Abstract
Objective:
The smr and qacA/B genes in Staphylococcus aureus confer tolerance to antiseptics and are associated with nosocomial acquisition of infection and underlying medical conditions. Such antiseptic tolerance (AT) genes have also been reported in coagulase-negative staphylococci (CoNS) and enterococci, however, little data are available regarding their prevalence. We sought to describe the frequency of AT genes among bloodstream isolates of S. aureus, CoNS and enterococci at Texas Children’s Hospital (TCH).
Methods:
Banked CoNS, S. aureus and enterococci isolated from blood cultures collected from 10/1/2016–10/1/2017 were obtained from the TCH clinical microbiology laboratory. All isolates underwent PCR for the qacA/B and smr genes. Medical records were reviewed for all cases.
Results:
103 CoNS, 19 Enterococcus spp. and 119 S. aureus isolates were included. 80.6% of CoNS possessed at least one AT gene compared to 37% of S. aureus and 43.8% of E. faecalis isolates (p< 0.001). Among CoNS bloodstream isolates, the presence of either AT gene was strongly associated with nosocomial infection (p<0.001). AT genes in S. aureus were associated with nosocomial infection (p=0.025) as well as the diagnosis of CLA-BSI (p=0.04) and recent hospitalizations (p<0.001). There was no correlation with genotypic AT in E. faecalis and any examined clinical variable.
Conclusions:
AT is common among bloodstream staphylococci and E. faecalis isolates at TCH. Among CoNS, the presence of AT genes is strongly correlated with nosocomial acquisition of infection consistent with previous studies in S. aureus. These data suggest that the healthcare environment contributes to AT among staphylococci.
Keywords: Coagulase negative staphylococci, S. aureus, chlorhexidine, antiseptic tolerance, bacteremia
Introduction
Gram-positive bacteria are the principal causative agents of healthcare associated infections (HAI) in both adults and children.1–3 Chlorhexidine gluconate (CHG)-based antiseptics are commonly employed in efforts to diminish the frequency of HAIs.4–8 These strategies have been endorsed in guidelines produced by the Centers for Disease Control and Prevention (CDC) and the Infectious Diseases Society of America (IDSA) for the prevention of HAIs.9–11
A number of efflux pump genes in staphylococci are associated with higher minimum inhibitory and bactericidal concentrations (MIC and MBC) to CHG and other antiseptics (such as benzalkonium chloride and cetrimide).12–15 In S. aureus, the plasmid-borne smr and qacA/B gene complexes have been most commonly implicated, with such organisms often being termed antiseptic tolerant.16 The incidence of antiseptic tolerant S. aureus has increased following widespread use of CHG in hospital units and these organisms are also associated with invasive infections.17–20 In a recent study by our group, the presence of genotypic antiseptic tolerance (AT) in S. aureus was independently associated with chronic medical comorbidities in the host and nosocomial acquisition of infection.21 The relative prevalence of these genes among S. aureus in pediatric populations varies widely, ranging from 1–44.5% depending on geographic location and the type of patients studied.20,22–24 Pediatric specific data are of relevance given the variable use of CHG in children’s hospitals, particularly in neonatal intensive care units, arising from concerns for systemic absorption and safety in young children.25,26 We have reported that among a random sample of S. aureus isolates at Texas Children’s Hospital (TCH) 32.8% possessed either smr or qacA/B,21 suggesting our center represents a high prevalence region for AT.
Other important bacterial contributors to HAIs in children include the coagulase-negative staphylococci (CoNS) and Enterococcus spp. CoNS and enterococci can carry smr and qacA/B and their presence has likewise been associated with elevations in antiseptic MICs.27–29 The current literature, however, regarding the prevalence and clinical significance of AT in these other important gram-positive pathogens is sparse.30,31 Among a general adult population in Hong Kong, 13.5% of CoNS colonizing isolates were positive for qacA/B.32 The prevalence of AT genes among pediatric clinical isolates of CoNS is limited to a report from a NICU in France30 as well as eighteen isolates in a study at Seattle Children’s Hospital.31 We sought to determine the frequency of carriage of smr and qacA/B among bloodstream isolates of CoNS and enterococci compared to that of S. aureus at TCH. In addition, we examined the relationship between the presence/absence of these genes and clinical factors in affected patients.
