Abstract
Purpose
Over 95% of human anal cancers are etiologically associated with high-risk HPVs, with HPV type 16 (HPV16) the genotype most commonly found. Activating mutations in the catalytic subunit of Phosphatidylinositol (3,4,5)-trisphosphate kinase (PI3K), encoded by the Pik3ca gene, are detected in approximately 20% of human anal cancers.
Experimental Design
We asked if common activating mutations in Pik3ca contribute to anal carcinogenesis using an established mouse model for anal carcinogenesis in which mice are topically treated with the chemical carcinogen 7,12-Dimethylbenz(a)anthracene (DMBA). Mice expressing in their anal epithelium one of two activating mutations in Pik3ca genes, Pik3caH1047R or Pik3caE545K, were monitored for anal carcinogenesis in the presence or absence of transgenes expressing the HPV16 E6 and E7 oncogenes.
Results
Both mutant forms of Pik3ca increased susceptibility to anal carcinogenesis in the absence of HPV16 oncogenes, and cooperated with HPV16 oncogenes to induce the highest level and earliest onset of anal cancers. The combination of HPV16 oncogenes and Pik3ca mutations led to anal cancers even in the absence of treatment with DMBA. We further observed that the investigational mTOR1/2 dual inhibitor, TAK-228, significantly reduced the size of anal cancer-derived tumor spheroids in vitro and reduced the growth rates of anal cancer-derived tumor grafts in vivo.
Conclusion
These data demonstrate that activating mutations in Pik3ca drive anal carcinogenesis together with HPV16 oncogenes, and that the PI3K/mTOR pathway is a relevant target for therapeutic intervention.
Keywords: HPV16, Pik3ca, anal cancer, mTOR pathway, sapanisertib(TAK-228)
INTRODUCTION
Anal cancer is a relatively rare cancer in humans accounting for approximately 0.5% of all new cancer cases in United States. Anal cancer incidence and deaths have been increasing over the last decade (1). The most common etiologic factor in anal cancer is high-risk human papillomavirus (HPV), implicated in other anogenital cancers and a growing fraction of head and neck cancers. HPV is detected in 95% of human anal cancers with HPV16 accounting for 89% of HPV positivity (2). Previously, we established a mouse model for human anal cancer using HPV16 E6/E7 transgenic mice treated topically with DMBA (7,12-Dimethylbenz(a)anthracene) (3). Studies using this animal model led to the discoveries that HPV16 E7 is the more potent oncogene in anal carcinogenesis (4) and that the mTOR (“mechanistic target of rapamycin” or “mammalian target of rapamycin”) signaling pathway is activated in anal squamous cell carcinoma arising in HPV16 transgenic mice as well as in human anal cancers (5). mTOR is a serine/threonine protein kinase commonly activated downstream of the phosphoinositide 3-kinase (PI3K) signaling pathway (6). mTOR forms two distinct complexes, mTORC1 and mTORC2, that regulate multiple cellular processes required for cell growth and metabolism (7). mTOR also acts as a downstream regulator of the PI3K-AKT signaling pathway; therefore mTOR signaling and its downstream pathway are also regulated by the PI3K/AKT signaling pathway (8). Alterations in PI3K/AKT/mTOR signaling caused by hyper-activation of PI3K are thought to contribute to cancers including HPV-associated cancers (9–13). Recently, mutations that lead to increased PI3K signaling were found in 36% of 12 types of human cancers (14). Of these mutations causing activation of PI3K signaling, the most prevalent mutations occurred in the Pik3ca gene that encodes p110α, the catalytic subunit of PI3K. Three hot-spot mutations in the Pik3ca gene, E542K and E545K (exon 9) in the helical domain and H1047R/L (exon 20) in the kinase domain of p110α, lead to the constitutive activation of PI3K signaling independent of the PI3K regulatory subunit p85. Such mutations in Pik3ca arise in approximately 20% of human anal cancers, 90% of which are in exon 9 (15). The importance of these mutations in anal cancer has not been determined.
Here, we asked if such activating mutations in Pik3ca contribute to anal carcinogenesis in the context of mice expressing or lacking HPV16 oncogenes, E6 and E7. We generated mice expressing a mutant form of Pik3ca, either Pik3caH1047R or Pik3caE545K, +/− the HPV16 oncogenes, E6 and E7, and treated them topically in the anus with the chemical carcinogen DMBA. We found these activating mutations in Pik3ca to drive anal carcinogenesis, and their oncogenic potential was synergistic with HPV16 E6 and E7, even in the absence of DMBA. We also found that tumor spheres and tumor grafts derived from these anal tumors were inhibited in their growth by TAK-228 (also known as sapanisertib, INK-128 and MLN0128), an investigational drug that inhibits both TORC1 and TORC2 (16). We conclude that activating mutations in Pik3ca contribute to anal carcinogenesis and that this pathway represents an important new target for therapeutic intervention.
MATERIALS AND METHODS
Mice
K14E6, K14E7, KRT14-cre/Esr1 (K14CreERtm) mice have been described previously (17,18). R26-Pik3caH1047R (heretofore referred to as Pik3caH1047R) and R26-Pik3caE545K (heretofore referred to as Pik3caE454K) mice were kindly provided by Dr. Dustin A Deming and described previously (The Jackson Laboratory; Stock Number 016977; (19)). Details on the breeding schemes used to generate the various strains of mice used in this study, an ethics statement, and methods used to induce expression of the Pik3ca mutants and treat mice with DMBA are provided in supplemental information.
Anal cancer cell isolation and spheroid culture
Anal tumors were resected, rinsed with sterile phosphate buffered saline (PBS), and placed in a chelation buffer on ice (20) for one hour. The tumor tissue was then digested with collagenase and dispase at 37°C. Cells were pelleted and the supernatant discarded. The pellet was then re-suspended in advanced DMEM/F12 (ADF, Invitrogen) and the resulting cell suspension was combined with Matrigel at a 1:1 ratio. The suspension was plated by placing 50 μl droplets into wells of a 24 well culture plate and incubated for 15 minutes at 37°C. The droplets were then covered with feeding medium consisting of ADF supplemented with murine epidermal growth factor (EGF, Invitrogen) to a final concentration of 50 ng/ml. Spheroids were passaged at least once prior to therapeutic investigations. Spheroids beyond 9 passages were not utilized. Therapeutic investigations were performed by exchanging feeding media containing the desired concentration of the agent over the spheroids suspended in Matrigel. An investigational oral dual TORC1/2 inhibitor, TAK-228 (#I-3344 LC Labs, Woburn, MA) was dissolved in dimethyl sulfoxide to make a 10mM stock. TAK-228 was then diluted 1:1000 in culture medium prior to diluting to the indicated final concentration. Spheroids were treated for 48 hours. To measure changes in cellular proliferation, spheroid diameters were measured using 4X bright-field microscopy at baseline and post-treatment. For western blot analysis to assess the efficacy of the drug on inhibiting the PI3K/AKT/mTOR signaling pathway, spheroids were recovered 24 hours post-treatment of TAK-228. Details on the staining of spheroids with H&E are provided in Supplemental Information.
Establishment of anal tumorgrafts and therapeutic treatment of tumorgrafts with TAK-228
Anal tumors from 4-OHT treated K14E6/E7/Pik3caE545K: K14CreERtm mice were rubbed with 10% providone-iodine (CareFusion) and/or isopropyl alcohol, rinsed with sterile phosphate buffered saline (PBS), and excised with sterile instruments. Tumor tissue was minced in sterile PBS and then combined with reduced growth factor Matrigel (BD Matrigel Basement Membrane Matrix, Growth Factor Reduced (GFR) Catalog no. 354230) at a 1:1 ratio. 200 μL of minced tumor mixed with Matrigel was injected subcutaneously into immunodeficient (NOD scid gamma) mice in their flanks (initial graft set as passage zero or P0). Tumors were passaged by injecting 200 μL of minced tumor pieces in PBS mixed 1:1 with Matrigel. Details on treatment of tumor grafts are provided in Supplemental Information.
Immunoblotting
Primary tissue and tumorgrafts were harvested, snap frozen, briefly sonicated in cold RIPA buffer with protease and phosphatase inhibitors, agitated 30 min at 4C, and spun for 30 min at 12,000 rpm in a microfuge. Supernatant was collected, and protein concentration determined using the Bradford assay. Spheroid cultures were collected following 24 hours culture in the presence or absence of TAK-228, snap frozen, protein extracted and immunoblotting performed as previously described (21). Details on the choice of antibodies used are provided in Supplemental Information.
Immunohistochemistry and TUNEL assay
Immunohistochemistry was performed as previously described (17,22). Details on the choice of antibodies/reagents used are provided in Supplemental Information.
