Abstract
The taxonomic status, biotechnological and ecological potential of several Micromonospora strains isolated from an extreme hyper arid Atacama Desert soil were determined. Initially, a polyphasic study was undertaken to clarify the taxonomic status of five micromonosporae, strains LB4, LB19, LB32T, LB39T and LB41, isolated from an extreme hyper-arid soil collected from one of the driest regions of the Atacama Desert. All of the isolates were found to have chemotaxonomic, cultural and morphological properties consistent with their classification in the genus Micromonospora. Isolates LB32T and LB39T were distinguished from their nearest phylogenetic neighbours and proposed as new species, namely as Micromonospora arida sp. nov. and Micromonospora inaquosa sp. nov., respectively. Eluted methanol extracts of all of the isolates showed activity against a panel of bacterial and fungal indicator strains, notably against multi-drug resistant Klebsiella pneumoniae ATCC 700603 while isolates LB4 and LB41 showed pronounced anti-tumour activity against HepG2 cells. Draft genomes generated for the isolates revealed a rich source of novel biosynthetic gene clusters, some of which were unique to individual strains thereby opening up the prospect of selecting especially gifted micromonosporae for natural product discovery. Key stress-related genes detected in the genomes of all of the isolates provided an insight into how micromonosporae adapt to the harsh environmental conditions that prevail in extreme hyper-arid Atacama Desert soils.
Introduction
New natural products, especially antibiotics, are needed to control the spread of multi-drug resistant (MDR) microbial pathogens, as exemplified by MDR-resistant Gram-negative bacteria that are associated with high mortality rates1,2. Amongst prokaryotes, filamentous bacteria in the class Actinobacteria3 of the phylum Actinobacteria4 have a unique track record as a source of novel specialised (secondary) metabolites5,6. Despite this, the costly, repeated rediscovery of known chemical entities from common filamentous actinobacteria contributed to the sharp decline in the search for new clinically relevant antibiotics towards the end of the last century7,8. However, the discovery that the genomes of filamentous actinobacteria contained many biosynthetic gene clusters (BGCs) that encode for biosynthetic pathways of known and predicted specialised metabolites sparked a renewed interest in these organisms as a source of new chemical scaffolds9,10. Especially “gifted” (sensu Baltz11) actinobacteria known to have large genomes (>8.0 Mb) rich in BGCs, include Streptomyces strains12–14 and representatives of historically understudied taxa, such as the genera Amycolatopsis15, Micromonospora16 and Saccharothrix17. New approaches to the selective isolation, dereplication and screening of novel filamentous actinobacteria from neglected and unusual habitats also contributed towards the revival of interest in these organisms as a source of new specialised metabolites18,19.
Novel filamentous actinobacteria, notably streptomycetes, isolated from arid Atacama Desert soils are a fruitful source of new specialised metabolites19–21 underscoring the premise that extreme environmental conditions give rise to a unique actinobacterial diversity that is the basis of a novel chemistry20,22,23. Complementary metagenomic surveys of Atacama Desert habitats have revealed an enormous actinobacterial diversity most of which went undetected in corresponding culture-dependent studies24,25. Improved selective isolation and growth procedures can be expected to address this disparity between culture-dependent and culture-independent data, as illustrated by the discovery that members of the genus Micromonospora can be isolated from Atacama Desert soils26. This is an interesting development as micromonosporae are second only to streptomycetes as a source of new specialised metabolites27,28.
Carro and colleagues16 found that the genomes of representative Micromonospora type strains are a source of novel BGCs, many of which are characteristic of either individual species or groups of phylogenetically related species. Comparative genomics have also revealed phylogenetically distributed patterns of new specialised metabolites amongst members of the genus Amycolatopsis29. These observations open up the prospect of prioritising representatives of novel and rare actinobacterial taxa in the search for new specialised metabolites using state-of-the-art technologies; such as genome mining procedures9,30, with particular emphasis on strategies designed to activate cryptic (silent) biosynthetic gene clusters11,31.
The present study was designed to establish the taxonomic provenance of five Micromonospora strains isolated from an extreme hyper-arid Atacama Desert soil with a particular focus on their biotechnological and ecological potential. The isolates are known to be genetically diverse and four of them, namely LB4, LB39T and LB19 and LB32T, were found to be most closely related to the type strains of Micromonospora chalcea32,33, the type species of the genus, Micromonospora chokoriensis34 and Micromonospora saelicesensis35, respectively, based on 16S rRNA gene sequence similarities26. The isolates and their closest phylogenetic neighbours were the subject of an extensive polyphasic taxonomic study which showed that isolates LB32T and LB39T represent new Micromonospora species for which the names Micromonospora arida and Micromonospora inaquosa are proposed. Genomes generated from all of the isolates were found to harbour many new BGCs, and carried genes adapted to deal with environmental stress that reflect their ability to adapt to extreme environmental conditions that prevail in the Atacama Desert.
Results and Discussion
Cultural, chemotaxonomic, morphological and genomic properties of the isolates
In general, the cultural, chemotaxonomic and morphological properties of the isolates were consistent with their classification in the genus Micromonospora16,28. The isolates were Gram-stain positive, formed extensively branched, non-fragmented substrate hyphae bearing single, non-motile spores, lacked aerial hyphae, produced orange colonies which turned brown-black on spore formation, contained meso-A2pm acid and glucose, mannose and xylose in whole-organism hydrolysates, branched chain fatty acids, hydrogenated menaquinones with nine and/or ten isoprene units and polar lipid patterns containing diphosphatidylglycerol, phosphatidylethanolamine (diagnostic lipid) and phosphatidylinositol (phospholipid type 2 sensu Lechevalier et al.36). The RAPD’s profiles of the isolates (Fig. S1) underpinned their genetic diversity; though isolates LB4 and LB41 gave similar profiles.
The draft genomes of isolates LB4, LB19, LB32T, LB39T and LB41 have been deposited in GenBank under accession numbers QGSX00000000, QDGB00000000, QGSY00000000, QGSZ00000000, QGTA00000000, respectively, and are publically available. Key characteristics of the genomes are shown in Table 1; the number of contigs ranges from 339 to 1725, and the number of genes from 4976 in isolate LB4 to 7013 in LB39T. RNA genes represented 1–2% of the whole genome sequences ranging from 56 genes in isolate LB32T to 68 in isolate LB19. The in silico DNA G + C contents of the genomes fell within a narrow range, namely 70.6 to 72.9%, as was the case with strains in an earlier study16. Isolates LB32T and LB39T presented similar values with 71.0 and 70.6%, while their closest type strains show values of 71.5 and 71.2 for M. chokoriensis and M. saelicesensis, respectively; results that shown coherence with values established for group IVa strains in the micromonosporal phylogenomic tree presented by Carro et al.16.
Table 1.
Genome characteristics of the isolates.
| Isolates | |||||
|---|---|---|---|---|---|
| LB4 | LB19 | LB32T | LB39T | LB41 | |
| Genome size | 5562359 | 7272101 | 7149998 | 7748704 | 6771706 |
| Coding sequences | 4976 | 6678 | 6560 | 7013 | 6031 |
| Number of RNAs | 60 | 68 | 56 | 58 | 59 |
| Number of contigs | 1725 | 442 | 345 | 408 | 339 |
| DNA GC content | 71.8 | 70.9 | 71.0 | 70.6 | 72.9 |
| Number of bioclusters | 28 | 64 | 54 | 64 | 63 |
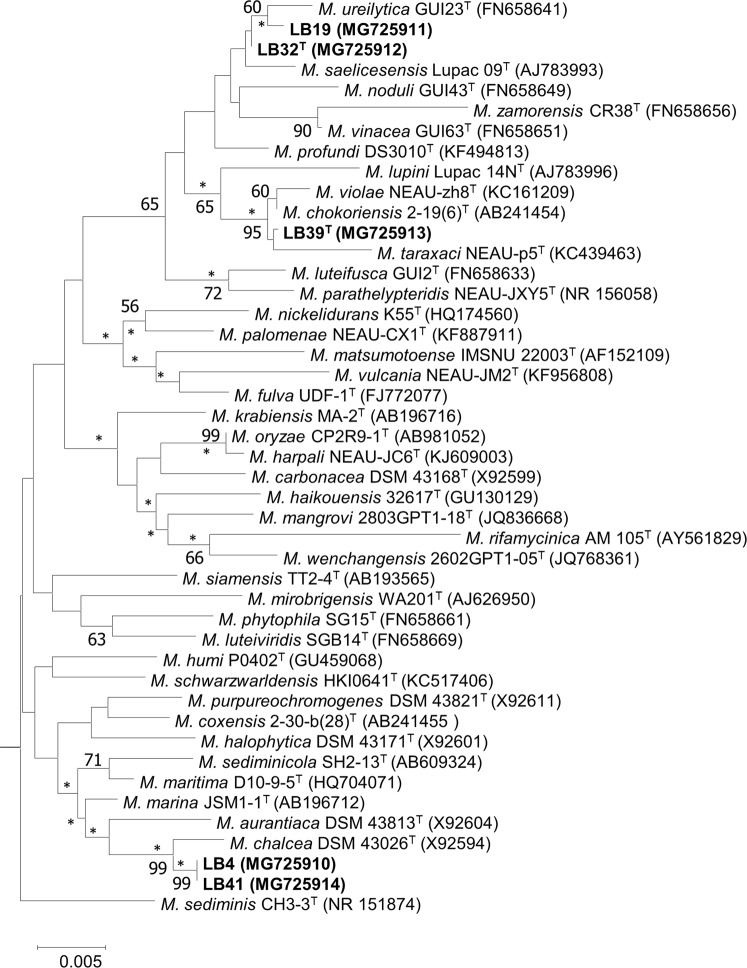
The positions of the isolates in the Micromonospora 16S rRNA gene tree are shown in Fig. S2 and their relationships with their closest phylogenetic neighbours in Fig. 1. The close relationships found between isolate LB4 and M. chalcea DSM 43026T, between isolate LB39T and the type strains of M. chokoriensis and Micromonospora violae37 and between isolates LB19 and LB32T and the type strains of M. saelicesensis and Micromonospora ureilytica38 are in good agreement with those reported by Carro et al.26. The remaining strain, isolate LB41, which was not included in the earlier analysis, was found to have an identical 16S rRNA gene sequence to isolate LB4; each of these isolates showed a corresponding sequence similarity with the type strain of M. chalcea of 99.6%. The taxonomic integrity of the M. chalcea clade is supported by a 99% bootstrap value and by the results from the maximum-likelihood and neighbour-joining analyses (Figs 1 and S1).
Figure 1.
Neighbour-joining phylogenetic tree based on 16S rRNA gene sequences showing relationships between the isolates and between them and closely related Micromonospora type strains. The numbers at the nodes indicate bootstrap values ≥50%. Asterisks indicate branches of the tree that were also recovered in the maximum-likelihood tree. Bar, 0.005 substitutions per nucleotide position.
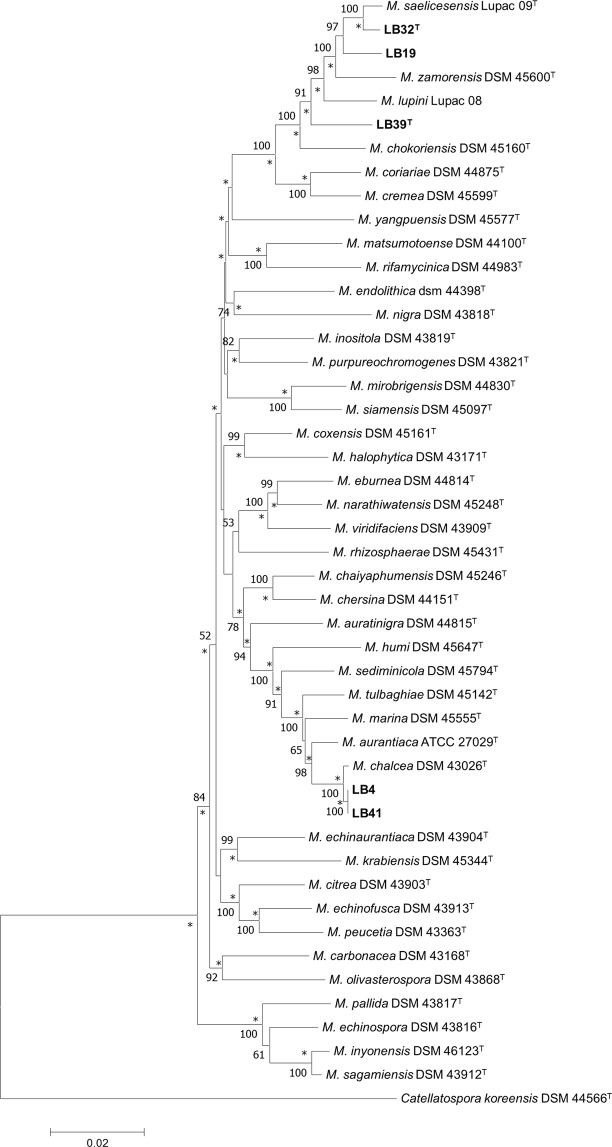
The isolates were recovered in two well supported clades based on the concatenated sequences of four housekeeping genes (atpD, gyrB, recA and rpoB) and corresponding 16S rRNA gene sequences (Fig. 2). Isolates LB4 and LB41 belong to a clade that encompasses the type strains of Micromonospora aurantiaca39, Micromonospora auratinigra40, Micromonospora chaiyaphumensis41, M. chalcea, Micromonospora chersina42, Micromonospora humi43, Micromonospora marina44, Micromonospora sediminicola45 and Micromonospora tulbaghia46; all of these validly named species were recovered in group 1a in the Micromonospora phylogenomic tree generated by Carro et al.16. Isolates LB4 and LB41 were found to have identical concatenated gene sequences and showed an MLSA genetic distance with M. chalcea DSM 43026T of 0.002% (Table S1), a value well below the species level threshold of ≤0.007 proposed by Rong and Huang47,48 and equivalent to the 70% DNA:DNA cut-off point recommended for the delineation of prokaryotic species49. In contrast, the two isolates shared genetic distances above the recommended threshold with all of the other closely related phylogenetic neighbours.
