Abstract
Sufficient connexin-mediated intercellular coupling is critical to maintain gap junctional communication for proper cardiac function. Alterations in connexin phosphorylation state, particularly dephosphorylation of connexin 43 (Cx43), may impact cell coupling and conduction in disease states. Cx43 dephosphorylation may be carried out by protein phosphatase activity. Here, we present an overview of the key phosphatases known to interact with Cx43 or modulators of Cx43, as well as some possible therapeutic targets to regulate phosphatase activity in the heart.
Keywords: Phosphatase, Connexin 43, Gap junction
1. Introduction
It has been well established that appropriate levels of protein phosphorylation are essential to maintain cardiac function and play an important role in the development of cardiac arrhythmias. Phosphorylation states of key substrates are modulated by protein kinases (PKs) and protein phosphatases (PPs) via posttranscriptional and posttranslational mechanisms [1–6]. Although the focus of studies on protein phosphorylation has been primarily on the role of PKs in cardiovascular diseases (CVDs) and their potential as therapeutic targets [3], more recent research has shown an emerging interest in the role of PPs and the potential of phosphatase-regulating drugs [7–19].
The most ubiquitous serine/threonine phosphatases, such as PP1, PP2A, and PP2B are known to contribute to the majority of phosphatase activity in the heart [4]. Dysregulation of these and other PPs have been found in numerous CVDs, including heart failure (HF), and may play a critical role in reduced intercellular coupling and arrhythmia development via connexin protein dephosphorylation [8–10].
Gap junctional channels, composed of connexins, are specialized membrane structures. These gap junction channels critically influence electrical and chemical signal propagation throughout the heart [20,21]. Conduction slowing arises from decreased depolarizing currents and/or decreased gap junctional coupling, which could underlie reentry occurring in various arrhythmias, such as during the transition from ventricular tachycardia to the fatal cardiac arrhythmia ventricular fibrillation [22–25] or during atrial fibrillation induction and/or maintenance in acute ischemia or HF [26,27]. Thus, the following review explores the importance of protein phosphatase regulation in connexin phosphorylation states, the impact of dysregulation in HF and altered conduction, and the implications for protein phosphatases as therapeutic targets.
2. Cardiac connexin dysregulation
Cell-to-cell electrical coupling in the heart occurs mainly via gap junctions. These membrane structures consist of intercellular hemi-channels formed from an assembly of connexins that connect adjacent cells and allow for electrical and chemical communication. Connexins are four-pass transmembrane proteins with two extracellular loops (EL), one cytosolic loop, and both the N-terminus and C-terminus towards the cytosol. Six connexin subunits assemble to form a connexon hemichannel, and interaction between the ELs of adjacent cells combines two hemichannels to form a gap junction channel. In addition to their primary role in hemichannel formation, connexins also interact with scaffolding proteins at the C-terminus, and may play a role in key signaling pathways and cell cycle regulation [11,12,28–30]. Connexin 43 in particular has been shown to interact with the scaffold protein zonula occludens-1 (ZO-1), which regulates gap junction formation and properties [21,31–35]. Thousands of gap junction channels may assemble together to form macromolecular complexes known as gap junction plaques, which facilitate electrical current propagation from cell to cell, enabling coordinated cardiomyocyte contraction. The hemichannels that comprise gap junctions may open or close in response to numerous triggers, including changes in transmembrane potential, changes in intracellular or extracellular ion concentrations, or alterations in phosphorylation status of connexin proteins [11,12,28–30].
Connexin 43 (Cx43) is the major connexin expressed in the ventricles, but is also present in atrial and endothelial cells. Connexin 40 (Cx40) and connexin 45 (Cx45) are also expressed in cardiac tissue, but are predominantly found in the atria [6,11] and atrioventricular conduction system [36,37], and are less abundant overall. The relative amounts, composition and distribution of these connexins have been shown to influence the conduction properties of cells [38,39].
Reduced Cx43 abundance is found in myocardial ischemia and HF. Downregulation of Cx43 expression occurs in myocardial ischemia in rat and rabbit hearts [40,41], as well as HF models in dog and rabbit, and in failing human hearts [8,20,42–45]. In left ventricular (LV) myocytes isolated from a rabbit model of nonischemic HF (combined aortic insufficiency and aortic constriction), we found that total Cx43 protein was decreased by 34% in HF compared to controls [8]. In further studies conducted in Cx43 knockdown rabbit myocytes with reduced expression but preserved phosphorylation state, we found reduced cell coupling, evaluated by Lucifer Yellow (LY) dye transfer, compared with controls. Cx43 was also overexpressed in HF rabbit myocytes to levels comparable with normal myocytes. Overexpression of Cx43 improved cell coupling in HF myocytes when compared with HF controls [42]. We recently also discovered that downregulated Cx43 also plays an important role in slowing of conduction and enhanced atrial arrhythmogenicity in the aged atrium [46]. These studies, in addition to studies in Cx43 heterozygous knockout mice, support the idea that decreased expression of Cx43 can result in slow conduction and increased susceptibility to cardiac arrhythmias [8,20,40–48].
