Abstract
Objective:
We assessed preferences for cancer risk management strategies for Lynch syndrome (LS) in LS-affected women.
Methods:
Women with LS aged ≥ 25 years evaluated 9 cancer risk management strategies using a visual analog scale (VAS) and modified standard gamble (SG). For the VAS, women ranked each strategy ranging from 0 (least preferred) to 100 (most preferred). VAS scores were calculated by dividing the corresponding number by 100. Scores closer to 1.0 reflected more favorable strategies. For the SG, participants were asked to specify their expected threshold of lifetime risk of endometrial or colorectal cancer, ranging from 0 to 100%, at which they would consider undertaking each strategy. Strategies included chemoprevention, cancer screening, and preventive surgery. Cancer worry and perceived cancer risk measures were collected on a subset of participants.
Results:
Sixty-one women completed preference assessments. By VAS, annual combined screening was the most preferred, followed by annual screenings and chemoprevention with oral contraceptives. By SG, women were the most willing to endorse oral contraceptives and biannual screening strategies at the lowest threshold of lifetime risk followed by annual screening strategies. Surgical interventions were the least preferred strategies using both VAS and SG. Women with a family history of gynecologic or colorectal cancer were less likely to consider prevention or screening options compared to women without a family history. Cancer worry was higher among women with a positive family history of LS cancer.
Conclusion:
Understanding women’s preferences may facilitate optimal use and adherence to cancer risk management strategies.
INTRODUCTION
Lynch Syndrome
Lynch syndrome (LS) is caused by germline mutations in DNA mismatch repair (MMR) genes (MLH1, MSH2, MSH6, and PMS2) and EPCAM. LS confers a high lifetime risk of developing colorectal cancer (CRC) or endometrial cancer (EC) specifically 40–80% (1–4) and 40%−60% (1, 3–6), respectively. Risks for ovarian cancer and other cancers in LS are also elevated, although not as markedly.
Consensus guidelines recommend that individuals with LS begin screening colonoscopy between 20 and 25 years of age or 2–5 years earlier than the youngest age at diagnosis of CRC in the family (7). Potential strategies for reducing endometrial and ovarian cancer risk include endometrial biopsy, transvaginal ultrasound, CA125 testing, prophylactic hysterectomy with bilateral salpingo-oophorectomy in women who have completed childbearing (8). While oral contraceptives have demonstrated a 50% decrease in endometrial cancer risk in the general population, they have not yet been proven to be efficacious in women with Lynch syndrome (9) and may only be appropriate in a select group of women (10).
Colonoscopy is a cost-effective screening strategy in LS; however, transvaginal ultrasounds alone have not been effective in identifying early cases of endometrial cancer (11, 12). Prophylactic hysterectomy with bilateral salpingo-oophorectomy (HYST/BSO) has potential negative quality of life implications (13, 14). Women’s preferences in the context of shared decision-making with their health care providers may influence the adoption of and/or adherence to gynecologic cancer risk management strategies for LS. Few studies have examined women’s preferences for the type and frequency of LS gynecologic cancer risk management options (15), nor have studies included psychosocial factors that may influence how women value or prioritize their management of LS cancer risk. Preference-sensitive decisions, such as which cancer risk management strategy to undertake, may be influenced by an individual’s perception of her own risk of developing cancer, as well as other factors including socioeconomic and demographic considerations, and experiences with family members who have been diagnosed with Lynch-related cancers. Using a preference assessment approach that incorporated psychosocial and family history variables in the analysis, this study evaluated preferences for LS cancer risk management strategies in LS-affected women with and without a personal history of cancer.
METHODS
Study participants
This study was approved by the University of Texas M.D. Anderson Cancer Center (MDACC) Institutional Review Board. Participants were recruited from a LS registry at MDACC, and through MDACC physician referral or self-referral. Women aged ≥25 years without a prior gynecologic cancer diagnosis were eligible if they were diagnosed with LS through genetic counseling and testing, or met the revised Amsterdam II criteria and thus had been clinically advised to follow Lynch syndrome screening recommendations.
Preference assessment
Preference assessment is the formal evaluation of the strength of a patient’s preference for different health states, such as clinical outcomes or cancer prevention strategies. Preference scores range from 0.0 to 1.0, where 0 represents “least preferred” and 1 represents “most preferred”. Results of preference assessment studies increase providers’ understanding of how patients interpret the risks and benefits of a given cancer treatment or screening strategy. Preference scores are used to quality-adjust outcomes of cost-effectiveness studies to arrive at cost per quality-adjusted life year (16–18).
Data collection
Data collection was guided by patient preference assessment methods to understand how patients interpret the risks and benefits of LS cancer risk management strategies (16–18).
Health states.
Brief written descriptions of 9 health states describing potential LS cancer risk management strategies were developed based on existing guidelines (7) with input from gastrointestinal physicians and gynecologic oncologists with clinical LS expertise. Participants were asked to evaluate the following health states:
3 anchor health states (perfect health, current health, and death), to establish benchmark preference scores and ensure participant comprehension.
6 cancer screening health states: annual and biannual colonoscopy; annual and biannual gynecologic cancer screening consisting of endometrial biopsy, transvaginal ultrasound, and CA125; and, annual and biannual combined colorectal and gynecologic cancer screening (colonoscopy, endometrial biopsy, transvaginal ultrasound, and CA125).