Figure 2.
Neighbour-joining phylogenetic tree based on multilocus sequence alignment of 16S rRNA, gyrB, rpoB, atpD, and recA gene sequences showing relationships between the isolates and between them and Micromonospora type strains. The numbers at the nodes are bootstrap support values when ≥50%. Asterisks indicate branches of the tree that were also recovered in the maximum-likelihood tree. Catellatospora koreensis DSM 44566T was used as the outgroup. Bar, 0.02 substitutions per nucleotide position.
Isolates LB19, LB32T and LB39T formed a well delineated clade in the MLSA tree together with the type strains of M. chokoriensis, Micromonospora coriariae50, Micromonospora cremea51, Micromonospora lupini, M. saelicesensis35 and M. zamorensis51; all of these validly named species were recovered in group IVa in the phylogenomic tree of Carro et al.16. The type strains of Micromonospora noduli, Micromonospora ureilytica and Micromonospora vinacea38 can be added to this group as they have been shown to be closely related both to one another and to the M. saelicesensis and M. zamorensis strains in phylogenetic tress based on gyrB, MLSA, and 16S rRNA gene sequences52,53.
Isolate LB32T formed a well-supported lineage in the MLSA tree together with M. saelicesensis Lupac09T; isolate LB19 was found at the periphery of this taxon, albeit as a distinct branch (Fig. 2). It is apparent from the MLSA distance score of 0.008 that isolate LB32T and M. saelicesensis are distinct, but sister, species (Table S1). In contrast, it is clear that isolate LB19 belongs to the species M. ureilytica as the two strains share a distance score of 0.005, well below the cut-off point for assigning strains to the same species47,48. On the same basis, it is evident from Table S1 that isolate LB39T shows distance scores with its nearest relatives above the 0.007 threshold and thereby merits consideration as a new Micromonospora species.
The isolates can be distinguished from one another by a broad range of phenotypic properties providing further evidence that they are not clones (Table 2). Excellent congruence was found between the standard phenotypic tests carried out in duplicate though this was not the case with some of the Biolog tests, many of which were weakly positive. The results of all of the phenotypic tests carried out on the isolates can be compared with those of the reference strains as the latter were recorded using the same media and methods. In general, all of the strains grew well from 20–37 °C, at pH 7 and 8, in the presence of 1% w/v sodium chloride, were catalase positive, active in the API-ZYM tests and oxidised a broad range of carbon compounds.
Table 2.
Phenotypic properties distinguishing the isolates from one another and from their closest phylogenetic neighbours.
| Isolate LB4 | Isolate LB41 | M. chalcea DSM 43026T | Isolate LB19 | M. ureilytica GUI23T* | Isolate LB32T | M. saelicesensis Lupac 09T | Isolate LB39T | M. chokoriensis JCM 13248T | |
|---|---|---|---|---|---|---|---|---|---|
| Biochemical tests: | |||||||||
| Catalase | + | + | − | + | + | + | + | + | + |
| Oxidase | − | − | − | − | + | − | + | + | + |
| API ZYM tests: | |||||||||
| Acid phosphatase | + | + | + | + | − | − | + | + | + |
| Alkaline phosphatase | + | + | + | − | + | − | − | + | + |
| α-Chymotrypsin | + | + | + | + | + | − | + | + | − |
| Cystine arylamidase | + | + | − | + | + | + | + | + | + |
| α-Galactosidase | + | + | + | + | − | + | + | + | + |
| N-acetyl-β-Glucosaminidase | − | − | + | + | + | + | + | + | − |
| α-Glucosidase | + | + | + | + | − | + | + | + | + |
| β-Glucosidase | + | + | + | + | − | + | + | + | + |
| β-Glucuronidase | + | + | + | + | − | + | − | + | + |
| α-Fucosidase | − | − | − | − | − | − | + | − | − |
| Lipase (C14) | + | + | + | + | − | + | + | + | + |
| Leucine arylamidase | + | + | − | + | + | + | + | + | + |
| α-Mannosidase | − | − | − | + | − | − | + | + | − |
| Valine arylamidase | + | + | − | + | + | + | + | + | + |
| GENIII BIOLOG microplate tests: | |||||||||
| (a) Oxidation of amino acids: | |||||||||
| L-Alanine | + | − | − | − | − | + | + | + | + |
| L-Arginine | + | + | + | − | − | + | − | + | − |
| D-Aspartic acid | + | + | + | − | − | − | − | − | − |
| Glycyl-L-proline | − | − | − | − | − | − | + | − | + |
| L-Histidine | − | − | + | − | − | − | − | − | − |
| D-Serine #2 | − | − | − | − | − | − | − | + | − |
| L-Serine | − | − | − | − | − | + | − | + | + |
| (b) Oxidation of sugars: | |||||||||
| D-Arabitol | − | − | − | + | + | − | − | − | − |
| D-Fucose | − | − | + | − | − | − | − | − | − |
| L-Fucose | − | + | + | + | + | + | + | + | + |
| 3-O-methyl-D-Glucose | − | + | + | + | + | + | + | + | + |
| N-acetyl-D-Galactosamine | + | − | + | + | + | + | + | + | + |
| Glucuronamide | − | − | − | − | − | − | − | − | + |
| Glycerol | − | + | + | − | − | + | − | + | + |
| myo-Inositol | − | − | − | − | − | + | + | − | − |
| α-D-Lactose | + | + | + | − | − | − | − | + | + |
| D-Mannitol | − | − | − | + | + | + | − | − | + |
| L-Rhamnose | − | − | + | − | − | + | + | + | + |
| D-Salicin | − | + | + | + | + | + | + | + | + |
| D-Sorbitol | − | − | + | + | + | + | + | − | + |
| (c) Oxidation of organic acids: | |||||||||
| Bromo-Succinic acid | − | − | − | + | + | + | + | + | − |
| Butyric acid | + | + | + | − | − | − | − | + | − |
| β-hydroxy-Butyric acid | + | + | − | + | + | + | + | − | + |
| γ-amino-n-Butyric acid | − | − | + | − | − | − | − | − | − |
| Citric acid | − | − | + | − | − | − | − | − | − |
| D-Galacturonic acid | + | − | + | + | + | + | − | + | + |
| β-methyl-D-Glucoside | − | − | + | + | + | + | + | + | + |
| D-Glucuronic acid | + | − | − | + | + | + | − | + | + |
| α-keto-Glutaric acid | + | − | + | + | + | + | − | − | − |
| D-Lactic acid methyl ester | + | + | - | + | + | + | + | + | + |
| D-Malic acid | − | + | + | − | − | − | − | − | + |
| L-Malic acid | + | + | − | + | + | + | + | + | + |
| N-acetyl-Neuraminic acid | + | + | + | − | − | + | − | − | − |
| L-Pyroglutamic acid | + | + | − | − | − | + | + | − | − |
| Quinic acid | + | + | − | − | − | − | − | − | + |
| D-Saccharic acid | − | − | − | + | + | + | + | + | + |
| (d) Oxidation of polymer: | |||||||||
| Pectin | + | + | + | + | + | + | + | − | + |
| Growth in the presence of: | |||||||||
| Inosine | + | + | + | + | + | + | + | + | − |
| Lithium chloride | + | + | − | − | − | − | + | − | − |
| Minocycline | − | − | − | − | − | − | − | + | − |
| Potassium tellurite | − | + | − | − | − | + | + | + | − |
| Sodium chloride (4%, w/v) | + | + | − | − | − | − | − | − | − |
| Sodium bromate | + | + | − | − | − | − | + | − | − |
| Sodium formate | − | − | − | − | − | − | + | − | − |
| Sodium lactate (1%, w/v) | + | + | − | − | − | − | + | − | − |
| Tolerance tests: | |||||||||
| Temperature range (°C) | 20–45 | 20–45 | 20–37 | 20–37 | 20–37 | 20–37 | 12–45 | 12–37 | 12–45 |
| pH range | 6–9 | 6–9 | 7–9 | 6–8 | 7–8 | 6–8 | 7–9 | 6–8 | 6–9 |
| Growth in the presence of NaCl (%, w/v) | 4 | 4 | 1 | 3 | 1 | 1 | 1 | 3 | 1 |
| Chemotaxonomy: | |||||||||
| Diaminopimelic acid | meso -A 2 pm | meso -A 2 pm | meso -A 2 pm* | meso - and OH-A 2 pm | meso -A 2 pm* | meso - and OH-A 2 pm | meso -A 2 pm* | meso - and OH-A 2 pm | meso -A 2 pm* |
| Fatty acids | iso-C16:0 (31.0%), iso-C15:0 (10.6%), iso-C17:0 (11.2%), anteiso-C17:0 (15.1%) | iso-C16:0 (38.5%), iso-C15:0 (7.7%), iso-C17:0 (6.4%), anteiso-C17:0 (11.0%) | iso-C16:0, iso-C15:0, anteiso-C17:0, iso-C17:1ω9c* | iso-C16:0 (11.0%), iso-C15:0 (27.0%), anteiso-C17:0 (10.4%) | iso-C15:0, iso-C17:1ω9c, iso-C17:0, anteiso-C17:0* | iso-C15:0 (23.8%), iso-C17:0 (14.6%), anteiso-C17:0 (12.1%) | iso-C16:0, iso-C15:0, C17:1 cis8* | iso-C15:0 (23.5%), iso-C16:0 (14.8%), 10-methyl C17:0 (14.0%), C17:0 (10.8%) | iso-C16:0, iso-C15:0, iso-C17:0, anteiso-C15:0* |
| Menaquinones | MK-9(H4,H6) (24.5, 17.6%) MK-10(H4,H6) (18.4, 16.6%) | MK-9 (H4,H6) (23.1, 10.2%) MK-10(H4,H6) (40.7, 17.6%) | MK-10(H4,H6)* | MK-10(H4,H6) (54.8, 18.1%) | MK-9 (H4) MK-10(H4,H6)* | MK-10(H4,H6,H8) (31.9, 28.9, 19.3%) | MK-10(H4,H6)* | MK-10(H4,H6,H8) (31.7, 24.3, 13.3%) | MK-9 (H4,H6) MK-10(H4,H6)* |
| Polar lipids | DPG, PE, PI, GL, 2PL | DPG, PE, PI, GL, 2PL | ND | DPG, PE, PI, GL, 5 L | DPG, PE, PI, GL* | DPG, PE, PI, GL, 2 L | DPG, PE, PI* | DPG, PE, PI, GL, 2PL | DPG, PE, PI, PIM* |
| Sugars | gal, glu, man, xyl | gal, glu, man, xyl | ND | gal, glu, man, rham, rib, xyl | gal, glu, man, rib, xyl* | gal, glu, man, rham, rib, xyl | ara, glu, man, rham, rib, xyl* | gal, glu, man, rham, rib, xyl | ara, gal, glu, man, rham, rib, xyl* |
+: positive, −:negative, abreviations: dpm: diaminopimelic acid, ara: arabinose, gal: galactose, glu: glucose, man: mannose, rha: rhamnose, rib: ribose, xyl: xylose, DPG: diphosphatidylglycerol, PE: phosphatidylethanolamine, PI: phosphatidylinositol, PIM: phosphatidylinositol mannosides, GL: glycolipids, PL: unknown polar lipids, L: unknown lipids. All tests are from this study unless indicated otherwise. *Data from Genilloud28, Ara and Kudo34, Carro et al.38 and Trujillo et al.35.
