Abstract
Rationale: Mediastinal lymph node (MLN) enlargement on chest computed tomography (CT) is prevalent in patients with interstitial lung disease (ILD) and may reflect immunologic activation and subsequent cytokine-mediated immune cell trafficking.
Objectives: We aimed to determine whether MLN enlargement on chest CT predicts clinical outcomes and circulating cytokine levels in ILD.
Methods: MLN measurements were obtained from chest CT scans of patients with ILD at baseline evaluation over a 10-year period. Patients with sarcoidosis and drug toxicity–related ILD were excluded. MLN diameter and location were assessed. Plasma cytokine levels were analyzed in a subset of patients. The primary outcome was transplant-free survival (TFS). Secondary outcomes included all-cause and respiratory hospitalizations, lung function, and plasma cytokine concentrations. Cox regression was used to assess mortality risk. Outcomes were assessed in three independent ILD cohorts.
Measurements and Main Results: Chest CT scans were assessed in 1,094 patients (mean age, 64 yr; 52% male). MLN enlargement (≥10 mm) was present in 66% (n = 726) and strongly predicted TFS (hazard ratio [HR], 1.53; 95% confidence interval [CI], 1.12–2.10; P = 0.008) and risk of all-cause and respiratory hospitalizations (internal rate of return [IRR], 1.52; 95% CI, 1.17–1.98; P = 0.002; and IRR, 1.71; 95% CI, 1.15–2.53; P = 0.008, respectively) when compared with subjects with MLN <10 mm. Patients with MLN enlargement had lower lung function and decreased plasma concentrations of soluble CD40L (376 pg/ml vs. 505 pg/ml, P = 0.001) compared with those without MLN enlargement. Plasma IL-10 concentration >45 pg/ml predicted mortality (HR, 4.21; 95% CI, 1.21–14.68; P = 0.024). Independent analysis of external datasets confirmed these findings.
Conclusions: MLN enlargement predicts TFS and hospitalization risk in ILD and is associated with decreased levels of a key circulating cytokine, soluble CD40L. Incorporating MLN and cytokine findings into current prediction models might improve ILD prognostication.
Keywords: interstitial lung disease, mediastinal lymph nodes, mortality, pulmonary fibrosis
At a Glance Commentary
Scientific Knowledge on the Subject
Enlarged mediastinal lymph nodes are prevalent in patients with interstitial lung disease. However, their prognostic value or association with circulating cytokine mediators in interstitial lung disease is unknown.
What This Study Adds to the Field
Mediastinal lymph node enlargement is associated with survival in patients with interstitial lung disease and might be useful for risk stratification. Additionally, mediastinal lymph node enlargement is associated with severity of lung function impairment, risk of respiratory and all-cause hospitalization, and decreased plasma levels of soluble CD40 ligand.
Mortality in patients with interstitial lung disease (ILD), which often results in pulmonary fibrosis, has doubled during the last three decades in the United States (1). Although several common ILDs lead to death within a few years of diagnosis, substantial variability within and between ILD subtypes makes outcome prognostication challenging (2, 3). A cornerstone of the ILD evaluation is high-resolution computed tomography (CT) of the chest (4), which can help differentiate ILD subtypes (5). Parenchymal features of ILD on high-resolution chest CT, including fibrosis extent, mosaic attenuation, and ground-glass opacities, have also been associated with differential survival risk (6–8).
Mediastinal lymph nodes (MLNs) are often enlarged on chest CT scans in patients with ILD, and they have been reported in up to 70% of patients with a usual interstitial pneumonia pattern (9, 10). The biology underpinning MLN enlargement remains unclear, but trafficking of immune cells from the peripheral circulation through MLNs to the lungs has been suggested as contributory to pulmonary fibrosis (11). Recent data also suggest that the background lung microbiome may influence the expression of genes with known immunologic function, including IL-6 and IL-10, which have been linked to ILD outcomes (12, 13). We hypothesized that radiologic enlargement of MLNs (≥10 mm) on chest CT scans has prognostic value in ILD. In this investigation, we aimed to determine whether features of MLNs on chest CT scans predict clinically relevant outcomes in patients with ILD. We then assessed whether MLN features are associated with differential levels of clinically relevant cytokines within the peripheral circulation of these patients.
Methods
Study Design and Patient Selection
Our analysis used data collected from subjects in the University of Chicago ILD Registry, a prospectively acquired ILD cohort. The University of Chicago Institutional Review Board approved this investigation (protocols nos. 14163-A and 16-1062), and all patients signed informed consent. Patients followed at our institution between 2006 and 2016 with multidisciplinary diagnosis of chronic ILD according to American Thoracic Society/European Respiratory Society criteria (7, 8, 14–16) were screened. Multidisciplinary diagnosis of ILD at our institution is performed in a rigorous fashion in conjunction with pulmonologists, dedicated chest radiologists, rheumatologists, and a thoracic pathologist (17). Subjects in whom chest CT scans were unavailable for review or of poor diagnostic quality for mediastinal assessment were excluded. Subjects with sarcoidosis, drug toxicity–related ILD, or confirmed or suspected malignancy were excluded. Subjects were eligible for study inclusion when they had a multidisciplinary diagnosis of ILD and a baseline chest CT scan obtained at ILD diagnosis available for review (see online supplement).
Data Collection
The electronic medical record was retrospectively reviewed to extract pertinent baseline variables from each patient’s initial clinic visit, including demographic data (age, race/ethnicity, and sex), tobacco use, comorbid disease conditions (coronary artery disease, diabetes mellitus, gastroesophageal reflux, and hypothyroidism), body mass index (BMI), antinuclear antibody (ANA) titer, pulmonary function tests (FVC, FEV1, FEV1/FVC, and DlCO)], total white blood cell (WBC) and absolute subset counts, and high-resolution CT imaging findings (honeycombing and emphysema). We constructed the sex/age/physiology-ILD score for study participants using the previously recommended point-score approach (18) that integrates patient-specific variables (sex, age), disease-specific variables (FVC, DlCO ), and ILD subtype variable to yield a total point score that has been shown to accurately predict mortality in idiopathic pulmonary fibrosis (IPF) and other chronic ILD subtypes at all stages of disease.
Procedures
Chest CT image interpretation and assessment of MLNs
Centralized analysis and interpretation of baseline chest CT scans obtained at ILD diagnosis was performed by investigators (P.N., W.K., S.M.M, and J.H.C.) at the University of Chicago (UCHICAGO) for the purpose of this study. To ascertain our study findings, we assessed three distinct ILD cohorts with differing populations of ILD subtypes for use as replication cohorts. Patients with independently adjudicated multidisciplinary diagnosis of ILD from four nontertiary hospital centers were assessed for use as a replication cohort (NONTERT) (see online supplement). All available anonymized chest CT images from patients enrolled in the INSPIRE (Effect of Interferon Gamma-1b on Survival in Patients with Idiopathic Pulmonary Fibrosis Trial) trial (19, 20), as well as from subjects in the University of California Davis (UCDAVIS) ILD Registry, a prospectively acquired ILD cohort, were also assessed as additional replication cohorts (see online supplement). All radiologists were blinded to clinical and outcomes data. Chest CT scans were provided to radiologists for evaluation of MLN features and to assess eligibility for participation in the study. Prespecified uniform criteria were used in performing all MLN assessments and across all studies. Radiologists underwent in-person hands-on training before study initiation utilizing nonstudy standard cases to solidify and deploy an equivalent methodology and scoring criteria.
Two radiologists (P.N. and W.K.) independently measured the MLN diameters to assess the reproducibility of these measurements in the primary cohort. Interobserver agreement was calculated using kappa statistics. For any discrepancies in categorization at the binary level, the individual node measurement and MLN location obtained by the radiologist with the greatest experience in pulmonary imaging was utilized. All images included were obtained from multidetector row CT scanners with contiguous images available for reconstruction in the transaxial plane at up to 1.0 mm thickness with an interval of ≤0.4 mm. The performing radiologist provided MLN measurements from the reformatted imaging data using virtual calipers. MLNs with a short-axis diameter ≥10 mm were reported as enlarged (21–23). MLN stations based on the International Association for the Study of Lung Cancer nomenclature were systematically assessed for enlarged lymph nodes (22). As our study objective was focused on lymph nodes in the mediastinum (stations 1–9), hilar lymph nodes (stations 10–14) were not assessed. Discrete lymph nodes were identified and exact measurements specified at each station. In cases of lymph node conglomeration, the whole station was measured.
Cytokine analysis
To elucidate the relationship between cytokine concentration and MLN features, we analyzed a randomly generated subset of patients with available plasma samples obtained at baseline evaluation of ILD. In this subset of patients followed at the UCHICAGO, patients without enlarged MLNs were matched 1:1 according to age, sex, race, and ILD subtype to patients with enlarged MLNs during the same time period (see online supplement). As many patients meeting criteria for interstitial pneumonia with autoimmune features (IPAF) are currently considered to be unclassifiable in clinical practice (24, 25), we grouped patients with IPAF and unclassifiable ILD together, and all other ILDs into a separate category, for further subgroup analyses of cytokines. Plasma samples were obtained and stored at −80°C until analysis. Plasma concentrations of immunomodulatory cytokines involved in innate and adaptive immune responses were analyzed by Multiplex bead array according to the manufacturer’s protocol (Millipore) and measurements were recorded (see online supplement).
