Abstract
Study Objectives
Aggression, substance misuse, and other health risk behaviors are common among combat veterans. We examined whether sleep quality and quantity predict the association between combat exposure, post-traumatic stress symptoms, and adverse health-related behaviors.
Methods
Soldiers (N = 2420) from a brigade combat team completed surveys assessing combat experiences, and psychological and behavioral health factors, approximately 3 months following deployment to Afghanistan in 2011.
Results
Respondents were 93.5% male; 73% were age 18–29 years old. The response rate was 80% (3076/3832); 94% (2876/3076) of the soldiers who attended the recruitment briefings consented to participate in this research. Complete data were available across the variables used in this study for up to 2420 soldiers. Sleep continuity disturbance accounted for the association of combat exposure with post-traumatic stress symptoms and aggression, alcohol use, and risky behavior. Moreover, for soldiers who reported sleep duration of <6 hr per day, the indirect association of combat exposure and post-traumatic stress on aggression, alcohol use, risky behavior, and opioid use was strongest.
Conclusions
This study is the first to model sleep problems as a predictor of the association between combat exposure and post-traumatic stress symptoms and frequently reported health-related behavior problems. Sleep disturbance is highly prevalent among Warfighters. While not fully preventable in operational contexts, these problems can be effectively mitigated postdeployment with appropriate policy and intervention resources. Improving the sleep characteristics of combat-exposed soldiers following deployment should reduce subsequent post-traumatic stress and related health compromising behavior, thereby enhancing force readiness.
Keywords: sleep disturbance, sleep continuity, poor sleep, combat, health behavior, opioid use, alcohol use, aggression, risk taking
Statement of Significance
Health-related behavioral problems such as aggression, alcohol use, opioid use, and high risk behaviors are frequently reported by war veterans. Sleep difficulties are also prevalent among veterans. The potential upstream role of sleep characteristics in the pathway from combat exposure to mental and behavioral health problems has not been explicitly examined. We observed that sleep problems were significantly associated with the pathway from combat exposure to post-traumatic stress symptoms and adverse health-related behaviors. Future studies using prospective panel designs will be necessary to delineate the temporal and causal characteristics of poor sleep in the link between combat, stress symptoms, and adverse health behaviors. Sleep health should be a cornerstone policy for force health and readiness.
Introduction
Post-traumatic stress and associated health-related behaviors such as aggression, alcohol misuse, drug use, and risk taking are problems reported in elevated numbers by war veterans [1–13]. Although the pathway by which combat exposure is related to health-related behaviors is likely multifaceted, post-traumatic stress appears to account for a significant proportion of the variance [2, 3, 8, 9, 14–18]. Post-traumatic stress disorder (PTSD) is prevalent in up to 23% of Operation Enduring Freedom and Operation Iraqi Freedom Veterans [19], making it a high prevalence psychiatric condition in this population. Even subclinical levels of post-traumatic stress are associated with myriad detrimental health-related behaviors in war veterans [15, 20–22]. Therefore, identifying potential antecedent or “upstream” variables that influence the link between combat exposure, post-traumatic stress, and health-related behaviors could provide healthcare providers, leadership, and policy makers with empirically determined targets for reducing combat-related stress and negative health-related behavior among combat veterans. We contend that sleep disturbance represents an important upstream mechanism of this pathway given that disturbances are prevalent, durable, and are associated with both post-traumatic stress symptoms and health-related behaviors.
Poor sleep appears to be prevalent and chronic among a large proportion of war veterans. In fact, 72% of soldiers in one sample (n = 3152) surveyed postdeployment from Iraq reported sleeping fewer than 6 hr per night [23]. Furthermore, soldiers with direct combat experience are 74% more likely to have trouble sleeping than those without exposure to combat; differences remain evident 12 months or more postdeployment [24].
A robust literature over the past decade suggests that sleep disturbance is often a causal factor in PTSD rather than an epiphenomenon [25]. First, those with PTSD report a higher rate of sleep disturbance than trauma-exposed controls in a wide array of populations [25] including veteran populations [26–28]. Second, treatments for sleep disturbance frequently reduce PTSD symptoms [29–33]; however, sleep disturbance often remains following treatment for PTSD [25]. Third, longitudinal research demonstrates that sleep disturbance following trauma temporally precedes PTSD in a wide array of samples including intensive care unit patients, natural disaster survivors, and veterans [25]. In particular, poor sleep has been shown to mediate the association between combat exposure and PTSD. A longitudinal study reported that postdeployment sleep problems temporally preceded and predicted post-traumatic stress severity in Iraq war veterans [34]. In particular, soldiers who reported short sleep duration (≤6 hr) were more likely to have PTSD and other health-related behavior problems [23, 34, 35]. In a related study, National Guard soldiers were surveyed both before and at three time points following a deployment to Iraq [36]. Predeployment daytime and nighttime sleep complaints significantly predicted PTSD symptoms above baseline levels up to 2 years following deployment. This pattern is typical for combat veterans. Indeed, data from 15 000 service members whose first deployment occurred between two waves of the Millennium Cohort Study survey suggest that predeployment insomnia significantly predicted increases in postdeployment PTSD [37].
Although the literature implicating sleep as an upstream variable to PTSD is robust, relatively little research has investigated how upstream sleep problems affect the direct link between the amount of combat exposure and post-traumatic stress symptoms, as well as the subsequent association between these symptoms and adverse health-related behaviors. A nascent literature has recently emerged to investigate how poor sleep may mediate and/or moderate trauma exposure, PTSD, and other health consequences. However, this literature is limited to studies that identify additional influential factors of this pathway for only narrow sets of health-related behaviors [9, 22, 38–41]. For example, some research suggests that sleep disturbance may moderate the effect of PTSD on aggression [25, 42–44] and depression in veterans [45]. But research beyond these specific outcomes is lacking. This begs an important question: is poor sleep associated with the relationship between PTSD and health behaviors in only a few domains, or is sleep robustly associated with this relationship across health outcomes broadly? To the best of our knowledge, no published study has examined the role of sleep disturbance as an upstream predictor in a path-based model of combat exposure, post-traumatic stress, and a robust set of health-related behaviors.