Statistical analysis
A Logrank test was used to determine the significance of differences in the onset of anal tumors between groups. A two-sided Fisher’s exact test was used to determine the significance of differences in the incidence of anal cancer between groups. To determine the significance of differences in the severity of disease, DNA synthesis level, growth change in spheroids and growth rate in tumorgraft between groups, two-sided Wilcoxon rank sum test was performed. All statistical analyses were performed using MSTAT statistical software version 6.1.4. (http://www.mcardle.wisc.edu/mstat).
RESULTS
An activating mutation in Pik3ca upregulates PI3K signaling and significantly increases the incidence of anal tumors
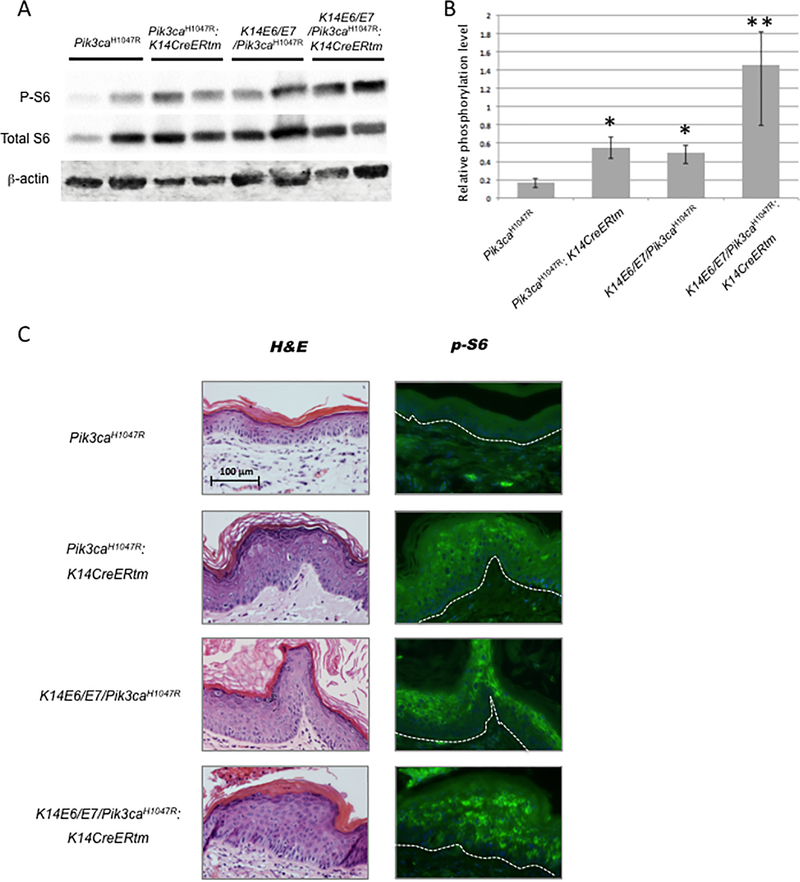
To determine whether activating mutations in Pik3ca found in human anal cancers contribute to anal carcinogenesis, we generated mice that conditionally express mutant forms of Pik3ca in anal, stratified squamous epithelium. We first used R26-Pik3caH1047R (Pik3caH1047R) mice, carrying a ROSA 26 locus knock-in allele of Pik3caH1047R positioned downstream of a floxed transcriptional STOP cassette upstream (23). To generate mice expressing this mutant form of Pik3ca in anal epithelium, K14CreERtm transgenic mice expressing the tamoxifen (or 4-hydroxy-tamoxifen: 4-OHT)-inducible CreERtm from the keratin14 (K14) promoter were bred to the Pik3caH1047R mice. To assess the efficiency of expression of the mutant form of Pik3ca, p110αH1047R, through inducible Cre-recombination in anal epithelium from Pik3caH1047R: K14CreERtm and K14E6/E7/Pik3caH1047R:K14CreERtm mice, we performed western blot analysis to monitor levels of phospho S6 Ribosomal Protein (p-S6) in lysates from the anoderm of these mice. These mice had been treated with 4-OHT 5 consecutive days to activate Cre and then anal tissues were collected at 6 weeks post-treatment. S6 Ribosomal Protein (S6) is a component of the 40s ribosomal subunit and also is known to be phosphorylated by activation of the PI3K-AKT-mTOR signaling pathway. As previously described, activating mutations of Pik3ca lead to an increase in phosphorylation of AKT and subsequent phosphorylation of S6 and 4E-BP1 (24,25). We have previously demonstrated that the HPV16 E6 and E7 oncogenes cause increased pS6 in the squamous cell carcinomas of mouse anus (5). In lysates from both Pik3caH1047R:K14CreERtm and K14E6/E7/Pik3caH1047R, phosphorylation of S6 was increased over that seen in Pik3caH1047R mice (Figure 1A/B). Not surprisingly, phosphorylation of S6 was more significantly increased in K14E6/E7/Pik3caH1047R:K14CreERtm mice compared to the other groups, Pik3caH1047R:K14CreERtm and K14E6/E7/Pik3caH1047R. A similar finding was observed when we analyzed levels of pS6 in these same tissues by immunofluorescence (Figure 1 C).
Figure 1. Induction of pS6 as a biomarker for activity of the PI3K/AKT/mTOR pathway.
Protein lysates of mouse anal tissue were analyzed for levels of ribosomal protein S6 (S6) and phospho-S6 (p-S6) by immunoblot analyses. A) Representative image of immunoblots. Top and middle, p-S6 and S6 as a biomarker for activation of PI3K-mTOR signaling. Bottom, beta-actin as a control. Blot showed the protein bands from mouse anal tissues’ lysates of following genotypes: Pik3caH1047R, Pik3caH1047R: K14CreERtm, K14E6/E7/Pik3caH1047R and K14E6/E7/Pik3caH1047R: K14CreERtm. B) Quantification of immunoblots. Intensities of p-S6 and S6 specific bands were quantified (n = 4 samples per genotype) and normalized to beta-actin expression. Single asterisk, *, indicates that phosphorylation of S6 in Pik3caH1047R: K14CreERtm as well as K14E6/E7/Pik3caH1047R significantly increased compared to Pik3caH1047R. Double asterisk, **, indicates that phosphorylation of S6 in K14E6/E7/Pik3caH1047R: K14CreERtm is significantly increased compared to either Pik3caH1047R: K14CreERtm or K14E6/E7/Pik3caH1047R. C) Immunofluorescne for pS6 in paraffin-embedded sections of anal epithelium from the same mouse strains analyzed in part A and B. Left panels show H&E stained sections. Right panels show pS6 imunofluorescence (in green).
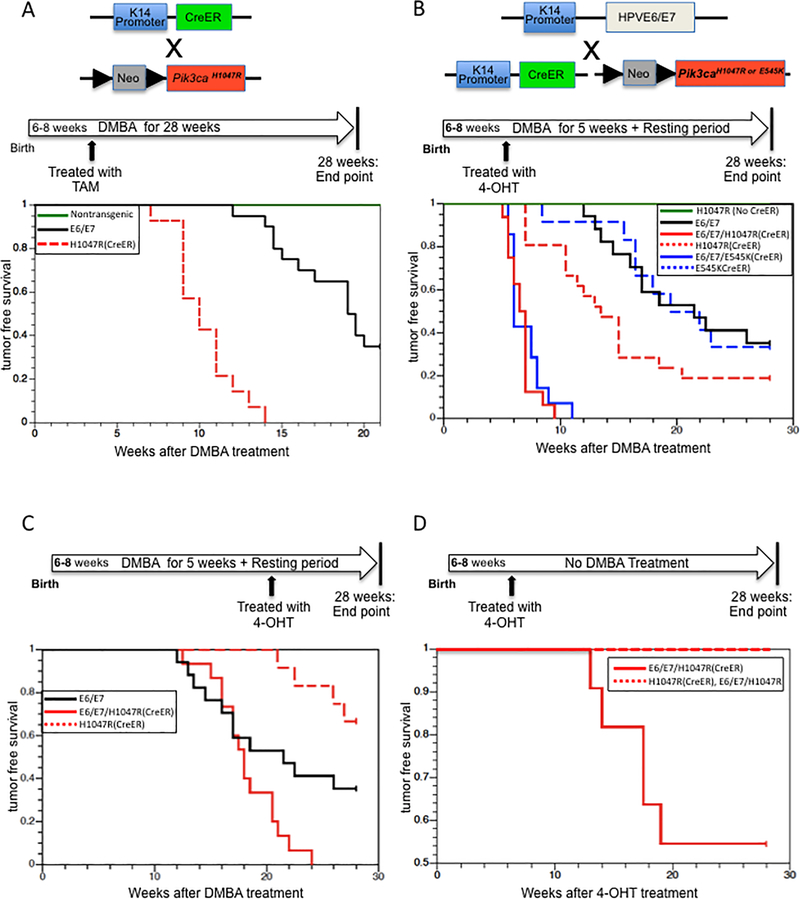
We then assessed the susceptibility of these mice to anal carcinogenesis using our previously established model, in which mice are treated with DMBA (0.12umol/week) for 20 weeks and then held an additional 8 weeks prior to sacrifice (5). Using this experimental design, we observed that the activating mutation in Pik3ca present in the Pik3caH1047R:K14CreERtm mice caused anal cancers (Figure 2A). Tamoxifen was first administered by intraperitoneal (I.P.) injection to systemically induce Cre-recombination thereby turning on expression of the mutant Pik3ca gene. Then we administered DMBA to the anal canal once a week for 20 weeks. All (n=14) of the Pik3caH1047R:K14CreERtm mice developed overt anal tumors with an average onset of 10.3 weeks after initial DMBA treatment, whereas none of the nontransgenic mice developed any overt tumors at this time point (Figure 2A). These observations indicate that the mutant form of Pik3ca, p110αH1047R, leads to increased susceptibility to anal tumors.