Methods
Microbiology and Molecular Biology
Blood culture isolates of S. aureus, CoNS and Enterococcus spp. obtained in the routine course of care from Oct 1, 2016- Oct 1, 2017 were procured from the TCH clinical microbiology laboratory. TCH is a freestanding children’s hospital and tertiary referral center in Houston, TX with 592 licensed inpatient beds and >20,000 admissions annually. All bloodstream isolates identified by the clinical microbiology laboratory are subcultured, frozen at −80°C and banked for at least one calendar year. Isolates for this study were subcultured and transferred to the Edward O Mason, Jr. Infectious Diseases Research Laboratory (IDRL) where they were assigned a strain number and additional analyses were performed. A portion of all isolates immediately underwent whole DNA extraction using QIAcube (Qiagen, Valencia, CA). For this study, only one bacterial isolate per episode of bacteremia was included; no colonization isolates were used in this study. CoNS and enterococci were identified to the species level in the clinical microbiology laboratory using MALDI-TOF mass spectrometry (Vitek MS, bioMerieux USA, Durham, NC). Only blood cultures obtained from patients < 19-years old were included in this study.
All isolates were subjected to PCR for the qacA/B and smr genes using previously published primers.20 A subset of PCR products from CoNS and Enterococcus isolates performed during the first PCR run underwent sequencing, were subjected to BLAST algorithms and compared against published gene sequences (Genebank JF817385 and JF817387) to confirm identity of PCR products. Laboratory personnel performing these studies were blinded to clinical data.
Antimicrobial susceptibility was performed by the TCH clinical microbiology laboratory in the routine course of care using Vitek-2 (bioMerieux USA, Durham, NC). Sixty CoNS isolates were chosen for CHG MIC determinations in the IDRL using the macrobroth dilution method;20 every sixth sequential isolate was chosen with this process repeated until a total of sixty isolates were selected for MIC determinations.
Corresponding medical records were reviewed for all patients with attention to underlying conditions, preceding CHG use,21 recent surgery or hospitalization, site of acquisition of infection and infectious diagnosis. Investigators reviewing medical records and abstracting clinical data were blinded to the results of the above molecular analyses which were performed in tandem with medical record review. This study was approved by the institutional review board of Baylor College of Medicine.
Infection Control Practices
CHG-containing products are utilized in a number of infection control practices at our institution.20 At TCH, CHG is included in central venous line (CVL) insertion and maintenance bundles. Daily CHG baths are employed at TCH for all hospitalized patients with a CVL in situ. All patients undergoing elective surgery at TCH are encouraged to take a CHG bath the night prior to operation and this agent is the skin cleanser of choice in our operating rooms immediately prior to incision. Additionally, daily CHG mouthwashes are routinely prescribed at TCH for hematopoietic stem cell transplant (HSCT) recipients and those with acute myeloid leukemia (AML). Notable exceptions to these rules include patients cared for in the neonatal intensive care unit (NICU) in which procedural CHG is only used in infants who are 1) at least 28 week corrected gestational age, 2) at least 7 days old and 3) weigh at least 1000 grams; iodophor preparations are used for procedural disinfection in infants not meeting these criteria. CHG bathes are only used in infants with corrected gestational age ≥ 48 weeks. Quaternary ammonium compound antiseptics are used for the cleaning of rooms and inanimate surfaces at TCH.
Definitions
Sites of acquisition of infection were considered as follows: community-acquired, community-onset healthcare-associated (CO-HCA) and nosocomial. Community-acquired infections were those occurring in otherwise healthy children with onset of signs and symptoms of infection in the community. CO-HCA infections were considered those in which signs and symptoms of infection developed in the outpatient setting in children with underlying medical conditions,21,33 excluding well-controlled asthma, eczema and allergic rhinitis; this definition was used to capture patients with underlying conditions that place them at higher risk for infection who may not develop infection in the hospital per se. Previous studies in S. aureus conducted by our group and others have shown that CO-HCA infections are distinct from community-acquired and hospital-acquired infections in terms of molecular features and antimicrobial susceptibility.33,34 Nosocomial infections were those in which the patient developed signs/symptoms of infection ≥ 72 hours after hospital admission.35 Patients were considered to have CHG exposure in the prior three months if they had documented use of any CHG preparation, surgery or CVL placement at TCH in the preceding three months or diagnosis of AML or HSCT.21 Patients who underwent surgery but did not have other indications for CHG use were considered to have one-time CHG use with others considered to have recurrent CHG use. CLA-BSI was defined in accordance with national guidelines;36 endocarditis was defined using the modified Duke criteria. For purposes of this study, CoNS isolates were regarded as true infections if isolated in more than one blood culture or if considered as a true infection by the treating physician. Length of stay was defined as time in calendar days from date of positive blood culture to hospital discharge. Recurrent infection was considered culture proven recurrence of infection due to an organism of the same species within 30 days of completing treatment.
