Abstract
To gain insight into possible underlying mechanism(s) of visual hallucinations (VH) in Parkinson's disease (PD), we explored changes in local oscillatory activity in different frequency bands with source-space magnetoencephalography (MEG). Eyes-closed resting-state MEG recordings were obtained from 20 PD patients with hallucinations (Hall+) and 20 PD patients without hallucinations (Hall-), matched for age, gender and disease severity. The Hall+ group was subdivided into 10 patients with VH only (unimodal Hall+) and 10 patients with multimodal hallucinations (multimodal Hall+). Subsequently, neuronal activity at source-level was reconstructed using an atlas-based beamforming approach resulting in source-space time series for 78 cortical and 12 subcortical regions of interest in the automated anatomical labeling (AAL) atlas. Peak frequency (PF) and relative power in six frequency bands (delta, theta, alpha1, alpha2, beta and gamma) were compared between Hall+ and Hall-, unimodal Hall+ and Hall-, multimodal Hall+ and Hall-, and unimodal Hall+ and multimodal Hall+ patients. PF and relative power per frequency band did not differ between Hall+ and Hall-, and multimodal Hall+ and Hall- patients. Compared to the Hall- group, unimodal Hall+ patients showed significantly higher relative power in the theta band (p = 0.005), and significantly lower relative power in the beta (p = 0.029) and gamma (p = 0.007) band, and lower PF (p = 0.011). Compared to the unimodal Hall+, multimodal Hall+ showed significantly higher PF (p = 0.007). In conclusion, a subset of PD patients with only VH showed slowing of MEG-based resting-state brain activity with an increase in theta activity, and a concomitant decrease in beta and gamma activity, which could indicate central cholinergic dysfunction as underlying mechanism of VH in PD. This signature was absent in PD patients with multimodal hallucinations.
Keywords: MEG, Visual hallucinations, Multimodal hallucinations, Cholinergic dysfunction, Parkinson's disease
Highlights
-
•
Although VH are common, multimodal hallucinations also occur in PD.
-
•
Spectral analyses showed no differences between Hall+ and Hall- patients.
-
•
Unimodal Hall+ patients showed higher θ-, and lower β- and γ-power, and higher PF.
-
•
Compared to unimodal Hall+ group, multimodal Hall+ group showed higher PF.
1. Introduction
Visual hallucinations (VH) are the most common type of hallucinations in Parkinson's disease (PD) with an overall prevalence of 22% to 38% (Fénelon, 2008; Goetz et al., 2011; Onofrj and Gilbert, 2018), followed by auditory (AH), olfactory (OH) and tactile (TH) hallucinations, which are less common with prevalence rates of 3–22% (Fénelon, 2008), 6–16% (Fénelon, 2008; Kulick et al., 2018), and 4–7% (Goetz et al., 2011; Kulick et al., 2018), respectively. Cognitive impairment in PD is strongly associated with VH (Fenelon and Alves, 2010; Hepp et al., 2013; Lenka et al., 2017). In contrast, multimodal hallucinations in PD are not necessarily associated with a greater risk of cognitive impairment (Inzelberg et al., 1998; Katzen et al., 2010). Hallucinations in PD are associated with higher caregiver burden and form a strong and independent risk factor for nursing home placement (Aarsland et al., 2000; Fenelon and Alves, 2010).
The majority of research examining the pathophysiology of hallucinations in PD involve studies on VH. In contrast, nonvisual hallucinations in PD, reported to accompany VH as a second modality experience (Goetz et al., 2011), remain understudied (Kulick et al., 2018). As such, dysfunctional activation of frontal (top-down) and posterior (bottom-up) brain regions have been reported in PD patients with VH (Boecker et al., 2007; Ffytche et al., 2017; Lenka et al., 2015; Nagano-Saito et al., 2004; Prell, 2018; Ramírez-Ruiz et al., 2008; Sanchez-Castaneda et al., 2010; Stebbins et al., 2004). In addition, multiple neurotransmitter systems have been related to hallucinations in PD: (1) the cholinergic system, (2) the dopaminergic system, and (3) the serotonergic system (Factor et al., 2017). First, the central cholinergic system is a modulator of the interaction between feedback or top-down and feedforward or bottom-up processing (Collerton et al., 2005; Friston, 2005), such that cholinergic dysfunction may increase the uncertainty in top-down activity resulting in incorrect scene representation, and thus hallucinations (Collerton et al., 2005; Friston, 2005). Support for this hypothesis of impaired bottom-up (i.e. reduced activation and metabolism in the visual pathways) and top-down (i.e. defective attentional) processing has been found in PD patients with VH and this dysfunctional top-down and bottom-up processing has also been associated with cognitive decline in PD (Boecker et al., 2007; Hepp et al., 2017; Matsui et al., 2006; Meppelink et al., 2009; Park et al., 2013; Stebbins et al., 2004). Second, drug-induced (mostly visual) hallucinations in PD, either or not accompanied with delusions, have been associated with dopaminergic treatment. Dopamine agonists have the highest risk of inducing this type of hallucinations, which are independent of cognitive decline and reverse with adjustment of dopaminergic drug treatment (Factor et al., 2017; Zahodne and Fernandez, 2008). Third, dysfunction of the serotonin system has been related to hallucinations in PD. In addition, response to pimavanserin (a 5-HT2A inverse-agonist), a novel antipsychotic for PD with no effect on dopamine receptors, underscores the role of serotonin in psychosis in PD (Factor et al., 2017; Kianirad and Simuni, 2017). It remains unclear why some PD patients develop only VH while others also develop hallucinations in other modalities. One hypothesis is that purely VH reflect a hypocholinergic status, while non-visual hallucinations may be related to other factors such as dopaminergic medication (Goetz et al., 1998; McAuley and Gregory, 2012). This is an interesting hypothesis, as it suggests different treatment options for both subtypes of hallucinations. In this study, we wish to investigate the underlying mechanisms of hallucinations in PD using magnetoencephalography (MEG).
MEG is a non-invasive technique to measure neuronal activity directly, and study normal and pathological (oscillatory) brain activity in health and disease (Stam and van Straaten, 2012). Activation of brain regions is often accompanied with decreases or increases in signal power in a particular frequency band due to changes in local synchrony in the underlying neuronal networks (Pfurtscheller and Lopes da Silva, 1999; Stam and van Straaten, 2012). Frequency specific neuronal oscillations provide insight into underlying neuronal network interactions (Donner and Siegel, 2011). Specifically, local cortical network interactions mainly involve oscillations above 30 Hz (i.e. gamma band) and mediate feedforward processing, whereas long-range interactions among distant brain regions are mediated through oscillations below 30 Hz (i.e. theta (4–8 Hz), alpha (8–13 Hz) and beta (13–30 Hz)) and facilitate integrative brain functions and feedback attentional processing (Bastos et al., 2015; Donner and Siegel, 2011; Siegel et al., 2012; Uhlhaas and Singer, 2013; von Stein and Sarnthein, 2000). Furthermore, both alpha and beta oscillations are boosted by cholinergic enhancement (Bauer et al., 2012) and play a role in feedback processes in the context of (visual) attention tasks (Bauer et al., 2012; Gross et al., 2004; Kamiński et al., 2012; Kopell et al., 2000; Lopes da Silva, 2013). In addition, the modulatory effect of acetylcholine on oscillatory brain activity is further supported by acetylcholine antagonists that induce a so-called ‘slowing’ of oscillatory brain activity with decrease in alpha and beta activity and increase in delta and theta activity (Bauer et al., 2012; Simpraga et al., 2017, 2018). Therefore, MEG may be of great value to provide an intrinsic temporal view of the brain in relation to hallucinations.
In the present study, in order to gain insight into the pathophysiological mechanism(s) underlying VH in PD, we used MEG to study frequency-specific neural oscillations in PD patients with unimodal VH (unimodal Hall+) and compared this with PD patients with multimodal hallucinations (multimodal Hall+) and PD patients without hallucinations (Hall-). Given the predominant occurrence of VH in PD, in this study, patients were recruited according to the criterium of presence or absence of hallucinations. After inclusion, patients with hallucinations were divided into a subgroup with only VH and a subgroup with multimodal hallucinations (see methods for details).
Since VH in PD are supposed to be related to cholinergic deficits, and the cholinergic system has been associated with top-down processing and enhancement of alpha and beta frequencies (involved in top-down processing), we expected to find ‘slowing’ (i.e. decrease in alpha and beta frequencies, and increase in delta and theta frequencies) of oscillatory brain activity in PD patients who experienced only VH.
2. Methods
2.1. Study population
Twenty PD patients with hallucinations (Hall+) and 20 without hallucinations (Hall-) were recruited from the Understanding Hallucinations (UH) study, and included in the Understanding Hallucinations – MEG (UH-MEG) study at the department of Clinical Neurophysiology of the VU University Medical Center (VUmc) in Amsterdam, The Netherlands. UH-MEG is a follow-up study of the UH study, which is an ongoing multicenter cross-sectional study that investigates phenomenology and underlying brain mechanisms of hallucinations across different neurological, psychiatric and perceptual disorders (clinicaltrials.gov identifier NCT02460965). Inclusion criteria of UH were age ≥ 18 years, mentally competent and PD diagnosis determined by the treating neurologist, fluent in Dutch language, and hallucinations experienced in at least the past month (i.e. Hall+) or no hallucination experiences in life (i.e. Hall-). Presence of hallucinations was assessed with the Questionnaire for Psychotic Experiences (QPE) (Sommer et al., 2018) and confirmed with the scale for outcomes in Parkinson's disease – psychiatric complications (SCOPA-PC) (Visser et al., 2007). As part of the UH-MEG, all patients underwent a 5-min eyes-closed MEG recording, followed by assessment of hallucinations with the QPE, loneliness by De Jong Gierveld Loneliness (DJGL) scale (de Jong-Gierveld and Kamphuls, 1985), depression by the Beck Depression Inventory-II (BDI-II) (Beck et al., 1996), and cognitive testing, which included the Mini-Mental State Examination (MMSE) as a measure of global cognitive functioning (Folstein et al., 1975), the Trail Making Test part A (TMT-A) as a measure of motor and visual processing speed (Reitan, 1958), and the TMT part B (TMT—B) (Crowe, 1998; Oosterman et al., 2010; Reitan, 1992) and forward condition of the Digit Span (Lindeboom and Matto, 1994) as measures of attention. A contrast score between TMT-B and TMT-A (TMTB-A: TMT-B minus TMT-A) was calculated as a measure of attentional set-shifting (i.e. cognitive flexibility to shift attention between things or tasks), which is an important cognitive problem in PD (Williams-Gray et al., 2008). Hall+ and Hall- patients were matched at the group level for age, gender, educational level, disease duration (i.e. years diagnosed with PD at enrollment), and disease severity assessed using the (modified) Hoehn and Yahr-scale (H&Y: range 0–5 with higher scores indicating more advanced disease severity) (Goetz et al., 2004).
All participants provided written informed consent. UH and UH-MEG were approved by the affiliated Institutional Review Board and conducted in accordance with the Declaration of Helsinki.
After assessing the phenomenology of hallucinations with the QPE, we observed a dichotomy in the presence of type of hallucinations within the Hall+ group. Ten patients within the Hall+ group experienced only VH, whereas ten patients experienced hallucinations in more than one modality. Therefore, we divided the Hall+ group into subgroups (i.e. patients with only VH (unimodal Hall+, N = 10) and patients with multimodal hallucinations (multimodal Hall+, N = 10)), to explore the specificity of changes in frequency-specific neural oscillations to the pathophysiology of VH. See the results section for a detailed description of the phenomenology of the Hall+ patients.
2.2. MEG acquisition and preprocessing
MEG data were recorded using a 306-channel (102 magnetometers, 204 gradiometers) whole-head MEG system (Elekta Neuromeg, Oy, Helsinki, Finland) with a sample frequency of 1250 Hz, online anti-aliasing filter of 410 Hz, and a high-pass filter of 0.1 Hz. Five minutes of (range 262–400 s) eyes-closed resting-state data were recorded with patients in supine position in the MEG-scanner inside a magnetically shielded room (Vacuumschmelze GmbH, Hanau, Germany).
The head position relative to the MEG sensors was recorded continuously using the signals from five head-localization coils. The positions of the head localization coils as well as the outline of the patient's scalp (∼500 points) were digitized using a 3D digitizer (Fastrak; Polhemus, Colchester, VT, U.S.A.).
