Abstract
Objective
Hyaline cartilage degenerative pathologies induce morphologic and biomechanical changes resulting in cartilage tissue damage. In pursuit of therapeutic options, electrical and mechanical stimulation have been proposed for improving tissue engineering approaches for cartilage repair. The purpose of this review was to highlight the effect of electrical stimulation and mechanical stimuli in chondrocyte behavior.
Design
Different information sources and the MEDLINE database were systematically revised to summarize the different contributions for the past 40 years.
Results
It has been shown that electric stimulation may increase cell proliferation and stimulate the synthesis of molecules associated with the extracellular matrix of the articular cartilage, such as collagen type II, aggrecan and glycosaminoglycans, while mechanical loads trigger anabolic and catabolic responses in chondrocytes.
Conclusion
The biophysical stimuli can increase cell proliferation and stimulate molecules associated with hyaline cartilage extracellular matrix maintenance.
Keywords: hyaline cartilage, articular cartilage, growth plate, electrical stimulation, mechanical stimuli
Introduction
Hyaline cartilage is an avascular tissue characterized by having abundant extracellular matrix (ECM) that is mainly composed of collagen type II and proteoglycans (PG).1-3 This tissue is formed by a unique cellular type, the chondrocyte, which is responsible for maintaining the integrity of the ECM.4,5 Within the skeletal system, hyaline cartilage is found in two specialized structures of the long bones: the articular cartilage and the growth plate.1,5 The first acts as a smooth, lubricated, low-friction surface that receives mechanical loads and facilitates motion between opposing joints6,7; while the second, which is located between the epiphysis and diaphysis, is responsible for the longitudinal growth and shape of long bones.8,9
Histologically, both articular and growth plate cartilages are tissues stratified in different zones according to chondrocytes phenotype and spatial arrangement of the ECM. Accordingly, articular cartilage is organized in 5 zones: superficial, intermediate, medium, deep, and calcified,10 whereas the growth plate is arranged into 3 zones: reserve, proliferative, and hypertrophic.11 In both tissues, the molecular structure of the ECM within the hyaline cartilage provides dimensionality, elasticity and strength to the tissue.3,10
Given its location, hyaline cartilage is exposed to mechanical loads that generate different signals implicated in the physiological regulation of chondrocytes behavior and several pathological processes, considering that chondrocytes sense and respond to different mechanical loads.2,12-14 For example, mechanical loading has been shown to affect cell deformation, fluid flow, nutrient and ion concentration gradients, pH changes, and anabolic and catabolic activity of ECM components and stimulation of growth factor synthesis.15-20 In turn, overweight or strong impact loading have been proven to cause damage in the ECM and chondrocyte apoptosis, resulting in dysfunction of the tissue and subsequent damage.6,13,21,22 Several studies have shown that hyaline cartilage behavior is also affected by electrical and electromagnetic stimuli, resulting in changes in cell dynamics, such as migration,23 differentiation,24-26 morphology,27-30 proliferation,31-36 and gene expression.37-50 This implies that mechanical, electrical, and electromagnetic stimulation may have an important role in the regulation of hyaline cartilage in both normal and pathological conditions.
Based on the above, understanding the influence of biophysical stimuli in the biology of hyaline cartilage not only provides useful information regarding their role in cartilage physiology in vivo but also supplies new tools for tissue engineering and regenerative medicine focused on the develop of new therapeutic approaches for the treatment of injuries in articular cartilage and growth plate. In this context, we performed a review of literature of the past 40 years of in vivo and in vitro studies, highlighting their results, advances, main limitations, and perspectives. The information presented summarizes the current understanding about the role of both the electrical stimulation over chondrocytes intended for articular cartilage recovery and the mechanical stimuli over growth plate and its influence on biological events during human bone development.
Electrical Stimulation for Tissue Engineering Hyaline Cartilage
Electric Fields
Electrical stimulation has been used to improve chondrocyte proliferation rate and the synthesis of characteristic ECM molecules. Electric fields (EFs) for cartilage regeneration have been used in in vivo and in vitro systems ( Table 1 ).31,35,38,46,51-53
Table 1.
Summary of Electric Field (EF) Application to In Vivo and In Vitro Systems.
| Study Model | Chondrocytes Isolation Source or Joint Treated | Tension | Current | Frequency (kHz) | Electric Field | Stimulation Time | Effect on Cell Proliferation | Effect on Matrix Synthesis | Reference |
|---|---|---|---|---|---|---|---|---|---|
| In vivo | Femoral condyles of New Zealand white rabbits | 15-500 mV | 6 nA | — | — | 1-9 weeks | Increase in cellular response, proliferation, and matrix production. | Baker et al.52 | |
| In vivo | Human knee with osteoarthritis | 0-12 V | — | 0.001 | — | From 16 to more than 600 days | Pain relief and a decrease in anti-inflammatory drug use. Moreover, the therapy delayed the need of surgical intervention in chronic patients | Farr et al.53 | |
| In vitro | Epiphysis of embryo chicken tibia | 1166 V for peer review | — | 0.005 | — | 6 hours | Cell membrane depolarization controls proliferation process | — | Rodan et al.54 |
| In vitro | Bovine costochondral joint | Part A (5-13, 5-33, 7-85) Vrms | 105, 300, 750, and 1890 mA/cm2 | 60 sinusoidal | 7, 20, 50, and 126 V/cm | 1 day | Increase in proliferation under an EF of 20 mV/cm | — | Brighton et al.32 |
| In vitro | Part B (13, 5) Vrms | 300 μA/cm2 | 60 sinusoidal | 20 mV/cm | 1 day | The cycles in an output signal (100%, 25%, 2%, and 0.25%) are no relevant parameters, hence changes in cell proliferation and matrix synthesis were no found | |||
| In vitro | Metacarpophalangeal epiphysis of a bovine | — | 2, 4, and 24 mA/cm2 | — | — | 6 hours | — | Increased levels of collagen type II and aggrecan with an EF of 20 mV/cm during 30 minutes | Wang et al.39 |
| In vitro | Distal and proximal extremities from humerus, scapulae and femurs of 2-day-old Wistar rats | 50 and 100 V | — | 60 sinusoidal | 4 and 8 mV/cm | 30 minutes, 1 and 5 hours during 1 week | Increase of proliferation with an EF of 4 mV/cm | Increase in glycosaminoglycans synthesis with an EF of 8 mV/cm | Vaca-González et al.55 |
| In vitro (cartilage explant) | Articular cartilage explant from the patellae of adult cows | — | — | 60 sinusoidal | 20 mV/cm | 1 hour and 30 minutes each for 6 hours | — | Increase in proteoglycan (34%) and collagen type II (74%) | Brighton et al.37 |
| In vitro (3-dimensional culture) | Metacarpophalangeal joint of steers + agarose gel | 0-12 V | — | 0.1 | — | 6 and 12 hours | There is no influence over protein synthesis, cell proliferation and mRNA expression levels | Akanji et al.46 | |
| In vivo (growth plate) | Epiphyseal plate from rabbit femur | 1.5 V | 20 μA | — | — | 6 weeks | Increased thickness of the proliferative zone and also the columnar cells arranged was longer compared with the controls | Forgon et al.56 | |
| In vivo (growth plate) | Proximal tibia of the rabbit | 2.5, 5, 10, 20 V | 1.24, 1.19, and 1.53 mA | 60 sinusoidal | 16.5, 33, 66, and 132 mV/cm | 48 hours | Increase in longitudinal growth of the proximal tibia with 3.3 mV/cm | Brighton et al.57 | |
In Vivo studies
The first reported use of electrical stimulation to influence cartilage was in 1974 by Baker et al.52 who assessed the influence of a bimetallic platinum electrochemical device in the recovery of full-thickness injuries of articular cartilage. The electrochemical device was implanted in the lateral femoral condyle at the location of the injury and voltages from 15 to 500 mV were applied. Results showed a 71% of increase in the production of ECM in treated animals.52 This finding set the basis for the application of electrical therapy in patients presenting with joint pathologies. The experience reported up to now in the literature has evidenced that the application of the EFs lead to pain improvement in patients with knee osteoarthritis. For instance, Farr et al.53 used a transcutaneous electric stimulator in 288 patients with knee osteoarthritis. Patients who received electrical stimulation for more than 750 hours (73% of total population) reported both pain relief and a decrease in anti-inflammatory drug use (45.3%). Moreover, the therapy delays the need of a surgical intervention in chronic patients.53
In Vitro Studies
The technique of delivering electrical stimulation to cell culture plates consists of an indirect coupling system that uses external parallel electrodes connected to a power supply ( Fig. 1A ).31,38,39,54 The custom-designed devices are noninvasive capacitive coupling systems that can be used to stimulate any cellular type, and they are based on the same principle used in medical stimulation devices.51,53,54
Figure 1.
Schematic diagram of an indirect coupling system to stimulate in vitro cell cultures. (A) Configuration to apply electric fields (EFs) to the culture medium between the parallel electrodes. (B) Configuration to apply electromagnetic fields (EMFs) to the culture medium between the parallel electrodes.
In vitro, the application of EFs to monolayer cultures has shown to increase cell population and to stimulate synthesis of main molecules that compound cartilage such as collagen type II, glycosaminoglycans (GAGs), and aggrecan. For instance, Rodan et al.55 demonstrated that increased chondrocyte proliferation observed after EFs stimulation was triggered by a depolarization in the cell membrane, it means that the Na+ and Ca2+ fluxes generated by the EFs trigger DNA synthesis of chondrocytes. More descriptive studies were carried out by Brighton et al.,34 in which they stimulated chondrocytes with different voltage values (10, 100, 250, and 1000 V) to a frequency of 60 kHz (sinusoidal-wave form). Their results indicated that voltages of 10 and 1000 V decreased cell proliferation; while a voltage of 250 V increased the GAGs synthesis.34 In these studies, no data about the magnitude of the EFs were reported.
