Abstract
It is estimated that as many as 10 million unnecessary antibiotic prescriptions are written each year for children. Children are more likely to receive antibiotics for an upper respiratory infection in an urgent care center compared with the primary care office. However, no study has examined the antibiotic prescribing practices of the same physicians in these settings. This retrospective chart review evaluated pediatricians’ antibiotic prescribing practices for patients with symptoms of an upper respiratory tract infection in the office setting and an urgent care setting. There was no difference in the total antibiotic prescribing rate by pediatricians in their primary care office versus an urgent care setting. Pediatricians who were high antibiotic prescribers in the office setting were also high prescribers in the urgent care. The highest prescribing physicians prescribed the appropriate recommended antibiotics for a particular diagnosis the lowest percentage of the time. Efforts to promote antimicrobial stewardship should be directed toward the individual physician and not toward the location where the patients are being evaluated.
Keywords: antibiotics, stewardship, general pediatrics
Background
Antibiotics are prescribed in 21% of all pediatric ambulatory visits in the United States, which results in almost 44 million antibiotic prescriptions annually.1 Respiratory conditions account for the majority of the visits in which antibiotics are prescribed.1 Unfortunately, antibiotics are not indicated in 23.4% of the visits in which they are prescribed for respiratory conditions, and these inappropriate prescriptions translate to over 10 million unnecessary antibiotic prescriptions in the pediatric population each year.1
It is well documented that antibiotic overuse and misuse leads to antimicrobial resistance, unnecessary adverse drug reactions, and increased costs.2-6 More recently, the literature has raised concerns regarding the association of antibiotics with the development of asthma, obesity, inflammatory bowel disease, juvenile idiopathic arthritis, and atopic dermatitis.7-13
A number of factors have been associated with an increased likelihood of children receiving an antibiotic prescription when they present with an upper respiratory tract infection (URI). The health care setting is one of these factors. Children are more likely to receive an antibiotic for a URI when they are seen in an urgent care setting (UC) or emergency department, as compared with the primary care physician’s office (PCPO).14,15 However, we could not find any studies examining the antibiotic prescribing practices of the same pediatricians who provide care in both the PCPO and in a pediatric UC.
This study compares the antibiotic prescribing practices of the same pediatricians in these 2 settings. The authors hypothesized that the antibiotic prescribing rates would be higher in the UC as compared with the PCPO. The study was also designed to determine which respiratory conditions most often resulted in an antibiotic prescription and whether the antibiotic choice was appropriate, as determined by published guidelines.
Methods
Setting
This study was conducted in the Department of Pediatrics at an academic institution located in Appalachia.
Study Design
This study is a retrospective chart review of 12 board-certified general pediatricians employed by an academic medical center who work in both their PCPO and an UC. These pediatricians finished their residency training 6 to 27 years ago. All sick visits for each physician in both the PCPO and UC starting October 1, 2013, were manually evaluated, and charts that included the words fever, nasal congestion/nasal discharge, earache, sore throat, and/or cough in the history of present illness met inclusion criteria. Charts were reviewed going forward from October 1, 2013. The first 100 visits in both the PCPO and an UC setting that met inclusion criteria were analyzed for each physician. The charts were electronic, and the authors reviewed the charts manually. The authors were not blinded to the review, and they did not review their own charts. Subjects were 0 to18 years of age and were patients of the academic practice or walk-ins to the UC. Patients prescribed an antibiotic for coexisting conditions not related to a URI were excluded. There were no other exclusions. Each visit was evaluated to determine if an antibiotic was prescribed. In addition, the appropriateness of the prescribed antibiotic was determined using current published guidelines for acute otitis media, sinusitis, or streptococcal pharyngitis.16-18 The diagnoses assigned by the prescribing physician were also recorded and included viral illness, croup, fever, upper respiratory illness, viral pharyngitis, cough, otitis media, sinusitis, streptococcal pharyngitis, bronchitis, and bronchiolitis.
All 12 pediatricians in the practice who work at both their PCPO and the UC were included in the analysis. One hundred charts were reviewed for each pediatrician in each setting. Therefore, a total of 1200 charts were reviewed in both the PCPO and UC setting for a total of 2400 charts reviewed overall.