Follow-up and Endpoint of the Study
The primary endpoint of the study was transplant-free survival (TFS) defined as time from initial ILD evaluation to death or lung transplantation, and it was evaluated during the first 10 years in the UCHICAGO and the NONTERT cohorts. All patients were followed up until occurrence of death, lung transplantation, end of study period, or loss to follow-up. Person-time was averaged at 30 days per month from initial ILD evaluation to study endpoint. Vital status was determined from review of medical records, as well as from the Social Security death index. Follow-up time was censored on July 31, 2016. In the UCDAVIS and INSPIRE replication cohorts, vital status was recorded from the time of performing the index CT scan to 3 years after study enrollment. The secondary endpoints included all-cause hospitalization and respiratory hospitalization, lung function, and plasma cytokine concentrations. Participants were adjudicated to have a respiratory hospitalization when the primary reason for hospitalization was one of the following: respiratory tract infection or pneumonia, respiratory failure (requiring mechanical ventilation), chronic obstructive pulmonary disease exacerbation, physician-diagnosed acute exacerbation of ILD, pneumothorax, aspiration event, pulmonary embolism, pulmonary hypertension, or other acute respiratory worsening presenting with increased dyspnea, hypoxia, or respiratory distress. All-cause hospitalization was adjudicated when participants had any hospitalization event including elective admission for lung biopsy, or elective admission for lung transplantation as previously described (26).
Statistical Analysis
Cox proportional hazard models were used to determine the association between MLN features on baseline chest CT scan and TFS. Subgroups of the entire ILD cohort based on MLN diameter (<10 mm and ≥10 mm) were constructed and analyzed using stratified unadjusted log-rank testing to assess primary endpoint-free survival between variable groups. These subcategories were based on clinically intuitive radiologic cutoffs. Cox models adjusted for covariates that were determined by stepwise selection of pertinent patient characteristics considered biologically relevant and that changed the point estimates of the univariate association with mortality by ≥10%. The reported effect estimates of the adjusted model are the coefficients after inclusion of covariates. These include terms for hospital center, sex, age, FVC, DlCO , ILD subtype, tobacco use, BMI, immunosuppression, and antifibrotic therapy, each measured at study enrollment. Sensitivity analyses were conducted testing the association between TFS and total MLN count per subject using univariate and multivariable Cox regression models. In addition, we tested the association between the primary endpoint and a unit change in MLN diameter (mm) using these Cox models. We further analyzed the association of MLN diameter with mortality within discrete ILD subcategories. Sensitivity analyses adjusting multivariable models for the presence or absence of radiographic honeycombing, as well as pulmonary artery (PA) diameter measurements as a surrogate for pulmonary hypertension, were also conducted.
The effects of mediastinal features on the secondary endpoints of hospitalization were determined using a negative binomial regression model. This model was chosen over the Poisson regression model because its mean structure contains an extra parameter to model the observed overdispersion within count data used to represent the secondary endpoint. Comparisons of patient characteristics were determined by analyses of variance, two-sided t tests, or chi-square tests, as appropriate. Confidence limits for Pearson chi-squared, t test, or F test coefficients assessing the relationship between MLN diameter and change in FVC, DlCO , and cytokine concentrations were based on 10,000 bootstrap replications to improve precision at the 95% confidence interval (CI) level. P values for cytokine comparisons between MLN subgroups were Bonferroni adjusted, and a correction for false discovery rates was applied to values obtained for the cytokine analysis (27). We tested the proportional hazards assumption by examining covariate effects over time and by regressing Schoenfeld residuals over time in the Cox survival models, and all models evaluated passed this test. All statistical analysis was conducted using Stata (2017 release 15; StataCorp).
Results
Study Cohort
The baseline characteristics of the 1,094 patients included in the study are shown in Table 1. Mean age was 64 (±12) years, 567 (52%) were men, and the mean percentage predicted FVC and DlCO were 65 (±19) and 51 (±22), respectively. Enlarged MLNs were present in 726 (66%) of patients (Table 1 and Figures E1 and E2 in the online supplement).
Table 1.
Baseline Characteristics of the Study Population
| Characteristics | Total Population (n = 1,094) | Mediastinal LN <10 mm (n = 368) | Mediastinal LN ≥10 mm (n = 726) | P Value |
|---|---|---|---|---|
| Age, mean (±SD) | 64.1 (12.0) | 61.6 (13.7) | 65.4 (10.8) | <0.001 |
| Male sex, n (%) | 567 (52.0) | 128 (35.1) | 439 (60.5) | <0.001 |
| White race, n (%) | 804 (73.7) | 247 (67.7) | 557 (76.7) | 0.001 |
| BMI, mean (±SD) | 30.0 (6.7) | 29.3 (6.7) | 30.3 (6.7) | 0.040 |
| Tobacco, pack-years, mean (±SD) | 16.5 (23 . 6) | 11.6 (20.6) | 19.0 (24.6) | <0.001 |
| Gastroesophageal reflux, n (%) | 453 (44.0) | 149 (43.3) | 304 (44.5) | 0.716 |
| CAD, n (%) | 172 (20.4) | 41 (15.8) | 131 (22.4) | 0.028 |
| Diabetes mellitus, n (%) | 161 (19.0) | 47 (12.9) | 114 (19.5) | 0.630 |
| Hypothyroidism, n (%) | 175 (17.0) | 64 (18.6) | 111 (16.3) | 0.344 |
| FVC (% predicted), mean (±SD) | 64.6 (18.5) | 67.5 (18.9) | 63.2 (18.1) | <0.001 |
| FEV1 (% predicted), mean (±SD) | 75.8 (20.9) | 77.0 (21.2) | 75.3 (20.7) | 0.245 |
| FEV1/FVC (% predicted), mean (±SD) | 83.5 (8.7) | 82.6 (9.0) | 83.9 (8.5) | 0.027 |
| DlCO (% predicted) (±SD) | 50.7 (21.5) | 57.8 (24.1) | 47.3 (19.2) | <0.001 |
| Positive ANA titer, n (%) | 498 (48.6) | 151 (45.1) | 347 (50.3) | 0.117 |
| WBC, mean (±SD) | 8.9 (5.6) | 8.4 (3.1) | 9.6 (6.5) | 0.009 |
| Granulocytes, mean (±SD) | 6.0 (2.7) | 5.7 (2.5) | 6.1 (2.7) | 0.063 |
| Lymphocytes, mean (±SD) | 1.7 (0.9) | 1.6 (0.8) | 1.8 (0.9) | 0.035 |
| Monocytes, mean (±SD) | 0.6 (0.4) | 0.6 (0.3) | 0.7 (0.5) | 0.281 |
| Eosinophils, mean (±SD) | 0.3 (0.5) | 0.2 (0.5) | 0.3 (0.6) | 0.225 |
| Basophils, mean (±SD) | 0.1 (0.5) | 0.0 (0.1) | 0.1 (0.5) | 0.307 |
| CRP, mean (±SD) | 10.9 (30.3) | 10.7 (25.6) | 10.9 (32.3) | 0.922 |
| Immunosuppressive therapy, n (%) | 706 (67.3) | 420 (67.4) | 286 (67.1) | 0.924 |
| Patients with HRCT honeycombing, n (%) | 403 (41.3) | 94 (29.6) | 309 (47.0) | <0.001 |
| Patients with HRCT emphysema, n (%) | 260 (24.6) | 79 (22.4) | 181 (25.6) | 0.261 |
| PA diameter, mean (±SD) | 30.0 (4.8) | 28.5 (4.4) | 30.7 (4.9) | <0.001 |
| Aorta diameter, mean (±SD) | 33.6 (4.3) | 32.4 (4.2) | 34.2 (4.2) | <0.001 |
| ILD subtype, n (%) | ||||
| IPF | 342 (31.3) | 84 (22.8) | 258 (35.5) | <0.001 |
| IPAF | 168 (15.3) | 36 (9.8) | 132 (18.2) | <0.001 |
| CHP | 136 (12.4) | 57 (15.5) | 79 (10.9) | 0.025 |
| CTD-ILD | 239 (21.8) | 111 (30.2) | 128 (17.6) | <0.001 |
| Unclassifiable | 209 (19.1) | 80 (21.7) | 129 (17.8) | 0.52 |
Definition of abbreviations: ANA = antinuclear antibody; BMI = body mass index; CAD = coronary artery disease; CHP = chronic hypersensitivity pneumonitis; CRP = C-reactive protein; CTD = chronic hypersensitivity pneumonitis; GER = gastroesophageal reflux; HRCT = high-resolution computed tomography; ILD = interstitial lung disease; IPAF = interstitial pneumonia with autoimmune features; IPF = idiopathic pulmonary fibrosis; LN = lymph node; PA = pulmonary artery; WBC = white blood cells.