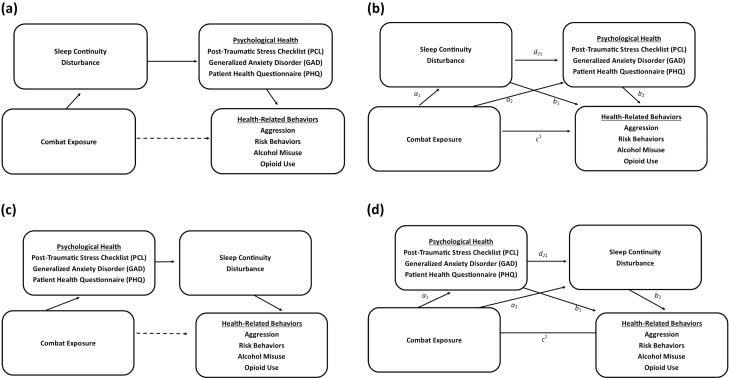
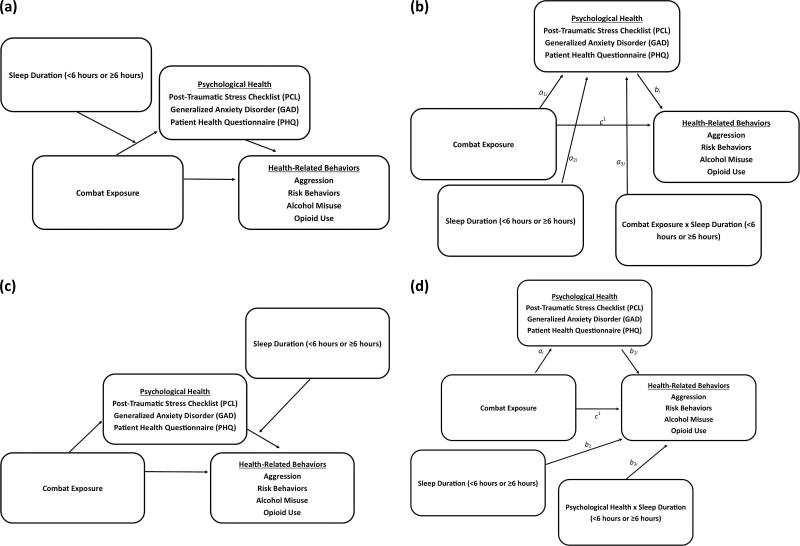
We hypothesize that poor sleep would be associated with the relationship between combat exposure, post-traumatic stress, and health-related behaviors. First, we predicted that sleep discontinuity and duration would statistically mediate the pathway from combat exposure, through post-traumatic stress symptoms, to health-related behaviors, including aggression, risk-behaviors, alcohol misuse, and opioid use (Figure 1a). Second, we predicted that the indirect association of combat exposure on health-related behaviors through post-traumatic stress would be stronger for those reporting <6 hr of sleep than ≥6 hr of sleep (Figure 2a). To test this hypothesis, we analyzed extant cross-sectional survey data that were collected 3 months following a 9 month deployment to Afghanistan among U.S. combat infantry soldiers. Furthermore, we tested whether these associations were unique to post-traumatic stress. We thus also examined alternative models to determine whether the pathway from combat exposure to sleep and downstream health-related behaviors was evident for depression and anxiety symptoms.
Figure 1.
(1a) Proposed model (conceptual). Combat exposure is indirectly associated with health-related behaviors through sleep continuity disturbance and psychological health, respectively. (1b) Proposed model (computational). Indirect effect of combat exposure, health-related behaviors, sleep continuity disturbance, and psychological health serially = a1d21b2. We predict that this indirect relationship will be most robust through sleep discontinuity and post-traumatic stress disorder symptoms. Lower-order effects of combat exposure on health-related behaviors through only sleep discontinuity (a1b1) and through only psychological health (a2b2) are also shown. The direct effect of combat exposure on health-related behaviors is represented by c1. (1c) Alternative “reverse” model (conceptual) of indirect association of combat exposure on health-related behaviors. (1d) Alternative “reverse” model (computational). Indirect effect of combat exposure health-related behaviors sleep continuity disturbance and psychological health serially = a1d21b2.
Figure 2.
(2a) Proposed conditional association of combat exposure on health-related behaviors through psychological health, based on different sleep durations (conceptual). Conceptually, this model compares the strength of the indirect effect of combat exposure on health-related behaviors through psychological health for each of the two sleep durations. We predict that this indirect association is stronger for shorter sleep duration (<6 hr) than for longer sleep duration (>6 hr). (2b) Proposed conditional association of combat exposure on health-related behaviors through psychological health, based on different sleep durations (computational). Statistically, this model is tested as (a1i + a3i * sleep duration) * bi. (2c) Alternative model (conceptual) of combat exposure on health-related behaviors through psychological health, based on different sleep durations. (2d) Alternative model (computational) of combat exposure on health-related behaviors through psychological health, based on different sleep durations. Conditional indirect association of combat exposure on health-related behaviors through psychological health = ai (b1i + b3i * sleep duration).
Methods
Sample
Data were collected as part of the Land Combat Study [11]. We examined extant survey data collected in 2011 from a brigade combat team 3 months postdeployment to Afghanistan. Soldiers attended recruitment briefings where they consented. Eighty per cent (3076/3832) of available soldiers attended the recruitment briefings. Soldiers absent during recruitment briefings were either on duty, ill, or on temporary duty elsewhere. Ninety-four per cent (2876/3076) of the soldiers who attended the recruitment briefings consented to participate. Missing data across all study measures ranged from .1% to 2.6%. The study was approved by the Walter Reed Army Institute of Research IRB.
Measures
Combat exposure
Combat exposure was measured with the 28-item Combat Experiences Scale (CES) [41]. The original response scale of 1 (never) to 4 (5 or more times) was recoded so that 0 represented never and 1 represented experiencing an event at least once. The recoded responses were summed to provide an overall index of combat exposure breadth [46].
Sleep
Sleep continuity was measured by three survey items each assessed on a three-point Likert-scale (from “Not bothered” to “Bothered a lot”): “During the past month, how much have you been bothered by…” “difficulty falling asleep,” “…difficulty staying asleep,” “…problems waking up too early.” These are the core sleep continuity items from the Insomnia Severity Index [47]. (Note that the Insomnia Severity Index does not use the term “bothered.” This phrasing could influence reporting in a population where the culture supports stoicism.) Responses on these items were summed to index the magnitude of reported sleep continuity disturbance. Sleep quantity was assessed by asking respondents “On average, how many hours of sleep have you gotten per day during the last week?” (3 or fewer, 4, 5, 6, 7, 8 or more). This variable was recoded into a dichotomous variable (≥6 vs. <6 hr) to represent adequate and short sleep durations, respectively [24, 34, 35].
Risky behavior
Risky behavior was assessed with four yes/no questions that asked participants if, during the past 3 months did/had they... “…drive or ride in a car without using a seatbelt,” “…driven a car or motorcycle recklessly,” “…driven a car or motorcycle more than 10 miles above the speed limit.” “…risked getting a sexually transmitted disease (STD), e.g., had sex with multiple partners or did not use a condom?” “Yes” responses were summed into an overall index of risk behavior.