Similar studies were performed by Armstrong and Brighton where chondrocytes were stimulated with several EFs (15, 22.5, 30, and 45 mV/cm). Their results demonstrated an increase in cell population when EFs of 15, 22.5, and 30 mV/cm were applied. An increase in GAGs synthesis (66%) was obtained after stimulation with EFs of 45 mV/cm.31,33 From these studies, it was possible to conclude that an EF close to 20 mV/cm stimulates chondrocyte dynamics. Brighton et al.32 in later studies reported that an EF of 20 mV/cm increases the cell proliferation by 50%; while Wang et al.39 in a more detailed study elucidated that this EF of 20 mV/cm increases aggrecan and collagen type II synthesis. On the contrary, researchers such as Nakasuji et al.35 have applied EFs of greater magnitude (50, 100, 250, and 500 mV/cm) to chondrocytes cultured in vitro. The results showed that EFs of greater magnitude also affect cell proliferation and ECM synthesis.35 Because of the discrepancy regarding the magnitude of the EFs required to stimulate the in vitro cultures, Vaca-González et al.54 implemented a new methodology to calculate EFs and modulate proliferation and GAGs synthesis. Results allowed to establish the dielectric constant of the cell culture medium (complex permittivity and conductivity), and also showed that EFs of 4 mV/cm applied during 30 minutes increment cell population; while EFs of 8 mV/cm applied during 5 hours maintain a stable GAGs synthesis.54
Studies Performed in Explants and 3-Dimensional Cartilage Constructs
The EFs have also been applied to cartilage explants and tridimensional constructs.56 Brighton et al.37 stimulated cartilage explants with EFs of 20 mV/cm. Results showed an increase in PG (34%) and collagen type II (71%) after 14 days of stimulation.37 Because EFs of 20 mV/cm have been shown to influence the cellular dynamics, the research group of Brighton studied the effect of the electrostimulation in osteoarthritic human cartilage explants. In this work, osteoarthritic explants stimulated for 14 days using 20 mV/cm EFs showed 1.4- and 1.5-fold increase in PG and collagen type II, respectively.38 EFs have been also applied to 3-dimensional (3D) cartilage constructs used for cartilage repair; however, to our knowledge, the reports in literature are limited and the results have been contradictory. For instance, Szasz et al.45 stimulated 3D chondrocyte-seeded agarose gels, obtaining increases in cell density and GAGs synthesis. In contrast, Akanji et al.46 investigated the effects of direct current on cell proliferation and matrix synthesis, using a 3D chondrocyte-agarose model system. Their results demonstrated that EFs have no influence over protein synthesis, cell proliferation, and mRNA expression levels.46
Effect of EFs Over the Growth Plate
Applying electrical stimulation across growth plate cartilage has little effect on composition,57 but can alter the thickness of zones within the cartilage,57,58 and ultimately the length of the bone.57,59 However, high voltage reduces bone growth and maintains cartilage in a quiescent state.59
Electromagnetic Fields
Similar to EFs, electromagnetic fields (EMFs) have been applied in in vivo and in vitro studies using cartilage and growth plate explants, and tridimensional structures ( Table 2 ).27,28,30,42,44,49,60 Similar to EFs, the technique of deliver EMFs consists of an indirect coupling system that uses external parallel coils connected to a function generator ( Fig. 1B ).28,29,47,60 This kind of noninvasive configuration has the advantage of generating small currents and potentials in the proximity of the targeted cells, and also it generates a homogeneous EMF across the cell culture.51
Table 2.
Summary of electromagnetic Field (EMF) Application to In Vivo and In Vitro Cartilage Explants and Scaffolds.
| Study Model | Chondrocytes Isolation Source | Frequency (Hz) | EMFs | Stimulation Time | Effect on Cell Proliferation | Effect on Matrix Synthesis | Reference |
|---|---|---|---|---|---|---|---|
| In vivo | Knee of Hartley guinea pigs | 1.5 | 1.0 G | 1 hour/day for 6 months | Articular cartilage morphology was preserved. The EMF retarded the development of osteoarthritic lesions | Ciombor et al.28 | |
| In vivo | Joint knee from Hartley guinea pigs | 75 | 1.5 mT | 6 hours a day for 3 months | The EMFs preserve the morphology of articular cartilage and the thickness of the medial tibia plateaus | Fini et al.61 | |
| In vitro | Femoral condyles of human knee | 10, 100, 370, 500 | 0.74, 0.85, 1.69, 3.14, 5.42 (mT peak) | 6 h | Magnetic field strengths of 1.7 and 3.1 mT peak with frequencies of 500 and 100 Hz maintained chondrocyte spherical morphology | Jahns et al.29 | |
| In vitro | Human articular cartilage chondrocytes | 75 | 2.3 mT | 1, 6, 9, and 18 hours | — | 9- and 18-hour stimulation leads to increased DNA and PG synthesis | De Mattei et al.36 |
| In vitro | Porcine articular cartilage | 5 pulses of 100 ns | 10 or 20 kV/cm | 1, 3, and 7 days | Significantly increase in cell proliferation | A decrease in glycosaminoglycan, collagen II, Sox9, and aggrecan gene expression was observed | Zhang et al.30 |
| In vitro (cartilage explant) | Metacarpophalangeal joints of bovine | 75 | 1.5 mT | 24 hours | — | 20% increase in proteoglycan synthesis | De Mattei et al.42 |
| In vitro (cartilage explant) | Metacarpophalangeal joints of bovine | 75 | 2.3 mT | 24 hours | — | EMFs are able to promote anabolic activities and proteoglycan synthesis | De Mattei et al.47 |
| In vitro (osteoarthritic cartilage explants) | Human femoral condyles | 75 | 1.6 mT | 4 hours daily for 4 days | Increment in DNA content | No changes in glycosaminoglycans were evidenced | Sadoghi et al.40 |
| In vitro (3-dmensional culture) | Human chondrocytes cultured in a type-I collagen gel | 16.7 | 2 mT | 1, 3, 7, and 14 days | — | Positive staining for collagen II and proteoglycan in the immediate pericellular region | Schmidt-Rohlfing et al.43 |
| In vivo (growth plate) | Male albino Wistar rats | 900 and 1800 MHz | — | 12 weeks | Stimulated rats experienced a more rapid weight gain and increase in length. Rats showed a loss in in the reserve zone, with an increase in thickness in the proliferative zone | Nisbet et al.67 | |
| In vivo (growth plate) | Right hind limb of a rabbit | — | 1.5 mT | 10 minutes per day over 15 days | EMFs exert a suppressive influence on chondrocyte proliferation | — | Arsen’ev et al.68 |
In Vivo Use of EMFs to Heal Osteoarthritis
In vivo animal models have been used to study the influence of EMFs in the recovery of knee osteoarthritis. Ciombor et al.28 showed that articular cartilage morphology was preserved and the development of osteoarthritic lesions were retarded when EMFs were applied. EMFs have also been implemented in clinical studies to treat knee osteoarthritis. Results have evidenced that electromagnetic stimulation contributes to preserve articular cartilage morphology,60,61 improvement in joint motion, tenderness, and a reduction in pain.62-65
In Vitro Studies Using Cell Cultures
The application of EMFs in monolayer cultures have shown preservation of chondrocytes morphology,29 increased DNA synthesis,36 and enhancement of proliferation and GAGs synthesis in human chondrocytes.66 It has also been demonstrated that EMFs significantly increase cell population, but decrease the synthesis of characteristic molecules of hyaline cartilage such as collagen type II, aggrecan, SOX 9, and GAGs.30
Studies Performed in Explants 3-Dimensional Cartilage Constructs
Healthy and pathological tissue explants, have also been treated with EMFs.40,42,49 The use of EMFs over cartilage explants have shown increase in PG synthesis and stimulation of anabolic activities,42,47 while the effect of EMFs in osteoarthritic explants have evidenced an improvement in osteoarthritis of grade I and III, increasing PG synthesis and counteracting catabolic activities.49 However, it was also shown that EMFs have no effect on DNA and PG synthesis.40 The application of EMFs has also been investigated in osteoarthritic chondrocytes cultured in 3D structures. Results showed that the PG concentration of osteoarthritic chondrocytes cultured in alginate scaffolds was stored in the cell culture medium after stimulation was finished.27 Normal articular chondrocytes cultured in 3D constructs were also stimulated with EMFs. In studies performed by Schmidt-Rohlfing et al.43 and Nicolin et al.,44 the chondrocytes that were cultured in a collagen membrane showed that this scaffold is a useful bioengineering construct since allowed increases in cell proliferation, collagen type II synthesis, and PG in the immediate pericellular region.
Effect of the EMFs Over the Growth Plate
Studies have been carried out to study the effects of EMFs over growth plate development. A recent study showed that male albino rats stimulated with EMFs experienced a more rapid weight gain and increase in length. Moreover, the stimulated rats showed a loss in cartilage matrix density in the reserve zone, with an increase in thickness of the reserve and proliferative zones. In addition, measurements of the growth plate thickness showed that the trabecular zone was thinnest and the reserve and proliferative zones were thickest. There were no significant differences between the groups with respect to the thickness of the hypertrophic zone.67 A similar study evidenced that EMFs exert a suppressive influence on chondrocyte proliferation in the growth plates and promote expansion of the differentiate fraction.68 The epiphyseal plate has been also stimulated through in vitro cultures; for instance, the costochondral junction growth plate of a rat was exposed to different EMFs and changes of temperature. Results indicated that longitudinal growth of the costochondral junction was only observed when EMFs were applied in an environment where the temperature increased.69
Mechanical Stimulation for Tissue Engineering Hyaline Cartilage
Compressive Loads
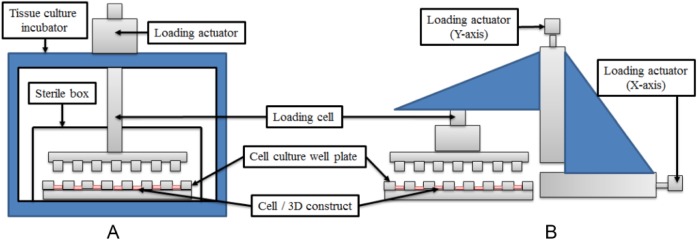
Because of the fact that hyaline cartilage, especially the one found in joints and growth plate, is mainly subjected to compressive mechanical stimuli,22,70,71 several in vivo and in vitro studies have been performed in order to identify the effects of mechanical compression in cell dynamics (proliferation, hypertrophy, and apoptosis),1,72-79 cell height,72,74,80 and the biosynthetic behavior of chondrocytes, assessed mainly in terms of expression of molecules of the ECM (collagen types II and X and aggrecan) and some enzymes (MMP 13 and ADAMTS-4/5).81,82 In addition, changes in the expression of metalloproteinase inhibitors (TIMP-1 and TIMP-2), growth factors (VEGF), and other molecules are influenced by load ( Table 3 ).16,79,83-85 Up to now the specific mechanisms involved in mechanotransduction are not well understood; however, 2 kinds of proteins have been involved in these processes: integral membrane proteins associated in ECM interactions (e.g., integrins) and mechanosensitive ion channels.86-89 Furthermore, signaling mediated by the primary cilium and MAPK pathway have also been implicated.90-95 Regarding the devices used to apply compressive loads, the technique of deliver mechanical loads consist of a system that uses loading actuators that exert an axial force ( Fig. 2A ).7,92,94 This devices present an advantage of maintain the cell cultures in atmospheric conditions of 37°C and 5% CO2 while mechanical loads are been applied.96
Table 3.
