Abstract
Background
Meningococcal outer membrane vesicle (OMV) vaccines are prepared with detergents to remove endotoxin, which also remove desirable antigens such as factor H binding protein (FHbp). Native OMV (NOMV) vaccines with genetically attenuated endotoxin do not require detergent treatment and elicit broader serum bactericidal antibody (SBA) responses than OMV or recombinant FHbp (rFHbp) vaccines.
Methods
We measured human complement–mediated SBA responses in mice immunized with NOMV with overexpressed FHbp subfamily B (NOMV-FHbp), NOMV with FHbp genetically inactivated (NOMV-KO), and/or a control rFHbp vaccine against meningococcal and gonococcal strains.
Results
Despite having 36-fold less FHbp per dose, the NOMV-FHbp vaccine elicited a ≥3-fold higher serum IgG anti-FHbp geometric mean titer than control vaccines containing rFHbp (P ≤ .003). Against 2 meningococcal outbreak strains with mismatched PorA and heterologous FHbp subfamily B sequence variants, the NOMV-FHbp vaccine produced ≥30-fold higher SBA titers than control vaccines. Mice immunized with NOMV-FHbp and NOMV-KO vaccines also elicited SBA against a gonococcal strain (P < .0001 vs the adjuvant-only control group). In contrast, 2 licensed meningococcal serogroup B vaccines, including one containing detergent-extracted OMV, did not produce gonococcal SBA in humans.
Conclusions
A meningococcal NOMV vaccine elicits SBA against gonococci and with overexpressed FHbp elicits SBA against meningococci.
Keywords: FHbp, gonococcus, meningococcus, Neisseria gonorrhoeae, NOMV, OMV, vaccine
In this study, a meningococcal native outer membrane vesicle (NOMV) vaccine with genetically attenuated endotoxin and over-expressed Factor H binding protein elicited bactericidal antibodies against both meningococcus and gonococcus, which represents a promising approach to develop a cross-protective Neisserial vaccine.
Meningococcal outer membrane vesicle (OMV) vaccines are treated with detergents to decrease endotoxin activity, and this treatment also removes certain desirable lipoprotein antigens, such as factor H binding protein (FHbp) [1] and neisserial heparin binding antigen [2]. As a result, detergent-extracted OMV (dOMV) vaccines elicit serum bactericidal antibody primarily against strains with PorA variable regions (VR1 and VR2) matched to the vaccine [3, 4]. Meningococcal dOMV vaccines have been used to control serogroup B epidemics and hyperendemic disease predominantly caused by clonal strains with PorA sequences matched to those of the vaccines [5–8]. A dOMV is also a principal component of a recently licensed serogroup B vaccine (Bexsero; referred to herein as “MenB-4C”), which also contains 3 recombinant protein antigens, including FHbp [9].
A potentially more promising strategy to confer broad protection against meningococcal disease is the use of native OMV (NOMV) vaccines prepared from meningococcal strains with genetically attenuated endotoxin and overexpressed FHbp [10, 11]. Since the NOMV vaccines are not prepared with a detergent, they retain protective detergent-soluble lipoprotein antigens [4]. NOMV vaccines also may have more native membrane protein conformations that might not be present in recombinant antigens.
Although the mechanism is not clear, recent epidemiologic studies suggest that an unexpected benefit of meningococcal vaccination with dOMV (MeNZB) or MenB-4C is partial protection against gonococcal disease [12–14]. Conceivably, components of the meningococcal dOMV, with or without recombinant protein antigens in the MenB-4C vaccine, might cross-react with antigens present in gonococcal strains and decrease the incidence and/or severity of gonococcal disease [15].
In the present study, we tested the immunogenicity in mice of a meningococcal NOMV vaccine with genetically attenuated endotoxin and overexpressed FHbp (NOMV-FHbp), which was produced for an infant primate study. The serum antibodies elicited in the mice had broader meningococcal bactericidal activity than those elicited in control mice immunized with a recombinant FHbp (rFHbp) vaccine or with a NOMV vaccine from a FHbp knockout (NOMV-KO) strain given individually or in combination with rFHbp. Unexpectedly, the anti-NOMV antibodies also elicited human complement–mediated SBA against the gonococcus. In contrast, sera from humans immunized with MenB-4C or a second licensed serogroup B vaccine (Trumenba; referred to herein as “MenB-FHbp”) did not elicit SBA against the gonococcal strain. Collectively, the data highlight the potential for a meningococcal NOMV vaccine to elicit protection against both meningococci and gonococci.
METHODS
Preparation of NOMV and rFHbp Vaccines
FHbp can be subdivided into 2 families, A and B, based on the relatedness of amino acid sequences and antigenic cross-reactivity [16]. For the NOMV-FHbp vaccine and control rFHbp vaccine, we used a subfamily B FHbp with amino acid sequence variant ID 1 (previously referred to as 1.1 from strain MC58 [1]). In addition, we introduced the R41S substitution that has approximately 100-fold decreased binding to human or nonhuman primate FH, compared with wild-type FHbp [17]. This mutation has a minimal effect on immunogenicity in mice whose FH does not bind to wild-type FHbp [18].
The NOMV vaccines were prepared from mutant strains described previously [18], which included inactivation of the LpxL1 gene to attenuate endotoxin activity [19], and overexpressing FHbp (NOMV-FHbp) or inactivating the gene encoding FHbp (NOMV-KO). For comparison of the amount of FHbp in each vaccine preparation, we also prepared a control NOMV from the parental strain H44/76 containing the wild-type FHbp gene and an empty pFP12 [10, 20] plasmid (NOMV-Empty). Modifications to the previous NOMV isolation procedure included (1) growth of the bacteria in Frantz medium [21] instead of Mueller-Hinton medium; (2) use of filtration (0.2 µm; Millipore), which removes residual bacteria, instead of phenol treatment, which kills residual bacteria; and (3) use of ultrafiltration (100-kDa molecular weight cutoff; Amicon) to concentrate the NOMV, instead of (NH4)2SO4 precipitation.