Statistical Analysis
With regards to CoNS infections, only true infections were included in analyses. Categorical variables were compared with Fisher’s exact test and continuous variables with Wilcoxon-Rank Sum or Kruskal-Wallis tests as appropriate with two-tailed p values <0.05 considered statistically significant. Clinical variables associated with the presence of antiseptic tolerance genes at the p<0.1 level were included in a multivariable logistic regression analysis. All statistical analyses were performed with STATA ver. 15 (STATA Corp, College Station, TX).
Results
During the study period, 404 viable isolates were obtained from 389 unique patients. Two-hundred fifty-four patients with blood cultures positive for CoNS had complete medical records and 151 of these were considered to be contaminants. After excluding these contaminants, 103 CoNS, 119 S. aureus and 19 Enterococcus isolates (totaling 241 unique isolates from 238 patients) were included in final analyses. The median age of studied patients was 1.3 years (Interquartile range [IQR]: 0.2–7.9 years) and 78.4% of patients had underlying medical conditions (Table 1). Significant differences in age, comorbidities and diagnoses existed between patients with bacteremia due to CoNS, S. aureus and enterococci.
Table 1.
General Characteristics of the Study Population
| N (%) |
S. aureus, n=119 |
CoNS, n=103 |
Entero- coccus spp., n=19 |
P value | |
|---|---|---|---|---|---|
| Median age, years (IQR)* | 1.3 (0.2-7.9) | 3.54 (0.3-11.5) | 0.91 (0.16-7.9) | 0.9 (0.12-5.8) | 0.01 |
| Age ≤ 28 days | 31 (12.9) | 13 (10.9) | 14 (13.5) | 4 (21.1) | 0.4 |
| Female Gender | 105 (43.5) | 49 (41.2) | 47 (45.6) | 9 (47.4) | 0.76 |
| Black Race | 45 (18.6) | 20 (16.8) | 22 (21.3) | 3 (15.8) | 0.67 |
| Hispanic Ethnicity | 102 (42.3) | 50 (42) | 44 (42.7) | 8 (42.1) | 1 |
| Underlying Medical Conditions† | 189 (78.4) | 77 (64.7) | 101 (98.1) | 11 (57.8) | <0.001 |
| Prematurity | 43 (17.8) | 17 (14.3) | 24 (23.3) | 2 (10.5) | 0.17 |
| Malignancy | 48 (19.9) | 12 (10.1) | 35 (33.9) | 1 (5.3) | <0.001 |
| Congenital Heart Disease | 30 (12.4) | 18 (15.1) | 12 (11.6) | 0 | 0.19 |
| Neurologic Conditions | 9 (3.7) | 3 (2.5) | 2 (1.9) | 4 (21.1) | 0.005 |
| Short Gut | 26 (10.7) | 10 (8.4) | 14 (13.6) | 2 (10.5) | 0.45 |
| Solid Organ Transplant | 8 (3.3) | 2 (1.7) | 6 (5.8) | 0 | 0.2 |
| End Stage Renal Disease | 4 (1.7) | 2 (1.7) | 2 (1.9) | 0 | 1 |
| Acquisition | <0.001 | ||||
| Community-acquired | 57 (23.6) | 48 (40.3) | 0 | 9 (47.4) | |
| Community-onset healthcare associated | 95 (39.4) | 33 (27.7) | 57 (55.3) | 5 (26.3) | |
| Nosocomial | 89 (36.9) | 38 (31.9) | 46 (44.6) | 5 (26.3) | |
| Source of Bacteremia | <0.001 | ||||
| Central Line Associated Bloodstream Infection (CLABSI) | 103 (42.7) | 28 (23.5) | 72 (69.9) | 3 (15.7) | |
| Endocarditis | 21 (8.7) | 16 (13.4) | 4 (3.9) | 1 (5.3) | |
| Musculoskeletal Infection | 36(14.9) | 36 (30.2) | 0 | 0 | |
| Skin and soft tissue infection | 12 (5) | 11 (9.2) | 1 (0.9) | 0 | |
| Surgical Site Infection | 6 (2.5) | 5 (4.2) | 1 (0.9) | 0 | |
| Urinary Tract Infection | 3 (1.2) | 0 | 1 (0.9) | 2 (10.5) | |
| Bacteremia Without a Focus | 41 (17) | 13 (10.9) | 16 (15.5) | 12 (63.1) | |
| Other | 20 (8.3) | 10 (8.4) | 9 (8.7) | 1 (5.3) |
IQR- interquartile range;
Most common underlying conditions listed, categories are not mutually exclusive
Seven different CoNS species were identified by MALDI including S. epidermidis (n=70), S. hominis (n=22), S. haemolyticus (n=4), S. capitis (n=3), S. warnerii (n=2), S. lugdenensis (n=1), and S. saprophyticus (n=1). The majority of enterococci were E. faecalis (n=16, 84.2%) with the remainder being E. faecium (n=3). Among S. aureus isolates, 25/119 (21%) were methicillin-resistant.
