Abstract
Objectives: Among opioid-exposed newborns, breastfeeding is associated with less severe withdrawal signs, yet breastfeeding rates remain low. We determined the extent to which hospital, maternal, and infant characteristics are associated with breastfeeding initiation and continuation among opioid-exposed dyads.
Materials and Methods: We examined breastfeeding initiation and continuation until infants' discharge among opioid-exposed dyads from 2006 to 2016. Among dyads meeting hospital breastfeeding guidelines, we assessed hospital (changes in breastfeeding guidelines and improvement initiatives [using delivery year as a proxy]), maternal (demographics, comorbid conditions, methadone versus buprenorphine treatment, and delivery mode), and infant (gestational age and birth weight) characteristics. We used multivariable logistic regression to examine independent associations of characteristics with breastfeeding initiation and continuation.
Results: Among 924 opioid-exposed dyads, 61% (564) met breastfeeding criteria. Overall, 50% (283/564) of dyads initiated and 33% (187/564) continued breastfeeding until discharge. Breastfeeding initiation and continuation rates increased from 38% and 8% in 2006, to 56% and 34% in 2016, respectively. In adjusted models, infants born after reducing restrictions in hospital breastfeeding guidelines and prenatal breastfeeding education (adjusted odds ratio, aOR 2.6 [95% confidence interval, CI 1.5–4.5]) had increased odds of receiving any maternal breast milk versus infants born with earlier hospital policies. Cesarean versus vaginal delivery (aOR 0.3 [95% CI 0.2–0.6]) and length of infant hospitalization (aOR 0.94 [95% CI 0.92–0.97]) were negatively associated with breastfeeding continuation.
Conclusions: Despite increasing breastfeeding rates among opioid-exposed dyads, rates remain suboptimal. Hospital-level factors were the greatest predictor of breastfeeding initiation. The findings suggest that changes in hospital guidelines and initiatives can impact breastfeeding initiation among this vulnerable population.
Keywords: : breastfeeding, opioid-exposed, perinatal substance use, neonatal abstinence syndrome, initiation and continuation
Introduction
Neonatal abstinence syndrome (NAS) is a postnatal drug withdrawal syndrome that can occur following in-utero opioid exposure. Amid rising rates of prescription opioids and heroin use, opioid use during pregnancy has also increased, and cases of NAS have risen fivefold in the past 15 years.1,2 Breastfeeding has been recognized as a highly effective nonpharmacologic treatment for NAS, which is associated with reduced need for pharmacologic treatment and shortened length of hospitalization.3,4 Professional organizations have strongly recommended breastfeeding among pregnant women in recovery receiving opioid agonist treatment, with adequate prenatal care, and without illicit drug use or human immunodeficiency virus (HIV).5–7 Despite these recommendations, breastfeeding rates remain low among mothers with opioid use disorder (OUD), and reasons for this are poorly understood.3,8
Evidence-based hospital practices that support breastfeeding include prenatal education, early initiation of breastfeeding after delivery, rooming-in, and removal of pacifiers or other artificial nipples. Adoption of these practices through quality improvement (QI) initiatives has led to improved breastfeeding among a range of hospitals serving a diverse range of patient populations.9 However, hospital-level initiatives supporting breastfeeding among opioid-exposed mothers have not been well described.
Maternal characteristics in the non-opioid exposed population associated with higher breastfeeding rates include non-Hispanic white race and Hispanic ethnicity, higher socioeconomic status, private insurance, adequate prenatal care, and absence of comorbid pregnancy conditions such as hypertension, lack of smoking, and vaginal delivery; infant characteristics include term delivery and normal infant birth weight (>2,500 g).10,11 It is unclear if these same factors are also associated with breastfeeding initiation and continuation among opioid-exposed dyads.
In addition, for pregnant women with OUD, receipt of either methadone or buprenorphine treatment is the standard of care.12,13 Women with OUD are more likely to have comorbid psychiatric diagnoses and hepatitis C.14 It is unknown whether methadone versus buprenorphine, comorbid psychiatric diagnoses or prescribed medications to treat such diagnoses, or hepatitis C impact breastfeeding initiation or continuation.
The first objective of this study was to examine rates of mothers who met criteria for breastfeeding per hospital guidelines and rates of breastfeeding initiation, continuation, and exclusivity until infants' discharge from 2006 until 2016. The second objective was to examine the extent to which the following characteristics were associated with breastfeeding initiation and continuation in opioid-exposed mother-infant dyads: (1) maternal and infant characteristics associated with breastfeeding in non-opioid exposed dyads; (2) maternal factors associated with women with OUD; and (3) hospital-level initiatives to improve breastfeeding rates.
Materials and Methods
Design, setting, and sample
We studied a retrospective cohort of mother-infant dyads exposed to opioids during pregnancy that delivered between 2006 and 2016 at Boston Medical Center (BMC), a large, urban, safety-net hospital in Boston, Massachusetts. During this time, pregnant women with OUD were cared for in a multidisciplinary program that provides obstetrical care, addiction treatment, and mental health services called Project RESPECT (Recovery, Empowerment, Social Services, Prenatal Care, Education, Community, and Treatment). Obstetrical providers in Project RESPECT have been partnering with local methadone clinics for more than 25 years and have been prescribing buprenorphine treatment since 2006. Approximately 2,800 infants are delivered at BMC annually, with 100 infants born to mothers receiving treatment for OUD (36 infants/1,000 live births). BMC has maintained Baby-Friendly designation since 1999, with more than 95% of non-opioid exposed dyads initiating breastfeeing.15,16 For this analysis, we restricted our cohort to the first delivery among opioid-exposed mother-infant dyads who delivered between January 1, 2006 and December 31, 2016. All variables were collected by retrospective medical chart review or pulled from the Boston University Clinical Data Warehouse and entered into a secure electronic database. The institutional review board at Boston University Medical Campus approved this study.
