Abstract
Background
Whether vitamin D supplementation reduces cancer or cardiovascular disease remains unclear, and randomized trial evidence is limited.
Methods
The VITamin D and OmegA-3 TriaL (VITAL) was a nationwide, randomized, placebo-controlled, 2X2 factorial trial of vitamin D3 (cholecalciferol, 2000 IU/day) and marine omega-3 fatty acids (1 g/day) for the prevention of cancer and cardiovascular disease. There were 25,871 U.S. men aged ≥50 and women aged ≥55, including 5,106 African Americans, who participated. Primary endpoints were total invasive cancer and major cardiovascular events (composite of myocardial infarction, stroke, and cardiovascular mortality). Secondary endpoints included site-specific cancers, cancer mortality, and additional cardiovascular events.
Results
Vitamin D supplementation did not reduce either of the primary endpoints. During a median 5.3 year intervention, 1,617 participants were diagnosed with cancer (793 assigned to vitamin D and 824 assigned placebo; hazard ratio [HR]=0.96; 95% confidence interval, 0.88–1.06; p-value=0.47); and 805 experienced a major cardiovascular event (396 assigned to vitamin D and 409 assigned to placebo; HR=0.97 [0.85–1.12]; p-value=0.69). For secondary endpoints, the hazard ratios and 95% confidence intervals comparing Vitamin D to placebo were: cancer deaths (n=341, HR 0.83 (0.67–1.02); breast cancer (1.02; 0.79–1.31); prostate cancer 0.88 (0.72–1.07); colorectal cancer 1.09 (0.73–1.62); expanded cardiovascular disease events 0.96 (0.86–1.08); myocardial infarction 0.96 (0.78–1.19); stroke 0.95 (0.76–1.20); and cardiovascular mortality 1.11 (0.88–1.40). The HR for all-cause deaths (n=978) was 0.99 (0.87–1.12). No excess risks of hypercalcemia or other adverse events were identified.
Conclusion
Vitamin D supplementation did not reduce invasive cancer incidence or cardiovascular events.
Long prescribed to prevent and treat bone-related disorders,1 supplemental vitamin D has been viewed in recent years as a potential strategy for preventing cancer and cardiovascular disease. In the United States, routine assessment of vitamin D status in primary care settings2 and use of vitamin D supplements3 have risen substantially. Ecologic studies have reported lower cancer and cardiovascular mortality in regions with greater sun exposure, which is necessary for cutaneous synthesis of vitamin D. Laboratory studies have documented the presence of vitamin D receptors in many tissues and have suggested plausible vitamin D pathways that may be related to cancer and cardiovascular disease; observational studies have shown associations between low serum 25-hydroxyvitamin D [25(OH)D] levels and increased cancer and cardiovascular risks.1, 4–6 Nevertheless, whether vitamin D supplementation prevents cancer or cardiovascular disease remains uncertain, as such results cannot establish causality.1, 4, 7, 8 For example, observational studies are susceptible to confounding by outdoor physical activity (which correlates with sun exposure), adiposity (which may decrease 25(OH)D bioavailability), general nutritional status, and other factors that may produce spurious protective associations.1, 4
Data from large-scale (n≥10,000) randomized trials of vitamin D in moderate or high doses and designed with cancer or cardiovascular disease as primary outcomes are lacking. Trials examining such outcomes, typically using secondary or post hoc analyses, have usually had null results, but use of low vitamin D doses, insufficient statistical power, short durations, and/or lack of rigorous endpoint adjudication limit conclusions.1, 4 However, meta-analyses9, 10 of randomized trial data suggest a stronger benefit of vitamin D on cancer mortality than cancer incidence. The U.S. Preventive Services Task Force recently concluded that data are insufficient to evaluate the effectiveness of vitamin D supplementation for cancer or cardiovascular disease prevention.7 Earlier, the Institute of Medicine also reached this conclusion and called for new trials of vitamin D (in amounts at least twice the current recommended dietary allowance of 600–800 IU/d for bone health) to clarify the benefit-risk balance.1 The VITamin D and OmegA-3 TriaL (VITAL), a large-scale, high-dose vitamin D trial with more than 5000 African Americans, for whom the question is particularly relevant due to lower cutaneous synthesis of vitamin D in response to solar radiation was designed to address these knowledge gaps.
