-PCR (A) and the presence of the encoded protein in the haemolymph plasma, determined by LC-MRM/MS (C), both under basal and immune challenged conditions. The presence of Sl gasmin in the incubation medium was essential to promote phagocytosis in vitro of fluorescent bacteria (Escherichia coli), as supported by the functional rescue of haemocytes unable to perform phagocytosis, obtained from Sl gasmin silenced larvae, when transferred to plasma of control larvae (B). The opsonizing role of Sl gasmin was demonstrated by LC-MRM/MS measurements of its amount recovered by proteolytic shaving of E. coli cells incubated in presence of haemolymph plasma (D). S. littoralis β-actin was used as reporter gene. The values reported are the mean ± SE. Different letters above each bar, in A and C, indicate significant differences (P<0.05, based on an One-Way ANOVA followed by Bonferroni’s test; *P<0.001, Student’s t test, in B and D).
The presence of Sl gasmin protein in the haemolymph plasma, associated with (1) the increase of its titer triggered by immune challenge, and (2) the phagocytosis failure in its absence strongly suggested a functional role of Sl gasmin in mediating pathogen recognition and subsequent phagocytosis by haemocytes. Indeed, Sl gasmin could act as an opsonization factor, i.e., a molecule that coats pathogens and mediates their recognition and suppression by immune cells.
To test this hypothesis, a rescue experiment of phagocytosis was carried out by incubating the haemocytes obtained from silenced larvae (unable to perform phagocytosis) in the plasma from control larvae: this restored phagocytosis of bacteria in vitro (t = 11.67, P<0.0001, n = 14, df = 26) (Fig 10C). In contrast, haemocytes obtained from control larvae did not show phagocytic activity in vitro when resuspended in the plasma obtained from silenced larvae, which contained much lower levels of Sl gasmin. This experiment allows to conclude that (1) Sl gasmin is an essential element required for pathogen recognition and subsequent phagocytosis by haemocytes, and (2) the haemocytes of both control and silenced larvae are fully functional.
To further clarify the role of Sl gasmin in the haemolymph, the hypothesis of a direct interaction between the protein and the pathogen was investigated by a proteolytic shaving approach coupled with LC-MRM/MS, which has previously been successfully used to identify host proteins interacting with invading bacteria [46]. For this purpose, intact E. coli cells were incubated with the plasma of immune primed S. littoralis larvae, which contain high levels of Sl gasmin. After incubation, the bacterial surface was proteolytically shaved to release putative Sl gasmin peptides, demonstrating the occurrence of a direct interaction of the protein with bacterial cells. The released peptide mixtures were then analysed by LC-MRM/MS, monitoring specific peptides selected from Sl gasmin sequence (S2 Table). The level of Sl gasmin adhering to E. coli cells was substantially and significantly higher (t = 7.969, P<0.0001, n = 5, df = 7) than that recorded in control plasma used for incubating the bacteria (Fig 10D). Hence, Sl gasmin present in the haemolymph plasma sticks on the surface of invading bacteria and is essential to mediate bacteria recognition and subsequent phagocytosis reaction by haemocytes. Bacteria incubated with buffer allowed the exclusion of false positive results.
Discussion
A homologue of the bracoviral gene BV2-5 was recently found in the genome of S. exigua (named gasmin) [25]. Here we have found an additional homologue of BV2-5 in the genome of S. littoralis (Sl gasmin), and observed that is highly expressed in larval stages exposed to an immune challenge. The results of the present study, based on complementary functional and molecular evidence, unequivocally demonstrate that this gene encodes an opsonizing factor triggering a fast phagocytic response. As already suggested [25], gasmin has been likely acquired by a basal ancestor of Spodoptera genus and maintained in distant species which separated about 24 MYA (S. littoralis and S. exigua) [47]. This suggests that there is a strong positive selection favoring the fixation of this gene, which, however, cannot be found in some related species (S. frugiperda), where the gene has been lost during evolution [25]. The important immune function of this gene, acquired through a mechanism of HGT mediated by a bracovirus, which acts as a unique gene vector [6, 25], reinforces the defense toolkit of S. littoralis. The integrative properties of BV have likely allowed the horizontal transfer of this viral gene. Indeed, parasitism on non-host larvae escaping death and accidental integration of BV sequences into the germ line allowed the stable integration into the moth genome and its transfer over generations [25]. This is the likely scenario in which several bracovirus genes have been acquired by the monarch butterfly (Danaus plexippus), the silkworm (Bombyx mori), the beet armyworm (S. exigua) and the fall armyworm (S. frugiperda), even though an adaptive advantage for these recipient insects has not been unequivocally demonstrated [25, 30]. To broaden the survey of gasmin-like genes present in insects and bracoviruses, we retrieved all candidate sequences available in the public databases, which showed high similarity to Sl gasmin (in total 17). These genes show great variability both in bracoviruses and insects, and the phylogenetic analyses fail to conclusively resolve any relationships (particularly for the S. litura homologue). The topology of the recovered phylogenetic tree might be the result of HGT and gene loss events, or other large modifications which led to a rapid evolution of the gene under positive selection, as expected for transferred genes response to new genetic and environmental contexts [10]. The gasmin genes are no exceptions, as most of the retrieved sequences have largely diverged. However, the striking similarity and conservation of the genomic and protein sequences present in S. littoralis and S. exigua strongly suggest a common origin. Further sampling of virus and insect gasmin homologues would be required to conclusively resolve this issue.
Collectively, the experimental evidence we present on Sl gasmin indicates that its transcription in the haemocytes is rapidly boosted by immune challenge, and the encoded protein is an opsonizing factor. Once in the haemolymph, Sl gasmin provides a better protection by mediating a fast phagocytic reaction by the haemocytes, which is abolished by RNAi-mediated gene silencing. However, this evidence raises the question about the role of the viral homologue, which is nearly identical and likely functionally equivalent. We may reasonably speculate that in the case of parasitized host larvae, where the BV genes are actively expressed, the viral homologue of Sl gasmin may limit the risk of accidental bacterial infection, which would be detrimental both for the host and the developing parasitoid progeny. Indeed, 24 h after oviposition by C. congregata, the viral gene BV2-5 is highly expressed in the fat body and in the haemocytes of parasitized Manduca sexta larvae, where, concurrently, antimicrobial peptides are up-regulated, while genes involved in the phenol oxidase cascade and cellular immune responses are strongly down-regulated [48, 49]. Then, our results and the evidence available in the literature both point out that the immunosuppression induced by the wasp is selective, and provides protection to its juveniles by disabling only the responses effective against large intruders, leaving unaltered or even potentiating the antimicrobial barriers, in order to prevent dangerous secondary infections by pathogens. This could have been one of the functional constraints driving the evolutionary pathway of integration of this gene in the BV genome. Indeed, reinforcing the clearing capacity of the host haemolymph by enhancing the phagocytic activity appears to be an effective strategy when, concurrently, other barriers are very rapidly disrupted by maternal secretions injected at the oviposition by the parasitic wasp. This is an interesting hypothesis worth of further research efforts. The effective importance in vivo of this mechanism of haemolymph clearance is supported by the reduced capacity to withstand Bt-induced septicaemia we observed in S. littoralis larvae exposed to RNAi-mediated silencing of Sl gasmin. This implies that the acquired opsonizing factor is important in the modulation of phagocytosis efficiency in vivo.