All entries represent original data without imputed values. Exception for baseline characteristics: BMI, n = 925; GER, n = 1029; CAD, n = 844; diabetes, n = 846; FVC, n = 986; FEV1, n = 977; FEV1/FVC, n = 965; DlCO, n = 968; ANA, n = 1,027; WBC, n = 695; CRP, n = 646; immunosuppressive therapy, n = 1,049; HRCT honeycombing, n = 979; HRCT emphysema, n = 1,062. Pack-years are presented for former and current smokers only. Positive ANA titer: ANA ≥ 1:320.
The overall interobserver agreement for measurements of mediastinal features was >80% for all variables assessed (P < 0.0001; Table E1). When compared with those without enlarged MLNs, study participants with enlarged MLNs were older (65 vs. 62 yr), more likely to be male (61% vs. 35%), had greater number of tobacco pack-years (19 vs. 12 pack-years), had higher total WBC and lymphocyte subset counts (9.6/μl vs. 8.4/μl and 1.8/μl vs. 1.6/μl, respectively), and had more high-resolution CT honeycombing (47% vs. 30%). Subjects with enlarged MLNs had greater prevalence of coronary artery disease (22% vs. 16%), greater PA diameter (31 vs. 29 mm), and greater aorta diameter (34 vs. 32 mm). Mean MLN diameters stratified by baseline demographic characteristics are shown in Table E2. MLN diameters were greatest (13 mm) in men, ever smokers, and patients with chronic hypersensitivity pneumonitis (Table E2).
When assessing individual ILD categories, MLN enlargement was highly prevalent among patients within the IPF (36% vs. 23%; P < 0.001) and IPAF diagnostic categories (18% vs. 10%; P < 0.001) when compared with those without MLN enlargement. Conversely, fewer patients had MLN enlargement within the diagnostic categories of chronic hypersensitivity pneumonitis (CHP; 11% vs. 16%; P < 0.025) and chronic hypersensitivity pneumonitis (CTD)–ILD (18% vs. 30%; P < 0.001). There was no significant difference in the prevalence of MLN enlargement among patients with unclassifiable ILD (18% vs. 22%; P = 0.152).
Primary Outcome Assessment
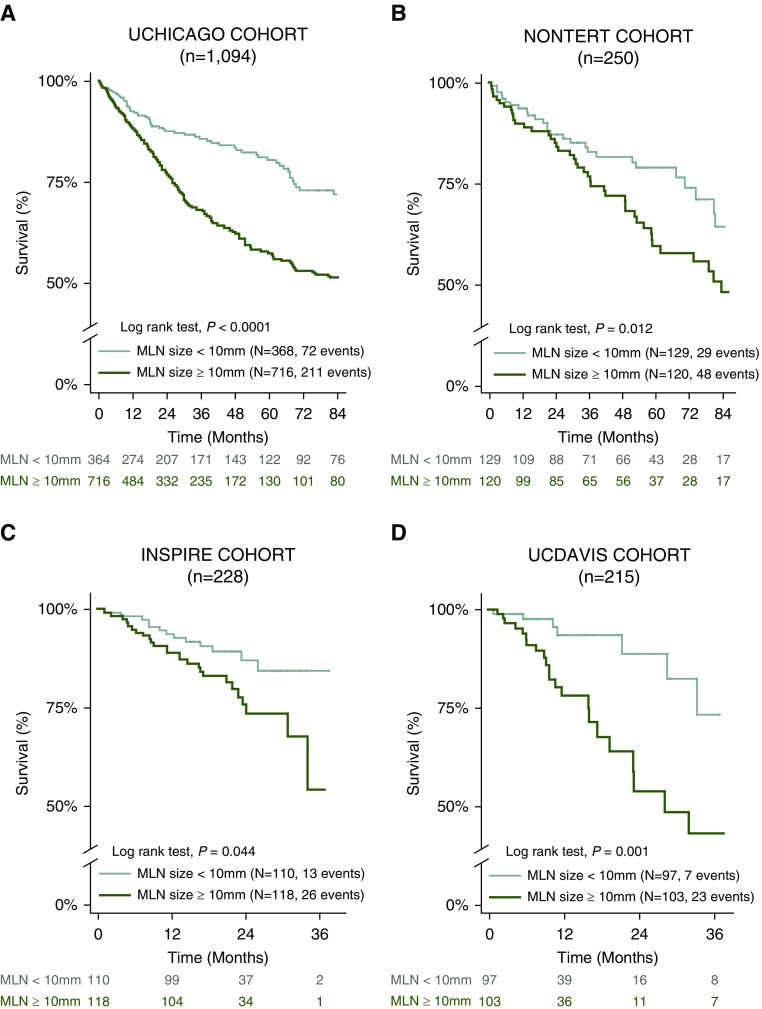
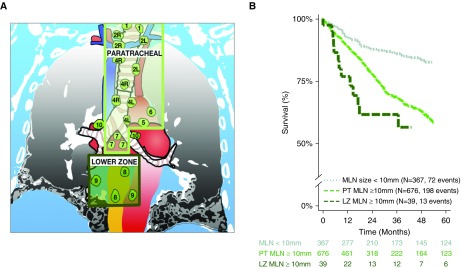
The risk of death was independently higher in study participants with enlarged MLNs (HR, 1.53; 95% CI, 1.12–2.10; P = 0.008) compared with subjects without enlarged MLNs (Table 2 and Figures 1 and 2). Substratification of the enlarged MLN cohort demonstrated a location-dependent effect on survival. After adjusting for study covariates, the increased mortality hazard differed between subjects with enlarged paratracheal MLNs (HR, 1.52; 95% CI, 1.11–2.09; P = 0.01) and subjects with lower zone MLNs (HR, 2.10; 95% CI, 1.01–4.35; P = 0.046) in comparison to subjects without enlarged MLNs (Table 2 and Figure 2). Study participants in the primary cohort with two or more enlarged MLNs had a 12% increase in the mortality hazard for each additional enlarged MLNs when compared with those without enlarged MLNs (HR, 1.12; 95% CI, 1.04–1.20; P = 0.003). These findings were replicated in three independent ILD cohorts, that is, NONTERT (a predominantly unclassifiable ILD population), INSPIRE (20) (an IPF population), and UCDAVIS (a predominantly IPF and CHP population) (Tables 2, E3–5, and Figure 1).
Table 2.
Mortality Risk by Mediastinal Lymph Node Characteristics in Patients with Interstitial Lung Disease
| Characteristic | Primary Cohort: UCHICAGO (n = 1,094) |
Replication Cohort 1: INSPIRE (n = 228) |
Replication Cohort 2: NONTERT (n = 250) |
Replication Cohort 3: UCDAVIS (n = 215) |
||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Univariate Cox regression | ||||||||
| MLN* ≥10 mm | 1.91 (1.46–2.50) | <0.001 | 1.96 (1.00–3.81) | 0.048 | 1.79 (1.13–2 . 84) | 0.013 | 3.78 (1.62–8.82) | 0.002 |
| PT MLN station* (stations 1–7) | 1.92 (1.46–2.52) | <0.001 | 1.82 (0.92–3.59) | 0.085 | 1.79 (1.13–2.84) | 0.013 | 3.53 (1.50–8.33) | 0.004 |
| LZ MLN station* (stations 8 and 9) | 2.57 (1.42–4.64) | 0.002 | 4.64 (1.32–16.4) | 0.017 | — | — | 5.27 (1.09–25.48) | 0.039 |
| MLN count* | 1.25 (1.17–1.33) | <0.001 | 1.33 (0.96–1.83) | 0.083 | 1.21 (1.00–1.45) | 0.046 | 1.51 (1.16–1.97) | 0.002 |
| Multivariable Cox regression† | ||||||||
| MLN* ≥10 mm | 1.53 (1.12–2.10) | 0.008 | 2.50 (1.01–6.22) | 0.048 | 2.12 (1. 09–4.13) | 0.028 | 3.54 (1.41–8.93) | 0.007 |
| PT MLN station* (stations 1–7) | 1.52 (1.11–2.09) | 0.010 | 2.15 (0.85–5.49) | 0.132 | 2.12 (1.09–4.13) | 0.028 | 3.51 (1.38–8.88) | 0.008 |
| LZ MLN station* (stations 8 and 9) | 2.10 (1.01–4.35 | 0.046 | 6.16 (1.53–24.7) | 0.010 | — | — | 3.50 (0.64–19.03) | 0.147 |
| MLN count* | 1.12 (1.04–1.20) | 0.003 | 1.34 (0.91–1.97) | 0.138 | 1.66 (0.86–3.23) | 0.132 | 1.52 (1.13–2.04) | 0.006 |
Definition of abbreviations: CI = confidence interval; HR = hazard ratio; ILD = interstitial lung disease; INSPIRE = Effect of Interferon Gamma-1b on Survival in Patients with Idiopathic Pulmonary Fibrosis Trial; LZ = lower zone; MLN = mediastinal lymph node; NONTERT = nontertiary hospital cohort with adjudicated multidisciplinary interstitial lung disease diagnosis; PT = paratracheal; UCDAVIS = University of California Davis; UCHICAGO = University of Chicago.