Aggression
Aggression was assessed using a four-item scale from previous studies with similar infantry populations [11, 48, 49]. The items asked respondents how often during the past month did they… “…get angry at someone and yell or shout at them,” “…get angry with someone and kick or smash something, slam the door, punch the wall, etc.,” “…threaten someone with physical violence.”, and “…get into a fight with someone and hit the person.” The response options ranged from “Never” to “Five or more times.” The items were summed to create an overall score where higher values indicate greater aggression.
Alcohol misuse
Alcohol misuse was assessed with the Alcohol Use Disorder Identification Test-Consumption (AUDIT-C) [50]. This measure has been used in previous research with U.S. Army soldiers [51]. Responses were recoded by subtracting one from individual scores and summed in a total index of alcohol misuse.
Opioid use
Respondents were asked how often in the past month (from “never” to “nearly every day”) they took prescription opiate/narcotic pain medication (e.g. OxyContin, Percocet, Vicodin, Tramadol, Tylenol with Codeine, Methadone). Responses were recoded into a dichotomous variable (yes/no opioid use during previous month) [52].
Post-traumatic stress
Post-traumatic stress symptoms were assessed using the 17-item Post-Traumatic Stress Disorder Checklist (PCL-C) [53]. We also calculated probable PTSD based on the following criteria: respondents not only had to report at least one intrusion, three avoidance, and two hyperarousal symptoms, but also have a score of at least 50 on a scale of 17 to 85 [11, 54]. Overall (summed) PCL-C scores were used in predictive models.
Depression and anxiety
Depression symptoms were assessed using the Patient Health Questionnaire (PHQ-8) [55]. PHQ-8 scores range from 0 to 24 generalized anxiety symptoms that were assessed using the Generalized Anxiety Disorder Screener (GAD-7) [56]. The GAD-7 scale score ranges from 0 to 21. Scores of 10 or higher indicate moderate impairment and scores of 15 or higher indicate severe impairment for both the PHQ-8 and GAD-7. Total (summed) GAD-7 and total (summed) PHQ-8 were each used in predictive models.
Statistics
Serial and conditional indirect associations of combat exposure on health-related behaviors were assessed using PROCESS [57]. PROCESS is a nonparametric, bias-corrected, bootstrapping procedure (k =1000 bootstraps) which computes 95% confidence intervals for the indirect effect of a predictor on an outcome through one or more mediators. In the case of conditional indirect associations, PROCESS computes 95% confidence intervals for the index of moderated mediation (IMM), which describes the change in the indirect effect at different levels of a third variable (moderator). This approach is advantageous to other structural equation modeling techniques as it is statistically powerful and robust against traditional normality assumptions [58, 59].
Figures 1b and 2b display the statistical models and path coefficient information for the hypothesized models. Conceptual models are displayed in Figures 1a and 2a. It is important to note that these models are nearly tantamount because a “mediator” (or indirect effect variable) requires variance, and sleep duration was coded as a dichotomous indirect variable. Hence, a conditional indirect effect model was appropriate for the sleep duration analysis, whereas a serial model was appropriate for the sleep continuity variable. The use of bootstrapped confidence intervals is preferred as point estimates for indirect effects do not conform to a normal sampling distribution. Bootstrapping obviates the assumption of normality by random sampling of k cases with replacement and produces unbiased point estimates and 95% confidence intervals to determine the statistical significance of a given indirect association.
We present the ratio of the indirect effect to the total highest order effects to demonstrate the magnitude of the indirect effect. We also report the partially standardized indirect effect size estimate for the highest order effects. This estimate changes in the criterion variable (represented by number of standard deviations) attributable to an original scaled single unit change in the predictor through the indirect effect variables. This effect size was chosen as each level of combat exposure represents the addition of a discreet type of combat experience, whereas our outcomes (health outcomes) represent a continuous level of severity. Lastly, given the cross-sectional nature of the data, we computed alternative models (Figures 1, c and d and 2, c and d) to determine whether sleep problems were more parsimoniously antecedent to psychological health in serial pathways.
Results
Descriptive
Survey respondents were 93.5% male, 57.1% junior enlisted, 32.6% Non-Commissioned Officers, and 10.4% Commissioned Officers and Warrant Officers. 42.5% of respondents were between the ages 18 and 24, 30.4% were 25–29, 22.4% were 30–39, and 4.7% were 40 or older.
The mean score on the 28-item measure of combat exposure was 12.49 (SD = 6.37). For 11 of the items, over 50% of respondents indicated that they had experienced the event (receiving incoming artillery, rocket, or mortar fire, 94%; being attacked or ambushed, 89%; receiving small arms fire, 89%; knowing someone seriously injured or killed, 82%; seeing dead bodies, 71%; shooting or directing fire at the enemy, 69%; seeing dead or seriously injured Americans, 68%; having a member of your own unit become a casualty, 67%; believe you would be seriously injured or killed, 69%; encountering sniper fire, 54%; and have a close buddy seriously injured or killed, 53%).
The majority (61.1%) of respondents reported sleeping fewer than 6 hr per day across the previous week. In reference to the past month, 54.1% reported being bothered by difficulty falling asleep, 54.3% by difficulty staying asleep, and 44.3% by waking up too early. The mean PCL-C score was 30.03 (SD = 13.47) and 9.5% of the sample met screening criteria for probable PTSD. The mean PHQ-8 depression score was 5.67 (SD = 5.02) with 6.5% meeting probable criteria for depression with significant functional impairment (overall score of 15 or higher), and the mean GAD-7 score was 5.40 (SD = 5.19) with 7.2% meeting probable criteria for generalized anxiety with severe functional impairment (overall score of 15 or higher).
The mean AUDIT-C score was 4.24 (SD = 3.05) with 57.3% meeting screening criteria for potentially hazardous drinking behavior. For opioid use, 16.9% reported using prescription opiate/narcotic pain medication during the previous month. The mean risk behaviors reported during the previous 3 months were .94 (SD = 1.07). The mean aggression score was 6.66 (SD = 3.08).
Serial and conditional indirect models
The results for the hypothesized and alternative serial and conditional indirect models are displayed in Tables 1 and 2, respectively. As displayed in Table 1, there was a statistically significant serial indirect association (95% CIs did not cross zero) between combat exposure and risky behavior, aggression, and alcohol use when PCL-C scores were included in the model (controlling for PHQ-8 and GAD-7). The overall hypothesized serial indirect effects of combat exposure mediated by sleep disturbance and PCL-C (not controlling for PHQ-8 or GAD-7) were statistically significant for risky behavior (bpartial = .0035, SE = .0007, 95% CI = .0021, .0049), aggression (bpartial = .0074, SE = .0009, 95% CI = .0058, .0092), and alcohol use (bpartial = .0022, SE = .0012, 95% CI = .0010, .0035). In other words, a soldier who experienced every measured combat exposure (compared with experiencing none) would be associated with indirect increases (via sleep and mental health) in risky behavior, aggression, and alcohol use of d = .10, .21, and .06, respectively. The percentage of the total effect of combat exposure accounted for by the hypothesized serial indirect effect (not controlling for PHQ-8 or GAD-7) for risky behavior, aggression, and alcohol use was 13.2%, 19.4%, and 7.6%, respectively. The serial indirect associations between combat exposure and health-related behaviors were not evident (95% CIs included zero) for any of the models that substituted PCL-C with either PHQ-8 or GAD-7 scores as an indirect factor (Table 1).
