Abstract
Background
Intracerebral hemorrhage (ICH) is associated with inflammation and disruption of the blood-brain barrier (BBB). Lipoxin A4 methyl ester (LXA4 ME), is a stable synthetic analog of lipoxin with anti-inflammatory properties. This study aimed to investigate the effects of LXA4 ME in a rat model of ICH.
Material/Methods
Male Sprague-Dawley rats (n=120), between 12–13 weeks of age, were divided into the sham group (sham-operated), the vehicle-treated group (ICH+vehicle), the LXA4 ME-L group (ICH+low-dose LXA4 ME, 10 ng/d), and the LXA4 ME-H group (ICH+high-dose LXA4 ME, 100 ng/d). The ICH model was created by injection of autologous blood into the right basal ganglia. LXA4 ME was injected into the ventricle 10 min after the development of ICH. A modified neurological severity score (mNSS), rotarod latencies, and brain water content were used to evaluate the rats. The TUNEL assay measured neuronal cell death. Western blot was used to measure protein expression of nuclear factor kappa B (NF-κB), matrix metalloproteinase-9 (MMP-9), zonula occludens-1 (ZO-1), and claudin-5.
Results
In the rat model of ICH, treatment with LXA4 ME reduced the levels of proinflammatory cytokines, improved neurologic function, reduced neuronal apoptosis, and reduced cerebral edema associated with damage to the BBB, and reduced the expression levels of NF-κB, MMP-9, ZO-1, and claudin-5.
Conclusions
In a rat model of ICH, treatment with LXA4 reduced early brain injury and protected the BBB by inhibiting the NF-κB-dependent MMP-9 pathway.
MeSH Keywords: Blood-Brain Barrier; Intracranial Hemorrhage, Hypertensive; Lipoxins; Matrix Metalloproteinase 9
Background
Spontaneous intracerebral hemorrhage (ICH) accounts for 15% of all cases of acute stroke and is associated with high rates of morbidity and mortality [1]. There is no effective treatment to prevent ICH-induced brain dysfunction. There is increasing evidence that inflammation contributes to the progression of ICH-induced secondary brain damage, leading to disruption of the blood-brain barrier (BBB) and cerebral edema, with expansion of the intracerebral hematoma [2,3]. The combination of disruption of the BBB and cerebral edema also results in neuronal apoptosis, brain swelling, and deterioration of neurologic function within the first 72 hours after ICH [4]. Therefore, treatment approaches that protect the integrity of the BBB by reducing inflammation may be a promising therapeutic strategy to reduce early brain injury following ICH.
Matrix metalloproteinases (MMPs) are a family of zinc endopeptidases that are can disrupt the integrity of the BBB by disruption of the microvascular extracellular matrix and protein components in certain pathological conditions [5]. In hemorrhagic stroke, the expression of MMP-9 has been shown to be increased, while levels of tight junction proteins and basal lamina proteins are decreased, leading to BBB disruption and cerebral edema in hemorrhagic stroke [6]. Previous studies have shown that increased activity of nuclear factor kappa B (NF-κB) can contribute to inflammatory reactions after ICH, which triggers the expression of genes for pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-6, all of which have a role in ICH-induced brain injury [7]. Also, NF-κB has been shown to directly regulate the transcription of MMP-9 [7]. Therefore, inhibition of NF-κB activity can result in reduced expression of MMP-9 and reduced inflammation, which could reduce disruption of the BBB and cerebral injury after ICH.
Lipoxins include endogenous lipoxygenase-derived eicosanoids and have been identified as the first endogenous signals to control inflammation in vivo. Lipoxin A4 methyl ester (LXA4 ME) is a relatively stable and potent endogenous lipoxin. Previous studies have shown that LXA4 ME suppressed NF-κB and pro-inflammatory cytokines in several inflammatory diseases, including autoimmune myocarditis, glomerulopathy, and arthritis [8–10]. Studies have also demonstrated the neuroprotective effects of LXA4 after traumatic brain injury and ischemic stroke [11,12]. LXA4 was reported to reduce brain injury in traumatic brain injury by inhibiting inflammation and reducing damage to the BBB with reduced cerebral edema in cerebral ischemia-reperfusion injury [13]. However, the potential protective effect of LXA4 on early brain injury and the effects on the BBB in ICH remain poorly understood.
Therefore, this study aimed to assess the role of LXA4 in early brain injury and the effects on the BBB by investigating apoptosis, the cerebral microvasculature, and the expression of inflammatory cytokines including NF-κB and MMP-9 in a rat model of ICH.
Material and Methods
A rat model of intracerebral hemorrhage (ICH)
Adult male Sprague-Dawley rats (n=140), between 12–13 weeks of age, were used in this study. The study and the experimental procedures were approved by the Animal Care and Use Committee of Hebei Medical University and were performed according to the Guide for the Chinese Council on Animal Protection. All rats were housed under a controlled environment with a 12-hour light and dark cycle, a humidity of 50–55%, and a temperature of 22–24°C. All animals were provided with free access to food and water before and after sham treatment or surgery.
The rats were anesthetized by intraperitoneal injection of 0.4% pentobarbital sodium. Following removal of 50 ml of autologous whole blood from the tail vein, autologous whole blood was injected into the right caudate putamen using stereotaxic guidance. The stereotactic coordinates were 3.0 mm lateral, 0.2 mm anterior, and 5.5 mm ventral to the bregma. The sham group was injected with sterile phosphate-buffered saline (PBS) instead of whole blood. The bone incision was sealed with bone wax and the wound was sutured and disinfected with iodine. In the course of the establishment of the rat model of ICH, seven rats died.