MEG channels with excessive artifacts were identified by visual inspection of the data by the first author (MD) and discarded. A maximum of 12 channels were excluded. Subsequently, an offline spatial filter, the temporal extension of Signal Space Separation (tSSS) (Taulu and Hari, 2009; Taulu and Simola, 2006) as implemented in MaxFilter software (Elekta Neuromeg Oy; version 2.2.15) with a sliding window of 10 s and a subspace correlation limit of 0.9 was used to remove artifacts (Hillebrand et al., 2013). The scalp surfaces of all patients were co-registered to T1-weighted templates with 1 mm resolution, grossly matched for head-size, using a surface matching procedure (see Supplemental Material for details). Visual inspection of the co-registration between digitized scalp surface and the co-registered template MRI was performed for all patients by MD. The sphere that best fitted the scalp surface as extracted from the co-registered template MRI was used as a volume conductor model for source reconstruction using the beamformer approach described below.
2.3. Source reconstruction using beamforming
Neuronal activity at source-level was reconstructed using an atlas-based beamforming approach (Hillebrand et al., 2012). The automated anatomical labeling (AAL) atlas was used to label the voxels in a patient's co-registered surrogate MRI in 78 cortical and 12 subcortical regions of interest (ROIs) (Tzourio-Mazoyer et al., 2002). Given the different number of voxels in each ROI, the centroid voxel (i.e. the voxel within the ROI that is nearest, in terms of Euclidean distance, to all other points in the ROI) was selected as representative for that ROI (Hillebrand et al., 2016). The neuronal activity for each centroid voxel, a so-called virtual electrode (VE), was reconstructed as the weighted sum of each MEG sensor's time-series. The (normalized) beamformer weights (Cheyne et al., 2007) were based on the forward solution (i.e. lead field for an equivalent current dipole in the spherical head model), and the broad-band (0.5–48 Hz) data covariance. On average, 286 s (range 262–394 s) of data was used to construct the covariance matrix. See (Hillebrand and Barnes, 2005; Hillebrand et al., 2005, 2012, 2016) for a detailed description of the beamforming approach.
The beamforming approach resulted in broad band (0.5–48 Hz) time-series for each centroid of the 90 ROIs. From these time-series 35 epochs of 4096 samples (3.2765 s) were visually selected by MD and independently evaluated on quality by one of the senior authors (CS). Epochs without consensus were replaced by new epochs. The selected epochs were converted to American Standard Code for Information Interchange (ASCII) format, and loaded into BrainWave software for further analysis (BrainWave version 0.9.152.12.5, C. J. Stam; available at http:/home.kpn.nl/stam7883/brainwave.html).
2.4. Spectral analysis
Peak frequency (i.e. frequency with the highest power in the 4–13 Hz range, PF), and relative power in the frequency bands delta (0.5–4 Hz), theta (4–8 Hz), alpha1 (8–10 Hz), alpha2 (10–13 Hz), beta (13–30 Hz), and gamma (30–48 Hz) were calculated as one average value per frequency band, and for each AAL region per epoch per patient by using the Fast Fourier Transformation. All components of the Fourier transform outside the pass band were set to zero, after which an inverse Fourier transform was performed to obtain the filtered time-series.
The PF and relative power values were averaged over the 35 artefact-free epochs per patient to obtain one value per patient per frequency band and per AAL region.
2.5. Statistical analysis
Statistical analyses were performed using IBM SPSS statistics 24.0. Patient characteristics and spectral measures were compared between the groups. Continuous data were tested for normality using the Shapiro-Wilk test. Normally distributed variables were compared using independent samples t-test. Data that did not follow a normal distribution were compared using nonparametric Mann-Whitney U test. Categorical data were compared using the chi-square test.
To explore the spatial distribution of relative power per frequency band and PF, we compared relative power and PF of different brain regions between the subgroups using repeated measures ANOVA with Greenhouse-Geiser correction for sphericity, with brain regions and frequency band as the within subject factor and group as the between subject factor, and FDR-correction for multiple comparisons. For this analysis the following brain regions per hemisphere were tested: frontal, central, parietal, occipital, temporal, limbic and subcortical. For frequency bands, only the bands/PF with significant difference between the groups in the main analysis, were included.
The False Discovery Rate (FDR) approach (Benjamini and Hochberg, 1995) with adjusted p value (i.e., q-value) of 0.05 was used to correct for multiple comparisons: (1) for the main analysis where one average value per frequency band/PF was calculated, correction was performed for the number of frequency bands and PF, (2) for the frequency bands/PF that revealed significant differences between the groups in the main analysis, power/PF was further explored regionally between the groups, and correction was performed for the number of brain regions. A p-value of <0.05 was considered significant.
Finally, Spearman correlation coefficients were calculated between each neuropsychological test and each relative power/PF per brain region that showed significant differences between any two groups.
3. Results
3.1. Hall+ vs. Hall- patients
3.1.1. Patient characteristics
Hall+ and Hall- patients did not differ at the group level for age, gender, educational level, disease duration, disease severity and medication use (Table 1), which indicates that matching was accurate. All Hall+ patients (n = 20, 100%) experienced VH (Table 1). Ten patients (n = 10/20, 50%) also experienced auditory hallucinations (AH). From this group (i.e. 10 patients with VH and AH), six patients experienced olfactory (OH) and tactile hallucinations (TH) (n = 6/20, 30%). All patients experienced recurrent complex VH containing people, animals and inanimate objects with and without movement. Twelve (60%) patients retained full insight, while six (30%) patients had partial insight into their hallucinations and doubted the real nature of the hallucinations. Two (10%) patients were fully convinced that their hallucinations were real (i.e. insight was absent). 80% (n = 16) of the patients had at least once interacted with their hallucinations. >50% of the patients also experienced minor hallucinations including visual illusions (i.e. seeing things differently than they actually are, e.g. seeing a face in a branch of a tree), passage hallucinations (i.e. seeing a person, animal or object passing in the peripheral visual field), and sensed presence hallucinations (i.e. sense that someone or something is present or nearby without being actually visible). One (5%) patient experienced delusions in the week preceding participation in the study (Table 1).
Table 1.
Patient characteristics.
| Hall+ (N = 20) | Hall- (N = 20) | |
|---|---|---|
| Age, yrs | 72.15 (6.22) | 70.50 (6.45) |
| Gender, female | 7 (35.0%) | 6 (30.0%) |
| Education level | 4 (3–7) | 7 (6–7) |
| Handedness, right | 18 (90.0%) | 15 (75.0%) |
| Disease duration, yrs | 7.71 (4.35–12.73) | 4.46 (2.75–9.38) |
| Hoehn & Yahr staging scale | 3.0 (3.0–4.0) | 3.0 (3.0–3.0) |
| LED, mg/day | 882.00 (628.75–1188.00) | 666.00 (547.25–1218.75) n = 18 |
| Type of hallucinations | ||
| VH | 20 (100.0%) | |
| AH | 10 (50.0%) | |
| OH | 6 (30.0%) | |
| TH | 6 (30.0%) | |
| Delusions | 1 (5.0%) | |
| BDI-II⁎⁎ | 15.00 (10.00–19.75) | 10.00 (5.00–14.75) |
| DJGL⁎ | 5.00 (1.00–6.00) | 1.00 (0.00–4.00) |
| Cognition | ||
| MMSE⁎⁎ | 26.0 (21.75–27.75) | 28.5 (27.0–29.0) |
| Digit Span forward | 8.20 (1.51) | 8.85 (1.66) |
| TMT-A | 96.47 (59.89) n = 19 | 65.73 (58.25) |
| TMT-B | 183.87 (113.35) n = 16 | 121.77 (67.52) n = 18 |
| TMTB-A | 101.69 (112.96) n = 16 | 74.13 (58.16) n = 18 |
Data are mean (SD), median (interquartile range), or n(%). Education level was assessed with the 7-item Verhage coding system for education (Verhage, 1964). Disease duration was calculated as the years diagnosed with PD at enrollment in the study. The Hoehn and Yahr staging scale was used to measure disease severity based on clinical features and functional disability. It ranges from 0 to 5 with higher scores indicating more advanced disease severity (Goetz et al., 2004). The total dose of dopaminergic medication (i.e. including dopaminomimetics and levodopa) was converted to a so-called levodopa equivalent dose in milligrams per day based on (Tomlinson et al., 2010). Depression was measured with the BDI-II. Loneliness was measured using the DJGL.
AH: Auditory Hallucinations; BDI-II: Beck Depression Inventory-II; DJGL: De Jong Gierveld Loneliness scale; Hall+: PD patients with hallucinations; Hall-: PD patients without hallucinations; LED: Levodopa Equivalent Dose; MMSE: Mini Mental State Examination; OH: Olfactory Hallucinations; PD: Parkinson's disease; TH: Tactile Hallucinations; TMT-A: Trail-Making Test part A; TMT—B: Trail-Making Test part B; TMTB-A: a contrast score between TMT-B and TMT-A calculated as a measure of attentional set-shifting; VH: Visual Hallucinations.
p < 0.05.
p ≤ 0.01.
Hall+ patients scored significantly lower on the MMSE and significantly higher on the BDI-II, and DJGL than the Hall- group (Table 1).
3.1.2. Spectral analysis
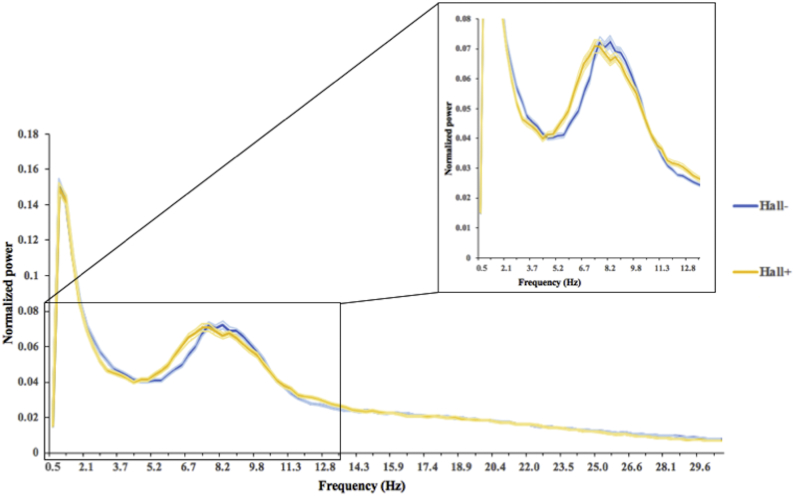
Fig. 1 shows the mean power spectrum for both patient groups. The Hall+ group showed slowing of resting state brain activity compared to Hall- group, but the groups did not differ in relative power or PF (Table 2).
Fig. 1.
Average power spectra over 90 AAL regions for Parkinson's disease patients with (Hall+: yellow) and without (Hall-: blue) hallucinations. Peak frequency (i.e. frequency with the most power in the 4–13 Hz range) is lower in Hall+ compared to Hall- patients. Filled area represents the standard error of the mean.
Table 2.
Relative power per frequency band in PD patients with and without hallucinations.
| Hall+ (n = 20) | Hall- (n = 20) | p-Value | |
|---|---|---|---|
| Delta | 0.262 (0.074) | 0.256 (0.038) | 0.758 |
| Theta | 0.207 (0.080) | 0.183 (0.054) | 0.285 |
| Alpha1 | 0.102 (0.026) | 0.104 (0.034) | 0.833 |
| Alpha2 | 0.101 (0.031) | 0.098 (0.018) | 0.758 |
| Beta | 0.257 (0.090) | 0.276 (0.064) | 0.453 |
| Gamma | 0.071 (0.019) | 0.082 (0.020) | 0.089 |
| Peak frequency | 7.97 (1.15) | 8.11 (0.70) | 0.643 |
Power is the relative power per frequency band (delta [0.5–4 Hz], theta [4–8 Hz], alpha1 [8–10 Hz], alpha2 [10–13 Hz], beta [13–30 Hz], and gamma [30–48 Hz]). Peak frequency is the frequency with highest power in range between 4 and 13 Hz. Hall+: Parkinson's disease patients with hallucinations; Hall-: Parkinson's disease patients without hallucinations.
