Abstract
Neuroendovascular procedures have led to breakthroughs in the treatment of ischemic stroke, intracranial aneurysms, and intracranial arteriovenous malformations. Due to these substantial successes, there is continuous development of novel and refined therapeutic approaches. Large animal models feature various conceptual advantages in translational research, which makes them appealing for the development of novel endovascular treatments. However, the availability and role of large animal models have not been systematically described so far. Based on comprehensive research in two databases, this systematic review describes current large animal models in neuroendovascular research including their primary use. It may therefore serve as a compact compendium for researchers entering the field or looking for opportunities to refine study concepts. It also describes particular applications for ischemic stroke and aneurysm therapy, as well as for the treatment of arteriovenous malformations. It focuses on most promising study designs and readout parameters, as well as on important pitfalls in endovascular translational research including ways to circumvent them.
Keywords: Endovascular, aneurysm, arteriovenous malformations, stroke, large animal models
Introduction
Neuroendovascular techniques are safe and effective for the treatment of acute ischemic stroke.1–3 Recently, these techniques proved to be effective even in an extended time window for selected patient populations,4,5 thereby substantially changing stroke treatment and research.6 Moreover, recent technical advancements such as flow-diverting stents, intra-aneurysmal flow-diversion, and new liquid-embolic agents have widened opportunities for endovascular treatment of cerebral aneurysms and intracranial vascular malformations (AVMs).7
However, preclinical research on endovascular technologies is more challenging as compared to conventional treatment opportunities. Rodent models offer numerous advantages such as wide-spread implementation, reproducibility, a broad spectrum of readout parameters and experimental imaging techniques, as well as the availability of transgenic animals.8,9 On the other hand, rodent models only allow to investigate basic aspects of neuroendovascular treatments due to size limitations and significant differences in brain and cerebrovascular anatomy.10
Large animal models are available for preclinical neuroendovascular research and offer numerous advantages including larger vessel sizes, compatibility with clinical magnetic resonance imaging (MRI),11 computed (CT)12 or positron emission tomography (PET),13 and a gyrencephalic brain with a gray-to-white-matter-ratio approximating that of humans.14 Despite minor anatomical differences in some species, cerebral blood supply and cerebrovascular architecture are very similar to the human situation.15 Large animal models are also feasible for long-term studies and extensive physiological monitoring. Behavioral tests for the assessment of functional outcomes are available, while international expert committees recommend confirmative research in large animal models prior to early stage clinical investigations.16,17 However, large animal models are not utilized widely yet, potentially due to special maintenance requirements, the necessity of for interdisciplinary research teams as well as profound knowledge on how to successfully employ these models, as well as some species-dependent anatomical differences to humans.9,10
Here, we review existing large animal models and their use in neuroendovascular research on stroke, aneurysms and AVMs. We also provide recommendations on most feasible study designs and readout strategies in order to capitalize on the advantages of large animal models while avoiding potential limitations.
Methodological approach
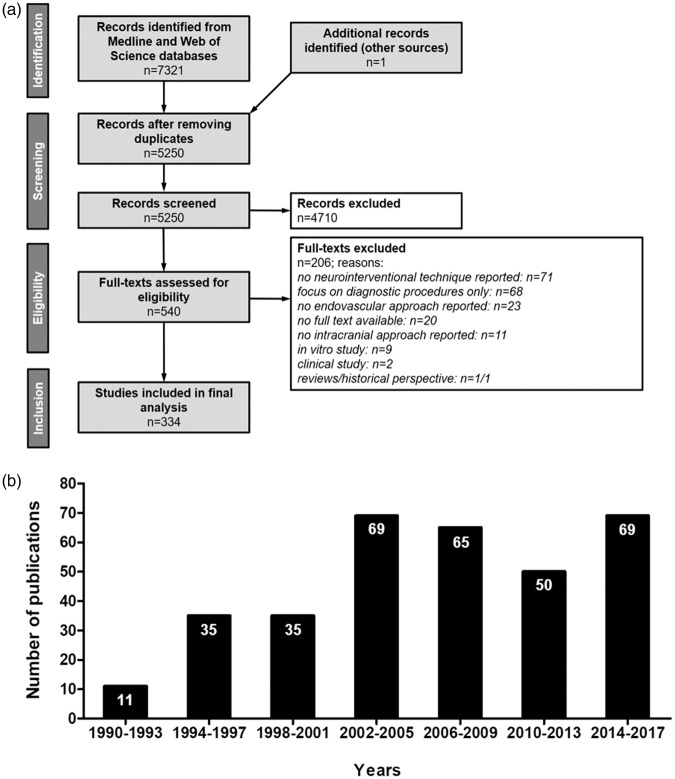
We conducted a systematic literature search for relevant publications according to the PRISMA guidelines. We accessed indexed and non-indexed Medline databases via Ovid search interface from Wolters Kluwer and Science Citation Index Expanded via Web of Science from Clarivate Analytics (Figure 1(a)). The Medline search strategy included 23 search steps of terms for the topic large animals and 37 search steps of terms for the topic cerebrovascular intervention. The databases were searched from 1990 up to the update status of the databases on November 24th 2017 (search date).
Figure 1.
Study data. (a) PRISMA flow chart of selection process; systematic research in Medline and Web of Science databases, inclusion of studies that address neurointerventional research, test endovascular techniques and were performed in large animals (b) Study numbers show a continuous increase from 1990 until 2005, followed by a slight decline and a recent increase to all-time highs.
Search strategies combined the aspects “large animals” and “neurological intervention” with AND. We also used keywords with synonyms and, if available, controlled vocabulary terms for Medline (for detailed search strategies, please see Supplementary Tables 1 and 2). Only articles published in English were included.
Additionally, we searched for large animal models that were developed to represent the vascular anatomy of humans as well as human neurovascular disorders. Studies utilizing extracranial vessels to simulate intracranial interventions and/or to establish a neurointerventional model were included, but discriminated from studies describing a neurointerventional procedure in the cerebrovasculature. Additionally, reference lists from identified papers of interest were screened to find other potentially relevant publications.
We further analyzed the application of these models in neurointerventional research focusing on endovascular approaches. Studies that (i) addressed neurointerventional research, (ii) tested endovascular techniques, and (iii) were performed in large animals were assessed.
We excluded articles due to the following criteria: no full text available (n = 20), focus on diagnostic procedures only (n = 68), no neurointerventional technique reported (n = 71), no endovascular approach (n = 23), clinical study (n = 2), review/historical perspective (n = 1/1), in vitro study (n = 9), and studies focusing on interventions in the peripheral circulation without modelling the cerebrovasculature (not intracranial, n = 11) (Figure 1(a)).
Results
Data set
We identified 5250 articles after removal of search result duplicates. From those, 4710 abstracts did not meet the inclusion criteria and were excluded. The full text of the remaining 540 articles was assessed, and data were finally extracted from 334 papers (Figure 1(a)).
For model description, 56 different large animal models using intracranial vessels were identified through full text assessment, and additional 18 models were derived by checking the reference lists of these papers. Moreover, we identified 26 models using extracranial vessels. One paper was excluded during the full text assessment due to an unclear methodological approach.
The number of publications reporting large animal trials in neurointerventional research continuously increased from the 1990s until 2005 (Figure 1(b)). The numbers of publications slightly declined thereafter, but increased from 2014 reaching all-time highs. This is potentially due to the recent breakthroughs in the neurointerventional field, prompting many academic and industry groups to intensify translational research on novel techniques and products.
Species and models
Table 1 provides a detailed overview on all species and models.
Table 1.
Large animal models for aneurysm, AVM, ischemic stroke, carotid siphon including models using extracranial vessels.
