ABSTRACT
Streptococcus pneumoniae (the pneumoccus) is the leading cause of otitis media, community-acquired pneumonia, and bacterial meningitis. The success of the pneumococcus stems from its ability to persist in the population as a commensal and avoid killing by immune system. This chapter first reviews the molecular mechanisms that allow the pneumococcus to colonize and spread from one anatomical site to the next. Then, it discusses the mechanisms of inflammation and cytotoxicity during emerging and classical pneumococcal infections.
INTRODUCTION
Streptococcus pneumoniae (the pneumococcus) is a leading cause of otitis media (OM), community-acquired pneumonia, bacteremia, and meningitis. The pneumococcus is a human-specific pathogen which colonizes the nasopharynx and spreads between hosts through aerosols and potentially through the contamination of objects with mucosal secretions if the bacteria is living within a biofilm (1–3). Rates of carriage vary from 5 to 10% of healthy adults to 20 to 40% of healthy children. However, these numbers can vary widely based on where the samples are collected (4–7). Risk factors associated with higher rates of carriage include race (particularly Australian Aboriginals and Native Americans) (8–12), infancy (13, 14), season, with higher carriage during winter months (13), and crowded areas such as childcare centers, with estimates suggesting that 40 to 60% of children who attend childcare are colonized (15). Duration of colonization decreases with age and varies from 2 weeks to 4 months (14, 16, 17). The introduction of pneumococcal conjugate vaccines has reduced carriage rates for serotypes covered by the vaccine, while nonvaccine serotypes have emerged to occupy this empty niche (18). Nasopharyngeal colonization is usually asymptomatic (19).
Invasive pneumococcal disease (IPD) occurs as a result of the spread of bacteria from the nasopharynx to other parts of the body, including the lungs, blood, and brain. Infants, the elderly, and immunocompromised individuals are at an increased risk for developing IPD (20–22). Pneumococcal models of invasive disease must account for not only the commensal nature of the bacteria, but also the wide spectrum of disease the pneumococcus is capable of causing. Colonization is a prerequisite for IPD, and while the incidence of infection is relatively low, high rates of colonization result in extensive morbidity and mortality that is a global concern. Worldwide, it is estimated that S. pneumoniae is responsible for 15 cases of IPD per 100,000 people per year (23) and over a million deaths annually. As of 2004, in the United States, it is estimated that the pneumococcus was responsible for more than 1.5 million cases of OM and 800,000 cases of pneumonia (24). Direct medical costs resulting from infections totaled $3.5 billion (24). The World Health Organization estimates that close to half a million children under the age of 5 years die annually as a result of S. pneumoniae infection (https://www.cdc.gov/pneumococcal/global.html). Pneumococcal bacteremia and meningitis are also responsible for significant mortality, particularly in the elderly, for whom rates may be as high as 60% and 80%, respectively (25).
In this article, we review the colonization and spread of S. pneumoniae from one anatomical site to another. We also discuss the mechanistic basis of inflammation and cytotoxicity resulting from invasive pneumococcal infection.
TRAFFICKING OF PNEUMOCOCCI THROUGH THE RESPIRATORY TRACT
Interactions with Epithelial Cells of the Nasopharynx
For over a century, S. pneumoniae has been categorized by serology, with distinct serotypes identified on the basis of the more than 90 immunologically and chemically distinct polysaccharide capsules that surround and protect the bacteria from phagocytosis (26). The capsular polysaccharide (CPS) is also the basis of the current pneumococcal vaccines. Prior to the introduction of the 13-valent pneumococcal conjugate vaccine in 2010, studies found that only a small subset of the many capsular types was responsible for the majority of IPD isolates (27). The vast majority of pneumococci colonize the nasopharynx for up to 6 weeks and are then cleared with no systemic symptoms in the host (1, 28). IPD is thought to occur most frequently early after the acquisition of a new capsular serotype, as evidenced by shifts in the strains most commonly isolated from IPD patients after vaccine introduction (22, 29–31). Furthermore, attack rates are higher for serotypes that are carried for shorter periods of time versus those that colonize for extended periods (28).
S. pneumoniae undergoes spontaneous phase variation alternating between a transparent and opaque colony phenotype which can be visualized microscopically by oblique transmitted light (32). The various phenotypes occupy different niches based on selection, with the transparent phenotype being the predominant phase in the nasopharynx (32), while the opaque phase is isolated from blood samples (33). The transparent phenotype expresses increased amounts of phosphorylcholine (ChoP) (34) and choline-binding protein A (CbpA) on the surface (35), both of which function as adhesins and contribute to the ability of the bacteria to colonize the nasopharynx. The opaque phenotype expresses increased levels of capsule and pneumococcal surface protein A (PspA), which are important factors for survival in the blood. Phase-variation is one of the mechanisms by which the pneumococcus alternates between an adhesive phenotype best suited for the nasopharynx and a phagocytosis-resistant phenotype that can survive in the blood.
ChoP decorates the cell wall (Fig. 1) and serves as a docking group for a set of 15 secreted proteins, termed choline-binding proteins (CBPs) (36). Among the CBPs, CbpA is a major pneumococcal adhesin (37) expressed predominantly in the transparent phenotype (35). Pneumococci lacking CbpA are not only largely unable to bind to the nasopharynx, but also have a diminished capacity to colonize the lower respiratory tract and cause pneumonia (35, 38). In vitro, CbpA mutants show decreased binding to both nasopharyngeal epithelial cells and activated type II human lung cell lines (37). Similarly, the CBPs LytB, LytC, CbpD, CbpE, and CbpG also contribute to nasopharyngeal colonization when tested in rat pups. LytB functions as a glucosaminidase (39), whereas LytC is a lysozyme (40) and CbpE is a choline esterase; all three enzymes are active on the bacterial cell wall (37). CbpG is a serine protease that in addition to contributing to nasopharyngeal colonization is required for development of high-grade bacteremia (41).
FIGURE 1.

Immunohistochemical and schematic depiction of the choline biology of the pneumococcal surface. Immunogold labeling of pneumococci with (A) TEPC-15 antibody recognizing free choline and (B) antiautolysin antibody. These two images contrast free (A) versus CBP-bound (B) choline. (C) Schematic view of the capsule (blue), cell wall (green), and membrane (red). The teichoic and lipoteichoic acids are indicated as dark blue lines bearing choline (circles). A proportion of these are capped by choline-binding proteins. Courtesy of K.G. Murti, St. Jude Electron Microscopy Core Facility.
Other virulence factors affecting the capacity of the pneumococcus to colonize the nasopharynx include sIgA1 proteases (42). IgA antibodies are the predominant type found at mucosal surfaces and serve to aggregate and opsonize pathogens. sIgA1 proteases neutralize the activity of sIgA by cleaving the Fc portion of human IgA1 (43). Hydrolyzed sIgA1 also contributes to pneumococcal adhesion. Fab fragments remaining on the surface of the bacteria after sIgA proteolysis neutralize the negative charge of the capsule and enhance adherence (42).
The two-component system CiaR/H is required for efficient colonization and regulates gene expression in response to oxidative stress (44). Among the many genes regulated by CiaR/H is htrA. HtrA, or high-temperature requirement A, is a heat-inducible serine protease that has both proteolytic and chaperone activities. HtrA assists in survival in the presence of environmental factors such as oxidative stress, osmotic stress, and elevated temperatures (45). Deletion of the CiaR/H operon results in a 25-fold decrease in levels of htrA expression and a 1,000-fold decrease in the number of bacteria colonizing the nasopharynx. Deletion of htrA alone results in a 100-fold decrease in nasopharyngeal colonization, indicating that CiaR/H regulates other factors that contribute to colonization (44). Microarray analysis during bacterial adhesion to epithelial cells in vitro has revealed a number of genes with enhanced expression, including CbpA and HtrA (46).
A second regulator important in nasopharyngeal colonization is RlrA, which regulates the transcription of the pilus-1 structural subunit genes rrg(A to C) (47, 48). Pili are found in only 20% of S. pneumoniae strains but are common in strains belonging to antibiotic-resistant clones (49, 50). Piliated strains show stronger adherence to lung epithelia cells and out-compete nonpiliated strains in mixed infection models (47). Furthermore, introduction of the pilus-1 locus into a nonpiliated strain increased adherence to the lung epithelial cells (47). Pilus-1 expression levels have been shown to change during the different stages of infection, with higher expression during early colonization and lower expression as the disease progresses (51).
Other pneumococcal factors that may aid adherence and colonization of the nasopharynx include PspK, SlrA, and PmpA. PspK, pneumococcal surface protein K, has been suggested to help in the colonization of the nasopharynx in unencapsulated strains by mediating adherence to epithelial cells (52). PspK has also been shown to bind the sIgA but does not appear to be involved in invasion (discussed in further detail below) (53). The surface-associated lipoproteins SlrA and PpmA have both been shown in mutagenesis studies to be important for nasopharynx colonization, but they do not appear to play a significant role in invasive disease (54, 55).
Another strategy employed by the bacteria to colonize and remain in the nasopharynx is formation of a biofilm. While the specific role of biofilm formation in pneumococcal infection is not well understood, it confers increased resistance to antimicrobial peptides and may promote sharing of genetic information between the bacteria as a result of proximity (56). Although bacteria in a biofilm are often metabolically dormant, when they are dispersed, they may be more virulent. Bacterial release from a biofilm can be triggered by changes in the microflora, inflammation, or viral infection (57). These released pneumococci were found to be phenotypically different from planktonic or biofilm bacteria and had an increased ability to disseminate and cause infection (58, 59). These release events represent a potential switch between asymptomatic colonization and invasive disease.
Ascension into the Middle Ear
OM is a highly prevalent pediatric disease and the primary cause of physician visits by small children. As many as 80% of children have presented with at least one case of OM (60). Along with Moraxella catarrhalis and Haemophilus influenzae, the pneumococcus is a primary cause of OM and is isolated in 30 to 40% of culture positive middle ear infusions (61–63). OM is thought to result when pneumococci in the nasopharynx ascend the eustachian tube and gain access to the middle ear (Fig. 2). Alternatively, it has also been suggested that OM is caused by the blocking of the eustachian tube resulting in a decrease of oxygen and increased dampness on the middle ear surface. While the mechanism is disputed, the transition from colonization of the nasopharynx to OM strongly correlates with accompanying viral infection (64). Typically, OM presents with earache, fever, nasal congestion, a feeling of fullness in the ear, and muffled hearing.
FIGURE 2.

Schematic depiction of the spread and progression of S. pneumonia infection. Carriage of the pneumococcus occurs in the nasopharynx and is usually asymptomatic in healthy individuals. The bacteria are spread by aerosol from the nasopharynx of carriers. The pneumococcus can spread from the nasopharynx to a number of different tissues. In children the bacteria usually causes otitis media. Invasive diseases generally start in the lungs and spread to the blood, with the most serious complication being meningitis. The switch from asymptomatic colonization to invasive disease in healthy individuals usually occurs when there is a disruption in the innate immune defenses. (This figure contains some artwork produced by Servier Medical Art [http://smart.servier.com/] under Creative Commons license 3.0).
The ability of S. pneumoniae to ascend the eustachian tubes involves neuraminidases (61, 65). S. pneumoniae encodes two neuraminidases, NanA and NanB, with NanA being the major neuraminidase (66, 67). Neuraminidases cleave N-acetylneuraminic acid from glycoproteins and glycolipids on the eukaryotic cell surface. Neuraminidases can also cleave mucin, reducing the viscosity of this barrier and permitting the bacteria to access the epithelium. At the epithelium, neuraminidase cleaves oligosaccharides on the host cell surface, exposing cryptic receptors and enhancing bacterial adherence (66, 67). Support for this mechanism is seen in chinchilla infection models whereby clearance of a neuraminidase-deficient mutant occurs twice as quickly as wild-type S. pneumoniae. Furthermore, structural changes in the cell surface carbohydrates of eustachian tube epithelial cells occur following infection with wild-type pneumococci compared to its isogenic nanA mutant (68, 69).