Cx43 is a phosphoprotein that is predominantly phosphorylated in the control state. Cx43 can be phosphorylated by a number of kinases and dephosphorylated by protein phosphatases such as PP1 and PP2A [6,10]. Posttranslational phosphorylation of Cx43 is thought to influence intercellular coupling through gap junction remodeling, and dysregulation of Cx43 phosphorylation occurs in disease states [8,11,12,28,29,42, 47–49]. Cx43 can be phosphorylated at at least 17 serine sites and two tyrosine sites located at the C-terminus via several kinases, including protein kinase A (PKA), protein kinase C (PKC), casein kinase 1 (CK1), mitogen-activated protein kinase (MAPK), Ca2+/calmodulin-dependent protein kinase II (CaMKII), and Src kinases (Fig. 1) [12,28,29,46, 49,50]. The level of kinase activation and related Cx43 expression and phosphorylation affects gap junctional conductance. PKA activation, in particular, has been shown to increase conductance and improve cell-to-cell communication [51], whereas increased activation of PKC decreases gap junctional communication [52]. Phosphorylation by CK1, MAPK, and Src kinases appears to influence intercellular communication by promoting Cx43 localization and gap junction assembly [50].
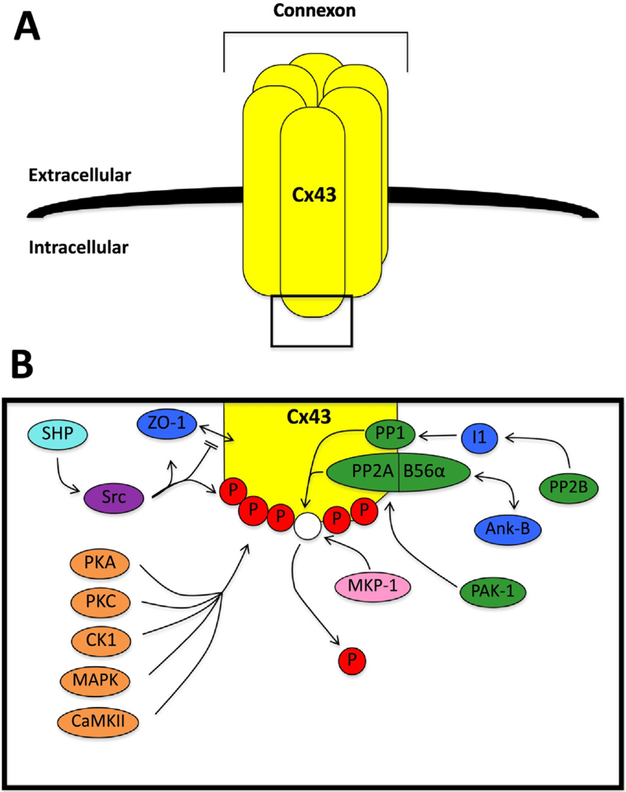
Fig. 1.
Connexin 43 phosphorylation overview. A. An overview of a connexon formed from connexin 43 (Cx43). B. A schematic of the interactions between Cx43 and key kinases and phosphatases. Shown are: a) serine/threonine kinases (orange), including protein kinase A (PKA), protein kinase C (PKC), casein kinase 1 (CK1), mitogen-activated protein kinase (MAPK), and Ca2+/calmodulin-dependent protein kinase II (CaMKII); b) serine/threonine phosphatases (green), including protein phosphatase 1 (PP1), protein phosphatase 2A (PP2A), calcineurin (PP2B), and p-21 activated kinase-1 (PAK-1); c) the tyrosine kinase (purple) Src kinase (Src); d) the tyrosine phosphatase (light blue) Src homology region 2 domain-containing phosphatase (SHP); e) the dual specificity phosphatase (pink) mitogen-activated protein kinase phosphatase 1 (MKP-1); and f) related proteins (dark blue) including zonula occludens-1 (ZO-1), inhibitor 1 (I1), and ankyrin-B (Ank-B). The phosphate groups are indicated by P. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Emerging evidence suggests that dephosphorylation of Cx43, in opposition to this kinase activity, leads to reduced gap junctional communication and increased arrhythmic susceptibility [8,12,29,41,42,47,48]. Dephosphorylation of Cx43 (by phosphatases) has been shown to decrease gap junctional communication, whether assessed in neonatal rat ventricular cell pairs with activation of endogenous phosphatases [9], or in perfused whole rat hearts during myocardial ischemia [40,41]. We found a 64% increase in nonphosphorylated Cx43 in HF rabbits compared to controls (in which Cx43 was primarily phosphorylated) [8]. Dephosphorylation of Cx43 has also been shown to occur in models of ischemia, and is associated with reduced gap junctional communication, slow conduction, and increased arrhythmogenicity [8,40–42,53]. Thus, connexin phosphorylation and dephosphorylation play an important role in regulating gap junction channel function and the development of cardiac arrhythmias in diseased hearts.
Findings on the effects of Cx43 phosphorylation at specific amino acid sites (primarily serine sites) have been contradictory. Prolonged ischemia, electrical uncoupling, and slow conduction have been associated with Cx43 dephosphorylation at Ser306 [54,55], Ser297 [54], Ser365 [56,57] and Ser368 [41,54], while phosphorylation at Ser279 and Ser282 has also been correlated with decreased conduction and dye coupling [58,59]. Phosphorylation by PKC, which may phosphorylate Ser365, Ser368, Ser369, Ser372, and Ser373 [60], has been associated with increased macroscopic electrical coupling [61], but has also been associated with reduced single channel conductance and a decrease in dye coupling [52,61]. Moreover, studies on the interactions between Cx43 phosphorylation sites have also found conflicting results. PKA phosphorylation at Ser364 and/or Ser365 has been found to enhance phosphorylation [60], yet other studies have found that dephosphorylation of Ser365 is necessary for PKC-induced phosphorylation of Ser368 [56, 57,62]. Decreased conduction, gap junction channel closure, and associated arrhythmias have also been linked to increased tyrosine phosphor-ylation of Cx43 [21,32,33,63,64], particularly phosphorylation of Tyr265 [65–67]. These findings, many of which seem contradictory, emphasize the complex role of Cx43 phosphorylation in the regulation of gap junctions.