All the isolates grew at pH 6 and in the presence of sodium chloride (1%, w/v) but not in the presence guanidine hydrochloride, niaproof, tetrazolium blue or tetrazolium violet. They were resistant to aztreonam, nalidixic acid, rifamycin SV, but not to fusidic acid, lincomycin, troleandomycin or vancomycin. All of the strains were positive for esterase (C4), esterase lipase (C8), β-galactosidase, naphtlol-AS-BI-phosphohydrolases and trypsin (API tests), oxidized acetic acid, acetoacetic acid, L-aspartic acid, D-gluconic acid, L-glutamic acid, and propionic acid (organic acids), D-cellobiose, D-fructose, D-fructose-6-phosphate, D-galactose, β-gentiobiose, N-acetyl-D-glucosamine, D-glucose, D-glucose-6-phosphate, D-maltose, N-acetyl-β-D-mannosamine, D-mannose, D-melibiose, D-raffinose, stachyose, sucrose, D-trehalose and D-turanose (sugars), degraded dextrin, gelatin and Tween 40. In contrast, none of the strains oxidized D-serine I (amino acid), α-hydroxi-butyric acid, α-keto-butyric acid, L-galactonic acid-γ-lactone, L-lactic acid, mucic acid and p-hydroxy-phenylacetic acid.
The close relationship recorded earlier between isolates LB4 and LB41 was underpinned by the results from the chemotaxonomic and phenotypic analyses (Table 2). The strains were found to have identical profiles for the biochemical, enzymatic and tolerance tests and showed a similar ability to oxidise organic acids and sugars. Whole organism hydrolysates of the isolates contained meso-A2pm, glucose, galactose, mannose and xylose; they were also shown to have identical polar lipid patterns. In addition, the major fatty acid of strains LB4 and LB41 was iso-C16:0 (31.0 and 38.5%, respectively) and the predominant isoprenologue MK-9 (H4) (24.5 and 23.1%). When compared with the profiles of the reference strains isolates LB4 and LB41 were most closely related to the type strain of M. chalcea showing overall phenotypic similarities with the latter of 76 and 77% indicating that all three strains belong to the same taxospecies54,55. These strains can be distinguished from all of the other organisms given their ability to metabolise D-aspartic acid and inability to oxidise D-saccharic acid. Similarly, the close relationship found earlier between isolate LB19 and the type strain of M. ureilytica is underpinned by the phenotypic data; these strains have many more unit characters in common than isolate LB19 has with the other reference type strains. Isolate LB19 and M. ureilytica GUI23T can be distinguished from all of the other organisms given their ability to oxidise D-arabitol. They also share similar whole organism hydrolysate and polar lipid patterns (Table 2).
Isolate LB32T can be separated readily from the type strain of M. saelicesensis, its closest phylogenetic neighbour, by a broad range of phenotypic properties, as exemplified by its ability to produce β-glucoronidase, oxidise L-serine, D-galacturonic acid, D-glucuronic acid, α-keto-glutaric acid, N-acetyl-neuraminic acid, glycerol and D-mannitol. In turn, M. saelicesensis strain Lupac 09T, unlike isolate LB32T, grew at pH 9.0 and 45 °C, showed much greater activity in the API-ZYM tests, was oxidase positive, oxidised glycyl-L-proline and D-sorbitol and grew in the presence of lithium chloride, sodium bromate and sodium formate. These differential characters are underscored by several chemotaxonomic traits, notably differences in fatty acid and whole cell sugar patterns (Table 2).
Isolate LB39T can be distinguished from the type strain of M. chokoriensis, its closest phylogenetic neighbour, using a combination of chemotaxonomic and other phenotypic features (Table 2). The former, unlike the latter, produces α-mannosidase, oxidises L-arginine, D-serine #2, butyric acid and bromo-succinic acid and grows in the presence of minocycline, sodium chloride (4%, w/v) and potassium tellurite. In contrast, only the reference strain grows at pH 9.0 and 45 °C, degrades pectin and oxidises L-histidine, α-hydroxy-butyric acid, D-malic acid, D-mannitol and D-sorbitol. The two organisms can also be distinguished using key chemical markers, as illustrated by differences in menaquinone, polar lipid and whole cell sugar composition.
ANI (average nucleotide identity) and dDDH (digital DNA-DNA hybridization) values were calculated between the isolates and between them and their closest phylogenetic neighbours, as shown in Table 3. It is apparent on both counts that isolates LB4 and LB41 are bona fide members of the species M. chalcea as they share ANI and dDDH values with the latter well above the 99.5–99.6%47,48 and 70% thresholds49 used to assign strains to the same genomic species. It is also apparent that isolates LB19 and LB39T are not closely related to one another or to any of the other strains included in these analyses. The situation with respect to isolate LB32T appears to be less clear cut as it shares a dDDH value with the type strain of M. saelicesensis of 68.2% though the corresponding ANI value, 96.2%, is above the ANI threshold. Similar anomalies have been observed between other closely related Micromonospora species, as with the type strains of Micromonospora carbonacea and Micromonospora haikouensis which shared dDDH and OrthoANI values of 59.9 and 95.2%, respectively; while the corresponding values for the type strains of Micromonospora inyonensis and Micromonospora sagamiensis were 68.9% and 96.5%16. Indeed, some species which have been conclusively shown to belong to different Micromonospora species sport higher dDDH and ANI values, as exemplified by the type strains of Micromonospora noduli and M. saelicesensis which share dDDH and OrthoANI values of 71.2 and 96.8%, respectively56. Corresponding data between isolate LB19 and the type strain of M. ureilytica cannot be determined until the whole genome sequence of the latter becomes available though the 99.6% MLSA value found between these strains indicates that they belong to the same genomic species, namely M. ureilytica38.
Table 3.
Average nucleotide indices and digital DNA:DNA hybridization values (%) between the isolates and between them and their closest phylogenetic neighbours.
| Isolate LB4 | Isolate LB19 | Isolate LB32T | Isolate LB39T | Isolate LB41 | M. aurantiaca ATCC 27029T | M. chalcea DSM 43026T | M. chokoriensis DSM 45160T | M. coriariae DSM 44875T | M. lupini Lupac 08 | M. marina DSM 45555T | M. noduli GUI43T | M. saelicesensis Lupac 09T | M. tulbaghiae DSM 45142T | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isolate LB4 | — | 81.9 | 82.1 | 81.9 | 99.8 | 93.4 | 98.5 | 81.8 | 82.6 | 82.2 | 92.5 | 93.3 | 82.2 | 80.8 |
| Isolate LB19 | 26.8 | — | 93.5 | 92.7 | 80.8 | 80.6 | 80.7 | 89.1 | 87.7 | 87.7 | 80.6 | 93.7 | 93.8 | 80.7 |
| Isolate LB32T | 26.8 | 54.1 | — | 92.7 | 81.0 | 80.9 | 80.9 | 89.3 | 87.8 | 87.6 | 80.8 | 95.8 | 96.2 | 80.8 |
| Isolate LB39T | 27.0 | 50.7 | 50.5 | — | 80.8 | 80.6 | 80.7 | 89.0 | 87.8 | 87.2 | 80.6 | 92.8 | 92.9 | 80.6 |
| Isolate LB41 | 99.2 | 24.9 | 24.8 | 25.0 | — | 93.2 | 98.7 | 80.6 | 81.4 | 81.1 | 92.2 | 80.9 | 81.1 | 92.9 |
| M. aurantiaca ATCC 27029 T | 54.6 | 24.7 | 24.7 | 24.7 | 52.4 | — | 93.0 | 80.5 | 81.3 | 80.9 | 94.1 | 80.8 | 80.9 | 94.5 |
| M. chalcea DSM 43026T | 89.2 | 24.8 | 24.7 | 24.9 | 89.7 | 51.5 | — | 80.4 | 81.3 | 81.0 | 92.1 | 80.8 | 80.9 | 92.9 |
| M. chokoriensis DSM 45160T | 26.9 | 38.3 | 38.3 | 37.8 | 24.5 | 24.3 | 24.3 | — | 87.4 | 87.1 | 80.4 | 80.8 | 89.1 | 80.8 |
| M. coriariae DSM 44875T | 27.5 | 35.6 | 35.6 | 35.7 | 25.3 | 25.3 | 25.1 | 34.4 | — | 87.9 | 81.3 | 87.8 | 87.9 | 81.3 |
| M. lupini Lupac 08 | 27.3 | 35.3 | 34.9 | 34.7 | 25.2 | 25.1 | 25.0 | 33.7 | 35.9 | — | 80.9 | 87.7 | 87.8 | 81.0 |
| M. marina DSM 45555T | 51.3 | 24.6 | 24.6 | 24.6 | 49.3 | 57.2 | 48.7 | 24.2 | 25.1 | 24.9 | — | 80.7 | 80.9 | 93.3 |
| M. noduli GUI43T | 26.8 | 55.3 | 66.1 | 50.4 | 24.7 | 24.6 | 24.6 | 37.9 | 35.6 | 35.0 | 24.5 | — | 96.6 | 80.8 |
| M. saelicesensis Lupac 09T | 26.9 | 55.6 | 68.4 | 50.7 | 24.7 | 24.5 | 24.5 | 37.9 | 35.7 | 34.9 | 24.5 | 71.2 | — | 80.9 |
| M. tulbaghiae DSM 45142T | 53.9 | 24.6 | 24.6 | 24.6 | 51.2 | 60.1 | 51.3 | 24.6 | 25.1 | 24.9 | 55.0 | 24.5 | 24.5 | — |
In summary, it can be concluded that isolates LB4 and LB41 exhibit a broad range of taxonomic properties consistent with their assignment to the validly named species, M. chalcea32,33, strains of which have been isolated from air, soil and aquatic habitats28. It is also evident that isolate LB19 belongs to the recently recognised species, M. ureilytica38, the sole representative of which came from a root nodule of Pisum sativum. These results provide further evidence that representatives of Micromonospora species are widely distributed in the environment16 though there is evidence that micromonosporae are a feature of extreme habitats57–59. It is also apparent that isolate LB32T, which forms a sister clade to the type strain of M. saelicesensis can be distinguished from the latter by a rich assortment of chemotaxonomic, genotypic and phenotypic data. Similarly, strain LB39T merits recognition as a novel Micromonospora species as a wealth of taxonomic data can be weighted to separate it from the type strain of M. chokoriensis. In light of these results it is proposed that isolates LB32T and LB39T be recognized as new Micromonospora species for which we propose the names Micromonospora arida sp. nov. and Micromonospora inaquosa sp. nov., respectively.
None of the isolates inhibited the growth of the B. subtilis, E. coli and P. fluorescens strains in previous plug assays26, possibly due to the use of an inadequate cultivation media. In contrast, extracts from all of the isolates were shown to be active against the bacterial and fungal indicator strains, as shown in Table 4 where extracts showing the greatest activity are given in bold. In general, the most pronounced activity was seen in fractions eluting at higher concentrations of methanol, as exemplified by the inhibition of the E. coli and K. pneumoniae strains. Extracts showed relatively little activity against the A. baumannii, A. fumigatus and P. fluorescens strains and only moderate inhibition of the methicillin-resistant and methicillin-sensitive strains of S. aureus. Similarly, little activity was found against the C. albicans strain with the exception of extracts from isolate LB41. Interestingly, only extracts from isolates LB4 and LB41 showed pronounced inhibition of human hepatocellular carcinoma (HepG2) cells. These results are not only promising, but also provide further evidence that novel and rare micromonosporae from previously unexplored habitats are a promising source of antimicrobial agents60,61.
Table 4.
