Abstract
Background
Pain is a common feature of childhood and adolescence around the world, and for many young people, that pain is chronic. The World Health Organization guidelines for pharmacological treatments for children's persisting pain acknowledge that pain in children is a major public health concern of high significance in most parts of the world. While in the past pain was largely dismissed and was frequently left untreated, views on children's pain have changed over time and relief of pain is now seen as important.
We designed a suite of seven reviews on chronic non‐cancer pain and cancer pain (looking at antidepressants, antiepileptic drugs, non‐steroidal anti‐inflammatory drugs, opioids, and paracetamol) in order to review the evidence for children's pain utilising pharmacological interventions.
As the leading cause of morbidity in the world today, chronic disease (and its associated pain) is a major health concern. Chronic pain (that is pain lasting three months or longer) can arise in the paediatric population in a variety of pathophysiological classifications (nociceptive, neuropathic, or idiopathic) from genetic conditions, nerve damage pain, chronic musculoskeletal pain, and chronic abdominal pain, as well as for other unknown reasons.
Antidepressants have been used in adults for pain relief and pain management since the 1970s. The clinical impression from extended use over many years is that antidepressants are useful for some neuropathic pain symptoms, and that effects on pain relief are divorced and different from effects on depression; for example, the effects of tricyclic antidepressants on pain may occur at different, and often lower, doses than those on depression. Amitriptyline is one of the most commonly used drugs for treating neuropathic pain in the UK.
Objectives
To assess the analgesic efficacy and adverse events of antidepressants used to treat chronic non‐cancer pain in children and adolescents aged between birth and 17 years, in any setting.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online, MEDLINE via Ovid, and Embase via Ovid from inception to 6 September 2016. We also searched the reference lists of retrieved studies and reviews, and searched online clinical trial registries.
Selection criteria
Randomised controlled trials, with or without blinding, of any dose and any route, treating chronic non‐cancer pain in children and adolescents, comparing any antidepressant with placebo or an active comparator.
Data collection and analysis
Two review authors independently assessed studies for eligibility. We planned to use dichotomous data to calculate risk ratio and number needed to treat for one additional event, using standard methods. We assessed the evidence using GRADE and created three 'Summary of findings' tables.
Main results
We included four studies with a total of 272 participants (6 to 18 years of age) who had either chronic neuropathic pain, complex regional pain syndrome type 1, irritable bowel syndrome, functional abdominal pain, or functional dyspepsia. All of the studies were small. One study investigated amitriptyline versus gabapentin (34 participants), two studies investigated amitriptyline versus placebo (123 participants), and one study investigated citalopram versus placebo (115 participants). Due to a lack of available data we were unable to complete any quantitative analysis.
Risk of bias for the four included studies varied, due to issues with randomisation and allocation concealment (low to unclear risk); blinding of participants, personnel, and outcome assessors (low to unclear risk); reporting of results (low to unclear risk); and size of the study populations (high risk). We judged the remaining domains, attrition and other potential sources of bias, as low risk of bias.
Primary outcomes
No studies reported our primary outcomes of participant‐reported pain relief of 30% or greater or 50% or greater, or Patient Global Impression of Change.
Secondary outcomes
All studies measured adverse events, with very few reported (11 out of 272 participants). All but one adverse event occurred in the active treatment groups (amitriptyline, citalopram, and gabapentin). Adverse events in all studies, across active treatment and comparator groups, were considered to be a mild reaction, such as nausea, dizziness, drowsiness, tiredness, and abdominal discomfort (very low‐quality evidence).
There were also very few withdrawals due to adverse events, again all but one from the active treatment groups (very low‐quality evidence).
No serious adverse events were reported across any of the studies (very low‐quality evidence).
There were few or no data for our remaining secondary outcomes.
Quality of evidence
For the outcomes with available data, we downgraded the quality of the evidence by three levels to very low‐quality due to too few data and the fact that the number of events was too small to be meaningful.
Authors' conclusions
We identified only a small number of studies with small numbers of participants and insufficient data for analysis.
As we could undertake no meta‐analysis, we are unable to comment about efficacy or harm from the use of antidepressants to treat chronic non‐cancer pain in children and adolescents. Similarly, we could not comment on our remaining secondary outcomes: Carer Global Impression of Change; requirement for rescue analgesia; sleep duration and quality; acceptability of treatment; physical functioning; and quality of life.
We know from adult randomised controlled trials that some antidepressants, such as amitriptyline, can provide some pain relief in certain chronic non‐cancer pain conditions.
Plain language summary
Antidepressants for chronic non‐cancer pain in children and adolescents
Bottom line
We are uncertain as to whether antidepressants provide pain relief for chronic non‐cancer pain in children and adolescents. We do not have evidence to suggest that one type of antidepressant is more effective than another.
Background
Children can experience chronic or recurrent pain related to genetic conditions, nerve damage, muscle or bone pain, stomach pain, as well as for unknown reasons. Chronic pain is pain that lasts three months or longer and is commonly accompanied by changes in lifestyle and functional abilities, as well as by signs and symptoms of depression and anxiety.
Antidepressants have been used for pain relief and pain management since the 1970s and are considered by clinicians to be useful for symptoms of nerve, menstrual, muscular, joint, and stomach pain. Examples of antidepressants that have been used to treat neuropathic pain include amitriptyline, milnacipran, and citalopram.
Study characteristics
In September 2016 we searched for clinical trials in which antidepressants were used to treat chronic nerve, menstrual, muscular, joint, or stomach pain. We found four trials with a total of 272 participants (aged 6 to 18 years old) who had nerve pain, general painful inflammation, stomach pain, or irritable bowel syndrome, for more than 3 months.
Key results
No studies reported on pain relief of 30% or greater, or 50% or greater. Side effects were uncommon, and occurred only as mild reactions such as nausea, dizziness, drowsiness, tiredness, and abdominal discomfort (4 due to amitriptyline, 5 due to citalopram, 1 due to gabapentin, and 1 due to placebo). These 11 participants withdrew from the study due to these mild side effects. There were no serious side effects.
Quality of the evidence
We rated the quality of the evidence from studies using four levels: very low, low, moderate, or high. Very low‐quality evidence means that we are very uncertain about the results. High‐quality evidence means that we are very confident in the results.
The available evidence in this review was of very low‐quality due to a lack of data and small study sizes.
Summary of findings
Background
Pain is a common feature of childhood and adolescence around the world, and for many young people, that pain is chronic. The World Health Organization guidelines for pharmacological treatments for persisting pain in children acknowledge that pain in children is a major public health concern of high significance in most parts of the world (WHO 2012). While in the past, pain was largely dismissed and was frequently left untreated, views on children's pain have changed over time, and relief of pain is now seen as important. Since the 1970s, studies comparing child and adult pain management have revealed a variety of responses to pain, fuelling the need for a more in‐depth focus on paediatric pain (Caes 2016).
Infants (zero to 12 months), children (1 to 9 years), and adolescents (10 to 18 years), WHO 2012, account for 27% (1.9 billion) of the world's population (United Nations 2015); the proportion of those aged 14 years and under ranges from 12% (in Hong Kong) to 50% (in Niger) (World Bank 2014). However, little is known about the pain management needs of this population. For example, in the Cochrane Library, approximately 12 reviews produced by the Cochrane Pain, Palliative and Supportive Care Review Group in the past 18 years have been specifically concerned with children and adolescents, compared to over 100 reviews specific to adults. Additional motivating factors for investigating children's pain include the vast amount of unmanaged pain in the paediatric population and the development of new technologies and treatments. We convened an international group of leaders in paediatric pain to design a suite of seven reviews in chronic pain and cancer pain (looking at antidepressants, antiepileptic drugs, non‐steroidal anti‐inflammatory drugs, opioids, and paracetamol as priority areas) in order to review the evidence under a programme grant for children's pain utilising pharmacological interventions in children and adolescents (Appendix 1).
This review is based on a template for reviews of pharmacotherapies used to relieve pain in infants, children, and adolescents. The aim is for all reviews to use the same methods, based on new criteria for what constitutes reliable evidence (Appendix 2) (Moore 2010a; Moore 2012). This review focused on antidepressants to treat chronic non‐cancer pain.
Description of the condition
This review focused on chronic non‐cancer pain experienced by children and adolescents as a result of any type of chronic disease that occurs throughout the global paediatric population. Children's level of pain can be mild, moderate, or severe, and pain management is an essential element of patient management during all care stages of chronic disease.
As the leading cause of morbidity in the world today, chronic disease (and its associated pain) is a major health concern. Chronic pain can arise in the paediatric population in a variety of pathophysiological classifications: nociceptive, neuropathic, or idiopathic Chronic pain is pain that lasts three months or longer and may be accompanied by changes in lifestyle, personality, and functional abilities, as well as by signs and symptoms of depression (Ripamonti 2008).
Whilst diagnostic and perioperative procedures performed to treat chronic diseases are a known common cause of pain in these patients, this review did not cover perioperative pain or adverse effects of treatments such as mucositis.
Description of the intervention
Antidepressants have been used for pain relief and pain management since the 1970s (Walsh 1983; Watson 1982). The clinical impression from extended use over many years is that antidepressants are useful for some neuropathic pain symptoms, and that effects on pain relief are divorced and different from effects on depression; for example, the effects of tricyclic antidepressants on pain may occur at different, and often lower, doses than those on depression. Amitriptyline is one of the most commonly used drugs for treating neuropathic pain in the UK (Hall 2013).
The antidepressants include:
tricyclic antidepressants: amitriptyline, nortriptyline, imipramine, desipramine, clomipramine, maprotiline, plus others;
serotonin noradrenaline (norepinephrine) reuptake inhibitors: duloxetine, venlafaxine, milnacipran;
selective serotonin reuptake inhibitors: fluoxetine, paroxetine, citalopram, sertraline; and
dopamine noradrenaline reuptake inhibitors: bupropion.
How the intervention might work
Pain pathways are complicated, with multiple possible points for actions of drugs (Dickenson 2007). Different antidepressant drugs have different mechanisms of action, thus producing a variety of neurological effects and analgesic outcomes.
Reinforcement of the descending inhibitory pathways by increasing the amount of norepinephrine (noradrenaline) and serotonin in the synaptic cleft at both supraspinal and spinal levels is considered to be a major mechanism, as well as blockage of sodium channels. Other suggested mechanisms include postsynaptic alpha‐adrenergic, H1‐histaminergic, and muscarinic cholinergic receptor‐blocking effects, and N‐methyl‐D‐aspartate (NMDA) antagonism (Dharmshaktu 2012).
Drugs differ in that they may have greater or lesser of each of these effects, or in some cases the effects may be absent. This means that while antidepressants may appear very similar to one another, they may have different effects on pain relief in different populations and individuals. For example, a comparison of amitriptyline with nortriptyline in one cross‐over study in postherpetic neuralgia found that out of 31 participants, 5 had mild or no pain with amitriptyline but moderate to severe pain with nortriptyline, while 4 had good pain relief with nortriptyline but none with amitriptyline (Watson 1998). Despite the diverse and poorly understood mechanisms of action, antidepressants such as amitriptyline and duloxetine are considered to be an essential component of the therapeutic strategy for treatment of many types of neuropathic pain (Finnerup 2015; Moulin 2014; NICE 2013).
Why it is important to do this review
The paediatric population is at risk of inadequate management of pain (AMA 2013). Some conditions that would be aggressively treated in adult patients are being managed with insufficient analgesia in younger populations (AMA 2013). Although there have been repeated calls for best evidence to treat children's pain, such as Eccleston 2003, there are no easily available summaries of the most effective paediatric pain relief.
This review formed part of a Programme Grant addressing the unmet needs of people with chronic pain, commissioned by the National Institute for Health Research (NIHR) in the UK. This topic was identified in June 2015 during consultation with experts in paediatric pain. Please see Appendix 1 for full details of the meeting. The standards used to assess evidence in chronic pain trials have changed substantially in recent years, with particular attention being paid to trial duration, withdrawals, and statistical imputation following withdrawal, all of which can substantially alter estimates of efficacy. The most important change was to encourage a move from using average pain scores, or average change in pain scores, to the number of people who have a large decrease in pain (by at least 50%). Pain intensity reduction of 50% or more has been shown to correlate with improvements in comorbid symptoms, function, and quality of life (Moore 2011a). These standards are set out in the reference guide for pain studies (AUREF 2012).
Objectives
To assess the analgesic efficacy and adverse events of antidepressants used to treat chronic non‐cancer pain in children and adolescents aged between birth and 17 years, in any setting.
Methods
Criteria for considering studies for this review
Types of studies
We only included randomised controlled trials, with or without blinding, and participant‐ or observer‐reported outcomes.
