Abstract
We herein report a case involving a 64-year-old Japanese woman with a pulmonary Mycobacterium abscessus infection complicated by reactive AA amyloidosis, which, to our knowledge, has not been reported to date. The patient underwent gastrointestinal endoscopy for diarrhea during the treatment of pulmonary M. abscessus infection and was diagnosed with AA amyloidosis according to the histopathological findings from the endoscopic specimen. She died four months later. The prognosis of AA amyloidosis associated with pulmonary M. abscessus infection may be very poor, and physicians should pay attention to this rare condition when difficult-to-treat diarrhea occurs in patients with pulmonary M. abscessus infection.
Keywords: Mycobacterium abscessus, non-tuberculous mycobacteria, reactive AA amyloidosis, poor prognosis
Introduction
Mycobacterium abscessus is globally localized in various environments and is a group IV member (rapidly growing mycobacteria; RGM) according to the Runyon classification (1). M. abscessus causes skin, soft tissue, and bone infection. Although pulmonary M. abscessus infection is a relatively rare respiratory infection (2), its incidence has been increasing in both immunocompetent and immunocompromised hosts (3-5). A large laboratory-based analysis in Japan demonstrated that pulmonary M. abscessus infection accounted for 2.6% of all pulmonary infections caused by nontuberculous mycobacteria (NTM), with an incidence rate similar to that of M. kansasii infection (2).
Amyloidosis is characterized by the deposition of amyloid protein in various systemic organs. AA amyloidosis is probably the most common type of amyloidosis worldwide, and it is often complicated by chronic systemic inflammation and infection (6). Various diseases, such as rheumatoid arthritis, ankylosing spondylitis, juvenile osteoporosis, Crohn's disease, Castleman's disease, neoplasms such as lymphoma and mesothelioma, and tuberculosis, may cause reactive AA amyloidosis (7). Among these diseases, tuberculosis has been the most common cause of reactive AA amyloidosis in the past; however, approximately 90% cases of reactive AA amyloidosis in recent years have been related to rheumatoid arthritis. Reactive AA amyloidosis associated with nontuberculous mycobacterial pulmonary infections is extremely rare and to our knowledge has never been reported in association with pulmonary M. abscessus infection.
We herein report a rare case involving a 64-year-old Japanese woman with pulmonary M. abscessus infection complicated by reactive AA amyloidosis and present a brief review of the relevant literature.
Case Report
A 64-year-old Japanese woman visited a hospital with a low-grade fever and productive cough in April 2015. Chest computed tomography indicated nontuberculous mycobacterial pulmonary infection, although no NTM were cultured in the sputum. Two months later, treatment with clarithromycin (400 mg/day), rifampicin (300 mg/day), and ethambutol (750 mg/day) was initiated to treat with a radiological suspicion of NTM infection because she did not agree to a bronchoscopic examination; however, her symptoms did not improve after 2 months of treatment, which caused appetite loss and nausea. Therefore, all three drugs were discontinued. M. abscessus was cultured and identified using the VITEK2 after three weeks from a sputum sample that had been obtained in June.
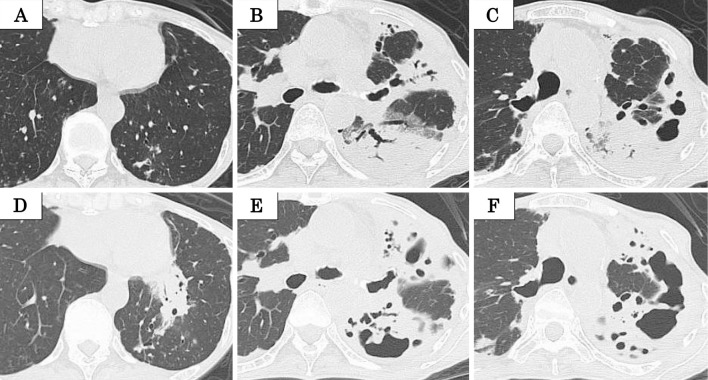
She was referred to our hospital due to the development of a high-grade fever (38-39 °C) in September 2015. Chest computed tomography (CT) (Fig. 2A-C) demonstrated consolidations with cavitary lesions and bronchiectasis in the left upper lobe. Bronchoscopy was performed, but culture of bronchial washing specimens showed no mycobacteria. Treatment with erythromycin (EM) (200 mg/day) was initiated, which led to a slight improvement in the high-grade fever. However, she developed abdominal pain and diarrhea (5-6/day) in November 2015, and EM was discontinued. Her abdominal symptoms did not improve, and she was readmitted to our hospital for the evaluation of her abdominal symptoms in January 2016.