Ability of different fractions of polar compounds extracted from the strains isolated from Lomas Bayas soil to inhibit: 1, Acetobacter baumannii CL5973; 2, Escherichia coli ATCC 25922; 3, Klebsiella pneumoniae ATCC 700603; 4, Pseudomonas aeruginosa MB5919; 5,6 methicillin resistant/sensitive Staphilococcus aureus MB5393; 6, Staphilococcus aureus ATCC 23213; 7, Aspergilllus fumigatus ATCC 46645; 8, human hepatocellular carcinoma (HepG2) cells. Values under 50% are marked in bold.
| Strain ID | Fraction | Conc. [ug/mL] | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % INH | SE | % INH | SE | % INH | SE | % INH | SE | % INH | SE | % INH | SE | % INH | SE | Conc. [mg/l] | Activity | SE | p-Value | |||
| LB4 | Water | 300 | 62.8 | 51.9 | −4.6 | 12.0 | −14.1 | 0.6 | 19.1 | 13.4 | 2.7 | 8.0 | 70.7 | 27.1 | −5.8 | 11.6 | 75 | −31.6 | 14.4 | 0.04 |
| LB4 | 25% methanol | 300 | −9.1 | 13.2 | −18.5 | 15.3 | −21.0 | 8.1 | −4.6 | 19.1 | −12.0 | 5.1 | −11.6 | 3.5 | −5.6 | 4.0 | 75 | 2.4 | 4.3 | 0.53 |
| LB4 | 50% methanol | 300 | −15.7 | 13.7 | −21.4 | 5.0 | −18.8 | 4.7 | −5.3 | 26.2 | −13.7 | 5.4 | −20.0 | 2.5 | −3.0 | 10.4 | 75 | 25.4 | 20.2 | 0.00 |
| LB4 | 75% methanol | 300 | 14.8 | 30.9 | −20.5 | 1.0 | −34.4 | 1.1 | 26.9 | 17.6 | −14.0 | 5.5 | −18.0 | 4.6 | 0.1 | 0.9 | 75 | 31.3 | 1.7 | 0.80 |
| LB4 | 100% methanol | 150 | 3.2 | — | −36.7 | 3.9 | −57.0 | 1.9 | 22.8 | — | −17.5 | 8.5 | −21.4 | 4.5 | −1.9 | 2.3 | 75 | −39.9 | 10.2 | 0.14 |
| LB4 | 100% methanol + 0.01% TFA | 150 | 58.0 | — | −28.5 | 6.5 | −53.9 | 2.3 | 21.5 | — | −13.6 | 10.6 | −11.8 | 1.7 | −0.1 | 2.5 | 75 | 27.6 | 2.7 | 0.70 |
| LB19 | Water | 300 | −8.6 | 17.5 | −4.3 | 6.7 | 3.2 | 30.9 | −4.8 | 20.6 | 3.0 | 20.3 | 50.0 | 33.2 | −1.4 | 5.3 | 75 | 10.8 | 13.8 | 0.05 |
| LB19 | 25% methanol | 300 | −17.5 | 7.5 | 5.1 | 5.8 | 1.8 | 12.4 | −6.2 | 21.4 | −15.2 | 3.3 | −10.5 | 1.4 | −7.3 | 10.3 | 75 | 10.9 | 5.3 | 0.45 |
| LB19 | 50% methanol | 300 | 62.0 | 34.7 | −9.8 | 0.2 | −20.1 | 0.0 | 23.0 | 24.4 | −13.9 | 6.1 | −2.2 | 4.0 | −3.0 | 7.1 | 75 | −24.3 | 13.0 | 0.06 |
| LB19 | 75% methanol | 300 | 67.2 | 21.9 | −18.4 | 1.6 | −36.3 | 2.7 | 31.6 | 32.7 | −12.6 | 6.4 | −18.5 | 1.4 | −4.5 | 3.7 | 75 | 18.6 | 12.8 | 0.07 |
| LB19 | 100% methanol | 300 | 83.4 | 24.4 | −29.4 | 3.5 | −46.1 | 5.7 | 39.4 | 42.6 | −17.7 | 5.8 | −15.9 | 11.1 | −2.4 | 8.9 | 75 | 17.1 | 7.5 | 0.28 |
| LB19 | 100% methanol + 0.01% TFA | 150 | 61.6 | — | −20.2 | 0.8 | −56.5 | 1.9 | 15.6 | — | −13.2 | 9.0 | −15.4 | 3.8 | −0.7 | 3.3 | 75 | −2.1 | 13.2 | 0.06 |
| LB32T | Water | 300 | 33.2 | 66.0 | −1.1 | 11.2 | −15.2 | 5.0 | −0.1 | 16.4 | 10.8 | 4.8 | 44.9 | 5.6 | −0.5 | 7.2 | 75 | −18.1 | 19.3 | 0.01 |
| LB32T | 25% methanol | 300 | −8.2 | 15.9 | 6.1 | 6.1 | −15.6 | 4.5 | −2.1 | 22.3 | −16.8 | 5.1 | −19.8 | 11.8 | −3.0 | 3.0 | 75 | 42.7 | 1.7 | 0.81 |
| LB32T | 50% methanol | 300 | 10.0 | 10.2 | −11.6 | 0.5 | −9.9 | 10.1 | 0.2 | 7.4 | −11.4 | 3.9 | −14.0 | 3.4 | 2.0 | 4.0 | 75 | 63.5 | 19.3 | 0.01 |
| LB32T | 75% methanol | 300 | −7.8 | 18.3 | −12.7 | 1.8 | −12.7 | 2.6 | −3.4 | 22.3 | −11.9 | 7.4 | −17.4 | 2.0 | −0.2 | 3.5 | 75 | 41.0 | 17.4 | 0.01 |
| LB32T | 100% methanol | 300 | 83.2 | 20.6 | −27.5 | 2.6 | −44.3 | 3.4 | 42.1 | 42.3 | −16.3 | 9.1 | −22.4 | 4.1 | −1.4 | 0.7 | 75 | 1.1 | 14.1 | 0.04 |
| LB32T | 100% methanol + 0.01% TFA | 300 | 60.6 | 17.7 | −29.6 | 0.2 | −58.6 | 0.8 | 33.2 | 25.3 | −18.2 | 6.0 | −20.0 | 3.2 | −6.3 | 1.9 | 75 | 31.2 | 6.2 | 0.37 |
| LB39T | Water | 300 | −8.7 | 17.3 | −9.4 | 3.1 | −26.1 | 3.0 | −2.9 | 23.2 | −12.5 | 8.5 | −12.5 | 2.9 | 1.3 | 3.2 | 75 | 14.3 | 8.9 | 0.20 |
| LB39T | 25% methanol | 300 | 3.8 | 41.7 | 1.7 | 0.3 | −16.6 | 3.8 | −12.4 | 16.7 | −16.7 | 5.2 | −18.1 | 0.5 | −3.4 | 5.3 | 75 | 35.0 | 0.9 | 0.89 |
| LB39T | 50% methanol | 300 | −6.5 | 15.7 | −11.2 | 4.3 | −21.9 | 2.8 | −3.8 | 17.2 | −13.1 | 6.8 | −20.9 | 0.4 | −1.7 | 2.6 | 75 | 25.5 | 20.8 | 0.00 |
| LB39T | 75% methanol | 300 | 57.5 | 54.9 | −27.5 | 0.2 | −30.9 | 1.9 | 10.5 | 15.8 | −12.8 | 4.2 | −11.9 | 3.1 | 1.9 | 6.5 | 75 | 26.0 | 10.4 | 0.13 |
| LB39T | 100% methanol | 300 | 75.2 | 26.1 | −26.7 | 3.3 | −37.8 | 1.6 | 32.5 | 38.7 | −17.1 | 7.8 | −18.2 | 0.0 | −1.8 | 4.1 | 75 | −5.1 | 0.9 | 0.90 |
| LB39T | 100% methanol + 0.01% TFA | 300 | 51.8 | 29.5 | −30.6 | 1.9 | −60.5 | 0.8 | 16.3 | 25.4 | −12.8 | 3.9 | −15.0 | 1.7 | −3.8 | 0.5 | 75 | 64.0 | 8.1 | 0.24 |
| LB41 | Water | 300 | −8.1 | 10.0 | −10.5 | 1.8 | −23.6 | 3.9 | −4.0 | 13.5 | −14.4 | 7.9 | −17.7 | 5.2 | 1.7 | 3.5 | 75 | −16.5 | 15.3 | 0.03 |
| LB41 | 25% methanol | 300 | −15.9 | 15.0 | −10.6 | 1.8 | −29.6 | 3.3 | −9.3 | 22.3 | −14.8 | 4.5 | −18.0 | 6.2 | −0.8 | 5.3 | 75 | 16.8 | 17.2 | 0.01 |
| LB41 | 50% methanol | 300 | 63.6 | 29.1 | −28.6 | 10.2 | −9.1 | 2.1 | 24.2 | 27.6 | −7.0 | 7.1 | −7.3 | 0.6 | −4.0 | 4.3 | 75 | 37.4 | 8.1 | 0.24 |
| LB41 | 75% methanol | 300 | 44.9 | 24.4 | −18.5 | 8.6 | −30.2 | 0.9 | 16.9 | 18.7 | −11.6 | 4.1 | −4.7 | 2.1 | −4.5 | 0.5 | 75 | 37.9 | 12.5 | 0.07 |
| LB41 | 100% methanol | 300 | 61.5 | 27.9 | −32.6 | 1.5 | −42.3 | 5.9 | 26.2 | 22.1 | −17.6 | 7.8 | −19.2 | 1.9 | −10.0 | 0.9 | 75 | −49.7 | 6.4 | 0.36 |
| LB41 | 100% methanol + 0.01% TFA | 300 | 30.1 | 62.3 | −14.4 | 2.1 | −6.7 | 1.2 | 0.9 | 18.6 | −10.7 | 8.4 | −17.3 | 3.7 | 1.4 | 2.7 | 75 | 47.1 | 0.3 | 0.97 |
| − | Negative Control | 150 | 9.0 | 10.9 | −8.0 | 1.1 | −2.0 | 5.8 | 17.4 | 1.8 | −8.0 | 1.9 | −8.0 | 1.5 | −5.0 | 2.8 | 500 | 4.8 | 14.6 | — |
| + | Positive Control (MMS) | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 4 | −99.9 | 0.2 | — |
% INH: percentage of inhibition; SE: Standard Error; TFA: trifluoroacetate; MMS: Methyl methane sulphonate.
Genetic potential of the isolates to produce specialised metabolites
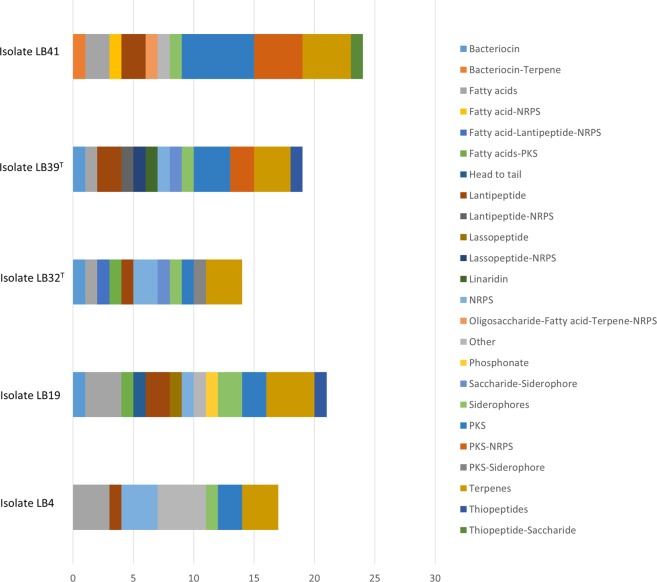
The draft genomes of all of the isolates were examined using the antiSMASH server to detect putative BGCs. The number of such bioclusters ranged from 28 in the genome of isolate LB4 to 64 in the genomes of isolates LB19 and LB41 though this lower number may be a function of a low quality genome, as shown by the relatively high number of contigs (Table 1). Even so, the number of BGCs found in the genomes of the isolates is well within the range found in those of the Micromonospora type strains examined by Carro et al.16. In contrast, the average number of BGCs detected in the genomes of the isolates, namely 54, is more than double the average number reported in the earlier study16. However, as in that study, the predominant BGCs types coded for lantipeptides, non-ribosomal peptide synthases, polyketide synthases, siderophores and terpenes (Table S2; Fig. 3).
Figure 3.
Biosynthetic gene clusters found in the genomes of isolates LB4, LB19, LB32T, LB39T, and LB41 using antiSMASH 4.0.
The genomes of the isolates contained 25 BGCs encoding for compounds that showed some degree of similarity to specialised metabolites not previously found in Micromonospora strains. The genomes of all of the isolates encode for BGCs that showed a genetic correspondence to coumermycin, an amino-coumarin antibiotic, produced by Streptomyces rishiriensis strain DSM 4048962, which is known to inhibit DNA gyrase and bacterial cell division63. In the same vein the genomes of all of the isolates code for a BGC that shows a similarity, ca. ∼40%, to lymphostin biosynthetic cluster, an immunosuppressant originally found in Streptomyces sp. KY1178364. Finally, all of the isolates have the capacity to produce compounds related to diazepinomicin, a small alkaloid molecule that binds to and inhibits Ras kinase with the potential to treat multiple solid tumours65; this compound was first detected in the marine actinobacterium, Micromonospora sp. DPJ1266. The genome of isolate LB32T contained a BGC that showed a relatively low similarity to that of algamycin I, an antibacterial 16-membered macrolide active against Micrococcus luteus and Salmonella typhimurium, a compound initially found in Streptomyces sp. KMA-01167.