| Type | Location | Species | References | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Aneurysm | |||||
| Elastase-induced Aneurysm | CCA | Dog, rabbit | 63,18 | Imitates biological and molecular environment, exhibits uninjured arterial walls | Requires time to mature, less consistent, comparatively small |
| Carotid-jugular arteriovenous fistula | Rabbit | 21 | |||
| Venous pouch Aneurysm | CCA-EJV | Dog, rabbit, pig, sheep | 64,65,22,66 | Reliable models with reproducible characteristics (consistent size, neck diameters) | Surgical trauma, presence of suture material and venous structure of aneurysmal wall |
| CCA-EJV plus curved implant | Dog | 49 | |||
| CCA-femoral vein | NHP (Japanese monkey) | 67 | |||
| ECA-EJV | Dog | 20 | |||
| Implant | Silicone aneurysm circuit, CCA-EJV | Pig | 19 | Good training model – endovascular treatments | Expensive, risk of thrombus development in implant |
| Amplatzer vascular plug, carotid-subclavian-renal arteries | Pig | 23 | |||
| AVM | |||||
| Carotid-jugular fistula | CCA-JV | Dog, NHP (rhesus monkey), cat | 68,69,70,71 | Easy induction | Does not provide AVM nidus |
| Rete mirabile (RM) | RM (anatomic arterial model) | Pig | 26 | Arterio-arterial malformation, suitable to study histopathological alterations | Not an arteriovenous connection, differs from real AVMs |
| Carotid-jugular fistula, RM as Nidus | Pig, sheep | 24,72 | Creates relevant morphological characteristics of human AVM | Can occlude spontaneously | |
| Rostral rete-cavernous sinus, RM as Nidus | Pig | 25 | |||
| Implant | Lingual artery-superior sagittal sinus (femoral artery) | Dog | 27 | Induced in cerebral circulation | Requires excellent surgical skills |
| MCA-superior sagittal sinus (superficial temporal artery, muscle graft) | Dog | 28 | |||
| Ischemic stroke, permanent occlusion | |||||
| Extravascular device | MCAO, transorbital, occluding device, ligation | Dog, cat | 38,73 | Reproducible infarct size, reproducible neurological deficits, highly consistent vessel occlusion | Analysis of reperfusion not possible, diathermy can increase blood-brain barrier permeability |
| Extravascular electrocoagulation | MCAO | Pig, NHP (marmoset), sheep | 32,34,30 | ||
| AchAO | Pig | 31 | |||
| Intravascular artificial embolization | MCAO | Dog, NHP (rhesus monkey) | 43,45 | Any required embolus shape, leads to more uniform infarcts, reducing variability | Final position of emboli depends on appropriate extent and quality of emboli, does not allow studying of thrombolysis |
| Ischemic stroke, transient occlusion | |||||
| Extravascular device | ACAO, transorbital, clip | NHP (baboon) | 74 | Enables to investigate reperfusion, Reliable, consistent occlusion, less traumatic | Slightly more volatile, no exact simulation of hemodynamic reperfusion characteristics |
| MCAO, transorbital, clip | NHP (squirrel monkey, baboon) | 36,35 | |||
| MCAO, clip | Dog, pig, sheep | 75,76,33 | |||
| AchAO, clip | Pig | 31 | |||
| Intravascular device | ACAO, coil | NHP (baboon) | 77 | Spatially and timely precise induction of the occlusion | Endovascular device placement technically challenging, requires surgical training, vasospasm is possible complication |
| MCAO, coil | Dog | 40 | |||
| PCAO, microcatheter | Rabbit | 39 | |||
| Intravascular blood clot | MCAO | Dog, rabbit, NHP (cynomolgus monkey) | 78,41,79 | Simulates pathological procedure of human stroke, minimally invasive, allow studying thrombolytic or anticoagulative interventions, blood vessels remain intact | Final destination of thrombus hardly predictable, preparation of thrombus critical – important for successful deployment |
| ICAO | Rabbit, pig, NHP (Rhesus or cynomolgus monkeys) | 80,12,42 | |||
| basilar artery occlusion | Dog | 81 | |||
| CCAO | Rabbit, pig | 82,83 | |||
| Intravascular artificial embolization | MCAO | Rabbit | 44 | Any required embolus shape, leads to more uniform infarcts, reducing variability | Final position of emboli depends on appropriate extent and quality of emboli, does not allow study of thrombolysis |
| Intravascular thrombin infusion | MCAO | Rabbit | 46 | Reliable occlusion, opportunity to study thrombolysis | Does not result in uniformly sized infarcts |
| Ischemic stroke, spontaneous | |||||
| Spontaneous stroke | MCAO, suspected embolus | Dog | 47 | Could play important role in research on stroke pathophysiology – similarities in neuroanatomy and clinical outcome, lack of confounding factors (anaesthesia, surgical trauma) | Low incidence of stroke in dogs, high variability, restricted to scenarios in which a maximal proximity to clinical situation is required |
| Artificial carotid siphon model | |||||
| Artificial carotid siphon model | tortuous artery model, plastic curved rod ICA | Dog | 49 | Realistic environment to assess endovascular devices | Significant phenotypic diversity of the human CS, creation requires huge effort |
| digital tube CCA | Dog | 84,85 | |||
| synthetic Dacron graft CCA | Pig | 48 | |||
| forelimb, brachial branch of subclavian artery | Pig | 50 | Simple, reproducible and technically less demanding | Vessel diameter does not approximate that of human ICA | |
| Models using extracranial vessels | |||||
| Stroke | Renal artery blood clot | Dog | 86 | Arterial access much easier, diameter comparable to intracranial vessels | High simplification, less tortuous, differs with regard to arterial wall composition |
| superficial femoral artery blood clot | Pig | 87 | |||
| iliofemoral bifurcation & kidney | Pig | 88 | |||
| diverse cervicocerebral arteries blood clot | Dog, rabbit, pig | 89,90,91,92,93,94,95 | |||
| subclavian artery blood clot | Pig | 96 | |||
| Branches of subclavian artery | Pig | 97 | |||
| Aneurysm/AVM | renal artery embolization | Dog, rabbit, pig | 51,98,99 | Suitable for recanalization studies | Differences in vascular characteristics as compared to cranial vessels |
| kidney embolization | Dog | 100 | |||
| subclavian artery and branches device | Pig | 101 | Easier surgical access, vessel diameters similar to human cerebral vessels | Cannot simulate the complex geometry of AVMs | |
| femoral + subclavian artery embolization | Rabbit | 102 | |||
| femoral artery device | Dog | 103 | |||
| Silicon shunt femoral artery-vein device | NHP (baboon) | 104 | |||
| branching vessel of forelimb device | Pig | 105 | |||
| abdominal aorta device | Rabbit | 106,107,108 | |||
| abdominal aorta embolization | Sheep | 109 | |||
| auricular artery embolization | Rabbit | 110 | |||
CCA: common carotid artery; EJV: external jugular vein; ECA: external carotid artery; JV: jugular vein; MCA: middle cerebral artery; MCAO: middle cerebral artery occlusion; AchAO: anterior choroidal artery occlusion; ACAO: anterior cerebral artery occlusion; PCAO: posterior cerebral artery occlusion; ICAO: internal carotid artery occlusion; CCAO: common carotid artery occlusion; ICAO: internal carotid artery occlusion.
Aneurysm models
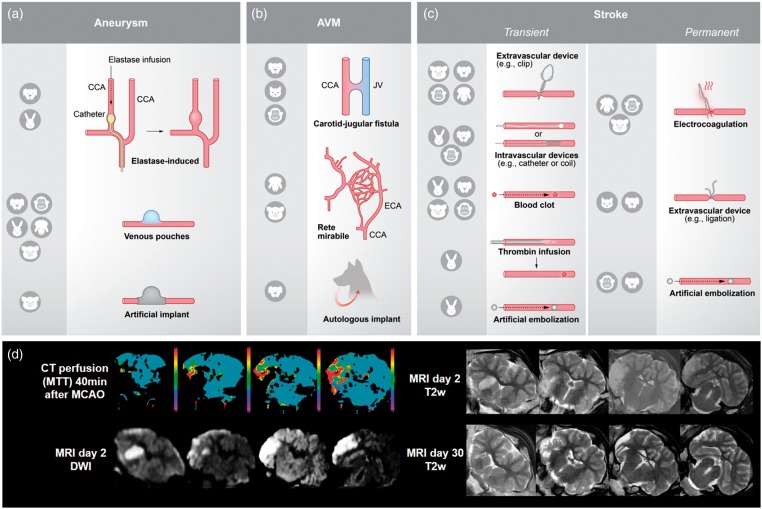
Three different ways to induce an aneurysm in large animals are described (Figure 2(a)): elastase-induced aneurysms, venous pouch aneurysms, and use of artificial implants. Elastase-induced aneurysms imitate the biological and molecular environment of naturally occurring aneurysms and exhibit uninjured arterial walls.18 On the other hand, they require time to mature,19 are less reproducible in appearance,20, and comparatively small.21 Aneurysms created by venous pouches are commonly used, reliable aneurysm models22 with reproducible characteristics such as consistent aneurysm size and neck diameters. Disadvantages are the surgical trauma required for induction, at least transient presence of suture threads, and the venous structure of the aneurysmal wall.20 Aneurysms emerging from artificial implants provide good training opportunities for endovascular treatments such as coiling,19 but are expensive23 and come at the risk of thrombus formation within the implant. Hybrid models connecting an artificial vascular tree with multiple aneurysms to an animal’s circulation are also available for neurointerventional training purpose.19
Figure 2.
Illustration of the different techniques for model induction and utilization of clinical imaging procedures. (a) There are three different ways to induce an aneurysm model: elastase-induced aneurysms (aneurysm develops in a closed vessel by elastase infusion), venous pouches aneurysms (preparation of a venous pouch and suturing to artery, usually CCA) and use of artificial implants. Abbreviations: CCA: common carotid artery. (b) AVM models can be created by a carotid-jugular fistula (shunt between CCA and JV), use of RM in pigs and sheep, and use of autologous implants. Abbreviations: JV: jugular vein; ECA: external carotid artery. (c) Ischemic stroke models are based on transient or permanent occlusion of cerebral arteries. Extravascular and intravascular occlusion methods can be discriminated. Extravascular occlusion comprises electrocoagulation and the use of ligation, or occluding devices such as aneurysm clips. Intravascular occlusion can be induced by intravascular devices, such as aneurysm coils, blood clots, thrombin infusion or artificial emboli. (d) A major advantage of large animal models is the compatibility with clinical imaging technologies. Sample images were taken in a sheep after transient MCAO by a surgical clip (3-h occlusion time). Abbreviations: MTT: mean transit time; DWI: diffusion-weighted imaging; T2w: T2-weighted imaging. False color scale indicates MTTS from 0 (purple) to 10 s (red). All images were taken on standard clinical scanner during an in-house study (data not published).
In the analyzed literature, therapies were tested immediately after aneurysm induction (30.4%, 68/224) or after a maturation time of up to two weeks (17.9%, 40/224), up to four weeks (38.4%, 86/224), up to eight weeks (4.4%, 10/224), or after more than eight weeks of maturation (1.3%, 3/224). Some studies (7.6%, 17/224) did not mention aneurysm maturation time.
Models of arterio-venous malformations
AVM models can be created by a carotid-jugular fistula, by using species exhibiting rete mirabile (RM), or by using autologous implants (Figure 2(b)). Carotid-jugular fistulas can be induced easily, but do not provide an AVM nidus.24 The RM in pigs and sheep can be used alternatively. The RM is the branching of the maxillary artery into a network of fine arteries, which then reunite to the internal carotid artery. Although not an arteriovenous connection,25 the RM is an often utilized natural AVM model and suitable to study post-interventional histopathological alterations.26 Artificial arteriovenous shunts utilizing the RM as the AVM nidus provide relevant morphological characteristics of human AVM, such as feeding artery, interposed nidus, and draining vein.24 However, they are prone to the risk of spontaneous occlusion.25 Further, AVMs can be induced by an autologous implant in the cerebral circulation, such as an artery or a muscle graft with related artery. This procedure has immediate effects on cerebral circulation and provides a good simulation of pathophysiological conditions observed clinically.27,28 However, excellent surgical skills are required to consistently create such AVM models.29
Therapy in AVM models is mainly tested immediately after AVM induction (72.7%, 24/33) or in some cases after a maturation time up to four weeks (18.2%, 6/33), and in rare cases even beyond that time (9.1%, 3/33).
Ischemic stroke models
Stroke models are based on permanent or transient occlusion of cerebral arteries (Figure 2(c)), mainly the middle cerebral artery (MCA). Permanent occlusions models result in reproducible functional outcome and lesion size30,31 but do not allow analysis of reperfusion.32 Transient occlusion models tend to produce more volatile results, but enable to investigate reperfusion.33
Stroke induction can be discriminated into extravascular and intravascular occlusion methods. Extravascular occlusion involves neurosurgical access to the target vessel followed by ligation, electrocoagulation or occluding devices (e.g. aneurysm clips). Electrocoagulation is relatively easy to perform, leads to reproducible neurological deficits,34 and highly consistent vessel occlusion. However, diathermy can increase blood–brain barrier permeability.32 Vessel occlusion using clips or by ligation induces a reliable and consistent occlusion, which is less traumatic.35 Transient vessel occlusion allows studying reperfusion effects, but hemodynamic characteristics are different from those seen in reperfusion.10 Moreover, clip and thread handling can be challenging36 and requires appropriate experimenter training. Extravascular occlusion needs transorbital access (mainly for the anterior or middle cerebral arteries37) or craniectomy (all major arteries accessible). Transorbital access only requires a small craniotomy in the orbita, and manipulation of surrounding tissue is minimized.38 Disadvantages of this approach are eye loss and related postoperative complications, including abnormal behavior caused by vision defects.32 Craniotomy results in a larger wound and is technically more demanding, but preserves the eye. Craniotomy is avoided when using intravascular occlusion models.