Figure 2. Tumor free survival of Pik3ca mutant mice in the absence and presence of HPV-16 E6/E7. A.
A. Anal tumors in Tamoxifen-treated Pik3caH1047R: K14CreERtm mice using a DMBA-induced carcinogenesis protocol. Green-colored line indicates the tumor free survival in non-transgenic mice, E6/E7 (black-colored line) indicates K14E6/E7 mice, and H1047R (red-colored dot) indicates Pik3caH1047R: K14CreERtm mice. The data of tumor onset in non-transgenic mice and K14E6/E7 mice was obtained from prior study (Marie K. Thomas et al, 2011, Virology). B. Anal tumors in 4-OHT-treated mice with the following genotypes: H1047R(No CreER)(Green-colored line) indicates Pik3caH1047R mice, E6/E7 (black-colored line) indicates K14E6/E7/Pik3caH1047R, H1047R(CreER) (red-colored dot) indicates Pik3caH1047R:K14CreERtm mice, E545K(CreER) (blue-colored dot) indicates Pik3caE545K:K14CreERtm mice, E6/E7/H1047R(CreER) (red-colored line) indicates K14E6/E7/Pik3caH1047R: K14CreERtm mice, and E6/E7/E545K(CreER) (blue-colored line) indicates K14E6/E7/Pik3caE545K:K14CreERtm mice. C. Tumor free survival in 4-OHT-treated mice of following genotypes: H1047R(CreER) (red-colored dot) indicates in Pik3caH1047R:K14CreERtm mice and E6/E7/H1047R(CreER) (red-colored line) indicates K14E6/E7/Pik3caH1047R:K14CreERtm mice. These mice first were administered DMBA for 5 weeks and then were treated with 4-OHT at the 20 weeks timepoint. As a reference, E6/E7 (black-colored line) indicates K14E6/E7/Pik3caH1047R mice. D. Tumor free survival in 4-OHT-treated mice of following genotypes: H1047R(CreER) (red-colored dotted line) indicates Pik3caH1047R: K14CreERtm mice and E6/E7/H1047R(CreER) (red-colored line) indicates K14E6/E7/Pik3caH1047R: K14CreERtm mice. None of these mice received DMBA.
Two different activating mutations in Pik3ca synergize with HPV16 E6 and E7 oncogenes to cause anal tumors
Next, we monitored the tumorigenic potential of the mutants of Pik3ca on anal carcinogenesis in context of HPV16 E6/E7 transgenic mice. Here, we used both Pik3caE545K mice as well as Pik3caH1047R mice. K14CreERtm mice were bred to either Pik3caH1047R or Pik3caE545K mice and the resulting Pik3caH1047R: K14CreERtm or Pik3caE545K:K14CreERtm mice were crossed to K14E6 and K14E7 mice. We generated 7 different groups of mice with the following genotypes: Pik3caH1047R, Pik3caE545K, Pik3caH1047R:K14CreERtm, Pik3caE545K:K14CreERtm, K14E6/E7/Pik3caH1047R, K14E6/E7/Pik3caH1047R:K14CreERtm, and K14E6/E7/Pik3caE545K:K14CreERtm.
To assess the tumorigenic contributions of expressing p110αH1047R or p110αE545K in combination with HPV16 E6/E7 oncoproteins, we modified our prior protocol in terms of timing and method of delivery of tamoxifen to induce Cre-recombination and the duration of administration with DMBA. Using our original protocol (5) with systemically induced expression of p110αH1047R, we observed not only the early onset of tumors (Figure 2A), but also morbidity issues (tumors arising in other epithelial tissues, edema) in Pik3caH1047R:K14CreERtm (data not shown). Therefore, instead of I.P. injection with tamoxifen, the anal canal was topically treated with 4-hydroxy-tamoxifen (4-OHT) to avoid those morbidities. Also, we applied DMBA for only 5 weeks, anticipating that the onset of tumorigenesis would be accelerated compared to that seen in the absence of the expression of the HPV16 oncogenes (Figure 2B). None of Pik3caH1047R or Pik3caE545K mice developed overt anal tumors reflective of the short duration of DMBA treatment (Figure 2B). In the K14E6/E7/Pik3caH1047R group, 11 out of 17 mice (64.7%) had overt tumors and these tumors arose between 11.5 and 26 weeks after initial administration with DMBA. 17 out of 21 Pik3caH1047R:K14CreERtm mice (80.8%) had overt anal tumors with onset of anal tumors between 7 and 20.5 weeks. The onset of overt tumors in Pik3caH1047R:K14CreERtm mice was significantly earlier compared with that observed in K14E6/E7/Pik3caH1047R mice (Figure 2B, logrank sum test P (one-sided)=0.0231), though the incidence of overt anal tumor was not significantly different between the two groups. In Pik3caE545K:K14CreERtm mice, 8 out of 12 mice (66.7%) developed overt anal tumors with onset of tumors between 8.5 and 23 weeks. These results further demonstrate the oncogenic potential of these activating mutations in Pik3ca contributes to anal carcinogenesis in the absence of HPV oncogenes, even when the duration of treatment with DMBA was reduced. Interestingly, both K14E6/E7/Pik3caH1047R:K14CreERtm and K14E6/E7/Pik3caE545K:K14CreERtm mice developed overt tumors significantly earlier compared with that seen in Pik3caH1047R:K14CreERtm (Figure 2B, log rank sum test P(two-sided) = 5.83 × 10−6), Pik3caE545K:K14CreERtm (P(two-sided) = 5.42×10−6) mice or K14E6/E7/Pik3caH1047R mice (P (two-sided) = 6.21 × 10−7, 5.92 × 10−7, respectively). The overt tumors in the K14E6/E7/Pik3caH1047R:K14CreERtm group arose between 5.5 and 9.5 weeks with an average age of onset of 6.7 weeks after DMBA treatment began. Similarly, K14E6/E7/Pik3caE545K:K14CreERtm mice developed overt tumors between 5.5 and 11 weeks with an average onset of 7.0 weeks after DMBA treatment began. These observations indicate that both activating mutations in Pik3ca strongly drive anal tumorigenesis in the presence of HPV16 E6/E7, and that they show synergistic effects with the viral oncogenes on anal tumorigenesis.
Late expression of activating mutations in Pik3ca accelerates anal carcinogenesis in the presence of HPV-16 oncogenes
To learn temporally how an activating mutation in Pik3ca contributes to anal carcinogenesis, we monitored the oncogenic potential of delayed expression of Pik3caH1047R in the context of HPV16 E6/E7 mice. Both Pik3caH1047R:K14CreERtm and K14E6/E7:Pik3caH1047R:K14CreERtm mice were administered DMBA to their anus for 5 weeks. 20 weeks post-administration of DMBA, 4-OHT was topically applied to the anus for 5 consecutive days. All of K14E6/E7/Pik3caH1047R:K14CreERtm mice developed anal tumors prior to 24 weeks (Figure 2C); whereas only 4 out of 12 Pik3caH1047R:K14CreERtm mice (33.4%) developed overt anal tumors. K14E6/E7/Pik3caH1047R:K14CreERtm mice developed anal tumors significantly earlier compared with K14E6/E7/Pik3caH1047R mice or Pik3caH1047R:K14CreERtm mice (Figure 2C, log rank sum test P (two-sided) = 0.045, 8.06 × 10−6, respectively). These observations indicate that an activating mutation in Pik3ca accelerates anal carcinogenesis in the presence of HPV16 oncogenes, even when onset of its expression is delayed.
