Abstract
Breast milk HIV-1 transmission is currently the predominant contributor to pediatric HIV infections. Yet, only ~10% of breastfeeding infants born to untreated HIV-infected mothers become infected. This study assessed the protective capacity of natural HIV envelope-specific antibodies isolated from milk of HIV-infected women in an infant rhesus monkey (RM), tier 2 SHIV oral challenge model. To mimic placental and milk maternal antibody transfer, infant RMs were i.v. infused and orally treated at the time of challenge with a single weakly-neutralizing milk monoclonal antibody (mAb), a tri-mAb cocktail with weakly neutralizing and ADCC functionalities, or an anti-influenza control mAb. Of these groups, the fewest tri-mAb-treated infants had SHIV detectable in plasma or tissues (2/6, 5/6, and 7/8 animals infected in tri-mAb, single-mAb, and control-mAb groups, respectively). Tri-mAb-treated infants demonstrated significantly fewer plasma transmitted/founder variants and reduced peripheral CD4+ T cell proviral loads at 8 weeks post-challenge compared to control mAb-treated infants. Abortive infection was observed as detectable CD4+ T cell provirus in non-viremic control mAb- and single mAb-, but not tri-mAb-treated animals. These results suggest that polyfunctional milk antibodies contribute to the natural inefficiency of HIV-1 transmission through breastfeeding and infant vaccinations eliciting non-neutralizing antibody responses could reduce postnatal HIV transmission.
Introduction
According to the 2016 UNAIDS report, approximately 150,000 pediatric infections occur annually, accounting for ~10% of new global HIV-1 infections 1. The benefits of breastfeeding to infant health are well recognized, yet vertical transmission of HIV-1 via breastfeeding results in nearly half of the annual mother-to-child-transmission (MTCT) occurrences 2. In resource-limited areas, formula-fed infants exhibit high mortality rates due to respiratory and diarrheal illnesses 3, 4 and thus, formula feeding is not a viable strategy to reduce pediatric HIV transmissions. While administration of antiretroviral therapy (ART) to HIV-1 infected, breastfeeding mothers reduces MTCT rates to below 5% 5, socioeconomic barriers to ART access and compliance 6, 7, as well as acute maternal infections make it unlikely that ART alone can achieve eradication of pediatric HIV-1 8–10. Therefore, developing effective immune-based prevention strategies, such as a maternal or infant vaccine to protect infants from oral HIV-1 acquisition during breastfeeding, may greatly contribute to the goal of achieving an HIV-free generation 11.
Despite repeated, daily mucosal HIV exposure during years of breastfeeding, only ~10% of breastfeeding infants of untreated HIV-infected mothers acquire HIV 11, suggesting the presence of protective factors in milk. The role of innate factors such as mucins 12, defensins 13, lactoferrin 14, long chain fatty acids 15, IL-15 16, and tenascin C 17 present in breast milk have been extensively studied for their anti-HIV activity. Additionally, the milk microbiome, particularly lactobacillus and pediococcal species, have been reported to inhibit HIV infection of target cells 18. In chronically HIV-infected mothers, breast milk also contains HIV-1 envelope (Env)-specific antibodies and Env-specific memory B cells 19, 20, which are primarily IgG1 isotype and are otherwise similar in specificity and function to those identified in blood of chronically infected individuals 21. While breast milk antibodies capable of ADCC have been associated with diminished vertical transmission rates 22 and reduced infant mortality after infection 23 in humans, the protective capabilities of polyfunctional milk antibodies remain unclear. Induction or passive infusion of broadly neutralizing antibodies (bNAbs) is an attractive immunologic strategy for global HIV control (reviewed in 24) including in the setting of postnatal HIV transmission 25, 26. Yet, bNAbs only develop naturally in fewer than 20% of individuals, typically take 2–4 years to develop after infection 27, and have been unable to be elicited through vaccination. Moreover, bNAbs have not been identified in breast milk 19, 28. Thus, the contribution of non- and weakly-neutralizing breast milk antibodies to the inefficiency of HIV-1 transmission through breastfeeding warrants further exploration.
In this study, we sought to define the impact of systemic and orally administered natural breast milk-derived maternal HIV Env-specific monoclonal antibodies (mAbs) with non- and weakly-neutralizing functions on infant oral virus acquisition and dissemination in the periphery and lymphoid tissues. MAbs selected for this study were isolated from milk B cells of a cohort of HIV-1-infected Malawian women and were intended to represent IgG antibodies with various antiviral functionalities and specificities of those commonly found in breast milk (ADCC, tier 1 and weak tier 2 neutralization, dendritic cell-virus binding inhibition, epithelial cell-virus binding inhibition, and C1, V3, CD4-blocking) 19, 28, 29. RMs were passively infused with the maternal breast milk mAbs to mimic antibody transfer via the placenta, and then repeatedly fed infant formula containing the maternal breast milk-derived mAbs, and low dose tier 2 chimeric simian/human immunodeficiency virus, SHIV-1157ipd3N4 30. Defining the contributions of non-broadly neutralizing breast milk-derived antibodies to protection against transmission of HIV-1 through breastfeeding may inform the design of maternal and infant vaccines aimed at eliminating postnatal HIV-1 infections and limiting the size of the latent viral reservoir in the setting of breakthrough infections.
Results
Selection of maternal breast milk mAbs for in vivo evaluation in infant monkeys and study design.
The HIV-1 Env-specific mAbs isolated from breast milk B cells of lactating, HIV-1-infected Malawian women 20 and selected for infusion into infant RMs in this study were initially characterized based on binding specificity, epithelial and dendritic cell-virus binding inhibition, ADCC, and neutralization against the tier 2 challenge virus in this study, SHIV-1157ipd3N4 30, as well as neutralization of several tier 1 HIV/SHIV variants (Figure 1A) 28. As previously reported, all of the mAbs isolated from milk B cells were IgG1, did not demonstrate broadly neutralizing activity, and had similar characteristics to those isolated from peripheral blood HIV-1 Env-specific B cells 20. The infusion mAbs were selected based on their diversity of functions and specificities; mAb DH378 demonstrated CD4-blocking capability associated with CD4 binding site (CD4bs)-specificity and mAb DH377 bound to linear and conformational variable loop 3 (V3) binding, specificities previously associated with decreased MTCT risk 31, 32, and mAb DH382 was constant region 1 (C1)-specific and competed with mAb A32-Env binding. MAbs DH378 and DH377 were able to block epithelial and dendritic cell-virus binding in vitro 28. Unsurprisingly, the A32-like mAb DH382 mediated potent ADCC activity against the tier 2 challenge SHIV-1157ipd3N4, with a maximum % specific killing of 40.7% and an endpoint concentration of <0.04µg/mL. Both DH378 and DH377 neutralized all tier 1 viruses tested, including SHIV SF162P4, SHIV BaL-P4, MW965.26, and SHIV-1157ipEL-p. Yet, DH378, but not DH377, demonstrated weak neutralization activity against the tier 2 clade C SHIV challenge virus when tested at high concentration (SHIV-1157ipd3N4 neutralization; DH378 IC50=79µg/mL, DH377 IC50>100 µg/mL).
Figure 1. Infant RM passive mAb infusion and oral SHIV challenge study design.
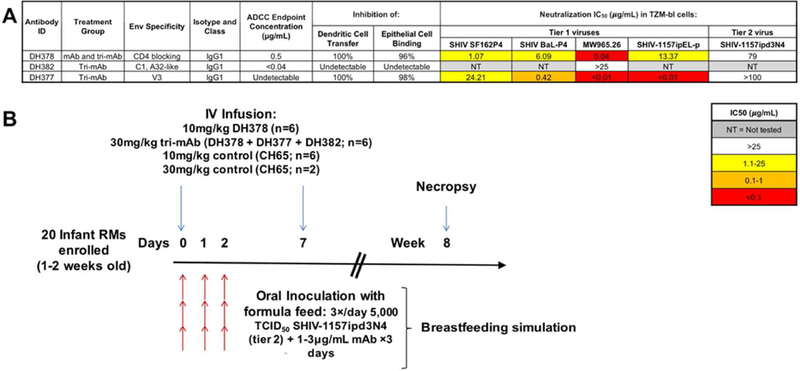
A) Functional characterization of maternal breast milk mAbs selected for passive infusion into infant RMs. MAbs were selected based on diverse functionality, including Env binding specificity and ADCC, inhibition of dendritic cell virus transfer, inhibition of epithelial cell-virus binding, and virus neutralization functionalities. B) Timeline for passive mAb infusion and SHIV-1157ipd3N4 challenge of infant RMs. Twenty infant RMs (1–2 weeks old) were systemically infused with the indicated mAbs and subsequently orally challenged after 1 hour with 5,000 TCID50 of the tier 2 SHIV-1157ipd3N4 in formula feed to mimic infant oral viral exposure during breast feeding. Animals were reinfused 7 days after the first mAb infusion with the same mAb dose to sustain systemic Ab titers. Animals were necropsied at 8 weeks after challenge and lymphatic and GI tract tissues were collected.