Once the pneumococcus is in the middle ear, inflammation is triggered by pneumolysin and cell wall components (70). Pneumolysin is a potent pore-forming toxin that directly kills host cells and activates complement. In addition, cell wall components activate Toll-like receptor (TLR) signaling and the alternative pathway of complement. Pneumolysin strongly contributes to hearing loss and cochlear damage during OM (71, 72). Guinea pigs infected with wild-type S. pneumoniae or a mutant deficient in neuraminidase demonstrated significant damage to the reticular lamina, sensory hair cells, and supporting cells of the organ of Corti. Guinea pigs infected with a pneumolysin-deficient mutant had no visible damage. The contribution of pneumolysin and cell wall to inflammation will be discussed in more detail later in this article.
CbpA/pIgR-Mediated Invasion in the Upper Respiratory Tract
Once attached to epithelial cells, the pneumococcus is able to translocate across the mucosal barrier by coopting the polymeric immunoglobulin receptor (pIgR) (73). Mucosal epithelial cells transport IgA and IgM in a vesicle moving from the basolateral to the apical surface by binding to pIgR. On the apical surface, pIgR is cleaved and immunoglobulins are secreted into the lumen. Cleaved pIgR is subsequently shuttled back to the basolateral surface (74). S. pneumoniae translocates through epithelial cells by hijacking pIgR on the apical side of the host cell, resulting in transport as the receptor is endocytosed and recycled to the basolateral surface (Fig. 3). The pneumococcus binds to pIgR by ligating the motif YRNYPT of CbpA (75). In vitro, CbpA alone is sufficient to mediate translocation across a cell as latex beads coated with CbpA are endocytosed (73). In vivo, this interaction manifests as decreased nasopharyngeal colonization in mice lacking pIgR and the reduced capacity for S. pneumoniae mutants lacking CbpA to enter the bloodstream (76).
FIGURE 3.
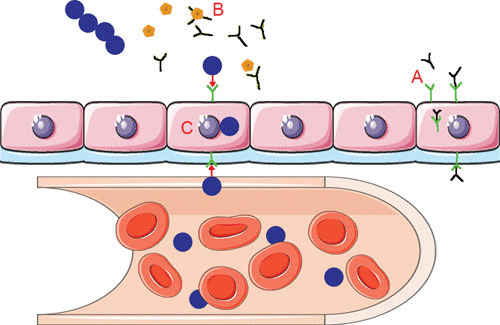
Schematic representation of the pneumococcus hijacking the pIgR/IgA system to cross the mucosal epithelia into the blood. (A) Mucosal epithelial cells transport IgA (black) from the basolateral to the apical surface using the receptor pIgR (green). This receptor is then endocytosed and recycled back to the basolateral surface to transport more IgA. (B) To protect itself from IgA, the pneumococcus produces the protease sIgA1 (yellow), which cleaves the host IgA into Fab fragments. (C) The choline-binding protein, CbpA (red), binds to the empty pIgR and shuttles the pneumococcus from the apical side to the basolateral side of the epithelial cells. (This figure contains some artwork produced by Servier Medical Art [http://smart.servier.com/] under Creative Commons license 3.0).
Accessing the Lower Respiratory Tract
Development of pneumonia is contingent on the ability of S. pneumoniae to establish a lower-respiratory-tract infection despite host defenses that either kill or clear the aspirated bacteria. The first barrier is the mucociliary escalator, which works mechanically to keep aspirated particles and microorganisms out of the lungs. As is true for many respiratory viruses, neuraminidase plays an important role in initiating bacterial pneumonia. As indicated, neuraminidase-deficient pneumococci do not cleave mucin efficiently and have a diminished capacity to adhere. Mutants deficient in nanA have a reduced capacity to bind to chinchilla tracheas ex vivo (77) and are attenuated in their ability to cause a lower-respiratory-tract infection following intranasal challenge (38).
The second step in adjusting to the environment of the lung involves changes to the bacterial surface that promote bacterial adherence to epithelium. The respiratory epithelium produces copious antibacterial peptides that kill bacteria on contact. Recent evidence indicates that pneumococci counteract this defense by shedding capsule, which results in relative resistance to the killing by antimicrobial peptides (78). This process is driven by the bacterial autolytic enzyme, LytA. Although LytA has long been known to promote autolysis in response to antibiotics, this new role of the enzyme drives capsule loss without bacterial lysis. Loss of capsule permits a close interaction of the bacteria with host cells, leading to successful initiation of infection.
Pneumococcal adhesion to eukaryotic lung cells is a two-step process that initially entails a loose interaction with host cell surface glycoconjugates followed by a tighter, more secure interaction with host cell protein receptors that promote internalization. During the initial stages of infection, S. pneumoniae and other respiratory tract pathogens such as Pseudomonas aeruginosa and H. influenzae, bind to N-acetylgalactosamine β1-3 galactose (79). Neuraminidases cleave the sialic acid and expose N-acetylgalactosamine β1-3 galactose and other ligands on the host cell surface (69, 80). The efficient removal of sialic acid by neuraminidases can be reduced by the amount and position of acetylation on sialic acid. It is thought that the major pneumococcal esterase, EstA, deacetylates the sialic acid and increases the release of sialic acid by NanA (81). In vitro, pneumococci adhere more efficiently to tissue culture cells treated with neuraminidase (77). The synergism observed in a pneumococcus and influenza coinfection may be the result of enhanced adherence mediated by neuraminidase activity (82). It has long been known that influenza primes the lungs for the development of a secondary bacterial infection. This synergism has been successfully modeled in mice, where pretreatment with influenza readily enhances severe pneumococcal pneumonia, leading to death despite challenge with a dose that is usually insufficient to infect an otherwise healthy mouse (83–85). Mechanistically, superinfection likely occurs as a result of neuraminidase expressed by influenza stripping sialic acid from the mucosa and exposing bacterial receptors. Oseltamivir, a neuraminidase inhibitor, has been shown to prevent pneumococcal superinfection in this postinfluenza pneumococcal challenge model (86).
Interactions in the Alveoli
As pneumonia progresses, the respiratory epithelium is denuded, exposing the underlying extracellular matrix of the bronchioles and alveoli. Surface-bound bacterial proteins such as PepO, PavA, PavB, PfbA, PclA, and PsrP all contribute to attachment through interactions with the extracellular matrix (87–90). Once established in the alveoli, inflammation is particularly intense, resulting in consolidation of the affected lobes. Consolidation progresses through stages of engorgement and red hepatization during which capillaries and epithelial cells become inflamed, and fluid and erythrocytes accumulate in the alveoli in a fibrin mesh (red hepatization). Subsequently, the lungs darken (gray hepatization) as leukocytes enter the lesion and the bacteria are engulfed by macrophages. Resolution continues for several days as capsule-specific antibodies provide efficient opsonization and inflammatory mediators dissipate (91).
Pneumococcal cell wall, pneumolysin, and hydrogen peroxide are the virulence determinants that mediate the greatest inflammation and cytotoxicity observed in the lungs (92–94). Challenge of mice with purified pneumolysin or cell wall TLR ligand products is sufficient to cause edema and influx of neutrophils that recapitulate pneumonia (95, 96). Multiple studies clearly demonstrate that deletion of the genes that encode autolysin, pneumolysin, or the enzyme that produces hydrogen peroxide greatly attenuate the ability of the bacteria to survive and replicate in the lungs (38, 92, 94, 96–98). The contribution of these virulence products is discussed in greater detail below.
INVASION OF PNEUMOCOCCI INTO THE BLOODSTREAM
Access to the Bloodstream
During red hepatization, infected alveoli overflow with bacteria, edema fluid, and erythrocytes wrapped in a fibrin mesh. Fibrin strands pass through interalveolar connections, also known as the pores of Kohn, from one alveolus to the next, and the lymphatics are dilated and filled with cells and fibrin. When infection reaches this stage, a mouse becomes bacteremic. Access to the bloodstream by S. pneumoniae may occur through several pathways, including via the lymphatics, via cell damage to the epithelial and endothelial cells, and via direct invasion of endothelial cells. Most likely, all three pathways contribute to bloodstream invasion in an infected animal.
Although the pneumococcus can directly invade cells, this occurs at relatively low efficiency compared to bacterial pathogens that are commonly thought of as invasive, such as Salmonella and Shigella species (99). Invasion is dependent on activation of the host cell by pneumococcal cell wall components and pneumolysin and results in de novo expression of host defenses such as platelet activating factor receptor (PAFr), C3, and other factors. PAFr, a receptor abundant on lung cells, recognizes ChoP on its natural ligand, the chemokine PAF. The surface of the bacterium mimics this by decorating the cell wall with ChoP. CV-1 origin SV40 (COS) cells expressing PAFr have been shown to bind more bacteria than COS cells not expressing PAFr (99). Studies have also colocalized PAFr with adherent bacteria on the surface of human cells (100). PAFr binding is not limited to the pneumococcus; other respiratory pathogens such as Haemophilus spp., Neisseria spp., and Pseudomonas spp. also express ChoP on their surfaces in a phase-variable manner (101, 102). The pneumococcus can also bind the PAFr on other tissues (103, 104). In defense against the widespread expression of ChoP on respiratory pathogens, the host deploys C-reactive protein, an acute-phase reactant that activates complement and opsonizes the bacteria (105). Thus, phase variation of the amount of surface-bound ChoP serves as a mechanism by which the pneumococcus switches from a more adherent form (high ChoP) suited for the mucosa to one that is more resistant to phagocytosis (low ChoP) and well adapted to the bloodstream.
Unlike PAF, the binding of pneumococcus to the PAFr does not result in the activation of a G-protein-mediated signal transduction pathway (100). Rather, pneumococcal uptake requires activation of extracellular signal-regulated kinases consistent with activation by β-arrestin. Uptake of the pneumococcus into a vacuole involves clathrin followed by recruitment of β-arrestin scaffold, Rab 5, and then Rab 7 and Rab 11. Rab 5 is involved in early endocytosis, Rab 7 is found in the late endosome, and Rab 11 is responsible for vacuole recycling (106). Overexpression of arrestin in endothelial cells enhances colocalization of the bacteria with Rab 7 and Rab 11 and increases survival of the pneumococci normally killed by the lysosome. Thus, it is currently thought that association of β-arrestin with the PAFr vacuole complex contributes to the successful translocation of the bacteria away from the lysosome (100).
The pilus-1 system may also be employed in the escape from the lungs and peritoneal cavity into the bloodstream. RrgA, the major adhesive determinant of the pilus, has been shown to facilitate the spread of the bacteria to the bloodstream (107). RrgA binds the complement receptor 3 on macrophages and increases intracellular survival (107). The severity and progression of disease were shown to be affected in mice lacking complement receptor 3 and suggests that phagocytosis by macrophages may facilitate the spread of infection to distal sites (107). This theory is possibly further supported by the fact that ubiquitously expressed pneumococcal protein PepO may lead to an increase in macrophage phagocytosis (108).
Recently, a new macropinocytosis pathway was described that is independent of the PAFr and does not require ChoP (109). This pathway relies on actin interactions with the uptake vesicle independent of dynamin, clathrin, and caveolin (109) and represents a potential alternative pathway for bacterial translocation. However, without the requirement of ChoP it could be used by a wider group of bacterial pathogens. In model systems, roughly half of adherent pneumococci enter epithelial cells via micropinocytosis and half via PAFr. Thus, while the pneumococcus is the prototypical extracellular pathogen, intracellular translocation is an important part of its pathogenesis.
Survival in the Bloodstream
Once the bacteria escape into the bloodstream, CPS becomes the most important virulence determinant and is responsible for inhibiting phagocytosis. The chemical structure (serotype) and amount of CPS present on the surface of the bacteria contribute to the differential ability of different serotypes to survive in the blood (110–113). Mutants lacking capsule are essentially avirulent (111), requiring 10,000- to 100,000-fold more bacteria to kill a mouse than the encapsulated parent strain following intraperitoneal injection. It is believed that CPS inhibits phagocytosis by preventing phagocytes from physically reaching opsonizing serum components, such as complement, C-reactive protein, mannose-binding proteins, and antibodies that are deposited on the cell wall and by giving the bacteria a negative charge which repels a close association with leukocytes (32, 114, 115). Formation of antibody to the serotype-specific CPS marks the initiation of clearance of the infection, because antibodies to CPS are highly opsonic and are protective against subsequent pneumococcal challenge with the same serotype (116). The fact that capsular antibodies are so effective forms the basis of current effective pneumococcal vaccines.