Summary of Some Representative Studies Using Compressive Loads on Hyaline Cartilage.
| Study Model | Chondrocytes Isolation Source | Load Magnitude | Frequency (Hz) | Application Scheme | Stimulation Time | Results | Reference |
|---|---|---|---|---|---|---|---|
| In vivo | Growth plates of young rats, rabbits, and calves | 0.1 MPa | — | Compression and distraction were applied over 1 week | — | Reduced growth rate with compression and increased growth rate with distraction | Stokes et al.74 |
| In vivo | Sprague-Dawley rats | 0.2 MPa | — | Cyclic compression was applied at 1, 8, or 15 days after seeding | 2 weeks | Loading reduced bone growth and no changes were observed in the synthesis of aggrecan, collagen II and collagen X | Cancel et al.82 |
| In vivo | Adult female New Zealand White rabbits | Between 1 and 2 MPa | 1 | 80 cumulative hours in 2-hour increments | 3 days a week for 14 weeks | Increment in cartilage deep zone and PG synthesis. No changes in collagen were observed | Saadat et al.84 |
| In vivo (growth plate) | Distal ulnae from 4-week-old swine | 1 N | — | A prestrain (5%) was first applied, followed by a stress relaxation test (10% strain) | — | Under compression the cell/matrix volume ratio decreased in the reserve and hypertrophic zones, whereas it increased in the proliferative zone | Amini et al.80 |
| In vivo (growth plate) | Distal ulnae of 4-week-old swine | Strain rate of 1.5E−03 s−1 | 0.1 | The samples were subjected to a 2% strain preload. The static loading samples underwent an additional 10% strain. The dynamic loading samples were submitted to an additional cyclic strain oscillating between 7% and 13% strain | 48 hours of stimulation | With static loading cellular columnar organization was preserved, but a loss in aggrecan, type II and type X collagens synthesis was denoted. With dynamic loading, a loss of columnar arrangement was observed in the proliferative and hypertrophic zones, but it contributed to the synthesis of aggrecan and type II collagen | Sergerie et al.81 |
| In vitro (cartilage explant) | Adult articular cartilage from the bovine humeral head | 10 kg | 0.001, 0.5, 1 | At 0.5 Hz and the sine wave amplitude was varied between 5% and 20% of the articular cartilage. The sine wave amplitude was fixed at 10%, while the frequency was varied between 0.001 and 1.0 Hz | 45 hours | The oscillatory loading increased protein synthesis, but had an inhibitory influence on PG synthesis. Static compression caused a significant increase in fibronectin synthesis | Wong et al.101 |
| In vitro (cartilage explant) | Articular cartilage explants from 18- to 24-month-old steers | 0.1, 1.0, 2.5, or 5.0 MPa | 0.001, 0.01, 0.1, or 0.5 | Continuously applied, uniaxial cyclic loading was applied for 2 hours | 1, 3, or 6 days | When the load was increased, the proteoglycan synthesis was decreased | Steinmeyer et al.102 |
| In vitro (3-dimensional culture) | Femoropatellar from 1- to 2-week-old calves | — | 0.001, 0.01, 0.1, and 1 | 6 hours under static compression; 10 hours under dynamic compression | From 2 to 43 days | Molecular synthesis decreased when static load was applied while with dynamic loading the synthesis was enhanced. Loading enhanced glycosaminoglycans production | Buschmann et al.104 |
| In vitro (3-dimensional culture) | Metacarpal-phalangeal joints of 2-t o 3-year-old free-range steers | — | 0.3 and 1 | Din unconfined compression for 30 minutes on and 90 minutes off for 16 hours | 2 weeks | Contrarily, deposition of larger matrix. Molecules of aggrecan and collagen II was either not affected or inhibited by loading | Farnsworth et al.106 |
Figure 2.
Schematic diagram of the devices used to apply mechanical loads to chondrocytes cultured in 3-dimensional (3D) structures. Both schemes have in common a loading cell, a cell culture well plate and the 3D construct. (A) Configuration to apply compressive loads to 3D cell cultures. This device uses a tissue culture incubator and a sterile box to maintain cell cultures in humidified atmosphere. (B) Configuration to apply compressive and shear stress to the chondrocyte culture. This device uses two loading actuators to apply loads in the X and Y axis.
In Vivo Studies
To understand the effect of mechanical loading on chondrocytes behavior within the tissue, some in vivo studies have been performed in both articular cartilage and growth plate. The studies performed in articular cartilage have mainly focused in analyzing the synthesis of ECM molecules as PG, where a direct correlation between loading in the joint and PG levels within the tissue was observed.7,16,84,95 Several studies performed in the growth plate have assessed the effect of mechanical loading as a regulator of bone growth and ossification. Such behavior has been recognized by the Hueter-Volkman law, which establishes that static compression is crucial for bone development; however, excessive loads may inhibit the normal bone growth. Moreover, alterations of mechanical modulation have been associated to several clinical conditions, including the angular progression deformities in tibia (Blount disease), and the development of scoliosis.72,73 Studies using chicks and rodents have demonstrated that mechanical stimulation of bone rudiments during embryological development plays an important role in normal growth and bone morphogenesis.97
At cellular level, studies using rodents have shown that mechanical loading induces histological changes within the growth plate, specifically in the width of the proliferative and hypertrophic zones.74-77,98 Additionally, it has been observed a decrease in collagen type II and aggrecan concentrations in growth plates subjected to long term static compressions.82
In Vitro Studies: Tissue Cultures
As an alternative model to study the influence of mechanical loading over the chondrocytes behavior, in vitro protocols applying compressive loads to articular cartilage and growth plate explants have been performed. For articular cartilage, the reported results of ECM molecules synthesis under dynamic loading are contradictory with some studies showing an increase while others indicate either a decrease or no changes after loading.70,71,79,84,99-102 For growth plate, studies using tissue explants have shown differential morphological changes of chondrocytes in the different zones under compression.80 Additionally, under short-term compressive loads, a decrease in aggrecan, collagen type II and X has been reported. In contrast, under dynamic loading no changes in ECM composition although changes in cell organization were observed.81
In Vitro Studies: Cell Cultures
Given the difficulties for analyzing the individual effects of mechanical stimuli to which cartilage is subjected in vivo, the response of chondrocytes has been evaluated in monolayer and 3D cultures.16,22,70,71,93,101,102 These analyses not only provide information about the cellular responses and the regulatory mechanisms associated, but also they allow to evaluate the use of mechanical stimuli in tissue engineering approaches.16 The effect of compressive loads in in vitro cultures of chondrocytes depends up on load’s intensity and duration 7. In this context, it has been observed that the application of static loads increased the production of several types of MMPs, and also inhibited the synthesis of collagen type II and PG.7,95 In turn, the effects of dynamic loading in cell proliferation and synthesis of PG, GAGs, and collagen type II are contradictory.7,70,71,79,84,95,96,99-118 This behavior may be related to the wide variability of the methodology used for each group, which can be related to different sources of chondrocytes, age of the animal model, characteristics of 3D matrix used, chondrocyte seeding density, frequency of the stimulus, load cycles, and initiation and duration of the stimulation.7,84,96,101,103-108,115
Tension
The chondrocytes response to tensile loading have also been addressed using in vitro monolayer cultures. Such cultures have been performed by seeding cells in membranes that are submitted to uniaxial or biaxial dynamic stretching. Most of the studies have evaluated chondrocyte dynamics in terms of cell proliferation and molecular synthesis of collagens and PG. Similar to that observed for compressive loading, the evidence available suggests that the response of chondrocytes to tensile loading also depends on the duration and intensity of the stimulation.18,119-121 In fact, anabolic effects in chondrocytes are induced after short-term simulation (less than 12 hours); while a decrease in the ECM molecules is only observed after a prolonged stimulation.18 In addition, some studies have reported an increase of other molecules such as proteases and their inhibitors (e.g., TIMPs, MMPs), soluble factors (e.g., TGFβ, VEGF, PTHrP), and pro-inflammatory factors (e.g., nitric oxide, prostaglandin E, cyclooxygenase 2) after tensile loading stimulation.18,93,119,121-123
However, it is difficult to extrapolate definitive conclusions regarding the stimulus characteristics and chondrocytes response considering that, similarly to compressive loading, there is a high variability in the stimulation protocol used among different studies in terms of strain magnitude, loading duration and frequency (reviewed in Bleuel et al.18).
Hydrostatic Pressure
In Vitro Studies: Tissue Cultures
Hydrostatic pressure (HP) has been extensively used as a stimulus to induce changes over hyaline cartilage such as protein expression and matrix production.124 However, the mechanism by which HP produce those changes is not well understood. HP provides a robust method of chondrocyte stimulation since it can be applied over cells cultured in monolayer, 3D engineered constructs or cartilage explants ( Table 4 ).125,126 Several studies have shown the relation between HP and biological response of hyaline cartilage, such as investigations performed by Parkkinnen et al. have shown a microtubule-dependent compaction of the Golgi apparatus and decrease in GAGs synthesis 127. In a similar study, Lammi et al.128 found that static HP resulted in a 37% decrease in GAGs synthesis, reduction of aggrecan mRNA levels and synthesis of atypically large aggrecan molecules. The alteration in aggrecan size demonstrates that HP may affect the production of ECM molecules at posttranslation levels.