Experimental groups and treatment with lipoxin A4 methyl ester (LXA4 ME)
There were 120 adult rats assigned to four study groups (n=30), the sham group (sham-operated), vehicle-treated group (ICH+vehicle), the low-dose lipoxin A4 methyl ester (LXA4 ME-L) group (ICH+10 ng/d), and the high-dose LXA4 ME-H group (ICH+100 ng/d). LXA4 ME, 10 ng or 100 ng in 1 μL of sterile PBS (Cayman Chemical, Ann Arbor, MI, USA) or vehicle (1 μL in sterile PBS) were injected into the cerebral ventricle at 1 h after induction of ICH. The treatment doses that were chosen to investigate the effect of LXA4 ME were according to previously reported doses [14,15].
The modified neurological severity score (mNSS)
The modified neurological severity score (mNSS) was used that consisted of sensory tests, motor tests, reflex testing, and beam balance tests. The neurological deficits of the rats on days 1–5 after ICH were evaluated. The higher the score, the greater the degree of neurological injury (normal score=0; maximal deficit score=18).
The rotarod performance test
In addition to the mNSS scores, an accelerating automated rotarod was used to measure the effects of therapeutic intervention on vestibulomotor function [16]. The speed was slowly increased from 5 to 40 rpm for 5 minutes. The rats were tested before the onset of ICH to obtain baseline values. Tests were performed to assess early effects on motor function on days 1–5 after ICH. As the speed increased within a short time (5 min), the average time that elapsed from the rotation of the cylinder was recorded. The experiment ended when the rats fell twice from the cylinder.
Blood-brain barrier (BBB) permeability
The permeability of the blood-brain barrier (BBB) was assessed by examining the extravasation of Evans blue (EB) dye at 6 h, 12 h, 18 h, 24 h, and 48h after ICH. The rats were anesthetized one hour before being euthanized and were injected intravenously with 2% EB in PBS at a final concentration of 100 μM. To clear the intravascular EB dye, rats were perfused through the left cardiac ventricle with saline. Then, the rats were euthanized by decapitation. The right cerebral hemispheres were quickly removed and placed into a test tube with 2 ml of PBS. Protein was isolated from the homogenized brain in 1 mL of 50% trichloroacetic acid solution and centrifuged at 14,000 rpm for 15 min. A spectrophotometer was used (λ=600 nm) to measure the absorbance of the supernatant.
Measurement of brain water content (cerebral edema)
Cerebral edema was evaluated using the wet weight (WW) to dry weight (DW) ratios. Rats were euthanized on days 1–3 after ICH and the cerebral hemispheres was quickly removed and weighed to obtain the WW. Then, brain tissues were dried in a 100°C oven for 24 h and then re-weighed to determine the DW. Tissue water content was calculated as: (WW-DW)/WW×100%.
Analysis of neuronal apoptosis by terminal deoxynucleotidyl transferase-mediated (dUTP) nick-end labeling (TUNEL) staining
Rats were anesthetized with chloral hydrate and underwent transcardial perfusion with ice-cold saline and 4% paraformaldehyde in 0.1 M PBS, and euthanized rapidly within 24 h following ICH. The rat brain containing the area of cerebral hemorrhage was fixed in 4% paraformaldehyde for 48 h, the brain tissue was embedded in paraffin and sectioned at 5 μm onto glass slides. After deparaffinization and rehydration, the tissue sections were rinsed twice in 0.1 M PBS and incubated with a solution of proteinase K in a humidified atmosphere for 15 min at 37°C. Tissue sections were then treated with green fluorescein-labeled dUTP solution (containing 10% TdT). Tissue sections were also incubated with 4′,6-diamidino-2-phenylindole (DAPI) for 5 min at room temperature to detect the nuclei, which stained blue. The slides were examined using fluorescence microscopy and quantified by randomly selecting five microscopic fields. Neural apoptosis was determined as TUNEL-positive cells/mm2 per field.
Detection of matrix metalloproteinase-9 (MMP-9)
The activity of MMP-9 was detected at 24 h after ICH using the MMP gelatin zymography electrophoretic analysis reagent kit (Genmed Scientifics Inc., Shanghai, China), according to the manufacturer’s instructions. Rat cerebral tissues were homogenized in lysis buffer and centrifuged at 12,000 rpm for 15 min at 4°C. The BCA assay (Beyotime, Shanghai, China) was used to determine the concentration of the proteins according to the BCA method. Then, 40μg of the samples were loaded into the lanes and separated by 8% Tris-tricine gel with 0.1% gelatin as a substrate. Following separated by electrophoresis and refolding, the gel was incubated with digestive buffer at 37°C for 36 h. The gel was stained with 0.5% Coomassie brilliant blue R-250 for 60 min (Sigma-Aldrich, St. Louis MO, USA). After destaining for 1 h, the development of the gel was terminated when a clear white band appeared on a blue background. To assess MMP-9 activity, the white band was quantified using Image J software (Bio-Rad, Hercules, CA, USA).