1 chemoprevention health state describing the use of oral contraceptives to prevent gynecologic cancers.
2 surgery health states to prevent or reduce the risk of gynecologic cancers consisting of premenopausal and postmenopausal hysterectomy and bilateral salpingo-oophorectomy (HYST/BSO). The health states stated the surgery could be performed either via laparoscopy or laparotomy. The premenopausal health state described symptoms that women would experience after surgery including flushing, night sweats, and vaginal dryness.
Visual analog scale.
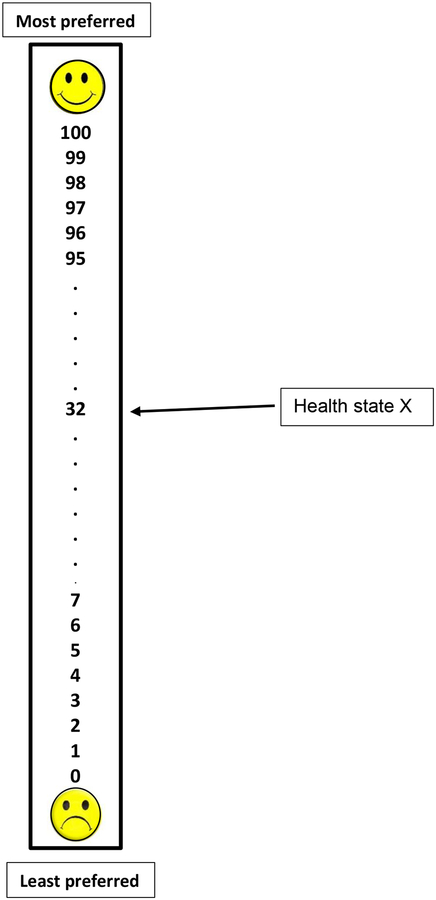
Using a “feeling thermometer”(19) (Fig. 1) adapted from prior studies (20), women were asked to read each health state description and rank each health state ranging from 0 (least preferred) to 100 (most preferred). Preference scores were calculated by dividing the number corresponding to health state rankings by 100 (i.e., 75/100 = .75). Scores closer to 1.0 reflect more favorable health states.
Fig. 1. VAS feeling thermometer.
Women were asked to rate each health state on the scale. Higher numbers indicate a more favorable rating for a given health state. The VAS score for the arrow depicting the sample health state X is 32/100 = .32.
Standard gamble.
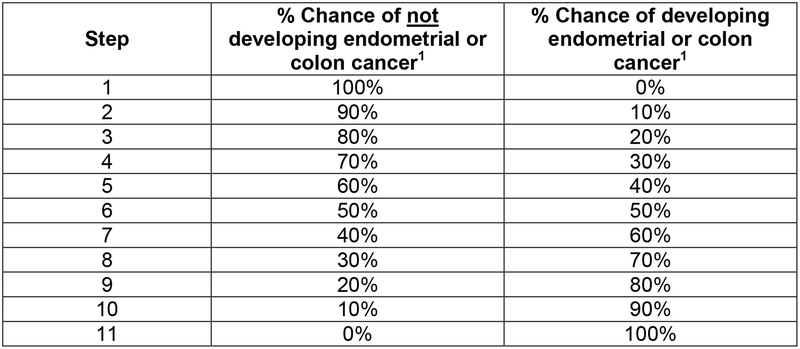
A modified standard gamble assessed womens’ expected threshold of lifetime risk of endometrial and colorectal cancer, ranging from 0 to 100%, at which they would consider undertaking each of the risk management strategies described in the health states. Using the table shown in Fig. 2, a research interviewer asked each woman how high her risk of colorectal or endometrial cancer would need to be in order to consider undergoing each of the risk management strategies depicted in the health state descriptions. Participants were instructed to consider colorectal cancer for the colonoscopy strategy, endometrial cancer was considered for gynecologic screening, and both cancers were considered with combined gynecologic and colorectal cancer screening health states. The standard gamble preference score for a particular health state was calculated by subtracting participants’ stated threshold of lifetime disease risk from 100% and dividing that difference by 100 to arrive at a score on a 0.0 to 1.0 scale. Scores closer to 1.0 reflect more favorable health states; however, for clinical relevance, the standard gamble scores are reported as lifetime risks.
Fig. 2. Modified standard gamble table showing percentage risk of developing and not developing cancer.
The research interviewer started at step 1 by asking the participant if she would elect the risk management strategy under evaluation if she had a 0% risk of endometrial or colorectal cancer. If the participant answered NO, then the interviewer moved to step 2 and asked the same question. This process continued until the participant answered YES to the electing the management strategy described in a given health state.
1 Colorectal cancer was the target cancer of interest when for any health state that involved colonoscopy; endometrial cancer was the target cancer of interest for health states that involved HYST/BSO, transvaginal ultrasound, and CA125, as well as the chemoprevention health state. Both cancers were considered with combined screening health states.
Cancer worry and perceived cancer risk.
A subgroup of participants (n=48) completed measures on cancer worry and perceived cancer risk as part of a concurrent questionnaire study of gynecologic screening in LS (21). This subgroup did not differ from the rest of study population in terms of demographic and family history characteristics. Three cancer worry items related to endometrial, ovarian, and colorectal cancer were adapted from prior cancer worry research (22). Three items assessed perceived risk of developing endometrial, ovarian, or colorectal cancer compared to other women (ranging from 1= much lower to 5= much higher), and also quantified risk on a 0 to 100 scale (0=no chance; 100=definitely).
