Abstract
Aim:
To compare relative efficacy and safety of mechanical compression devices (AutoPulse and LUCAS) with manual compression in patients with cardiac arrest undergoing cardiopulmonary resuscitation (CPR).
Methods:
For this Bayesian network meta-analysis, seven randomized controlled trials (RCTs) were selected using PubMed/Medline, EMBASE, and CENTRAL (Inception- 31 October 2017). For all the outcomes, median estimate of odds ratio (OR) from the posterior distribution with corresponding 95% credible interval (Cr I) was calculated. Markov chain Monte Carlo (MCMC) modeling was used to estimate the relative ranking probability of each intervention based on surface under the cumulative ranking curve (SUCRA).
Results:
In analysis of 12, 908 patients with cardiac arrest [AutoPulse (2, 608 patients); LUCAS (3, 308 patients) and manual compression (6, 992 patients)], manual compression improved survival at 30 days or hospital discharge (OR, 1.40, 95% Cr 1,1.09–1.94), and neurological recovery (OR, 1.51, 95% Cr 1,1.06–2.39) compared to AutoPulse. There were no differences between LUCAS and AutoPulse with regards to survival to hospital admission, neurological recovery or return of spontaneous circulation (ROSC). Manual compression reduced the risk of pneumothorax (OR, 0.56, 95% Cr I, 0.33–0.97); while, both manual compression (OR, 0.15, 95% Cr I, 0.01–0.73) and LUCAS (OR, 0.07, 95% Cr I, 0.00–0.43) reduced the risk of hematoma formation compared to AutoPulse. Probability analysis ranked manual compression as the most effective treatment for improving survival at 30 days or hospital discharge (SUCRA, 84%).
Conclusions:
Manual compression is more effective than AutoPulse and comparable to LUCAS in improving survival at 30 days or hospital discharge and neurological recovery. Manual compression had lesser risk of pneumothorax or hematoma formation compared to AutoPulse.
Keywords: Compression devices, Cardiac arrest, Network meta-analysis
Introduction
Sudden cardiac arrest accounts for substantial mortality and morbidity worldwide. The estimated incidence of out of hospital cardiac arrest (OHCA) is more than 350,000 per year in the Unites States (US) [1], and more than 270,000 in the European Union [2]. The estimated overall survival rate in the US is as low as 12% [1]. Early initiation of high quality chest compressions is considered the essential component of successful cardiopulmonary resuscitation (CPR) for enhancing survival among cardiac arrest victims [3,4]. The European Resuscitation Council (ERC) and the American Heart Association (AHA) recommend quality CPR with chest compressions delivered at a rate of 100–120/ min with a depth of at least 5 cm [1,2]. These requirements are usually difficult to meet due to limited man power, fatigue, competing tasks and access to the patient, which consequently may lead to suboptimal CPR.
To meet the required specifications, the Food and Drug Administration (USA) approved two mechanical compression devices: AutoPulse (Zoll Medical Corporation, Chelmsford, MA, USA) and LUCAS (Physio-Control/Jolife AB, Lund, Sweden) to perform chest compression. There is a noticeable inconsistency in the published literature with regards to efficacy of these devices. Various randomized controlled trials (RCTs) could not demonstrate a survival benefit of mechanical compression over manual compression, whereas, there is substantial observational data which suggested that mechanical CPR could improve survival to hospital admission rates [5]. Furthermore, there is paucity of data related to safety profiles of these devices. This discrepancy in literature calls for assessment of relative efficacy and safety of mechanical compression devices and manual compression in patients with cardiac arrest. To fill this knowledge gap, we performed a Bayesian network meta-analysis to compare AutoPulse, LUCAS and manual compression in this subset of patients.
Methods
This meta-analysis followed the Cochrane Collaboration group, and PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) Extension for Network Meta-analyses guidelines [6,7].