Phagocytosis is one of the first, most rapid and effective barriers of protection against microorganisms. Indeed, haemocytes immediately start their phagocytic activity in response to intrusion of pathogens into the haemolymph, and each cell is able to engulf hundreds of bacteria [41]. Phagocytosis initiation can be either direct, via specific haemocyte-surface receptors, or indirect, via opsonins that label pathogens, and thus make them recognizable by haemocyte-surface phagocytic receptors [40, 41, 50]. Opsonization factors in insects are still poorly studied [51–53]. In Diptera, opsonization-dependent phagocytosis is mainly mediated by thioester-containing proteins (TEP), a group of proteins that includes the α2-macroglobulins and complement factors that mediate phagocytosis in vertebrates, which have been reported both for Anopheles and Drosophila species [50–52, 54, 55]. TEP are proteins that specifically promote the phagocytosis of different sets of pathogens [52]. In Lepidoptera, several opsonins secreted by different tissues have been described [53, 56–58] and, in all cases, opsonization factors showed the capacity to bind a broad range of microbes, such as both Gram-positive and Gram-negative bacteria, and yeasts. Here we present evidence that Sl gasmin is an additional opsonization factor present in Lepidoptera, which has been transferred through a BV associated with a parasitic wasp attacking a non-permissive ancestor of Spodoptera species. We speculate that this passage of Sl gasmin confers effective and fast protection to the wasp eggs and juveniles soon after parasitization. This is relevant from a functional point of view, as the insertion of the ovipositor into the host body can be a route of infection, besides any other risk of accidental infection. A future comparative analysis of opsonizing factors active in the immune response by Spodoptera species lacking gasmin-like factors will likely shed light on the adaptive mechanisms that have favoured the fixation of this gene during evolution. We can reasonably speculate that the addition of gasmin to the array of pre-existing opsonizing factors results in a faster reaction and a broader capacity to engulf different types of microorganisms. These are likely possibilities worth of further research efforts.
The contrasting functional evidence provided for S. exigua gasmin, generated, as already said above, by a biased experimental approach [25, 30], is problematic to interpret both from a mechanistic and evolutionary point of view. It is not easy to imagine that a protein triggering cytoskeleton disruption associated with reduced viral infection does not affect a number of other functions, with possible trades-off counteracting the fixation of its coding gene. This seems to be the case, as the proposed putative benefit of protection against viruses brings in reduced protection against other pathogens; indeed, haemocytes from an European strain of S. exigua, which do not produce functional gasmin, as it bears a truncated gasmin gene, fails to engulf bacteria by phagocytosis when infected with the recombinant baculovirus expressing gasmin [30]. This does not fit with what we have observed in the present study and it is difficult to imagine that we have so different functions for proteins sharing a very high level of sequence identity. While we performed a functional analysis by RNAi mediated gene silencing in vivo, for S. exigua gasmin a recombinant baculovirus was used for in vitro functional studies in an insect population that does not have genes encoding gasmin. This could have generated uncontrolled phenotypic responses, partly due to the concurrent effect of viral infection and the overexpression of a heterologous protein, that we do not even know if it is secreted outside of the infected cells [25, 30]. Moreover, data derived from these in vitro studies are somewhat difficult to interpret from an in vivo functional perspective. In particular, it is not easy to justify why gasmin, considered as an immune disrupter, is up-regulated in response to an immune challenge [30]; conversely, the results we present here are highly consistent with the proper immune role of Sl gasmin.
In conclusion, our results demonstrate that in insects an immune function has been reinforced by HGT of a viral gene. The route of acquisition of the immune gene Sl gasmin, which is mediated by a viral symbiont of a parasitoid wasp attacking moth larvae, sheds light on a completely novel evolutionary pathway [6, 25]. The integrative properties of BV, which are powerful natural genetic engineers, pave the way for the transfer of sequences among completely different evolutionary lineages. In the case of immune genes, these may undergo intense selection in the context of the complex immune interplay among parasitic wasps harbouring the BV, their hosts and associated microbiota, eventually favouring the most efficient traits conferring protection to the system. The “jump” of this genetic material in moth species generates the addition of a very effective function, paradoxically obtained with the help of a parasitoid. Then our study demonstrates that, in multicellular organisms, essential physiological functions can be acquired and/or shaped by HGT and do not always derive from evolution of pre-existing genes.
Materials and methods
Insects
S. littoralis larvae were reared on artificial diet (47.3 g/l wheat germ, 67.3 g/l brewer’s yeast, 189 g/l corn meal, 6.8 g/l ascorbic acid, 0.75 g/l cholesterol, 0.5 g/l propyl 4-hydroxybenzoate, 3 g/l methyl 4-hydroxybenzoate, 1.3 g/l wheat germ oil, 33.8 g/l agar and 3 g/l vitamin mix (1.2 g/Kg vitamin B1, 2.6 g/Kg vitamin B2, 2.5 g/Kg vitamin B6, 40 g/Kg choline, 10 g/Kg pantothenic acid, 32 g/Kg inositol, 0.25 g/Kg biotin, 2.5 g/Kg folic acid, 5 g/Kg 4-aminobenzoic acid, 0.5 mg/Kg vitamin B12, 10 g/Kg glutathione, 2.1 g/Kg vitamin A, 0.25 g/Kg vitamin D3, 24 g/Kg vitamin E, 0.25 g/Kg vitamin K, 25 g/Kg vitamin C in dextrose)), at 25 ± 1°C, 70 ± 5% R.H., and under a 16:8 h light/dark period.
Tissue sample collection and RNA/DNA extraction
S. littoralis larvae were anaesthetized on ice and surface-sterilized with 70% ethanol prior to dissection. Larval haemolymph was collected from a cut of the leg and haemocytes were separated from plasma by centrifugation for 5 min, at 500 × g, at 4°C. Midgut and fat body were isolated after cutting the larval body lengthwise, and the remaining body carcass separately collected. These samples (i.e., haemocytes, midgut, fat body and carcass) were immediately transferred into TRIzol reagent (Life Technologies, Carlsbad, CA, USA) and kept at -80°C until total RNA extraction, that was performed according to manufacturer’s instructions. DNA was extracted from haemocytes using the protocol described elsewhere [59], with minor modifications. The concentration of extracted RNA or DNA was assessed by measuring the absorbance at 260 nm, with a Varioskan Flash Multimode Reader (Thermo Scientific, Waltham, MA, USA), and sample purity was evaluated by assessing 260/280 nm absorbance ratio. RNA quality was checked by electrophoresis on 1% agarose gel.
