Abstract
Nature has bestowed mankind with surplus resources (natural products) on land and water. Natural products have a significant role in the prevention of disease and boosting of health in humans and animals. These natural products have been experimentally documented to possess various biological properties such as antioxidant, anti-inflammatory and anti-apoptotic activities. In vitro and in vivo studies have further established the usefulness of natural products in various preclinical models of neurodegenerative disorders. Natural products include phytoconstituents, like polyphenolic antioxidants, found in herbs, fruits, nuts, vegetables and also in marine and freshwater flora. These phytoconstituents may potentially suppress neuro-degeneration and improve memory as well as cognitive functions of the brain. Also, they are known to play a pivotal role in the prevention and cure of different neurodegenerative diseases, such as Alzheimer’s disease, epilepsy, Parkinson’s disease and other neuronal disorders. The large-scale neuro-pharmacological activities of natural products have been documented due to the result of either the inhibition of inflammatory processes, or the up-regulation of various cell survival proteins or a combination of both. Due to the scarcity of human studies on neuroprotective effects of natural products, this review focuses on the various established activities of natural products in in vitro and in vivo preclinical models, and their potential neuro-therapeutic applications using the available knowledge in the literature.
Keywords: Neurological disorders, neuroprotection, plant products, nutraceuticals, natural compounds, chronic neurodegenerative diseases
1. INTRODUCTION
Most commonly, products obtained from natural sources have been used by human beings as a leading source of medicinal agents so as to bring relief from many diseases, illnesses and frail. At the same time, people using these medicines are unaware of the side effects and poisonous nature of some natural products. The therapeutic use of plants containing these medicinal products can be traced back to the Sumerian and Akkadians civilization [1]. Natural products have played an important role in ancient traditional medicine systems, such as Unani, Chinese and Ayurveda which are still in common use today. Plants are the best source for the isolation of secondary metabolites demonstrating significant structural diversity and offer a wide range of new and exciting pharmacophores. There are about less than 1% of approximately 250,000 higher plants that have been explored in-depth for their phytochemistry or pharmacological potential [2]. According to the World Health Organization (WHO), 75% of the world population still depends on plant-based traditional medicines for primary health care, which chiefly involve the use of plant extracts or their bioactive secondary metabolites. For the betterment of human health, a limitless source of molecules is present in nature in the form of herbs, spices and foods. Phytochemicals from plants are known to exert additive, synergistic or antagonistic effects on the body.
Neuroprotection refers to the mechanisms and strategies employed to defend the central nervous system (CNS) against injury due to both acute (e.g. trauma or stroke) and chronic neurodegenerative disorders (e.g. Dementia, Parkinson's, Alzheimer's, Epilepsy etc.) [3]. Herbal medicine and nutraceuticals represent an important and valuable source in prevention rather than treatment of neurological disorders [4]. In various experimental models of neurological diseases, phytoconstituents have reportedly shown to have modulatory effects on the nervous system [5]. The pathogenesis of nervous system disorders is not completely understood yet but most of the studies on different nervous disorder models mimicking key features of disease have highlighted important factors such as oxidative stress, mitochondrial dysfunction, neuro-inflammation etc [6, 7]. The models for neurotoxicity have been found to be an important tool for developing novel therapeutic strategies and assessing the efficacy and adverse effects of symptomatic treatments [8].
In addition to the above mentioned properties; neuro-protective natural products have been reported to modulate multiple signalling pathways via direct effects on enzymes, such as kinases, regulatory receptors and proteins [9, 10]. Many published reports suggest that natural products exert a number of their biological effects via remodelling of chromatin and epigenetic modifications [11]. This wide band of pharmacological or biological activities has made them suitable candidates for the treatment of neurological disorders and neurodegenerative diseases [12, 13].
Natural products and nutraceuticals work via a different mechanism to impart their neuroprotective effect. Many classes of chemical constituents are known to interact with the GABAA receptor [14] e.g. diterpenes and cyclodepsipeptides selectively inhibit its activity [15]. Alkaloids, on the other hand, positively modulate the binding of muscimol to GABA receptor complex [16, 17]. Similarly, some flavonoids have proven tendency for binding to benzodiazepine site on the GABAA receptor [18, 19 and also act as an scavenger to pro-inflammatory and neurotoxic species [20]. Several plants like Arisaema amurense, Biota orientalis, Mentha arvensis, Salvia miltiorrhiza, Albizia julibrissin, Astragalus membranaceus, Glycyrrhiza uralensis have been found to have an inhibitory effect on mono-amine oxidase-B (MAO-B)enzyme activity [21].
Oxidative stress, necrosis, cytotoxicity, ions imbalance, mitochondria dysfunction, cellular inflammation, apoptosis, increased blood–brain permeability, and morphological changes are pathological alterations in response to injuries, which aggravate medical conditions and give hints to screen alternative neuroprotection approaches [22].
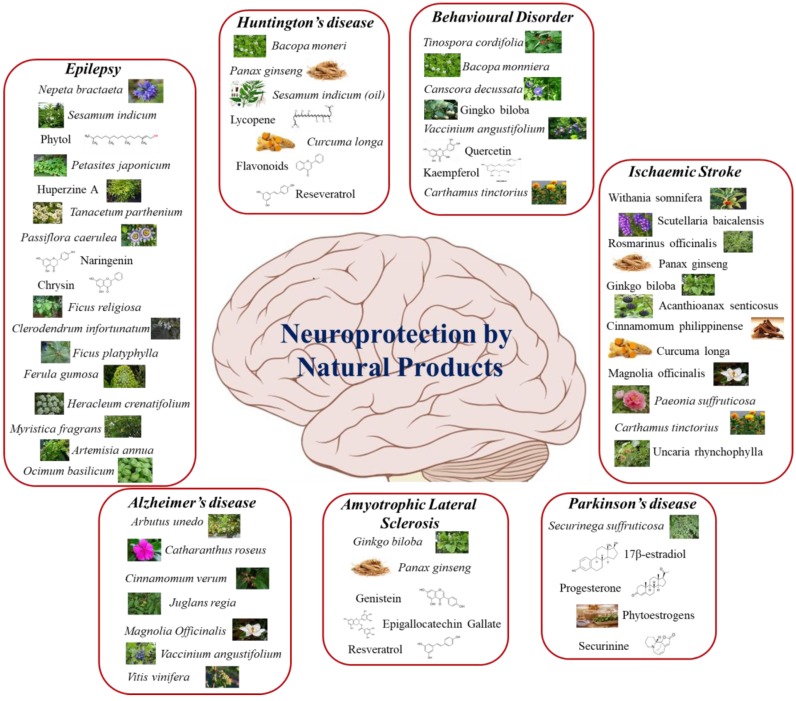
Extensive research on identification and discovery of novel neuroprotective drugs has shown that plant extracts and their bioactive compounds together with nutraceuticals can have tremendous potential as neuroprotective candidates against several types of neurodegenerative disorders. In this review, we briefly discuss some neurodegenerative diseases, with a focus on their prevention by natural products and nutraceuticals. We present an ethnobiological strategy, stressing on natural products for their role in neuroprotection (Fig. 1).
Fig. (1).
Neuroprotection by various natural products. (The color version of the figure is available in the electronic copy of the article).
2. METHODOLOGY
Database searches using Google scholar, PubMed, and science direct were conducted until May 2018 to include up to date documented information in the present review. The search was limited to English language papers. For data mining, the following MeSH words were used in the databases mentioned above: neuroprotection, prevention, natural product, phytoconstituents, natural products Alzheimer's, natural products brain, natural products Parkinson’s, natural products Amyotrophic lateral sclerosis, natural products Huntington’s disease, natural products epilepsy, natural product ischemic brain injury, natural product peripheral nerve injury, natural products motor behaviour disorders, in vivo and in vitro studies for prevention of nervous disorders. In almost all cases, the original articles were obtained and the relevant data was extracted.
3. NEUROPROTECTIVE ROLE OF VARIOUS NATURAL PRODUCTS IN DIFFERENT NEUROLOGICAL DISORDERS
3.1. Cognitive and Motor Behavioural Disorder
Aging or pathologic state can progress to worsening of cognitive and motor functions. They share pathways of impairment which eventually cause a decline in neuronal survival [23]. The cognitive loss may emerge alone as a developmental deficit or with a set of neuropsychiatric complaints and hence claiming utilization of nootropics so as to boost cognitive potential. For the reason of their marginal side effects, medicinal plants are enormously investigated throughout the world [24]. One of the key factors promoting cognitive decline is the disruption of cholinergic neurotransmission in the brain [25]. With the growth in elderly population and inflation in life expectancy, cognitive and memory impairments linked to age-related neurodegenerative disorders that have become a considerable public health issue [26]. Neurotoxic factors which are responsible for progressive cognitive dysfunction and dementia are inflammatory cytokines, mitochondrial dysfunction, oxidative stress and excitotoxicity [27, 28].
Enrichment of cognition takes place by immunostimulation and amplification of acetylcholine synthesis [29]. In normal and cognitive deficit animals, Tinospora cordifolia has been found to supplement the cognition when evaluated for behavioural test [30]. Likewise, another plant Bacopa monnieri (BM) is a renowned nootropic and supplementation of alcoholic extract of BM has been found to improve both cognitive function and retention capacity with a decrease in retrograde amnesia in rats. Moreover, it has been shown to protect from phenytoin-induced cognitive deficit as well [31, 32]. Thus, it is utilized in the treatment of memory and attention disorders [33]. Canscora decussata, commonly known as Shankhpushpi, has been found to inhibit acetylcholinesterase (AChE) enzyme which is involved in the hydrolysis of acetylcholine (neurotransmitter), into choline and acetic acid, thus, AChE inhibition leads to protect the loss of acetylcholine (neurotransmitter). Acetylcholine is directly responsible for cognitive function [34]. The cognitive and memory enhancing activity of Canscora decussata can be attributed to mangiferin. Besides mangiferin, a significant proportion of xanthones has been found which is responsible for the synergistic activity [35]. Cerebral ischemia and neuronal damage resulting from Alzheimer’s disease (AD) and multi-infarct dementia (MID) may lead to the progression of symptoms of cognitive deficits [36]. Extensive studies have been carried out on gingko extract for its effects on cognition and memory in patients with dementia related to AD or cerebral insufficiency [37].
One of the richest sources of cytoprotective polyphenolic antioxidant is Vaccinium angustifolium (blueberry) belonging to the class of anthocyanins. Preclinical studies reported that blueberry supplementation is associated with enhanced memory and motor performance in aged animals [38-42]. Since blueberry polyphenols protect muscle tissues from oxidative damage in-vitro they, therefore, could contribute to the amelioration of motor performance. Low levels of released lactate dehydrogenase (LDH) and creatine kinase (CK) have also been reported [43]. Blueberry supplementation in aged rats has led to amelioration of cognitive and motor performance deficits [44, 45].
Withania somnifera is a plant of medical importance which possesses several biological properties. Withanone is one of the natural compounds present in Withania somnifera root extract which exerts significant exhibited improvement in cognitive decline by the inhibition of amyloid β-42 in Wistar rat model. Moreover, withanone alleviates IL-6, IL-1β, MCP-1, TNF-α and NO levels as well as enzymatic activities of β- secretase and γ- secretase. Treatment with withanone also increased acetylcholine and GSH level and inhibited the expression of Th1 and Th2 cytokine in peripheral blood. Overall withanone exhibited improvement in cognitive decline by ameliorating inflammation and oxidative stress [46].
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is a neurotoxin that has been widely used to create animal models of Parkinson’s disease [47]. MPTP was accidentally discovered by a chemistry student in 1976 [48]. However, he abruptly identified the effects of MPTP administration in non-human primates and explained the mutilations that resembled the motor disabilities of idiopathic Parkinson’s disease [49]. Till date, several active plant constituents have been studied to assess the neuroprotective effects against MPTP-induced motor defects. Quercetin is an important natural compound found in many plants and has been reported to improve motor defects induced by MPTP. This therapeutic effect has been associated with protection of dopaminergic neurons. Another study conducted by Li and Pu, 2011 reported a marked improvement in motor defects upon pretreatment with kaempferol. It has been reported that kaempferol recovers memory dysfunction and oxidative stress in a multi-infarct dementia model and reduces ischemic brain injury by upregulating endothelial nitric oxide synthase activity in transient focal cerebral ischemia [50]. In another study by Lu et al., post-ischemic treatment with kaempferol inhibits ischemic brain damage and neuroinflammation by inhibition of STAT3 and NF-kB activation and could be used in the treatment for the neuroinflammation associated diseases [51]. Rutong et al. confirmed that kaempferol 3-O-rutinoside improves behavioural performances in a 6-hydroxydopamine persuaded rodent model of Parkinson’s disease, partially via the suppression of α-synuclein overexpression or aggregation, as well as the suppression of reactive astrogliosis [52].
Almost similar kind of results have been observed with hydroxysafflor yellow A (HSYA) from Carthamus tinctorius [53, 54] baicalein & baicalin [55, 56], nobiletin [57] and acacetin [58] (Table 1).
Table 1.
Functions of constituents of natural products for cognitive decline.
| S. No. |
Natural Products-
Cognitive Decline |
Common Name | Constituent | Functions | Refs. |
|---|---|---|---|---|---|
| 1 | Bacopa monnieri | Brahmi | Bacosides, Bacopasides, or Bacopasaponins | Boost in retaining capability | [31] |
| 2 | Canscora decussata | Shankh Pushpi | Mangiferin | Inhibition of AChE | [34, 35] |
| 3 | Carthamus tinctorius | Safflower | Hydroxysafflor yellow A | Overcome motor deficiencies | [53, 54] |
| 4 | Tinospora cordifolia | Gulbel | Whole plant/ Ethanolic extract | Enhancement of cognition | [29, 215] |
| 5 | Vaccinium angustifolium | Lowbush Blueberry | Anthocyanins | Enriched memory and motor performance | [39, 41] |
| 6 | Withania somnifera | Indian ginseng | Withanone | Withanone exhibited improvement in cognitive decline by ameliorating inflammation and oxidative stress | [46] |
3.2. Epilepsy
Epilepsy is a serious neurological disorder identified by repetitive seizures precipitated by superfluous discharges of cerebral neurons [59]. Different limitations have been associated with the use of conventional anti-epileptic drugs (AEDs) like, inadequate seizure control, side effects, cost, potentiation of epilepsy-induced co-morbidities, etc., which limit their use as a comprehensive therapy. The focus has now shifted to use natural substances (phytoconstituents) for the treatment of epilepsy. Phytoconstituents, obtained from traditional herbs, have been reported to be promising anti-epileptic drugs which can interact with most of the possible targets involved in the pathogenesis of epilepsy and can be useful in different types of epilepsy. Due to their negligible toxicity, low cost, fewer side effects compared to conventional AEDs, they have been found to be very useful and indispensable in different types of epilepsy and thus can prove to be very potent drugs for management of seizures. A dose-dependent delay in the onset of seizure and decrease in tonic hindlimb extension has been reported in pentylenetetrazole (PTZ)-induced seizure model upon treatment with aqueous and methanolic extracts of Nepeta bracteata [60]. Its anticonvulsant activity may be attributed to modification of glutaminergic and GABAergic transmission or blockade of sodium channels [61]. Another constituent from chlorophyll namely phytol has been reported to decrease seizure percentage and increase the latency of onset of a seizure [62, 63]. A study conducted by Costa, et al., reveals the protective effect against pilocarpine-induced epilepsy and a subsequent decline in the rate of mortality. Almost similar kinds of results were obtained with sesamin, an active constituent from Sesamum indicum seeds. Sesamin, a free radical scavenger and antioxidant, is known to decrease reactive oxygen species (ROS) and malondialdehyde (MDA) in epileptic kainic acid-treated mice and rats and its treatment reduces the mortality rate from 22% to 0% as well [64]. The extract of shrub Petasites japonicum (BMP) native to Europe is also known to possess some anticonvulsant activity. Kainic acid-treated mice when subjected to Petasites japonicum BMP exhibited a decline in mortality rate by 50% and decreased convulsive seizures. A remarkable decline in the loss of neurons has also been found in CA1 and CA3 regions of the hippocampus [65]. As reported by Steven et al., 2009, the Swiss Webster mice when subcutaneously injected with PTZ developed seizures which were controlled by oral administration of Huperzine A, a sesquiterpene alkaloid [66].
Fruits, plant-derived beverages, herbs, vegetables and nuts are rich sources of flavonoids. Most of the flavonoids reported to have strong antioxidant potential and thus shield the cells from oxidative injury [67]. A special attention is being paid to medicinal plants as a defence against oxidative stress and epilepsy [68]. A flavone glycoside baicalin preserves levels of endogenous enzymes, increases levels of GABA in the brain in addition to the suppression of oxidative stress [69]. In adult Sprague Dawley rats, it has shown positive results as an anticonvulsant and neuroprotective against pilocarpine-induced epilepsy [70]. A well-known flavone apigenin found to be present in Tanacetum parthenium has reported to decrease locomotor activity and latency of the onset of a seizure in picrotoxin mice model [71]. Studies conducted on chrysin, a constituent of Passiflora caerulea (passion flowers) have exhibited anticonvulsant properties in vivo. Previous reports have recorded the abolition of PTZ-induced tonic-clonic seizures upon intracerebroventricular injection of chrysin [72]. Naringenin, a very potent flavanone, has a wide range of biological activities and exerts tremendous positive activity on benzodiazepine site of GABA receptor by finding its way to cross the blood-brain barrier [73]. Naringenin could be a promising drug for the prevention of pilocarpine-induced epilepsy due to its potential to limit oxidative stress. It has shown to decrease seizure severity, increase latency of onset of a seizure and has shown protective effects at cerebral level [69]. In the ailments that emerge as epileptic co-morbidity and the disorders that enhance epilepsy, saponins have known to play a critical role [41, 75]. For the management of epilepsy, the traditional methods of medicine utilized the adventitious roots of Ficus religiosa for centuries. In PTZ-induced kindling epileptic mouse model, Damanpreet et al., reported the amendment in depression associated to kindling. This discovery has been supported by the alteration of cerebral neurotransmitter levels [76]. An extract of saponin rich fraction of Ficus religiosa has found to have anticonvulsant effects and moreover, several saponins have been labelled to acquire seizure abolishing properties [74, 77-79]. Saponins from Clerodendrum infortunatum have reported to decrease the time interval of seizures and they have shown a dose-dependent effects against convulsions induced by leptazol [80]. The possible mechanism behind the analgesic and anticonvulsant activity can be the elevation in serotonin and GABA levels of the brain [81-83]. The saponin-rich fraction obtained from Ficus platyphylla stem bark has exhibited protection against strychnine and pentylenetetrazole-induced seizures. A momentous delay has been observed in the onset of tonic seizures and myoclonic jerks. Impairment of membrane excitability, a property demonstrated by most anti-epileptics predominantly by the voltage-gated sodium channel (VGSC) obstructing drugs, has also been reported [84].