Table 4.
Summary of Some Representative Studies Using Hydrostatic Pressure on Hyaline Cartilage.
| Study Model | Chondrocytes Isolation Source | Load Magnitude | Frequency (Hz) | Application Scheme | Stimulation Time | Results | Reference |
|---|---|---|---|---|---|---|---|
| In vitro | Bovine chondrocytes | 0.8 atm | — | 5 minutes on and 30 minutes off | 10 times | 40% increase in proteoglycan synthesis, enhanced aggrecan mRNA | Suh et al.126 |
| In vitro | Adult bovine chondrocyte | 10 MPa | 1 | 2, 4, 8, 12, and 24 hours | 1 day | Increased aggrecan mRNA up to 24 h, maximal increase in collagen II mRNA with 4- and 8-h application. | Smith et al.132 |
| In vitro | Adult bovine chondrocyte | 10 MPa | 1 | 4 hours | 4 days | Significant increase in aggrecan and collagen II mRNA. | Smith et al.133 |
| In vitro | Adult bovine chondrocyte | 1, 5, 10 MPa | 1 | 4 hours | 4 days | Enhanced aggrecan expression with all treatments, enhanced collagen II expression only with 5 and 10 MPa, for 4 days | Ikenoue et al.134 |
| In vitro (3-dimensional culture) | Adult P3 equine chondrocytes | 3.44 or 6.87 | 0.25 | 20 minutes every 4 hours | 3, 4, and 5 weeks | Both magnitudes increased glycosaminoglycan production; only 6.87 MPa increased collagen production. | Carver and Heath129 |
| In vitro (3-dimensional culture) | Immature bovine chondrocytes | 10 | 1 | 4 hours per day | 8 weeks | Increased collagen production at 4 weeks and 8 weeks | Hu and Athanasiou125 |
| In vivo (cartilage explant) | Adult bovine chondrocytes | 5 | 0.5 | 1.5 hours | — | Enhanced sulfated glycosaminoglycan incorporation | Parkkinen et al.131 |
Studies Performed in Cartilage Explants and 3-Dimensional Constructs
HP also has been applied over 3D scaffolds and explants. For instance, Carver and Heath129,130 showed that chondrocytes cultured in poly glycolic acid (PGA) meshes and stimulated with 6.87 MPa experienced an increase in GAGs and collagen production in adult cells, whereas the same magnitude of HP had an increase only collagen synthesis in juvenile cells. There are no differences of applying HP either in cartilage explants or in chondrocytes cultured in vitro; however, the magnitude of the pressure and the frequency are relevant factors to stimulate PG synthesis.131
Shear Stress
Within the joint and growth plate chondrocytes are exposed either to compressive HP or deviatory stresses such as shear stress.135 Previous studies have indicated that chondrocytes are exposed to different levels of fluid flow within the tissue,136,137 suggesting that mechanical shear stress has a pathophysiologic relevance in cartilage biology.138 Within this context, several studies have analyzed the influence of such stimuli on chondrocyte behavior. For instance, Mohtai et al.139 applied shear stress over human articular chondrocytes seeded in a high monolayer culture. Cell cultures were stimulated with loads of 0.16, 0.41, 0.82, and 1.64 Pa at rotating velocities of 20, 50, 100, and 200 rpm. Results indicated that this stimulation scheme increased the release of pro-inflammatory mediators and nitric oxide, decreased the expression of aggrecan and collagen type II, and induced molecular changes associated with apoptosis.139 Additionally, it is known that hyaline cartilage tissue engineering constructs, are also affected by shear stress, revealing that this stimulus may alter the intercellular signaling pathways in chondrocytes.129,134 Some reports suggest that fluid shear stress reduces expression of aggrecan and collagen type II.140
Other studies, like those developed by Waldman et al.141 used a stimulation scheme based on 2% of shear stress amplitude at 1 Hz for 400 cycles every second day, demonstrate that an intermittent application of dynamic shearing forces during 4-week periods improved the quality of the cartilaginous tissue formed in vitro. These data indicate that the nature and magnitude of shear stress may play a significant role in the homeostasis of the structure and function of hyaline cartilage. For this reason, the cellular mechanisms underlying the responses of articular chondrocytes to mechanical stresses are important for understanding the pathogenesis of several hyaline cartilage diseases.138 Similar to compressive loads, the technique of deliver the shear stress consists of a loading actuator that generate forces along the X and Y axis at the same time ( Fig. 2B ).139,141 In this context, this configuration allows one to apply precise strains on multiple axis similar to the mechanical environment supported by the joint.142
Cellular Mechanisms Involved in Transduction of Biophysical Stimuli
In chondrocytes, biophysical stimuli are sensed mainly by membrane proteins involved in ECM-cell interactions and ionic channels.7,143,144In addition, some stimuli may induce changes in the cytoskeleton.23,145
ECM-cell interactions are associated mainly with the transduction of mechanical stimuli, since they allow cells to sense conformational changes of the ECM that trigger, intracellularly, the activation of different cell signaling cascades leading to specific cell responses.7,143 The main proteins associated with ECM-cell interaction are integrins, integral membrane proteins that bind to several ECM molecules with different affinities.146-148 The main integrins expressed by chondrocytes are α1β1, α3β1, α5β1, α10β1, αVβ3, and αVβ5, which act as receptors for collagen types VI and II, matrilin-1, fibronectin, osteopontin, COMP, and vitronectin, respectively.87,88,147-149 Cytoplasmic domains of integrins are coupled to kinases that have been implicated in signal transduction through Ras, Rho, and Rac pathways.93,95 In addition to integrins, other membrane proteins like CD44 and annexin V (also known as anchorin CII) have also been associated in ECM-cell interactions in chondrocytes. CD44 is a transmembrane hyaluronan-binding glycoprotein that is involved not only in anchoring but also in sensing and signaling functions.149-151 Annexin V is a member of a family of calcium and phospholipid binding proteins that binds mainly with collagens present in the PCM and ECM, mainly type II and with lesser affinity to types V, IX, X, and XI.150-152
On the other hand, ion channels have been associated to both mechanical and electrical signal transduction. Although a plethora of ion channels have identified in chondrocytes membranes, calcium channels are the main type of ion channels associated to transduction of biophysical stimulation.86,89,95 The calcium channels most associated to these processes include voltage-gated sodium channels, voltage-gated calcium channels, and stretch activated ion channels. In addition, various members of the TRPV (transient receptor potential vanilloid) nonselective cationic channels family, especially TRPV4, have been associated to osmotic stress responses.86,89,95 In fact, it has been described that in chondrocytes, cell deformations caused either by changes in cell volume or due to mechanical loading, lead to activation of different kinds of ionic channels, and some studies have evidenced changes in intracellular calcium levels in chondrocytes after mechanical loading.86,91,92,94,95 Electric signals applied either in direct or indirect contact with the cells, exert their effect on the cell membrane by activating the voltage-gated calcium channels leading to increase in the intracellular Ca2+ levels.51,144,153
Finally, as previously mentioned, some stimuli are associated to conformational changes of the cytoskeleton. That is the case of electrical stimulation, which has proven to induce changes in actin filaments causing cytoplasm elongation followed by a perpendicular alignment with regard to the applied EFs.145 Furthermore, electric stimulation has shown to promote cell movement, process known as galvanotaxis.23
Perspectives
This review focuses on the effect of biophysical stimuli in chondrocyte behavior, showing that electric and mechanical stimulation have important roles in physiology of hyaline cartilage. In addition, biophysical stimuli display interesting potential as additional tools for both tissue engineering and regenerative medicine, toward the development of new therapeutic alternatives for cartilage pathologies. The effects of biophysical stimulation on chondrocytes dynamics have been explored in vivo and using several in vitro approximations. In monolayer cell cultures, stimulation with EFs, EMFs, HP, and tension can promote chondrocyte proliferation and molecular synthesis.18,51,61,132,134 Taking this into account, with the current use of scaffolds combined with the application of biophysical stimuli it is possible to obtain tissue constructs that mimic in vivo characteristics of cartilage.
For both electric and mechanical stimuli, different frequencies, intensities, and duration of stimulation schemes have been tested; however, the magnitudes of the stimuli that directly stimulate chondrocytes in those cultures have been poorly explored. Regarding electric stimulation, at present, calculations of cell culture media dielectric constants have been recently reported. These electric constants, such as permittivity and conductivity, are essential parameters to calculate electric and EMFs.54,154 Thus, this leads to a new research area that will allow to implement a better methodology to calculate the EFs and EMFs in a more detailed way. In electrical stimulations, there are many discrepancies in the obtained results because of the fact that one of the limitations is the required high voltage to generate the EFs, the stimulation time to achieve the best rate of proliferation and molecular synthesis, as well as the amount of days that cell cultures need to be under stimulation. For this reason, electrical stimulation for tissue engineering hyaline cartilage still is an open research area. On the other hand, little is known of the mechanical environment and the effects on fluid flow and nutrient availability within cartilage or in vitro 3D cultures of the different types of mechanical stimulation. Recently, there have been some approaches that use computational modeling to approach such issues.155-159 This is a promising field that may improve the understanding of the effects of mechanical stimuli on chondrocyte behavior as well as provide powerful tools for scaffold designs for 3D in vitro culture systems and mechanical stimulation scheme selection. Furthermore, biophysical stimuli could help to enhance cell proliferation, differentiation, and ECM deposition, opening up new possibilities in 3D articular cartilage and growth plate tissues, and allowing to design novel tissue-engineering constructs similar to real life.
Given the good results obtained using some protocols of electrical and mechanical stimulation independently for in vitro hyaline cartilage tissue engineering, the integration of these both methods is currently been studied.50 This leads to a new field of research that will focus on stimulation of cartilage explants or 3D constructs with 2 biophysical stimuli simultaneously. As a novel and potential tool, it would be possible to create special devices that generate distinct electrical and mechanical intensities according to the necessity of a particular treatment. This contributes to tissue engineering because it is noteworthy that biophysical stimuli are the basis for the next generation of cartilage regeneration technology, since those electrical and mechanical stimuli help the development of biomimetic samples that recreate an environment where the functional, structural, and biological features of hyaline cartilage remain stable at the time of cartilage replacement.