Though not as prevalent or extensively studied, Cx40 and Cx45 may also play a role in cardiac gap junctional communication. Like Cx43, Cx40 is regulated by posttranslational phosphorylation [68,69], and may be phosphorylated by PKA and PKC [69]. Cx40 phosphorylation by PKA in SKHep1 cells resulted in increased gap junction conductance and metabolic coupling [69]. Decreased Cx40 phosphorylation in micro-vascular endothelial cells, both during sepsis and with PKA inhibition, has been associated with decreased electrical coupling, which can be prevented by PKA activation [70]. Taken together, these studies suggest that Cx40 phosphorylation state may influence gap junctional conduction and could play a role in atrial arrhythmias. Indeed, recent studies have started to investigate the role of Cx40 in atrial fibrillation, and have found multiple Cx40 mutations associated with altered conduction properties [71–76]. The relationship between these mutations, altered conduction, and connexin phosphorylation remains to be explored.
Cx45 has been shown to be serine phosphorylated by CaMKII, CK1, PKA, and MAPK in HeLa cells [77,78]. Phosphorylation by PKA and MAPK were associated with decreased junctional conductance [78], suggesting that phosphorylation of Cx45 may influence conduction properties. Overall, the roles of both Cx40 and Cx45 phosphorylation in gap junctional communication, particularly in myocardium, merits further research.
3. Phosphatases and dephosphorylation of Cx43
Phosphorylation state of gap junctions is critical in regulating gap junction coupling between adjacent myocytes. Although phosphorylation occurs by protein kinase activity, the phosphorylation status of Cx43 is counteracted by protein phosphatases (Fig. 1). Since Cx43 phosphorylation sites are mostly serine sites at the C-terminus, dephosphorylation of Cx43 occurs mainly via serine/threonine protein phosphatases (PPs), particularly PP1 and PP2A in cardiac tissue [28,29,49].
Increased PP activity has been associated with CVDs, including HF, and atrial and ventricular arrhythmias [8,26,27,42,43]. We demonstrated that both PP1 and PP2A colocalize with Cx43 in cardiac tissue, and that the level of PP2A colocalized with Cx43 increased 2.5-fold in HF compared to controls, while the level of PP1 in HF remained the same [8]. This increased PP2A activity at the level of Cx43 in HF was associated with slow conduction and reduced intercellular coupling by slower LY dye transfer [8,42,43]. Non-phosphorylatable Cx43 gap junctions in S3A knock-in mice showed slow conduction and increased ventricular arrhythmias, while phosphatase-resistant Cx43 gap junctions in S3E knock-in mice were resistant to conduction slowing and less susceptible to arrhythmogenesis [12]. Thus, the mechanisms underlying protein phosphatase regulation of connexins are worth further investigation.
4. Phosphatase regulation
Inhibiting phosphatase activity, particularly PP2A, may improve intercellular coupling in HF, ischemia, and other cardiac conditions by increasing Cx43 expression and phosphorylation state. Intrinsic or extrinsic PP inhibition, as well as modulation of upstream regulators of PP activity, may be effective therapeutic approaches to reduce PP activity, improve Cx43 dysregulation, and ultimately modify conduction.
4.1. PP1 and PP2A
PP1 and PP2A can be directly inhibited by small molecule serine/threonine phosphatase inhibitors. Although some of these inhibitors are selective for PP1 and PP2A, they all may also inhibit PP4, PP5, and PP6 to an extent, which may have undesirable effects [13]. The most selective and widely available PP inhibitors are okadaic acid, calyculin A, and fostriecin.
Studies suggest that okadaic acid at a concentration (10 nmol/L) inhibits PP2A, but not PP1 [79,80]. We used okadaic to successfully inhibit PP2A in vitro, enhance Cx43 phosphorylation, and improve intercellular coupling in HF [8,43].
Fostriecin has been reported to be highly selective for PP2A over PP1 [14]. However, okadaic acid and calyculin A, but not fostriecin, were found to reduce ischemia-induced Cx43 dephosphorylation [15]. Fostriecin is of particular interest since it is being investigated as a cancer treatment to inhibit PP2A. Fostriecin has been safely administered to patients in pharmacokinetic studies and clinical trials, supporting its possible use in clinical applications [19].
Overall, studies on the effects of small molecule PP inhibitors on PP2A at the level of Cx43 have been limited, but evidence to date supports the value for additional research to explore their potential clinical utility as antiarrhythmic agents. However, further investigation is necessary to identify more selective means to target PPs with higher specificity in order to elucidate the activity of particular phosphatases.
4.2. PAK-1
PP2A may be indirectly inhibited by modulation of upstream regulators, such as p21-activated kinases (PAKs). PAKs are a family of six serine-threonine kinases (PAK-1 to −6) that phosphorylate a variety of substrates [81–83]. PAK-1, −2, and −4 have been shown to play important roles in cardiac function [81–84]. PAK-1 in particular has been shown to co-localize with PP2A and to modulate PP2A activity [43,85–87]. We showed that PAK-1 associates with Cx43, and that both total and activated PAK-1 were enhanced at the level of Cx43 in HF in rabbit and human LV [43]. Our further study in myocytes overexpressing active PAK-1 suggests that enhanced PAK-1 contributes to increased PP2A activation, resulting in increased Cx43 dephosphorylation and associated with decreased intercellular coupling [43]. PAK-1-mediated regulation of Cx43 through PP2A may therefore be a therapeutic target for prevention of ventricular arrhythmias in HF through improved inter-cellular coupling.