Figure 1.
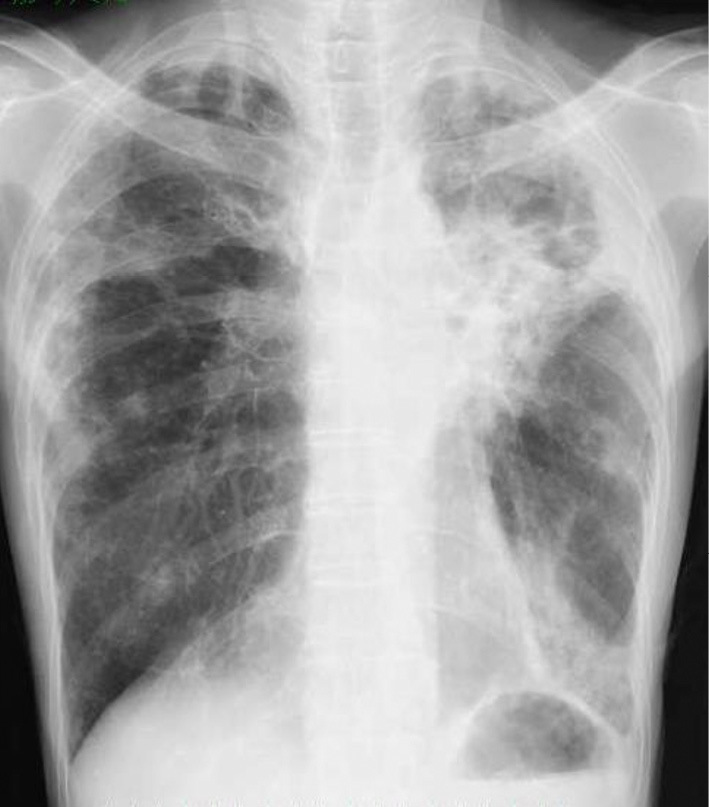
Chest radiograph obtained on admission shows infiltration in the left lung.
Figure 2.
Chest computed tomography (CT) performed in February 2016 (D, E, F) demonstrates consolidations with cavitary lesions and bronchiectasis in the left upper lobe, which appear to have worsened on chest CT performed in September 2015 (A, B, C).
On admission, a physical examination revealed the following: height, 155 cm; body weight, 29.0 kg; body mass index (BMI), 12.0 kg/m2; body temperature, 36.1 ℃; heart rate, 109 beats/min; blood pressure, 107/63 mmHg; and oxygen saturation, 98% in room air. On auscultation, respiratory and cardiac sounds were normal, while hyper bowel sounds were audible. She did not have crimped edema. She also reported tenderness over the left lower abdomen. Laboratory tests (Table 1) revealed an elevated white blood cell count (19,200 /μL) and serum C-reactive protein (6.7 mg/dL) level and hypoalbuminemia (1.9 g/dL). Rheumatoid factor and antinuclear antibody were within normal limits, but the serum amyloid AA level was elevated (384 μg/mL; normal range, <10 μg/mL). In addition, there were no obvious abnormal urinary findings. A chest radiograph (Fig. 1) showed infiltrations in the left lung, and chest CT (Fig. 2D-F) demonstrated slightly worsening consolidation with cavitary lesions and bronchiectasis in the left upper lobe.
Table 1.
Findings of Peripheral Blood and Urine Analysis at Admission.