Antiseptic Tolerance Genes
Overall, 80.6% (83/103) of CoNS isolates possessed either qacA/B or smr compared to 37% (44/119) of S. aureus and 43.8% (7/16) of E. faecalis and none (0/3) of the E. faecium (p<0.001, Figure 1). qacA/B was detected in 13.4% (16/119) of S. aureus, 70.8% (73/103) of CoNS and 43.8% (7/16) of E. faecalis (p<0.001). smr was detected in 23.5% (28/119) of S. aureus, 33% (34/103) of CoNS and 25% (4/16, p=0.28) of E. faecalis. Both AT genes were detected in 23.3% (24/103) of CoNS, 25% (4/16) of E. faecalis and none of the S. aureus (p<0.001). The proportion of CoNS isolates with AT genes was similar among isolates considered true infections (83/103, 80.6%) and contaminants (128/151, 84.7%, p=0.39). Sequenced qacA/B- and smr-PCR products obtained from CoNS and E. faecalis were identical to published sequences of these respective genes in S. aureus.
Figure 1. Comparison of Proportion of Isolates with Antiseptic Tolerance Genes in Staphylococci and Enterococci, n=241.
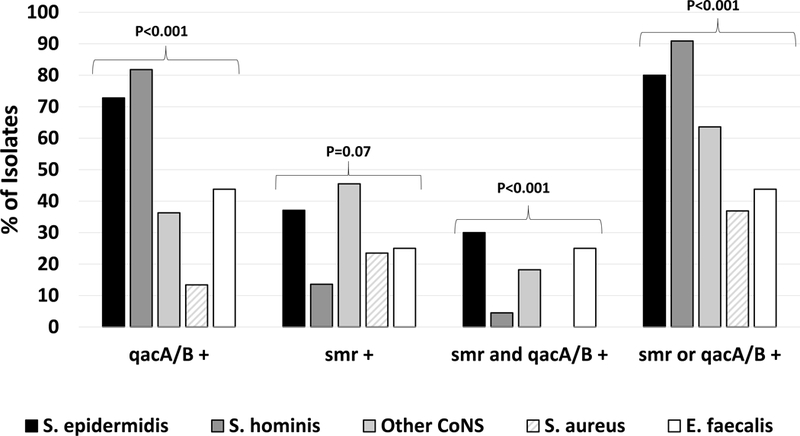
Simple proportions of isolates bearing genes of interest were compared using Fisher’s exact test.
Antiseptic Tolerance
Sixty CoNS isolates had CHG MICs determined by broth dilution. Isolates with both qacA/B and smr had higher MICs than those without these genes (MIC90: 0.25 vs. 0.03 μg/ml, p=0.06, Supplemental Table 1).
Antiseptic Tolerance and Clinical Variables
Among patients with CoNS infections, a greater proportion of qacA/B-positive strains were associated with nosocomial infections (41/73, 56.1% vs. 5/30, 16.7%, p<0.001, Table 2) than qacA/B-negative isolates. smr-positive CoNS infections were more often associated with nosocomial acquisition (21/34, 61.8% vs. 25/69, 36.2%, p=0.02) and a longer median length of hospital stay (14.5 vs. 6.5 days p=0.02, Table 3). When considered as a group, antiseptic tolerant CoNS were associated with a higher rate of nosocomial acquisition of infection (46/83, 55.4% vs. 0/20, p<0.001, Supplemental Table 2); there were no differences in any other examined clinical variable between antiseptic tolerant and susceptible CoNS.
Table 2.