Sl gasmin cloning and bioinformatics analysis
A partial Sl gasmin cDNA (FQ973054.1) was identified by BLAST analysis [60, 61] in a public database of expressed sequence tags (EST) from S. littoralis female antenna, using as query the full length cDNA sequence of S. exigua gasmin (KP406767.1).
To isolate Sl gasmin ORF, total RNA extracted from haemocytes of S. littoralis 6th instar larvae was subjected to retro-transcription (Ambion RETROscript® kit—Life Technologies). Given the very high level of sequence identity to gasmin (Fig 1), a cDNA was obtained by PCR, using Phusion High-Fidelity DNA Polymerase (Fisher Scientific, Pittsburgh, PA, USA) and primers designed to amplify the whole gasmin ORF (gasmin ORF forward primer ATGTTGCCTATTACCATACTAACG, gasmin ORF reverse primer ATACTGGAATTGGACATATTTGAGC). PCR conditions were programmed for 30 s at 98°C; 40 cycles of [10 s 98°C, 30 s 60°C, 1 min 72°C] and 15 min at 72°C. After amplification, the obtained PCR product was separated by gel electrophoresis and the visible band of the expected size was purified with a Quick gel extraction & PCR purification COMBO Kit (Invitrogen, Carlsbad, CA, USA). The PCR product was cloned into Zero-Blunt TOPO vector (Zero-Blunt TOPO PCR Cloning Kit, Invitrogen), according to the manufacturer’s instructions. After transformation of One Shot TOP10 chemically competent E. coli cells (Invitrogen), the transformants were incubated overnight at 37°C on LB plates containing 50 μg/ml kanamycin. Bacterial colonies containing the fragment of the appropriate size were selected by colony PCR, using Phusion High-Fidelity DNA Polymerase (Fisher Scientific) and M13 Forward (-20)/M13 reverse primers (Invitrogen), and grown overnight in LB medium containing 50 μg/ml kanamycin. The plasmid DNA was extracted from 4 ml of bacterial culture using a Charge Switch-Pro plasmid miniprep Kit (Invitrogen), as instructed by the manufacturer and sequenced.
The presence of an intron into Sl gasmin sequence was determined by PCR amplification of the total DNA extracted from S. littoralis haemocytes using Phusion High-Fidelity DNA Polymerase (Thermo Fischer Scientific) with specific primers (gasmin ORF forward primer ATGTTGCCTATTACCATACTAACG; gasmin intron reverse primer CAGGTGTCCGCATTCCACTGA). The length of PCR products was checked by electrophoresis on 1% agarose gels, before sequencing.
Extensive similarity searches of complete and high-throughput genome sequence databases hosted by NCBI using TBLASTN, as well as of non-redundant protein databases (BLASTP), using the inferred S. exigua and S. littoralis protein sequences, allowed the identification of numerous potential homologues. These were manually annotated to identify probable coding and intronic regions. Preliminary alignments of inferred amino acid sequences (351 aa) were performed using Muscle [62] and manually filtered to identify homologues, which could be aligned essentially contiguously with the Spodoptera protein sequences. Alignments were manually refined, and ambiguously aligned regions were excluded using the program GBlocks [63], leaving XX amino acid positions for phylogenetic reconstruction using PhyML [64] as implemented in the program SEAVIEW [65], under the JTT amino acid substitution matrix, with 4 gamma distributed substitution rate categories (see S2 File). Bootstrap proportions were estimated using parameters optimized on the original ML tree (rate distribution parameter (alpha) = 1.45).
To identify signatures of selection the FEL (Fixed Effects Likelihood) method was used [66], implemented at the DataMonkey website (http://datamonkey.org/) [67]. Nucleotide sequences of S. exigua, S. littoralis and C. congregata virus circle 25, including the intron, were aligned and the alignment was manually curated to maintain in-frame codon alignment. Selection was tested in the S. exigua—S. littoralis branches.
Sl gasmin expression analysis by qRT-PCR
Total RNA used for transcriptional analysis was isolated as described above. Relative expression of studied genes was measured by one-step qRT-PCR, using the SYBR Green PCR Kit (Applied Biosystems, Carlsbad, CA, USA), according to the manufacturer’s instructions. S. littoralis β-actin gene (Z46873) was used as endogenous control for RNA loading. Primer Express 1.0 software (Applied Biosystems) was used to design the primers used (S3 Table). Relative gene expression data were analyzed using the ΔΔCt method [68–70]. qRT-PCR for measurement of Sl gasmin expression was carried out using specific primers (S3 Table), designed to detect a region of Sl gasmin mRNA not included in the sequence targeted by the dsRNA (see “dsRNA synthesis” paragraph). For validation of the ΔΔCt method, the difference between the Ct value of Sl gasmin and the Ct value of β-actin transcripts [ΔCt = Ct (Sl gasmin)-Ct (β-actin)] was plotted versus the log of ten-fold serial dilutions (2000, 200, 20, 2 and 0.2 ng) of the purified RNA samples. The plot of log total RNA input versus ΔCt displayed a slope less than 0.1 (Y = 1.149+0.0133X, R2 = 0.0493), indicating that the efficiencies of the two amplicons were approximately equal.
Expression profiles of Sl gasmin in response to different pathogens
To analyze Sl gasmin expression in response to microbial challenge, S. littoralis 5th instar larvae, surface-sterilized with 70% ethanol and chilled on ice, received an intra-haemocoelic injection of 2 × 107 E. coli, 3 × 108 S. aureus or 2 × 107 S. cerevisiae cells, suspended in 5 μl of PBS (Phosphate Buffered Saline: 137 mM NaCl, 2.7 mM KCl, 10 mM phosphate buffer, pH 7.4). Injections were performed through the neck membrane, using a Hamilton Microliter 1701RN syringe (10 μl, gauge 26s, length 51 mm, needle 3). At the injection and at different time points after injection, experimental larvae (n = 12 for each experimental treatment) were dissected and haemocytes were collected and processed for total RNA extraction as described above; the relative expression of Sl gasmin was assessed by qRT-PCR.
dsRNA synthesis
Total RNA extracted from haemocytes of S. littoralis 6th instar larvae was retro-transcribed (Ambion RETROscript kit, Life Technologies) and a 789 bp long Sl gasmin cDNA fragment was obtained by PCR, using the Sl gasmin dsRNA forward primer (GCCGGCATGTTGTCTATTACC) in combination with the Sl gasmin dsRNA reverse primer (TCCTTCCAGCTTCTGAGTCA). This cDNA fragment was used as template for a nested-PCR reaction, performed with primers containing at their 5’ ends the T7 polymerase promoter sequence (T7-Sl gasmin forward TAATACGACTCACTATAGGGAG-TTCGAGGATACAAGCAGAG; T7-Sl gasmin reverse TAATACGACTCACTATAGGGAG-GGATGCTCAGGATATCTGTTAC). The resulting PCR product was used as template to synthesize Sl gasmin dsRNA (522 bp long), using the Ambion MEGAscript RNAi Kit (Life Technologies), according to the manufacturer’s instructions. Control dsRNA, 500 bp long, was obtained from a control template supplied by the kit used. dsRNA preparations were quantified by measuring their absorbance at 260 nm with a Varioskan Flash Multimode Reader, and purity was evaluated by assessing 260/280 nm absorbance ratios. Products were run on 1% agarose gels to confirm their integrity.