4.3. B56α and ankyrin-B
Another possible target for indirect inhibition of PP2A is the interaction between B56α and ankyrin-B. B56α is a regulatory subunit of PP2A [16–18]. Cardiomyocyte-directed overexpression of B56α was associated with enhanced PP2A activation [17], and mice deficient in B56α showed increased PP2A activity, slow conduction and increased heart rate variability [18]. B56α binds ankyrin-B in vivo, and B56α and ankyrin-B co-localize in cardiomyocytes. Reduced ankyrin-B has been associated with disorganized B56α distribution [16]. Although the role of B56α-PP2A and its association with ankyrin-B at the level of Cx43 is unknown, existing research implicates ankyrin-B in the localization of B56α-PP2A, which could have effects on PP2A activity and, subsequently, Cx43 phosphorylation. The interaction between ankyrin-B and B56α-PP2A may therefore be another attractive target for therapeutic intervention to reduce Cx43 dephosphorylation and improve electrical coupling.
4.4. Calcineurin (PP2B)
Calcineurin (PP2B), a calcium dependent phosphatase, may indirectly contribute to Cx43 dephosphorylation through dephosphorylation of Inhibitor 1 (I1) and subsequent activation of PP1 [88]. Increased calcium in guinea pig myocardium resulted in decreased Cx43 phosphorylation at specific sites and PP1 activation, with reduced gap junctional communication and slow conduction [56]. Furthermore, inhibition of calcineurin A by cyclosporine A in rats prevented Cx43 dephosphorylation after myocardial ischemia [40]. Calcineurin may therefore be another target to reduce Cx43 dephosphorylation indirectly through PP1 regulation.
4.5. Src and tyrosine phosphatases
While phosphorylation state of serine residues is known to be critical in regulating the function of gap junction channels, tyrosine residues have also been found to be involved in gap junction coupling. A study in a canine model of myocardial infarction (MI) found that downregulation of Cx43 is associated with an upregulation of phosphorylated c-Src, a tyrosine kinase, through competition for a binding site on ZO-1 [31]. Increased c-Src activity has also been associated with increased tyrosine phosphorylated Cx43 and reduced gap junctional conduction in cardiomyopathy [32]. Inhibition of c-Src with Src inhibitors, such as the pyrazolopyrimidine PP1, has been shown to increase Cx43 expression, improve conduction, and reduce arrhythmic inducibility [21,33] implicating c-Src and its role in tyrosine phosphorylation as a potential therapeutic target to mitigate Cx43 dysregulation.
The tyrosine kinase v-Src has also been implicated in the closure of gap junction channels, both by direct tyrosine phosphorylation of Cx43, and by indirect serine phosphorylation of Cx43 via MAPK and PKC activation at sites that are not phosphorylated in normal gap junctions [63,64]. Phosphorylation at these sites has been correlated with decreased coupling and conduction [58,59]. Recently, it has been shown that the T-cell protein tyrosine phosphatase (TC-PTP, or PTPN2) can interact with Cx43 directly to decrease v-Src-induced phosphorylation and increase gap junctional communication. However, TCPTP had no effect on MAPK phosphorylation and therefore could not completely reverse the gap junctional effects of v-Src [89]. Cx43 dephosphorylation that occurs during chemical ischemia in astrocytes has been associated with c-Src and ERK phosphatase MKP-1 [90]. Though it is not clear whether MKP-1 interacts directly with Cx43, it is reasonable that MKP-1 may be a potential target to regulate Cx43 phosphorylation, either through direct interaction with Cx43, or through MAPK signaling. While Src kinases and their effects on tyrosine phosphorylation and MAPK signaling contribute to reduced Cx43 expression and phosphorylation [21,31–33,58,59,63,64,89,90], many details of their interactions remain unknown. Further studies are needed to elucidate the relationship between Cx43, Src kinases and MAPK, which may reveal other potential targets to regulate the phosphorylation of Cx43.
5. Conclusion
Investigations into the role of protein phosphatases in connexin-mediated gap junctional communication have been much more limited than studies of protein kinases. However, there is growing evidence that protein phosphatases play a significant role in the gap junctional conduction changes that occur in disease states, particularly HF and ischemia. Further explorations into the role of these phosphatases and their associations with Cx43 may provide mechanistic insights that highlight potential therapeutic targets to develop novel therapies to reverse Cx43 dephosphorylation and improve intercellular coupling in CVDs.
Acknowledgments
This research was supported by National Institutes of Health (HL113640 to XA and HL80101 to SMP) and the American Heart Association (3770030 & 12050478 to XA and 25860028 to SMP).