Full journal publication was required, with the exception of online clinical trial results, summaries of otherwise unpublished clinical trials, and abstracts with sufficient data for analysis. We included studies published in any language. We excluded abstracts (usually meeting reports) or unpublished data, non‐randomised studies, studies of experimental pain, case reports, and clinical observations.
Types of participants
We included studies of infants, children, and adolescents, aged from birth to 17 years old, with chronic or recurrent pain (lasting for three months or longer), arising from genetic conditions, neuropathy, or other conditions. These included but were not limited to chronic musculoskeletal pain and chronic abdominal pain.
We excluded studies of perioperative pain, acute pain, cancer pain, headache, migraine, and pain associated with primary disease or its treatment.
We included studies of participants with more than one type of chronic pain, and then analysed results according to the primary condition.
Types of interventions
We included studies reporting interventions prescribing antidepressants for the relief of chronic non‐cancer pain, by any route, in any dose, with comparison to a placebo or any active comparator.
Types of outcome measures
In order to be eligible for inclusion in this review, studies had to report pain assessment, as well as meeting the other selection criteria.
We included trials measuring pain intensity and pain relief assessed using validated tools such as numerical rating scale (NRS), visual analogue scale (VAS), Faces Pain Scale ‐ Revised (FPS‐R), Colour Analogue Scale (CAS), or any other validated numerical rating scale.
We were particularly interested in Pediatric Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (PedIMMPACT) definitions for moderate and substantial benefit in chronic pain studies (PedIMMPACT 2008). These are defined as: at least 30% pain relief over baseline (moderate); at least 50% pain relief over baseline (substantial); much or very much improved on Patient Global Impression of Change (PGIC) scale (moderate); very much improved on PGIC (substantial).
These outcomes differ from those used in most earlier reviews, concentrating as they do on dichotomous outcomes where pain responses do not follow a normal (Gaussian) distribution. People with chronic pain desire high levels of pain relief, ideally more than 50% pain intensity reduction, and ideally having no worse than mild pain (Moore 2013a; O'Brien 2010).
We also recorded any reported adverse events. We reported the timing of outcome assessments.
Primary outcomes
Participant‐reported pain relief of 30% or greater
Participant‐reported pain relief of 50% or greater
PGIC much or very much improved
In the absence of self reported pain, we considered the use of 'other‐reported' pain, typically an observer such as a parent, carer, or healthcare professional (Stinson 2006; von Baeyer 2007).
Secondary outcomes
We identified the following with reference to the PedIMMPACT recommendations, which suggest core outcome domains and measures for consideration in paediatric acute and chronic/recurrent pain clinical trials (PedIMMPACT 2008).
Carer Global Impression of Change
Requirement for rescue analgesia
Sleep duration and quality
Acceptability of treatment
Physical functioning as defined by validated scales
Quality of life as defined by validated scales
Any adverse events
Withdrawals due to adverse events
Any serious adverse event. Serious adverse events typically include any untoward medical occurrence or effect that at any dose results in death, is life‐threatening, requires hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability or incapacity, is a congenital anomaly or birth defect, is an 'important medical event' that may jeopardise the patient, or may require an intervention to prevent one of the above characteristics or consequences.
Search methods for identification of studies
We developed the search strategy based on previous strategies used by the Cochrane Pain, Palliative and Supportive Care Review Group and carried out the searches.
Electronic searches
We searched the following databases:
Cochrane Central Register of Controlled Trials (CENTRAL) (via the Cochrane Register of Studies Online) searched on 6 September 2016;
MEDLINE (via Ovid) 1947 to week 2 September 2016, searched on 6 September 2016;
Embase (via Ovid) 1947 to week 2 September 2016, searched on 6 September 2016.
We used medical subject headings (MeSH) or equivalent and text word terms. We restricted our search to randomised controlled trials and clinical trials. There were no language or date restrictions. The focus of the keywords in our search terms was on chronic non‐cancer pain and antidepressants. We tailored searches to individual databases. The search strategies for MEDLINE, Embase, and CENTRAL are in Appendix 3, Appendix 4, and Appendix 5, respectively.
Searching other resources
We searched ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch/) on 6 September 2017 for ongoing trials. In addition, we checked reference lists of reviews and retrieved articles for additional studies, and performed citation searches on key articles. We planned to contact experts in the field for unpublished and ongoing trials. We planned to contact study authors for additional information where necessary.
Data collection and analysis
We planned to perform separate analyses according to particular chronic pain conditions. We planned to combine different chronic pain conditions in analyses for exploratory purposes only.
Selection of studies
Two review authors independently determined study eligibility by reading the abstract of each study identified by the search. Review authors independently eliminated studies that clearly did not satisfy the inclusion criteria, and obtained full copies of the remaining studies. Two review authors independently read these studies to select those that met the inclusion criteria, a third review author adjudicating in the event of disagreement. We did not anonymise the studies in any way before assessment. We included a PRISMA flow chart (Figure 1) to illustrate the results of the search and the process of screening and selecting studies for inclusion in the review (Moher 2009), as recommended in section 11.2.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We included studies in the review irrespective of whether measured outcome data were reported in a ‘usable’ way.
1.
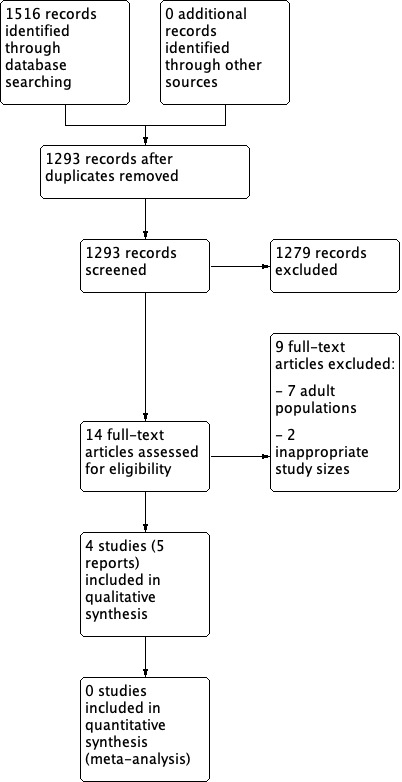
Study flow diagram.
Data extraction and management
We obtained full copies of the studies and two review authors independently carried out data extraction. Where this information was available, we extracted data on pain condition, number of participants treated, drug and dosing regimen, study design (placebo or active control), study duration and follow‐up, analgesic outcome measures and results, withdrawals, and adverse events (participants experiencing any adverse event or serious adverse event). We collated multiple reports of the same study, so that each study rather than each report was the unit of interest in the review. We collected characteristics of the included studies in sufficient detail to populate a ‘Characteristics of included studies' table.
We used a template data extraction form and checked for agreement before entry into Cochrane's statistical software Review Manager 5 (RevMan 2014).
If a study had more than two intervention arms, we only included the data from the intervention and control groups that met the eligibility criteria. If we included multi‐arm studies, we planned to analyse multiple intervention groups in an appropriate way that avoided arbitrary omission of relevant groups and double‐counting of participants.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We completed a 'Risk of bias' table for each included study using the Cochrane 'Risk of bias' tool in Review Manager 5 (RevMan 2014).
We assessed the following for each study. Any disagreements were resolved by discussion between review authors or by consulting a third review author when necessary.
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (i.e. any truly random process, e.g. random number table; computer random number generator); or unclear risk of bias (when the method used to generate the sequence is not clearly stated). We excluded studies that used a non‐random process and were therefore at high risk of bias (e.g. odd or even date of birth; hospital or clinic record number).
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of, or during, recruitment, or changed after assignment. We assessed the methods as: low risk of bias (e.g. telephone or central randomisation; consecutively numbered, sealed, opaque envelopes); or unclear risk of bias (when the method is not clearly stated). We excluded studies that did not conceal allocation and were therefore at a high risk of bias (e.g. open list).
Blinding of participants and personnel (checking for possible performance bias). We assessed any methods used to blind the participants and personnel from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study states that the participants and personnel involved were blinded to treatment groups); unclear risk of bias (study does not state whether or not participants and personnel were blinded to treatment groups); or high risk of bias (participants or personnel were not blinded) (as stated in Types of studies, we included trials with or without blinding, and participant‐ or observer‐reported outcomes).
Blinding of outcome assessment (checking for possible detection bias). We assessed any methods used to blind the outcome assessors from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (e.g. study states that it was single‐blinded and describes the method used to achieve blinding of the outcome assessor); unclear risk of bias (study states that outcome assessors were blinded but does not provide an adequate description of how this was achieved); or high risk of bias (outcome assessors were not blinded) (as stated in Types of studies, we included trials with or without blinding, and participant‐ or observer‐reported outcomes).
Incomplete outcome data (checking for possible attrition bias due to the amount, nature, and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as: low risk of bias (i.e. less than 10% of participants did not complete the study or used 'baseline observation carried forward' (BOCF) analysis, or both); unclear risk of bias (used 'last observation carried forward' (LOCF) analysis); or high risk of bias (used 'completer' analysis).
Selective reporting (checking for possible reporting bias). We assessed the methods used to report the outcomes of the study as: low risk of bias (if all planned outcomes in the protocol or methods were reported in the results); unclear risk of bias (if there was not a clear distinction between planned outcomes and reported outcomes); or high risk of bias (if some planned outcomes from the protocol or methods were clearly not reported in the results).
Size of study (checking for possible biases confounded by small size) (Dechartres 2013; Dechartres 2014; McQuay 1998; Nüesch 2010; Thorlund 2011). We assessed studies as being at low risk of bias (200 participants or more per treatment arm); unclear risk of bias (50 to 199 participants per treatment arm); or high risk of bias (fewer than 50 participants per treatment arm).
Other bias. We assessed studies for any additional sources of bias as low, unclear, or high risk of bias, and provided rationale.
Measures of treatment effect
Where dichotomous data were available, we planned to calculate a risk ratio (RR) with 95% confidence interval (CI) and meta‐analyse the data as appropriate. We planned to calculate numbers needed to treat for an additional beneficial outcome (NNTBs) where appropriate (McQuay 1998); for unwanted effects the NNTB becomes the number needed to treat for an additional harmful outcome (NNTH) and is calculated in the same manner. Where continuous data were reported, we planned to use appropriate methods to combine these data in the meta‐analysis.
Unit of analysis issues
We accepted randomisation to the individual participant only. We split the control treatment arm between active treatment arms in a single study if the active treatment arms were not combined for analysis. We only accepted studies with minimum 10 participants per treatment arm.
Dealing with missing data
We planned to use intention‐to‐treat analysis where the intention‐to‐treat population consisted of participants who were randomised, took at least one dose of the assigned study medication, and provided at least one postbaseline assessment. We planned to assign missing participants zero improvement wherever possible.
Assessment of heterogeneity
We planned to identify and measure heterogeneity as recommended in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We planned to deal with clinical heterogeneity by combining studies that examined similar conditions. We planned to undertake and present a meta‐analysis only if we judged participants, interventions, comparisons, and outcomes to be sufficiently similar to ensure a clinically meaningful answer. We planned to assess statistical heterogeneity visually and by using the I² statistic (L'Abbé 1987). When I² was greater than 50%, we planned to consider the possible reasons.
Assessment of reporting biases
We assessed the risk of reporting bias, as recommended in chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
The aim of this review was to use dichotomous outcomes of known utility and of value to patients (Hoffman 2010; Moore 2010b; Moore 2010c; Moore 2010d; Moore 2013a). This review did not depend on what the authors of the original studies chose to report or not, though clearly difficulties would arise in studies failing to report any dichotomous results. We extracted and planned to use continuous data, but these are useful for illustrative purposes only.
We assessed publication bias using a method designed to detect the amount of unpublished data with a null effect required to make any result clinically irrelevant (usually taken to mean a number needed to treat (NNT) of 10 or higher) (Moore 2008).
Data synthesis
We planned to use a fixed‐effect model for meta‐analysis. We planned to use a random‐effects model for meta‐analysis if there was significant clinical heterogeneity and we considered it appropriate to combine studies. We planned to conduct our analysis using the primary outcomes of pain and adverse events, and to calculate the NNTHs for adverse events. We planned to use the Cochrane software program Review Manager 5 (RevMan 2014).
Quality of the evidence
To analyse data, two review authors independently rated the quality of each outcome. We used the GRADE approach to assess the quality of the body of evidence related to each of the key outcomes, and reported our judgement in a 'Summary of findings' table per Chapter 12 of the Cochrane Handbook (Appendix 6) (Higgins 2011).
In addition, there may be circumstances where the overall rating for a particular outcome would need to be adjusted as recommended by GRADE guidelines (Guyatt 2013a). For example, if there were so few data that the results were highly susceptible to the random play of chance, or if studies used LOCF imputation in circumstances where there were substantial differences in adverse event withdrawals, one would have no confidence in the result, and would need to downgrade the quality of the evidence by three levels, to very low quality. In addition, in circumstances where no data were reported for an outcome, we planned to report that there was no evidence to support or refute (Guyatt 2013b).