Statistics and Data Analysis
Continuous variables were compared using the Student’s t test. Categorical variables were compared using χ2 or Fisher’s exact tests when appropriate. Chi square or Fisher’s exact tests were used for comparisons in Figures 1 and 2, and error bars were derived using the exact binomial distribution. We compared antibiotic prescribing frequency by the providers’ years since their training was completed using simple linear regression. All statistical analyses were performed using Stata 14 (College Station, TX). All P values were derived with a 2-tailed α of .05.
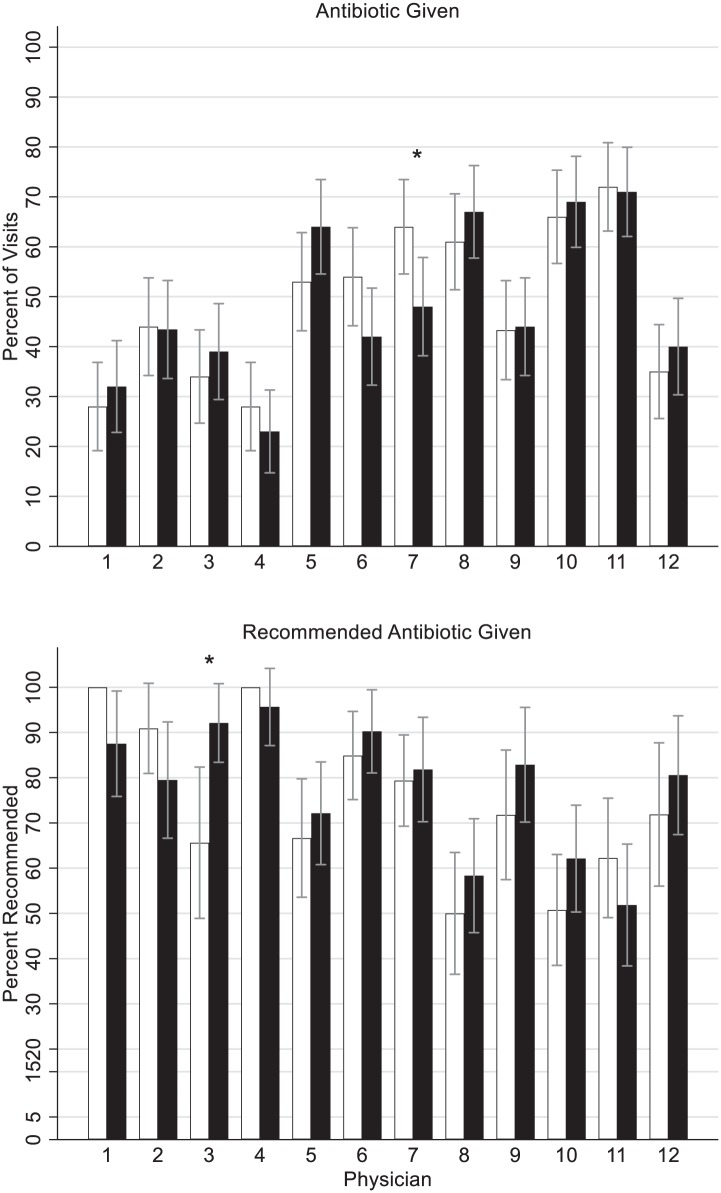
Figure 1.
Antibiotic given and correct (appropriate) antibiotic for approved indication by setting (white bars = primary care physician’s office, black bars = urgent care setting) and physician. P < .001 for overall between physician comparison for antibiotic given and correct antibiotic given. *P < .05 for difference between settings within physician.