3.2. Subgroup analyses
3.2.1. Patient characteristics
As described above, given the dichotomy in the presence of type of hallucinations within the Hall+ group, namely n = 10 patients with only VH and n = 10 patients with multimodal hallucinations, we performed exploratory subgroup analyses and compared PD patients with only VH (unimodal Hall+) with PD patients with multimodal hallucinations (multimodal Hall+), and both these subgroups separately with Hall- patients, to explore the specificity of spectral changes to the pathophysiology of VH.
Unimodal Hall+, multimodal Hall+ and Hall- patients did not differ at the group level for age, gender, educational level, disease duration, disease severity and medication use (Table 3). Unimodal Hall+ patients performed significantly worse on MMSE, TMT-A, TMT—B, and experienced more depressive symptoms and loneliness than Hall- patients. Multimodal Hall+ patients experienced more depressive symptoms compared to Hall- patients (Table 3). Unimodal Hall+ and multimodal Hall+ patients did not differ on cognition, DJGL or BDI-II (Table 3).
Table 3.
Patient characteristics in Parkinson's disease patients with unimodal, multimodal, and without hallucinations.
| Unimodal Hall+ (N = 10) | Multimodal Hall+ (N = 10) | Hall- (N = 20) | |
|---|---|---|---|
| Age, yrs | 74.20 (5.85) | 70.10 (6.17) | 70.50 (6.45) |
| Gender, female | 2 (20.0%) | 5 (50.0%) | 6 (30.0%) |
| Education level | 6.50 (3.75–7.0) | 4.00 (2.75–5.50) | 7.00 (6.0–7.0) |
| Handedness, right | 9 (90.0%) | 9 (90.0%) | 15 (75.0%) |
| Disease duration, yrs | 8.13 (4.81–19.79) | 6.46 (4.02–11.19) | 4.46 (2.75–9.38) |
| Hoehn & Yahr staging scale | 3.5 (3.0–4.0) | 3.0 (2.38–4.0) | 3.0 (3.0–3.0) |
| LED, mg/day | 922.0 (575.25–1459.75) | 860.0 (587.50–1056.75) | 666.0 (547.25–1218.75) n = 18 |
| BDI-II⁎, § | 16.0 (9.75–21.25) | 14.50 (9.75–18.50) | 10.0 (5.0–14.75) |
| DJGL⁎ | 5.0 (2.50–6.0) | 3.50 (1.0–6.0) | 1.00 (0.0–4.0) |
| Type of hallucinations | |||
| VH | 10 (100.0%) | 10 (100.0%) | |
| AH | 0 | 10 (100.0%) | |
| OH | 0 | 6 (60.0%) | |
| TH | 0 | 6 (60.0%) | |
| Distress from hallucinations | 2 (20.0%) | 5 (50.0%) | |
| Emotional valence of hallucinations | 1 (10.0%) | 4 (40.0%) | |
| Delusions | 0 | 1 (10.0%) | |
| Cognition | |||
| MMSE⁎⁎ | 24.5 (16.25–27.0) | 27.0 (24.75–28.25) | 28.5 (27.0–29.0) |
| Digit Span forward | 7.90 (1.97) | 8.50 (0.85) | 8.85 (1.66) |
| TMT-A⁎ | 118.89 (66.10) n = 9 | 76.30 (48.33) | 65.73 (58.25) |
| TMT-B⁎⁎ | 242.43 (121.84) n = 7 | 155.63 (75.08) | 121.77 (67.52) n = 18 |
| TMTB-A | 136.29 (138.46) n = 7 | 97.88 (57.42) | 74.13 (58.16) n = 18 |
Data are mean (SD), median (interquartile range), or n(%).Education level was assessed with the 7-item Verhage coding system for education (Verhage, 1964). Disease duration was calculated as the years diagnosed with PD at enrollment in the study. The Hoehn and Yahr staging scale was used to measure disease severity based on clinical features and functional disability. It ranges from 0 to 5 with higher scores indicating more advanced disease severity (Goetz et al., 2004). The total dose of dopaminergic medication (i.e. including dopaminomimetics and levodopa) was converted to a so-called levodopa equivalent dose in milligrams per day based on (Tomlinson et al., 2010). Depression was measured with the BDI-II. Loneliness was measured using the DJGL.
BDI-II: Beck Depression Inventory-II; DJGL: De Jong Gierveld Loneliness scale; Hall-: PD patients without hallucinations; LED: Levodopa Equivalent Dose; MMSE: Mini Mental State Examination; Multimodal Hall+: PD patients with multimodal hallucinations; PD: Parkinson's disease; TMT-A: Trail-Making Test part A; TMT—B: Trail-Making Test part B; TMTB-A: a contrast score between TMT-B and TMT-A calculated as a measure of attentional set-shifting; Unimodal Hall+: PD patients with only visual hallucinations;
p < 0.05; significantly different between unimodal Hall+ and Hall- patients.
p < 0.01; significantly different between unimodal Hall+ and Hall- patients.
p < 0.05; significantly different between multimodal Hall+ and Hall- patients.
Although not significantly different, the emotional valence of hallucinations was more severe (i.e. content was more often negative) in multimodal Hall+ patients and they also experienced more often distress from their hallucinations than unimodal Hall+ patients (Table 3).
3.2.2. Spectral analysis
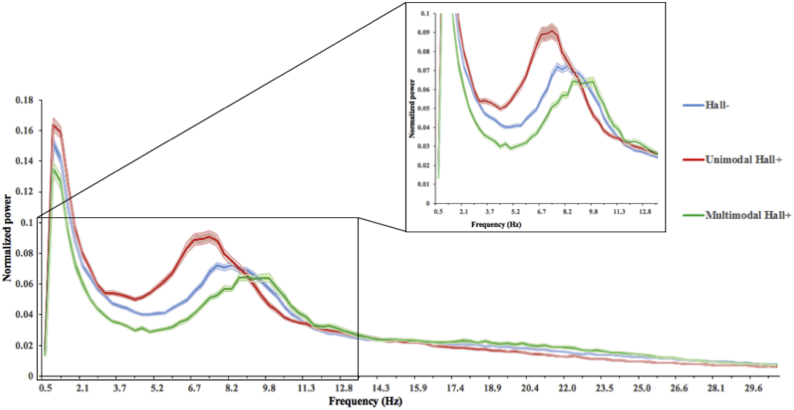
Fig. 2 shows the mean power spectrum for the unimodal Hall+ and multimodal Hall+ patients in relation to the Hall- group. For both relative power and PF, the unimodal Hall+ and multimodal Hall+ group deviated in opposite direction compared to the Hall- group (Table 4). Compared to the Hall- group, unimodal Hall+ patients showed significantly higher relative power in the theta band (p = 0.005), and significantly lower relative power in the beta (p = 0.029) and gamma (p = 0.007) band, and lower PF (p = 0.011). The relative power per AAL region for the theta, beta and gamma frequency band, as well as PF per AAL region, are shown in Tables S1–S4. After correcting for multiple comparisons, theta, beta, and gamma band relative power, as well as PF (Tables S1–S4), were significantly different for several AAL regions. Fig. 3 displays the mean relative power for the theta, beta and gamma frequency band, as well as the mean PF, for each cortical ROI for both the unimodal Hall+ and Hall- patients.
Fig. 2.
Average power spectra over 90 AAL regions for Parkinson's disease patients with only VH (unimodal Hall+: red), with multimodal (multimodal Hall+: green) and without (Hall-: blue) hallucinations. Peak frequency (i.e. frequency with the most power in the 4–13 Hz range) is lowest in unimodal Hall+ patients. Filled area represents the standard error of the mean.
VH: Visual Hallucinations.
Table 4.
Relative power per frequency band in Parkinson's disease patients with unimodal, multimodal, and without hallucinations.
| Unimodal Hall+ (n = 10) | Multimodal Hall+ (n = 10) | Hall- (n = 20) | p-Value, Unimodal Hall + vs. Hall- | p-Value, Multimodal Hall + vs. Hall- | p-Value, Unimodal Hall + vs. Multimodal Hall+ | |
|---|---|---|---|---|---|---|
| Delta | 0.286 (0.093) | 0.238 (0.060) | 0.256 (0.038) | 0.296 | 0.169 | 0.315 |
| Theta | 0.247 (0.056) | 0.166 (0.082) | 0.183 (0.054) | 0.005⁎ | 0.267 | 0.019 |
| Alpha1 | 0.095 (0.027) | 0.109 (0.025) | 0.104 (0.034) | 0.484 | 1.000 | 0.280 |
| Alpha2 | 0.090 (0.021) | 0.111 (0.036) | 0.098 (0.018) | 0.303 | 0.328 | 0.218 |
| Beta | 0.219 (0.062) | 0.295 (0.101) | 0.276 (0.064) | 0.029a | 0.619 | 0.063 |
| Gamma | 0.061 (0.014) | 0.081 (0.019) | 0.082 (0.020) | 0.007a | 0.948 | 0.023 |
| Peak frequency | 7.31 (0.88) | 8.63 (1.01) | 8.11 (0.70) | 0.011a | 0.091 | 0.007a |
Power is the relative power per frequency band (delta [0.5–4 Hz], theta [4–8 Hz], alpha1 [8–10 Hz], alpha2 [10–13 Hz], beta [13–30 Hz], and gamma [30–48 Hz]), averaged over all 90 AAL regions. Peak frequency is the frequency with highest power in range between 4 and 13 Hz, averaged over all 90 AAL regions.
Hall-: Parkinson's disease patients without hallucinations; Multimodal Hall+: Parkinson's disease patients with multimodal hallucinations; Unimodal Hall+: Parkinson's disease patients with only visual hallucinations.
Bold indicates p<.05 or p<.01
Significantly different between the groups after FDR-correction.
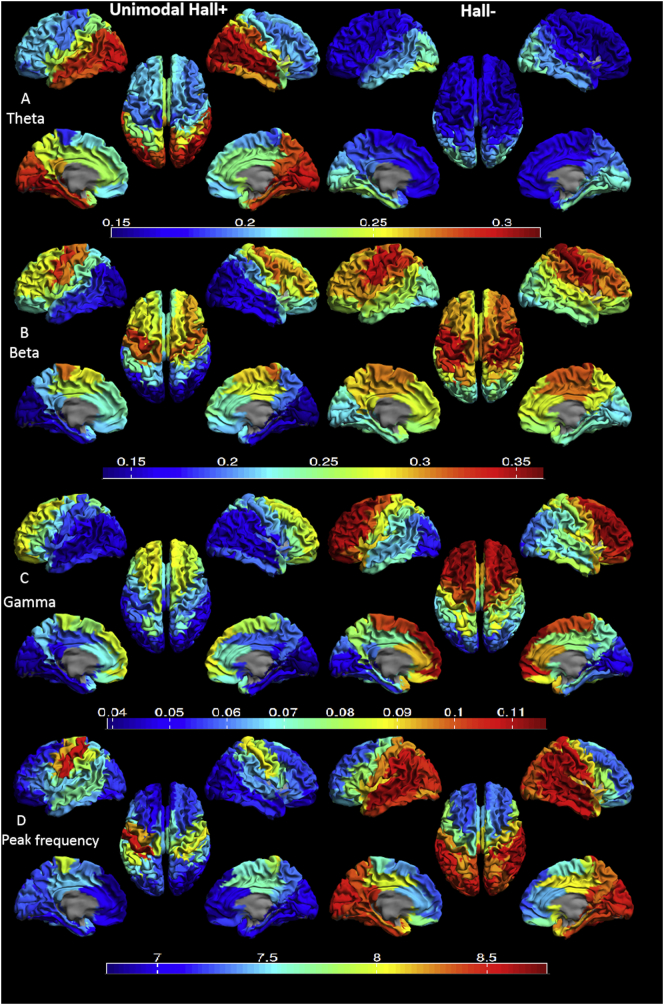
Fig. 3.