It is also interesting that 18 out of these 25 BGCs were discontinuously distributed across the genomes of the isolates: 1, 5, 3, 2 and 7 in strains LB4, LB19, LB32T, LB39T and LB41, respectively (Table S2; Fig. 3); the hybrid system NRPS-PKS was only found in isolates LB39T and LB41. Secondary metabolite related genes detected using the SEED server were also discontinuously distributed, as exemplified by genes associated with lanthionine synthases which varied from 4 in isolate LB4 to 16 in isolate LB39T (Table 5) while all of the isolates, apart from strain LB32T, contained genes related to the synthesis of thiazole-oxazole-modified microcins, ribosomally produced peptides with post-translationally installed heterocycles derived from cysteine, serine and threonine residues68. In turn, only the genome of isolate LB19 harboured a gene associated with the synthesis of clavulanic acid, which encodes for a clavaldehyde dehydrogenase (contig 9) according to a RAST analysis. In this context it is also interesting that compounds extracted from the isolates varied in their ability to inhibit a variety of indicator micro-organisms and the HepG2 cells (Table 4).
Table 5.
Genes implicated in secondary metabolism in the strains of study and closest type strains detected by RAST subsystems.
| Isolate LB4 | Isolate LB19 | Isolate LB32T | Isoalte LB39T | Isolate LB41 | M. chalcea DSM 43026T | M. saelicesensis Lupac 09T | ||
|---|---|---|---|---|---|---|---|---|
| Secondary metabolism | 9 | 10 | 6 | 19 | 17 | 10 | 4 | |
| Thiazole- oxazole-modified microcin (TOMM) synthesis | 5 | 4 | 0 | 3 | 7 | 3 | 0 | |
| TOMM biosynthesis dehydrogenase (protein B) | 1 | 1 | 0 | 1 | 2 | 1 | 0 | |
| TOMM biosynthesis cyclodehydratase (protein C) | 2 | 1 | 0 | 1 | 2 | 1 | 0 | |
| TOMM biosynthesis docking scaffold (protein D) | 2 | 1 | 0 | 1 | 2 | 1 | 0 | |
| FIG214983: hypothetical protein | 0 | 0 | 0 | 0 | 1 | 0 | 0 | |
| SagD family docking scaffold | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| Lanthionine synthetases | 4 | 5 | 6 | 16 | 6 | 7 | 4 | |
| LanB | Lanthionine biosynthesis protein LanB | 1 | 1 | 0 | 2 | 1 | 1 | 0 |
| LanC | Lanthionine biosynthesis cyclase LanC | 0 | 1 | 0 | 2 | 0 | 1 | 0 |
| MT | O-methyltransferase clustered with LanBC | 1 | 0 | 0 | 1 | 1 | 0 | 0 |
| IsoAspMT | Protein-L-isoaspartate O-methyltransferase (EC 2.1.1.77) | 2 | 2 | 3 | 8 | 3 | 3 | 1 |
| LanL | Lanthionine biosynthesis protein LanL | 0 | 1 | 0 | 1 | 1 | 1 | 1 |
| LanM | Lanthionine biosynthesis protein LanM | 0 | 0 | 3 | 0 | 0 | 0 | 2 |
| HP1 | Hypothetical protein associated with LanBC | 0 | 0 | 0 | 2 | 0 | 1 | 0 |
| Clavulanic acid biosynthesis | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| CAD | Clavaldehyde dehydrogenase | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
The genomes of the M. chalcea strains were found to harbour 38 different BGCs that presented some similarity with known compounds, out of which 10 have not been detected previously in Micromonospora strains; 7 of these BGCs were only present in the genome of isolate LB41 and the other three in the other LB strains (LB4, LB32T, LB39T and LB41). In addition, 26 out of the 38 BGCs were only found in the genomes of one out of the three M. chalcea strains, 9 in two of them and the remaining three in all of them. These results provide further evidence that M. chalcea strains are a good source of novel antibiotics, notably aminoglycosides, lactones and macrolides61. However, none of the M. chalcea strains had the capacity to synthesize tetrocarcin A, a spirotetronate antibiotic produced by M. chalcea NRRL 1128969 or chalcidin or neomycin produced by M. chalcea sp.70 and M. chalcea B9-68371, respectively though the taxonomic provenance of these strains is questionable.
The results of this study taken together with those reported by Carro et al.16 show that the genomes of Micromonospora strains are a unique source of BGCs that have the potential to synthesise an array of completely novel and uncharacterised specialised metabolites. It is particularly interesting that the genomes of the novel micromonosporae from the extreme hyper-arid Lomas Bayas soil have the capacity to synthesise a broad range of new bioactive compounds. It is also encouraging that M. chalcea strains LB4 and LB41 showed moderate to pronounced antitumour activity and that M. ureilytica strain LB19 and the putative type strains of M. arida (LB32T) and M. inaquosa (LB39T) showed promise in restricting the growth of the MRD K. pneumoniae strain. Gifted actinobacterial isolates such as these have a role to play in the search and discovery of new chemical scaffolds using state-of-the-art genome tools9, including ones designed to induce the expression of silent BGCs31,72. Indeed, novel micromonosporae should feature much more prominently in the search and discovery of new classes of specialised metabolites that are needed to control MRD pathogens which currently threaten to take humankind back to the pre-antibiotic days of medicine73,74. The search for additional novel and rare gifted micromonosporae from Atacama Desert habitats should include functional metagenomics and the use of isolation procedures known to target members of this taxon26,60,61 and improved characterisation procedures, notably ones for acquiring reliable phenotypic data56.
Stress-related genes encoded in the genome of the strains
The genomes of all of the isolates contained between 97 and 131 putative genes known to be associated with stress responses, notably ones coding for carbon starvation, heat shock responses, osmoregulation and oxidative stress (Table S3). The genomes of isolates LB19, LB32T and LB39T contained osmY the expression of which is known to be induced under hyperosmotic stress75. The expression of this gene is associated with the induction of the glycine betaine binding protein (proU), which was found in all of the isolated strains (with an average of 4 genes). The genomes of isolates LB19, LB32T and LB41 harboured genes involved in mycothiol biosynthesis (mshA, mshB, mshC, mshD), an analogue of glutathione that acts as an electron acceptor/donor and serves as a cofactor in detoxification reactions for alkylating agents, free radicals and xenobiotics76. Catalase and peroxidase genes were found in all the genomes confirming the results of the laboratory tests (Table S3). However, the type strain of M. chalcea, which gave a negative result has the capacity to produce catalase16. The genomes of all the isolates encode for several RNA polymerase Sigma factors and serine phosphatases that acts as regulators, it is known that Sigma B controls a general stress regulon which is induced when cells encounter growth-limiting conditions77.
The world’s highest levels of surface ultraviolet (UV) irradiance have been reported from the Atacama Desert78 hence it is particularly interesting that the genomes of all of the isolates included genes associated with protection against UV-radiation; we have previously shown that these strains grew on M65 agar following exposure to UV light at 100 mJoules/second for 30 minutes26. The genomes of all of the isolates contained genes belonging to the uvrABC DNA repair system, associated with excision proteins which have been reported in several bacteria79. Specific desiccation stress genes were not detected in any of the genomes though several genes associated with the biosynthesis and uptake of trehalose were present, this sugar has been linked with tolerance to heat and desiccation in bacteria80. The assortment of stress related genes outlined above provide an insight into how micromonosporae are able to adapt to severe environmental conditions that prevail in arid Atacama Desert soils. However, a similar complement of stress related genes have been found in the genomes of representative Micromonospora taxa isolated from diverse habitats16 thereby supporting the view that micromonoporae per se have the capacity to colonize multiple microhabitats28, including ones associated with extreme biomes58,81. In this context it is also interesting that the genomes of all of the isolates contained genes associated with the production of a range of growth promoters of potential value in phytostimulation16. The genomes of all of the isolates also contained methylglyoxal detoxification genes (gloA and gloB) which are associated with increases in plant tolerance to abiotic and biotic stress82.
Description of Micromonospora arida sp. nov
Micromonospora arida (a’ri.da L. fem. adj. arida, dry, referring to the isolation of the strain from an extreme hyper-arid soil).
Aerobic, Gram-stain-positive, chemoorganotrophic actinobacterium which produces non-motile, single spores on extensively branched substrate hyphae, but does not form aerial hyphae. Colonies are orange on ISP2 agar turning brown-black on sporulation. Grows between 20–37 °C, optimally ~28 °C, at pH 7.0 and 8.0, optimally ~pH 7.0, and in the presence of 1% w/v sodium chloride. Casein and starch are degraded. Catalase positive and oxidase negative. Cystine arylamidase, esterase (C4), esterase lipase (C8), N-acetyl-β-glucosaminidase, α- and β-galactosidase, α- and β-glucosidase, β-glucuronidase, leucine arylamidase, lipase (C14), naphthol-AS-BI-phosphohydrolase, trypsin and valine arylamidase are produced, but not acid or alkaline phosphatase, α-chymotrypsin, α-fucosidase or α-mannosidase. Metabolises L-alanine, L-arginine and L-serine (amino-acids); acetic acid, acetoacetic acid, L-aspartic acid, β-hydroxy-butyric acid, D-galacturonic acid, D-gluconic acid, L-glutamic acid, D-glucoronic acid, α-keto-glutaric acid, D-lactic acid methyl ester, L-malic acid, N-acetyl-neuraminic acid, methyl pyruvate, propionic acid, L-pyroglutamic acid, D- saccharic acid and bromo-succinic acid (organic acids); D-cellobiose, D-fructose, D-fructose-6-phosphate, L-fucose, D-galactose, D-glucose, D-glucose-6-phosphate, N-acetyl-D-galactosamine, N-acetyl-D-glucosamine, 3-O-methyl-D-glucose, glycerol, myo-inositol, D-mannitol, N-acetyl-B-D-mannose, D-salicin, D-sorbitol, stachyose, sucrose, D-trehalose and D-turanose (sugars) and dextrin, gelatin and pectin (polymers), but not D-aspartic acid, L-histidine or D-serine (amino acids), γ-amino-n-butyric acid, butyric acid, α- and α-keto-butyric acid, citric acid, D-malic acid, L-galacturonic acid-γ-lactate, L-lactic acid, mucic acid, p-hydroxy-phenylacetic acid and quinic acid (organic acids), or D-arabitol, D-fucose, glucoranimide or α-D-lactose (sugars). Resistant to aztreonam, nalidixic acid and rifamycin SV, but sensitive to fusidic acid, minocycline, troleandomycin and vancomycin. Does not grow at pH 5.0 or in the presence of guanidine hydrochloride, inosine, lithium chloride, potassium tellurite, sodium bromate, sodium lactate, tetrazolium blue or tetrazolium violet. Whole cell hydrolysates contain hydroxy- and meso-A2pm, galactose, glucose, mannose, rhamnose, ribose and xylose. The major fatty acids are iso-C15:0, iso-C17:0, anteiso-C17:0 and C17:0 and the predominant isoprenologues MK-10 (H4, H6, H8); the polar lipid profile contains diphosphatidylglycerol, phosphatidylethanolamine and phospatidylinositol together with unidentified components. The dDNA G + C content is 71.0 mol% and the genome size ~7.1 Mbp.
The type strain, LB32T (=CECT 9662T = LMG 30765T) was isolated from an extreme hyper-arid surface soil (2 cm) collected from the Lomas Bayas region of the Atacama Desert soil in Chile. The genome accession number is QGSY00000000.
Description of Micromonospora inaquosa sp. nov
Micromonospora inaquosa (in.a.quo’sa. L. fem. adj. inaquosa without water, referring to the isolation of the strain from an extreme hyper-arid soil).