Because of the presence of volatile oils and other chemical components, a number of classes of aromatic herbs are being investigated for therapeutic purpose. A number of aromatic herbs have been utilized conventionally as they possess strong CNS activities in addition to anti-epileptic action. In mice, the supplementation of the essential oil from fruitlets of Ferula gumosa has been shown to reduce tonic seizures induced by pentylenetetrazole. As the effective dose is near to LD50, therefore it has shown to cause neurotoxic effects. The anti-epileptic effects of the oil may be attributed to pinene while as the neurotoxicity might be credited to α-thujene [85]. Tosun et al., [86] detailed the protective effects of octyl acetate and octano from fruits of Heracleum crenatifolium. The essential oil has exhibited noteworthy abolition of seizures induced by maximal electroshock (MES) in mice. The oil from kernels of Myristica fragrans (nutmeg oil) has reported to abolish the tonic hindlimb extension prompted by maximal electroshock (MES). A substantial postponement in strychnine induced tonic extensor jerks of the hind limb has been affirmed. Against pentylenetetrazole-induced tonic seizures, nutmeg oil has shown dose-dependent anticonvulsant activity. The exact mechanism is still unknown but Wahab et al. reported that nutmeg oil exerts anti-convulsant effects through induction of neurotransmission mediated by GABA and reported that it interacts with sodium channels of the neurons. Hence, the nutmeg oil has proved to be an anticonvulsant at lower doses [87]. There has been a major deferral in latency of onset in pilocarpine and picrotoxin-induced seizures by using the essential oil of Artemisia annua and ethanolic extract acquired from fresh leaves of Artemisia annua. But the onset of seizures induced by strychnine and pentylenetetrazole has reported to be prevented using essential oil as well as ethanolic extract of fresh leaves of Artemisia annua [88]. Both essential oil and extract do not have the ability to bind glycine receptors as agonists, but can bind as glycine receptors antagonists, thus enhance the onset of convulsion. Alternatively, the latency time for the onset of convulsion onset can be increased due to depressive activity on the central nerves system but not related with GABA mechanisms [88].
Ocimum basilicum is an essential oil (EO) and the composition of eugenol is around 9% [89]. Koutroumanidou et al. and Okoli et al. reported that EO isolated from Ocimum basilicum plants exerts anticonvulsant effects against pentylenetetrazol-induced seizures and seizure latency in mice model [90, 91]. The treatment with EO of Ocimum basilicum isolated from leaves exerts CNS depressant activity. EO of Ocimum basilicum also enhances the latency for the onset of convulsions in pentylenetetrazol- and picotoxin-induced seizures in mice model. The effects of EO of Ocimum basilicum were reversed by flumazenil which is an agonist of GABAA receptors thus supporting a finding that Ocimum basilicum EO exerts anti-convulsant effects by targeting GABAergic neurotransmission mechanism. Furthermore, Ocimum basilicum EO has not shown anti-convulsant effects using strychnine-induced seizures in mice model as strychnine induces convulsions by antagonizing glycine receptors activity and enhancing postsynaptic excitability and ongoing activity in the spinal cord and brain stem but not related to GABA receptor. Thus, Ocimum basilicum EO produces anti-convulsant effects by modulating GABAergic neurotransmission [92].
A natural compound alpha-asarone (α-asarone) obtained from a Chinese herbal medicinal plant that is Acorus gramineus which is widely used clinically for the treatment of epilepsy. It has also been shown to possess neuroprotective property and also reported to induce glutamate uptake and decrease excitation of synapses. It is clinically already proven that α-asarone induces sedative and anticonvulsant effects on the central nervous system (CNS). A study was conducted using in-vitro and in-vivo models to understand the anti-epileptic effects of α-asarone via elucidating the mechanism of action and therapeutic targets of α-asarone. It was found that α-asarone is an inhibitor of the hippocampal neuronal activity and thus induces anti-epileptic effects in the CNS by increasing tonic GABAergic neuronal inhibition [93].
Another plant source natural product, Cannabidiol isolated from marijuana plant is a non-psychoactive compound. In a study conducted by Hess et al. [82] to assess the efficacy, safety and tolerability of Cannabidiol as an adjunct therapy along with anti-epileptic drugs on the patients suffering from tuberous sclerosis complex whose most common neurological symptom is epilepsy. In this clinical trial study, after treatment with Cannabidiol, responder rate of the patients suffering from refractory seizures was 50%. After the 3 months of the treatment with Cannabidiol, patients exhibited reduced occurrence of weekly seizure by 48.8 median percent. Furthermore, cognitive enhancement in 85.7% cases and behavioural improvement in 66.7% cases have been reported by the parents of all the patients. Besides the efficacy of Cannabidiol, most of the adverse effects associated with Cannabidiol were transient in nature and resolved by dose change of Cannabidiol [94].
Flavonoids are the natural polyphenols found to be present abundantly in plants, fruits and vegetables. They have been reported to have promising anti-seizure and anti-epileptic effects which are mainly attributed due to their allosteric modulation of GABAA receptors and anti-inflammatory effects. But the potency of the flavonoids is generally hindered due to low oral bioavailability and low metabolic stability. It has been reported that chemical modification by methylation of the free hydroxyl (OH-) group of the flavonoids can drastically improve absorption, bioavailability, membrane transport and metabolic stability. Naringenin, kaempferol and different methylated forms naringenin and kaempferol (naringenin 7-O-methyl ether, naringenin 4',7-dimethyl ether, and kaempferide (4'-O-methyl kaempferol)) were used to assess the anti-epileptic effects using pentylenetetrazole-induced seizure model of zebrafish. It was found that only naringenin 7-O-methyl ether and naringenin 4',7-dimethyl ether are very effective against pentylenetetrazole-induced seizure in larval zebrafish while naringenin, kaempferol and kaempferide have insufficient anti-seizure effects. Additionally, naringenin 4',7-dimethyl ether is also effective against acute seizure mice models (timed intravenous pentylenetetrazole convulsive seizure model and 6 Hertz (Hz) psychomotor seizure model). Thus methylation of naringenin leads to enhance the efficacy against seizures thus has anti-epileptic effects [95] (Table 2).
Table 2.
Functions of constituents of natural products for epilepsy.
| S. No. | Natural Products-Epilepsy | Common name | Constituent | Functions | Refs. |
|---|---|---|---|---|---|
| 1 | Artemisia annua | Sweet wormwood | Essential oil assimilated from fresh leaves | Escalation in latency of onset of seizures | [88] |
| 2 | Chlorophyll | Chlorophyll | Phytol | Increased latency of seizure onset | [62, 63] |
| 3 | Clerodendruminfortunatum | Hill glory bower | Saponins | Diminished time interval of seizures | [80] |
| 4 | Citrus fruits | - | Naringenin | Drop in seizure severity | [73] |
| 5 | Ferula gumosa | Galbanum | Pinene | Protection against PTZ provoked seizures | [82] |
| 6 | Ficus platyphylla | Broadleaf fig | Saponin rich fraction | Defense in response to strychnine & PTZ incited seizures | [84] |
| 7 | Ficus religiosa | Sacred fig | Saponin rich fraction | Modification of cerebral neurotransmitter levels | [76] |
| 8 |
Fruits, plant derived beverages, herbs |
- | Flavonoids | Antioxidant activity | [72,95] |
| 9 | Heracleumcrenatifolium | Cow parsnip | octyl acetate; octano | Termination of maximal electroshock (MES) induced seizures | [86] |
| 10 | Myristica fragrans | Nutmeg | oil from kernels | Anticonvulsant activity against MES, Strychnine and PTZ stimulated seizure | [87] |
| 11 | Nepeta bracteata | Catmint | Flower extracts | Alteration of glutaminergic and GABAergic conduction | [61] |
| 12 | Acorus gramineus | Japanese Sweet Flag | α-asarone | Inhibit hippocampal neuronal activity | [90] |
| 13 | Cannabis sativa | Marijuana plant | Cannabidiol | Suppressed weekly seizure, cognitive enhancement, behavioural improvement | [91] |
| 14 | Citrus fruits, and other plants | -------------- | Naringenin 4',7-dimethyl ether | Anti-epileptic effects by suppressing seizures in zebrafish and mice model | [79, 95] |
3.3. Alzheimer’s Disease
Alzheimer’s disease (AD) is a severe, chronic and progressive neurodegenerative disease characterized by impairment in memory and cognitive function. It is usually an age-related problem. The disease was first diagnosed and described by a German physician “Alois Alzheimer” in 1906 [96]. Alzheimer’s disease is affecting the aging population worldwide and has been expected to reach 106.8 million by 2050 [97]. The main pathogenesis of the Alzheimer’s disease is the formation of amyloid-β (Aβ) fibrils which contain β-amyloid peptides. The exact mechanism for the formation of amyloid plaques is unclear but the plausible mechanism is thought to be that Aβ is generally produced and aggregated within the extracellular matrix to form plaques. These Aβ plaques are reported to be toxic for the adjacent neurons. The Aβ also reported to be accumulated within the neurons which lead to synaptic dysfunction, cognitive impairment and Aβ plaques formation [98]. Various effective therapeutic molecules have been reported to suppress the formation of Aβ plaques for the treatment of Alzheimer’s disease [99, 100]. Keeping in view the cost of treatment for Alzheimer’s disease and the limitations associated with the previous preventive techniques, natural products including nutraceuticals have gained paramount importance [5]. The natural products have been found to restore memory and cognitive deficits in the brain. The curative effects of these products are mainly due to the action of phytonutrients on distinct signalling pathways associated with protein folding and neuroinflammation. Several studies conducted on patients with AD have revealed the oxidative stress in the affected parts of the brain [101]. Reports from Pappolla, et al., [102]. Heo and Lee., [103] reveal that the constituents from Arbutus unedo, have a very strong antioxidant potential against hydrogen peroxide-induced neurotoxicity in PC12 cells. This antioxidant potential may be endorsed to various phytoconstituents like anthocyanins, gallic acid, tannins, vitamin C, vitamin E and carotenoids. Anthocyanins from Vaccinium angustifolium exhibited protection from oxidative injury. Likewise, resveratrol from Vitis vinifera has exhibited alleviation of inflammation and oxidative stress through activation of sirtuin-1 and hence reduced expression of NF-κB [104]. Neuropathology of AD is characterized by the formation of neurofibrillary tangles which results from the deposition of extracellular amyloid plaques and accumulation of intracellular hyperphosphorylated neuronal microtubule-associated protein known as “tau proteins” [105]. Therefore, AD is the result of mutations in the gene that forms amyloid precursor protein (APP) thus leading to altered production of peptide and contribution in loss of neurons and synaptic connections. Juglans regia is known to aid in the inhibition of Aβ fibril formation, defibrillation of the preformed Aβ and amyloidogenic action. The constituents responsible for this action are fatty acids, α-tocopherol, vitamin and polyphenols, especially ellagic acid present in it [106]. Almost a similar type of activity has been reported by Frydman, et al., [107] in Cinnamomum verum; cinnamaldehyde, eugenol, cinnamyl acetate and cinnamyl alcohol are largely responsible for its anti-Alzheimer’s disease activity through blockade of oligomer and amyloid fibril formation.
Bromelain is a proteolytic enzyme found profusely in fruit and stem of pineapple. It has bee reported that bromelain interacts and degrades synthetic amyloid-β42 monomers and oligomers. Cerebro-spinal fluid (CSF), isolated from the Alzheimer's disease patients when incubated with bromelain leads to decreased level of amyloid-β42 monomers and low and oligomers thus bromelain has a strong potential to be used as a therapeutic compound for the treatment of Alzheimer's disease [108].
A recent therapeutic progress in the field of AD is the discovery of acetylcholinesterase (AChe) inhibitor. The basis of this finding is thought to be the affected cholinergic pathways in the cerebral cortex and basal forebrain in AD [109] and therefore the consequential cholinergic insufficiency adds to the cognitive damage [110]. Caffeoylquinic acid, flavonoids, citric acids from Catharanthus roseus can prove to be beneficial in AD patients because of their property to inhibit AChE enzyme [111]. Likewise, AChE inhibitory and memory boosting capacity of Magnolia officinalis has been attributed to the presence of magnolol, honokiol, obovatol and 4-O-methylhonokiol [112] (Table 3).
Table 3.
Functions of constituents of natural products for alzheimer’s disease.
| S. No. | Natural Products-Alzheimer’s Disease | Common Name | Constituent | Functions | Refs. |
|---|---|---|---|---|---|
| 1 | Arbutus unedo | Strawberry tree | Anthocyannins, gallic acid, tannins, vitamin C, vitamin E and carotenoids |
Antioxidant potential against hydrogen peroxide induced neurotoxicity in PC12 cells, improvement of memory and augmentation of spatial learning | [105, 103] |
| 2 | Catharanthusroseus | Madagaskar periwinkle | Caffeoylquinic acid, flavonoids, citric acids | Inhibition of AChE | [111] |
| 3 | Cinnamomumverum | Cinnamon | Cinnamaldehyde, Eugenol, Cinnamyl acetate, and cinnamyl alcohol |
Blockade of oligomer and amyloid fibril formation | [107] |
| 4 | Juglans regia | Persian walnut | Fatty acids, alpha tocopherol, vitamin and polyphenols, especially ellagic acid | Inhibition of Aβ fibril formation, defibrillation of the preformed Aβ and amyloidogenic action | [104] |
| 5 | Magnolia officinalis | Houpo | Magnolol, honokiol, obovatol and 4-O-methylhonokiol | AChE inhibitory and memory boosting capacity |
[112] |
| 7 |
Vaccinium angustifolium |
Lowbush blueberry | Anthocyanins | Lowering of oxidative injury and drop in the expression of age-linked protein, such as NF-kB | [216] |
| 8 | Vitis vinifera | Grape vine | Resveratrol | Mitigation of inflammation and oxidative stress via activation of sirtuin 1and therefore weakening of NF-kB activity and apoptotic activity of FOXO proteins | [217] |
| 9 | Ananas comosus | Pine Apple | Bromelain | Degrades amyloid-β42 monomers and oligomers in CSF of AD patients. and also degrades synthetic forms. | [108] |
3.4. Parkinson’s Disease
Parkinson’s disease (PD) is the second most common chronic neurodegenerative disorder, which affects movements. Loss of dopaminergic neurons in the region of substantia nigra of the basal ganglia is a characteristic feature of PD which results in postural instability, tremors, bradykinesia, rigidity and progressive loss of muscular coordination and balance [113]. The pathological hallmark of the disease is a protein called as α-synuclein that binds to ubiquitin and forms cytoplasmatic inclusions referred to as “Lewy bodies” which are round eosinophilic intraneuronal filamentous inclusions [114] thereby causing degeneration of the dopaminergic neurons [115]. The disease has been found to be associated with several biochemical abnormalities in the brain including deficiency of mitochondrial complex I, depletion of intracellular thiols and increased nigral iron content [116].
PD is difficult to cure and available treatments are only symptomatic. The gold standard treatment for PD uses dopamine precursor l-3,4-dihydroxyphenylalanine (L-DOPA) which decreases the motor symptoms. However, it is associated with complications including L-DOPA-induced dyskinesias and others [117]. To overcome the side effects associated with the synthetic products, scientists have nowadays shifted to the use of different natural products.
Estrogenic compounds such as 17β-estradiol and progesterone are reported to have neuroprotective effects [118]. 17β-estradiol and naturally occurring plant-derived compounds like phytoestrogens found to have neuroprotective effects via activation of receptors like estrogen receptor-α (ERα), ERβ, or G-protein coupled receptor-1 (GPER1) and stimulation of the signaling cascades including (a) extracellular regulated kinase-1 (ERK1)/ERK2, (b) PI3K/Akt, and (c) c-Jun amino-terminal kinase (JNK), which regulate various downstream transcription factors implicated in the survival of the neurons [119, 120]. ERα, in the active form, can bind to insulin-like growth factor (IGF)-1 receptor and thus regulate the signalling pathway involved in the neuroprotective action that is PI3K/Akt–glycogen synthase kinase-3β (GSK3β)–β-catenin [119, 121]. The expression of the anti-apoptotic proteins such as Bcl-2, Bcl-xL and Bcl-W was enhanced while the expression of the pro-apoptotic proteins such as BCL2L4 and BAD was reduced by the activated ERK1/ERK2 and PI3K/Akt signalling pathways [62, 122, 123]. 17β-estradiol leads to the activation of JNK pathway which results in enhanced expression of anti-apoptotic proteins such as Bcl-W and BCL2L11 (BIM) [124]. The common downstream molecule of ERK1/ERK2 and PI3K/Akt signalling pathways is GSK3β which gets activated and induces neuronal cell death, therefore, inhibition of GSK3β promotes the survival of neurons [125].
Different spices including turmeric, ginger, pepper, cloves have been found to possess neuroprotective effects against PD [126, 127]. Consumption of green tea is beneficial against age-related neurological conditions and delayed onset of PD [128]. Securinine, a major natural alkaloid product from the root of the plant Securinega suf-fruticosa, has been reported to be a potential candidate for the treatment of neurodegenerative diseases related to neuroinflammation including Parkinson’s disease [129]. Neuroprotection is due to anti-inflammatory effects of securinine in glial cells. Securinine causes inhibition of the inflammatory mediator NF-κB, mitogen-activated protein kinases (MAPK) and also causes inhibition of interferon-γ- (IFN-γ), reduces mRNA expression for inducible nitric oxide synthase (iNOS) and decreases the level of nitric oxide (NO) level. Probiotics are live microorganisms that when administered in adequate amounts, confer health benefits to the host [130]. Potential probiotics on brain functions have been shown to be dependent on vagal activation [131]. Probiotics have been found to increase the concentration of dopamine by the production of L-DOPA, a precursor of dopamine that can cross the protective blood-brain barrier which then gets converted into dopamine with the help of DOPA decarboxylase enzyme [132] (Table 4).
Table 4.
Functions of constituents of natural products for parkinson’s disease.
| S. No. |
Natural Products-
Parkinson’s Disease |
Common Name | Constituent | Functions | Refs. |
|---|---|---|---|---|---|
| 1 | Estrogenic compounds | - | 17β-estradiol and progesterone | Neuroprotective effects | [118] |
| 2 | Natural polyphenolic compounds | - | Phytoestrogens | Neuroprotection via activation of receptors | [119, 120] |
| 3 | Securinega suffruticosa | Yi ye qiu | Securinine | Neuroprotection due to anti-inflammatory effects | [129] |
| 4 | Sex hormones | - | 17β-estradiol | Neuroprotection via of receptor activation | [118, 119, 121, 122] |
3.5. Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS) is a fatal disease and the exact cause of ALS is unknown which is characterized by gradual progressive deterioration of upper motor neurons (UMNs) and lower motor neurons (LMN) [133]. The UMN findings include hyperreflexia, extensor planar response and spasticity. This results from the degeneration of the lateral corticospinal tract in the spinal cord. While the LMN findings include hyporeflexia, weakness, atrophy, and fasciculation. ALS is eventually fatal because of respiratory muscle weakness. Until recently, riluzole is the only Food and Drug Administration (FDA) approved medication available and also recommended by the Institute for Clinical Excellence. It can marginally ameliorate respiratory failure and enhance the survival time up to 3 months only. On the other hand, it shows no beneficial effect on muscle wasting, atrophy, spasticity, weakness, dysarthria, dysphagia and life quality of ALS patients [134].