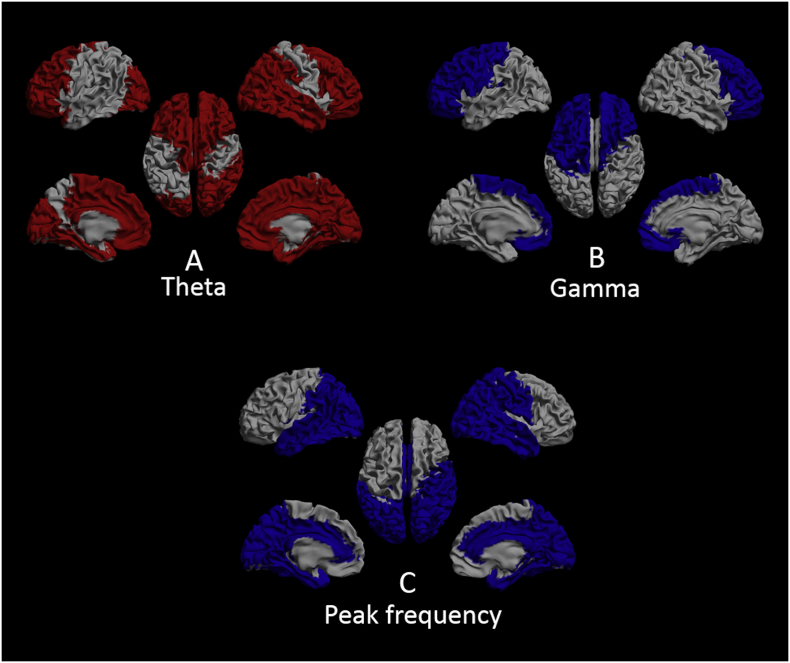
Mean relative power for each region of interest (ROI) in unimodal Hall+ (left) and Hall- (right) patients displayed as a color-coded map on a parcellated template brain viewed from, in clockwise order, the left, top, right, right-midline and left-midline. Panel A: relative power in the theta band. Panel B: relative power in the beta band. Panel C: relative power in the gamma band. Panel D: Peak frequency. Hot and cold colors indicate higher and lower relative power/peak frequency, respectively. See table S1-S4 for the subcortical regions per frequency band that showed significant difference between the groups and for all the relative power and peak frequency values in the two groups.
Hall-: Parkinson's disease patients without hallucinations; unimodal Hall+: Parkinson's disease patients with only visual hallucinations.
In the theta band, unimodal Hall+ patients showed higher relative power in 74 (82.2%) out of 90 AAL regions compared to the Hall- group. These regions were spread across the entire brain and included all the regions in the limbic lobes and all the subcortical regions in both hemispheres (Table S1). In the beta band, relative power was lower in 14 (15.6%) out of 90 AAL regions in unimodal Hall+ patients. These regions were mainly located in the parietal and occipital lobes (Table S2). In the gamma band, unimodal Hall+ patients showed lower relative power in 47 (52.2%) out of 90 AAL regions. These regions included mainly the frontal and limbic lobes, and subcortical regions, but not the temporal, parietal and occipital lobes (Table S3). PF was lower in 51 (56.7%) out of 90 AAL regions in unimodal Hall+ patients compared to Hall- patients. These regions comprised almost the entire brain except (mainly) the frontal lobes (Table S4).
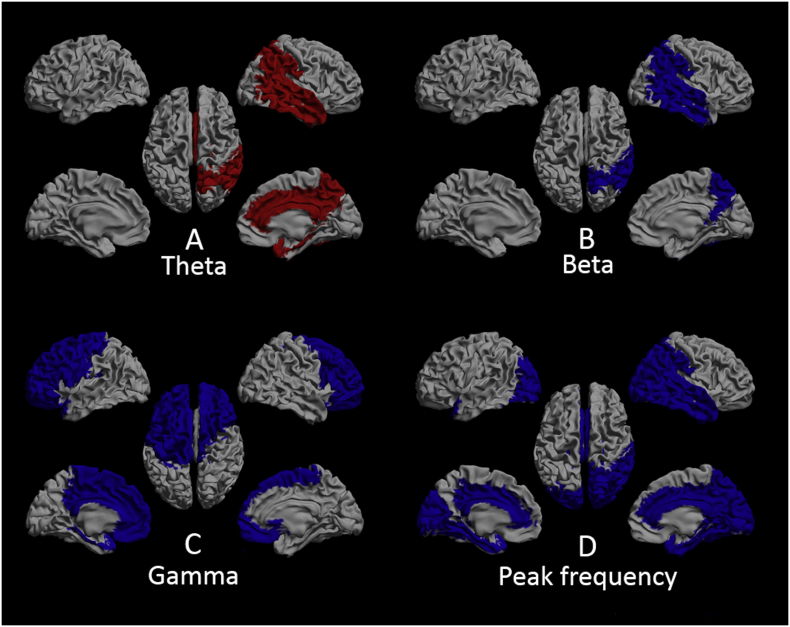
To explore whether a potential spatial pattern could be found for the AAL regions that revealed significant difference that might be related to VH, we performed regional analysis and found significant interaction effects for brain regions per frequency band between the groups F(8.50, 157.16) = 4.70, p < 0.001. In the theta band, brain regions that showed significant difference between unimodal Hall+ and Hall- patients were all located across the right hemisphere, and comprised the parietal, temporal, limbic and subcortical brain regions (Tables S5 and S6, Fig. 4A). In the beta band, the parietal and temporal brain region in the right hemisphere showed significant difference between the unimodal Hall+ and Hall- group (Tables S5 and S6, Fig. 4B). In the gamma band, relative power in the bilateral frontal, left central and limbic brain region differed significantly between unimodal Hall+ and Hall- patients (Tables S5 and S6, Fig. 4C). Brain regions that revealed significant difference between unimodal Hall+ and Hall- patients in terms of PF included left occipital and limbic brain region, whereas in the right hemisphere, all but the fronto-central brain regions showed significant difference between the groups (Tables S5 and S6, Fig. 4D).
Fig. 4.
Distribution of the brain regions that showed significant difference between unimodal Hall+ and Hall- patients, displayed as in Fig. 3, for the theta (panel A), beta (panel B), and gamma (panel C) band, and for peak frequency (panel D). Red: higher relative power in unimodal Hall+ patients. Blue: lower relative power/peak frequency in unimodal Hall+ patients. Gray: brain regions that did not differ between the groups. Note: subcortical regions are not shown in this figure. See table S6 for the subcortical regions per frequency band that showed significant difference between the two groups and table S5 for the mean relative power/peak frequency values in the two groups.
Hall-: Parkinson's disease patients without hallucinations; unimodal Hall+: Parkinson's disease patients with only visual hallucinations.
The multimodal Hall+ and Hall- groups did not differ in relative power or PF (Table 4). The unimodal Hall+ and multimodal Hall+ groups differed significantly in relative power in the theta (p = 0.19) and gamma (p = 0.023) frequency band and in PF (p = 0.007). After FDR-correction, only PF between the groups remained significantly different with multimodal Hall+ patients showing higher PF than unimodal Hall+ patients (Table 4). The relative power per AAL region for the theta and gamma band and PF are shown in Tables S7–S9.
Further regional exploration showed that in the theta band, all but the bilateral central, left parietal and temporal brain region were significantly different between the unimodal Hall+ and multimodal Hall+ group (Tables S5 and S6, Fig. 5A). In the gamma band, relative power in the bilateral frontal and left central brain region differed significantly between unimodal Hall+ and multimodal Hall+ patients (Tables S5 and S6, Fig. 5B). With regard to PF, unimodal Hall+ and multimodal Hall+ differed significantly in all but the bilateral frontal and left central brain region (Tables S5 and S6, Fig. 5C).
Fig. 5.
Distribution of the brain regions that showed significant difference between unimodal Hall+ and multimodal Hall+ patients, displayed as in Fig. 3, for the theta (panel A), and gamma (panel B) band, and for peak frequency (panel C). Red: higher relative power in unimodal Hall+ patients. Blue: lower relative power/peak frequency in unimodal Hall+ patients. Gray: brain regions that did not differ between the groups. Note: subcortical regions are not shown in this figure. See table S6 for the subcortical regions per frequency band that showed significant difference between the two groups and table S5 for the mean relative power/peak frequency values in the two groups.
Multimodal Hall+: Parkinson's disease patients with multimodal hallucinations; unimodal Hall+: Parkinson's disease patients with only visual hallucinations.
Although the subgroups did not differ in use of medication (Table 3), we redid the main analyses in the subgroups (Table 4) with medication (LED) as a covariate in order to exclude a potential effect of medication on our results, and found that the corrected model still showed the same effects (see Table S10).
3.2.3. Correlation with neuropsychological tests
3.2.3.1. MMSE
In the Hall- group, a negative correlation was found between relative power in the right parietal and limbic brain region in the theta band and MMSE (Table 5). In the beta band, MMSE was positively correlated with relative power in the right parietal brain region (Table 5). MMSE was positively correlated with PF in all but the right occipital brain region (Table 5). No significant correlations were found for the gamma band or the unimodal Hall+ group.
Table 5.
Correlation between relative power/peak frequency per significantly differing brain region and neuropsychological tests in Parkinson's disease patients without hallucinations.
| Brain region | Neuropsychological test | N | Spearman rho (ρ) | p-Value |
|---|---|---|---|---|
| Theta band | ||||
| Right parietal | MMSE | 20 | −0.45 | 0.048 |
| TMT-A | 20 | 0.51 | 0.023 | |
| TMT-B | 18 | 0.53 | 0.025 | |
| Right temporal | MMSE | 20 | −0.44 | 0.054 |
| TMT-A | 20 | 0.51 | 0.020 | |
| TMT-B | 18 | 0.51 | 0.032 | |
| Right limbic | MMSE | 20 | −0.45 | 0.049 |
| TMT-A | 20 | 0.53 | 0.016 | |
| TMT-B | 18 | 0.47 | 0.049 | |
| Right subcortical | MMSE | 20 | −0.43 | 0.062 |
| TMT-A | 20 | 0.55 | 0.011 | |
| TMT-B | 18 | 0.51 | 0.029 | |
| Beta band | ||||
| Right parietal | MMSE | 20 | 0.51 | 0.021 |
| TMT-A | 20 | −0.61 | 0.004 | |
| TMT-B | 18 | −0.60 | 0.008 | |
| Right temporal | MMSE | 20 | 0.44 | 0.054 |
| TMT-A | 20 | −0.49 | 0.029 | |
| TMT-B | 18 | −0.62 | 0.007 | |
| Gamma band | ||||
| Left frontal | MMSE | 20 | −0.01 | 0.966 |
| TMT-A | 20 | 0.10 | 0.669 | |
| TMT-B | 18 | 0.18 | 0.464 | |
| Right frontal | MMSE | 20 | −0.05 | 0.824 |
| TMT-A | 20 | 0.19 | 0.433 | |
| TMT-B | 18 | 0.20 | 0.432 | |
| Left central | MMSE | 20 | −0.18 | 0.436 |
| TMT-A | 20 | 0.33 | 0.160 | |
| TMT-B | 18 | 0.14 | 0.569 | |
| Left limbic | MMSE | 20 | −0.05 | 0.844 |
| TMT-A | 20 | 0.13 | 0.599 | |
| TMT-B | 18 | 0.12 | 0.641 | |
| Peak frequency | ||||
| Right parietal | MMSE | 20 | 0.55 | 0.012 |
| TMT-A | 20 | −0.55 | 0.012 | |
| TMT-B | 18 | −0.44 | 0.069 | |
| Left occipital | MMSE | 20 | 0.51 | 0.023 |
| TMT-A | 20 | −0.56 | 0.010 | |
| TMT-B | 18 | −0.59 | 0.011 | |
| Right occipital | MMSE | 20 | 0.41 | 0.076 |
| TMT-A | 20 | −0.47 | 0.035 | |
| TMT-B | 18 | −0.50 | 0.034 | |
| Right temporal | MMSE | 20 | 0.46 | 0.042 |
| TMT-A | 20 | −0.56 | 0.010 | |
| TMT-B | 18 | −0.46 | 0.056 | |
| Left limbic | MMSE | 20 | 0.57 | 0.008 |
| TMT-A | 20 | −0.66 | 0.001 | |
| TMT-B | 18 | −0.48 | 0.043 | |
| Right limbic | MMSE | 20 | 0.64 | 0.002 |
| TMT-A | 20 | −0.65 | 0.002 | |
| TMT-B | 18 | −0.44 | 0.068 | |
| Right subcortical | MMSE | 20 | 0.57 | 0.009 |
| TMT-A | 20 | −0.61 | 0.005 | |
| TMT-B | 18 | −0.33 | 0.184 | |
MMSE: Mini-Mental State Examination; TMT-A: Trail-Making Test part A; TMT—B: Trail-Making Test part B.