Aerobic, Gram-stain-positive, chemoorganotrophic actinobacterium which produces non-motile, single spores on extensively branched substrate hyphae, but does not form aerial hyphae. Colonies are orange on ISP2 agar turning brown-black on sporulation. Grows between 12–37 °C, optimally ~28 °C, at pH 7.0 and 8.0, optimally ~pH 7.0 and in the presence of 1% w/v sodium chloride. Casein and starch are degraded. Catalase and oxidase positive. Acid and alkaline phosphatase, α-chymotrypsin, cystine arylamidase, esterase (C4), esterase lipase (C8), N-acetyl-β-glucosaminidase, α- and β-galactosidase, α- and β-glucosidase, β-glucuronidase, leucine arylamidase, lipase (C14), α-mannosidase, naphthol-AS-BI-phosphohydrolase, trypsin, and valine arylamidase are produced, but not α-fucosidase. Metabolises L-alanine, L-arginine, and D- and L-serine (amino-acids); acetic acid, acetoacetic acid, L-aspartic acid, butyric acid, D-galacturonic acid, D-gluconic acid, L-glutamic acid, D-glucuronic acid, D-lactic acid methyl ester, L-malic acid, methyl pyruvate, propionic acid, D- saccharic acid and bromo-succinic acid (organic acids); D-cellobiose, D-fructose, D-fructose-6-phosphate, L-fucose, D-galactose, D-glucose, D-glucose-6-phosphate, N-acetyl-D-galactosamine, N-acetyl-D-glucosamine, 3-O-methyl-D-glucose, glycerol, N-acetyl-β-D-mannose, D-salicin, stachyose, sucrose, D-trehalose and D-turanose (sugars) and dextrin and gelatin (polymers), but not L-histidine (amino acids); D-aspartic acid, γ-amino-n-butyric acid, α- and β-hydroxy-butyric acid, α-keto-butyric acid, citric acid, α-keto-glutaric acid, D-malic acid, L-galacturonic acid-γ-lactate, L-lactic acid, mucic acid, p-hydroxy-phenyl acetic acid, L-pyroglutamic acid, and quinic acid (organic acids); pectin (polymer) or glucoranimide, myo-inositol, D-mannitol or D-sorbitol (sugars). Resistant to aztreonam, minocycline, nalidixic acid and rifamycin SV, but sensitive to fusidic acid, troleandomycin and vancomycin. Does not grow at pH 5.0 or in the presence of guanidine hydrochloride, inosine, lithium chloride, potassium tellurite, sodium bromate, sodium lactate, tetrazolium blue or tetrazolium violet. Whole cell hydrolysates contain hydroxy- and meso-A2pm, galactose, glucose, mannose, rhamnose, ribose and xylose. The major fatty acids are iso-C15:0, iso-C16:0, 10-methyl C17:0 and C17:0, the predominant isoprenologues MK-10 (H4, H6, H8); the polar lipid profile consists of diphosphatidylglycerol, phosphatidylethanolamine together with unidentified components. The dDNA G + C content is 70.6 mol% and the genome size ~7.8 Mbp.
The type strain, LB39T (=CECT 9663T = LMG 30766T) was isolated from an extreme hyper-arid surface soil (2 cm) collected from the Lomas Bayas region of the Atacama Desert soil in Chile. The genome accession number is QGSZ00000000.
Methods
Selective isolation
All of the strains (isolates LB4, LB19, LB32T, LB39T and LB41) were recovered from the surface (2 cm) of an extreme hyper-arid soil collected from the Lomas Bayas region of the Atacama Desert (23° 24′ 27″ S, 69°31′03″ W 24.02.2014) by Professor Luis Cáceres (University of Antofagasta) as previously described26. Briefly, when transferred to the UK the sample was stored at 4 °C. The strains were isolated using the selective isolation procedure devised by Makkar and Cross83; to this end, aliquots (100 µl) of the 10−1/2 and 10−1 dilutions of the soil in ¼ strength Ringer’s solution were spread over the surface of starch-casein agar plates84 supplemented with sterile cycloheximide, nystatin and novobiocin (each at 25 µg/ml). Three replicate plates were prepared per dilution and incubated at 28 °C for 3 weeks when five characteristic orange-coloured Micromonospora colonies were detected. The isolates were maintained on M65 (DSMZ medium) agar plates and as mixtures of hyphal fragments and spores in 20% v/v glycerol at −80 °C.
Extraction of DNA and determination of RAPD profiles
Genetic profiles were generated by PCR using the primer M13 (5′-GAGGGTGGCGGTTCT-3′)85. DNA was extracted from all of the isolates using a REDExtract-N.Amp kit (Sigma) and amplified following the manufacturer’s recommendations to give a final volume of 20 μl per reaction; the thermal cycling parameters were: 7 min at 95 °C, 35 cycles of 1 min at 94 °C, 1 min at 45 °C and 2 min at 72 °C, followed by a 6 min final extension at 72 °C. A 1.5% agarose gel containing ethidium bromide was loaded with 5 µl of each of the PCR products and electrophoresis run at 85 V for 90 minutes in freshly prepared 1x TBE-EDTA buffer at pH 8.0 using a Bio-Rad PowerPac 300 power supply; a DNA molecular weight marker (1 kbp) was used as a molecular size standard. Photographs of the electrophoresis results recovered as TIFF files were aligned using BioNumerics package 6.0 into similarity groups.
Phylogenetic analysis
Genomic DNA extraction, PCR-mediated amplification and 16S rRNA gene sequencing were performed as described by Carro et al.26. Universal primers 27 F and 1522R86 were used for PCR amplification in a final volume of 50 μl using Bioline 2x MiFiTM mix following instructions of the manufacturer. The PCR products were purified and sequenced using the EZseq Barcode Service (Macrogen). The manually aligned sequences were compared with those of their closest neighbours retrieved from the EzBioCloud server87. Maximum-likelihood88 and neighbour-joining89 algorithms were used to generate the phylogenetic trees. In addition, a multilocus sequence analysis (MLSA) based on 16S rRNA, atpD, gyrB, recA and rpoB gene sequences retrieved from whole-genome sequences of the isolates was carried out using established procedures52 and a micromonosporal MLSA tree generated from the 9165 nucleotides using the neighbour-joining and maximum-likelihood algorithms.
Phenotypic profiles
The isolates were examined for micromorphological, Gram-stain and motility using a phase-contrast microscope (Leica; CTR MIC) and 7-day-old cultures grown on GYM Streptomyces agar (DSMZ medium 6590). They were also examined for their ability to grow in the presence of various concentrations of sodium chloride (1, 2, 5, 7 and 9% w/v) and over a range of pH (4.0–9.0 at one unit intervals) and temperature regimes (4, 10, 20, 28 37 and 40 °C) using GYM as the basal medium. All of these tests were recorded on duplicated cultures after 14 days of incubation. Enzymatic activities of the isolates were determined using API ZYM kits (bioMerieux) according to the manufacturer’s instructions. The ability of the isolates to oxidise diverse carbon and nitrogen sources and to show resistance to inhibitory compounds was determined using GEN III microplates in an Omnilog device (BIOLOG Inc., Haywood, USA) and the exported data of the duplicated samples analysed using the opm package for R version 1.0691,92. Other phenotypic analyses were determined following Carro et al.93.
Biomass for the chemotaxonomic analyses carried out on each of the isolates was prepared in shake flasks (180 rpm) in ISP2 broth94 following incubation at 28 °C for 14 days, washed twice in sterile saline solution, and freeze-dried. Standard procedures were used to detect the isomers of diaminopimelic acid (A2pm)95, menaquinones96, polar lipids97 and whole cell sugar composition98, using appropriate controls. Cellular fatty acids were extracted, methylated, examined by gas chromatography (Agilent Technologies mod. 7890 A) and analysed using the protocol of the Sherlock Microbial Identification (MIDI) system, version 6.399. The resultant peaks were named using the RTSBA6 database.
Whole-genome sequencing and genomic analyses
A single colony of each of the isolates was used to inoculate 50 ml aliquots of M65 broth and the resultant preparations incubated at 28 °C for 7 days when cells were centrifuged prior to sending to Microbes NG (Birmingham, UK). Genomic DNA extracted from each of the preparations was sequenced on an Illumina HiSeq 2500 instrument with 2 × 250 bp paired-end reads. All of the strains were analysed using a standard pipeline and identified with their closest reference genome using Kraken100 and by mapping the reads using BWA-MEM101. The reads were assembled into contigs using SPAdes 3.90102 and contigs <500 bp discarded. Variant calling performed on the draft assemblies using VarScan were reordered and reoriented relative to a reference genome based on a MUMmer whole-genome alignment. An automated annotation was performed using Prokka103 while antiSMASH 4.0 was used to determine and compare BGCs encoding for natural products104. The presence of other genes was detected using the SEED viewer105 following RAST annotation of the genomes106,107.
Digital DNA:DNA hybridisation (dDDH) values between the genomes of the isolates and between them and available genomes of their phylogenetic neighbours were calculated using the genome-to-genome distance calculator, GGDC 2.0, using formula 2 of the GGDC web server available at http://ggds.dsmz.de/ggdc.php. In addition, ANI values were determined between the strains using OAT version 0.93.1108.
Bioassays with the extract of the isolates
Each of the isolates was shaken in 50 ml of ISP 2 broth94 at 180 revolutions per minute (rpm) with resin beads (Amberlite XAD-16N, Sigma) at 28 °C for 14 days. Each preparation was centrifuged (4100 rpm) for 15 min and the biomass and XAD-16N resin beads soaked overnight in methanol and filtered through glass wool prior to evaporation of the methanol fraction at 40 °C by nitrogen sparging to generate the extracts. They were fractionated with Solid Phase Extraction (SPE) cartridges, using either 2 g or 5 g of a C18 resin (55 µm, 70 Å, from Strata) depending on the weight of the extract; four column volumes of the following solvents were sequentially used for the fractionation of the samples: 100% water, 25%, 50, 75 and 100% methanol and 100% methanol + 0.01% TFA. The eluted fractions were screened using liquid chromatography–mass spectrometry (LCMS).
Inhibition tests were carried out on each of the fractions using a concentration of 300 µg/ml in 96 well-plates containing a total incubation volume of 200 µl. The screening assays were carried out using a range of indicator microorganisms, namely MRD strains of Acinetobacter baumannii (CL5973), Escherichia coli (ATCC 25922), Klebsiella pneumoniae (ATCC 700603), Pseudomonas aeruginosa (MB5919), Staphylococcus aureus MB5393 (methicillin-resistant) and ATCC 29213 (methicillin-sensitive), as well as MDR Aspergillus fumigatus ATCC 46645. Negative controls were included in the plates for each microorganism tested without extracts. Anti-tumour activity against human hepatocellular carcinoma HepG2 cells was determined in the same system using concentrations of 75 mg/ml for each fraction. Negative and positive controls were included containing dimethyl sulfoxide (DMSO) and methyl methane sulphonate (MMS), respectively. Dexorubicin was used as standard at several concentrations (0.11, 0.34, 1, 3, 9, 28, 83, 250 μg/l).
Accession numbers for NCBI deposited genome sequences: LB4 (QGSX00000000), LB19 (QDGB00000000), LB32T (QGSY00000000), LB39T (QGSZ00000000), LB 41 (QGTA00000000) and SRA accession numbers are: LB4 (SRR8278219), LB19 (SRR8278244), LB32T (SRR8278835), LB39T (SRR8278845), LB41 (SRR8278854).
Supplementary information
Acknowledgements
This project was partly funded by a UK-Newton Project for UK-Chile collaboration (JIC CA 586) and by support from the Basal Programme of CONICYT for the Centre for Biotechnology and Bioengineering (CeBiB) (project FB0001). IN and LC thank Newcastle University for postdoctoral fellowships and ATB and MG are indebted to the Leverhulme Trust for Emeritus Fellowships. LC thanks the University of Salamanca for a postdoctoral fellowship.
Author Contributions
L.C., M.G. and H.P.K. designed the project. A.T.B., M.G. and J.A.A. collected the soil samples. C.P. isolated the strains, L.C. and I.N. carried out the taxonomic characterization of the strains, and J.M.I. generated the fatty acids profiles. L.C. and J.F.C. obtained the bacteria extracts that were analysed by J.F.C. and J.M. V.R. carried on antibacterial plug assays. L.C. was responsible for the genome sequencing, annotation, and genomic analyses. L.C. and M.G. drafted the manuscript which was critically reviewed by all the authors.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-019-38789-z.
References
- 1.Doorduijn DJ, Rooijakkers SHM, van Schaik W, Bardoel BW. Complement resistance mechanisms of Klebsiella pneumoniae. Immunobiology. 2016;221:1102–1109. doi: 10.1016/j.imbio.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 2.Genilloud O. Actinomycetes: still a source of novel antibiotics. Nat. Prod. Rep. 2017;34:1203–1232. doi: 10.1039/C7NP00026J. [DOI] [PubMed] [Google Scholar]
- 3.Goodfellow, M. “Actinobacteria”, in Bergey’s Manual of Systematics of Archaea and Bacteria, (eds Whitman, W. B., Rainey, F., Kämpfer, P., Trujillo, M. & Chun, J. P. et al.). 10.1002/9781118960608.cbm00004 (Wiley, 2015).
- 4.Goodfellow, M. “Actinobacteria phyl. nov.”, in Bergey’s Manual of Systematics of Archaea and Bacteria (eds Whitman, W. B., Rainey, F., Kämpfer, P., Trujillo, M. & Chun, J. P. et al.). 10.1002/9781118960608.pbm00002 (Wiley, 2015).