References
- [1].Rapundalo ST, Cardiac protein phosphorylation: functional and pathophysiological correlates, Cardiovasc. Res 38 (3) (1998) 559–588. [DOI] [PubMed] [Google Scholar]
- [2].Cohen P, The origins of protein phosphorylation, Nat. Cell Biol 4 (5) (2002) E127–E130. [DOI] [PubMed] [Google Scholar]
- [3].Cohen P, Protein kinases–the major drug targets of the twenty-first century? Nat. Rev. Drug Discov 1 (4) (2002) 309–315. [DOI] [PubMed] [Google Scholar]
- [4].MacDougall LK, Jones LR, Cohen P, Identification of the major protein phosphatases in mammalian cardiac muscle which dephosphorylate phospholamban, Eur.J. Biochem 196 (1991) 725–734. [DOI] [PubMed] [Google Scholar]
- [5].Weber S, Meyer-Roxlau S, Wagner M, Dobrev D, El-Armouche A, Counteracting protein kinase activity in the heart: the multiple roles of protein phosphatases, Front. Pharmacol 6 (2015) 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hervé JC, Sarrouilhe D, Protein phosphatase modulation of the intercellular junctional communication: importance in cardiac myocytes, Prog. Biophys. Mol. Biol 90 (1–3) (2006) 225–248. [DOI] [PubMed] [Google Scholar]
- [7].Popescu I, Galice S, Mohler PJ, Despa S, Elevated local [Ca2+] and CaMKII promote spontaneous Ca2+ release in ankyrin-B-deficient hearts, Cardiovasc. Res 111 (3) (2016) 287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ai X, Pogwizd SM, Connexin 43 downregulation and dephosphorylation in nonischemic heart failure is associated with enhanced colocalized protein phosphatase type 2A, Circ. Res 96 (1) (2005) 54–63. [DOI] [PubMed] [Google Scholar]
- [9].Duthe F, Plaisance I, Sarrouilhe D, Herve JC, Endogenous protein phosphatase 1 runs down gap junctional communication of rat ventricular myocytes, Am. J. Physiol. Cell Physiol 281 (2001) C1648–C1656. [DOI] [PubMed] [Google Scholar]
- [10].DeGrande ST, Little SC, Nixon DJ, Wright P, Snyder J, Dun W, et al. , Molecular mechanisms underlying cardiac protein phosphatase 2A regulation in heart, J. Biol. Chem 288 (2) (2013) 1032–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Axelsen LN, Calloe K, Holstein-Rathlou NH, Nielsen MS, Managing the complexity of communication: regulation of gap junctions by post-translational modification, Front. Pharmacol 4 (2013) 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Remo BF, Qu J, Volpicelli FM, Giovannone S, Shin D, Lader J, et al. , Phosphatase-resistant gap junctions inhibit pathological remodeling and prevent arrhythmias, Circ. Res 108 (12) (2011) 1459–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Swingle M, Ni L, Honkanen RE, Small molecule inhibitors of Ser/thr protein phosphatases: specificity, use and common forms of abuse, Methods Mol. Biol 365 (2007) 23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Walsh AH, Cheng A, Honkanen RE, Fostriecin, an antitumor antibiotic with inhibitory activity against serine/threonine protein phosphatases types 1 (PP1) and 2A (PP2A), is highly selective for PP2A, FEBS Lett. 416 (3) (1997) 230–234. [DOI] [PubMed] [Google Scholar]
- [15].Jeyaraman M, Tanguy S, Fandrich RR, Lukas A, Kardami E, Ischemia-induced dephosphorylation of cardiomyocyte connexin-43 is reduced by okadaic acid and calyculin A but not fostriecin, Mol. Cell. Biochem 242 (1–2) (2003) 129–134. [PubMed] [Google Scholar]
- [16].Bhasin N, Cunha SR, Mudannayake M, Gigena MS, Rogers TB, Mohler PJ, Molecular basis for PP2A regulatory subunit B56alpha targeting in cardiomyocytes, Am. J. Physiol. Heart Circ. Physiol 293 (1) (2007) H109–H119. [DOI] [PubMed] [Google Scholar]
- [17].Kirchhefer U, et al. , Cardiac function is regulated by B56α-mediated targeting of protein phosphatase 2A (PP2A) to contractile relevant substrates, J. Biol. Chem 289 (49) (2014) 33862–33873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Little SC, Curran J, Makara MA, Kline CF, Ho HT, Xu Z, et al. , Protein phosphatase 2A regulatory subunit B56α limits phosphatase activity in the heart, Sci. Signal 8 (386) (2015) ra72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lê LH, Erlichman C, Pillon L, Thiessen JJ, Day A, Wainman N, et al. , Phase I and pharmacokinetic study of fostriecin given as an intravenous bolus daily for five consecutive days, Investig. New Drugs 22 (2) (2004) 159–167. [DOI] [PubMed] [Google Scholar]
- [20].Kostin S, Rieger M, Dammer S, Hein S, Richter M, Klovekorn WP, et al. , Gap junction remodeling and altered connexin43 expression in the failing human heart, Mol. Cell. Biochem 242 (2003) 135–144. [PubMed] [Google Scholar]
- [21].Rutledge CA, Ng FS, Sulkin MS, Greener ID, Sergeyenko AM, Liu H, et al. , C-Src kinase inhibition reduces arrhythmia inducibility and connexin43 dysregulation after myocardial infarction, J. Am. Coll. Cardiol 63 (9) (2014) 928–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Packer M, Sudden unexpected death in patients with congestive heart failure: a second frontier, Circulation 72 (1985) 681–685. [DOI] [PubMed] [Google Scholar]
- [23].Pogwizd SM, Nonreentrant mechanisms underlying spontaneous ventricular arrhythmias in a model of nonischemic heart failure in rabbits, Circulation 92 (1995) 1034–1048. [DOI] [PubMed] [Google Scholar]