Inclusion criteria
Eligible studies were RCTs which compared AutoPulse, LUCAS and manual Compression in subjects with cardiac arrest (both OHCA and In Hospital Cardiac Arrest (IHCA)). The studies had to report at least one clinical event among desired outcomes in adult population. There were no restrictions on sample size, comorbidities, initial rhythm or follow up duration. Two authors (MUK and ST) screened the search results based on priori criteria. The entire process was done under the supervision of third author (SUK).
Data sources and searches
Two authors (ANL and MUK) searched MEDLINE, EMBASE and CENTRAL from Inception to 31 October 2017. The review of the bibliographies of the relevant articles was also performed. The search was restricted to full text articles, humans and RCTs. There was no restriction on language or publication year. The key search words were: “cardiopulmonary resuscitation”, “CPR”, “cardiac arrest”, “mechanical compression devices”, “AutoPulse” and “LUCAS”. The search results were downloaded to Endnote (Thompson ISI ResearchSoft, Philadelphia, Pennsylvania, USA) and duplicates were removed manually and through EndNote.
Data extraction and quality assessment
Two authors (ST and MZK) performed data abstraction on a prespecified data collection form. The following information was extracted: baseline characteristics of the participants, events, non-events, sample size, and follow-up duration. We preferred outcomes from intention to treat analyses. When available, adjusted estimates were extracted. We also reviewed study protocols and appendices for additional information. The accuracy of data was appraised by third author, ANL. The Cochrane bias risk assessment tool was used for quality assessment and bias risk assessment was done at study level [8] (Supplementary Table S1).
Outcome measures
The primary outcome was survival at 30 days or hospital discharge. The secondary outcomes were survival to hospital admission, return of spontaneous circulation (ROSC), neurological recovery, visceral damage, sternal or rib fracture, pneumothorax, and hematoma formation. There was variation in definition of neurological recovery. Three studies reported improvement in neurological function through cerebral performance category (CPC), while one study assessed neurological improvement by modified Rankin Scale (mRS). We defined neurological recovery as CPC score 1 or 2, or mRS ≤ 3. The definitions of the other endpoints were taken as reported in the trials.
Statistical analysis
The Bayesian network meta-analysis is a superior statistical approach to traditional meta-analysis due to its ability to pool data related to multiple treatments concurrently, which allows greater flexibility to use complex models with a more natural interpretation [9]. This strategy can rank treatments according to their relative efficacy and safety, facilitating predictive statements to be made regarding a specific problem and consequently improving evidence-based decision making.
The Bayesian network meta-analysis was performed using NetMetaXL 1.6.1 (Canadian Agency for Drugs and Technologies in Health; Ottawa, Canada) and winBUGS 1.4.3 (MRC Biostatistics Unit; Cambridge, United Kingdom). The random effects model was selected for interpretation of results for its more conservative estimates. The analyses were conducted with vague priors and informative priors separately to assess for the appropriateness of the model. For random effects vague priors, we assumed use the following priors: sd~dunif (0,2); where dunif is the density function of the uniform distribution, sd is the vector of standard deviations, and 0 and 2 describe minimum and maximum vector of quantiles, respectively. For informative variance prior, all-cause mortality informative priors were selected based on non-pharmacological intervention with objective outcomes.
NetMetaXL uses these selections and bases the informative variance priors on evidence on the extent of heterogeneity noticed in prior metaanalyses, as reported in Turner et al [10]. For all analyses, we assumed vague priors on baseline [dnorm (0, 10,000)] and basic parameters [dnorm (0, 10,000)], where function “dnorm” return the value of the probability density function for the normal distribution based on given parameters. Since informative priors, when used properly, can improve modeling efficiency by providing solutions to computational issues, we ultimately applied predictive distributions (informative variance priors) to random effects analyses [10,11]. For all the outcomes, we achieved convergence at 20,000 iterations and autocorrelation was checked and confirmed. The inconsistency was assessed by comparing the deviance residuals and DIC statistics in fitted consistency and inconsistency models [12].