To assess the degree to which common HIV Env-specific polyfunctional milk antibodies contribute to protection against oral virus acquisition and replication, 20 infant RMs were divided into 2 anti-HIV-1 Env-specific breast milk mAb treatment groups (n=6) and a control mAb treatment group (n=8) for passive mAb infusion and oral SHIV-1157ipd3N4 challenge (Figure 1B). In this study scheme, systemic passive infusion was intended to simulate placental IgG transfer, while the oral inoculum was intended to represent oral exposure to the combination of milk HIV Env-specific antibodies at physiologic levels with infectious virus 19. The first treatment group consisted of 6 animals treated with DH378, the weakly neutralizing mAb against the tier 2 challenge SHIV. The second group consisted of 6 animals treated with an equimolar mixture of mAbs DH378, DH377, and DH382—a mixture containing mAbs with binding specificities including a specificity previously associated with decreased MTCT (V3) 31, 32, ADCC activity, dendritic and epithelial cell-virus binding inhibition, and weak tier 2 neutralization against the challenge SHIV. Anti-influenza hemagglutinin (HA) mAb CH65 was employed as a control mAb treatment in 8 animals, including total mAb dose controls for each anti-HIV mAb treatement group (10mg/kg CH65 in n=6 animals; 30mg/kg CH65 in n=2 animals). To simulate placental maternal Ab transfer, infants were infused intravenously with the mAbs 1 hour prior to the first of 9 oral SHIV-1157ipd3N4/mAb combination challenges in formula (3 times/day for 3 consecutive days). Animals were reinfused with the same mAb dose on day 7 to sustain detectable systemic mAb levels for at least 2 weeks after virus challenge. SHIV-challenged infant monkeys were then followed for plasma mAb kinetics, systemic cell-free and cell-associated virus loads, and were necropsied at 8 weeks for assessment of lymphoid and GI tract tissue viral loads (Figure 1B).
Pharmacokinetics of maternal milk HIV-1 mAb infusion of infant RM serum and saliva.
Serum mAb concentrations at longitudinal time points through 8 weeks post SHIV-challenge were measured by ELISA to determine the kinetics of the mAb persistence. Serum mAb levels in animals from all treatment groups were similar through 2 weeks post-infusion, achieving peak concentrations of 9.2×104- 4.5×106 ng/mL and variable rates of mAb clearance, presumably due to variability of the infant immune response against the recombinant mAb and clearance rates (Figure 2A-C; Figure S1).
Figure 2. The kinetics of passively infused mAbs and anti-SHIV Ab function in passively breast milk mAb-infused, SHIV-challenged infant RM serum and saliva.
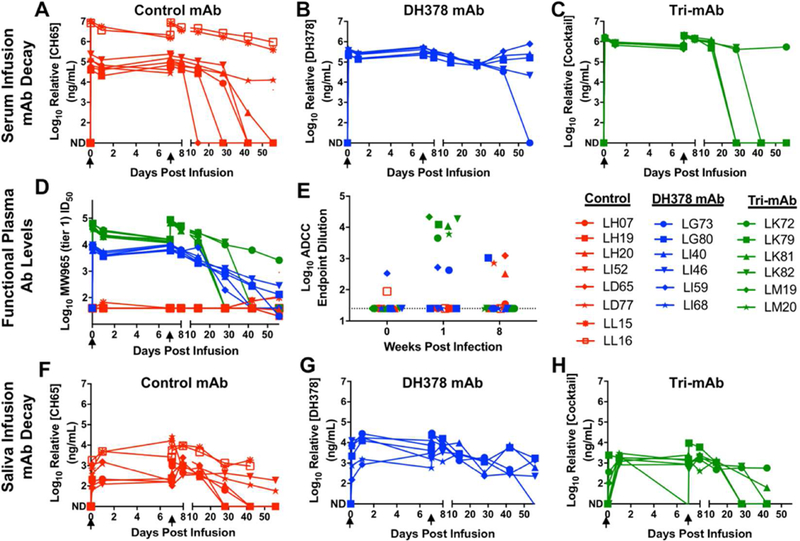
Concentrations of infused mAbs in A-C) serum and F-H) saliva from pre-infusion to 8 weeks post infusion are depicted for control mAb-treated (A,F; CH65; red), DH378 mAb-treated (B,G; DH378; blue), and tri-mAb-treated (C,H; DH378, DH377, DH382; green) infant RMs. D) Neutralizing Ab levels in serum reported as ID50 against the tier-1 virus MW965 from pre-infusion to 8 weeks post challenge. E) ADCC-mediating Ab levels in serum reported as endpoint titer against SHIV-1157ipd3N4 at preinfusion, 1 week (1 day after 2nd mAb infusion), and 8 weeks post challenge. Black arrows indicate systemic mAb infusions at days 0 and 7. ND indicates not detectable.
Plasma neutralizing antibody (NAb) and ADCC levels were measured to assess in vivo functionality of the infused treatment mAbs and the development of host humoral responses (Figure 2B, C). Predictably, NAb responses against the tier 1 clade C virus MW965 were high in all DH378- and tri-mAb-treated animals early after mAb infusion, and the magnitude of the responses declined to undetectable in some animals as early as 6 weeks post challenge when circulating mAb levels declined (Figure 2D). Interestingly, several control animals (LH20, LI52, LD65, LD77, LL15) developed tier 1, MW965 NAb responses as early as 6 weeks post-challenge. None of the animals had detectable plasma neutralization against either the tier 2 SHIV-1157ipd3N4 challenge virus or the related tier 1 virus SHIV-1157ipEL-p 33 at peak mAb concentrations or 8 weeks post challenge, which was unsurprising as serum mAb concentrations never surpassed that of the in vitro IC50 (Figure 1A). Serum ADCC endpoint titers against SHIV-1157ipd3N4-infected CD4+ T cells were measured preinfusion, at peak infusion mAb concentration (1 day after second infusion), and at 8 weeks post infection for control and mAb-treated RMs (Figure 2E). As expected, serum from tri-mAb-treated animals exhibited high magnitude ADCC function (mean endpoint dilution=12,303), which deteriorated by 8 weeks post initial infusion to undetectable levels. Two of 6 DH378-treated animals demonstrated low magnitude ADCC function detectable at peak mAb levels (mean endpoint dilution=175), comparable to that of background levels detected in some infants (Figure 2E). Notably, 2 of 8 control mAb-treated and 1 of 6 DH378-treated animals, but none of the tri-mAb-infused infant RMs, developed detectable autologous ADCC-mediating mAbs against the challenge SHIV strain after infection at 8 weeks post initial infusion, presumably related to virus load at 8 weeks of infection.
Saliva mAb concentrations were also measured prior to and following oral challenge (Figure 2F-H). All animals demonstrated detectable mAb levels in saliva at 1 hour post infusion, the time of the first SHIV challenge. Saliva mAb levels peaked around 1 day post infusion in all animals and only slightly declined by 7 days post initial infusion in most animals.
Effect of maternal milk HIV-1 antibody treatment on SHIV acquisition and plasma viremia in orally challenged infant RMs.