Administration of dsRNA to S. littoralis larvae and silencing of Sl gasmin
S. littoralis 4th instar larvae (1st day) were anaesthetized on ice and 1 μl of Sl gasmin dsRNA or control dsRNA (see above), dissolved in PBS, was poured into the lumen of the foregut by means of a Hamilton Microliter 1701RN syringe (10 μl, gauge 26s, length 51 mm, needle 2). dsRNA treatments consisted of one oral administration of 150 ng per day, for 3 days (from 4th to 5th instar). After the last dsRNA administration and prior to any experiment, haemocytes from 3–4 treated larvae were used for qRT-PCR analysis, to confirm the occurrence of gene silencing.
Cry1Ca bioassay and assessment of bacterial load
Purified Cry1Ca protein was produced in a recombinant B. thuringiensis strain EG1081 (Ecogen Inc.). Prior to use, Cry1Ca was dialyzed overnight, at 4°C in 50 mM sodium carbonate buffer, pH 9.0. After dialysis, toxin concentration was determined by the Bradford assay [71], using bovine serum albumin as standard.
Silenced and control larvae were singly isolated in multi-well plastic trays (Bio-Ba-32, Color-Dec, Italy), containing artificial diet, covered with perforated plastic lids (Bio-Cv-4, Color-Dec), and maintained under the rearing conditions reported above. For the first 3 days, the upper surface (1 cm2) of the artificial diet (0.3 cm3) was uniformly overlaid with 50 μl of purified Cry1Ca toxin, dissolved in 50 mM sodium carbonate buffer at pH 9.0. Control larvae were reared on artificial diet overlaid with 50 μl sodium carbonate buffer. Experimental larvae were maintained on artificial diet, replaced every 24 h, and daily inspected for survival, until pupation. To determine the 50% lethal concentration (LC50) of Cry1Ca toxin, the bioassay was carried out at 5 different concentrations of toxin and using 16 larvae for each experimental condition and control. Probit analysis [72], to determine LC50 values at day 10, 90% fiducial limits and toxicity increase ratio (TI) for each experimental treatment, was performed with the POLO-PC program (LeOra Software, Berkeley, CA).
The assessment of the bacterial loads was performed as previously described [32]. Briefly, 6 h after the last Sl gasmin or GFP dsRNA administration, newly molted 5th instar larvae of S. littoralis, were exposed for 3 days to 2.7 μg/cm2 (corresponding to the LC50 of Cry1Ca in Sl gasmin silenced larvae). Both experimental groups included internal controls maintained on a toxin-free diet. On day 7, larvae were transferred to an untreated diet and, 24 h later, the midgut and haemolymph were separately collected under a horizontal laminar flow hood as described above. Experimental samples were obtained by pooling 10 larvae. The experiment was repeated 3 times. Changes over time in the relative bacterial load in the midgut (n = 7 for each sampling point) and haemolymph (n = 8 for each sampling point) samples were determined by qRT-PCR, measuring the transcript level of 16S rRNA (AJ567606.1) to assess the impact of Bt toxin and Sl gasmin silencing on bacterial proliferation. The qRT-PCR was performed as described above, using S. littoralis β-actin as reporter gene (primers used are reported in S3 Table).
Cellular and humoral immune assays
The impact of gene silencing on cellular immune responses was assessed by scoring its effect on encapsulation, nodulation and phagocytosis. Encapsulation and nodulation responses were assessed as previously described [31, 32]. CM Sepharose fast flow chromatography beads (Pharmacia), suspended in PBS, were injected into the haemocoel of S. littoralis larvae using a Hamilton Microliter 1702 RN syringe (25 μl, gauge 22s, length 55 mm, needle 3). After 24 h, beads were recovered upon larval dissection and scored to evaluate their encapsulation rate, which was expressed with the encapsulation index (E.I. = [Σ (encapsulation degree × total beads of this degree)/ total beads × 4] × 100), that takes into account both the encapsulation degree of each recovered bead (0—no cells adherent to the beads, 1—up to 10 adherent cells, 2—more than 10 adherent cells but no complete layer around the bead, 3—one or more complete layers without melanization, 4—one or more complete layers with melanization) and the relative abundance of beads with a given encapsulation degree [73]. For the nodulation assay, 12 h after the last dsRNA administration, S. littoralis larvae, surface-sterilized with 70% ethanol and chilled on ice, received an intra-haemocoelic injection of 5 μl of a PBS suspension of 2 × 106 E. coli cells, or 2 × 107 S. cerevisiae cells. Injections were performed through the neck membrane, using a Hamilton 1701 RN SYR (10 μl, 26s gauge, 55 mm long, point style 3). A thoracic leg was cut 18 h after injection, and the exuding haemolymph was collected and immediately diluted into an equal volume of ice-cold MEAD anticoagulant buffer (98 mM NaOH, 145 mM NaCl, 17 mM EDTA, and 41 mM citric acid, pH 4.5). The haemocyte nodules occurring in the haemolymph samples were counted under a light transmitted microscope at 400× magnification (Axioskop 20, Carl Zeiss Microscopy, Germany), using a Bürker chamber. When an intense immune response gave rise to large aggregates of nodules difficult to count separately, the number of distinct nodules observed was arbitrarily doubled, because the percentage of nonwhite pixels measured (ZEN software; Carl Zeiss Microscopy) on the large aggregates was on average twice that measured on a bright-microscopy field containing discrete nodules and free haemocytes.
To measure phagocytosis competence of S. littoralis haemocytes, an in vitro assay was performed as described in [32] with minor modifications. Briefly, haemolymph samples were collected from a cut of the leg into ice-cold PBS (1:1 v/v) and added with an equal volume of a PBS suspension of 2 × 106 fluorescein conjugated E. coli cells (K-12 strain BioParticles, fluorescein conjugate, Invitrogen) or 2 × 107 S. aureus (Wood strain, BioParticles fluorescein conjugate, Invitrogen). After incubation with E. coli (10 min) or with S. aureus (30 min), samples were loaded into a Bürker chamber, where total and fluorescent haemocytes were counted under a fluorescence microscope (Axioskop 20). Prior starting incubation experiments, vital staining with trypan blue was used to routinely check the viability of collected haemocytes. A haemolymph aliquot was mixed with 0.4% (w/v) trypan blue (2:1 v/v), prior to count viable and dead cells under a light transmitted microscope (Axioskop 20), using a Bürker chamber. Haemocyte samples with a viability rate lower than 98% were discarded.