Inclusion criteria
Breastfeeding eligibility was determined from the electronic medical record, including prenatal, pediatric, and lactation specialist notes, and was based on specified hospital guidelines (Table 1). The first written institutional guidelines specifying recommendations for breastfeeding among mothers with OUD were developed in 2006, and were subsequently updated in 2010 and again in 2015, based on hospital experience and recommendations by the Academy of Breastfeeding Medicine and American College of Obstetrics and Gynecology.5,7,16 Criteria specified participation in an addiction recovery program, time spent participating in prenatal care, and lack of illicit or nonprescribed substances on urine toxicology testing (Table 1). Additional contraindications to breastfeeding since 2006 have included mothers with HIV, open herpes lesions on the breast, hepatitis C positive with cracked bleeding nipples, or other prescribed medications known to be harmful in breastfeeding.
Table 1.
Timeline of Hospital-Level Initiatives to Improve the Care of Opioid-Exposed Mother-Infant Dyads
| Time period | Participation in addiction treatment program required | Prenatal care requirements | Urine toxicology requirements: number of weeks without aberrant testing before deliverya | Breastfeeding eligibility guidelines and institution breastfeeding improvements |
|---|---|---|---|---|
| 01/2006–12/2009 | Yes | None | 12 | • 2006 First Written Breastfeeding Guidelines |
| 01/2010–12/2012 | Yes | ≥12 weeks before delivery, no more than two missed visits | 10 | • 2010 Revised Written Breastfeeding Guidelines |
| • 2010 Brochure on benefits of breastfeeding in OUD developed and distributed passively in prenatal clinic | ||||
| 01/2013–03/2015 | Yes | ≥12 weeks before delivery, no more than two missed visits | 10 | • 2013 NAS QI Committee formed; statewide QI collaborative started |
| • 2013–14 NAS QI Committee focuses on standardization of monitoring of NAS infants and early pharmacologic treatment | ||||
| • Early 2015 NAS QI Committee reviewed breastfeeding guidelines | ||||
| 04/2015–12/2016 | Yes | Attendance at ≥50% or ≥5 prenatal visits | 4 | • April 2015 revised breastfeeding guidelines approved |
| • 04/2015 Started active face-to-face prenatal breastfeeding education | ||||
| • 2015 NAS QI Committee focuses on: (1) skin-to-skin, (2) rooming-in, and (3) lactation support, after mothers are discharged, but infant still admitted. |
Aberrant toxicology test defined as any nonprescribed opioids, benzodiazepines, amphetamines, or other illicit substances.
NAS, neonatal abstinence syndrome; OUD, opioid use disorder; QI, quality improvement.
Main outcomes
The main outcomes were as follows: (1) breastfeeding initiation, defined as any breast milk given or direct breastfeeding during the hospitalization; (2) continuation until discharge, defined as any breast milk or direct breastfeeding in the 24 hours before infant discharge; and (3) exclusive breastfeeding, defined as only breast milk given or direct breastfeeding during the entire infants' hospitalization.
Main exposures
We assessed hospital, maternal, and infant characteristics as exposures. Hospital characteristics were measured using delivery period as a proxy for the implementation of specific hospital-based initiatives focused on breastfeeding support for substance-exposed dyads and changes to criteria in breastfeeding guidelines, outlined in detail in Table 1. In addition to breastfeeding guideline changes highlighted above, there were several hospital initiatives implemented during our study period. First, in 2010, a brochure listing the benefits of breastfeeding was distributed to expectant mothers with OUD in Project RESPECT. Next, in early 2013, a multidisciplinary committee began working on improving outcomes for infants with NAS, in coordination with a statewide QI collaborative.17 This led to greater awareness of the importance of nonpharmacologic treatments for NAS, such as breastfeeding. Starting in 2015, the committee actively focused on several breastfeeding initiatives, including further revision of breastfeeding guidelines (enacted April 2015), a mandatory online educational module on breastfeeding in the setting of OUD for all physician and nursing staff caring for this population, and active face-to-face prenatal education with mothers where medical students discussed breastfeeding benefits and determined breastfeeding intent. The committee also worked to promote nonpharmacologic measures, including time spent skin-to-skin and rooming-in, and improve ongoing lactation support after the mother was discharged, but the infant was still admitted, to target breastfeeding continuation.
Maternal demographics included age at the time of delivery, race/ethnicity, maternal insurance, hypertension during pregnancy, smoking, opioid medication treatment at delivery, psychiatric diagnosis, psychiatric medications, hepatitis C status, and mode of delivery. Infant characteristics included birth weight, gestational age at birth, and length of hospitalization (days).
Data analysis
Data were analyzed in SAS Version 9.3. Rates of breastfeeding eligibility, initiation, continuation, and exclusivity until infants' discharge by delivery year were calculated. Hospital, maternal, and infant characteristics among dyads that did and did not initiate and continue breastfeeding until discharge were compared using chi-square tests for categorical variables and t-tests for continuous variables. Logistic regression was used to examine associations between breastfeeding initiation and continuation using three different models. Unadjusted and adjusted odds ratios (aORs) and 95% confidence intervals (CIs) were calculated. Model 1 evaluated unadjusted associations. Model 2 examined factors known to be predictors of breastfeeding in non-opioid exposed mothers and factors associated with OUD. Model 3 included all the variables in Model 2 plus four time intervals from 2006 to 2016 (1/2006–12/2009, 1/2010–12/2012, 1/2013–3/2015, and 4/2015–12/2016), representing specific changes in hospital breastfeeding eligibility guidelines and QI initiatives described above. No variables were found to be collinear (variance inflation factors all <1.5); thus, all variables were included in our regression models.