Pneumococcal proteins also contribute to resistance to defenses in the serum. PspA has been demonstrated to inhibit complement activation mediated via the classical pathway on the bacteria surface (117, 118). Mutants deficient in PspA are cleared more rapidly from the bloodstream of XID mice compared to wild-type mice. XID mice lack the ability to form an antibody response to polysaccharides, and thus, clearance correlates with the amount of complement on the bacterial surface (119). PspA also protects bacteria from the bactericidal peptides of apolactoferrin; blocking PspA with antibodies enhances apolactoferrin killing (120). CbpA also has the ability to inhibit complement deposition by binding to C3 which blocks C3 cleavage and by binding to factor H (121, 122). PspA allows the bacteria to reduce C3 deposition, whereas factor H, a negative regulator of the alternative pathway, leads to interference in the formation of the C3 convertase. Factor H can also be bound by the pneumococcal surface protein Tuf (123). Furthermore, resistance to opsonophagocytic activity of neutrophils is mediated through the action of the exoglycosidases NanA, BgaA, and StrH, which decrease the amount of C3 being deposited onto the pneumococcal surface (124).
TRAFFICKING OF PNEUMOCOCCI INTO THE HEART, BRAIN, AND FETUS
Interactions with the Heart
Once in the bloodstream, pneumococci disseminate widely into many organs by the binding of bacterial CbpA to endothelial laminin receptor and ChoP to the PAFr. For instance, a common complication of severe bacterial pneumonia is cardiac dysfunction. Pneumonia and cardiac events may be closely associated with heart disease predisposing people to pneumonia or, potentially, pneumonia increasing the risk of heart disease; further studies are needed (125). Bacteremia delivers pneumococci to the cardiac vascular endothelium, where CbpA/laminin receptor-mediated translocation into the cardiac tissue occurs. In mice and nonhuman primates, cardiac damage ensues in the form of microscopic lesions in the myocardium (126, 127) caused by the release of pneumolysin and H2O2, with added pathology resulting from the influx of immune cells (126, 128). Immunization of mice with the CbpA protects the animals from cardiac damage (126). Importantly, pneumococcal cell wall products are also inhibitory to cardiac contractility (129), as is pneumolysin, which disrupts Ca++ signaling due to pore formation even if cells are not immediately killed (130). Once in the heart of the mouse, the bacteria use clathrin-mediated endocytosis to invade cardiomyocytes (131). Interaction between the pneumococcus and the heart is an emerging field.
Interactions at the Blood-Brain Barrier
Pneumococcal meningitis is by far the most devastating complication of IPD. Some estimates suggest that approximately one-third of affected individuals die (132–134) and half of the survivors suffer from permanent neurological sequelae (135–137). Severe damage occurs in the hippocampus, particularly in the dentate gyrus, with survivors suffering from hippocampal atrophy and defects in learning and memory (138). Neuronal damage is, in part, mediated by the response of the host defenses to bacterial products (139); for example, cell wall components are detected by host cells, and the influx of leukocytes leads to extensive inflammation (140, 141). Inflammation in the cerebrospinal fluid (CSF) exacerbates neuronal damage by releasing matrix metalloproteases such as MMP-9 (142), neurotoxic free radicals such as peroxynitrate (143), and proinflammatory cytokines that recruit more leukocytes (141). Ultimately, the overexuberant host response triggers caspase-dependent and -independent apoptosis of neurons (139). Blocking leukocyte entry into the CSF duringmeningitis decreases damage by approximately 50% (144, 145) and as such is the basis for treating individuals with pneumococcal meningitis with dexamethasone prior to antibiotic therapy. The other half of neuronal damage is directly due to cytotoxic compounds such as pneumolysin and hydrogen peroxide that damage the mitochondria and initiate apoptosis (146). These factors are reviewed later in the article. The extent of the host response to damage can be seen by the fact that introduction of purified cell wall alone into the CSF can lead to signs and symptoms of meningitis and ultimately to neuronal damage (147).
Pneumococcal invasion from blood into the CSF is thought to occur either in the choroid plexus or by crossing the blood-brain barrier in the cerebral capillaries that traverse the subarachnoid space. The pneumococcus is thought to initially bind to the blood-brain barrier endothelium through interactions of the NEEK motif of CbpA with the laminin receptor (148). Such binding to the laminin receptor is a common strategy of meningeal pathogens, including H. influenzae, meningococcus, prions, and several viruses. Studies with mice have determined that once bound to the endothelium, translocation across the barrier is dependent on the interaction between ChoP and PAFr (148–151). PAFr knockout mice were resistant to development of meningitis (100). Likewise, CbpA mutants were unable to cross the blood-brain barrier despite bacterial titers in the blood of 108 CFU/ml (38). Thus, the two-step process of recognition of laminin receptor on the cerebrovascular endothelium by homologs of CbpA followed by translocation across the barrier using surface ChoP binding to PAFr is a conserved process of invasion of the central nervous system that is shared by the most successful meningeal pathogens.
Interactions with the Placenta and Fetus
PAFr is found on a number of tissues, including the placenta during pregnancy. Infection and inflammation during pregnancy were shown to lead to postnatal cognitive deficiencies in a number of studies reviewed by Loughran et al. 2016 (103). The pneumococcus itself does not cross the placenta to the fetus. However, fragments of cell wall released during antibiotic treatment cross the placenta in a PAFr-dependent manner and accumulate in the developing fetal cortex in mice. Rather than neuronal death, as seen in postnatal meningitis in mice, the fetal brain responds with an increase in neuroproliferation (104). Neuroproliferation is increased by interfering with the levels of the cytostatic transcription factor FoxG1 as a result of cell wall interaction with TLR2. The abnormal brain architecture is associated with behavioral abnormalities in the postnatal period in mouse models. This leads to the possibility that bacterial products encountered during pregnancy may be associated with cognitive disorders in children.
INFLAMMATION AND CYTOTOXICITY
Intense inflammation is a hallmark of pneumococcal disease, and the pneumococcus serves as a prototype for understanding the molecular mechanisms of inflammation in response to Gram-positive bacteria (152, 153). Inflammatory components released by the pneumococcus include peptidoglycan, teichoic acid, pneumolysin, hydrogen peroxide, and a number of other secreted proteins. Alone, several of these factors have been shown to trigger inflammation and are cytotoxic (see above). In concert, these factors trigger inflammation through multiple inflammatory cascades, including the TLR pathway, the chemokine/cytokine cascade, the complement cascade, and the coagulation cascade. In a naive host lacking serotype-specific antibodies, these cascades hasten the accumulation of leukocytes, which are ineffective in phagocytosing pneumococci coated in capsule. The increased white blood cells at the site of infection lead to an even greater release of proinflammatory mediators without efficient clearance of the bacteria.
Inflammation
The key surface component recognized by the innate immune system is the cell wall (Fig. 4). The pneumococcal cell wall is composed of the peptidoglycan network with teichoic acid attached to roughly every third N-acetylmuramic acid residue (154). When tested in animals, peptidoglycan and teichoic acid can elicit inflammation and recapitulate many of the symptoms of pneumonia, otitis media, and meningitis (147, 152, 155). Although CPS is effective in protecting the bacteria, the CPS itself is not inflammatory (156). Components of the bacterial cell wall can mediate inflammation through multiple pathways. At the cellular level, peptidoglycan and teichoic acid bind to pattern recognition molecules such as lipopolysaccharide-binding protein and peptidoglycan recognition proteins. These complexes in turn bind to TLR-2 on the surface of epithelial and endothelial cells, monocytes, and macrophages (157, 158). Cross-linking of TLR-2 triggers intracellular signaling that activates transcriptional regulators such as NF-κB. NF-κB expression then results in production of proinflammatory cytokines such as interleukin-1β (IL-1β), IL-6, IL-8, and tumor necrosis factor (159). Nod receptors in the cytoplasm may also modulate inflammation by binding to intracellular peptidoglycan (160, 161). The importance of Nod receptor activation for resolution of the infection is supported by the need for the expression of the chemokine receptor CCR2 to efficiently recruit macrophages to the site of infection for bacterial clearance (162). Activation of epithelial and endothelial cells results in recruitment of effector cells such as neutrophils and macrophages, altered vascular permeability, and creation of a serous exudate.
FIGURE 4.

Structure of the pneumococcal cell wall and its relationship to inflammation. (A) Penicillin induces cell wall degradation by the autolysin releasing cell wall fragments such as lipoteichoic acid, glycan polymers with and without teichoic acid, and small stem peptides. All teichoicated species contain ChoP, a key component increasing inflammatory activity. (B) All of these components interact with a variety of human cells, which in turn produce inflammatory mediators. Particularly important in this response is the platelet activating factor (PAFr). These mediators combine to produce the symptomatology of pneumococcal infection, including changes in blood flow, fluid balance in the tissue, and leukocytosis. Glc, glucose; TDH, trideoxyhexose; NAcGaln, N-acetylgalctosamine; Galn, galactosamine; l-Ala, l-alanine; d-Glu, d-glucose; l-Lys, l-lysine; TNF, tumor necrosis factor; NO, nitric oxide; PGE2, prostaglandin E2; IC pressure, intracranial pressure; MIPS, macrophage inflammatory protein.
Cell wall components not only cause inflammation through interaction with cells but also activate several complement pathways. Antibodies specific to bacterial proteins on the cell surface activate the classical pathway (117). Similarly, CPS and cell walls bind to hydrolyzed C3 and activate the alternative complement pathway (156, 163, 164). The classical and alternative pathways result in release of C3a and C5a, both of which are chemoattractants and potent anaphylactic molecules leading to inflammation. Cell wall also activates complement via the lectin-binding pathway. Mannose-binding lectin, a member of the collectin family, binds to carbohydrates such as N-acetyl-glucosamine, a constituent of peptidoglycan (165). The binding of mannose-binding lectin to the bacterial cell results in the formation of a C3 convertase on the surface of the bacteria and deposition of C3b. Mannose-binding lectin deficiency is associated with an increased risk of invasive pneumococcal infection (166). Finally, C-reactive protein bound to cell wall ChoP also activates the classical complement cascade by binding C1q (167). This results in additional opsonization and further release of the C3a and C5a. Pneumococcal PepO can disrupt this process by binding to C1q, increasing bacterial survival (168). However, PepO expression in the lungs has been suggested to trigger release of the chemoattractant IL-8 and IP-10, which leads to neutrophil recruitment and contributes to the host response pathology (169).
Complement activation is not limited to the surface of the bacteria; cell wall fragments released by the bacteria following lysis and during cell wall turnover are also capable of activating complement (170). Likewise, pneumolysin released into the milieu also activates complement (171). Pneumolysin binds to the Fc portion of immunoglobulins and activates the classical pathway. It has been suggested that cell wall components, pneumolysin, and other proteins such as PepO are released by the bacteria to deplete complement (168, 172, 173). This would have the most direct impact during blood stream infections and in the lungs. Release of cell wall components is mediated by the murein hydrolase, LytA. LytA is responsible for pneumococcal lysis in the stationary phase as well as in the presence of antibiotics (174). Furthermore, it has been shown that LytA is important for the release of capsule in response to antimicrobial peptides found on the epithelial surface (78). Autolysin-mediated lysis is responsible for the spike in inflammation observed immediately following antibiotic treatment of meningitis. Autolysin-negative mutants may have reduced virulence compared to the wild type as a result of the inability of the mutant to release cell wall (CW) or the inability to shed the capsule, leaving the bacterium sensitized to the antimicrobial peptides (78, 175).
Although complement is crucial for the opsonization of the bacteria, it also leads to the formation of the membrane attack complex (MAC) formed through the action of C5, C7, and C9, which form a hole in cellular membranes in a fashion similar to pneumolysin. The pneumococcal glycolytic enzyme phosphoglycerate kinase has been shown to inhibit MAC formation by blocking C9 polymerization (176). Gram-positive bacteria, such as Streptococcus, are also in general more resistant to MAC killing than their Gram-negative counterparts since they lack an outer membrane, leaving MAC to form on the thick peptidoglycan wall.