Western blot
Brain tissues surrounding the hematoma in the ICH model groups and in the sham group were excised. After the tissue was mechanically homogenized in radioimmunoprecipitation assay (RIPA) lysis buffer, the sample lysates were centrifuged at 12,000 rpm for 15 min at 4°C. The BCA protein assay kit (Beyotime, Shanghai, China) was used to estimate protein concentration. Forty micrograms of protein were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred onto a nitrocellulose membrane. After blocked with dried skimmed milk powder, the membrane was then incubated overnight with rabbit anti-β-actin (1: 1000) (Santa Cruz Biotechnology Inc., Dallas, TX, USA), rabbit anti-TNF-α (1: 1000) (Santa Cruz Biotechnology Inc., Dallas, TX, USA), rabbit anti-IL-6 (1: 1000) (Santa Cruz Biotechnology Inc., Dallas, TX, USA), rabbit anti-IL-1β (1: 1000) (Santa Cruz Biotechnology Inc., Dallas, TX, USA), rabbit anti-occludin (1: 1000) (Santa Cruz Biotechnology Inc., Dallas, TX, USA), rabbit anti-zonula occluden-1 (ZO-1) (1: 1000) (Santa Cruz Biotechnology Inc., Dallas, TX, USA), rabbit anti-NF-κB (1: 1000) (Santa Cruz Biotechnology Inc., Dallas, TX, USA), and rabbit anti-MMP-9 (1: 1000) (Abcam, Cambridge, UK) at 4°C. Then the membranes were labeled with secondary antibodies for 1.5 h at room temperature. The protein density was detected using a Bio-Rad imaging system and analyzed by the Image J software (Bio-Rad, Hercules, CA, USA). Relative protein levels were expressed as the ratio of target band to the β-actin band integrated optical density (IOD) values.
Statistical analysis
Data were expressed as the mean ±SEM and were analyzed using SPSS version 24.0 software (IBM, Chicago, IL, USA). Statistical analysis of the data was performed using one-way analysis of variance (ANOVA), followed by Tukey’s post hoc analysis. Differences with P<0.05 were considered significant.
Results
Lipoxin A4 methyl ester (LXA4 ME) reduced intracerebral hemorrhage (ICH)-induced neurological deficits
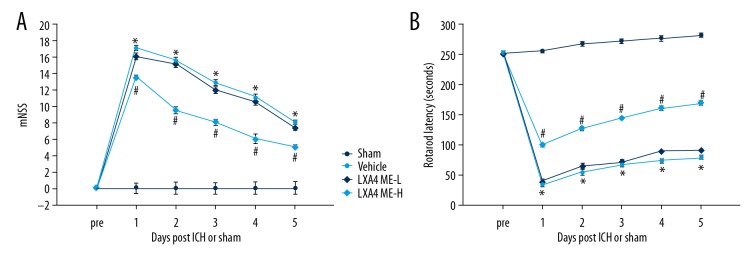
The rotarod latencies and the modified neurological severity score provided the evaluation of neurological functions following intracerebral hemorrhage (ICH) in the rat model. The mNSS scores were determined over 5 days after ICH. Compared with the sham group, the mNSS scores in all rats that underwent ICH were significantly improved (P<0.05) (Figure 1A). Treatment with lipoxin A4 methyl ester (LXA4 ME) significantly reduced the mNSS scores and resulted in improved neural function when compared with the untreated vehicle group (P<0.05) (Figure 1A). Although LXA4 ME-L (low-dose LXA4 ME, 10 ng/d) reduced the neurological deficit scores, no statistically significant difference was observed between LXA4 ME-L group and the vehicle group (P>0.05) (Figure 1A). The rotarod test showed significantly increased impairment in motor function in the ICH model rats compared with the sham group (P<0.05) (Figure 1B). The rats in the ICH model groups that received LXA4 ME-H (high-dose LXA4 ME, 100 ng/d) showed significantly increased rotarod latencies when compared with the vehicle group (P<0.05). However, although LXA4 ME-L partly increased rotarod latencies and improved motor function, there was no statistically significant difference between the vehicle group and LXA4 ME-L group (P>0.05).
Figure 1.
The neuroprotective effect of lipoxin A4 methyl ester (LXA4 ME) on behavior testing in the rat model of intracerebral hemorrhage (ICH). Recovery of neurobehavioral function based on mNSS (A). Vestibulomotor function recovery based on rotarod testing (B). * P<0.05 compared with sham; # P<0.05 compared with vehicle; & P<0.05 compared with LXA4 ME-L. (n=6–8 per group).
LXA4 ME reduced ICH-induced damage to the blood-brain barrier (BBB) and cerebral edema
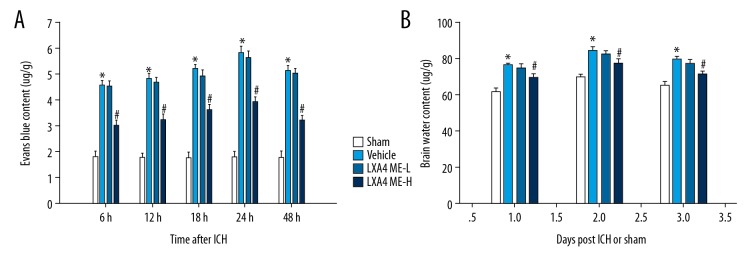
The permeability of the BBB in the injured rat cerebral hemispheres was assessed at 6 h, 12 h, 18 h, 24 h, and 48 h after ICH. BBB permeability was significantly changed at different times. The Evans blue content in the area around the hematoma was lowest in the 6 h group and highest in the 24 h group. There was a significant increase in extravasation of EB in the vehicle group compared with the sham group. LXA4 ME-H treatment significantly reduced the EB content when compared with the vehicle-treated group (P<0.05) (Figure 2A). Also, LXA4 ME-L treatment did not have a significant effect on the inhibition of extravasation of EB when compared with the vehicle-treated group.
