Abstract
Despite advances in the diagnosis and management of asthma, uncontrolled disease is still associated with a substantial mortality and morbidity burden. Patients often overestimate their level of asthma control while also reporting that asthma symptoms affect their quality of life and ability to work or study. There is some evidence of success with primary prevention measures in high-risk children and the secondary prevention of asthma in sensitized individuals or those at risk of developing occupational asthma. There are challenges with diagnosis – with under- and overdiagnosis and misdiagnosis being common – and in the treatment of asthma, despite clear treatment guidelines. In particular, severe asthma presents a huge challenge to the clinician, and its complex and heterogeneous nature warrants a personalized medicine approach to match therapies to individual patients. However, the tools for this are currently lacking in primary care. This article reviews the current unmet need in the diagnosis and clinical management of asthma, and provides an overview of the limitations of current therapies.
Keywords: β2-agonists, anticholinergics, inhaled corticosteroids, respiratory disease, unmet need, asthma management
Video abstract
Introduction
The global prevalence of clinical asthma has increased over the past 60 years, particularly in children,1 with estimates of more than 300 million people of all ages affected worldwide.2,3 Although clinical advances in diagnosis and management have led to a reduction in the number of asthma-related deaths, there remains no cure and a substantial mortality burden still exists. Between 1990 and 2015, the age-standardized asthma-related death rate fell by 58.8%, with 397,000 deaths due to asthma in 2015.4 The National Review of Asthma Deaths, a UK-wide investigation of asthma deaths occurring between 2012 and 2013, reported that >50% of deaths occurred in patients treated for mild or moderate asthma, and, importantly, factors that could have prevented deaths were identified in 46% of cases.5
In addition to mortality, uncontrolled asthma is associated with substantial morbidity, despite the availability of effective treatments, and patients often overestimate their level of asthma control. For example, >80% of respondents to the Europe-wide Recognize Asthma and Link to Symptoms and Experience survey who had experienced acute exacerbations in the previous year considered their asthma to be controlled, despite only a fifth of respondents having Global Initiative for Asthma (GINA)-defined asthma control.6 There is thus a need to improve the assessment of control from both a patient and physician perspective through education measures. Furthermore, among patients treated with an inhaled corticosteroid (ICS) and a long-acting β2-agonist (LABA), poor asthma control is associated with worse health-related quality of life compared with those with well-controlled asthma when considering mental and physical component scores and health utility.7 Additionally, compared with well-controlled asthma, poorly controlled asthma is associated with increased loss of work productivity, including higher rates of presenteeism and overall work impairment.7 In 2016, 24% of patients participating in Asthma UK’s Annual Asthma Survey reported that asthma symptoms affected their ability to work or study, with more than a quarter reporting at least 1 week off work or education as a result of their asthma in the previous year.8
Scope
There have been several articles on unmet needs in asthma. These have been mainly secondary care-based,9,10 but some also consider the patient’s perspective.11 This review aims to provide an up-to-date review of the literature, together with the author’s primary care perspective. Although this was not intended to be a systematic literature review, literature searches were conducted on PubMed from August to November 2017 using the keywords “asthma prevention”, “asthma diagnosis” and “asthma management”. Further relevant articles were obtained from the reference lists of reviewed articles. This review forms the first part of a thematic series, which reviews the use of anticholinergics in asthma.
Asthma prevention
Primary prevention of asthma
Asthma is a heterogeneous disease, and its development and persistence are associated with gene–environment interactions. Findings from intervention studies suggest that a “window of opportunity” may exist in early life, perhaps even in utero, during which targeted interventions can be used to minimize the risk of future asthma development. The aim of primary prevention measures is to prevent the onset of asthma in high-risk individuals, such as those with a family history of atopy or asthma, through the use of targeted interventions that minimize exposure to dietary, environmental, and/or pharmacologic allergens.1,12
A number of factors have been associated with an increased risk of developing asthma in young children who wheeze; however, evidence is mostly inconclusive. Positive associations with future asthma development have been reported for maternal factors during gestation (smoking, cesarean delivery, stress, high body mass index [BMI]), familial factors (genetics, family history of atopy), infections (respiratory syncytial virus, rhinovirus, pertussis), medication (paracetamol, β2-agonists, antibiotics), diet, and inhalation exposure (tobacco smoke, indoor and outdoor air pollution, house dust mite, mold).1 Conversely, factors found to be negatively associated with the development of childhood asthma include agricultural subsistence, lifestyle, day care, diet, endotoxin, and farm animal exposure.1
Primary prevention measures have investigated intervention and avoidance programs that focus on individual risk factors; however, these have generally yielded inconclusive results. Based on the heterogeneous nature of asthma development and the interplay of multiple environmental and genetic factors, another approach is to implement multifaceted prevention programs that target high-risk children during gestation and/or early life. A number of multifaceted programs have been trialed, with varying results. For example, the Canadian Asthma Primary Prevention Study aimed to minimize exposure to allergens (house dust mites and pet allergens) and environmental tobacco smoke, and encouraged breastfeeding during the first year of life. These measures successfully reduced the prevalence of pediatric allergist-diagnosed asthma and asthma symptoms among high-risk children up to 7 years of age by 56% compared with the control group.13,14 Similarly, the Isle of Wight Prevention Study was successful in reducing the development of allergic disease, including asthma, among high-risk children during the first 8 years of life by reducing exposure to food and house dust mite allergens in infancy.15 A Cochrane review found that multifaceted rather than monofaceted intervention was preferable to usual care for the primary prevention of asthma in children aged <5 or ≥5 years; however, no significant differences were found between mono- and multifaceted interventions in reducing the frequency of asthma diagnosis in children aged <5 or ≥5 years or in reducing the likelihood of nocturnal coughing.16 Despite the promising findings of these studies, other multifaceted prevention programs in primary care settings, such as the PREVASC study, have not been effective at reducing asthma symptoms in high-risk infants up to 2 years of age.17 Selected primary prevention studies are detailed in Table 1.
Table 1.