Characterization of NOMV Vaccines
The relative amount of FHbp and the identity of protein antigens present in the NOMV preparations were determined by liquid chromatography tandem mass spectrometry (LC-MS/MS), performed at the University of California–Davis Proteomics Core Facility. Total protein in the NOMV preparations was processed for mass spectrometry as described in protocols provided by the University of California–Davis Proteomics Core Facility. Cysteine residues were reduced and carboxyamidated, and the mixture was digested with Lys-C/trypsin (Promega). Also, we approximated the amount of the major outer membrane protein antigens by determining the relative amount of PorB, using sodium dodecyl sulfate–polyacrylamide gel electrophoresis and densitometry of Coomassie blue–stained gel (SimplyBlue SafeStain; Thermo Scientific) and then dividing the peak area for the most abundant peptide for each protein by the peak area for the most abundant PorB peptide. The results are presented in Table 1. The identity of the proteins in each NOMV preparation are presented in Supplementary Table 1. Sequence coverage for all of the major outer membrane proteins except immunoglobulin A1 (IgA1) protease was ≥90%.
Table 1.
Relative Amounts of Candidate Vaccine Proteins Measured by Mass Spectrometry
| Protein | Molecular Weight, kDa |
Protein Level per 5 µg of NOMVa | ||
|---|---|---|---|---|
| Empty, µgb | Knockout, µgc | FHbp, µgc | ||
| FHbp | 23 | 0.02 | 0.00 | 0.40 |
| PorA | 42 | 0.25 | 0.15 | 0.18 |
| PorB | 34 | 0.53 | 0.70 | 0.75 |
| RmpM | 26 | 0.38 | 0.21 | 0.54 |
| IgA1 protease | 203 | 0.08 | 0.05 | 0.03 |
| FetA | 74 | 0.23 | 0.17 | 0.43 |
| Opa | 25 | 0.02 | 0.10 | 0.03 |
| TbpA | 99 | 0.06 | 0.05 | 0.17 |
| Omp85 | 88 | 0.07 | 0.04 | 0.10 |
| Opc | 28 | 0.01 | 0.01 | 0.09 |
| NspA | 18 | 0.01 | 0.00 | 0.01 |
Abbreviations: FHbp, factor H binding protein; IgA1, immunoglobulin A1; NOMV, native meningococcal outer membrane vesicle.
aThe amount was calculated as described in Methods.
bControl NOMV was used for analytical purposes, which was prepared from strain H44/76 with natural wild-type expression of FHbp ID 1 and transformed with a negative control plasmid that did not contain the gene encoding FHbp.
cNOMV vaccines used to immunize mice.
Mouse Immunogenicity
Five groups of 3-week-old female CD-1 mice (14–16 per group) were immunized intraperitoneally at 3-week intervals, and terminal collection of blood specimens was done 2 weeks after the third dose. The vaccines tested were NOMV-FHbp, NOMV-KO, rFHbp, a combination of rFHbp and NOMV-KO (rFHbp/NOMV-KO), and aluminum hydroxide adjuvant (Al(OH)3) alone (Alhydrogel, Brenntag Biosector). The dose of NOMV was 5 μg, and the dose of rFHbp was 10 μg, with each antigen adsorbed with 600 µg of Al(OH)3. One mouse in each of the NOMV-FHbp and NOMV-KO/rFHbp groups died before the conclusion of the immunogenicity experiment. For each vaccine group, we made 4 serum pools (3–4 mice per pool), which were stored at −80°C prior to performing antibody assays. Mouse immunogenicity experiments were conducted under a protocol approved by the Institutional Animal Care and Use Committee at the University of California–San Francisco Benioff Children’s Hospital Oakland.
Human Sera
SBA against the gonococcus was performed on stored sera from healthy adults immunized with the recommended 2 doses of MenB-4C or 3 doses of MenB-FHbp as part of 2 previously published immunogenicity studies [22, 23]. For this study, all human serum samples were deidentified; investigators were aware of the vaccine assignment and the pre- or post-immunization status associated with each sample.
Serologic Analysis
Complement activity was inactivated in mouse or human sera by incubation at 56°C for 30 minutes. Serum immunoglobulin G (IgG) anti-FHbp antibody titers were measured by ELISA using rFHbp ID 1 (2 µg/ml diluted in phosphate-buffered saline) as the antigen on the plate. Bound mouse IgG was detected with goat anti-mouse IgG (whole molecule) conjugated to alkaline phosphatase (Sigma-Aldrich; 1:10 000 dilution) and para-nitrophenyl phosphate substrate (Sigma-Aldrich; 1 mg/mL in 50 mM NaCO3 and 1 mM MgCl2 [pH 9.8]). Titers were assigned from the interpolated reciprocal serum dilution that yielded an absorbance at 405 nm of 2.0.
Meningococcal SBA titers were measured as previously described using mid–logarithmic phase meningococci grown in liquid culture in Frantz medium [21] containing 4 mM D,L-lactate and 2 mM CMP-NANA (Sigma-Aldrich). The complement was a mixture of serum specimens that were collected from 3 healthy human subjects and had been depleted of IgG antibodies by using a protein G column (HiTrap Protein G, 5 ml; GE Life Sciences) as previously described [17]. Informed consent was obtained from the subjects who provided complement, under a protocol approved by the Institutional Review Board of the University of California–San Francisco Benioff Children’s Hospital Oakland. SBA titers of the mouse sera were assigned from the interpolated serum dilution that resulted in 50% survival of the bacteria after incubation at 37°C for 60 minutes as compared to negative control wells at t = 60 minutes.