| <Blood cell counts> | <Blood chemistry> | <Serology> | <Urinalysis> | |||||||||
| WBC | 19,200 | /μL | TP | 6.4 | g/dL | CRP | 6.7 | mg/dL | ||||
| Neut | 87.1 | % | Albumin | 1.9 | g/dL | Specific gravity | 1.024 | |||||
| Lymph | 7.9 | % | T-bil | 0.7 | mg/dL | RF | 9.5 | U/dL | pH | 6.0 | ||
| Eo | 0.2 | % | AST | 21 | IU/L | Anti-nuclear antibody | <40 | Urine sugar | (-) | mg/dL | ||
| RBC | 429×104 | /μL | ALT | 14 | IU/L | TSH | 1.63 | µIU/mL | Urine protein | 30 | mg/dL | |
| Hb | 10.8 | g/dL | LDH | 244 | IU/L | FT4 | 1.65 | ng/dL | Urine protein (day) | 0.1 | g/day | |
| Ht | 34.1 | % | ALP | 330 | IU/L | T-SPOT.TB | (-) | |||||
| Plt | 59.4×104 | /μL | γ-GTP | 22 | IU/L | MAC Ab. | (-) | |||||
| T-cho | 164 | mg/dL | β-D-glucan | (-) | ||||||||
| LDL-cho | 90 | mg/dL | Aspergillus Ag. | (-) | ||||||||
| BUN | 8 | mg/dL | Amyloid AA | 384 | μg/mL | |||||||
| Cre | 0.48 | mg/dL | ||||||||||
| Na | 133 | mEq/L | ||||||||||
| K | 4.6 | mEq/L | ||||||||||
| Cl | 94 | mEq/L | ||||||||||
WBC: white blood cell, RBC: red blood cell, Hb: hemoglobin, Ht: hematocrit, Plt: platelet, TP: total protein, T-bil: total bilirubin, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, ALP: alkaline phosphatase, γ-GTP: gamma-glutamyl transpeptidase, BUN: blood urea nitrogen, Cre: creatinine, CRP: C-reactive protein, RF: rheumatoid factor, TSH: thyroid stimulating hormone, FT4: free thyroxine
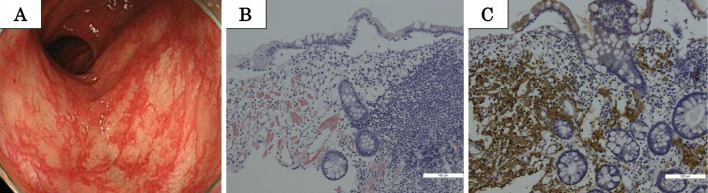
After admission, lower gastrointestinal endoscopy revealed red flare with hypervascularity of the mucosal membrane from the terminal ileum to the rectum (Fig. 3A). A histopathological examination of biopsied intestinal mucosa demonstrated positive Congo red staining, which was indicative of amyloid deposition, while immunostaining revealed anti-AA antibody-positive cells in the mucosa (Fig. 3B, C). These findings suggested AA amyloidosis. In addition, M. abscessus was cultured twice in sputum samples obtained at the time of admission. No other diseases that could cause reactive AA amyloidosis were evident, and the patient was diagnosed with pulmonary M. abscessus infection complicated by reactive AA amyloidosis.
Figure 3.
Macroscopic findings in lower gastrointestinal endoscopy (A) performed for a 64-year-old woman with pulmonary Mycobacterium abscessus infection complicated by reactive AA amyloidosis. Histopathological and immunohistochemical findings for specimens obtained from mucosal lesions in the large intestine indicate amyloid deposition (positive Congo red staining) and anti-AA amyloid antibody-positive cells (B, C).
Unfortunately, there were no treatment options for her condition, and she died four months after the diagnosis of reactive AA amyloidosis due to the aggravation of respiratory failure caused by the progression of M. abscessus infection.
Discussion
Reactive AA amyloidosis associated with infection by NTM is extremely rare, and only six cases have been reported to date (6-11). To our knowledge, the present case is the first of reactive AA amyloidosis associated with M. abscessus infection.
Table 2 shows the characteristics of the reported patients, including our own (6-11). The previously reported cases were middle-aged (60-70 years old), with a male:female ratio of 3:4. Patients with NTM infection in Japan have been predominantly middle-aged women (2,5), so there may be no marked sex- or age-related differences between patients with NTM infection with reactive AA amyloidosis and those with NTM infection without reactive AA amyloidosis, although the number of cases is few.
Table 2.
Reported Cases of Nontuberculous Mycobacterial Pulmonary Infection with Reactive AA Amyloidosis.