Selected mono- and multifaceted studies investigating primary and secondary prevention measures in asthma
| Study | Interventions | Key findings |
|---|---|---|
| Primary prevention measures16 | ||
| Monofaceted interventions | ||
| Manchester Allergy and Allergy Study (NACMAAS)50 | • Randomized controlled trial investigating whether a low-allergen environment reduces the risk of primary sensitization and development of atopic disease in high-risk children • HDM allergen avoidance vs no intervention |
• Reduction of some respiratory symptoms in the first year of life in high-risk infants in the intervention group vs the control51 • Increased risk of mite sensitization, but lower specific airway resistance in high-risk children in the intervention group vs the control at 3 years of age52 |
| Prevention and Incidence of Asthma and Mite Allergy (PIAMA) study53 | • Population-based study in the Netherlands with follow-up until the age of 18 years investigating the influence of lifestyle and environment on the development of asthma and allergy • Reduced exposure to HDM, beginning during the prenatal period |
• The intervention had no effect on sensitization or atopy up to the age of 4 years, or on asthma and allergy outcomes up to the age of 8 years53,54 |
| Study of Prevention of Allergy in Children in Europe (SPACE)56 | • European population-based study that aimed to prevent sensitization to HDM and food allergens, and the development of atopic symptoms, during infancy in high-risk children • HDM reduction strategies and education on the reduction of food and inhaled allergens |
• The rate of sensitization to aero- and food allergens was decreased in the intervention group vs the control at 1 year of age55 • At 2 years of age, there was no protective effect of HDM avoidance on the development of sensitization or symptomatic allergy vs the control group56 • In 5- to 7-year-olds, HDM sensitization was reduced in the intervention group vs the control group57 |
| Zeiger study58 | • A prospective, randomized, controlled study of food allergen avoidance in infancy • Reduced exposure to allergenic food during the last trimester of pregnancy; staged introduction of foods in infancy |
• A significant reduction in food allergy and milk sensitization was found before the age of 2 years • At age 7 years, there was no difference between the intervention and control groups in food allergy, atopic dermatitis, allergic rhinitis, asthma, any atopic disease, lung function, food/aeroallergen sensitization, serum IgE level, or the presence of nasal eosinophils or nasal basophilic cells58 |
| Multifaceted interventions | ||
| Canadian Asthma Primary Prevention Study (CAPPS)14 | • The study aimed to minimize exposure to allergens (HDM, pet allergens, food allergens) and environmental tobacco smoke • Breastfeeding was encouraged in the first year of life (diet supplemented with hypoallergenic formula if required) • Strict elimination of allergenic foods to 12 months |
• At 2 years of age, significantly fewer children had asthma in the intervention group compared with the control group (16.3% vs 23.0%)14 • At 7 years, there was a 56% reduction in the prevalence of pediatric asthma among high-risk children13 • Reduced risk of physician-diagnosed asthma, but not of other allergic outcomes, among females aged 15 years (RR 0.37, 95% CI 0.13–0.90)59 |
| Isle of Wight Prevention Study60 | • Study aimed to minimize exposure to food allergens during the prenatal period and to food and pet allergens, as well as HDM during infancy • Stepwise introduction of allergenic foods |
• Demonstrated that allergic diseases (asthma, atopic dermatitis, rhinitis, atopy) can be reduced for at least the first 8 years of life by combined food- and HDM-allergen avoidance in infancy60 |
| Prevention of Asthma in Children (PREVASC) study17 | • Aimed to reduce allergen (HDM, food allergens, and indoor pet allergens) exposure during the prenatal period and during early infancy • Exclusive breastfeeding to 6 months was encouraged, with the nonstandardized introduction of solid food thereafter |
• No significant differences in total and specific IgE were found between the groups • The intervention was not effective at reducing asthma-like symptoms in high-risk children during the first 2 years of life, although it was modestly effective at 2 years17 |
| Secondary prevention measures | ||
| Prevention of Early Asthma in Kids (PEAK) study61 | • Children (aged 2 and 3 years) who had a positive asthma predictive index score received either fluticasone (88 μg) or placebo bid for 2 years, followed by 1 year with no study medication | • No disease-modifying effect on asthma symptoms or lung function was observed during the treatment-free period61 |
| Inhaled Fluticasone Propionate in Wheezy Infants (IFWIN) study62 | • Infants received fluticasone propionate (100 μg bid) and were followed up until 5 years of age | • Early use of inhaled fluticasone propionate in preschool children with wheeze had no effect on the development of asthma or wheeze later in childhood62 |
| Preventive Allergy Treatment (PAT) study63 | • This study evaluated the preventive effect of SIT on asthma development over 7 years | • A 3-year course of SIT with standardized allergen extracts may prevent asthma development in children with allergic rhinoconjunctivitis up to 7 years after treatment63 |
Abbreviations: bid, bis in die (twice daily); CI, confidence interval; HDM, house dust mite; IgE, immunoglobulin E; RR, risk reduction; SIT, specific immunotherapy.
It is possible that primary intervention programs have limited generalizability to other populations beyond those studied, who may have different lifestyles and are thus exposed to different risk factors. Additionally, the essential components of multifaceted programs remain unknown.12 Nonetheless, there is compelling evidence to suggest that reducing exposure to multiple allergens reduces the likelihood of an asthma diagnosis in children versus usual care; however, further studies are warranted.
Secondary prevention of asthma
For sensitized individuals, secondary prevention measures aim to prevent the subsequent development of chronic and persistent disease, and include the avoidance of indoor and outdoor environmental pollution and allergens. For example, active and passive smoking in childhood and adolescence substantially increases the incidence of wheeze and the risk for new-onset asthma, particularly in nonallergic children and in those exposed in utero to maternal smoking.18 Additionally, exposure to outdoor allergens in the form of environmental traffic-related air pollution during early and late childhood has been associated with a significant increase in the incidence of childhood asthma and allergic disease up to the age of 12 years.19
Occupational asthma refers to new-onset asthma caused directly by exposure to substances in the workplace, and represents the most common work-related respiratory disease in industrialized countries.20 Notably, it is estimated that ≥10% of cases of new or recurrent adult asthma are work-related, although the exact frequency is unknown.20,21 Although guidelines recommend that individuals with suspected occupational asthma be asked whether their symptoms improve when away from work, this approach may lack diagnostic accuracy. Therefore, the use of an objective test, such as a specific allergen challenge, is recommended.21,22 In cases of confirmed occupational asthma, further exposure should be prevented as early as possible to reduce the risk of developing established disease and to promote recovery.20
In summary, interventions to improve primary and secondary prevention of asthma have been disappointing. However, research in this area remains a priority. In 2017, the European Asthma Research and Innovation Partnership project included the following statement in the top 15 research priorities in asthma: “Understand the increase in asthma (both childhood asthma and different types of asthma, such as allergic and hyper-responsive asthma) to help develop primary and secondary prevention strategies”.23 It is hoped that further research in this area can help improve understanding of the risk factors associated with asthma development so that appropriate and successful prevention measures can be implemented in a timely manner.
Unmet needs in asthma diagnosis
A recent cross-sectional study found that patients diagnosed with asthma exhibit heterogeneous patterns of airway dysfunction, despite having similar symptom scores, highlighting the need for accurate asthma diagnosis before treatment initiation, particularly in primary care.24 Currently, there is no single gold-standard diagnostic test for asthma, and guidelines recommend the use of an initial structured clinical assessment to determine the probability of asthma in individuals with respiratory symptoms, with consideration of a personal or family history of atopy.21,22 When asthma is suspected, objective tests are then recommended to assess pulmonary function and demonstrate airway obstruction; these include spirometry, bronchodilator reversibility, peak expiratory flow variability, assessment of airway inflammation, and airway hyperreactivity.21,22 Nevertheless, the tests have varying degrees of false positive and false negative values underlying the premise that there is no one gold standard test, and although positive findings on objective tests only indicate the probability of asthma, these tests are not being carried out, resulting in high rates of false-positive and false-negative findings.22 Similarly, in patients with suspected asthma, outcomes of objective tests and the presence of respiratory symptoms may vary over time, reflecting the heterogeneous nature of the disease. Therefore, guidelines recommend that testing is repeated and compared in patients during symptomatic and asymptomatic periods.22 The sensitivity and specificity of objective tests used in the diagnosis of asthma are summarized in Table 2.
Table 2.