We calculated median estimate of odds ratio (OR) from the posterior distribution and reported it with 2.5th to the 97.5th centiles of the distribution [95% credible interval (Cr I)]. The assessment of between- study variances was interpreted as suggested by Turner et al: low (τ2 = 0.04), moderate (τ2 = 0.14) and high (τ2 = 0.40) [10]. Markov chain Monte Carlo (MCMC) modeling was used to calculate the relative ranking probability of each intervention. “Rankograms” along with surface under the cumulative ranking curve (SUCRA) were provided to compare hierarchy of efficacy and safety of the interventions [13]. The SUCRA is a numeric presentation of the overall ranking and demonstrates a single number associated with each treatment. The SUCRA values range from 0 to 100%. The higher the SUCRA value, and the closer to 100%, the higher the likelihood that a therapy is in the top rank or highly effective; the closer to 0 the SUCRA value, the more likely that a therapy is in the bottom rank or ineffective.
Results
A total of 1994 articles were retrieved after electronic data base search and review of bibliographies; of which —1159 were duplicates, and 828 were removed based on title, abstract, study design, unwanted comparisons or undesired outcomes. Ultimately seven trials were incorporated into this meta-analysis (Fig. 1). In total 12,908 cardiac arrest patients [AutoPulse (2, 608 patients); LUCAS (3, 308 patients) and manual compression (6, 992 patients)] participated in this meta-analysis. The mean age of the participants was 68 ± 3 years, 64% were men, 28% had cardiac arrest due to ventricular arrhythmia, 25% had pulseless electrical activity and 37% had asystole. The trial by Koster et al. was the only study which assessed the interventions in both OHCA and IHCA patients [14], while the rest of the studies enrolled exclusively subjects succumbing to OHCA (Table 1).
Fig. 1.
PRISMA flow diagram showing study selection process
Table 1.
Baseline characteristics of the studies, ASPIRE (AutoPulse Assisted Prehospital International Resuscitation); CIRC (Circulation Improving Resuscitation Care); CPR (Cardiopulmonary Resuscitation); LINC (LUCAS in Cardiac Arrest); N/A (Not Available); PARAMEDIC (The Prehospital Randomized Assessment of A Mechanical Compression Device in Cardiac Arrest); VF (Ventricular Fibrillation); VT (Ventricular Tachycardia); PEA (Pulseless Electrical Activity).