| References | Age/ sex |
Common symptoms | Time from diagnosis of NTM to that of AA amyloidosis | Time from diagnosis of AA amyloidosis to death | Mycobacteria |
|---|---|---|---|---|---|
| 7 | 73/M | Diarrhea/Stomach ache | 2 years 6 months | Unknown | M. simiae |
| 8 | 73/M | Diarrhea | 1 week | 5 months | M. intracellulare |
| 9 | 61/F | Diarrhea/Stomach ache/Edema | 8 years | 2 months | M. avium-intracellulare complex |
| 10 | 69/F | Diarrhea/Stomach ache | 5 years | 4 months | M. avium-intracellulare complex |
| 6 | 75/M | Edema | 6 years | 13 months | Unknown |
| 11 | 75/F | Diarrhea/Stomach ache | 7 years | 3 years 2 months | M. intracellulare M. avium |
| Present case | 64/F | Diarrhea/Stomach ache/Edema | 10 months | 4 months | M. abscessus |
The major symptoms of patients with NTM infection complicated by reactive AA amyloidosis, including the present patient, were abdominal pain, stomachache, and diarrhea (Table 2). Cough, sputum, hemosputum, and weight loss are common symptoms of NTM infection (5,12,13), although abdominal symptoms, such as diarrhea, are uncommon. In addition, intestinal NTM infection is generally quite rare, although Huh et al. reported that M. avium-intracellulare complex (MAC) was one of the common causes of intestinal infection in patients with acquired immunodeficiency syndrome, and that intestinal MAC infection may lead to diarrhea (14,15). The side effects of macrolide, a key drug for the treatment of NTM infection, also include abdominal symptoms, such as diarrhea. Thus, physicians should consider reactive AA amyloidosis associated with NTM infection as a differential diagnosis for diarrhea in patients with NTM infection. In addition, the digestive tract should be inspected before the discontinuation of macrolide in patients with refractory digestive symptoms.
Patients with reactive AA amyloidosis exhibit a relatively short mean survival time (24 months) (16), while patients with pulmonary M. abscessus infection also exhibit a poor prognosis and high mortality rate (15.0-16.7%) (1). The reported interval between the diagnosis of NTM and that of AA amyloidosis is approximately 5-8 years (6). Four of seven reported patients died within six months after the diagnosis of AA amyloidosis (Table 2). A low BMI, bilateral lung involvement, and the fibrocavitary type were predictors of a poor prognosis in patients with pulmonary M. abscessus infection (13). Reported factors for a poor prognosis in patients with AA amyloidosis include a decreased serum albumin level (<2.5 g/dL), end-stage renal failure at baseline, and an increased serum AA amyloid level during the follow-up period (16,17), and Lachmann et al. reported that the serum AA amyloid level is strongly associated with the outcome in patients with AA amyloidosis (17). Our patient had a low BMI, hypoalbuminemia, and increased serum AA amyloid level, although her creatinine level was normal and the change in the serum AA amyloid level over time was not evaluated. Although various factors can increase the risk of a poor prognosis in M. abscessus infection and/or AA amyloidosis, reactive AA amyloidosis, especially in patients with an increased serum AA amyloid level, may be a factor for a poor prognosis in patients with NTM infection.
According to the American Thoracic Society/Infectious Disease Society of America guidelines, a combination of clarithromycin or azithromycin, intravenous amikacin, and cefoxitin or imipenem should be used for the treatment of M. abscessus infection, although a definite effective treatment regimen has not been established (18). We chose to treat the present patient with EM, expecting an anti-inflammatory effect (19), as she did not agree to undergo inpatient treatment or intravenous medication due to strong concerns over the side effects of intravenous antibiotic drugs. However, it has been suggested that EM monotherapy can cause macrolide-resistant M. abscessus infection (20,21), and whether or not treatment with EM is appropriate for M. abscessus infection remains controversial. Controlling the underlying diseases is the most effective treatment for achieving stabilization or even regression of amyloid deposition (22); therefore, treatment with clarithromycin, imipenem, and amikacin should probably have been administered to this patient. A further investigation is needed to determine the appropriate treatment regimen for patients with AA amyloidosis associated with pulmonary M. abscessus infection.