Table 1.
Serial indirect models of combat exposure with health-related behaviors: model coefficients and bias-corrected bootstrapped (k = 1000 bootstraps) 95% confidence intervals
| 95% CI | n | |||||
|---|---|---|---|---|---|---|
| Criterion | Mediator(s) | b | SE | LL | UL | |
| Hypothesis model | ||||||
| Risk Behaviors | ||||||
| Sleep Disturbance → PCL-C† | .0003* | .0001 | .0001 | .0005 | 2354 | |
| Sleep Disturbance† | .0009* | .0004 | .0002 | .0017 | 2354 | |
| PCL-C† | .0041* | .0008 | .0027 | .0057 | 2354 | |
| Sleep Disturbance → PHQ-8‡ | .0001 | .0001 | −.0001 | .0003 | 2354 | |
| Sleep Disturbance‡ | .0001 | .0002 | −.0001 | .0006 | 2354 | |
| PHQ-8‡ | −.0010* | .0004 | −.0019 | −.0003 | 2354 | |
| Sleep Disturbance → GAD-7§ | .0000 | .0000 | −.0001 | .0000 | 2354 | |
| Sleep Disturbance | .0003 | .0003 | .0000 | .0010 | 2354 | |
| GAD-7§ | −.0004 | .0003 | −.0011 | .0002 | 2354 | |
| Aggression | ||||||
| Sleep Disturbance → PCL-C† | .0019* | .0005 | .0010 | .0031 | 2344 | |
| Sleep Disturbance† | .0022* | .0011 | .0005 | .0050 | 2344 | |
| PCL-C† | .0261* | .0031 | .0201 | .0323 | 2344 | |
| Sleep Disturbance → PHQ-8‡ | .0003 | .0005 | −.0006 | .0013 | 2344 | |
| Sleep Disturbance‡ | .0006 | .0008 | −.0009 | .0025 | 2344 | |
| PHQ-8‡ | −.0050* | .0017 | −.0088 | −.0018 | 2344 | |
| Sleep Disturbance → GAD-7§ | .0000 | .0001 | −.0002 | .0001 | 2344 | |
| Sleep Disturbance§ | .0011 | .0008 | −.0002 | .0030 | 2344 | |
| GAD-7§ | −.0021 | .0020 | −.0059 | .0018 | 2344 | |
| Alcohol Use | ||||||
| Sleep Disturbance → PCL-C† | .0005* | .0002 | .0002 | .0011 | 2360 | |
| Sleep Disturbance† | .0017 | .0011 | −.0002 | .0042 | 2360 | |
| PCL-C† | .0073* | .0020 | .0035 | .0115 | 2360 | |
| Sleep Disturbance → PHQ-8‡ | .0001 | .0002 | −.0002 | .0006 | 2360 | |
| Sleep Disturbance‡ | .0003 | .0005 | −.0003 | .0017 | 2360 | |
| PHQ-8‡ | −.0017* | .0008 | −.0037 | −.0005 | 2360 | |
| Sleep Disturbance → GAD-7§ | .0000 | .0000 | −.0001 | .0000 | 2360 | |
| Sleep Disturbance§ | .0007 | .0006 | .0000 | .0024 | 2360 | |
| GAD-7§ | −.0006 | .0006 | −.0021 | .0003 | 2360 | |
| Opioid Use | ||||||
| Sleep Disturbance → PCL-C† | .0002 | .0001 | .0000 | .0005 | 2384 | |
| Sleep† | .0047* | .0014 | .0023 | .0079 | 2384 | |
| PCL-C† | .0032* | .0015 | .0003 | .0065 | 2384 | |
| Sleep Disturbance → PHQ-8‡ | .0000 | .0001 | .0000 | .0003 | 2384 | |
| Sleep Disturbance‡ | .0012 | .0012 | −.0010 | .0037 | 2384 | |
| PHQ-8‡ | −.0005 | .0004 | −.0015 | .0002 | 2384 | |
| Sleep Disturbance → GAD-7§ | .0000 | .0000 | .0000 | .0001 | 2384 | |
| Sleep Disturbance§ | .0018 | .0011 | .0000 | .0043 | 2384 | |
| GAD-7§ | −.0004 | .0004 | −.0014 | .0001 | 2384 | |
| Reverse-order model | ||||||
| Risk Behaviors | ||||||
| PCL-C→ Sleep Disturbance† | .0003 | .0002 | −.0002 | .0008 | 2354 | |
| Sleep Disturbance† | .0002 | .0002 | −.0001 | .0006 | 2354 | |
| PCL-C† | .0015 | .0011 | −.0007 | .0037 | 2354 | |
| Aggression | ||||||
| PCL-C→ Sleep Disturbance† | .0009 | .0007 | −.0004 | .0022 | 2344 | |
| Sleep Disturbance† | .0005 | .0005 | −.0003 | .0017 | 2344 | |
| PCL-C† | .0161* | .0039 | .0089 | .0243 | 2344 | |
| Alcohol Use | ||||||
| PCL-C→ Sleep Disturbance† | .0007 | .0007 | −.0006 | .0021 | 2360 | |
| Sleep Disturbance† | .0004 | .0005 | −.0004 | .0017 | 2360 | |
| PCL-C† | .0032 | .0032 | −.0030 | .0094 | 2360 |
Coefficients (b) are unstandardized betas.
†Controlling for PHQ-8 (Patient Health Questionnaire) and GAD-7 (Generalized Anxiety Disorder).
‡Controlling for PCL-C (Post-traumatic Stress Disorder (PTSD) Checklist) and GAD-7.
§Controlling for PCL-C and PHQ-8; CE (Combat exposure): Sum of 28-item combat exposure checklist; Poor Disturbance: Sum of three sleep disturbance items; PCL-C: PTSD checklist sum. Risk Behaviors: Sum of four yes/no risk behavior questions; Aggression: Sum of four aggression items; Alcohol Misuse: Recoded AUDIT-C (Alcohol Use Disorder Identification Test- C). Opioid use is logistic regression on single yes/no item on use of opiates. Confidence intervals were used to determine the statistical significance of indirect effects with bootstrapping. t-test statistics are not appropriate as the indirect tests do not conform to normality assumptions. Confidence intervals (CI): 95% not crossing “0” are considered statistically significant.
SE = standard error; LL = lower limit; UL = upper limit; n = sample size.
Table 2.