Analysis
Descriptive statistics were used to characterize the study sample. Mann-Whitney tests were used to evaluate differences in preference scores between participant groups. Chi-square and Fisher’s exact tests were used to compare participant characteristics. All tests were 2-sided and p<.05 was considered to be statistically significant. Subset analyses examined differences in visual analog scale and standard gamble scores by age (< 35 years vs. older), race/ethnicity (White vs. other), education (college graduate vs. other), marital status (married vs. other), having children (children vs. none), having a first-degree relative with colorectal cancer, as well as having any family history of endometrial or ovarian cancer. These subset analyses were also completed on the sub-sample of respondents with no prior history of colorectal cancer. Score differences also were evaluated in those with a personal history of colorectal cancer vs. those with no prior history. IBM SPSS version 23.0 was used for statistical analysis (Armonk, NY: IBM Corp).
RESULTS
Participant characteristics
Table 1 shows the demographic and clinical characteristics of the study population (N=61). The mean age was 38.1 years (range, 24.7 – 57.9; 95%CI [36.3, 39.8]). Patients were more likely to be White (p<.001), married with children (p<.001), and have completed a college education (p=.01). Almost half (49.2%) had MSH2 mutations (23) and few (16%) had been previously diagnosed with colorectal cancer. Most had at least one first degree relative with colorectal cancer and just over one-third had a first degree relative with endometrial cancer.
Table 1.
Demographic and clinical characteristics (N=61)
| Characteristic | |
|---|---|
| Mean age, years (min, max) | 38.1 (24.7, 57.9) |
| n (%) | |
| Race/ethnicity | |
| White | 54 (88.5) |
| Hispanic | 4 (6.6) |
| Black | 2 (3.3) |
| Other | 1 (1.6) |
| Education level | |
| High school | 6 (9.8) |
| Some college | 14 (23.0) |
| College degree or higher | 40 (65.6) |
| Not stated | 1 (1.6) |
| Married | 47 (77.0) |
| Prior children | 49 (80.3) |
| Mutation status | |
| MLH1 | 21 (34.4) |
| MSH2 | 30 (49.2) |
| MSH6 | 2 (3.3) |
| Other1 | 8 (13.1) |
| Personal history of cancer | |
| Colorectal | 10 (16.4%) |
| Any family history2: | |
| Endometrial cancer and/or ovarian cancer3 | 37 (60.7) |
| Colorectal cancer | 59 (96.7) |
| First degree relative4: | |
| Colorectal cancer | 49 (80.3) |
| Endometrial or ovarian cancer5 | 21 (34.4) |
8 women (13.1%) were deemed clinically high risk for Lynch syndrome and advised to follow Lynch syndrome risk management recommendations, due to personal and/or family cancer history and/or tumor studies
37 women had a family history of both colorectal and endometrial or ovarian cancer
26 women had a family history of endometrial cancer; 6 women had a family history of ovarian cancer; and 5 women had a family history of endometrial and ovarian cancer
14 women had first degree relatives with colorectal cancer and endometrial or ovarian cancer
18 women had a first degree relative with endometrial cancer; 2 women had a first degree relative with ovarian cancer; 1 woman had a first degree relative with both endometrial and ovarian cancer
Preference outcomes
Visual analog scale scores.
Median visual analog scale (VAS) scores for each LS risk management strategy are shown in Table 2. The anchor health states for perfect health, current health, and death were evaluated using only the VAS, and responses indicated participants comprehended the exercise (perfect health rated highest at 1.0, death rated lowest at 0.0). Participants rated the annual gynecologic and colorectal cancer combined screening health state as the highest (most preferred) with a VAS score of .98. Other highly ranked health states included annual colonoscopy (VAS=.90), gynecologic cancer screening (.90), chemoprevention with oral contraceptives (0.85), biannual colonoscopy (0.85), biannual combined screening (0.85), and postmenopausal prophylactic HYST/BSO (.85). The least favorable cancer risk management strategy was premenopausal prophylactic HYST/BSO, with a VAS score of .50.
Table 2.