We measured SBA against 4 invasive meningococcal serogroup B strains (Table 2). Strain H44/76 is a case isolate from an epidemic in Norway [6]. This strain also was used to construct the mutant strains from which the NOMV vaccines were prepared. The test strain therefore is matched for PorA VR 7,16 in the NOMV-FHbp and NOMV-KO vaccines and for FHbp ID 1 in the NOMV-FHbp and rFHbp vaccines. Strain SK106 is a case isolate from a patient hospitalized in Ohio in 2003 [24] and is mismatched for the NOMV PorA and matched for FHbp ID 1 in the vaccines. Strains CH819 and CH860 are invasive case isolates from outbreaks at a university in New Jersey [25, 26] and in Quebec, Canada [27, 28], respectively. Both test strains are mismatched for the PorA VR type in the NOMV vaccines and matched for the FHbp subfamily (B). CH819 expresses FHbp ID 276 with 96% amino acid sequence identity to FHbp ID 1 in the rFHbp or NOMV-FHbp vaccines. CH860 expresses FHbp ID 15 with 87% identity to the FHbp ID 1 in the vaccines.
Table 2.
Neisserial Strains Used for Measurement of Serum Bactericidal Activity
| Species | Strain | PorA VRa | FHbp IDb | Reference(s) |
|---|---|---|---|---|
| NmB | H44/76 | 7, 16 | ID 1 | [6, 43] |
| NmB | SK106 | 19, 15 | ID 1 | [24] |
| NmB | CH819 | 5, 2-2 | ID 276 | [25, 26] |
| NmB | CH860 | 19, 15-11 | ID 15 | [27, 28] |
| Ng | FA1090 | NA | NA | Available at: https://www.ncbi.nlm. nih.gov/nuccore/NC_002946.2 |
Abbreviations: FHbp, factor H binding protein; NA, not applicable; Ng, Neisseria gonorrhoeae; NmB, Neisseria meningitidis serogroup B; VR, variable region.
aSequence type of PorA based on VR1 and VR2 as designated on the public database (available at: https://pubmlst.org/neisseria/PorA/).
bFHbp amino acid sequence identification number as designated in a public database (available at: https://pubmlst.org/neisseria/fHbp/). All FHbp variants were assigned to subfamily B.
For gonococcal SBA, we tested heat-inactivated serum at a 1:5 dilution with 20% exogenous normal human serum as a complement source and used gonococcal strain FA1090. For the mouse sera, we depleted IgM antibodies to remove naturally acquired SBA. In brief, the sera were mixed with equal volumes of anti-mouse IgM sepharose (Sigma) and incubated at room temperature for 30 minutes. The IgM-depleted sera were collected following centrifugation at 1000 × g for 1 minute. The resin was washed with 1 volume of Hank’s balanced saline solution (HBSS++), and the wash solution was added to the IgM-depleted sera. Sera were sterilized using a cellulose acetate filter (Costar) and stored at 4°C prior to use. For the human sera, depletion of IgM was not needed because FA1090 is resistant to killing by normal human serum [29–31] and preimmune sera did not show any killing (see Results).
Statistical Analyses
Serum antibody reciprocal titers were transformed to log10 values for calculation of geometric means. Titers below the lowest dilution tested were assigned half of the value (eg, a titer of <1:10 was assigned a value of 1:5). Statistical tests were unpaired, 2-tailed Mann-Whitney t tests. A χ2 test was used to compare the percentages of mice in each group with gonococcal SBA titers of <1:5 or ≥1:5. P values of ≤.05 were considered to be statistically significant.
RESULTS
Serum IgG Anti-FHbp Antibody Responses
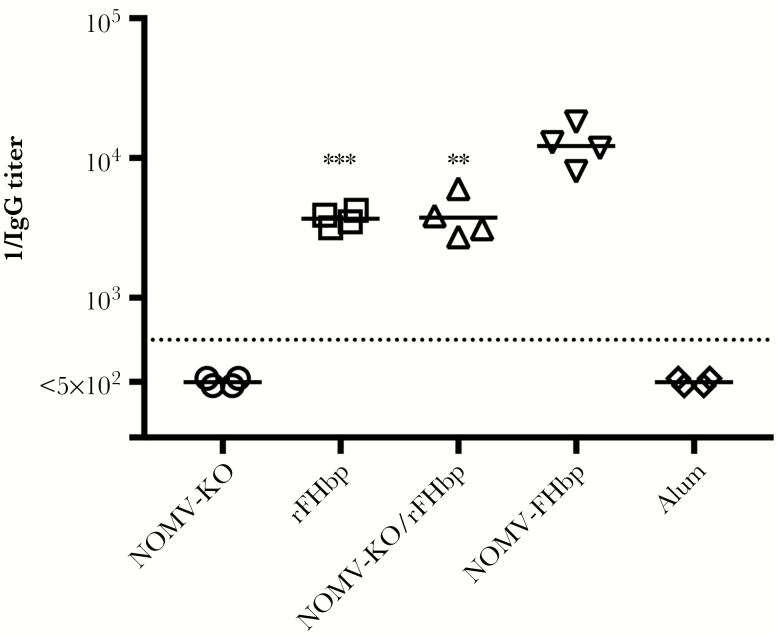
Serum IgG anti-FHbp antibody titers were tested in 4 serum pools per vaccine group (3–4 mice per pool). Despite a 36-fold lower amount of FHbp per dose of the NOMV-FHbp vaccine, the reciprocal geometric mean titer (GMT) of the mice immunized with the NOMV-FHbp vaccine was 3.2–3.3-fold higher than those of mice immunized with the rFHbp/NOMV-KO combination vaccine or the rFHbp vaccine (reciprocal GMT, 12196 vs 3755 or 3677, respectively; P ≤ .003; Figure 1). The reciprocal IgG anti-FHbp GMTs of mice immunized with the NOMV-KO vaccine or aluminum adjuvant without a vaccine antigen were <500 (the lowest dilution tested).