| Studies (Year) | Setting | Groups | n | Age | Men (%) | CPR by bystander (%) | VF /VT (%) | PEA (%) | Asystole (%) |
|---|---|---|---|---|---|---|---|---|---|
| ASPIRE [17] | OHCA | Manual | 373 | 66 | 66 | 35 | 32 | 25 | 40 |
| AutoPulse | 394 | 67 | 64 | 32 | 31 | 20 | 42 | ||
| Axelsson et al. [22] | OHCA | Manual | 169 | 71 | 66 | 42 | 32 | 12 | 34 |
| LUCAS | 159 | 71 | 63 | 45 | 30 | 18 | 34 | ||
| Smekal et al. [23] | OHCA | Manual | 73 | 71 | 68 | 31 | N/A | N/A | N/A |
| LUCAS | 75 | 75 | 68 | 34 | N/A | N/A | N/A | ||
| LINC [18] | OHCA | Manual | 1289 | 69 | 66 | 55 | 30 | 20 | 46 |
| LUCAS | 1300 | 69 | 67 | 57 | 29 | 20 | 47 | ||
| CIRC [19] | OHCA | Manual | 2132 | 66 | 61 | 47 | 24 | NA | NA |
| AutoPulse | 2099 | 66 | 61 | 49 | 21 | NA | NA | ||
| PARAMEDIC [20] | OHCA | Manual | 2819 | 72 | 63 | 44 | 22 | 25 | 49 |
| LUCAS | 1652 | 71 | 63 | 43 | 23 | 24 | 50 | ||
| Koster et al. [14] | OHCA/ IHCA | Manual | 137 | 66 | 64 | NA | 25 | 39 | 24 |
| AutoPulse | 115 | 65 | 65 | NA | 26 | 38 | 23 | ||
| LUCAS | 122 | 63 | 67 | NA | 34 | 34 | 18 | ||
In the network meta-analysis, manual compression improved survival at 30 days or hospital discharge (OR, 1.40, 95% Cr I, 1.09–1.94) and neurological recovery (OR, 1.51, 95% Cr I, 1.06–2.39) when compared to AutoPulse. There were no differences between manual compression and LUCAS or among LUCAS and AutoPulse with regards to survival at 30 days or hospital discharge or neurological recovery. All three interventions showed identical benefits with regards to survival to hospital admission or ROSC (Fig. 2). Compared to AutoPulse, manual compression was associated with 44% relative risk reduction of pneumothorax (OR, 0.56, 95% Cr I, 0.33–0.97) and 85% lesser risk of hematoma formation (OR, 0.15, 95% Cr I, 0.01–0.73). LUCAS had significant 93% reduced risk of hematoma formation compared to AutoPulse (OR, 0.07, 95% Cr I, 0.00–0.43) (Fig. 3). All of the three interventions could not demonstrate differences with regards to visceral damage, tension pneumothorax or rib or sternal fractures.
Fig. 2.
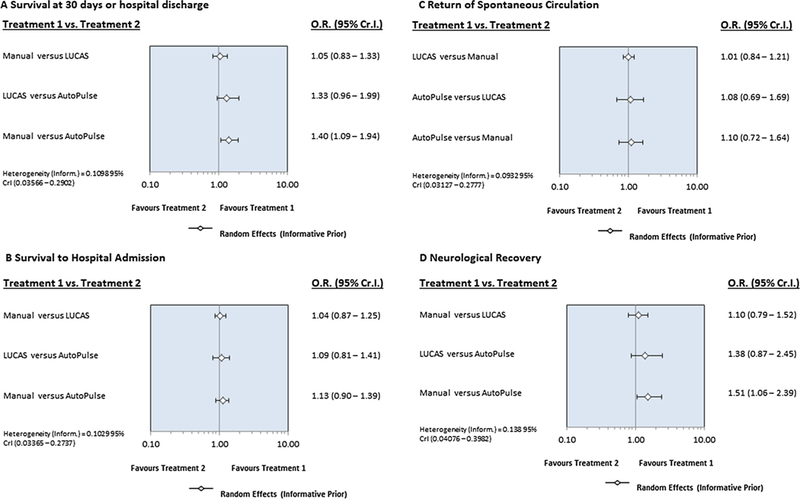
Forest plot showing comparison of intervention with regards to efficacy outcomes.
Fig. 3.
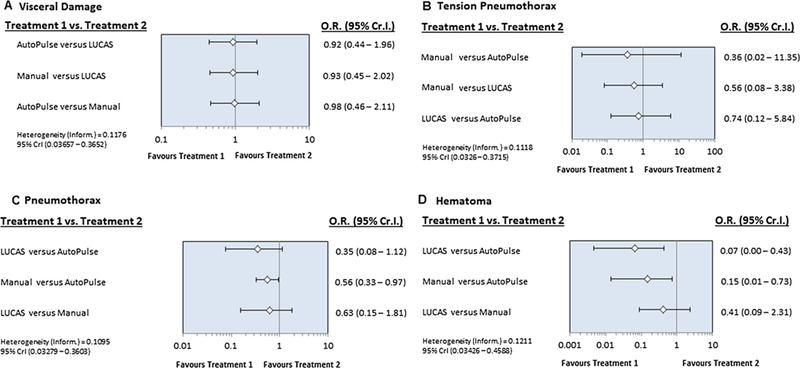
Rankogram showing comparative ranking of each interventions for efficacy outcomes.