In the INSPIRE cohort, 228 patients had high-resolution computed tomography of acceptable quality available for mediastinal evaluation.
Compared to patients without enlarged MLN (i.e., MLN <10 mm).
Adjusted for sex, age, FVC, DlCO, ILD subtype, tobacco pack-years, body mass index, immunosuppressive therapy, antifibrotic therapy, and hospital center.
Figure 1.
Mediastinal lymph node (MLN) features in interstitial lung disease and distinctive survival patterns. (A–D) Survival pattern by MLN diameter in University of Chicago (UCHICAGO) cohort (A), nontertiary medical centers (NONTERT) (B), INSPIRE cohort (C), and University of California Davis (UCDAVIS) cohort (D). In the INSPIRE cohort, 228 patients had high-resolution computed tomography scans of acceptable quality for mediastinal evaluation. INSPIRE = Effect of Interferon Gamma-1b on Survival in Patients with Idiopathic Pulmonary Fibrosis Trial.
Figure 2.
Mediastinal lymph node (MLN) location predicts mortality risk. (A) Digital representation of enlarged mediastinal lymph nodes stations >10 mm. (B) Survival pattern by MLN station. LZ = lower zone (International Association for the Study of Lung Cancer Lymph node stations 8–9); PT = paratracheal (International Association for the Study of Lung Cancer lymph node stations 1–7).
Sensitivity analyses testing the linear association between TFS and MLN diameter demonstrated a 5% rise in mortality with every 1-mm increase in MLN diameter when >10 mm (HR, 1.05; 95% CI, 1.04–1.08; P < 0.001). The increased mortality hazard remained consistent after adjusting for study covariates including hospital center, sex, age, FVC, DlCO , ILD subtype, tobacco pack-years, BMI, hypothyroidism, immunosuppression, and antifibrotic therapy (HR, 1.05; 95% CI, 1.03–1.07; P < 0.001). Cohort subclassification by discrete ILD subtypes demonstrated consistency in the trend to increased mortality with enlarged MLNs (Figure E3). Sensitivity analyses adjusting for smoking demonstrated that between-group differences in soluble CD40 ligand (sCD40L) concentrations occurred independent of smoking (UCHICAGO, 1,322 vs. 529, P = 0.015; UCDAVIS, 490 vs. 327, P = 0.034). Analyses adjusting for the presence or absence of radiographic honeycombing demonstrated that the presence of MLN enlargement predicts TFS (HR, 1.45; 95% CI, 1.06–1.99; P = 0.022) and respiratory hospitalization (internal rate of return [IRR], 1.62; 95% CI, 1.09–2.41; P = 0.017) independent of radiographic honeycombing. Additional sensitivity analyses adjusting for PA diameter measurements as a surrogate for pulmonary hypertension (28–30) demonstrated that presence of MLN enlargement predicts TFS and respiratory hospitalization independent of PA diameter (HR, 1.53; 95% CI, 1.11–2.10, P = 0.009 and IRR, 1.57, 95% CI, 1.05–2.34, P = 0.028, respectively) or PA/aorta ratio (HR, 1.51; 95% CI, 1.10–2.08; P = 0.012 and IRR, 1.69; 95% CI, 1.14–2.51; P = 0.009, respectively).
Inclusion of MLN enlargement into our statistical risk prediction models demonstrated a modest improvement in model discrimination for the combined cohorts (Table E6).
Secondary Outcome Assessment
All-cause hospitalizations, respiratory hospitalizations, and pulmonary function
When assessing the secondary study endpoints, all-cause and respiratory hospitalization risks rose with increasing MLN diameter (Figure E4). In comparison to subjects without enlarged MLNs, those with enlarged MLNs had increased risks of all-cause and respiratory hospitalization (IRR, 1.54; 95% CI, 1.22–1.95; P < 0.001 and IRR, 1.55; 95% CI, 1.10–2.19; P = 0.012, respectively) even after adjusting for study covariates (IRR, 1.52; 95% CI, 1.17–1.98; P = 0.002 and IRR, 1.71; 95% CI, 1.15–2.53; P = 0.008, respectively; Table 3 and Figure E4). The presence of abnormal MLNs in “paratracheal” or “lower zone” MLN stations also independently predicted all-cause hospitalization (IRR, 1.46; 95% CI, 1.12–1.90; P = 0.005 and IRR, 3.52; 95% CI, 2.02–6.13; P < 0.001, respectively) and respiratory hospitalization (IRR, 1.59; 95% CI, 1.07–2.36; P = 0.021 and IRR, 4.58; 95% CI, 2.11–9.96; P < 0.001, respectively). Patients with two or more enlarged MLNs had increased risk of all-cause and respiratory hospitalization (IRR, 1.51; 95% CI, 1.19–1.93; P = 0.001 and IRR, 1.63; 95% CI, 1.14–2.33; P = 0.007, respectively) when compared with patients with fewer than two MLNs (Table 3). When assessing lung function, patients with enlarged MLNs had substantially worse lung function compared with those without enlarged MLNs (FVC, 63% vs. 68%; P < 0.001; DlCO, 47% vs. 58%; P < 0.001; Table 1 and Figure E5). However, total count of enlarged MLNs per patient demonstrated a weak association with baseline FVC (r = −0.12, P = 0.0001) and DlCO (r = −0.27, P < 0.0001).
Table 3.
Risk of All-Cause and Respiratory Hospitalization in Patients with Interstitial Lung Disease
| Characteristic | Unadjusted (n = 1,094) |
Adjusted (n = 880) |
||||
|---|---|---|---|---|---|---|
| IRR | 95% CI | P Value | IRR | 95% CI | P Value | |
| All-cause hospitalization | ||||||
| MLN* ≥10 mm | 1.54 | 1.22–1.95 | <0.001 | 1.52 | 1.17–1.98 | 0.002 |
| PT MLN station* (stations 1–7) | 1.48 | 1.17–1.88 | 0.001 | 1.46 | 1.12–1.90 | 0.005 |
| LZ MLN station* (stations 8 and 9) | 2.67 | 1.62–4.41 | <0.001 | 3.52 | 2.02–6.13 | <0.001 |
| MLN count† | 1.55 | 1.25–1.93 | <0.001 | 1.51 | 1.19–1.93 | 0.001 |
| Respiratory hospitalization | ||||||
| MLN* ≥10 mm | 1.55 | 1.10–2.19 | 0.012 | 1.71 | 1.15–2.53 | 0.008 |
| PT MLN station* (stations 1–7) | 1.46 | 1.03–2.06 | 0.034 | 1.59 | 1.07–2.36 | 0.021 |
| LZ MLN station* (stations 8 and 9) | 3.19 | 1.61–6.30 | 0.001 | 4.58 | 2.11–9.96 | <0.001 |
| MLN count† | 1.63 | 1.18–2.24 | 0.003 | 1.63 | 1.14–2.33 | 0.007 |
Definition of abbreviations: CI = confidence interval; ILD = interstitial lung disease; IRR = incidence rate ratio; LZ = lower zone; MLN = mediastinal lymph node; PT = paratracheal.
Results are adjusted for sex, age, FVC, DlCO, ILD subtype, tobacco pack-years, body mass index, immunosuppressive therapy, and hospital center.
Compared to patients without any enlarged MLN (i.e., MLN <10 mm).
Comparing subjects with ≥2 MLN to subjects with <2 MLN.
Plasma cytokine concentration
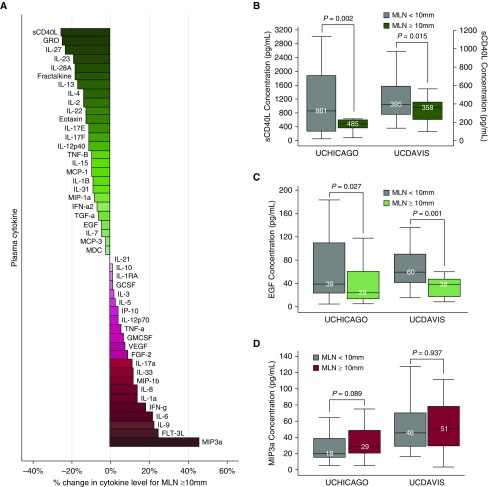
The randomly generated patient subset with available baseline plasma samples consisted of 116 participants. Plasma levels of cytokine sCD40L differed significantly between patients with and without enlarged MLNs (Tables E7 and E8). Median plasma sCD40L concentration was decreased in patients with MLN enlargement compared with patients without enlarged MLNs (376 vs. 505 pg/ml, P = 0.0015; Tables 7 and 8). Subgroup analyses demonstrated a more profound decrease in plasma sCD40L concentration within the subset with unclassifiable ILD or IPAF (861.14 vs. 484.80 pg/ml, P = 0.002) than in the subset with IPF, CHP, or CTD-ILD (409.87 vs. 356.35 pg/ml, P = 0.070; Figure 3 and Tables E9 and E10). Similarly, the plasma concentration of epidermal growth factor (EGF) was decreased in patients with MLN enlargement compared with patients without enlarged MLNs within the subset with unclassifiable ILD or IPAF (38.82 vs. 29.26 pg/ml, P = 0.027), unlike in the subset with IPF, CHP, or CTD-ILD (26.46 vs. 28.77 pg/ml, P = 0.487; Figure 3 and Table E10). Conversely, median plasma concentration of macrophage inflammatory protein-3 (MIP3a, or CCL20) was increased although not statistically significant (21 vs. 31 pg/ml, P = 0.137), even when assessing the subset with unclassifiable ILD or IPAF (P = 0.847), and within the subset with IPF, CHP, or CTD-ILD (P = 0.089; Figure 3). These findings were independently replicated in the UCDAVIS cohort (Figure 3 and Tables E9 and E10). Stratification by MLN location within the chest revealed no differences in plasma cytokine levels (Tables E11 and E12).