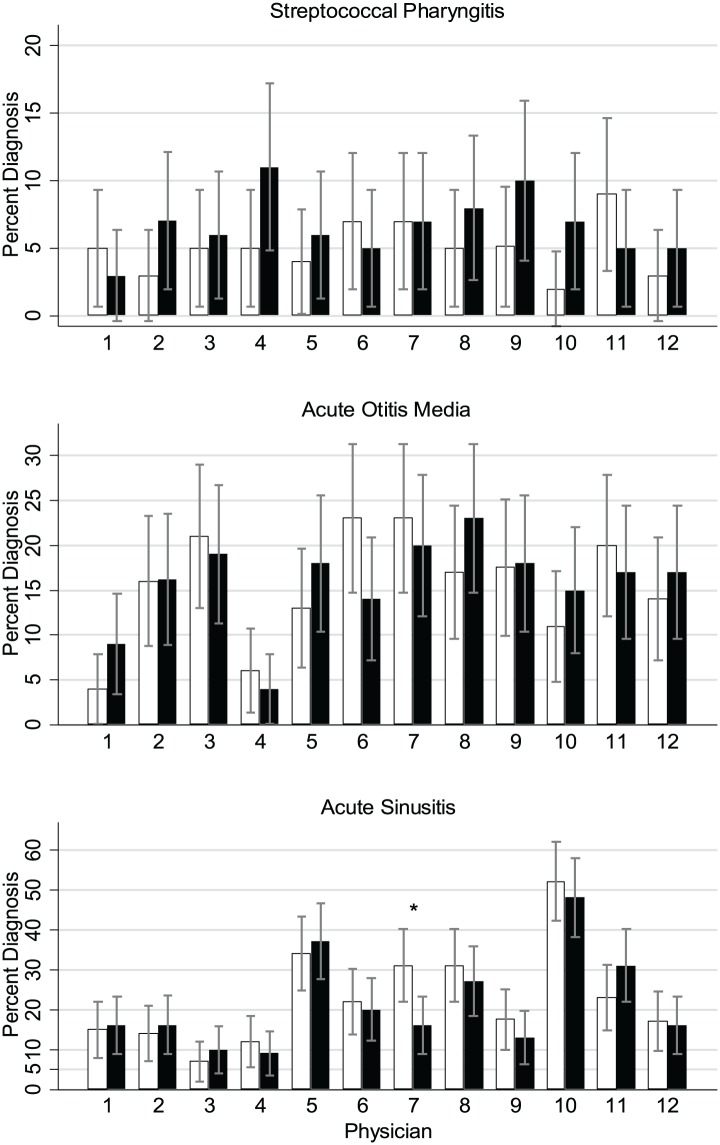
Figure 2.
Diagnosis by setting (white bars = primary care physician’s office, black bars = urgent care setting) and physician. Overall between physician comparison for diagnosis: streptococcal pharyngitis, P = .75; acute otitis media, P < .001; acute sinusitis, P < .001. *P < .05 for difference between settings within physician.
Ethical Approval and Informed Consent
The institution’s institutional review board approved the study (IRBNet ID# 583612-1).
Results
Median age, distribution of symptoms, and diagnoses are reported in Table 1.
Table 1.
Demographic Characteristics of Study Population.
| Characteristics | UC (n = 1200) | PCPO (n = 1200) | P |
|---|---|---|---|
| Age, mean (SD) | 5.3 (4.5) | 5.8 (4.8) | .007 |
| Nasal symptoms, % (n) | 79.6 (954) | 76.4 (915) | .06 |
| Fever, % (n) | 45.3 (543) | 34.2 (409) | <.001 |
| Sore throat, % (n) | 32.8 (393) | 32.1 (384) | .72 |
| Cough, % (n) | 77.7 (932) | 74.9 (897) | .11 |
| Headache, % (n) | 16.6 (199) | 10.1 (121) | <.001 |
| Earache, % (n) | 18.8 (225) | 16.2 (194) | .10 |
| Otitis media, % (n) | 15.8 (190) | 15.5 (185) | .78 |
| Sinusitis, % (n) | 21.6 (259) | 23.0 (275) | .42 |
| Streptococcal pharyngitis, % (n) | 6.7 (80) | 5.0 (60) | .08 |
Abbreviations: UC, urgent care; PCPO, primary care physician’s office.
The percentage of antibiotics given for all patient encounters meeting the inclusion criteria was not significantly different in the 2 settings (Table 2): 48.5% (581/1200) of the included patients in PCPO and 48.5% (582/1200) in the UC received antibiotics (P = .999). In addition, only 1 pediatrician prescribed a significantly different number of antibiotics in the 2 settings, and this physician prescribed significantly more antibiotics in the PCPO than the UC. All other physicians had no significant difference in their antibiotic prescribing frequencies between the 2 settings (Figure 1).