Bold indicates p<.05 or p<.01
3.2.3.2. TMT-A and TMT-B
In the Hall- group, Spearman correlation showed a positive correlation between both the TMT-A and TMT-B and relative power in all brain regions in the theta band, and negative correlations with relative power in brain regions in the beta band (Table 5). For PF, a negative correlation was found between relative power in all brain regions and TMT-A, whereas for TMT—B, a significant negative correlation was found with relative power in the bilateral occipital and left limbic brain region (Table 5). No significant correlations were found for the gamma band or the unimodal Hall+ group.
4. Discussion
4.1. Main findings
This study is the first to explore neurophysiological markers of hallucinations in PD by using source-space MEG. Although we hypothesized to find slowing of resting-state oscillatory brain activity in PD patients who experienced visual hallucinations, the primary analysis revealed no significant differences in relative power or PF between PD patients with and without hallucinations. However, remarkable results were found when exploratory subgroup analyses were performed after dissecting the hallucinating group into purely visual hallucinations (unimodal Hall+) and hallucinations also in other modalities (multimodal Hall+). Compared to patients without hallucinations, patients with only VH showed slowing of resting-state oscillatory brain activity, with spatial distributions characterized by an increase in theta power in all but the fronto-central and occipital brain region in the right hemisphere, and concomitant decrease in beta power in the right temporoparietal brain region, and decrease in gamma power in the bilateral frontal and left limbic brain region, and lowering of PF in almost all but the frontal brain regions. These deviations were absent in the patient group with multimodal hallucinations compared to patients without hallucinations. Compared to patients with only VH, patients with multimodal hallucinations showed a significant and diffuse increase in PF in all but the frontal brain regions.
Analysis of relative power/PF in relation to performance on neuropsychological tests showed, only in patients without hallucinations, a correlation between higher theta power and worse performance on the MMSE, better performance on MMSE and higher beta power in the right parietal region and higher PF in all but the right occipital brain regions. Lower theta and higher beta power were associated with a better performance on both TMT-A and TMT—B, whereas a diffuse higher PF was associated with a better performance on TMT-A, and higher PF in bilateral occipital and left limbic brain region was associated with better performance on the TMT-B test.
4.2. Underlying mechanism(s) of unimodal visual and multimodal hallucinations in PD
4.2.1. Unimodal visual hallucinations
The cholinergic system is seen as a modulator of the cortical signal-to-noise ratio (Collerton et al., 2005). Slowing in resting-state brain activity (increased power in delta and theta frequencies and decreased power in alpha and beta frequencies) has been associated with impaired cholinergic function (Bauer et al., 2012; Simpraga et al., 2018). As mentioned earlier, the central cholinergic system has been involved in the integration of top-down attentional and bottom-up sensory processing such that cholinergic dysfunction (results in decrease in signal-to-noise ratio) may increase the uncertainty in top-down activity resulting in incorrect scene representation, and thus hallucinations (Collerton et al., 2005; Friston, 2005). Indeed, impaired bottom-up (i.e. reduced activation of the visual pathways) and top-down (i.e. defective attentional) processing, and thus cholinergic dysfunction, has frequently been reported in PD patients who experience VH (Boecker et al., 2007; Hepp et al., 2017; Matsui et al., 2006; Meppelink et al., 2009; Park et al., 2013; Stebbins et al., 2004). Recently, Hepp et al. proposed that impaired bottom-up visual processing in combination with defective top-down attentional processing may underlie VH in PD (Hepp et al., 2017). In accordance with these findings, our results provide neurophysiological evidence that VH in PD may emerge due to central cholinergic dysfunction.
With respect to spatial distribution, compared to patients without hallucinations, patients with unimodal VH showed notable findings in the theta and beta band. In both frequency bands, brain regions that revealed significant difference between the groups were located in the right hemisphere and comprised the temporoparietal brain areas, among others. The right hemisphere has been shown to play a role in arousal and attentional processes and mediate top-down attentional processing (Levy and Wagner, 2011; Posner, 1994; Sacchet et al., 2015). The temporoparietal brain regions form part of the ventral attentional network (VAN, also named salience network), which is lateralized to the right hemisphere and involved in shifting attention in the presence of salient stimuli (Corbetta et al., 2002; Vossel et al., 2014). Moreover, the right temporoparietal brain regions have been involved in source monitoring or ‘self-other’ distinction (i.e. discrimination between external perceptions and internally generated information) (Sowden and Catmur, 2015), and deficits in source monitoring have been reported in PD patients with VH (Barnes et al., 2003; Muller et al., 2014).
Beta band activity has been associated with long-range feedback or top-down processing and attention (Gross et al., 2004; Kamiński et al., 2012; Kopell et al., 2000; Michalareas et al., 2016). Theta band activity has also been proposed in top-down processing with a key inhibitory role in working memory to suppress task-irrelevant or distracting information in situations that demand cognitive control (Klimesch, 1999; Nigbur et al., 2011). Moreover, increase in theta oscillations is observed during lower vigilance and states of drowsiness (Strijkstra et al., 2003). In patients without hallucinations, we found that higher power in the right temporoparietal regions in the theta band was correlated with worse performance on the tests for visual processing speed (TMT-A) and attention (TMT—B), whereas higher power in the right temporoparietal regions in the beta band was associated with better performance on both tests (Table 5). These correlations were lacking in patients with only VH, which might be due to the small sample size of the group (data available in n = 9 for TMT-A and n = 7 for TMT—B). Taken together, our results provide support for alterations in top-down attentional processing in PD patients with VH.
Gamma band activity is generated in early sensory cortices, and involved in feedforward or bottom-up processing (Bastos et al., 2015; Herrmann et al., 2010; Kopell et al., 2000). The lack of significant differences in gamma power in the posterior brain regions between PD patients with unimodal VH and PD patients without hallucinations suggests that there may be no alterations in bottom-up processing in PD patients with only VH. Less straightforward is the interpretation of decreased gamma power in the frontal brain regions in PD patients with only VH compared to PD patients without hallucinations. Gamma oscillations are modulated by various cognitive processes such as attention and working memory, and are therefore supposed to reflect integration mechanisms of the brain. Particularly, gamma oscillations are involved in working memory storage that can be controlled by beta oscillations, such that beta rhythm regulates the access of sensory information into working memory and controls its maintenance (Herrmann et al., 2010; Miller et al., 2018). Hence, decreased gamma power in the frontal brain regions might be a consequence of decreased beta power and thus top-down processing. However, several other brain regions also showed higher power in the theta and lower power in the gamma band, and lower PF in patients with only VH, hence our results with respect to spatial distribution may not provide sufficient insight into the exact role of specific brain regions in the underlying pathophysiological mechanisms of VH and should be interpreted with caution. Future work to evaluate MEG-based functional connectivity and brain network organization may be of additional value in exploring the exact role of multiple brain regions and networks - involved in attention and perception - in the pathophysiology of VH.
Another possible explanation for slowing of resting-state brain activity in patients with only VH as opposed to patients with multimodal hallucinations and patients without hallucinations may be sought in the patient characteristics of the groups. Although not significantly different, patients with only VH were somewhat older, had somewhat longer disease duration at enrollment and were slightly more cognitively impaired than patients with multimodal hallucinations and patients without hallucinations, indicating a slightly more advanced disease stage in patients with only VH. For decades, diffuse and local slowing of resting state oscillatory brain activity, involving increases in theta power and decreases in beta and gamma power, has been a consistently reported feature in PD patients, with severity of slowing increasing with advancing disease, and predicting risk of future dementia (Bosboom et al., 2006; Caviness et al., 2007; Fonseca et al., 2009; Klassen et al., 2011; Neufeld et al., 1994; Olde Dubbelink et al., 2014; Olde Dubbelink et al., 2013; Serizawa et al., 2008; Soikkeli et al., 1991; Stoffers et al., 2007).
4.2.2. Multimodal hallucinations
Patients with multimodal hallucinations experienced both VH and AH (with similar prevalences) and hallucinations in other modalities but did not show more slowing of resting-state brain activity than patients with only VH or patients without hallucinations. Patients with multimodal hallucinations rather showed faster, although not significantly so, resting-state brain activity than patients with only VH and patients without hallucinations, which indicates the complexity of the pathophysiology of hallucinations in PD. In addition, given the extensive differences in spatial distribution in the different frequency bands/PF between patients with multimodal hallucinations and patients with unimodal VH, it is difficult to draw conclusions about the involvement of specific brain regions in the pathophysiology of hallucinations in PD.
A likely candidate to explain changes in spectral power in PD patients with multimodal hallucinations may be the dopaminergic system. Research on the effect of dopaminergic neurotransmission on resting-state oscillatory brain activity in PD is scarce. Nonetheless, a few studies have examined the effect of exposure to dopaminergic agents (i.e. dopaminomimetics or dopamine precursor levodopa (L-dopa)) on resting-state brain activity in PD patients and found contradicting results (Babiloni et al., 2018; Melgari et al., 2014; Stoffers et al., 2007; Yaar and Shapiro, 1983). A previous quantitative EEG study examined 25 PD patients on chronic L-dopa therapy and found a spatially confined increase in power in all frequency bands over the left-occipital brain region (Yaar and Shapiro, 1983). In addition, Melgari et al. (2014) obtained resting-state source-space EEG recordings in 24 PD patients before and after an oral dose of L-dopa and found a significant increase in alpha and beta power over centro-parietal electrodes (Melgari et al., 2014). This is in contrast with findings from a recent study by Babiloni et al. (2018), who studied resting-state EEG activity in PD patients with normal (N = 35) and impaired cognition (N = 85) before and after L-dopa intake and compared these data with EEGs from healthy individuals (N = 50). Compared to the healthy individuals, the PD groups with and without cognitive decline showed a diffuse increase in delta power and decrease in alpha power in the posterior brain regions. In relation to PD patients with normal cognition, cognitively impaired PD patients showed greater increase in delta power, greater reduction in occipital alpha power with concomitant increase in alpha power in the frontal, central and temporal brain regions (Babiloni et al., 2018). Notably, an MEG-study by Stoffers et al. in non-demented PD patients (N = 37) did not find any significant effect of acute L-dopa administration on spectral power (Stoffers et al., 2007). Thus, there is considerable variability in the reported relation between resting-state brain activity and dopaminergic neurotransmission, which could be related to the demographics of the patient groups or methodological differences between the studies.
In our study, the daily L-dopa equivalent dose (LED) did not differ significantly between patients with only VH, patients with multimodal hallucinations, and patients without hallucinations (Table 3). Nonetheless, psychosis has frequently been reported as a non-motor adverse effect of dopaminergic treatment in both early-stage and late-stage PD (Barrett et al., 2017; Morgante et al., 2012; Ravina et al., 2007; Stowe et al., 2008). Moreover, recent positive findings show that hallucinations in PD can be alleviated with subthalamic deep brain stimulation, which could probably be related to the reduction of dopaminergic medication (Lhommée et al., 2018). Considering the findings that dopaminergic treatment in PD may lead to psychosis, and that restoration of brain dopamine levels by drug treatment may (at least partly) restore normal patterns of oscillatory brain activity, suggest that hyperdopaminergic neurotransmission may underlie psychosis in PD and does not induce slowing in resting-state oscillatory brain activity.
A highly speculative explanation for the increase in signal power in patients with multimodal hallucinations may be sought in the decreased output from the nigrostriatal dopaminergic system to connected brain areas. The dopamine depleted nigro-striatal-thalamo-cortical circuit in PD may lead to reduced modulatory control on connected cortical brain regions (Melgari et al., 2014; Rodriguez-Oroz et al., 2009). In response, connected brain areas may lower their detection threshold for neuronal firing, which may result in hyper-excitability (due to increased sensitivity of neurons towards incoming signals) within the connected brain regions. This hyper-excitability may result in false-positive neuronal firing, which may be perceived as an experience without the presence of an external source; a hallucination (dependent on the modality-specific brain region). This mechanism is known as cortical deafferentiation (Carter and ffytche, 2015). Dysregulation of neural circuits due to imbalance between excitation and inhibition as a general model of hallucinations has been proposed in both neurological and psychiatric disorders, with greater evidence for inhibitory deficits in hallucinations (Jardri et al., 2016).