Sensitivity and specificity of objective tests in asthma diagnosis
| Strategy | Description* | Parameter* | Range of predictive values* (note that a single value indicates datum from a single study)
|
Comments** | |||
|---|---|---|---|---|---|---|---|
| Sensi | Specii | PPViii | NPViv | ||||
| Clinical assessment | |||||||
| Symptoms and signs | The commonest symptoms assessed were cough and wheeze, and, in adults, shortness of breath | Cough in adults Wheeze in adults Dyspnea in adults Cough in schoolchildren21 Wheeze in children21 Cough in preschool children Wheeze in preschool children Shortness of breath in preschool children |
16%–66% 9%–76% 11%–73% 63% 59% 88% 54% 76% |
26%–64% 34%–87% 38%–71% 75% 93% 7% 57% 52% |
8%–44% 10%–81% 41%–59% 14% 34% 76% 80% 84% |
18%–92% 28%–94% 26%–70% 97% 97% 15% 27% 40% |
As isolated symptoms cough, wheeze and shortness of breath are neither sensitive, nor specific for asthma. Most children with asthma have intermittent cough, wheeze and exercise-induced symptoms, but only about a quarter of children with these symptoms have asthma. Note that the single study in preschool children compared current symptoms with a diagnosis of asthma two years later. |
| Symptom variability | Episodic symptoms in adults Diurnal symptoms in adults Symptoms after exercise in adults Episodic symptoms in children22,23 Symptoms after exercise in children22,23 Nocturnal symptoms in children22.23 |
9%–40% 30%–56% 5%–40% 36%–93% 82%–94% 57%–84% |
36%–91% 36%–83% 32%–93% 35%–93% 59%–73% 58%–78% |
14%–86% 48%–76% 5%–81% 40%–94% 54%–86% 64%–85% |
18%–93% 18%–67% 58%–84% 62%–90% 79%–91% 57%–82% |
Asking about episodic symptoms improves the positive predictive values in children compared to current symptoms. | |
| Combinations of symptoms (typically cough, wheeze, chest tightness, dyspnea, exercise symptoms) | Symptom scores in adults Symptom scores in chiidren21–23 Symptoms of cough and wheeze in preschool children |
60% 45%–83% 49% |
66% 85%–97% 59% |
44%–94% 80% |
66%–97% 51% |
Combinations of symptoms are clinically more helpful than isolated symptoms, especially in children. For example, two thirds of children with a cluster of cough, wheeze, chest tightness, dyspnea and exercise symptoms have asthma. Asthma is unlikely if a child does not have at least some of these symptoms. | |
| History of atopy |
Personal/family history of atopic/allergic diseases | Personal history of atopy in adults Personal history of rhinitis/eczema in preschool children Family history of atopy in adults Family history of atopy in children |
54%–55% 47%–62% 26%–60% 43%–44% |
68%–74% 20%–75% 56%–83% 57%–70% |
46%–76% 72%–86% 44%–74% 51%–77% |
45%–79% 14%–30% 38%–70% 24%–62% |
History (personal or family) of atopic disease has poor sensitivity and specificity for asthma. |
| Strategies for demonstrating airway obstruction | |||||||
| Spirometry | Regard an FEV1:FVC ratio of less than 70% as a positive test for obstructive airway disease | Obstructive spirometry in adults Obstructive spirometry in children (5–18 years) |
23%–47% 52% |
31%–100% 73% |
45%–100% 75% |
18%–73% 49% |
In the four larger studies (adults and children), the NPV was between 18% and 54%, which means that more than half of patients being investigated who have normal spirometry will have asthma (ie, false negatives). |
| Strategies for demonstrating variability in airway obstruction | |||||||
| Bronchodilator reversibility | In adults, regard an improvement in FEV1 of ≥12% and ≥200 mL as a positive test. In children regard an improvement in FEV1 of ≥l2% as a positive test. |
Bronchodilator reversibility in adults Bronchodilator reversibility in schoolchildren (using a threshold of 9% change in FEV1)71 |
17%–69% 50% |
55%–81% 86% |
53%–82% | 22%–68% | In these secondary care populations, about one in three people with a positive reversibility test will not have asthma (the cohorts all included people with COPD); and at least one in three people with a negative bronchodilator reversibility test will have asthma. |
| Challenge tests | Regard a PC20 value of 8 mg/mL or less as a positive test | Methacholine challenge in adults Methacholine challenge in children31,43,72 |
51%–100% 47%–86% |
39%–100% 36%–97% |
60%–100% 20% |
46%–100% 94% |
Challenge tests are a good indicator for those with a definitive diagnosis of asthma already (based upon clinical judgment, signs and symptoms and response to antiasthma therapy). |
| Fall in FEV1 ≥15% at cumulative dose of ≤635 mg is positive Exercise challenge |
Mannitol in adults Mannitol in children Exercise challenge in adults Exercise challenge in children |
56% 63% 26%–80% 69%–72% |
75% 81% 100% 69%–72% |
80% 100% 90%–99% |
49% 0% 5%–73% |
These data are from a single study in adults and children with symptoms of asthma on questionnaire. The studies in adults had very small sample sizes. The larger study in children had a false positive rate of 1% (PPV 99%). |
|
| Peak flow charting | Monitor peak flows for 2–4 weeks, calculate mean variability. Regard ≥20% variability as a positive test |
PEF charting in adults in a population study – using mean variability of >20% – using mean variability of >15% – using diurnal variation >15% on >3 days/week PEF charting in children – using variation >12.3% (95th centile) |
46% 3%–5% 20% 50% |
80% 98%–99% 97% 72% |
97% 60%–67% 82% 48% |
10% 60% 64% 74% |
It is not clear whether the patients in these studies were symptomatic at the time of the charting, and results may not reflect clinical use in symptomatic populations. One study concluded that the number of days with diurnal variation was more accurate than calculating the mean variation. |
| Strategies for detecting eosinophilic inflammation or atopy | |||||||
| FeNO | Adults: Regard an FeNO level of 40 ppb or more as a positive test Children 5–16 years: regard an FeNO level of 35 ppb or more as a positive test. |
FeNO in adults FeNO in schoolchildren |
43%–88% 57% |
60%–92% 87% |
54%–95% 90% |
65%–93% 49% |
These studies are all in secondary care populations. Approximately one in five adults with a positive FeNO test will not have asthma (ie, false positives) and one in five adults with a negative FeNO test will have asthma (ie, false negatives). |
| Blood eosinophils | Suggested thresholds for blood eosinophils: Adults >4.15% Children ≥4%65 |
Blood eosinophils in adults Blood eosinophils in children |
15%–36% 55%–62% |
39%–100% 67%–84% |
39%–100% 56%–69% |
27%–65% 73% |
Elevated blood eosinophil level is poorly predictive. The threshold varies in these studies from 4.0% to 6.3%. |
| IgE | Any allergen-specific IgE >0.35 kU/l in adults Total IgE in adults >100 kU/I |
54%–93% 57% |
67%–73% 78% |
5%–14% 5% |
95%–99% 99% |
A normal IgE substantially reduces the probability of asthma in adults with a false negative rate of less than one in 10, although a positive result is poorly predictive. | |
| Skin-prick testing | Any positive test (wheal ≥3 mm) in adults Any positive test (wheal ≥3 mm) in children |
61%–62% 44%–79% |
63%–69% 56%–92% |
14%–81% 65%–92% |
39%–96% 36%–79% |
||
Notes:
Data derived from NICE evidence tables unless otherwise specified.64 Only studies reporting sensitivity, specificity, PPV, and NPV included here;
comments have been added by the guideline development group as an aid to interpretation of the data presented;
probability of test being positive when asthma present;
probability of test being negative when asthma absent;
proportion of patients with positive test who actually have asthma (100 minus PPV is the proportion of patients with a false positive test);
proportion of patients with negative test who do not have asthma (100 minus NPV is the proportion of patients with asthma, but in whom test was negative). In most studies, the reference test was spirometry plus either bronchodilator reversibility or a challenge test, although some studies also included a “typical history of attacks” or diurnal variation, or used physician diagnosis. Studies evaluating methacholine-challenge tests used physician diagnosis or bronchodilator reversibility and/or diurnal peak flow variability. In children, reference tests used were physician-diagnosed asthma plus spirometry or documented history of wheeze on at least two occasions and variability in FEV1 over time or on exercise testing. This table is reproduced from BTS/SIGN British Guideline on the management of asthma by kind permission of the British Thoracic Society.22
Abbreviations: COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FeNO, fractional exhaled nitric oxide; FVC, forced vital capacity; NICE, National Institute for Health and Care Excellence; NPV, negative predictive value; PC20, provocative concentration causing a 20% fall in FEV1; PEF, peak expiratory flow; PPV, positive predictive value; Sens, sensitivity; Spec, specificity.