Figure 2.
Treatment with lipoxin A4 methyl ester (LXA4 ME) reduced intracerebral hemorrhage (ICH)-induced brain edema and damage to the blood-brain barrier (BBB). (A) Evans blue (EB) dye extravasation shows that leakage of EB in the hematoma and area around the hematoma in the LXA4 ME-H group (ICH+high-dose LXA4 ME, 100 ng/d) was significantly less than the sham-operated group. (B) Brain water content was significantly reduced in LXA4 ME-H group but not the LXA4 ME-L group (ICH+low-dose LXA4 ME, 10 ng/d), compared with vehicle-treated rats on days 1–3 after ICH. There was no significant difference between the LXA4 ME-L group and vehicle group. Values are expressed as the mean ±SEM. * P<0.05 vs. the sham group; # P<0.05 vs. the vehicle-treated group; & P<0.05 vs. the LXA4 ME-L group (ICH+low-dose LXA4 ME, 10 ng/d). (n=4–5 per group).
Similar results were found in brain edema measurements. The cerebral water content of the affected hemispheres was measured on days 1–3 after ICH. Compared with rats on the first day, the water content in the ipsilateral cerebral hemispheres was significantly increased in rats sacrificed on the second day after ICH. In the rats sacrificed on the third day after ICH, the water content decreased slightly when compared with rats sacrificed on the second day after ICH. ICH significantly increased cerebral edema when compared with the sham group (P<0.05). There was a significant decrease in brain water content in the LXA4 ME-H group compared with the vehicle group and the LXA4 ME-L group (P<0.05) (Figure 2B). However, no significant difference was found between the vehicle group and the LXA4 ME-L group.
LXA4 ME treatment reduced the number of apoptotic cells at 24 h after ICH
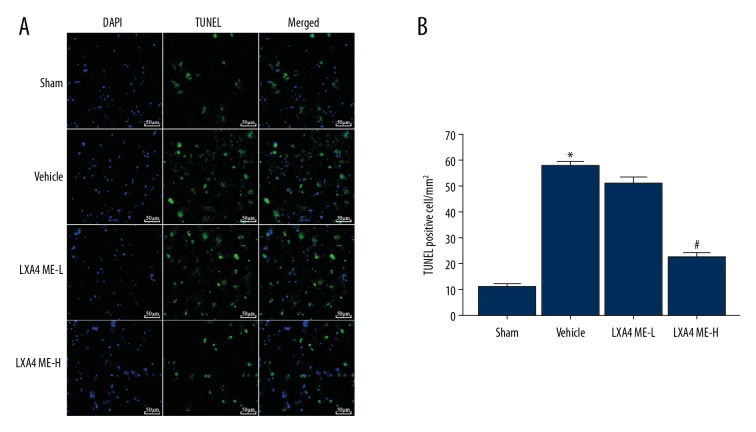
Apoptosis of neurons around the area of intracerebral hemorrhage was evaluated by TUNEL and 4′,6-diamidino-2-phenylindole (DAPI) staining. In the sham group, there was no significant apoptosis of neurons. TUNEL-positive cells increased significantly in the vehicle group. Quantitative analysis showed that LXA4 ME treatment significantly reduced neuronal apoptosis around the hematoma at 24 h following ICH in the LXA4 ME-H group. The density of positively stained cells in LXA4 ME-L group was not found to be increased compared with those in the sham group (P<0.05) (Figure 3).
Figure 3.
Treatment with lipoxin A4 methyl ester (LXA4 ME) reduced the number of TUNEL-positive apoptotic cells in the area around the hematoma after intracerebral hemorrhage (ICH). (A) Rat brain tissue sections were stained with TUNEL (green) and 4′,6-diamidino-2-phenylindole (DAPI) (blue). (B) Quantification of the percentage of TUNEL-positive cells. Scale bar 50 μm. Values are expressed as the mean ±SEM. * P<0.05 vs. the sham group; # P<0.05 vs. the vehicle-treated group; & P<0.05 vs. the LXA4 ME-L group (ICH+low-dose LXA4 ME, 10 ng/d). (n=4–5 per group).
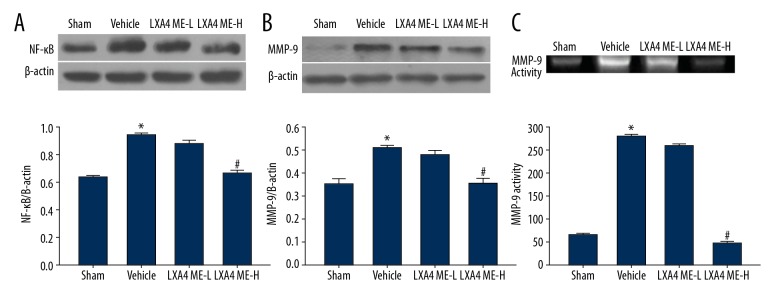
LXA4 ME inhibited activation of the NF-κB-dependent MMP-9 pathway induced by ICH
The expression levels of NF-κB and MMP-9 were significantly increased in the vehicle-treated group (P<0.05). LXA4 ME treatment down-regulated the expression of NF-κB and MMP-9 in the LXA4 ME-H group (Figure 4A, 4B). No significant difference was found between the vehicle group and the LXA4 ME-L group. Similar results were also found for MMP-9 activity (Figure 4C).