- 5.Demain AL. Importance of microbial natural products and the need to revitalize their discovery. J Ind. Microbiol. Biotech. 2014;41:185–201. doi: 10.1007/s10295-013-1325-z. [DOI] [PubMed] [Google Scholar]
- 6.Barka EA, et al. Taxonomy, physiology, and natural products of actinobacteria. Microbiol. Mol. Biol. Rev. 2016;80:1–43. doi: 10.1128/MMBR.00019-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shoichet BK. Drug discovery: nature’s pieces. Nat. Chem. 2013;5:9–10. doi: 10.1038/nchem.1537. [DOI] [PubMed] [Google Scholar]
- 8.Wright GD. Something old, something new: revisiting natural products in antibiotic drug discovery. Can. J. Microbiol. 2014;60:147–154. doi: 10.1139/cjm-2014-0063. [DOI] [PubMed] [Google Scholar]
- 9.Katz, L. & Baltz, R. H. Natural product discovery: past, present, and future. J. Ind. Microbiol. Biotechnol. 43, 155–176 (2016). [DOI] [PubMed]
- 10.Gomez-Escribano, J. P., Alt, S., Bibb, M. J. Next generation sequencing of actinobacteria for the discovery of novel natural products. Mar. Drugs. 14, 10.3390/md14040078 (2016). [DOI] [PMC free article] [PubMed]
- 11.Baltz, R. H. Synthetic biology, genome mining, and combinatorial biosynthesis of NRPS-derived antibiotics: a prespective. J. Ind. Microbiol. Biotechnol, 10.1007/s10295-017-1999-8 (2017). [DOI] [PubMed]
- 12.Harrison J, Studholme DJ. Recently published Streptomyces genome sequences. Microbial. Biotechnol. 2014;7:373–380. doi: 10.1111/1751-7915.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tian X, et al. Comparative genomics analysis of Streptomyces species reveals their adaptation to the marine environment and their diversity at the genomic level. Front. Microbiol. 2016;7:998. doi: 10.3389/fmicb.2016.00998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castro JF, et al. The “gifted” actinomycete Streptomyces leeuwenhoekii. Antonie van Leeuwenhoek. 2018;111:1433–1448. doi: 10.1007/s10482-018-1034-8. [DOI] [PubMed] [Google Scholar]
- 15.Tang B, et al. A systematic study of the whole genome sequence of Amycolatopsis methanolica strain 239T provides an insight into its physiological and taxonomic properties which correlate with its position in the genus. Synth. Syst. Biotechnol. 2016;1:169–186. doi: 10.1016/j.synbio.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carro L, et al. Genome-based classification of micromonosporae with a focus on their biotechnological and ecological potential. Sci. Rep. 2018;8:525. doi: 10.1038/s41598-017-17392-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mo X, et al. Identification of nocamycin biosynthetic gene cluster from Saccharothrix syringae NRRL B-16468 and generation of new nocamycin derivatives by manipulating gene cluster. Microb. Cell Fact. 2017;16:100. doi: 10.1186/s12934-017-0718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodfellow M, Fiedler HP. A guide to successful bioprospecting: informed by actinobacterial systematics. Antonie Van Leeuwenhoek. 2010;98:119–142. doi: 10.1007/s10482-010-9460-2. [DOI] [PubMed] [Google Scholar]
- 19.Goodfellow M, Nouioui I, Sanderson R, Xie F, Bull AT. Rare taxa and dark microbial matter: novel bioactive actinobacteria abound in Atacama Desert soils. Antonie van Leeuwenhoek. 2018;111:1315–1332. doi: 10.1007/s10482-018-1088-7. [DOI] [PubMed] [Google Scholar]
- 20.Bull AT, Asenjo JA, Goodfellow M, Gomez-Silva B. The Atacama Desert: Technical resources and the growing importance of novel microbial diversity. Annu Rev Microbiol. 2016;70:215–234. doi: 10.1146/annurev-micro-102215-095236. [DOI] [PubMed] [Google Scholar]
- 21.Rateb ME, Ebel R, Jaspars M. Natural product diversity of actinobacteria in the Atacama Desert. Antonie van Leewenhoek. 2018;111:1467–1477. doi: 10.1007/s10482-018-1030-z. [DOI] [PubMed] [Google Scholar]
- 22.Bull, A. T. “Actinobacteria of the extremobiosphere”, in Extremophiles Handbook, (ed. Horikoshi, K.), 1203–1240 (Springer, 2011).
- 23.Bull AT, Asenjo JA. Microbiology of hyper-arid environments: recent insights from the Atacama Desert, Chile. Antonie van Leeuwenhoek. 2013;103:1173–1179. doi: 10.1007/s10482-013-9911-7. [DOI] [PubMed] [Google Scholar]
- 24.Bull AT, et al. High altitude, hyper-arid soils of the Central-Andes harbor mega-diverse communities of actinobacteria. Extremophiles. 2017;22:47–57. doi: 10.1007/s00792-017-0976-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Idris H, Goodfellow M, Sanderson R, Asenjo JA, Bull AT. Actinobacterial rare biospheres and dar matter revelaed in habitats of the Chilean Atacama Desert. Sci. Rep. 2017;7:8373. doi: 10.1038/s41598-017-08937-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carro L, et al. Hunting for cultivable Micromonospora strains in soils of the Atacama Desert. Antonie van Leeuwenhoek. 2018;111:1375–1387. doi: 10.1007/s10482-018-1049-1. [DOI] [PubMed] [Google Scholar]
- 27.Berdy J. Bioactive microbial metabolites. J Antibiot (Tokyo) 2005;58:1–26. doi: 10.1038/ja.2005.1. [DOI] [PubMed] [Google Scholar]
- 28.Genilloud, O. “Micromonospora,” in Bergey’s Manual of Systematics of Archaea and Bacteria, (eds Whitman, W. B., Rainey, F., Kämpfer, P., Trujillo, M. & Chun, J. P. et al.), 10.1002/9781118960608.gbm00148 (Wiley 2015).
- 29.Adamek M, et al. Comparative genomes reveal phylogenetic distribution patterns of secondary metabolites in Amycolatopsis. BMC Genomes. 2018;19:426. doi: 10.1186/s12864-018-4809-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harvey AL, Edrada-Ebel R, Quinn RJ. The- re-emergence of natural products for drugs discovery in the genomics era. Nature Rev. Drug Disco. 2015;14:111–129. doi: 10.1038/nrd4510. [DOI] [PubMed] [Google Scholar]
- 31.Zhang, M. M. et al. CRISPC-Cas9 strategy for activation of silent Streptomyces biosynthetic gene clusters. Nature Chem Biol, 10.1038/nchembio.2341 (2017). [DOI] [PMC free article] [PubMed]
- 32.Foulerton, A. New species of Streptothrix isolated from the air. Lancet, 1199–1200 (1905).
- 33.Ørskov, J. Investigations into the Morphology of the Ray Fungi. Levin and Munksgaard (1923).
- 34.Ara I, Kudo T. Two new species of the genus Micromonospora: Micromonospora chokoriensis sp. nov. and Micromonospora coxensis sp. nov., isolated from sandy soil. J. Gen. Appl. Microbiol. 2007;53:29–37. doi: 10.2323/jgam.53.29. [DOI] [PubMed] [Google Scholar]
- 35.Trujillo ME, Kroppenstedt RM, Fernández-Molinero C, Schumann P, Martínez-Molina E. Micromonospora lupini sp. nov. and Micromonospora saelicesensis sp. nov., isolated from root nodules of Lupinus angustifolius. Int. J. Syst. Evol. Microbiol. 2007;57:2799–2804. doi: 10.1099/ijs.0.65192-0. [DOI] [PubMed] [Google Scholar]
- 36.Lechevalier MP, De Biévre C, Lechevalier HA. Chemotaxonomy of aerobic actinomycetes: phosphoipid composition. Biochem Syst Ecol. 1977;5:249–260. doi: 10.1016/0305-1978(77)90021-7. [DOI] [Google Scholar]
- 37.Zhang Y, et al. Micromonospora violae sp. nov., isolated from a root of Viola philippica Car. Antonie van Leeuwenhoek. 2014;106:219–225. doi: 10.1007/s10482-014-0184-6. [DOI] [PubMed] [Google Scholar]
- 38.Carro L, Riesco R, Spöer C, Trujillo M. Micromonospora ureilytica sp. nov., Micromonospora noduli sp. nov. and Micromonospora vinacea sp. nov., isolated from Pisum sativum nodules. Int. J. Syst. Evol. Microbiol. 2016;66:3509–3514. doi: 10.1099/ijsem.0.001231. [DOI] [PubMed] [Google Scholar]
- 39.Sveshnikova MA, Maksimova TS, Kudrina ES. The species belonging to the genus Micromonospora Ørskov, 1923 and their taxonomy. Mikrobiologiya. 1969;38:883–893. [PubMed] [Google Scholar]
- 40.Thawai C, Tanasupawat S, Itoh T, Suwanborirux K, Kudo T. Micromonospora aurantionigra sp. nov., isolated from a peat swamp forest in Thailand. Actinomycetologica. 2004;18:8–14. doi: 10.3209/saj.18_8. [DOI] [Google Scholar]
- 41.Jongrungruangchok S, Tanasupawat S, Kudo T. Micromonospora chaiyaphumensis sp. nov., isolated from Thai soils. Int. J. Syst. Evol. Microbiol. 2008;58:924–928. doi: 10.1099/ijs.0.65594-0. [DOI] [PubMed] [Google Scholar]
- 42.Tomita K, Hoshino Y, Ohkusa N, Tsuno T, Miyaki T. Micromonospora chersina sp. nov. Actinomycetologica. 1992;6:21–28. doi: 10.3209/saj.6_21. [DOI] [Google Scholar]
- 43.Songsumanus A, Tanasupawat S, Thawai C, Suwanborirux K, Kudo T. Micromonospora humi sp. nov., isolated from peat swamp forest soil. Int. J. Syst. Evol. Microbiol. 2011;61:1176–1181. doi: 10.1099/ijs.0.024281-0. [DOI] [PubMed] [Google Scholar]
- 44.Tanasupawat S, Jongrungruangchok S, Kudo T. Micromonospora marina sp. nov., isolated from sea sand. Int. J. Syst. Evol. Microbiol. 2010;60:648–652. doi: 10.1099/ijs.0.014068-0. [DOI] [PubMed] [Google Scholar]
- 45.Supong K, et al. Micromonospora sediminicola sp. nov., isolated from marine sediment. Int. J. Syst. Evol. Microbiol. 2013;63:570–575. doi: 10.1099/ijs.0.041103-0. [DOI] [PubMed] [Google Scholar]
- 46.Kirby BM, Meyers PR. Micromonospora tulbaghiae sp. nov., isolated from the leaves of wild garlic, Tulbaghia violacea. Int. J. Syst. Evol. Microbiol. 2010;60:1328–133. doi: 10.1099/ijs.0.013243-0. [DOI] [PubMed] [Google Scholar]
- 47.Rong X, Huang Y. Taxonomic evaluation of the Streptomyces hygroscopicus clade using multilocus sequence analysis and DNA–DNA hybridization, validating the MLSA scheme for systematics of the whole genus. Syst. Appl. Microbiol. 2012;35:7–18. doi: 10.1016/j.syapm.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 48.Rong X, Huang Y. Multi-locus sequence analysis: taking prokaryotic systematics to the next level. Methods Microbiol. 2014;41:221–251. doi: 10.1016/bs.mim.2014.10.001. [DOI] [Google Scholar]
- 49.Wayne LG, et al. Report of the ad hoc committee on reconciliation of approaches to bacterial systematics. Int. J. Syst. Evol. Microbiol. 1987;37:463–464. doi: 10.1099/00207713-37-4-463. [DOI] [Google Scholar]
- 50.Trujillo ME, Kroppenstedt RM, Schumann P, Carro L, Martinez-Molina E. Micromonospora coriariae sp. nov., isolated from root nodules of Coriaria myrtifolia. Int. J. Syst. Evol. Microbiol. 2006;56:2381–2385. doi: 10.1099/ijs.0.64449-0. [DOI] [PubMed] [Google Scholar]
- 51.Carro L, Pukall R, Spröer C, Kroppenstedt RM, Trujillo ME. Micromonospora cremea sp. nov. and Micromonospora zamorensis sp. nov., isolated from the rhizosphere of Pisum sativum. Int. J. Syst. Evol. Microbiol. 2012;62:2971–2977. doi: 10.1099/ijs.0.038695-0. [DOI] [PubMed] [Google Scholar]
- 52.Carro L, Sproer C, Alonso P, Trujillo ME. Diversity of Micromonospora strains isolated from nitrogen fixing nodules and rhizosphere of Pisum sativum analyzed by multilocus sequence analysis. Syst. Appl. Microbiol. 2012;35:73–80. doi: 10.1016/j.syapm.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 53.Zhao S, et al. Micromonospora parathelypteridis sp. nov., an endophytic actinomycete with antifungal activity isolated from the root of Parathelypteris beddomei (Bak.) Ching. Int. J. Syst. Evol. Microbiol. 2017;67:268–274. doi: 10.1099/ijsem.0.001614. [DOI] [PubMed] [Google Scholar]
- 54.Goodfellow M, Weaver CR, Minnikin DE. Numerial classification of some rhodococci, corynebacteria and related organisms. J. Gen. Microbiol. 1982;128:731–745. doi: 10.1099/00221287-128-4-731. [DOI] [PubMed] [Google Scholar]
- 55.Williams JGK, Kubelic AR, Livak KJ, Rafalski JA, Tingey SV. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic. Acids. Res. 1990;18:6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Riesco R, et al. Defining the species Micromonospora saelicesensis and Micromonospora noduli under the framework of genomics. Front Microbiol. 2018;9:1360. doi: 10.3389/fmicb.2018.01360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hirsch P, Mevs U, Kroppenstedt RM, Schumann P, Stackebrandt E. Cryptoendolithic actinomycetes from antarctic sandstone rock samples: Micromonospora endolithica sp. nov. and two isolates related to Micromonospora coerulea Jensen 1932. Syst. Appl. Microbiol. 2004;27:166–74. doi: 10.1078/072320204322881781. [DOI] [PubMed] [Google Scholar]
- 58.Norovsurén ZH, Oborotov GV, Zenova GM, Aliev RA, Zviagintsev DG. Haloalkaliphilic actinomycetes in soils of Mongolian desert steppes. Izv. Akad. Nauk. Ser. Biol. 2007;4:501–507. [PubMed] [Google Scholar]
- 59.Lubsanova DA, Zenova GM, Kozhevin PA, Manucharova NA, Shvarov AP. Filamentous actinobacteria of the saline soils of arid territories. Moscow Univ. Soil Sci. Bull. 2014;69:88–92. doi: 10.3103/S0147687414020057. [DOI] [Google Scholar]
- 60.Talukdar M, et al. Bioprospecting Micromonospora from Kaziranga Natural Park of India and their anti-infective potential. World. J. Microbiol. Biotechnol. 2012;28:2703–2712. doi: 10.1007/s11274-012-1080-8. [DOI] [PubMed] [Google Scholar]
- 61.Talukdar, M., Bora, T. C. & Jha, D. K. “Micromonospora: a potential source of antibiotic”, in Bioprospecting of Indigenous Bioresources of North-East India, (ed. Purkayastha, J.) pp. 195–213 (Springer Science, 2016).