Alterations in serotonin neurotransmission have also been proposed in the pathophysiology of hallucinations in PD, with a specific role for the serotonin 2 receptor (Factor et al., 2017). Treatment with pimavanserin, a serotonin 2A inverse-agonist, has been shown to alleviate psychosis in both PD patients with normal and impaired cognitive functioning (Espay et al., 2018; Kianirad and Simuni, 2017). To date, only one study has examined in vivo changes in serotonin receptor binding in PD with positron emission tomography (PET) and found increased serotonin binding in the ventral visual pathway in PD patients with VH compared to patients without hallucinations (Ballanger et al., 2010). The use of selective serotonin reuptake inhibitors (SSRIs) (increasing the extracellular level of serotonin) has been associated with changes in rhythmic brain activity in the delta, theta and alpha band in prefrontal brain regions, with decreases in the delta and theta band and increases in the alpha band (Bares et al., 2008; Leuchter et al., 2017; Leuchter et al., 2009). In this study, we did not find significant differences between the groups in the delta or alpha band. Significant differences in the theta band were widespread throughout the brain in both patients with only VH and patients with multimodal hallucinations. Specifically, patients with multimodal hallucinations showed lower relative power in the theta band compared to both patients with only VH and patients without hallucinations, hinting that serotonergic dysfunction may play a role in multimodal hallucinations in PD.
Nevertheless, the above-mentioned neurophysiological evidence is insufficient to provide strong support for the notion that dopaminergic or serotonergic dysfunction may induce faster resting-state brain activity in PD patients with multimodal hallucinations. Future studies investigating different modalities of hallucinations within PD are needed to gain insight into other potential underlying mechanisms.
4.3. Strengths and limitations
A strength of this study is that it investigated hallucinations in PD with source-space MEG, which made it possible to find regionally-specific differences between the groups. Second, both patients with and without hallucinations, as well as, patients with only VH and patients with multimodal hallucinations, were carefully matched for age, gender, educational level, disease duration, disease stage, and use of medication, which makes our findings robust for the influence of these general and disease related factors.
This study also has limitations. First, by performing subgroup analyses we reduced the sample size of the hallucination group, and therefore, the results should be interpreted with caution. However, by dividing patients with hallucinations in subgroups based on the modality of hallucinations, we were able to explore the specificity of possible group differences to the pathophysiology of VH. Second, cholinesterase inhibitors are known to influence spectral power and entail increases in high frequency power with simultaneous decreases in low frequency power (Fogelson et al., 2003). We observed the opposite pattern in our patients. In our study, only two patients (N = 1 in the unimodal Hall+ group and N = 1 in the Hall- group) used the cholinesterase inhibitor rivastigmine. Therefore, it is unlikely that the use of cholinesterase inhibitors has influenced our findings. In addition, atypical antipsychotics (e.g. clozapine, quetiapine) are also used to treat psychosis in PD (Wilby et al., 2017), and have been shown to increase power in lower frequencies and decrease power in higher frequencies (Hyun et al., 2011; Maccrimmon et al., 2012). In our study, only two patients with multimodal hallucinations used atypical antipsychotics (N = 1 clozapine, and N = 1 quetiapine). As we found decreased power in the delta and theta band, and increases in power in the alpha and beta band in patients with multimodal hallucinations, it is unlikely that the use of atypical antipsychotics has influenced findings in this patient group.
5. Conclusion
Source-space MEG shows distinct spectral differences between Parkinson's disease patients with unimodal visual hallucinations and patients without hallucinations. Slowing of resting-state brain activity with increases in theta activity, and concomitant decreases in beta and gamma activity indicates central cholinergic dysfunction as underlying mechanism of visual hallucinations in Parkinson's disease. Future work to evaluate functional connectivity and brain network organization is needed in order to explore the exact role of multiple brain regions and networks - involved in attention and perception – in the pathophysiology of visual hallucinations in Parkinson's disease.
Funding
This study was supported by ZONMW TOP grant 40-00812-98-13009.
Declarations of interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2019.101752.
Appendix A. Supplementary data
Supplementary material
References
- Aarsland D., Larsen J.P., Tandberg E., Laake K. Predictors of nursing home placement in Parkinson's disease: a population-based, prospective study. J. Am. Geriatr. Soc. 2000;48:938–942. doi: 10.1111/j.1532-5415.2000.tb06891.x. [DOI] [PubMed] [Google Scholar]
- Babiloni C., Del Percio C., Lizio R., Noce G., Lopez S., Soricelli A., Ferri R., Pascarelli M.T., Catania V., Nobili F., Arnaldi D., Famà F., Orzi F., Buttinelli C., Giubilei F., Bonanni L., Franciotti R., Onofrj M., Stirpe P., Fuhr P., Gschwandtner U., Ransmayr G., Fraioli L., Parnetti L., Farotti L., Pievani M., D'Antonio F., De Lena C., Güntekin B., Hanoğlu L., Yener G., Emek-Savaş D.D., Triggiani A.I., Taylor J.P., McKeith I., Stocchi F., Vacca L., Frisoni G.B., De Pandis M.F. Levodopa may affect cortical excitability in Parkinson's disease patients with cognitive deficits as revealed by reduced activity of cortical sources of resting state electroencephalographic rhythms. Neurobiol. Aging. 2018:73. doi: 10.1016/j.neurobiolaging.2018.08.010. [DOI] [PubMed] [Google Scholar]
- Ballanger B., Strafella A.P., van Eimeren T., Zurowski M., Rusjan P.M., Houle S., Fox S.H. Serotonin 2A receptors and visual hallucinations in Parkinson disease. Arch. Neurol. 2010;67:416–421. doi: 10.1001/archneurol.2010.35. [DOI] [PubMed] [Google Scholar]
- Bares M., Brunovsky M., Kopecek M., Novak T., Stopkova P., Kozeny J., Sos P., Krajca V., Hoschl C. Early reduction in prefrontal theta QEEG cordance value predicts response to venlafaxine treatment in patients with resistant depressive disorder. Eur. Psychiatry. 2008;23:350–355. doi: 10.1016/j.eurpsy.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Barnes J., Boubert L., Harris J., Lee A., David A.S. Reality monitoring and visual hallucinations in Parkinson's disease. Neuropsychologia. 2003;41:565–574. doi: 10.1016/s0028-3932(02)00182-3. [DOI] [PubMed] [Google Scholar]
- Barrett M.J., Smolkin M.E., Flanigan J.L., Shah B.B., Harrison M.B., Sperling S.A. Characteristics, correlates, and assessment of psychosis in Parkinson disease without dementia. Park. Relat. Disord. 2017;43:56–60. doi: 10.1016/j.parkreldis.2017.07.011. [DOI] [PubMed] [Google Scholar]
- Bastos A.M., Vezoli J., Bosman C.A., Schoffelen J.M., Oostenveld R., Dowdall J.R., DeWeerd P., Kennedy H., Fries P. Visual areas exert feedforward and feedback influences through distinct frequency channels. Neuron. 2015;85:390–401. doi: 10.1016/j.neuron.2014.12.018. [DOI] [PubMed] [Google Scholar]
- Bauer M., Kluge C., Bach D., Bradbury D., Heinze H.J., Dolan R.J., Driver J. Cholinergic enhancement of visual attention and neural oscillations in the human brain. Curr. Biol. 2012;22:397–402. doi: 10.1016/j.cub.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Brown G.K. Beck depression inventory-II. San Antonio. 1996;78:490–498. [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B. 1995:289–300. [Google Scholar]
- Boecker H., Ceballos-Baumann A.O., Volk D., Conrad B., Forstl H., Haussermann P. Metaboic alterations in patients with Parkinson disease and visual hallucinations. Arch. Neurol. 2007;64:984–988. doi: 10.1001/archneur.64.7.984. [DOI] [PubMed] [Google Scholar]
- Bosboom J.L.W., Stoffers D., Stam C.J., van Dijk B.W., Verbunt J., Berendse H.W., Wolters E.C. Resting state oscillatory brain dynamics in Parkinson's disease: an MEG study. Clin. Neurophysiol. 2006;117:2521–2531. doi: 10.1016/j.clinph.2006.06.720. [DOI] [PubMed] [Google Scholar]
- Carter R., ffytche D.H. On visual hallucinations and cortical networks: a trans-diagnostic review. J. Neurol. 2015;262:1780–1790. doi: 10.1007/s00415-015-7687-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness J.N., Hentz J.G., Evidente V.G., Driver-Dunckley E., Samanta J., Mahant P., Connor D.J., Sabbagh M.N., Shill H.A., Adler C.H. Both early and late cognitive dysfunction affects the electroencephalogram in Parkinson's disease. Parkinsonism Relat. Disord. 2007;13:348–354. doi: 10.1016/j.parkreldis.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Cheyne D., Bostan A.C., Gaetz W., Pang E.W. Event-related beamforming: a robust method for presurgical functional mapping using MEG. Clin. Neurophysiol. 2007;118:1691–1704. doi: 10.1016/j.clinph.2007.05.064. [DOI] [PubMed] [Google Scholar]
- Collerton D., Perry E., McKeith I. Why people see things that are not there: a novel perception and attention deficit model for recurrent complex visual hallucinations. Behav. Brain Sci. 2005;28:737–757. doi: 10.1017/S0140525X05000130. discussion 757–794. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Kincade J.M., Shulman G.L. Neural systems for visual orienting and their relationships to spatial working memory. J. Cogn. Neurosci. 2002;14:508–523. doi: 10.1162/089892902317362029. [DOI] [PubMed] [Google Scholar]
- Crowe S.F. The differential contribution of mental tracking, cognitive flexibility, visual search, and motor speed to performance on parts a and B of the trail making test. J. Clin. Psychol. 1998;54:585–591. doi: 10.1002/(sici)1097-4679(199808)54:5<585::aid-jclp4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- de Jong-Gierveld J., Kamphuls F. The development of a Rasch-type loneliness scale. Appl. Psychol. Meas. 1985;9:289–299. [Google Scholar]
- Donner T.H., Siegel M. A framework for local cortical oscillation patterns. Trends Cogn. Sci. 2011;15:191–199. doi: 10.1016/j.tics.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Espay A.J., Guskey M.T., Norton J.C., Coate B., Vizcarra J.A., Ballard C., Factor S.A., Friedman J.H., Lang A.E., Larsen N.J., Andersson C., Fredericks D., Weintraub D. Pimavanserin for Parkinson's disease psychosis: effects stratified by baseline cognition and use of cognitive-enhancing medications. Mov. Disord. 2018;1–8 doi: 10.1002/mds.27488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Factor S.A., McDonald W.M., Goldstein F.C. The role of neurotransmitters in the development of Parkinson's disease-related psychosis. Eur. J. Neurol. 2017;24:1244–1254. doi: 10.1111/ene.13376. [DOI] [PubMed] [Google Scholar]
- Fénelon G. Psychosis in Parkinson's disease: phenomenology, frequency, risk factors, and current understanding of pathophysiologic mechanisms. CNS Spectr. 2008;13:18–25. doi: 10.1017/s1092852900017284. [DOI] [PubMed] [Google Scholar]