An activating mutation in Pik3ca is sufficient to drive anal carcinogenesis in the presence of HPV16 oncogenes even without administration of DMBA
HPV16 E6/E7 mice do not develop anal cancers without administration of DMBA (4). We asked if an activating mutation in Pik3ca, in the context of expression of these HPV16 oncogenes, drives anal carcinogenesis without treatment with DMBA. Pik3caH1047R:K14CreERtm and K14E6/E7/Pik3caH1047R:K14CreERtm mice were treated topically with 4-OHT on the anus for 5 consecutive days, but not treated with DMBA. After 28 weeks, anal tissues were recovered and histologically evaluated to determine the extent of neoplasia. Strikingly, 5 out of 10 K14E6/E7/Pik3caH1047R:K14CreERtm mice developed overt anal tumors without DMBA treatment; whereas, none of the Pik3caH1047R:K14CreERtm mice developed tumors (Figure 2D). As seen previously with K14E6/E7 mice (4), the K14E6/E7/Pik3caH1047R mice did not develop anal tumors in the absence of DMBA treatment. The onset of overt tumors in K14E6/E7/Pik3caH1047R:K14CreERtm mice was significantly different than that observed in Pik3caH1047R:K14CreERtm (Figure 2D, log rank sum test P (two-sided)=0.0056) or in K14E6/E7/Pik3caH1047R mice. These observations indicate that expression of an activating mutant form of Pik3ca in the presence of HPV16 oncogenes is sufficient to drive anal tumorigenesis in mice.
Activating mutations in Pik3ca led to more aggressive neoplastic disease in the context of mice expressing HPV16 oncogenes
To determine whether the mutant forms of Pik3ca cause an increased severity of neoplastic disease in the anal region, we performed detailed histopathological analysis of the tissue harvested from 4-OHT and DMBA-treated mice. Tissues from the mice of each genotype were harvested, fixed, paraffin-embedded, and sectioned, and then the sections stained with H&E and subjected to detailed histopathologic analysis (Table 1). None of Pik3caH1047R mice developed a malignant disease, but did develop low-grade dysplasia. In contrast, 7 out of 17 K14E6/E7/Pik3caH1047R mice (41.1%) had malignant anal tumors. The severity of disease (in which we give a score from 1–7 for the worst grade of disease in each mouse, with normal histology given a score of 1, low grade dysplasia a score of 2, high grade dysplasia a score of 3, verrucous carcinoma a score of 4, and grades 1–3 squamous cell carcinoma scores of 5–7, respectively) in this genotype was significantly worse than in Pik3caH1047R mice. In the Pik3caH1047R:K14CreERtm group, 14 out of 21 mice (66.7%) developed anal cancer. The incidence of cancer and severity of disease were significantly greater than for the Pik3caH1047R mice, but were not significantly different from the K14E6/E7/Pik3caH1047R mice. Interestingly, most of the cancers (12 out of 14) in Pik3caH1047R:K14CreERtm mice were verrucous carcinoma, which is an uncommon variant of squamous cell carcinoma known as a very well-differentiated squamous malignancy with minimal atypia. In Pik3caE545K:K14CreERtm mice, 4 out of 9 mice (44.4%) developed anal cancer, with 2 out of these 4 mice having verrucous carcinoma. Consistent with the observation of overt tumors in Figure 2B, all K14E6/E7/Pik3caH1047R:K14CreERtm mice had anal cancers, with half developing poorly-differentiated invasive squamous cell carcinomas. Incidence of cancer and severity of disease in K14E6/E7/Pik3caH1047R:K14CreERtm mice were significantly greater than in the Pik3caH1047R:K14CreERtm and K14E6/E7/Pik3caH1047R mice. Similarly, 12 out of 13 K14E6/E7/Pik3caE545K:K14CreERtm mice developed malignant tumors. These data indicate that activating mutations in Pik3ca and expression of HPV16 E6/E7 synergize to increase the severity of anal carcinogenesis.
Table 1.
| Within Normal Limit | Dysplasia | Verrucous Carcinoma | Squamous Cell Carcinoma | Cancer Incidence (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Low | High | Grade 1 | Grade 2 | Grade 3 | ||||
| Pik3caH1047R | 13 | 0 | ||||||
| Pik3caH1047R: K14CreERtm | 7 | 12 | 1 | 1 | 66.7 | |||
| Pik3caE545K: K14CreERtm | 2 | 2 | 1 | 2 | 1 | 1 | 44.4 | |
| K14E6/E7/Pik3caH1047R | 10A | 4 | 2 | 1 | 41.1 | |||
| K14E6/E7/Pik3caH1047R: K14CreERtm | 2 | 5 | 4 | 3 | 100 | |||
| K14E6/E7/Pik3caE545K: K14CreERtm | 1B | 1 | 4 | 3 | 4 | 92.3 | ||
| K14E6/E7 (NO DMBA treatment) | 10C | 0 | ||||||
| Pik3caH1047R: K14CreERtm (NO DMBA treatment) | 14 | 0 | ||||||
| K14E6/E7/Pik3caH1047R: K14CreERtm (NO DMBA treatment) | 4D | 6 | 60 | |||||
Two animals developed a sebaceous carcinoma.
One animal developed a sebaceous carcinoma and was sacrificed earlier than the end point of study (28 weeks) due to morbidity
Data from prior study (Marie K. Stelzer et al. Cancer Prev. Res. 2010 : A mouse model for human anal cancer).
All of these animal developed carcinoma in situ.
Anal cancers arising in mice expressing activating mutations in Pik3ca in the presence of HPV16 oncogenes differed histopathologically from cancers arising in the absence of HPV16 oncogenes
We observed anal cancers from five genotypes: Pik3caH1047R:K14CreERtm, Pik3caE545K:K14CreERtm, K14E6/E7/Pik3caH1047R, K14E6/E7/Pik3caH1047R:K14CreERtm and K14E6/E7/Pik3caE545K:K14CreERtm. As shown in Table 1, the majority of anal cancers in Pik3caH1047R:K14CreERtm mice were verrucous carcinomas (12 out of 14 mice, 85.7%). Most verrucous carcinomas arose around the anus and were observed as overt, exophytic tumors. By histopathological analyses, 15 out of 16 tumors (93.8%) developed below the anorectal junction in Pik3caH1047R:K14CreERtm mice (Supplemental Figure S1). In biomarker studies, MCM7 was strongly expressed in cancer from Pik3caH1047R:K14CreERtm mice, but its expression was restricted to the basal and parabasal layer cells (Supplemental Figure S2, third panel). As expected, p-S6 was robustly detected in the cytoplasm of anal cancer cells. Similarly, half of the tumors (2 of 4) in Pik3caE545K:K14CreERtm mice were verrucous carcinoma and all of these tumors developed from below the recto-anal junction but still within the anus. In contrast, 3 out of 7 K14E6/E7/Pik3caH1047R mice (42.9 %) were scored to have squamous cell carcinoma, with 4 out of 9 tumors (44.5%) arising at the anorectal junction or above the junction, where HPV-associated anal cancer in human patients is commonly found (Supplemental Figure S1). In K14E6/E7/Pik3caH1047R mice, the expression of MCM7 was highly upregulated in suprabasal layer cells as well as basal cells (Supplemental Figure S2, third panel). Consistent with the observation in epithelium (Figure 1C), p-S6 was detected in the cytoplasm of cancer cells (Supplemental Figure S2, fourth panel). The cancer phenotypes from K14E6/E7/Pik3caH1047R:K14CreERtm and K14E6/E7/Pik3caE545K:K14CreERtm mice were more similar to that observed in the K14E6/E7/Pik3caH1047R than in the Pik3caH1047R:K14CreERtm mice. Unlike the anal cancers in Pik3caH1047R:K14CreERtm mice, 12 out of 14 K14E6/E7/Pik3caH1047R:K14CreERtm mice (85.7%) developed invasive squamous cell carcinoma, and about half of the cancers (18 out of 35, 51.4%) originated at the anorectal junction or above the junction (Supplemental Figure S1). Similarly, 11 out of 12 K14E6/E7/Pik3caE545K:K14CreERtm mice (91.7.%) developed invasive squamous cell carcinoma and 5 out of 13 tumors were found at the anorectal junction (Supplemental Figure S1).