Conditional indirect models of combat exposure on health-related behaviors for different sleep durations: model coefficients and bias-corrected bootstrapped (k = 1000 bootstraps) 95% confidence intervals
| 95% CI | n | ||||||
|---|---|---|---|---|---|---|---|
| Mediator | Criterion | Sleep quantity | Coefficient | SE | LL | UL | |
| Hypothesis model | |||||||
| PCL-C † | Risk Behaviors | CIA | −.0019* | .0009 | −.0036 | −.0003 | 2368 |
| ≥6 hr | .0041* | .0008 | .0027 | .0058 | 2368 | ||
| <6 hr | .0060* | .0009 | .0044 | .0078 | 2368 | ||
| Aggression | CIA | −.0119* | .0045 | −.0208 | −.0032 | 2350 | |
| ≥6 hr | .0225* | .0037 | .0159 | .0303 | 2350 | ||
| <6 hr | .0344* | .0037 | .0281 | .0429 | 2350 | ||
| Alcohol Misuse | CIA | −.0036* | .0017 | −.0073 | −.0007 | 2374 | |
| ≥6 hr | .0076* | .0017 | .0047 | .0113 | 2374 | ||
| <6 hr | .0112* | .0022 | .0073 | .0158 | 2374 | ||
| Opioid Use | CIA | −.0030* | .0013 | −.0059 | −.0006 | 2375 | |
| ≥6 hr | .0064* | .0014 | .0041 | .0097 | 2375 | ||
| <6 hr | .0094* | .0017 | .0065 | .0134 | 2375 | ||
| PHQ-8 ‡ | Risk Behaviors | CIA | −.0009 | .0009 | −.0026 | .0008 | 2368 |
| ≥6 hr | −.0017 | .0006 | −.0032 | −.0005 | 2368 | ||
| <6 hr | −.0009 | .0006 | −.0020 | .0002 | 2368 | ||
| Aggression | CIA | −.0034 | .0038 | −.0110 | .0040 | 2350 | |
| ≥6 hr | −.0080* | .0030 | −.0140 | −.0025 | 2350 | ||
| <6 hr | −.0046 | .0026 | −.0101 | .0002 | 2350 | ||
| Alcohol Misuse | CIA | −.0014 | .0015 | −.0047 | .0014 | 2374 | |
| ≥6 hr | −.0030* | .0012 | −.0059 | −.0008 | 2374 | ||
| <6 hr | −.0016 | .0010 | −.0038 | .0003 | 2374 | ||
| Opioid Use | CIA | −.0012 | .0012 | −.0035 | .0013 | 2375 | |
| ≥6 hr | −.0024* | .0010 | −.0046 | −.0006 | 2375 | ||
| <6 hr | −.0012 | .0009 | −.0033 | .0003 | 2375 | ||
| GAD-7§ | Risk Behaviors | CIA | .0004 | .0007 | −.0010 | .0018 | 2368 |
| ≥6 hr | −.0002 | .0005 | −.0013 | .0008 | 2368 | ||
| <6 hr | −.0007 | .0005 | −.0018 | .0004 | 2368 | ||
| Aggression | CIA | .0023 | .0042 | −.0056 | .0113 | 2350 | |
| ≥6 hr | −.0010 | .0030 | −.0070 | .0050 | 2350 | ||
| <6 hr | −.0033 | .0031 | −.0094 | .0027 | 2350 | ||
| Alcohol Misuse | CIA | .0007 | .0014 | −.0021 | .0036 | 2374 | |
| ≥6 hr | −.0004 | .0010 | −.0025 | .0017 | 2374 | ||
| <6 hr | −.0012 | .0010 | −.0034 | .0006 | 2374 | ||
| Opioid Use | CIA | .0007 | .0012 | −.0019 | .0030 | 2375 | |
| ≥6 hr | −.0004 | .0009 | −.0024 | .0012 | 2375 | ||
| <6 hr | −.0011 | .0009 | −.0029 | .0007 | 2375 | ||
| Alternative model (Figure 2, c and d) | |||||||
| PCL-C† | Risk Behaviors | CIA | −.0015 | .0013 | −.0043 | .0010 | 2368 |
| ≥6 hr | .0036* | .0012 | .0015 | .0062 | 2368 | ||
| <6 hr | .0050* | .0009 | .0035 | .0071 | 2368 | ||
| Aggression | CIA | .0059 | .0046 | −.0030 | .0153 | 2350 | |
| ≥6 hr | .0333* | .0048 | .0247 | .0440 | 2350 | ||
| <6 hr | .0274* | .0032 | .0214 | .0343 | 2350 | ||
| Alcohol Misuse | CIA | .0056 | .0046 | −.0039 | .0143 | 2374 | |
| ≥6 hr | .0132* | .0041 | .0056 | .0215 | 2374 | ||
| <6 hr | .0076* | .0021 | .0035 | .0121 | 2374 | ||
| Opioid Use | CIA | .0030 | .0031 | −.0032 | .0090 | 2375 | |
| ≥6 hr | .0103* | .0030 | .0040 | .0164 | 2375 | ||
| <6 hr | .0072* | .0016 | .0042 | .0105 | 2375 |
Coefficients are unstandardized betas. Confidence intervals (CI) were used to determine the statistical significance of indirect effects with bootstrapping as standard parametric statistics are not appropriate as the tests do not conform to normality assumptions. CI (95%) not crossing “0” are considered statistically significant.
†Controlling for PHQ-8 (Patient Health Questionnaire) and GAD-7 (Generalized Anxiety Disorder).
‡Controlling for PCL-C (Post-traumatic Stress Disorder (PTSD) Checklist) and GAD-7.
§Controlling for PCL-C and PHQ-8; PCL-C = Post-traumatic Stress Disorder Checklist. PHQ-8 = Patient Health Questionnaire.
GAD-7 = Generalized Anxiety Disorder; CIA = Conditional Indirect Association; SE = standard error; LL = lower limit; UL = upper limit; n = sample size.
Finally, we examined alternative, “reverse order,” models for significant serial indirect associations (Figure 1c). As shown in Table 1, these alternative “reverse order” models were not statistically significant.
For the conditional indirect models (Figures 2, a and b and Table 2), sleep quantity statistically moderated the indirect association between combat exposure and all of the health-related behaviors. For those reporting <6 hr of sleep, the indirect association of combat exposure through PCL-C was 46.3%, 52.8%, 47.3%, and 46.8% larger for risk behaviors, aggression, alcohol use, and opioid use, respectively, than for those reporting ≥6 hr of sleep per day (Table 2). In other words, for respondents reporting <6 hr of sleep per day, the association between combat exposure and health-related behaviors through PCL-C scores was statistically stronger (Table 2) than those who reported ≥6 hr of sleep per day. Conditional indirect models with PHQ-8 and GAD-7 scores did not reveal statistically significant conditional indirect associations between combat exposure and health-related behaviors through PCL-C scores. Lastly, alternative “reverse order” conditional indirect models (Figures 2, c and d) were not statistically significant for any health-related behavior (Table 2).