Visual Analog Scale and Standard Gamble Scores for Screening and Prevention Health States (N=61 women)
| Health state | Visual analog scale1 | Standard Gamble2 | ||||
|---|---|---|---|---|---|---|
| Score | Score | % risk of developing cancer | ||||
| Median (range) | SD | Median (range) | SD | Median (range) | SD | |
| Perfect Health (VAS only) | 1.00 (.80, 1.00) | .05 | N/A | N/A | N/A | N/A |
| Current Health (VAS only) | .85 (.40, .99) | .11 | N/A | N/A | N/A | N/A |
| Death (VAS only) | .00 (.00, 1.00) | .11 | N/A | N/A | N/A | N/A |
| Chemoprevention with oral contraceptives | .85 (.00, 1.00) | .29 | .80 (.00, 1.00) | .30 | 20.0 (0.0, 100.0) | 29.79 |
| Annual colonoscopy | .90 (.10, 1.00) | .22 | .78 (0.00, 1.00) | .24 | 22.5 (0.0, 100.0) | 24.15 |
| Biannual colonoscopy | .85 (.00, 1.00) | .23 | .80 (.10, 1.00) | .20 | 20.0 (0.00, 90.0) | 19.62 |
| Annual endometrial biopsy, transvaginal ultrasound, CA 125 | .90 (.10, 1.00) | .20 | .70 (.30, 1.00) | .20 | 30.0 (0.0, 70.0) | 19.70 |
| Biannual endometrial biopsy, transvaginal ultrasound, CA 125 | .80 (.00, 1.00) | .21 | .80 (.40, 1.00) | .17 | 20.0 (0.0, 60.0) | 17.21 |
| Annual combined screening consisting of colonoscopy and endometrial biopsy, transvaginal ultrasound, CA 125 | .98 (.10, 1.00) | .16 | .75 (.20, 1.00) | .23 | 22.5 (0.0, 80.0) | 23.27 |
| Biannual combined screening colonoscopy and endometrial biopsy, transvaginal ultrasound, CA 125 | .85 (.00, 1.00) | .22 | .80 (.30, 1.00) | .18 | 20.0 (0.0, 70.0) | 18.27 |
| Prophylactic HYST/BSO (pre-menopausal) | .50 (.00, 1.00) | .32 | .40 (0.00, 1.00) | .28 | 60.0 (0.0, 100.0) | 28.34 |
| Prophylactic HYST/BSO (post-menopausal) | .85 (.00, 1.00) | .26 | .60 (0.00, 1.00) | .26 | 40.0 (0.0, 100.0) | 26.18 |
SD=standard deviation
Visual analog scale: Health states were ranked from 0 (least preferred) to 100 (most preferred). Preference scores were calculated by dividing the number corresponding to health state rankings by 100 (i.e., 75/100 = .75). Scores closer to 1.0 reflect more favorable health states.
Standard gamble: Scores for health states were calculated by subtracting patients’ stated threshold of lifetime disease risk from 100% and dividing that difference by 100 to arrive at a score on a 0.0 to 1.0 scale (0.0 = least favorable; 1.0=most favorable). Percent risk of developing cancer was defined as the threshold of lifetime risk of endometrial and colorectal (0% to 100%) at which patients would consider undertaking the risk management strategy depicted in each health state.
Some differences in rankings of screening and surgical strategies were observed in subgroup analyses by demographic and clinical variables [refer to Appendix A, Supplemental Data]. Compared to non-White respondents, those who were White had lower median VAS scored for biannual screening strategies, including biannual colonoscopy (median VAS score=.80 for White respondents vs. 1.0 for non-White, p=.003), biannual gynecologic screening (0.8 vs. .98, p=.01), and biannual combined screening (.85 vs. .92, p=.04). Women with children rated postmenopausal HYST/BSO more favorably compared to those without children (VAS=.85 vs. .63, p=.038). Women with a college degree or higher rated premenopausal HYST/BSO lower than women with less than a college degree (VAS .40 vs. .78, p=.01).
When women with a personal history of colorectal cancer were excluded from the analysis, those with a college degree also rated biannual colonoscopy more favorably (.85 vs. .80, p=.01). In addition, younger women rated annual colonoscopy less favorably than women over age 35 (.85 vs .95; p=.038). Women with a first-degree relative affected by a LS gynecologic cancer rated biannual gynecologic cancer screening less favorably (VAS= .75) compared to women who did not have a similar family history (VAS=.87) (p=.04).
Standard gamble scores.
The standard gamble median scores and corresponding lifetime risk levels are shown in Table 2. Chemoprevention with oral contraceptives, biannual colonoscopy, biannual gynecologic cancer screening, and biannual combined gynecologic and colorectal cancer screening were endorsed at a threshold of 20% endometrial or colorectal cancer lifetime risk. Annual colonoscopy was endorsed at 22% lifetime risk and annual combined gynecologic and colorectal cancer screening were endorsed at 25% lifetime risk. Participants would consider annual gynecologic cancer screening if their lifetime risk was 30%, postmenopausal HYST/BSO at a lifetime risk of 40%, and premenopausal HYST/BSO if their lifetime risk was at least 60%.
Subgroup analyses identified differences in preferences for premenopausal HYST/BSO by age. Women older than 35 years were more willing to consider premenopausal HYST/BSO (at 50% lifetime risk) compared to women age 35 or younger (at 80% lifetime risk) (p=.018). A consistent response pattern was observed after excluding those with personal history of colorectal cancer from the analysis. Younger women would not consider premenopausal HYST/BSO unless their lifetime risk was 77.5%, compared to women over 35 years who would opt for this strategy at 45% lifetime risk (p=.02).
Compared to women who had never been diagnosed with cancer, women with a personal history of colorectal cancer indicated they would consider biannual gynecologic screening if their lifetime risk for endometrial cancer was 10%, while women without cancer who would not choose biannual screening unless their risk was 27.5% (p=.038). Both groups of women had similar attitudes towards premenopausal HYST/BSO and would only consider premenopausal HYST/BSO if their life risk for endometrial cancer was 60% (p=.72). Similarly, women with a personal history of colorectal cancer would opt for postmenopausal HYST/BSO if their lifetime risk for endometrial cancer was 45%, and women without cancer would choose this strategy at a lifetime risk of 40% (p=.73).