Cytotoxicity
In addition to the pathology derived from inflammation, the pneumococcus can directly damage eukaryotic cells. The principal mediators of cytotoxicity are pneumolysin and hydrogen peroxide (72, 92, 93, 146, 177). Pneumolysin has long been recognized as a principal virulence factor of the pneumococcus (Fig. 5). Studies using a variety of challenge routes and animal models have convincingly demonstrated that pneumolysin-deficient mutants are drastically attenuated (38, 92, 96, 177). The mechanism responsible for pneumolysin secretion from the bacteria is poorly understood. While it was initially thought that pneumolysin was only released as a result of autolysis (178), it has also been shown to have an independent route, because mutants lacking the autolytic enzyme, LytA, show the same pattern of release as the wild-type bacteria (179, 180). Pneumolysin is a pore-forming toxin that kills cells via necroptosis, a programmed mode of necrosis. At high concentrations, pneumolysin, like other pore-forming toxins, triggers necroptosis due to ion dysregulation (181, 182). Critically, pneumolysin-mediated cell death has been shown to be preventable using drugs that block RIPK1, RIPK3, or MLKL, the signaling pathway involved. The toxin binds to cholesterol on the surface of the host cell and oligomerizes to form pores as large as 30 nm in diameter (183). This results in Ca++ and K+ dysregulation. At lower concentrations, the toxin has a variety of effects on different cell types. Pneumolysin has been demonstrated to slow ciliary beating of epithelial cells (184), disrupt tight junctions (185), and inhibit the capacity of neutrophils and macrophages to kill by inhibiting oxidative burst (186, 187). Disruption of the alveoli-capillary barrier contributes to the leakage that allows serous exudates to enter the lungs and the bacteria to cross into the blood stream (188). In the middle ear, pneumolysin is responsible for damage to the cochlea and hair cells, contributing to hearing loss (72). During meningitis, pneumolysin causes neuronal damage mediated by an influx of extracellular calcium triggering apoptosis (146). Chelating extracellular calcium inhibits the release of apoptosis inducing factor (AIF) and protects cells from pneumolysin-induced apoptosis in vitro.
FIGURE 5.

Domain structure of pneumolysin. Pneumolysin has three functionally separate domains: one activating complement, one causing hemolysis, and the other binding to cholesterol. Site-specific mutations alter these properties individually (191, 192).
Hydrogen peroxide (H2O2) is a major product of pneumococcal metabolism and damages host tissues. H2O2 is the result of the activity of the enzyme pyruvate oxidase (SpxB), which decarboxylates pyruvate to produce acetyl phosphate, H2O2, and CO2 (38, 97). Mutation of spxB dramatically attenuates virulence in the respiratory tract but not in the blood stream (38). Studies of the cytotoxic effects of H2O2 are not as comprehensive as those for pneumolysin. Nonetheless, H2O2 also contributes to mitochondrial damage of neurons, resulting in apoptosis (146), and inhibits beating of ciliated ependymal cells lining the ventricular system of the brain and cerebral aqueducts (189, 190). It is also required for cardiac damage (131).
CONCLUDING REMARKS
The ability to invade and cause pathology in such varied organ systems while also avoiding killing by the immune system makes S. pneumoniae a highly successful pathogen. Asymptomatic colonization allows the pneumococcus to persist in the population, and extensive serotype diversity complicates the development of effective vaccines. The successful invasion strategy of ChoP/PAFr and CbpA/laminin receptor shared by a number of major respiratory pathogens coupled with cytotoxic pneumolysin makes the pneumococcus a prototypic pathogen for studying host pathogen interactions.
REFERENCES
- 1.Austrian R. 1986. Some aspects of the pneumococcal carrier state. J Antimicrob Chemother 18(Suppl A):35–45. [PubMed] [DOI] [PubMed] [Google Scholar]
- 2.Marks LR, Reddinger RM, Hakansson AP. 2014. Biofilm formation enhances fomite survival of Streptococcus pneumoniae and Streptococcus pyogenes. Infect Immun 82:1141–1146 10.1128/IAI.01310-13.[PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musher DM. 2003. How contagious are common respiratory tract infections? N Engl J Med 348:1256–1266 10.1056/NEJMra021771. [PubMed] [DOI] [PubMed] [Google Scholar]
- 4.Melegaro A, Gay NJ, Medley GF. 2004. Estimating the transmission parameters of pneumococcal carriage in households. Epidemiol Infect 132:433–441 10.1017/S0950268804001980. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Regev-Yochay G, Raz M, Dagan R, Porat N, Shainberg B, Pinco E, Keller N, Rubinstein E. 2004. Nasopharyngeal carriage of Streptococcus pneumoniae by adults and children in community and family settings. Clin Infect Dis 38:632–639 10.1086/381547. [PubMed] [DOI] [PubMed] [Google Scholar]
- 6.Wyllie AL, Rümke LW, Arp K, Bosch AA, Bruin JP, Rots NY, Wijmenga-Monsuur AJ, Sanders EA, Trzciński K. 2016. Molecular surveillance on Streptococcus pneumoniae carriage in non-elderly adults; little evidence for pneumococcal circulation independent from the reservoir in children. Sci Rep 6:34888 10.1038/srep34888. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wyllie AL, Wijmenga-Monsuur AJ, van Houten MA, Bosch AA, Groot JA, van Engelsdorp Gastelaars J, Bruin JP, Bogaert D, Rots NY, Sanders EA, Trzciński K. 2016. Molecular surveillance of nasopharyngeal carriage of Streptococcus pneumoniae in children vaccinated with conjugated polysaccharide pneumococcal vaccines. Sci Rep 6:23809 10.1038/srep23809. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davidson M, Parkinson AJ, Bulkow LR, Fitzgerald MA, Peters HV, Parks DJ. 1994. The epidemiology of invasive pneumococcal disease in Alaska, 1986-1990: ethnic differences and opportunities for prevention. J Infect Dis 170:368–376 10.1093/infdis/170.2.368. [PubMed] [DOI] [PubMed] [Google Scholar]
- 9.Torzillo PJ, Hanna JN, Morey F, Gratten M, Dixon J, Erlich J. 1995. Invasive pneumococcal disease in central Australia. Med J Aust 162:182–186. [PubMed] [DOI] [PubMed] [Google Scholar]
- 10.Morris PS, Leach AJ, Silberberg P, Mellon G, Wilson C, Hamilton E, Beissbarth J. 2005. Otitis media in young Aboriginal children from remote communities in Northern and Central Australia: a cross-sectional survey. BMC Pediatr 5:27 10.1186/1471-2431-5-27. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackenzie GA, Leach AJ, Carapetis JR, Fisher J, Morris PS. 2010. Epidemiology of nasopharyngeal carriage of respiratory bacterial pathogens in children and adults: cross-sectional surveys in a population with high rates of pneumococcal disease. BMC Infect Dis 10:304 10.1186/1471-2334-10-304. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith-Vaughan H, Marsh R, Mackenzie G, Fisher J, Morris PS, Hare K, McCallum G, Binks M, Murphy D, Lum G, Cook H, Krause V, Jacups S, Leach AJ. 2009. Age-specific cluster of cases of serotype 1 Streptococcus pneumoniae carriage in remote indigenous communities in Australia. Clin Vaccine Immunol 16:218–221 10.1128/CVI.00283-08. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray BM, Turner ME, Dillon HC Jr. 1982. Epidemiologic studies of Streptococcus pneumoniae in infants. The effects of season and age on pneumococcal acquisition and carriage in the first 24 months of life. Am J Epidemiol 116:692–703 10.1093/oxfordjournals.aje.a113452. [PubMed] [DOI] [PubMed] [Google Scholar]
- 14.Gray BM, Converse GM III, Dillon HC Jr. 1980. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J Infect Dis 142:923–933 10.1093/infdis/142.6.923. [PubMed] [DOI] [PubMed] [Google Scholar]
- 15.Dunais B, Pradier C, Carsenti H, Sabah M, Mancini G, Fontas E, Dellamonica P. 2003. Influence of child care on nasopharyngeal carriage of Streptococcus pneumoniae and Haemophilus influenzae. Pediatr Infect Dis J 22:589–592 10.1097/01.inf.0000073203.88387.eb. [PubMed] [DOI] [PubMed] [Google Scholar]
- 16.Smith T, Lehmann D, Montgomery J, Gratten M, Riley ID, Alpers MP. 1993. Acquisition and invasiveness of different serotypes of Streptococcus pneumoniae in young children. Epidemiol Infect 111:27–39 10.1017/S0950268800056648. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Högberg L, Geli P, Ringberg H, Melander E, Lipsitch M, Ekdahl K. 2007. Age- and serogroup-related differences in observed durations of nasopharyngeal carriage of penicillin-resistant pneumococci. J Clin Microbiol 45:948–952 10.1128/JCM.01913-06. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis SM, Deloria-Knoll M, Kassa HT, O’Brien KL. 2013. Impact of pneumococcal conjugate vaccines on nasopharyngeal carriage and invasive disease among unvaccinated people: review of evidence on indirect effects. Vaccine 32:133–145 10.1016/j.vaccine.2013.05.005. [PubMed] [DOI] [PubMed] [Google Scholar]
- 19.Nunes S, Sá-Leão R, Carriço J, Alves CR, Mato R, Avô AB, Saldanha J, Almeida JS, Sanches IS, de Lencastre H. 2005. Trends in drug resistance, serotypes, and molecular types of Streptococcus pneumoniae colonizing preschool-age children attending day care centers in Lisbon, Portugal: a summary of 4 years of annual surveillance. J Clin Microbiol 43:1285–1293 10.1128/JCM.43.3.1285-1293.2005. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butler JC. 2004. Epidemiology of pneumococcal disease, p 148–168. In Tuomanen EI, Mitchell TJ, Morrison DA, Spratt BG (ed), The Pneumococcus. ASM Press, Washington, DC. [Google Scholar]
- 21.Grau I, Ardanuy C, Calatayud L, Rolo D, Domenech A, Liñares J, Pallares R. 2012. Invasive pneumococcal disease in healthy adults: increase of empyema associated with the clonal-type Sweden(1)-ST306. PLoS One 7:e42595 10.1371/journal.pone.0042595. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehmann D, Willis J, Moore HC, Giele C, Murphy D, Keil AD, Harrison C, Bayley K, Watson M, Richmond P. 2010. The changing epidemiology of invasive pneumococcal disease in aboriginal and non-aboriginal western Australians from 1997 through 2007 and emergence of nonvaccine serotypes. Clin Infect Dis 50:1477–1486 10.1086/652440. [PubMed] [DOI] [PubMed] [Google Scholar]
- 23.Fedson DS, Musher DM, Eskola J. 1998. Pneumococcal vaccine. In Plotkin SA, Ordenstein WA (ed), Vaccines, 3rd ed. WB Saunders, Philadelphia, PA. [Google Scholar]
- 24.Huang SS, Johnson KM, Ray GT, Wroe P, Lieu TA, Moore MR, Zell ER, Linder JA, Grijalva CG, Metlay JP, Finkelstein JA. 2011. Healthcare utilization and cost of pneumococcal disease in the United States. Vaccine 29:3398–3412 10.1016/j.vaccine.2011.02.088. [PubMed] [DOI] [PubMed] [Google Scholar]
- 25.Kramer MR, Rudensky B, Hadas-Halperin I, Isacsohn M, Melzer E. 1987. Pneumococcal bacteremia: no change in mortality in 30 years: analysis of 104 cases and review of the literature. Isr J Med Sci 23:174–180. [PubMed] [PubMed] [Google Scholar]