Activation of PI3K pathway in anal epithelium induces DNA synthesis in basal cells
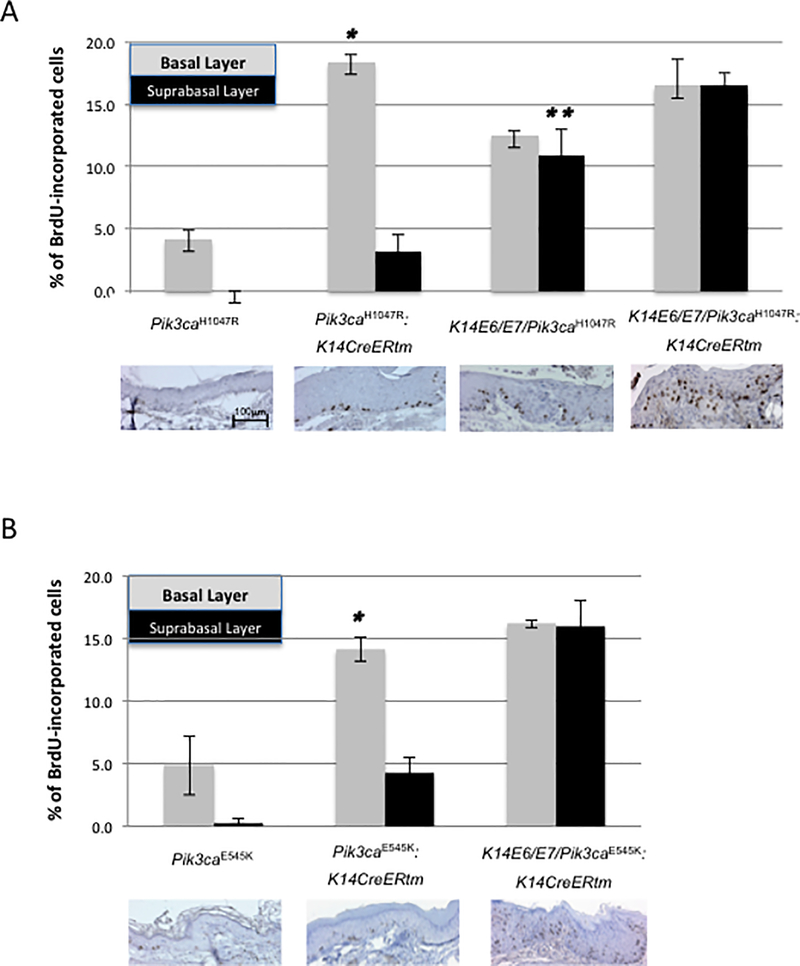
HPV16 E6/E7 induce DNA synthesis in the suprabasal compartment of anal epithelium as well as the skin (31), cervix (30,32) and lingual/esophageal epithelium (33). To determine whether activating mutations in Pik3ca induce DNA synthesis in anal epithelium, we analyzed by immunohistochemistry the frequency of BrdU-positive basal and suprabasal cells in sections of tissue from mice injected with this nucleoside analog 1 hour before sacrifice (30). Expression of p110αH1047R as well as p110αE545K significantly induced DNA synthesis in basal cells of the anal epithelium, and also to a lesser degree in suprabasal cells (Figure 3). In both 4-OHT-treated Pik3caH1047R:K14CreERtm mice and Pik3caE545K:K14CreERtm mice, the frequency of basal cells supporting DNA synthesis was significantly higher than in the control group (Pik3caH1047R:K14CreERtm vs Pik3caH1047R and Pik3caE545K:K14CreERtm mice vs Pik3caE545K, P = 0.02857 and P = 0.05714, respectively) or K14E6/E7/Pik3ca mutant mice (Pik3caH1047R:K14CreERtm or Pik3caE545K:K14CreERtm vs K14E6/E7/Pik3caH1047R, P=0.03 for both comparisons). There was no significant difference in the frequency of BrdU positivity in basal cells between the Pik3caH1047R: K14CreERtm and Pik3caE545K:K14CreERtm mice. Suprabasal DNA synthesis in Pik3caH1047R:K14CreERtm mice as well as Pik3caE545K:K14CreERtm mice also was significantly increased compared to the control group (Pik3caH1047R and Pik3caE545K, P = 0.01972 and P = 0.05714, respectively). However, this induction of suprabasal DNA synthesis was significantly lower than that observed in K14E6/E7/Pik3ca mutant mice (Pik3caH1047R:K14CreERtm vs K14E6/E7/Pik3caH1047R, Pik3caE545K:K14CreERtm vs K14E6/E7/Pik3caE545K, P=0.02857, respectively).
Figure 3. Level of proliferation in anal epithelium conferred by mutant forms of Pik3ca and HPV16 oncogenes.
Panels A and B, show the quantification of the percentage of BrdU-positive cells in the basal and suprabasal cells in the anal epithelium of DMBA-treated mice of each genotype (n=4) injected with BrdU one hour prior to sacrifice. A: Analysis of mice with Pik3caH1047R and HPV-16 E6/E7. The asterisk indicates that the extent of proliferation in the basal compartment in Pik3caH1047R:K14CreERtm mice, as measured by BrdU incorporation, is statistically significant compared with that observed in K14E6/E7/Pik3caH1047R mice (p=0.02857, two-sided Wilcoxon rank sum test). The double asterisks indicate that the frequency of cells undergoing DNA synthesis in suprabasal compartment in K14E6/E7/Pik3caH1047R mice is significantly different compared with either Pik3caH1047R: K14CreERtm or Pik3caE545K: K14CreERtm mice (p=0.02857, two-sided Wilcoxon rank sum test). B: Analysis of mice with Pik3caE545K and HPV-16 E6/E7. The asterisk indicates that the frequency of cells undergoing DNA synthesis in the basal compartment in Pik3caE545K:K14CreERtm mice is statistically significant compared to K14E6/E7/Pik3caH1047R mice (p=0.02857, two-sided Wilcoxon rank sum test).
The dual TORC1/2 inhibitor, TAK-228, causes regression of mouse anal tumor spheroids in culture
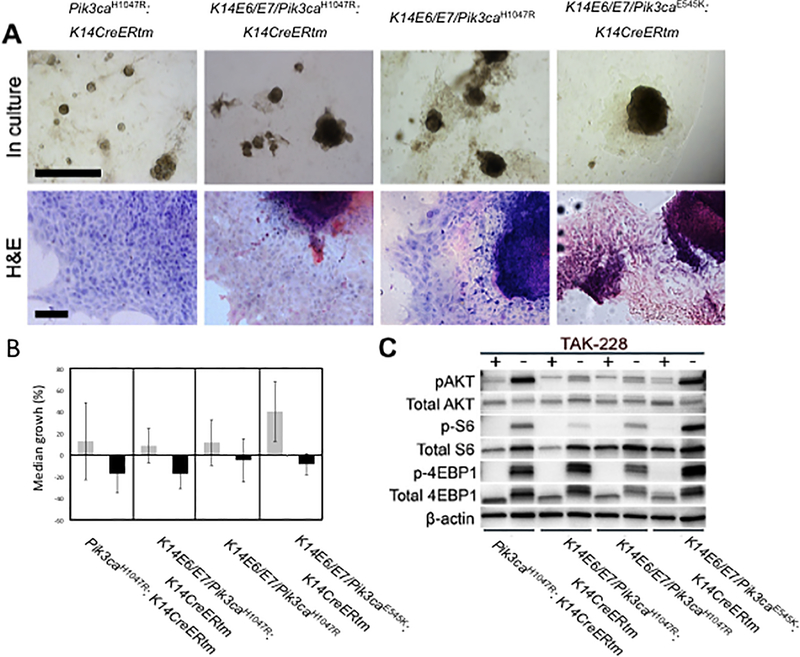
To assess the effect of inhibiting the mTOR pathway on the growth of mouse anal cancers, we tested an investigational dual TORC1/2 inhibitor, TAK-228, on spheroid cultured tumor cells. After isolating primary mouse anal tumors from K14E6/E7, K14E6/E7/Pik3caH1047R:K14CreERtm, and K14E6/E7/Pik3caE545K:K14CreERtm mice, tumor cells were combined with Matrigel and plated in 24 well plates, allowing us to culture these tumors as spheroids (Figure. 4A). Prior to treatment with TAK-228, we measured the diameter of each spheroid in the experimental and control groups. Spheroids in the experimental group were cultured in medium containing 10 μM of TAK-228. After 48 hours, the diameter of spheroids from both groups were again measured (Figure. 4B). All tumor spheroids treated with TAK-228 showed a significant reduction in growth compared to the control group not treated with the drug. Interestingly, spheroids from the K14E6/E7 mice treated with TAK-228 also showed significantly reduced cell growth, consistent with our prior observations (5) and those shown above (Figure 1, Supplemental Figure S2) that mTOR pathway is activated in anal tumors arising in K14E6/E7 mice. However, TAK228 caused a greater reduction in the growth of cells expressing a mutant form of Pik3ca. To verify inhibition of the mTOR pathway in TAK-228 treated spheroids, we performed western blot analysis on protein lysates from spheroids using three biomarkers of the PI3K-AKT-mTOR signaling pathway (Figure 4C). In contrast to the control group, phosphorylation of AKT, S6 and 4-EBP1 was diminished in all drug-treated groups, consistent with TAK-228 causing the inhibition of growth in tumor spheroids through the inhibition of the PI3K-AKT-mTOR signaling pathway. These data confirmed that TAK-228 efficiently restricts the activation of both the TORC1 (phosphorylation of both S6 and 4-EBP1) and the TORC2 (phosphorylation of AKT) pathways.