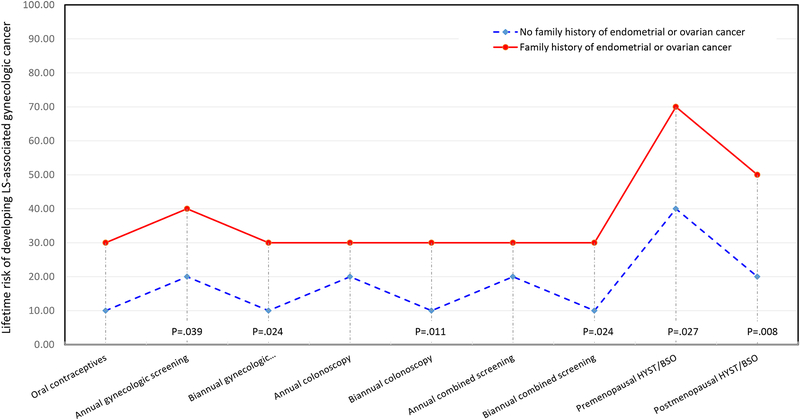
Several subgroup differences were observed when comparing respondents with and without family histories of gynecologic cancers. Women with any family history of endometrial or ovarian cancer were less likely to choose the screening and prevention strategies compared to women who had no family history of LS gynecologic cancer (Fig. 3). For annual gynecologic cancer screening, women with a family history would opt for this strategy at a 40% lifetime risk of developing a LS gynecologic cancer, while women without a family history would select this option with only a 20% lifetime risk (p=.04). Similarly, women with a family history of gynecologic cancer chose biannual gynecologic cancer at a 30% lifetime risk level while women without a family history only needed a 10% lifetime risk in order to choose this strategy (p=.02). The pattern held for women with a family history compared to those without for biannual colonoscopy (30% vs 10% lifetime risk, p=.01), biannual combined gynecologic cancer screening (30% vs 10% lifetime risk, p=.02), premenopausal HYST/BSO (70% vs 40% lifetime risk, p=.03), and postmenopausal HYST/BSO (50% vs 20% lifetime risk, p=.008). When examining women with and without a first degree relative affected by a LS gynecologic cancer, those with first degree relatives were less likely to choose biannual gynecologic cancer screening, opting for this strategy at a 30% lifetime risk of cancer compared to a 20% lifetime risk for women who did not have a first degree relative with a gynecologic cancer (p=.03).
Fig. 3.
Median Lifetime Risk Threshold by Family History of Endometrial or Ovarian Cancer
Cancer Worry and Risk Perception
For the subgroup who completed measures of cancer worry and perceived cancer risk, 59.6%, 37% and 27.7% were characterized as having “a lot” of worry about colorectal, endometrial and ovarian cancer risks, respectively. The median perceived risks of developing colorectal, endometrial, and ovarian cancers were 75% (interquartile range 60% - 80%), 60% (interquartile range 42.5% - 75%), and 30% (interquartile range 20% - 70%), respectively.
Among women with at least one first degree relative with colorectal cancer, 61.1%, 33.0%, and 27.8% reported being “a lot” worried about being diagnosed with colorectal, endometrial cancer, and ovarian cancer, respectively. The median perceived risks of developing colorectal, endometrial, and ovarian cancers were 77.5%, 60.0%, 40.0% respectively.
Among women with first degree relatives with gynecologic cancer, 58.8%, 58.8%, and 23.3% reported being “a lot” worried about being diagnosed with colorectal, endometrial cancer, and ovarian cancer, respectively. The median perceived risks of developing colorectal, endometrial, and ovarian cancers were 75.0%, 60.0%, and 30.0%, respectively.
Among women with a family history of gynecologic cancer, 56.7%, 46.7%, and 26.7% were reported being “a lot” worried about being diagnosed with colorectal, endometrial cancer, and ovarian cancer, respectively. The median perceived risks of developing colorectal, endometrial, and ovarian cancers were 70.0%, 55.0%, and 30.0% respectively.
DISCUSSION
To the best of our knowledge, this is one of the first studies that has systematically evaluated preferences for LS screening and risk management strategies using direct preference assessment methods such as VAS and SG. This study also was unique in its incorporation of psychosocial variables, cancer worry and perceived cancer risk, as well as LS family cancer history to better understand preference for risk management strategies.
Our findings with regard to overall VAS scores showed that annual screening strategies were the most preferred, with chemoprevention using oral contraceptives and biannual screenings rated almost as favorably. Using the SG approach, women were most willing to consider oral contraceptives and biannual screening strategies at the lowest threshold of lifetime risk, with annual screening strategies considered at slightly higher lifetime risk levels. Surgical interventions were the least preferred using both VAS and SG, with premenopausal HYST/BSO rated as the least preferred intervention. These findings suggest that women may consider screening strategies as more preferable in comparison to risk-reducing hysterectomy, although there is more definitive evidence regarding the efficacy of surgery in managing gynecological cancer risk in women with LS.
Younger women were less likely to consider premenopausal HYST/BSO and preferred this strategy only if their lifetime risk level was higher compared to women over age 35. These findings are consistent with prior research that indicated negative quality of life outcomes among younger women who undergo risk-reducing HYST/BSO, including rapid onset of menopausal symptoms with increased severity and frequency compared to women who elect surgery after menopause (24–26).