METHODS
Study Design
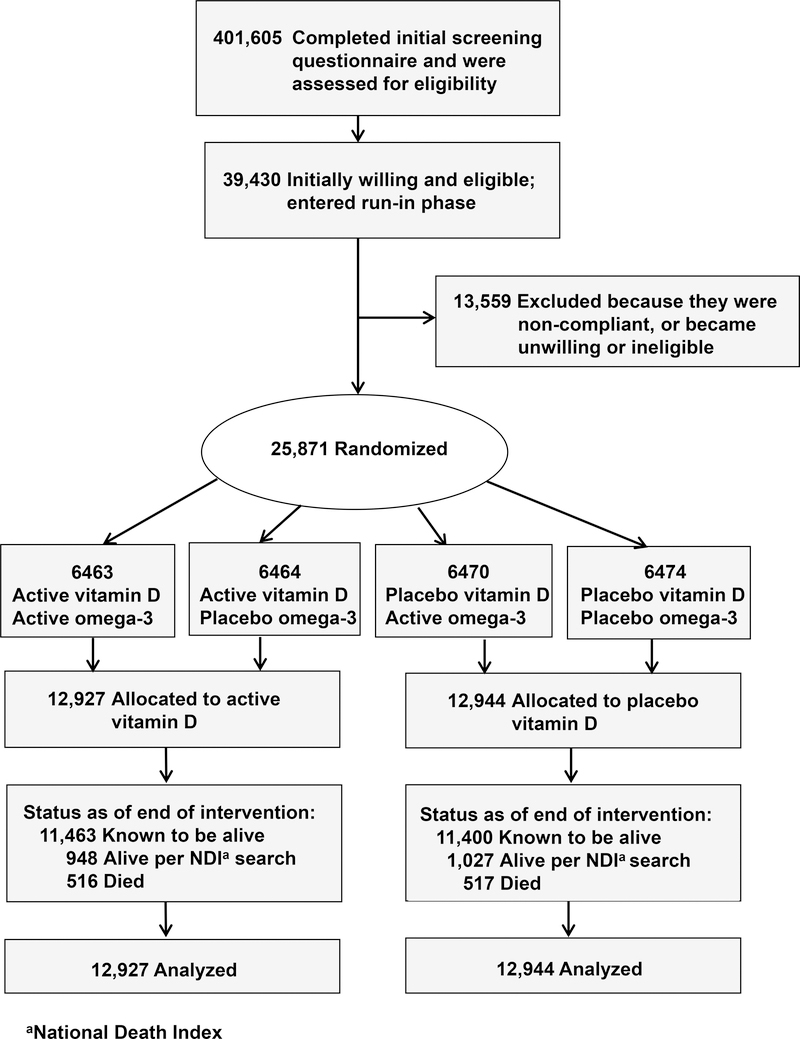
The present study was a randomized, double-blind, placebo-controlled, 2X2 factorial trial that examined the benefits and risks of vitamin D3 (cholecalciferol, 2000 IU/day) and marine omega-3 fatty acids (1 g/day) for primary prevention of cancer and cardiovascular disease among 25,871 men aged ≥50 and women aged ≥55. Study protocol has been described elsewhere, 4, 11 and the protocol may be found at NEJM.org. Participants were recruited throughout the U.S., balanced by sex, and with a goal to include at least 5000 African Americans. Eligible participants had no history of cancer (except non-melanoma skin cancer) or cardiovascular disease at study entry, and were required to agree to limit vitamin D from all supplemental sources, including multivitamins, to 800 IU/day; and to complete a three-month placebo run-in phase. Safety exclusions included renal failure or dialysis, cirrhosis, history of hypercalcemia, or other serious conditions that would preclude participation. The recruitment flow diagram appears in Figure 1. Randomization to vitamin D, omega-3 fatty acids, both active agents, or both placebos took place from November 2011 to March 2014 and was computer generated within sex, race, and five-year age groups in blocks of eight. Participants provided written informed consent. Study medication ended as planned on December 31, 2017, yielding a median 5.3-year (range 3.8–6.1 years) intervention period. The trial was approved by the institutional review board of Brigham and Women’s Hospital, Boston, and was monitored by an external Data and Safety Monitoring Board.
Figure 1:
Flow Diagram of Enrollment in the Vitamin D Component of the Trial.
Baseline questionnaires collected data on risk factors for cancer, cardiovascular disease, and other conditions, and included a food frequency questionnaire. Participants received follow-up questionnaires at 6 months and 1 year after randomization and annually thereafter to collect information on adherence to randomized treatments, use of nonstudy vitamin D supplements, development of major illnesses, updates on risk factors, and potential side effects of the study agents. Study capsules were mailed with questionnaires to participants.
Baseline blood samples were collected during the run-in from all willing participants, including 16,956 of 25,871 randomized (65.5%). Quest Diagnostics performed serum 25(OH)D assays on all analyzable samples using liquid chromatography-tandem mass spectrometry. Our study participated in the vitamin D standardization program of the Centers for Disease Control and Prevention.12
Study Endpoints
Primary endpoints were total invasive cancer and major cardiovascular events (composite of myocardial infarction, stroke, and cardiovascular mortality). Secondary cancer endpoints were incident colorectal, breast, and prostate cancers, and total cancer mortality. Secondary cardiovascular endpoints were an expanded composite of major cardiovascular events plus coronary revascularization and the individual components of major cardiovascular events. Participants reporting an endpoint were asked to sign a release for medical records, which were reviewed for confirmation by an Endpoints Committee of physicians blinded to treatment assignment. Cancer was confirmed with histologic or cytologic data,13 MI and stroke using established criteria,14, 15 coronary revascularization by medical record review, and cardiovascular death by convincing evidence of a cardiovascular event from all available sources. Analyses included only confirmed endpoints.
For deaths reported by family members, the next-of-kin was asked for permission to obtain medical records and a copy of the death certificate. Alternatively, the latter was obtained from the state vital records bureau. The Endpoints Committee reviewed the records to assign cause of death. If records were unavailable (or participants lost to follow-up), the National Death Index (NDI) Plus was searched for cause of death based on death-certificate information. Deaths were defined using all these sources; a secondary analysis of cause-specific mortality required medical records or other adjudication of cause of death beyond NDI coding.
Statistical Analysis
Treatment-effect analyses compared randomized groups based on the intention-to-treat principle (all randomized participants were analyzed) VITAL was designed to have >85% power to detect observed hazard ratios (HR) of 0.85 and 0.80 for the primary cancer and cardiovascular endpoints, respectively.4 Initial analyses compared baseline characteristics of participants by randomized treatment assignment using t-tests or chi-square tests. Primary analyses compared main effects of vitamin D on cancer and cardiovascular disease using Cox proportional hazards models controlling for age, sex, and omega-3 randomization group. Person-time was counted from randomization to the endpoint, to death, or to end of the trial on December 31, 2017. Cumulative incidence plots and interactions with time examined whether treatment effects varied over time. Prespecified analyses of the primary outcomes excluding events occurring during the first year and first two years of follow-up assessed latent treatment effects. Adherence effects were estimated by censoring follow-up when the participant stopped study capsules or began taking >800 IU/day of outside vitamin D.