For rescue experiments with haemocytes from silenced larvae, haemolymph samples were extracted from S. littoralis larvae chilled on ice, 24 h after the last dsRNA administration (Sl gasmin dsRNA or control dsRNA). Samples were centrifuged 5 min at 500 × g, at 4°C. The plasma was kept on ice, haemocytes were resuspended in PBS and centrifuged as previously described. PBS was then removed and haemocytes from larvae treated with Sl gasmin dsRNA were resuspended in the plasma isolated from larvae treated with control dsRNA, while haemocytes from larvae treated with control dsRNA were resuspended in the plasma isolated from larvae treated with Sl gasmin dsRNA. Then, the phagocytosis by haemocytes was evaluated as described above.
The humoral immune response, as affected by gene silencing, was assessed by measuring the transcript level of genes encoding antimicrobial peptides and lysozyme, in response to injections of different microorganisms, as previously described [32]. Briefly, 6 h after the last dsRNA administration, S. littoralis larvae, surface-sterilized with 70% ethanol and chilled on ice, received an intra-haemocoelic injection of 2 × 107 E. coli or S. aureus cells, or 3 × 108 S. cerevisiae cells, suspended in 5 μl of PBS. Injections were performed through the neck membrane with a Hamilton 1701 RN SYR (10 μl, gauge 26s, length 55 mm, needle 3). At the time of injection and 18 h after injection, larvae (n = 8 for each experimental sample) were dissected and haemocytes, midgut, and fat body were collected and processed for total RNA extraction, as described above. The relative expression of attacin 1 (FQ971100.1), gloverin (FQ965511.1), and lysozyme 1a (FQ961692.1) were thus assessed by q-RT-PCR as described above. Primers used are reported in S3 Table.
Detection of actin filaments in haemocytes
Newly moulted 5th instar larvae of S. littoralis, treated with Sl gasmin dsRNA or control dsRNA, as described above, were surface-sterilized with 70% ethanol and chilled on ice. Larval haemolymph from individual larvae was collected from a cut of the leg and placed on glass slides for 10 min, to allow the haemocytes to settle and attach to the glass. Haemolymph was then carefully removed and haemocytes rinsed 3 times with PBS. Attached cells were fixed for 10 min in 4% paraformaldehyde in PBS, washed 3 times in PBS and permeabilized for 4 min with 0.1% Triton-X100 in PBS. Haemocytes were washed 3 times in PBS and then incubated for 20 min with 4 μg/ml TRITC-phalloidin (Tetramethylrhodamine B isothiocyanate-phalloidin). After 3 rinses in PBS, the samples were mounted in Vectashield Mounting Medium with DAPI (Vector Laboratories) and examined under a fluorescence microscope (ZEISS Axiophot 2 epifluorescence microscope). The observations have been performed in 3 different experiments and considering at least 10 randomly selected microscopic fields for each experimental condition.
Multiple reaction monitoring (MRM) targeted proteomic approach
To detect the presence of Sl gasmin in the plasma, haemolymph was centrifuged as described above to remove the haemocytes, and the supernatant (plasma) was stored at -80°C. Samples were then dissolved in denaturant buffer (6 M urea, 10 mM EDTA, 300 mM Tris, pH 8.0) containing dithiothreitol (10-fold molar excess on the Cys residues) at 37 °C for 2 h, before the addition of iodoacetamide (IAM) to perform carboamidomethylation, using 5-fold molar excess of alkylating agent on thiol residues. The mixture was incubated in the dark at room temperature for 30 minutes and the product was purified by Chloroform/Methanol/H2O precipitation. Supernatants were removed and the pellets were dried. Digestion of the protein mixture was carried out in 10 mM ammonium bicarbonate (AMBIC), using trypsin at a 50:1 protein:enzyme mass ratio. The samples were incubated at 37°C for 16 h and dried after acidification (10% HCOOH in water). To eliminate any impurity, samples were suspended in 200 μl of 100 mM AMBIC, filtrated by centrifugal filter units (0.22 μm) and dried in a speed-vac concentrator. Samples were evaporated and suspended in 50 μl of 0.1% HCOOH in water. Peptide mixtures were analyzed by LC-MRM/MS analysis using a Xevo TQ-S (Waters, Milford, MA, USA) with an IonKey UPLC Microflow Source coupled to an UPLC Acquity System (Waters), using an IonKey device. For each run, 1 μl peptide mixture was separated on a TS3 1.0 mm × 150 mm analytical RP column (Waters) at 60°C, with a flow rate of 3 μl/min using 0.1% HCOOH in water (LC-MS grade) as eluent A, and 0.1% HCOOH in acetonitrile as eluent B. Peptides were eluted (starting 1 min after injection) with a linear gradient of eluent B in A, from 7% to 95% in 55 min. The column was re-equilibrated at initial conditions for 4 min. The MRM mass spectrometric analyses were performed in positive ion mode using a MRM detection window of 0.5–1.6 min per peptide; the duty cycle was set to automatic and dwell times were minimal 5 ms. Cone voltage was set to 35V. The selected transitions and the collision energy for each Sl gasmin peptide are reported in S2 Table.
To determine whether Sl gasmin is able to bind to the surface of bacteria, plasma samples obtained from S. littoralis 5th instar larvae (n = 20) were added to an equal volume of MEAD and incubated with an equal volume of E. coli suspension in PBS (4 × 106 cells for each μl of haemolymph) for 1 h. The suspension was then centrifuged for 10 min, at 12,000 × g, at 4°C and the pellet resuspended in 2 ml of 10 mM phosphate buffer, 45 mM NaCl, pH 7.4. Centrifugation and resuspension were repeated and the bacterial pellet as well as supernatants were frozen in liquid nitrogen and stored at -80°C until use. In control experiments bacteria were incubated with PBS and MEAD (1:1:1 v/v/v). Samples were submitted to reduction, alkylation and tryptic digestion as described above. After the preparation step they were processed and analyzed by LC-MRM/MS, as previously described. LC-MRM/MS analyses were performed on 3 technical replicates for each biological replicate and the average of these multiple measurements was used for data analysis. The data obtained represent the average value of total ion current associated to each transitions for the selected peptides.
Reagents
Unless differently indicated, all reagents were provided by Sigma-Aldrich, Italy.