In conclusion, we encountered a rare case of M. abscessus infection complicated by reactive AA amyloidosis. The findings from this case indicate that physicians should consider reactive AA amyloidosis associated with NTM infection when difficult-to-treat digestive symptoms, such as diarrhea, are observed in patients with NTM infection.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Pasipanodya JG, Ogbonna D, Ferro BE, et al. Systematic review and meta-analyses of the effect of chemotherapy on pulmonary Mycobacterium abscessus outcomes and disease recurrence. Antimicrob Agents Chemother 61: 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morimoto K, Hasegawa N, Izumi K, et al. A laboratory-based analysis of nontuberculous mycobacterial lung disease in Japan from 2012 to 2013. Ann Am Thorac Soc 14: 49-56, 2017. [DOI] [PubMed] [Google Scholar]
- 3.Lai CC, Hsueh PR. Diseases caused by nontuberculous mycobacteria in Asia. Future Microbiol 9: 93-106, 2014. [DOI] [PubMed] [Google Scholar]
- 4.Benwill JL, Wallace RJ Jr. Mycobacterium abscessus: challenges in diagnosis and treatment. Curr Opin Infect Dis 27: 506-510, 2014. [DOI] [PubMed] [Google Scholar]
- 5.Nagano H, Kinjo T, Nei Y, Yamashiro S, Fujita J, Kishaba T. Causative species of nontuberculous mycobacterial lung disease and comparative investigation on clinical features of Mycobacterium abscessus complex disease: a retrospective analysis for two major hospitals in a subtropical region of Japan. PLoS One 12: e0186826, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsuji K, Arai H, Furusu A, et al. A case of membranoproliferative glomerulonephritis and AA amyloidosis complicated with pulmonary nontuberculous mycobacterial infection. CEN Case Rep 4: 24-30, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aharon A, Langevitz P, Maran R, Blank-Porat D, Shtrasburg S, Livneh A. Reactive amyloidosis in a patient with Mycobacterium simiae pulmonary infection. Respir Med 92: 123-124, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Zaizen Y, Arita K, Kawano H, et al. [A Case Report of AA Amyloidosis associated with Cystic Infection during the Course of Non-tuberculous Mycobacteriosis]. Respiration & Circulation 55: 237-241, 2007(in Japanese). [Google Scholar]
- 9.Shinozuka N, Kasamatsu N, Seto T, Yasui T, Nakamura A, Hashizume I. [A fatal case of pulmonary non-tuberculous mycobacteriosis with reactive AA amyloidosis]. Nihon Kokyuki Gakkai Zasshi 45: 636-642, 2007(in Japanese). [PubMed] [Google Scholar]
- 10.Haga T, Kasamatsu N, Kobayashi T, Shibata M, Ogasawara T, Hashizume I. [A case of pulmonary nontuberculous Mycobacteriosis with reactive AA amyloidosis exacerbated in spite of various autimicrobial therapy]. The Japanese Jounal of Chest Diseases 68: 245-251, 2009(in Japanese). [Google Scholar]
- 11.Higuchi D, Nakamoto A. [A case of refractory diarrhea due to gastrointestinal AA amyloidosis occurred in nontuberculous mycobacteriosis with bronchiectasis]. The Jounal of National Okinawa Hospital 34: 35-39, 2014(in Japanese). [Google Scholar]
- 12.Huang HL, Cheng MH, Lu PL, et al. Epidemiology and predictors of NTM pulmonary infection in Taiwan - a retrospective, five-year multicenter study. Sci Rep 7: 16300, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park J, Cho J, Lee CH, Han SK, Yim JJ. Progression and treatment outcomes of lung disease caused by Mycobacterium abscessus and Mycobacterium massiliense. Clin Infect Dis 64: 301-308, 2017. [DOI] [PubMed] [Google Scholar]
- 14.Yamazaki R, Mori T, Nakazato T, et al. Non-tuberculous mycobacterial infection localized in small intestine developing after allogeneic bone marrow transplantation. Intern Med 49: 1191-1193, 2010. [DOI] [PubMed] [Google Scholar]
- 15.Huh JG, Kim YS, Lee JS, et al. Mycobacterium ulcerans infection as a cause of chronic diarrhea in an AIDS patient: a case report. World J Gastroenterol 14: 808-811, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gertz MA, Kyle RA. Secondary systemic amyloidosis: response and survival in 64 patients. Medicine (Baltimore) 70: 246-256, 1991. [PubMed] [Google Scholar]
- 17.Lachmann HJ, Goodman HJ, Gilbertson JA, et al. Natural history and outcome in systemic AA amyloidosis. N Engl J Med 356: 2361-2371, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175: 367-416, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Komiya K, Kurashima A, Ihi T, et al. Long-term, low-dose erythromycin monotherapy for Mycobacterium avium complex lung disease: a propensity score analysis. Int J Antimicrob Agents 44: 131-135, 2014. [DOI] [PubMed] [Google Scholar]
- 20.Nash KA, Brown-Elliott BA, Wallace RJ Jr. A novel gene, erm(41), confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob Agents Chemother 53: 1367-1376, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Renna M, Schaffner C, Brown K, et al. Azithromycin blocks autophagy and may predispose cystic fibrosis patients to mycobacterial infection. J Clin Invest 121: 3554-3563, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Real de Asua D, Costa R, Galvan JM, Filigheddu MT, Trujillo D, Cadinanos J. Systemic AA amyloidosis: epidemiology, diagnosis, and management. Clin Epidemiol 6: 369-377, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]