Figure 4.
Lipoxin A4 methyl ester (LXA4 ME) reduced intracerebral hemorrhage (ICH)-induced activation of the nuclear factor kappa B (NF-κB)-dependent matrix metalloproteinase-9 (MMP-9) pathway and MMP-9 gelatinase activity. Representative Western blots and densitometric quantification of the nuclear NF-κB/β-actin and cytosolic NF-κB/β-actin at 24 h after ICH (A, B). Quantification of pro-MMP-9 levels at 24 h after ICH (C). Values are expressed as the mean ±SEM. * P<0.05 vs. the sham group; # P<0.05 vs. the vehicle-treated group; & P<0.05 vs. the LXA4 ME-L group (ICH+low-dose LXA4 ME, 10 ng/d). (n=4–6 per group).
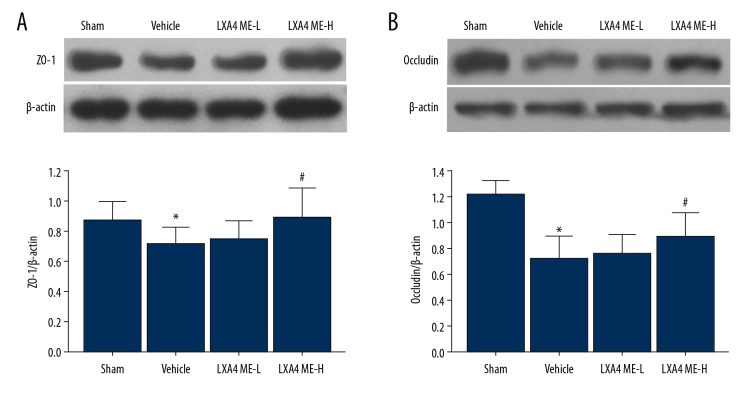
LXA4 ME prevented the degradation of zonula occludens-1 (ZO-1) and claudin-5
As shown in Figure 5, proteins associated with the integrity of the BBB were investigated in the rat model. The ICH-induced decrease in ZO-1 and claudin-5 protein expression was inhibited by LXA4 ME. Both low and high concentrations of LXA4 ME inhibited degradation of ZO-1 and claudin-5. However, there was no significant degradation of ZO-1 and claudin-5 between the vehicle group and the LXA4 ME-L group.
Figure 5.
Treatment with lipoxin A4 methyl ester (LXA4 ME) increased the tight junction proteins zonula occludens-1 (ZO-1) and claudin-5 at 24 hour following intracerebral hemorrhage (ICH). Representative Western blots and densitometric quantification of nuclear ZO-1/β-actin and claudin/β-actin at 24h following ICH (A, B). Values are expressed as the mean ±SEM. * P<0.05 vs. the sham group; # P<0.05 vs. the vehicle-treated group; & P<0.05 vs. the LXA4 ME-L group (ICH+low-dose LXA4 ME, 10 ng/d). (n=4–6 per group).
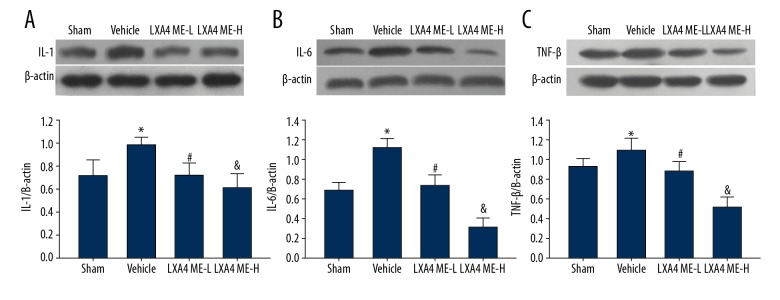
LXA4 ME treatment reduced the protein expression levels of interleukin I (L-1), IL-6 and tumor necrosis factor-β (TNF-β)
In the vehicle group, increased expression of IL-1, IL-6, and TNF-β was observed in the ipsilateral cerebral hemisphere. Intraventricular administration of LXA4 ME decreased ICH-induced overexpression of IL-1, IL-6, and TNF-β (Figure 6). Also, treatment with LXA4 ME decrease the protein expression levels of IL-1, IL-6, and TNF-β in a dose-dependent manner.
Figure 6.
The levels of interleukin-1 (IL-1), IL-6, and tumor necrosis factor-β (TNF-β) after treatment with lipoxin A4 methyl ester (LXA4 ME). Overexpression of IL-1 (A), IL-6 (B), and TNF-β (C) induced by intracerebral hemorrhage (ICH) were significantly reduced after treatment with LXA4 ME. Values are expressed as the mean ± SEM. * P<0.05 vs. the sham group; # P<0.05 vs. the vehicle-treated group; & P<0.05 vs. the LXA4 ME-L group (ICH+low-dose LXA4 ME, 10 ng/d). (n=4–6 per group).
Discussion
Neurological damage resulting from intracerebral hemorrhage (ICH) involves complex pathogenic processes and includes primary brain injury and secondary brain injury associated with inflammation and apoptosis [17]. There is increasing evidence to indicate that inflammation following ICH is involved in secondary brain injury that impairs neurological outcome during the acute stage following ICH [18]. Inflammatory mechanisms mainly contribute to the progression of disruption of the blood-brain barrier (BBB), cerebral edema, and neuronal apoptosis, which lead to neurological impairment [19]. The suppression of inflammatory responses at the early stage following ICH might provide an effective therapeutic method for reducing cerebral dysfunction.