Overall, the use of biophysical techniques provides not only new tools to create in vitro biomimetic tissues, but they can also be novel therapeutic approaches that may be implemented in patients with several chronic hyaline cartilage pathologies. The methodologies used to stimulate cartilage regeneration are getting closer and closer to develop technologies that satisfy the requirements of a successful cartilage healing and make that the concept of cartilage tissue engineering can be extrapolated to a purely clinical environment.
Footnotes
Acknowledgments and Funding: The authors gratefully thank the research support from the Biotechnology Institute of the Universidad Nacional de Colombia and the Administrative Department of Science, Techonology and Innovation in Colombia (COLCIENCIAS).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1. Mackie EJ, Tatarczuch L, Mirams M. The skeleton: a multi-functional complex organ: the growth plate chondrocyte and endochondral ossification. J Endocrinol. 2011;211:109-21. [DOI] [PubMed] [Google Scholar]
- 2. Landinez N, Garzón-Alvarado DA, Acosta JCV. Phenomenology work setting for articular cartilage damage. Rev Cubana Ortop Traumatol. 2009;23:1-28. [Google Scholar]
- 3. Quintero M, Monfort J, Mitrovic DR. Osteoartrosis: biología, fosopatología, clínica y tratamiento. Primera ed. Madrid, Spain: Médica Panamericana; 2009. [Google Scholar]
- 4. Ahmed TA, Hincke MT. Strategies for articular cartilage lesion repair and functional restoration. Tissue Eng Part B Rev. 2010;16:305-29. [DOI] [PubMed] [Google Scholar]
- 5. Becerra J, Andrades JA, Guerado E, Zamora-Navas P, Lopez-Puertas JM, Reddi AH. Articular cartilage: structure and regeneration. Tissue Eng Part B Rev. 2010;16:617-627. [DOI] [PubMed] [Google Scholar]
- 6. Bhosale AM, Richardson JB. Articular cartilage: structure, injuries and review of management. Br Med Bull. 2008;87:77-95. [DOI] [PubMed] [Google Scholar]
- 7. Lee C, Grad S, Wimmer M, Alini M. The influence of mechanical stimuli on articular cartilage tissue engineering. In: Ashammakhi N, Reis RL. editors. Topics in tissue engineering. 2006. p. 1-32. E-book. http://www.oulu.fi/spareparts/ebook_topics_in_t_e_vol2/abstracts/alini_0102.pdf
- 8. Karsenty G, Kronenberg HM, Settembre C. Genetic control of bone formation. Annu Rev Cell Dev Biol. 2009;25:629-48. [DOI] [PubMed] [Google Scholar]
- 9. Burdan F, Szumiło J, Korobowicz A, Farooquee R, Patel S, Patel A, et al. Morphology and physiology of the epiphyseal growth plate. Folia Histochem Cytobiol. 2009;47:5-16. [DOI] [PubMed] [Google Scholar]
- 10. Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009;1:461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ham AW, Cormack DH. Ham’s histology. Philadelphia, PA; Lippincott; 1987. [Google Scholar]
- 12. Stemberger R, Kerschan-Schindl K. Osteoarthritis: physical medicine and rehabilitation—nonpharmacological management. Wien Med Wochenschr. 2013;163:228-35. [DOI] [PubMed] [Google Scholar]
- 13. Lories RJ, Luyten FP. The bone-cartilage unit in osteoarthritis. Nat Rev Rheumatol. 2011;7:43-9. [DOI] [PubMed] [Google Scholar]
- 14. Hochberg ZE. Clinical physiology and pathology of the growth plate. Best Pract Res Clin Endocrinol Metab. 2002;16:399-419. [DOI] [PubMed] [Google Scholar]
- 15. Stoltz JF, de Isla N, Huselstein C, Bensoussan D, Muller S, Decot V. Mechanobiology and cartilage engineering: the underlying pathophysiological phenomena. Biorheology. 2006;43:171-80. [PubMed] [Google Scholar]
- 16. Yao H, Wu Y, Lu X. Cartilage mechanobiology. In: Nagatomi JE, editor. Mechanobiology Handbook. Boca Raton, FL: CRC Press; 2011. p. 229-52. [Google Scholar]
- 17. Heegaard JH, Beaupre GS, Carter DR. Mechanically modulated cartilage growth may regulate joint surface morphogenesis. J Orthop Res. 1999;17:509-17. [DOI] [PubMed] [Google Scholar]
- 18. Bleuel J, Zaucke F, Bruggemann GP, Niehoff A. Effects of cyclic tensile strain on chondrocyte metabolism: a systematic review. PLoS One. 2015;10:e0119816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gray ML, Pizzanelli AM, Grodzinsky AJ, Lee RC. Mechanical and physiochemical determinants of the chondrocyte biosynthetic response. J Orthop Res. 1988;6:777-92. [DOI] [PubMed] [Google Scholar]
- 20. Guilak F, Ratcliffe A, Mow VC. Chondrocyte deformation and local tissue strain in articular cartilage: a confocal microscopy study. J Orthop Res. 1995;13:410-21. [DOI] [PubMed] [Google Scholar]
- 21. Kuszel L, Trzeciak T, Richter M, Czarny-Ratajczak M. Osteoarthritis and telomere shortening. J Appl Genet. 2015;56:169-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martel-Pelletier J, Boileau C, Pelletier JP, Roughley PJ. Cartilage in normal and osteoarthritis conditions. Best Pract Res Clin Rheumatol. 2008;22:351-84. [DOI] [PubMed] [Google Scholar]
- 23. Chao PH, Roy R, Mauck RL, Liu W, Valhmu WB, Hung CT. Chondrocyte translocation response to direct current electric fields. J Biomech Eng. 2000;122:261-7. [DOI] [PubMed] [Google Scholar]
- 24. Ciombor DM, Lester G, Aaron RK, Neame P, Caterson B. Low frequency EMF regulates chondrocyte differentiation and expression of matrix proteins. J Orthop Res. 2002;20:40-50. [DOI] [PubMed] [Google Scholar]
- 25. Mayer-Wagner S, Passberger A, Sievers B, Aigner J, Summer B, Schiergens TS, et al. Effects of low frequency electromagnetic fields on the chondrogenic differentiation of human mesenchymal stem cells. Bioelectromagnetics. 2011;32:283-90. [DOI] [PubMed] [Google Scholar]
- 26. Esposito M, Lucariello A, Costanzo C, Fiumarella A, Giannini A, Riccardi G, et al. Differentiation of human umbilical cord-derived mesenchymal stem cells, WJ-MSCs, into chondrogenic cells in the presence of pulsed electromagnetic fields. In Vivo. 2013;27:495-500. [PubMed] [Google Scholar]
- 27. Fioravanti A, Nerucci F, Collodel G, Markoll R, Marcolongo R. Biochemical and morphological study of human articular chondrocytes cultivated in the presence of pulsed signal therapy. Ann Rheum Dis. 2002;61:1032-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ciombor DM, Aaron RK, Wang S, Simon B. Modification of osteoarthritis by pulsed electromagnetic field—a morphological study. Osteoarthritis Cartilage. 2003;11:455-62. [DOI] [PubMed] [Google Scholar]
- 29. Jahns ME, Lou E, Durdle NG, Bagnall K, Raso VJ, Cinats D, et al. The effect of pulsed electromagnetic fields on chondrocyte morphology. Med Biol Eng Comput. 2007;45:917-25. [DOI] [PubMed] [Google Scholar]
- 30. Zhang K, Guo J, Ge Z, Zhang J. Nanosecond pulsed electric fields (nsPEFs) regulate phenotypes of chondrocytes through Wnt/β-catenin signaling pathway. Sci Rep. 2014;4:5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Armstrong PF, Brighton CT, Star AM. Capacitively coupled electrical stimulation of bovine growth plate chondrocytes grown in pellet form. J Orthop Res. 1988;6:265-71. [DOI] [PubMed] [Google Scholar]
- 32. Brighton CT, Jensen L, Pollack SR, Tolin BS, Clark CC. Proliferative and synthetic response of bovine growth plate chondrocytes to various capacitively coupled electrical fields. J Orthop Res. 1989;7:759-65. [DOI] [PubMed] [Google Scholar]
- 33. Brighton CT, Townsend PF. Increased cAMP production after short-term capacitively coupled stimulation in bovine growth plate chondrocytes. J Orthop Res. 1988;6:552-8. [DOI] [PubMed] [Google Scholar]
- 34. Brighton CT, Unger AS, Stambough JL. In vitro growth of bovine articular cartilage chondrocytes in various capacitively coupled electrical fields. J Orthop Res. 1984;2:15-22. [DOI] [PubMed] [Google Scholar]
- 35. Nakasuji S, Morita Y, Tanaka K, Tanaka T, Nakamachi E. Effect of pulse electric field stimulation on chondrocytes. Paper presented at: Asian Pacific Conference for Materials and Mechanics; November 2009; Yokohama, Japan. [Google Scholar]
- 36. De Mattei M, Caruso A, Pezzetti F, Pellati A, Stabellini G, Sollazzo V, et al. Effects of pulsed electromagnetic fields on human articular chondrocyte proliferation. Connect Tissue Res. 2001;42:269-79. [DOI] [PubMed] [Google Scholar]
- 37. Brighton CT, Wang W, Clark CC. Up-regulation of matrix in bovine articular cartilage explants by electric fields. Biochem Biophys Res Commun. 2006;342:556-61. [DOI] [PubMed] [Google Scholar]
- 38. Brighton CT, Wang W, Clark CC. The effect of electrical fields on gene and protein expression in human osteoarthritic cartilage explants. J Bone Joint Surgery Am. 2008;90:833-48. [DOI] [PubMed] [Google Scholar]
- 39. Wang W, Wang Z, Zhang G, Clark CC, Brighton CT. Up-regulation of chondrocyte matrix genes and products by electric fields. Clin Orthop Relat Res. 2004;427(suppl):S163-73. [DOI] [PubMed] [Google Scholar]
- 40. Sadoghi P, Leithner A, Dorotka R, Vavken P. Effect of pulsed electromagnetic fields on the bioactivity of human osteoarthritic chondrocytes. Orthopedics. 2013;36:e360-5. [DOI] [PubMed] [Google Scholar]
- 41. Cecen B, Kozaci D, Boylu T, et al. Effects of electromagnetic fields on chondrocytes cells of human seeded onto 3D collagen-PLLA scaffolds and Chondro-gide membrane. Sakarya Univ J Sci. 2012;16:213-20. [Google Scholar]
- 42. De Mattei M, Pellati A, Pasello M, Ongaro A, Setti S, Massari L, et al. Effects of physical stimulation with electromagnetic field and insulin growth factor-I treatment on proteoglycan synthesis of bovine articular cartilage. Osteoarthritis Cartilage. 2004;12:793-800. [DOI] [PubMed] [Google Scholar]
- 43. Schmidt-Rohlfing B, Silny J, Woodruff S, Gavenis K. Effects of pulsed and sinusoid electromagnetic fields on human chondrocytes cultivated in a collagen matrix. Rheumatol Int. 2008;28:971-7. [DOI] [PubMed] [Google Scholar]