- Fenelon G., Alves G. Epidemiology of psychosis in Parkinson's disease. J. Neurol. Sci. 2010;289:12–17. doi: 10.1016/j.jns.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Ffytche D.H., Creese B., Politis M., Chaudhuri K.R., Weintraub D., Ballard C., Aarsland D. The psychosis spectrum in Parkinson disease. Nat. Rev. Neurol. 2017;13:81–95. doi: 10.1038/nrneurol.2016.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelson N., Kogan E., Korczyn A.D., Giladi N., Shabtai H., Neufeld M.Y. Effects of rivastigmine on the quantitative EEG in demented parkinsonian patients. Acta Neurol. Scand. 2003;107:252–255. doi: 10.1034/j.1600-0404.2003.00081.x. [DOI] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fonseca L.C., Tedrus G.M.A.S., Letro G.H., Bossoni A.S. Dementia, mild cognitive impairment and quantitative EEG in patients with Parkinson's disease. Clin. EEG Neurosci. 2009;40:168–172. doi: 10.1177/155005940904000309. [DOI] [PubMed] [Google Scholar]
- Friston K.J. Hallucinations and perceptual inference. Behav. Brain Sci. 2005;28:764–766. [Google Scholar]
- Goetz C.G., Vogel C., Tanner C.M., Stebbins G.T. Early dopaminergic drug-induced hallucinations in parkinsonian patients. Neurology. 1998;51:811–814. doi: 10.1212/wnl.51.3.811. [DOI] [PubMed] [Google Scholar]
- Goetz C.G., Poewe W., Rascol O., Sampaio C., Stebbins G.T., Counsell C., Giladi N., Holloway R.G., Moore C.G., Wenning G.K., Yahr M.D., Seidl L. Movement Disorder Society task force report on the Hoehn and Yahr staging scale: status and recommendations. Mov. Disord. 2004;19:1020–1028. doi: 10.1002/mds.20213. [DOI] [PubMed] [Google Scholar]
- Goetz C.G., Stebbins G.T., Ouyang B. Visual plus nonvisual hallucinations in Parkinson's disease: development and evolution over 10 years. Mov. Disord. 2011;26:2196–2200. doi: 10.1002/mds.23835. [DOI] [PubMed] [Google Scholar]
- Gross J., Schmitz F., Schnitzler I., Kessler K., Shapiro K., Hommel B., Schnitzler A. Modulation of long-range neural synchrony reflects temporal limitations of visual attention in humans. Proc. Natl. Acad. Sci. U. S. A. 2004;101:13050–13055. doi: 10.1073/pnas.0404944101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepp D.H., da Hora C.C., Koene T., Uitdehaag B.M., van den Heuvel O.A., Klein M., van de Berg W.D., Berendse H.W., Foncke E.M. Cognitive correlates of visual hallucinations in non-demented Parkinson's disease patients. Park. Relat. Disord. 2013;19:795–799. doi: 10.1016/j.parkreldis.2013.04.026. [DOI] [PubMed] [Google Scholar]
- Hepp D.H., Foncke E.M.J., Olde Dubbelink K.T.E., van de Berg W.D.J., Berendse H.W., Schoonheim M.M. Loss of functional connectivity in patients with Parkinson disease and visual hallucinations. Radiology. 2017;000:170438. doi: 10.1148/radiol.2017170438. [DOI] [PubMed] [Google Scholar]
- Herrmann C.S., Frund I., Lenz D. Human gamma-band activity: a review on cognitive and behavioral correlates and network models. Neurosci. Biobehav. Rev. 2010;34:981–992. doi: 10.1016/j.neubiorev.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Hillebrand A., Barnes G.R. Beamformer analysis of MEG data. Int. Rev. Neurobiol. 2005;68:149–171. doi: 10.1016/S0074-7742(05)68006-3. [DOI] [PubMed] [Google Scholar]
- Hillebrand A., Singh K.D., Holliday I.E., Furlong P.L., Barnes G.R. A new approach to neuroimaging with magnetoencephalography. Hum. Brain Mapp. 2005;25:199–211. doi: 10.1002/hbm.20102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand A., Barnes G.R., Bosboom J.L., Berendse H.W., Stam C.J. Frequency-dependent functional connectivity within resting-state networks: an atlas-based MEG beamformer solution. Neuroimage. 2012;59:3909–3921. doi: 10.1016/j.neuroimage.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand A., Fazio P., de Munck J.C., van Dijk B.W. Feasibility of clinical magnetoencephalography (MEG) functional mapping in the presence of dental artefacts. Clin. Neurophysiol. 2013;124:107–113. doi: 10.1016/j.clinph.2012.06.013. [DOI] [PubMed] [Google Scholar]
- Hillebrand A., Tewarie P., van Dellen E., Yu M., Carbo E.W.S., Douw L., Gouw A.A., van Straaten E.C.W., Stam C.J. Direction of information flow in large-scale resting-state networks is frequency-dependent. Proc. Natl. Acad. Sci. U. S. A. 2016 doi: 10.1073/pnas.1515657113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun J., Baik M.J., Kang U.G. Effects of psychotropic drugs on quantitative EEG among patients with schizophrenia-spectrum disorders. Clin. Psychopharmacol. Neurosci. 2011;9:78–85. doi: 10.9758/cpn.2011.9.2.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzelberg R., Kipervasser S., Korczyn A.D. Auditory hallucinations in Parkinson's disease. J. Neurol. Neurosurg. Psychiatry. 1998;64:533–535. doi: 10.1136/jnnp.64.4.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardri R., Hugdahl K., Hughes M., Brunelin J., Waters F., Alderson-Day B., Smailes D., Sterzer P., Corlett P.R., Leptourgos P., Debbané M., Cachia A., Denève S. Are hallucinations due to an imbalance between excitatory and inhibitory influences on the brain? Schizophr. Bull. 2016;42:1124–1134. doi: 10.1093/schbul/sbw075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiński J., Brzezicka A., Gola M., Wróbel A. Beta band oscillations engagement in human alertness process. Int. J. Psychophysiol. 2012;85:125–128. doi: 10.1016/j.ijpsycho.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Katzen H., Myerson C., Papapetropoulos S., Nahab F., Gallo B., Levin B. Multi-modal hallucinations and cognitive function in Parkinson's disease. Dement. Geriatr. Cogn. Disord. 2010;30:51–56. doi: 10.1159/000314875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kianirad Y., Simuni T. Pimavanserin, a novel antipsychotic for management of Parkinson's disease psychosis. Expert. Rev. Clin. Pharmacol. 2017;10:1161–1168. doi: 10.1080/17512433.2017.1369405. [DOI] [PubMed] [Google Scholar]
- Klassen B.T., Hentz J.G., Shill H.A., Driver-Dunckley E., Evidente V.G.H., Sabbagh M.N., Adler C.H., Caviness J.N. Quantitative EEG as a predictive biomarker for Parkinson disease dementia. Neurology. 2011;77:118–124. doi: 10.1212/WNL.0b013e318224af8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res. Brain Res. Rev. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Kopell N., Ermentrout G.B., Whittington M.A., Traub R.D. Gamma rhythms and beta rhythms have different synchronization properties. Proc. Natl. Acad. Sci. U. S. A. 2000;97:1867–1872. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulick C.V., Montgomery K.M., Nirenberg M.J. Comprehensive identification of delusions and olfactory, tactile, gustatory, and minor hallucinations in Parkinson's disease psychosis. Parkinsonism Relat. Disord. 2018;54:40–45. doi: 10.1016/j.parkreldis.2018.04.008. [DOI] [PubMed] [Google Scholar]
- Lenka A., Jhunjhunwala K.R., Saini J., Pal P.K. Structural and functional neuroimaging in patients with Parkinson's disease and visual hallucinations: a critical review. Parkinsonism Relat. Disord. 2015;21:683–691. doi: 10.1016/j.parkreldis.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Lenka A., Hegde S., Arumugham S.S., Pal P.K. Pattern of cognitive impairment in patients with Parkinson's disease and psychosis: a critical review. Parkinsonism Relat. Disord. 2017;37:11–18. doi: 10.1016/j.parkreldis.2016.12.025. [DOI] [PubMed] [Google Scholar]
- Leuchter A.F., Cook I.A., Gilmer W.S., Marangell L.B., Burgoyne K.S., Howland R.H., Trivedi M.H., Zisook S., Jain R., Fava M., Iosifescu D., Greenwald S. Effectiveness of a quantitative electroencephalographic biomarker for predicting differential response or remission with escitalopram and bupropion in major depressive disorder. Psychiatry Res. 2009;169:132–138. doi: 10.1016/j.psychres.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Leuchter A.F., Hunter A.M., Jain F.A., Tartter M., Crump C., Cook I.A. Escitalopram but not placebo modulates brain rhythmic oscillatory activity in the first week of treatment of major depressive disorder. J. Psychiatr. Res. 2017;84:174–183. doi: 10.1016/j.jpsychires.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Levy B.J., Wagner A.D. Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Ann. N. Y. Acad. Sci. 2011;1224:40–62. doi: 10.1111/j.1749-6632.2011.05958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeboom J., Matto D. Digit series and Knox cubes as concentration tests for elderly subjects. Tijdschr. Gerontol. Geriatr. 1994;25:63–68. [PubMed] [Google Scholar]
- Lopes da Silva F. EEG and MEG: Relevance to neuroscience. Neuron. 2013;80:1112–1128. doi: 10.1016/j.neuron.2013.10.017. [DOI] [PubMed] [Google Scholar]
- Maccrimmon D., Brunet D., Criollo M., Galin H., Lawson J.S. Clozapine augments delta, theta, and right frontal EEG alpha power in schizophrenic patients. ISRN Psychiatry. 2012;2012:596486. doi: 10.5402/2012/596486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui H., Nishinaka K., Oda M., Hara N., Komatsu K., Kubori T., Udaka F. Hypoperfusion of the visual pathway in parkinsonian patients with visual hallucinations. Mov. Disord. 2006;21:2140–2144. doi: 10.1002/mds.21140. [DOI] [PubMed] [Google Scholar]
- McAuley J.H., Gregory S. Prevalence and clinical course of olfactory hallucinations in idiopathic Parkinson's disease. J. Park. Dis. 2012;2:199–205. doi: 10.3233/JPD-2012-012086. [DOI] [PubMed] [Google Scholar]
- Melgari J.M., Curcio G., Mastrolilli F., Salomone G., Trotta L., Tombini M., Di Biase L., Scrascia F., Fini R., Fabrizio E., Rossini P.M., Vernieri F. Alpha and beta EEG power reflects L-dopa acute administration in Parkinsonian patients. Front. Aging Neurosci. 2014;6:1–7. doi: 10.3389/fnagi.2014.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meppelink A.M., de Jong B.M., Renken R., Leenders K.L., Cornelissen F.W., van Laar T. Impaired visual processing preceding image recognition in Parkinson's disease patients with visual hallucinations. Brain. 2009;132:2980–2993. doi: 10.1093/brain/awp223. [DOI] [PubMed] [Google Scholar]
- Michalareas G., Vezoli J., van Pelt S., Schoffelen J.M., Kennedy H., Fries P. Alpha-Beta and Gamma rhythms subserve feedback and feedforward influences among human visual cortical areas. Neuron. 2016;89:384–397. doi: 10.1016/j.neuron.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E.K., Lundqvist M., Bastos A.M. Working memory 2.0. Neuron. 2018;100:463–475. doi: 10.1016/j.neuron.2018.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgante L., Colosimo C., Antonini A., Marconi R., Meco G., Pederzoli M., Pontieri F.E., Cicarelli G., Abbruzzese G., Zappulla S., Ramat S., Manfredi M., Bottacchi E., Abrignani M., Berardelli A., Cozzolino A., Paradiso C., De Gaspari D., Morgante F., Barone P. Psychosis associated to Parkinson's disease in the early stages: relevance of cognitive decline and depression. J. Neurol. Neurosurg. Psychiatry. 2012;83:76–82. doi: 10.1136/jnnp-2011-300043. [DOI] [PubMed] [Google Scholar]
- Muller A.J., Shine J.M., Halliday G.M., Lewis S.J.G. Visual hallucinations in Parkinson's disease: theoretical models. Mov. Disord. 2014;29:1591–1598. doi: 10.1002/mds.26004. [DOI] [PubMed] [Google Scholar]
- Nagano-Saito A., Washimi Y., Arahata Y., Iwai K., Kawatsu S., Ito K., Nakamura A., Abe Y., Yamada T., Kato T., Kachi T. Visual hallucination in Parkinson's disease with FDG PET. Mov. Disord. 2004;19:801–806. doi: 10.1002/mds.20129. [DOI] [PubMed] [Google Scholar]
- Neufeld M.Y., Blumen S., Aitkin I., Parmet Y., Korczyn A.D. EEG frequency analysis in demented and nondemented parkinsonian patients. Dementia. 1994;5:23–28. doi: 10.1159/000106690. [DOI] [PubMed] [Google Scholar]