Gingko Biloba contains beneficial terpenes trilactones known as ginkgolides and sesquiterpene trilactones bilobalide. Ferrante et al., [133] examined the gender-specific neuroprotective effect of oral administration of Gingko biloba extract against mitochondrial damage and oxidative stress in transgenic mice model of ALS. The extract of the Gingko biloba exhibited significant improvement in motor performance and survival and ameliorated the loss of anterior motor neurons in male mice. It also showed a significant improvement in weight loss in both the genders thereby providing the strong evidences of its neuroprotective effects [133]. Similarly Ginseng has around 20 ginsenosides like protopanaxatriol and protopanaxadiol which are pharmacologically active constituents of ginseng. Jiang et al., 2000 described the beneficial effects of ginseng in transgenic mice in motor impairment and survival associated with ALS. It showed the prolongation in the onset of signs and survival and provided evidence for the ameliorative effects of ginseng in ALS [135].
Another study by Trieu et al., studied the effects of genistein treatment for its protection against pathological conditions like ALS and stroke. Genistein exhibits protection against singlet-oxygen induced cerebral damage in vivo by delaying the onset and reducing the mortality rate in G93A mice [136]. Epigallocatechin gallate (EGCG) from green tea is a bioactive natural constituent having a number of pharmacological actions like anticancer, anti-inflammatory, antioxidants etc. Koh et al., studied the role of EGCG in oxidative stress-injured motor neurons via PI3K/Akt and GSK-3 pathways. Pretreatment of the cells with EGCG before oxidative exposure increased the cell viability by enhancing the survival signals. They proved that EGCG induces the activation of PI3K/Akt and inhibits GSK-3 and may be a potential therapeutic strategy for ALS [137]. Another pharmacologically active compound, resveratrol, found in red wine, peanuts, grapes and berries etc. has antioxidant, anti-aging, anti-Alzheimer’s disease, anti-inflammatory and neuroprotective properties. Yanez et al., described the protective effects of resveratrol against neurotoxic effects produced by cerebrospinal fluid in cortical motor neurons primary culture. Resveratrol mitigated the Ca2+ elevation produced by ALS and acts as a neuroprotective compound with potential therapeutic application in ALS patients [138] (Table 5).
Table 5.
Functions of constituents of natural products for amyotrophic lateral sclerosis.
| S. No. | Natural Products-Amyotrophic Lateral Sclerosis | Common Name | Constituent | Functions | Refs. |
|---|---|---|---|---|---|
| 1 | Ginkgo biloba | Ginkgo | Terpenestrilactones (ginkgolides A,B,C and J) Sesquiterpene trilactones (bilobalides), flavonol glycosides (myricetinkaempherol and quercetin) |
Cognitive / memory enhancement | [197, 199, 218] |
| 2 | Panax ginseng and other species | Ginseng | Ginsenosides (protopanaxadiol and protopanaxatriol) | Antioxidant, anti-inflammatory | [135, 219] |
| 3 | Genistein | Genistein | 4,5,7-trihydroxyisoflavone | Anti-viral, anti-angiogenic | [136, 220] |
| 4 | Epigallocatechin Gallate (EGCG) | Catechin | Flavan-3-ol | Antioxidant, anti-inflammatory | [137, 221] |
| 5 | Resveratrol | Resveratrol | 3,5,4-trihydroxy trans stilbene | Anti-aging, anti-ischemic | [138, 223] |
3.6. Huntington’s Disease
Huntington’s chorea or Huntington’s disease (HD) was initially defined by an Ohio physician named George Huntington. Falling into the class of inherent autosomal dominant neurodegenerative disorder, it is differentiated by augmented motor impairment along with chorea and dystonia, emotional imbalance, memory, weight loss, personality changes and diminished capability to think clearly [139-142]. With juveniles being chiefly afflicted, it can transpire between 30 and 50-year populations [143]. The pathological backbone of neurodegenerative illness is oxidative stress (OS). Degeneration of antioxidant processes and elevated markers of reactive oxygen species (ROS) production initiate death of neuronal cells [144, 145]. Mother nature is an unbeaten and unparalleled expert having cure to probably maximum ailments of a man. Of all the medicinally relevant/active plants, several have complete pharmacological influence over the body. In neurodegenerative diseases, the beneficial therapeutic effects of these plants are manifested as antioxidant, anti-inflammatory, anti-apoptotic, calcium antagonization and modulation of neurological function [146, 147]. Numerous drugs dominant to CNS have been recognized as bright anti-Huntington's disease competitors some of which include Bacopa monnieri, Cannabis sativa, Centella asiatica, Gastrodia elata, Ginkgo biloba, Panax ginseng, Withania somnifera etc. Neuroprotective compounds such as curcumin, EGCG, ginsenosides, kaempferol, naringin, resveratrol and S-allylcysteine have been certified of their anti-Huntington’s disease property [148]. Bacopa monnieri (BM) can prove efficacious in HD therapy as its potential for being a powerful antioxidant and preventive action in opposition to stress-mediated neuronal impairment. Studies conducted on dietary BM additives have shown to produce a significant impact on brain against oxidative damage instigated by neurotoxicants [149]. The other compounds from plant sources which can be used in HD as antioxidants are sesamol (from sesame oil), celastrol and lycopene. Celastrol is a triterpenoid quinine methide while lycopene is a renowned carotenoid found in substantial amounts in tomatoes and tomato-based products [150, 151]. Sesamol has shown to produce protective effects against 3-nitropropionic acid (3-NP)-induced HD [152]. An additional nitric acid pathway in neuroprotection has been reported to be traced by lycopene [153, 154]. Flavonoids are also known to inhibit nitric oxide synthase and therefore delaying progression of HD [152-157].
Operating as an irreversible inhibitor of succinic acid dehydrogenase (SDH), in rats, the neuronal disruptions incited by 3-Nitropropionic acid are very similar to disorders in patients with Huntington’s disease [158]. Curcumin, a substance in turmeric has reportedly improved body weight, abolished motor defects and inflated SDH activity in 3-NP treated rats [159]. Resveratrol, a naturally occurring phytoalexin, can hamper or modify the development of neurological ailments like HD and ischemic stroke. The 3-NP instigated cognitive and motor defect can also be reversed by resveratrol [155, 160]. Upon treatment with resveratrol, improvement of locomotor activity and maze performance has been reported in animal model of HD [160].
In addition to excitotoxicity, an increase in malondialdehyde (MDA), heme-oxygenase, 3-nitrotyrosine has been detected in the brains of HD patients and rodents [161-163]. Ginseng root is a well-known herbal drug. Besides possessing antioxidant properties, it maintains the constitutional stability of neurons, improves cognitive capacity, restricts excitotoxicity and excess inflow of Ca2+ in neurons. There has been also a reduction in lipid peroxidation [164, 165]. A reducing sugar trehalose can be employed in emerging a novel therapeutic drug for HD as it possesses both the properties of inducing autophagy and chemical chaperone [166] (Table 6).
Table 6.
Functions of constituents of natural products for huntington’s disease.
| S. No. | Natural Products-Huntington’s Disease | Common Name | Constituent | Functions | Refs. |
|---|---|---|---|---|---|
| 1 | Bacopa monnieri | Brahmi | Leaf powder | Protective effect on brain against oxidative damage | [149] |
| 2 | Panax ginseng | Five fingers | Root | Maintenance of neuronal constitutional stability, improvement of cognitive capacity | [164, 165] |
| 3 | Sesamum indicum (oil) | Sesame | Sesamol | Protective effects against 3-NP induced HD | [64] |
| 4 | Tomatoes and tomato-based products | - | Lycopene | Antioxidant activity, additional nitric acid pathway in neuroprotection | [153, 154] |
| 5 | Curcuma longa | Turmeric | Curcumin | Obliteration of motor defects and inflation of SDH activity | [126, 163, 203] |
| 6 | Several fruits and vegetables | - | Flavonoids | Inhibition of nitric oxide synthase | [156, 157] |
| 7 | Several plants particularly grapevines | - | Resveratrol | Improvement of locomotor activity and maze performance | [138, 160, 222, 223] |
3.7. Peripheral Nerve Injury
The peripheral nervous system (PNS) is a highly complex system composed of the cranial and the spinal nerves which project from the spinal cord and then pass through the intervertebral foramina of the vertebrae [167]. Peripheral nerves are present in nearly all parts of the human body and are composed of both motor and sensory neurons [168]. Fractures, lacerations, crush injuries, scars, compression or some iatrogenic ways result in the injury of these peripheral nerves [169]. If such nerve injuries are left untreated, there may be a partial or total loss of sensory, motor and autonomic functions resulting in restricted activity or lifelong disability [170]. Desirable motor and sensory recovery after peripheral nerve injury is a clinical challenge and uses different neurotrophic drugs like methylprednisolone and gabapentin, steroids, hormones, and even low-dose radiation has been reported [171, 172]. However, their adverse effects are a major limitation associated with their clinical use.
High chemical diversity, biochemical specificity and several other molecular properties of the drugs obtained from natural products make them favourable for the treatment of nerve injuries and associated symptoms such as neuropathic pain [173, 174]. Primarily, peripheral nerve injury is followed by secondary ischemic injury and is dominated by inflammation. Vitamin B12, also called cobalamin, is naturally present in fishes and animal products like meat, eggs, milk and milk products [175]. It is a water-soluble vitamin with multiple functions in organisms, although it is required in minute quantities. Vitamin B12 acts as a coenzyme and facilitates the synthesis of nucleic acids and proteins via conversion of homocysteine to methionine. It accelerates the formation of the myelin sheath, promotes the neuraxon’s skeleton protein transportation and improves nerve conduction velocity [176]. Moreover, vitamin B12 has been reported to cause proliferation of Schwann cells and increases the diameter of axons [177]. Also, vitamin B12 is a good scavenger of reactive oxygen species and is well-known for antioxidant properties and neuroprotective role. In addition, vitamin B12 promotes regeneration and functional recovery of injured nerves through increased expression of brain-derived neurotrophic factor (BDNF) at both mRNA and protein levels [178]. Thioctic acid, also known as α-lipoic acid, is another biological antioxidant and detoxifying agent. It is proposed to be good for treating diabetic neuropathy, cognitive and neuromuscular deficits, countering age-associated cardiovascular and as a modulator of various inflammatory signalling pathways [179, 180]. Thioctic acid’s role as an agent interfering in liver metabolism and disease was also reviewed [181]. Due to the presence of an asymmetric carbon C3, thioctic acid exists in two enantiomers, namely, (+) and (−). The (−) enantiomer probably represents the active form of the compound which elicits the biological effects and is located intracellularly. Thioctic acid is a registered drug used worldwide as a nutraceutical and it is marketed mainly in the racemic (+/−) form for stability reasons. Thioctic acid has also been found to be effective against the central nervous system lesions which are consequent to peripheral nerve injury [181, 182].
Acetyl-L-carnitine (ALCAR) is a physiological peptide which also has some inherent antioxidant properties and ability to enhance the nerve growth factor binding capacity to receptors present on sensory neuronal populations and is integral to high-energy substrate oxidative metabolism within mitochondria [183]. In a preliminary study, ALCAR has been reported to reduce HIV-associated peripheral neuropathy as it leads to morphological regeneration of cutaneous nerves [184, 185]. N-acetyl-cysteine has also been shown to have a motor and sensory neuroprotective capacity. NAC has some cell-cycle regulatory potential as well as antioxidant properties and it also exerts complex effects on neurotrophic factor signalling pathways within neurons, such as blocking activation of JNK in trophin-deprived PC12 cells and uncoupling NGF activation of MAPK from that of Ras [186, 187]. Glutathione depletion, particularly intra-mitochondrial, increases the susceptibility of neurons to a variety of toxic stimuli like trophic factor withdrawal and oxidative stress [188]. However, N-acetyl-cysteine stimulates the synthesis of glutathione which is the principal renewable free radical scavenger within neurons. Unlike glutathione, cysteine is the rate-limiting precursor and can readily be taken up as NAC which crosses the blood-brain barrier, hence also have the advantage to be used as a therapeutic agent [189].
3.8. Ischaemic Stroke
The global root of mortality and persisting illness is the ischemic stroke and till now, no effective therapy is available for the treatment of cerebral ischemia. Recombinant plasminogen activator is the sole medicament utilized clinically and its use is restricted only to meager populations [190]. The prehistoric practice of therapeutics has given an account of plant-derived drugs for the cure of numerous disorders. In the immediate past, they have earned profuse approval due to its cost-effectiveness, appreciably higher therapeutic window and infrequent side effects. For prophylactic management of stroke, they are promising candidates. According to Siesjo et al., several pathways form high volumes of free radicals during ischemia/reperfusion eventually leading to the death of cell. This state accompanied by the decreased antioxidant defence, triggers oxidative stress which plays a critical role in the pathogenesis of neuronal injury linked to stroke. The recovery of brain damage after ischemia/reperfusion through inhibition of ROS gushes is the focus of present-day in order to develop new strategies for overcoming damage provoked by stroke [191].
Withania somnifera popularly recognized as ‘Aswagandha’ is a neuroprotective herb used in disorders of CNS [192]. Withanolides are the active principles of Withania somnifera. When administered repeatedly, they have exhibited amplification in the expression of the antioxidant enzymes [193]. Flavonoids from Scutellaria baicalensis have shown a substantial protective effect against cerebral ischemia and ischemia/reperfusion-induced brain injury. The fundamental mechanism behind the protective effect of Scutellaria baicalensis flavonoids is the scavenging potential of free radicals. There has been also a reduction in MDA level in injured brain tissues and enhanced SOD enzyme activity in brain tissues after cerebral hypoxia [183, 194]. Carnosic acid (CA) is a constituent of rosemary herb. It is acquired from Rosmarinus officinalis. CA may correspond to a novel kind of neuroprotective electrophilic compound as it is able to cross the blood-brain barrier and move into the brain, upsurges the concentration of reduced glutathione in-vivo, and protects the brain against ischemia/reperfusion brain injury using middle cerebral artery occlusion (MCAO) model [195]. The active principle ginsenosides of Panax ginseng reside in its root. By provoking endogenous neural stem cells activation, ginsenosides total saponins (GTS) can help in the recovery of neurological insufficiencies after focal cerebral ischemia. Thus, they boost restoration of the adult central nervous system [196]. A chemical compound isolated from the traditional Chinese herb known as ginseng 20(S)-GinsenosideRg reduces lipid peroxides, scavenges free radicals and boosts energy metabolism. In a rat model, these biological effects of ginseng 20(S)-GinsenosideRg exert neuroprotection against the cerebral ischemia brain injury [197].
A traditional Chinese herb extensively employed in the clinical treatment of stroke is Ginkgo biloba. Its standard extract EGb761 has exhibited substantial therapeutic outcomes on ischemic stroke. The likely reason behind it is believed to be activation of Akt–cyclic AMP-responsive element binding protein 1-Brain-derived neurotrophic factor (Akt-CREB–BDNF) pathway [198]. It has shown an improvement in behavioural neurologic outcome. The resultant neuroprotection can be ascribed to the shrinkage of brain infarct volume induced by perpetual and temporary middle cerebral artery (MCA) obstruction [199].
Acanthopanax polysaccharides are extracted from the root of Acanthopanax senticosus. They are comprised of chiisanoside, eleutheroside, saponin, senticoside, syringin, triterpenic and flavone compounds [200]. They have been reported to possess a wide variety of effects like anti-histaminic, antioxidant, anti-stress, hypolipidemic, immunomodulatory and sedative [201, 202]. The symptoms induced by cerebral ischemia/reperfusion injury in rat model can be improved by the treatment with Acanthopanax polysaccharides. They can also reduce the water content of the brain and infarct volume. In the brain tissue of rats with the cerebral ischemia/reperfusion injury, a decline in MDA, IL-1 and TNF-α levels with subsequent elevation of SOD and GSH-Px activities and IL-10 level has been reported after the supplementation with Acanthopanax polysaccharides [201]. Cinnamophilin (sequestered from Cinnamomum philippinense) and curcuma oil (isolated from powdered rhizomes of Curcuma longa Linn.) [203] when administered prior to cerebral ischemia has demonstrated neuroprotective effects but the underlying targets and mechanisms have not been completely understood [204].
Honokiol, a constituent of the herb Magnolia officinalis, can reduce the production of synaptosomal reactive oxygen groups, decrease infarct volume and attenuate Na+, K+-ATPase, mitochondrial membrane potential, mitochondrial metabolic activity in mice before and after stimulation of the brain ischaemia with middle cerebral artery occlusion. For the patients who experience ischaemic stroke, honokiol epitomizes as an encouraging therapy [205]. A common secondary effect often appearing after stroke is dementia. Primarily, there is formation and accumulation of β-amyloid that lead to progressive neuronal apoptosis. One of the phenolic phytochemicals, paeonol isolated from the Chinese herb Paeonia suffruticosa protects the memory loss resulting from cerebral ischemia. Paeonol treatment resulted in the reduced level of amyloid precursor protein (APP), β-secretase enzyme immunopositive cells and apoptosis [206]. A study conducted by Hsieh, et al., [207] revealed that paeonol lessens neuro-insufficiency and reduces cerebral infarction in rat model. It has also shown to complicate microglia activation and IL-1 besides scavenging superoxide anion in ischemia/ reperfusion injury in rat brain. Based on the in-vitro studies, the methanolic extract of Uncaria rhynchophylla has reported to inhibit nitric oxide (NO) and TNF-α production in BV-2 mouse microglial cells. The ischemic induction of COX-2 expression and the death of CA1 neurons in hippocampus, after global ischemia have also been prevented. These findings imply that in neuroprotective activities, there is the contribution of anti-inflammatory effects [208]. Another natural compound Hydroxysafflor yellow A (HSYA) isolated from the flower of the Carthamus tinctorius (safflower plant), has been shown to reduce apoptosis partially via PI3K/Akt/ GSK3β signalling pathway in rats pointing to the fact that HSYA protects against cerebral ischaemia/reperfusion injury [209]. Hydroxysafflor yellow B (SYB) has also exhibited neuroprotective actions by recuperating the energy metabolism, scavenging free radicals and decreasing lipid peroxides in the brain tissue [210].
Rhizoma Pinelliae pedatisectae is a Chinese herbal medicine which is a dried tuber of Pinelliapedatisecta Schott. It has a neuroprotective action against various disorders, therefore we further investigated the effects of n-butyl alcohol extracts of Rhizoma Pinelliae pedatisectae against cerebral ischemic/reperfusion injury using middle cerebral artery occlusion (MCAO) ischemic rat model. The extract of Rhizoma Pinelliae pedatisectae exhibited protective action in a dose-dependent manner and reported to enhance antioxidant enzyme, superoxide dismutase (SOD) activity and reduce malondialdehyde (lipid membrane damage marker/oxidative stress marker), and other inflammatory markers such as TNF-α and IL-1β. Cerebral ischemic/reperfusion injury normally leads to apoptotic neuronal cell death and prophylaxis treatment with n-butyl alcohol extracts of Rhizoma Pinelliae pedatisectae that reduced neuronal cell death as shown by increased Bcl-2 (anti-apoptotic marker) and decreased Bax (apoptotic marker). Thus, Rhizoma Pinelliae pedatisectae extract exhibited neuroprotective effects against cerebral ischemia in MCAO rat model by ameliorating oxidative stress, inflammatory and apoptotic responses [211].