Lipoxin A4 methyl ester (LXA4 ME), is a stable synthetic analog of lipoxin with anti-inflammatory properties that is derived from arachidonic acid, a ω-6 polyunsaturated fatty acid (PUFA) generated from linoleic acid. As the proportion of arachidonic acid in the cell membranes of inflammatory cells is much higher than resolvins and protectins that originate from the ω-3 PUFAs, the substrate availability for metabolism of arachidonic acid by cyclooxygenase (COX) or lipoxygenase (LOX) isozymes is greater than that found in the metabolism of ω-3 PUFAs, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) [20]. A previously published study has shown that LXA4 analogs and LXA-ME had improved biological stability [21]. In this study, LXA4 ME was chosen for its potent anti-inflammatory properties at an optimal dose determined by a previous study [22]. In the present study, the LXA4-ME dose was set according to previous reports and preliminary experimental results. Preliminary studies showed that there was no significant difference between the ICH group and ICH+1 ng LXA4 ME-treated group. With the increase in LXA4-ME dose, the modified neurological severity score (mNSS) and the extravasation of Evans blue were significantly reduced. The rotarod latency was found to be increased in the ICH+10 ng LXA4 ME group. Also, preliminary results indicated that there was an increased risk of mortality in the rat model with 1000 ng of LXA4 ME when compared with the 100 ng LXA4 ME-treated ICH rat model. Therefore, according to the preliminary experimental results and previous studies, 10 ng and 100 ng were identified as the study doses of LXA4-ME. Also, a previous study showed that LXA4 ME could upregulate the expression of anti-inflammatory cytokines, IL-10 and TGF-β1, and reduce the expression of the pro-inflammatory cytokines, TNF-α and IL-1β, in the ischemic brain [23]. However, the specific mechanisms for the neuroprotective effects of LXA4 ME need to be investigated further.
Neuroinflammation leads to disruption of the BBB and cerebral edema, which can be fatal. Damage to the BBB is associated with acute and chronic inflammation and hemorrhage. Edema fluid passively diffuses into the brain parenchyma, which further exacerbates cerebral edema and neuronal death and results in secondary brain injury [24]. The degree of the post-traumatic cerebral edema determines neurological outcome and survival after ICH. In the present study, ICH resulted in neurobehavioral dysfunction and upregulation of neuroinflammation in the vehicle-treated group in the rat model of ICH and compared with the sham-operated rats, a significant accumulation of Evans Blue stain was presented in the ipsilateral cerebral hemisphere. Also, treatment with LXA4 ME reduced the permeability of the BBB, reduced cerebral edema, and improved neurobehavioral function. LXA4 was recently reported to play a protective role in traumatic brain injury (TBI) by reducing cerebral edema, which may occur by reducing inflammation and permeability of the BBB [11].
Occludin is a transmembrane protein that is localized in the tight junctions [25]. Zonula occludens-1 (ZO-1) is involved in the organization of proteins within the tight junction [26]. The expression of ZO-1 and occludin are associated with the integrity of the BBB. The findings of the present study showed that LXA4 ME restored the protein levels of claudin-5 and ZO-1, indicating that LXA4 ME might block the down-regulation of tight junction proteins, resulting in. preservation of the BBB. Therefore, LXA4 ME protects neurological function in ICH by reducing inflammation and BBB permeability, which might indicate a potential role for this compound in improving prognosis after ICH.
In the present study, the effect of LXA4 ME on the activity of matrix metalloproteinase-9 (MMP-9) and nuclear factor kappa B (NF-κB) were investigated. LXA4 ME inhibited the ICH-induced increase in MMP-9 and the NF-κB/MMP-9 pathway. These findings suggested that LXA4 ME blocked the increase in NF-κB-dependent MMP-9, which may lead explain the reduction in cerebral inflammation. Previous studies have shown that NF-κB is an important transcription factor that triggers pro-inflammatory gene expression and that MMP-9 production is associated with the expression of TNF-α and IL-1β [27]. Other studies have shown that MMP-9 is involved in the degradation of tight junction proteins and microvascular basal lamina proteins, leading to a breakdown in the BBB and to cerebral edema [28]. Given that NF-κB regulates transcriptional activation of multiple inflammatory cytokines, it might be expected that LXA4 ME targets NF-κB to inhibit MMP-9 transcription and to improve the integrity of the BBB. Wu et al. demonstrated that LXA4 ME reduced neurologic damage by improving BBB permeability in a rat model of cerebral infarction following middle cerebral artery occlusion (MCAO), partly by reducing the expression of MMP-9 [29].
Previous studies have shown that neuronal apoptosis was commonly found in the areas of cerebral tissue surrounding the hematoma in ICH [30]. LXA4 has recently been shown to confer neuroprotective effects via antioxidation and anti-apoptosis in chronic cerebral hypoperfusion [31]. The present study showed that the inhibition of MMP-9 by NF-κB suppression reduced neuronal damage and neuronal apoptosis after ischemia [32]. The pathophysiological role of MMPs in cerebral ischemia is the neuronal and endothelial cell apoptosis, which is induced by the degradation of cell and matrix interactions in ICH [33]. In the present study, ICH resulted in increased NF-κB activation in the cerebral hematoma and the area around the hematoma that was significantly reduced by LXA4 ME treatment and was associated with reduced numbers of TUNEL-positive apoptotic cells. Therefore, it is possible that the anti-apoptotic effect of LXA4 ME might be associated with the inhibition of the NF-κB/MMP-9 pathway.