Figure 3.
(A) Bar graph shows percentage change in plasma cytokine concentrations for mediastinal lymph nodes (MLNs) ≥10 mm within the University of Chicago (UCHICAGO) primary cohort (n = 116). The color of each bar represents the directionality of the change (red indicates positive change; green indicates negative change). (B) Soluble CD40 ligand (pg/ml) stratified by MLN diameter in patients with unclassifiable interstitial lung disease (ILD) or interstitial pneumonia with autoimmune features (IPAF) within the UCHICAGO primary cohort (n = 36) and the University of California Davis (UCDAVIS) replication cohort (n = 34). (C) Epidermal growth factor (pg/ml) stratified by MLN diameter in patients with unclassifiable ILD or IPAF within the UCHICAGO primary cohort (n = 36) and the UCDAVIS replication cohort (n = 34). (D) MIP3a (pg/ml) stratified by MLN diameter in patients with idiopathic pulmonary fibrosis, chronic hypersensitivity pneumonitis, or chronic hypersensitivity pneumonitis–ILD within the UCHICAGO primary cohort (n = 80) and the UCDAVIS replication cohort (n = 84). Group comparisons for patients with MLNs ≥10 mm (green or red) to patients with MLNs <10 mm (gray) were conducted using the Wilcoxon signed-rank test for matched nonparametric data. All comparisons of cytokine concentrations were conducted in 10,000 bootstrap replications to improve precision at the 95% confidence interval level. EGF = epidermal growth factor; sCD40L = soluble CD40 ligand.
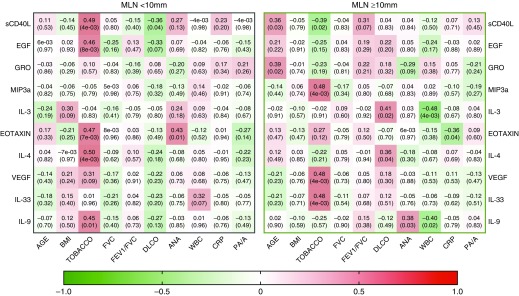
Analysis of the top differing cytokines by MLN enlargement revealed differences in correlation between these inflammatory modulators and key clinical variables (Figure 4). Among subjects without enlarged MLNs, tobacco exposure correlated positively with sCD40L, EGF, eotaxin, IL-4, and IL-9 (r = 0.49, P = 0.003; r = 0.46, P = 0.008; r = 0.47, P = 0.007; r = 0.50, P = 0.004; and r = 0.45, P = 0.01, respectively) whereas serum ANA titers correlated positively with eotaxin alone (r = 0.43, P = 0.01). Conversely, among subjects with enlarged MLNs, tobacco exposure correlated negatively with circulating levels of sCD40L (r = –0.39, P = 0.02) but positively with MIP-3a, vascular endothelial growth factor, and IL-33 (r = 0.48, P = 0.004; r = 0.48, P = 0.004; and r = 0.48, P = 0.004, respectively). Within this subgroup, total WBC count also correlated negatively with IL-3 and IL-9 (r = –0.48, P = 0.004 and r = 0.40, P = 0.02, respectively). Importantly, only elevated plasma IL-10 >45 pg/ml independently predicted mortality (HR, 4.21; 95% CI, 1.21–14.68; P = 0.024), and this finding was independently replicated in the UCDAVIS cohort (Figure E6 and Table E13).
Figure 4.
Correlation of top cytokines with clinically acquired biologic variables stratified by mediastinal lymph node (MLN) size. Left panel depicts correlation matrix for MLNs <10 mm, whereas right panel depicts correlation matrix for MLNs ≥10 mm. Cytokine correlation with clinical variable was determined by Pearson’s correlation algorithm and is displayed in the corresponding box (coefficient of r value on top and P value in parentheses). The color of each box represents the directionality of the correlation (red indicates positive correlation; green indicates negative correlation). The bar below scales the degree of correlation. Pearson’s correlation was used to determine the significance of correlation (P < 0.05) between the concentrations of individual cytokines with clinical variables as shown above. ANA = antinuclear antibody; BMI = body mass index; CRP = C-reactive protein; PA/A = pulmonary artery/aorta ratio; WBC = white blood cell.
There were no significant differences (P = 0.68) in the use of immunosuppression between patients with and without enlarged MLNs. Furthermore, results remained consistent after excluding all subjects who had received immunosuppressive therapy in the cytokine subset. We also did not find immunosuppressive therapy use as an independent predictor of MLN enlargement in the cytokine subset or in the overall UCHICAGO, NONTERT, and UCDAVIS replication cohorts (P = 0.26, 0.75, and 0.88, respectively, multivariable regression model).
Discussion
The central finding of this study is that identification of enlarged MLNs on chest CT at baseline ILD evaluation is associated with increased mortality, worse lung function, and increased risk of hospitalization. We also report for the first time that increased MLN diameter is associated with a greater proportion of circulating lymphocytes, lower plasma concentration of cytokine sCD40L, as well as a change in correlation between key immunomodulatory cytokines and baseline variables of importance in ILD. This raises the possibility that clinically significant increase in MLN size may be reflective of underlying immunologic phenomena in patients with advanced ILD. Clinical outcomes varied with the size, location, and number of enlarged MLNs, indicating significant involvement of the immune system in the pathobiology of ILD. Importantly, patients with the greatest circulating levels of IL-10 in our study had the worst survival. In addition, we demonstrate the consistency of these findings across tertiary and nontertiary medical centers, and in an independent nationally acquired ILD dataset.
Several factors support the hypothesis that enlarged MLNs are of prognostic value in ILD. First, there is a strong biologic rationale for MLN assessment in thoracic disease. Pulmonary disorders with a robust immunologic response are frequently characterized by enlarged MLNs (31–37), which are a hallmark of ongoing immune response within the tissue. We have recently demonstrated that IPF lungs contain high numbers of immune cells, especially T cells, and that these T cells have distinct phenotypic markers when compared with lung T cells from patients without fibrotic lung disease (11). We found these same phenotypic differences in the MLNs, which suggests the trafficking of effector immune cells from the lungs to the MLNs (11). These findings suggest that enlargement and changes in cellular composition of MLNs may contribute to the pathophysiology of disease progression in pulmonary fibrosis. However, MLN enlargement may also be a marker of disease severity or a hallmark of a protective immune response that increases with disease severity. Second, the total number of enlarged MLNs has previously been linked to the severity of pulmonary fibrosis in ILD (38). Third, radiologic MLN assessment has been repeatedly demonstrated to be critical in staging and prognostication of numerous pulmonary diseases, including sarcoidosis and lung cancer (22, 39–42). Increased MLN size has been strongly linked to more advanced stages of malignancy and greater mortality (43, 44). Fourth, there is abundant evidence in health and disease that noninvasive CT assessment of MLN enlargement correlates fairly well with more invasive techniques such as endobronchial ultrasound measurements (21, 45). Thus, preliminary CT evaluation is almost always used to guide MLN sampling and tissue procurement for diagnosis and planning therapy. Taken together, these findings strongly suggest that MLN assessment is an important determinant of outcomes.
The results of this investigation support the utility of noninvasive MLN assessment on routinely obtained CT at baseline ILD evaluation. We highlight the value of this technique for predicting ILD subpopulations with differential survival patterns, hospitalization risk, and differences in lung function severity at baseline. Importantly, our results show that the absence of MLN enlargement identifies a subset of patients with ILD who have a significant survival advantage and possibly a unique clinical phenotype. The consistency of our findings in diverse ILDs and across tertiary and nontertiary centers provides evidence for the potential inclusion of MLN assessment into risk prediction models as a powerful and reliable index providing added value to established prognostic metrics such as the sex/age/physiology score (18, 46). Although our results demonstrated consistency across all major ILD categories, the MLN findings were most striking within the IPAF and unclassifiable ILD subgroups, both of which are heterogeneous disease categories that likely include patients spanning the spectrum of more IPF-like to more non–IPF-like. The stronger association of MLN enlargement with outcomes in IPF compared with CHP or CTD-ILD may indicate the potential value of MLN enlargement and plasma cytokines (sCD40L and EGF) in the subclassification of ILD subtypes currently deemed unclassifiable.