'Summary of findings' table
We included a 'Summary of findings' table as set out in the Cochrane Pain, Palliative and Supportive Care Review Group’s author guide (AUREF 2012), and recommended in section 4.6.6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We planned to justify and document all assessments of the quality of the body of evidence.
In an attempt to interpret reliability of the findings for this systematic review, we planned to assess the summarised data using the GRADE guidelines (Appendix 6) to rate the quality of the body of evidence of each of the key outcomes listed in Types of outcome measures per Chapter 12 of the Cochrane Handbook (Guyatt 2011; Higgins 2011), as appropriate. Utilising the explicit criteria against study design, risk of bias, imprecision, inconsistency, indirectness, and magnitude of effect, we planned to summarise the evidence in an informative, transparent, and succinct 'Summary of findings' table or 'Evidence profile' table (Guyatt 2011).
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses where a minimum number of data were available (at least 200 participants per treatment arm). We planned to analyse according to age group; type of drug; geographical location or country; type of control group; baseline measures; frequency, dose, and duration of drugs; and nature of drug.
We planned to investigate whether the results of subgroups were significantly different by inspecting the overlap of confidence intervals and by performing the test for subgroup differences available in Review Manager 5.
Sensitivity analysis
We did not plan to carry out any sensitivity analysis because the evidence base is known to be too small to allow reliable analysis; we did not plan to pool results from chronic pain of different origins in the primary analyses. We planned to examine details of dose escalation schedules in the unlikely circumstance that this could provide some basis for a sensitivity analysis.
Results
Description of studies
Results of the search
A PRISMA flow diagram of the search results is shown in Figure 1.
The three main database searches revealed 1516 records of titles and abstracts, of which 223 duplicates were removed. Our searches of ClinicalTrials.gov and the WHO ICTRP yielded no additional eligible studies.
We screened the remaining 1293 titles and abstracts for eligibility, removing 1279 as ineligible studies.
We retrieved the full‐text reports of the 14 remaining studies. Nine were ineligible. We identified no ongoing studies. Four studies (five reports) fulfilled the eligibility criteria and provided data. Due to these studies investigating different antidepressant drugs and type of chronic pain condition, we could enter none into a quantitative meta‐analysis.
Included studies
See Characteristics of included studies.
Bahar 2008 investigated 33 participants in a single‐centre, randomised, double‐blind, placebo‐controlled, parallel‐group study. Participants were adolescents diagnosed with irritable bowel syndrome (IBS) based on Rome II criteria (see full article for details of the criteria) (Bahar 2008). Participants were 12 to 18 years old, and 73% were female. Participants received oral capsules of amitriptyline 10 to 30 mg/day (depending on body weight), or an oral placebo capsule, once per day for eight weeks. People were excluded if they were currently receiving any concurrent pharmacotherapy for depression, anxiety, or chronic pain syndromes.
Brown 2016 investigated 34 participants in a single‐centre, randomised, double‐blind, active comparator‐controlled, parallel‐group study. Participants had a diagnosis of complex regional pain syndrome type 1 (CRPS‐I) or neuropathic pain and had been recommended for pharmacological treatment with either gabapentin or amitriptyline by clinical physician. Participants were 7 to 18 years old, and 82% were female. A fixed dose of oral amitriptyline was administered in doses of 10 mg/day, and a fixed dose of oral gabapentin in doses of 900 mg/day (300 mg x 3), both for six weeks. People were excluded if they were lactose intolerant; pregnant; previously using either gabapentin or amitriptyline for the treatment of CRPS‐I or neuropathic pain; or had health conditions requiring the regular use of anticholinergics, antihypertensives, anticonvulsants, H2 receptor antagonists, antidepressants, sympathomimetics, thyroid replacements, antacids, or analgesics.
Roohafza 2014 investigated 115 participants in a single‐centre, randomised, double‐blind, placebo‐controlled, parallel‐group study. Participants were school‐aged children who fulfilled Rome III diagnostic criteria for functional abdominal pain. Participants were 6 to 18 years old with 74.4% female in the intervention group and 55.8% female in the placebo group. Participants received oral capsules of citalopram 10 mg/day or an oral placebo capsule, once per day, for the first week, then citalopram 20 mg/day, or an oral placebo capsule, once per day, for the second, third, and fourth weeks. People were excluded if they had other concomitant gastrointestinal disorders or a history of receiving psychotropic drugs, antibiotics, or probiotics in the preceding two months.
Saps 2009 investigated 90 participants in a multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group study. Participants had a diagnosis of functional abdominal pain, functional dyspepsia, and IBS according to the Rome II criteria. Participants were 8 to 17 years old, and 73% were female. Participants received oral capsules of amitriptyline 10 mg/day (or 20 mg/day if > 35 kg), or an oral placebo capsule, once per day, for four weeks. People were excluded if they were diagnosed with an organic disease, plotted below the fifth percentile for weight or height, had abnormal testing (electrocardiogram, complete blood count, erythrocyte sedimentation rate, albumin, pancreatic and liver enzymes, urine analysis, stool examination for occult blood, ova, and parasites, tissue transglutaminase), had a positive lactose breath test or had a history of symptoms resolving after two weeks of a lactose‐free diet.
Excluded studies
See Characteristics of excluded studies.
We excluded nine studies in this review. Upon reading the full texts, we discovered seven were adult populations (Agius 2013; Alencar 2014; Chappell 2009; Guler 2005; Johnson 1997; Kalita 2014; Talaei 2009), one study had fewer than 10 participants in the treatment arm (Arnold 2015), and one study was an N‐of‐1 trial (Huber 2007).
Risk of bias in included studies
A summary of the 'Risk of bias' assessment is shown in Figure 2. Full details of 'Risk of bias' assessments are found in the Characteristics of included studies tables.
2.
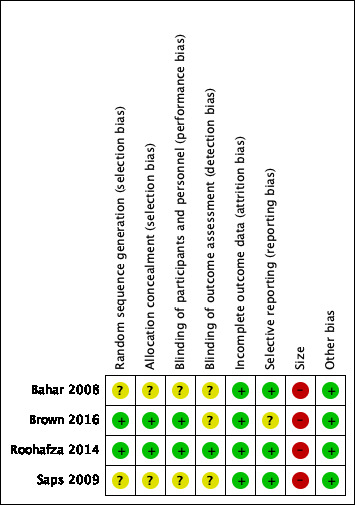
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
All four studies randomly allocated their participants to the intervention and control groups.
Two studies adequately described their randomisation methods (Brown 2016; Roohafza 2014). We judged these two studies as at low risk of selection bias for random sequence generation.
Two studies did not adequately describe their methods of randomisation (Bahar 2008; Saps 2009). We judged these two studies as at unclear risk of selection bias for random sequence generation.
No studies displayed a high risk of selection bias for random sequence generation.
Allocation concealment
All four studies claimed to conceal allocation of the intervention and control groups to their participants.
Two studies adequately described their randomisation methods (Brown 2016; Roohafza 2014). We judged these two studies as at low risk of selection bias for allocation concealment.
Two studies did not adequately describe their methods of randomisation (Bahar 2008; Saps 2009). We judged these two studies as at unclear risk of selection bias for allocation concealment.
No studies displayed a high risk of selection bias for allocation concealment.
Blinding
Performance bias
Two studies adequately described their methods of double‐blinding participants and personnel (Brown 2016; Roohafza 2014). We judged these two studies as at low risk of performance bias.
Two studies did not adequately describe methods used to double blind participants and personnel (Bahar 2008; Saps 2009). We judged these two studies as at unclear risk of performance bias.
No studies displayed a high risk of performance bias.
Detection bias
One study adequately described the blinding of their outcome assessment measurements (Roohafza 2014). We judged this study as at low risk of detection bias.
Three studies did not adequately describe blinding of their outcome assessment measurements (Bahar 2008; Brown 2016; Saps 2009). We judged these three studies as at unclear risk of detection bias.
No studies displayed a high risk of detection bias.
Incomplete outcome data
In all four included studies, all participants were accounted for in terms of withdrawals and completion of treatment, with reasons provided (Bahar 2008; Brown 2016; Roohafza 2014; Saps 2009). We judged these four studies as at low risk of attrition bias.
No studies displayed an unclear or high risk of attrition bias.
Selective reporting
In three studies, the planned outcomes listed in the methods section were appropriately reported in the results section (Bahar 2008; Roohafza 2014; Saps 2009). We judged these three studies as at low risk of selective reporting bias.
Brown 2016 reported unclear results that were difficult to link to the planned primary and secondary outcomes (e.g. the outcome disruption of school, social, and sports was not clearly identified in the published paper). We judged this study as at unclear risk of reporting bias.
No studies displayed a high risk of reporting bias.
Other potential sources of bias
Size
Three studies investigated fewer than 50 participants per treatment arm (Bahar 2008 total of 33 participants, Brown 2016 total of 34 participants, and Saps 2009 total of 90 participants). We judged these three studies as at high risk of bias for size.
Roohafza 2014 investigated a total of 115 participants, however fewer than 50 per treatment arm (43 in each treatment group) completed the study. We also judged this study as at high risk of bias for size.
No studies displayed a low or unclear risk of bias for size.
Other
We found no other potential sources of bias in any of the included studies. We judged these studies as at low risk of other bias.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Amitriptyline compared with gabapentin for chronic non‐cancer pain.
| Amitriptyline compared with gabapentin for chronic non‐cancer pain | ||||||
|
Patient or population: children and adolescents (birth to 17 years) with chronic non‐cancer pain Settings: single‐centre, chronic pain clinic, Canada Intervention: amitriptyline Comparison: gabapentin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Gabapentin | Amitriptyline | |||||
| Participant‐reported pain relief of 30% or greater | No data | No data | No data | No data | No evidence to support or refuteb | |
| Participant‐reported pain relief of 50% or greater | No data | No data | No data | No data | No evidence to support or refuteb | |
| Patient Global Impression of Change: much improved or very much improved | No data | No data | No data | No data | No evidence to support or refuteb | |
| Adverse events | 1/17 | 2/17 | N/A | 34 participants (1 study) |
⊕⊝⊝⊝ very lowa | |
| Serious adverse events | 0/17 | 0/17 | N/A | 34 participants (1 study) |
⊕⊝⊝⊝ very lowa | |
| Withdrawals due to adverse events | 2/17 | 1/17 | N/A | 34 participants (1 study) |
⊕⊝⊝⊝ very lowa | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; N/A: not applicable; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded three levels due to too few data and number of events were too small to be meaningful.
bNo data available for this outcome, and therefore no GRADE rating has been applied and there is no evidence to support or refute.
Summary of findings 2. Amitriptyline compared with placebo for chronic non‐cancer pain.
| Amitriptyline compared with placebo for chronic non‐cancer pain | ||||||
|
Patient or population: children and adolescents (birth to 17 years) with chronic non‐cancer pain Settings: single‐ and multicentre; (1) private outpatient practice paediatric gastroenterology clinic, California, USA; (2) paediatric gastroenterology clinics, (6) tertiary care centres, USA Intervention: amitriptyline Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Placebo | Amitriptyline | |||||
| Participant‐reported pain relief of 30% or greater | No data | No data | No data | No data | No evidence to support or refuteb | |
| Participant‐reported pain relief of 50% or greater | No data | No data | No data | No data | No evidence to support or refuteb | |
| Patient Global Impression of Change: much improved or very much improved | No data | No data | No data | No data | No evidence to support or refuteb | |
| Adverse events | 1/61 | 2/62 | N/A | 123 participants (2 studies) |
⊕⊝⊝⊝ very lowa | |
| Serious adverse events | 0/61 | 0/62 | N/A | 123 participants (2 studies) |
⊕⊝⊝⊝ very lowa | |
| Withdrawals due to adverse events | 1/61 | 2/62 | N/A | 123 participants (2 studies) |
⊕⊝⊝⊝ very lowa | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; N/A: not applicable; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded three levels due to too few data and number of events were too small to be meaningful.
bNo data available for this outcome, and therefore no GRADE rating has been applied and there is no evidence to support or refute.