The lack of a single well-defined gold-standard diagnostic measure for patients with suspected asthma means that both over- and underdiagnosis are common. For example, in one study, no evidence of asthma was found in a third of participants who had previously received a physician diagnosis of asthma upon serial assessment of symptoms, lung function, and airway reversibility.25 In addition, undiagnosed asthma (defined as the presence of asthma-like symptoms with one or more obstructive airway abnormalities in the absence of physician-diagnosed asthma) was reported in a third of adolescents; underdiagnosis was independently associated with self-reported family problems, daily exposure to indoor tobacco smoke, low physical activity, high BMI and the absence of serial sneezing.26 It is likely that the problem of asthma underdiagnosis also reflects the underrepresentation of respiratory symptoms to primary care physicians, despite the presence of decreased lung function in some individuals.27
The overdiagnosis of asthma may in part explain the increasing prevalence of asthma observed in recent years, whereas its underdiagnosis and subsequent undertreatment may be reflected in the reported morbidity and mortality associated with asthma. In the Netherlands, an asthma diagnostic consultancy service was established with the aim of assisting general practitioners in their diagnostic process. Subsequently, an asthma diagnosis was excluded in approximately half the patients referred to this service, and a change in maintenance therapy was recommended in three-quarters.28
In addition to the under- and overdiagnosis of asthma, misdiagnosis is also common, and has implications for the correct management of patients diagnosed with asthma who may have another respiratory condition that is neither appropriately investigated nor treated. Young children commonly present with sporadic respiratory symptoms; however, diagnosing asthma in children aged <5 years is challenging, as the routine assessment of airflow limitation is not possible in young children.21 For example, recurrent viral wheeze is common among this population, and usually occurs in combination with an upper respiratory tract infection. However, a diagnosis of asthma based on the initial presentation of wheeze is difficult.12 In this population, “trial by treatment” is the diagnostic approach commonly used, with reviews recommended every 2–3 months to assess response to controller treatment and temporal patterns of symptoms. In addition, a diagnosis of asthma in young children should consider any family history of atopy.12 GINA recommend that symptoms be treated based upon the physician’s clinical judgment and objective tests performed once the child reaches an appropriate age.12
Despite advances in the understanding of asthma pathophysiology and an increased awareness of patient risk factors, there is still a need for improved diagnostic measures and procedures. Therefore, asthma diagnosis should be considered a key unmet need, as improvements in this area will ensure timely and accurate diagnosis of patients with suspected asthma, and allow them subsequently to receive appropriate management.
Unmet needs in asthma management
According to GINA recommendations, the long-term aims of asthma management in patients of all ages are to achieve control of asthma symptoms and to reduce the risk of future exacerbations and fixed airflow limitation.12,22 In control-based asthma management, which involves a continuous cycle of assessment, treatment adjustment, and review, both symptoms and future exacerbation risk should be considered when initiating therapy and establishing a management plan.12 However, even with the availability of different treatment options and guidelines to aid clinical decision-making, a high proportion of patients with asthma fail to obtain guideline-defined control and remain symptomatic.29
Poor asthma control can arise through a combination of patient and physician factors. Patient factors associated with poor asthma control include poor adherence to treatment, poor perception, lower annual household income, previous exacerbation or emergency room visit, psychoemotional factors, and poor inhaler technique, including errors and comorbidities (rhinitis, obesity, depression).5,7,30 In the UK, almost two-thirds of individuals who pay for their asthma medication stated that this impacted their finances, with the highest impact on patients in northeast England.8
A review of local, national, and multinational survey data revealed inadequate patient education in terms of both their underlying disease and its treatment, underreporting of symptoms, and a mismatch between patient perception of their asthma control and symptoms. Importantly, patients often tolerated poor asthma control and underreported symptoms, with direct implications on disease classification and management.29–31 For example, >65% of patients questioned in the Asthma UK survey reported symptoms in the previous week, although >80% considered their asthma to be “controlled”, highlighting the disconnection between the patients’ perception of their disease and its control.32 Similarly, in the Asthma Insights and Reality in Europe study, only 5.3% of participants achieved the goals set forth in the GINA recommendations.29 Furthermore, in Asthma UK’s 2016 Annual Asthma Survey, 82% of participants reported having poorly controlled asthma, which affected the everyday life of more than half the patients.8
The Global Asthma Physician and Patient (GAPP) survey aimed to identify differences in opinions on the management of asthma between patients and physicians, and identified a direct link between patient–physician communication and treatment compliance.11 Notably, there was a mismatch between the perception of education provision, with 87% of physicians stating that up to 50% of the time spent in office visits was devoted to education, compared with 64% of patients who, on average, stated that only 25% of the appointment was spent discussing educational issues.11 Similarly, there was a mismatch in the perception of compliance, with patients overstating their level of treatment compliance compared with that reported by the physician.11 There was a direct association between better patient–physician communication and greater patient-reported compliance.
Physician factors associated with poor asthma control include noncompliance with guidelines, lack of action plans, and no/poor assessment of asthma control. Basic asthma care includes an annual asthma review, an action plan, and an assessment of inhaler technique; however, only a third of patients surveyed received these three aspects of care.8,22 Notably, 42% of patients received an asthma action plan in 2016, compared with 24% in 2013; therefore, although this figure almost doubled between 2013 and 2016, more than half the patients surveyed did not have an asthma action plan, despite more than three-quarters (78%) attending an annual asthma review.8 In the INSPIRE study, only 28% of participants had well-controlled asthma (defined by Asthma Control Questionnaire [ACQ] score), despite 70% receiving treatment with an ICS with or without a LABA. Consistently with previous reports, there was a disconnect between patient-reported and ACQ-defined asthma control.31
In summary, there is encouraging evidence that asthma management can be improved by measures to enhance physician and patient education on supported self-management and developing better inhaler techniques. A detailed review of inhaler devices is covered by Dr Omar Usmani (Imperial College London) elsewhere in this thematic review series.