Figure 1.
Immunoglobulin G (IgG) anti-FHbp antibody titers elicited in mice by native meningococcal outer membrane vesicle (NOMV) and/or recombinant factor H binding protein (rFHbp). Each symbol represents pooled serum from 3–4 mice. Values less than the lowest dilution tested (1:500) were assigned half that value. ***P = .0006 and **P = .003, by a 2-tailed t test, compared with NOMV-FHbp. KO, knockout.
Meningococcal Serum Bactericidal Antibody Responses
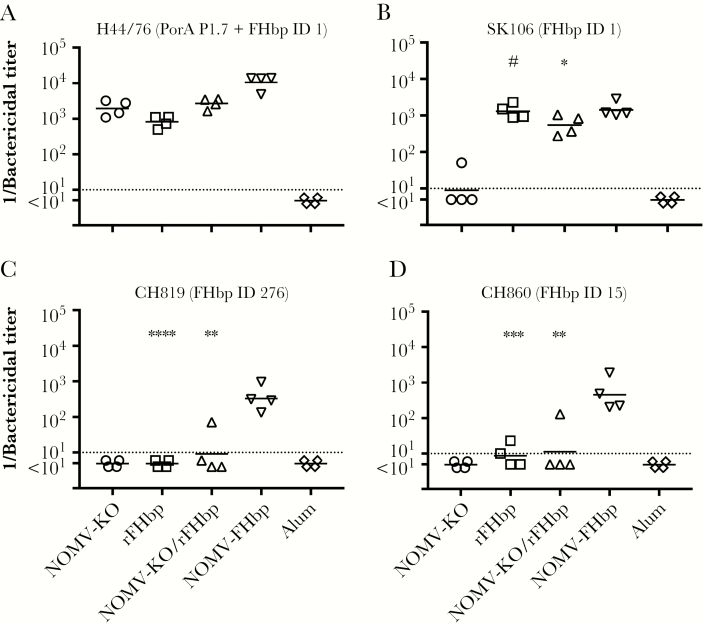
In previous studies, the principal antigenic targets of SBA elicited by NOMV-FHbp vaccines were PorA and FHbp [4, 10, 32]. For strain H44/76, which matched the vaccines to those 2 and other antigens, the reciprocal GMTs for mice immunized with the rFHbp, NOMV-KO, or rFHbp/NOMV-KO combination vaccines were 815, 1939, and 2695, respectively (P ≥ .33, by pair-wise t tests). In contrast, the reciprocal GMT of mice immunized with the NOMV-FHbp vaccine (10589) was 3.8-fold to 5.5-fold higher than for the other vaccine groups (P ≤ .006, by pair-wise t tests; Figure 2A). All of the titers of the negative control pools from mice immunized with aluminum hydroxide alone were <1:10.
Figure 2.
Human complement-mediated serum bactericidal activity (SBA) against 4 meningococcal strains. Each symbol represents the titer of a serum pool from 3 to 4 mice. A, SBA against wild-type strain H44/76, which has PorA and factor H binding protein (FHbp) ID 1 antigens matched to the vaccine. ***P = .0002 and *P = .005, by a 2-tailed t test, compared with native meningococcal outer membrane vesicle (NOMV)–FHbp. B, SBA against SK106, which has a mismatched PorA antigen and a matched FHbp ID 1 antigen. #P = .79 and *P = .052, compared with NOMV-FHbp. C, SBA against outbreak strain CH819, which has a mismatched PorA and a mismatched FHbp ID 276 (96% sequence identity with ID 1). ****P < .0001 and **P = .004, compared with NOMV-FHbp. D, SBA against outbreak strain CH860, which has mismatched PorA and a mismatched FHbp ID 15 (87% sequence identity with ID 1). ***P = .0008 and **P = .009, compared with NOMV-FHbp. KO, knockout.
Serogroup B strain SK106 was mismatched for PorA in the NOMV vaccine and matched with FHbp ID 1 for the rFHbp and NOMV-FHbp vaccines (Table 2). Mice immunized with the NOMV-KO vaccine had a reciprocal GMT of 9 (Figure 2B). In contrast, mice immunized with the rFHbp vaccine, the combination rFHbp/NOMV-KO vaccine, or the NOMV-FHbp vaccine had a reciprocal GMT of 1299, 547, and 1420, respectively (P≥ .052; Figure 2B). Thus, when the test strain had a mismatch for PorA and an exact match with FHbp ID 1 in the rFHbp or NOMV-FHbp vaccines, SBA titers were high. With the NOMV-KO vaccine, titers were high for strain H44/76 with PorA that matched the vaccine but not for strain SK016 with a heterologous PorA VR type.
In general, SBA responses to FHbp are subfamily specific [16, 33]. The FHbp vaccine antigen was FHbp ID 1 in subfamily B. We measured SBA responses against 2 additional serogroup B strains responsible for outbreaks in North America. Both strains were mismatched for both PorA and had FHbp subfamily B sequence variants that were not exactly matched to that in the NOMV-FHbp and rFHbp vaccines. For strain CH819, the reciprocal GMT was 329 for the NOMV-FHbp vaccine versus <10 for the rFHbp, the rFHbp/NOMV-KO combination, or the NOMV-KO vaccines (P ≤ .004; Figure 2C). For strain CH860, the reciprocal GMT was 452 for the NOMV-FHbp vaccine versus ≤11 for the other vaccines (P ≤ .009; Figure 2D).