Our study demonstrates key pathobiologic differences between patients with and without enlarged MLNs. Notably, tobacco exposure and positive ANA titers are prevalent in patients with ILD (47), and the observed subgroup-related differences in plasma cytokine correlation implicates specific cytokine responses in these exposure-linked processes. This investigation also revealed differences in the plasma concentrations of multiple cytokines among patients with enlarged MLNs. Of these, the most significant change was the reduction in plasma concentration of sCD40L, a well-known immunomodulatory factor (48). The CD40/CD40L signaling pathway is fundamental to dendritic cell activity with an immunosuppressive effect in chronic disease states (48, 49). sCD40L is released after cleavage of CD40L from the cell surface of activated T cells and can induce activation and differentiation of B cells (40).
In recent studies, circulating levels of sCD40L and IL-12 increased after treatment with rituximab, a chimeric monoclonal anti-CD20 antibody that depletes peripheral B cells (50, 51). Likewise, a rituximab-mediated increase in the acute production and release of IL-6 has been demonstrated in vitro (52). Interestingly, all three cytokines were decreased in our study cohort with enlarged MLNs, and this patient subgroup had concurrently worse clinical outcomes. An ongoing study is currently evaluating the beneficial therapeutic effect of VAY736, a fully human IgG1 monoclonal antibody that, similar to rituximab, targets B cells in patients with IPF and coexistent MLN enlargement, lending credence to the hypothesis that elevating plasma levels of sCD40L, IL-12p70, and IL-6 in the circulation might be protective in fibrotic ILD and lead to more favorable prognosis (ClinicalTrials.gov no. NCT03287414).
EGF is a mitogenic protein with high affinity for its tyrosine kinase receptor—EGF receptor (EGFR) (53). Alveolar upregulation of EGFR occurs in several ILD subtypes, including IPF, fibrotic NSIP, and cryptogenic organizing pneumonia, suggesting an important role for EGFR in the pathogenesis of abnormal reepithelialization (54–56). Furthermore, inhibition of EGFR has been strongly linked to ILD pathogenesis, with EGFR inhibitor therapy frequently resulting in the development and worsening of ILD (57–59). Our findings of reduced plasma EGF concentration in the ILD subset with enlarged MLNs further strengthen the pathobiology linking the EGFR pathway to ILD risk and disease severity.
Also, we observed a detrimental effect of elevated IL-10 plasma levels in both patients with MLN enlargement and those without enlarged MLNs. The anti-inflammatory cytokine IL-10 regulates proinflammatory responses and is deemed critically important to the pathologic processes that determine chronicity of pulmonary fibrosis (60–62). Circulating fibrocytes involved in tissue fibrosis are significantly elevated in patients with IPF, and preclinical models have associated the overexpression of circulating IL-10 with fibrocyte recruitment (61, 63). In addition, hyperplastic alveolar epithelial cells within lung biopsies from patients with IPF are a prominent source of IL-10, and continued cellular production could amplify the processes leading to pulmonary fibrosis (64). Our study demonstrates increased mortality in patients with elevated circulating levels of IL-10, underscoring its potential utility as a cytokine biomarker for disease progression in ILD.
The expansion of circulating lymphocytes in the subpopulation with enlarged MLNs supports the concept that altered gene expression patterns of immunologic pathways initiate and amplify the processes that determine progression of pulmonary fibrosis (13, 65). As dysregulated immune signaling and aberrant innate immune responses characterize disease progression and are associated with altered circulating leukocyte phenotypes, the enlarged MLNs in pulmonary fibrosis may be structural evidence of the increased lymphocyte trafficking that occurs during this process. Interestingly, differences in circulating levels of MIP3a between both subgroups were remarkable and approached statistical significance. MIP3a is a chemokine expressed in lymph nodes and is strongly chemotactic for lymphocytes and dendritic cells (66, 67). The increased plasma level of MIP3a suggests its involvement in the processes leading to MLN enlargement in patients with ILD. Our findings are consistent with previous studies that negatively correlated increased gene expression of specific immunomodulatory pathways with TFS in fibrotic ILD (13). Interestingly, the top canonical pathways with upregulated genes enriched in IPF include IL-10, IL-17A, and IL-6 signaling pathways (13, 65); these same cytokines were among the top immunomodulatory mediators that differed when comparing our study subpopulation with MLN enlargement to those without enlarged MLNs.
Our study had several limitations. First, although most CT scans available for review were high-resolution ILD protocol images, baseline chest CT scans for a few patients were standard chest CT scans, and we acknowledge the differences in these imaging modalities for evaluating parenchymal abnormalities. However, all included CT scans in our study had appropriate image quality for MLN assessment. Furthermore, our study encompasses a longitudinal ILD cohort acquired during a decade and the variation in CT image quality may reflect advances in radiologic diagnostic techniques during this period. Second, for consistency in terminology, our MLN assessment was strictly restricted to thoracic lymph node stations within the mediastinum (superior mediastinal, inferior mediastinal, para-aortic, and subaortic nodal stations) as defined by International Association for the Study of Lung Cancer nomenclature (68). Thus, hilar and interlobar lymph nodes were not assessed. Third, our observations of intrathoracic MLN enlargement might have been concurrent with extrathoracic lymphadenopathy as part of a generalized process. However, our study was focused on thoracic lymph nodes and not designed to assess other regional lymph nodes for enlargement. Fourth, the results of this investigation are from analyses of data acquired from healthcare settings that may potentially be affected by selection biases and not represent the general population. However, the prevalence of MLN enlargement in the general population is unknown, and most data in ILD are obtained from subjects evaluated within the healthcare setting. To minimize the influence of healthcare-related effect modifiers on MLN characteristics, we utilized only baseline variables and CT scans obtained at initial ILD evaluation for assessing MLN features. The replication of our results across tertiary and nontertiary medical centers, as well as in an independent nationally acquired ILD cohort, strengthens the external validity of these findings.
Conclusions
MLN enlargement strongly predicts clinical outcomes in patients with ILD. Targeting MLN parameters similar to those of healthy subjects may improve prognosis in ILD; therefore, future studies are needed to determine the value of MLN assessment in predicting response to therapy.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the support staff of the University of Chicago Respiratory Clinical Research Unit, the Interstitial Lung Disease Clinic (Nancy Trojan, Catherine Brown, and Spring Holland), and NorthShore University HealthSystem in Evanston, IL (Mohammad Imran Beig, Paul Chung, Kathleen M. Biblowitz, Daisy Zhu, and Naomi BenIsrael Olive), for assistance with this study. The authors also extend gratitude to the patients with ILD who made these research endeavors possible.
Footnotes
Supported by grants from the NIH (R21AI126031, K12HL119995, K23HL138190, R01AI125644, and R01HL130796)). The Kohn and Mitchell Family Foundation also provided support for this study. Data from this study were provided by the Clinical Research Data Warehouse maintained by the Center for Research Informatics at University of Chicago. The Center for Research Informatics is funded by the Biological Sciences Division, the Institute for Translational Medicine/Clinical and Translational Science Award (NIH grant UL1 TR000430) at the University of Chicago.