Intravascular occlusion requires endovascular access to the target vessel. It is induced by devices such as coils, blood clots, artificial emboli, or thrombin infusion. Intravascular devices such as coils or catheters enable a spatially and temporally precise stroke induction,39 but endovascular device placement is technically challenging and requires intensive training to generate reproducible results. Moreover, vasospasm is a possible complication,40 effecting many relevant outcome parameters. Using blood clots for vessel occlusion perfectly simulates the pathological mechanism of human stroke, is minimally invasive, and allows studying thrombolytic or anticoagulative interventions.41 Another advantage is that blood vessels remain physically undamaged after thrombus deposition.42 The major disadvantage is that the occlusion site is hardly predictable, making the outcome highly heterogeneous. Another critical factor is preparation of the thrombus, which critically influences its characteristics.41 Artificial emboli made of silicone rubber or silicone-coated filaments provide a standardized but artificial alternative. These emboli could be of any required shape,43 helping to define occlusion site and thereby leading to more uniform infarcts. This reduces variability,44,45 but studying recanalization is not possible. Endovascular approaches are not applicable for species exhibiting an RM.30,32 Another possibility to produce a vessel occlusion also beyond the RM is thrombin infusion and subsequent thrombus formation. This method provides reliable occlusion and the opportunity to study recanalization, but induced infarcts are heterogeneous in size.46
Spontaneous stroke occurrence is reported in dogs and might play an important role in the investigation of stroke pathophysiology. There are no experimental confounding factors such as anesthesia or surgical trauma.47 However, the low incidence of stroke in dogs, along with a high variability, restricts this model to scenarios in which a maximal proximity to the clinical situation is required in spite of inter-subject variability.
Carotid siphon models
The human carotid siphon (CS) has unique anatomical bends which remain critical structures for endovascular catheter access to the intracranial vessels, in particular when using large-bore (5F or 6F) intermediate catheters for clot aspiration in acute stroke treatment or for facilitated navigation of flow-diverting stents in aneurysm therapy. In particular in elderly patients with increased CS tortuosity (Supplementary Figure 1), vascular access with these catheters may be impaired. On the other hand, too forceful manipulation during catheterization attempts may result into complications such as endothelial injury with vasospasm, dissection, thrombosis or even vessel perforation. Thus, new designs for intermediate cranial access catheters require testing using vascular models of the CS to evaluate important parameters like steerability/torquability, lubricity, stiffness, and durability under realistic in vivo conditions. Surgical CS models are created by using different implants and offer a realistic environment including pulsating blood flow and vascular responses for the assessment of endovascular devices in terms of vascular navigation.48 However, there is a significant diversity in human CS anatomy49 and each model can only represent one particular formation. Moreover, surgical implantation and creation of these models are technically challenging and require a recovery period before device testing. Alternatively, the human CS anatomy can be easily modelled by maximally flexing the porcine forelimb.50 This creates a brachial artery tortuosity, resulting in an anatomical vessel configuration similar to that of the human CS. This method is simple, reproducible, and technically less demanding, but vessel diameter does not approximate that of the human ICA.50
Models using extracranial vessels
Animal modeling of large vessel occlusion exclusively in the extracranial circulation has informed the thrombectomy device design (Table 1), leading to efficient and effective technologies for treating stroke patients. For instance, research on thrombolysis and thrombectomy is sometimes performed on renal arteries, the superficial femoral artery, the iliofemoral bifurcation, the subclavian artery or its branches (mainly in pigs), and on different cervicocerebral arteries. The latter includes the lingual artery, maxillary artery, cervical arteries, external carotid artery (ECA), or ascending pharyngeal artery (APA). Aneurysm or AVM therapy is often simulated using renal arteries, which are suitable for recanalization studies.51
Simple metrics such as the angiographic evidence of recanalization are used to assess device efficacy. Importantly, the trauma caused to the vessel wall can be studied as well as the consequence of distal52 emboli to a downstream organ (e.g., renal artery vascular occlusion models53). However, there are important limitations to these models that must be appreciated when interpreting the data. The first obvious point is that arteries of the brain have significant differences in structure,54 mechanics55 and function as compared to systemic arteries. Importantly, intradural arteries are not tethered or constrained in the perivascular environment. Additionally, the tortuosity of the human intracranial circulation is not seen in existing animal vascular occlusion models. It is for these reasons that an important complement to animal modeling includes in vitro vascular occlusion modeling in phantoms of patient-specific or population-based vascular replicas.56–61 This combined approach has led to the development and ultimate regulatory approval of highly effective thrombectomy devices such as stent-retrievers and aspiration catheters.
However, the lack of the target organ of interest, namely the brain, is a critical limitation for the next evolution in stroke care.10 Perhaps the optimal treatment will involve pre-thrombectomy neuroprotection,62 direct to angiosuite mechanical thrombectomy for emergent large vessel occlusions, catheter-based delivery of thrombolytics to treat shed microemboli or other agents to further limit/alter the course of neuronal injury. When considering such a complex treatment solution, the variables are further compounded when considering dose of each treatment or route of delivery.
Benefits and challenges of large animal models in neurointerventional research
Large animal models offer several advantages and a higher similarity to the human situation compared to small animal models, which make them particularly attractive for neurointerventional research (Table 2).
Table 2.
Advantages of large animal models for neurointerventional research.
| Small animal | Large animal | Human | References | ||
|---|---|---|---|---|---|
| Brain | Size | Small | Large | Large | 10,14,113 |
| Encephalization quotient (EQ) | 0.4 (rat), 0.5 (mouse) | 0.8 (sheep), 1.2 (dog) and 2.1 (rhesus monkey) | 6.56 | 113,114 | |
| Anatomy | Lissencephalic Mouse: GM/WM: 90%/10% Rat: GM/WM: 88%/12% Soft tentorium cerebelli | Gyrencephalic Dog: GM/WM: 63%/37% Sheep: GM/WM: 70%/30% rhesus monkey: GM/WM: 68%/32% Rigid tentorium cerebelli | Gyrencephalic Gray matter: 55% White matter: 45% Rigid tentorium cerebelli | 14,10,115–117,112 | |
| Vascular supply | Similar to humans | Dog, rabbit, NHP: similar to humans tortuosity of canine ICA Sheep, pig, cat: rete mirabile (sheep, cat: ICA, pig: ascending pharyngeal artery) | Internal carotid artery (ICA), basiliary artery | 40,46,42,72,118,119 | |
| Vessel | Size | Rat: MCA diameter 0.35–0.58 mm Diameter often too small for endovascular approaches | Similar to humans e.g. dog: MCA diameter 2–3.5 mm Diameter large enough for endovascular access | MCA diameter 2.7–3.5 mm | 89,95,10,12,120–124 |
| physiology | Similar to humans | Similar to humans, e.g.: degree of vasospasm (dog) fibrinolytic response (rabbit) platelet response (NHP) | 89,121,42 | ||
| Circle of Willis (CW) | Rat, mouse: similar to humans, except missing anterior communicating artery | Dog, rabbit, monkey: similar to humans, except missing anterior communicating artery and single median anterior cerebral artery (ACA) Sheep and swine: different to humans: internal carotid artery forms large part of CW, irregular anterior communicating artery | Main arteries: internal carotid artery, vertebral artery contributing arteries: anterior cerebral artery, anterior communicating artery, posterior cerebral artery, posterior communicating artery, basilar artery Incidence of complete circle of Willis: 37.1% (overalls); 43.8% (females); 31.2% (male) Most frequent of 28 variations of CW: absent posterior communicating artery (right side: 15.3%; left side: 10.9%; bilaterally: 17.1%) | 125–129 | |
| Imaging | Dedicated small animal scanners required | Clinical systems applicable | Clinical systems available | ||
| X-ray-based | Possible (angiography, CT) | Possible (angiography, fluoroscopy, CT) | Standard routine | 130,77,12,131,132 | |
| Radionuclide | Possible (PET, SPECT) | Possible (PET, SPECT) | Standard routine | 133,134 | |
| Nuclear magnetic resonance | Possible (NMR, MRI) | Possible (NMR, MRI) | Standard routine | 135,77 | |
| Ultrasound | Possible, but rarely performed | Possible | Standard routine | 136 | |
| Physiological parameter assessment | Recording | More challenging | Easy | Standard routine | 76,137,9,138 |
| Instrumentation | Requires specialized device configuration | Human appliances can be used | Standard routine | 76,137,9,138 | |
| Simultaneous recording | Restricted | Possible, multiple parameters | Standard routine | 76,137,9,138 |
ICA: internal carotid artery; NHP: non-human-primate; ACA: anterior cerebral artery; CT: computed tomography; PET: positron emission tomography; SPECT: single photon emission computed tomography; MRI: magnetic resonance imaging; NMR: nuclear magnetic resonance; GM: grey matter; WM: white matter.
In contrast to the situation in rodents, large animal and human brain anatomy share many similarities. Large animal brains are mostly gyrencephalic and exhibit higher white matter content. This is important since white matter is more resilient to ischemia and therapeutic approaches targeting white matter are believed to have a wider therapeutic time window.111 Moreover, white matter is important for higher brain function and plasticity. Another example is the comparatively rigid tentorium cerebelli in most large animal species, as compared to the soft structure in rodents. This plays a crucial role in the effects of edema and can aggravate consequences of intracerebral pressure112 in humans and large animals alike. Moreover, large animals models can be used easily with clinical imaging equipment (Figure 2(d)).
Individual anatomical variations can impair model induction or the therapeutic approaches. Large animal strains are often outbred, increasing the likelihood of such variances. For instance, collateral vasculature or tortuous blood vessels as observed in pigs,76 dogs,75 and cats139 can cause challenges in experimental interventions. Moreover, some species-specific anatomic prerequisites must be taken into consideration, the most prominent one being the RM.
Large animal experiments are often more complex than small animal studies. Training and pilot studies are therefore warranted to optimize the results of the main trial. The time and resource “loss” caused by such pilot experiments are often compensated by increased reliability and decreased variability in the main trial. This is of particular importance because large animal stroke models, such as the situation in human patients, tend to be more variable in outcome than their rodent counterparts. Pilot studies are also important to reveal at least basic information on effect sizes and thus required group/sample sizes in cases where small animal data are not available. This also helps to balance the number of required animals per group to maintain sufficient statistical power against ethical and financial constraints.
Pilot studies can further reveal whether model optimization can increase the amount or quality of information derived from the main experiment. In cases where an entirely new technical field is entered, pilot studies, together with consulting expert colleagues in large animal experimentation, help to select the appropriate large animal model.