Gonococcal Serum Bactericidal Antibody Responses
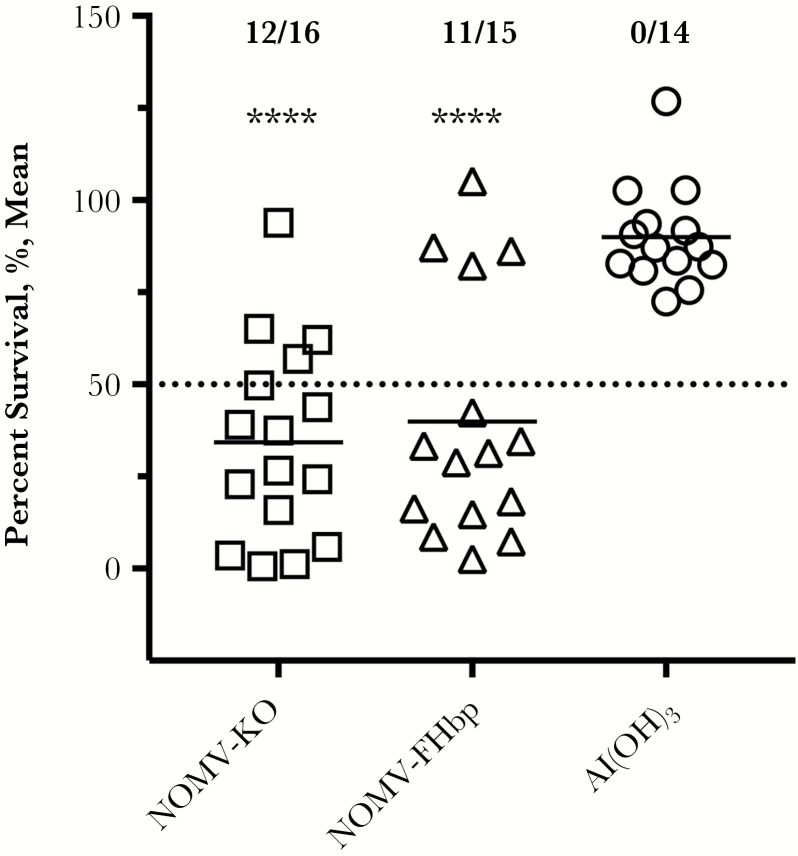
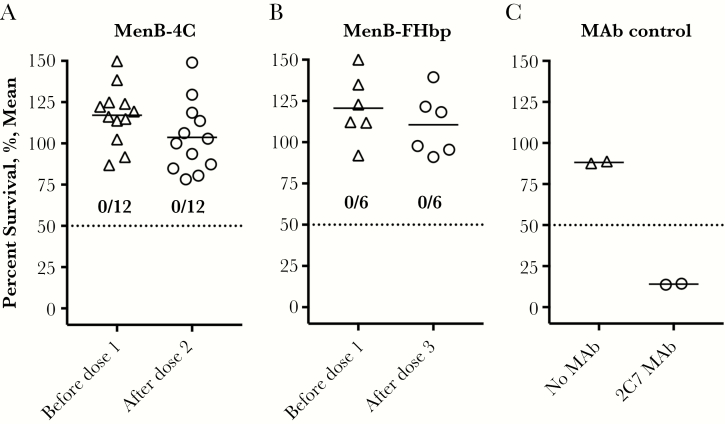
For measurement of gonococcal SBA responses, we focused on the sera from mice immunized with NOMV-FHbp or NOMV-KO vaccines, since gonococci do not express FHbp on the bacterial surface [34]. Mice immunized with aluminum hydroxide adjuvant without an antigen served as a negative control. Eleven of 15 mice immunized with the NOMV-FHbp vaccine and 12 of 16 immunized with the NOMV-KO vaccine had SBA titers of ≥1:5, compared with 0 of 14 immunized with the aluminum hydroxide adjuvant without antigen (P < .0001, by the χ2 test; Figure 3). Since the dOMV vaccine used in New Zealand and the MenB-4C vaccine, which contains dOMV, were reported to provide protection against gonococcus [12, 35], we tested SBA in stored sera obtained before dose 1 and 1 month after dose 2 or dose 3 from healthy adults immunized in previous studies with meningococcal serogroup B vaccines [22, 23]. It was noteworthy that 0 of 12 subjects immunized with MenB-4C or 0 of 6 immunized with MenB-FHbp had non-detectable SBA in sera obtained before or after immunization (titers, <1:5; Figure 4A and 4B). A positive control showed that antigonococcal lipooligosaccharide monoclonal antibody 2C7 [36] was bactericidal under the same assay conditions (Figure 4C).
Figure 3.
Serum bactericidal activity (SBA) measured against Neisseria gonorrhoeae strain FA1090. Bacteria were incubated with individual mouse serum samples at a 1:5 dilution, after which normal human serum (20%) was added as a complement source. SBA was defined by <50% survival of bacteria after incubation for 30 minutes at 37°C. A total of 12 of 16 mice immunized with native meningococcal outer membrane vesicle–knockout (NOMV-KO) and 11 of 15 mice immunized with NOMV–factor H binding protein (FHbp) had SBA titers of ≥1:5, compared with 0 of 14 mice immunized with aluminum hydroxide (Al(OH)3) given without an antigen (P < .0001, by the χ2 test).
Figure 4.