Probability analysis ranked manual compression as the most effective intervention for having the highest probability of survival at 30 days or hospital discharge (SUCRA, 84%), survival to hospital admission (SUCRA, 77%), or neurological improvement (SUCRA, 87%) (Fig. 4). With regards to safety profile, AutoPulse had the lowest probability of having visceral damage (SUCRA, 56%), whereas, manual compression was ranked safest with regards to tension pneumothorax (SUCRA, 71%) (Fig. 5).
Fig. 4.
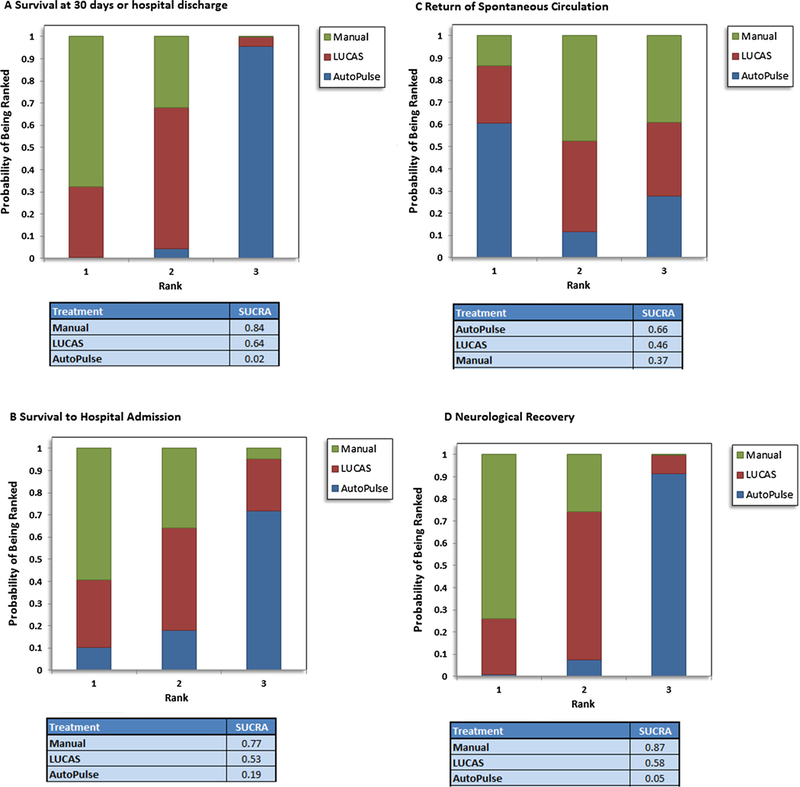
Forest plot showing comparison of intervention with regards to safety outcomes.
Fig. 5.
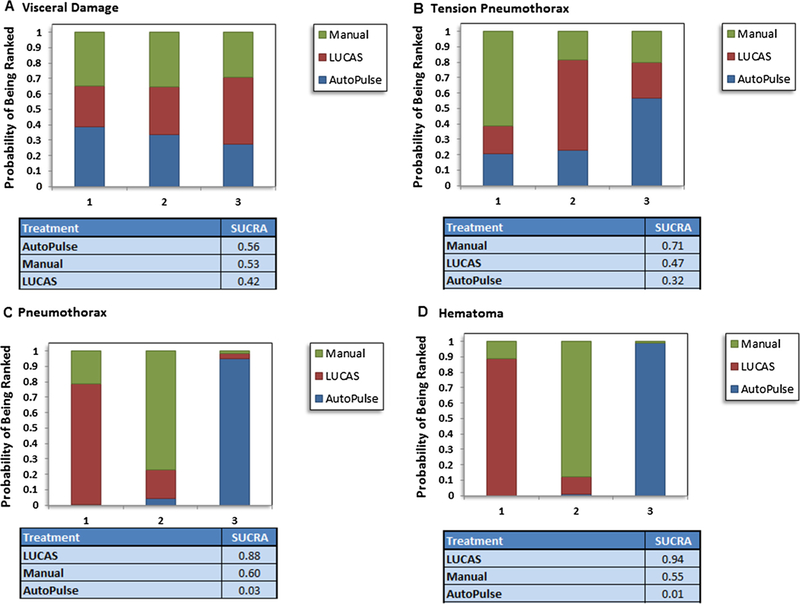
Rankogram showing comparative ranking of each interventions for safety outcomes.