Possible variations in treatment effect by race/ethnicity, age, sex, body mass index (BMI), baseline 25(OH)D level, concurrent omega-3 randomization, outside use of vitamin D supplements, and baseline risk factors for cancer and cardiovascular disease were specified a priori. However, analyses were not controlled for multiple hypothesis testing, with no formal adjustment to the p-values or confidence intervals. Thus, results for secondary and exploratory outcomes, and for subgroups, should be interpreted with caution. The incidence of potential side effects by randomized group was also compared.
RESULTS
Table 1 shows baseline characteristics of the trial participants (further details in Table S1, Supplementary Appendix). Of the 25,871 participants, 51% were women and mean age was 67.1 years. The cohort was racially diverse, with 71% self-declared non-Hispanic whites, 20% African Americans, and the rest, members of other racial/ethnic groups. Randomization balanced participant characteristics between groups. Among the 15,787 participants with analyzable blood samples, the mean serum total 25(OH)D level at baseline was 30.8 (SD, 10.0) ng/mL, with 12.7% and 32.2% having levels <20 ng/ml and 20-<30 ng/mL, respectively (to convert from ng/ml to nmol/L, multiply by 2.5). In a subset of 1,644 participants with repeat measurements after 1 year, mean 25(OH)D levels increased from 29.8 ng/mL at baseline to 41.8 ng/mL at 1 year (40% increase) in the vitamin D group and changed minimally (mean, −0.7 ng/mL) in the placebo group. Baseline 25(OH)D levels varied by age, sex, race, and BMI but most groups achieved 25(OH)D levels close to, or above, 40 ng/mL at 1 year (Supplementary Appendix, Figs. S1a and S1b).
Table 1.
Baseline Characteristics of the 25,871 Study Participants, According to Randomized Vitamin D Assignmenta
| Baseline Characteristic | All Participants | Vitamin D | |
|---|---|---|---|
| Active | Placebo | ||
| N | 25,871 | 12,927 | 12,944 |
| Sex, % female | 13085 (50.6) | 6547 (50.6) | 6538 (50.5) |
| Mean age ± SD, years | 67.1 ± 7.1 | 67.1 ± 7.0 | 67.1 ± 7.1 |
| ≥75 | 3318 (12.8) | 1658 (12.8) | 1660 (12.8) |
| Race/ethnicity, % | |||
| Non-Hispanic White | 18046 (71.3) | 9013 (71.3) | 9033 (71.4) |
| African American | 5106 (20.2) | 2553 (20.2) | 2553 (20.2) |
| Hispanic (not African American) | 1013 ( 4.0) | 516 ( 4.1) | 497 ( 3.9) |
| Asian/Pacific Islander | 388 ( 1.5) | 188 ( 1.5) | 200 ( 1.6) |
| American Indian/Alaskan Native | 228 ( 0.9) | 118 ( 0.9) | 110 ( 0.9) |
| Other/unknown | 523 ( 2.1) | 259 ( 2.0) | 264 ( 2.1) |
| Mean body mass index ± SD, kg/m2 | 28.1 (5.7) | 28.1 (5.7) | 28.1 (5.8) |
| Current smoking, % | 1836 ( 7.2) | 921 ( 7.2) | 915 ( 7.2) |
| Hypertension, treated with medication, % | 12791 (49.8) | 6352 (49.5) | 6439 ( 50.1) |
| Cholesterol-lowering medication (current use), % | 9524 (37.5) | 4822 (38.0) | 4702 (36.9) |
| Diabetes, % | 3549 (13.7) | 1812 (14.0) | 1737 (13.4) |
Abbreviations: SD = standard deviation. There were no significant differences in the baseline characteristics between the groups.
The mean response rate to questionnaires was 93.1%, and mortality follow-up was >98% over the 5.3-year follow-up. Adherence to randomized treatment (percent taking ≥2/3 of their study capsules) in the active and placebo groups averaged 82.0% and 80.3%, respectively, during this time (Table S2, Supplementary Appendix). The prevalence of outside vitamin D use (>800 IU/day) was 3.8% vs. 5.6% at two years and 6.4% vs 10.8% at five years in the active and placebo groups, respectively, likely reflecting outside-of-study screening for 25(OH)D and initiation of supplementation in some participants with low levels.
Cancer
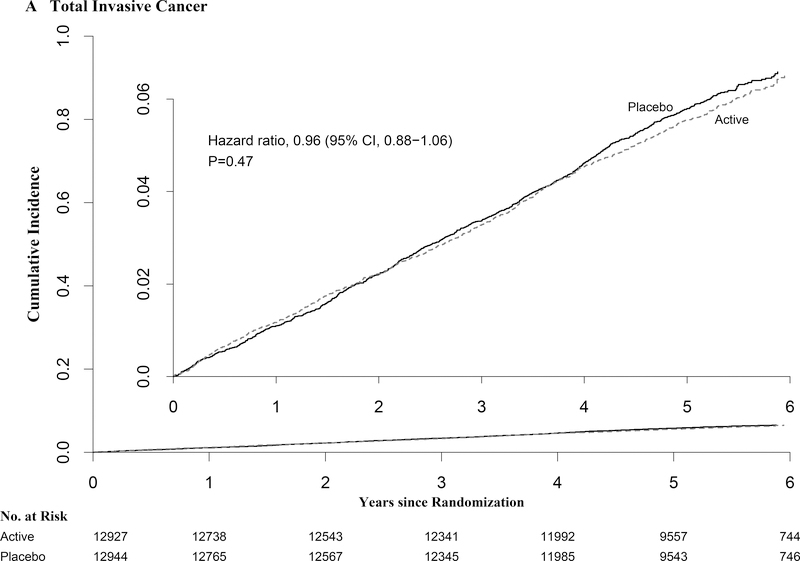
A total of 1,617 participants developed the primary endpoint of total invasive cancer, with event rates similar in the vitamin D and placebo group (793 vs. 824 cancers; HR=0.96 [95% confidence interval, 0.88–1.06]; p-value=0.47) (Table 2). The incidence of site-specific cancers (of breast, prostate, and colorectum) also did not differ significantly between groups. During follow-up, 154 participants in the vitamin D group and 187 in the placebo group died from cancer (HR=0.83 [0.67–1.02]).