Statistical analysis
Data were analyzed using Prism (GraphPad Software Inc. version 6.0b, San Diego, CA, USA) and SPSS (IBM SPSS Statistics, Version 21, Armonk, NY) software. The comparison between 2 experimental groups was done using the unpaired Student’s t test, while in the case of more than 2 experimental groups, One-Way ANOVA. Two-Way ANOVA was carried out on AMP and lysozyme 1A immune induction experiments, with RNAi treatment and bacterial injection as factors, while a Three-Way ANOVA was carried out for bacteria relative quantification, with dsRNA treatment, time and Cry1Ca toxin exposure as factors. When necessary transformation of data was carried out, to meet the assumption of normality. Levene’s test was carried out to test the homogeneity of variance. When significant effects were observed (P value<0.05), Bonferroni’s post-hoc test was used. When one of the assumptions was not met, even after the transformation of the data, Kruskal-Wallis one-way ANOVA (non-parametric ANOVA) test was employed.
Supporting information
Statistical analysis performed on the relative quantification of bacterial load by qRT-PCR and the humoral immune response by Spodoptera littoralis larvae as affected by RNAi.
(DOCX)
Specific tryptic peptides from Sl gasmin sequence were selected for LC-MS/MS analyses in MRM mode. Individual transitions from the parent ions to the most intense fragments and the corresponding collision energies are reported.
(DOCX)
Sequences of the primers used for qRT-PCR analyses (F: Forward, R: Reverse).
(DOCX)
Aligned potential homologue protein sequences retrieved form extensive similarity searches of complete and high-throughput genome sequence databases hosted by NCBI.
(TXT)
Log of the FEL analysis aimed at identifying selective pressure on Spodoptera littoralis and Spodoptera exigua sequences. The program uses a maximum-likelihood approach to infer nonsynoymous (dN) and synonymous (dS) substitution rates on a per-site basis for a given coding alignment and corresponding phylogeny.
(TXT)
Acknowledgments
We thank Michael Strand (University of Georgia, Athens, GA, USA), Angharad Gatehouse (University of Newcastle, UK), Salvador Herrero (University of Valencia, Spain) and Jean-Michel Drezen (IRBI, CNRS-University of Tours, France) for their comments on the manuscript. Purified Cry1Ca protein was kindly donated by Salvador Herrero (University of Valencia, Spain).
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by the Ministero dell’Istruzione, dell’Università e della Ricerca, Futuro in Ricerca 2013 (RBFR13PMT1) (to SC) and by the European Union’s Horizon 2020 research and innovation programme, under grant agreement no. 773554 (EcoStack) (to FP). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gogarten JP, Townsend JP. Horizontal gene transfer, genome innovation and evolution. Nat Rev Microbiol. 2005;3:679–687. 10.1038/nrmicro1204 [DOI] [PubMed] [Google Scholar]
- 2.Jain R, Rivera MC, Lake JA. Horizontal gene transfer among genomes: the complexity hypothesis. Proc Natl Acad Sci U S A. 1999;96:3801–3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi S-Y, Cai X-H, Ding D-f. Identification and Categorization of Horizontally Transferred Genes in Prokaryotic Genomes. Acta Biochim Biophys Sin. 2005;37:561–566. [DOI] [PubMed] [Google Scholar]
- 4.Dunning Hotopp JC. Horizontal gene transfer between bacteria and animals. Trends Genet. 2011;27: 157–163. 10.1016/j.tig.2011.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crisp A., Boschetti C, Perry M, Tunnacliffe A, Micklem G. Expression of multiple horizontally acquired genes is a hallmark of both vertebrate and invertebrate genomes. Genome Biol. 2015;16:50 10.1186/s13059-015-0607-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drezen J-M, Gauthier J, Josse T, Bézier A, Herniou E, Huguet E. Foreign DNA acquisition by invertebrate genomes. J Invertebr Pathol. 2017;147:157–168. 10.1016/j.jip.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 7.Peccoud J, Loiseau V, Cordaux R, Gilbert C. Massive horizontal transfer of transposable elements in insects. Proc Natl Acad Sci U S A. 2017;114:4721–4726. 10.1073/pnas.1621178114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sieber KB, Bromley RE, Dunning Hotopp JC. Lateral gene transfer between prokaryotes and eukaryotes. Exp Cell Res. 2017;358:421–426. 10.1016/j.yexcr.2017.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dotto BR, Carvalho EL, Silva AF, Silva LFD, Pinto PM, Ortiz MF, et al. HTT-DB: horizontally transferred transposable elements database. Bioinformatics. 2015;31:2915–2917. 10.1093/bioinformatics/btv281 [DOI] [PubMed] [Google Scholar]
- 10.Sun BF, Xiao JH, He SM, Liu L, Murphy RW, Huang DW. Multiple ancient gene transfers and duplications in lepidopteran species. Insect Mol Biol. 2013;22:72–87. 10.1111/imb.12004 [DOI] [PubMed] [Google Scholar]
- 11.Boto L. Horizontal gene transfer in the acquisition of novel traits by metazoans. Proc R Soc B. 2014;281:20132450 10.1098/rspb.2013.2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danchin EG, Rosso M-N, Vieira P, de Almeida-Englera J, Coutinhob PM, Henrissat B, et al. Multiple lateral gene transfers and duplications have promoted plant parasitism ability in nematodes. Proc Natl Acad Sci U S A. 2010;107:17651–17656. 10.1073/pnas.1008486107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danchin EG, Guzeeva EA, Mantelin S, Berepiki A, Jones JT. Horizontal gene transfer from bacteria has enabled the plant-parasitic nematode Globodera pallida to feed on host-derived sucrose. Mol Biol Evol. 2016;33:1571–1579. 10.1093/molbev/msw041 [DOI] [PubMed] [Google Scholar]
- 14.Danchin EGJ, Perfus-Barbeoch L, Rancurel C, Thorpe P, Da Rocha M, Bajew S, et al. The transcriptomes of Xiphinema index and Longidorus elongatus suggest independent acquisition of some plant parasitism genes by horizontal gene transfer in early-branching nematodes. Genes. 2017;8:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haegeman A, Jones JT, Danchin EGJ. Horizontal gene transfer in nematodes: a catalyst for plant parasitism? Mol Plant Microbe Interact. 2011;24:879–887. 10.1094/MPMI-03-11-0055 [DOI] [PubMed] [Google Scholar]