Discussion
In this network meta-analysis of seven trials involving 12,908 patients subjected to CPR following cardiac arrest, manual compression when compared to AutoPulse improved the rates of survival at 30 days or hospital discharge by 60% and neurological recovery by 49%. LUCAS and AutoPulse shared similar efficacy profile in terms of survival at 30 days or hospital discharge, survival to hospital admission, ROSC and neurological recovery. Similarly, there were no differences between manual compression and LUCAS with regards to efficacy. Manual compression had lesser risk of pneumothorax or hematoma formation compared to AutoPulse; while, LUCAS showed superior safety in terms of hematoma formation compared to AutoPulse. Probability analysis ranked manual compression as the most effective strategy to improve survival at 30 days or hospital discharge, survival to hospital admission, and neurological recovery, followed by LUCAS as the second best strategy. Manual compression had the lowest probability of causing pneumothorax, whereas AutoPulse had the lowest probability of being safe in terms of pneumothorax, tension pneumothorax, hematoma formation and rib or sternal fractures.
The AHA and ERC consider mechanical compression acceptable for continuing CPR during transportation or during coronary revascularization [15,16]. However, RCTs have failed to demonstrate survival benefit with mechanical compression devices. The possible explanations for this observation are multiple. Firstly, compared to manual compression, device positioning interrupts the continuity of chest compression and can potentially prolong the time to first shock delivery. In the pioneer ASPIRE (AutoPulse Assisted Prehospital International Resuscitation) trial, which was discontinued prematurely due to unfavorable neurological and survival outcomes with AutoPulse, the mean time to first shock in ventricular fibrillation was prolonged by 2.1 min in the AutoPulse group [17]. In the CIRC (Circulation Improving Resuscitation Care) trial and LINC (LUCAS in Cardiac Arrest) trial the delay in first shock delivery was 1–1.5 min longer with device than with manual compression [18,19]. The prolongation of both the compression free duration and the time to first shock may compromise the cerebral and cardiac perfusion and consequently result in poor neurological and survival outcomes.
Second, not all patients can be fitted into the available mechanical devices. In LINC trial, 3.5% patients could not fit the device due to either increased body habitus (2.3%) or being too small (1.2%); and only 95% patients were able to receive LUCAS [18]. This factor might further delay the deployment of the device and thus compromise the outcomes. Third, the CPR quality feedback was not up to the mark in some of the studies. CPR feedback devices are critical component to assess quality of CPR during cardiac arrest and helps in adjustment of chest compressions at the bed side. The PARAMEDIC trial (The Prehospital Randomized Assessment of A Mechanical Compression Device in Cardiac Arrest) cited this as a major limitation and highlights the sparsity of data with regards to quality assessment of these devices [20].
Fourth, there was a substantial variability in study specified protocols which might have had some effects on the observed outcomes. To address the issue of interruptions to CPR, the LINC and CIRC trials trained providers with specific attention to reducing the interruption to CPR that occurs while deploying the device. Differences in protocol driven sequences might bias the effects of the interventions.
Finally, the majority of trials did not comment on the safety hazards of these devices. This issue was brought to attention by Koster and colleagues [14]. Their study was the first RCT powered to assess the safety outcomes among AutoPulse, LUCAS and manual compression. Total of three patients died due to resuscitation related hazards: two patients in LUCAS arm had liver rupture and massive hemorrhage and one patient with AutoPulse had tension pneumothorax with air embolism causing the stroke. Furthermore, a higher rate of serious visceral injuries occurred with AutoPulse (11.6%), followed by LUCAS (7.4%) or manual compression (6.4%). These findings raise safety concerns since these complications can further compromise an already severely jeopardized hemodynamic state; and may contribute to increased mortality.