Figure 4. Dual TORC1/2 inhibitor, TAK-228, inhibits the growth of anal tumor spheroids.
A. Representative images of anal tumor spheroids in culture (top row of images) with the following genotypes: Pik3caH1047R: K14CreERtm, K14E6/E7Pik3caH1047R: K14CreERtm, K14E6/E7Pik3caH1047R, K14E6/E7Pik3caE545K: K14CreERtm, respectively. Black-colored size bar indicates 1 mm. H&E stained tumor spheroids (bottom row of images). Black-colored size bar indicates 100 μm. B. Median growth of anal cancer spheroids on TAK-228 treatment. Gray-colored box indicates vehicle-only treatment on spheroids as control arm, while black-colored box indicates tumor spheroids treated with TAK-228. After treatment of TAK-228, the growth of tumor spheroids was clearly inhibited. The triple asterisks indicate that growth of tumor spheroids were significantly decreased compared to control group, in Pik3caH1047R: K14CreERtm (p (two-sided) = 8.32×10−7), K14E6/E7Pik3caH1047R: K14CreERtm (p (two-sided)=1.43×10−19), K14E6/E7Pik3caH1047R (p (two-sided)=9.81×10−6), K14E6/E7Pik3caE545K: K14CreERtm (p (two-sided)=1.00×10−26). C. Western blot analysis of lysates from tumor spheroids probed with biomarkers of the mTOR signaling pathway. When compared with the control group, the mTOR pathway was inhibited in all TAK-228 treated groups.
TAK-228 treatment led to reduced growth of tumorgrafts from K14E6/E7/ Pik3caE545K: K14CreERtm mice
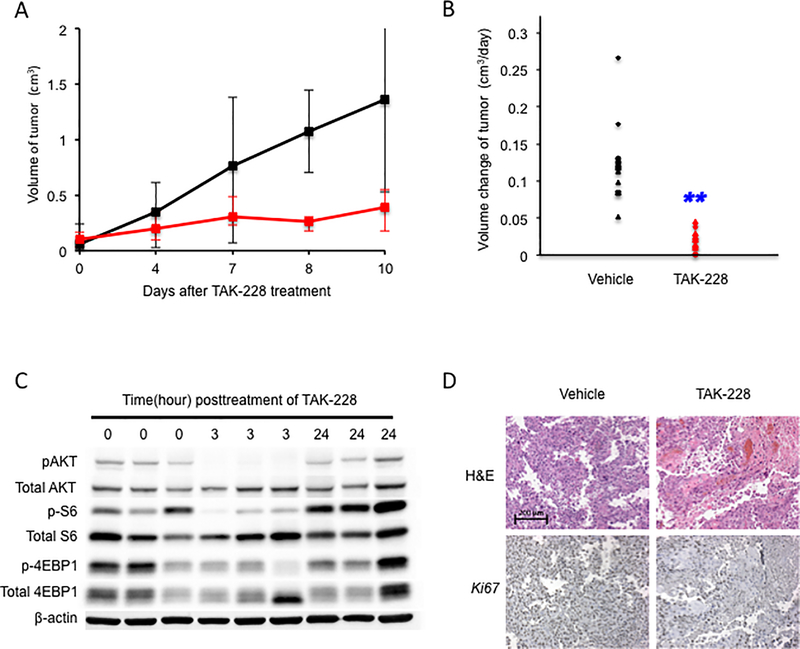
To examine the effect of TAK-228 treatment in vivo, we generated tumor grafts in immunodeficient mice (NSG mice). Here we were interested in testing the efficacy of TAK-228 on anal squamous cell carcinoma, as opposed to benign tumors. For this reason, tumor grafts from anal tumors arising in K14E6/E7/Pik3caE545K:K14CreERtm mice were first established in NSG mice and verified by histology to be squamous cell carcinomas. Tumor grafts (passage 3) that were verified to be squamous cell carcinoma were then injected subcutaneously into the flanks of 12 NSG mice. In the treatment arm, 6 randomized mice received 1mg/kg of TAK-228 dissolved in vehicle, while the other 6 mice in the control arm were provided vehicle only, in both cases delivered by oral gavage daily for 8–9 days (Figure 5A). The growth rate of tumors in treatment group was significantly decreased compared to that seen in the control group (P (two-sided) = 1.08 × 10−5, Figure 5B). This observation is similar to the result obtained with the spheroids, indicating that TAK-228 was effective in controlling the growth of tumors arising in mice expressing an activating mutation in Pik3ca and the HPV16 oncogenes. To assess inhibition of the PI3K/AKT/mTOR pathway in TAK-228 treated tumorgrafts, we performed western blot analysis on protein lysates from tumorgrafts harvested at 0 hour, 3 hours and 24 hours post treatment with TAK-228 (Figure 5C). TAK-228 efficiently inhibited the phosphorylation of AKT, S6 and 4-EBP1 at 3 hours post-treatment but not at 24 hours post-treatment, consistent with the known pharmacokinetics of the drug (16).
Figure 5. TAK-228 significantly reduced the growth rate of anal tumors in vivo.
A. Graphic depiction of the change in tumor volume in NSG mice treated with TAK-228. Black-colored line indicates the control arm, while the red-colored line indicates treatment arm (TAK-228). B. Daily change of tumors’ volume in NSG mice. The double blue-colored asterisks indicate a significant reduction in tumorgraft growth rate in TAK-228 treated group compared to the control arm. C. Western blot analysis performed on lysates from tumorgrafts harvested at 0 hour, 3 hours and 24 hours post treatment with TAK-228. D. Representative sections of tumorgraft from both control arm and treatment arm were stained with Hematoxylin and Eosin (H&E, top panel) and Ki-67 immunohistochemistry (bottom panel). Brown stain indicated, positive DAB staining, while blue is the hematoxylin counterstain. Treatment with TAK-228 results in inhibition of tumor cell proliferation based on Ki-67 immunohistochemistry (bottom right panel).
TAK-228 inhibits cell proliferation and induces apoptosis (34,35). To determine whether these phenotypes are affected by treatment with TAK-228 in our hands, we analyzed sections of paraffin-embedded tissues from animals in both the control and treatment arms. To determine whether proliferation is changed after TAK-228 treatment, we carried out Ki67 immunohistochemistry (Figure 5D). In TAK-228 treated tumor grafts, the frequency of Ki67-positive cells was decreased compared to vehicle-treated tumor grafts. Using the TUNEL assay, we observed a large number of cells with fragmented DNA indicative of apoptosis in the TAK-228-treated tumor grafts, whereas such cells were rarely detected in the vehicle-treated tumor grafts (Supplemental Figure S3). We also performed immunofluorescence for K10, a marker of the spinous layer, and K14, a marker of basal cells, to examine any changes in the epithelial differentiation/maturation status following treatment with TAK-228. (Supplemental Figure S4). Compared to vehicle-treated tissue, the expression of K10 was slightly increased in TAK-228-treated tumor grafts, while expression of K14 was decreased. These observations indicate that TAK-228 might also induce differentiation/maturation of anal tumor cells.
DISCUSSION
In this study, we demonstrate the oncogenic potential of Pik3ca mutations in driving anal carcinogenesis in the presence or absence of HPV16 oncogenes, and show that an investigational dual TORC1/2 inhibitor now in clinical trials is effective at inhibiting the growth of tumor spheroids in vitro and tumor grafts in vivo. These data establish the importance of the PIK/AKT/mTOR pathway in anal carcinogenesis, and the potential therapeutic value of targeting this pathway in treating human anal cancers.