Some genetically modified large animal strains exist and can be used to address special research questions. For instance, transgenic NHPs140 or pigs141 expressing green fluorescent protein have been reported. Transgenic large animal models are also available for other neurological disorders.142
Types of interventions and therapies in the literature
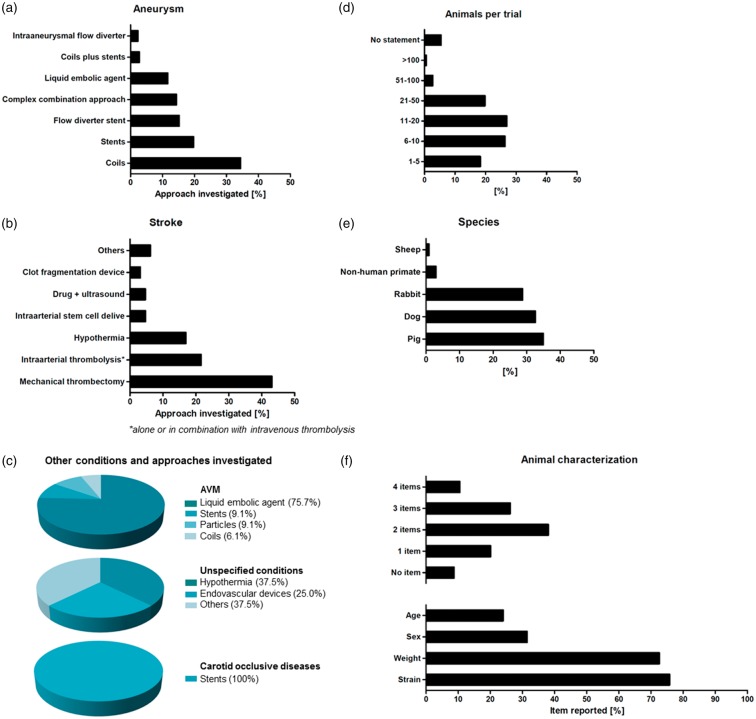
Experimental aneurysm therapies were reported by two-third (67.0%; 224/334) of all studies, 19.5% (65/334) assessed stroke therapies, and 9.9% (33/334) AVM treatments. This is reasonable because aneurysm, stroke and AVMs are frequent and severe neurological disorders. Figure 3(a) to (c) provides detailed information on investigated approaches; 2.4% (8/334) of studies investigated conditions that were not defined in more detail; these included “intracranial lesions,” “abnormalities in central nervous system” or “brain injury” (referred to as “unspecified conditions” in Figure 3(c)); 1.2% (4/334) simulated carotid artery occlusive diseases.
Figure 3.
Applied therapies in analyzed studies. (a) Aneurysms: main focus was on coils, stents and flow diverter stents (b) stroke: dominant therapy was mechanical thrombectomy, a recognized alternative to intravenous thrombolysis (c) AVMs: majority of studies investigated liquid embolic agents; unspecified conditions (e.g. “intracranial lesions,” “abnormalities in central nervous system” or “brain injury”): focus was on hypothermia and endovascular devices; carotid occlusive diseases: all studies investigated stents. Information about animals: (d) Number of animals: almost half of the studies used only up to 10 animals. (e) Occurrence of different species: most commonly used species were pigs and dogs, followed by rabbits (f) Animal characteristics: only a minority of studies reported all four items – strain, weight, sex and age.
Most interventional aneurysm therapies focused on coils (34.4%, 77/224), followed by stents (19.6%, 44/224) and flow diverter stents (15.2%, 34/224). Studies also investigated liquid embolic agents or intraaneurysmal flow diverters, reflecting the technical progress in aneurysm therapy. Some therapeutic studies on aneurysms reported combined approaches. Those, for instance, comprised coils or stents in combination with additional effector substances (such as Onyx or fibroblasts,143,144 6.3%, 14/224), combined approaches using neck bridging devices (1.8%, 4/224), and microcatheter-based delivery of stem cells,145 hydrogels,146 and other biomaterials147 (5.4%, 12/224). Some combination approaches were only employed by very few studies, as is the case with unconventional approaches such as magnetic microparticles148 (0.9%, 2/224).
Liquid embolic agents were primarily investigated in AVM therapy studies (75.8%, 25/33; Figure 3(c)). Expectedly, mechanical thrombectomy was the predominant technique investigated in stroke therapy studies (43.1%, 28/65; Figure 3(b)). Intraarterial thrombolysis was investigated by 21.5% (14/65) of the studies. A significant proportion of all studies on stroke therapy investigated hypothermia (16.9%, 11/65), which may improve the results of recanalization by neuroprotective effects.76 Only a small part of the studies investigated the effects of intraarterially administered stem cells (4.6%, 3/65), an approach believed to promote neurological regeneration.
Interestingly, we also identified one paper that reported the off-label use of a stent-retriever to remove a misplaced stent.149 This represents an innovative complication management approach that deserves further testing in animal studies for proving and refinement prior to use in patients.
Study design
Therapeutic efficacy was the most important endpoint assessed either alone or in combination with secondary endpoints. Almost one-third of all studies focused solely on efficacy (29.6%, 99/334), directly followed by studies assessing safety and efficacy (24.2%, 81/334), or feasibility and efficacy (17.7%, 59/334). The minority of studies focused on feasibility (11.1%, 37/334) or safety (6.9%, 23/334) alone, some studies assessed safety and feasibility (7.5%, 25/334). The minority of studies (3%, 10/334) investigated the triad of feasibility, safety and efficacy simultaneously.
Some papers (23.7%, 79/334) reported acute studies (no reawakening and survival of experimental subjects), 70% (234/334) reported survival studies, and 6.3% (21/334) featured both, non-survival and survival experiments. The follow-up periods were up to one day (1.2%, 3/255), up to three days (0.4%, 1/255), up to one week (2.7%, 7/255), up to two weeks (9%, 23/255), up to one month (21.2%, 54/255), between one and two months (15.7%, 40/255), between three and six months (36.5%, 93/255), and over six months (10.6%, 27/255). Some studies did not report the follow-up period (2.7%, 7/255).
Study objectives define study duration and follow-up periods. For instance, acute studies are adequate for feasibility and safety assessments, as potential adverse events such as tissue damage can be investigated immediately. On the other hand, survival studies allow observation of short- and long-term effects including possible disadvantages of an approach that emerge at later stages. The stroke academic and industry round table (STAIR) expert consortium generally recommends long-term studies in efficacy-oriented stroke research.16 The situation may slightly differ in neurointerventional studies in which for instance successful recanalization may serve as the major efficacy endpoint. Long-term observation is nevertheless advantageous when identifying delayed effects or complications such as aneurysm recanalization in experimental aneurysm therapy,150 and provide a more complete context of effects. Life span of large animals allows for observation times of a year or even more.
Lack of randomization and blinding is believed to account for a significant proportion of false-positive study results.151 Surprisingly, most large animal studies we assessed were neither designed in randomized nor blinded fashion (65.9%, 220/334). Only 12.6% (42/334) randomized experimental subjects and only 4.1% (14/334) featured a completely blinded study design; 17.4% (58/334) studies only blinded analysis of selected endpoints.
Since more investigators are usually required to perform large animal studies, complete blinding might indeed be challenging. A potential solution is to separate model induction from testing the diagnostic or therapeutic paradigm, for instance by randomization right after stroke induction, as well as to strictly separate the experimenters acquiring data from those who perform data analysis.
Animal numbers and animal characterization
Almost half of the studies only used up to 10 animals in total (44.6%, 149/334), making inter-group comparisons statistically challenging. In contrast, only a minority used more than 50 animals (3.3%, 9/334) (Figure 3(d)). Very few studies described details of animal housing and care (5.7%, 19/334). More than four fifths (82%, 274/334) of all studies reported some details on the applied anesthesia protocol. The majority gave precise information about used anesthetics (63.4%, 212/334), but some studies only provide unspecific or basic information (18.6%, 62/334). Nearly 20% of studies did not provide any information on medication at all, including anesthesia (18%, 60/334). Not even 10% of all studies (9.3%, 31/334) did report details on the use of analgesics.
Animal strain was reported most frequently (75.8%, 253/334), followed by weight (72.2%, 241/334) and sex (31.4%, 105/334) (Figure 3(f)). Age was reported by less than a fourth of all studies (24%, 80/334), and from those about one-third (27/80) only provided an unspecific classification (e.g. “adult” or “juvenile”). More than half of the studies reported one to three characteristics (60.2%, 201/334), but only 10.5% (35/334) reported all four items (strain, weight, sex, age), while 8.7% (29/334) did not provide any of such information. Information about the animals is not only essential for reproducibility of a study, but also of relevance for study result interpretation since strain, age and sex can influence both the model and the intervention.16
No studies reported the use of subjects exhibiting comorbidities. This can be explained by the fact that comorbidities in large animals are not genetically induced, but naturally occur with age, distress, malnutrition, and other factors as is the case in humans. Therefore, comorbid large animals exhibit extremely heterogeneous phenotypes and subjects require a long time to develop these comorbidities, impairing their use.
Selection criteria
Only very few studies applied a priori defined selection criteria (4.8%, 16/334). Of these, 50% (8/16) reported specific inclusion criteria, 37.5% (6/16) reported the use of inclusion criteria but did not specify them, and 12.5% (2/16) reported exclusion criteria. None of the studies used both inclusion and exclusion criteria. Similarly, just a few studies mentioned post hoc exclusion of animals (11.1%, 37/334) with all, but one study providing the specific reason for subject exclusion. Some studies reported animal death prior to study conclusion, but inclusion of these cases into analysis (2.1%, 7/334). Information on inclusion and exclusion criteria is essential to ensure reproducibility. These criteria should be determined at the beginning of the study and mentioned in the publication. Animal health condition should be covered by inclusion criteria.