Serum bactericidal activity (SBA) measured against Neisseria gonorrhoeae strain FA1090. Bacteria were incubated with heat-inactivated individual human serum samples at a 1:5 dilution and with normal human serum (20%) as a source of complement. A, Sera obtained before dose 1 or after dose 2 from humans immunized with 2 doses of MenB-4C in a previous study [22]. B, Sera from humans immunized with 3 doses of MenB–factor H binding protein (FHbp) in previous studies [23]. None of the immunized humans had gonococcal SBA titers of ≥1:5 in sera obtained before immunization dose 1 or 1 month after dose 2 or dose 3. C, Positive control monoclonal antibody (MAb) 2C7 (50 µg/mL) showing bactericidal activity against strain FA1090.
Mass spectrometry of the protein composition of the meningococcal NOMV vaccines identified 249 proteins (Supplementary Table 1). Forty of these had >80% amino acid sequence identity based on the whole-genome sequence from gonococcal strain FA1090, which identifies them as potential antigenic targets of SBA.
DISCUSSION
In the present study, we tested the immunogenicity in mice of 1 lot of an NOMV vaccine with genetically attenuated endotoxin and an overexpressed FHbp subfamily B mutant R41S with decreased binding of human FH. The main purpose of the study was to confirm the immunogenicity of the NOMV-FHbp vaccine lot before testing the vaccine in an infant primate study. One of our observations was that mice vaccinated with NOMV-FHbp had 3-fold higher serum IgG anti-FHbp antibody titers than mice immunized with a control rFHbp vaccine or a control rFHbp vaccine administered in combination with a NOMV-FHbp KO vaccine. The NOMV-FHbp vaccine also elicited ≥30-fold higher SBA titers against 2 meningococcal test strains with heterologous PorA VR types, compared with the NOMV-FHbp vaccine and FHbp amino acid sequence variants with 87% or 96% identity to the vaccine FHbp antigen. These results are noteworthy because the NOMV-FHbp vaccine contained 36-fold less FHbp per dose than the rFHbp vaccine. The reason for the higher anti-FHbp antibody responses to the NOMV-FHbp vaccine is not simply an effect of adjuvant properties in the NOMV, since administering the rFHbp in combination with a NOMV-FHbp KO vaccine did not enhance serum anti-FHbp antibody responses, compared with the rFHbp vaccine alone. It is possible that the higher responses to FHbp in the NOMV-FHbp result from more-optimal presentation of FHbp in the context of the NOMV and/or the lipid moiety present on FHbp in the NOMV.
Several meningococcal NOMV vaccines that have genetically attenuated endotoxin and contain FHbp have been tested in human clinical studies [37, 38]. Although these vaccines appeared to be safe and well tolerated, the breadth and magnitude of the SBA responses were modest. One possible reason is that the NOMV vaccines tested had minimal overexpression of FHbp (estimated to be approximately 2-fold higher than that for the parent wild-type strain from which the mutant vaccine strain was derived). Data from wild-type mice (whose mouse FH does not bind to FHbp) indicated that optimal NOMV-FHbp immunogenicity required at least 3–10-fold higher expression of FHbp as compared to strain H44/76 [10, 11], which has naturally high expression of FHbp [39]. In contrast, the NOMV-FHbp vaccine in the present study had approximately 20-fold higher FHbp expression than NOMV from wild-type strain H44/76. Also, the wild-type FHbp antigens used in the human clinical studies bound human FH. Data from human FH transgenic mice and infant rhesus monkeys whose macaque FH binds to wild-type FHbp indicated that binding of FH to FHbp vaccines impaired SBA responses [17, 40, 41, 42]. Further, mutant FHbp antigens with low binding to human or macaque FH, such as the R41S mutant used in the present study, elicited higher SBA responses than control FHbp vaccines that bound FH [18].
Both the NOMV-FHbp and NOMV-FHbp KO vaccines unexpectedly elicited SBA against Neisseria gonorrhoeae. In contrast, adult humans immunized in previous studies with licensed meningococcal serogroup B vaccines showed no evidence of gonococcal SBA in preimmunization or postimmunization sera. Although one cannot compare directly the antibody responses of mice given the NOMV vaccines and humans given the licensed serogroup B vaccines, the lack of SBA responses in humans indicates that the presence of SBA responses in mice is not the result of an overly sensitive SBA method. Furthermore, the lack of gonococcal SBA responses in humans immunized with the MenB-4C vaccine are particularly noteworthy, given the results of retrospective epidemiologic analyses indicating that the meningococcal dOMV (MeNZB) given alone in New Zealand [12] or as part of the MenB-4C vaccine in Quebec [35] may have decreased the incidence of gonococcal disease in both populations. Thus, if these dOMV vaccines confer protection against gonococcal disease, the protection would appear to be independent of eliciting SBA against the FA1090 test strain used in the present study.
For NOMV vaccines against meningococci, the most important antibodies for SBA are against PorA, when the VR type of the target strain matches that of the vaccine, and against FHbp, when that in the target strain is in the same subfamily as that in the vaccine. In contrast, the gonococcal SBA did not require antibodies to FHbp because the NOMV-KO vaccine elicited activity similar to that of the NOMV-FHbp vaccine. Mass spectrometry of the NOMV vaccines provides insight on potential meningococcal antigens that might cross-react with gonococci. However, further studies are necessary to determine whether one or more of these antigens elicits protection against gonococcal disease. Our hypothesis is that an effective combination meningococcal-gonococcal vaccine can be developed based on meningococcal NOMV overexpressing the cross-reactive gonococcal antigen(s).
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Howard Kim, Kelsey Sharkey, and Vianca Vianzon, University of California–San Francisco Benioff Children’s Hospital Oakland, for excellent technical assistance.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (grants R01 AI046464 [to D. M. G.], R01 AI114701 [to D. M. G. and P. T. B.], R01 AI099125 [to P. T. B.], R41 AI124759 [to G. R. M.], and R01 AI114790 and U01 AI118161 [to S. R. and L. A. L.]) and the National Center for Research Resources (grant C06 RR016226), National Institutes of Health.