A natural compound, 11-keto-β-boswellic acid, was isolated from the plant extract of Boswelliaserrata. Oxidative stress plays a key role in the cerebral ischemic injury. 11-keto-beta-boswellic acid is reported to be an activator of Nrf-2 which is a transcription factor that gets activated in oxidative stress and regulates detoxification pathway. Post-treatment with 11-keto-β-boswellic acid in MCAO rat model for cerebral ischemia suppressed the cerebral infarction as well as apoptotic neuronal cell death. It also reduced MDA level, alleviated SOD activity and enhanced protein expression of Nrf-2 and hemeoxygenase-1 (HO-1) in the brain. 11-keto-β-boswellic acid exerts neuroprotective effects against cerebral ischemia by alleviating oxidative stress through modulating Nrf-2/HO-1 axis pathway [212].
Osthole is a plant-derived bioactive coumarin compound which is found to be present in various plants such as Cnidiummonnieri. Pretreatment with osthole has been shown to exert neuroprotective effects against cerebral ischemia-reperfusion injury in MCAO rat model. Osthole protects the brain tissue against cerebral ischemia-reperfusion injury as shown by histological analysis and cerebral function. Moreover, osthole also reduced Bax and activated caspase-3 as well as enhanced Bcl-2 protein expression in the brain tissue along with a reduced number of apoptotic cells. Thus, osthole exhibited neuroprotective action against cerebral ischemia due to its anti-apoptotic effects [213].
Caffeic acid is a natural compound present in several products such as tea, coffee, fruits and vegetables. It has various biological properties like anti-oxidant, anti-microbial, anti-inflammatory, etc. It was reported that the inhibition of 5-lipoxygenase (LOX) enzyme has been shown to protect the brain from ischemic injury in a rat model. Caffeic acid, an inhibitor of LOX enzyme, has been shown neuroprotective effects against global cerebral ischemia-reperfusion injury using bilateral carotid artery occlusion (BCAO) rat model. Treatment with caffeic acid suppressed the escape latency performed for spatial learning and memory using Morris water maze. Caffeic acid treatment reduced hippocampus neuronal damage, increased number of neuron, reduced NF-κB expression as well as MDA level, and enhanced SOD activity in the hippocampus region of the brain. Treatment with caffeic acid significantly decreased LOX expression, thus caffeic acid exhibited neuroprotective effects mainly through LOX inhibition [214] (Table 7).
Table 7.
Functions of constituents of natural products for ischaemic stroke.
| S.No. | Natural Products-Ischemic Stroke | Common Name | Constituent | Functions | Refs. |
|---|---|---|---|---|---|
| 1 | Acanthopanax senticosus | Devil’s bush | Acanthopanax polysaccharides | Lowering of brain water content and infarct volume | [200, 201] |
| 2 | Cinnamomum philippinense | Philippine cinnamon | Cinnamophilin | Neuroprotection | [107, 202] |
| 3 | Curcuma longa | Turmeric | Oil isolated from powdered rhizomes | Neuroprotection | [203] |
| 4 | Ginkgo biloba | Maidenhair tree | Extract EGb761 | Launch of Akt–CREB–BDNF pathway | [197] |
| 5 | Ginseng/ Panax ginseng | Five fingers/ Radix ginseng | 20(S)-GinsenosideRg/ Ginsenosides | Hunting of free radicals, energy metabolism rejuvenation/ Trigger of endogenous neural stem cells activation | [195, 196] |
| 6 | Magnolia officinalis | Houpou | Honokiol | The decline in production of synaptosomal reactive oxygen groups | [204] |
| 7 | Paeonia suffruticosa | Tree peony | Paeonol | Protection of memory loss developing from ischemia of the brain | [205, 206] |
| 8 | Rosmarinus officinalis | Rosemary | Carnosic acid | Improvements in reduced glutathione concentration | [194] |
| 9 | Carthamus tinctorius | Safflower | Hydroxysafflor yellow A (HSYA) Hydroxysafflor yellow B (SYB) | Protection in response to cerebral ischaemic reperfusion injury | [208] |
| 10 | Scutellaria baicalensis | Baikal skullcap | Flavonoids | Eradication of free radicals | [193] |
| 11 | Withania somnifera | Ashwagandha | Hydroalcoholic extract | Augmentation in the antioxidant enzyme expression | [191] |
| 12 | Rhizoma Pinelliae pedatisectae | Pinellia tuber | n-butyl alcohol extract of tuber | Enhanced antioxidant enzyme, suppressed inflammatory and apoptotic responses | [210] |
| 13 | Boswellia serrata | Indian Olibanum, Indian Frankincense | 11-keto-β-boswellic acid | Modulator of Nrf-2/HO-1 pathway | [211] |
| 14 | Cnidium monnieri | Monnier’ssnowparsley | Osthole | Regulate apoptosis pathway | [212] |
| 15 |
Coffee, Tea, Fruits & Vegetables |
__ | Caffeic acid | Inhibit lipoxygenase enzyme, modulate oxidative stress |
[221] |
CONCLUSION
This review summarizes that the facts are comprehensive and deeply informative about the various established activities of natural products in in vitro and in vivo preclinical models, and their potential neuro-therapeutic applications using the available knowledge in the literature. Therefore, this review would assist as a reference for current advances in the study on natural products for neuroprotective.
ACKNOWLEDGEMENTS
This work is supported by SERB, Department of Science and Technology, Grant No. YSS/2015/001851 and SR/NM/ NB-1044/2016(G). Anas Ahmad is thankful to INST for providing fellowship.
LIST OF ABBREVIATIONS
- 3-NP
3-nitropropionic acid
- ACh
Acetylcholine
- AChE
Acetylcholine Esterase
- AD
Alzheimer’s Disease
- AED
Anti-Epileptic Drugs
- Akt
Protein kinase B
- ALCAR
Acetyl-L-Carnitine
- APP
Amyloid Precursor Protein
- APP
Amyloid Precursor Protein
- BACE
Beta-site APP Cleaving Enzyme
- BAD
Bcl-2-Associated Death promoter
- Bax
Bcl-2-like protein 4
- Bcl-2
B-cell lymphoma-2
- Bcl-W
Bcl-2-like protein
- Bcl-xL
B-cell lymphoma-extra-large
- BDNF
Brain Derived Neurotrophic Factor
- BIM
Bcl-2-like protein 11
- BM
Bacopa monnieri
- BMP
Petasites japonicum
- CA
Cornuammonis
- CA
Carnosicacid
- CK
Creatine Kinase
- CNS
Central Nervous System
- COX-2
Cycloxygenase-2
- CREB
cAMP Response Element Binding protein
- DA
Dopamine
- DOPA
Dihydroxyphenylalanine
- ER
Estrogen Receptor
- ERK
Extracellular signal-Regulated Kinases
- FAO
Food and Agriculture Organisation
- GABA
Gamma aminobutyric acid
- GPER
G Protein-Coupled Estrogen Receptor
- GSH-Px
Glutathione peroxidase
- GSK3β
Glycogen synthase kinase 3 beta
- GTS
Ginsenosides Total Saponins
- HD
Huntington’s Disease
- HIV
Human Immunodeficiency Virus
- HSYA
Hydroxysafflor Yellow A
- IFN-𝛾
Interferon gamma
- IGF
Insulin-like Growth Factor
- IL-1
Interleukin-1
- iNOS
Inducible Nitric Oxide Synthase
- JNK
c-Jun amino-terminal Kinase
- L-DOPA
Levo-dihydroxyphenylalanine
- LD
Lethal Dose
- LDH
Lactate Dehydrogenase
- MAO
Monoamine Oxidase
- MAPK
Mitogen-Activated Protein Kinase
- MeSH
Medical Subject Heading
- MCA
Middle Cerebral Artery
- MDA
Malondialdehyde
- MES
Maximal Electroshock
- MID
Multi-Infarct Dementia
- MPTP
1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine
- NAC
N-Acetyl-Cysteine
- NF-𝜅B
Nuclear Factor-kappa B
- NGF
Nerve Growth Factor
- NO
Nitric Oxide
- OS
Oxidative Stress
- PD
Parkinson’s Disease
- PI3K
Phosphoinositide 3-Kinase
- PNS
Peripheral Nervous System
- PTZ
Pentylenetetrazole
- Ras
Retrovirus-Associated DNA Sequences
- ROS
Reactive Oxygen Species
- SD
Sprague Dawley
- SDH
Succinic Acid Dehydrogenase
- SN
Substantia Nigra
- SOD
Superoxide Dismutase
- SYB
Hydroxysafflor Yellow B
- THLE
Tonic Hind Limb Extensor
- TNF
Tumor Necrosis Factor
- TUNEL
Terminal deoxy nucleotidyl transferase-mediated dUTP-biotin nick end labelling
- VGSC
Voltage-Gated Sodium Channel
- WHO
World Health Organization
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Borchardt J.K. The beginnings of drug therapy: Ancient mesopotamian medicine. Drug News Perspect. 2002;15(3):187–192. doi: 10.1358/dnp.2002.15.3.840015. [http://dx.doi.org/10.1358/dnp.2002.15.3.840015]. [PMID: 12677263]. [DOI] [PubMed] [Google Scholar]
- 2.Petlevski R., Hadžija M., Slijepčević M., Juretić D. Effect of ‘antidiabetis’ herbal preparation on serum glucose and fructosamine in NOD mice. J. Ethnopharmacol. 2001;75(2-3):181–184. doi: 10.1016/s0378-8741(01)00177-5. [http://dx.doi.org/10.1016/S0378-8741(01)00177-5]. [PMID: 11297848]. [DOI] [PubMed] [Google Scholar]
- 3.Bhat S.A., Kamal M.A., Yarla N.S., Ashraf G.M. Synopsis on managment strategies for neurodegenerative disorders: Challenges from bench to bedside in successful drug discovery and development. Curr. Top. Med. Chem. 2017;17(12):1371–1378. doi: 10.2174/1568026616666161222121229. [http://dx.doi.org/10.2174/1568026616666161222121229]. [PMID: 28017151]. [DOI] [PubMed] [Google Scholar]
- 4.Dadhania V.P., Trivedi P.P., Vikram A., Tripathi D.N. Nutraceuticals against neurodegeneration: A mechanistic insight. Curr. Neuropharmacol. 2016;14(6):627–640. doi: 10.2174/1570159X14666160104142223. [http://dx.doi.org/ 10.2174/1570159X14666160104142223]. [PMID: 26725888]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar G.P., Khanum F. Neuroprotective potential of phytochemicals. Pharmacogn. Rev. 2012;6(12):81–90. doi: 10.4103/0973-7847.99898. [http://dx.doi. org/10.4103/0973-7847.99898]. [PMID: 23055633]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh S., Dikshit M. Apoptotic neuronal death in Parkinson’s disease: involvement of nitric oxide. Brain Res. Brain Res. Rev. 2007;54(2):233–250. doi: 10.1016/j.brainresrev.2007.02.001. [http://dx.doi.org/10.1016/j.brainresrev.2007. 02.001]. [PMID: 17408564]. [DOI] [PubMed] [Google Scholar]
- 7.Vasant M.S., Kumar H., Kim I.-S., Koppulla S., Kim B.-W., Choi D.-K. Strategic selection of neuroinflammatory models in Parkinson's disease: evidence from experimental studies CNS Neurol. Disord. Drug Targets, (Formerly Current Drug Targets-CNS Neurological Disorders) 2013;12(5):680–697. doi: 10.2174/18715273113129990059. [DOI] [PubMed] [Google Scholar]
- 8.Fox S.H., Brotchie J.M. The MPTP-lesioned non-human primate models of Parkinson’s disease. Past, present, and future In Prog. Brain Res. Elsevier. 2010;184:133–157. doi: 10.1016/S0079-6123(10)84007-5. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z.Y., Liu J.Y., Yang C.B., Malampati S., Huang Y.Y., Li M.X., Li M., Song J.X. Neuroprotective natural products for the treatment of parkinson’s disease by targeting the autophagy-lysosome pathway: A systematic review. Phytother. Res. 2017;31(8):1119–1127. doi: 10.1002/ptr.5834. [http://dx.doi.org/10.1002/ptr.5834]. [PMID: 28504367]. [DOI] [PubMed] [Google Scholar]
- 10.Bagli E., Goussia A., Moschos M.M., Agnantis N., Kitsos G. Natural compounds and neuroprotection: Mechanisms of action and novel delivery systems. In Vivo. 2016;30(5):535–47. [PubMed] [Google Scholar]
- 11.Rahman I., Chung S. Dietary polyphenols, deacetylases and chromatin remodeling in inflammation. J. Nutrigenet. Nutrigenomics. 2010;3(4-6):220–230. doi: 10.1159/000324358. [http://dx.doi.org/10.1159/000324358]. [PMID: 21474953]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harvey A.L., Clark R.L., Mackay S.P., Johnston B.F. Current strategies for drug discovery through natural products. Expert Opin. Drug Discov. 2010;5(6):559–568. doi: 10.1517/17460441.2010.488263. [http://dx.doi.org/10.1517/ 17460441.2010.488263]. [PMID: 22823167]. [DOI] [PubMed] [Google Scholar]
- 13.Kimura I. Medical benefits of using natural compounds and their derivatives having multiple pharmacological actions. Yakugaku Zasshi. 2006;126(3):133–143. doi: 10.1248/yakushi.126.133. [http://dx.doi.org/10.1248/yakushi. 126.133]. [PMID: 16508237]. [DOI] [PubMed] [Google Scholar]
- 14.Wang F., Shing M., Huen Y., Tsang S.Y., Xue H. Neuroactive flavonoids interacting with GABAA receptor complex. Curr. Drug Targets CNS Neurol. Disord. 2005;4(5):575–585. doi: 10.2174/156800705774322030. [http://dx.doi. org/10.2174/156800705774322030]. [PMID: 16266290]. [DOI] [PubMed] [Google Scholar]
- 15.Luk K-C., Stern L., Weigele M., O’Brien R.A., Spirt N. Isolation and identification of “diazepam-like” compounds from bovine urine. J. Nat. Prod. 1983;46(6):852–861. doi: 10.1021/np50030a005. [http://dx.doi.org/10. 1021/np50030a005]. [PMID: 6330305]. [DOI] [PubMed] [Google Scholar]
- 16.Häberlein H., Tschiersch K-P., Boonen G., Hiller K-O. Chelidonium majus L.: components with in vitro affinity for the GABAA receptor. Positive cooperation of alkaloids. Planta Med. 1996;62(3):227–231. doi: 10.1055/s-2006-957865. [http://dx.doi.org/10.1055/s-2006-957865]. [PMID: 8693034]. [DOI] [PubMed] [Google Scholar]
- 17.Leung W.C., Zheng H., Huen M., Law S.L., Xue H. Anxiolytic-like action of orally administered dl-tetrahydropalmatine in elevated plus-maze. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27(5):775–779. doi: 10.1016/s0278-5846(03)00108-8. [http://dx.doi.org/10.1016/S0278-5846(03) 00108-8]. [PMID: 12921909]. [DOI] [PubMed] [Google Scholar]
- 18.Liao J-F., Wang H-H., Chen M-C., Chen C-C., Chen C-F. Benzodiazepine binding site-interactive flavones from Scutellaria baicalensis root. Planta Med. 1998;64(6):571–572. doi: 10.1055/s-2006-957517. [http://dx.doi. org/10.1055/s-2006-957517]. [PMID: 9776664]. [DOI] [PubMed] [Google Scholar]
- 19.Hui K.M., Wang X.H., Xue H. Interaction of flavones from the roots of Scutellaria baicalensis with the benzodiazepine site. Planta Med. 2000;66(1):91–93. doi: 10.1055/s-0029-1243121. [http://dx.doi.org/10.1055/s-0029-1243121]. [PMID: 10705749]. [DOI] [PubMed] [Google Scholar]
- 20.Spencer J.P. The impact of flavonoids on memory: physiological and molecular considerations. Chem. Soc. Rev. 2009;38(4):1152–1161. doi: 10.1039/b800422f. [http://dx.doi.org/10.1039/b800422f]. [PMID: 19421586]. [DOI] [PubMed] [Google Scholar]
- 21.Lin R-D., Hou W.C., Yen K.Y., Lee M.H. Inhibition of monoamine oxidase B (MAO-B) by Chinese herbal medicines. Phytomedicine. 2003;10(8):650–656. doi: 10.1078/0944-7113-00324. [http://dx.doi.org/10.1078/0944-7113-00324]. [PMID: 14692725]. [DOI] [PubMed] [Google Scholar]
- 22.Lin X., Zhang N. Berberine: Pathways to protect neurons. Phytother. Res. 2018;32(8):1501–1510. doi: 10.1002/ptr.6107. [http://dx.doi.org/10.1002/ ptr.6107]. [PMID: 29732634]. [DOI] [PubMed] [Google Scholar]
- 23.Giacalone M., Di Sacco F., Traupe I., Pagnucci N., Forfori F., Giunta F. Blueberry polyphenols and neuroprotection. Bioactive Nutraceuticals and Dietary Supplements in Neurological and Brain Disease; Elsevier. 2015:17–28. [Google Scholar]
- 24.Kulkarni R., Girish K.J., Kumar A. Nootropic herbs (Medhya Rasayana) in Ayurveda: An update. Pharmacogn. Rev. 2012;6(12):147–153. doi: 10.4103/0973-7847.99949. [http://dx.doi.org/10.4103/0973-7847.99949]. [PMID: 23055641]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vinutha B., Prashanth D., Salma K., Sreeja S.L., Pratiti D., Padmaja R., Radhika S., Amit A., Venkateshwarlu K., Deepak M. Screening of selected Indian medicinal plants for acetylcholinesterase inhibitory activity. J. Ethnopharmacol. 2007;109(2):359–363. doi: 10.1016/j.jep.2006.06.014. [http://dx.doi.org/10.1016/j.jep.2006.06.014]. [PMID: 16950584]. [DOI] [PubMed] [Google Scholar]
- 26.Itua I., Naderali E.K. Review: omega-3 and memory function: to eat or not to eat. Am. J. Alzheimers Dis. Other Demen. 2010;25(6):479–482. doi: 10.1177/1533317510376943. [http://dx.doi.org/10.1177/1533317510376943]. [PMID: 20702502]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daulatzai M.A. Neurotoxic saboteurs: Straws that break the hippo’s (hippocampus) back drive cognitive impairment and Alzheimer’s Disease. Neurotox. Res. 2013;24(3):407–459. doi: 10.1007/s12640-013-9407-2. [http://dx.doi.org/10.1007/s12640-013-9407-2]. [PMID: 23820984]. [DOI] [PubMed] [Google Scholar]