Discussion
Sleep difficulties displayed a robust indirect association with combat exposure, PTSD, and health-related behaviors. The findings in this sample of combat-exposed soldiers were specific to PTSD. This is perhaps not surprising given the nature of the stressor in this population. The significant decrease in effect size of the indirect models when sleep was moved out of an antecedent role suggests that targeting postdeployment sleep problems should be a first-line target to reduce health-compromising behaviors with potentially significant personal, social, and occupational costs. Indeed, even relatively small effect sizes could represent a meaningful concern when modest effects are aggregated across a large fighting force. Thus, although some of the effect sizes in this research are small, the societal impact of these effects grows when multiplied across the high number of combat Soldiers and veterans.
Although other postcombat variables are certainly associated with the pathway we studied, sleep problems are particularly salient in military populations. Indeed, sleep difficulties are largely unavoidable in an operational context (e.g. rocket fire, limited space, incoming aircraft, and shift changes) [60]. Yet, sleep problems following deployment are persistent for many combat veterans [23]. However, if targeted, they are highly mutable. Pharmaceutical (e.g. Prazosin) and cognitive behavioral techniques are effective treatments for sleep difficulties and post-traumatic stress in combat veterans [61–63]. To the extent that sleep is associated with the behavioral health of the force, this study underscores postdeployment sleep as a critical target for providers and policy makers alike.
Negative health-related behaviors exact a significant cost to soldiers and to the federal government. For example, alcohol misuse among active duty soldiers cost the Department of Defense an estimated US$892 million in 2006 and rendered over 10 000 active duty soldiers unable to deploy with their units [64]. Moreover, the personal impacts of negative health-related behaviors on the lives of service members are myriad. For instance, combat veterans with PTSD are 2.5 times more likely to use opioids for pain than veterans without PTSD [9].
There are several feasible options for military leaders to improve sleep in service members [65]. First, sleeping conditions could be improved for soldiers returning from combat [66]. Second, pharmaceutical treatments and strategic use of stimulants may assist treatment and sleep control [65]. Third, sleep education and behavior treatments that emphasize stimulus control, sleep hygiene, and improved sleep scheduling are effective and longer lasting than pharmaceutical approaches [65–69]. Leadership views and behaviors concerning sleep have a salutatory effect on the sleep and health of soldiers [70]. Fourth, cognitive-behavioral therapy for insomnia (CBT-I) has demonstrated success in service members, though deploying CBT-I at-large in the military will require increased training for nonspecialty providers (e.g. medics), or the development of telemedicine and virtual health technologies, both of which are current priorities [65, 71, 31]. Sleep duration in the military is often affected by external forces (imposed schedules, unpredictable nature of combat, etc.). Nevertheless, compared with other risk factors (e.g. combat exposure), sleep disturbance and duration are accessible and malleable targets for intervention and prevention in the military.
This research suggests several future directions. Future research should investigate how external forces such as leadership demands affect sleep behavior that affects health. Relatedly, future research should consider the extent that the influence of active duty status on the models tested here by attempting to generalize these findings to combat veterans who have separated from active duty. Finally, future research should consider the role that injuries and pain conditions have on the association between sleep, mental health, and opioid use in active duty samples. It is plausible that the indirect effect of combat experiences on opioid use through poor sleep and mental health may be stronger for soldiers who sustained physical injury.
This study has several limitations. First, these data were cross-sectional and correlational. As such, we cannot conclusively infer causality or temporal order of sleep, PTSD, and health behaviors. Thus, it is important to consider alternative directional hypotheses. Sleep disturbance is a well-documented consequence of post-traumatic stress [37] and other mental health problems [72] (e.g. depression). It is possible that sleep difficulties are a byproduct of combat-related stress symptoms rather than a cause. Sleep difficulties are more efficaciously and effectively treated than PTSD, and treating sleep often ameliorates mental health symptoms [72, 73]. The indirect pathway models reported in this paper were also more robust with sleep preceding PTSD. Therefore, it would appear that considering sleep as antecedent to PTSD is the most parsimonious interpretation of the results. Still, this cannot be firmly established given the cross-sectional and correlational study design. Notwithstanding this limitation, poor sleep may be viewed as a risk factor for worse health behaviors in conjunction with PTSD. Second, the generalizability of these findings outside of a land combat context is unknown. Importantly, the demographics and observed rates of probable clinical PTSD and depression, and prevalence of anger/aggression in the present study were comparable to the Army at-large, which mitigates this concern [73]. Third, this research relied exclusively on self-report. Given the stigma surrounding mental health in the military [54], underreporting was possible. All soldiers completing the survey were assured that responses would remain confidential.
These findings are relevant for decision-makers outside of the military as well. There are now over 2.2 million active duty and reserve military personnel and over 20 million veterans in the United States [74]. As such, a greater understanding of modifiable risk factors that link combat exposure to post-traumatic stress symptoms and health-compromising behaviors is valuable to clinicians, scholars, and research scientists in psychiatry, mental health, behavioral science, and allied fields. This research can guide and provide empirical support for national defense policy and recommendations for sleep and health in populations exposed to combat and potentially other traumatic and stressful contexts.
Health-compromising behaviors burden many combat veterans and their families. Identifying robust mechanisms linking combat exposure and PTSD to health-related behaviors is important for both national defense strategy and public health. This is the first study to investigate the association of poor sleep with the link between combat and health-compromising behaviors. Our findings provide a robust empirical underpinning for policy recommendations with sleep health as a central focus for soldiers returning from combat. Continuing to do so could enhance force health, retention, and readiness.
Funding
This project was funded by the Military Operational Medicine Research Area Directorate, U.S. Army Medical Research and Materiel Command, Ft. Detrick, Maryland. Additional support was provided by the NIH/NIDA K23DA035915. All authors have no other financial and nonfinancial disclosures.
Conflict of interest statement. Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the author and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense. The investigators have adhered to the policies for protection of human participants as prescribed in AR 70–25.
Acknowledgments
We would like to acknowledge the soldiers and scientists who have supported the WRAIR Land Combat Study.