To characterize the effectiveness of the mAb treatments in preventing SHIV acquisition, SHIV plasma viral RNA loads of orally-challenged infant RMs were measured in longitudinal samples by qRT-PCR. 6/8 (75%) CH65-treated control animals, 3/6 (50%) DH378-treated animals, and 2/6 (33%) tri-mAb-treated animals became detectably viremic after 9 oral SHIV challenges resulting in statistically similar systemic SHIV acquisition rates between controls and each treatment group (Figure 3A; FDR corrected Fisher’s exact test; Controls vs. DH378 p=0.58, tri-mAb cocktail p=0.55). Peak and set point viral RNA loads in plasma were largely similar between CH65-treated control animals (median and range; peak=6.2×106copies/mL, 9.3×105-2.7×107copies/mL; set point=4.4×105copies/mL, 5.4×104-9.5×107copies/mL) and tri-mAb-treated animals (median and range; peak=2.1×106copies/mL, 5.3×105-3.6×106copies/mL; set point=3.0×105copies/mL, 1.2×104-5.9×105copies/mL) when excluding animals with undetectable plasma viral RNA loads (Figure 3B). Interestingly, the tri-mAb cocktail-treated animal LK81 emerged as an outlier with the lowest plasma peak and set point viral RNA loads observed in a viremic animal from this study (peak=5.3×105copies/mL; set point=1.2×104copies/mL). While set point viral RNA loads in plasma were similar between viremic CH65-treated control animals and viremic DH378-treated animals (median and range; peak=7.7×107copies/mL, 7.4×107- 1.1×108copies/mL; set point=5.1×105copies/mL, 3.5×105-8.9×106copies/mL), the peak viral RNA loads in plasma from viremic DH378-treated animals was significantly higher than that from viremic controls (Figure 3B; FDR-corrected Wilcoxon test; FDR-corrected p=0.05). Given the ranges of peak and set point viral RNA loads, MHC typing was performed to rule out unbalanced MHC types associated with natural control of viremia. Several infants possessed MHC alleles highly (Mamu-A001, Mamu-B017) or weakly (Mamu-A002, Mamu-B047) associated with low set point viral loads and/or longer survival lengths (Table 1) 34–41. Yet, these protective alleles were not more common in any single treatment group (67% for control mAb-, and tri-mAb-treated animals, and 80% for DH378 mAb-treated animals had least 1 protective MHC allele). Additionally, all groups also contained animals with the Mamu-A004 allele, which is associated with increased set point viral loads 42. All viremic, DH378 mAb-treated animals possessed this allele, which could contribute to the significantly higher plasma viral loads observed in these animals (Figure 3B).
Figure 3. Infection rates and plasma viral loads of passively breast milk mAb-infused, SHIV-challenged infant RMs.
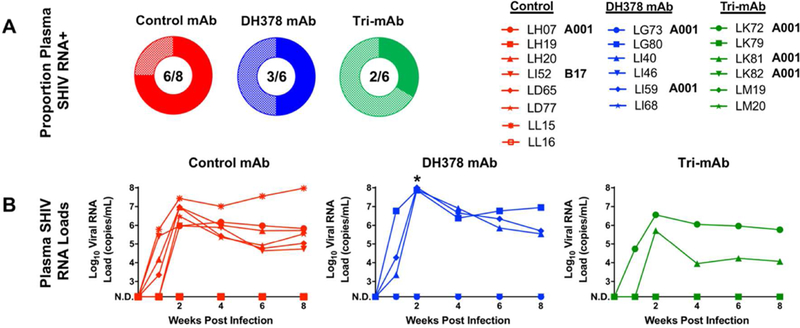
A) Proportion of viremic RMs with 6/8 control mAb-treated RMs (red), 3/6 DH378 mAb-treated RMs (blue; Fisher’s exact test; FDR-corrected p=0.58), and 2/6 tri-mAb-treated RMs (green; Fisher’s exact test; FDR-corrected p=0.55) demonstrating viremia as defined by a detectable plasma viral RNA load at any tested time point. B) Plasma viral RNA loads in mAb treated, orally SHIV challenged infant RMs. * indicates significantly higher 2 week post infection plasma viral RNA loads in DH378 mAb-treated RMs compared to CH65-treated RMs (Wilcoxon test; FDR-corrected p=0.05). Animals with HLA type A001 or B17 are annotated appropriately in the legend.
Table 1.
MHC alleles present in control mAb-, DH378 mAb-, and tri-mAb-treated and SHIV-1157ipd3N4-challenged infant RMs.
| Animal ID | Treatment Group | Infection Status | Mamu-A | Mamu-B | Mamu-DRB | |||
|---|---|---|---|---|---|---|---|---|
| Haplotype 1 | Haplotype 2 | Haplotype 1 | Haplotype 2 | Haplotype 1 | Haplotype 2 | |||
| LH07 | Control mAb | Viremic | A001 | A008 | B055 | B024a | DR01a | DR14a |
| LH20 | A002a | A004 | B001a | B001a | DR01a | DR016 | ||
| LI52 | A006 | A224a | B015a | B017a | DR03f | DR016 | ||
| LL15 | A007 | A008 | B001a | B055 | DR04a | DR06 | ||
| LH19 | Non- viremic | A004 | A008 | B021a | B047a | DR01a | DR06 | |
| LL16 | A004 | A008 | B012b | B080 | DR03a | DR11a | ||
| LG80 | DH378 mAb | Viremic | A004 | A011 | B055 | B012b | DR11c | DR02’ |
| LI59 | A001 | A004 | B024a | B048 | DR03a | DR03f | ||
| LI40 | A002a | A004 | B012a | B012a | DR03a | DR03f | ||
| LG73 | Non- viremic | A001 | A011 | B055 | B001a | DR01a | DR04a | |
| LI46 | A002a | A002a | B048 | B048 | DR04a | DR03f | ||
| LK72 | Tri-mAb | Viremic | A001 | A002a | B024a | B012a | DR03f | DR03f |
| LK81 | A001 | A008 | B043a | B047a | DR01a | DR13a | ||
| LK79 | Non-viremic | A002a | A004 | B001a | B012a | DR11a | DR03f | |
| LK82 | A002a | A001 | B001a | B001a | DR11a | DR01a | ||
| LM19 | A004 | A004 | B001a | B002 | DR03a | DR13a | ||
| LM20 | A004 | A008 | B015a | B015b | DR03a | DR01a | ||
Cell-associated SHIV load in blood and tissues from breast milk mAb-treated, orally SHIV-challenged infant RMs.
To determine whether the presence of pre-existing HIV-1 Env-specific mAbs impacted the cell-associated viral load in blood and tissue compartments following oral challenge in mAb-infused infant RMs, tissue mononuclear cell proviral loads, proportions of viral RNA producing T cells, and tissue-associated infectious virus titers were measured in PBMCs, GI tract, and lymphoid tissues (Figure 4). SHIV provirus in CD4+ T cells in PBMCs (preinfection, 2 and 8 weeks post-infection), GI, and lymphoid tissues at 8 weeks post challenge in viremic infants demonstrated widely variable, but routinely detectable proviral loads (104-107 gag copies/million CD4+ T cells) independent of tissue type or mAb treatment (Figure 4A). SHIV provirus was undetectable in PBMCs or any tissues isolated from tri-mAb-treated animal LK81, which exhibited detectable, albeit relatively low plasma SHIV RNA loads.
Figure 4. Tissue-associated SHIV measures in mononuclear cells isolated from lymphoid and GI tissues of viremic infant RMs 8 weeks post passive breast milk mAb infusion and oral SHIV challenge.
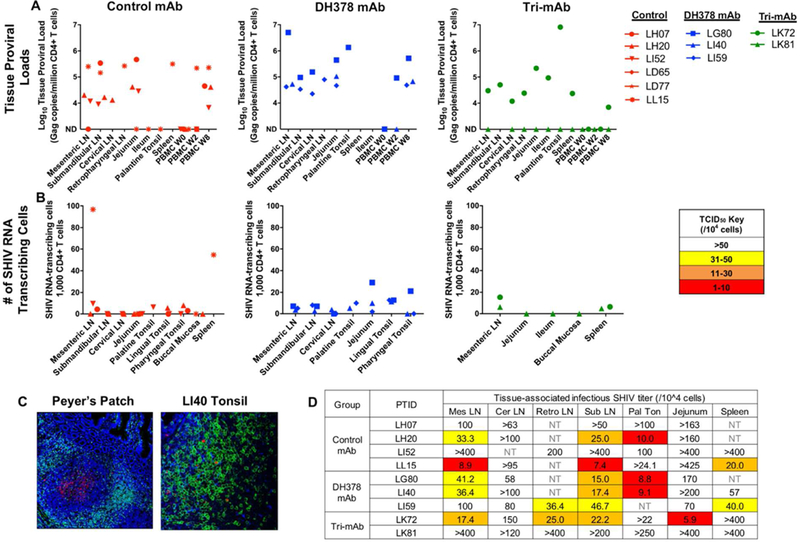
A) CD4+ T cell proviral DNA loads reported as copy number/million CD4+ T cells in lymphoid and GI tissue mononuclear cells and B) number of SHIV RNA producing CD3+ T cells in lymphoid and GI tissue blocks reported as the number of SHIV Gag RNA+ cells per 1,000 CD3+ T cell were measured for control mAb-treated (red), DH378 mAb-treated (blue), and tri-mAb-treated RMs (green) through ddPCR and in situ hybridization, respectively. ND indicates not detectable. C) Representative images of stained tissue blocks demonstrating active SHIV RNA transcription within a Peyer’s Patch (LI40 jejunum) and in CD3+ T cells in lymphoid tissue (LI40 lingual tonsil) employed to assess the number of SHIV RNA transcribing cells within lymphoid and GI tissues. Blue, green, and red depict nuclear, CD3, and SHIV Gag RNA staining, respectively. D) Tissue-associated infectious SHIV-1157ipd3N4 titers measured through tissue mononuclear cell coculture with TZM-bl reporter cells. Reported titers represent the estimated minimum number of mononuclear cells required to yield detectable infection (cutoff defined by mononuclear cells from unchallenged RM negative controls) of the TZM-bl cells in 50% of replicates. The cutoff for The limit of detection varied for each sample and was determined by mononuclear cell availability. NT indicates untested samples.