- [24].Anderson KP, Walker R, Urie P, Ershler PR, Lux RL, Karwandee SV, Myocardial electrical propagation in patients with idiopathic dilated cardiomyopathy, J. Clin. Invest 92 (1993) 122–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shaw RM, Rudy Y, Ionic mechanisms of propagation in cardiac tissue: roles of the sodium and L-type calcium currents during reduced excitability and decreased gap junction coupling, Circ. Res 81 (1997) 727–741. [DOI] [PubMed] [Google Scholar]
- [26].Heijman J, Voigt N, Nattel S, Dobrev D, Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression, Circ. Res 114 (2014) 1483–1499. [DOI] [PubMed] [Google Scholar]
- [27].Heijman J, Ghezelbash S, Wehrens XH, Dobrev D, Serine/threonine phosphatases in atrial fibrillation, J. Mol. Cell. Cardiol 103 (2017) 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kwak BR, Jongsma H, Regulation of cardiac gap junction channel permeability and conductance by several phosphorylating conditions, Mol. Cell. Biochem 157 (1996) 93–99. [DOI] [PubMed] [Google Scholar]
- [29].Schulz R, Görge PM, Görbe A, Ferdinandy P, Lampe PD, Leybaert L, Connexin 43 is an emerging therapeutic target in ischemia/reperfusion injury, cardioprotection and neuroprotection, Pharmacol. Ther 153 (2015) 90–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nielsen MS, Axelsen LN, Sorgen PL, Verma V, Delmar M, Holstein-Rathlou NH, Gap junctions, Compr. Physiol 2 (3) (2012) 1981–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kieken F, Mutsaers N, Dolmatova E, Virgil K, Wit AL, Kellezi A, et al. , Structural and molecular mechanisms of gap junction remodeling in epicardial border zone myocytes following myocardial infarction, Circ. Res 104 (9) (2009) 1103–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Tada M, Hori M, Functional role of c-Src in gap junctions of the cardiomyopathic heart, Circ. Res 85 (1999) 672–681. [DOI] [PubMed] [Google Scholar]
- [33].Sovari AA, Iravanian S, Dolmatova E, Jiao Z, Liu H, Zandieh S, et al. , Inhibition of c-Src tyrosine kinase prevents angiotensin II-mediated connexin-43 remodeling and sudden cardiac death, J. Am. Coll. Cardiol 58 (2011) 2332–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lau AF, c-Src: bridging the gap between phosphorylation- and acidification-induced gap junction channel closure, Sci. STKE 291 (2005) pe33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rhett JM, Jourdan J, Gourdie RG, Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1, Mol. Biol. Cell 22 (9) (2011) 1516–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gourdie RG, Severs NJ, Green CR, Rothery S, Germroth P, Thompson RP, The spatial distribution and relative abundance of gap-junctional connexin40 and connexin43 correlate to functional properties of components of the cardiac atrioventricular conduction system, J. Cell Sci 105 (Pt 4) (1993) 985–991. [DOI] [PubMed] [Google Scholar]
- [37].Coppen SR, Dupont E, Rothery S, Severs NJ, Connexin45 expression is preferentially associated with the ventricular conduction system in mouse and rat heart, Circ. Res 82 (2) (1998) 232–243. [DOI] [PubMed] [Google Scholar]
- [38].Kumar NM, Gilula NB, The gap junction communication channel, Cell 84 (1996) 381–388. [DOI] [PubMed] [Google Scholar]
- [39].Saffitz JE, Davis LM, Darrow BJ, Kanter HL, Laing JG, Beyer EC, The molecular basis of anisotropy: role of gap junctions, J. Cardiovasc. Electrophysiol 6 (1995) 498–510. [DOI] [PubMed] [Google Scholar]
- [40].Hatanaka K, Kawata H, Toyofuku T, Yoshida K, Down-regulation of connexin43 in early myocardial ischemia and protective effect by ischemic preconditioning in rat hearts in vivo, Jpn. Heart J 45 (2004) 1007–1019. [DOI] [PubMed] [Google Scholar]
- [41].Beardslee MA, Lerner DL, Tadros PN, Laing JG, Beyer EC, Yamada KA, et al. , Dephosphorylation and intracellular redistribution of ventricular connexins 43 during electrical uncoupling induced by ischemia, Circ. Res 87 (2000) 656–662. [DOI] [PubMed] [Google Scholar]
- [42].Ai X, Zhao W, Pogwizd SM, Connexin43 knockdown or overexpression modulates cell coupling in control and failing rabbit left ventricular myocytes, Cardiovasc. Res 85 (4) (2010) 751–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ai X, Jiang A, Ke Y, Solaro RJ, Pogwizd SM, Enhanced activation of p21-activated kinase 1 in heart failure contributes to dephosphorylation of connexin 43, Cardiovasc. Res 92 (1) (2011) 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Poelzing S, Rosenbaum DS, Altered connexin43 expression produces arrhythmia substrate in heart failure, Am. J. Physiol. Heart Circ. Physiol 287 (2004) H1762–H1770. [DOI] [PubMed] [Google Scholar]
- [45].Akar FG, Nass RD, Hahn S, Cingolani E, Shah M, Hesketh GG, et al. , Dynamic changes in conduction velocity and gap junction properties during development of pacing-induced heart failure, Am. J. Physiol. Heart Circ. Physiol 293 (2007) H1223–H1230. [DOI] [PubMed] [Google Scholar]