On the basis of a previous study [14], intraventricular injection was chosen as the method of drug delivery method, which resulted in a therapeutic effect in the rat model of ICH in the present study. In this study, treatment of rats in the ICH model with high-dose LXA4 ME significantly reduced inflammation, neurobehavioral injury, cerebral edema, and neuronal apoptosis, in a dose-dependent manner. LXA4, which is a ω-6 PUFA generated from linoleic acid, has the potential to resolve inflammation by signaling metabolic, cellular, and tissue events in ICH. These preliminary findings in a rat model of ICH are of interest and warrant further studies to investigate whether resolvins and protectins, which are derived from ω-3 PUFAs and EPA and DHA might have a therapeutic effect in ICH. Further studies are also required to determine whether intraventricularly injection of LXA4-ME enters the systemic circulation by passing through the BBB.
Conclusions
In a rat model of intracerebral hemorrhage (ICH), intraventricular treatment with lipoxin A4 methyl ester (LXA4 ME) reduced early brain injury and protected the blood-brain barrier (BBB) by inhibiting the nuclear factor kappa B (NF-κB)-dependent matrix metalloproteinase-9 (MMP-9) pathway. Further studies are required to determine the potential role of LXA4 ME in cerebral ischemia and infarction.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Bobinger T, Burkardt P, Huttner BH, Manaenko A. Programmed cell death after intracerebral hemorrhage. Curr Neuropharmacol. 2018;16(9):1267–81. doi: 10.2174/1570159X15666170602112851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ducruet AF, Zacharia BE, Hickman ZL, et al. The complement cascade as a therapeutic target in intracerebral hemorrhage. Exp Neurol. 2009;219(2):398–403. doi: 10.1016/j.expneurol.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macdonald RL, de Oliveira Manoel AL. Neuroinflammation as a Target for intervention in Subarachnoid Hemorrhage. Front Neurol. 2018;9:292. doi: 10.3389/fneur.2018.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katsu M, Niizuma K, Yoshioka H, et al. Hemoglobin-induced oxidative stress contributes to matrix metalloproteinase activation and blood–brain barrier dysfunction in vivo. J Cereb Blood Flow Metab. 2010;30(12):1939–50. doi: 10.1038/jcbfm.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Florczak-Rzepka M, Grond-Ginsbach C, Montaner J, et al. Matrix metalloproteinases in human spontaneous intracerebral hemorrhage: An update. Cerebrovasc Dis. 2012;34(4):249–62. doi: 10.1159/000341686. [DOI] [PubMed] [Google Scholar]
- 6.Ding R, Feng L, He L, et al. Peroxynitrite decomposition catalyst prevents matrix metalloproteinase-9 activation and neurovascular injury after hemoglobin injection into the caudate nucleus of rats. Neuroscience. 2015;297:182–93. doi: 10.1016/j.neuroscience.2015.03.065. [DOI] [PubMed] [Google Scholar]
- 7.Han L, Liu DL, Zeng QK, et al. The neuroprotective effects and probable mechanisms of Ligustilide and its degradative products on intracerebral hemorrhage in mice. Int Immunopharmacol. 2018;63:43–57. doi: 10.1016/j.intimp.2018.06.045. [DOI] [PubMed] [Google Scholar]
- 8.Shi Y, Pan H, Zhang HZ, et al. Lipoxin A4 mitigates experimental autoimmune myocarditis by regulating inflammatory response, NF-κB and PI3K/Akt signaling pathway in mice. Eur Rev Med Pharmacol Sci. 2017;21(8):1850–59. [PubMed] [Google Scholar]
- 9.Guo YP, Jiang HK, Jiang H, et al. Lipoxin A4 may attenuate the progression of obesity-related glomerulopathy by inhibiting NF-κB and ERK/p38 MAPK-dependent inflammation. Life Sci. 2018;198:112–18. doi: 10.1016/j.lfs.2018.02.039. [DOI] [PubMed] [Google Scholar]
- 10.Machado FS, Johndrow JE, Esper L, et al. Anti-inflammatory actions of lipoxin A4 and aspirin-triggered lipoxin are SOCS-2 dependent. Nat Med. 2006;12(3):330–34. doi: 10.1038/nm1355. [DOI] [PubMed] [Google Scholar]
- 11.Luo CL, Li QQ, Chen XP, et al. Lipoxin A4 attenuates brain damage and down-regulates the production of pro-inflammatory cytokines and phosphorylated mitogen-activated protein kinases in a mouse model of traumatic brain injury. Brain Res. 2013;1502:1–10. doi: 10.1016/j.brainres.2013.01.037. [DOI] [PubMed] [Google Scholar]
- 12.Hawkins KE, DeMars KM, Alexander JC, et al. Targeting resolution of neuroinflammation after ischemic stroke with a lipoxin A4 analog: Protective mechanisms and long-term effects on neurological recovery. Brain Behav. 2017;7(5):e00688. doi: 10.1002/brb3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hawkins KE, DeMars KM, Singh J, et al. Neurovascular protection by post-ischemic intravenous injections of the lipoxin A4 receptor agonist, BML-111, in a rat model of ischemic stroke. J Neurochem. 2014;129(1):130–42. doi: 10.1111/jnc.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin W, Jia Y, Huang L, et al. Lipoxin A4 methyl ester ameliorates cognitive deficits induced by chronic cerebral hypoperfusion through activating ERK/Nrf2 signaling pathway in rats. Pharmacol Biochem Behav. 2014;124:145–52. doi: 10.1016/j.pbb.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 15.Wu Y, Ye XH, Guo PP, et al. Neuroprotective effect of lipoxin A4methyl ester in a rat model of permanent focal cerebral ischemia. J Molec Neurosci. 2010;42(2):226–34. doi: 10.1007/s12031-010-9355-8. [DOI] [PubMed] [Google Scholar]