Table 2.
Hazard Ratios (HR) and 95% Confidence Intervals (CI) of the Primary, Secondary, and Other Outcomes by Randomized Vitamin D Assignment in Intention-To-Treat Analyses
| No. of Events | ||||
|---|---|---|---|---|
| Vitamin D (N = 12,927) | Placebo (N = 12,944) | HR | 95% CI | |
| Outcomes (1° and 2°) Total invasive cancera | 793 | 824 | 0.96 | 0.88–1.06 |
| Breast | 124 | 122 | 1.02 | 0.79–1.31 |
| Prostate | 192 | 219 | 0.88 | 0.72–1.07 |
| Colorectal | 51 | 47 | 1.09 | 0.73–1.62 |
| Cancer death | 154 | 187 | 0.83 | 0.67–1.02 |
| Cardiovascular disease (CVD) outcomes | ||||
| Major CVD eventsa,b | 396 | 409 | 0.97 | 0.85–1.12 |
| Expanded CVD eventsc | 536 | 558 | 0.96 | 0.86–1.08 |
| Myocardial infarction | 169 | 176 | 0.96 | 0.78–1.19 |
| Stroke | 141 | 149 | 0.95 | 0.76–1.20 |
| Cardiovascular mortality | 152 | 138 | 1.11 | 0.88–1.40 |
| Other vascular outcomesd | ||||
| Coronary artery bypass graft (CABG) | 73 | 98 | 0.75 | 0.55–1.01 |
| Percutaneous coronary intervention (PCI) | 182 | 188 | 0.97 | 0.79–1.19 |
| Myocardial infarction death | 24 | 15 | 1.60 | 0.84–3.06 |
| Stroke death | 19 | 23 | 0.84 | 0.46–1.54 |
| All-cause mortality | 485 | 493 | 0.99 | 0.87–1.12 |
| Excluding the first two years of follow-up: | ||||
| Total invasive cancer | 490 | 522 | 0.94 | 0.83–1.06 |
| Cancer death | 112 | 149 | 0.75 | 0.59–0.96 |
| Major cardiovascular events | 274 | 296 | 0.93 | 0.79–1.09 |
| All-cause mortality | 368 | 384 | 0.96 | 0.84–1.11 |
Primary outcome.
A composite of myocardial infarction, stroke, and cardiovascular mortality.
A composite of major cardiovascular events plus coronary revascularization (coronary artery bypass graft + percutaneous coronary intervention).
Not prespecified as primary or secondary outcomes.
From Cox regression models controlling for age, sex, and omega-3 randomization group. Analyses are not adjusted for multiple comparisons.
The cumulative incidence for total invasive cancer and cancer mortality did not differ significantly between groups (Fig. 2 and Table 2). This was also true in preplanned analyses of the primary outcomes excluding the first 1 and 2 years of follow-up. However, the test for proportionality over time was statistically significant for cancer mortality. In an analysis excluding 1 and 2 years of follow-up, and that was not specified in the protocol, cancer mortality was significantly reduced in both (HR=0.79 [0.63–0.99] and HR=0.75 [0.59–0.96], respectively). In analyses restricted to cancer deaths with medical records or other adjudication of cause of death beyond the NDI coding (n=153), the HRs were 0.72 (0.52–1.00) over total follow-up and 0.63 (0.43–0.92) after excluding the first 2 years. Preliminary analyses of cancer stage at diagnosis showed slightly fewer advanced and/or metastatic cancers among those assigned to vitamin D than to placebo, but differences were not statistically significant (data not shown).
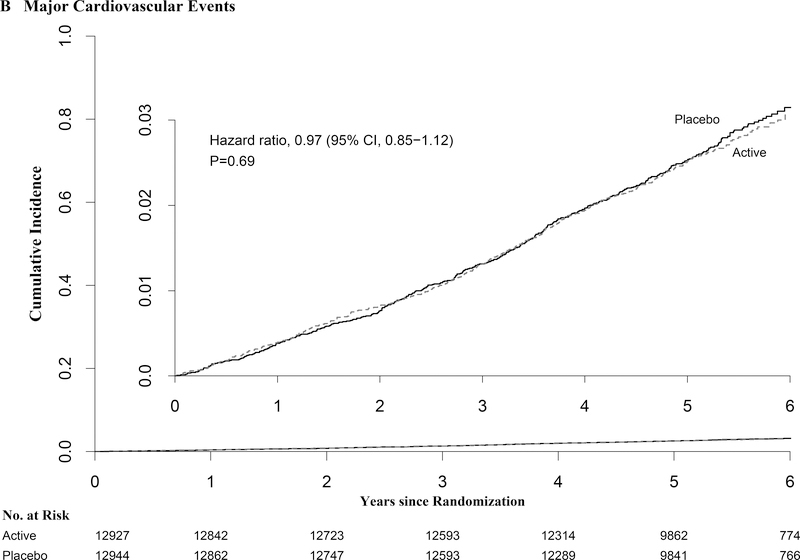
Figure 2:
Cumulative Incidence Rates of A) Total Invasive Cancer, and B) Major Cardiovascular Events, by Year of Follow-up. From Cox regression models controlling for age, sex, and omega-3 randomization group (intention-to-treat analyses).