- 44. Nicolin V, Ponti C, Baldini G, Gibellini D, Bortul R, Zweyer M, et al. In vitro exposure of human chondrocytes to pulsed electromagnetic fields. Eur J Histochem. 2007;51:203-12. [PubMed] [Google Scholar]
- 45. Szasz N, Hung H, Sen S, Grodzinsky A. Electric field regulation of chondrocyte biosynthesis in agarose gel constructs. Paper presented at: The 49th Annual Meeting of the Orthopaedic Research Society; 2003; New Orleans, LA. [Google Scholar]
- 46. Akanji OO, Lee DA, Bader DA. The effects of direct current stimulation on isolated chondrocytes seeded in 3D agarose constructs. Biorheology. 2008;45:229-43. [PubMed] [Google Scholar]
- 47. De Mattei M, Pasello M, Pellati A, Stabellini G, Massari L, Gemmati D, et al. Effects of electromagnetic fields on proteoglycan metabolism of bovine articular cartilage explants. Connect Tissue Res. 2003;44:154-9. [PubMed] [Google Scholar]
- 48. Macginitie LA, Gluzband YA, Grodzinsky AJ. Electric field stimulation can increase protein synthesis in articular cartilage explants. J Orthop Res. 1994;12:151-60. [DOI] [PubMed] [Google Scholar]
- 49. Ongaro A, Pellati A, Masieri FF, Caruso A, Setti S, Cadossi R, et al. Chondroprotective effects of pulsed electromagnetic fields on human cartilage explants. Bioelectromagnetics. 2011;32:543-51. [DOI] [PubMed] [Google Scholar]
- 50. Hilz FM, Ahrens P, Grad S, Stoddart MJ, Dahmani C, Wilken FL, et al. Influence of extremely low frequency, low energy electromagnetic fields and combined mechanical stimulation on chondrocytes in 3-D constructs for cartilage tissue engineering. Bioelectromagnetics. 2014;35:116-28. [DOI] [PubMed] [Google Scholar]
- 51. Balint R, Cassidy NJ, Cartmell SH. Electrical stimulation: a novel tool for tissue engineering. Tissue Eng Part B Rev. 2013;19:48-57. [DOI] [PubMed] [Google Scholar]
- 52. Baker B, Spadaro J, Marino A, Becker RO. Electrical stimulation of articular cartilage regeneration. Ann N Y Acad Sci. 1974;238:491-9. [DOI] [PubMed] [Google Scholar]
- 53. Farr J, Mont MA, Garland D, Caldwell JR, Zizic TM. Pulsed electrical stimulation in patients with osteoarthritis of the knee: follow up in 288 patients who had failed non-operative therapy. Surg Technol Int. 2006;15:227-33. [PubMed] [Google Scholar]
- 54. Vaca-González JJ, Guevara JM, Vega JF, Garzón-Alvarado DA. An in vitro chondrocyte electrical stimulation framework: a methodology to calculate electric fields and modulate proliferation, cell death and glycosaminoglycan synthesis. Cell Mol Bioeng. 2016;9:116-126. [Google Scholar]
- 55. Rodan GA, Bourret LA, Norton LA. DNA synthesis in cartilage cells is stimulated by oscillating electric fields. Science. 1978;199:690-2. [DOI] [PubMed] [Google Scholar]
- 56. Hronik-Tupaj M, Kaplan DL. A review of the responses of two- and three-dimensional engineered tissues to electric fields. Tissue Eng Part B Rev. 2012;18:167-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Forgon M, Vámhidy V, Kellényi L. Bone growth accelerated by stimulation of the epiphyseal plate with electric current. Arch Orthop Trauma Surg. 1985;104:121-4. [DOI] [PubMed] [Google Scholar]
- 58. Sato O, Akai M. Effect of direct-current stimulation on the growth plate. Arch Orthop Trauma Surg. 1989;109:9-13. [DOI] [PubMed] [Google Scholar]
- 59. Brighton CT, Pfeffer GB, Pollack SR. In vivo growth plate stimulation in various capacitively coupled electrical fields. J Orthop Res. 1983;1:42-9. [DOI] [PubMed] [Google Scholar]
- 60. Fini M, Giavaresi G, Carpi A, Nicolini A, Setti S, Giardino R. Effects of pulsed electromagnetic fields on articular hyaline cartilage: review of experimental and clinical studies. Biomed Pharmacother. 2005;59:388-94. [DOI] [PubMed] [Google Scholar]
- 61. Fini M, Giavaresi G, Torricelli P, Cavani F, Setti S, Canè V, et al. Pulsed electromagnetic fields reduce knee osteoarthritic lesion progression in the aged Dunkin Hartley guinea pig. J Orthop Res. 2005;23:899-908. [DOI] [PubMed] [Google Scholar]
- 62. Trock DH, Bollet AJ, Dyer RH, Jr, Fielding LP, Miner WK, Markoll R. A double-blind trial of the clinical effects of pulsed electromagnetic fields in osteoarthritis. J Rheumatol. 1993;20:456-60. [PubMed] [Google Scholar]
- 63. Trock DH, Bollet AJ, Markoll R. The effect of pulsed electromagnetic fields in the treatment of osteoarthritis of the knee and cervical spine. Report of randomized, double blind, placebo controlled trials. J Rheumatol. 1994;21:1903-11. [PubMed] [Google Scholar]
- 64. Pipitone N, Scott DL. Magnetic pulse treatment for knee osteoarthritis: a randomised, double-blind, placebo-controlled study. Curr Med Res Opin. 2001;17:190-6. [DOI] [PubMed] [Google Scholar]
- 65. Nicolakis P, Kollmitzer J, Crevenna R, Bittner C, Erdogmus CB, Nicolakis J. Pulsed magnetic field therapy for osteoarthritis of the knee–a double-blind sham-controlled trial. Wien Klin Wochenschr. 2002;114:678-84. [PubMed] [Google Scholar]
- 66. Sakai A, Suzuki K, Nakamura T, Norimura T, Tsuchiya T. Effects of pulsing electromagnetic fields on cultured cartilage cells. Int Orthop. 1991;15:341-6. [DOI] [PubMed] [Google Scholar]
- 67. Nisbet HO, Akar A, Nisbet C, Gulbahar MY, Ozak A, Yardimci C, et al. Effects of electromagnetic field (1.8/0.9 GHz) exposure on growth plate in growing rats. Res Vet Sci. 2016;104:24-9. [DOI] [PubMed] [Google Scholar]
- 68. Arsen’ev AV, Dudin MG, Mikhailov VM. Influence of pulsed magnetic field on the bone growth plates in rabbits. Biophysics. 2010;55:619-22. [PubMed] [Google Scholar]
- 69. Iannacone WM, Pienkowski D, Pollack SR, Brighton CT. Pulsing electromagnetic field stimulation of the in vitro growth plate. J Orthop Res. 1988;6:239-47. [DOI] [PubMed] [Google Scholar]
- 70. Sauerland K, Raiss RX, Steinmeyer J. Proteoglycan metabolism and viability of articular cartilage explants as modulated by the frequency of intermittent loading. Osteoarthritis Cartilage. 2003;11:343-50. [DOI] [PubMed] [Google Scholar]
- 71. Torzilli PA, Grigiene R, Huang C, Friedman SM, Doty SB, Boskey AL, et al. Characterization of cartilage metabolic response to static and dynamic stress using a mechanical explant test system. J Biomech. 1997;30:1-9. [DOI] [PubMed] [Google Scholar]
- 72. Stokes IA. Mechanical effects on skeletal growth. J Musculoskelet Neuronal Interact. 2002;2:277-80. [PubMed] [Google Scholar]
- 73. Villemure I, Stokes IA. Growth plate mechanics and mechanobiology. A survey of present understanding. J Biomech. 2009;42:1793-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Stokes IA, Clark KC, Farnum CE, Aronsson DD. Alterations in the growth plate associated with growth modulation by sustained compression or distraction. Bone. 2007;41:197-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Niehoff A, Kersting UG, Zaucke F, Morlock MM, Bruggemann GP. Adaptation of mechanical, morphological, and biochemical properties of the rat growth plate to dose-dependent voluntary exercise. Bone. 2004;35:899-908. [DOI] [PubMed] [Google Scholar]
- 76. Apte SS, Kenwright J. Physeal distraction and cell proliferation in the growth plate. J Bone Joint Surg Br. 1994;76:837-43. [PubMed] [Google Scholar]
- 77. Valteau B, Grimard G, Londono I, Moldovan F, Villemure I. In vivo dynamic bone growth modulation is less detrimental but as effective as static growth modulation. Bone. 2011;49:996-1004. [DOI] [PubMed] [Google Scholar]
- 78. Ohashi N, Robling AG, Burr DB, Turner CH. The effects of dynamic axial loading on the rat growth plate. J Bone Miner Res. 2002;17:284-92. [DOI] [PubMed] [Google Scholar]
- 79. Ryan JA, Eisner EA, DuRaine G, You Z, Reddi AH. Mechanical compression of articular cartilage induces chondrocyte proliferation and inhibits proteoglycan synthesis by activation of the ERK pathway: implications for tissue engineering and regenerative medicine. J Tissue Eng Regen Med. 2009;3:107-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Amini S, Veilleux D, Villemure I. Tissue and cellular morphological changes in growth plate explants under compression. J Biomech. 2010;43:2582-8. [DOI] [PubMed] [Google Scholar]
- 81. Sergerie K, Parent S, Beauchemin PF, Londono I, Moldovan F, Villemure I. Growth plate explants respond differently to in vitro static and dynamic loadings. J Orthop Res. 2011;29:473-80. [DOI] [PubMed] [Google Scholar]
- 82. Cancel M, Grimard G, Thuillard-Crisinel D, Moldovan F, Villemure I. Effects of in vivo static compressive loading on aggrecan and type II and X collagens in the rat growth plate extracellular matrix. Bone. 2009;44:306-15. [DOI] [PubMed] [Google Scholar]
- 83. Pufe T, Lemke A, Kurz B, Petersen W, Tillmann B, Grodzinsky AJ, et al. Mechanical overload induces VEGF in cartilage discs via hypoxia-inducible factor. Am J Pathol. 2004;164:185-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Saadat E, Lan H, Majumdar S, Rempel DM, King KB. Long-term cyclical in vivo loading increases cartilage proteoglycan content in a spatially specific manner: an infrared microspectroscopic imaging and polarized light microscopy study. Arthritis Res Ther. 2006;8:R147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Pickvance EA, Oegema TR, Jr, Thompson RC., Jr. Immunolocalization of selected cytokines and proteases in canine articular cartilage after transarticular loading. J Orthop Res. 1993;11:313-23. [DOI] [PubMed] [Google Scholar]
- 86. Lee C, Grad S, Wimmer MA, Alini M. The influence of mechanical stimuli on articular cartilage tissue engineering. In: Ashammakhi N, Reis RL, editors. Topics in tissue engineering. 2006. http://www.oulu.fi/spareparts/ebook_topics_in_t_e_vol2/abstracts/alini_0102.pdf.E-book.