Parity also may have influenced women’s preferences for prophylactic surgery. Women who did not have children rated postmenopausal HYST/BSO more favorably by VAS compared to those with children, which may suggest a desire to preserve one’s ability to have children compared to those who may have completed childbearing. In counseling women about LS risk management strategies, it may be important to balance the discussion of individual preferences for specific strategies along with a consideration of their efficacy and impact on quality of life and reproductive decisions.
Women with a family history of LS gynecologic cancer were significantly less likely to consider nearly all gynecologic risk reduction strategies. Those who had first degree relatives with LS gynecologic cancer also specified higher lifetime risk levels before they would consider many of the screening and prevention strategies. These preferences may reflect participants’ observations of and experiences with their relatives’ LS-associated gynecologic cancers, the most common of which is endometrial cancer, which generally has a relatively favorable prognosis (29). Symptoms associated with endometrial cancer allow for earlier diagnosis when the disease is curable, often with surgery alone (30), possibly leading to the impression that endometrial cancer is survivable when diagnosed at the time symptoms present. The relative reluctance to endorse gynecologic screening may also be partly attributed to the dislike of undergoing routine endometrial biopsies, which many women find painful and inconvenient (31, 32). In contrast, women in the sample with a prior diagnosis of colorectal cancer had a significantly higher likelihood of endorsing LS screening strategies compared to those without a personal cancer history, as well as higher likelihood of endorsing colorectal cancer screening. This finding may reflect a greater awareness of increased risks of second primary cancers, as lifetime risks of endometrial and colorectal cancer may be similar in LS-affected women (1, 33).
A subgroup of women (78%) participated in a concurrent study that evaluated perceptions of gynecologic screening for LS, and provided responses to items that assessed cancer worry and perceived cancer risk. As expected, cancer worry was higher among those with a positive family history of LS gynecologic cancer or with first-degree relative affected by colorectal or LS gynecologic cancer. However, the perceived risk of developing cancer remained unchanged for endometrial cancer and relatively stable for ovarian and colorectal cancers.
Despite higher levels of cancer worry and self-perceived risk of LS cancers by women with a family history of or a first degree relative with LS gynecologic cancers, preferences elicited using the modified standard gamble revealed these women were not as willing to choose LS cancer screening and prevention strategies as women who did not have a family history of or a first degree relatives with LS gynecologic cancers. In other words, women who had a family history of or first degree relative with LS gynecologic cancers stipulated a higher level of individual lifetime risk of cancer before choosing most of the risk management strategies. At first glance, these results seem counterintuitive, as one might think women with higher cancer worry levels and higher levels of self-perceived risk would be more risk averse to their chances of being diagnosed with cancer and thus more willing to choose screening and prevention strategies (e.g. lower tolerance regarding lifetime risk). While the underlying reasons are likely complex and varied, it is possible that having high levels of cancer worry and knowing their own level of risk may not translate into more risk averse choices in screening and prevention strategies.
This study offers insight regarding women’s potential perceptions and preferences for risk management options in LS that may be important to incorporate in shared decision-making when counseling LS-affected women about managing their cancer risk. Although there is evidence regarding the efficacy of premenopausal HYST/BSO after completion of childbearing, this option was often among the least preferred options. This preference may be influenced by an awareness of the detrimental impact on quality of life as a result of premature menopausal symptoms caused by this surgery (13, 14), and may have influenced women to elect frequent and life-long surveillance instead. Moreover, less favorable premenopausal surgery preferences of women without children may indicate the desire to preserve one’s ability to have children at a future date. While the evidence for gynecologic screening strategies in LS is limited and providers may be reluctant to recommend such strategies as a result, it is important for providers to understand how different subgroups of women with LS may view preferences for risk management, and possible factors influencing those preferences, when counseling about women about their options. In addition, experiences with family history have been shown to influence genomic decision-making. In this study, we showed that the experience of having a family history of the most common LS cancers may also influence preferences for risk management options. With the increasing use of genomic screening methods in LS, there may be varying levels of reliance on initially identifying at-risk individuals based on cancer family history (34). Thus, having a nuanced understanding of the influence of family cancer history may facilitate a more informed discussion about risk management strategies in women affected by LS.
While the findings of this study may prove useful to clinicians who counsel women about LS risk management, there were imitations. The description of gynecologic cancer screening strategies used in the VAS and SG assessments included ovarian cancer screening with CA125 and transvaginal ultrasound along with endometrial biopsy. It is possible that some women may have been aware that CA125 and transvaginal ultrasound are not proven screening methods method in detecting ovarian cancer, which in turn may have influenced their responses to preference assessment exercises. Many of our participants, or their family members, had been involved in previous LS studies, and may have been more experienced or better informed about managing LS risk, and their responses may differ compared to more information-naïve patients seen clinically. We did not evaluate health literacy or numeracy skills prior to the VAS or SG exercises, thus we are not certain of participants’ accurate comprehension of concepts such lifetime risks of cancer. Finally, while we did observe differences based on race/ethnicity and education levels, the study population was largely White and well-educated, and results may not be generalizable to more diverse groups of LS-affected women.