Unmet needs in the treatment of asthma
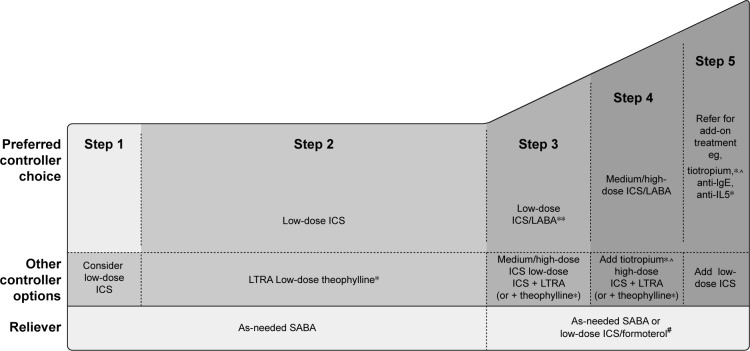
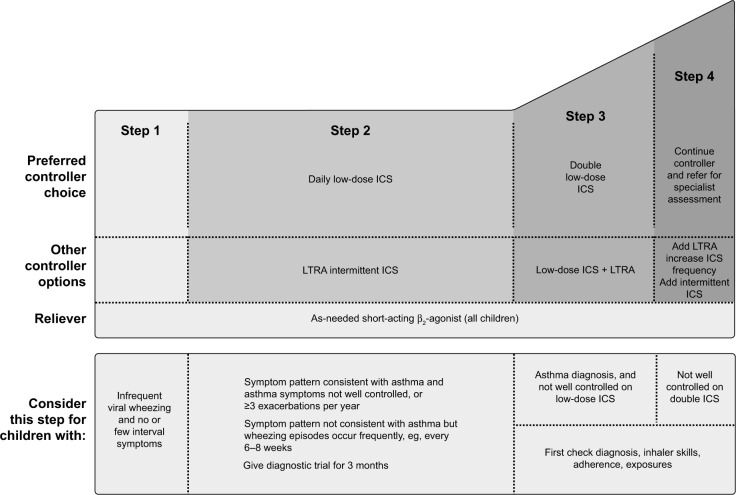
According to GINA recommendations, asthma treatment decisions should be based on assessing and adjusting treatment, as well as reviewing the treatment response.12 Before stepping up treatment, the following should be considered: is the diagnosis of asthma correct? Are there any other significant comorbidities contributing to the worsening of symptoms? Is there compliance with the existing medication? Is the inhaler technique correct? Are there any other confounding factors, such as smoking or exposure to asthma triggers, at work?12 The treatment can then be adjusted up or down in a stepwise approach, as shown in Figures 1 and 2. However, ineffective asthma treatment, such as inappropriate use of therapy, remains and can thus be considered a primary unmet need.
Figure 1.
Stepwise asthma management in adults, adolescents, and children aged 6–11 years.
Notes: *Not for children aged <12 years; **for children aged 6–11 years (preferred step 3 treatment medium-dose ICS); #for patients prescribed BDP/formoterol or BUD/ formoterol maintenance and reliever therapy; ^tiotropium by mist inhaler is an add-on treatment for patients aged ≥12 years with a history of exacerbations. Copyright ©2018 Global Initiative for Asthma. Reproduced with permission from. Global Initiative for Asthma. Global strategy for asthma management and prevention. 2018. Available from: http://ginasthma.org/2018-gina-report-global-strategy-for-asthma-management-and-prevention/.12
Abbreviations: ICS, inhaled corticosteroid; BDP, beclomethasone dipropionate; BUD, budesonide; LABA, long-acting β2-agonist; LTRA, leukotriene receptor antagonist; SABA, short-acting β2-agonist.
Figure 2.
Stepwise asthma management in children aged ≤5 years.
Note: Copyright ©2018 Global Initiative for Asthma. Reproduced with permission from. Global Initiative for Asthma. Global strategy for asthma management and prevention. 2018. Available from: http://ginasthma.org/2018-gina-report-global-strategy-for-asthma-management-and-prevention/.12
Abbreviations: ICS, inhaled corticosteroid; LTRA, leukotriene-receptor antagonist.
Inhaled corticosteroids
Both physicians and patients believe there is a need for new medication options for the treatment of asthma.11 A key element of asthma pathophysiology is airway inflammation. First-line asthma controller treatment with a low-dose ICS targets eosinophilic airway inflammation and is recommended early in the disease course to reduce the risk of future exacer-bations.12 However, despite high agreement among physicians that ICS represents the gold standard for asthma treatment, the results of the GAPP survey reported an underuse in first-line ICS for asthma patients. Notably, first-line treatment for asthma is not prescribed in accordance with GINA recommendations, with physicians often prescribing a LABA only, despite the lack of efficacy of this drug class on airway inflammation.11 Canonica et al concluded that the underuse of ICSs may be explained by physician dissatisfaction with this drug class and lack of adherence to treatment guidelines.11
However, in the 3-year Inhaled Steroid Treatment as Regular Therapy in Early Asthma (START) study, early intervention with low-dose ICS (budesonide 200 or 400 μg/ daily) in children and adults with recent-onset mild persistent asthma reduced the risk of a severe asthma exacerbation versus placebo by 44%.33 Importantly, ICS treatment was safe and well tolerated, with no unexpected unfavorable adverse events reported.34 Although guidelines recommend the addition of a LABA to first-line ICS in asthma patients who remain symptomatic (Figure 1), uncertainty exists regarding the optimal dose of ICS.35 The dose–response relationship of fluticasone propionate in terms of improvement of symptoms and lung function begins to plateau at around 100–200 μg/day, with maximum efficacy achieved with doses of approximately 500 μg/day in adults and adolescents for lung function and symptom control, although data on the use of this dose in patients with asthma are limited.36 Consistently with this, a meta-analysis has shown that if add-on therapy is warranted in patients with asthma who remain symptomatic despite the use of ICS, the addition of a LABA to moderate-dose ICS (approximately 200 μg/day fluticasone or equivalent) confers greater clinical benefit than doubling the dose of ICS.35
In most patients with asthma, symptoms can be controlled by low-dose ICSs; however, some patients require high doses or oral steroids (steroid-insensitive asthma), while others are completely resistant to the effects of steroids regardless of dose (steroid-resistant asthma).37 Additionally, patients with more severe asthma are less responsive to ICS than those with mild asthma. ICS resistance can occur through exposure to risk factors, such as smoking,38 or through genotype effects. Evidence suggests that familial steroid resistance is linked to abnormalities in the glucocorticoid receptor (GR), including reduced GR-binding affinity, reduced nuclear translocation, and decreased GR expression in peripheral blood mononuclear cells.37,39 Additionally, abnormal histone acetylation and transcription factor activation may be mechanisms of steroid resistance in patients with asthma.37 Additional studies on peripheral blood mononuclear cells isolated from steroid-resistant individuals have demonstrated that corticosteroids fail to inhibit the secretion of interleukin-2 (IL2) and interferon-γ (IFNγ) in these patients.37 Therefore, steroid resistance represents an area of unmet need in asthma management, particularly in severe asthma, and additional therapies are required that target the underlying airway inflammation. Interestingly, there is some evidence to suggest that LABAs promote GR nuclear translocation and increase sensitivity to corticosteroids, and may thus enhance the function of glucocorticoids and assist in restoring their function in steroid-insensitive/resistant patients.37,40,41
β2-Agonists
Since β2-agonists do not target airway inflammation in asthma, patients should only be treated with a β2-agonist alone in exceptional circumstances, including in those with mild intermittent asthma and infrequent symptoms.12 Although LABAs in the absence of an ICS may be effective at reducing asthma symptoms, evidence from meta-analyses suggests that they may increase the risk of adverse outcomes in adult patients, including severe exacerbations and death.42 Therefore, an as-needed short-acting β2-agonist is recommended as reliever therapy in GINA step 1 (Figures 1 and 2),12 rather than regular use of a LABA. Patients have reported that suboptimal asthma control substantially affected their health-related quality of life, despite receiving maintenance treatment with an ICS with or without a LABA.31 Therefore, such patients may benefit from additional bronchodilation, such as that provided by an anticholinergic (also known as a long-acting muscarinic antagonist).
Leukotriene receptor antagonists
Leukotriene receptor antagonists, such as montelukast, may be effective in some patient populations, including those with exercise-induced asthma, virus-induced wheeze, and asthma associated with allergic rhinitis.43 However, no routine laboratory tests are currently available to predict leukotriene-receptor-antagonist responsiveness in individual patients.