Summary of findings 3. Citalopram compared with placebo for chronic non‐cancer pain.
| Citalopram compared with placebo for chronic non‐cancer pain | ||||||
|
Patient or population: children and adolescents with functional abdominal pain Settings: single‐centre, tertiary outpatient clinic of paediatric gastroenterology, Iran Intervention: citalopram Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Placebo | Citalopram | |||||
| Participant‐reported pain relief of 30% or greater | No data | No data | No data | No data | No evidence to support or refuteb | |
| Participant‐reported pain relief of 50% or greater | No data | No data | No data | No data | No evidence to support or refuteb | |
| Patient Global Impression of Change: much improved or very much improved | No data | No data | No data | No data | No evidence to support or refuteb | |
| Adverse events | 0/56 | 5/59 | N/A | 115 participants (1 study) |
⊕⊝⊝⊝ very lowa | |
| Serious adverse events | 0/56 | 0/59 | N/A | 115 participants (1 study) |
⊕⊝⊝⊝ very lowa | |
| Withdrawals due to adverse events | 0/56 | 5/59 | N/A | 115 participants (1 study) |
⊕⊝⊝⊝ very lowa | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; N/A: not applicable; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded three levels due to too few data and number of events are too small to be meaningful.
bNo data available for this outcome, and therefore no GRADE rating has been applied and there is no evidence to support or refute.
Results and outcomes of the individual studies are shown in Appendix 7 and Appendix 8.
Comparison 1: Antidepressants versus an active comparator
One study, Brown 2016, investigated amitriptyline versus gabapentin in people with CRPS‐I or neuropathic pain.
Primary outcomes
No data were reported for our three primary outcomes: participant‐reported pain relief of 30% or greater; participant‐reported pain relief of 50% or greater; and PGIC much or very much improved.
Due to the lack of evidence, we were unable to judge the quality (no evidence to support or refute).
Secondary outcomes
Sleep duration and quality
Brown 2016 reported the average decrease in sleep score on a 5‐point Likert scale (mean (±standard deviation) (m (±SD)). For participants who completed the study, the mean decrease in sleep score for 12 amitriptyline participants was 1.25 (±1.86) and for 14 gabapentin participants was 0.46 (±1.60), P = 0.75 . For all participants, the mean decrease in sleep score for 17 amitriptyline participants was 0.88 (±1.69) and for 17 gabapentin participants was 0.38 (±1.45), P = 0.77 (very low‐quality evidence).
Any adverse event
Brown 2016 reported the number of participants who experienced at least 1 adverse event: 1 for gabapentin (1 event) and 2 for amitriptyline (1 event per participant). Adverse events in all studies, across active treatment and comparator groups, were considered to be mild reactions such as nausea, dizziness, drowsiness, tiredness, and abdominal discomfort (very low‐quality evidence).
Withdrawals due to adverse events
Total withdrawals were low: 2 (12%) in the gabapentin group and 1 (5.8%) in the amitriptyline group (very low‐quality evidence) (Brown 2016).
Withdrawals due to adverse events were low: 2 (12%) in the gabapentin group and 1 (5.8%) in the amitriptyline group (very low‐quality evidence) (Brown 2016).
Serious adverse event
No serious adverse events were reported in Brown 2016 (very low‐quality evidence).
Other secondary outcomes
No data were reported for our remaining secondary outcomes: Carer Global Impression of Change; requirement for rescue analgesia; acceptability of treatment; physical functioning; and quality of life.
Due to the lack of evidence, we were unable to judge the quality (no evidence to support or refute).
Quality of the evidence
There is no evidence to support or refute the use of antidepressants versus an active comparator across our primary outcomes and some of our secondary outcomes.
The quality of evidence for antidepressants versus an active comparator across our remaining secondary outcomes is very low‐quality, due to too few data and the fact that the number of events was too small to be meaningful.
See Table 1.
Comparison 2: Antidepressants versus placebo
Three studies investigated antidepressants compared with a placebo.
Bahar 2008 investigated amitriptyline versus placebo in people with IBS. Saps 2009 investigated amitriptyline versus placebo in people with IBS and functional abdominal pain. Roohafza 2014 investigated citalopram versus placebo in people with functional abdominal pain. Consequently, no two studies were comparable by pain condition or by type of drug with adequate data, and we were unable to perform any quantitative analysis of our outcomes.
Primary outcomes
No data were reported for our three primary outcomes: participant‐reported pain relief of 30% or greater; participant‐reported pain relief of 50% or greater; and PGIC much or very much improved.
Due to the lack of evidence, we were unable to judge the quality (no evidence to support or refute).
Bahar 2008 reported no significant reduction for a variety of symptoms including abdominal pain. Saps 2009 reported a significant decrease (P < 0.05) of pain in both groups (amitriptyline and placebo), and therefore no difference between the two treatments. Roohafza 2014 reported that the intention‐to‐treat analysis found no difference between citalopram and placebo.
Secondary outcomes
Quality of life as defined by validated scales
Bahar 2008 reported a quality of life score as a mean difference overall (dysphoria + interference with activity + health worry + food avoidance) from baseline to end of week 13. The mean difference was 126.2 in the amitriptyline group and 129.8 in the placebo group (P = 0.002) (very low‐quality evidence).
Any adverse events
Adverse events were minimal and mild across the three studies: amitriptyline 0, placebo 0 (Bahar 2008); citalopram 5, placebo 0 (Roohafza 2014); amitriptyline 2, placebo 1 (Saps 2009). Adverse events in all studies, across active treatment and comparator groups, were considered to be mild reactions such as nausea, dizziness, drowsiness, tiredness, and abdominal discomfort (very low‐quality evidence).
Withdrawals due to adverse events
All three studies reported withdrawals due to adverse events: amitriptyline 0, placebo 0 (Bahar 2008); citalopram 5, placebo 0 (Roohafza 2014); amitriptyline 2, placebo 1 (Saps 2009) (very low‐quality evidence).
Serious adverse events
No serious adverse events occurred in any of the three studies (very low‐quality evidence).
Other secondary outcomes
No data were reported for our remaining secondary outcomes: Carer Global Impression of Change; requirement for rescue analgesia; sleep duration and quality; acceptability of treatment; and physical functioning.
Quality of the evidence
There is no evidence to support or refute the use of antidepressants versus an active comparator across our primary outcomes and some of our secondary outcomes.
The quality of evidence for antidepressants versus an active comparator across our remaining secondary outcomes is very low‐quality, due to too few data and the fact that the number of events was too small to be meaningful.
Discussion
Summary of main results
We included four studies reporting data from 272 participants (6 to 18 years of age), comparing amitriptyline with gabapentin; amitriptyline versus placebo; or citalopram versus placebo.
Of the two studies comparing the same treatments (amitriptyline versus placebo), in the same condition (IBS) (Bahar 2008; Saps 2009), Bahar 2008 did not provide pain‐related outcome data, therefore no two studies were comparable by type of pain condition and by type of antidepressant. With no outcome data available for the two studies, we were unable to perform any quantitative analysis of our outcomes.
Risk of bias for the four included studies varied, due to issues with randomisation and allocation concealment (low to unclear risk); blinding of participants, personnel, and outcome assessors (low to unclear risk); reporting of results (low to unclear risk); and size of the study populations (high risk). We judged the remaining domains, attrition and other potential sources of bias, as low risk of bias.
We found no evidence from randomised controlled trials to suggest that antidepressants are effective in treating chronic non‐cancer pain in children or adolescents, nor do we have evidence to suggest that one antidepressant is more effective than another. We were unable to comment on harm.
Overall completeness and applicability of evidence
This review identified only a small number of studies, with insufficient data for analysis. Bahar 2008 and Saps 2009 were the only two studies to compare amitriptyline with placebo in the same condition (IBS); however, Bahar 2008 did not provide pain‐related outcome data.
As we could undertake no meta‐analysis, we are unable to make a judgement about the efficacy or harm from the use of antidepressants to treat chronic non‐cancer pain in children and adolescents. Similarly, we cannot comment on our remaining secondary outcomes: Carer Global Impression of Change; requirement for rescue analgesia; sleep duration and quality; acceptability of treatment; physical functioning; and quality of life.
There is evidence from adult randomised controlled trials that some antidepressants, such as amitriptyline, can provide some pain relief in certain chronic non‐cancer pain conditions (Moore 2015).
The suite of reviews
This review is part of a suite of reviews on pharmacological interventions for chronic pain and cancer‐related pain in children and adolescents (Appendix 1). Taking a broader view on this suite of reviews, some pharmacotherapies (investigated in our other reviews) are likely to provide more data than others. The results were thus as expected considering that randomised controlled trials in children are known to be limited. The results have the potential to inform policymaking decisions for funding future clinical trials into antidepressant treatment of child and adolescent pain, therefore any results (large or small) are important in order to capture a snapshot of the current evidence for antidepressants.
Quality of the evidence
Of the four included studies, only two clearly described the randomisation methods, one clearly described the double‐blinding method, and all studies provided information about withdrawals, dropouts, and adverse events (Figure 2).
The studies recruited participants with adequate baseline pain but did not report clinically useful outcome measures.
The studies themselves were of moderate quality and validity, but the number of studies and sample sizes for these comparisons were somewhat limited, given what is known about study size and estimates of effect for outcomes derived from studies with few participants and events (Dechartres 2013; Dechartres 2014; McQuay 1998; Nüesch 2010; Thorlund 2011).
There was no evidence to support or refute the use of antidepressants, versus an active comparator or a placebo, across our primary outcomes and some of our secondary outcomes. Across the remaining secondary outcomes, the quality of the evidence is very low, due to too few data and the fact that the number of events was too small to be meaningful.
Potential biases in the review process
We carried out extensive searches of major databases using broad search criteria, and also searched two large clinical trial registries. We consider it to be unlikely that we have missed relevant studies.
Agreements and disagreements with other studies or reviews
We were not able to identify any published systematic reviews on this topic.
Authors' conclusions
Implications for practice.
General
We identified four randomised controlled trials, however we were unable to analyse these to determine whether to support or refute the use of antidepressants to treat chronic non‐cancer pain in children and adolescents. There is evidence from adult randomised controlled trials that some antidepressants, such as amitriptyline, can provide some pain relief in certain chronic neuropathic pain conditions (Moore 2015).
This is disappointing as children and adolescents with chronic non‐cancer pain have specific needs for analgesia.
In current practice, despite the lack of evidence on effectiveness and safety, and despite lack of licensing for use in pain below 18 years of age, clinicians prescribe antidepressants to children and adolescents when medically necessary, based on extrapolation from adult guidelines. However, extrapolating from adult data may be unreliable, and risks compromising safety in children and adolescents.
The only current guidelines are from the World Health Organization on the pharmacological treatment of persisting pain in children with medical illnesses (WHO 2012).
For children and adolescents with chronic non‐cancer pain
The amount and quality of evidence around the use of antidepressants for treating chronic non‐cancer pain is very low. This means that at present, treatment is based on clinical experience and advice from respected authorities. We could make no judgement about adverse events or withdrawals.
For clinicians
The amount and quality of evidence for the use of antidepressants for treating chronic non‐cancer pain is very low. This means that at present, treatment is based on clinical experience and advice from respected authorities. We could make no judgement about adverse events or withdrawals.
For policymakers
The amount and quality of evidence for the use of antidepressants for treating chronic non‐cancer pain is very low. This means that at present, treatment is based on clinical experience and advice from respected authorities. We could make no judgement about adverse events or withdrawals.
For funders
The amount and quality of evidence for the use of antidepressants for treating chronic non‐cancer pain is very low. This means that at present, treatment is based on clinical experience and advice from respected authorities. We could make no judgement about adverse events or withdrawals.
Implications for research.
General
The lack of robust evidence of efficacy and safety found in this review highlighted the need to design and fund high quality and clinically relevant research on this topic.
Overall, there appears to be a gap between how antidepressant drugs are used in clinical practice to treat chronic non‐cancer pain in children and adolescents, and how this drug class has been investigated in prospective clinical trials.
The challenge is to develop clinician‐ and patient‐informed trial protocols examining clinically‐meaningful, patient‐centred outcomes.
Design
Several methodological issues stand out.
The first is the use of outcomes of value to children with chronic non‐cancer pain. Existing trials tend to be designed more for purposes of registration and marketing than informing and improving clinical practice, that is the outcomes are often average pain scores or statistical differences, and rarely how many individuals achieve satisfactory pain relief. In the case where pain is initially mild or moderate, consideration needs to be given to what constitutes a satisfactory outcome.
The second issue is the time taken to achieve good pain relief. We have no information about what constitutes a reasonable time to achieve a satisfactory result. This may best be approached initially with a Delphi methodology.
The third issue is design. Studies with a cross‐over design often have significant attrition, therefore parallel‐group designs may be preferable.
The fourth issue is size. The studies need to be suitably powered to ensure adequate data after the effect of attrition due to various causes. Much larger studies of several hundred participants or more are needed.
There are some other design issues that might be addressed. Most important might well be a clear decision concerning the gold‐standard treatment comparator.
An alternative approach may be to design large registry studies. This could provide an opportunity to foster collaboration among paediatric clinicians and researchers, in order to create an evidence base.
Measurement (endpoints)
Trials need to consider the additional endpoint of 'no worse than mild pain' as well as the standard approaches to pain assessment.