Author Contributions: Conception and design: A.A., J.M.O., A.I.S., I.N., M.E.S., and J.H.C. Acquisition of data for the work: A.A., J.M.O., C.B., C.H., P.N., W.K., S.B., U.M., K.T., J.V.P., A.N.H., S.M.M., C.M.S., R.V., A.I.S., I.N., M.E.S., and J.H.C. Analysis and interpretation: A.A., J.M.O., C.B., C.H., P.N., W.K., S.B., U.M., K.T., J.V.P., A.N.H., S.M.M., C.M.S., R.V., A.I.S., I.N., M.E.S., and J.H.C. Drafting the manuscript for important intellectual content: A.A., J.M.O., C.B., C.H., P.N., W.K., S.B., U.M., K.T., J.V.P., A.N.H., S.M.M., C.M.S., R.V., A.I.S., I.N., M.E.S., and J.H.C. Critical revision for important intellectual content: all authors.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201804-0761OC on September 14, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs RW, Morozoff C, Shirude S, Naghavi M, et al. Trends and patterns of differences in chronic respiratory disease mortality among US counties, 1980–2014. JAMA. 2017;318:1136–1149. doi: 10.1001/jama.2017.11747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raghu G. Epidemiology, survival, incidence and prevalence of idiopathic pulmonary fibrosis in the USA and Canada. Eur Respir J. 2017;49:1602384. doi: 10.1183/13993003.02384-2016. [DOI] [PubMed] [Google Scholar]
- 3.Raghu G, Chen SY, Hou Q, Yeh WS, Collard HR. Incidence and prevalence of idiopathic pulmonary fibrosis in US adults 18–64 years old. Eur Respir J. 2016;48:179–186. doi: 10.1183/13993003.01653-2015. [DOI] [PubMed] [Google Scholar]
- 4.Walsh SL. Multidisciplinary evaluation of interstitial lung diseases: current insights: number 1 in the series “Radiology” edited by Nicola Sverzellati and Sujal Desai. Eur Respir Rev. 2017;26:170002. doi: 10.1183/16000617.0002-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung JH, Chawla A, Peljto AL, Cool CD, Groshong SD, Talbert JL, et al. CT scan findings of probable usual interstitial pneumonitis have a high predictive value for histologic usual interstitial pneumonitis. Chest. 2015;147:450–459. doi: 10.1378/chest.14-0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung JH, Zhan X, Cao M, Koelsch TL, Manjarres DCG, Brown KK, et al. Presence of air trapping and mosaic attenuation on chest computed tomography predicts survival in chronic hypersensitivity pneumonitis. Ann Am Thorac Soc. 2017;14:1533–1538. doi: 10.1513/AnnalsATS.201701-035OC. [DOI] [PubMed] [Google Scholar]
- 7.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 9.Souza CA, Müller NL, Lee KS, Johkoh T, Mitsuhiro H, Chong S. Idiopathic interstitial pneumonias: prevalence of mediastinal lymph node enlargement in 206 patients. AJR Am J Roentgenol. 2006;186:995–999. doi: 10.2214/AJR.04.1663. [DOI] [PubMed] [Google Scholar]
- 10.Lynch DA, Sverzellati N, Travis WD, Brown KK, Colby TV, Galvin JR, et al. Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society White Paper. Lancet Respir Med. 2018;6:138–153. doi: 10.1016/S2213-2600(17)30433-2. [DOI] [PubMed] [Google Scholar]
- 11.Adegunsoye A, Hrusch CL, Bonham CA, Jaffery MR, Blaine KM, Sullivan M, et al. Skewed lung CCR4 to CCR6 CD4+ T cell ratio in idiopathic pulmonary fibrosis is associated with pulmonary function. Front Immunol. 2016;7:516. doi: 10.3389/fimmu.2016.00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Lesko M, Badri MH, Kapoor BC, Wu BG, Li Y, et al. Lung microbiome and host immune tone in subjects with idiopathic pulmonary fibrosis treated with inhaled interferon-γ. ERJ Open Res. 2017;3:00008-2017. doi: 10.1183/23120541.00008-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang Y, Ma SF, Espindola MS, Vij R, Oldham JM, Huffnagle GB, et al. COMET-IPF Investigators. Microbes are associated with host innate immune response in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2017;196:208–219. doi: 10.1164/rccm.201607-1525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Travis WD, Costabel U, Hansell DM, King TE, Jr, Lynch DA, Nicholson AG, et al. ATS/ERS Committee on Idiopathic Interstitial Pneumonias. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer A, Antoniou KM, Brown KK, Cadranel J, Corte TJ, du Bois RM, et al. “ERS/ATS Task Force on Undifferentiated Forms of CTD-ILD”. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur Respir J. 2015;46:976–987. doi: 10.1183/13993003.00150-2015. [DOI] [PubMed] [Google Scholar]
- 16.Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, et al. American Thoracic Society European Respiratory society Japanese Respiratory Society Latin American Thoracic Association An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline Am J Respir Crit Care Med 2015192e3–e19.[Published erratum appears in Am J Respir Crit Care Med 2015;192:644.]26177183 [Google Scholar]
- 17.Adegunsoye A, Oldham JM, Chung JH, Montner SM, Lee C, Witt LJ, et al. Phenotypic clusters predict outcomes in a longitudinal interstitial lung disease cohort. Chest. 2018;153:349–360. doi: 10.1016/j.chest.2017.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryerson CJ, Vittinghoff E, Ley B, Lee JS, Mooney JJ, Jones KD, et al. Predicting survival across chronic interstitial lung disease: the ILD-GAP model. Chest. 2014;145:723–728. doi: 10.1378/chest.13-1474. [DOI] [PubMed] [Google Scholar]
- 19.Peljto AL, Zhang Y, Fingerlin TE, Ma SF, Garcia JG, Richards TJ, et al. Association between the MUC5B promoter polymorphism and survival in patients with idiopathic pulmonary fibrosis. JAMA. 2013;309:2232–2239. doi: 10.1001/jama.2013.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King TE, Jr, Albera C, Bradford WZ, Costabel U, Hormel P, Lancaster L, et al. INSPIRE Study Group. Effect of interferon gamma-1b on survival in patients with idiopathic pulmonary fibrosis (INSPIRE): a multicentre, randomised, placebo-controlled trial. Lancet. 2009;374:222–228. doi: 10.1016/S0140-6736(09)60551-1. [DOI] [PubMed] [Google Scholar]
- 21.Udoji TN, Phillips GS, Berkowitz EA, Berkowitz D, Ross C, Bechara RI. Mediastinal and hilar lymph node measurements. comparison of multidetector-row computed tomography and endobronchial ultrasound. Ann Am Thorac Soc. 2015;12:914–920. doi: 10.1513/AnnalsATS.201312-430OC. [DOI] [PubMed] [Google Scholar]
- 22.Walker CM, Chung JH, Abbott GF, Little BP, El-Sherief AH, Shepard JA, et al. Mediastinal lymph node staging: from noninvasive to surgical. AJR Am J Roentgenol. 2012;199:W54–W64. doi: 10.2214/AJR.11.7446. [DOI] [PubMed] [Google Scholar]
- 23.Glazer GM, Gross BH, Quint LE, Francis IR, Bookstein FL, Orringer MB. Normal mediastinal lymph nodes: number and size according to American Thoracic Society mapping. AJR Am J Roentgenol. 1985;144:261–265. doi: 10.2214/ajr.144.2.261. [DOI] [PubMed] [Google Scholar]
- 24.Guler SA, Ellison K, Algamdi M, Collard HR, Ryerson CJ. Heterogeneity in unclassifiable interstitial lung disease. a systematic review and meta-analysis. Ann Am Thorac Soc. 2018;15:854–863. doi: 10.1513/AnnalsATS.201801-067OC. [DOI] [PubMed] [Google Scholar]
- 25.Collins BF, Spiekerman CF, Shaw MA, Ho LA, Hayes J, Spada CA, et al. Idiopathic interstitial pneumonia associated with autoantibodies: a large case series followed over 1 year. Chest. 2017;152:103–112. doi: 10.1016/j.chest.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adegunsoye A, Oldham JM, Bellam SK, Chung JH, Chung PA, Biblowitz KM, et al. African-American race and mortality in interstitial lung disease: a multicentre propensity-matched analysis. Eur Respir J. 2018;51:1800255. doi: 10.1183/13993003.00255-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
- 28.Shin S, King CS, Puri N, Shlobin OA, Brown AW, Ahmad S, et al. Pulmonary artery size as a predictor of outcomes in idiopathic pulmonary fibrosis. Eur Respir J. 2016;47:1445–1451. doi: 10.1183/13993003.01532-2015. [DOI] [PubMed] [Google Scholar]
- 29.Gleason JB, Patel KB, Hernandez F, Hadeh A, Highland KB, Rahaghi F, et al. Pulmonary artery dimensions as a prognosticator of transplant-free survival in scleroderma interstitial lung disease. Hai. 2017;195:403–409. doi: 10.1007/s00408-017-0005-6. [DOI] [PubMed] [Google Scholar]
- 30.Chin M, Johns C, Currie BJ, Weatherley N, Hill C, Elliot C, et al. Pulmonary artery size in interstitial lung disease and pulmonary hypertension: association with interstitial lung disease severity and diagnostic utility. Front Cardiovasc Med. 2018;5:53. doi: 10.3389/fcvm.2018.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomlinson GS, Thomas N, Chain BM, Best K, Simpson N, Hardavella G, et al. Transcriptional profiling of endobronchial ultrasound-guided lymph node samples aids diagnosis of mediastinal lymphadenopathy. Chest. 2016;149:535–544. doi: 10.1378/chest.15-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeong YJ, Lee KS. Pulmonary tuberculosis: up-to-date imaging and management. AJR Am J Roentgenol. 2008;191:834–844. doi: 10.2214/AJR.07.3896. [DOI] [PubMed] [Google Scholar]
- 33.Kirchner J, Kirchner EM, Goltz JP, Obermann A, Kickuth R. Enlarged hilar and mediastinal lymph nodes in chronic obstructive pulmonary disease. J Med Imaging Radiat Oncol. 2010;54:333–338. doi: 10.1111/j.1754-9485.2010.02179.x. [DOI] [PubMed] [Google Scholar]