The proportion of SHIV RNA transcribing cells as measured by in situ hybridization assays was also similar in lymphoid and GI tissues from animals in all treatment groups. However, mesenteric lymph node and spleen from control mAb-treated animal LL15 exhibited a notably higher prevalence of SHIV RNA positive cells compared to other SHIV-infected infants (Figure 4B). Extensive SHIV RNA production was observed within and surrounding Peyer’s patches of the small intestine as well as in CD3+ T cells of lymphoid tissues (Figure 4C). Additionally, SHIV RNA detection within the Peyer’s patches largely failed to colocalize with CD3 expression, indicating viral association with dendritic cells and/or other APCs within these germinal centers.
To assess the level of cell-associated infectious SHIV within oral-associated lymphoid and GI tissues at 8 weeks post-challenge, tissue mononuclear cells were cocultured with TZM-bl reporter cells with luminescence output indicating the level of infectious SHIV production (Figure S2). In general, tissue-associated infectious SHIV titers were similar between treatment groups (Figure 4D). However, the palatine tonsil exhibited the highest cell-associated infectious titers of any tissue in 4 of 7 infants with sufficient tonsil tissue for the assay. Furthermore, this assay employed total mononuclear cells and flow cytometric analysis revealed that proportions of CD4+ T cells of total CD45+ mononuclear cells were lower in palatine tonsil than other lymphoid tissues (Figure S3), indicating a potential highly infectious tissue-associated virus titer in palatine tonsil. Additionally, two viremic animals, CH65-treated animal LH07 and tri-mAb-treated animal LK81, exhibited undetectable tissue-associated infectious virus titers.
Proviral loads, tissue-associated infectious virus titers, and tissue viral RNA levels in PBMCs (preinfection, 2 and 8 weeks post-infection), GI tract tissues, and lymphoid tissues in non-viremic, SHIV-challenged infant RMs were also measured to assess the possibilities of abortive infection or low level replication in the non-viremic infant RMs (Figure 5). Several animals with undetectable plasma SHIV RNA loads throughout the study including DH378-treated animals LG73 and LI46, and CH65-treated animal LH19, had detectable SHIV proviral DNA loads in various tissues at similar levels to those measured from animals with detectable plasma SHIV RNA loads (Figure 5A). To confirm the presence of tissue-associated virus in these non-viremic animals, the SHIV proviral envelopes were amplified by PCR of genomic DNA (gDNA) from submandibular LN CD4+ T cells of LH19 and LG73, sequenced to demonstrate the presence of full-length envelope open reading frames, and expressed as pseudovirus in the SG3∆env backbone to assess env infectious functionality (Figure 5B). Both pseudoviruses were capable of infecting TZM-bl reporter cells with the LH19 and LG73 env pseudoviruses eliciting luminescence magnitudes of 571,294 RLU and 176,820 RLU, respectively, which was appreciably higher than that of cell only controls (~500 RLU).
Figure 5. Tissue-associated SHIV DNA in CD4+ T cells isolated from lymphoid and GI tissues of non-viremic infant RMs 8 weeks post passive breast milk mAb infusion and oral SHIV challenge.
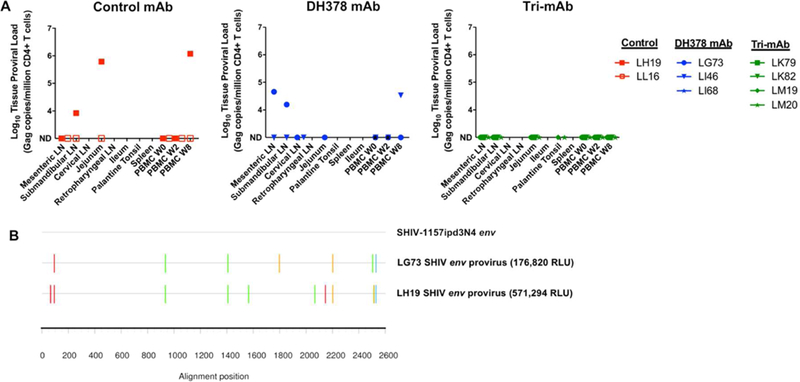
A) Proviral DNA loads reported as copy number/million CD4+ T cells in lymphoid and GI tissue mononuclear cells were measured for control mAb-treated (red), DH378 mAb-treated (blue), and tri-mAb-treated RMs (green) through ddPCR. ND indicates not detectable. B) Highlighter plot alignments of 2 amplified SHIV env sequences obtained through SGA of extracted DNA from CD4+ T cells isolated from LH19 and LG73 submandibular lymph nodes compared to the SHIV-1157ipd3N4 env stock sequence. Colored lines indicate nucleotide mismatches compared to the SHIV stock. These envs were cloned and subsequently incorporated into pseudoviruses with a SG3∆env backbone through co-transfection in 293T cells. Pseudovirus infectivity was estimated in TZM-bl reporter cells as RLUs compared to cell-only controls (<500 RLU). Pseudoviruses expressing env isolated from LH19 and LG73 demontstrated infectious capability with 571,294 RLU and 176,820 RLU magnitudes, respectively.
Thus, 4 infants, at least 1 from each mAb treatment group, had discordance between the presence of detectable plasma viral RNA and CD4+ T cell provirus (Figure 6A). Provirus was observed in CD4+ T cells isolated from non-viremic animals LG73, LI46, and LH19. Alternatively, provirus was not detectable in the viremic animal LK81. Notably, prior to correction for multiple comparisons, tri-mAb-treated infants, but not DH378-treated infants, demonstrated lower magnitude proviral loads in 8 week PBMCs compared to control mAb-treated animals (Wilcoxon test; raw p=0.03; FDR-corrected p=0.18). Taken together, 7/8 CH65- treated control animals, 5/6 DH378-treated animals, and 2/6 tri-mAb-treated animals demonstrated either detectable viremia or proviral loads following oral SHIV challenge (Figure 6B).
Figure 6. Summary of SHIV detection and proportion of animals with detectable cell-free or cell-associated SHIV in breast milk mAb-infused, orally SHIV-challenged infant RMs.
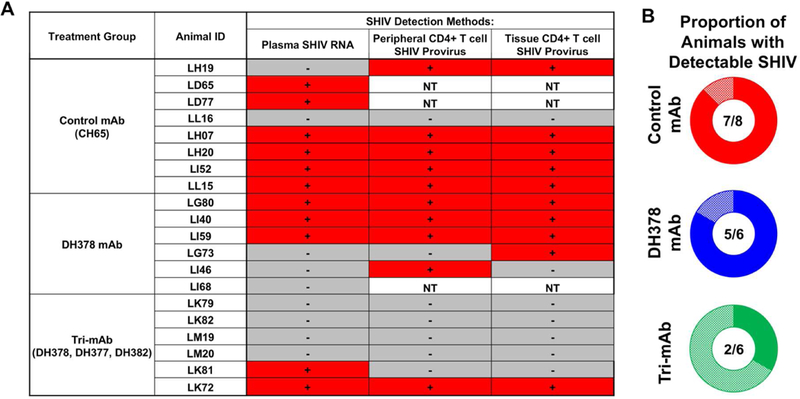
A) Binary indication of detectable plasma SHIV RNA, peripheral CD4+ T cell SHIV provirus, and tissue CD4+ T cell SHIV provirus detection. For each animal, SHIV detection methods with detectable SHIV in any tested tissue or at any timepoint were indicated with a red “+”, while those with undetectable values in all tested tissues and at all timepoints were indicated with a grey “-”. B) Proportion of animals with detectable cell-free or cell-associated SHIV as defined by detectable SHIV plasma RNA, SHIV provirus in tissues or PBMCs, tissue mononuclear cell-associated infectious SHIV titers, or SHIV RNA-transcribing cells.