References
- 1. Bray RM, et al. Department of Defense Survey of Health Related Behaviors Among Active Duty Military Personnel. Research Triangle Park, NC: Research Triangle Inst (RTI); 2009. [Google Scholar]
- 2. Elbogen EB, et al. Protective factors and risk modification of violence in Iraq and Afghanistan War veterans. J Clin Psychiatry. 2012;73(6):e767–e773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McFall ME, et al. Combat-related posttraumatic stress disorder and severity of substance abuse in Vietnam veterans. J Stud Alcohol. 1992;53(4):357–363. [DOI] [PubMed] [Google Scholar]
- 4. Ramchand R, et al. Prevalence of, risk factors for, and consequences of posttraumatic stress disorder and other mental health problems in military populations deployed to Iraq and Afghanistan. Curr Psychiatry Rep. 2015;17(5):37. [DOI] [PubMed] [Google Scholar]
- 5. Renshaw KD, et al. Relationship distress in partners of combat veterans: the role of partners’ perceptions of posttraumatic stress symptoms. Behav Ther. 2012;43(2):416–426. [DOI] [PubMed] [Google Scholar]
- 6. Santiago PN, et al. Screening for alcohol misuse and alcohol-related behaviors among combat veterans. Psychiatr Serv. 2010;61(6):575–581. [DOI] [PubMed] [Google Scholar]
- 7. Sayer NA, et al. Reintegration problems and treatment interests among Iraq and Afghanistan combat veterans receiving VA medical care. Psychiatr Serv. 2010;61(6):589–597. [DOI] [PubMed] [Google Scholar]
- 8. Seal KH, et al. Substance use disorders in Iraq and Afghanistan veterans in VA healthcare, 2001-2010: implications for screening, diagnosis and treatment. Drug Alcohol Depend. 2011;116(1–3):93–101. [DOI] [PubMed] [Google Scholar]
- 9. Seal KH, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940–947. [DOI] [PubMed] [Google Scholar]
- 10. Shin HJ, et al. Longitudinal correlates of aggressive behavior in help-seeking U.S. veterans with PTSD. J Trauma Stress. 2012;25(6):649–656. [DOI] [PubMed] [Google Scholar]
- 11. Thomas JL, et al. Prevalence of mental health problems and functional impairment among Active Component and National Guard soldiers 3 and 12 months following combat in Iraq. Arch Gen Psychiatry. 2010;67(6):614–623. [DOI] [PubMed] [Google Scholar]
- 12. Wheeler DP, et al. Bringing it all back home: social work and the challenge of returning veterans. Health Soc Work. 2007;32(4):297–300. [DOI] [PubMed] [Google Scholar]
- 13. Widome R, et al. Health risk behaviors of Afghanistan and Iraq war veterans attending college. Am J Health Promot. 2011;26(2):101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cook J, et al. Influence of PTSD symptom clusters on smoking status among help-seeking Iraq and Afghanistan veterans. Nicotine Tob Res. 2009;11(10):1189–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jakupcak M, et al. Anger, hostility, and aggression among Iraq and Afghanistan War veterans reporting PTSD and subthreshold PTSD. J Trauma Stress. 2007;20(6):945–954. [DOI] [PubMed] [Google Scholar]
- 16. Schnurr PP, et al. Combat exposure, posttraumatic stress disorder symptoms, and health behaviors as predictors of self-reported physical health in older veterans. J Nerv Ment Dis. 1999;187(6):353–359. [DOI] [PubMed] [Google Scholar]
- 17. Svetlicky V, et al. Combat exposure, posttraumatic stress symptoms and risk-taking behavior in veterans of the Second Lebanon War. Isr J Psychiatry Relat Sci. 2010;47(4):276–283. [PubMed] [Google Scholar]
- 18. Taft CT, et al. Assessment and treatment of posttraumatic anger and aggression: a review. J Rehabil Res Dev. 2012;49(5):777–788. [DOI] [PubMed] [Google Scholar]
- 19. Fulton JJ, et al. The prevalence of posttraumatic stress disorder in Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veterans: a meta-analysis. J Anxiety Disord. 2015;31:98–107. [DOI] [PubMed] [Google Scholar]
- 20. Kuhn E, et al. Aggressive and unsafe driving in male veterans receiving residential treatment for PTSD. J Trauma Stress. 2010;23(3):399–402. [DOI] [PubMed] [Google Scholar]
- 21. Ouimette P, et al. Modeling associations between posttraumatic stress symptoms and substance use. Addict Behav. 2010;35(1):64–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stappenbeck CA, et al. The effects of alcohol problems, PTSD, and combat exposure on nonphysical and physical aggression among Iraq and Afghanistan war veterans. Psychol Trauma. 2014;6(1):65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seelig AD, et al. ; Millennium Cohort Study Team. Sleep patterns before, during, and after deployment to Iraq and Afghanistan. Sleep. 2010;33(12):1615–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wright KM, et al. Insomnia as predictor versus outcome of PTSD and depression among Iraq combat veterans. J Clin Psychol. 2011;67(12):1240–1258. [DOI] [PubMed] [Google Scholar]
- 25. Cox RC, et al. Sleep disturbance in posttraumatic stress disorder: epiphenomenon or causal factor? Curr Psychiatry Rep. 2017;19(4):22. [DOI] [PubMed] [Google Scholar]
- 26. Cohen DJ, et al. Quantitative electroencephalography during rapid eye movement (REM) and non-REM sleep in combat-exposed veterans with and without post-traumatic stress disorder. J Sleep Res. 2013;22(1):76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Macera CA, et al. Do sleep problems mediate the relationship between traumatic brain injury and development of mental health symptoms after deployment? Sleep. 2013;36(1):83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van Liempt S, et al. Impact of impaired sleep on the development of PTSD symptoms in combat veterans: a prospective longitudinal cohort study. Depress Anxiety. 2013;30(5):469–474. [DOI] [PubMed] [Google Scholar]
- 29. Galovski TE, et al. Augmenting cognitive processing therapy to improve sleep impairment in PTSD: a randomized controlled trial. J Consult Clin Psychol. 2016;84(2):167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lipinska G, et al. Pharmacology for sleep disturbance in PTSD. Hum Psychopharmacol. 2016;31(2):156–163. [DOI] [PubMed] [Google Scholar]
- 31. Margolies SO, et al. Efficacy of a cognitive-behavioral treatment for insomnia and nightmares in Afghanistan and Iraq veterans with PTSD. J Clin Psychol. 2013;69(10):1026–1042. [DOI] [PubMed] [Google Scholar]
- 32. Rusch HL, et al. Effect of acute sleep disturbance and recovery on insulin-like growth factor-1 (IGF-1): possible connections and clinical implications. J Clin Sleep Med. 2015;11(10):1245–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Talbot LS, et al. Cognitive behavioral therapy for insomnia in posttraumatic stress disorder: a randomized controlled trial. Sleep. 2014;37(2):327–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Luxton DD, et al. Prevalence and impact of short sleep duration in redeployed OIF soldiers. Sleep. 2011;34(9):1189–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Swinkels CM, et al. The association of sleep duration, mental health, and health risk behaviors among U.S. Afghanistan/Iraq era veterans. Sleep. 2013;36(7):1019–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Koffel E, et al. Pre-deployment daytime and nighttime sleep complaints as predictors of post-deployment PTSD and depression in National Guard troops. J Anxiety Disord. 2013;27(5):512–519. [DOI] [PubMed] [Google Scholar]