We compare our report with previous traditional meta-analyses which grouped mechanical compression devices together and hence could not assess the individual risks associated with these devices. Hui L and colleagues pooled 12 studies (11,162 patients) and showed no difference between manual compression and mechanical compression devices in terms of neurological outcomes, survival to hospital admission or discharge [21]. This study had certain short comings. First, the authors included eight RCTs, three prospective cohort studies and one descriptive controlled trial; and hence, the study was subjected to bias inherent to observational data (selection, attrition and calculation bias). Moreover, they combined Thumper and vest CPR studies along with LUCAS and AutoPulse, whereas, we focused on contemporary FDA approved devices in this review. Another review by Bonnes et al. included 20 studies (21,363 patients), out of which 15 were non randomized studies and 5 were RCTs [5]. The authors concluded that although observational data endorsed mechanical compression, high quality RCTs did not favor mechanical compression over manual CPR. This study did not assess safety outcomes among different devices. Our meta-analysis is the only network meta-analysis, to our knowledge, which has not only assessed the safety profile of these devices, but we also utilized the superior Bayesian statistical approach to compare the interventions by keeping relevant outcomes in focus.
This study also has certain limitations. First, like any meta-analysis, there is noticeable heterogeneity with regards to baseline characteristics of the participants, co-morbidities, study specific resuscitation protocols, definition of the outcomes and follow up duration. Second, as reported earlier there was variation in the timing of device application, quality of CPR, lack of CPR feed-back and post resuscitation management. Finally, these studies are affected by performance bias due to open label design.
In conclusion, CPR with manual compression showed better survival at 30 days or hospital discharge and neurological outcomes than AutoPulse; while manual compression had similar efficacy profile to LUCAS. These benefits may be attributable to CPR interruptions, suboptimal mechanical device fit and device related adverse events such as pneumothorax or hematoma formation. These findings question the routine applicability of the devices during CPR and strongly endorse the notion that appropriate training of the providers with conventional chest compressions might achieve superior outcomes with lesser complications compared to mechanical compression. However, the authors also believe that this study should provide industry with the incentive to engage in device improvement and address the shortcoming of current devices. It is also possible that enhanced device training with dedicated device personal can remedy CPR interruptions and delays even with current devices.
Supplementary Material
Footnotes
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.resuscitation.2018.05.005.
Conflicts of interest
None.
References
- [1].Hazinski MF, Nolan JP, Aickin R, Bhanji F, Billi JE, Callaway CW, et al. Part 1: executive summary: 2015 International consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation 2015;132:S2–39. [DOI] [PubMed] [Google Scholar]
- [2].Perkins GD, Handley AJ, Koster RW, Castren M, Smyth MA, Olasveengen T, et al. European resuscitation council guidelines for resuscitation 2015: section 2. Adult basic life support and automated external defibrillation. Resuscitation 2015;95:81–99. [DOI] [PubMed] [Google Scholar]
- [3].Berdowski J, Berg RA, Tijssen JG, Koster RW. Global incidences of out-of-hospital cardiac arrest and survival rates: systematic review of 67 prospective studies. Resuscitation 2010;81:1479–87. [DOI] [PubMed] [Google Scholar]
- [4].Christenson J, Andrusiek D, Everson-Stewart S, Kudenchuk P, Hostler D, Powell J, et al. Chest compression fraction determines survival in patients with out-of-hospital ventricular fibrillation. Circulation 2009;120:1241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bonnes JL, Brouwer MA, Navarese EP, Verhaert DV, Verheugt FW, Smeets JL, et al. Manual cardiopulmonary resuscitation versus CPR including a mechanical chest compression device in out-of-hospital cardiac arrest: a comprehensive meta-analysis from randomized and observational studies. Ann Emerg Med 2016;67 pp. 349–360.e3. [DOI] [PubMed] [Google Scholar]
- [6].Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0: updated March 2011.