Plasma SHIV variant diversity in mAb-treated and orally SHIV-infected infant RMs
To assess the potential impact of maternal mAb passive infusion of infants on the genetic bottleneck of mucosal virus transmission and SHIV env diversification by peak viremia (2 weeks post challenge), single SHIV variants were isolated from infant RM plasma using single genome amplification (SGA). Of note, the median viral diversity of the SHIV-1157ipd3N4 challenge virus stock was 0.02% (range=0–0.07%), considerably lower than the diversity typically observed in chronically HIV-1-infected humans (>1% diversity). Viral diversity within each animal was visualized with phylogenetic tree and highlighter plot (Figure S4). Distinct SHIV transmitted/founder (T/F) variants present in plasma isolated at peak viremia (2 weeks post challenge) were enumerated using previously established criteria 43, requiring that a genotypically distinct variant contain 2 novel mutations (to minimize the possibility of recombination yielding false results), and that these mutations be observed in at least 2 SGAs (to minimize the effects of PCR-related mutation). A similar number of distinct SHIV variants were present in plasma at peak viremia between control mAb-treated RMs (Table 2; n=8; median variants isolated=2.5; range=0–4) and DH378 mAb-treated animals (n=6; median variants isolated=0.5; range=0–2; Wilcoxon test; raw p=0.16). However, plasma SHIV diversity at peak viremia was lower in tri-mAb-treated RMs (n=6; median variants isolated=0; range=0–1; Wilcoxon test; raw p=0.05), indicating a possible sieve effect of the tri-mAb treatment on oral SHIV acquisition. SHIV env amplicons from all infants in a single tree rooted to the SHIV- 1157ipd3N4 challenge virus env revealed that the tri-mAb-treated RMs LK72 and LK81 are more homogenous compared to the other 2 infusion groups (Figure S5).
Table 2.
Diversity of SHIV-1157ipd3N4 variants in serum isolated 1 week post initial oral challenge from control mAb-, DH378 mAb-, or tri-mAb-treated infant RMs.
| Control mAb (Animal ID) | # of Variants | SGA Sequences per Animal | DH378 mAb (Animal ID) | # of Variants | SGA Sequences per Animal | Tri-mAb (Animal ID) | # of Variants | SGA Sequences per Animal |
|---|---|---|---|---|---|---|---|---|
| LI52 | 4 | 33 | LG80 | 2 | 30 | LK72 | 1 | 25 |
| LD65 | 3 | 36 | LI59 | 2 | 18 | LK81 | 1 | 32 |
| LH20 | 3 | 23 | LI40 | 1 | 21 | LK79 | 0 | - |
| LL15 | 3 | 27 | LI46 | 0 | - | LK82 | 0 | - |
| LH07 | 2 | 16 | LG73 | 0 | - | LM19 | 0 | - |
| LD77 | 1 | 42 | LI68 | 0 | - | LM20 | 0 | - |
| LH19 | 0 | - | ||||||
| LL16 | 0 | - | ||||||
| Median (Range) # Variants | 2.5 (0–4) | Median (Range) # Variants | 0.5 (0–2) | Median (Range) # Variants | 0 (0–1) | |||
Discussion
Despite daily chronic oral exposure to HIV for up to 2 years, only ~10% of breastfeeding infants of untreated HIV-infected mothers acquire HIV 11. Furthermore, postnatally HIV-infected infants demonstrate slower disease progression compared to in utero or peripartum infected infants 44. Therefore, a better understanding of the naturally protective components in breast milk of HIV-infected mothers, including milk antibodies, could aid in the development of effective vaccination strategies aimed at reducing MTCT and other modes of HIV acquisition. HIV-specific neutralizing antibodies have been shown to reduce chronic viral loads and enhance host anti-HIV B cell responses in orally challenged, infected RM infants 45. Additionally, post-exposure infusion of an anti-HIV bNAb cocktail within 24hrs after infection was capable of SHIV clearance in infant RMs 26. While tier 1 virus weakly-neutralizing antibodies predicted reduced MTCT rate 32, maternal plasma HIV-specific antibody potency and breadth has recently been associated with increased incidence of vertical transmission 46. Yet, HIV-specific ADCC-mediating antibody levels have been associated with reduced vertical transmission rates and infant mortality 22, 23. Thus, the potential protective effects of maternal breast milk antibodies lacking neutralization breadth and potency remains uncertain. In this study, we sought to probe the protective capabilities of several weakly or non-neutralizing, polyfunctional milk mAbs previously isolated from a cohort of HIV-infected Malawian women in an infant RM passive infusion and oral SHIV challenge model. The three maternal mAbs selected for inclusion in this study were intended to represent mAbs exhibiting various anti-HIV functionalities (ADCC, tier 1 and weak tier 2 neutralization, dendritic cell-virus binding inhibition, epithelial cell-virus binding inhibition) and HIV Env specificities (C1, V3, CD4-blocking). The treatment groups in this study were designed to assess whether a CD4-blocking mAb with tier 1 and weak autologous tier 2 neutralization (DH378 mAb treatment group), or a polyclonal mAb infusion with multiple antiviral functionalities (tri-mAb treatment group) could contribute to protection.
As expected, serum from groups treated with mAbs DH377 and/or DH378 exhibited neutralization against tier 1 virus, MW965, and serum from groups treated with mAb DH382 exhibited ADCC against the challenge SHIV. The lack of SHIV-1157ipd3N4 neutralization by DH378-infused animals was expected as mAb levels of DH378 did not reach the IC50 for SHIV-1157ipd3N4 neutralization (79µg/mL), given the limitation of the infusion volume in infant RMs. Of note, this in vitro estimation of neutralization potency in TZM-bl reporter cells does not necessarily directly predict the in vivo effect of infused mAbs 47. Several viremic animals treated with the control mAb developed neutralization responses against MW965 and ADCC responses against the challenge SHIV by week 8. Yet, only one viremic animals from both anti-HIV mAb-treated groups exhibited adaptive ADCC responses at week 8, which could indicate mAb blocking of immunogenic epitopes.
Plasma viremia was assessed to classify the extent to which anti-HIV breast milk mAb-treated animals were protected from systemic SHIV acquisition relative to the control mAb CH65-infused animals. Importantly, as maternal mAbs employed in this study did not coevolve with the challenge virus, which would be the case in the natural setting of infant HIV-1 exposure, the observed protection facilitated by these mAbs may underrepresent that which would occur against autologous viral strains. Both the DH378-treatment and the tri-mAb cocktail treatment groups had a lower number of viremic animals compared to the control mAb-infused group (6/8 animals viremic), with only 3/6 and 2/6 animals becoming viremic, respectively (Figure 3). Yet, this potential partial protection mediated by milk mAbs lacked statistical significance when compared to controls. Additionally, peak VLs, but not set point VLs, in DH378-treated, viremic animals were significantly higher than that of control animals and this difference was not attributable to more protective MHC-types in the control animals (Table 1). This apparent mAb effect on plasma VL could be due to selection of more fit variants with subtle differences in time to peak VL not appreciated by our sampling schedule, and/or antibody- dependent enhancement of viral infection of target cells . Antibody-dependent enhancement of HIV infection, observed as increased T/F variants and/or higher infection rates, has been previously associated with the presence of poorly-neutralizing, HIV-specific antibodies similar to mAb DH378 48.. However, peak VLs in the tri-mAb-treated, viremic animals were not elevated over those in control animals, indicating that a cocktail of functionally diverse mAbs may negate the potential effects of a single mAb on viral replication. Additionally, tri-mAb-treated animal LK81 demonstrated the lowest peak and set point viral loads observed in any viremic animal (Figure 3). This enhanced viral control post-acquisition may be attributable to the animal MHC background with 2 protective MHC alleles, Mamu-A001 and Mamu-B047a 34–41, however, other viremic animals sharing the Mamu-A001 allele, including LK72 with 2 protective alleles, did not demonstrate notably lower viral loads (Table 1). Furthermore, these MHC alleles are not associated with decreased peak viral loads 34, 40.
Tissue-associated virus was assessed in viremic animals at 8 weeks via multiple approaches to fully characterize the state of the tissue-associated virus load within lymphoid and GI tract tissues (Figure 4). The size of the resting memory cell latent reservoir in these infant RMs was not directly assessed due to limitations of cell numbers. While limited differences in measures of virus-infected cells were observed between most infant RMs regardless of treatment groups, tri-mAb-treated animal LK81 did not have detectable proviral loads or tissue mononuclear cell-associated infectious viral titers, and only low levels of active viral transcription in only 2 of 5 tissues tested. This finding is consistent with the low plasma viremia observed in this animal, and potentially attributable to the pre-existing tri-mAb anti-HIV functionalities. While our characterizations of tissue-associated virus were otherwise similar between treatment groups, tissue mononuclear cell-associated infectious viral titers indicated that the tonsillar tissue had heightened infectious SHIV levels compared to other lymphoid and GI tissues. This result aligns with previous reports indicating the susceptibility of tonsillar tissue as a portal of HIV entry and amplification 49, and further suggests that the tonsils may be a key anatomical site for strategies seeking to prevent or treat HIV infections, particularly through the oral route.