Challenges of severe asthma
Severe asthma is defined by GINA as asthma that requires step 4 or 5 treatment to maintain symptom control (Figure 1), and may present similarly to asthma that is poorly controlled due to lack of treatment.12 Although severe asthma only affects 5%–10% of the asthma population,44 it is responsible for a high proportion of total asthma-related deaths (39% in the recent National Review of Asthma Deaths survey).5 Many patients with severe asthma are treated with oral corticosteroids, although these are associated with side effects in the majority of patients, as well as poor adherence and reduced quality of life.45 Therefore, there is a need for effective treatments in this population of patients, and urgent solutions are required. A major unmet need in severe asthma is the development of therapies that match the individual phenotype of the patient. Biological therapies, such as omalizumab and mepolizumab, are now available for a more targeted approach to therapy.46 However, in the absence of patient phenotyping, treatment options with efficacy over the range of asthma severities, including patients with severe asthma, are needed.
Limitations of current asthma therapies
Randomized clinical trials analyze group mean data; however, asthma is a heterogeneous disease, and different phenotypes (observable features) and endotypes (molecular mechanisms of pathogenesis) exist that may respond differently to therapy.47 Additionally, real-world populations include both responders and nonresponders. Current diagnostic labels, including those for airway disease, are based on an outdated understanding of physiological mechanisms, and do not consider the heterogeneity of the disease, which may lead to suboptimal management.48
The complex and heterogeneous nature of asthma warrants a precision medicine approach. Agusti et al48 introduced the concept of “treatable traits” as a label-free precision-medicine strategy for chronic airway diseases, such as asthma and chronic obstructive pulmonary disease. Within the adult population, treatable traits are based upon phenotypes or an understanding of the underlying endotype, and include airflow limitation, which may be treated with a LABA and/or long-acting anticholinergic, and eosinophil airway inflammation, for which an ICS is recommended.48 While this approach is certainly attractive, the tools required for precision medicine are currently lacking in clinical practice, especially in primary care. Additionally, the definitions proposed by Agusti et al48 exclude children, who represent a large population of patients in need of improved diagnostic measures and treatment options. Until such time as the tools for precision medicine become universally available and pediatric patients are considered, there remains a need for asthma therapy with proven efficacy and safety over the range of disease severities and patients. Recently, the efficacy of tiotropium, a long-acting anticholinergic approved for the treatment of symptomatic patients at risk of exacerbation despite treatment with an ICS plus a LABA, was demonstrated to be independent of baseline characteristics and allergic status in patients with asthma.49 This suggests that an anticholinergic such as tiotropium may be appropriate for targeting a broad population of patients. The evidence for the role of anticholinergics in adults, children, and preschool children is covered by several review articles in this series, and is thus not included here.
Conclusion
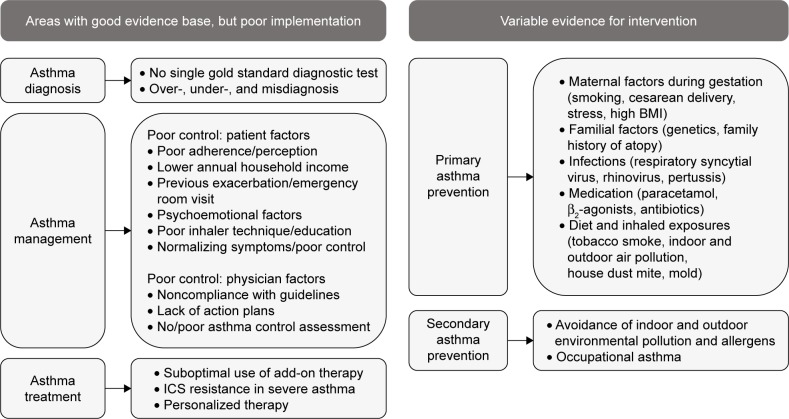
Asthma remains a disease with significant morbidity and preventable mortality. Figure 3 outlines the key areas of unmet need. Despite increased recognition of environmental and genetic factors, which are linked to the development of asthma, there is limited evidence of successful primary prevention measures. Similarly, there is a huge unmet need in reducing factors known to aggravate established asthma, such as vehicle pollution and tobacco smoke. Evidence for successful intervention has been variable to date. Under-and overdiagnosis of asthma is common, and there is a need for accurate objective tests that can be used widely in primary care, in addition to quality-assured implementation of guideline-recommended diagnostic procedures.
Figure 3.
Key areas of unmet need in asthma.
Abbreviations: BMI, body mass index; ICS, inhaled corticosteroid.
ICS therapy remains the cornerstone of asthma management, but compliance is still poor. Potential solutions include improved patient education/self-management and use of integral inhaler device monitors, which may improve outcomes. However, asthma is a heterogeneous disease, and there is a need to target treatment to the individual patient. Therefore, it may be beneficial to adopt the precision medicine approach, particularly given that the use of targeted biological therapy in patients with severe asthma is indeed progressing rapidly. Nevertheless, for the majority of patients with asthma, many of whom are treated in primary care, this approach is far from being established. Therefore, there is an important role for such agents as long-acting anticholinergics, which are effective across a broad spectrum of severity and age groups.
Acknowledgments
The author would like to thank Kjeld Hansen, a member of the Patient Ambassador Group for the European Lung Foundation, for his input to the video summary for this manuscript. Medical writing assistance in the form of the preparation and revision of the draft manuscript was supported financially by Boehringer Ingelheim and provided by Lisa Jolly, PhD, of MediTech Media, under the author’s conceptual direction and based on feedback from the author. Boehringer Ingelheim was given the opportunity to review the manuscript for factual accuracy only. The author takes full responsibility for the scope, direction, content, and editorial decisions relating to the manuscript, was involved at all stages of development, and has approved the submitted manuscript.
Footnotes
Disclosure
As a general practitioner, the author has provided a primary care perspective on the review topic. The author has acted as a medical consultant and spoken on behalf of Astra-Zeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Mundipharma/Napp, Nutricia, Pfizer, and Teva. The author reports no other conflicts of interest in this work.
References
- 1.Beasley R, Semprini A, Mitchell EA. Risk factors for asthma: is prevention possible? Lancet. 2015;386(9998):1075–1085. doi: 10.1016/S0140-6736(15)00156-7. [DOI] [PubMed] [Google Scholar]
- 2.Global Burden of Disease 2016 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bousquet J, Khaltaev N. Global surveillance, prevention and control of chronic respiratory diseases: a comprehensive approach. Geneva: World Health Organization; 2007. [Google Scholar]
- 4.GBD 2013 Mortality and Causes of Death Collaborators Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease study 2013. Lancet. 2015;385(9963):117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Royal College of Physicians . Why Asthma Still Kills: The National Review of Asthma Deaths (NRAD) London: RCP; 2014. [Google Scholar]
- 6.Price D, Fletcher M, van der Molen T. Asthma control and management in 8,000 European patients: the Recognise Asthma and Link to Symptoms and Experience (REALISE) survey. NPJ Prim Care Respir Med. 2014;24:14009. doi: 10.1038/npjpcrm.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pavord ID, Mathieson N, Scowcroft A, Pedersini R, Isherwood G, Price D. The impact of poor asthma control among asthma patients treated with inhaled corticosteroids plus long-acting β2-agonists in the United Kingdom: a cross-sectional analysis. NPJ Prim Care Respir Med. 2017;27(1):17. doi: 10.1038/s41533-017-0014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asthma UK Annual asthma survey: 2016 report. 2016. [Accessed November 01, 2017]. Available from: https://www.asthma.org.uk/globalassets/get-involved/external-affairs-campaigns/publications/annual-asthma-care-survey/annualasthmasur-vey2016final.pdf.