Endpoint assessment and readout parameters
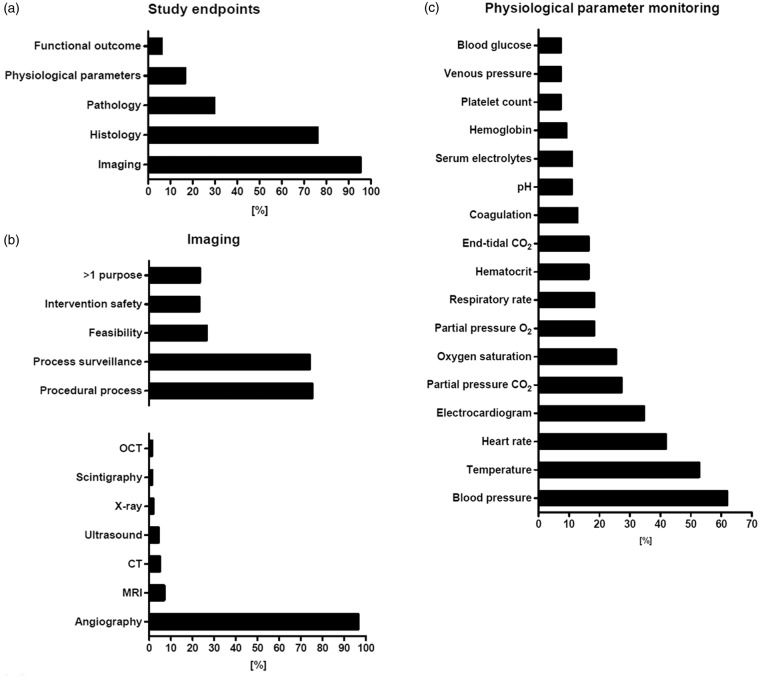
Imaging endpoints dominated the evaluation process (95.1%, 318/334; referred to as “study endpoints” in Figure 4(a)), what reflects the uncomplicated use of clinical imaging modalities with large animal models as well as the high importance of imaging technologies and imaging-based endpoints in clinical neurointerventional routine and research. Imaging has been used in most cases to prove therapeutic (and sometimes model) efficacy (73.9%, 235/318; referred to as “process surveillance” in Figure 4(b)), for instance to detect revascularization after vessel occlusion or occlusion of an aneurysm. More than two-third of studies using imaging for outcome assessment have also used imaging to control for model performance, adequate model handling, and lesion induction (75.2%, 239/318; referred to as “procedural process” in Figure 4(b)). Angiography is particularly suitable for endovascular procedures, as it can be used to visualize blood flow and hemodynamic changes in the blood vessel, while simultaneously allowing potential interventions. Hence, it is not surprising that angiography was by far the most frequently used imaging procedure (96.5%, 307/318). Other modalities were used with much lower frequency. Examples comprise MRI (6.9%, 22/318), CT (5%, 16/318), ultrasound (4.4%, 14/318), or conventional X-rays and fluoroscopy (1.9%, 6/318; Figure 4(b)). The lower number of studies using such modalities can be explained by the fact that MRI and in particular diffusion weighted imaging may be important in some areas such as stroke research and treatment, but are less important for aneurysm or AVM assessment as the major domains of large animal models in neuroendovascular research. There is still a lack of data reporting variability of imaging endpoints such as ischemic volume on MRI following MCA occlusion. However, such information is important for defining sample sizes for efficacy endpoints.
Figure 4.
Overview of endpoint assessment in analyzed studies. (a) Evaluation process: frequent use of imaging (reflecting uncomplicated use in large animals) and histology. Only 16.5% evaluated physiological parameters. Behavioral tests are applied in stroke research (b) Data about imaging: clearly dominated by angiography (well qualified for use in neurointerventional research). Most common use for imaging was to prove efficacy and for procedural process. (c) Presentation of physiological parameters: dominated by blood pressure, temperature, heart rate and electrocardiogram. CT: computed tomography; MRI: magnetic resonance imaging; OCT: optical coherence tomography.
Histological examination was the second most frequent method employed for evaluation (76.1%, 254/334; Figure 4(a)). Histology is useful for safety assessment, for example to evaluate the inflammatory response or vessel wall damage, and almost half of the studies (41.6%, 139/334) indeed assessed safety alone or in combination with other endpoints. Moreover, histology can assess efficacy aspects such as endothelialization or neointima formation after coil placement.
Behavioral tests can be performed on pigs,31 dogs,152 rabbits,153 NHPs,154 and sheep.30 However, only a small proportion of the studies used behavioral endpoints (6%, 20/334; Figure 4(a)). Interestingly, all of these studies focused on stroke. In turn, almost a third of studies focusing on stroke included behavioral tests (30.7%, 20/65). Behavioral tests are a valid option to investigate functional effects of stroke lesion induction and therapy. The STAIR committee16,17 recommends at least two outcome measurements allowing both functional and morphological assessment in stroke research, what shall also account for large animal models.
Some studies measured brain temperature (3.9%, 13/334) or intra-aneurysmal pressure (1.5%, 5/334), while others specifically investigated mortality and morbidity (1.2%, 4/334). Only few studies reported special aspects such as radiation dosimetry (0.6%, 2/334), coil insertion pressure, stereographic photography, liquid scintillation counting (0.3% each, 1/334), drawing blood samples (0.6%, 2/334), pressure transducers in the RM, cerebrospinal fluid sampling, or cerebral blood flow (0.3% each, 1/334).
Not even one-fifth of all studies evaluated physiological parameters (16.5%, 55/334). This is surprising, because recording of physiological parameters in large animal models is even less complicated than in rodents, and a broad spectrum of physiological parameters may be recorded easily, and simultaneously. Blood pressure was the predominant parameter recorded (61.8%, 34/55), followed by temperature (52.7%, 29/55), heart rate (41.8%, 23/55), and electrocardiogram (34.6%, 19/55; Figure 4(c)). End-tidal isoflurane, end-tidal O2, body weight, cardiac output, intracranial pressure, electroencephalogram, d-dimer (3.6% each, 2/55), hemograms, renal function during surgery, motor-evoked potentials, and auditory brain stem response (1.8% each, 1/55) were investigated rarely. Some studies 5.5% (3/55) reported measuring of physiological parameters, but without any further details including the particular parameters monitored.
STAIR16,17 recommends rigorous physiological monitoring to control for potential side effects of surgery, and to reduce or at least explain infarct size variability. This recommendation can be extended to neurointerventional research using large animals. Reporting of some core physiological parameters such as blood pressure, temperature, and heart rate should be mandatory. Recording of additional physiological depends on the respective study objective. For example, in studies featuring hypothermia, monitoring of blood gases is essential as these are influenced by body temperature.155 Any study should record physiological parameters not only at the beginning and the end of an experiment, but also in the meaningful intervals throughout the procedure. Measuring physiological parameters is an elegant way to derive the maximum of information from a single experimental subject, facilitating result interpretation and, if necessary, providing a thorough basis for subject exclusion.
Only 0.6% (2/334) of the studies clearly defined study endpoints, but these featured both primary and secondary end points. This is nevertheless a surprisingly low number. Large animal models are primarily used in confirmative and translational research, often as the last experimental step prior to clinical application, and thus critically depend on a clear definition of study objectives and, thereby, primary and secondary endpoints.
Conclusion
Large animal models offer many important benefits – foremost to mention their similarities to human brain and vascular anatomy – that make them attractive for neurointerventional research. New endovascular approaches can be developed and refined in large animal experiments, which may improve minimal-invasive neurointerventional therapy for patients in future. On the other hand, large animal studies are laborious and expensive. Careful study planning, deriving maximum information from each study subject, and transparently reporting negative data or pitfalls are therefore essential. Tapping the full potential of large animal models critically requires careful outweighing the individual advantages and disadvantages of animal models, carefully selecting the model best suitable to answer the research question, and implementation of improvements where required, as well as maximally precise study design.
Supplemental Material
Supplemental Material for Large animals in neurointerventional research: A systematic review on models, techniques and their application in endovascular procedures for stroke, aneurysms and vascular malformations by Andrea M Herrmann, Stephan Meckel, Matthew J Gounis, Leona Kringe, Edith Motschall, Christoph Mülling and Johannes Boltze in Journal of Cerebral Blood Flow & Metabolism
Acknowledgements
The authors wish to explicitly thank Dr. Larisa Bulavina for providing the professional illustrations in Figure 2(a) to (c).
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: only intramural funds were used for this work.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: SM: consultancy and honoraria as member of the scientific advisory board (Acandis GmbH); travel support (Covidien/Medtronic; Microvention; Stryker); study grant (money paid to institution; Bracco S.p.A.); JB: consultancy and honoraria (Acandis GmbH). All other authors declare that they have no conflict of interest.
Availability of data and material
The datasets generated and/or analyzed during the current study are available from the corresponding author on request.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Campbell BCV, Donnan GA, Lees KR, et al. Endovascular stent thrombectomy: the new standard of care for large vessel ischaemic stroke. Lancet Neurol 2015; 14: 846–854. [DOI] [PubMed] [Google Scholar]
- 2.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 3.Campbell BCV, Hill MD, Rubiera M, et al. Safety and efficacy of solitaire stent thrombectomy: individual patient data meta-analysis of randomized trials. Stroke 2016; 47: 798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018; 378: 708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018; 378: 11–21. [DOI] [PubMed] [Google Scholar]
- 6.Jovin TG, Albers GW, Liebeskind DS. Stroke treatment academic industry roundtable: the next generation of endovascular trials. Stroke 2016; 47: 2656–2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kallmes DF, Hanel R, Lopes D, et al. International retrospective study of the pipeline embolization device: a multicenter aneurysm treatment study. AJNR Am J Neuroradiol 2015; 36: 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ginsberg MD, Busto R. Rodent models of cerebral ischemia. Stroke 1989; 20: 1627–1642. [DOI] [PubMed] [Google Scholar]
- 9.Traystman RJ. Animal models of focal and global cerebral ischemia. ILAR J 2003; 44: 85–95. [DOI] [PubMed] [Google Scholar]
- 10.Mehra M, Henninger N, Hirsch JA, et al. Preclinical acute ischemic stroke modeling. J Neurointerv Surg 2012; 4: 307–313. [DOI] [PubMed] [Google Scholar]
- 11.Xu X-Q, Cheng Q-G, Zu Q-Q, et al. Comparative study of the relative signal intensity on DWI, FLAIR, and T2 images in identifying the onset time of stroke in an embolic canine model. Neurol Sci 2014; 35: 1059–1065. [DOI] [PubMed] [Google Scholar]