For genomic testing to yield the greatest health benefit, patient and provider adoption and utilization of cancer risk management recommendations for those at highest risk are essential. A key factor in the adoption of such recommendations is understanding preferences for the use of recommended strategies (35), particularly when more than one option is available, as in managing gynecologic cancer risk in LS. Findings from studies such as ours may facilitate a better understanding of women’s preferences for important decisions regarding the management of LS cancer risk, and may ultimately facilitate optimal use and adherence to risk management strategies.
Supplementary Material
Acknowledgements:
This work was supported in part by: 1) MD Cancer Center Support Grant (P30CA016672, Pl: Pisters, MD Anderson Cancer Center) from the National Cancer Institute/National Institutes of Health, which also supports the Assessment, Intervention and Measurement (AIM) Shared Resource; 2) MD Anderson Gynecologic SPORE for Uterine Cancer, (2P50 CA098258, PI: Lu), from the National Cancer Institute, National Institutes of Health; and 3) the Duncan Family Institute for Cancer Prevention and Risk Assessment.
This work was presented in part at the American Society of Clinical Oncology Annual Meeting 2006 and the Society of Gynecologic Oncology Annual Meeting on Women’s Cancer 2008.
Footnotes
CONFLICT OF INTEREST STATEMENT
Dr. Sun reports grant funding from AstraZeneca.
Dr. Meyer reports other support from AstraZeneca and Clovis Oncology.
Ms. Daniels reports no conflicts of interest.
Dr. Bodurka reports no conflicts of interest.
Dr. Nebgen reports no conflicts of interest.
Dr. Burton-Chase reports no conflicts of interest.
Dr. Peterson reports no conflicts of interest.
Dr. Lu reports no conflicts of interest.
REFERENCES
- 1.Aarnio M, Mecklin JP, Aaltonen LA, Nystrom-Lahti M, Jarvinen HJ. Life-time risk of different cancers in hereditary non-polyposis colorectal cancer (HNPCC) syndrome. International journal of cancer. 1995;64(6):430–3. [DOI] [PubMed] [Google Scholar]
- 2.Diergaarde B, Braam H, Vasen HF, Nagengast FM, van Muijen GN, Kok FJ, et al. Environmental factors and colorectal tumor risk in individuals with hereditary nonpolyposis colorectal cancer. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2007;5(6):736–42. [DOI] [PubMed] [Google Scholar]
- 3.Moller P, Seppala T, Bernstein I, Holinski-Feder E, Sala P, Evans DG, et al. Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: first report from the prospective Lynch syndrome database. Gut. 2017;66(3):464–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moller P, Seppala TT, Bernstein I, Holinski-Feder E, Sala P, Gareth Evans D, et al. Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: a report from the Prospective Lynch Syndrome Database. Gut. 2018;67(7):1306–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aaltonen MH, Staff S, Mecklin JP, Pylvanainen K, Maenpaa JU. Comparison of lifestyle, hormonal and medical factors in women with sporadic and Lynch syndrome-associated endometrial cancer: A retrospective case-case study. Molecular and clinical oncology. 2017;6(5):758–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lynch H L P, Lanspa S, Snyder C, Lynch J, Boland C. . Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin Genet. 2009;76(1):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Colorectal (Version 1.2018 - July 12, 2018). https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf. Accessed December 3, 2018.
- 8.Schmeler KM, Lynch HT, Chen LM, Munsell MF, Soliman PT, Clark MB, et al. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. The New England journal of medicine. 2006;354(3):261–9. [DOI] [PubMed] [Google Scholar]
- 9.Daniels MS, Lu KH. Genetic predisposition in gynecologic cancers. Semin Oncol. 2016;43(5):543–7. [DOI] [PubMed] [Google Scholar]
- 10.Davidson BA, Moorman PG. Risk-benefit assessment of the combined oral contraceptive pill in women with a family history of female cancer. Expert opinion on drug safety. 2014;13(10):1375–82. [DOI] [PubMed] [Google Scholar]
- 11.Dove-Edwin I, Boks D, Goff S, Kenter GG, Carpenter R, Vasen HF, et al. The outcome of endometrial carcinoma surveillance by ultrasound scan in women at risk of hereditary nonpolyposis colorectal carcinoma and familial colorectal carcinoma. Cancer. 2002;94(6):1708–12. [DOI] [PubMed] [Google Scholar]
- 12.Rijcken FEM, Mourits MJE, Kleibeuker JH, Hollema H, van der Zee AGJ. Gynecologic screening in hereditary nonpolyposis colorectal cancer. Gynecologic Oncology. 2003;91(1):74–80. [DOI] [PubMed] [Google Scholar]
- 13.Chapman JS, Powell CB, McLennan J, Crawford B, Mak J, Stewart N, et al. Surveillance of survivors: follow-up after risk-reducing salpingo-oophorectomy in BRCA 1/2 mutation carriers. Gynecol Oncol. 2011;122(2):339–43. [DOI] [PubMed] [Google Scholar]
- 14.Etchegary H, Dicks E, Tamutis L, Dawson L. Quality of life following prophylactic gynecological surgery: experiences of female Lynch mutation carriers. Familial Cancer. 2018;17(1):53–61. [DOI] [PubMed] [Google Scholar]