Table 2.
Antibiotics Given and Appropriateness by Setting.
| UC Antibiotics Given | PCPO Antibiotics Given | P | |
|---|---|---|---|
| Total antibiotic given, % (n) | 48.5 (582) | 48.5 (581) | 1.0 |
| Appropriate antibiotic given, % (n) | 68.9 (401) | 66.1 (384) | .31 |
Abbreviations: UC, urgent care; PCPO, primary care physician’s office.
Although the intraprescriber antibiotic rates were similar between settings for most of the pediatricians, there was variation in the interprescriber antibiotic rates between the individual pediatricians. In the PCPO, the antibiotic prescribing percentage ranged from 28% to 72%, and in the UC, the range was from 23% to 71%. In other words, patients seeing the highest prescribing pediatrician were 2.4 (95% confidence interval = 1.6-3.8) times more likely in the PCPO and 2.6 (95% confidence interval = 1.7-4.0) times more likely in the UC to receive an antibiotic than if they were seen by the lowest prescribing pediatrician.
The appropriateness of the antibiotic prescribed based on published guidelines in each setting is reported in Table 2. The appropriateness of the antibiotic prescribed by each physician is shown in Figure 1. The appropriateness was similar between settings for each individual physician but showed great interphysician variability. The recommended antibiotic prescribing ranged from 50% to 100% in the PCPO and from 52% to 96% in the UC. The lower antibiotic prescribing physicians were more likely to prescribe the recommended antibiotic. For example, doctors 1 and 4 were the lowest antibiotic prescribing physicians. They were also the most likely to prescribe the recommended antibiotics. Doctors 1 and 4 prescribed the recommended antibiotic in the PCPO 100% of the time and 88% and 96% of the time, respectively, in the UC. Conversely, the highest prescribing physicians, doctors 8, 10, and 11, prescribed the recommended antibiotics the lowest percentage of the time. These 3 physicians prescribed the recommended antibiotics 50%, 51%, and 62% of the time, respectively, in the PCPO and 58%, 62%, and 52% of the time, respectively, in the UC.
The data were analyzed to compare diagnoses per physician as well (Figure 2). A total of 92.3% of antibiotics prescribed in the study were linked to diagnoses (otitis media, streptococcal pharyngitis, and sinusitis) for which antibiotic treatment is frequently recommended. However, the physicians varied considerably in their frequency of both otitis media and sinusitis diagnoses. In the 200 total charts reviewed per physician, the number of patients diagnosed with otitis media varied from a low of 10 (doctor 4) to a high of 42 (doctor 10). The number of diagnoses of sinusitis varied from 17 (doctor 3) to 99 (doctor 11). However, the number of diagnoses of streptococcal pharyngitis was similar between physicians ranging from 7 (doctor 12) to 15 (doctor 7).
Sixty-three percent of the time when antibiotics were prescribed for illnesses usually not requiring antibiotics the patient’s diagnosis was bronchitis.
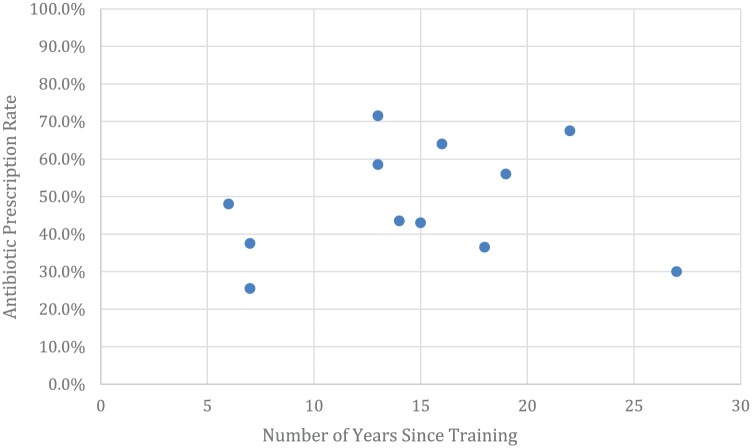
There was no correlation (coefficient 0.003; P = .69) between the number of years removed from residency training and the pediatricians’ antibiotic prescribing rates (Figure 3). For example, the pediatrician with the most years since completing training prescribed the second fewest antibiotics.