- 12.Gralla J, Schroth G, Remonda L, et al. A dedicated animal model for mechanical thrombectomy in acute stroke. AJNR Am J Neuroradiol 2006; 27: 1357–1361. [PMC free article] [PubMed] [Google Scholar]
- 13.Kuge Y, Yokota C, Tagaya M, et al. Serial changes in cerebral blood flow and flow-metabolism uncoupling in primates with acute thromboembolic stroke. J Cereb Blood Flow Metab 2001; 21: 202–210. [DOI] [PubMed] [Google Scholar]
- 14.Boltze J, Nitzsche F, Jolkkonen J, et al. Concise review: increasing the validity of cerebrovascular disease models and experimental methods for translational stem cell research. Stem Cells 2017; 35: 1141–1153. [DOI] [PubMed] [Google Scholar]
- 15.Sorby-Adams AJ, Vink R, Turner RJ. Large animal models of stroke and traumatic brain injury as translational tools. Am J Physiol Regul Integr Comp Physiol 2018; 315: R165–R190. [DOI] [PubMed] [Google Scholar]
- 16.Stroke Therapy Academic Industry Roundtable (STAIR). Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke 1999; 30: 2752–2758. [DOI] [PubMed] [Google Scholar]
- 17.Fisher M, Feuerstein G, Howells DW, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 2009; 40: 2244–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altes TA, Cloft HJ, Short JG, et al. 1999 ARRS Executive Council Award. Creation of saccular aneurysms in the rabbit: a model suitable for testing endovascular devices. American Roentgen Ray Society. AJR Am J Roentgenol 2000; 174: 349–354. [DOI] [PubMed] [Google Scholar]
- 19.Namba K, Mashio K, Kawamura Y, et al. Swine hybrid aneurysm model for endovascular surgery training. Interv Neuroradiol 2013; 19: 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raymond J, Salazkin I, Metcalfe A, et al. Lingual artery bifurcation aneurysms for training and evaluation of neurovascular devices. AJNR Am J Neuroradiol 2004; 25: 1387–1390. [PMC free article] [PubMed] [Google Scholar]
- 21.Ding Y, Dai D, Kadirvel R, et al. Creation of large elastase-induced aneurysms: presurgical arterial remodeling using arteriovenous fistulas. AJNR Am J Neuroradiol 2010; 31: 1935–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guglielmi G, Ji C, Massoud TF, et al. Experimental saccular aneurysms. II. A new model in swine. Neuroradiology 1994; 36: 547–550. [DOI] [PubMed] [Google Scholar]
- 23.Mühlenbruch G, Nikoubashman O, Steffen B, et al. Endovascular broad-neck aneurysm creation in a porcine model using a vascular plug. Cardiovasc Intervent Radiol 2013; 36: 239–244. [DOI] [PubMed] [Google Scholar]
- 24.Massoud TF, Ji C, Viñuela F, et al. An experimental arteriovenous malformation model in swine: anatomic basis and construction technique. AJNR Am J Neuroradiol 1994; 15: 1537–1545. [PMC free article] [PubMed] [Google Scholar]
- 25.Chaloupka JC, Viñuela F, Robert J, et al. An in vivo arteriovenous malformation model in swine: preliminary feasibility and natural history study. AJNR Am J Neuroradiol 1994; 15: 945–950. [PMC free article] [PubMed] [Google Scholar]
- 26.Lylyk P, Viñuela F, Vinters HV, et al. Use of a new mixture for embolization of intracranial vascular malformations. Preliminary experimental experience. Neuroradiology 1990; 32: 304–310. [DOI] [PubMed] [Google Scholar]
- 27.Yamada M, Miyasaka Y, Irikura K, et al. A canine model of intracranial arteriovenous shunt with acute cerebral venous hypertension. Neurol Res 1998; 20: 73–78. [DOI] [PubMed] [Google Scholar]
- 28.Pietilä TA, Zabramski JM, Thèllier-Janko A, et al. Animal model for cerebral arteriovenous malformation. Acta Neurochir 2000; 142: 1231–1240. [DOI] [PubMed] [Google Scholar]
- 29.Xu M, Xu H, Qin Z. Animal models in studying cerebral arteriovenous malformation. Biomed Res Int 2015; 2015: 178407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boltze J, Förschler A, Nitzsche B, et al. Permanent middle cerebral artery occlusion in sheep: a novel large animal model of focal cerebral ischemia. J Cereb Blood Flow Metab 2008; 28: 1951–1964. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka Y, Imai H, Konno K, et al. Experimental model of lacunar infarction in the gyrencephalic brain of the miniature pig: neurological assessment and histological, immunohistochemical, and physiological evaluation of dynamic corticospinal tract deformation. Stroke 2008; 39: 205–212. [DOI] [PubMed] [Google Scholar]
- 32.Imai H, Konno K, Nakamura M, et al. A new model of focal cerebral ischemia in the miniature pig. J Neurosurg 2006; 104: 123–132. [DOI] [PubMed] [Google Scholar]
- 33.Wells AJ, Vink R, Blumbergs PC, et al. A surgical model of permanent and transient middle cerebral artery stroke in the sheep. PLoS One 2012; 7: e42157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marshall JW, Ridley RM. Assessment of functional impairment following permanent middle cerebral artery occlusion in a non-human primate species. Neurodegeneration 1996; 5: 275–286. [DOI] [PubMed] [Google Scholar]
- 35.Spetzler RF, Selman WR, Weinstein P, et al. Chronic reversible cerebral ischemia: evaluation of a new baboon model. Neurosurgery 1980; 7: 257–261. [DOI] [PubMed] [Google Scholar]
- 36.Hudgins WR, Garcia JH. Transorbital approach to the middle cerebral artery of the squirrel monkey: a technique for experimental cerebral infarction applicable to ultrastructural studies. Stroke 1970; 1: 107–111. [DOI] [PubMed] [Google Scholar]
- 37.Huang J, Mocco J, Choudhri TF, et al. A modified transorbital baboon model of reperfused stroke. Stroke 2000; 31: 3054–3063. [DOI] [PubMed] [Google Scholar]
- 38.Diaz FG, Mastri AR, Ausman JI, et al. Acute cerebral revascularization: part I. Cerebral ischemia experimental animal model. Surg Neurol 1979; 12: 353–362. [PubMed] [Google Scholar]
- 39.English JD, Hetts SW, El-Ali A, et al. A novel model of large vessel ischemic stroke in rabbits: microcatheter occlusion of the posterior cerebral artery. J Neurointerv Surg 2015; 7: 363–366. [DOI] [PubMed] [Google Scholar]
- 40.Rink C, Christoforidis G, Abduljalil A, et al. Minimally invasive neuroradiologic model of preclinical transient middle cerebral artery occlusion in canines. Proc Natl Acad Sci U S A 2008; 105: 14100–14105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng L, Liu J, Chen J, et al. Establishing a model of middle cerebral artery occlusion in rabbits using endovascular interventional techniques. Exp Ther Med 2013; 6: 947–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qureshi AI, Suri MFK, Ali Z, et al. Intraarterial reteplase and intravenous abciximab for treatment of acute ischemic stroke. A preliminary feasibility and safety study in a non-human primate model. Neuroradiology 2005; 47: 845–854. [DOI] [PubMed] [Google Scholar]
- 43.Molinari GF. Experimental cerebral infarction. I. Selective segmental occlusion of intracranial arteries in the dog. Stroke 1970; 1: 224–231. [DOI] [PubMed] [Google Scholar]
- 44.Yang J-P, Liu H-J, Liu R-C. A modified rabbit model of stroke: evaluation using clinical MRI scanner. Neurol Res 2009; 31: 1092–1096. [DOI] [PubMed] [Google Scholar]
- 45.Molinari GF, Moseley JI, Laurent JP. Segmental middle cerebral artery occlusion in primates: an experimental method requiring minimal surgery and anesthesia. Stroke 1974; 5: 334–339. [DOI] [PubMed] [Google Scholar]
- 46.Jahan R, Stewart D, Vinters HV, et al. Middle cerebral artery occlusion in the rabbit using selective angiography: application for assessment of thrombolysis. Stroke 2008; 39: 1613–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomsen BB, Gredal H, Wirenfeldt M, et al. Spontaneous ischaemic stroke lesions in a dog brain: neuropathological characterisation and comparison to human ischaemic stroke. Acta Vet Scand 2017; 59: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Georganos SA, Guilbert F, Salazkin I, et al. Surgical construction of an in vivo carotid siphon model to test neurovascular devices. Neurosurgery 2004; 54: 1239–1243; discussion 1243. [DOI] [PubMed] [Google Scholar]
- 49.Nakayama Y, Satow T, Funayama M, et al. Construction of 3 animal experimental models in the development of honeycomb microporous covered stents for the treatment of large wide-necked cerebral aneurysms. J Artif Organs 2016; 19: 179–187. [DOI] [PubMed] [Google Scholar]
- 50.Carniato S, Mehra M, King RM, et al. Porcine brachial artery tortuosity for in vivo evaluation of neuroendovascular devices. AJNR Am J Neuroradiol 2013; 34: E36–E38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sadato A, Taki W, Ikada Y, et al. Experimental study and clinical use of poly(vinyl acetate) emulsion as liquid embolisation material. Neuroradiology 1994; 36: 634–641. [DOI] [PubMed] [Google Scholar]
- 52.Nogueira RG, Levy EI, Gounis M, et al. The Trevo device: preclinical data of a novel stroke thrombectomy device in two different animal models of arterial thrombo-occlusive disease. J Neurointerv Surg 2012; 4: 295–300. [DOI] [PubMed] [Google Scholar]
- 53.Jahan R. Solitaire flow-restoration device for treatment of acute ischemic stroke: safety and recanalization efficacy study in a swine vessel occlusion model. AJNR Am J Neuroradiol 2010; 31: 1938–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stehbens WE. Pathology of the cerebral blood vessels, Saint Louis: Mosby, 1972. [Google Scholar]
- 55.Monson KL, Goldsmith W, Barbaro NM, et al. Axial mechanical properties of fresh human cerebral blood vessels. J Biomech Eng 2003; 125: 288–294. [DOI] [PubMed] [Google Scholar]
- 56.Chueh JY, Wakhloo AK, Gounis MJ. Effectiveness of mechanical endovascular thrombectomy in a model system of cerebrovascular occlusion. AJNR Am J Neuroradiol 2012; 33: 1998–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chueh J-Y, Kühn AL, Puri AS, et al. Reduction in distal emboli with proximal flow control during mechanical thrombectomy: a quantitative in vitro study. Stroke 2013; 44: 1396–1401. [DOI] [PubMed] [Google Scholar]
- 58.Gounis MJ, Wakhloo AK, Chueh J-Y. Preclinical investigations for thrombectomy devices – does it translate to humans? Stroke 2013; 44: S7–S10. [DOI] [PubMed] [Google Scholar]
- 59.Chueh J-Y, Puri AS, Wakhloo AK, et al. Risk of distal embolization with stent retriever thrombectomy and ADAPT. J Neurointerv Surg 2016; 8: 197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fennell VS, Setlur Nagesh SV, Meess KM, et al. What to do about fibrin rich ‘tough clots'? Comparing the Solitaire stent retriever with a novel geometric clot extractor in an in vitro stroke model. J Neurointerv Surg 2018; 10: 907–910. [DOI] [PubMed] [Google Scholar]