Sensitivity analysis
To examine whether maternal and infant characteristics changed over the 11-year study period, which could explain differences in breastfeeding initiation and continuation, rather than hospital-based practices that changed over the same period, we compared the characteristics during each of the four time intervals using chi-square tests for categorical variables and ANOVA tests for continuous variables. For all characteristics with statistically significant differences, a plausible interaction between time period and characteristic was considered. If a potential pathway for effect modification was identified, an interaction term was assessed, and if found to be significant, stratified models evaluating the characteristic within each time period were computed.
Results
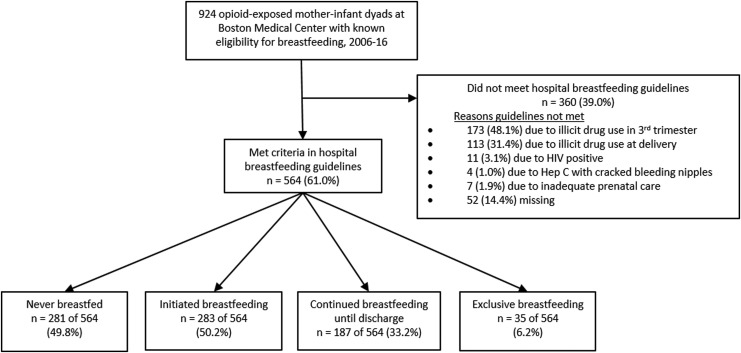
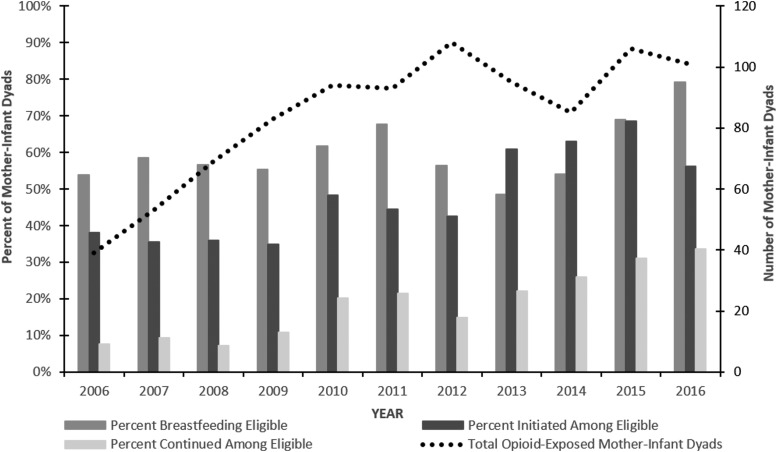
Figure 1 shows a schematic of the study sample. Among 924 pregnant women receiving opioid agonist treatment at the time of delivery from 2006 to 2016, 564 (61%) met criteria to breastfeed per institution guidelines. Of those, 283 (50%) initiated breastfeeding, 187 (33%) continued breastfeeding until discharge, and 35 (6%) exclusively breastfed during the infants' hospitalization. Figure 2 shows proportion meeting breastfeeding guidelines, initiating, and continuing breastfeeding trending upwards from 2006 until 2016, as changes in hospital guidelines and initiatives to support breastfeeding for women with OUD were implemented. In 2006, only 54% met hospital guidelines and 21% initiated breastfeeding, compared with 79% who met guidelines and 45% who initiated breastfeeding in 2016.
FIG. 1.
Schema of breastfeeding initiation, continuation, and exclusivity until discharge, 2006–2016.
FIG. 2.
Trends in breastfeeding eligibility, initiation, and continuation among 924 opioid-exposed mother-infant dyads, January 2006–December 2016.
Overall, our cohort of women with OUD who met hospital breastfeeding guidelines were overwhelmingly of non-Hispanic white race (88%), received public insurance (94%), had high rates of co-occurring psychiatric diagnoses (70%), and smoked cigarettes during pregnancy (77%). More than half of mothers had a diagnosis of hepatitis C (62%). Just over half received methadone (54%) as the primary medication treatment for their OUD, and the remainder received buprenorphine (46%) at the time of delivery. Table 2 presents the characteristics of the sample, comparing opioid-exposed mother-infant dyads who chose to initiate breastfeeding and continue until hospital discharge with those who did not.
Table 2.