- 16.Cui CM, Gao JL, Cui Y, et al. Chloroquine exerts neuroprotection following traumatic brain injury via suppression of inflammation and neuronal autophagic death. Mol Med Rep. 2015;12(2):2323–28. doi: 10.3892/mmr.2015.3611. [DOI] [PubMed] [Google Scholar]
- 17.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5(1):53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 18.Askenase MH, Sansing LH. Stages of the inflammatory response in pathology and tissue repair after intracerebral hemorrhage. Semin Neurol. 2016;36(3):288–97. doi: 10.1055/s-0036-1582132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan L, He P, Peng Y, et al. Mdivi-1 ameliorates early brain injury after subarachnoid hemorrhage via the suppression of inflammation-related blood–brain barrier disruption and endoplasmic reticulum stress-based apoptosis. Free Radic Biol Med. 2017;112:336–49. doi: 10.1016/j.freeradbiomed.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Calder PC. Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids. 2006;75(3):197–202. doi: 10.1016/j.plefa.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Clish CB, O’Brien JA, Gronert K, et al. Local and systemic delivery of a stable aspirin-triggered lipoxin prevents neutrophil recruitment in vivo. Proc Natl Acad Sci USA. 1999;96(14):8247–52. doi: 10.1073/pnas.96.14.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Svensson CI, Zattoni M, Serhan CN. Lipoxins and aspirin-triggered lipoxin inhibit inflammatory pain processing. J Exp Med. 2007;204(2):245–52. doi: 10.1084/jem.20061826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Q, Shao L, Hu X, et al. Lipoxin A4 preconditioning and postconditioning protect myocardial ischemia/reperfusion injury in rats. Mediators Inflamm. 2013;2013 doi: 10.1155/2013/231351. 231351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schoknecht K, Shalev H. Blood–brain barrier dysfunction in brain diseases: Clinical experience. Epilepsia. 2012;53:7–13. doi: 10.1111/j.1528-1167.2012.03697.x. [DOI] [PubMed] [Google Scholar]
- 25.Anderson JM, Van Itallie CM. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol. 1995;269(4 Pt 1):G467–75. doi: 10.1152/ajpgi.1995.269.4.G467. [DOI] [PubMed] [Google Scholar]
- 26.Anderson JM, Fanning AS, Lapierre L, Van Itallie CM. Zonula occludens (ZO)-1 and ZO-2: Membrane-associated guanylate kinase homologues (MAGuKs) of the tight junction. Biochem Soc Trans. 1995;23(3):470–75. doi: 10.1042/bst0230470. [DOI] [PubMed] [Google Scholar]
- 27.Jiang K, Chen X, Zhao G, et al. IFN-τ plays an anti-inflammatory role in Staphylococcus aureus-induced endometritis in mice through the suppression of NF-κB pathway and MMP9 expression. J Interferon Cytokine Res. 2017;37(2):81–89. doi: 10.1089/jir.2016.0058. [DOI] [PubMed] [Google Scholar]
- 28.Zhang HT, Zhang P, Gao Y, et al. Early VEGF inhibition attenuates blood-brain barrier disruption in ischemic rat brains by regulating the expression of MMPs. Mol Med Rep. 2017;15(1):57–64. doi: 10.3892/mmr.2016.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Y, Wang YP, Guo P, et al. A lipoxin A4 analog ameliorates blood–brain barrier dysfunction and reduces MMP-9 expression in a rat model of focal cerebral ischemia – reperfusion injury. J Mol Neurosci. 2012;46(3):483–91. doi: 10.1007/s12031-011-9620-5. [DOI] [PubMed] [Google Scholar]
- 30.Lim-Hing K, Rincon F. Secondary hematoma expansion and perihemorrhagic edema after intracerebral hemorrhage: From bench work to practical aspects. Front Neurol. 2017;8:74. doi: 10.3389/fneur.2017.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin W, Jia Y, Huang L, et al. Lipoxin A4 methyl ester ameliorates cognitive deficits induced by chronic cerebral hypoperfusion through activating ERK/Nrf2 signaling pathway in rats. Pharmacol Biochem Behav. 2014;124:145–52. doi: 10.1016/j.pbb.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 32.Chen XJ, Zhang JG, Wu L. Plumbagin inhibits neuronal apoptosis, intimal hyperplasia and also suppresses TNF-α/NF-κB pathway induced inflammation and matrix metalloproteinase-2/9 expression in rat cerebral ischemia. Saudi J Biol Sci. 2018;25(6):1033–39. doi: 10.1016/j.sjbs.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang JJ, Emanuel BA, Mack WJ, et al. Matrix metalloproteinase-9: Dual role and temporal profile in intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2014;23(10):2498–505. doi: 10.1016/j.jstrokecerebrovasdis.2014.07.005. [DOI] [PubMed] [Google Scholar]