Primary outcomes need to be outcomes of value to children with chronic non‐cancer pain and their families. To help them make decisions about drug treatment, families seek to know what chance their child has of achieving relief that is meaningful to them. There is as yet no patient‐defined level of pain relief, or improvement in function, that is considered meaningful. This could be addressed in future using Delphi methodology.
As a surrogate, expert consensus recommends reporting the proportion of participants achieving at least 30% or 50% pain relief, but none of the included studies did so. Consideration could also be given to reporting the proportion of participants achieving the endpoint of ‘no worse than mild pain’ and ‘no pain’. The endpoint might depend on whether participants are selected for having severe pain at baseline, or whether children whose pain is mild‐moderate are included. For fluctuating conditions, endpoints might be proportion of pain‐free days, or days in which pain does not reach a specific level (PedIMMPACT 2008).
Time to achieve benefit was not reported, yet this is of great interest to children and families. Where treatments have equal efficacy, a treatment with earlier onset might still be considered ‘superior’ by consumers. Future research should measure and report the time to achieve outcome and the longevity of benefits.
Other
The obvious study design of choice is the prospective randomised trial, but other pragmatic designs may be worth considering. Studies could incorporate initial randomisation but a pragmatic design in order to provide immediately relevant information on effectiveness and costs. Such designs in pain conditions have been published (Moore 2010e).
What's new
| Date | Event | Description |
|---|---|---|
| 7 June 2019 | Amended | We amended the GRADE methods for assessing no evidence, for consistency with the other reviews in this series. |
| 18 March 2019 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 2, 2017 Review first published: Issue 8, 2017
| Date | Event | Description |
|---|---|---|
| 5 July 2018 | Amended | Searches updated with terms relating to 'infants'. We did not identify any new studies. |
| 14 August 2017 | Amended | References for some reviews from the suite amended to reflect correct publication Issue. |
| 20 February 2017 | Amended | References for cancer pain protocols updated. |
Notes
A restricted search in March 2019 did not identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be re‐assessed for updating in five years. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitates major revisions.
Acknowledgements
We acknowledge the contribution of Christopher Eccleston to the template protocol.
We thank Nanna Brix Finnerup, Andrew Gray, Sebastian Straube, and Andrew Moore for peer reviewing.
Cochrane Review Group funding acknowledgement: the National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pain, Palliative and Supportive Care Review Group. Disclaimer: the views and opinions expressed herein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS), or the Department of Health.
Appendices
Appendix 1. Meeting for NIHR Programme Grant agenda on pain in children
Date
Monday 1st June 2015
Location
International Association of the Study of Pain (IASP) Conference, Seattle, USA
Delegates
Allen Finlay, Anna Erskine, Boris Zernikow, Chantal Wood, Christopher Eccleston, Elliot Krane, George Chalkaiadis, Gustaf Ljungman, Jacqui Clinch, Jeffrey Gold, Julia Wager, Marie‐Claude Gregoire, Miranda van Tilburg, Navil Sethna, Neil Schechter, Phil Wiffen, Richard Howard, Susie Lord.
Purpose
National Institute for Health Research (NIHR) (UK) Programme Grant ‐ Addressing the unmet need of chronic pain: providing the evidence for treatments of pain.
Proposal
Nine reviews in pharmacological interventions for chronic pain in children and adolescents: Children (5 new, 1 update, 1 overview, and 2 rapid) self‐management of chronic pain is prioritised by the planned NICE guideline. Pain management (young people and adults) with a focus on initial assessment and management of persistent pain in young people and adults.
We propose titles in paracetamol, ibuprofen, diclofenac, other NSAIDs, and codeine, an overview review on pain in the community, 2 rapid reviews on the pharmacotherapy of chronic pain, and cancer pain, and an update of psychological treatments for chronic pain.
Key outcomes
The final titles: (1) opioids for cancer‐related pain (Wiffen 2017a), (2) opioids for chronic non‐cancer pain (Cooper 2017a), (3) antiepileptic drugs for chronic non‐cancer pain (Cooper 2017b), (4) antidepressants for chronic non‐cancer pain (this review), (5) non‐steroidal anti‐inflammatory drugs (NSAIDs) for chronic non‐cancer pain (Eccleston 2017), (6) non‐steroidal anti‐inflammatory drugs (NSAIDs) for cancer‐related pain (Cooper 2017c), (7) paracetamol for chronic non‐cancer pain (Cooper 2017d).
PICO
Patients: children, aged 3 to 12, chronic pain defined as pain persisting for 3 months (NB: now changed to: birth to 17 years to include infants, children and adolescents).
Interventions: by drug class including antiepileptic drugs, antidepressants, opioids, NSAIDs, paracetamol.
Comparisons: maintain a separation of cancer and non‐cancer, exclude headache, in comparison with placebo and or active control.
Outcomes: we will adopt the IMMPACT criteria.
Appendix 2. Methodological considerations for chronic pain
There have been several recent changes in how the efficacy of conventional and unconventional treatments is assessed in chronic painful conditions. The outcomes are now better defined, particularly with new criteria for what constitutes moderate or substantial benefit (Dworkin 2008); older trials may only report participants with 'any improvement'. Newer trials tend to be larger, avoiding problems from the random play of chance. Newer trials also tend to be of longer duration, up to 12 weeks, and longer trials provide a more rigorous and valid assessment of efficacy in chronic conditions. New standards have evolved for assessing efficacy in neuropathic pain, and we are now applying stricter criteria for the inclusion of trials and assessment of outcomes, and are more aware of problems that may affect our overall assessment. We summarise some of the recent insights that must be considered in this new review.
Pain results tend to have a U‐shaped distribution rather than a bell‐shaped distribution. This is true in acute pain (Moore 2011a; Moore 2011b), back pain (Moore 2010d), and arthritis (Moore 2010c), as well as in fibromyalgia (Straube 2010); in all cases average results usually describe the experience of almost no one in the trial. Data expressed as averages are potentially misleading, unless they can be proven to be suitable.
As a consequence, we have to depend on dichotomous results (the individual either has or does not have the outcome) usually from pain changes or patient global assessments. The Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) group has helped with their definitions of minimal, moderate, and substantial improvement (Dworkin 2008). In arthritis, trials of less than 12 weeks' duration, and especially those shorter than eight weeks, overestimate the effect of treatment (Moore 2010c); the effect is particularly strong for less effective analgesics, and this may also be relevant in neuropathic‐type pain.
The proportion of patients with at least moderate benefit can be small, even with an effective medicine, falling from 60% with an effective medicine in arthritis to 30% in fibromyalgia (Moore 2009; Moore 2010c; Moore 2013b; Moore 2014b; Straube 2008; Sultan 2008). A Cochrane review of pregabalin in neuropathic pain and fibromyalgia demonstrated different response rates for different types of chronic pain (higher in diabetic neuropathy and postherpetic neuralgia and lower in central pain and fibromyalgia) (Moore 2009). This indicates that different neuropathic pain conditions should be treated separately from one another, and that pooling should not be done unless there are good grounds for doing so.
Individual patient analyses indicate that patients who get good pain relief (moderate or better) have major benefits in many other outcomes, affecting quality of life in a significant way (Moore 2010b; Moore 2014a).
Imputation methods such as last observation carried forward (LOCF), used when participants withdraw from clinical trials, can overstate drug efficacy, especially when adverse event withdrawals with drug are greater than those with placebo (Moore 2012).
Appendix 3. MEDLINE search strategy (via Ovid)
exp Child/
exp Adolescent/
exp Infant/
(child* or boy* or girl* or adolescen* or teen* or toddler* or preschooler* or pre‐schooler* or baby or babies or infant*).tw
1 or 2 or 3 or 4
exp Antidepressive Agents/
(amitriptyline or clomipramine or doxepin or imipramine or nortriptyline or trimipramine or mianserin or trazadone or citalopram or fluoxetine or fluvoxamine or sertraline).mp.
6 or 7
exp Pain/
pain*.tw
9 or 10
5 and 8 and 11
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
13 or 14 or 15 or 16 or 17 or 18 or 19 or 20
exp animals/ not humans.sh.
21 not 22
12 and 23
Appendix 4. Embase search strategy (via Ovid)
exp Child/
exp Adolescent/
exp Infant/
(child* or boy* or girl* or adolescen* or teen* or toddler* or preschooler* or pre‐schooler* or baby or babies or infant*).tw
1 or 2 or 3 or 4
exp antidepressive agents/
(amitriptyline or clomipramine or doxepin or imipramine or nortriptyline or trimipramine or mianserin or trazadone or citalopram or fluoxetine or fluvoxamine or sertraline).mp.
6 or 7
exp Pain/
pain*.tw
9 or 10
5 and 8 and 11
random$.tw.
factorial$.tw.
crossover$.tw.
cross over$.tw.
cross‐over$.tw.
placebo$.tw.
(doubl$ adj blind$).tw.
(singl$ adj blind$).tw.
assign$.tw.
allocat$.tw.
volunteer$.tw.
Crossover Procedure/
double‐blind procedure.tw.
Randomized Controlled Trial/
Single Blind Procedure/
or13/27
(animal/ or nonhuman/) not human/
28 not 29
12 and 30
Appendix 5. CENTRAL search strategy (via Cochrane Register of Studies Online)
MESH DESCRIPTOR Child EXPLODE ALL TREES
MESH DESCRIPTOR Adolescent
MESH DESCRIPTOR Infant EXPLODE ALL TREES
(child* or boy* or girl* or adolescen* or teen* or toddler* or preschooler* or pre‐schooler* or baby or babies or infant*):TI,AB,KY
#1 OR #2 OR #3 or #5
MESH DESCRIPTOR Antidepressive Agents EXPLODE ALL TREES
(amitriptyline or clomipramine or doxepin or imipramine or nortriptyline or trimipramine or mianserin or trazadone or citalopram or fluoxetine or fluvoxamine or sertraline):TI,AB,KY
#6 OR #7
MESH DESCRIPTOR Pain EXPLODE ALL TREES
pain*
#9 or #10
#5 AND #8 AND #11
Appendix 6. GRADE guidelines
Some advantages of utilising the GRADE process are (Guyatt 2008):
transparent process of moving from evidence to recommendations;
clear separation between quality of evidence and strength of recommendations;
explicit, comprehensive criteria for downgrading and upgrading quality of evidence ratings; and
clear, pragmatic interpretation of strong versus weak recommendations for clinicians, patients, and policymakers.
The GRADE system uses the following criteria for assigning grades of evidence:
high: we are very confident that the true effect lies close to that of the estimate of the effect;
moderate: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different;
low: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect; and
very low: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
We will decrease the grade if there is:
serious (‐1) or very serious (‐2) limitation to study quality;
important inconsistency (‐1);
some (‐1) or major (‐2) uncertainty about directness;
imprecise or sparse data (‐1); or
high probability of reporting bias (‐1).
We will increase the grade if there is:
strong evidence of association ‐ significant risk ratio of > 2 (< 0.5) based on consistent evidence from two or more observational studies, with no plausible confounders (+1);
very strong evidence of association ‐ significant risk ratio of > 5 (< 0.2) based on direct evidence with no major threats to validity (+2);
evidence of a dose response gradient (+1); or
all plausible confounders would have reduced the effect (+1).
"In addition, there may be circumstances where the overall rating for a particular outcome would need to be adjusted per GRADE guidelines (Guyatt 2013a). For example, if there were so few data that the results were highly susceptible to the random play of chance, or if studies used LOCF imputation in circumstances where there were substantial differences in adverse event withdrawals, one would have no confidence in the result, and would need to downgrade the quality of the evidence by three levels, to very low quality. In circumstances where no data were reported for an outcome, we planned to report the level of evidence as 'no evidence to support or refute' (Guyatt 2013b)."