Results of prespecified subgroup analyses are presented in Table 3. The findings suggest that BMI may have modified the effect of vitamin D treatment on cancer, with reductions in cancer incidence.
Table 3.
Hazard Ratios (HR) and 95% Confidence Intervals (CI) of the Primary Outcomes Comparing Vitamin D (Vit D) and Placebo Groups, According to Baseline Characteristics (Prespecified Subgroups) in Intention-To-Treat Analyses
| Total Invasive Cancer | Major Cardiovascular Events | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| N Events | Inter-action P-value | N Events | Inter-action P-value | ||||||
| Subgroup | Total | Vit D | Placebo | HR (95%CI) | Vit D | Placebo | HR (95%CI) | P-value | |
| Age (years) | 25871 | 0.73 | 0.31 | ||||||
| <median (66.7) | 12859 | 302 | 322 | 0.95 (0.81–1.11) | 140 | 131 | 1.07 (0.85–1.36) | ||
| ≥median (66.7) | 13012 | 491 | 502 | 0.98 (0.86–1.11) | 256 | 278 | 0.93 (0.78–1.10) | ||
| Sex | 25871 | 0.38 | 0.57 | ||||||
| Male | 12786 | 452 | 488 | 0.93 (0.82–1.06) | 223 | 223 | 1.01 (0.84–1.21) | ||
| Female | 13085 | 341 | 336 | 1.02 (0.87–1.18) | 173 | 186 | 0.93 (0.76–1.14) | ||
| Race | 25304 | 0.21 | 0.37 | ||||||
| Non-Hispanic White | 18046 | 626 | 632 | 0.99 (0.89–1.11) | 280 | 301 | 0.93 (0.79–1.10) | ||
| African American | 5106 | 98 | 126 | 0.77 (0.59–1.01) | 69 | 76 | 0.91 (0.65–1.26) | ||
| Other | 2152 | 53 | 52 | 1.03 (0.70–1.51) | 32 | 24 | 1.36 (0.80–2.31) | ||
| Body Mass Index (kg/m2) | 25254 | 0.002 | 0.66 | ||||||
| <25 | 7843 | 206 | 278 | 0.76 (0.63–0.90) | 117 | 115 | 1.07 (0.83–1.38) | ||
| 25–<30 | 10122 | 338 | 323 | 1.04 (0.90–1.21) | 152 | 162 | 0.93 (0.74–1.16) | ||
| ≥30 | 7289 | 228 | 199 | 1.13 (0.94–1.37) | 120 | 120 | 0.98 (0.76–1.26) | ||
| Body Mass Index (kg/m2) | 25254 | 0.026 | 0.89 | ||||||
| <median (27.1) | 12582 | 361 | 421 | 0.86 (0.75–0.99) | 189 | 193 | 0.99 (0.81–1.21) | ||
| ≥median (27.1) | 12672 | 411 | 379 | 1.08 (0.94–1.24) | 200 | 204 | 0.97 (0.80–1.18) | ||
| Baseline Serum 25(OH)Da | 15787 | 0.99 | 0.75 | ||||||
| <20 ng/mL | 2001 | 58 | 63 | 0.97 (0.68–1.39) | 34 | 34 | 1.09 (0.68–1.76) | ||
| ≥20 ng/mL | 13786 | 459 | 464 | 0.98 (0.86–1.12) | 218 | 216 | 1.00 (0.83–1.21) | ||
| Baseline serum 25(OH)Da | 15787 | 0.57 | 0.42 | ||||||
| <cohort median | 7812 | 251 | 252 | 1.02 (0.86–1.21) | 128 | 139 | 0.94 (0.74–1.20) | ||
| ≥cohort median | 7975 | 266 | 275 | 0.95 (0.80–1.12) | 124 | 111 | 1.09 (0.84–1.41) | ||
| Baseline Vitamin D useb | 25871 | 0.64 | 0.71 | ||||||
| Yes | 11030 | 370 | 376 | 0.99 (0.86–1.14) | 165 | 164 | 1.00 (0.81–1.25) | ||
| No | 14841 | 423 | 448 | 0.94 (0.83–1.08) | 231 | 245 | 0.95 (0.79–1.14) | ||
| Omega-3 Fatty Acids randomization statusc | 25871 | 0.56 | 0.56 | ||||||
| Placebo group | 12938 | 385 | 412 | 0.94 (0.82–1.08) | 210 | 209 | 1.01 (0.83–1.22) | ||
| Omega-3 group | 12933 | 408 | 412 | 0.99 (0.87–1.14) | 186 | 200 | 0.93 (0.76–1.14) | ||
Serum 25-hydroxyvitamin D levels: to convert 25(OH)D unit from ng/ml to nmol/L, multiply by 2.5. For median analyses, cutpoint was at 31 mg/mL.
Use of out-of-study vitamin D supplements at baseline (restricted to ≤800 IU/d from all sources combined, including individual supplements and multivitamins).
Randomization status in the trial.
From Cox regression models controlling for age, sex, and omega-3 randomization group. Analyses are not adjusted for multiple comparisons.