- 15.Wang G, Kuppermann M, Kim B, Phillips KA, Ladabaum U. Influence of patient preferences on the cost-effectiveness of screening for lynch syndrome. Journal of oncology practice. 2012;8(3 Suppl):e24s–30s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blinman P, King M, Norman R, Viney R, Stockler MR. Preferences for cancer treatments: an overview of methods and applications in oncology. Annals of oncology : official journal of the European Society for Medical Oncology. 2012;23(5):1104–10. [DOI] [PubMed] [Google Scholar]
- 17.Drummond MF OBB, Stoddard GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes, Second Edition: Oxford Medical Publications; 1997. [Google Scholar]
- 18.Stiggelbout AM, de Haes JC. Patient preference for cancer therapy: an overview of measurement approaches. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19(1):220–30. [DOI] [PubMed] [Google Scholar]
- 19.Furlong William DF, George W. Torrance, Ronald Barr, and John Horsman. “Guide to design and development of health-state utility instrumentation.” McMaster University Centre for Health Economics and Policy Analysis Working Paper No 90–9, 1990. 1990 [Available from: http://www.chepa.org/Files/Working%20Papers/WP%2090-9.pdf. [Google Scholar]
- 20.Sun C, Brown AJ, Jhingran A, Frumovitz M, Ramondetta L, Bodurka DC. Patient preferences for side effects associated with cervical cancer treatment. Int J Gynecol Cancer. 2014;24(6):1077–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burton-Chase AM, Hovick SR, Sun CC, Boyd-Rogers S, Lynch PM, Lu KH, et al. Gynecologic cancer screening and communication with health care providers in women with Lynch syndrome. Clin Genet. 2014;86(2):185–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stefanek M, Enger C, Benkendorf J, Flamm Honig S, Lerman C. Bilateral prophylactic mastectomy decision making: A vignette study. Preventive medicine. 1999;29(3):216–21. [DOI] [PubMed] [Google Scholar]
- 23.Dowty JG, Win AK, Buchanan DD, Lindor NM, Macrae FA, Clendenning M, et al. Cancer risks for MLH1 and MSH2 mutation carriers. Human mutation. 2013;34(3):490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Domchek SM, Rebbeck TR. Prophylactic oophorectomy in women at increased cancer risk. Current opinion in obstetrics & gynecology. 2007;19(1):27–30. [DOI] [PubMed] [Google Scholar]
- 25.Elit L, Esplen MJ, Butler K, Narod S. Quality of life and psychosexual adjustment after prophylactic oophorectomy for a family history of ovarian cancer. Fam Cancer. 2001;1(3–4):149–56. [DOI] [PubMed] [Google Scholar]
- 26.Etchegary H, Dicks E, Watkins K, Alani S, Dawson L. Decisions about prophylactic gynecologic surgery: a qualitative study of the experience of female Lynch syndrome mutation carriers. Hered Cancer Clin Pract. 2015;13(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crijnen TE, Janssen-Heijnen ML, Gelderblom H, Morreau J, Nooij MA, Kenter GG, et al. Survival of patients with ovarian cancer due to a mismatch repair defect. Fam Cancer. 2005;4(4):301–5. [DOI] [PubMed] [Google Scholar]
- 28.Grindedal EM, Renkonen-Sinisalo L, Vasen H, Evans G, Sala P, Blanco I, et al. Survival in women with MMR mutations and ovarian cancer: a multicentre study in Lynch syndrome kindreds. Journal of medical genetics. 2010;47(2):99–102. [DOI] [PubMed] [Google Scholar]
- 29.Vasen HF, Watson P, Mecklin JP, Jass JR, Green JS, Nomizu T, et al. The epidemiology of endometrial cancer in hereditary nonpolyposis colorectal cancer. Anticancer research. 1994;14(4b):1675–8. [PubMed] [Google Scholar]
- 30.Dinkelspiel HE, Wright JD, Lewin SN, Herzog TJ. Contemporary clinical management of endometrial cancer. Obstet Gynecol Int. 2013;2013:583891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang M, Sun C, Boyd-Rogers S, Burzawa J, Milbourne A, Keeler E, et al. Prospective study of combined colon and endometrial cancer screening in women with lynch syndrome: a patient-centered approach. Journal of oncology practice. 2011;7(1):43–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nebgen DR, Lu KH, Rimes S, Keeler E, Broaddus R, Munsell MF, et al. Combined colonoscopy and endometrial biopsy cancer screening results in women with Lynch syndrome. Gynecol Oncol. 2014;135(1):85–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dunlop MG, Farrington SM, Carothers AD, Wyllie AH, Sharp L, Burn J, et al. Cancer risk associated with germline DNA mismatch repair gene mutations. Human molecular genetics. 1997;6(1):105–10. [DOI] [PubMed] [Google Scholar]
- 34.Hampel H, Pearlman R, Beightol M, Zhao W, Jones D, Frankel WL, et al. Assessment of Tumor Sequencing as a Replacement for Lynch Syndrome Screening and Current Molecular Tests for Patients With Colorectal Cancer. JAMA oncology. 2018;4(6):806–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phillips KA, Liang S-Y, Van Bebber S, The CANPERS (Cancer and Personalized Medicine) Research Group, Afable-Munsuz A, Elkin E, … Walsh J . Challenges To The Translation Of Genomic Information Into Clinical Practice And Health Policy: Utilization, Preferences, And Economic Value. Current Opinion in Molecular Therapeutics. 2008;10(3):260–6. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.