- 28.Neumann J.T., Cohan C.H., Dave K.R., Wright C.B., Perez-Pinzon M.A. Global cerebral ischemia: Synaptic and cognitive dysfunction. Curr. Drug Targets. 2013;14(1):20–35. doi: 10.2174/138945013804806514. [http://dx.doi. org/10.2174/138945013804806514]. [PMID: 23170794]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mishra R., Manchanda S., Gupta M., Kaur T., Saini V., Sharma A., Kaur G. Tinospora cordifolia ameliorates anxiety-like behavior and improves cognitive functions in acute sleep deprived rats. Sci. Rep. 2016;6:25564. doi: 10.1038/srep25564. [http://dx.doi.org/10.1038/srep25564]. [PMID: 27146164]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yalla R.Y., Mohana L.S., Saravana K. Review on effect of natural memory enhancing drugs on dementia. Int. J. Phytopharmacol. 2010;1:1–7. [Google Scholar]
- 31.Singh H., Dhawan B. Neuropsychopharmacological effects of the Ayurvedic nootropic Bacopa monniera Linn.(Brahmi). Indian J. Pharmacol. 1997;29(5):359. [Google Scholar]
- 32.Vohora D., Pal S.N., Pillai K.K. Protection from phenytoin-induced cognitive deficit by Bacopa monniera, a reputed Indian nootropic plant. J. Ethnopharmacol. 2000;71(3):383–390. doi: 10.1016/s0378-8741(99)00213-5. [http:// dx.doi.org/10.1016/S0378-8741(99)00213-5]. [PMID: 10940574]. [DOI] [PubMed] [Google Scholar]
- 33.Shukia B., Khanna N.K., Godhwani J.L. Effect of Brahmi Rasayan on the central nervous system. J. Ethnopharmacol. 1987;21(1):65–74. doi: 10.1016/0378-8741(87)90095-x. [http://dx.doi.org/10.1016/0378-8741(87)90095-X]. [PMID: 3695557]. [DOI] [PubMed] [Google Scholar]
- 34.Sethiya N.K., Nahata A., Dixit V., Mishra S. Cognition boosting effect of Canscora decussata (a South Indian Shankhpushpi). Eur. J. Integr. Med. 2012;4(1):e113–e121. [http://dx.doi.org/10.1016/ j.eujim.2011.11.003]. [Google Scholar]
- 35.Urbain A., Marston A., Grilo L.S., Bravo J., Purev O., Purevsuren B., Batsuren D., Reist M., Carrupt P-A., Hostettmann K. Xanthones from Gentianella amarella ssp. acuta with acetylcholinesterase and monoamine oxidase inhibitory activities. J. Nat. Prod. 2008;71(5):895–897. doi: 10.1021/np070690l. [http://dx.doi.org/10.1021/ np070690l]. [PMID: 18336006]. [DOI] [PubMed] [Google Scholar]
- 36.Cummings J.L., Vinters H.V., Cole G.M., Khachaturian Z.S. Alzheimer’s disease: etiologies, pathophysiology, cognitive reserve, and treatment opportunities. Neurology. 1998;51(1) Suppl. 1:S2–S17. doi: 10.1212/wnl.51.1_suppl_1.s2. [http://dx.doi.org/10.1212/WNL.51.1_Suppl_1.S2]. [PMID: 9674758]. [DOI] [PubMed] [Google Scholar]
- 37.Assemi M. Herbs affecting the central nervous system: gingko, kava, St. John’s wort, and valerian. Clin. Obstet. Gynecol. 2001;44(4):824–835. doi: 10.1097/00003081-200112000-00020. [http://dx.doi.org/10.1097/00003081-200112000-00020]. [PMID: 11600863]. [DOI] [PubMed] [Google Scholar]
- 38.Dias G.P., Cavegn N., Nix A., do Nascimento Bevilaqua M.C., Stangl D., Zainuddin M.S.A., Nardi A.E., Gardino P.F., Thuret S. The role of dietary polyphenols on adult hippocampal neurogenesis: molecular mechanisms and behavioural effects on depression and anxiety. Oxid. Med. Cell. Longev. 2012 doi: 10.1155/2012/541971. [http:// dx.doi.org/10.1155/2012/541971]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joseph J.A., Shukitt-Hale B., Denisova N.A., Bielinski D., Martin A., McEwen J.J., Bickford P.C. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J. Neurosci. 1999;19(18):8114–8121. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [http://dx.doi. org/10.1523/JNEUROSCI.19-18-08114.1999]. [PMID: 10479711]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Youdim K.A., Shukitt-Hale B., Martin A., Wang H., Denisova N., Bickford P.C., Joseph J.A. Short-term dietary supplementation of blueberry polyphenolics: Beneficial effects on aging brain performance and peripheral tissue function. Nutr. Neurosci. 2000;3(6):383–397. [http://dx.doi.org/10.1080/1028415X.2000.11747338]. [Google Scholar]
- 41.Casadesus G., Shukitt-Hale B., Stellwagen H.M., Zhu X., Lee H-G., Smith M.A., Joseph J.A. Modulation of hippocampal plasticity and cognitive behavior by short-term blueberry supplementation in aged rats. Nutr. Neurosci. 2004;7(5-6):309–316. doi: 10.1080/10284150400020482. [http:// dx.doi.org/10.1080/10284150400020482]. [PMID: 15682927]. [DOI] [PubMed] [Google Scholar]
- 41.Shukitt-Hale B., Lau F.C., Carey A.N., Galli R.L., Spangler E.L., Ingram D.K., Joseph J.A. Blueberry polyphenols attenuate kainic acid-induced decrements in cognition and alter inflammatory gene expression in rat hippocampus. Nutr. Neurosci. 2008;11(4):172–182. doi: 10.1179/147683008X301487. [http://dx.doi.org/10.1179/147683008X301487]. [PMID: 18681986]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hurst R.D., Wells R.W., Hurst S.M., McGhie T.K., Cooney J.M., Jensen D.J. Blueberry fruit polyphenolics suppress oxidative stress-induced skeletal muscle cell damage in vitro. Mol. Nutr. Food Res. 2010;54(3):353–363. doi: 10.1002/mnfr.200900094. [http://dx.doi.org/10.1002/mnfr. 200900094]. [PMID: 19885847]. [DOI] [PubMed] [Google Scholar]
- 44.Shukitt-Hale B., Carey A., Casadesus G., Galli R., Joseph J. Mechanisms involved in blueberry enhancements of motor and cognitive function in young and old rats. Soc. Neurosci. Abs. 2003;29:63314. [Google Scholar]
- 45.Shukitt-Hale B., Galli R.L., Meterko V., Carey A., Bielinski D.F., McGhie T., Joseph J.A. Dietary supplementation with fruit polyphenolics ameliorates age-related deficits in behavior and neuronal markers of inflammation and oxidative stress. Age (Dordr.) 2005;27(1):49–57. doi: 10.1007/s11357-005-4004-9. [http://dx.doi.org/10.1007/s11357-005-4004-9]. [PMID: 23598603]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pandey A., Bani S., Dutt P., Kumar S.N., Avtar S.K., Nabi Q.G. Multifunctional neuroprotective effect of Withanone, a compound from Withania somnifera roots in alleviating cognitive dysfunction. Cytokine. 2018;102:211–221. doi: 10.1016/j.cyto.2017.10.019. [http://dx.doi.org/10.1016/j.cyto. 2017.10.019]. [PMID: 29108796]. [DOI] [PubMed] [Google Scholar]
- 47.Visanji N. P., Brotchie J. M. Current protocols in pharmacology . 2005:5.42. 1–5.42. 13. doi: 10.1002/0471141755.ph0542s29. [DOI] [PubMed] [Google Scholar]
- 48.Davis G.C., Williams A.C., Markey S.P., Ebert M.H., Caine E.D., Reichert C.M., Kopin I.J. Chronic Parkinsonism secondary to intravenous injection of meperidine analogues. Psychiatry Res. 1979;1(3):249–254. doi: 10.1016/0165-1781(79)90006-4. [http://dx.doi.org/10.1016/0165-1781(79) 90006-4]. [PMID: 298352]. [DOI] [PubMed] [Google Scholar]
- 49.Langston J.W., Forno L.S., Rebert C.S., Irwin I. Selective nigral toxicity after systemic administration of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyrine (MPTP) in the squirrel monkey. Brain Res. 1984;292(2):390–394. doi: 10.1016/0006-8993(84)90777-7. [http://dx.doi.org/10.1016/0006-8993(84)90777-7]. [PMID: 6607092]. [DOI] [PubMed] [Google Scholar]
- 50.Huang J-L., Fu S-T., Jiang Y-Y., Cao Y-B., Guo M-L., Wang Y., Xu Z. Protective effects of Nicotiflorin on reducing memory dysfunction, energy metabolism failure and oxidative stress in multi-infarct dementia model rats. Pharmacol. Biochem. Behav. 2007;86(4):741–748. doi: 10.1016/j.pbb.2007.03.003. [http://dx.doi.org/10.1016/j.pbb.2007.03.003]. [PMID: 17448528]. [DOI] [PubMed] [Google Scholar]
- 51.Yu L., Chen C., Wang L-F., Kuang X., Liu K., Zhang H., Du J-R. Neuroprotective effect of kaempferol glycosides against brain injury and neuroinflammation by inhibiting the activation of NF-κB and STAT3 in transient focal stroke. PLoS One. 2013;8(2):e55839. doi: 10.1371/journal.pone.0055839. [http://dx.doi.org/10.1371/journal.pone.0055839]. [PMID: 23437066]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ren R., Shi C., Cao J., Sun Y., Zhao X., Guo Y., Wang C., Lei H., Jiang H., Ablat N., Xu J., Li W., Ma Y., Qi X., Ye M., Pu X., Han H. Neuroprotective effects of a standardized flavonoid extract of safflower against neurotoxin-induced cellular and animal models of Parkinson’s disease. Sci. Rep. 2016;6:22135. doi: 10.1038/srep22135. [http:// dx.doi.org/10.1038/srep22135]. [PMID: 26906725]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang Z., Yang J., Jia Y., Tian Y., Wen A. Pharmacokinetic properties of hydroxysafflor yellow A in healthy Chinese female volunteers. J. Ethnopharmacol. 2009;124(3):635–638. doi: 10.1016/j.jep.2009.02.026. [http://dx. doi.org/10.1016/j.jep.2009.02.026]. [PMID: 19570628]. [DOI] [PubMed] [Google Scholar]
- 54.Han B., Zhao H. Effects of hydroxysafflor yellow A in the attenuation of MPTP neurotoxicity in mice. Neurochem. Res. 2010;35(1):107–113. doi: 10.1007/s11064-009-0035-4. [http://dx.doi.org/10.1007/s11064-009-0035-4]. [PMID: 19680807]. [DOI] [PubMed] [Google Scholar]
- 55.Gao L., Li C., Yang R-Y., Lian W-W., Fang J-S., Pang X-C., Qin X-M., Liu A-L., Du G-H. Ameliorative effects of baicalein in MPTP-induced mouse model of Parkinson’s disease: A microarray study. Pharmacol. Biochem. Behav. 2015;133:155–163. doi: 10.1016/j.pbb.2015.04.004. [http://dx.doi.org/10.1016/j.pbb.2015.04.004]. [PMID: 25895692]. [DOI] [PubMed] [Google Scholar]
- 56.Xue X., Liu H., Qi L., Li X., Guo C., Gong D., Qu H. Baicalein ameliorated the upregulation of striatal glutamatergic transmission in the mice model of Parkinson’s disease. Brain Res. Bull. 2014;103:54–59. doi: 10.1016/j.brainresbull.2014.02.004. [http://dx.doi.org/10.1016/j.brainresbull.2014.02. 004]. [PMID: 24576689]. [DOI] [PubMed] [Google Scholar]
- 57.Nakajima A., Aoyama Y., Nguyen T-T.L., Shin E-J., Kim H-C., Yamada S., Nakai T., Nagai T., Yokosuka A., Mimaki Y., Ohizumi Y., Yamada K. Nobiletin, a citrus flavonoid, ameliorates cognitive impairment, oxidative burden, and hyperphosphorylation of tau in senescence-accelerated mouse. Behav. Brain Res. 2013;250:351–360. doi: 10.1016/j.bbr.2013.05.025. [http://dx.doi.org/10.1016/j.bbr.2013.05.025]. [PMID: 23714077]. [DOI] [PubMed] [Google Scholar]
- 58.Kim H.G., Ju M.S., Ha S.K., Lee H., Lee H., Kim S.Y., Oh M.S. Acacetin protects dopaminergic cells against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neuroinflammation in vitro and in vivo. Biol. Pharm. Bull. 2012;35(8):1287–1294. doi: 10.1248/bpb.b12-00127. [http://dx.doi.org/10.1248/bpb.b12-00127]. [PMID: 22863927]. [DOI] [PubMed] [Google Scholar]
- 59.Adeyemi O.O., Akindele A.J., Yemitan O.K., Aigbe F.R., Fagbo F.I. Anticonvulsant, anxiolytic and sedative activities of the aqueous root extract of Securidaca longepedunculata Fresen. J. Ethnopharmacol. 2010;130(2):191–195. doi: 10.1016/j.jep.2010.04.028. [http://dx.doi.org/10. 1016/j.jep.2010.04.028]. [PMID: 20435127]. [DOI] [PubMed] [Google Scholar]
- 60.Juergens U.R., Dethlefsen U., Steinkamp G., Gillissen A., Repges R., Vetter H. Anti-inflammatory activity of 1.8-cineol (eucalyptol) in bronchial asthma: a double-blind placebo-controlled trial. Respir. Med. 2003;97(3):250–256. doi: 10.1053/rmed.2003.1432. [http://dx.doi.org/10.1053/ rmed.2003.1432]. [PMID: 12645832]. [DOI] [PubMed] [Google Scholar]
- 61.Bhat J.U., Parray S.A., Aslam M., Ansari S., Nizami Q., Khanam R., Siddiqui A., Ahmad M.A. Anti-seizure activity of flower extracts of Nepeta bractaeta in Swiss albino mice. EXCLI J. 2012;11:531–537. [PMID: 27540346]. [PMC free article] [PubMed] [Google Scholar]
- 62.Baxter J.H., Steinberg D., Mize C.E., Avigan J. Absorption and metabolism of uniformly 14C-labeled phytol and phytanic acid by the intestine of the rat studied with thoracic duct cannulation. Biochim. Biophys. Acta. 1967;137(2):277–290. doi: 10.1016/0005-2760(67)90103-8. [http://dx.doi.org/ 10.1016/0005-2760(67)90103-8]. [PMID: 4167617]. [DOI] [PubMed] [Google Scholar]
- 63.Baxter J.H. Absorption of chlorophyll phytol in normal man and in patients with Refsum’s disease. J. Lipid Res. 1968;9(5):636–641. [PMID: 4177872]. [PubMed] [Google Scholar]
- 64.Hsieh P.F., Hou C-W., Yao P-W., Wu S-P., Peng Y-F., Shen M-L., Lin C-H., Chao Y-Y., Chang M-H., Jeng K-C. Sesamin ameliorates oxidative stress and mortality in kainic acid-induced status epilepticus by inhibition of MAPK and COX-2 activation. J. Neuroinflammation. 2011;8(1):57. doi: 10.1186/1742-2094-8-57. [http://dx.doi.org/10.1186/ 1742-2094-8-57]. [PMID: 21609430]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sok D.E., Oh S.H., Kim Y.B., Kang H.G., Kim M.R. Neuroprotection by extract of Petasites japonicus leaves, a traditional vegetable, against oxidative stress in brain of mice challenged with kainic acid. Eur. J. Nutr. 2006;45(2):61–69. doi: 10.1007/s00394-005-0565-8. [http://dx.doi.org/10. 1007/s00394-005-0565-8]. [PMID: 15997340]. [DOI] [PubMed] [Google Scholar]
- 66.Schachter S.C. Botanicals and herbs: a traditional approach to treating epilepsy. Neurotherapeutics. 2009;6(2):415–420. doi: 10.1016/j.nurt.2008.12.004. [http:// dx.doi.org/10.1016/j.nurt.2008.12.004]. [PMID: 19332338]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nassiri-Asl M., Naserpour Farivar T., Abbasi E., Sadeghnia H.R., Sheikhi M., Lotfizadeh M., Bazahang P. Effects of rutin on oxidative stress in mice with kainic acid-induced seizure. J. Integr. Med. 2013;11(5):337–342. doi: 10.3736/jintegrmed2013042. [http://dx.doi.org/10.3736/jintegrmed 2013042]. [PMID: 24063781]. [DOI] [PubMed] [Google Scholar]
- 68.Xie T., Wang W.P., Mao Z.F., Qu Z.Z., Luan S.Q., Jia L.J., Kan M.C. Effects of epigallocatechin-3-gallate on pentylenetetrazole-induced kindling, cognitive impairment and oxidative stress in rats. Neurosci. Lett. 2012;516(2):237–241. doi: 10.1016/j.neulet.2012.04.001. [http://dx.doi.org/10. 1016/j.neulet.2012.04.001]. [PMID: 22521706]. [DOI] [PubMed] [Google Scholar]
- 69.Wang F., Xu Z., Ren L., Tsang S.Y., Xue H. GABA A receptor subtype selectivity underlying selective anxiolytic effect of baicalin. Neuropharmacology. 2008;55(7):1231–1237. doi: 10.1016/j.neuropharm.2008.07.040. [http://dx. doi.org/10.1016/j.neuropharm.2008.07.040]. [PMID: 18723037]. [DOI] [PubMed] [Google Scholar]
- 70.Liu Y-F., Gao F., Li X-W., Jia R-H., Meng X-D., Zhao R., Jing Y-Y., Wang Y., Jiang W. The anticonvulsant and neuroprotective effects of baicalin on pilocarpine-induced epileptic model in rats. Neurochem. Res. 2012;37(8):1670–1680. doi: 10.1007/s11064-012-0771-8. [http://dx.doi. org/10.1007/s11064-012-0771-8]. [PMID: 22528832]. [DOI] [PubMed] [Google Scholar]
- 71.Jäger A.K., Krydsfeldt K., Rasmussen H.B. Bioassay-guided isolation of apigenin with GABA-benzodiazepine activity from Tanacetum parthenium. Phytother. Res. 2009;23(11):1642–1644. doi: 10.1002/ptr.2816. [http://dx.doi.org/10.1002/ptr.2816]. [PMID: 19441011]. [DOI] [PubMed] [Google Scholar]
- 72.Medina J.H., Viola H., Wolfman C., Marder M., Wasowski C., Calvo D., Paladini A.C. Neuroactive flavonoids: new ligands for the Benzodiazepine receptors. Phytomedicine. 1998;5(3):235–243. doi: 10.1016/S0944-7113(98)80034-2. [http://dx.doi.org/10.1016/S0944-7113(98)80034-2]. [PMID: 23195847]. [DOI] [PubMed] [Google Scholar]