- 87. DeLise AM, Fischer L, Tuan RS. Cellular interactions and signaling in cartilage development. Osteoarthritis Cartilage. 2000;8:309-34. [DOI] [PubMed] [Google Scholar]
- 88. Huselstein C, Netter P, de Isla N, Wang Y, Gillet P, Decot V, et al. Mechanobiology, chondrocyte and cartilage. Biomed Mater Eng. 2008;18:213-20. [PubMed] [Google Scholar]
- 89. Barrett-Jolley R, Lewis R, Fallman R, Mobasheri A. The emerging chondrocyte channelome. Front Physiol. 2010;1:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ding L, Heying E, Nicholson N, Stroud NJ, Homandberg GA, Buckwalter JA, et al. Mechanical impact induces cartilage degradation via mitogen activated protein kinases. Osteoarthritis Cartilage. 2010;18:1509-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Han SK, Wouters W, Clark A, Herzog W. Mechanically induced calcium signaling in chondrocytes in situ. J Orthop Res. 2012;30:475-81. [DOI] [PubMed] [Google Scholar]
- 92. Madden RM, Han SK, Herzog W. The effect of compressive loading magnitude on in situ chondrocyte calcium signaling. Biomech Model Mechanobiol. 2015;14:135-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Perera PM, Wypasek E, Madhavan S, et al. Mechanical signals control SOX-9, VEGF, and c-Myc expression and cell proliferation during inflammation via integrin-linked kinase, B-Raf, and ERK1/2-dependent signaling in articular chondrocytes. Arthritis Res Ther. 2010;12:R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Raizman I, De Croos JN, Pilliar R, Kandel RA. Calcium regulates cyclic compression-induced early changes in chondrocytes during in vitro cartilage tissue formation. Cell Calcium. 2010;48:232-42. [DOI] [PubMed] [Google Scholar]
- 95. Ramage L, Nuki G, Salter DM. Signalling cascades in mechanotransduction: cell-matrix interactions and mechanical loading. Scand J Med Sci Sports. 2009;19:457-69. [DOI] [PubMed] [Google Scholar]
- 96. Lee DA, Noguchi T, Frean SP, Lees P, Bader DL. The influence of mechanical loading on isolated chondrocytes seeded in agarose constructs. Biorheology. 2000;37:149-61. [PubMed] [Google Scholar]
- 97. Nowlan NC, Sharpe J, Roddy KA, Prendergast PJ, Murphy P. Mechanobiology of embryonic skeletal development: Insights from animal models. Birth Defects Res Embryo Today. 2010;90:203-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ohashi N, Robling AG, Burr DB, Turner CH. The effects of dynamic axial loading on the rat growth plate. J Bone Miner Res. 2002;17:284-92. [DOI] [PubMed] [Google Scholar]
- 99. Sah RL, Kim YJ, Doong JY, Grodzinsky AJ, Plaas AH, Sandy JD. Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res. 1989;7:619-36. [DOI] [PubMed] [Google Scholar]
- 100. Buschmann MD, Kim YJ, Wong M, Frank E, Hunziker EB, Grodzinsky AJ. Stimulation of aggrecan synthesis in cartilage explants by cyclic loading is localized to regions of high interstitial fluid flow. Arch Biochem Biophys. 1999;366:1-7. [DOI] [PubMed] [Google Scholar]
- 101. Wong M, Siegrist M, Cao X. Cyclic compression of articular cartilage explants is associated with progressive consolidation and altered expression pattern of extracellular matrix proteins. Matrix Biol. 1999;18:391-9. [DOI] [PubMed] [Google Scholar]
- 102. Steinmeyer J, Knue S. The proteoglycan metabolism of mature bovine articular cartilage explants superimposed to continuously applied cyclic mechanical loading. Biochem Biophys Res Commun. 1997;240:216-21. [DOI] [PubMed] [Google Scholar]
- 103. Bian L, Fong JV, Lima EG, Stoker AM, Ateshian GA, Cook JL, et al. Dynamic mechanical loading enhances functional properties of tissue-engineered cartilage using mature canine chondrocytes. Tissue Eng Part A. 2010;16:1781-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Buschmann MD, Gluzband YA, Grodzinsky AJ, Hunziker EB. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Sci. 1995;108 (pt 4):1497-508. [DOI] [PubMed] [Google Scholar]
- 105. Demarteau O, Wendt D, Braccini A, Jakob M, Schäfer D, Heberer M, et al. Dynamic compression of cartilage constructs engineered from expanded human articular chondrocytes. Biochem Biophys Res Commun. 2003;310:580-8. [DOI] [PubMed] [Google Scholar]
- 106. Farnsworth NL, Antunez LR, Bryant SJ. Dynamic compressive loading differentially regulates chondrocyte anabolic and catabolic activity with age. Biotechnol Bioeng. 2013;110:2046-57. [DOI] [PubMed] [Google Scholar]
- 107. Hunter CJ, Imler SM, Malaviya P, Nerem RM, Levenston ME. Mechanical compression alters gene expression and extracellular matrix synthesis by chondrocytes cultured in collagen I gels. Biomaterials. 2002;23:1249-59. [DOI] [PubMed] [Google Scholar]
- 108. Hung CT, Mauck RL, Wang CC, Lima EG, Ateshian GA. A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Ann Biomed Eng. 2004;32:35-49. [DOI] [PubMed] [Google Scholar]
- 109. Hunter CJ, Mouw JK, Levenston ME. Dynamic compression of chondrocyte-seeded fibrin gels: effects on matrix accumulation and mechanical stiffness. Osteoarthritis Cartilage. 2004;12:117-30. [DOI] [PubMed] [Google Scholar]
- 110. Kisiday JD, Jin M, DiMicco MA, Kurz B, Grodzinsky AJ. Effects of dynamic compressive loading on chondrocyte biosynthesis in self-assembling peptide scaffolds. J Biomech. 2004;37:595-604. [DOI] [PubMed] [Google Scholar]
- 111. Wang PY, Tsai WB. Modulation of the proliferation and matrix synthesis of chondrocytes by dynamic compression on genipin-crosslinked chitosan/collagen scaffolds. J Biomater Sci Polym Ed. 2013;24:507-19. [DOI] [PubMed] [Google Scholar]
- 112. Lee CR, Grodzinsky AJ, Spector M. Biosynthetic response of passaged chondrocytes in a type II collagen scaffold to mechanical compression. J Biomed Mater Res A. 2003;64:560-9. [DOI] [PubMed] [Google Scholar]
- 113. Lima EG, Bian L, Mauck RL, Byers BA, Tuan RS, Ateshian GA, et al. The effect of applied compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Conf Proc IEEE Eng Med Biol Soc. 2006;1:779-82. [DOI] [PubMed] [Google Scholar]
- 114. Lima EG, Bian L, Ng KW, Mauck RL, Byers BA, Tuan RS, et al. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Osteoarthritis Cartilage. 2007;15:1025-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Mauck RL, Seyhan SL, Ateshian GA, Hung CT. Influence of seeding density and dynamic deformational loading on the developing structure/function relationships of chondrocyte-seeded agarose hydrogels. Ann Biomed Eng. 2002;30:1046-56. [DOI] [PubMed] [Google Scholar]
- 116. Nebelung S, Gavenis K, Lüring C, Zhou B, Mueller-Rath R, Stoffel M, et al. Simultaneous anabolic and catabolic responses of human chondrocytes seeded in collagen hydrogels to long-term continuous dynamic compression. Ann Anat. 2012;194:351-8. [DOI] [PubMed] [Google Scholar]
- 117. Nebelung S, Gavenis K, Rath B, Tingart M, Ladenburger A, Stoffel M, et al. Continuous cyclic compressive loading modulates biological and mechanical properties of collagen hydrogels seeded with human chondrocytes. Biorheology. 2011;48:247-61. [DOI] [PubMed] [Google Scholar]
- 118. Nicodemus GD, Bryant SJ. Mechanical loading regimes affect the anabolic and catabolic activities by chondrocytes encapsulated in PEG hydrogels. Osteoarthritis Cartilage. 2010;18:126-37. [DOI] [PubMed] [Google Scholar]
- 119. Honda K, Ohno S, Tanimoto K, Ijuin C, Tanaka N, Doi T, et al. The effects of high magnitude cyclic tensile load on cartilage matrix metabolism in cultured chondrocytes. Eur J Cell Biol. 2000;79:601-9. [DOI] [PubMed] [Google Scholar]
- 120. Ueki M, Tanaka N, Tanimoto K, Nishio C, Honda K, Lin YY, et al. The effect of mechanical loading on the metabolism of growth plate chondrocytes. Ann Biomed Eng. 2008;36:793-800. [DOI] [PubMed] [Google Scholar]
- 121. Huang J, Ballou LR, Hasty KA. Cyclic equibiaxial tensile strain induces both anabolic and catabolic responses in articular chondrocytes. Gene. 2007;404:101-9. [DOI] [PubMed] [Google Scholar]