Interestingly, we observed high proviral loads in a range of tissues from several control and DH378 mAb-treated non-viremic animals (Figure 5). These findings indicate either abortive or very low level SHIV infection. Generation of pseudoviruses containing 2 proviral envelope genes (env) isolated from these animals demonstrated that they were capable of infection in vitro, indicating functional envelope genes of these proviruses. However, the viability of the full length provirus was not assessed due to inability to amplify the full genome. Notably, detectable provirus was not observed in CD4+ T cells isolated from any tissues of all non-viremic, tri-mAb-treated animals. This finding indicates that the tri-mAb cocktail successfully prevented or eliminated even abortive infection in these animals. Alternatively, Liu et al. recently demonstrated that passive bNAb infusion mediating complete protection from subsequent intravaginal SHIV challenge in RMs did not prevent initial virus acquisition and proliferation in distal tissues, but rather that the bNAb successfully cleared this infection by around 10 days post-challenge 50. Similarly, Hessell et al reported that 24-hour post exposure passive infusion of an anti-HIV bNab cocktail in SHIV-challenged infant RMs cleared virus from blood and tissues 26. Hessell et al also demonstrated that passive infusion of RMs with a V2i-specific mAb prior to repeated SHIV challenges reduced blood viral loads and lymphoid tissue proviral loads 51. Thus, perhaps the various anti-SHIV functionalities mediated by the tri-mAb treatment in this study similarly cleared the initial infection of distal tissues. To determine whether the tri-mAb treatment prevented initial SHIV acquisition in these animals or mediated clearance of SHIV in distal tissues, additional work characterizing the viral populations present in distal lymphoid tissues at various early time points post-challenge is necessary. Regardless of the mechanism leading to uninfected distal tissues, we speculate that the potent ADCC-mediating mAb, DH382, may have played a pivotal role since the weakly-neutralizing DH378 mAb treatment alone failed to similarly limit tissue-associated proviral loads.
Finally, the SHIV diversity at peak viremia in infant RMs was measured to assess the capability of these milk mAbs to limit the number of transmitted/founder (T/F) variants transmitted across the mucosa and/or early SHIV diversification after oral challenge (Table 2). The fewer distinct SHIV variants isolated from tri-mAb-treated animals compared to control mAb-treated animals indicates that this cocktail of maternal mAbs successfully limited the number of SHIV variants capable of sustaining infection in infant RMs. As DH378 mAb-treated infant RMs exhibited similar numbers of SHIV plasma variants compared to control mAb-treated animals, again the combination of distinct antibody functions, with a particular emphasis on ADCC, is likely important for reducing SHIV variant transmission. Of note, as only day 14 post challenge SHIV variants were isolated to assess viral diversity, the mechanism of mAb-mediated control of SHIV diversity may include limiting the number of initial T/F variants, minimizing post infection diversification, or a combination of both.
In conclusion, a combination of mAbs with diverse Env epitope specificity, weak neutralization capabilities, and ADCC functionality, appeared effective at lowering the number of T/F SHIV variants, and potentially SHIV levels in circulating CD4+ T cells and tissues in SHIV-challenged infant RMs. Taken together, these trends suggest that polyclonal non-broadly neutralizing maternal mAbs with multiple functionalities, and particularly ADCC, may contribute to the naturally limited MTCT during breastfeeding, potentially through inhibition of initial virus acquisition at mucosal sites or through rapid viral clearance in the tissues. A better understanding of the mechanism of this protection as well as other maternal and/or infant factors that contribute to the relative protection of infants in the setting of breastfeeding may lead to more effective pediatric HIV therapeutic and prophylactic vaccine design strategies.
Materials and Methods
More detailed description of experimental methods provided in Supplemental Methods.
Study Design
Twenty infant rhesus macaques (RM; 1–2 weeks old) were IV infused with either 10mg/kg anti-HIV Env gp120 monoclonal antibody (mAb) DH378 (n=6), 10mg/kg anti-influenza HA mAb CH65 (n=6), 30mg/kg α-HA mAb CH65 (n=2), or 30mg/kg of a tri-mAb cocktail composed of stoichiometric equivalents of 3 anti-HIV Env gp120 mAbs (n=6)—DH377, DH378, and DH382—one hour prior to the first oral SHIV challenge. Animals were subjected to 3 oral challenges per day for 3 consecutive days consisting of 5,000 TCID50 SHIV-1157ipd3N4 (NIH AIDS Reagent Program) incubated in 1mL of RPMI containing 1–3µg/mL DH378, CH65, or tri-mAb cocktail for 15 minutes, followed by dilution in ~10mL formula feed to simulate oral acquisition via breastfeeding. Animals were re-infused with their respective antibody infusions one week after the initial infusion. Blood and saliva (via weck cell sponges) were collected before each infusion, 1 hour after each infusion, 1 day after each infusion, and at weeks 2, 4, 6, and 8 of the study. All animals were necropsied at week 8 of the study and tissues were collected.
Production of infusion mAbs
Infusion mAbs were obtained through antigen-specific B cell sorting and Ig variable gene amplification, as previously described 20, and produced through transient transfection either by the manufacturer Catalent (DH378; Catalent) or at the Duke Human Vaccine Institute in Expi293 cells (DH377, DH382, CH65), as previously described 52.
HIV-1 Neutralization in TZM-bl Cells
Neutralizing antibody titers were measured by the reduction in Tat-regulated Luc reporter gene expression in a TZM-bl (NIH AIDS Reagent Program) reporter cell assay, as previously described 53.
Tissue Mononuclear Cell Viral Coculture
Tissue-associated infectious virus titer was assessed through Tat-regulated Luc-F reporter gene expression to quantify infection of TZM-bl reporter cells after coculture with serial dilutions of tissue mononuclear cells isolated from RMs.
Plasma Viral RNA Load Quantification
Reverse transcriptase quantitative PCR was performed to determine the SHIV-1157ipd3N4 RM plasma RNA load, as previously described 54.
Mononuclear Cell Provirus Quantification
RM CD4+ T cell-associated genomic DNA (gDNA) was isolated from various GI and lymphoid tissues with the QIAaMP DNA/RNA extraction kit (Qiagen) and quantified using the Biorad QX200 droplet digital PCR System according to the manufacturer instructions (Biorad) with SHIV gag specific primers and probe and a commercially available human TERT-specific reference assay (Biorad). The SHIV proviral load in SHIV copies/million CD4+ T cells was calculated by dividing the SHIV DNA copy number by the TERT copy number divided by 2 multiplied by 106 cells.
Measurement of virus-specific IgG levels in plasma and saliva
Antibody concentrations in plasma and saliva were identified with Enzyme-linked immunosorbent assays (ELISA), as previously described 28.
Antibody Dependent Cellular-Cytotoxicity
ADCC activity of the purified mAbs and peripheral serum samples was determined by a luciferase-based cell killing assay with Renilla luciferase reporter gene encoding SHIV1157-ipd3N4-IMC, CEM.NKRCCR5 target cells (NIH AIDS Reagent Program), and PBMCs from a healthy HIV-seronegative donor, as previously described 29, 32.
Flow Cytometry
RM PBMCs or tissue mononuclear cells were stained with a panel of fluorochrome-conjugated antibodies and either phenotyped using an LSRII flow cytometer or bulk sorted into CD4+ and CD8+ T cells using a FACS Aria II cytometer (BD Biosciences). CD4+ and CD8+ T cells were positively selected from isolated tissue mononuclear cells by sequential selection of lymphocytes, FSC and SSC singlets, viable cells, CD45+ leukocytes, CD3+ T cells, and CD4+ vs CD8+ T cells. Data analysis was performed using FlowJo software (TreeStar).
In Situ Hybridization
In situ hybridization for the detection and quantification of SHIV gag RNA in formalin-fixed tissue blocks was performed using the Affymetrix protocol according to manufacturer instruction (Affymetrix). Tissue blocks were stained for CD3, DAPI, and SHIV gag RNA and imaged on slides with a Leica TCS SP8 confocal microscope. The number of SHIV RNA producing cells was reported as the number of SHIV gag RNA+ cells per 1,000 CD3+ T cells.
Transmitted/Founder Analysis
Transmitted/Founder (T/F) viral sequences in plasma were obtained by single genome amplification (SGA) through nested reverse transcriptase PCR with subsequent direct amplicon sequencing, as previously described 55. Sequence alignments and phylogenetic trees were constructed using clustalW and Highlighter plots were created using the tool at www.lanl.gov.