Cardiovascular Disease and All-Cause Mortality
For major cardiovascular events (myocardial infarction, stroke, and cardiovascular death), 805 cases occurred during follow-up; event rates were similar in the vitamin D and placebo groups (396 vs. 409 events; HR=0.97 [0.85–1.12]; p-value=0.69) (Table 2). Vitamin D supplementation also did not affect risk of secondary cardiovascular endpoints (Table 2). There were no treatment differences in the cumulative incidence of major cardiovascular events (Fig. 2), and no significant effect modification by baseline characteristics or randomization to the omega-3 intervention (Table 3) or by traditional cardiovascular risk factors (Table S3, Supplementary Appendix). All-cause mortality was similar in the vitamin D and placebo groups (485 vs 493 deaths: HR=0.99 [0.87–1.12]). Analyses censoring for nonadherence did not materially alter results. No meaningful change in cardiovascular or all-cause mortality results occurred after excluding the first 2 years of follow-up (Table 2).
Adverse Events
There were no significant increases in diagnoses of hypercalcemia, kidney stones, or gastrointestinal symptoms between treatment groups (Table S4, Supplementary Appendix).
DISCUSSION
In this large primary prevention trial, vitamin D3 supplementation (2000 IU/day) did not significantly reduce total invasive cancer or the composite of major cardiovascular events (myocardial infarction, stroke, and cardiovascular mortality). The intervention also did not reduce the incidence of total cancer mortality or breast, prostate, or colorectal cancer.
Treatment effects did not vary by baseline serum 25(OH)D levels. Vitamin D did not significantly reduce any secondary cardiovascular endpoints or all-cause mortality in the overall cohort or in subgroups.
In analyses excluding early follow up, there was also no significant between group difference in total cancer incidence or major cardiovascular events. A post hoc analysis of cancer mortality suggested a possible reduction in total cancer mortality after exclusion of early follow-up, based on an unadjusted 95% confidence interval that does not include 1.
The results of subgroup analyses raise the possibility of differential effects on cancer incidence according to BMI with normal-weight participants experiencing a possible treatment-associated reductions in cancer incidence. However, these analyses should be considered hypothesis generating, in the context of the negative findings for the primary outcome measures and given that they are not adjusted for multiple comparisons.
The present trial, due to its size and long duration (≥5 years), had sufficient power to examine the effect of high-dose vitamin D on cancer and cardiovascular risk. Previous vitamin D trials testing doses from 400 to 1100 IU/day and administered with or without calcium have suggested, in aggregate, no significant benefit for cancer incidence but significant benefit for cancer mortality that would need to be confirmed. A 2014 meta-analysis of four such trials16–19 yielded summary relative risks of 1.00 (0.94–1.06) for cancer incidence and 0.88 (0.78–0.98) for cancer mortality.9 Another meta-analysis had similar results.10 Two high-dose vitamin D trials have recently been completed. One 4-year trial20 tested daily vitamin D (2000 IU) plus calcium (1500 mg) vs. placebo for cancer prevention in 2303 Nebraskan women and found a suggestive but nonsignificant 30% reduction in cancer incidence. The 3.3-year Vitamin D Assessment Study (ViDA),21 which tested monthly vitamin D (100,000 IU) vs. placebo for cardiovascular disease prevention in 5110 New Zealanders, reported null results for cancer outcomes. However, these trials had shorter durations and fewer cancer deaths than did our trial, as well as few black participants. Also, ViDA used intermittent bolus dosing, associated with nonphysiological fluctuations in vitamin D blood levels.22
Data from laboratory and animal studies support mechanisms whereby vitamin D may inhibit carcinogenesis and slow tumor progression, including promotion of cell differentiation, inhibition of cancer cell proliferation, and anti-inflammatory, immunomodulatory, proapoptotic, and antiangiogenic effects.1, 23 Vitamin D may decrease tumor invasiveness and propensity to metastasize, leading to reduced cancer mortality.23 Higher 25(OH)D levels at diagnosis or treatment have been linked to longer survival in cancer patients.9 Observational studies suggest that vitamin D may confer greater protection against cancer mortality than cancer incidence, albeit with reductions in both endpoints,5 with strongest inverse relationships between 25(OH)D levels and colorectal cancer.24–26 Our power for site-specific analyses was limited. Additionally, given the long latency for cancer development, extended follow-up is necessary to fully ascertain potential treatment effects.
The observed lack of benefit of vitamin D supplementation for cardiovascular outcomes in our trial is consistent with results of prior vitamin D trials,16, 19, 27–32 even for moderate or high doses.31 Most recently, monthly high-dose vitamin D did not reduce cardiovascular disease in ViDA.30 Neither our trial nor ViDA30 found that vitamin D reduced all-cause mortality; lower-dose vitamin D trials have shown neutral effects or at most modest reductions in this endpoint.32–34 However, detection of reduced all-cause mortality, if present, may require longer follow-up.
Prior research points to possible mechanisms through which vitamin D supplementation might reduce cancer risk in normal-weight but not overweight or obese participants. Parathyroid hormone appears to be suppressed at lower 25(OH)D levels in overweight/obese persons,35 which would be consistent with obesity-related hormonal dysregulation with less supplementation benefit. Alternatively, because of volumetric dilution36 or decreased bioactivity of vitamin D, overweight/obese persons may require higher doses to derive cancer benefit, analogous to body size differences in aspirin dosage requirements.37 However, in our trial, there was only a modest variation in mean 25(OH)D response to the tested dose according to BMI group (Figure S2b). Finally, vitamin D supplementation is unlikely to affect all mechanistic pathways linking obesity to numerous cancers.38 These hypothesis-generating issues require further investigation.
The finding of a possible vitamin D-associated reduction in cancer among African Americans — a group with modest vitamin D requirements for bone health relative to whites (lower fracture risk despite lower 25(OH)D levels than whites)1—may imply that optimal vitamin D status may vary by organ system/tissue. We speculate that the possible treatment-associated cancer reductions among normal-weight participants and suggestive reductions among African Americans, which contrast with the null cardiovascular findings in these groups, may be explained by different vitamin D requirements for these outcomes.