Figure 3.
Antibiotic prescription rate by years of physician experience.
Discussion
There was no difference in the total antibiotic prescribing rate by pediatricians in their primary care office versus a UC. The authors of this study had predicted to see more antibiotic prescriptions in the UC. Previously published studies have shown that antibiotic prescribing rates in UC are typically higher.14 UC physicians may often feel rushed due to a higher patient load and are likely not as familiar with the patients. This could lead to an increased antibiotic prescribing rate. Also, pediatricians may have had the opportunity for more patient and family education about appropriate use of antibiotics in their own primary care offices. However, these factors did not cause an increase in the number of antibiotics prescribed in the UC in our study. One factor to consider is that the UC in this study is associated with an academic center, which may not be representative of all UC settings. However, the large patient load and lack of familiarity with patients in the UC in this study would be similar to other settings.
One of the pediatricians prescribed more antibiotics for URIs in their PCPO than in the UC. The reasons for this are less compelling. Some physicians may feel more pressure to please their own patients in their PCPO than the patients they see transiently in the UC. This may lead to a higher prescribing rate in the physicians’ own offices. Also, pediatricians who practice with a group of high antibiotic prescribing physicians may feel more pressure to prescribe antibiotics as they work next to their colleagues in their PCPO.
There was high variability of antibiotic prescribing rates between the physicians. However, individual physicians’ antibiotic prescribing rates were consistent between the 2 locations. Only 1 physician had a significant difference in antibiotic prescribing between the 2 locations. Pediatricians who were high antibiotic prescribers in the PCPO were also high prescribers in the UC. The same was true for low antibiotic prescribers.
Higher prescribing physicians were also less likely in both settings to use the recommended antibiotic for the diagnoses. This suggests that the higher prescribing practitioners would likely benefit from education regarding guidelines for the treatment of otitis media, sinusitis, and streptococcal pharyngitis.
The most common diagnoses resulting in an antibiotic prescription were otitis media, sinusitis, and streptococcal pharyngitis. There was a great deal of interprescriber variability in the number of patients diagnosed with otitis media and sinusitis (Figure 2). However, there was much less variation in the frequency of diagnosing streptococcal pharyngitis. One explanation for this variation could be that these diagnoses were accurate and the doctors diagnosing more otitis media and sinusitis actually saw more patients with these conditions. However, the lack of significant variation in the diagnosis of streptococcal pharyngitis suggests that our sample size was large enough that there should not be this much variation in diagnoses. While there is some subjectivity regarding which patients need testing, the diagnosis of streptococcal pharyngitis can be made by an objective test (Group A streptococcal rapid antigen test and culture). However, otitis media and sinusitis are diagnosed by history and physical examination, with no assistance of an objective test. This variation in diagnoses contributed to the wide range of antibiotic prescribing rates between physicians. These data suggest that the physicians who diagnosed otitis media and sinusitis more frequently used less stringent diagnostic criteria to make these diagnoses.
A unique strength of this study is that all charts were reviewed for symptoms in the history of present illness, instead of simply reviewing charts based on diagnostic codes. Reviewing all charts of patients presenting with fever, nasal congestion/nasal discharge, earache, sore throat, and/or cough prevented any coding shifts to justify antibiotic use. It helped answer the question the authors were asking—how often is the patient presenting with any combination of the above-mentioned symptoms indicative of an URI leaving either the PCPO or UC with a prescription for an antibiotic? One limitation is that it is a single-center study, which may limit its applicability in other settings. Another is that the charts reviewed were a convenience sample and thus may not represent the practice population exactly.
The results of this study suggest that efforts to promote antimicrobial stewardship should be directed toward individual physicians regardless of the location where they are caring for patients. Based on our study, these efforts could include the use of more stringent diagnostic criteria for the diagnoses of otitis media and sinusitis, education regarding recommended antibiotics for the treatment of otitis media, sinusitis, and streptococcal pharyngitis as well as education regarding the lack of indication for the use of antibiotics in the treatment of bronchitis.