To identify and enumerate T/F variants, clusters of related sequences were visually analyzed using phylogenetic trees (Figtree v1.4) and sequences containing <2 mutations were considered a single variant. Variants containing ≥2 mutations were considered as progeny of distinct T/F genomes. Potential G-A hymermutations caused by APOBEC 3G/3F were identified using Hypermut algorithm 2.0 and were reverted for analysis if there were ≤ 2 present. Sequences that had >3 potential APOBEC 3G/3F mutations were not considered for T/F analysis (Hypermut, http://www.hiv.lanl.gov). Sequence clusters of ≥ 2 sequences with ≥2 shared mutations were considered as distinct T/F variants.
Provirus env cloning and pseudovirus preparation
SHIV env amplification from genomic DNA (gDNA) extracted from infant RM CD4+ T cells was done through bulk nested PCR and cloning into pcDNA3.1/V5-His-Topo (Invitrogen). Pseudovirus was produced via cotransfection of the SHIV env plasmid and a plasmid containing a subtype B env deficient HIV genome (SG3∆env) in 293T cells (Invitrogen) as previously described 53. Pseudotyped virus infectivity was screened by TZM-bl cell infection and Tat-regulated luminescence with the Bright-Glo luciferase reagent (Promega).
Statistical Analysis
Statistical tests were performed with SAS v9.4 (SAS Institute). Comparisons of viral load, proviral load (copies/million cells), and the number of T/F variants in infants from each mAb treatment group were performed using the exact Wilcoxon test. The proviral load (binary designation), and the number of infants with detectable SHIV in each mAb treatment group were compared with Fisher’s exact test. False discovery rate (FDR) p-value correction was used to correct for multiple comparisons. A p-value of <0.05 (two-tailed) was considered as significant for all analyses.
Supplementary Material
Acknowledgements
We thank Ruth Ruprect and the Dana Farber Cancer Institute for generously permitting the use of the SHIV-1157ipd3N4 for challenging the animals in this study through the NIH AIDS reagent Program. We also thank the Duke Human Vaccine Institute Protein Production Facility for help with mAb production, the Duke University Sequencing and Genomic Technology core facility for help with the ddPCR and Fluidigm assays, Barton Haynes and Kevin Saunders for providing protein antigens used in ELISAs, Tori Huffman and R. Whitney Edwards for technical assistance on ADCC data collection, David O’Connor and Roger Wiseman at the Wisconson National Primate Center for performing the MHC-typing, and the NIH HIV Research and Design (HIVRAD) and Research Project Grant (R01) programs for funding. TZM-bl cells and SG3∆env were provided by John Kappes and Xiaoyun Wu through the NIH AIDS Reagent Program.
Funding Sources: HHS | National Institutes of Health (R01AI1063980, P01AI117915)
This work was funded by HHS | National Institutes of Health (R01AI1063980, P01AI117915). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Disclosures
No conflicts of interest to declare.
References
- 1.UNAIDS. Children and HIV: Fact Sheet July 2016. Available from “http://www.unaids.org/sites/default/files/media_asset/FactSheet_Children_en.pdf”. 2016.
- 2.Kourtis AP, Butera S, Ibegbu C, Belec L, Duerr A. Breast milk and HIV-1: vector of transmission or vehicle of protection? Lancet Infect Dis 2003; 3(12): 786–793. [DOI] [PubMed] [Google Scholar]
- 3.Kuhn L, Aldrovandi G. Survival and health benefits of breastfeeding versus artificial feeding in infants of HIV-infected women: developing versus developed world. Clin Perinatol 2010; 37(4): 843–862, x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Academy of Pediatrics Work Group on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics 100:1035–1039. Available from “ 10.1542/peds.100.6.1035”. 1997. [DOI] [PubMed] [Google Scholar]
- 5.WHO Mother-to-Child Transmission of HIV. 2017. Available from “http://www.who.int/hiv/topics/mtct/about/en/”. 2017.
- 6.Nachega JB, Uthman OA, Anderson J, Peltzer K, Wampold S, Cotton MF et al. Adherence to antiretroviral therapy during and after pregnancy in low-income, middle-income, and high-income countries: a systematic review and meta-analysis. Aids 2012; 26(16): 2039–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Psaros C, Remmert JE, Bangsberg DR, Safren SA, Smit JA. Adherence to HIV care after pregnancy among women in sub-Saharan Africa: falling off the cliff of the treatment cascade. Curr HIV/AIDS Rep 2015; 12(1): 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drake AL, Wagner A, Richardson B, John-Stewart G. Incident HIV during pregnancy and postpartum and risk of mother-to-child HIV transmission: a systematic review and meta-analysis. PLoS Med 2014; 11(2): e1001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moodley D, Esterhuizen T, Reddy L, Moodley P, Singh B, Ngaleka L et al. Incident HIV infection in pregnant and lactating women and its effect on mother-to-child transmission in South Africa. J Infect Dis 2011; 203(9): 1231–1234. [DOI] [PubMed] [Google Scholar]
- 10.Moodley D, Esterhuizen TM, Pather T, Chetty V, Ngaleka L. High HIV incidence during pregnancy: compelling reason for repeat HIV testing. Aids 2009; 23(10): 1255–1259. [DOI] [PubMed] [Google Scholar]
- 11.Fouda GG, Permar SR. Immune-based interventions to prevent postnatal HIV-1 transmission. Trends Microbiol 2014; 22(8): 425–427. [DOI] [PubMed] [Google Scholar]
- 12.Saeland E, de Jong MA, Nabatov AA, Kalay H, Geijtenbeek TB, van Kooyk Y. MUC1 in human milk blocks transmission of human immunodeficiency virus from dendritic cells to T cells. Mol Immunol 2009; 46(11–12): 2309–2316. [DOI] [PubMed] [Google Scholar]
- 13.Kuhn L, Trabattoni D, Kankasa C, Semrau K, Kasonde P, Lissoni F et al. Alpha-defensins in the prevention of HIV transmission among breastfed infants. J Acquir Immune Defic Syndr 2005; 39(2): 138–142. [PMC free article] [PubMed] [Google Scholar]
- 14.Harmsen MC, Swart PJ, de Bethune MP, Pauwels R, De Clercq E, The TH et al. Antiviral effects of plasma and milk proteins: lactoferrin shows potent activity against both human immunodeficiency virus and human cytomegalovirus replication in vitro. J Infect Dis 1995; 172(2): 380–388. [DOI] [PubMed] [Google Scholar]
- 15.Villamor E, Koulinska IN, Furtado J, Baylin A, Aboud S, Manji K et al. Long-chain n-6 polyunsaturated fatty acids in breast milk decrease the risk of HIV transmission through breastfeeding. Am J Clin Nutr 2007; 86(3): 682–689. [DOI] [PubMed] [Google Scholar]
- 16.Walter J, Ghosh MK, Kuhn L, Semrau K, Sinkala M, Kankasa C et al. High concentrations of interleukin 15 in breast milk are associated with protection against postnatal HIV transmission. J Infect Dis 2009; 200(10): 1498–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mansour RG, Stamper L, Jaeger F, McGuire E, Fouda G, Amos J et al. The Presence and Anti-HIV-1 Function of Tenascin C in Breast Milk and Genital Fluids. PLoS One 2016; 11(5): e0155261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin V, Maldonado A, Fernandez L, Rodriguez JM, Connor RI. Inhibition of human immunodeficiency virus type 1 by lactic acid bacteria from human breastmilk. Breastfeed Med 2010; 5(4): 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fouda GG, Yates NL, Pollara J, Shen X, Overman GR, Mahlokozera T et al. HIV-specific functional antibody responses in breast milk mirror those in plasma and are primarily mediated by IgG antibodies. Journal of virology 2011; 85(18): 9555–9567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sacha CR, Vandergrift N, Jeffries TL Jr., McGuire E, Fouda GG, Liebl B et al. Restricted isotype, distinct variable gene usage, and high rate of gp120 specificity of HIV-1 envelope-specific B cells in colostrum compared with those in blood of HIV-1-infected, lactating African women. Mucosal Immunol 2015; 8(2): 316–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuaillon E, Valea D, Becquart P, Al Tabaa Y, Meda N, Bollore K et al. Human milk-derived B cells: a highly activated switched memory cell population primed to secrete antibodies. Journal of immunology 2009; 182(11): 7155–7162. [DOI] [PubMed] [Google Scholar]
- 22.Mabuka J, Nduati R, Odem-Davis K, Peterson D, Overbaugh J. HIV-specific antibodies capable of ADCC are common in breastmilk and are associated with reduced risk of transmission in women with high viral loads. PLoS pathogens 2012; 8(6): e1002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milligan C, Richardson BA, John-Stewart G, Nduati R, Overbaugh J. Passively acquired antibody-dependent cellular cytotoxicity (ADCC) activity in HIV-infected infants is associated with reduced mortality. Cell Host Microbe 2015; 17(4): 500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mascola JR, Haynes BF. HIV-1 neutralizing antibodies: understanding nature’s pathways. Immunol Rev 2013; 254(1): 225–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baba TW, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S et al. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med 2000; 6(2): 200–206. [DOI] [PubMed] [Google Scholar]