Disclaimer. The authors were solely responsible for the investigation, design, data analysis, and writing of the manuscript.
Potential conflicts of interest. P. T. B., L. A. L., S. R., G. R. M., and D. M. G. are inventors on patent applications or on issued patents in the area of gonococcal or meningococcal vaccines. Rights to these inventions have been assigned to the University of California–San Francisco Benioff Children’s Hospital Oakland or the University of Massachusetts. E. I. certifies no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 21st International Pathogenic Neisseria Conference, Pacific Grove, California, September 2018.
References
- 1. Masignani V, Comanducci M, Giuliani MM, et al. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J Exp Med 2003; 197:789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Serruto D, Spadafina T, Ciucchi L, et al. Neisseria meningitidis GNA2132, a heparin-binding protein that induces protective immunity in humans. Proc Natl Acad Sci U S A 2010; 107:3770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tappero JW, Lagos R, Ballesteros AM, et al. Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile. JAMA 1999; 281:1520–7. [DOI] [PubMed] [Google Scholar]
- 4. Koeberling O, Seubert A, Granoff DM. Bactericidal antibody responses elicited by a meningococcal outer membrane vesicle vaccine with overexpressed factor H-binding protein and genetically attenuated endotoxin. J Infect Dis 2008; 198:262–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bovre K, Holten E, Vik-Mo H, et al. Neisseria meningitidis infections in Northern Norway: an epidemic in 1974–1975 due mainly to group B organisms. J Infect Dis 1977; 135:669–72. [DOI] [PubMed] [Google Scholar]
- 6. Caugant DA, Frøholm LO, Bøvre K, et al. Intercontinental spread of a genetically distinctive complex of clones of Neisseria meningitidis causing epidemic disease. Proc Natl Acad Sci U S A 1986; 83:4927–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O’Hallahan J, Lennon D, Oster P, et al. From secondary prevention to primary prevention: a unique strategy that gives hope to a country ravaged by meningococcal disease. Vaccine 2005; 23:2197–201. [DOI] [PubMed] [Google Scholar]
- 8. Bilal A, Taha MK, Caeymaex L, et al. Neonatal meningococcal meningitis in France from 2001 to 2013. Pediatr Infect Dis J 2016; 35:1270–2. [DOI] [PubMed] [Google Scholar]
- 9. Findlow J, Borrow R, Snape MD, et al. Multicenter, open-label, randomized phase II controlled trial of an investigational recombinant meningococcal serogroup B vaccine with and without outer membrane vesicles, administered in infancy. Clin Infect Dis 2010; 51:1127–37. [DOI] [PubMed] [Google Scholar]
- 10. Hou VC, Koeberling O, Welsch JA, Granoff DM. Protective antibody responses elicited by a meningococcal outer membrane vesicle vaccine with overexpressed genome-derived neisserial antigen 1870. J Infect Dis 2005; 192:580–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koeberling O, Seubert A, Santos G, et al. Immunogenicity of a meningococcal native outer membrane vesicle vaccine with attenuated endotoxin and over-expressed factor H binding protein in infant rhesus monkeys. Vaccine 2011; 29:4728–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Petousis-Harris H, Paynter J, Morgan J, et al. Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: a retrospective case-control study. Lancet 2017; 390:1603–10. [DOI] [PubMed] [Google Scholar]
- 13. Pérez O, del Campo J, Cuello M, et al. Mucosal approaches in Neisseria vaccinology. VacciMonitor 2009; 18:53–5. [Google Scholar]
- 14. Whelan J, Kløvstad H, Haugen IL, Holle MR, Storsaeter J. Ecologic study of meningococcal B vaccine and Neisseria gonorrhoeae infection, Norway. Emerg Infect Dis 2016; 22:1137–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Régnier SA, Huels J. Potential impact of vaccination against Neisseria meningitidis on Neisseria gonorrhoeae in the United States: results from a decision-analysis model. Hum Vaccin Immunother 2014; 10:3737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fletcher LD, Bernfield L, Barniak V, et al. Vaccine potential of the Neisseria meningitidis 2086 lipoprotein. Infect Immun 2004; 72:2088–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beernink PT, Shaughnessy J, Braga EM, et al. A meningococcal factor H binding protein mutant that eliminates factor H binding enhances protective antibody responses to vaccination. J Immunol 2011; 186:3606–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beernink PT, Shaughnessy J, Pajon R, Braga EM, Ram S, Granoff DM. The effect of human factor H on immunogenicity of meningococcal native outer membrane vesicle vaccines with over-expressed factor H binding protein. PLoS Pathog 2012; 8:e1002688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van der Ley P, Steeghs L, Hamstra HJ, ten Hove J, Zomer B, van Alphen L. Modification of lipid A biosynthesis in Neisseria meningitidis lpxL mutants: influence on lipopolysaccharide structure, toxicity, and adjuvant activity. Infect Immun 2001; 69:5981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pagotto F, Dillon JA. Multiple origins and replication proteins influence biological properties of beta-lactamase-producing plasmids from Neisseria gonorrhoeae. J Bacteriol 2001; 183:5472–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frasch CE, van Alphen L, Holst J, Poolman JT, Rosenqvist E. Outer membrane vesicle vaccines for meningococcal disease. In: Pollard AJ, Maiden MC, eds. Meningococcal vaccines: methods and protocols. Totawa, N.J: Humana Press, 2001:81–7. [DOI] [PubMed] [Google Scholar]