- [8].Higgins JPT, Altman DG, G0tzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ades AE, Sculpher M, Sutton A, Abrams K, Cooper N, Welton N, et al. Bayesian methods for evidence synthesis in cost-effectiveness analysis. Pharmacoeconomics 2006;24:1–19. [DOI] [PubMed] [Google Scholar]
- [10].Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta-analysis, using empirical data from the cochrane database of systematic reviews. Int J Epidemiol 2012;41:818–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Thorlund K, Thabane L, Mills EJ. Modelling heterogeneity variances in multiple treatment comparison meta-analysis - are informative priors the better solution? BMC Med Res Methodol 2013;13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making 2013;33:641–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Salanti G, Ades AE, Ioannidis JPA. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163–71. [DOI] [PubMed] [Google Scholar]
- [14].Koster RW, Beenen LF, van der Boom EB, Spijkerboer AM, Tepaske R, van der Wal AC, et al. Safety of mechanical chest compression devices AutoPulse and LUCAS in cardiac arrest: a randomized clinical trial for non-inferiority. Eur Heart J 2017;38:3006–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Brooks SC, Anderson ML, Bruder E, Daya MR, Gaffney A, Otto CW, et al. Part 6: alternative techniques and ancillary devices for cardiopulmonary resuscitation: 2015 American heart association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015;132:S436–43. [DOI] [PubMed] [Google Scholar]
- [16].Truhlar A, Deakin CD, Soar J, Khalifa GE, Alfonzo A, Bierens JJ, et al. European resuscitation council guidelines for resuscitation 2015: section 4. Cardiac arrest in special circumstances. Resuscitation 2015;95:148–201. [DOI] [PubMed] [Google Scholar]
- [17].Hallstrom A, Rea TD, Sayre MR, Christenson J, Anton AR, Mosesso VN Jr, et al. Manual chest compression vs use of an automated chest compression device during resuscitation following out-of-hospital cardiac arrest: a randomized trial. JAMA 2006;295:2620–8. [DOI] [PubMed] [Google Scholar]
- [18].Rubertsson S, Lindgren E, Smekal D, Ostlund O, Silfverstolpe J, Lichtveld RA, et al. Mechanical chest compressions and simultaneous defibrillation vs conventional cardiopulmonary resuscitation in outofhospital cardiac arrest: the LINC randomized trial. JAMA 2014;311:53–61. [DOI] [PubMed] [Google Scholar]
- [19].Wik L, Olsen JA, Persse D, Sterz F, Lozano M Jr, Brouwer MA, et al. Manual vs. integrated automatic load-distributing band CPR with equal survival after out of hospital cardiac arrest. The randomized CIRC trial. Resuscitation 2014;85:741–8. [DOI] [PubMed] [Google Scholar]
- [20].Perkins GD, Lall R, Quinn T, Deakin CD, Cooke MW, Horton J, et al. Mechanical versus manual chest compression for out-of-hospital cardiac arrest (PARAMEDIC): a pragmatic, cluster randomised controlled trial. Lancet 2015;385:947–55. [DOI] [PubMed] [Google Scholar]
- [21].Li H, Wang D, Yu Y, Zhao X, Jing X. Mechanical versus manual chest compressions for cardiac arrest: a systematic review and meta-analysis. Scand J Trauma Resusc Emerg Med 2016;24:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Axelsson C, Nestin J, Svensson L, Axelsson AB, Herlitz J. Clinical consequences of the introduction of mechanical chest compression in the EMS system for treatment of out-of-hospital cardiac arrest-a pilot study. Resuscitation 2006;71:47–55. [DOI] [PubMed] [Google Scholar]
- [23].Smekal D, Johansson J, Huzevka T, Rubertsson S. A pilot study of mechanical chest compressions with the LUCAS device in cardiopulmonary resuscitation. Resuscitation 2011;82:702–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