Maternal and Infant Characteristics by Breastfeeding Initiation and Continuation Status Among Eligible Mother-Infant Dyads at Boston Medical Center 2006–2016
| Initiated breastfeeding, n (%) | Continued to discharge, n (%) | |||||
|---|---|---|---|---|---|---|
| No (n = 281) | Yes (n = 283) | pa | No (n = 129) | Yes (n = 190) | pa | |
| Delivery year [n (%)] | <0.001 | 0.001 | ||||
| 01/2006–12/2009 | 88 (31.3) | 49 (17.3) | 27 (20.8) | 22 (11.8) | ||
| 01/2010–12/2012 | 100 (35.6) | 82 (29.0) | 27 (20.8) | 55 (29.4) | ||
| 01/2013–03/2015 | 39 (13.9) | 67 (23.7) | 17 (13.1) | 50 (26.7) | ||
| 04/2015–12/2016 | 54 (19.2) | 85 (30.0) | 59 (45.4) | 60 (32.1) | ||
| Maternal pregnancy characteristics [n (%)] | ||||||
| Age | 0.66 | 0.67 | ||||
| <25 years | 69 (24.6) | 78 (27.6) | 31 (23.9) | 51 (27.3) | ||
| 25–34 years | 181 (64.4) | 172 (60.8) | 83 (63.9) | 110 (58.8) | ||
| ≥35 years | 31 (11.0) | 33 (11.7) | 16 (12.3) | 26 (13.9) | ||
| Non-Hispanic white race/ethnicity | 253 (90.0) | 241 (85.2) | 0.08 | 115 (88.5) | 155 (82.9) | 0.17 |
| Public insurance (Medicaid) | 266 (95.3) | 255 (93.4) | 0.43 | 125 (97.7) | 163 (91.1) | 0.06 |
| Any hypertension | 34 (12.1) | 38 (13.5) | 0.63 | 32 (24.6) | 33 (17.7) | 0.13 |
| Any diabetes | 38 (13.5) | 36 (12.7) | 0.78 | 36 (27.7) | 28 (15.0) | 0.006 |
| Any smoking | 231 (82.8) | 200 (71.2) | 0.005 | 96 (74.4) | 131 (70.8) | 0.73 |
| Opioid agonist therapy | 0.002 | 0.32 | ||||
| Methadone | 169 (60.1) | 134 (47.3) | 65 (50.0) | 83 (44.4) | ||
| Buprenorphine | 112 (39.9) | 149 (52.7) | 65 (50.0) | 104 (55.6) | ||
| Any psychiatric diagnosis | 192 (69.3) | 201 (71.5) | 0.57 | 97 (74.6) | 138 (74.6) | 0.99 |
| Any psych medication | 136 (48.4) | 112 (39.6) | 0.04 | 61 (46.9) | 71 (38.0) | 0.11 |
| Hepatitis C diagnosis | 197 (70.4) | 149 (53.0) | <0.001 | 72 (55.8) | 94 (50.5) | 0.21 |
| Mode of delivery | 0.06 | <0.001 | ||||
| Vaginal | 167 (59.4) | 190 (67.1) | 64 (49.2) | 135 (72.2) | ||
| Cesarean | 114 (40.6) | 93 (32.9) | 66 (50.8) | 54 (27.8) | ||
| Infant characteristics [n (%)] | ||||||
| Gestational age | 0.006 | 0.92 | ||||
| ≤33 6/7 weeks | 13 (4.6) | 10 (3.5) | 5 (3.9) | 6 (3.2) | ||
| 34 0/7–36 6/7 weeks | 37 (13.2) | 16 (5.7) | 8 (6.2) | 13 (7.0) | ||
| ≥37 0/7 weeks | 231 (82.2) | 257 (90.8) | 117 (90.0) | 168 (89.8) | ||
| Birth weight (grams) | 0.006 | 0.20 | ||||
| <2500 | 59 (21.0) | 35 (12.4) | 21 (16.2) | 21 (11.2) | ||
| ≥2500 | 222 (79.0) | 248 (87.6) | 109 (83.9) | 166 (88.8) | ||
| Length of stay (days) [Mean (SD)] | 20.6 (12.2) | 17.6 (11.5) | 0.002 | 20.9 (12.7) | 15.0 (9.5) | <0.001 |
Chi-squared for categorical variables, t-test for continuous variables.
SD, standard deviation.
Table 3 presents the results of our unadjusted and adjusted models. In the unadjusted logistic regression models, maternal buprenorphine treatment (versus methadone) was positively associated, and infant preterm birth <34 weeks (versus ≥37 weeks) and low birth weight <2,500 g (versus ≥2,500 g) were negatively associated with breastfeeding initiation, but these did not reach significance in our final adjusted model. In addition, in the first adjusted model (Model 2), maternal white race was negatively associated with breastfeeding initiation, but this did not retain significance once delivery year was added into the model. In the final adjusted model (Model 3), deliveries of infants born after 2013 were positively associated with breastfeeding initiation. In the final model, there was a 2.84 times greater odds (95% CI 1.64–7.93) of breastfeeding initiation among dyads cared for at BMC from 2013 to March 2015 after our NAS quality collaborative had been developed and 2.60 times greater odds (95% CI 1.51–4.50) of breastfeeding initiation among dyads delivered from April 2015 to December 2016 after guidelines relaxed restrictions on breastfeeding eligibility and breastfeeding education among opioid-exposed dyads was promoted, compared to dyads delivered between 2006 and 2009.
Table 3.
Associations of Hospital Delivery Year, and Maternal and Infant Characteristics with Breastfeeding Initiation and Continuation Among Eligible Mother-Infant Dyads at Boston Medical Center 2006–2016
| Initiated breastfeeding | Continued to discharge | |||||
|---|---|---|---|---|---|---|
| Unadjusted OR Model 1 (95% CI) | aOR Model 2a(95% CI) | aOR Model 3b(95% CI) | Unadjusted OR Model 1 (95% CI) | aOR Model 2a(95% CI) | aOR Model 3b(95% CI) | |
| Hospital characteristics, delivery year as proxy | ||||||
| Period 1 (01/2006–12/2009) | Ref. | n/a | Ref. | Ref. | n/a | Ref. |
| Period 2 (01/2010–12/2012) | 1.47 (0.93–2.33) | n/a | 1.15 (0.70–1.91) | 2.50 (1.21–5.17)c | n/a | 2.20 (0.94–5.13) |
| Period 3 (01/2013–03/2015) | 3.09 (1.82–5.23)c | n/a | 2.84 (1.61–5.02)c | 3.61 (1.64–7.93)c | n/a | 3.11 (1.29–7.55)c |
| Period 4 (04/2015–12/2016) | 2.83 (1.74–4.61)c | n/a | 2.60 (1.51–4.50)c | 1.25 (0.64–2.43) | n/a | 1.38 (0.58–3.29) |
| Maternal pregnancy characteristics | ||||||
| Age | ||||||
| <25 years | 1.19 (0.81–1.75) | 1.24 (0.81–1.88) | 1.38 (0.89–2.12) | 1.24 (0.73–2.11) | 1.13 (0.60–2.12) | 1.12 (0.59–2.13) |