Appendix 7. Summary of efficacy in individual studies
| Study | Treatment | Pain outcome | Other efficacy outcome |
| Bahar 2008 |
Intervention group (N = 16): amitriptyline oral capsule 10 to 30 mg/day before bed (10 mg: < 50 kg, 20 mg: 50 to 80 kg, 30 mg: > 80 kg) Control group (N = 17): oral placebo tablet Study duration: 13 weeks: enrolment and symptom scoring, 8 weeks of intervention, washout and symptom scoring |
Participant‐reported pain relief of 30% or greater: Not reported. Participant‐reported pain relief of 50% or greater: Not reported. PGIC much or very much improved: Not reported. |
Carer Global Impression of Change: no data Requirement for rescue analgesia: no data Sleep duration and quality: no data Acceptability of treatment: no data Physical functioning: no data Quality of life: Mean difference overall (dysphoria + interference with activity + health worry + food avoidance) from baseline to end of week 13: Amitriptyline: 126.2 Placebo: 129.8 P = 0.002 Right lower quadrant pain % changes in symptoms from week 0: @ week 6 Amitriptyline: ‐25 ± 14.4 Placebo: 17.6 ± 9.5 P = 0.014 @ week 10 Amitriptyline: ‐12.5 ± 8.5 Placebo: 11.8 ± 8.1 P = 0.039 @ week 13 Amitriptyline: ‐25 ± 11.2 Placebo: 17.6 ± 9.5 P = 0.004 Periumbilical pain % changes in symptoms from week 0: @ week 6 Amitriptyline: ‐18.8 ± 10.1 Placebo: 5.9 ± 10.4 P = 0.089 @ week 10 Amitriptyline: ‐12.5 ± 8.5 Placebo: 17.6 ± 9.5 P = 0.018 @ week 13 Amitriptyline: ‐12.5 ± 12.5 Placebo: 17.6 ± 9.5 P = 0.055 |
| Brown 2016 |
Intervention group (N = 17): oral amitriptyline 10 mg/day Control group (N = 17): oral gabapentin 900 mg/day (300 x 3) Study duration: 6 weeks' duration |
Participant‐reported pain relief of 30% or greater: no data Participant‐reported pain relief of 50% of greater: no data PGIC much or very much improved: no data |
Patient Global Impression of Change: no data Carer Global Impression of Change: no data Requirement for rescue analgesia: no data Sleep duration and quality: Average decrease in sleep score on 5‐point Likert scale Completed participants m (SD) Amitriptyline: 1.25 (1.86); n = 12 Gabapentin: 0.46 (1.60); n = 14 All participants m (SD) Amitriptyline: 0.88 (1.69); n = 17 Gabapentin: 0.38 (1.45); n = 17 Acceptability of treatment: no data Physical functioning: no data Quality of life: no data |
| Roohafza 2014 |
Intervention group (N = 59): oral tablet citalopram 10 mg/day (week 1); 20 mg/day (weeks 2, 3, and 4) Control group (N = 56): placebo tablet/day Study duration: 4 weeks' duration |
Participant‐reported pain relief of 30% or greater: Not reported. Participant‐reported pain relief of 50% or greater: Not reported. PGIC much or very much improved: Not reported. |
Patient Global Impression of Change: see next below Physician‐reported Global Impression of Change: Change in Clinical Global Impression‐Severity Score @ wk 4 Citalopram: ‐3.66 ± 1.40 Placebo: ‐3.09 ± 1.73 Change in Clinical Global Impression‐Severity Score @ wk 12 Citalopram: ‐3.94 ± 1.39 Placebo: ‐3.17 ± 1.55 INTENTION‐TO‐TREAT ANALYSIS Change in Clinical Global Impression‐Severity Score @ wk 4 Citalopram: ‐2.55 ± 2.06 Placebo: ‐2.45 ± 1.99 Change in Clinical Global Impression‐Severity Score @ wk 12 Citalopram: ‐2.89 ± 1.96 Placebo: ‐3.15 ± 1.48 Carer Global Impression of Change: no data Requirement for rescue analgesia: no data Sleep duration and quality: no data Acceptability of treatment: no data Physical functioning: no data Quality of life: no data Change in pain @ wk 4 Citalopram: ‐1.95 ± 1.30 Placebo: ‐1.62 ± 1.52 Change in pain @ wk 12 Citalopram: ‐2.48 ± 1.29 Placebo: ‐1.81 ± 1.46 INTENTION‐TO‐TREAT ANALYSIS Change in pain @ wk 4 Citalopram: ‐1.44 ± 1.40 Placebo: ‐1.29 ± 1.51 Change in pain @ wk 12 Citalopram: ‐1.84 ± 1.56 Placebo: ‐1.44 ± 1.50 |
| Saps 2009 |
Intervention group (N = 46): amitriptyline oral capsule, 10 mg/day < 35 kg or 20 mg/day > 35 kg for 4 weeks Control group (N = 44): oral placebo tablet for 4 weeks Study duration: 5 weeks' duration |
Participant‐reported pain relief of 30% or greater: no data Participant‐reported pain relief of 50% or greater: no data PGIC much or very much improved: no data |
Patient Global Impression of Change: no data Carer Global Impression of Change: no data Requirement for rescue analgesia: no data Sleep duration and quality: no data Acceptability of treatment: no data Physical functioning: no data Quality of life: no data |
| m: mean; N: number of participants; PGIC: Patient Global Impression of Change;SD: standard deviation; | |||
Appendix 8. Summary of adverse events and withdrawals in individual studies
| Study | Treatment | Adverse events | Withdrawals |
| Bahar 2008 |
Intervention group (N = 16): amitriptyline oral capsule 10 to 30 mg/day before bed (10 mg: < 50 kg, 20 mg: 50 to 80 kg, 30 mg: > 80 kg) Control group (N = 17): oral placebo tablet Study duration: 13 weeks: enrolment and symptom scoring, 8 weeks of intervention, washout and symptom scoring |
Total adverse events: Amitriptyline: 0/16 Placebo: 0/17 No. participants with any adverse event: Amitriptyline: 0/16 Placebo: 0/17 No. participants with serious adverse events: Amitriptyline: 0/16 Placebo: 0/17 Specific adverse events: Amitriptyline: 0/16 Placebo: 0/17 No specific side effects were reported. |
Total all‐cause withdrawals: Amitriptyline: 0/16 Placebo: 0/17 (3 withdrawals from intervention group before administration) Withdrawals due to adverse events: Amitriptyline: 0/16 Placebo: 0/17 |
| Brown 2016 |
Intervention group (N = 54): oral amitriptyline 10 mg/day Control group (N = 53): oral gabapentin 900 mg/day (300 x 3) Study duration: 6 weeks' duration |
Total adverse events: Amitriptyline: 1 Gabapentin: 2 No. participants with any adverse event: Completed participants Amitriptyline: 2/14 (14.2%) Gabapentin: 1/15 (6.6%) All participants Amitriptyline: 2/17 (11.7%) Gabapentin: 1/17 (5.9%) No. participants with serious adverse events: Amitriptyline: 0 Gabapentin: 0 Specific adverse events: Not reported. |
Total all‐cause withdrawals: Amitriptyline: 3/17 Gabapentin: 2/17 Withdrawals due to adverse events: Amitriptyline: 1 Gabapentin: 2 |
| Roohafza 2014 |
Intervention group (N = 59): oral tablet citalopram 10 mg/day (week 1); 20 mg/day (weeks 2, 3, and 4) Control group (N = 56): placebo tablet/day Study duration: 4 weeks' duration |
Total adverse events: Citalopram: 5/59 (8%) Placebo: 0/56 (0%) No. participants with any adverse event: Citalopram: 5/59 (8%) Placebo: 0/56 (0%) No. participants with serious adverse events: Citalopram: 0/59 (0%) Placebo: 0/56 (0%) Specific adverse events: Citalopram (n = 43) Insomnia: 2 Nausea: 3 Drowsiness: 16 Dry mouth: 19 Diarrhoea: 0 Vomiting: 0 Fatigue: 8 Headache: 3 Dizziness: 1 Allergic reaction: 1 Loss of appetite: 14 Placebo (n = 43) Insomnia: 1 Nausea: 1 Drowsiness: 7 Dry mouth: 10 Diarrhoea: 0 Vomiting: 0 Fatigue: 6 Headache: 1 Dizziness: 2 Allergic reaction: 0 Loss of appetite: 8 |
Total all‐cause withdrawals: Citalopram: 16/59 (27%) Placebo: 13/56 (23%) (A total of 86 participants completed the 4‐week medication period.) Withdrawals due to adverse events: Citalopram: 5/59 (8%) Placebo: 0/56 (0%) |
| Saps 2009 |
Intervention group (N = 46): amitriptyline oral capsule, 10 mg/day < 35 kg or 20 mg/day > 35 kg for 4 weeks Control group (N = 44): oral placebo tablet for 4 weeks Study duration: 5 weeks' duration |
Total adverse events: Amitriptyline: 2/46 Placebo: 1/44 No. participants with any adverse event: Amitriptyline: 2/46 Placebo: 1/44 No. participants with serious adverse events: Amitriptyline: 0/46 Placebo: 0/44 Specific adverse events: Amitriptyline: 0/46 Placebo: 0/44 |
Total all‐cause withdrawals: Total = 7 Amitriptyline: 2/46 Placebo: 1/44 Unstated which group: 4 Withdrawals due to adverse events: Amitriptyline: 2/46 Placebo: 1/44 |
| N: number of participants | |||
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bahar 2008.
| Methods |
Allocation: randomised Blinding: double‐blind Controlled: placebo Centre: single Arm: 2 arms, parallel groups Study dates: 4‐year trial (2002 to 2005) |
|
| Participants |
Inclusion criteria: adolescents newly diagnosed with IBS based on Rome II criteria Exclusion criteria: currently receiving any concurrent pharmacotherapy for depression, anxiety, or chronic pain syndromes Baseline characteristics N = 33 Age: 12 to 18 years Gender: male (9); female (24) Number randomised: intervention (16); placebo (17) Setting and location: private outpatient practice paediatric gastroenterology clinic, California, USA |
|
| Interventions |
Intervention group (N = 16): amitriptyline oral capsule (10 mg: < 50 kg, 20 mg: 50 to 80 kg, 30 mg: > 80 kg) Control group (N = 17): oral placebo tablet Study duration: 13 weeks' duration: enrolment and symptom scoring, 8 weeks of intervention, washout and symptom scoring |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Sources of funding: Brooks Medical Research Foundation of the California Community Foundation (Los Angeles, California), and AstraZeneca, LP (Wayne, Pennsylvania) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "subjects were randomized in a double‐blind, placebo controlled fashion to receive amitriptyline or placebo" |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk |
Quote: "double‐blind study" Comment: insufficient information provided regarding observer or reporter knowledge. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Insufficient information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: All participants stated to have completed the trial. |
| Selective reporting (reporting bias) | Low risk | Comment: All planned outcomes listed in the methods were reported in the results. |
| Size | High risk | Comment: Total participants = 33. Participants < 50 per treatment arm. |
| Other bias | Low risk | Comment: No other potential sources of bias |
Brown 2016.
| Methods |
Allocation: randomised Blinding: double‐blind Controlled: active comparator Centre: single Arm: 2 arms, parallel groups Study dates: 38‐month trial (April 2006 to July 2010) |
|
| Participants |
Inclusion criteria: CRPS‐I or neuropathic pain and recommendation for pharmacological treatment with gabapentin or amitriptyline by a clinic physician during the patient's intake appointment. Exclusion criteria: unable to speak English, lactose intolerant, pregnant, previously using either gabapentin or amitriptyline for the treatment of CRPS‐I or neuropathic pain or if they were unable to swallow a size “0” gelatin capsule. Children were also excluded if study medications were contraindicated by additional health conditions or the treatment of such conditions, including the regular use of any of the following medications or classes of medications: anticholinergics, antihypertensives, anticonvulsants, H2 receptor antagonists, antidepressants, sympathomimetics, thyroid replacements, antacids, and analgesics. Baseline characteristics N = 34 Age: 7 to 18 years Gender: male (6); female (28) Number randomised: intervention (17); active comparator (17) Number completed: intervention (15); active comparator (14) Setting and location: Chronic Pain Clinic (The Hospital for Sick Children, Toronto, Canada) |
|
| Interventions |
Intervention group (N = 17): oral amitriptyline 10 mg/day Control group (N = 17): oral gabapentin 900 mg/day (300 x 3) Study duration: 6 weeks' duration |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Sources of funding: Canadian Institutes of Health Research (CIHR) New Emerging Team (NET) Grant (GHL‐63209) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "The randomization sequence generation was completed by the research support service pharmacist (not involved in patient care) and the allocation list was concealed from the participants and the study team" Quote: "Since some neuropathic pain conditions disproportionately affect boys and girls, randomization was stratified by sex to ensure that equivalent numbers of boys and girls were randomized to each treatment group. The randomization sequence of 1:1 ratio of amitriptyline to gabapentin was a block 4 design with the possible sequence combinations (e.g., AABB, ABAB) assigned a number and then a point on a page of printed random numbers picked" |
| Allocation concealment (selection bias) | Low risk | Quote: "The research support pharmacy held the allocation sequence schedule, with a copy of participant‐specific medications in sealed manila envelopes available to the research coordinator for emergency purposes or unblinding at the end of the study period" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk |
Quote: "To maintain blinding due to differences in dosing frequency for the two study drugs, participants were prescribed one capsule at night (∼20:00 h) for the first 3 days, then added a second capsule in the morning (∼08:00 h) for the next 3 days and then added a third capsule mid‐afternoon (∼14:00 h) for the remainder of the trial. Children randomized to the amitriptyline group received amitriptyline in the evening pill and placebo in the morning and afternoon pills; while children randomized to gabapentin received 300 mg of gabapentin in each pill." Quote: "Both study and placebo medications were made to be similar in composition, odour, colour and taste by over encapsulating the untouched original dosage form with a larger opaque hard gelatin capsule (7.34 ml in length) and filling any space with lactose powder." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Insufficient information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: All participants were accounted for. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Not all planned outcomes were reported; disruption of school, social, and sports not reported. |
| Size | High risk | Comment: Total participants = 34. Participants < 50 per treatment arm. |
| Other bias | Low risk | Comment: No other potential sources of bias |
Roohafza 2014.