- 9.Scal P, Davern M, Ireland M, Park K. Transition to adulthood: delays and unmet needs among adolescents and young adults with asthma. J Pediatr. 2008;152(4):471.e1–475.e1. doi: 10.1016/j.jpeds.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Worstell M. Asthma: individual patient perspective and current unmet needs. Clin Exp Allergy. 2000;30(Suppl 1):11–15. doi: 10.1046/j.1365-2222.2000.00090.x. [DOI] [PubMed] [Google Scholar]
- 11.Canonica GW, Baena-Cagnani CE, Blaiss MS, Dahl R, Kaliner MA, Valovirta EJ. Unmet needs in asthma: Global Asthma Physician and Patient (GAPP) survey: global adult findings. Allergy. 2007;62(6):668–674. doi: 10.1111/j.1398-9995.2007.01352.x. [DOI] [PubMed] [Google Scholar]
- 12.Global Initiative for Asthma Global strategy for asthma management and prevention. 2018. [Accessed May 21, 2018]. Available from: http://ginasthma.org/2018-gina-report-global-strategy-for-asthma-management-and-prevention/
- 13.Chan-Yeung M, Ferguson A, Watson W, et al. The Canadian Childhood Asthma Primary Prevention study: outcomes at 7 years of age. J Allergy Clin Immunol. 2005;116(1):49–55. doi: 10.1016/j.jaci.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 14.Becker A, Watson W, Ferguson A, Dimich-Ward H, Chan-Yeung M. The Canadian Asthma Primary Prevention study: outcomes at 2 years of age. J Allergy Clin Immunol. 2004;113(4):650–656. doi: 10.1016/j.jaci.2004.01.754. [DOI] [PubMed] [Google Scholar]
- 15.Arshad SH, Bateman B, Sadeghnejad A, Gant C, Matthews SM. Prevention of allergic disease during childhood by allergen avoidance: the Isle of Wight prevention study. J Allergy Clin Immunol. 2007;119(2):307–313. doi: 10.1016/j.jaci.2006.12.621. [DOI] [PubMed] [Google Scholar]
- 16.Maas T, Kaper J, Sheikh A, et al. Mono and multifaceted inhalant and/or food allergen reduction interventions for preventing asthma in children at high risk of developing asthma. Cochrane Database Syst Rev. 2009;3:CD006480. doi: 10.1002/14651858.CD006480.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Schönberger HJ, Dompeling E, Knottnerus JA, et al. The PREVASC study: the clinical effect of a multifaceted educational intervention to prevent childhood asthma. Eur Respir J. 2005;25(4):660–670. doi: 10.1183/09031936.05.00067704. [DOI] [PubMed] [Google Scholar]
- 18.Gilliland FD, Islam T, Berhane K, et al. Regular smoking and asthma incidence in adolescents. Am J Respir Crit Care Med. 2006;174(10):1094–1100. doi: 10.1164/rccm.200605-722OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bowatte G, Lodge C, Lowe AJ, et al. The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization: a systematic review and a meta-analysis of birth cohort studies. Allergy. 2015;70(3):245–256. doi: 10.1111/all.12561. [DOI] [PubMed] [Google Scholar]
- 20.Nicholson PJ, Cullinan P, Taylor AJ, Burge PS, Boyle C. Evidence based guidelines for the prevention, identification, and management of occupational asthma. Occup Environ Med. 2005;62(5):290–299. doi: 10.1136/oem.2004.016287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Institute for Health and Care Excellence . Asthma: Diagnosis and Monitoring of Asthma in Adults, Children and Young People. London: NICE; 2017. [PubMed] [Google Scholar]
- 22.British Thoracic Society (BTS)/Scottish Intercollegiate Guidelines Network (SIGN) British Guideline on the management of asthma. Edinburgh: SIGN; 2016. [Accessed November 01, 2017]. (QRG 153). [cited November 2017] Available from: http://www.sign.ac.uk. [Google Scholar]
- 23.Masefield S, Edwards J, Hansen K, et al. The future of asthma research and development: a roadmap from the European Asthma Research and Innovation Partnership (EARIP) Eur Respir J. 2017;49(5):1602295. doi: 10.1183/13993003.02295-2016. [DOI] [PubMed] [Google Scholar]
- 24.Shaw D, Green R, Berry M, et al. A cross-sectional study of patterns of airway dysfunction, symptoms and morbidity in primary care asthma. Prim Care Respir J. 2012;21(3):283–287. doi: 10.4104/pcrj.2012.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179(11):1121–1131. doi: 10.1503/cmaj.081332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siersted HC, Boldsen J, Hansen HS, Mostgaard G, Hyldebrandt N. Population based study of risk factors for underdiagnosis of asthma in adolescence: Odense schoolchild study. BMJ. 1998;316(7132):651–657. [PMC free article] [PubMed] [Google Scholar]
- 27.van Schayck CP, van der Heijden FM, van den Boom G, Tirimanna PR, van Herwaarden CL. Underdiagnosis of asthma: is the doctor or the patient to blame? The DIMCA project. Thorax. 2000;55(7):562–565. doi: 10.1136/thorax.55.7.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gillis RM, van Litsenburg W, van Balkom RH, Muris JW, Smeenk FW. The contribution of an asthma diagnostic consultation service in obtaining an accurate asthma diagnosis for primary care patients: results of a real-life study. NPJ Prim Care Respir Med. 2017;27(1):35. doi: 10.1038/s41533-017-0027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rabe KF, Vermeire PA, Soriano JB, Maier WC. Clinical management of asthma in 1999: the Asthma Insights and Reality in Europe (AIRE) study. Eur Respir J. 2000;16(5):802–807. doi: 10.1183/09031936.00.16580200. [DOI] [PubMed] [Google Scholar]
- 30.Holgate ST, Price D, Valovirta E. Asthma out of control? A structured review of recent patient surveys. BMC Pulm Med. 2006;6(Suppl 1):S2. doi: 10.1186/1471-2466-6-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Partridge MR, van der Molen T, Myrseth SE, Busse WW. Attitudes and actions of asthma patients on regular maintenance therapy: the INSPIRE study. BMC Pulm Med. 2006;6:13. doi: 10.1186/1471-2466-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hyland ME, Ståhl E. Asthma treatment needs: a comparison of patients’ and health care professionals’ perceptions. Clin Ther. 2004;26(12):2141–2152. doi: 10.1016/j.clinthera.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 33.Pauwels RA, Pedersen S, Busse WW, et al. Early intervention with budesonide in mild persistent asthma: a randomised, double-blind trial. Lancet. 2003;361(9363):1071–1076. doi: 10.1016/S0140-6736(03)12891-7. [DOI] [PubMed] [Google Scholar]
- 34.Sheffer AL, Silverman M, Woolcock AJ, Diaz PV, Lindberg B, Lindmark B. Long-term safety of once-daily budesonide in patients with early-onset mild persistent asthma: results of the Inhaled Steroid Treatment as Regular Therapy in Early Asthma (START) study. Ann Allergy Asthma Immunol. 2005;94(1):48–54. doi: 10.1016/S1081-1206(10)61285-9. [DOI] [PubMed] [Google Scholar]
- 35.Masoli M, Weatherall M, Holt S, Beasley R. Systematic review of the dose-response relation of inhaled fluticasone propionate. Arch Dis Child. 2004;89(10):902–907. doi: 10.1136/adc.2003.035709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holt S, Suder A, Weatherall M, Cheng S, Shirtcliffe P, Beasley R. Dose-response relation of inhaled fluticasone propionate in adolescents and adults with asthma: meta-analysis. BMJ. 2001;323(7307):253–256. doi: 10.1136/bmj.323.7307.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barnes PJ. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2013;131(3):636–645. doi: 10.1016/j.jaci.2012.12.1564. [DOI] [PubMed] [Google Scholar]