| 25–34 years | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| ≥35 years | 1.12 (0.66–1.91) | 1.06 (0.60–1.88) | 1.04 (0.58–1.86) | 1.23 (0.62–2.43) | 1.26 (0.57–2.78) | 1.19 (0.53–2.64) |
| Non-Hispanic white race/ethnicity | 0.64 (0.38–1.06) | 0.53 (0.31–0.91)c | 0.59 (0.34–1.04) | 0.63 (0.33–1.22) | 0.53 (0.25–1.11) | 0.55 (0.26–1.17) |
| Medicaid versus non-Medicaid | 1.36 (0.65–2.87) | 1.21 (0.54–2.9) | 1.22 (0.53–2.79) | 3.83 (1.09–13.53)c | 4.22 (0.93–19.14) | 3.32 (0.83–17.51) |
| Any hypertension | 1.15 (0.74–1.79) | 1.17 (0.72–1.88) | 1.06 (0.68–1.67) | 0.66 (0.38–1.14) | 0.78 (0.40–1.51) | 0.89 (0.44–1.75) |
| Any smoking | 1.01 (0.98–1.03) | 1.01 (0.98–1.03) | 1.01 (0.98–1.03) | 1.00 (0.97–1.02) | 1.00 (0.98–1.03) | 1.01 (0.98–1.04) |
| Buprenorphine versus methadone treatment | 1.68 (1.20–2.34)c | 1.54 (1.05–2.25)c | 1.43 (0.95–2.13) | 1.25 (0.80–1.96) | 0.81 (0.46–1.43) | 0.77 (0.44–1.37) |
| Any psychiatric diagnosis | 1.11 (0.77–1.60) | 1.38 (0.90–2.12 | 1.06 (0.68–1.67) | 1.00 (0.60–1.67) | 1.23 (0.64–2.40) | 1.17 (0.59–2.34) |
| Any psychiatric medication | 0.70 (0.50–0.98) | 0.73 (0.49–1.09) | 0.86 (0.57–1.31) | 0.69 (0.44–1.09) | 0.86 (0.48–1.54) | 0.76 (0.41–1.42) |
| Hepatitis C diagnosis | 1.00 (0.99–1.02) | 1.00 (0.99–1.02) | 1.00 (0.99–1.02) | 0.98 (0.96–1.01) | 0.98 (0.96–1.01) | 0.98 (0.96–1.01) |
| Cesarean versus vaginal delivery | 0.72 (0.51–1.0) | 0.70 (0.48–1.02) | 0.69 (0.47–1.02) | 0.37 (0.23–0.60)c | 0.32 (0.19–0.57)c | 0.33 (0.19–0.57)c |
| Infant characteristics | ||||||
| Gestational age <37 0/7 weeks versus ≥37 0/7 weeks | 0.47 (0.28–0.78)c | 0.65 (0.34–1.23) | 0.71 (0.37–1.37) | 1.02 (0.48–2.1) | 2.10 (0.74–5.96) | 2.20 (0.94–6.86) |
| Birth weight <2500 g versus ≥2500 g | 0.53 (0.34–0.84)c | 0.74 (0.42–1.33) | 0.71 (0.40–1.02) | 0.66 (0.34–1.26) | 1.03 (0.42–2.5) | 0.94 (0.37–2.34) |
| Length of stay (days) | 0.98 (0.96–0.99)c | 0.99 (0.98–1.01) | 0.99 (0.98–1.01) | 0.95 (0.93–0.97)c | 0.94 (0.92–0.97)c | 0.94 (0.92–0.97)c |
Includes all maternal and infant characteristics.
Includes all maternal and infant characteristics PLUS delivery year.
p-value <0.05.
aOR, adjusted odds ratio CI, confidence interval.
With respect to breastfeeding continuation until infant discharge, in our unadjusted models, private insurance was positively associated, but did not reach significance in our multivariable models. In the final adjusted model, longer duration of infant's hospitalization in days (aOR 0.94, [95% CI 0.92–0.97]) and cesarean section delivery (versus vaginal) (aOR 0.33, [95% CI 0.19–0.57]) were associated with lower odds of breastfeeding continuation. With respect to delivery year, delivering between January 2013 and March 2015 was positively associated with breastfeeding continuation (aOR 3.11, [95% CI 1.29–7.55]), compared to delivering in 2006–2009.
In our sensitivity analysis evaluating the four different study periods, there were statistically significant differences in maternal age, race/ethnicity, psychiatric conditions and treatment, medication for addiction treatment, and infant length of stay (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/bfm). However, only for type of maternal opioid agonist treatment during pregnancy could a plausible mechanism for effect modification be hypothesized. In the middle of our study period, the results of a multicenter randomized control trial were published finding improved neonatal withdrawal outcomes using buprenorphine compared with methadone.18 This likely contributed to the increased buprenorphine use during pregnancy at our institution, from 20% before 2010 to over 50% in 2015–2016 (Supplementary Table S1). Given this plausible mechanism for effect modification, an interaction term comparing type of medication treatment and study period was added to the model, which was found to be significant in the model of breastfeeding initiation only. Thus, four stratified models by period for breastfeeding initiation were evaluated. During the first study period between 1/2006 and 12/2009, mothers receiving buprenorphine had 4.5 times the odds of initiating breastfeeding compared with moms receiving methadone. After 2010, there was no significant difference in breastfeeding initiation by type of medication treatment (Supplementary Table S2).
Discussion
In our study of more than 500 opioid-exposed mother-infant dyads who met institutional criteria for breastfeeding, only half initiated and a third continued breastfeeding until their infant's discharge. These rates increased over the study period in the setting of multiple revisions in hospital breastfeeding guidelines and concerted efforts to improve breastfeeding support for mothers with OUD. Hospital-level changes and initiatives, measured using delivery year as a proxy, were most strongly associated with breastfeeding initiation. After adjusting for delivery period, cesarean delivery and length of stay were the only characteristics predictive of breastfeeding continuation, while other characteristics associated with breastfeeding among non-opioid exposed populations, including race and gestational age, and factors associated with OUD such as type of opioid agonist medication and hepatitis C status were not.