| Methods |
Allocation: randomised Blinding: double‐blind Controlled: placebo Centre: single Arm: 2 arms, parallel groups Study dates: 11‐month trial (February 2013 to December 2013) |
|
| Participants |
Inclusion criteria: school‐aged children who fulfilled Rome III diagnostic criteria for functional abdominal pain Exclusion criteria: other concomitant gastrointestinal disorders and those with a history of receiving psychotropic drugs, antibiotics, or probiotics in the preceding 2 months Baseline characteristics N = 115 Age: 6 to 18 years Gender: male (30); female (56) Number randomised: intervention (59); placebo (56) Number completed: intervention (43); placebo (43) Setting and location: tertiary outpatient clinic of paediatric gastroenterology, Isfahan, Iran |
|
| Interventions |
Intervention group (N = 56): oral tablet citalopram 10 mg/day (week 1); 20 mg/day (weeks 2, 3, and 4) Control group (N = 59): placebo tablet/day Study duration: 4 weeks' duration |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Sources of funding: support from the Isfahan University of Medical Sciences, grant #391299 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... coded by a pharmacist using random numbers generated by the random allocation software" |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation was concealed and the attending physician, participants, and outcome assessor were unaware of the drug codes." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The placebo group received placebo tablets (similar in shape, colour, and size with citalopram) in a same order." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Quote: "The interviewer was blinded to the allocation sequence and study arms." Quote: "Allocation was concealed and the attending physician, participants, and outcome assessor were unaware of the drug codes." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: All participants were accounted for. |
| Selective reporting (reporting bias) | Low risk | Comment: All planned outcomes listed in the methods were reported in the results. |
| Size | High risk | Comment: Total participants = 115. Participants > 50 per treatment randomised; participants < 50 per treatment arm completed. |
| Other bias | Low risk | Comment: No other potential sources of bias |
Saps 2009.
| Methods |
Allocation: randomised Blinding: double‐blind Controlled: placebo Centre: multicentre Arm: 2 arms, parallel groups Study dates: 42‐month trial (January 2003 to August 2006) |
|
| Participants |
Inclusion criteria: diagnosis of functional abdominal pain, functional dyspepsia, or IBS according to the Rome II criteria Exclusion criteria: diagnosed with an organic disease, plotted below the 5th percentile for weight or height, had abnormal testing (electrocardiogram, complete blood count, erythrocyte sedimentation rate, albumin, pancreatic and liver enzymes, urine analysis, stool examination for occult blood and ova and parasites, tissue transglutaminase), a positive lactose breath test or a history of symptoms resolving after 2 weeks of a lactose‐free diet Baseline characteristics N = 90 Age: 8 to 17 years Gender: male (24); female (66) Number randomised: intervention (46); placebo (44) Number completed: intervention (43); placebo (40) Setting and location: paediatric gastroenterology clinics of 6 tertiary care centres geographically dispersed in the USA |
|
| Interventions |
Intervention group (N = 46): amitriptyline oral capsule, 10 mg/day < 35 kg or 20 mg/day > 35 kg for 4 weeks Control group (N = 44): oral placebo tablet for 4 weeks Study duration: 5 weeks' duration |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Sources of funding: supported in part by: (a) the 2003 Clinical Research Award of the American College of Gastroenterology, (b) the CHP19596 RA501 grant and the grants M01 RR‐00048, M01 RR‐00084, and MO1 RR‐02172 from the National Center for Research Resources, National Institutes of Health | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized to receive either placebo or amitriptyline" |
| Allocation concealment (selection bias) | Unclear risk | Quote: Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk |
Quote: "double‐blind study" Comment: Insufficient information provided regarding observer or reporter knowledge. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Insufficient information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: All participants were accounted for. |
| Selective reporting (reporting bias) | Low risk | Comment: All planned outcomes listed in the methods were reported in the results. |
| Size | High risk | Comment: Total participants = 90. Participants < 50 per treatment arm randomised and completed study. |
| Other bias | Low risk | Comment: No other potential sources of bias |
CRPS‐I: complex regional pain syndrome; IBS: irritable bowel syndrome
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agius 2013 | Participants: adult population |
| Alencar 2014 | Participants: adult population |
| Arnold 2015 | Analysis: intervention arm had < 10 participants |
| Chappell 2009 | Participants: adult population |
| Guler 2005 | Participants: adult population |
| Huber 2007 | Intervention: N‐of‐1 trial |
| Johnson 1997 | Participants: adult population |
| Kalita 2014 | Participants: adult population |
| Talaei 2009 | Participants: adult population |
Differences between protocol and review
We did not consider studies with fewer than 10 participants per treatment arm for inclusion in this review, as is standard practice for this group.
In the protocol we stated the age inclusion criterion as birth to 17 years old. Three trials reported participants as being between 6 and 18 years of age. We decided to include the studies rather than miss data on participants who were under 18 years of age.
Contributions of authors
TC and PW registered the title.
TC, PW, and Christopher Eccleston wrote the template protocol for the suite of children's reviews of which this review is a part.
All authors contributed to writing the protocol, and all authors agreed on the final version.
All authors were responsible for data extraction, analysis, and writing of the Discussion for the full review.
All authors will be responsible for the completion of updates.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
NIHR Programme Grant, Award Reference Number: 13/89/29 (Addressing the unmet need of chronic pain: providing the evidence for treatments of pain)
Declarations of interest
PW: none known.
TC: none known.
LH: none known.
JC: none known; JC is a specialist paediatric pain physician and treats patients with complex pain.
JG: none known; JG is a paediatric psychologist specialising in the assessment/evaluation and treatment of children and adolescents with chronic pain.
RH: none known; RH is a specialist paediatric pain clinician and treats patients with chronic pain.
SL: none known; SL is a specialist paediatric pain clinician and treats patients with chronic pain.
NS: none known; NS is a developmental paediatrician and treats children and adolescents with pain; NS directs the Chronic Pain Clinic at Boston Children’s Hospital and is on the faculty at Harvard Medical School.
CW: none known; CW is a paediatrician and anaesthesiologist; CW specialises in pain and treats children and adults presenting chronic pain; CW also treats patients in palliative care.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Bahar 2008 {published data only}
- Bahar RJ, Collins BS, Steinmetz B, Ament ME. Double‐blind placebo‐controlled trial of amitriptyline for the treatment of irritable bowel syndrome in adolescents. Journal of Pediatrics 2008;152(5):685‐9. [DOI] [PubMed] [Google Scholar]
Brown 2016 {published data only}
- Brown SC, Johnston BC, Amaria K, Watkins J, Campbell F, Pehora C, et al. A randomized controlled trial of amitriptyline versus gabapentin for complex regional pain syndrome type I and neuropathic pain in children. Scandinavian Journal of Pain 2016;13:156‐63. [DOI] [PubMed] [Google Scholar]
Roohafza 2014 {published data only}
- Roohafza H, Pourmoghaddas Z, Saneian H, Gholamrezaei A. Citalopram for pediatric functional abdominal pain: a randomized, placebo‐controlled trial. Neurogastroenterology & Motility 2014;26(11):1642‐50. [DOI] [PubMed] [Google Scholar]
Saps 2009 {published data only}
- Chogle A, Saps M. Electrocardiograms changes in children with functional gastrointestinal disorders on low dose amitriptyline. World Journal of Gastroenterology 2014;20(32):11321‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saps M, Youssef NN, Miranda A, Nurko S, Hyman PE, Cocjin JT, et al. A multicenter randomized double blind placebo controlled study on the efficacy of amitriptyline in functional abdominal pain in children: effect of seasonal variation. Gastroenterology 2009;136(5 Suppl 1):A157. [Google Scholar]
References to studies excluded from this review
Agius 2013 {published data only}
- Agius AM, Jones NS, Muscat R. A randomized controlled trial comparing the efficacy of low‐dose amitriptyline, amitriptyline with pindolol and surrogate placebo in the treatment of chronic tension‐type facial pain. Rhinology 2013;51(2):143‐53. [DOI] [PubMed] [Google Scholar]
Alencar 2014 {published data only}
- Alencar FGP Jr, Viana PG, Zamperini C, Becker A. Patient education and self‐care for the management of jaw pain upon awakening: a randomized controlled clinical trial comparing the effectiveness of adding pharmacologic treatment with cyclobenzaprine or tizanidine. Journal of Oral & Facial Pain and Headache 2014;28(2):119‐27. [DOI] [PubMed] [Google Scholar]
Arnold 2015 {published data only}
- Arnold LM, Bateman L, Palmer RH, Lin Y. Preliminary experience using milnacipran in patients with juvenile fibromyalgia: lessons from a clinical trial program. Pediatric Rheumatology 2015;13(1):no pagination. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chappell 2009 {published data only}
- Chappell AS, Littlejohn G, Kajdasz DK, Scheinberg M, D'Souza DN, Moldofsky H. A 1‐year safety and efficacy study of duloxetine in patients with fibromyalgia. Clinical Journal of Pain 2009;25(5):365‐75. [DOI] [PubMed] [Google Scholar]
Guler 2005 {published data only}
- Guler N, Durmus E, Tuncer S. Long‐term follow‐up of patients with atypical facial pain treated with amitriptyline. New York State Dental Journal 2005;71(4):38‐42. [PubMed] [Google Scholar]
Huber 2007 {published data only}
- Huber AM, Tomlinson GA, Koren G, Feldman BM. Amitriptyline to relieve pain in juvenile idiopathic arthritis: a pilot study using Bayesian metaanalysis of multiple N‐of‐1 clinical trials. Journal of Rheumatology 2007;31(5):1125‐32. [PubMed] [Google Scholar]
Johnson 1997 {published data only}
- Johnson SP. Fluoxetine and amitriptyline in the treatment of fibromyalgia. Journal of Family Practice 1997;44(2):128‐30. [PubMed] [Google Scholar]
Kalita 2014 {published data only}
- Kalita J, Kohat AK, Misra UK, Bhoi SK. An open labeled randomized controlled trial of pregabalin versus amitriptyline in chronic low backache. Journal of the Neurological Sciences 2014;342(1‐2):127‐32. [DOI] [PubMed] [Google Scholar]
Talaei 2009 {published data only}
- Talaei A, Siavash M, Majidi H, Chehrei A. Vitamin B12 may be more effective than nortriptyline in improving painful diabetic neuropathy. International Journal of Food Sciences and Nutrition 2009;60 Suppl 5:71‐6. [DOI] [PubMed] [Google Scholar]
Additional references
AMA 2013
- American Medical Association. Pediatric pain management. https://www.ama‐assn.org/ (accessed 25 January 2016).
AUREF 2012
- Cochrane Pain, Palliative and Supportive Care Group. PaPaS author and referee guidance. papas.cochrane.org/papas‐documents (accessed 16 July 2016).