- 26.Brugger SD, Troxler LJ, Rüfenacht S, Frey PM, Morand B, Geyer R, Mühlemann K, Höck S, Thormann W, Furrer J, Christen S, Hilty M. 2016. Polysaccharide capsule composition of pneumococcal serotype 19A subtypes is unaltered among subtypes and independent of the nutritional environment. Infect Immun 84:3152–3160 10.1128/IAI.00474-16. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fiore AE, Levine OS, Elliott JA, Facklam RR, Butler JC. 1999. Effectiveness of pneumococcal polysaccharide vaccine for preschool-age children with chronic disease. Emerg Infect Dis 5:828–831 10.3201/eid0506.990616. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crook DW, Brueggemann AB, Sleeman KL, Peto TEA. 2004. Pneumococcal carriage, p 136–147. In Tuomanen EI, Mitchell TJ, Morrison DA, Spratt BG (ed), The Pneumococcus. ASM Press, Washington D.C. [Google Scholar]
- 29.Rodenburg GD, de Greeff SC, Jansen AG, de Melker HE, Schouls LM, Hak E, Spanjaard L, Sanders EA, van der Ende A. 2010. Effects of pneumococcal conjugate vaccine 2 years after its introduction, the Netherlands. Emerg Infect Dis 16:816–823 10.3201/eid1605.091223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feikin DR, Kagucia EW, Loo JD, Link-Gelles R, Puhan MA, Cherian T, Levine OS, Whitney CG, O’Brien KL, Moore MR, Serotype Replacement Study Group. 2013. Serotype-specific changes in invasive pneumococcal disease after pneumococcal conjugate vaccine introduction: a pooled analysis of multiple surveillance sites. PLoS Med 10:e1001517 10.1371/journal.pmed.1001517. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flasche S, Van Hoek AJ, Sheasby E, Waight P, Andrews N, Sheppard C, George R, Miller E. 2011. Effect of pneumococcal conjugate vaccination on serotype-specific carriage and invasive disease in England: a cross-sectional study. PLoS Med 8:e1001017 10.1371/journal.pmed.1001017. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiser JN, Austrian R, Sreenivasan PK, Masure HR. 1994. Phase variation in pneumococcal opacity: relationship between colonial morphology and nasopharyngeal colonization. Infect Immun 62:2582–2589. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JO, Weiser JN. 1998. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae. J Infect Dis 177:368–377 10.1086/514205. [PubMed] [DOI] [PubMed] [Google Scholar]
- 34.Kim JO, Romero-Steiner S, Sørensen UB, Blom J, Carvalho M, Barnard S, Carlone G, Weiser JN. 1999. Relationship between cell surface carbohydrates and intrastrain variation on opsonophagocytosis of Streptococcus pneumoniae. Infect Immun 67:2327–2333. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenow C, Ryan P, Weiser JN, Johnson S, Fontan P, Ortqvist A, Masure HR. 1997. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol Microbiol 25:819–829 10.1111/j.1365-2958.1997.mmi494.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 36.Fernández-Tornero C, López R, García E, Giménez-Gallego G, Romero A. 2001. A novel solenoid fold in the cell wall anchoring domain of the pneumococcal virulence factor LytA. Nat Struct Biol 8:1020–1024 10.1038/nsb724. [PubMed] [DOI] [PubMed] [Google Scholar]
- 37.Vollmer W, Tomasz A. 2001. Identification of the teichoic acid phosphorylcholine esterase in Streptococcus pneumoniae. Mol Microbiol 39:1610–1622 10.1046/j.1365-2958.2001.02349.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 38.Orihuela CJ, Gao G, Francis KP, Yu J, Tuomanen EI. 2004. Tissue-specific contributions of pneumococcal virulence factors to pathogenesis. J Infect Dis 190:1661–1669 10.1086/424596. [PubMed] [DOI] [PubMed] [Google Scholar]
- 39.García P, González MP, García E, López R, García JL. 1999. LytB, a novel pneumococcal murein hydrolase essential for cell separation. Mol Microbiol 31:1275–1281 10.1046/j.1365-2958.1999.01238.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 40.García P, Paz González M, García E, García JL, López R. 1999. The molecular characterization of the first autolytic lysozyme of Streptococcus pneumoniae reveals evolutionary mobile domains. Mol Microbiol 33:128–138 10.1046/j.1365-2958.1999.01455.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 41.Gosink KK, Mann ER, Guglielmo C, Tuomanen EI, Masure HR. 2000. Role of novel choline binding proteins in virulence of Streptococcus pneumoniae. Infect Immun 68:5690–5695 10.1128/IAI.68.10.5690-5695.2000. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weiser JN, Bae D, Fasching C, Scamurra RW, Ratner AJ, Janoff EN. 2003. Antibody-enhanced pneumococcal adherence requires IgA1 protease. Proc Natl Acad Sci U S A 100:4215–4220 10.1073/pnas.0637469100. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kilian M, Mestecky J, Russell MW. 1988. Defense mechanisms involving Fc-dependent functions of immunoglobulin A and their subversion by bacterial immunoglobulin A proteases. Microbiol Rev 52:296–303. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sebert ME, Palmer LM, Rosenberg M, Weiser JN. 2002. Microarray-based identification of htrA, a Streptococcus pneumoniae gene that is regulated by the CiaRH two-component system and contributes to nasopharyngeal colonization. Infect Immun 70:4059–4067 10.1128/IAI.70.8.4059-4067.2002. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ibrahim YM, Kerr AR, McCluskey J, Mitchell TJ. 2004. Role of HtrA in the virulence and competence of Streptococcus pneumoniae. Infect Immun 72:3584–3591 10.1128/IAI.72.6.3584-3591.2004. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Orihuela CJ, Radin JN, Sublett JE, Gao G, Kaushal D, Tuomanen EI. 2004. Microarray analysis of pneumococcal gene expression during invasive disease. Infect Immun 72:5582–5596 10.1128/IAI.72.10.5582-5596.2004. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barocchi MA, Ries J, Zogaj X, Hemsley C, Albiger B, Kanth A, Dahlberg S, Fernebro J, Moschioni M, Masignani V, Hultenby K, Taddei AR, Beiter K, Wartha F, von Euler A, Covacci A, Holden DW, Normark S, Rappuoli R, Henriques-Normark B. 2006. A pneumococcal pilus influences virulence and host inflammatory responses. Proc Natl Acad Sci U S A 103:2857–2862 10.1073/pnas.0511017103. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.LeMieux J, Hava DL, Basset A, Camilli A. 2006. RrgA and RrgB are components of a multisubunit pilus encoded by the Streptococcus pneumoniae rlrA pathogenicity islet. Infect Immun 74:2453–2456 10.1128/IAI.74.4.2453-2456.2006. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sjöström K, Blomberg C, Fernebro J, Dagerhamn J, Morfeldt E, Barocchi MA, Browall S, Moschioni M, Andersson M, Henriques F, Albiger B, Rappuoli R, Normark S, Henriques-Normark B. 2007. Clonal success of piliated penicillin nonsusceptible pneumococci. Proc Natl Acad Sci U S A 104:12907–12912 10.1073/pnas.0705589104. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moschioni M, Donati C, Muzzi A, Masignani V, Censini S, Hanage WP, Bishop CJ, Reis JN, Normark S, Henriques-Normark B, Covacci A, Rappuoli R, Barocchi MA. 2008. Streptococcus pneumoniae contains 3 rlrA pilus variants that are clonally related. J Infect Dis 197:888–896 10.1086/528375. [PubMed] [DOI] [PubMed] [Google Scholar]
- 51.Pancotto L, De Angelis G, Bizzarri E, Barocchi MA, Del Giudice G, Moschioni M, Ruggiero P. 2013. Expression of the Streptococcus pneumoniae pilus-1 undergoes on and off switching during colonization in mice. Sci Rep 3:2040 10.1038/srep02040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park IH, Kim KH, Andrade AL, Briles DE, McDaniel LS, Nahm MH. 2012. Nontypeable pneumococci can be divided into multiple cps types, including one type expressing the novel gene pspK. MBio 3:3 10.1128/mBio.00035-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keller LE, Jones CV, Thornton JA, Sanders ME, Swiatlo E, Nahm MH, Park IH, McDaniel LS. 2013. PspK of Streptococcus pneumoniae increases adherence to epithelial cells and enhances nasopharyngeal colonization. Infect Immun 81:173–181 10.1128/IAI.00755-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hermans PW, Adrian PV, Albert C, Estevão S, Hoogenboezem T, Luijendijk IH, Kamphausen T, Hammerschmidt S. 2006. The streptococcal lipoprotein rotamase A (SlrA) is a functional peptidyl-prolyl isomerase involved in pneumococcal colonization. J Biol Chem 281:968–976 10.1074/jbc.M510014200. [DOI] [PubMed] [Google Scholar]
- 55.Cron LE, Bootsma HJ, Noske N, Burghout P, Hammerschmidt S, Hermans PW. 2009. Surface-associated lipoprotein PpmA of Streptococcus pneumoniae is involved in colonization in a strain-specific manner. Microbiology 155:2401–2410 10.1099/mic.0.026765-0. [DOI] [PubMed] [Google Scholar]
- 56.Marks LR, Reddinger RM, Hakansson AP. 2012. High levels of genetic recombination during nasopharyngeal carriage and biofilm formation in Streptococcus pneumoniae. MBio 3:e00200-12 10.1128/mBio.00200-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chao Y, Marks LR, Pettigrew MM, Hakansson AP. 2015. Streptococcus pneumoniae biofilm formation and dispersion during colonization and disease. Front Cell Infect Microbiol 4:194 10.3389/fcimb.2014.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marks LR, Davidson BA, Knight PR, Hakansson AP. 2013. Interkingdom signaling induces Streptococcus pneumoniae biofilm dispersion and transition from asymptomatic colonization to disease. MBio 4:e00438-13 10.1128/mBio.00438-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pettigrew MM, Marks LR, Kong Y, Gent JF, Roche-Hakansson H, Hakansson AP. 2014. Dynamic changes in the Streptococcus pneumoniae transcriptome during transition from biofilm formation to invasive disease upon influenza A virus infection. Infect Immun 82:4607–4619 10.1128/IAI.02225-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Teele DW, Klein JO, Rosner B. 1989. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J Infect Dis 160:83–94 10.1093/infdis/160.1.83. [DOI] [PubMed] [Google Scholar]
- 61.Block SL. 1997. Causative pathogens, antibiotic resistance and therapeutic considerations in acute otitis media. Pediatr Infect Dis J 16:449–456 10.1097/00006454-199704000-00029. [DOI] [PubMed] [Google Scholar]
- 62.Sierra A, Lopez P, Zapata MA, Vanegas B, Castrejon MM, Deantonio R, Hausdorff WP, Colindres RE. 2011. Non-typeable Haemophilus influenzae and Streptococcus pneumoniae as primary causes of acute otitis media in Colombian children: a prospective study. BMC Infect Dis 11:4 10.1186/1471-2334-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosenblut A, Napolitano C, Pereira A, Moreno C, Kolhe D, Lepetic A, Ortega-Barria E. 2017. Etiology of acute otitis media and serotype distribution of Streptococcus pneumoniae and Haemophilus influenzae in Chilean children <5 years of age. Medicine (Baltimore) 96:e5974 10.1097/MD.0000000000005974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pettigrew MM, Gent JF, Pyles RB, Miller AL, Nokso-Koivisto J, Chonmaitree T. 2011. Viral-bacterial interactions and risk of acute otitis media complicating upper respiratory tract infection. J Clin Microbiol 49:3750–3755 10.1128/JCM.01186-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leibovitz E. 2003. Acute otitis media in pediatric medicine: current issues in epidemiology, diagnosis, and management. Paediatr Drugs 5(Suppl 1):1–12. [PubMed] [Google Scholar]
- 66.Stahl WL, O’Toole RD. 1972. Pneumococcal neuraminidase: purification and properties. Biochim Biophys Acta 268:480–487 10.1016/0005-2744(72)90343-9. [DOI] [PubMed] [Google Scholar]