- 61.Nawka MT, Fiehler J, Spallek J, et al. Current status of training environments in neuro-interventional practice: are animal models still contemporary? J Neurointerv Surg Epub ahead of print 26 July 2018. DOI: 10.1136/neurintsurg-2018-014036. [DOI] [PubMed] [Google Scholar]
- 62.Fisher M, Saver JL. Future directions of acute ischaemic stroke therapy. Lancet Neurol 2015; 14: 758–767. [DOI] [PubMed] [Google Scholar]
- 63.Shi W-Y, Li M-H, Yan L, et al. Creation of carotid fusiform aneurysm in a canine model. Neurosurg Quarter 2012; 22: 255–260. [Google Scholar]
- 64.German WJ, Black SP. Experimental production of carotid aneurysms. N Engl J Med 1954; 250: 104–106. [DOI] [PubMed] [Google Scholar]
- 65.Forrest MD, O'Reilly GV. Production of experimental aneurysms at a surgically created arterial bifurcation. AJNR Am J Neuroradiol 1989; 10: 400–402. [PMC free article] [PubMed] [Google Scholar]
- 66.Stehbens WE. Histological changes in chronic experimental aneurysms surgically fashioned in sheep. Pathology 1997; 29: 374–379. [DOI] [PubMed] [Google Scholar]
- 67.Tenjin H, Ueda S, Fushiki S, et al. Experimental production of carotid artery aneurysms in Japanese monkey. J Kyoto Pref Univ Med 1994; 103: 715–720. [Google Scholar]
- 68.Geremia G, Bakon M, Brennecke L, et al. Experimental arteriovenous fistulas: treatment with porous metallic stents. AJNR Am J Neuroradiol 1995; 16: 1965–1973. [PMC free article] [PubMed] [Google Scholar]
- 69.Scott BB, McGillicuddy JE, Seeger JF, et al. Vascular dynamics of an experimental cerebral arteriovenous shunt in the primate. Surg Neurol 1978; 10: 34–38. [PubMed] [Google Scholar]
- 70.Spetzler RF, Wilson CB, Weinstein P, et al. Normal perfusion pressure breakthrough theory. Clin Neurosurg 1978; 25: 651–672. [DOI] [PubMed] [Google Scholar]
- 71.Tokiwa K, Miyasaka Y, Irikura K, et al. The effects of a carotid-jugular fistula on cerebral blood flow in the cat: an experimental study in the chronic period. Neurol Res 1995; 17: 297–300. [DOI] [PubMed] [Google Scholar]
- 72.Qian Z, Climent S, Maynar M, et al. A simplified arteriovenous malformation model in sheep: feasibility study. AJNR Am J Neuroradiol 1999; 20: 765–770. [PMC free article] [PubMed] [Google Scholar]
- 73.Hayakawa T, Waltz AG. Intracranial pressure, blood pressure, and pulse rate after occlusion of a middle cerebral artery in cats. J Neurosurg 1975; 43: 399–407. [DOI] [PubMed] [Google Scholar]
- 74.Liu XG, Branston NM, Kawauchi M, et al. A model of acute focal ischemia in the territory of the anterior cerebral artery in baboons. Stroke 1992; 23: 40–44. [DOI] [PubMed] [Google Scholar]
- 75.Mullan JC, Korosue K, Heros RC. The use of somatosensory evoked potential monitoring to produce a canine model of uniform, moderately severe stroke with permanent arterial occlusion. Neurosurgery 1993; 32: 967–973; discussion 973. [DOI] [PubMed] [Google Scholar]
- 76.Mattingly TK, Denning LM, Siroen KL, et al. Catheter based selective hypothermia reduces stroke volume during focal cerebral ischemia in swine. J Neurointerv Surg 2016; 8: 418–422. [DOI] [PubMed] [Google Scholar]
- 77.Schwartz AE, Pile-Spellman J. New model of reperfused stroke by occlusion of the anterior cerebral artery in baboons. Acta Neurochir 2011; 153: 327–331. [DOI] [PubMed] [Google Scholar]
- 78.Chung D-J, Choi C-B, Lee S-H, et al. Intraarterially delivered human umbilical cord blood-derived mesenchymal stem cells in canine cerebral ischemia. J Neurosci Res 2009; 87: 3554–3567. [DOI] [PubMed] [Google Scholar]
- 79.Kito G, Nishimura A, Susumu T, et al. Experimental thromboembolic stroke in cynomolgus monkey. J Neurosci Methods 2001; 105: 45–53. [DOI] [PubMed] [Google Scholar]
- 80.Benes V, Zabramski JM, Boston M, et al. Effect of intra-arterial tissue plasminogen activator and urokinase on autologous arterial emboli in the cerebral circulation of rabbits corrected. Stroke 1990; 21: 1594–1599. [DOI] [PubMed] [Google Scholar]
- 81.Qureshi AI, Boulos AS, Hanel RA, et al. Randomized comparison of intra-arterial and intravenous thrombolysis in a canine model of acute basilar artery thrombosis. Neuroradiology 2004; 46: 988–995. [DOI] [PubMed] [Google Scholar]
- 82.Lapchak PA, Araujo DM, Pakola S, et al. Microplasmin: a novel thrombolytic that improves behavioral outcome after embolic strokes in rabbits. Stroke 2002; 33: 2279–2284. [DOI] [PubMed] [Google Scholar]
- 83.Jiang Y, Li Y, Xu X, et al. An in vitro porcine model evaluating a novel stent retriever for thrombectomy of the common carotid artery. Catheter Cardiovasc Interv 2016; 87: 457–464. [DOI] [PubMed] [Google Scholar]
- 84.Xie J, Li M-H, Tan H-Q, et al. Establishment of an experimental intracranial internal carotid artery model and the application in covered-stent navigability testing. AJNR Am J Neuroradiol 2009; 30: 1041–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tan H-Q, Li M-H, Zhu Y-Q, et al. Surgical construction of a novel simulated carotid siphon in dogs. J Neurosurg 2008; 109: 1173–1178. [DOI] [PubMed] [Google Scholar]
- 86.Jung SC, Yoon B-R, Oh JS, et al. Development of endovascular vibrating polymer actuator probe for mechanical thrombolysis: in vivo study. ASAIO J 2012; 58: 503–508. [DOI] [PubMed] [Google Scholar]
- 87.Gory B, Bresson D, Kessler I, et al. Histopathologic evaluation of arterial wall response to 5 neurovascular mechanical thrombectomy devices in a swine model. AJNR Am J Neuroradiol 2013; 34: 2192–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grad Y, Sievert H, Nishri B, et al. A novel endovascular device for emboli rerouting: part I: evaluation in a Swine model. Stroke 2008; 39: 2860–2866. [DOI] [PubMed] [Google Scholar]
- 89.Levy EI, Sauvageau E, Hanel RA, et al. Self-expanding versus balloon-mounted stents for vessel recanalization following embolic occlusion in the canine model: technical feasibility study. AJNR Am J Neuroradiol 2006; 27: 2069–2072. [PMC free article] [PubMed] [Google Scholar]
- 90.Park S, Hwang SM, Song JS, et al. Evaluation of the Solitaire system in a canine arterial thromboembolic occlusion model: is it safe for the endothelium? Interv Neuroradiol 2013; 19: 417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Haider T, Plasenzotti R, Bergmeister H, et al. New mechanical thrombectomy model in the rabbit: a feasibility study. J Neurosci Methods 2016; 271: 139–142. [DOI] [PubMed] [Google Scholar]
- 92.Yuki I, Kan I, Golshan A, et al. A swine model to analyze arterial structural changes induced by mechanical thrombectomy. AJNR Am J Neuroradiol 2013; 34: E87–E90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhu L, Shao Q, Li T, et al. Evaluation of the JRecan device for thrombus retrieval: efficacy and safety in a swine model of acute arterial occlusion. J Neurointerv Surg 2016; 8: 526–530. [DOI] [PubMed] [Google Scholar]
- 94.Yuki I, Kan I, Vinters HV, et al. The impact of thromboemboli histology on the performance of a mechanical thrombectomy device. AJNR Am J Neuroradiol 2012; 33: 643–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ringer AJ, Guterman LR, Hopkins LN. Site-specific thromboembolism: a novel animal model for stroke. AJNR Am J Neuroradiol 2004; 25: 329–332. [PMC free article] [PubMed] [Google Scholar]
- 96.Roth C, Junk D, Papanagiotou P, et al. A comparison of 2 stroke devices: the new Aperio clot-removal device and the solitaire AB/FR. AJNR Am J Neuroradiol 2012; 33: 1317–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wainwright JM, Jahan R. Solitaire FR revascularization device 4×40: safety study and effectiveness in preclinical models. J Neurointerv Surg 2016; 8: 710–713. [DOI] [PubMed] [Google Scholar]
- 98.Sadato A, Wakhloo AK, Hopkins LN. Effects of a mixture of a low concentration of n-butylcyanoacrylate and ethiodol on tissue reactions and the permanence of arterial occlusion after embolization. Neurosurgery 2000; 47: 1197–1203; discussion 1204–1205. [DOI] [PubMed] [Google Scholar]
- 99.Takao H, Murayama Y, Ebara M, et al. New thermoreversible liquid embolic agent for embolotherapy: technical report. Neuroradiology 2009; 51: 95–98. [DOI] [PubMed] [Google Scholar]
- 100.Nishi S, Nakayama Y, Hashimoto N, et al. Basic fibroblast growth factor impregnated hydrogel microspheres for embolization of cerebral arteriovenous malformations. ASAIO J 1998; 44: M405–410. [DOI] [PubMed] [Google Scholar]
- 101.Nikoubashman O, Pjontek R, Brockmann M-A, et al. Retrieval of migrated coils with stent retrievers: an animal study. AJNR Am J Neuroradiol 2015; 36: 1162–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gounis MJ, Lieber BB, Wakhloo AK, et al. Effect of glacial acetic acid and ethiodized oil concentration on embolization with N-butyl 2-cyanoacrylate: an in vivo investigation. AJNR Am J Neuroradiol 2002; 23: 938–944. [PMC free article] [PubMed] [Google Scholar]
- 103.Wellman BJ, Loftus CM, Noh D, et al. A combined surgical-endovascular device concept for giant aneurysm neck occlusion. Neurosurgery 1998; 42: 1364–1368; discussion 1368–1369. [DOI] [PubMed] [Google Scholar]
- 104.Hagen MW, Girdhar G, Wainwright J, et al. Thrombogenicity of flow diverters in an ex vivo shunt model: effect of phosphorylcholine surface modification. J Neurointerv Surg 2017; 9: 1006–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nonn A, Kirschner S, Figueiredo G, et al. Feasibility, safety, and efficacy of flow-diverting stent-assisted microsphere embolization of fusiform and sidewall aneurysms. Neurosurgery 2015; 77: 126–135; discussion 135–136. [DOI] [PubMed] [Google Scholar]
- 106.Dai D, Ding YH, Kadirvel R, et al. Patency of branches after coverage with multiple telescoping flow-diverter devices: an in vivo study in rabbits. AJNR Am J Neuroradiol 2012; 33: 171–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kallmes DF, Ding YH, Dai D, et al. A new endoluminal, flow-disrupting device for treatment of saccular aneurysms. Stroke 2007; 38: 2346–2352. [DOI] [PubMed] [Google Scholar]
- 108.Masuo O, Terada T, Walker G, et al. Study of the patency of small arterial branches after stent placement with an experimental in vivo model. AJNR Am J Neuroradiol 2002; 23: 706–710. [PMC free article] [PubMed] [Google Scholar]