Footnotes
Author Contributions: RH and JE: contributed to conception and design, drafted the manuscript, gave final approval and agree to be accountable for all aspects of the work.
BM, SL, JS and JG: contributed to the design, critically revised the manuscript, gave final approval and agree to be accountable for all aspects of the work.
TWG: contributed to the analysis, critically revised the manuscript, gave final approval and agrees to be accountable for all aspects of the work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Rebecca Hayes  https://orcid.org/0000-0002-1019-334X
https://orcid.org/0000-0002-1019-334X
References
- 1. Hersh AL, Shapiro DJ, Pavia AT, Shah SS. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics. 2011;128:1053-1061. [DOI] [PubMed] [Google Scholar]
- 2. Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P T. 2015;40:277-283. [PMC free article] [PubMed] [Google Scholar]
- 3. Jick H. Lifting the fog of health care delivery and costs. Pharmacotherapy. 2014;34:1227-1229. [DOI] [PubMed] [Google Scholar]
- 4. Suda KJ, Hicks LA, Roberts RM, Hunkler RJ, Danziger L. A national evaluation of antibiotic expenditures by healthcare setting in the United States, 2009. J Antimicrob Chemother. 2013;68:715-718. [DOI] [PubMed] [Google Scholar]
- 5. Bourgeois FT, Mandl KD, Valim C, Shannon MW. Pediatric adverse drug events in the outpatient setting: an 11-year national analysis. Pediatrics. 2009;124:e744-e750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention. Biggest threats and data: antibiotic resistance threats in the United States, 2013. http://www.cdc.gov/drugresistance/threat-report-2013. Accessed October 17, 2017.
- 7. Ong MS, Umetsu DT, Mandl KD. Consequences of antibiotics and infections in infancy: bugs, drugs, and wheezing. Ann Allergy Asthma Immunol. 2014;112:441-445.e1. [DOI] [PubMed] [Google Scholar]
- 8. Marra F, Marra CA, Richardson K, et al. Antibiotic use in children is associated with increased risk of asthma. Pediatrics. 2009;123:1003-1010. [DOI] [PubMed] [Google Scholar]
- 9. Kronman MP, Zaoutis TE, Haynes K, Feng R, Coffin SE. Antibiotic exposure and IBD development among children: a population-based cohort study. Pediatrics. 2012;130:e794-e803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tsakok T, McKeever TM, Yeo L, Flohr C. Does early life exposure to antibiotics increase the risk of eczema? A systemic review. Br J Dermatol. 2013;169:983-991. [DOI] [PubMed] [Google Scholar]
- 11. Bailey LC, Forrest CB, Zhang P, Richards TM, Livshits A, DeRusso PA. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr. 2014;168:1063-1069. [DOI] [PubMed] [Google Scholar]
- 12. Saari A, Virta LJ, Sankilampi U, Dunkel L, Saxen H. Antibiotic exposure in infancy and risk of being overweight in the first 24 months of life. Pediatrics. 2015;135:617-626. [DOI] [PubMed] [Google Scholar]
- 13. Horton DB, Scott FI, Haynes K, et al. Antibiotic exposure and juvenile idiopathic arthritis: a case-control study. Pediatrics. 2015;136:e333-e342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pennie RA. Prospective study of antibiotic prescribing for children. Can Fam Physician. 1998;44:1850-1856. [PMC free article] [PubMed] [Google Scholar]
- 15. Ahmed MN, Muyot MM, Begum S, Smith P, Little C, Windemuller FJ. Antibiotic prescription pattern for viral respiratory illness in emergency room and ambulatory care settings. Clin Pediatr (Phila). 2010;49:542-547. [DOI] [PubMed] [Google Scholar]
- 16. Lieberthal AS, Carroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics. 2013;131:e964-e999. [DOI] [PubMed] [Google Scholar]
- 17. Wald ER, Applegate KE, Bordley C, et al. Clinical practice guideline for the diagnosis and management of acute bacterial sinusitis in children aged 1 to 18 years. Pediatrics. 2013;132:e262-e280. [DOI] [PubMed] [Google Scholar]
- 18. Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55:e86-e102. [DOI] [PMC free article] [PubMed] [Google Scholar]