- 67.Berry AM, Lock RA, Paton JC. 1996. Cloning and characterization of nanB, a second Streptococcus pneumoniae neuraminidase gene, and purification of the NanB enzyme from recombinant Escherichia coli. J Bacteriol 178:4854–4860 10.1128/jb.178.16.4854-4860.1996. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tong HH, Blue LE, James MA, DeMaria TF. 2000. Evaluation of the virulence of a Streptococcus pneumoniae neuraminidase-deficient mutant in nasopharyngeal colonization and development of otitis media in the chinchilla model. Infect Immun 68:921–924 10.1128/IAI.68.2.921-924.2000. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tong HH, Grants I, Liu X, DeMaria TF. 2002. Comparison of alteration of cell surface carbohydrates of the chinchilla tubotympanum and colonial opacity phenotype of Streptococcus pneumoniae during experimental pneumococcal otitis media with or without an antecedent influenza A virus infection. Infect Immun 70:4292–4301 10.1128/IAI.70.8.4292-4301.2002. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tuomanen EI. 2000. Pathogenesis of pneumococcal inflammation: otitis media. Vaccine 19(Suppl 1):S38–S40 10.1016/S0264-410X(00)00276-0. [DOI] [PubMed] [Google Scholar]
- 71.Winter AJ, Comis SD, Osborne MP, Tarlow MJ, Stephen J, Andrew PW, Hill J, Mitchell TJ. 1997. A role for pneumolysin but not neuraminidase in the hearing loss and cochlear damage induced by experimental pneumococcal meningitis in guinea pigs. Infect Immun 65:4411–4418. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Comis SD, Osborne MP, Stephen J, Tarlow MJ, Hayward TL, Mitchell TJ, Andrew PW, Boulnois GJ. 1993. Cytotoxic effects on hair cells of guinea pig cochlea produced by pneumolysin, the thiol activated toxin of Streptococcus pneumoniae. Acta Otolaryngol 113:152–159 10.3109/00016489309135784. [PubMed] [DOI] [PubMed] [Google Scholar]
- 73.Zhang JR, Mostov KE, Lamm ME, Nanno M, Shimida S, Ohwaki M, Tuomanen E. 2000. The polymeric immunoglobulin receptor translocates pneumococci across human nasopharyngeal epithelial cells. Cell 102:827–837 10.1016/S0092-8674(00)00071-4. [DOI] [PubMed] [Google Scholar]
- 74.Kaetzel CS. 2001. Polymeric Ig receptor: defender of the fort or Trojan horse? Curr Biol 11:R35–R38 10.1016/S0960-9822(00)00041-5. [DOI] [PubMed] [Google Scholar]
- 75.Lu L, Lamm ME, Li H, Corthesy B, Zhang JR. 2003. The human polymeric immunoglobulin receptor binds to Streptococcus pneumoniae via domains 3 and 4. J Biol Chem 278:48178–48187 10.1074/jbc.M306906200. [PubMed] [DOI] [PubMed] [Google Scholar]
- 76.Luton F, Vergés M, Vaerman JP, Sudol M, Mostov KE. 1999. The SRC family protein tyrosine kinase p62yes controls polymeric IgA transcytosis in vivo. Mol Cell 4:627–632 10.1016/S1097-2765(00)80213-0. [DOI] [PubMed] [Google Scholar]
- 77.Tong HH, McIver MA, Fisher LM, DeMaria TF. 1999. Effect of lacto-N-neotetraose, asialoganglioside-GM1 and neuraminidase on adherence of otitis media-associated serotypes of Streptococcus pneumoniae to chinchilla tracheal epithelium. Microb Pathog 26:111–119 10.1006/mpat.1998.0257. [PubMed] [DOI] [PubMed] [Google Scholar]
- 78.Kietzman CC, Gao G, Mann B, Myers L, Tuomanen EI. 2016. Dynamic capsule restructuring by the main pneumococcal autolysin LytA in response to the epithelium. Nat Commun 7:10859 10.1038/ncomms10859. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Krivan HC, Roberts DD, Ginsburg V. 1988. Many pulmonary pathogenic bacteria bind specifically to the carbohydrate sequence GalNAc beta 1-4Gal found in some glycolipids. Proc Natl Acad Sci U S A 85:6157–6161 10.1073/pnas.85.16.6157. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Howie AJ, Brown G. 1985. Effect of neuraminidase on the expression of the 3-fucosyl-N-acetyllactosamine antigen in human tissues. J Clin Pathol 38:409–416 10.1136/jcp.38.4.409. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kahya HF, Andrew PW, Yesilkaya H. 2017. Deacetylation of sialic acid by esterases potentiates pneumococcal neuraminidase activity for mucin utilization, colonization and virulence. PLoS Pathog 13:e1006263 10.1371/journal.ppat.1006263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peltola VT, McCullers JA. 2004. Respiratory viruses predisposing to bacterial infections: role of neuraminidase. Pediatr Infect Dis J 23(Suppl):S87–S97 10.1097/01.inf.0000108197.81270.35. [PubMed] [DOI] [PubMed] [Google Scholar]
- 83.McCullers JA, Bartmess KC. 2003. Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae. J Infect Dis 187:1000–1009 10.1086/368163. [PubMed] [DOI] [PubMed] [Google Scholar]
- 84.Peltola VT, Murti KG, McCullers JA. 2005. Influenza virus neuraminidase contributes to secondary bacterial pneumonia. J Infect Dis 192:249–257 10.1086/430954. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nakamura S, Davis KM, Weiser JN. 2011. Synergistic stimulation of type I interferons during influenza virus coinfection promotes Streptococcus pneumoniae colonization in mice. J Clin Invest 121:3657–3665 10.1172/JCI57762. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McCullers JA. 2004. Effect of antiviral treatment on the outcome of secondary bacterial pneumonia after influenza. J Infect Dis 190:519–526 10.1086/421525. [PubMed] [DOI] [PubMed] [Google Scholar]
- 87.Agarwal V, Kuchipudi A, Fulde M, Riesbeck K, Bergmann S, Blom AM. 2013. Streptococcus pneumoniae endopeptidase O (PepO) is a multifunctional plasminogen- and fibronectin-binding protein, facilitating evasion of innate immunity and invasion of host cells. J Biol Chem 288:6849–6863 10.1074/jbc.M112.405530. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Löfling J, Vimberg V, Battig P, Henriques-Normark B. 2011. Cellular interactions by LPxTG-anchored pneumococcal adhesins and their streptococcal homologues. Cell Microbiol 13:186–197 10.1111/j.1462-5822.2010.01560.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 89.Jensch I, Gámez G, Rothe M, Ebert S, Fulde M, Somplatzki D, Bergmann S, Petruschka L, Rohde M, Nau R, Hammerschmidt S. 2010. PavB is a surface-exposed adhesin of Streptococcus pneumoniae contributing to nasopharyngeal colonization and airways infections. Mol Microbiol 77:22–43 10.1111/j.1365-2958.2010.07189.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 90.Pracht D, Elm C, Gerber J, Bergmann S, Rohde M, Seiler M, Kim KS, Jenkinson HF, Nau R, Hammerschmidt S. 2005. PavA of Streptococcus pneumoniae modulates adherence, invasion, and meningeal inflammation. Infect Immun 73:2680–2689 10.1128/IAI.73.5.2680-2689.2005. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tuomanen E. 2004. Attachment and invasion of the respiratory tract, p 221–237. In Tuomanen E, Mitchell T, Morrison DA, Spratt BG (ed), The Pneumococcus. ASM Press, Washington, DC. [Google Scholar]
- 92.Berry AM, Paton JC. 2000. Additive attenuation of virulence of Streptococcus pneumoniae by mutation of the genes encoding pneumolysin and other putative pneumococcal virulence proteins. Infect Immun 68:133–140 10.1128/IAI.68.1.133-140.2000. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Feldman C, Anderson R, Cockeran R, Mitchell T, Cole P, Wilson R. 2002. The effects of pneumolysin and hydrogen peroxide, alone and in combination, on human ciliated epithelium in vitro. Respir Med 96:580–585 10.1053/rmed.2002.1316. [PubMed] [DOI] [PubMed] [Google Scholar]
- 94.Canvin JR, Marvin AP, Sivakumaran M, Paton JC, Boulnois GJ, Andrew PW, Mitchell TJ. 1995. The role of pneumolysin and autolysin in the pathology of pneumonia and septicemia in mice infected with a type 2 pneumococcus. J Infect Dis 172:119–123 10.1093/infdis/172.1.119. [PubMed] [DOI] [PubMed] [Google Scholar]
- 95.Tuomanen E, Rich R, Zak O. 1987. Induction of pulmonary inflammation by components of the pneumococcal cell surface. Am Rev Respir Dis 135:869–874 10.1164/arrd.1987.135.4.869. [PubMed] [DOI] [PubMed] [Google Scholar]
- 96.Rubins JB, Charboneau D, Paton JC, Mitchell TJ, Andrew PW, Janoff EN. 1995. Dual function of pneumolysin in the early pathogenesis of murine pneumococcal pneumonia. J Clin Invest 95:142–150 10.1172/JCI117631. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Spellerberg B, Cundell DR, Sandros J, Pearce BJ, Idanpaan-Heikkila I, Rosenow C, Masure HR. 1996. Pyruvate oxidase, as a determinant of virulence in Streptococcus pneumoniae. Mol Microbiol 19:803–813 10.1046/j.1365-2958.1996.425954.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 98.Berry AM, Paton JC, Hansman D. 1992. Effect of insertional inactivation of the genes encoding pneumolysin and autolysin on the virulence of Streptococcus pneumoniae type 3. Microb Pathog 12:87–93 10.1016/0882-4010(92)90111-Z. [DOI] [PubMed] [Google Scholar]
- 99.Cundell DR, Gerard NP, Gerard C, Idanpaan-Heikkila I, Tuomanen EI. 1995. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature 377:435–438 10.1038/377435a0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 100.Radin JN, Orihuela CJ, Murti G, Guglielmo C, Murray PJ, Tuomanen E. 2005. B-arrestin 1 participates in platelet-activating factor receptor-mediated endocytosis of Streptococcus pneumoniae. Infect Immu 73:7827–7835. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Weiser JN, Goldberg JB, Pan N, Wilson L, Virji M. 1998. The phosphorylcholine epitope undergoes phase variation on a 43-kilodalton protein in Pseudomonas aeruginosa and on pili of Neisseria meningitidis and Neisseria gonorrhoeae. Infect Immun 66:4263–4267. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Weiser JN, Shchepetov M, Chong ST. 1997. Decoration of lipopolysaccharide with phosphorylcholine: a phase-variable characteristic of Haemophilus influenzae. Infect Immun 65:943–950. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Loughran AJ, Tuomanen EI. 2016. Blood borne: bacterial components in mother’s blood influence fetal development. Inflamm Cell Signal 3:e1421. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Humann J, Mann B, Gao G, Moresco P, Ramahi J, Loh LN, Farr A, Hu Y, Durick-Eder K, Fillon SA, Smeyne RJ, Tuomanen EI. 2016. Bacterial peptidoglycan traverses the placenta to induce fetal neuroproliferation and aberrant postnatal behavior. Cell Host Microbe 19:388–399 10.1016/j.chom.2016.02.009. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gould JM, Weiser JN. 2001. Expression of C-reactive protein in the human respiratory tract. Infect Immun 69:1747–1754 10.1128/IAI.69.3.1747-1754.2001. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Seachrist JL, Ferguson SS. 2003. Regulation of G protein-coupled receptor endocytosis and trafficking by Rab GTPases. Life Sci 74:225–235 10.1016/j.lfs.2003.09.009. [PubMed] [DOI] [PubMed] [Google Scholar]