How activating mutations in Pik3ca arise in HPV-associated cancers
Activating mutations in Pik3ca arise in 20~25% of human anal cancers (15,36), and 40~55% of HPV-positive human head and neck cancers (11). The most frequent mutations are found in exon 9 (E542K, E545K) and are consistent with the hypothesis that HPV16 drives APOBEC (“apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like”)-mediated mutagenesis by cytosine deamination. Cytidine deaminase activity of APOBEC family members is argued to cause oncogenic mutations in many types of cancers (37–40). APOBEC-mediated cytosine deamination of the PIK3CA helical domain (Exon 9) has been linked to HPV-associated carcinogenesis (41). HPV oncogenes E6 and E7 can induce the expression of APOBEC3 family members (42,43), which can explain how HPV oncogenes drive APOBEC-mediated DNA mutagenesis. A mutational signature of the APOBEC family is frequently detected in human anal cancers (36). Thus HPV may drive the accumulation of certain activating mutations in Pik3ca through activation of APOBECs. The Pik3caH1047R mutation is more oncogenic than the Pik3caE545K mutation in anal carcinogenesis in the absence of HPV oncogenes (Figure 2B and Table 1), as seen previously in a mouse mammary cancer model (19). However, in the presence of HPV16 oncogenes, this difference in potency was lost (Figure 2B and Table 1), suggesting that HPV16 oncogenes potentiate the oncogenic activities of these two Pik3ca activating mutations similarly. This together with the fact that HPV drives APOBEC activity may explain why H1047R mutations are less common in HPV-positive human cancers.
HPV16 oncogenes activate the PI3K-AKT-mTOR signaling pathway, yet activating mutations in Pik3ca accelerate anal carcinogenesis
HPV16 E6 oncogene activates the PI3K-AKT-mTOR signaling pathway in tissue culture (44), consistent with our prior (5) and current (Figure 1) data that this pathway is activated in the anal epithelium of HPV16 transgenic mice in the absence of expression of activating mutations in Pik3ca. Nevertheless, activating mutations in HPV-associated human cancers are frequently observed, which led us to the current study assessing the importance of such mutations in anal carcinogenesis. Our data clearly indicate that such mutations help drive anal carcinogenesis even in the presence of HPV16 oncogenes. The PI3K-AKT-mTOR signaling pathway was clearly enhanced when both an activating mutation in Pik3ca and HPV16 oncogenes were expressed (Figure 1) providing insight into why such activating mutations in Pik3ca contribute to HPV-associated anal cancers.
How activating mutations in Pik3ca contribute to anal carcinogenesis
Activating mutations in Pik3ca play an important role in Ras-driven carcinogenesis in mice. Disruption of the interaction of p110α with oncogenic Ras causes a defect in AKT activation and reduced transformation efficiency by H-Ras (45) In this study, we observed that early onset expression of an activating mutant form of p110α together with HPV16 oncogenes drove anal carcinogenesis even in the absence of DMBA, a carcinogen known to drive initiation of carcinogenesis (Fig. 1). This leads us to hypothesize that activating mutations in Pik3ca contribute to the initiation phase of carcinogenesis, with HPV16 oncogenes contributing to the promotion phase, as we have clearly demonstrated previously (46). Consistent with this concept, activating mutations in Pik3ca have been shown to promote tumor initiation in breast and lung carcinogenesis (47,48) and drive rapid onset of colon cancers (24). Even when an activating mutation in Pik3ca is expressed late in the context of expression of HPV oncogenes, it led to rapid onset of anal carcinogenesis (Figure 2C), which is further evidence that such mutations are strong drivers of anal carcinogenesis regardless of when they arise.
Dual TORC1/2 inhibitor, TAK-228, has an inhibitory effect on cell growth in anal tumor spheroids and tumor grafts by restricting mTOR pathway activation induced by Pik3ca mutations and HPV16 oncogenes
Currently, concurrent chemotherapy and radiation (CRT) is the standard therapy for patients with anal cancers and is associated with a high degree of morbidity (49). Treatment of recurrent anal cancer, which is common, remains a challenge. In this study, the dual TORC1/2 inhibitor, TAK-228, proved highly effective in treating mouse anal tumor grafts as well as tumor spheroids suggesting that this or similar drugs might be effective new options, alone or in combination with other therapies, in the treatment of primary or recurrent anal cancers in humans.
Supplementary Material
TRANSLATIONAL SIGNIFICANCE.
Human anal cancers are etiologically associated with human papillomaviruses (HPVs) however a large fraction contains mutations that activate the PI3K/AKT/mTOR signaling pathway. This same pathway is activated by HPV oncogenes. Herein we investigate the role in anal cancer of common, activating mutations in Pik3ca, which encodes the catalytic subunit of PI3K, using a mouse model for anal carcinogenesis. We find that these mutations make mice susceptible to anal cancers by themselves, and synergize with HPV16 oncogenes, E6 and E7, to drive rapid onset and highest severity of anal neoplastic disease. We further demonstrate that a drug that inhibits the mTOR pathway downstream of PI3K and that is in clinical trials is highly effective at inhibiting the growth of anal tumors both in vitro and in vivo. These findings demonstrate that the PI3K/AKT/mTOR pathway is a relevant target for therapeutic intervention in treating patients with anal cancer.
ACKNOWLEDGEMENTS
This study was supported by grants from the National Cancer Institute to P.F.L. (CA022443, CA210807, CA171873) and D.A.D. (CA226526). Research in the lab of M.B-A. is supported by the European Research Council, the Swiss National Science Foundation, the Krebsliga Beider Basel, the Swiss Cancer League, the Department of Surgery of the University Hospital of Basel and the Swiss Initiative for Systems Biology (SystemsX.ch). We thank Tobias Eichlisberger (FMI) for technical advice and shipping of the animals.
Footnotes
The authors have no conflicts of interest to report.
REFERENCES
- 1.American Cancer Society. Cancer Facts & Figures 2017. 2017.
- 2.Baricevic I, He X, Chakrabarty B, Oliver AW, Bailey C, Summers J, et al. High-sensitivity human papilloma virus genotyping reveals near universal positivity in anal squamous cell carcinoma: different implications for vaccine prevention and prognosis. Eur J Cancer 2015;51(6):776–85 doi 10.1016/j.ejca.2015.01.058. [DOI] [PubMed] [Google Scholar]
- 3.Stelzer MK, Pitot HC, Liem A, Schweizer J, Mahoney C, Lambert PF. A mouse model for human anal cancer. Cancer Prev Res (Phila) 2010;3(12):1534–41 doi 10.1158/1940-6207.CAPR-10-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas MK, Pitot HC, Liem A, Lambert PF. Dominant role of HPV16 E7 in anal carcinogenesis. Virology 2011;421(2):114–8 doi 10.1016/j.virol.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stelzer MK, Pitot HC, Liem A, Lee D, Kennedy GD, Lambert PF. Rapamycin inhibits anal carcinogenesis in two preclinical animal models. Cancer Prev Res (Phila) 2010;3(12):1542–51 doi 10.1158/1940-6207.CAPR-10-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abraham RT. PI 3-kinase related kinases: ‘big’ players in stress-induced signaling pathways. DNA Repair (Amst) 2004;3(8–9):883–7 doi 10.1016/j.dnarep.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 2011;12(1):21–35 doi 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature 2006;441(7092):424–30 doi 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 9.Koncar RF, Feldman R, Bahassi EM, Hashemi Sadraei N. Comparative molecular profiling of HPV-induced squamous cell carcinomas. Cancer Med 2017;6(7):1673–85 doi 10.1002/cam4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer 2009;9(8):550–62 doi 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 11.Cancer Genome Atlas N Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015;517(7536):576–82 doi 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma YY, Wei SJ, Lin YC, Lung JC, Chang TC, Whang-Peng J, et al. PIK3CA as an oncogene in cervical cancer. Oncogene 2000;19(23):2739–44 doi 10.1038/sj.onc.1203597. [DOI] [PubMed] [Google Scholar]