While the rates of breastfeeding improved at our institution over time, starting at 38% in 2006 and increasing to 56% in 2016, these rates remain significantly lower than national estimates for all mothers of greater than 80%.19 Even more stark was the rate of breastfeeding exclusivity of 6% at the time of infants' discharge, which is considerably lower than the national estimate for all mothers of 40% at 3 months.19 These suboptimal rates occurred within a Baby-Friendly designated hospital, with long standing adoption of breastfeeding support practices. Rates of breastfeeding among opioid-exposed dyads at our institution fit within the range of previously published U.S.-based studies on opioid-exposed dyads, all based on dyads from a single hospital, clinic, or addiction treatment program, with ranges of breastfeeding initiation of 24–81%.20–23 This wide range is similar to published studies from Canada and Europe, with initiation rates ranging from 17% to 77%.4,24–28 Fewer studies have reported data on continuation of breastfeeding until or past an infant's hospital discharge, but published rates range from 11% to 66%.20,23,26,29
Given the substantial health benefits for infants with NAS who receive breast milk, identification of factors associated with breastfeeding success in this vulnerable population represents a key opportunity for improved infant outcomes. In our multivariable model, hospital characteristics, measured using delivery year as a proxy, were the strongest predictor of breastfeeding initiation, suggesting that the hospital environment can have a substantial impact. We speculate that the confluence of expanded criteria for encouraging breastfeeding for pregnant women with OUD, hospital QI efforts to improve outcomes for infants with NAS, and structured, face-to-face prenatal breastfeeding education at our multidisciplinary prenatal clinic have contributed to the increasing breastfeeding rates over the past decade.
The creation and updating of breastfeeding eligibility guidelines over time required collaboration of investment across multiple disciplines. Decisions to update the guidelines were prompted by frustration or lack of clarity in current practice. Professional organization guidelines recommend a range of time between 30 and 90 days without illicit drug use before delivery, as the current literature does not conclusively support one time period over another.5,7 We hypothesize that the process of reviewing and updating the guidelines to relax restrictions in illicit use from 90 days before delivery down to 30 days before delivery led to engagement by staff and further education on the importance of breastfeeding in this population, which translated to a more supportive hospital culture focused on breastfeeding success.16 Grossman and colleagues recently changed their breastfeeding guidelines to encourage breastfeeding among all mothers with OUD who had no measure of illicit drug use at the time of delivery only. They found, as we did, that changes in guidelines led to substantial increases in breastfeeding, as infants receiving at least 50% breast milk at the time of discharge increased from 20% to 42%.23 Additional research is needed to understand the impact of discouraging women who may be motivated to breastfeed, but do not meet strict hospital breastfeeding guidelines based on illicit use before delivery. There is a need to carefully balance the benefits of in-hospital breastfeeding initiation in reducing NAS severity, with concern for potential postpartum illicit drug use in mothers recently in recovery.
In addition, our involvement with a statewide QI effort yielded a heightened level of engagement by our hospital to improve the care of opioid-exposed mother-infant dyads. Our team focused on improvements in prenatal education, continued skin-to-skin care, rooming-in, and lactation support after the mothers are discharged. Our success in promoting these efforts was aided by our multidisciplinary QI team and a dedicated, comprehensive prenatal care model where prenatal education efforts could be concentrated, rather than requiring education across multiple providers and practices. Unfortunately, our institution did not track individual receipt of prenatal education and was not able to make comparisons to evaluate our educational interventions at an individual level. Crook and colleagues, however, evaluated the impact of Baby-Friendly status and a three-session, group-based, prenatal education program for pregnant women with OUD on the breastfeeding rates at their institution. They found no statistically significant differences in rates of any breastfeeding among the three groups, but may not have been underpowered to see an effect.30
We found that the proportion of women receiving buprenorphine treatment at delivery increased during the study period, likely influenced by changing prescribing patterns following the results of a study that showed improved neonatal outcomes with buprenorphine.18 In our sensitivity analysis, buprenorphine treatment was associated with increased odds of breastfeeding initiation compared to methadone treatment in the earliest study period, but this finding was not significant in later years or in the final multivariable model. This finding is consistent with some,31 but not all previous studies of opioid-exposed mother-infant cohorts.4 In our cohort, it is also possible that some of this effect is due to unmeasured confounders of maternal addiction severity, rather than the difference in the treatment medications themselves,32 as methadone treatment has been the preferred treatment option for mothers who have struggled in their recovery during pregnancy at our institution.33 Thus, the decision to not initiate breastfeeding may have been more related to length of time in treatment or time since last relapse, which are markers for stability in recovery.34 In addition, before 2001, the American Academy of Pediatrics did not recommend breastfeeding for mothers taking more than 20 mg of methadone, which excluded the majority of women receiving methadone treatment for OUD.35 Misperceptions about the safety profile of breastfeeding while taking methadone may persist.
With respect to maternal and infant characteristics, only cesarean section and increasing length of infant's hospitalization were negatively associated with breastfeeding continuation, while a host of other maternal demographic and medical factors were not. We hypothesize that, in the opioid-exposed population, factors that contribute to breastfeeding initiation and continuation are even more complex than the non-opioid exposed population, and many of these factors may not have been captured in our analysis. For example, among opioid-exposed dyads, the time each mother spends at the infants' bedside after her discharge, while her infant is still admitted, performing skin-to-skin and directly breastfeeding depended on various factors, including competing demands from treatment programs.36 Next, breastfeeding success may be dependent on an infant's ability to latch successfully, which can be impaired if withdrawal symptoms are severe.37 In addition, rates of prior trauma in the form of domestic violence and/or sexual abuse among women with OUD are high, which may adversely affect views and success of breastfeeding.38,39 Finally, negative staff perceptions and stigma toward mothers with OUD, even in our Baby-Friendly institution with rooming-in for opioid-exposed dyads and a prenatal clinic caring for pregnant women with OUD, may have led mothers to feel less welcome at the bedside and contributed to early discontinuation of breastfeeding.