- 107.Orrskog S, Rounioja S, Spadafina T, Gallotta M, Norman M, Hentrich K, Fälker S, Ygberg-Eriksson S, Hasenberg M, Johansson B, Uotila LM, Gahmberg CG, Barocchi M, Gunzer M, Normark S, Henriques-Normark B. 2012. Pilus adhesin RrgA interacts with complement receptor 3, thereby affecting macrophage function and systemic pneumococcal disease. MBio 4:e00535-12 10.1128/mBio.00535-12. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yao H, Zhang H, Lan K, Wang H, Su Y, Li D, Song Z, Cui F, Yin Y, Zhang X. 2017. Purified Streptococcus pneumoniae endopeptidase O (PepO) enhances particle uptake by macrophages in a Toll-like receptor 2- and miR-155-dependent manner. Infect Immun 85:e01012-16 10.1128/IAI.01012-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Loh LN, Gao G, Tuomanen EI. 2017. Dissecting bacterial cell wall entry and signaling in eukaryotic cells: an actin-dependent pathway parallels platelet-activating factor receptor-mediated endocytosis. MBio 8:e02030-16 10.1128/mBio.02030-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Abeyta M, Hardy GG, Yother J. 2003. Genetic alteration of capsule type but not PspA type affects accessibility of surface-bound complement and surface antigens of Streptococcus pneumoniae. Infect Immun 71:218–225 10.1128/IAI.71.1.218-225.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Magee AD, Yother J. 2001. Requirement for capsule in colonization by Streptococcus pneumoniae. Infect Immun 69:3755–3761 10.1128/IAI.69.6.3755-3761.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kelly T, Dillard JP, Yother J. 1994. Effect of genetic switching of capsular type on virulence of Streptococcus pneumoniae. Infect Immun 62:1813–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Briles DE, Crain MJ, Gray BM, Forman C, Yother J. 1992. Strong association between capsular type and virulence for mice among human isolates of Streptococcus pneumoniae. Infect Immun 60:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fine DP. 1975. Pneumococcal type-associated variability in alternate complement pathway activation. Infect Immun 12:772–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hostetter MK. 1986. Serotypic variations among virulent pneumococci in deposition and degradation of covalently bound C3b: implications for phagocytosis and antibody production. J Infect Dis 153:682–693 10.1093/infdis/153.4.682. [DOI] [PubMed] [Google Scholar]
- 116.Centers for Disease Control and Prevention. 1997. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 46:1–25. [Google Scholar]
- 117.Ren B, Szalai AJ, Hollingshead SK, Briles DE. 2004. Effects of PspA and antibodies to PspA on activation and deposition of complement on the pneumococcal surface. Infect Immun 72:114–122 10.1128/IAI.72.1.114-122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ren B, Szalai AJ, Thomas O, Hollingshead SK, Briles DE. 2003. Both family 1 and family 2 PspA proteins can inhibit complement deposition and confer virulence to a capsular serotype 3 strain of Streptococcus pneumoniae. Infect Immun 71:75–85 10.1128/IAI.71.1.75-85.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tu AH, Fulgham RL, McCrory MA, Briles DE, Szalai AJ. 1999. Pneumococcal surface protein A inhibits complement activation by Streptococcus pneumoniae. Infect Immun 67:4720–4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shaper M, Hollingshead SK, Benjamin WH Jr, Briles DE. 2004. PspA protects Streptococcus pneumoniae from killing by apolactoferrin, and antibody to PspA enhances killing of pneumococci by apolactoferrin [corrected]. Infect Immun 72:5031–5040 10.1128/IAI.72.9.5031-5040.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Duthy TG, Ormsby RJ, Giannakis E, Ogunniyi AD, Stroeher UH, Paton JC, Gordon DL. 2002. The human complement regulator factor H binds pneumococcal surface protein PspC via short consensus repeats 13 to 15. Infect Immun 70:5604–5611 10.1128/IAI.70.10.5604-5611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dave S, Pangburn MK, Pruitt C, McDaniel LS. 2004. Interaction of human factor H with PspC of Streptococcus pneumoniae. Indian J Med Res 119(Suppl):66–73. [PubMed] [Google Scholar]
- 123.Mohan S, Hertweck C, Dudda A, Hammerschmidt S, Skerka C, Hallström T, Zipfel PF. 2014. Tuf of Streptococcus pneumoniae is a surface displayed human complement regulator binding protein. Mol Immunol 62:249–264 10.1016/j.molimm.2014.06.029. [DOI] [PubMed] [Google Scholar]
- 124.Dalia AB, Standish AJ, Weiser JN. 2010. Three surface exoglycosidases from Streptococcus pneumoniae, NanA, BgaA, and StrH, promote resistance to opsonophagocytic killing by human neutrophils. Infect Immun 78:2108–2116 10.1128/IAI.01125-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ramirez JA, Wiemken TL, Peyrani P, Arnold FW, Kelley R, Mattingly WA, Nakamatsu R, Pena S, Guinn BE, Furmanek SP, Persaud AK, Raghuram A, Fernandez F, Beavin L, Bosson R, Fernandez-Botran R, Cavallazzi R, Bordon J, Valdivieso C, Schulte J, Carrico RM, University of Louisville Pneumonia Study Group. 2017. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis 65:1806–1812 10.1093/cid/cix647. [DOI] [PubMed] [Google Scholar]
- 126.Brown AO, Mann B, Gao G, Hankins JS, Humann J, Giardina J, Faverio P, Restrepo MI, Halade GV, Mortensen EM, Lindsey ML, Hanes M, Happel KI, Nelson S, Bagby GJ, Lorent JA, Cardinal P, Granados R, Esteban A, LeSaux CJ, Tuomanen EI, Orihuela CJ. 2014. Streptococcus pneumoniae translocates into the myocardium and forms unique microlesions that disrupt cardiac function. PLoS Pathog 10:e1004383 10.1371/journal.ppat.1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Musher DM, Rueda AM, Kaka AS, Mapara SM. 2007. The association between pneumococcal pneumonia and acute cardiac events. Clin Infect Dis 45:158–165 10.1086/518849. [DOI] [PubMed] [Google Scholar]
- 128.Reyes LF, Restrepo MI, Hinojosa CA, Soni NJ, Anzueto A, Babu BL, Gonzalez-Juarbe N, Rodriguez AH, Jimenez A, Chalmers JD, Aliberti S, Sibila O, Winter VT, Coalson JJ, Giavedoni LD, Dela Cruz CS, Waterer GW, Witzenrath M, Suttorp N, Dube PH, Orihuela CJ. 2017. Severe pneumococcal pneumonia causes acute cardiac toxicity and subsequent cardiac remodeling. Am J Respir Crit Care Med 196:609–620 10.1164/rccm.201701-0104OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fillon S, Soulis K, Rajasekaran S, Benedict-Hamilton H, Radin JN, Orihuela CJ, El Kasmi KC, Murti G, Kaushal D, Gaber MW, Weber JR, Murray PJ, Tuomanen EI. 2006. Platelet-activating factor receptor and innate immunity: uptake of Gram-positive bacterial cell wall into host cells and cell-specific pathophysiology. J Immunol 177:6182–6191 10.4049/jimmunol.177.9.6182. [DOI] [PubMed] [Google Scholar]
- 130.Alhamdi Y, Neill DR, Abrams ST, Malak HA, Yahya R, Barrett-Jolley R, Wang G, Kadioglu A, Toh CH. 2015. Circulating pneumolysin is a potent inducer of cardiac injury during pneumococcal infection. PLoS Pathog 11:e1004836 10.1371/journal.ppat.1004836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brissac T, Shenoy AT, Patterson LA, Orihuela CJ, Pirofski L. 2017. Cell invasion and pyruvate oxidase derived H2O2 are critical for Streptococcus pneumoniae mediated cardiomyocyte killing. Infect Immun 86:IAI.00569-17 10.1128/IAI.00569-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Durand ML, Calderwood SB, Weber DJ, Miller SI, Southwick FS, Caviness VS Jr, Swartz MN. 1993. Acute bacterial meningitis in adults. A review of 493 episodes. N Engl J Med 328:21–28 10.1056/NEJM199301073280104. [DOI] [PubMed] [Google Scholar]
- 133.Thigpen MC, Whitney CG, Messonnier NE, Zell ER, Lynfield R, Hadler JL, Harrison LH, Farley MM, Reingold A, Bennett NM, Craig AS, Schaffner W, Thomas A, Lewis MM, Scallan E, Schuchat A, Emerging Infections Programs Network. 2011. Bacterial meningitis in the United States, 1998-2007. N Engl J Med 364:2016–2025 10.1056/NEJMoa1005384. [DOI] [PubMed] [Google Scholar]
- 134.Hameed N, Tunkel AR. 2010. Treatment of drug-resistant pneumococcal meningitis. Curr Infect Dis Rep 12:274–281 10.1007/s11908-010-0110-7. [DOI] [PubMed] [Google Scholar]
- 135.de Gans J, van de Beek D, European Dexamethasone in Adulthood Bacterial Meningitis Study Investigators. 2002. Dexamethasone in adults with bacterial meningitis. N Engl J Med 347:1549–1556 10.1056/NEJMoa021334. [DOI] [PubMed] [Google Scholar]
- 136.Hoffmann O, Mahrhofer C, Rueter N, Freyer D, Bert B, Fink H, Weber JR. 2007. Pneumococcal cell wall-induced meningitis impairs adult hippocampal neurogenesis. Infect Immun 75:4289–4297 10.1128/IAI.01679-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gerber J, Pohl K, Sander V, Bunkowski S, Nau R. 2003. Rifampin followed by ceftriaxone for experimental meningitis decreases lipoteichoic acid concentrations in cerebrospinal fluid and reduces neuronal damage in comparison to ceftriaxone alone. Antimicrob Agents Chemother 47:1313–1317 10.1128/AAC.47.4.1313-1317.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Free SL, Li LM, Fish DR, Shorvon SD, Stevens JM. 1996. Bilateral hippocampal volume loss in patients with a history of encephalitis or meningitis. Epilepsia 37:400–405 10.1111/j.1528-1157.1996.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 139.Braun JS, Novak R, Herzog KH, Bodner SM, Cleveland JL, Tuomanen EI. 1999. Neuroprotection by a caspase inhibitor in acute bacterial meningitis. Nat Med 5:298–302 10.1038/6514. [DOI] [PubMed] [Google Scholar]
- 140.Hanisch UK, Prinz M, Angstwurm K, Häusler KG, Kann O, Kettenmann H, Weber JR. 2001. The protein tyrosine kinase inhibitor AG126 prevents the massive microglial cytokine induction by pneumococcal cell walls. Eur J Immunol 31:2104–2115 . [DOI] [PubMed] [Google Scholar]
- 141.Freyer D, Manz R, Ziegenhorn A, Weih M, Angstwurm K, Döcke WD, Meisel A, Schumann RR, Schönfelder G, Dirnagl U, Weber JR. 1999. Cerebral endothelial cells release TNF-alpha after stimulation with cell walls of Streptococcus pneumoniae and regulate inducible nitric oxide synthase and ICAM-1 expression via autocrine loops. J Immunol 163:4308–4314. [PubMed] [Google Scholar]
- 142.Meli DN, Christen S, Leib SL. 2003. Matrix metalloproteinase-9 in pneumococcal meningitis: activation via an oxidative pathway. J Infect Dis 187:1411–1415 10.1086/374644. [DOI] [PubMed] [Google Scholar]
- 143.Kastenbauer S, Koedel U, Pfister HW. 1999. Role of peroxynitrite as a mediator of pathophysiological alterations in experimental pneumococcal meningitis. J Infect Dis 180:1164–1170 10.1086/315048. [DOI] [PubMed] [Google Scholar]
- 144.Weber JR, Angstwurm K, Bürger W, Einhäupl KM, Dirnagl U. 1995. Anti ICAM-1 (CD 54) monoclonal antibody reduces inflammatory changes in experimental bacterial meningitis. J Neuroimmunol 63:63–68 10.1016/0165-5728(95)00131-X. [DOI] [PubMed] [Google Scholar]
- 145.Tuomanen EI, Saukkonen K, Sande S, Cioffe C, Wright SD. 1989. Reduction of inflammation, tissue damage, and mortality in bacterial meningitis in rabbits treated with monoclonal antibodies against adhesion-promoting receptors of leukocytes. J Exp Med 170:959–969 10.1084/jem.170.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Braun JS, Sublett JE, Freyer D, Mitchell TJ, Cleveland JL, Tuomanen EI, Weber JR. 2002. Pneumococcal pneumolysin and H(2)O(2) mediate brain cell apoptosis during meningitis. J Clin Invest 109:19–27 10.1172/JCI12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Orihuela CJ, Fillon S, Smith-Sielicki SH, El Kasmi KC, Gao G, Soulis K, Patil A, Murray PJ, Tuomanen EI. 2006. Cell wall-mediated neuronal damage in early sepsis. Infect Immun 74:3783–3789 10.1128/IAI.00022-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Orihuela CJ, Mahdavi J, Thornton J, Mann B, Wooldridge KG, Abouseada N, Oldfield NJ, Self T, Ala’Aldeen DA, Tuomanen EI. 2009. Laminin receptor initiates bacterial contact with the blood brain barrier in experimental meningitis models. J Clin Invest 119:1638–1646 10.1172/JCI36759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ring A, Weiser JN, Tuomanen EI. 1998. Pneumococcal trafficking across the blood-brain barrier. Molecular analysis of a novel bidirectional pathway. J Clin Invest 102:347–360 10.1172/JCI2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Banerjee A, Van Sorge NM, Sheen TR, Uchiyama S, Mitchell TJ, Doran KS. 2010. Activation of brain endothelium by pneumococcal neuraminidase NanA promotes bacterial internalization. Cell Microbiol 12:1576–1588 10.1111/j.1462-5822.2010.01490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Uchiyama S, Carlin AF, Khosravi A, Weiman S, Banerjee A, Quach D, Hightower G, Mitchell TJ, Doran KS, Nizet V. 2009. The surface-anchored NanA protein promotes pneumococcal brain endothelial cell invasion. J Exp Med 206:1845–1852 10.1084/jem.20090386. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tomasz A, Saukkonen K. 1989. The nature of cell wall-derived inflammatory components of pneumococci. Pediatr Infect Dis J 8:902–903 10.1097/00006454-198912000-00034. [PubMed] [DOI] [PubMed] [Google Scholar]