- [46].Yan J, Kong W, Zhang Q, Beyer EC, Walcott G, Fast VG, et al. , C-Jun N-terminal kinase activation contributes to reduced connexin43 and development of atrial arrhythmias, Cardiovasc. Res 97 (3) (2013) 589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].McCain ML, Desplantez T, Geisse NA, Rothen-Rutishauser B, Oberer H, Parker KK, et al. , Cell-to-cell coupling in engineered pairs of rat ventricular cardiomyocytes: relation between Cx43 immunofluorescence and intercellular electrical conductance, Am. J. Physiol. Heart Circ. Physiol 302 (2) (2012) H443–H450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Boulaksil M, Bierhuizen MF, Engelen MA, Stein M, Kok BJ, van Amersfoorth SC, et al. , Spatial heterogeneity of Cx43 is an arrhythmogenic substrate of polymorphic ventricular tachycardias during compensated cardiac hypertrophy in rats, Front. Cardiovasc. Med 3 (2016) 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Palatinus JA, Rhett JM, Gourdie RG, The connexin43 carboxyl terminus and cardiac gap junction organization, Biochim. Biophys. Acta 1818 (8) (2012) 1831–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Solan JL, Lampe PD, Connexin 43 phosphorylation – structural changes and biological effects, Biochem. J 419 (2) (2009) 261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Matsumura K, Mayama T, Lin H, Sakamoto Y, Ogawa K, Imanaga I, Effects of cyclic AMP on the function of the cardiac gap junction during hypoxia, Exp. Clin. Cardiol 11 (4) (2006) 286–293. [PMC free article] [PubMed] [Google Scholar]
- [52].Lampe PD, TenBroek EM, Burt JM, Kurata WE, Johnson RG, Phosphorylation of Connexin43 on Serine368 by protein kinase C regulates gap junctional communication, J. Cell Biol 149 (2000) 1503–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Jeyaraman MM, Srisakuldee W, Nickel BE, Kardami E, Connexin43 phosphorylation and cytoprotection in the heart, Biochim. Biophys. Acta 1818 (8) (2012) 2009–2013. [DOI] [PubMed] [Google Scholar]
- [54].Axelsen LN, Stahlhut M, Mohammed S, Larsen BD, Nielsen MS, Holstein-Rathlou NH, et al. , Identification of ischemia-regulated phosphorylation sites in connexin43: a possible target for the antiarrhythmic peptide analogue rotigaptide (ZP123), J. Mol. Cell. Cardiol 40 (2006) 790–798. [DOI] [PubMed] [Google Scholar]
- [55].Procida K, Jørgensen L, Schmitt N, Delmar M, Taffet SM, Holstein-Rathlou NH, et al. , Phosphorylation of connexin43 on serine 306 regulates electrical coupling, Heart Rhythm. 6 (2009) 1632–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Jabr RI, Hatch FS, Salvage SC, Orlowski A, Lampe PD, Fry CH, Regulation of gap junction conductance by calcineurin through Cx43 phosphorylation: implications for action potential conduction, Pflugers Arch. (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Nassal MM, Werdich AA, Wan X, Hoshi M, Deschênes I, Rosenbaum DS, et al. , Phosphorylation at connexin43 serine-368 is necessary for myocardial conduction during metabolic stress, J. Cardiovasc. Electrophysiol 27 (1) (2016) 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Warn-Cramer BJ, Lampe PD, Kurata WE, Kanemitsu MY, Loo LWM, Eckhart W, et al. , Characterization of the mitogen-activated protein kinase phosphorylation sites on the Connexin-43 gap junction protein, J. Biol. Chem 271 (1996) 3779–3786. [DOI] [PubMed] [Google Scholar]
- [59].Warn-Cramer BJ, Cottrell GT, Burt JM, Lau AF, Regulation of connexin-43 gap junctional intercellular communication by mitogen activated protein kinase, J. Biol. Chem 273 (1998) 9188–9196. [DOI] [PubMed] [Google Scholar]
- [60].Shah MM, Martinez AM, Fletcher WH, The connexin43 gap junction protein is phosphorylated by protein kinase a and protein kinase C: in vivo and in vitro studies, Mol. Cell. Biochem 238 (2002) 57–68. [DOI] [PubMed] [Google Scholar]
- [61].Kwak BR, van Veen TAB, Analbers LJS, Jongsma HJ, TPA increases conductance but decreases permeability in neonatal rat cardiomyocyte gap junction channels, Exp. Cell Res 220 (1995) 456–463. [DOI] [PubMed] [Google Scholar]
- [62].Solan JL, Marquez-Rosado L, Sorgen PL, Thornton PJ, Gafken PR, Lampe PD, Phosphorylation at S365 is a gatekeeper event that changes the structure of Cx43 and prevents down-regulation by PKC, J. Cell Biol 179 (2007) 1301–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Mitra SS, Xu J, Nicholson BJ, Coregulation of multiple signaling mechanisms in pp60v-Src-induced closure of Cx43 gap junction channels, J. Membr. Biol 245 (8) (2012) 495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Solan JL, Lampe PD, Connexin 43 in LA-25 cells with active v-src is phosphorylated on Y247, Y265, S262, S279/282, and S368 via multiple signaling pathways, Cell Commun. Adhes 15 (1) (2008) 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Giepmans BNG, Hengeveld T, Postma FR, Moolenaar WH, Interaction of c-Src with gap junction protein Connexin-43, J. Biol. Chem 276 (2001) 8544–8549. [DOI] [PubMed] [Google Scholar]
- [66].Postma FR, Hengeveld T, Alblas J, Giepmans BNG, Zondag GCM, Jalink K, et al. , Acute loss of cell-cell communication caused by G protein-coupled receptors: a critical role for c-Src, J. Cell Biol 140 (1998) 1199–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Toyofuku T, Akamatsu Y, Zhang H, Kuzuya T, Tada M, Hori M, c-Src regulates the interaction between Connexin-43 and ZO-1 in cardiac myocytes, J. Biol. Chem 276 (2001) 1780–1788. [DOI] [PubMed] [Google Scholar]
- [68].Traub O, Eckert R, Lichtenberg-Fraté H, Elfgang C, Bastide B, Scheidtmann KH, et al. , Immunochemical and electrophysiological characterization of murine connexin40 and −43 in mouse tissues and transfected human cells, Eur. J. Cell Biol 64 (1) (1994) 101–112. [PubMed] [Google Scholar]