- 109.Mottu F, Rüfenacht DA, Laurent A, et al. Iodine-containing cellulose mixed esters as radiopaque polymers for direct embolization of cerebral aneurysms and arteriovenous malformations. Biomaterials 2002; 23: 121–131. [DOI] [PubMed] [Google Scholar]
- 110.Momeni A, Valliant EM, Brennan-Pierce EP, et al. Developing an in situ forming polyphosphate coacervate as a new liquid embolic agent: from experimental design to pilot animal study. Acta Biomater 2016; 32: 286–297. [DOI] [PubMed] [Google Scholar]
- 111.Falcao ALE, Reutens DC, Markus R, et al. The resistance to ischemia of white and gray matter after stroke. Ann Neurol 2004; 56: 695–701. [DOI] [PubMed] [Google Scholar]
- 112.Vink R. Large animal models of traumatic brain injury. J Neurosci Res 2018; 96: 527–2. [DOI] [PubMed] [Google Scholar]
- 113.Roth G, Dicke U. Evolution of the brain and intelligence. Trends Cogn Sci 2005; 9: 250–257. [DOI] [PubMed] [Google Scholar]
- 114.Cairó O. External measures of cognition. Front Hum Neurosci 2011; 5: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Atchaneeyasakul K, Guada L, Ramdas K, et al. Large animal canine endovascular ischemic stroke models: a review. Brain Res Bull 2016; 127: 134–140. [DOI] [PubMed] [Google Scholar]
- 116.Zhang K, Sejnowski TJ. A universal scaling law between gray matter and white matter of cerebral cortex. Proc Natl Acad Sci U S A 2000; 97: 5621–5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nitzsche B, Boltze J, Ludewig E, et al. A stereotaxic breed-averaged, symmetric T2w canine brain atlas including detailed morphological and volumetrical data sets. Neuroimage Epub ahead of print 31 January 2018. DOI: 10.1016/j.neuroimage.2018.01.066. [DOI] [PubMed] [Google Scholar]
- 118.Frackowiak H, Godynicki S. Brain basal arteries in various species of Felidae. Pol J Vet Sci 2003; 6: 195–200. [PubMed] [Google Scholar]
- 119.Yamori Y, Horie R, Handa H, et al. Pathogenetic similarity of strokes in stroke-prone spontaneously hypertensive rats and humans. Stroke 1976; 7: 46–53. [DOI] [PubMed] [Google Scholar]
- 120.Wey H-Y, Kroma GM, Li J, et al. MRI of perfusion-diffusion mismatch in non-human primate (baboon) stroke: a preliminary report. Open Neuroimag J 2011; 5: 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gounis MJ, Nogueira RG, Mehra M, et al. A thromboembolic model for the efficacy and safety evaluation of combined mechanical and pharmacologic revascularization strategies. J Neurointerv Surg 2013; 5(Suppl 1): i85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sommer CJ. Ischemic stroke: experimental models and reality. Acta Neuropathol 2017; 133: 245–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rai AT, Hogg JP, Cline B, et al. Cerebrovascular geometry in the anterior circulation: an analysis of diameter, length and the vessel taper. J Neurointerv Surg 2013; 5: 371–375. [DOI] [PubMed] [Google Scholar]
- 124.Fukuyama N, Tsukamoto Y, Takizawa S, et al. Altered blood flow in cerebral perforating arteries of rat models of diabetes: a synchrotron radiation microangiographic study toward clinical evaluation of white matter hyperintensities. Geriatr Gerontol Int 2015; 15(Suppl 1): 74–80. [DOI] [PubMed] [Google Scholar]
- 125.Kapoor K, Kak VK, Singh B. Morphology and comparative anatomy of circulus arteriosus cerebri in mammals. Anat Histol Embryol 2003; 32: 347–355. [DOI] [PubMed] [Google Scholar]
- 126.Förschler A, Boltze J, Waldmin D, et al. MR-Bildgebung eines experimentellen Schlaganfallmodells beim Schaf. Rofo 2007; 179: 516–524. [DOI] [PubMed] [Google Scholar]
- 127.Dorr A, Sled JG, Kabani N. Three-dimensional cerebral vasculature of the CBA mouse brain: a magnetic resonance imaging and micro computed tomography study. Neuroimage 2007; 35: 1409–1423. [DOI] [PubMed] [Google Scholar]
- 128.Lee RM. Morphology of cerebral arteries. Pharmacol Ther 1995; 66: 149–173. [DOI] [PubMed] [Google Scholar]
- 129.Zaninovich OA, Ramey WL, Walter CM, et al. Completion of the circle of Willis varies by gender, age, and indication for computed tomography angiography. World Neurosurg 2017; 106: 953–963. [DOI] [PubMed] [Google Scholar]
- 130.van der Bom IMJ, Mehra M, Walvick RP, et al. Quantitative evaluation of C-arm CT cerebral blood volume in a canine model of ischemic stroke. AJNR Am J Neuroradiol 2012; 33: 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Badea CT, Drangova M, Holdsworth DW, et al. In vivo small-animal imaging using micro-CT and digital subtraction angiography. Phys Med Biol 2008; 53: R319–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lin M de, Ning L, Badea CT, et al. A high-precision contrast injector for small animal x-ray digital subtraction angiography. IEEE Trans Biomed Eng 2008; 55: 1082–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Eich T, Eriksson O, Sundin A, et al. Positron emission tomography: a real-time tool to quantify early islet engraftment in a preclinical large animal model. Transplantation 2007; 84: 893–898. [DOI] [PubMed] [Google Scholar]
- 134.Sinharay S, Lee D, Shah S, et al. Cross-sectional and longitudinal small animal PET shows pre and post-synaptic striatal dopaminergic deficits in an animal model of HIV. Nucl Med Biol 2017; 55: 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li H, Yan J-Z, Chen Y-J, et al. Non-invasive quantification of age-related changes in the vertebral endplate in rats using in vivo DCE-MRI. J Orthop Surg Res 2017; 12: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cha S-H, Han MH, Choi YH, et al. Vascular responses in normal canine carotid arteries: comparison between various self-expanding stents of the same unconstrained size. Invest Radiol 2003; 38: 95–101. [DOI] [PubMed] [Google Scholar]
- 137.Mack WJ, Huang J, Winfree C, et al. Ultrarapid, convection-enhanced intravascular hypothermia: a feasibility study in nonhuman primate stroke. Stroke 2003; 34: 1994–1999. [DOI] [PubMed] [Google Scholar]
- 138.Cai B, Wang N. Large animal stroke models vs. rodent stroke models, pros and cons, and combination? Acta Neurochir Suppl 2016; 121: 77–81. [DOI] [PubMed] [Google Scholar]
- 139.Lee KC, Joo JY, Huh JS, et al. Effects of repeated short versus single long episodes of focal ischemia on somatosensory evoked potentials and development of cerebral infarction in cats. Neurol Med Chir 1997; 37: 447–451. discussion 451–452. [DOI] [PubMed] [Google Scholar]
- 140.Seita Y, Tsukiyama T, Iwatani C, et al. Generation of transgenic cynomolgus monkeys that express green fluorescent protein throughout the whole body. Sci Rep 2016; 6: 24868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Park K-E, Park C-H, Powell A, et al. Targeted gene knockin in porcine somatic cells using CRISPR/Cas ribonucleoproteins. Int J Mol Sci 2016; 17: 810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schuldenzucker V, Schubert R, Muratori LM, et al. Behavioral testing of minipigs transgenic for the Huntington gene – a three-year observational study. PLoS One 2017; 12: e0185970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Murayama Y, Viñuela F, Tateshima S, et al. Endovascular treatment of experimental aneurysms by use of a combination of liquid embolic agents and protective devices. AJNR Am J Neuroradiol 2000; 21: 1726–1735. [PMC free article] [PubMed] [Google Scholar]
- 144.Marx WE, Cloft HJ, Helm GA, et al. Endovascular treatment of experimental aneurysms by use of biologically modified embolic devices: coil-mediated intraaneurysmal delivery of fibroblast tissue allografts. AJNR Am J Neuroradiol 2001; 22: 323–333. [PMC free article] [PubMed] [Google Scholar]
- 145.Adibi A, Eesa M, Wong JH, et al. Combined endovascular coiling and intra-aneurysmal allogeneic mesenchymal stromal cell therapy for intracranial aneurysms in a rabbit model: a proof-of-concept study. J Neurointerv Surg 2017; 9: 707–712. [DOI] [PubMed] [Google Scholar]
- 146.Killer M, Kallmes DF, McCoy MR, et al. Angiographic and histologic comparison of experimental aneurysms embolized with hydrogel filaments. AJNR Am J Neuroradiol 2009; 30: 1488–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Metcalfe A, Desfaits A-C, Salazkin I, et al. Cold hibernated elastic memory foams for endovascular interventions. Biomaterials 2003; 24: 491–497. [DOI] [PubMed] [Google Scholar]
- 148.Oechtering J, Kirkpatrick PJ, Ludolph AGK, et al. Magnetic microparticles for endovascular aneurysm treatment: in vitro and in vivo experimental results. Neurosurgery 2011; 68: 1388–1397; discussion 1397–1398. [DOI] [PubMed] [Google Scholar]
- 149.Meyer T, Nikoubashman O, Kabelitz L, et al. Endovascular stentectomy using the snare over stent-retriever (SOS) technique: an experimental feasibility study. PLoS One 2017; 12: e0178197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ding Y, Dai D, Kallmes DF, et al. Preclinical testing of a novel thin film nitinol flow-diversion stent in a rabbit elastase aneurysm model. AJNR Am J Neuroradiol 2016; 37: 497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bebarta V, Luyten D, Heard K. Emergency medicine animal research: does use of randomization and blinding affect the results? Acad Emerg Med 2003; 10: 684–687. [DOI] [PubMed] [Google Scholar]
- 152.Lu S-S, Liu S, Zu Q-Q, et al. In vivo MR imaging of intraarterially delivered magnetically labeled mesenchymal stem cells in a canine stroke model. PLoS One 2013; 8: e54963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Feng L, Liu J, Liu Y, et al. Tirofiban combined with urokinase selective intra-arterial thrombolysis for the treatment of middle cerebral artery occlusion. Exp Ther Med 2016; 11: 1011–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Susumu T, Yoshikawa T, Akiyoshi Y, et al. Effects of intra-arterial urokinase on a non-human primate thromboembolic stroke model. J Pharmacol Sci 2006; 100: 278–284. [DOI] [PubMed] [Google Scholar]
- 155.van der Worp HB, Macleod MR, Kollmar R. Therapeutic hypothermia for acute ischemic stroke: ready to start large randomized trials? J Cereb Blood Flow Metab 2010; 30: 1079–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Large animals in neurointerventional research: A systematic review on models, techniques and their application in endovascular procedures for stroke, aneurysms and vascular malformations by Andrea M Herrmann, Stephan Meckel, Matthew J Gounis, Leona Kringe, Edith Motschall, Christoph Mülling and Johannes Boltze in Journal of Cerebral Blood Flow & Metabolism
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on request.