- 153.Tuomanen EI, Austrian R, Masure HR. 1995. Pathogenesis of pneumococcal infection. N Engl J Med 332:1280–1284 10.1056/NEJM199505113321907. [PubMed] [DOI] [PubMed] [Google Scholar]
- 154.Moreillon P, Majcherczyk PA. 2003. Proinflammatory activity of cell-wall constituents from Gram-positive bacteria. Scand J Infect Dis 35:632–641 10.1080/00365540310016259. [PubMed] [DOI] [PubMed] [Google Scholar]
- 155.Tuomanen E, Tomasz A, Hengstler B, Zak O. 1985. The relative role of bacterial cell wall and capsule in the induction of inflammation in pneumococcal meningitis. J Infect Dis 151:535–540 10.1093/infdis/151.3.535. [PubMed] [DOI] [PubMed] [Google Scholar]
- 156.Winkelstein JA, Tomasz A. 1978. Activation of the alternative complement pathway by pneumococcal cell wall teichoic acid. J Immunol 120:174–178. [PubMed] [PubMed] [Google Scholar]
- 157.Weber JR, Freyer D, Alexander C, Schröder NW, Reiss A, Küster C, Pfeil D, Tuomanen EI, Schumann RR. 2003. Recognition of pneumococcal peptidoglycan: an expanded, pivotal role for LPS binding protein. Immunity 19:269–279 10.1016/S1074-7613(03)00205-X. [DOI] [PubMed] [Google Scholar]
- 158.Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, Aderem A. 2000. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proc Natl Acad Sci U S A 97:13766–13771 10.1073/pnas.250476497. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Spellerberg B, Rosenow C, Sha W, Tuomanen EI. 1996. Pneumococcal cell wall activates NF-kappa B in human monocytes: aspects distinct from endotoxin. Microb Pathog 20:309–317 10.1006/mpat.1996.0029. [PubMed] [DOI] [PubMed] [Google Scholar]
- 160.Girardin SE, Boneca IG, Carneiro LA, Antignac A, Jéhanno M, Viala J, Tedin K, Taha MK, Labigne A, Zähringer U, Coyle AJ, DiStefano PS, Bertin J, Sansonetti PJ, Philpott DJ. 2003. Nod1 detects a unique muropeptide from Gram-negative bacterial peptidoglycan. Science 300:1584–1587 10.1126/science.1084677. [PubMed] [DOI] [PubMed] [Google Scholar]
- 161.Watanabe T, Kitani A, Murray PJ, Strober W. 2004. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat Immunol 5:800–808 10.1038/ni1092. [PubMed] [DOI] [PubMed] [Google Scholar]
- 162.Davis KM, Nakamura S, Weiser JN. 2011. Nod2 sensing of lysozyme-digested peptidoglycan promotes macrophage recruitment and clearance of S. pneumoniae colonization in mice. J Clin Invest 121:3666–3676 10.1172/JCI57761. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hostetter MK, Thomas ML, Rosen FS, Tack BF. 1982. Binding of C3b proceeds by a transesterification reaction at the thiolester site. Nature 298:72–75 10.1038/298072b0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 164.Hummell DS, Berninger RW, Tomasz A, Winkelstein JA. 1981. The fixation of C3b to pneumococcal cell wall polymers as a result of activation of the alternative complement pathway. J Immunol 127:1287–1289. [PubMed] [PubMed] [Google Scholar]
- 165.Weis WI, Drickamer K, Hendrickson WA. 1992. Structure of a C-type mannose-binding protein complexed with an oligosaccharide. Nature 360:127–134 10.1038/360127a0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 166.Roy S, Knox K, Segal S, Griffiths D, Moore CE, Welsh KI, Smarason A, Day NP, McPheat WL, Crook DW, Hill AV, Oxford Pneumoccocal Surveillance Group. 2002. MBL genotype and risk of invasive pneumococcal disease: a case-control study. Lancet 359:1569–1573 10.1016/S0140-6736(02)08516-1. [DOI] [PubMed] [Google Scholar]
- 167.Holzer TJ, Edwards KM, Gewurz H, Mold C. 1984. Binding of C-reactive protein to the pneumococcal capsule or cell wall results in differential localization of C3 and stimulation of phagocytosis. J Immunol 133:1424–1430. [PubMed] [PubMed] [Google Scholar]
- 168.Agarwal V, Sroka M, Fulde M, Bergmann S, Riesbeck K, Blom AM. 2014. Binding of Streptococcus pneumoniae endopeptidase O (PepO) to complement component C1q modulates the complement attack and promotes host cell adherence. J Biol Chem 289:15833–15844 10.1074/jbc.M113.530212. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zou J, Zhou L, Hu C, Jing P, Guo X, Liu S, Lei Y, Yang S, Deng J, Zhang H. 2017. IL-8 and IP-10 expression from human bronchial epithelial cells BEAS-2B are promoted by Streptococcus pneumoniae endopeptidase O (PepO). BMC Microbiol 17:187 10.1186/s12866-017-1081-8. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nau R, Eiffert H. 2002. Modulation of release of proinflammatory bacterial compounds by antibacterials: potential impact on course of inflammation and outcome in sepsis and meningitis. Clin Microbiol Rev 15:95–110 10.1128/CMR.15.1.95-110.2002. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mitchell TJ, Andrew PW, Saunders FK, Smith AN, Boulnois GJ. 1991. Complement activation and antibody binding by pneumolysin via a region of the toxin homologous to a human acute-phase protein. Mol Microbiol 5:1883–1888 10.1111/j.1365-2958.1991.tb00812.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 172.Alcantara RB, Preheim LC, Gentry-Nielsen MJ. 2001. Pneumolysin-induced complement depletion during experimental pneumococcal bacteremia. Infect Immun 69:3569–3575 10.1128/IAI.69.6.3569-3575.2001. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Alcantara RB, Preheim LC, Gentry MJ. 1999. Role of pneumolysin’s complement-activating activity during pneumococcal bacteremia in cirrhotic rats. Infect Immun 67:2862–2866. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tomasz A, Albino A, Zanati E. 1970. Multiple antibiotic resistance in a bacterium with suppressed autolytic system. Nature 227:138–140 10.1038/227138a0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 175.Jedrzejas MJ. 2001. Pneumococcal virulence factors: structure and function. Microbiol Mol Biol Rev 65:187–207 10.1128/MMBR.65.2.187-207.2001. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Blom AM, Bergmann S, Fulde M, Riesbeck K, Agarwal V. 2014. Streptococcus pneumoniae phosphoglycerate kinase is a novel complement inhibitor affecting the membrane attack complex formation. J Biol Chem 289:32499–32511 10.1074/jbc.M114.610212. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Stringaris AK, Geisenhainer J, Bergmann F, Balshüsemann C, Lee U, Zysk G, Mitchell TJ, Keller BU, Kuhnt U, Gerber J, Spreer A, Bähr M, Michel U, Nau R. 2002. Neurotoxicity of pneumolysin, a major pneumococcal virulence factor, involves calcium influx and depends on activation of p38 mitogen-activated protein kinase. Neurobiol Dis 11:355–368 10.1006/nbdi.2002.0561. [PubMed] [DOI] [PubMed] [Google Scholar]
- 178.Berry AM, Lock RA, Hansman D, Paton JC. 1989. Contribution of autolysin to virulence of Streptococcus pneumoniae. Infect Immun 57:2324–2330. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Balachandran P, Hollingshead SK, Paton JC, Briles DE. 2001. The autolytic enzyme LytA of Streptococcus pneumoniae is not responsible for releasing pneumolysin. J Bacteriol 183:3108–3116 10.1128/JB.183.10.3108-3116.2001. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Price KE, Greene NG, Camilli A. 2012. Export requirements of pneumolysin in Streptococcus pneumoniae. J Bacteriol 194:3651–3660 10.1128/JB.00114-12. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.González-Juarbe N, Bradley KM, Shenoy AT, Gilley RP, Reyes LF, Hinojosa CA, Restrepo MI, Dube PH, Bergman MA, Orihuela CJ. 2017. Pore-forming toxin-mediated ion dysregulation leads to death receptor-independent necroptosis of lung epithelial cells during bacterial pneumonia. Cell Death Differ 24:917–928 10.1038/cdd.2017.49. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.González-Juarbe N, Gilley RP, Hinojosa CA, Bradley KM, Kamei A, Gao G, Dube PH, Bergman MA, Orihuela CJ. 2015. Pore-forming toxins induce macrophage necroptosis during acute bacterial pneumonia. PLoS Pathog 11:e1005337 10.1371/journal.ppat.1005337. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gilbert RJ, Jiménez JL, Chen S, Tickle IJ, Rossjohn J, Parker M, Andrew PW, Saibil HR. 1999. Two structural transitions in membrane pore formation by pneumolysin, the pore-forming toxin of Streptococcus pneumoniae. Cell 97:647–655 10.1016/S0092-8674(00)80775-8. [DOI] [PubMed] [Google Scholar]
- 184.Steinfort C, Wilson R, Mitchell T, Feldman C, Rutman A, Todd H, Sykes D, Walker J, Saunders K, Andrew PW. 1989. Effect of Streptococcus pneumoniae on human respiratory epithelium in vitro. Infect Immun 57:2006–2013. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rayner CF, Jackson AD, Rutman A, Dewar A, Mitchell TJ, Andrew PW, Cole PJ, Wilson R. 1995. Interaction of pneumolysin-sufficient and -deficient isogenic variants of Streptococcus pneumoniae with human respiratory mucosa. Infect Immun 63:442–447. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Paton JC, Ferrante A. 1983. Inhibition of human polymorphonuclear leukocyte respiratory burst, bactericidal activity, and migration by pneumolysin. Infect Immun 41:1212–1216. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cockeran R, Theron AJ, Feldman C, Mitchel TJ, Anderson R. 2004. Pneumolysin potentiates oxidative inactivation of alpha-1-proteinase inhibitor by activated human neutrophils. Respir Med 98:865–871 10.1016/j.rmed.2004.02.014. [PubMed] [DOI] [PubMed] [Google Scholar]
- 188.Maus UA, Srivastava M, Paton JC, Mack M, Everhart MB, Blackwell TS, Christman JW, Schlöndorff D, Seeger W, Lohmeyer J. 2004. Pneumolysin-induced lung injury is independent of leukocyte trafficking into the alveolar space. J Immunol 173:1307–1312 10.4049/jimmunol.173.2.1307. [PubMed] [DOI] [PubMed] [Google Scholar]
- 189.Hirst RA, Rutman A, Sikand K, Andrew PW, Mitchell TJ, O’Callaghan C. 2000. Effect of pneumolysin on rat brain ciliary function: comparison of brain slices with cultured ependymal cells. Pediatr Res 47:381–384 10.1203/00006450-200003000-00016. [PubMed] [DOI] [PubMed] [Google Scholar]
- 190.Hirst RA, Sikand KS, Rutman A, Mitchell TJ, Andrew PW, O’Callaghan C. 2000. Relative roles of pneumolysin and hydrogen peroxide from Streptococcus pneumoniae in inhibition of ependymal ciliary beat frequency. Infect Immun 68:1557–1562 10.1128/IAI.68.3.1557-1562.2000. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Rossjohn J, Feil SC, McKinstry WJ, Tweten RK, Parker MW. 1997. Structure of a cholesterol-binding, thiol-activated cytolysin and a model of its membrane form. Cell 89:685–692 10.1016/S0092-8674(00)80251-2. [DOI] [PubMed] [Google Scholar]
- 192.Saunders FK, Mitchell TJ, Walker JA, Andrew PW, Boulnois GJ. 1989. Pneumolysin, the thiol-activated toxin of Streptococcus pneumoniae, does not require a thiol group for in vitro activity. Infect Immun 57:2547–2552. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]