- 13.Guertin DA, Sabatini DM. An expanding role for mTOR in cancer. Trends Mol Med 2005;11(8):353–61 doi 10.1016/j.molmed.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, et al. Mutational landscape and significance across 12 major cancer types. Nature 2013;502(7471):333–9 doi 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cacheux W, Rouleau E, Briaux A, Tsantoulis P, Mariani P, Richard-Molard M, et al. Mutational analysis of anal cancers demonstrates frequent PIK3CA mutations associated with poor outcome after salvage abdominoperineal resection. Br J Cancer 2016;114(12):1387–94 doi 10.1038/bjc.2016.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burris HA 3rd, Kurkjian CD, Hart L, Pant S, Murphy PB, Jones SF, et al. TAK-228 (formerly MLN0128), an investigational dual TORC1/2 inhibitor plus paclitaxel, with/without trastuzumab, in patients with advanced solid malignancies. Cancer Chemother Pharmacol 2017;80(2):261–73 doi 10.1007/s00280-017-3343-4. [DOI] [PubMed] [Google Scholar]
- 17.Shin MK, Pitot HC, Lambert PF. Pocket proteins suppress head and neck cancer. Cancer Res 2012;72(5):1280–9 doi 10.1158/0008-5472.CAN-11-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riley RR, Duensing S, Brake T, Munger K, Lambert PF, Arbeit JM. Dissection of human papillomavirus E6 and E7 function in transgenic mouse models of cervical carcinogenesis. Cancer Res 2003;63(16):4862–71. [PubMed] [Google Scholar]
- 19.Meyer DS, Koren S, Leroy C, Brinkhaus H, Muller U, Klebba I, et al. Expression of PIK3CA mutant E545K in the mammary gland induces heterogeneous tumors but is less potent than mutant H1047R. Oncogenesis 2013;2:e74 doi 10.1038/oncsis.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xue X, Shah YM. In vitro organoid culture of primary mouse colon tumors. J Vis Exp 2013(75):e50210 doi 10.3791/50210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foley TM, Payne SN, Pasch CA, Yueh AE, Van De Hey DR, Korkos DP, et al. Dual PI3K/mTOR Inhibition in Colorectal Cancers with APC and PIK3CA Mutations. Mol Cancer Res 2017;15(3):317–27 doi 10.1158/1541-7786.MCR-16-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin MK, Balsitis S, Brake T, Lambert PF. Human papillomavirus E7 oncoprotein overrides the tumor suppressor activity of p21Cip1 in cervical carcinogenesis. Cancer Res 2009;69(14):5656–63 doi 10.1158/0008-5472.CAN-08-3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams JR, Xu K, Liu JC, Agamez NM, Loch AJ, Wong RG, et al. Cooperation between Pik3ca and p53 mutations in mouse mammary tumor formation. Cancer Res 2011;71(7):2706–17 doi 10.1158/0008-5472.CAN-10-0738. [DOI] [PubMed] [Google Scholar]
- 24.Leystra AA, Deming DA, Zahm CD, Farhoud M, Olson TJ, Hadac JN, et al. Mice expressing activated PI3K rapidly develop advanced colon cancer. Cancer Res 2012;72(12):2931–6 doi 10.1158/0008-5472.CAN-11-4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yueh AE, Payne SN, Leystra AA, Van De Hey DR, Foley TM, Pasch CA, et al. Colon Cancer Tumorigenesis Initiated by the H1047R Mutant PI3K. PLoS One 2016;11(2):e0148730 doi 10.1371/journal.pone.0148730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blow JJ, Hodgson B. Replication licensing--defining the proliferative state? Trends Cell Biol 2002;12(2):72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishida S, Huang E, Zuzan H, Spang R, Leone G, West M, et al. Role for E2F in control of both DNA replication and mitotic functions as revealed from DNA microarray analysis. Mol Cell Biol 2001;21(14):4684–99 doi 10.1128/MCB.21.14.4684-4699.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Attwooll C, Lazzerini Denchi E, Helin K. The E2F family: specific functions and overlapping interests. EMBO J 2004;23(24):4709–16 doi 10.1038/sj.emboj.7600481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brake T, Connor JP, Petereit DG, Lambert PF. Comparative analysis of cervical cancer in women and in a human papillomavirus-transgenic mouse model: identification of minichromosome maintenance protein 7 as an informative biomarker for human cervical cancer. Cancer Res 2003;63(23):8173–80. [PubMed] [Google Scholar]
- 30.Balsitis S, Dick F, Dyson N, Lambert PF. Critical roles for non-pRb targets of human papillomavirus type 16 E7 in cervical carcinogenesis. Cancer Res 2006;66(19):9393–400 doi 10.1158/0008-5472.CAN-06-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gulliver GA, Herber RL, Liem A, Lambert PF. Both conserved region 1 (CR1) and CR2 of the human papillomavirus type 16 E7 oncogene are required for induction of epidermal hyperplasia and tumor formation in transgenic mice. J Virol 1997;71(8):5905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shai A, Brake T, Somoza C, Lambert PF. The human papillomavirus E6 oncogene dysregulates the cell cycle and contributes to cervical carcinogenesis through two independent activities. Cancer Res 2007;67(4):1626–35 doi 10.1158/0008-5472.CAN-06-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strati K, Pitot HC, Lambert PF. Identification of biomarkers that distinguish human papillomavirus (HPV)-positive versus HPV-negative head and neck cancers in a mouse model. Proc Natl Acad Sci U S A 2006;103(38):14152–7 doi 10.1073/pnas.0606698103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gokmen-Polar Y, Liu Y, Toroni RA, Sanders KL, Mehta R, Badve S, et al. Investigational drug MLN0128, a novel TORC1/2 inhibitor, demonstrates potent oral antitumor activity in human breast cancer xenograft models. Breast Cancer Res Treat 2012;136(3):673–82 doi 10.1007/s10549-012-2298-8. [DOI] [PubMed] [Google Scholar]
- 35.Slotkin EK, Patwardhan PP, Vasudeva SD, de Stanchina E, Tap WD, Schwartz GK. MLN0128, an ATP-competitive mTOR kinase inhibitor with potent in vitro and in vivo antitumor activity, as potential therapy for bone and soft-tissue sarcoma. Mol Cancer Ther 2015;14(2):395–406 doi 10.1158/1535-7163.MCT-14-0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cacheux W, Dangles-Marie V, Rouleau E, Lazartigues J, Girard E, Briaux A, et al. Exome sequencing reveals aberrant signalling pathways as hallmark of treatment-naive anal squamous cell carcinoma. Oncotarget 2018;9(1):464–76 doi 10.18632/oncotarget.23066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kosumi K, Baba Y, Ishimoto T, Harada K, Nakamura K, Ohuchi M, et al. APOBEC3B is an enzymatic source of molecular alterations in esophageal squamous cell carcinoma. Med Oncol 2016;33(3):26 doi 10.1007/s12032-016-0739-7. [DOI] [PubMed] [Google Scholar]
- 38.Burns MB, Temiz NA, Harris RS. Evidence for APOBEC3B mutagenesis in multiple human cancers. Nat Genet 2013;45(9):977–83 doi 10.1038/ng.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts SA, Lawrence MS, Klimczak LJ, Grimm SA, Fargo D, Stojanov P, et al. An APOBEC cytidine deaminase mutagenesis pattern is widespread in human cancers. Nat Genet 2013;45(9):970–6 doi 10.1038/ng.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature 2013;500(7463):415–21 doi 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henderson S, Chakravarthy A, Su X, Boshoff C, Fenton TR. APOBEC-mediated cytosine deamination links PIK3CA helical domain mutations to human papillomavirus-driven tumor development. Cell Rep 2014;7(6):1833–41 doi 10.1016/j.celrep.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 42.Vieira VC, Leonard B, White EA, Starrett GJ, Temiz NA, Lorenz LD, et al. Human papillomavirus E6 triggers upregulation of the antiviral and cancer genomic DNA deaminase APOBEC3B. MBio 2014;5(6) doi 10.1128/mBio.02234-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Warren CJ, Xu T, Guo K, Griffin LM, Westrich JA, Lee D, et al. APOBEC3A functions as a restriction factor of human papillomavirus. J Virol 2015;89(1):688–702 doi 10.1128/JVI.02383-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spangle JM, Munger K. The human papillomavirus type 16 E6 oncoprotein activates mTORC1 signaling and increases protein synthesis. J Virol 2010;84(18):9398–407 doi 10.1128/JVI.00974-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gupta S, Ramjaun AR, Haiko P, Wang Y, Warne PH, Nicke B, et al. Binding of ras to phosphoinositide 3-kinase p110alpha is required for ras-driven tumorigenesis in mice. Cell 2007;129(5):957–68 doi 10.1016/j.cell.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 46.Song S, Liem A, Miller JA, Lambert PF. Human papillomavirus types 16 E6 and E7 contribute differently to carcinogenesis. Virology 2000;267(2):141–50 doi 10.1006/viro.1999.0106. [DOI] [PubMed] [Google Scholar]
- 47.Green S, Trejo CL, McMahon M. PIK3CA(H1047R) Accelerates and Enhances KRAS(G12D)-Driven Lung Tumorigenesis. Cancer Res 2015;75(24):5378–91 doi 10.1158/0008-5472.CAN-15-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheen MR, Marotti JD, Allegrezza MJ, Rutkowski M, Conejo-Garcia JR, Fiering S. Constitutively activated PI3K accelerates tumor initiation and modifies histopathology of breast cancer. Oncogenesis 2016;5(10):e267 doi 10.1038/oncsis.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mouw KW, Cleary JM, Reardon B, Pike J, Braunstein LZ, Kim J, et al. Genomic Evolution after Chemoradiotherapy in Anal Squamous Cell Carcinoma. Clin Cancer Res 2017;23(12):3214–22 doi 10.1158/1078-0432.CCR-16-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.