- 73.Shakeel S., Rehman M.U., Tabassum N., Amin U., Mir M.U.R. Effect of naringenin (A naturally occurring flavanone) against pilocarpine-induced status epilepticus and oxidative stress in mice. Pharmacogn. Mag. 2017;13(Suppl. 1):S154–S160. doi: 10.4103/0973-1296.203977. [http://dx. doi.org/10.4103/0973-1296.203977]. [PMID: 28479741]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mathew J., Paul J., Nandhu M.S., Paulose C.S. Bacopa monnieri and Bacoside-A for ameliorating epilepsy associated behavioral deficits. Fitoterapia. 2010;81(5):315–322. doi: 10.1016/j.fitote.2009.11.005. [http://dx.doi.org/ 10.1016/j.fitote.2009.11.005]. [PMID: 19944749]. [DOI] [PubMed] [Google Scholar]
- 75.Joh E.H., Lee I.A., Kim D.H. Kalopanaxsaponins A and B isolated from Kalopanax pictus ameliorate memory deficits in mice. Phytother. Res. 2012;26(4):546–551. doi: 10.1002/ptr.3596. [http://dx.doi.org/10. 1002/ptr.3596]. [PMID: 21928370]. [DOI] [PubMed] [Google Scholar]
- 76.Singh D., Mishra A., Goel R.K. Effect of saponin fraction from Ficus religiosa on memory deficit, and behavioral and biochemical impairments in pentylenetetrazol kindled mice. Epilepsy Behav. 2013;27(1):206–211. doi: 10.1016/j.yebeh.2012.11.004. [http://dx.doi.org/10.1016/j.yebeh.2012.11. 004]. [PMID: 23332444]. [DOI] [PubMed] [Google Scholar]
- 77.Kar A. Glycosides, in Pharmacognosy and pharmacobiotechnology. New DelhiNew Age International (P) Limited: 2003. [Google Scholar]
- 78.Kokate C.K., Purohit A.P., Gokhale S.B. Pharmacognosy. Pune: Nirali Prakashan; 2003. [Google Scholar]
- 79.Pal D., Sahoo M., Mishra A.K. Analgesic and anticonvulsant effects of saponin isolated from the stems of Opuntia vulgaris Mill in mice. Eur Bull Drug Res. 2005;13:91–97. [Google Scholar]
- 80.Pal D., Sannigrahi S., Mazumder U. K. Analgesic and anticonvulsant effects of saponin isolated from the leaves of Clerodendrum infortunatum Linn. in mice. 2009 [PubMed] [Google Scholar]
- 81.Gupta M. kanti Mazumder, U.; Chakrabarti, S., CNS activities of methanolic extract of Moringa oleifera root in mice. Fitoterapia. 1999;70(3):244–250. [http://dx.doi.org/10.1016/S0367-326X(99) 00029-5]. [Google Scholar]
- 82.Mazumder U., Gupta M., Rath N. CNS activities of Cassia fistula in mice. Phytother. Res. 1998;12(7):520–522. [http://dx.doi.org/ 10.1002/(SICI)1099-1573(199811)12:7<520:AID-PTR345>3.0. CO;2-O]. [Google Scholar]
- 83.Gupta M., Mazumder U.K., Pal D., Bhattacharya S., Chakrabarty S. Studies on brain biogenic amines in methanolic extract of Cuscuta reflexa Roxb. and Corchorus olitorius Linn. seed treated mice. Acta Pol. Pharm. 2003;60(3):207–210. [PMID: 14556490]. [PubMed] [Google Scholar]
- 84.Chindo B.A., Anuka J.A., McNeil L., Yaro A.H., Adamu S.S., Amos S., Connelly W.K., Lees G., Gamaniel K.S. Anticonvulsant properties of saponins from Ficus platyphylla stem bark. Brain Res. Bull. 2009;78(6):276–282. doi: 10.1016/j.brainresbull.2008.12.005. [http://dx.doi.org/10.1016/j. brainresbull.2008.12.005]. [PMID: 19111909]. [DOI] [PubMed] [Google Scholar]
- 85.Sayyah M., Kamalinejad M., Bahrami Hidage R., Rustaiyan A. Antiepileptic potential and composition of the fruit essential oil of Ferula gummosa boiss. Iran. Biomed. J. 2001;5(2):69–72. [Google Scholar]
- 86.Tosun F., Kızılay Ç.A., Erol K., Kılıç F.S., Kürkçüoğlu M., Başer K.H.C. Anticonvulsant activity of furanocoumarins and the essential oil obtained from the fruits of Heracleum crenatifolium. Food Chem. 2008;107(3):990–993. [http://dx.doi.org/10.1016/j. foodchem.2007.08.085]. [Google Scholar]
- 87.Wahab A., Ul Haq R., Ahmed A., Khan R.A., Raza M. Anticonvulsant activities of nutmeg oil of Myristica fragrans. Phytother. Res. 2009;23(2):153–158. doi: 10.1002/ptr.2548. [http://dx.doi.org/10.1002/ptr. 2548]. [PMID: 19067329]. [DOI] [PubMed] [Google Scholar]
- 88.Perazzo F.F., Carvalho J.C., Carvalho J.E., Rehder V.L. Central properties of the essential oil and the crude ethanol extract from aerial parts of Artemisia annua L. Pharmacol. Res. 2003;48(5):497–502. doi: 10.1016/s1043-6618(03)00216-0. [http://dx.doi.org/10.1016/S1043-6618(03)00216-0]. [PMID: 12967596]. [DOI] [PubMed] [Google Scholar]
- 89.Koutroumanidou E., Kimbaris A., Kortsaris A., Bezirtzoglou E., Polissiou M., Charalabopoulos K., Pagonopoulou O. Increased seizure latency and decreased severity of pentylenetetrazol-induced seizures in mice after essential oil administration. Epilepsy Res. Treat. 2013;2013:532657. doi: 10.1155/2013/532657. [http://dx.doi.org/10.1155/2013/ 532657]. [PMID: 23819045]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Okoli C., Ezike A., Agwagah O., Akah P. Anticonvulsant and anxiolytic evaluation of leaf extracts of Ocimum gratissimum, a culinary herb pharmacognosy research. 2010;2(1):36–40. doi: 10.4103/0974-8490.60580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oliveira J.S., Porto L.A., Estevam C.S., Siqueira R.S., Barreto P.B., Niculau E.S., Blank A.F., Almeida R.N.d., Marchioro M., Quintans-Júnior L.J. Phytochemical screening and anticonvulsant property of Ocimum basilicum leaf essential oil. Bol. Latinoam. Caribe Plantas Med. Aromat. 2009;8:195–202. [Google Scholar]
- 92.Ismail M. Central Properties and Chemical Composition of Ocimum basilicum. Essential Oil. Pharm. Biol. 2006;44(8):619–626. [http://dx.doi.org/10.1080/13880200600897544]. [Google Scholar]
- 93.Huang C., Li W.G., Zhang X.B., Wang L., Xu T.L., Wu D., Li Y. α-asarone from Acorus gramineus alleviates epilepsy by modulating A-type GABA receptors. Neuropharmacology. 2013;65:1–11. doi: 10.1016/j.neuropharm.2012.09.001. [http://dx.doi.org/10.1016/j.neuropharm.2012.09.001]. [PMID: 22975146]. [DOI] [PubMed] [Google Scholar]
- 94.Hess E.J., Moody K.A., Geffrey A.L., Pollack S.F., Skirvin L.A., Bruno P.L., Paolini J.L., Thiele E.A. Cannabidiol as a new treatment for drug-resistant epilepsy in tuberous sclerosis complex. Epilepsia. 2016;57(10):1617–1624. doi: 10.1111/epi.13499. [http://dx.doi.org/10.1111/ epi.13499]. [PMID: 27696387]. [DOI] [PubMed] [Google Scholar]
- 95.Copmans D., Orellana-Paucar A.M., Steurs G., Zhang Y., Ny A., Foubert K., Exarchou V., Siekierska A., Kim Y., De Borggraeve W., Dehaen W., Pieters L., de Witte P.A.M. Methylated flavonoids as anti-seizure agents: Naringenin 4′,7-dimethyl ether attenuates epileptic seizures in zebrafish and mouse models. Neurochem. Int. 2018;112:124–133. doi: 10.1016/j.neuint.2017.11.011. [http://dx.doi.org/10.1016/ j.neuint.2017.11.011]. [PMID: 29174382]. [DOI] [PubMed] [Google Scholar]
- 96.Berchtold N.C., Cotman C.W. Evolution in the conceptualization of dementia and Alzheimer’s disease: Greco-Roman period to the 1960s. Neurobiol. Aging. 1998;19(3):173–189. doi: 10.1016/s0197-4580(98)00052-9. [http://dx.doi. org/10.1016/S0197-4580(98)00052-9]. [PMID: 9661992]. [DOI] [PubMed] [Google Scholar]
- 97.Brookmeyer R., Johnson E., Ziegler-Graham K., Arrighi H.M. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2007;3(3):186–191. doi: 10.1016/j.jalz.2007.04.381. [http://dx.doi.org/10.1016/j.jalz. 2007.04.381]. [PMID: 19595937]. [DOI] [PubMed] [Google Scholar]
- 98.Takahashi R.H., Nagao T., Gouras G.K. Plaque formation and the intraneuronal accumulation of β-amyloid in Alzheimer’s disease. Pathol. Int. 2017;67(4):185–193. doi: 10.1111/pin.12520. [http://dx.doi.org/ 10.1111/pin.12520]. [PMID: 28261941]. [DOI] [PubMed] [Google Scholar]
- 99.Fazili N.A., Naeem A., Ashraf G.M., Hua G.S., Kamal M.A. Therapeutic Interventions for the Suppression of Alzheimer’s Disease: Quest for a Remedy. Curr. Drug Metab. 2015;16(5):346–353. doi: 10.2174/1389200215999141125115749. [http://dx.doi.org/10.2174/1389200215999141125115749]. [PMID: 25429669]. [DOI] [PubMed] [Google Scholar]
- 100.Szeto J.Y., Lewis S.J.J., Lewis J.G. S., Current treatment options for Alzheimer’s disease and Parkinson’s disease dementia. Curr. Neuropharmacol. 2016;14(4):326–338. doi: 10.2174/1570159X14666151208112754. [http://dx.doi.org/ 10.2174/1570159X14666151208112754]. [PMID: 26644155]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Behl C., Moosmann B. Antioxidant neuroprotection in Alzheimer’s disease as preventive and therapeutic approach. Free Radic. Biol. Med. 2002;33(2):182–191. doi: 10.1016/s0891-5849(02)00883-3. [http://dx.doi.org/10.1016/ S0891-5849(02)00883-3]. [PMID: 12106814]. [DOI] [PubMed] [Google Scholar]
- 102.Pappolla M.A., Chyan Y.J., Omar R.A., Hsiao K., Perry G., Smith M.A., Bozner P. Evidence of oxidative stress and in vivo neurotoxicity of beta-amyloid in a transgenic mouse model of Alzheimer’s disease: A chronic oxidative paradigm for testing antioxidant therapies in vivo. Am. J. Pathol. 1998;152(4):871–877. [PMID: 9546346]. [PMC free article] [PubMed] [Google Scholar]
- 103.Heo H.J., Lee C.Y. Strawberry and its anthocyanins reduce oxidative stress-induced apoptosis in PC12 cells. J. Agric. Food Chem. 2005;53(6):1984–1989. doi: 10.1021/jf048616l. [http://dx.doi.org/10.1021/jf048616l]. [PMID: 15769124]. [DOI] [PubMed] [Google Scholar]
- 104.Ma T., Tan M-S., Yu J-T., Tan L. Resveratrol as a therapeutic agent for Alzheimer’s disease. Bio. Med. Res. Inter; 2014. [http:// dx.doi.org/10.1155/2014/350516] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chauhan V., Chauhan A. Oxidative stress in Alzheimer’s disease. Pathophysiology. 2006;13(3):195–208. doi: 10.1016/j.pathophys.2006.05.004. [http://dx.doi.org/10. 1016/j.pathophys.2006.05.004]. [PMID: 16781128]. [DOI] [PubMed] [Google Scholar]
- 106.Chauhan N., Wang K.C., Wegiel J., Malik M.N. Walnut extract inhibits the fibrillization of amyloid beta-protein, and also defibrillizes its preformed fibrils. Curr. Alzheimer Res. 2004;1(3):183–188. doi: 10.2174/1567205043332144. [http://dx.doi.org/10.2174/1567205043332144]. [PMID: 15975066]. [DOI] [PubMed] [Google Scholar]
- 107.Frydman-Marom A., Levin A., Farfara D., Benromano T., Scherzer-Attali R., Peled S., Vassar R., Segal D., Gazit E., Frenkel D., Ovadia M. Orally administrated cinnamon extract reduces β-amyloid oligomerization and corrects cognitive impairment in Alzheimer’s disease animal models. PLoS One. 2011;6(1):e16564. doi: 10.1371/journal.pone.0016564. [http://dx.doi.org/10.1371/journal.pone.0016564]. [PMID: 21305046]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sancesario G.M., Nuccetelli M., Cerri A., Zegeer J., Severini C., Ciotti M.T., Pieri M., Martorana A., Caltagirone C., Nistico R., Bernardini S. Bromelain degrades Aβ1-42 monomers and soluble aggregates: An in vitro study in cerebrospinal fluid of alzheimer’s disease patients. Curr. Alzheimer Res. 2018;15(7):628–636. doi: 10.2174/1567205015666180123124851. [http://dx.doi.org/10.2174/1567205015666180123124851]. [PMID: 29359669]. [DOI] [PubMed] [Google Scholar]
- 109.Katzman R., Saitoh T. Advances in Alzheimer’s disease. FASEB J. 1991;5(3):278–286. [http://dx.doi.org/10.1096/fasebj.5.3.2001787]. [PMID: 2001787]. [PubMed] [Google Scholar]
- 110.Becker R., Giacobini E., editors. Cholinergic Basis of Alzheimer’s Disease. Boston: Birkhauser; 1991. [Google Scholar]
- 111.Pereira D.M., Ferreres F., Oliveira J., Valentão P., Andrade P.B., Sottomayor M. Targeted metabolite analysis of Catharanthus roseus and its biological potential. Food Chem. Toxicol. 2009;47(6):1349–1354. doi: 10.1016/j.fct.2009.03.012. [http://dx.doi.org/10.1016/j.fct.2009.03.012]. [PMID: 19298840]. [DOI] [PubMed] [Google Scholar]
- 112.Lee Y.K., Yuk D.Y., Kim T.I., Kim Y.H., Kim K.T., Kim K.H., Lee B.J., Nam S-Y., Hong J.T. Protective effect of the ethanol extract of Magnolia officinalis and 4-O-methylhonokiol on scopolamine-induced memory impairment and the inhibition of acetylcholinesterase activity. J. Nat. Med. 2009;63(3):274–282. doi: 10.1007/s11418-009-0330-z. [http://dx.doi.org/10.1007/s11418-009-0330-z]. [PMID: 19343477]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ansari R., Mahta A., Mallack E., Luo J.J. Hyperhomocysteinemia and neurologic disorders: a review. J. Clin. Neurol. 2014;10(4):281–288. doi: 10.3988/jcn.2014.10.4.281. [http://dx.doi.org/10.3988/jcn.2014.10.4.281]. [PMID: 25324876]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Braak H., Ghebremedhin E., Rüb U., Bratzke H., Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318(1):121–134. doi: 10.1007/s00441-004-0956-9. [http://dx.doi. org/10.1007/s00441-004-0956-9]. [PMID: 15338272]. [DOI] [PubMed] [Google Scholar]
- 115.Sanders L.H., McCoy J., Hu X., Mastroberardino P.G., Dickinson B.C., Chang C.J., Chu C.T., Van Houten B., Greenamyre J.T. Mitochondrial DNA damage: Molecular marker of vulnerable nigral neurons in Parkinson’s disease. Neurobiol. Dis. 2014;70:214–223. doi: 10.1016/j.nbd.2014.06.014. [http://dx.doi.org/10.1016/j.nbd.2014.06.014]. [PMID: 24981012]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sayre L.M., Smith M.A., Perry G. Chemistry and biochemistry of oxidative stress in neurodegenerative disease. Curr. Med. Chem. 2001;8(7):721–738. doi: 10.2174/0929867013372922. [http://dx.doi.org/10.2174/0929867013372922]. [PMID: 11375746]. [DOI] [PubMed] [Google Scholar]
- 117.Mercuri N.B., Bernardi G. The ‘magic’ of L-dopa: why is it the gold standard Parkinson’s disease therapy? Trends Pharmacol. Sci. 2005;26(7):341–344. doi: 10.1016/j.tips.2005.05.002. [http://dx.doi.org/10.1016/j.tips.2005.05. 002]. [PMID: 15936832]. [DOI] [PubMed] [Google Scholar]
- 118.Callier S., Morissette M., Grandbois M., Pélaprat D., Di Paolo T. Neuroprotective properties of 17β-estradiol, progesterone, and raloxifene in MPTP C57Bl/6 mice. Synapse. 2001;41(2):131–138. doi: 10.1002/syn.1067. [http://dx.doi.org/10.1002/syn.1067]. [PMID: 11400179]. [DOI] [PubMed] [Google Scholar]
- 119.Arevalo M-A., Azcoitia I., Garcia-Segura L.M. The neuroprotective actions of oestradiol and oestrogen receptors. Nat. Rev. Neurosci. 2015;16(1):17–29. doi: 10.1038/nrn3856. [http://dx.doi.org/10.1038/nrn3856]. [PMID: 25423896]. [DOI] [PubMed] [Google Scholar]
- 120.Bourque M., Dluzen D.E., Di Paolo T. Neuroprotective actions of sex steroids in Parkinson’s disease. Front. Neuroendocrinol. 2009;30(2):142–157. doi: 10.1016/j.yfrne.2009.04.014. [http://dx.doi.org/10.1016/j.yfrne.2009.04.014]. [PMID: 19410597]. [DOI] [PubMed] [Google Scholar]
- 121.Cardona-Gomez P., Perez M., Avila J., Garcia-Segura L.M., Wandosell F. Estradiol inhibits GSK3 and regulates interaction of estrogen receptors, GSK3, and beta-catenin in the hippocampus. Mol. Cell. Neurosci. 2004;25(3):363–373. doi: 10.1016/j.mcn.2003.10.008. [http://dx.doi.org/ 10.1016/j.mcn.2003.10.008]. [PMID: 15033165]. [DOI] [PubMed] [Google Scholar]
- 122.Garcia-Segura L.M., Azcoitia I., DonCarlos L.L. Neuroprotection by estradiol. Prog. Neurobiol. 2001;63(1):29–60. doi: 10.1016/s0301-0082(00)00025-3. [http://dx.doi. org/10.1016/S0301-0082(00)00025-3]. [PMID: 11040417]. [DOI] [PubMed] [Google Scholar]
- 123.Mattson M.P. Apoptosis in neurodegenerative disorders. Nat. Rev. Mol. Cell Biol. 2000;1(2):120–129. doi: 10.1038/35040009. [http://dx.doi.org/10.1038/ 35040009]. [PMID: 11253364]. [DOI] [PubMed] [Google Scholar]
- 124.Yao M., Nguyen T-V.V., Pike C.J. Estrogen regulates Bcl-w and Bim expression: role in protection against β-amyloid peptide-induced neuronal death. J. Neurosci. 2007;27(6):1422–1433. doi: 10.1523/JNEUROSCI.2382-06.2007. [http://dx.doi.org/10.1523/JNEUROSCI.2382-06.2007]. [PMID: 17287517]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Morissette M., Al Sweidi S., Callier S., Di Paolo T. Estrogen and SERM neuroprotection in animal models of Parkinson’s disease. Mol. Cell. Endocrinol. 2008;290(1-2):60–69. doi: 10.1016/j.mce.2008.04.008. [http://dx.doi. org/10.1016/j.mce.2008.04.008]. [PMID: 18515001]. [DOI] [PubMed] [Google Scholar]