Limitations
Our retrospective cohort comes from a single-site, urban academic center, with a unique multidisciplinary prenatal program and large volume of infants with NAS, which may limit the generalizability of our findings; however, existing studies also predominately come from single centers. We were not able to closely track process measures that were part of our breastfeeding initiatives such as maternal receipt of active or passive prenatal education, staff and parent perceptions of breastfeeding, time spent in skin-to-skin or rooming-in, and lactation specialist support following maternal discharge. Rather, since these changes took place at a hospital level, we relied on delivery period as a proxy measure for these initiatives. At our institution, delivery period was a good proxy for hospital-level initiatives because of the dedicated prenatal clinic caring for mothers with OUD, ensuring prenatal education, and policy-driven newborn practice with a small number of providers involved, ensuring practices were universally adopted. Other factors that also contribute to breastfeeding success that we did not measure included breastfeeding intent, measures of infant's ability to latch and feed, or impact of a previous breastfeeding experience. Despite these limitations, we were able to track breastfeeding initiation, continuation, and exclusivity among a large sample of opioid-exposed dyads over a relatively long time period. We were also able to examine a diverse range of maternal and infant factors, including opioid agonist received during pregnancy.
Conclusion
Given the current opioid epidemic and specific health benefits of breastfeeding opioid-exposed infants, this study serves as an early investigation of hospital-, maternal-, and infant-level predictors of initiation and continuation of breastfeeding. Focusing on implementation of hospital guidelines that encourage breastfeeding among opioid-exposed mother-infant dyads, and specialized programs to support breastfeeding among this population may have the most impact on improving low initiation and continuation rates among women with OUD.
Supplementary Material
Acknowledgments
Data collection for this project was made possible through support from Boston University CTSI 1UL1TR001430. Dr. Schiff was supported by HRSA Training Grant T32HP10028. Ms. Joseph was supported by the Boston University Medical Student Summer Research Program. Dr. Parker was supported by the W.K. Kellogg Foundation (P3031871).
Disclosure Statement
No competing financial interests exist.
References
- 1. Patrick SW, Schumacher RE, Benneyworth BD, et al. . Neonatal abstinence syndrome and associated health care expenditures: United States, 2000–2009. JAMA 2012;307:1934–1940 [DOI] [PubMed] [Google Scholar]
- 2. Ko JY, Patrick SW, Tong VT, et al. . Incidence of neonatal abstinence syndrome—28 States, 1999–2013. MMWR Morb Mortal Wkly Rep 2016;65:799–802 [DOI] [PubMed] [Google Scholar]
- 3. Tsai LC, Doan TJ. Breastfeeding among mothers on opioid maintenance treatment: A literature review. J Hum Lact 2016;32:521–529 [DOI] [PubMed] [Google Scholar]
- 4. Welle-Strand GK, Skurtveit S, Jansson LM, et al. . Breastfeeding reduces the need for withdrawal treatment in opioid-exposed infants. Acta Paediatr 2013;102:1060–1066 [DOI] [PubMed] [Google Scholar]
- 5. ACOG Committee on Health Care for Underserved Women; American Society of Addiction Medicine. ACOG Committee Opinion No. 524: Opioid abuse, dependence, and addiction in pregnancy. Obstet Gynecol 2012;119:1070–1076 [DOI] [PubMed] [Google Scholar]
- 6. Hudak ML, Tan RC; COMMITTEE ON DRUGS; COMMITTEE ON FETUS AND NEWBORN; American Academy of Pediatrics. Neonatal drug withdrawal. Pediatrics 2012;129:e540–e560 [DOI] [PubMed] [Google Scholar]
- 7. Reece-Stremtan S, Marinelli KA. ABM clinical protocol #21: Guidelines for breastfeeding and substance use or substance use disorder, revised 2015. Breastfeed Med 2015;10:135–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Short VL, Gannon M, Abatemarco DJ. The association between breastfeeding and length of hospital stay among infants diagnosed with neonatal abstinence syndrome: A population-based study of in-hospital births. Breastfeed Med 2016;11:343–349 [DOI] [PubMed] [Google Scholar]
- 9. Beake S, Pellowe C, Dykes F, et al. . A systematic review of structured compared with non-structured breastfeeding programmes to support the initiation and duration of exclusive and any breastfeeding in acute and primary health care settings. Matern Child Nutr 2012;8:141–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anstey EH. Racial and geographic differences in breastfeeding—United States, 2011–2015. MMWR Morb Mortal Wkly Rep 2017;66:DOI: 10.15585/mmwr.mm6627a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pitonyak JS, Jessop AB, Pontiggia L, et al. . Life course factors associated with initiation and continuation of exclusive breastfeeding. Matern Child Health J 2016;20:240–249 [DOI] [PubMed] [Google Scholar]
- 12. Oza-Frank R, Chertok I, Bartley A. Differences in breast-feeding initiation and continuation by maternal diabetes status. Public Health Nutr 2015;18:727–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Committee on Obstetric Practice. Committee Opinion No. 711: Opioid use and opioid use disorder in pregnancy. Obstet Gynecol 2017;130:e81–e94 [DOI] [PubMed] [Google Scholar]
- 14. Patrick SW, Schiff DM, COMMITTEE ON SUBSTANCE USE AND PREVENTION. A public health response to opioid use in pregnancy. Pediatrics 2017;139:e20164070. [DOI] [PubMed] [Google Scholar]