- 34.Barker R, Kazmi F, Bower M. Imaging in multicentric Castleman’s disease. J HIV Ther. 2008;13:72–74. [PubMed] [Google Scholar]
- 35.Brown JR, Skarin AT. Clinical mimics of lymphoma. Oncologist. 2004;9:406–416. doi: 10.1634/theoncologist.9-4-406. [DOI] [PubMed] [Google Scholar]
- 36.Odoardi F, Sie C, Streyl K, Ulaganathan VK, Schläger C, Lodygin D, et al. T cells become licensed in the lung to enter the central nervous system. Nature. 2012;488:675–679. doi: 10.1038/nature11337. [DOI] [PubMed] [Google Scholar]
- 37.Franquet T. Imaging of pulmonary viral pneumonia. Radiology. 2011;260:18–39. doi: 10.1148/radiol.11092149. [DOI] [PubMed] [Google Scholar]
- 38.Jung JI, Kim HH, Jung YJ, Park SH, Lee JM, Hahn ST. Mediastinal lymphadenopathy in pulmonary fibrosis: correlation with disease severity. J Comput Assist Tomogr. 2000;24:706–710. doi: 10.1097/00004728-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Dooms C, Tournoy KG, Schuurbiers O, Decaluwe H, De Ryck F, Verhagen A, et al. Endosonography for mediastinal nodal staging of clinical N1 non-small cell lung cancer: a prospective multicenter study. Chest. 2015;147:209–215. doi: 10.1378/chest.14-0534. [DOI] [PubMed] [Google Scholar]
- 40.Fischer BM, Mortensen J, Hansen H, Vilmann P, Larsen SS, Loft A, et al. Multimodality approach to mediastinal staging in non-small cell lung cancer. Faults and benefits of PET-CT: a randomised trial. Thorax. 2011;66:294–300. doi: 10.1136/thx.2010.154476. [DOI] [PubMed] [Google Scholar]
- 41.Koo HJ, Kim MY, Shin SY, Shin S, Kim SS, Lee SW, et al. Evaluation of mediastinal lymph nodes in sarcoidosis, sarcoid reaction, and malignant lymph nodes using CT and FDG-PET/CT. Medicine (Baltimore) 2015;94:e1095. doi: 10.1097/MD.0000000000001095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boujaoude Z, Dahdel M, Pratter M, Kass J. Endobronchial ultrasound with transbronchial needle aspiration in the diagnosis of bilateral hilar and mediastinal lymphadenopathy. J Bronchology Interv Pulmonol. 2012;19:19–23. doi: 10.1097/LBR.0b013e3182442b89. [DOI] [PubMed] [Google Scholar]
- 43.Rusch VW, Crowley J, Giroux DJ, Goldstraw P, Im JG, Tsuboi M, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the N descriptors in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2007;2:603–612. doi: 10.1097/JTO.0b013e31807ec803. [DOI] [PubMed] [Google Scholar]
- 44.Hung JJ, Jeng WJ, Hsu WH, Lin SF, Hsieh CC, Huang BS, et al. Prognostic factors in pathological stage IB nonsmall cell lung cancer greater than 3 cm. Eur Respir J. 2010;36:1355–1361. doi: 10.1183/09031936.00014109. [DOI] [PubMed] [Google Scholar]
- 45.Dhooria S, Agarwal R, Aggarwal AN, Gupta N, Gupta D, Behera D. Agreement of mediastinal lymph node size between computed tomography and endobronchial ultrasonography: a study of 617 patients. Ann Thorac Surg. 2015;99:1894–1898. doi: 10.1016/j.athoracsur.2015.02.055. [DOI] [PubMed] [Google Scholar]
- 46.Ley B, Ryerson CJ, Vittinghoff E, Ryu JH, Tomassetti S, Lee JS, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012;156:684–691. doi: 10.7326/0003-4819-156-10-201205150-00004. [DOI] [PubMed] [Google Scholar]
- 47.Bauer PR, Kalra S, Osborn TG, St Sauver J, Hanson AC, Schroeder DR, et al. Influence of autoimmune biomarkers on interstitial lung diseases: A tertiary referral center based case-control study. Respir Med. 2015;109:397–405. doi: 10.1016/j.rmed.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jenabian MA, Patel M, Kema I, Vyboh K, Kanagaratham C, Radzioch D, et al. Soluble CD40-ligand (sCD40L, sCD154) plays an immunosuppressive role via regulatory T cell expansion in HIV infection. Clin Exp Immunol. 2014;178:102–111. doi: 10.1111/cei.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daoussis D, Andonopoulos AP, Liossis SN. Targeting CD40L: a promising therapeutic approach. Clin Diagn Lab Immunol. 2004;11:635–641. doi: 10.1128/CDLI.11.4.635-641.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Myers RP, Swain MG, Lee SS, Shaheen AA, Burak KW. B-cell depletion with rituximab in patients with primary biliary cirrhosis refractory to ursodeoxycholic acid. Am J Gastroenterol. 2013;108:933–941. doi: 10.1038/ajg.2013.51. [DOI] [PubMed] [Google Scholar]
- 51.Brunekreeft KL, Strohm C, Gooden MJ, Rybczynska AA, Nijman HW, Grigoleit GU, et al. Targeted delivery of CD40L promotes restricted activation of antigen-presenting cells and induction of cancer cell death. Mol Cancer. 2014;13:85. doi: 10.1186/1476-4598-13-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones JD, Hamilton BJ, Skopelja S, Rigby WF. Induction of interleukin-6 production by rituximab in human B cells. Arthritis Rheumatol. 2014;66:2938–2946. doi: 10.1002/art.38798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys. 2004;59(Suppl):21–26. doi: 10.1016/j.ijrobp.2003.11.041. [DOI] [PubMed] [Google Scholar]
- 54.Baughman RP, Lower EE, Miller MA, Bejarano PA, Heffelfinger SC. Overexpression of transforming growth factor-alpha and epidermal growth factor-receptor in idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:57–61. [PubMed] [Google Scholar]
- 55.Plantier L, Crestani B, Wert SE, Dehoux M, Zweytick B, Guenther A, et al. Ectopic respiratory epithelial cell differentiation in bronchiolised distal airspaces in idiopathic pulmonary fibrosis. Thorax. 2011;66:651–657. doi: 10.1136/thx.2010.151555. [DOI] [PubMed] [Google Scholar]
- 56.Tzouvelekis A, Ntolios P, Karameris A, Vilaras G, Boglou P, Koulelidis A, et al. Increased expression of epidermal growth factor receptor (EGF-R) in patients with different forms of lung fibrosis. BioMed Res Int. 2013;2013:654354. doi: 10.1155/2013/654354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li C, Wei R, Jones-Hall YL, Vittal R, Zhang M, Liu W. Epidermal growth factor receptor (EGFR) pathway genes and interstitial lung disease: an association study. Sci Rep. 2014;4:4893. doi: 10.1038/srep04893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shi L, Tang J, Tong L, Liu Z. Risk of interstitial lung disease with gefitinib and erlotinib in advanced non-small cell lung cancer: a systematic review and meta-analysis of clinical trials. Lung Cancer. 2014;83:231–239. doi: 10.1016/j.lungcan.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 59.Kudoh S, Kato H, Nishiwaki Y, Fukuoka M, Nakata K, Ichinose Y, et al. Japan Thoracic Radiology Group. Interstitial lung disease in Japanese patients with lung cancer: a cohort and nested case-control study. Am J Respir Crit Care Med. 2008;177:1348–1357. doi: 10.1164/rccm.200710-1501OC. [DOI] [PubMed] [Google Scholar]
- 60.Rothman N, Skibola CF, Wang SS, Morgan G, Lan Q, Smith MT, et al. Genetic variation in TNF and IL10 and risk of non-Hodgkin lymphoma: a report from the InterLymph Consortium. Lancet Oncol. 2006;7:27–38. doi: 10.1016/S1470-2045(05)70434-4. [DOI] [PubMed] [Google Scholar]
- 61.Sun L, Louie MC, Vannella KM, Wilke CA, LeVine AM, Moore BB, et al. New concepts of IL-10-induced lung fibrosis: fibrocyte recruitment and M2 activation in a CCL2/CCR2 axis. Am J Physiol Lung Cell Mol Physiol. 2011;300:L341–L353. doi: 10.1152/ajplung.00122.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kolahian S, Fernandez IE, Eickelberg O, Hartl D. Immune mechanisms in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2016;55:309–322. doi: 10.1165/rcmb.2016-0121TR. [DOI] [PubMed] [Google Scholar]
- 63.Moeller A, Gilpin SE, Ask K, Cox G, Cook D, Gauldie J, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:588–594. doi: 10.1164/rccm.200810-1534OC. [DOI] [PubMed] [Google Scholar]
- 64.Millar AB. IL-10: another therapeutic target in idiopathic pulmonary fibrosis? Thorax. 2006;61:835–836. doi: 10.1136/thx.2006.060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herazo-Maya JD, Noth I, Duncan SR, Kim S, Ma SF, Tseng GC, et al. Peripheral blood mononuclear cell gene expression profiles predict poor outcome in idiopathic pulmonary fibrosis. Sci Transl Med. 2013;5:205ra136. doi: 10.1126/scitranslmed.3005964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hieshima K, Imai T, Opdenakker G, Van Damme J, Kusuda J, Tei H, et al. Molecular cloning of a novel human CC chemokine liver and activation-regulated chemokine (LARC) expressed in liver. Chemotactic activity for lymphocytes and gene localization on chromosome 2. J Biol Chem. 1997;272:5846–5853. doi: 10.1074/jbc.272.9.5846. [DOI] [PubMed] [Google Scholar]
- 67.Rossi DL, Vicari AP, Franz-Bacon K, McClanahan TK, Zlotnik A. Identification through bioinformatics of two new macrophage proinflammatory human chemokines: MIP-3alpha and MIP-3beta. J Immunol. 1997;158:1033–1036. [PubMed] [Google Scholar]
- 68.Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P Members of IASLC Staging Committee. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4:568–577. doi: 10.1097/JTO.0b013e3181a0d82e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.