- 126.Mythri R.B., Bharath M.M. Curcumin: a potential neuroprotective agent in Parkinson’s disease. Curr. Pharm. Des. 2012;18(1):91–99. doi: 10.2174/138161212798918995. [http://dx.doi.org/10.2174/138161212798918995]. [PMID: 22211691]. [DOI] [PubMed] [Google Scholar]
- 127.Mythri R.B., Harish G., Bharath M.M. Therapeutic potential of natural products in Parkinson’s disease. Recent Pat. Endocr. Metab. Immune Drug Discov. 2012;6(3):181–200. doi: 10.2174/187221412802481793. [http://dx.doi.org/ 10.2174/187221412802481793]. [PMID: 22827714]. [DOI] [PubMed] [Google Scholar]
- 128.Kandinov B., Giladi N., Korczyn A.D. Smoking and tea consumption delay onset of Parkinson’s disease. Parkinsonism Relat. Disord. 2009;15(1):41–46. doi: 10.1016/j.parkreldis.2008.02.011. [http://dx.doi.org/10.1016/j.parkreldis. 2008.02.011]. [PMID: 18434232]. [DOI] [PubMed] [Google Scholar]
- 129.Leonoudakis D., Rane A., Angeli S., Lithgow G.J., Andersen J.K., Chinta S.J. Smoking and tea consumption delay onset of Parkinson’s disease. Parkinsonism Relat. Disord. 2009;15(1):41–46. doi: 10.1016/j.parkreldis.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 130.Fijan S. Microorganisms with claimed probiotic properties: an overview of recent literature. Int. J. Environ. Res. Public Health. 2014;11(5):4745–4767. doi: 10.3390/ijerph110504745. [http://dx.doi.org/10.3390/ijerph110504745]. [PMID: 24859749]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G., Bienenstock J., Cryan J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA. 2011;108(38):16050–16055. doi: 10.1073/pnas.1102999108. [http://dx.doi.org/10.1073/ pnas.1102999108]. [PMID: 21876150]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Surwase S.N., Jadhav J.P. Bioconversion of L-tyrosine to L-DOPA by a novel bacterium Bacillus sp. JPJ. Amino Acids. 2011;41(2):495–506. doi: 10.1007/s00726-010-0768-z. [http://dx.doi.org/10.1007/s00726-010-0768-z]. [PMID: 20963458]. [DOI] [PubMed] [Google Scholar]
- 133.Ferrante R.J., Klein A.M., Dedeoglu A., Beal M.F. Therapeutic efficacy of EGb761 (Gingko biloba extract) in a transgenic mouse model of amyotrophic lateral sclerosis. J. Mol. Neurosci. 2001;17(1):89–96. doi: 10.1385/jmn:17:1:89. [http://dx.doi.org/10.1385/JMN:17:1:89]. [PMID: 11665866]. [DOI] [PubMed] [Google Scholar]
- 134.Nabavi S.F., Daglia M., D’Antona G., Sobarzo-Sánchez E., Talas Z.S., Nabavi S.M. Natural compounds used as therapies targeting to amyotrophic lateral sclerosis. Curr. Pharm. Biotechnol. 2015;16(3):211–218. doi: 10.2174/1389201016666150118132224. [http://dx.doi.org/10.2174/1389201016666 150118132224]. [PMID: 25601606]. [DOI] [PubMed] [Google Scholar]
- 135.Jiang F., DeSilva S., Turnbull J. Beneficial effect of ginseng root in SOD-1 (G93A) transgenic mice. J. Neurol. Sci. 2000;180(1-2):52–54. doi: 10.1016/s0022-510x(00)00421-4. [http://dx.doi.org/10.1016/S0022-510X(00)00421-4]. [PMID: 11090864]. [DOI] [PubMed] [Google Scholar]
- 136.Trieu V.N., Uckun F.M. Genistein is neuroprotective in murine models of familial amyotrophic lateral sclerosis and stroke. Biochem. Biophys. Res. Commun. 1999;258(3):685–688. doi: 10.1006/bbrc.1999.0577. [http://dx. doi.org/10.1006/bbrc.1999.0577]. [PMID: 10329446]. [DOI] [PubMed] [Google Scholar]
- 137.Koh S-H., Kwon H., Kim K.S., Kim J., Kim M-H., Yu H-J., Kim M., Lee K-W., Do B.R., Jung H.K., Yang K.W., Appel S.H., Kim S.H. Epigallocatechin gallate prevents oxidative-stress-induced death of mutant Cu/Zn-superoxide dismutase (G93A) motoneuron cells by alteration of cell survival and death signals. Toxicology. 2004;202(3):213–225. doi: 10.1016/j.tox.2004.05.008. [http://dx.doi.org/10.1016/j.tox. 2004.05.008]. [PMID: 15337584]. [DOI] [PubMed] [Google Scholar]
- 138.Yáñez M., Galán L., Matías-Guiu J., Vela A., Guerrero A., García A.G. CSF from amyotrophic lateral sclerosis patients produces glutamate independent death of rat motor brain cortical neurons: protection by resveratrol but not riluzole. Brain Res. 2011;1423:77–86. doi: 10.1016/j.brainres.2011.09.025. [http://dx.doi.org/10.1016/j.brainres.2011.09.025]. [PMID: 21983205]. [DOI] [PubMed] [Google Scholar]
- 139.Krobitsch S., Kazantsev A.G. Huntington’s disease: From molecular basis to therapeutic advances. Int. J. Biochem. Cell Biol. 2011;43(1):20–24. doi: 10.1016/j.biocel.2010.10.014. [http://dx.doi.org/10.1016/j.biocel.2010.10.014]. [PMID: 21056115]. [DOI] [PubMed] [Google Scholar]
- 140.Kumar P., Kalonia H., Kumar A. Huntington’s disease: pathogenesis to animal models. Pharmacol. Rep. 2010;62(1):1–14. doi: 10.1016/s1734-1140(10)70238-3. [http://dx.doi.org/10.1016/S1734-1140(10)70238-3]. [PMID: 20360611]. [DOI] [PubMed] [Google Scholar]
- 141.Sawa A., Tomoda T., Bae B-I. Mechanisms of neuronal cell death in Huntington’s disease. Cytogenet. Genome Res. 2003;100(1-4):287–295. doi: 10.1159/000072864. [http://dx.doi.org/10.1159/000072864]. [PMID: 14526190]. [DOI] [PubMed] [Google Scholar]
- 142.Singhal A.K., Naithani V., Bangar O.P. Medicinal plants with a potential to treat Alzheimer and associated symptoms. International Journal of Nutrition, Pharmacology. Neurological Diseases. 2012;2(2):84. [http://dx.doi.org/10.4103/2231-0738.95927]. [Google Scholar]
- 143.Farrer L.A., Cupples L.A., Wiater P., Conneally P.M., Gusella J.F., Myers R.H. The normal Huntington disease (HD) allele, or a closely linked gene, influences age at onset of HD. Am. J. Hum. Genet. 1993;53(1):125–130. [PMID: 8317477]. [PMC free article] [PubMed] [Google Scholar]
- 144.Farooqui T., Farooqui A.A. Aging: an important factor for the pathogenesis of neurodegenerative diseases. Mech. Ageing Dev. 2009;130(4):203–215. doi: 10.1016/j.mad.2008.11.006. [http://dx.doi.org/10.1016/j.mad.2008.11.006]. [PMID: 19071157]. [DOI] [PubMed] [Google Scholar]
- 145.Dong X.X., Wang Y., Qin Z.H. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol. Sin. 2009;30(4):379–387. doi: 10.1038/aps.2009.24. [http:// dx.doi.org/10.1038/aps.2009.24]. [PMID: 19343058]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wu P.F., Zhang Z., Wang F., Chen J.G. Natural compounds from traditional medicinal herbs in the treatment of cerebral ischemia/reperfusion injury. Acta Pharmacol. Sin. 2010;31(12):1523–1531. doi: 10.1038/aps.2010.186. [http://dx.doi.org/10.1038/aps.2010.186]. [PMID: 21127495]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sandhya S., Vinod K., Kumar S. Herbs used for brain disorders. Hygeia J Drugs Med. 2010;2:38–45. [Google Scholar]
- 148.Dey A., De J.N. Neuroprotective therapeutics from botanicals and phytochemicals against Huntington’s disease and related neurodegenerative disorders. J. Herb. Med. 2015;5(1):1–19. [http:// dx.doi.org/10.1016/j.hermed.2015.01.002]. [Google Scholar]
- 149.Shinomol G.K. Muralidhara, Bacopa monnieri modulates endogenous cytoplasmic and mitochondrial oxidative markers in prepubertal mice brain. Phytomedicine. 2011;18(4):317–326. doi: 10.1016/j.phymed.2010.08.005. [http://dx. doi.org/10.1016/j.phymed.2010.08.005]. [PMID: 20850955]. [DOI] [PubMed] [Google Scholar]
- 150.Allison A.C., Cacabelos R., Lombardi V.R., Alvarez X.A., Vigo C. Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2001;25(7):1341–1357. doi: 10.1016/s0278-5846(01)00192-0. [http://dx. doi.org/10.1016/S0278-5846(01)00192-0]. [PMID: 11513350]. [DOI] [PubMed] [Google Scholar]
- 151.Visioli F., Riso P., Grande S., Galli C., Porrini M. Protective activity of tomato products on in vivo markers of lipid oxidation. Eur. J. Nutr. 2003;42(4):201–206. doi: 10.1007/s00394-003-0415-5. [http://dx.doi.org/10.1007/ s00394-003-0415-5]. [PMID: 12923651]. [DOI] [PubMed] [Google Scholar]
- 152.Baba N.H., Antoniades K., Habbal Z. Effects of dietary canola, olive, and linolenic acid enriched olive oils on plasma lipids, lipid peroxidation and lipoprotein lipase activity in rats. Nutr. Res. 1999;19(4):601–612. [http://dx.doi.org/10.1016/S0271-5317(99) 00025-1]. [Google Scholar]
- 153.Hsiao G., Fong T. H., Tzu N. H., Lin K. H., Chou D. S., Sheu J. R. A potent antioxidant, lycopene, affords neuroprotection against microglia activation and focal cerebral ischemia in rats in vivo, 2004;18(3):351–356. [PubMed] [Google Scholar]
- 154.Kumar P., Kumar A. Effect of lycopene and epigallocatechin-3-gallate against 3-nitropropionic acid induced cognitive dysfunction and glutathione depletion in rat: a novel nitric oxide mechanism. Food Chem. Toxicol. 2009;47(10):2522–2530. doi: 10.1016/j.fct.2009.07.011. [http://dx.doi. org/10.1016/j.fct.2009.07.011]. [PMID: 19616597]. [DOI] [PubMed] [Google Scholar]
- 155.Kumar P., Padi S.S., Naidu P.S., Kumar A. Cyclooxygenase inhibition attenuates 3-nitropropionic acid-induced neurotoxicity in rats: possible antioxidant mechanisms. Fundam. Clin. Pharmacol. 2007;21(3):297–306. doi: 10.1111/j.1472-8206.2007.00485.x. [http://dx.doi.org/10.1111/j.1472-8206.2007. 00485.x]. [PMID: 17521299]. [DOI] [PubMed] [Google Scholar]
- 156.Raso G.M., Meli R., Di Carlo G., Pacilio M., Di Carlo R. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 expression by flavonoids in macrophage J774A.1. Life Sci. 2001;68(8):921–931. doi: 10.1016/s0024-3205(00)00999-1. [http://dx.doi.org/10.1016/S0024-3205(00)00999-1]. [PMID: 11213362]. [DOI] [PubMed] [Google Scholar]
- 157.Ishige K., Schubert D., Sagara Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic. Biol. Med. 2001;30(4):433–446. doi: 10.1016/s0891-5849(00)00498-6. [http://dx.doi.org/10.1016/ S0891-5849(00)00498-6]. [PMID: 11182299]. [DOI] [PubMed] [Google Scholar]
- 158.Nishimura M., Okimura Y., Fujita H., Yano H., Lee J., Suzaki E., Inoue M., Utsumi K., Sasaki J. Mechanism of 3-nitropropionic acid-induced membrane permeability transition of isolated mitochondria and its suppression by L-carnitine. Cell Biochem. Funct. 2008;26(8):881–891. doi: 10.1002/cbf.1521. [http://dx.doi.org/10.1002/ cbf.1521]. [PMID: 18942062]. [DOI] [PubMed] [Google Scholar]
- 159.Kumar P., Padi S.S., Naidu P.S., Kumar A. Possible neuroprotective mechanisms of curcumin in attenuating 3-nitropropionic acid-induced neurotoxicity. Methods Find. Exp. Clin. Pharmacol. 2007;29(1):19–25. doi: 10.1358/mf.2007.29.1.1063492. [http://dx.doi.org/10.1358/mf.2007.29.1. 1063492]. [PMID: 17344940]. [DOI] [PubMed] [Google Scholar]
- 160.Kumar P., Padi S.S.V., Naidu P.S., Kumar A. Effect of resveratrol on 3-nitropropionic acid-induced biochemical and behavioural changes: possible neuroprotective mechanisms. Behav. Pharmacol. 2006;17(5-6):485–492. doi: 10.1097/00008877-200609000-00014. [http://dx.doi.org/10.1097/00008877-200609000-00014]. [PMID: 16940769]. [DOI] [PubMed] [Google Scholar]
- 161.Chen C-M. Mitochondrial dysfunction, metabolic deficits, and increased oxidative stress in Huntington’s disease. Chang Gung Med. J. 2011;34(2):135–152. [PMID: 21539755]. [PubMed] [Google Scholar]
- 162.Kumar A., Ratan R.R. Oxidative stress and Huntington’s disease: The good, the bad, and the ugly. J. Huntingtons Dis. 2016;5(3):217–237. doi: 10.3233/JHD-160205. [http://dx.doi.org/10.3233/JHD-160205]. [PMID: 27662334]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Choudhary S., Kumar P., Malik J. Plants and phytochemicals for Huntington’s disease. Pharmacogn. Rev. 2013;7(14):81–91. doi: 10.4103/0973-7847.120505. [http://dx.doi.org/10.4103/0973-7847.120505]. [PMID: 24347915]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Keum Y-S., Park K-K., Lee J-M., Chun K-S., Park J.H., Lee S.K., Kwon H., Surh Y-J. Antioxidant and anti-tumor promoting activities of the methanol extract of heat-processed ginseng. Cancer Lett. 2000;150(1):41–48. doi: 10.1016/s0304-3835(99)00369-9. [http://dx.doi.org/10.1016/S0304-3835(99)00369-9]. [PMID: 10755385]. [DOI] [PubMed] [Google Scholar]
- 165.Radad K., Gille G., Liu L., Rausch W-D. Use of ginseng in medicine with emphasis on neurodegenerative disorders. J. Pharmacol. Sci. 2006;100(3):175–186. doi: 10.1254/jphs.crj05010x. [http://dx.doi.org/10.1254/ jphs.CRJ05010X]. [PMID: 16518078]. [DOI] [PubMed] [Google Scholar]
- 166.Sarkar S., Davies J.E., Huang Z., Tunnacliffe A., Rubinsztein D.C. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and α-synuclein. J. Biol. Chem. 2007;282(8):5641–5652. doi: 10.1074/jbc.M609532200. [http://dx.doi.org/10.1074/ jbc.M609532200]. [PMID: 17182613]. [DOI] [PubMed] [Google Scholar]
- 167.McCance K.L., Heuther S.E. Pathophysiology: The Biologic Basis for Disease in Adults and Children. Philadelphia, USA: Elsevier Mosby; 2006. p. 411. [Google Scholar]
- 168.Geuna S., Tos P., Titolo P., Ciclamini D., Beningo T., Battiston B. Update on nerve repair by biological tubulization. J. Brachial Plex. Peripher. Nerve Inj. 2014;9(1):3. doi: 10.1186/1749-7221-9-3. [http://dx.doi.org/10.1186/ 1749-7221-9-3]. [PMID: 24606921]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Noble J., Munro C.A., Prasad V.S., Midha R. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J. Trauma. 1998;45(1):116–122. doi: 10.1097/00005373-199807000-00025. [http://dx.doi.org/10.1097/00005373-199807000-00025]. [PMID: 9680023]. [DOI] [PubMed] [Google Scholar]
- 170.Burnett M.G., Zager E.L. Pathophysiology of peripheral nerve injury: a brief review. Neurosurg. Focus. 2004;16(5):E1. doi: 10.3171/foc.2004.16.5.2. [http://dx.doi.org/10.3171/foc.2004.16.5.2]. [PMID: 15174821]. [DOI] [PubMed] [Google Scholar]
- 171.Gustafsson H., Flood K., Berge O-G., Brodin E., Olgart L., Stiller C-O. Gabapentin reverses mechanical allodynia induced by sciatic nerve ischemia and formalin-induced nociception in mice. Exp. Neurol. 2003;182(2):427–434. doi: 10.1016/s0014-4886(03)00097-9. [http://dx.doi.org/10.1016/ S0014-4886(03)00097-9]. [PMID: 12895453]. [DOI] [PubMed] [Google Scholar]
- 172.Schenker M., Riederer B.M., Kuntzer T., Barakat-Walter I. Thyroid hormones stimulate expression and modification of cytoskeletal protein during rat sciatic nerve regeneration. Brain Res. 2002;957(2):259–270. doi: 10.1016/s0006-8993(02)03607-7. [http://dx.doi.org/10.1016/S0006-8993(02) 03607-7]. [PMID: 12445968]. [DOI] [PubMed] [Google Scholar]
- 173.Quintans J.S., Antoniolli Â.R., Almeida J.R., Santana-Filho V.J., Quintans-Júnior L.J. Natural products evaluated in neuropathic pain models - a systematic review. Basic Clin. Pharmacol. Toxicol. 2014;114(6):442–450. doi: 10.1111/bcpt.12178. [http://dx.doi.org/10.1111/bcpt.12178]. [PMID: 24252102]. [DOI] [PubMed] [Google Scholar]
- 174.Ren Z-L., Zuo P-P. Neural regeneration: role of traditional Chinese medicine in neurological diseases treatment. J. Pharmacol. Sci. 2012;120(3):139–145. doi: 10.1254/jphs.12r06cp. [http://dx.doi.org/10.1254/jphs. 12R06CP]. [PMID: 23099323]. [DOI] [PubMed] [Google Scholar]
- 175.McDowell L.R. Vitamins in Animal and Human Nutrition. USA: Iowa State University Pres; 2008. [Google Scholar]
- 176.Wang S.A., Yang J., Zhang G.B., Feng Y.H., Wang F., Zhou P.Y. Effect of mecobalamin treatment on the recovery of patients with posterior communicating artery aneurysm inducing oculomotor nerve palsy after operation. Eur. Rev. Med. Pharmacol. Sci. 2015;19(14):2603–2607. [PMID: 26221889]. [PubMed] [Google Scholar]