- Nigbur R., Ivanova G., Stürmer B. Theta power as a marker for cognitive interference. Clin. Neurophysiol. 2011;122:2185–2194. doi: 10.1016/j.clinph.2011.03.030. [DOI] [PubMed] [Google Scholar]
- Olde Dubbelink K.T.E., Stoffers D., Deijen J.B., Twisk J.W.R., Stam C.J., Berendse H.W. Cognitive decline in Parkinson's disease is associated with slowing of resting-state brain activity: a longitudinal study. Neurobiol. Aging. 2013;34:408–418. doi: 10.1016/j.neurobiolaging.2012.02.029. [DOI] [PubMed] [Google Scholar]
- Olde Dubbelink K.T.E., Hillebrand A., Twisk J.W.R., Deijen J.B., Stoffers D., Schmand B.A., Stam C.J., Berendse H.W. Predicting dementia in Parkinson disease by combining neurophysiologic and cognitive markers. Neurology. 2014;82:263–270. doi: 10.1212/WNL.0000000000000034. [DOI] [PubMed] [Google Scholar]
- Onofrj M., Gilbert G.J. GABA and hallucinations in Parkinson disease. Neurology. 2018;91:293–294. doi: 10.1212/WNL.0000000000005985. [DOI] [PubMed] [Google Scholar]
- Oosterman J.M., Vogels R.L.C., Van Harten B., Alida A., Poggesi A., Scheltens P., Kessels R.P.C., Scherder E.J.A., Gouw A.A., Poggesi A., Scheltens P., Kessels R.P.C., Scherder E.J.A. Assessing mental flexibility: neuroanatomical and neuropsychological correlates of the trail making test in elderly people. 2010:4046. doi: 10.1080/13854040903482848. [DOI] [PubMed] [Google Scholar]
- Park H.K., Kim J.S., Im K.C., Kim M.J., Lee J.-H., Lee M.C., Kim J., Chung S.J. Visual hallucinations and cognitive impairment in Parkinson's disease. Can. J. Neurol. Sci. 2013;40:657–662. doi: 10.1017/s0317167100014888. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G., Lopes da Silva F.H. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin. Neurophysiol. 1999;110:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Posner M.I. Attention: the mechanisms of consciousness. Proc. Natl. Acad. Sci. 1994;91:7398–7403. doi: 10.1073/pnas.91.16.7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prell T. Structural and functional brain patterns of non-motor syndromes in Parkinson's disease. Front. Neurol. 2018;9:1–9. doi: 10.3389/fneur.2018.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Ruiz B., Martí M.J., Tolosa E., Falcón C., Bargalló N., Valldeoriola F., Junqué C. Brain response to complex visual stimuli in Parkinson's patients with hallucinations: a functional magnetic resonance imaging study. Mov. Disord. 2008;23:2335–2343. doi: 10.1002/mds.22258. [DOI] [PubMed] [Google Scholar]
- Ravina B., Marder K., Fernandez H.H., Friedman J.H., McDonald W., Murphy D., Aarsland D., Babcock D., Cummings J., Endicott J., Factor S., Galpern W., Lees A., Marsh L., Stacy M., Gwinn-Hardy K., Voon V., Goetz C. Diagnostic criteria for psychosis in Parkinson's disease: report of an NINDS, NIMH work group. Mov. Disord. 2007;22:1061–1068. doi: 10.1002/mds.21382. [DOI] [PubMed] [Google Scholar]
- Reitan R.M. Validity of the trail making test as an indicator of organic brain damage. Percept. Mot. Skills. 1958;8:271–276. [Google Scholar]
- Reitan R.M. 1992. Trail Making Test: Manual for Administration and Scoring. [Google Scholar]
- Rodriguez-Oroz M.C., Jahanshahi M., Krack P., Litvan I., Macias R., Bezard E., Obeso J.A. Initial clinical manifestations of Parkinson's disease: features and pathophysiological mechanisms. Lancet Neurol. 2009;8:1128–1139. doi: 10.1016/S1474-4422(09)70293-5. [DOI] [PubMed] [Google Scholar]
- Sacchet M.D., LaPlante R.A., Wan Q., Pritchett D.L., Lee A.K.C., Hamalainen M., Moore C.I., Kerr C.E., Jones S.R. Attention drives synchronization of alpha and Beta rhythms between right inferior frontal and primary sensory neocortex. J. Neurosci. 2015;35:2074–2082. doi: 10.1523/JNEUROSCI.1292-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Castaneda C., Rene R., Ramirez-Ruiz B., Campdelacreu J., Gascon J., Falcon C., Calopa M., Jauma S., Juncadella M., Junque C. Frontal and associative visual areas related to visual hallucinations in dementia with lewy bodies and Parkinson's disease with dementia. Mov. Disord. 2010;25:615–622. doi: 10.1002/mds.22873. [DOI] [PubMed] [Google Scholar]
- Serizawa K., Kamei S., Morita A., Hara M., Mizutani T., Yoshihashi H., Yamaguchi M., Takeshita J., Hirayanagi K. Comparison of quantitative EEGs between Parkinson disease and age-adjusted normal controls. J. Clin. Neurophysiol. 2008;25:361–366. doi: 10.1097/WNP.0b013e31818f50de. [DOI] [PubMed] [Google Scholar]
- Siegel M., Donner T.H., Engel A.K. Spectral fingerprints of large-scale neuronal interactions. Nat. Rev. Neurosci. 2012;13:121–134. doi: 10.1038/nrn3137. [DOI] [PubMed] [Google Scholar]
- Simpraga S., Alvarez-Jimenez R., Mansvelder H.D., van Gerven J.M.A., Groeneveld G.J., Poil S.-S., Linkenkaer-Hansen K. EEG machine learning for accurate detection of cholinergic intervention and Alzheimer's disease. Sci. Rep. 2017;7:5775. doi: 10.1038/s41598-017-06165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpraga S., Mansvelder H.D., Groeneveld G.J., Prins S., Hart E.P., Poil S.S., Linkenkaer-Hansen K. An EEG nicotinic acetylcholine index to assess the efficacy of pro-cognitive compounds. Clin. Neurophysiol. 2018;129:2325–2332. doi: 10.1016/j.clinph.2018.08.014. [DOI] [PubMed] [Google Scholar]
- Soikkeli R., Partanen J., Soininen H., Paakkonen A., Riekkinen P.S. Slowing of EEG in Parkinson's disease. Electroencephalogr. Clin. Neurophysiol. 1991;79:159–165. doi: 10.1016/0013-4694(91)90134-p. [DOI] [PubMed] [Google Scholar]
- Sommer I.E., Kleijer H., Hugdahl K. Toward personalized treatment of hallucinations. Curr. Opin. Psychiatry. 2018;31:237–245. doi: 10.1097/YCO.0000000000000416. [DOI] [PubMed] [Google Scholar]
- Sowden S., Catmur C. The role of the right temporoparietal junction in the control of imitation. Cereb. Cortex. 2015;25:1107–1113. doi: 10.1093/cercor/bht306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam C.J., van Straaten E.C.W. The organization of physiological brain networks. Clin. Neurophysiol. 2012;123:1067–1087. doi: 10.1016/j.clinph.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Stebbins G.T., Goetz G.G., Carrillo M.C., Bangen K.J., Turner D.A., Glover G.H., Gabrieli J.D.E. Altered cortical visual processing in PD with hallucinations: an fMRI study. Neurology. 2004;63:1409–1416. doi: 10.1212/01.wnl.0000141853.27081.bd. [DOI] [PubMed] [Google Scholar]
- Stoffers D., Bosboom J.L.W., Deijen J.B., Wolters E.C., Berendse H.W., Stam C.J. Slowing of oscillatory brain activity is a stable characteristic of Parkinson's disease without dementia. Brain. 2007;130:1847–1860. doi: 10.1093/brain/awm034. [DOI] [PubMed] [Google Scholar]
- Stowe R.L., Ives N.J., Clarke C., van Hilten J., Ferreira J., Hawker R.J., Shah L., Wheatley K., Gray R. Dopamine agonist therapy in early Parkinson's disease. Cochrane Database Syst. Rev. 2008 doi: 10.1002/14651858.CD006564.pub2. [DOI] [PubMed] [Google Scholar]
- Strijkstra A.M., Beersma D.G.M., Drayer B., Halbesma N., Daan S. Subjective sleepiness correlates negatively with global alpha (8-12 Hz) and positively with central frontal theta (4-8 Hz) frequencies in the human resting awake electroencephalogram. Neurosci. Lett. 2003;340:17–20. doi: 10.1016/s0304-3940(03)00033-8. [DOI] [PubMed] [Google Scholar]
- Taulu S., Hari R. Removal of magnetoencephalographic artifacts with temporal signal-space separation: demonstration with single-trial auditory-evoked responses. Hum. Brain Mapp. 2009;30:1524–1534. doi: 10.1002/hbm.20627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taulu S., Simola J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys. Med. Biol. 2006;51:1759. doi: 10.1088/0031-9155/51/7/008. [DOI] [PubMed] [Google Scholar]
- Tomlinson C.L., Stowe R., Patel S., Rick C., Gray R., Clarke C.E. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov. Disord. 2010;25:2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uhlhaas P.J., Singer W. High-frequency oscillations and the neurobiology of schizophrenia. Dialogues Clin. Neurosci. 2013;15:301–313. doi: 10.31887/DCNS.2013.15.3/puhlhaas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhage F. 1964. Intelligentie en leeftijd: Onderzoek bij Nederlanders van twaalf tot zevenenzeventig jaar. [Google Scholar]
- Visser M., Verbaan D., van Rooden S.M., Stiggelbout A.M., Marinus J., van Hilten J.J. Assessment of psychiatric complications in Parkinson's disease: the SCOPA-PC. Mov. Disord. Off. J. Mov. Disord. Soc. 2007;22:2221–2228. doi: 10.1002/mds.21696. [DOI] [PubMed] [Google Scholar]
- von Stein A.U., Sarnthein J. Different frequencies for different scales of cortical integration: from local gamma to long range alphartheta synchronization. Int. J. Psychophysiol. 2000;38:301–313. doi: 10.1016/s0167-8760(00)00172-0. [DOI] [PubMed] [Google Scholar]
- Vossel S., Geng J.J., Fink G.R. Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist. 2014;20:150–159. doi: 10.1177/1073858413494269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilby K.J., Johnson E.G., Johnson H.E., Ensom M.H.H. Evidence-based review of pharmacotherapy used for Parkinson's disease psychosis. Ann. Pharmacother. 2017;51:682–695. doi: 10.1177/1060028017703992. [DOI] [PubMed] [Google Scholar]
- Williams-Gray C.H., Hampshire A., Barker R.A., Owen A.M. Attentional control in Parkinson's disease is dependent on COMT val 158 met genotype. Brain. 2008;131:397–408. doi: 10.1093/brain/awm313. [DOI] [PubMed] [Google Scholar]
- Lhommée E., Wojtecki L., Czernecki V., Witt K., Maier F., Tonder L., Timmermann L., Hälbig T.D., Pineau F., Durif F., Witjas T., Pinsker M., Mehdorn M., Sixel-Döring F., Kupsch A., Krüger R., Elben S., Chabardès S., Thobois S., Brefel-Courbon C., Ory-Magne F., Regis J.M., Maltête D., Sauvaget A., Rau J., Schnitzler A., Schüpbach M., Schade-Brittinger C., Deuschl G., Houeto J.L., Krack P., Negovanska V., Welter M.L., Corvol J.C., Agid Y., Navarro S., Meier N., Hartmann A., Hesekamp H., Cornu P., Möller B., Nebel A., Raethjen J., Knudsen K., Volkmann J., Falk D., Paschen S., Meister I., Kuhn J., Donner K., Kessler J., Barbe M., Fink G., Maarouf M., Kühn A., Müller B., Faust K., Gruber D., Schneider G.H., Seigneuret E., Pollak P., Fraix V., Kistner A., Rascol O., Arbus C., Danet L., Chaynes P., Groiss S.J., Hartmann C., Südmeyer M., Partowinia-Peters M., Vesper J., Ledily S., Damier P., Raoul S., Trenkwalder C., Richter-Dreske W., Wächter T., Weiss D., Eusebio A., Azulay J.P., Polo G., Pinto S., Levin J., Dornier S., Pene F., Hourton D., Quintin M., Hoffart-Jourdain C., Brocvielle H., Balthasar K., Stein M., Harnisch S., Reuss A., Aminossadati B., Nasemann C., Oertel W., Bataille B., Hellwig D., Gharabaghi A., Amtage F., Mertens P., Kloss M., Post B., Speelman H. Behavioural outcomes of subthalamic stimulation and medical therapy versus medical therapy alone for Parkinson's disease with early motor complications (EARLYSTIM trial): secondary analysis of an open-label randomised trial. Lancet Neurol. 2018;17:211–212. doi: 10.1016/S1474-4422(18)30035-8. [DOI] [PubMed] [Google Scholar]
- Yaar I., Shapiro M.B. A quantitative study of the electroencephalographic response to levodopa treatment in parkinsonian patients. Clin. Electroencephalogr. 1983;14:82–85. doi: 10.1177/155005948301400207. [DOI] [PubMed] [Google Scholar]
- Zahodne L.B., Fernandez H.H. Pathophysiology and treatment of psychosis in Parkinson's disease: a review. Drugs Aging. 2008;25:665–682. doi: 10.2165/00002512-200825080-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material