- [69].van Rijen HV, van Veen TA, Hermans MM, Jongsma HJ, Human connexin40 gap junction channels are modulated by cAMP, Cardiovasc. Res 45 (4) (2000) 941–951. [DOI] [PubMed] [Google Scholar]
- [70].Bolon ML, Peng T, Kidder GM, Tyml K, Lipopolysaccharide plus hypoxia and reoxygenation synergistically reduce electrical coupling between microvascular endothelial cells by dephosphorylating connexin40, J. Cell. Physiol 217 (2) (2008) 350–359. [DOI] [PubMed] [Google Scholar]
- [71].Santa Cruz A, Meşe G, Valiuniene L, Brink PR, White TW, Valiunas V, Altered conductance and permeability of Cx40 mutations associated with atrial fibrillation, J. Gen. Physiol 146 (5) (2015) 387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Gemel J, Simon AR, Patel D, Xu Q, Matiukas A, Veenstra RD, et al. , Degradation of a connexin40 mutant linked to atrial fibrillation is accelerated, J. Mol. Cell. Cardiol 74 (2014) 330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Patel D, Gemel J, Xu Q, Simon AR, Lin X, Matiukas A, et al. , Atrial fibrillation-associated connexin40 mutants make hemichannels and synergistically form gap junction channels with novel properties, FEBS Lett. 588 (8) (2014) 1458–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Sun Y, Tong X, Chen H, Huang T, Shao Q, Huang W, et al. , An atrial-fibrillation-linked connexin40 mutant is retained in the endoplasmic reticulum and impairs the function of atrial gap-junction channels, Dis. Model. Mech 7 (5) (2014) 561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Lübkemeier I, Andrié R, Lickfett L, Bosen F, Stöckigt F, Dobrowolski R, et al. , The Connexin40A96S mutation from a patient with atrial fibrillation causes decreased atrial conduction velocities and sustained episodes of induced atrial fibrillation in mice, J. Mol. Cell. Cardiol 65 (2013) 19–32. [DOI] [PubMed] [Google Scholar]
- [76].Gemel J, Levy AE, Simon AR, Bennett KB, Ai X, Akhter S, et al. , Connexin40 abnormalities and atrial fibrillation in the human heart, J. Mol. Cell. Cardiol 76 (2014) 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Bao M, Kanter EM, Huang RY, Maxeiner S, Frank M, Zhang Y, et al. , Residual Cx45 and its relationship to Cx43 in murine ventricular myocardium, Channels (Austin) 5(6) (2011) 489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].van Veen TA, van Rijen HV, Jongsma HJ, Electrical conductance of mouse connexin45 gap junction channels is modulated by phosphorylation, Cardiovasc. Res 46 (3) (2000) 496–510. [DOI] [PubMed] [Google Scholar]
- [79].Herzig S, Neuman J, Effects of serine/threonine protein phosphatases on ion channels in excitable membranes, Physiol. Rev 80 (2000) 173–210. [DOI] [PubMed] [Google Scholar]
- [80].Bialojan C, Takai A, Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics, Biochem. J 256 (1988) 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Ke Y, Lei M, Solaro RJ, Regulation of cardiac excitation and contraction by p21 activated kinase-1, Prog. Biophys. Mol. Biol 98 (2–3) (2008) 238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Ke Y, Lei M, Wang X, Solaro RJ, Unique catalytic activities and scaffolding of p21 activated kinase-1 in cardiovascular signaling, Front. Pharmacol 4 (2013) 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Kelly ML, Astsaturov A, Chernoff J, Role of p21-activated kinases in cardiovascular development and function, Cell. Mol. Life Sci 70 (22) (2013) 4223–4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Wang R, Wang Y, Lin WK, Zhang Y, Liu W, Huang K, et al. , Inhibition of angiotensin II-induced cardiac hypertrophy and associated ventricular arrhythmias by a p21 activated kinase 1 bioactive peptide, PLoS One 9 (7) (2014) e101974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Ke Y, Wang L, Pyle WG, de Tombe PP, Solaro RJ, Intracellular localization and functional effects of P21-activated kinase-1 (Pak1) in cardiac myocytes, Circ. Res 94 (2004) 194–200. [DOI] [PubMed] [Google Scholar]
- [86].Taglieri DM, Monasky MM, Knezevic I, Sheehan KA, Lei M, Wang X, et al. , Ablation of p21-activated kinase-1 in mice promotes isoproterenol-induced cardiac hypertrophy in association with activation of Erk1/2 and inhibition of protein phosphatase 2A, J. Mol. Cell. Cardiol 51 (6) (2011) 988–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Ke Y, Wang X, Jin XY, Solaro RJ, Lei M, PAK1 is a novel cardiac protective signaling molecule, Front. Med 8 (4) (2014) 399–403. [DOI] [PubMed] [Google Scholar]
- [88].El-Armouche A, Bednorz A, Pamminger T, Ditz D, Didié M, Dobrev D, et al. , Role of calcineurin and protein phosphatase-2A in the regulation of phosphatase inhibitor-1 in cardiac myocytes, Biochem. Biophys. Res. Commun 346 (3) (2006) 700–706. [DOI] [PubMed] [Google Scholar]
- [89].Li H, Spagnol G, Naslavsky N, Caplan S, Sorgen PL, TC-PTP directly interacts with connexin43 to regulate gap junction intercellular communication, J. Cell Sci 127 (Pt15) (2014) 3269–3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Li W, Hertzberg EL, Spray DC, Regulation of connexin43-protein binding in astrocytes in response to chemical ischemia/hypoxia, J. Biol. Chem 280 (9) (2005) 7941–7948. [DOI] [PubMed] [Google Scholar]