In observational studies, the 25(OH)D levels associated with lowest risks tend to be above 30 ng/mL for cancer (at least colorectal cancer)25 but between 20–25 ng/mL for cardiovascular disease.6 Thus, vitamin D requirements for cardiovascular health may have already been met for most participants. Although neither our trial nor ViDA found significant cardiovascular benefit for vitamin D among participants with low 25(OH)D at baseline, it remains possible that a trial among persons with extremely low vitamin D (i.e., well below the 20 ng/mL level recommended for bone health1) would show stronger risk reductions. However, maintaining participants in a vitamin D deficient state and circumventing real-world clinical care for 5 years would be neither ethical nor feasible.
Our trial has many strengths, including a large general population sample with racial/ethnic and geographic diversity; daily vitamin D dosing; high follow-up rates and pill-taking adherence; rigorously adjudicated endpoints; baseline and follow-up blood collections in many participants; and achieved mean 25(OH)D levels in the targeted range. Ancillary studies addressing treatment effects on diabetes, heart failure, cognition, autoimmune disorders, and other outcomes, will inform the overall benefit-risk balance of high-dose supplementation. Our trial also has limitations. Median treatment duration was 5.3 years. The trial tested only one vitamin D dose. Ongoing trials39 will add information regarding other doses, although some are using bolus dosing. A 2-year post-intervention follow-up of our cohort is ongoing to capture latency effects and increase statistical power to assess endpoints.
In summary, daily high-dose vitamin D supplementation for 5 years among initially healthy adults did not reduce incidence of cancer or major cardiovascular events.
Supplementary Material
ACKNOWLEDGMENTS
VITAL Investigators, Staff, and Study Participants
The authors thank the VITAL investigators, staff, and the trial participants for their outstanding dedication and commitment.
Drs. Manson, Cook, Lee, and Buring had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analyses.
Funding/Support: VITAL was supported by grants U01 CA138962 and R01 CA138962, which included support from the National Cancer Institute, National Heart, Lung and Blood Institute, Office of Dietary Supplements, National Institute of Neurological Disorders and Stroke, and the National Center for Complementary and Integrative Health. The ancillary studies are supported by grants from multiple Institutes, including the National Heart, Lung and Blood Institute; the National Institute of Diabetes and Digestive and Kidney Diseases; the National Institute on Aging; the National Institute of Arthritis and Musculoskeletal and Skin Diseases; the National Institute of Mental Health; and others.
Pharmavite LLC of Northridge, California (vitamin D) and Pronova BioPharma of Norway and BASF (Omacor fish oil) donated the study agents, matching placebos, and packaging in the form of calendar packs. Quest Diagnostics (San Juan Capistrano, CA) measured serum 25-hydroxyvitamin D at no cost to the study.
VITAL was approved by the Institutional Review Board of Partners Healthcare/Brigham and Women’s Hospital, and the study agents have received Investigational New Drug Approval from the U.S. Food and Drug Administration.
Role of the Sponsor: The National Institutes of Health sponsors of VITAL had a collaborative role in the design and conduct of the study and interpretation of the data. Final decisions concerning the above, however, as well as data collection, management, analysis, review or approval of the manuscript, and decision to submit the manuscript for publication resided with study investigators and the study research group. The opinions expressed in the manuscript are those of the study authors and do not necessarily represent the views of the Department of Health and Human Services/National Institutes of Health.
Footnotes
Conflict of Interest Disclosures: All authors completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
VITAL is registered at clinicaltrials.gov (NCT01169259). The VITAL website is www.vitalstudy.org.
The VITAL investigator group is listed in the Supplementary Appendix, available at NEJM.org.
Contributor Information
JoAnn E. Manson, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, Department of Epidemiology.
Nancy R. Cook, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, Department of Epidemiology.
I-Min Lee, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, Department of Epidemiology.
William Christen, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
Shari S. Bassuk, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
Samia Mora, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
Heike Gibson, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
David Gordon, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
Trisha Copeland, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
Denise D’Agostino, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
Georgina Friedenberg, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
Claire Ridge, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
Vadim Bubes, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
Edward L. Giovannucci, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA.
Walter C. Willett, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, Department of Epidemiology, Department of Nutrition (ELG, WCW), Harvard T.H. Chan School of Public Health, Boston, MA.
Julie E. Buring, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, Department of Epidemiology.