- 37. Gehrman P, et al. Predeployment sleep duration and insomnia symptoms as risk factors for new-onset mental health disorders following military deployment. Sleep. 2013;36(7):1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dyches KD, et al. Modeling the indirect association of combat exposure with anger and aggression during combat deployment: the moderating role of perceived unit morale. Mil Psychol. 2017;29(4):260. [Google Scholar]
- 39. Jacobson IG, et al. Alcohol use and alcohol-related problems before and after military combat deployment. JAMA. 2008;300(6):663–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stander VA, et al. Etiology of depression comorbidity in combat-related PTSD: a review of the literature. Clin Psychol Rev. 2014;34(2):87–98. [DOI] [PubMed] [Google Scholar]
- 41. Wilk JE, et al. Relationship of combat experiences to alcohol misuse among U.S. soldiers returning from the Iraq war. Drug Alcohol Depend. 2010;108(1–2):115–121. [DOI] [PubMed] [Google Scholar]
- 42. Babson KA, et al. Anxiety sensitivity and sleep quality: independent and interactive predictors of posttraumatic stress disorder symptoms. J Nerv Ment Dis. 2013;201(1):48–51. [DOI] [PubMed] [Google Scholar]
- 43. LaMotte AD, et al. Sleep problems and physical pain as moderators of the relationship between PTSD symptoms and aggression in returning veterans. Psychol Trauma. 2017;9(1):113–116. [DOI] [PubMed] [Google Scholar]
- 44. Short NA, et al. Insomnia and emotion dysregulation: independent and interactive associations with posttraumatic stress symptoms among trauma-exposed smokers. J Affect Disord. 2014;165:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Borders A, et al. Sleep problems may mediate associations between rumination and PTSD and depressive symptoms among OIF/OEF veterans. Psychol Trauma. 2015;7(1):76–84. [DOI] [PubMed] [Google Scholar]
- 46. Britt TW, et al. Morale as a moderator of the combat exposure-PTSD symptom relationship. J Trauma Stress. 2013;26(1):94–101. [DOI] [PubMed] [Google Scholar]
- 47. Morin CM, et al. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cabrera OA, et al. Aggressiveness and perceived marital quality: the moderating role of a family-supportive work climate. Mil Psychol. 2010;22(1):57. [Google Scholar]
- 49. Killgore WD, et al. Post-combat invincibility: violent combat experiences are associated with increased risk-taking propensity following deployment. J Psychiatr Res. 2008;42(13):1112–1121. [DOI] [PubMed] [Google Scholar]
- 50. Bush K, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol use disorders identification test. Arch Intern Med. 1998;158(16):1789–1795. [DOI] [PubMed] [Google Scholar]
- 51. Jennings KS, et al. Characterizing the health and attitudes of rear detachment soldiers. Mil Behav Health. 2017;5(2):189–201. [Google Scholar]
- 52. Toblin RL, et al. Chronic pain and opioid use in US soldiers after combat deployment. JAMA Intern Med. 2014;174(8):1400–1401. [DOI] [PubMed] [Google Scholar]
- 53. Weathers FW, et al. The PTSD Checklist (PCL): reliability, validity, and diagnostic utility.Presented at: Annual Convention of the International Society for Traumatic Stress Studies; 1993. [Google Scholar]
- 54. Hoge CW, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351(1):13–22. [DOI] [PubMed] [Google Scholar]
- 55. Kroenke K, et al. The PHQ-8: a new depression diagnostic and severity measure. Psychiatr Annals. 2002;32(9):509–515. [Google Scholar]
- 56. Spitzer RL, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. [DOI] [PubMed] [Google Scholar]
- 57. Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York: Guilford Press; 2013. [Google Scholar]
- 58. Hayes AF. Beyond Baron and Kenny: statistical mediation analysis in the new millennium. Commun Monogr. 2009;76(4):408–420. [Google Scholar]
- 59. Hayes AF, et al. Examining mechanisms and their contingencies: process versus structural equation modeling. Australas Mark J. 2017;25:76–81. [Google Scholar]
- 60. Wesensten NJ, et al. The challenge of sleep management in military operations. U.S. Army Med Dep J. 2013;109–118. [PubMed] [Google Scholar]
- 61. Germain A, et al. Placebo-controlled comparison of prazosin and cognitive-behavioral treatments for sleep disturbances in US Military Veterans. J Psychosom Res. 2012;72(2):89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Raskind MA, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160(2):371–373. [DOI] [PubMed] [Google Scholar]
- 63. Ruff RL, et al. Improving sleep: initial headache treatment in OIF/OEF veterans with blast-induced mild traumatic brain injury. J Rehabil Res Dev. 2009;46(9):1071–1084. [DOI] [PubMed] [Google Scholar]
- 64. Harwood HJ, et al. Economic implications of reduced binge drinking among the military health system’s TRICARE Prime plan beneficiaries. Mil Med. 2009;174(7):728–736. [DOI] [PubMed] [Google Scholar]
- 65. Troxel WM, et al. Sleep in the military: promoting healthy sleep among U.S. service members. Rand Health Q. 2015;5(2):19. [PMC free article] [PubMed] [Google Scholar]
- 66. Peterson AL, et al. Sleep disturbance during military deployment. Mil Med. 2008;173(3):230–235. [DOI] [PubMed] [Google Scholar]
- 67. Hryshko-Mullen AS, et al. Behavioral treatment of insomnia: the Wilford Hall insomnia program. Mil Med. 2000;165(3):200–207. [PubMed] [Google Scholar]
- 68. Lichstein KL, et al. Behavioral assessment and treatment of insomnia: a review with an emphasis on clinical application. Behav Ther. 1994;25(4):659–688. [Google Scholar]
- 69. Smith MT, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159(1):5–11. [DOI] [PubMed] [Google Scholar]
- 70. Gunia BC, et al. Sleep leadership in high-risk occupations: an investigation of soldiers on peacekeeping and combat missions. Mil Psychol. 2015;27(4):197–211. [Google Scholar]
- 71. Gellis LA, et al. Cognitive behavioral treatment for insomnia in veterans with long-standing posttraumatic stress disorder: a pilot study. J Aggress Maltreat Trauma. 2011;20(8):904–916. [Google Scholar]
- 72. Manber R, et al. Insomnia and depression: a multifaceted interplay. Curr Psychiatry Rep. 2009;11(6):437–442. [DOI] [PubMed] [Google Scholar]
- 73. Milliken CS, et al. Longitudinal assessment of mental health problems among active and reserve component soldiers returning from the Iraq war. JAMA. 2007;298(18):2141–2148. [DOI] [PubMed] [Google Scholar]
- 74. Affairs USDoV. The Veteran Population Projection Model. 2016. https://www.data.va.gov/dataset/veteran-population-projection-2014 [Google Scholar]