Caes 2016
- Caes L, Boemer KE, Chambers CT, Campbell‐Yeo M, Stinson J, Birnie KA, et al. A comprehensive categorical and bibliometric analysis of published research articles on pediatric pain from 1975 to 2010. Pain 2016;157(2):302‐13. [DOI: 10.1097/j.pain.0000000000000403] [DOI] [PubMed] [Google Scholar]
Cooper 2017a
- Cooper TE, Fisher E, Gray A, Krane E, Sethna NF, Tilburg M, et al. Opioids for chronic non‐cancer pain in children and adolescents. Cochrane Database of Systematic Reviews 2017, Issue 7. [DOI: 10.1002/14651858.CD012538.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 2017b
- Cooper TE, Heathcote L, Clinch J, Howard R, Krane E, Wiffen PJ. Antiepileptic drugs for chronic non‐cancer pain in children and adolescents. Cochrane Database of Systematic Reviews 2017, Issue 8. [DOI: 10.1002/14651858.CD012536.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 2017c
- Cooper TE, Heathcote L, Anderson B, Gregoire MC, Ljungman G, Eccleston C. Non‐steroidal anti‐inflammatory drugs (NSAIDs) for cancer‐related pain in children and adolescents. Cochrane Database of Systematic Reviews 2017, Issue 7. [DOI: 10.1002/14651858.CD012563.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 2017d
- Cooper TE, Fisher E, Anderson B, Wilkinson N, Williams G, Eccleston C. Paracetamol (acetaminophen) for chronic non‐cancer pain in children and adolescents. Cochrane Database of Systematic Reviews 2017, Issue 8. [DOI: 10.1002/14651858.CD012539.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dechartres 2013
- Dechartres A, Trinquart L, Boutron I, Ravaud P. Influence of trial sample size on treatment effect estimates: meta‐epidemiological study. BMJ 2013;346:f2304. [DOI: 10.1136/bmj.f2304] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dechartres 2014
- Dechartres A, Altman DG, Trinquart L, Boutron I, Ravaud P. Association between analytic strategy and estimates of treatment outcomes in meta‐analyses. JAMA 2014;312:623‐30. [DOI: 10.1001/jama.2014.8166] [DOI] [PubMed] [Google Scholar]
Dharmshaktu 2012
- Dharmshaktu P, Tayal V, Kalra BS. Efficacy of antidepressants as analgesics: a review. Journal of Clinical Pharmacology 2012;52(1):6‐17. [DOI] [PubMed] [Google Scholar]
Dickenson 2007
- Dickenson AH, Ghandehari J. Anti‐convulsants and anti‐depressants. Handbook of Experimental Pharmacology 2007;177:145‐77. [DOI] [PubMed] [Google Scholar]
Dworkin 2008
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. Journal of Pain 2008;9(2):105‐21. [DOI: 10.1016/j.jpain.2007.09.005] [DOI] [PubMed] [Google Scholar]
Eccleston 2003
- Eccleston C, Malleson PM. Management of chronic pain in children and adolescents (Editorial). BMJ 2003;326:1408‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eccleston 2017
- Eccleston C, Cooper TE, Fisher E, Anderson B, Wilkinson N. Non‐steroidal anti‐inflammatory drugs (NSAIDs) for chronic non‐cancer pain in children and adolescents. Cochrane Database of Systematic Reviews 2017, Issue 8. [DOI: 10.1002/14651858.CD012537.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Finnerup 2015
- Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta‐analysis. Lancet Neurology 2015;14(2):162‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924‐6. [DOI: 10.1136/bmj.39489.470347.AD] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64:383‐94. [DOI: 10.1016/j.jclinepi.2010.04.026] [DOI] [PubMed] [Google Scholar]
Guyatt 2013a
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso‐Coelle P, et al. Making an overall rating of the confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66(2):151‐7. [DOI: 10.1016/j.jclinepi.2012.01.006] [DOI] [PubMed] [Google Scholar]
Guyatt 2013b
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables ‐ binary outcomes. Journal of Clinical Epidemiology 2013;66(2):158‐72. [DOI: 10.1016/j.jclinepi.2012.01.012] [DOI] [PubMed] [Google Scholar]
Hall 2013
- Hall GC, Morant SV, Carroll D, Gabriel ZL, McQuay HJ. An observational descriptive study of the epidemiology and treatment of neuropathic pain in a UK general population. BMC Family Practice 2013;14:28. [DOI: 10.1186/1471-2296-14-28] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hoffman 2010
- Hoffman DL, Sadosky A, Dukes EM, Alvir J. How do changes in pain severity levels correspond to changes in health status and function in patients with painful diabetic peripheral neuropathy?. Pain 2010;149(2):194‐201. [DOI: 10.1016/j.pain.2009.09.017] [DOI] [PubMed] [Google Scholar]
L'Abbé 1987
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107:224‐33. [DOI] [PubMed] [Google Scholar]
McQuay 1998
- McQuay H, Moore R. An Evidence‐based Resource for Pain Relief. Oxford (UK): Oxford University Press, 1998. [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, the PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2008
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle (WA): IASP Press, 2008:15‐24. [ISBN: 978‐0‐931092‐69‐5] [Google Scholar]
Moore 2009
- Moore RA, Straube S, Wiffen PJ, Derry S, McQuay HJ. Pregabalin for acute and chronic pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007076.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2010a
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al. "Evidence" in chronic pain ‐ establishing best practice in the reporting of systematic reviews. Pain 2010;150(3):386‐9. [DOI: 10.1016/j.pain.2010.05.011] [DOI] [PubMed] [Google Scholar]
Moore 2010b
- Moore RA, Straube S, Paine J, Phillips CJ, Derry S, McQuay HJ. Fibromyalgia: moderate and substantial pain intensity reduction predicts improvement in other outcomes and substantial quality of life gain. Pain 2010;149(2):360‐4. [DOI] [PubMed] [Google Scholar]
Moore 2010c
- Moore RA, Moore OA, Derry S, Peloso PM, Gammaitoni AR, Wang H. Responder analysis for pain relief and numbers needed to treat in a meta‐analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Annals of the Rheumatic Diseases 2010;69(2):374‐9. [DOI: 10.1136/ard.2009.107805] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2010d
- Moore RA, Smugar SS, Wang H, Peloso PM, Gammaitoni A. Numbers‐needed‐to‐treat analyses ‐ do timing, dropouts, and outcome matter? Pooled analysis of two randomized, placebo‐controlled chronic low back pain trials. Pain 2010;151(3):592‐7. [DOI: 10.1016/j.pain.2010.07.2013] [DOI] [PubMed] [Google Scholar]
Moore 2010e
- Moore RA, Derry S, McQuay HJ, Straube S, Aldington D, Wiffen P, et al. ACTINPAIN writing group of the IASP Special Interest Group (SIG) on Systematic Reviews in Pain Relief. Clinical effectiveness: an approach to clinical trial design more relevant to clinical practice, acknowledging the importance of individual differences. Pain 2010;149:173‐6. [PUBMED: 19748185] [DOI] [PubMed] [Google Scholar]
Moore 2011a
- Moore RA, Straube S, Paine J, Derry S, McQuay HJ. Minimum efficacy criteria for comparisons between treatments using individual patient meta‐analysis of acute pain trials: examples of etoricoxib, paracetamol, ibuprofen, and ibuprofen/paracetamol combinations after third molar extraction. Pain 2011;152(5):982‐9. [DOI: 10.1016/j.pain.2010.11.030] [DOI] [PubMed] [Google Scholar]
Moore 2011b
- Moore RA, Mhuircheartaigh RJ, Derry S, McQuay HJ. Mean analgesic consumption is inappropriate for testing analgesic efficacy in post‐operative pain: analysis and alternative suggestion. European Journal of Anaesthesiology 2011;28(6):427‐32. [DOI: 10.1097/EJA.0b013e328343c569] [DOI] [PubMed] [Google Scholar]
Moore 2012
- Moore RA, Straube S, Eccleston C, Derry S, Aldington D, Wiffen P, et al. Estimate at your peril: imputation methods for patient withdrawal can bias efficacy outcomes in chronic pain trials using responder analyses. Pain 2012;153(2):265‐8. [DOI: 10.1016/j.pain.2011.10.004] [DOI] [PubMed] [Google Scholar]
Moore 2013a
- Moore RA, Straube S, Aldington D. Pain measures and cut‐offs ‐ 'no worse than mild pain' as a simple, universal outcome. Anaesthesia 2013;68(4):400‐12. [DOI: 10.1111/anae.12148] [DOI] [PubMed] [Google Scholar]
Moore 2013b
- Moore A, Derry S, Eccleston C, Kalso E. Expect analgesic failure; pursue analgesic success. BMJ 2013;346:f2690. [DOI: 10.1136/bmj.f2690] [DOI] [PubMed] [Google Scholar]
Moore 2014a
- Moore RA, Derry S, Taylor RS, Straube S, Phillips CJ. The costs and consequences of adequately managed chronic non‐cancer pain and chronic neuropathic pain. Pain Practice 2014;14(1):79‐94. [DOI] [PubMed] [Google Scholar]
Moore 2014b
- Moore RA, Cai N, Skljarevski V, Tölle TR. Duloxetine use in chronic painful conditions ‐ individual patient data responder analysis. European Journal of Pain 2014;18(1):67‐75. [DOI: 10.1002/j.1532-2149.2013.00341.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2015
- Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for neuropathic pain in adults. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD008242.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moulin 2014
- Moulin D, Boulanger A, Clark AJ, Clarke H, Dao T, Finley GA, et al. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Research and Management 2014;19(6):328‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2013
- National Institute for Health and Care Excellence (NICE). Neuropathic pain ‐ pharmacological management: the pharmacological management of neuropathic pain in adults in non‐specialist settings. www.nice.org.uk/guidance/cg173 (accessed 17 October 2016).
Nüesch 2010
- Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Tschannen B, Altman DG, et al. Small study effects in meta‐analyses of osteoarthritis trials: meta‐epidemiological study. BMJ 2010;341:c3515. [DOI: 10.1136/bmj.c3515] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Brien 2010
- O'Brien EM, Staud RM, Hassinger AD, McCulloch RC, Craggs JG, Atchinson JW, et al. Patient‐centered perspective on treatment outcomes in chronic pain. Pain Medicine 2010;11(1):6‐15. [DOI: 10.1111/j.1526-4637.2009.00685.x] [DOI] [PubMed] [Google Scholar]
PedIMMPACT 2008
- McGrath PJ, Walco GA, Turk DC, Dworking RH, Brown MT, Davidson K, et al. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT. Journal of Pain 2008;9(9):771‐83. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ripamonti 2008
- Ripamonti C, Bandieri E. Pain therapy. Critical Reviews in Oncology/Hematology 2008;70:145‐59. [DOI: 10.1016/j.critrevonc.2008.12.005] [DOI] [PubMed] [Google Scholar]
Stinson 2006
- Stinson JN, Kavanagh T, Yamada J, Gill N, Stevens B. Systematic review of the psychometric properties, interpretability and feasibility of self‐report pain intensity measures for use in clinical trials in children and adolescents. Pain 2006;125(1‐2):143‐57. [DOI: 10.1016/j.pain.2006.05.006] [DOI] [PubMed] [Google Scholar]
Straube 2008
- Straube S, Derry S, McQuay HJ, Moore RA. Enriched enrolment: definition and effects of enrichment and dose in trials of pregabalin and gabapentin in neuropathic pain. A systematic review. British Journal of Clinical Pharmacology 2008;66(2):266‐75. [DOI: 10.1111/j.1365-2125.2008.03200.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Straube 2010
- Straube S, Derry S, Moore RA, Paine J, McQuay HJ. Pregabalin in fibromyalgia ‐ responder analysis from individual patient data. BMC Musculoskeletal Disorders 2010;11:150. [DOI: 10.1186/1471-2474-11-150] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sultan 2008
- Sultan A, Gaskell H, Derry S, Moore RA. Duloxetine for painful diabetic neuropathy and fibromyalgia pain: systematic review of randomised trials. BMC Neurology 2008;8:29. [DOI: 10.1186/1471-2377-8-29] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Imberger G, Walsh M, Chu R, Gluud C, Wetterslev J, et al. The number of patients and events required to limit the risk of overestimation of intervention effects in meta‐analysis ‐ a simulation study. PLoS ONE 2011;6(10):e25491. [DOI: 10.1371/journal.pone.0025491] [DOI] [PMC free article] [PubMed] [Google Scholar]
United Nations 2015
- United Nations. World population prospects 2015 ‐ population indicators. esa.un.org/unpd/wpp/Download/Standard/Population/ (accessed 29 February 2016).
von Baeyer 2007
- Baeyer CL, Spagrud LJ. Systematic review of observational (behavioural) measures of pain for children and adolescents aged 3 to 18 years. Pain 2007;127(1‐2):140‐50. [DOI: 10.1016/j.pain.2006.08.014] [DOI] [PubMed] [Google Scholar]
Walsh 1983
- Walsh TD. Antidepressants in chronic pain. Clinical Neuropharmacology 1983;6(4):271‐95. [PubMed] [Google Scholar]
Watson 1982
- Watson CP, Evans RJ, Reed K, Merskey H, Goldsmith L, Warsh J. Amitriptyline versus placebo in postherpetic neuralgia. Neurology 1982;32(6):671‐3. [DOI] [PubMed] [Google Scholar]
Watson 1998
- Watson CP, Vernich L, Chipman M, Reed K. Nortriptyline versus amitriptyline in postherpetic neuralgia: a randomized trial. Neurology 1998;51(4):1166‐71. [DOI] [PubMed] [Google Scholar]
WHO 2012
- World Health Organization. WHO Guidelines on the Pharmacological Treatment of Persisting Pain in Children with Medical Illnesses. Geneva: WHO Press, World Health Organization, 2012. [ISBN 978 92 4 154812 0] [PubMed] [Google Scholar]
Wiffen 2017a
- Wiffen P, Cooper T, Anderson AK, Gray A, Gregoire MC, Ljungman G, et al. Opioids for cancer‐related pain in children and adolescents. Cochrane Database of Systematic Reviews 2017, Issue 7. [DOI: 10.1002/14651858.CD012564.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
World Bank 2014
- World Bank. Data ‐ population ages 0‐14 (% of total). data.worldbank.org/indicator/SP.POP.0014.TO.ZS (accessed 29 February 2016).