- 16.Cotton JA, Lilley CJ, Jones LM, Kikuchi T, Reid AJ, Thorpe P, et al. The genome and life-stage specific transcriptomes of Globodera pallida elucidate key aspects of plant parasitism by a cyst nematode. Genome Biol. 2014;5:R43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smant G, Stokkermans JP, Yan Y, de Boer JM, Baum TJ, Wang X, et al. Endogenous cellulases in animals: isolation of beta-1, 4-endoglucanase genes from two species of plant-parasitic cyst nematodes. Proc Natl Acad Sci U S A. 1998;95:4906–4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Acuña R, Padilla BE, Flórez-Ramos CP, Rubio JD, Herrera JC, Benavides P, et al. () Adaptive horizontal transfer of a bacterial gene to an invasive insect pest of coffee. Proc Natl Acad Sci U S A. 2012;109:4197–202. 10.1073/pnas.1121190109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pauchet Y, Heckel DG. The genome of the mustard leaf beetle encodes two active xylanases originally acquired from bacteria through horizontal gene transfer. Proc R Soc B. 2013;280:20131021 10.1098/rspb.2013.1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moran NA, Jarvik T. Lateral transfer of genes from fungi underlies carotenoid production in aphids. Science. 2010;328:624–627. 10.1126/science.1187113 [DOI] [PubMed] [Google Scholar]
- 21.Wybouw N, Dermauw W, Tirry L, Stevens C, Grbić M, Feyereisen R, et al. A gene horizontally transferred from bacteria protects arthropods from host plant cyanide poisoning. eLife. 2014;3:e02365 10.7554/eLife.02365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinson EO, Martinson VG, Edwards R, Mrinalini, Werren JH. Laterally transferred gene recruited as a venom in parasitoid wasps. Mol Biol Evol. 2015;33:1042–1052. 10.1093/molbev/msv348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moran Y, Fredman D, Szczesny P, Grynberg M, Technau U. Recurrent horizontal transfer of bacterial toxin genes to eukaryotes. Mol Biol Evol. 2012;29:2223–2230. 10.1093/molbev/mss089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chou S, Daugherty MD, Peterson SB, Biboy J, Yang Y, Jutras BL, et al. Transferred interbacterial antagonism genes augment eukaryotic innate immune function. Nature. 2015;518:98–101. 10.1038/nature13965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gasmi L, Boulain H, Gauthier J, Hua-Van A, Musset K, Jakubowska AK, et al. Recurrent domestication by Lepidoptera of genes from their parasites mediated by Bracoviruses. PLoS Genet. 2015;11:e1005470 10.1371/journal.pgen.1005470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pennacchio F, Strand MR. Evolution of developmental strategies in parasitic hymenoptera. Annu Rev Entomol. 2006;51:233–258. 10.1146/annurev.ento.51.110104.151029 [DOI] [PubMed] [Google Scholar]
- 27.Strand MR, Burke GR. Polydnavirus-wasp associations: evolution, genome organization, and function. Curr Opin Virol. 2013;3:587–594. 10.1016/j.coviro.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 28.Strand MR, Burke GR. Polydnaviruses: from discovery to current insights. Virology. 2015;479–480:393–402. 10.1016/j.virol.2015.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pennacchio F, Caccia S, Digilio MC. Host regulation and nutritional exploitation by parasitic wasps. Curr Opin Insect Sci. 2014;6:74–79. [DOI] [PubMed] [Google Scholar]
- 30.Gasmi L, Jakubowska AK, Herrero S. Gasmin (BV2-5), a polydnaviral-acquired gene in Spodoptera exigua. Trade-off in the defense against bacterial and viral infections. Dev Comp Immunol. 2016;56:37–45. 10.1016/j.dci.2015.11.014 [DOI] [PubMed] [Google Scholar]
- 31.Di Lelio I, Varricchio P, Di Prisco G, Marinelli A, Lasco V, Caccia S, et al. Functional analysis of an immune gene of Spodoptera littoralis by RNAi. J Insect Physiol. 2014;64:90–97. 10.1016/j.jinsphys.2014.03.008 [DOI] [PubMed] [Google Scholar]
- 32.Caccia S, Di Lelio I, La Storia A, Marinelli A, Varricchio P, Franzetti E, et al. Midgut microbiota and host immunocompetence underlie Bacillus thuringiensis killing mechanism. Proc Natl Acad Sci U S A. 2016;113:9486–9491. 10.1073/pnas.1521741113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eleftherianos I, Millichap PJ, ffrench-Constant RH, Reynolds SE. RNAi suppression of recognition protein mediated immune responses in the tobacco hornworm Manduca sexta causes increased susceptibility to the insect pathogen Photorhabdus. Dev Com Immunol. 2006;30:1099–1107. [DOI] [PubMed] [Google Scholar]
- 34.Eleftherianos I, Boundy S, Joyce SA, Aslam S, Marshall JW, Cox RJ, et al. An antibiotic produced by an insect-pathogenic bacterium suppresses host defenses through phenoloxidase inhibition. Proc Natl Acad Sci U S A. 2007;104:2419–2424. 10.1073/pnas.0610525104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim ZX, Robinson KE, Jain RG, Chandra GS, Asokan R, Asgari S, et al. Diet-delivered RNAi in Helicoverpa armigera—Progresses and challenges. J Insect Physiol. 2016;85:86–93. 10.1016/j.jinsphys.2015.11.005 [DOI] [PubMed] [Google Scholar]
- 36.KonDo Y, Yoda S, Mizoguchi T, Ando T, Yamaguchi J, Yamamoto K, et al. Toll ligand Spätzle3 controls melanization in the stripe pattern formation in caterpillars. Proc Natl Acad Sci U S A. 2017;114:8336–8341. 10.1073/pnas.1707896114 Erratum in: Proc Natl Acad Sci U S A. 2017 Sep 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sadekuzzaman M, Gautam N, Kim Y. A novel calcium-independent phospholipase A2 and its physiological roles in development and immunity of a lepidopteran insect, Spodoptera exigua. Dev Comp Immunol. 2017;77:210–220. 10.1016/j.dci.2017.08.014 [DOI] [PubMed] [Google Scholar]
- 38.Xie M, Ren NN, You YC, Chen WJ, Song QS, You MS. Molecular characterisation of two α-esterase genes involving chlorpyrifos detoxification in the diamondback moth, Plutella xylostella. Pest Manag Sci. 2017;73:1204–1212. 10.1002/ps.4445 [DOI] [PubMed] [Google Scholar]
- 39.Terenius O, Papanicolaou A, Garbutt JS, Eleftherianos I, Huvenne H, Kanginakudru S, et al. RNA interference in Lepidoptera: an overview of successful and unsuccessful studies and implications for experimental design. J Insect Physiol. 2011;57:231–245. 10.1016/j.jinsphys.2010.11.006 [DOI] [PubMed] [Google Scholar]
- 40.Marmaras VJ, Lampropoulou M. Regulators and signalling in insect haemocyte immunity. Cell Signal. 2009;21:186–195. 10.1016/j.cellsig.2008.08.014 [DOI] [PubMed] [Google Scholar]
- 41.Hillyer JF. Insect immunology and hematopoiesis. Dev Comp Immunol. 2016;58:102–118. 10.1016/j.dci.2015.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurtz J. Phagocytosis by invertebrate hemocytes: causes of individual variation in Panorpa vulgaris scorpionflies. Microsc Res Techniq. 2002;57:456–468. [DOI] [PubMed] [Google Scholar]
- 43.Hillyer JF, Strand MR. Mosquito hemocyte-mediated immune responses. Curr Opin Insect Sci. 2014;3:14–21. 10.1016/j.cois.2014.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Ann Rev Immunol. 2007;25:697–743. [DOI] [PubMed] [Google Scholar]