- 122. Beckmann R, Houben A, Tohidnezhad M, Kweider N, Fragoulis A, Wruck CJ, et al. Mechanical forces induce changes in VEGF and VEGFR-1/sFlt-1 expression in human chondrocytes. Int J Mol Sci. 2014;15:15456-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Tanaka N, Ohno S, Honda K, Tanimoto K, Doi T, Ohno-Nakahara M, et al. Cyclic mechanical strain regulates the PTHrP expression in cultured chondrocytes via activation of the Ca2+ channel. J Dent Res. 2005;84:64-8. [DOI] [PubMed] [Google Scholar]
- 124. Weightman B, Kempson G. Load carriage. In: Freeman MAR, editor. Adult articular cartilage. London, England: Pitman Medical; 1979. p. 293-341. [Google Scholar]
- 125. Hu JC, Athanasiou KA. The effects of intermittent hydrostatic pressure on self-assembled articular cartilage constructs. Tissue Eng. 2006;12:1337-44. [DOI] [PubMed] [Google Scholar]
- 126. Suh JK, Baek GH, Arøen A, Malin CM, Niyibizi C, Evans CH, et al. Intermittent sub-ambient interstitial hydrostatic pressure as a potential mechanical stimulator for chondrocyte metabolism. Osteoarthritis Cartilage. 1999;7:71-80. [DOI] [PubMed] [Google Scholar]
- 127. Parkkinen JJ, Lammi MJ, Inkinen R, Jortikka M, Tammi M, Virtanen I, et al. Influence of short-term hydrostatic pressure on organization of stress fibers in cultured chondrocytes. J Orthop Res. 1995;13:495-502. [DOI] [PubMed] [Google Scholar]
- 128. Lammi MJ, Inkinen R, Parkkinen JJ, et al. Expression of reduced amounts of structurally altered aggrecan in articular cartilage chondrocytes exposed to high hydrostatic pressure. Biochem J. 1994;304(pt 3):723-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Carver SE, Heath CA. Increasing extracellular matrix production in regenerating cartilage with intermittent physiological pressure. Biotechnol Bioeng. 1999;62:166-74. [PubMed] [Google Scholar]
- 130. Carver SE, Heath CA. Semi-continuous perfusion system for delivering intermittent physiological pressure to regenerating cartilage. Tissue Eng. 1999;5:1-11. [DOI] [PubMed] [Google Scholar]
- 131. Parkkinen JJ, Ikonen J, Lammi MJ, Laakkonen J, Tammi M, Helminen HJ. Effects of cyclic hydrostatic pressure on proteoglycan synthesis in cultured chondrocytes and articular cartilage explants. Arch Biochem Biophys. 1993;300:458-65. [DOI] [PubMed] [Google Scholar]
- 132. Smith RL, Lin J, Trindade MC, Shida J, Kajiyama G, Vu T, et al. Time-dependent effects of intermittent hydrostatic pressure on articular chondrocyte type II collagen and aggrecan mRNA expression. J Rehabil Res Dev. 2000;37:153-61. [PubMed] [Google Scholar]
- 133. Smith RL, Rusk SF, Ellison BE, et al. In vitro stimulation of articular chondrocyte mRNA and extracellular matrix synthesis by hydrostatic pressure. J Orthop Res. 1996; 14: 53-60. [DOI] [PubMed] [Google Scholar]
- 134. Ikenoue T, Trindade MC, Lee MS, Lin EY, Schurman DJ, Goodman SB, et al. Mechanoregulation of human articular chondrocyte aggrecan and type II collagen expression by intermittent hydrostatic pressure in vitro. J Orthop Res. 2003;21:110-6. [DOI] [PubMed] [Google Scholar]
- 135. Kwan MK, Woo SL-Y. Biomechanical properties of healing cartilage. In: Mow VC, Ratcliffe A, Woo SL-Y, editors. Biomechanics of diarthrodial joints. New York, NY: Springer; 1990. p. 391-9. [Google Scholar]
- 136. Duggan DJ, Bittner M, Chen Y, Meltzer P, Trent JM. Expression profiling using cDNA microarrays. Nat Genet. 1999;21:10-4. [DOI] [PubMed] [Google Scholar]
- 137. Ramsay G. DNA chips: state-of-the art. Nat Biotechnol. 1998;16:40-4. [DOI] [PubMed] [Google Scholar]
- 138. Yeh CC, Chang SF, Huang TY, Chang HI, Kuo HC, Wu YC, et al. Shear stress modulates macrophage-induced urokinase plasminogen activator expression in human chondrocytes. Arthritis Res Ther. 2013;15:R53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Mohtai M, Gupta MK, Donlon B, Ellison B, Cooke J, Gibbons G, et al. Expression of interleukin-6 in osteoarthritic chondrocytes and effects of fluid-induced shear on this expression in normal human chondrocytes in vitro. J Orthop Res. 1996;14:67-73. [DOI] [PubMed] [Google Scholar]
- 140. Lee JH, Fitzgerald JB, Dimicco MA, Grodzinsky AJ. Mechanical injury of cartilage explants causes specific time-dependent changes in chondrocyte gene expression. Arthritis Rheum. 2005;52:2386-95. [DOI] [PubMed] [Google Scholar]
- 141. Waldman SD, Spiteri CG, Grynpas MD, Pilliar RM, Kandel RA. Long-term intermittent shear deformation improves the quality of cartilaginous tissue formed in vitro. J Orthop Res. 2003;21:590-6. [DOI] [PubMed] [Google Scholar]
- 142. Bilgen B, Chu D, Stefani R, Aaron RK. Design of a biaxial mechanical loading bioreactor for tissue engineering. J Vis Exp. 2013;(74):e50387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Gao Y, Liu S, Huang J, Guo W, Chen J, Zhang L, et al. The ECM-cell interaction of cartilage extracellular matrix on chondrocytes. Biomed Res Int. 2014;2014:648459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Xu J, Wang W, Clark CC, Brighton CT. Signal transduction in electrically stimulated articular chondrocytes involves translocation of extracellular calcium through voltage-gated channels. Osteoarthritis Cartilage. 2009;17:397-405. [DOI] [PubMed] [Google Scholar]
- 145. Li X, Kolega J. Effects of direct current electric fields on cell migration and actin filament distribution in bovine vascular endothelial cells. J Vasc Res. 2002;39:391-404. [DOI] [PubMed] [Google Scholar]
- 146. Kim SH, Turnbull J, Guimond S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol. 2011;209:139-51. [DOI] [PubMed] [Google Scholar]
- 147. Loeser RF. Integrins and chondrocyte-matrix interactions in articular cartilage. Matrix Biol. 2014;39:11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Gao Y, Liu S, Huang J, Guo W, Chen J, Zhang L, et al. The ECM-cell interaction of cartilage extracellular matrix on chondrocytes. Biomed Res Int. 2014;2014:648459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Knudson W, Loeser RF. CD44 and integrin matrix receptors participate in cartilage homeostasis. Cell Mol Life Sci. 2002;59:36-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Kurtis MS, Tu BP, Gaya OA, Mollenhauer J, Knudson W, Loeser RF, et al. Mechanisms of chondrocyte adhesion to cartilage: role of beta1-integrins, CD44, and annexin V. J Orthop Res. 2001;19:1122-30. [DOI] [PubMed] [Google Scholar]
- 151. Knudson W, Chow G, Knudson CB. CD44-mediated uptake and degradation of hyaluronan. Matrix Biol. 2002;21:15-23. [DOI] [PubMed] [Google Scholar]
- 152. von der Mark K, Mollenhauer J. Annexin V interactions with collagen. Cell Mol Life Sci. 1997;53:539-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Mycielska ME, Djamgoz MB. Cellular mechanisms of direct-current electric field effects: galvanotaxis and metastatic disease. J Cell Sci. 2004;117:1631-9. [DOI] [PubMed] [Google Scholar]
- 154. Moncada ME, Saldarriaga MP, Bravo AF, Pinedo CR. Medición de Impedancia Eléctrica en Tejido Biológico – Revisión. Tecno Lógicas. 2010;25:51-76. [Google Scholar]
- 155. Khayyeri H, Checa S, Tagil M, O’Brien FJ, Prendergast PJ. Tissue differentiation in an in vivo bioreactor: in silico investigations of scaffold stiffness. J Mater Sci Mater Med. 2010;21:2331-6. [DOI] [PubMed] [Google Scholar]
- 156. Hossain MS, Bergstrom DJ, Chen XB. Modelling and simulation of the chondrocyte cell growth, glucose consumption and lactate production within a porous tissue scaffold inside a perfusion bioreactor. Biotechnol Rep. 2015;5:55-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Sengers BG, van Donkelaar CC, Oomens CW, Baaijens FP. Computational study of culture conditions and nutrient supply in cartilage tissue engineering. Biotechnol Prog. 2005;21:1252-61. [DOI] [PubMed] [Google Scholar]
- 158. Szafranski JD, Grodzinsky AJ, Burger E, Gaschen V, Hung HH, Hunziker EB. Chondrocyte mechanotransduction: effects of compression on deformation of intracellular organelles and relevance to cellular biosynthesis. Osteoarthritis Cartilage. 2004;12:937-46. [DOI] [PubMed] [Google Scholar]
- 159. Guilak F. Compression-induced changes in the shape and volume of the chondrocyte nucleus. J Biomech. 1995;28:1529-41. [DOI] [PubMed] [Google Scholar]