- 38.Chalmers GW, Macleod KJ, Little SA, Thomson LJ, McSharry CP, Thomson NC. Influence of cigarette smoking on inhaled corticosteroid treatment in mild asthma. Thorax. 2002;57(3):226–230. doi: 10.1136/thorax.57.3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sher ER, Leung DY, Surs W, et al. Steroid-resistant asthma: cellular mechanisms contributing to inadequate response to glucocorticoid therapy. J Clin Invest. 1994;93(1):33–39. doi: 10.1172/JCI116963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Usmani OS, Ito K, Maneechotesuwan K, et al. Glucocorticoid receptor nuclear translocation in airway cells after inhaled combination therapy. Am J Respir Crit Care Med. 2005;172(6):704–712. doi: 10.1164/rccm.200408-1041OC. [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi Y, Mercado N, Miller-Larsson A, Barnes PJ, Ito K. Increased corticosteroid sensitivity by a long acting β2 agonist formoterol via β2 adrenoceptor independent protein phosphatase 2A activation. Pulm Pharmacol Ther. 2012;25(3):201–207. doi: 10.1016/j.pupt.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Salpeter SR, Buckley NS, Ormiston TM, Salpeter EE. Meta-analysis: effect of long-acting β-agonists on severe asthma exacerbations and asthma-related deaths. Ann Intern Med. 2006;144(12):904–912. doi: 10.7326/0003-4819-144-12-200606200-00126. [DOI] [PubMed] [Google Scholar]
- 43.Marcello C, Carlo L. Asthma phenotypes: the intriguing selective intervention with montelukast. Asthma Res Pract. 2016;2:11. doi: 10.1186/s40733-016-0026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Busse WW, Banks-Schlegel S, Wenzel SE. Pathophysiology of severe asthma. J Allergy Clin Immunol. 2000;106(6):1033–1042. doi: 10.1067/mai.2000.111307. [DOI] [PubMed] [Google Scholar]
- 45.Asthma UK Severe asthma: the unmet need and the global challenge. 2017. [Accessed November 01, 2017]. Available from: https://www.asthma.org.uk/globalassets/get-involved/external-affairs-campaigns/publications/severe-asthma-report/auk_severeasthma_2017.pdf.
- 46.Menzella F, Galeone C, Bertolini F, Castagnetti C, Facciolongo N. Innovative treatments for severe refractory asthma: how to choose the right option for the right patient? J Asthma Allergy. 2017;10:237–247. doi: 10.2147/JAA.S144100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lötvall J, Akdis CA, Bacharier LB, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011;127(2):355–360. doi: 10.1016/j.jaci.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 48.Agusti A, Bel E, Thomas M, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J. 2016;47(2):410–419. doi: 10.1183/13993003.01359-2015. [DOI] [PubMed] [Google Scholar]
- 49.Kerstjens HA, Moroni-Zentgraf P, Tashkin DP, et al. Tiotropium improves lung function, exacerbation rate, and asthma control, independent of baseline characteristics including age, degree of airway obstruction, and allergic status. Respir Med. 2016;117:198–206. doi: 10.1016/j.rmed.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 50.International Standard Randomised Controlled Trial Number Manchester Asthma and Allergy study: primary prevention of asthma and allergy by allergen avoidance in high risk infants. 2005. [Accessed November 01, 2017]. Available from: http://www.isrctn.com/ISRCTN63558189.
- 51.Custovic A, Simpson BM, Simpson A, Kissen P, Woodcock A. Effect of environmental manipulation in pregnancy and early life on respiratory symptoms and atopy during first year of life: a randomised trial. Lancet. 2001;358(9277):188–193. doi: 10.1016/S0140-6736(01)05406-X. [DOI] [PubMed] [Google Scholar]
- 52.Woodcock A, Lowe LA, Murray CS, et al. Early life environmental control: effect on symptoms, sensitization, and lung function at age 3 years. Am J Respir Crit Care Med. 2004;170(4):433–439. doi: 10.1164/rccm.200401-083OC. [DOI] [PubMed] [Google Scholar]
- 53.Prevention and Incidence of Asthma and Mite Allergy The PIAMA research project. [Accessed November 01, 2017]. Available from: http://piama.iras.uu.nl/index-en.php.
- 54.Corver K, Kerkhof M, Brussee JE, et al. House dust mite allergen reduction and allergy at 4 yr: follow up of the PIAMA study. Pediatr Allergy Immunol. 2006;17(5):329–336. doi: 10.1111/j.1399-3038.2006.00410.x. [DOI] [PubMed] [Google Scholar]
- 55.Halmerbauer G, Gartner C, Schierl M, et al. Study on the Prevention of Allergy in Children in Europe (SPACE): allergic sensitization at 1 year of age in a controlled trial of allergen avoidance from birth. Pediatr Allergy Immunol. 2003;14(1):10–17. doi: 10.1034/j.1399-3038.2003.02069.x. [DOI] [PubMed] [Google Scholar]
- 56.Horak F, Jr, Matthews S, Ihorst G, et al. Effect of mite-impermeable mattress encasings and an educational package on the development of allergies in a multinational randomized, controlled birth-cohort study: 24 months results of the Study of Prevention of Allergy in Children in Europe. Clin Exp Allergy. 2004;34(8):1220–1225. doi: 10.1111/j.1365-2222.2004.02024.x. [DOI] [PubMed] [Google Scholar]
- 57.Arshad SH, Bojarskas J, Tsitoura S, et al. Prevention of sensitization to house dust mite by allergen avoidance in school age children: a randomized controlled study. Clin Exp Allergy. 2002;32(6):843–849. doi: 10.1046/j.1365-2222.2002.01378.x. [DOI] [PubMed] [Google Scholar]
- 58.Zeiger RS, Heller S. The development and prediction of atopy in high-risk children: follow-up at age seven years in a prospective randomized study of combined maternal and infant food allergen avoidance. J Allergy Clin Immunol. 1995;95(6):1179–1190. doi: 10.1016/s0091-6749(95)70074-9. [DOI] [PubMed] [Google Scholar]
- 59.Ramsey C, Chan E, Chooniedass R, et al. The Canadian Asthma Primary Prevention Study (CAPPS): outcomes at 15 years of age. Am J Resp Crit Care Med. 2013;181:A3520. [Google Scholar]
- 60.Arshad SH, Bateman B, Sadeghnejad A, Gant C, Matthews SM. Prevention of allergic disease during childhood by allergen avoidance: the Isle of Wight prevention study. J Allergy Clin Immunol. 2007;119(2):307–313. doi: 10.1016/j.jaci.2006.12.621. [DOI] [PubMed] [Google Scholar]
- 61.Guilbert TW, Morgan WJ, Zeiger RS, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354(19):1985–1997. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
- 62.Murray CS, Woodcock A, Langley SJ, Morris J, Custovic A. Secondary prevention of asthma by the use of Inhaled Fluticasone propionate in Wheezy Infants (IFWIN): double-blind, randomised, controlled study. Lancet. 2006;368(9537):754–762. doi: 10.1016/S0140-6736(06)69285-4. [DOI] [PubMed] [Google Scholar]
- 63.Jacobsen L, Niggemann B, Dreborg S, et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007;62(8):943–948. doi: 10.1111/j.1398-9995.2007.01451.x. [DOI] [PubMed] [Google Scholar]
- 64.National Institute for Health and Care Excellence . Asthma: Diagnosis, Monitoring and Chronic Asthma Management. London: NICE; 2017. [PubMed] [Google Scholar]