References
- 1.Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 2.LeFevre ML; U.S. Preventive Services Task Force. Screening for vitamin D deficiency in adults: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;162:133–40. [DOI] [PubMed] [Google Scholar]
- 3.Kantor ED, Rehm CD, Du M, White E, Giovannucci EL. Trends in dietary supplement use among US adults from 1999–2012. JAMA. 2016;316:1464–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manson JE, Bassuk SS, Lee IM, et al. The VITamin D and OmegA-3 TriaL (VITAL): Rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2012;33:159–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin L, Ordonez-Mena JM, Chen T, Schottker B, Arndt V, Brenner H. Circulating 25-hydroxyvitamin D serum concentration and total cancer incidence and mortality: a systematic review and meta-analysis. Preventive Medicine. 2013;57:753–64. [DOI] [PubMed] [Google Scholar]
- 6.Zhang R, Li B, Gao X, et al. Serum 25-hydroxyvitamin D and the risk of cardiovascular disease: dose-response meta-analysis of prospective studies. Am J Clin Nutr. 2017;105:810–819. [DOI] [PubMed] [Google Scholar]
- 7.Moyer VA; U.S. Preventive Services Task Force. Vitamin, mineral, and multivitamin supplements for the primary prevention of cardiovascular disease and cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:558–64. [DOI] [PubMed] [Google Scholar]
- 8.Manson JE, Bassuk SS. Vitamin D research and clinical practice: at a crossroads. JAMA. 2015;313:1311–2. [DOI] [PubMed] [Google Scholar]
- 9.Keum N, Giovannucci E. Vitamin D supplements and cancer incidence and mortality: a meta-analysis. Br J Cancer. 2014;111:976–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst Rev. 2014;6:CD007469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bassuk SS, Manson JE, Lee IM, et al. Baseline characteristics of participants in the VITamin D and OmegA-3 TriaL (VITAL). Contemp Clin Trials. 2016;47:235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Binkley N, Carter GD. Toward clarity in clinical vitamin D status assessment: 25(OH)D assay standardization. Endocrinol Metab Clin North Am. 2017;46:885–899. [DOI] [PubMed] [Google Scholar]
- 13.Fritz AG, Percy C, Jack A, et al. International Classification of Diseases for Oncology (ICD-O), Third Edition. Geneva: World Health Organization; 2000. [Google Scholar]
- 14.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD; Writing Group on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–35.22923432 [Google Scholar]
- 15.Adams HP Jr., Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 16.Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ. 2003;326:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wactawski-Wende J, Kotchen JM, Anderson GL, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354:684–96. [DOI] [PubMed] [Google Scholar]
- 18.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85:1586–91. [DOI] [PubMed] [Google Scholar]
- 19.Avenell A, MacLennan GS, Jenkinson DJ, et al. Long-term follow-up for mortality and cancer in a randomized placebo-controlled trial of vitamin D(3) and/or calcium (RECORD trial). J Clin Endocrinol Metab. 2012;97:614–22. [DOI] [PubMed] [Google Scholar]
- 20.Lappe J, Watson P, Travers-Gustafson D, et al. Effect of vitamin D and calcium supplementation on cancer incidence in older women: a randomized clinical trial. JAMA. 2017;317:1234–1243. [DOI] [PubMed] [Google Scholar]
- 21.Scragg R, Khaw KT, Toop L, et al. Monthly high-dose vitamin D supplementation and cancer risk: a post hoc analysis of the Vitamin D Assessment randomized clinical trial. JAMA Oncol. 2018:e182178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hollis BW, Wagner CL. Clinical review: The role of the parent compound vitamin D with respect to metabolism and function: Why clinical dose intervals can affect clinical outcomes. J Clin Endocrinol Metab. 2013;98:4619–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14:342–57. [DOI] [PubMed] [Google Scholar]
- 24.Mondul AM, Weinstein SJ, Layne TM, Albanes D. Vitamin D and cancer risk and mortality: state of the science, gaps, and challenges. Epidemiol Rev. 2017;39:28–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCullough ML, Zoltick ES, Weinstein SJ, et al. Circulating vitamin D and colorectal cancer risk: an international pooling project of 17 cohorts. J Natl Cancer Inst. 2018. [e-pub 2018 Jun 14] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bauer SR, Hankinson SE, Bertone-Johnson ER, Ding EL. Plasma vitamin D levels, menopause, and risk of breast cancer: dose-response meta-analysis of prospective studies. Medicine. 2013;92:123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsia J, Heiss G, Ren H, et al. Calcium/vitamin D supplementation and cardiovascular events. Circulation. 2007;115:846–54. [DOI] [PubMed] [Google Scholar]
- 28.Sanders KM, Stuart AL, Williamson EJ, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303:1815–22. [DOI] [PubMed] [Google Scholar]
- 29.Ford JA, MacLennan GS, Avenell A, Bolland M, Grey A, Witham M; RECORD Trial Group. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr. 2014;100:746–55. [DOI] [PubMed] [Google Scholar]
- 30.Scragg R, Stewart AW, Waayer D, et al. Effect of monthly high-dose vitamin D supplementation on cardiovascular disease in the Vitamin D Assessment Study : a randomized clinical trial. JAMA Cardiol. 2017;2:608–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elamin MB, Abu Elnour NO, Elamin KB, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:1931–42. [DOI] [PubMed] [Google Scholar]
- 32.Rejnmark L, Bislev LS, Cashman KD, et al. Non-skeletal health effects of vitamin D supplementation: A systematic review on findings from meta-analyses summarizing trial data. PloS One. 2017;12:e0180512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2014:CD007470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bolland MJ, Grey A, Gamble GD, Reid IR. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta-analysis. Lancet Diabetes & Endocrinology. 2014;2:307–20. [DOI] [PubMed] [Google Scholar]
- 35.Shapses SA, Lee EJ, Sukumar D, Durazo-Arvizu R, Schneider SH. The effect of obesity on the relationship between serum parathyroid hormone and 25-hydroxyvitamin D in women. J Clin Endocrinol Metab. 2013;98:E886–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drincic AT, Armas LA, Van Diest EE, Heaney RP. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity (Silver Spring). 2012;20:1444–8. [DOI] [PubMed] [Google Scholar]
- 37.Rothwell PM, Cook NR, Gaziano JM, et al. Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: analysis of individual patient data from randomised trials. Lancet. 2018;392(10145):387–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Renehan AG, Zwahlen M, Egger M. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat Rev Cancer. 2015;15:484–98. [DOI] [PubMed] [Google Scholar]
- 39.Bassuk SS, Manson JE. Chapter 66: Randomized clinical trials of vitamin D, with a focus on the VITamin D and OmegA-3 TriaL (VITAL) In: Feldman D, Pike JW, Bouillon R, Giovannucci E, Goltzman D and Hewison M, eds. Vitamin D, Volume 2: Health, Disease, and Therapeutics, Fourth Edition San Diego CA: Academic Press; 2018: 167–176. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.