- 22. Giuntini S, Lujan E, Gibani MM, et al. Serum bactericidal antibody responses of adults immunized with the MenB-4C vaccine against genetically diverse serogroup B meningococci. Clin Vaccine Immunol 2017; 5:e00430–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lujan E, Partridge E, Giuntini S, Ram S, Granoff DM. Breadth and duration of meningococcal serum bactericidal activity in health care workers and microbiologists immunized with the MenB-FHbp vaccine. Clin Vaccine Immunol 2017; 24. doi: 10.1128/CVI.00121-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beernink PT, Welsch JA, Harrison LH, Leipus A, Kaplan SL, Granoff DM. Prevalence of factor H-binding protein variants and NadA among meningococcal group B isolates from the United States: implications for the development of a multicomponent group B vaccine. J Infect Dis 2007; 195:1472–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rossi R, Beernink PT, Giuntini S, Granoff DM. Susceptibility of meningococcal strains responsible for two serogroup B outbreaks on U.S. university campuses to serum bactericidal activity elicited by the MenB-4C Vaccine. Clin Vaccine Immunol 2015; 22:1227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Basta NE, Mahmoud AA, Wolfson J, et al. Immunogenicity of a meningococcal B vaccine during a university outbreak. N Engl J Med 2016; 375:220–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Law DK, Lefebvre B, Gilca R, et al. Characterization of invasive Neisseria meningitidis strains from Quebec, Canada, during a period of increased serogroup B disease, 2009–2013: phenotyping and genotyping with special emphasis on the non-carbohydrate protein vaccine targets. BMC Microbiol 2015; 15:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Law DK, Lorange M, Ringuette L, et al. Invasive meningococcal disease in Quebec, Canada, due to an emerging clone of ST-269 serogroup B meningococci with serotype antigen 17 and serosubtype antigen P1.19 (B:17:P1.19). J Clin Microbiol 2006; 44:2743–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lewis LA, Shafer WM, Dutta Ray T, Ram S, Rice PA. Phosphoethanolamine residues on the lipid A moiety of Neisseria gonorrhoeae lipooligosaccharide modulate binding of complement inhibitors and resistance to complement killing. Infect Immun 2013; 81:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ram S, Cullinane M, Blom AM, et al. C4bp binding to porin mediates stable serum resistance of Neisseria gonorrhoeae. Int Immunopharmacol 2001; 1:423–32. [DOI] [PubMed] [Google Scholar]
- 31. Gulati S, Agarwal S, Vasudhev S, Rice PA, Ram S. Properdin is critical for antibody-dependent bactericidal activity against Neisseria gonorrhoeae that recruit C4b-binding protein. J Immunol 2012; 188:3416–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Koeberling O, Delany I, Granoff DM. A critical threshold of meningococcal factor H binding protein expression is required for increased breadth of protective antibodies elicited by native outer membrane vesicle vaccines. Clin Vaccine Immunol 2011; 18:736–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jiang HQ, Hoiseth SK, Harris SL, et al. Broad vaccine coverage predicted for a bivalent recombinant factor H binding protein based vaccine to prevent serogroup B meningococcal disease. Vaccine 2010; 28:6086–93. [DOI] [PubMed] [Google Scholar]
- 34. Jongerius I, Lavender H, Tan L, et al. Distinct binding and immunogenic properties of the gonococcal homologue of meningococcal factor h binding protein. PLoS Pathog 2013; 9:e1003528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Longtin J, Dion R, Simard M, et al. Possible impact of wide-scale vaccination against serogroup B Neisseria meningitidis on gonorrhea incidence rates in one region of Quebec, Canada. ID Week 2017. San Diego, California: Oxford University Press, 2017:LB-3. [Google Scholar]
- 36. Gulati S, McQuillen DP, Mandrell RE, Jani DB, Rice PA. Immunogenicity of Neisseria gonorrhoeae lipooligosaccharide epitope 2C7, widely expressed in vivo with no immunochemical similarity to human glycosphingolipids. J Infect Dis 1996; 174:1223–37. [DOI] [PubMed] [Google Scholar]
- 37. Keiser PB, Biggs-Cicatelli S, Moran EE, et al. A phase 1 study of a meningococcal native outer membrane vesicle vaccine made from a group B strain with deleted lpxL1 and synX, over-expressed factor H binding protein, two PorAs and stabilized OpcA expression. Vaccine 2011; 29:1413–20. [DOI] [PubMed] [Google Scholar]
- 38. Keiser PB, Gibbs BT, Coster TS, et al. A phase 1 study of a group B meningococcal native outer membrane vesicle vaccine made from a strain with deleted lpxL2 and synX and stable expression of opcA. Vaccine 2010; 28:6970–6. [DOI] [PubMed] [Google Scholar]
- 39. Pajon R, Beernink PT, Harrison LH, Granoff DM. Frequency of factor H-binding protein modular groups and susceptibility to cross-reactive bactericidal activity in invasive meningococcal isolates. Vaccine 2010; 28:2122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Costa I, Pajon R, Granoff DM. Human factor H (FH) impairs protective meningococcal anti-FHbp antibody responses and the antibodies enhance FH binding. MBio 2014; 5:e01625–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Granoff DM, Costa I, Konar M, Giuntini S, Van Rompay KK, Beernink PT. Binding of complement factor H (FH) decreases protective anti-FH binding protein antibody responses of infant rhesus macaques immunized with a meningococcal serogroup B vaccine. J Infect Dis 2015; 212:784–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Granoff DM, Giuntini S, Gowans FA, Lujan E, Sharkey K, Beernink PT. Enhanced protective activity to a mutant meningococcal factor H-binding protein vaccine with low-factor H binding. JCI Insight 2016; 1:e88907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Piet JR, Huis in ‘t Veld RA, van Schaik BD, et al. Genome sequence of Neisseria meningitidis serogroup B strain H44/76. J Bacteriol 2011; 193:2371–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.