- 177.Lopatina T., Kalinina N., Karagyaur M., Stambolsky D., Rubina K., Revischin A., Pavlova G., Parfyonova Y., Tkachuk V. Adipose-derived stem cells stimulate regeneration of peripheral nerves: BDNF secreted by these cells promotes nerve healing and axon growth de novo. PLoS One. 2011;6(3):e17899. doi: 10.1371/journal.pone.0017899. [http://dx.doi.org/ 10.1371/journal.pone.0017899]. [PMID: 21423756]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sun H., Yang T., Li Q., Zhu Z., Wang L., Bai G., Li D., Li Q., Wang W. Dexamethasone and vitamin B(12) synergistically promote peripheral nerve regeneration in rats by upregulating the expression of brain-derived neurotrophic factor. Arch. Med. Sci. 2012;8(5):924–930. doi: 10.5114/aoms.2012.31623. [http://dx.doi.org/10.5114/aoms.2012.31623]. [PMID: 23185205]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shay K.P., Moreau R.F., Smith E.J., Smith A.R., Hagen T.M. Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Biochim. Biophys. Acta. 2009;1790(10):1149–1160. doi: 10.1016/j.bbagen.2009.07.026. [http://dx.doi.org/10.1016/j.bbagen.2009.07.026]. [PMID: 19664690]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Biewenga G., Haenen G.R., Bast A. The role of lipoic acid in the treatment of diabetic polyneuropathy. Drug Metab. Rev. 1997;29(4):1025–1054. doi: 10.3109/03602539709002242. [http://dx.doi.org/10.3109/03602539709002242]. [PMID: 9421684]. [DOI] [PubMed] [Google Scholar]
- 181.Bustamante J., Lodge J.K., Marcocci L., Tritschler H.J., Packer L., Rihn B.H. α-lipoic acid in liver metabolism and disease. Free Radic. Biol. Med. 1998;24(6):1023–1039. doi: 10.1016/s0891-5849(97)00371-7. [http://dx.doi.org/10. 1016/S0891-5849(97)00371-7]. [PMID: 9607614]. [DOI] [PubMed] [Google Scholar]
- 182.Zhang Y., Wang X., Wang X., Xu Z., Liu Z., Ni Q., Chu X., Qiu M., Zhao A., Jia W. Protective effect of flavonoids from Scutellaria baicalensis Georgi on cerebral ischemia injury. J. Ethnopharmacol. 2006;108(3):355–360. doi: 10.1016/j.jep.2006.05.022. [http://dx.doi.org/10. 1016/j.jep.2006.05.022]. [PMID: 16829002]. [DOI] [PubMed] [Google Scholar]
- 183.De Grandis D., Santoro L., Di Benedetto P. L-acetylcarnitine in the treatment of patients with peripheral neuropathies. Clin. Drug Investig. 1995;10(6):317–322. doi: 10.2165/00044011-199510060-00001. [http://dx.doi.org/10.2165/ 00044011-199510060-00001]. [PMID: 27519331]. [DOI] [PubMed] [Google Scholar]
- 184.Hart A.M., Wilson A.D., Montovani C., Smith C., Johnson M., Terenghi G., Youle M. Acetyl-l-carnitine: a pathogenesis based treatment for HIV-associated antiretroviral toxic neuropathy. AIDS. 2004;18(11):1549–1560. doi: 10.1097/01.aids.0000131354.14408.fb. [http://dx.doi.org/10.1097/01.aids.0000 131354.14408.fb]. [PMID: 15238773]. [DOI] [PubMed] [Google Scholar]
- 185.Kamata H., Tanaka C., Yagisawa H., Matsuda S., Gotoh Y., Nishida E., Hirata H. Suppression of nerve growth factor-induced neuronal differentiation of PC12 cells. N-acetylcysteine uncouples the signal transduction from ras to the mitogen-activated protein kinase cascade. J. Biol. Chem. 1996;271(51):33018–33025. doi: 10.1074/jbc.271.51.33018. [http://dx.doi.org/10.1074/jbc.271.51.33018]. [PMID: 8955147]. [DOI] [PubMed] [Google Scholar]
- 186.Park D.S., Stefanis L., Yan C.Y.I., Farinelli S.E., Greene L.A. Ordering the cell death pathway. Differential effects of BCL2, an interleukin-1-converting enzyme family protease inhibitor, and other survival agents on JNK activation in serum/nerve growth factor-deprived PC12 cells. J. Biol. Chem. 1996;271(36):21898–21905. doi: 10.1074/jbc.271.36.21898. [http://dx.doi.org/10.1074/jbc.271.36.21898]. [PMID: 8702992]. [DOI] [PubMed] [Google Scholar]
- 187.Drukarch B., Schepens E., Jongenelen C.A., Stoof J.C., Langeveld C.H. Astrocyte-mediated enhancement of neuronal survival is abolished by glutathione deficiency. Brain Res. 1997;770(1-2):123–130. doi: 10.1016/s0006-8993(97)00790-7. [http://dx.doi.org/10.1016/S0006-8993(97)00790-7]. [PMID: 9372211]. [DOI] [PubMed] [Google Scholar]
- 188.Dringen R., Hamprecht B. N-acetylcysteine, but not methionine or 2-oxothiazolidine-4-carboxylate, serves as cysteine donor for the synthesis of glutathione in cultured neurons derived from embryonal rat brain. Neurosci. Lett. 1999;259(2):79–82. doi: 10.1016/s0304-3940(98)00894-5. [http://dx.doi. org/10.1016/S0304-3940(98)00894-5]. [PMID: 10025562]. [DOI] [PubMed] [Google Scholar]
- 189.Kaste M. Thrombolysis in ischaemic stroke -- present and future: role of combined therapy. Cerebrovasc. Dis. 2001;11(Suppl. 1):55–59. doi: 10.1159/000049126. [http://dx.doi.org/10.1159/000049126]. [PMID: 11244201]. [DOI] [PubMed] [Google Scholar]
- 190.Siesjö B.K. Pathophysiology and treatment of focal cerebral ischemia. Part I: Pathophysiology. J. Neurosurg. 1992;77(2):169–184. doi: 10.3171/jns.1992.77.2.0169. [http://dx.doi.org/10.3171/jns.1992.77.2.0169]. [PMID: 1625004]. [DOI] [PubMed] [Google Scholar]
- 191.Chaudhary G., Sharma U., Jagannathan N.R., Gupta Y.K. Evaluation of Withania somnifera in a middle cerebral artery occlusion model of stroke in rats. Clin. Exp. Pharmacol. Physiol. 2003;30(5-6):399–404. doi: 10.1046/j.1440-1681.2003.03849.x. [http://dx.doi.org/10.1046/j.1440-1681. 2003.03849.x]. [PMID: 12859433]. [DOI] [PubMed] [Google Scholar]
- 192.Bhattacharya S.K., Bhattacharya D., Sairam K., Ghosal S. Effect of Withania somnifera glycowithanolides on a rat model of tardive dyskinesia. Phytomedicine. 2002;9(2):167–170. doi: 10.1078/0944-7113-00089. [http://dx. doi.org/10.1078/0944-7113-00089]. [PMID: 11995951]. [DOI] [PubMed] [Google Scholar]
- 193.Gaire B.P., Moon S-K., Kim H. Scutellaria baicalensis in stroke management: nature’s blessing in traditional Eastern medicine. Chin. J. Integr. Med. 2014;20(9):712–720. doi: 10.1007/s11655-014-1347-9. [http://dx.doi.org/ 10.1007/s11655-014-1347-9]. [PMID: 24752475]. [DOI] [PubMed] [Google Scholar]
- 194.Satoh T., Kosaka K., Itoh K., Kobayashi A., Yamamoto M., Shimojo Y., Kitajima C., Cui J., Kamins J., Okamoto S., Izumi M., Shirasawa T., Lipton S.A. Carnosic acid, a catechol-type electrophilic compound, protects neurons both in vitro and in vivo through activation of the Keap1/Nrf2 pathway via S-alkylation of targeted cysteines on Keap1. J. Neurochem. 2008;104(4):1116–1131. doi: 10.1111/j.1471-4159.2007.05039.x. [http://dx.doi.org/10.1111/j.1471-4159.2007.05039.x]. [PMID: 17995931]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zheng G.Q., Cheng W., Wang Y., Wang X.M., Zhao S.Z., Zhou Y., Liu S.J., Wang X.T. Ginseng total saponins enhance neurogenesis after focal cerebral ischemia. J. Ethnopharmacol. 2011;133(2):724–728. doi: 10.1016/j.jep.2010.01.064. [http://dx.doi.org/10.1016/j.jep.2010.01. 064]. [PMID: 21073942]. [DOI] [PubMed] [Google Scholar]
- 196.Tian J., Fu F., Geng M., Jiang Y., Yang J., Jiang W., Wang C., Liu K. Neuroprotective effect of 20(S)-ginsenoside Rg3 on cerebral ischemia in rats. Neurosci. Lett. 2005;374(2):92–97. doi: 10.1016/j.neulet.2004.10.030. [http://dx.doi.org/10.1016/j.neulet.2004.10.030]. [PMID: 15644271]. [DOI] [PubMed] [Google Scholar]
- 197.Zhang Z., Peng D., Zhu H., Wang X. Experimental evidence of Ginkgo biloba extract EGB as a neuroprotective agent in ischemia stroke rats. Brain Res. Bull. 2012;87(2-3):193–198. doi: 10.1016/j.brainresbull.2011.11.002. [http://dx. doi.org/10.1016/j.brainresbull.2011.11.002]. [PMID: 22100334]. [DOI] [PubMed] [Google Scholar]
- 198.Lee E.J., Chen H.Y., Wu T.S., Chen T.Y., Ayoub I.A., Maynard K.I. Acute administration of Ginkgo biloba extract (EGb 761) affords neuroprotection against permanent and transient focal cerebral ischemia in Sprague-Dawley rats. J. Neurosci. Res. 2002;68(5):636–645. doi: 10.1002/jnr.10251. [http://dx.doi.org/10.1002/jnr.10251]. [PMID: 12111854]. [DOI] [PubMed] [Google Scholar]
- 199.Deyama T., Nishibe S., Nakazawa Y. Constituents and pharmacological effects of Eucommia and Siberian ginseng. Acta Pharmacol. Sin. 2001;22(12):1057–1070. [PMID: 11749801]. [PubMed] [Google Scholar]
- 200.Xie Y., Zhang B., Zhang Y. Protective effects of Acanthopanax polysaccharides on cerebral ischemia-reperfusion injury and its mechanisms. Int. J. Biol. Macromol. 2015;72:946–950. doi: 10.1016/j.ijbiomac.2014.09.055. [http://dx. doi.org/10.1016/j.ijbiomac.2014.09.055]. [PMID: 25451748]. [DOI] [PubMed] [Google Scholar]
- 201.Li W., Liu M., Feng S., Wu B., Zhang S., Yang W., Liu G.J. Acanthopanax for Acute Ischemic Stroke. Stroke. 2010;41(11):e582–e583. [http://dx.doi.org/10.1161/STROKEAHA.110.593855]. [PMID: 20847317]. [Google Scholar]
- 202.Lee E-J., Chen H-Y., Lee M-Y., Chen T-Y., Hsu Y-S., Hu Y-L., Chang G-L., Wu T-S. Cinnamophilin reduces oxidative damage and protects against transient focal cerebral ischemia in mice. Free Radic. Biol. Med. 2005;39(4):495–510. doi: 10.1016/j.freeradbiomed.2005.04.004. [http://dx.doi.org/ 10.1016/j.freeradbiomed.2005.04.004]. [PMID: 16043021]. [DOI] [PubMed] [Google Scholar]
- 203.Rathore P., Dohare P., Varma S., Ray A., Sharma U., Jagannathan N.R., Ray M. Curcuma oil: reduces early accumulation of oxidative product and is anti-apoptogenic in transient focal ischemia in rat brain. Neurochem. Res. 2008;33(9):1672–1682. doi: 10.1007/s11064-007-9515-6. [http://dx.doi.org/10.1007/s11064-007-9515-6]. [PMID: 17955367]. [DOI] [PubMed] [Google Scholar]
- 204.Chen C.M., Liu S.H., Lin-Shiau S.Y. Honokiol, a neuroprotectant against mouse cerebral ischaemia, mediated by preserving Na+, K+-ATPase activity and mitochondrial functions. Basic Clin. Pharmacol. Toxicol. 2007;101(2):108–116. doi: 10.1111/j.1742-7843.2007.00082.x. [http://dx.doi.org/10. 1111/j.1742-7843.2007.00082.x]. [PMID: 17651312]. [DOI] [PubMed] [Google Scholar]
- 205.Su S-Y., Cheng C-Y., Tsai T-H., Hsieh C-L. Paeonol protects memory after ischemic stroke via inhibiting β-secretase and apoptosis. Evid. Based Complement. Alternat. Med. 2012 doi: 10.1155/2012/932823. [http://dx.doi.org/10.1155/2012/932823]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hsieh C-L., Cheng C-Y., Tsai T-H., Lin I.H., Liu C-H., Chiang S-Y., Lin J-G., Lao C-J., Tang N-Y. Paeonol reduced cerebral infarction involving the superoxide anion and microglia activation in ischemia-reperfusion injured rats. J. Ethnopharmacol. 2006;106(2):208–215. doi: 10.1016/j.jep.2005.12.027. [http://dx.doi.org/10.1016/j.jep.2005.12.027]. [PMID: 16458462]. [DOI] [PubMed] [Google Scholar]
- 207.Suk K., Kim S.Y., Leem K., Kim Y.O., Park S.Y., Hur J., Baek J., Lee K.J., Zheng H.Z., Kim H. Neuroprotection by methanol extract of Uncaria rhynchophylla against global cerebral ischemia in rats. Life Sci. 2002;70(21):2467–2480. doi: 10.1016/s0024-3205(02)01534-5. [http://dx. doi.org/10.1016/S0024-3205(02)01534-5]. [PMID: 12173411]. [DOI] [PubMed] [Google Scholar]
- 208.Chen L., Xiang Y., Kong L., Zhang X., Sun B., Wei X., Liu H. Hydroxysafflor yellow A protects against cerebral ischemia-reperfusion injury by anti-apoptotic effect through PI3K/Akt/ GSK3β pathway in rat. Neurochem. Res. 2013;38(11):2268–2275. doi: 10.1007/s11064-013-1135-8. [http://dx.doi.org/10.1007/s11064-013-1135-8]. [PMID: 23990223]. [DOI] [PubMed] [Google Scholar]
- 209.Wang C., Zhang D., Li G., Liu J., Tian J., Fu F., Liu K. Neuroprotective effects of safflor yellow B on brain ischemic injury. Exp. Brain Res. 2007;177(4):533–539. doi: 10.1007/s00221-006-0705-2. [http://dx.doi.org/10. 1007/s00221-006-0705-2]. [PMID: 17006684]. [DOI] [PubMed] [Google Scholar]
- 210.Ye Y., Li J., Cao X., Chen Y., Ye C., Chen K. Protective effect of n-butyl alcohol extracts from Rhizoma Pinelliae Pedatisectae against cerebral ischemia-reperfusion injury in rats. J. Ethnopharmacol. 2016;188:259–265. doi: 10.1016/j.jep.2016.04.046. [http://dx.doi.org/10.1016/j.jep.2016. 04.046]. [PMID: 27132713]. [DOI] [PubMed] [Google Scholar]
- 211.Ding Y., Chen M., Wang M., Li Y., Wen A. Posttreatment with 11-Keto-β-Boswellic Acid Ameliorates Cerebral Ischemia-Reperfusion Injury: Nrf2/HO-1 Pathway as a Potential Mechanism. Mol. Neurobiol. 2015;52(3):1430–1439. doi: 10.1007/s12035-014-8929-9. [http://dx.doi.org/ 10.1007/s12035-014-8929-9]. [PMID: 25452227]. [DOI] [PubMed] [Google Scholar]
- 212.Li K., Ding D., Zhang M. Neuroprotection of Osthole against Cerebral Ischemia/Reperfusion Injury through an Anti-apoptotic Pathway in Rats. Biol. Pharm. Bull. 2016;39(3):336–342. doi: 10.1248/bpb.b15-00699. [http:// dx.doi.org/10.1248/bpb.b15-00699]. [PMID: 26934926]. [DOI] [PubMed] [Google Scholar]
- 213.Liang G., Shi B., Luo W., Yang J. The protective effect of caffeic acid on global cerebral ischemia-reperfusion injury in rats. Behav. Brain Funct. 2015;11:18. doi: 10.1186/s12993-015-0064-x. [http://dx.doi.org/10.1186/s12993-015-0064-x]. [PMID: 25907417]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Singh H., Rastogi R., Srimal R., Dhawan B. Effect of bacosides A and B on avoidance responses in rats. Phytother. Res. 1988;2(2):70–75. [http://dx.doi.org/10.1002/ptr.2650020205]. [Google Scholar]
- 215.Gupta A., Raj H., Karchuli M.S., Upmanyu N. Comparative evaluation of ethanolic extracts of Bacopa monnieri, Evolvulus alsinoides, Tinospora cordifolia and their combinations on cognitive functions in rats. Curr. Aging Sci. 2013;6(3):239–243. doi: 10.2174/18746098112059990036. [http:// dx.doi.org/10.2174/18746098112059990036]. [PMID: 23866011]. [DOI] [PubMed] [Google Scholar]
- 216.Papandreou M.A., Dimakopoulou A., Linardaki Z.I., Cordopatis P., Klimis-Zacas D., Margarity M., Lamari F.N. Effect of a polyphenol-rich wild blueberry extract on cognitive performance of mice, brain antioxidant markers and acetylcholinesterase activity. Behav. Brain Res. 2009;198(2):352–358. doi: 10.1016/j.bbr.2008.11.013. [http://dx.doi.org/10. 1016/j.bbr.2008.11.013]. [PMID: 19056430]. [DOI] [PubMed] [Google Scholar]
- 217.Vingtdeux V., Dreses-Werringloer U., Zhao H., Davies P., Marambaud P. Therapeutic potential of resveratrol in Alzheimer’s disease. BMC Neurosci. 2008;9(2) Suppl. 2:S6. doi: 10.1186/1471-2202-9-S2-S6. [http://dx.doi. org/10.1186/1471-2202-9-S2-S6]. [PMID: 19090994]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Singh B., Kaur P., Gopichand; Singh R.D., Ahuja P.S. Biology and chemistry of Ginkgo biloba. Fitoterapia. 2008;79(6):401–418. doi: 10.1016/j.fitote.2008.05.007. [http://dx.doi.org/10.1016/j.fitote.2008.05.007]. [PMID: 18639617]. [DOI] [PubMed] [Google Scholar]
- 219.Nakaya T-A., Kita M., Kuriyama H., Iwakura Y., Imanishi J. Panax ginseng induces production of proinflammatory cytokines via toll-like receptor. J. Interferon Cytokine Res. 2004;24(2):93–100. doi: 10.1089/107999004322813336. [http://dx.doi.org/10.1089/107999004322813336]. [PMID: 14980073]. [DOI] [PubMed] [Google Scholar]
- 220.Polkowski K., Mazurek A.P. Biological properties of genistein. A review of in vitro and in vivo data. Acta Poloniae Pliarmaceutica—. Drug Res. 2000;57(2):l35–l55. [PubMed] [Google Scholar]
- 221.Khan N., Afaq F., Saleem M., Ahmad N., Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol (-)-epigallocatechin-3-gallate. Cancer Res. 2006;66(5):2500–2505. doi: 10.1158/0008-5472.CAN-05-3636. [http://dx.doi.org/10.1158/0008-5472.CAN-05-3636]. [PMID: 16510563]. [DOI] [PubMed] [Google Scholar]
- 222.Baur J.A., Sinclair D.A. Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discov. 2006;5(6):493–506. doi: 10.1038/nrd2060. [http://dx.doi.org/10.1038/nrd2060]. [PMID: 16732220]. [DOI] [PubMed] [Google Scholar]
- 223.Langcake P., Pryce R. The production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiol. Plant Pathol. 1976;9(1):77–86. [http://dx. doi.org/10.1016/0048-4059(76)90077-1]. [Google Scholar]