- 45.Yi H-Y, Chowdhury M, Huang Y-D, Yu X-Q. Insect antimicrobial peptides and their applications. Appl Microbiol Biotechnol. 2014;98:5807–5822. 10.1007/s00253-014-5792-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boleij A, Laarakkers CM, Gloerich J, Swinkels DW, Tjalsma H. Surface-affinity profiling to identify host-pathogen interactions. Infect Immun. 2011;79:4777–4783. 10.1128/IAI.05572-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kergoat GJ, Prowell DP, Le Ru BP, Mitchell A, Dumas P, Clamens AL, et al. Disentangling dispersal, vicariance and adaptive radiation patterns: a case study using armyworms in the pest genus Spodoptera (Lepidoptera: Noctuidae). Mol Phylogenet Evol. 2012;65:855–870. 10.1016/j.ympev.2012.08.006 [DOI] [PubMed] [Google Scholar]
- 48.Chevignon G, Thézé J, Cambier S, Poulain J, Da Silva C, Bézier A, et al. Functional annotation of Cotesia congregata bracovirus: identification of the viral genes expressed in parasitized host immune tissues. J Virol. 2014;88:8795–8812. 10.1128/JVI.00209-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chevignon G, Cambier S, Da Silva C, Poulain J, Drezen JM, Huguet E, et al. Transcriptomic response of Manduca sexta immune tissues to parasitization by the bracovirus associated wasp Cotesia congregata. Insect Biochem Mol Biol. 2015;62:86–99. 10.1016/j.ibmb.2014.12.008 [DOI] [PubMed] [Google Scholar]
- 50.Browne N, Heelan M, Kavanagh K. An analysis of the structural and functional similarities of insect hemocytes and mammalian phagocytes. Virulence. 2013;4:597–603. 10.4161/viru.25906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levashina EA, Moita LF, Blandin S, Vriend G, Lagueux M, Kafatos FC. Conserved role of a complement-like protein in phagocytosis revealed by dsRNA knockout in cultured cells of the mosquito, Anopheles gambiae. Cell. 2001;104:709–718. [DOI] [PubMed] [Google Scholar]
- 52.Stroschein-Stevenson SL, Foley E, O’Farrell PH, Johnson AD. Identification of Drosophila gene products required for phagocytosis of Candida albicans. PLoS Biol. 2006;4:e4 10.1371/journal.pbio.0040004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim CH, Shin YP, Noh MH, Jo YH, Han YS, Seong YS, et al. An insect multiligand recognition protein functions as an opsonin for the phagocytosis of microorganisms. J Biol Chem. 2006;285:25243–5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ulvila J, Vanha-Aho LM, Rämet M. Drosophila phagocytosis—still many unknowns under the surface. Acta Pathol Microbiol Immunol Scand. 2011;119:651–662. [DOI] [PubMed] [Google Scholar]
- 55.Vlisidou I, Wood W. Drosophila blood cells and their role in immune responses. The FEBS J. 2015;282:1368–1382. 10.1111/febs.13235 [DOI] [PubMed] [Google Scholar]
- 56.Tian YY, Liu Y, Zhao XF, Wang JX. Characterization of a C-type lectin from the cotton bollworm, Helicoverpa armigera. Dev Comp Immunol. 2009;33:772–779. 10.1016/j.dci.2009.01.002 [DOI] [PubMed] [Google Scholar]
- 57.Li L, Li YP, Song CX, Xiao M, Wang JL, Liu XS. Identification and functional characterization of a peptidoglycan recognition protein from the cotton bollworm, Helicoverpa armigera. Arch Insect Biochem Physiol. 2014;86:240–258. 10.1002/arch.21174 [DOI] [PubMed] [Google Scholar]
- 58.Zhan MY, Shahzad T, Yang PJ, Liu S, Yu XQ, Rao XJ. A single-CRD C-type lectin is important for bacterial clearance in the silkworm. Dev Comp Immunol. 2016;65:330–339. 10.1016/j.dci.2016.08.004 [DOI] [PubMed] [Google Scholar]
- 59.Cubero OF, Crespo A, Fatehi J, Bridge PD. DNA extraction and PCR amplification method suitable for fresh, herbarium-stored, lichenized, and other fungi. Plant Syst Evol. 1999;216:243–249. [Google Scholar]
- 60.Gish W, States DJ. Identification of protein coding regions by database similarity search. Nat Genet. 1993;3:266–272. 10.1038/ng0393-266 [DOI] [PubMed] [Google Scholar]
- 61.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol. 2000;17:540–552. 10.1093/oxfordjournals.molbev.a026334 [DOI] [PubMed] [Google Scholar]
- 64.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst Biol. 2010;59:307–321. 10.1093/sysbio/syq010 [DOI] [PubMed] [Google Scholar]
- 65.Gouy M, Guindon S, Gascuel O. SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol. 2010;27:221–224. 10.1093/molbev/msp259 [DOI] [PubMed] [Google Scholar]
- 66.Pond SL, Frost SD. Datamonkey: rapid detection of selective pressure on individual sites of codon alignments. Bioinformatics. 2005;21:2531–2533. 10.1093/bioinformatics/bti320 [DOI] [PubMed] [Google Scholar]
- 67.Weaver S, Shank SD, Spielman SJ, Li M, Muse SV, Kosakovsky Pond SL. Datamonkey 2.0: a modern web application for characterizing selective and other evolutionary processes. Mol Biol Evol. 2018;35:773–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 69.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29: 2002–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST(C)) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. [DOI] [PubMed] [Google Scholar]
- 72.Finney DJ Probit analysis. Cambridge University Press, Cambridge, United Kingdom; 1971. [Google Scholar]
- 73.Li Y, Jian-Feng L, Feng C, Ke X, Fu W. Role of venom and ovarian proteins in immune suppression of Ostrinia furnacalis (Lepidoptera: Pyralidae) larvae parasitized by Macrocentrus cingulum (Hymenoptera: Braconidae), a polyembryonic parasitoid. Insect Sci. 2007;14:93–100. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Statistical analysis performed on the relative quantification of bacterial load by qRT-PCR and the humoral immune response by Spodoptera littoralis larvae as affected by RNAi.
(DOCX)
Specific tryptic peptides from Sl gasmin sequence were selected for LC-MS/MS analyses in MRM mode. Individual transitions from the parent ions to the most intense fragments and the corresponding collision energies are reported.
(DOCX)
Sequences of the primers used for qRT-PCR analyses (F: Forward, R: Reverse).
(DOCX)
Aligned potential homologue protein sequences retrieved form extensive similarity searches of complete and high-throughput genome sequence databases hosted by NCBI.
(TXT)
Log of the FEL analysis aimed at identifying selective pressure on Spodoptera littoralis and Spodoptera exigua sequences. The program uses a maximum-likelihood approach to infer nonsynoymous (dN) and synonymous (dS) substitution rates on a per-site basis for a given coding alignment and corresponding phylogeny.
(TXT)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.