- 62.Kawaguchi H, et al. Studies on coumermycin, a new antibiotic. I. Production, isolation and characterization of coumermycin A1. J. Antibiot. Ser. A. 1965;18:1–10. [PubMed] [Google Scholar]
- 63.Anderle C, et al. Biological activies of novel gyrase inhibitors of the aminocoumarin class. Antimicrob Agents Cremother. 2008;52:1982–1990. doi: 10.1128/AAC.01235-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nagata H, et al. Lymphostin (LK6-A), a novel immunosuppressant from Streptomyces sp. KY11783: taxonomy of the producing organism, fermentation, isolation and biological activities. J Antibiot (Tokyo). 1997;50:537–42. doi: 10.7164/antibiotics.50.537. [DOI] [PubMed] [Google Scholar]
- 65.Mason WP, et al. A phase II study of the Ras-MAPK signaling pathway inhibitor TLN-4601 in patients with gliobastoma at first progresssion. J. Neurooncol. 2012;107:243–249. doi: 10.1007/s11060-011-0747-6. [DOI] [PubMed] [Google Scholar]
- 66.Charan RD, et al. Diazepinomicin, a new antimicrobial alkaloid from a marine Micromonospora sp. J. Nat. Prod. 2004;67:1431–1433. doi: 10.1021/np040042r. [DOI] [PubMed] [Google Scholar]
- 67.Park JS, Yang HO, Kwon HC. Aldgamycin I, an antibacterial 16-membered macrolide from the abandoned mine bacterium, Streptomyces sp. KMA-001. J. Antibiot. 2009;62:171–175. doi: 10.1038/ja.2009.6. [DOI] [PubMed] [Google Scholar]
- 68.Melby JO, Nard NJ, Mitchell DA. Thiazole/oxazole-modified microcins: complex natural products from ribosomal templates. Curr. Opin. Chem. Biol. 2011;15:369–378. doi: 10.1016/j.cbpa.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fang J, et al. Cloning and characterization of the tetrocarcin A gene cluster from Micromonospora chalcea NRRL 11289 reveals a highly conserved strategy for tetronate biosynthesis in spirotetronate antibiotics. J. Bacteriol. 2008;190:6014–6025. doi: 10.1128/JB.00533-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gauze GF. Production of a new antibiotic, chalcidin, from Micromonospora chalcea culture. Antibiotiki. 1970;5:483–486. [PubMed] [Google Scholar]
- 71.Salauze D, Davies J. Isolation and characterisation of an aminoglycoside phosphotransferase from enomycin-producing Micromonospora chalcea; comparison with that of Streptomyces fradiae and other producers of 4,6-disubstituted 2.deoxystreptamine antibiotics. J Antibio. 1991;44:1432–1443. doi: 10.7164/antibiotics.44.1432. [DOI] [PubMed] [Google Scholar]
- 72.Zimert N, Alanjary M, Weber T. The evolution of genome mining microbes – a review. Nat. Prod. Rep. 2016;33:988–1005. doi: 10.1039/C6NP00025H. [DOI] [PubMed] [Google Scholar]
- 73.Roca I, et al. The global threat of antimicrobial resistance: science for intervention. New Microbes New Infect. 2015;6:22–29. doi: 10.1016/j.nmni.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ventosa CL. The antibiotic resistance crisis. Pharma. Therapeu. 2015;40:277–283. [PMC free article] [PubMed] [Google Scholar]
- 75.Yim HH, Villarejo M. osmY, a new hyperosmotically inducible gene, encodes a periplasmic protein in. Escherichia coli. J. Bacteriol. 1992;174:3637–3644. doi: 10.1128/jb.174.11.3637-3644.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Newton GL, Buchmeier N, Rahey RC. Biosynthesis and functions of mycothiol, the unique protective thiol of Actinobacteria. Microbiol. Mol. Biol. Rev. 2008;72:471–494. doi: 10.1128/MMBR.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boylan SA, Redfield AR, Brody MS, Price CW. Stress-induced activation of the sigma B transcription factor of Bacillus subtilis. J. Bacteriol. 1993;175:7931–7937. doi: 10.1128/jb.175.24.7931-7937.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cordero RR, et al. Ultraviolet radiation in the Atacama Desert. Antonie van Leeuwenhoek. 2018;111:1301–1313. doi: 10.1007/s10482-018-1075-z. [DOI] [PubMed] [Google Scholar]
- 79.Goosen N, Moolenaar GE. Repair of UV damage in bacteria. DNA Repair. 2008;7:353–374. doi: 10.1016/j.dnarep.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 80.Reina-Bueno M, et al. Role of trehalose in heat and desiccation tolerance in the soil bacterium Rhizobium etli. BMC Microbiol. 2013;12:207–223. doi: 10.1186/1471-2180-12-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Makhalanyane TP, et al. Microbial ecology of hot desert edaphic systems. FEMS Microbiology Reviews. 2015;39:203–221. doi: 10.1093/femsre/fuu011. [DOI] [PubMed] [Google Scholar]
- 82.Melvin P, Bankapalli K, D’Silva P, Shivaprasad PV. Methylglyoxal detoxification by a DJ-1 family protein provides dual abiotic and biotic stress tolerance in transgenic plants. Plant. Mol. Biol. 2017;94:381–397. doi: 10.1007/s11103-017-0613-9. [DOI] [PubMed] [Google Scholar]
- 83.Makkar NS, Cross T. Actinoplanetes in soil and on plant litter from freshwater habitats. J. Appl. Microbiol. 1982;52:209–218. [Google Scholar]
- 84.Küster E, Williams ST. Selection of media for isolation of streptomycetes. Nature. 1964;202:928–929. doi: 10.1038/202928a0. [DOI] [PubMed] [Google Scholar]
- 85.Williams ST, et al. A probability matrix for identification of some stresptomycetes. J. Gen. Microbiol. 1983;129:1315–1330. doi: 10.1099/00221287-129-6-1815. [DOI] [PubMed] [Google Scholar]
- 86.Lane, D. J. “16S/23S rRNA sequencing”, in Nucleic Acid Techniques in Bacterial Systematics, (eds. Stackebrandt, E. & Goodfellow, M.), 115–175 (Wiley, 1991).
- 87.Yoon SH, et al. Introducing EzBioCloud: a taxonomically united database of 16S rRNA and whole genome assemblies. Int. J. Syst. Evol. Microbiol. 2016;67:1613–1617. doi: 10.1099/ijsem.0.001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Felsenstein J. Evolutionary trees from DNA sequences: a maximum likelihood approach. J. Mol. Evol. 1981;17:368–376. doi: 10.1007/BF01734359. [DOI] [PubMed] [Google Scholar]
- 89.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 90.Atlas, R. M. Handbook of Microbiological Media, 3rd edn. (CRC Press Boca Raton, 2004).
- 91.Vaas LAI, Sikorski J, Michael V, Göker M, Klenk H-P. Visualization and curve-parameter estimation strategies for efficient exploration of phenotype microarray kinetics. PLoS One. 2012;7:e34846. doi: 10.1371/journal.pone.0034846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vaas LAI, et al. opm: an R package for analysing OmniLog_ phenotype microarray data. Bioinformatics. 2013;29:1823–1824. doi: 10.1093/bioinformatics/btt291. [DOI] [PubMed] [Google Scholar]
- 93.Carro L, et al. Micromonospora phytophila sp. nov. and Micromonospora luteiviridis sp. nov., isolated as natural inhabitants of plant nodules. Int. J. Syst. Evol. Microbiol. 2018;68:248–253. doi: 10.1099/ijsem.0.002490. [DOI] [PubMed] [Google Scholar]
- 94.Shirling EB, Gottlieb D. Methods for characterization of Streptomyces species. Int. J. Syt. Evol. Microbiol. 1966;16:313–340. [Google Scholar]
- 95.Staneck JL, Roberts GD. Simplified approach to the identification of aerobic actinomycetes by thin layer chromatography. Appl Microbiol. 1974;28:226–231. doi: 10.1128/am.28.2.226-231.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Collins MD, Goodfellow M, Minnikin DE, Alderson G. Menaquinone composition of mycolic acid-containing actinomycetes and some sporoactinomycetes. J. Appl. Bacteriol. 1985;58:77–86. doi: 10.1111/j.1365-2672.1985.tb01431.x. [DOI] [PubMed] [Google Scholar]
- 97.Minnikin DE, et al. An integrated procedure for extracting bacterial isoprenoid quinones and polar lipids. J. Microbiol. Methods. 1984;2:233–241. doi: 10.1016/0167-7012(84)90018-6. [DOI] [Google Scholar]
- 98.Lechevalier MP, Lechevalier HA. Chemical composition as a criterion in the classification of aerobic actinomycetes. Int. J. Syst. Evol. Microbiol. 1970;20:435–443. [Google Scholar]
- 99.Sasser, M. J. Identification of bacteria by gas chromatography of cellular fatty acids. (Del: Microbial ID Inc, Newark, 1990).
- 100.Wood DE, Salzberg SL. Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol. 2014;15:R46. doi: 10.1186/gb-2014-15-3-r46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler Transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bankevich A, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 104.Blin K, Medema MH, Kottmann R, Lee SY, Weber T. The antiSMASH database, a comprehensive database of microbial secondary metabolite biosynthetic gene clusters. Nucleic Acids Res. 2017;45:D555–D559. doi: 10.1093/nar/gkw960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Overbeek R, et al. The SEED and the Rapid Annotation of Microbial Genomes using Subsystems Technology (RAST) Nucleic. Acids Research. 2014;42:D206–D214. doi: 10.1093/nar/gkt1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Aziz RK, et al. The RAST Server: Rapid Annotations using Subsystems Technology. BMC Genomics. 2008;9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brettin, T. et al. RASTtk: A modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. 5, 8365, 10.1038/srep08365 (2015). [DOI] [PMC free article] [PubMed]
- 108.Lee I, Ouk Kim Y, Park SC, Chun J. OrthoANI: An improved algorithm and software for calculating average nucleotide identity. Int. J. Syst. Evol. Microbiol. 2016;66:1100–1103. doi: 10.1099/ijsem.0.000760. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.