- 15. Arnaudo CL, Andraka-Christou B, Allgood K. Psychiatric co-morbidities in pregnant women with opioid use disorders: Prevalence, impact, and implications for treatment. Curr Addict Rep 2017;4:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baby Friendly USA. The gold standard of care. Baby-Friendly USA. Published 2012. Available at https://www.babyfriendlyusa.org (accessed August4, 2017)
- 17. Wachman EM, Saia K, Humphreys R, et al. . Revision of breastfeeding guidelines in the setting of maternal opioid use disorder: One institution's experience. J Hum Lact 2016;32:382–387 [DOI] [PubMed] [Google Scholar]
- 18. NeoQIC. Neonatal quality improvement collaborative of Massachusetts—Substance exposed newborns. Published 2017. Available at www.neoqic.org/sen (accessed August8, 2017)
- 19. Centers for Disease Control and Prevention. Breastfeeding report card: Progressing toward national breastfeeding goals, United States, 2016. Published 2016. Available at https://www.cdc.gov/breastfeeding/pdf/2016breastfeedingreportcard.pdf (accessed July25, 2017)
- 20. O'Connor AB, Collett A, Alto WA, et al. . Breastfeeding rates and the relationship between breastfeeding and neonatal abstinence syndrome in women maintained on buprenorphine during pregnancy. J Midwifery Womens Health 2013;58:383–388 [DOI] [PubMed] [Google Scholar]
- 21. McCarthy JJ, Leamon MH, Willits NH, et al. . The effect of methadone dose regimen on neonatal abstinence syndrome. J Addict Med 2015;9:105–110 [DOI] [PubMed] [Google Scholar]
- 22. Backes CH, Backes CR, Gardner D, et al. . Neonatal abstinence syndrome (NAS): transitioning methadone treated infants from an inpatient to an outpatient setting. J Perinatol 2012;32:425–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grossman MR, Berkwitt AK, Osborn RR, et al. . An initiative to improve the quality of care of infants with neonatal abstinence syndrome. Pediatrics 2017;139:e20163360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ordean A, Kahan M, Graves L, et al. . Obstetrical and neonatal outcomes of methadone-maintained pregnant women: A canadian multisite cohort study. J Obstet Gynaecol Can 2015;37:252–257 [DOI] [PubMed] [Google Scholar]
- 25. Abrahams RR, Kelly SA, Payne S, et al. . Rooming-in compared with standard care for newborns of mothers using methadone or heroin. Can Fam Physician 2007;53:1722–1730 [PMC free article] [PubMed] [Google Scholar]
- 26. Dryden C, Young D, Hepburn M, et al. . Maternal methadone use in pregnancy: Factors associated with the development of neonatal abstinence syndrome and implications for healthcare resources. BJOG 2009;116:665–671 [DOI] [PubMed] [Google Scholar]
- 27. Smirk CL, Bowman E, Doyle LW, et al. . Home-based detoxification for neonatal abstinence syndrome reduces length of hospital admission without prolonging treatment. Acta Paediatr 2014;103:601–604 [DOI] [PubMed] [Google Scholar]
- 28. Abdel-Latif ME, Pinner J, Clews S, et al. . Effects of breast milk on the severity and outcome of neonatal abstinence syndrome among infants of drug-dependent mothers. Pediatrics 2006;117:e1163–e1169 [DOI] [PubMed] [Google Scholar]
- 29. O'Connor A, Alto W, Musgrave K, et al. . Observational study of buprenorphine treatment of opioid-dependent pregnant women in a family medicine residency: Reports on maternal and infant outcomes. J Am Board Fam Med 2011;24:194–201 [DOI] [PubMed] [Google Scholar]
- 30. Crook K, Brandon D. Prenatal Breastfeeding Education: Impact on infants with neonatal abstinence syndrome. Adv Neonatal Care 2017;17:299–305 [DOI] [PubMed] [Google Scholar]
- 31. Pritham UA, Paul JA, Hayes MJ. Opioid dependency in pregnancy and length of stay for neonatal abstinence syndrome. J Obstet Gynecol Neonatal Nurs 2012;41:180–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brogly SB, Saia K, Hernández-Diaz S, et al. . The comparative safety of buprenorphine versus methadone in pregnancy-what about confounding? Addiction 2016;111:2130–2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saia K, Bagley SM, Wachman EM, et al. . Prenatal treatment for opioid dependency: Observations from a large inner-city clinic. Addict Sci Clin Pract 2017;12:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McLellan AT, Lewis DC, O'Brien CP, et al. . Drug dependence, a chronic medical illness: Implications for treatment, insurance, and outcomes evaluation. JAMA 2000;284:1689–1695 [DOI] [PubMed] [Google Scholar]
- 35. American Academy of Pediatrics Committee on Drugs. Transfer of drugs and other chemicals into human milk. Pediatrics 2001;108:776–789 [PubMed] [Google Scholar]
- 36. Howard MB, Schiff DM, Penwill N, et al. . Impact of parental presence at infants' bedside on neonatal abstinence syndrome. Hosp Pediatr 2017;7:63–69 [DOI] [PubMed] [Google Scholar]
- 37. Jansson LM, Velez M, Harrow C. Methadone maintenance and lactation: A review of the literature and current management guidelines. J Hum Lact 2004;20:62–71 [DOI] [PubMed] [Google Scholar]
- 38. Cottler LB, Nishith P, Compton WM. Gender differences in risk factors for trauma exposure and post-traumatic stress disorder among inner-city drug abusers in and out of treatment. Compr Psychiatry 2001;42:111–117 [DOI] [PubMed] [Google Scholar]
- 39. Jansson LM, Velez ML, Butz AM. The effect of sexual abuse and prenatal substance use on successful breastfeeding. J Obstet Gynecol Neonatal Nurs 2017;46:480–484 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.