- 26.Hessell AJ, Jaworski JP, Epson E, Matsuda K, Pandey S, Kahl C et al. Early short-term treatment with neutralizing human monoclonal antibodies halts SHIV infection in infant macaques. Nat Med 2016; 22(4): 362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gray ES, Madiga MC, Hermanus T, Moore PL, Wibmer CK, Tumba NL et al. The neutralization breadth of HIV-1 develops incrementally over four years and is associated with CD4+ T cell decline and high viral load during acute infection. Journal of virology 2011; 85(10): 4828–4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeffries TL Jr., Sacha CR, Pollara J, Himes J, Jaeger FH, Dennison SM et al. The function and affinity maturation of HIV-1 gp120-specific monoclonal antibodies derived from colostral B cells. Mucosal Immunol 2016; 9(2): 414–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pollara J, McGuire E, Fouda GG, Rountree W, Eudailey J, Overman RG et al. Association of HIV-1 Envelope-Specific Breast Milk IgA Responses with Reduced Risk of Postnatal Mother-to-Child Transmission of HIV-1. Journal of virology 2015; 89(19): 9952–9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song RJ, Chenine AL, Rasmussen RA, Ruprecht CR, Mirshahidi S, Grisson RD et al. Molecularly cloned SHIV-1157ipd3N4: a highly replication- competent, mucosally transmissible R5 simian-human immunodeficiency virus encoding HIV clade C Env. Journal of virology 2006; 80(17): 8729–8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez DR, Vandergrift N, Douglas AO, McGuire E, Bainbridge J, Nicely NI et al. Maternal binding and neutralizing IgG responses targeting the C terminal region of the V3 loop are predictive of reduced peripartum HIV-1 transmission risk. Journal of virology 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Permar SR, Fong Y, Vandergrift N, Fouda GG, Gilbert P, Parks R et al. Maternal HIV-1 envelope-specific antibody responses and reduced risk of perinatal transmission. The Journal of clinical investigation 2015; 125(7): 2702–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siddappa NB, Watkins JD, Wassermann KJ, Song R, Wang W, Kramer VG et al. R5 clade C SHIV strains with tier 1 or 2 neutralization sensitivity: tools to dissect env evolution and to develop AIDS vaccines in primate models. PloS one 2010; 5(7): e11689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loffredo JT, Maxwell J, Qi Y, Glidden CE, Borchardt GJ, Soma T et al. Mamu-B*08-positive macaques control simian immunodeficiency virus replication. Journal of virology 2007; 81(16): 8827–8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mothe BR, Weinfurter J, Wang C, Rehrauer W, Wilson N, Allen TM et al. Expression of the major histocompatibility complex class I molecule Mamu-A*01 is associated with control of simian immunodeficiency virus SIVmac239 replication. Journal of virology 2003; 77(4): 2736–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muhl T, Krawczak M, Ten Haaft P, Hunsmann G, Sauermann U. MHC class I alleles influence set-point viral load and survival time in simian immunodeficiency virus-infected rhesus monkeys. Journal of immunology 2002; 169(6): 3438–3446. [DOI] [PubMed] [Google Scholar]
- 37.O’Connor DH, Mothe BR, Weinfurter JT, Fuenger S, Rehrauer WM, Jing P et al. Major histocompatibility complex class I alleles associated with slow simian immunodeficiency virus disease progression bind epitopes recognized by dominant acute-phase cytotoxic-T-lymphocyte responses. Journal of virology 2003; 77(16): 9029–9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pal R, Venzon D, Letvin NL, Santra S, Montefiori DC, Miller NR et al. ALVAC-SIV-gag-pol-env-based vaccination and macaque major histocompatibility complex class I (A*01) delay simian immunodeficiency virus SIVmac-induced immunodeficiency. Journal of virology 2002; 76(1): 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sauermann U, Siddiqui R, Suh YS, Platzer M, Leuchte N, Meyer H et al. Mhc class I haplotypes associated with survival time in simian immunodeficiency virus (SIV)-infected rhesus macaques. Genes Immun 2008; 9(1): 69–80. [DOI] [PubMed] [Google Scholar]
- 40.Yant LJ, Friedrich TC, Johnson RC, May GE, Maness NJ, Enz AM et al. The high-frequency major histocompatibility complex class I allele Mamu-B*17 is associated with control of simian immunodeficiency virus SIVmac239 replication. Journal of virology 2006; 80(10): 5074–5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang ZQ, Fu TM, Casimiro DR, Davies ME, Liang X, Schleif WA et al. Mamu-A*01 allele-mediated attenuation of disease progression in simian-human immunodeficiency virus infection. Journal of virology 2002; 76(24): 12845–12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Albrecht C, Malzahn D, Brameier M, Hermes M, Ansari AA, Walter L. Progression to AIDS in SIV-Infected Rhesus Macaques is Associated with Distinct KIR and MHC class I Polymorphisms and NK Cell Dysfunction. Front Immunol 2014; 5: 600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santra S, Tomaras GD, Warrier R, Nicely NI, Liao HX, Pollara J et al. Human Non-neutralizing HIV-1 Envelope Monoclonal Antibodies Limit the Number of Founder Viruses during SHIV Mucosal Infection in Rhesus Macaques. PLoS pathogens 2015; 11(8): e1005042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Becquet R, Marston M, Dabis F, Moulton LH, Gray G, Coovadia HM et al. Children who acquire HIV infection perinatally are at higher risk of early death than those acquiring infection through breastmilk: a meta-analysis. PloS one 2012; 7(2): e28510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ng CT, Jaworski JP, Jayaraman P, Sutton WF, Delio P, Kuller L et al. Passive neutralizing antibody controls SHIV viremia and enhances B cell responses in infant macaques. Nat Med 2010; 16(10): 1117–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghulam-Smith M, Olson A, White LF, Chasela CS, Ellington SR, Kourtis AP et al. Maternal but Not Infant Anti-HIV-1 Neutralizing Antibody Response Associates with Enhanced Transmission and Infant Morbidity. MBio 2017; 8(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Polonis VR, Brown BK, Rosa Borges A, Zolla-Pazner S, Dimitrov DS, Zhang MY et al. Recent advances in the characterization of HIV-1 neutralization assays for standardized evaluation of the antibody response to infection and vaccination. Virology 2008; 375(2): 315–320. [DOI] [PubMed] [Google Scholar]
- 48.Gorlani A, Forthal DN. Antibody-dependent enhancement and the risk of HIV infection. Curr HIV Res 2013; 11(5): 421–426. [DOI] [PubMed] [Google Scholar]
- 49.Stahl-Hennig C, Steinman RM, Tenner-Racz K, Pope M, Stolte N, Matz-Rensing K et al. Rapid infection of oral mucosal-associated lymphoid tissue with simian immunodeficiency virus. Science 1999; 285(5431): 1261–1265. [DOI] [PubMed] [Google Scholar]
- 50.Liu J, Ghneim K, Sok D, Bosche WJ, Li Y, Chipriano E et al. Antibody-mediated protection against SHIV challenge includes systemic clearance of distal virus. Science 2016; 353(6303): 1045–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hessell AJ, Shapiro MB, Powell R, Malherbe DC, McBurney SP, Pandey S et al. Reduced cell-associated DNA and improved viral control in macaques following passive transfer of a single anti-V2 monoclonal antibody and repeated SHIV challenges. Journal of virology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bonsignori M, Kreider EF, Fera D, Meyerhoff RR, Bradley T, Wiehe K et al. Staged induction of HIV-1 glycan-dependent broadly neutralizing antibodies. Sci Transl Med 2017; 9(381). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M et al. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. Journal of virology 2005; 79(16): 10108–10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilks AB, Perry JR, Ehlinger EP, Zahn RC, White R, Gauduin MC et al. High cell-free virus load and robust autologous humoral immune responses in breast milk of simian immunodeficiency virus-infected african green monkeys. Journal of virology 2011; 85(18): 9517–9526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keele BF, Li H, Learn GH, Hraber P, Giorgi EE, Grayson T et al. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. The Journal of experimental medicine 2009; 206(5): 1117–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