Comparison of Patients with Bacteremia due to qacA/B-Positive vs. qacA/B–Negative CoNS
|
qacA/B-Positive CoNS, n=73 |
qacA/B-Negative CoNS, n=30 |
P value | |
|---|---|---|---|
| Median Age, years (IQR) | 1.4 (0.23-9.4) | 0.5 (0.08-6.4) | 0.32 |
| Age ≤ 28 days | 9 (12.3) | 5 (16.7) | 0.54 |
| Median Duration of Bacteremia, days (IQR) | 1 (1-2) | 1 (1-2) | 0.65 |
| CLABSI | 53 (72.6) | 21 (70) | 1 |
| Line Removed | 24/53 (45.3) | 11/21 (52.4) | 0.62 |
| CHG Use in Prior 3 Months | 57 (78.1) | 22 (73.3) | 0.62 |
| One-Time CHG Use | 6 (8.2) | 2 (6.6) | 1 |
| Recurrent CHG Use | 51 (69.9) | 20 (66.6) | 0.86 |
| CVL in situ | 63 (86.3) | 28 (93.3) | 0.5 |
| Hospital Admission in Prior 3 Month | 69 (94.5) | 29 (96.7) | 1 |
| Surgery In Prior 3 Months | 53 (72.6) | 21 (70) | 0.8 |
| Underlying Conditions | 70 (95.9) | 30 (100) | 0.5 |
| Nosocomial Acquisition of Infection* | 41 (56.1) | 5 (16.7) | <0.001 |
| Antibiotics in Prior 3 Months | 68 (93.1) | 27 (90) | 0.69 |
| Infection Recurrence | 1 (1.4) | 2 (6.7) | 0.2 |
| Mortality | 1 (1.4) | 1 (3.3) | 0.5 |
| Median Length of Stay, days (IQR) | 9 (4-33.5) | 8 (5-23) | 0.92 |
No patients with CoNS bacteremia had a community-acquired infection
Table 3.
Comparison of Patients with Bacteremia due to smr-Positive versus smr-Negative CoNS
|
smr-Positive CoNS, n=34 |
smr-Negative CoNS, n=69 |
P value | |
|---|---|---|---|
| Median Age, years (IQR) | 0.69 (0.13-8.8) | 1.24 (0.23-6.24) | 0.99 |
| Age ≤ 28 days | 5 (14.7) | 9 (13) | 1 |
| Median Duration of Bacteremia, days (IQR) | 1 (1-2) | 1 (1-2) | 0.39 |
| CLABSI | 24 (70.6) | 50 (72.4) | 1 |
| Line Removed | 11/24 (45.8) | 24/50 (48) | 1 |
| CHG Use in Prior 3 Months | 27 (79.4) | 52 (75.3) | 0.81 |
| One-Time CHG Use | 7 (20.5) | 1 (1.4) | 0.002 |
| Recurrent CHG Use | 20 (58.8) | 51 (73.9) | 0.17 |
| CVL in situ | 29 (85.3) | 62 (89.8) | 0.53 |
| Hospital Admission in Prior 3 Month | 32 (94.1) | 66 (95.6) | 1 |
| Surgery In Prior 3 Months | 25 (73.5) | 49 (71) | 1 |
| Underlying Conditions | 32 (94.1) | 68 (98.5) | 0.25 |
| Nosocomial Acquisition of Infection* | 21 (61.8) | 25 (36.2) | 0.02 |
| Antibiotics in Prior 3 Months | 27 (79.4) | 64 (92.7) | 0.06 |
| Infection Recurrence | 0 | 2 (2.9) | 1 |
| Mortality | 0 | 2 (2.9) | 1 |
| Median Length of Stay, days (IQR) | 14.5 (7-54) | 6.5 (3-19.5) | 0.02 |
No patients with CoNS bacteremia had a community-acquired infection
Antiseptic tolerant and susceptible S. aureus were also compared (Table 4). Patients with antiseptic tolerant S. aureus infections were younger, had more medical comorbidities, recent hospitalizations, exposure to CHG and antibiotics, more often acquired infection in the hospital and more often experienced infection recurrence. Additionally, antiseptic tolerant S. aureus isolates were more often associated with the diagnosis of CLA-BSI (15/44, 34.1% vs. 13/75, 17.3%, p=0.046) and less often with the diagnosis of musculoskeletal infection (1/44, 2.3% vs. 31/75, 41.3%, p<0.001) than antiseptic susceptible infections. In multivariable analyses, nosocomial/CO-HCA infections were more often associated with AT in S. aureus while musculoskeletal infections were less often associated with AT. Among enterococci, there was no significant association with AT genes and any examined clinical variable.
Table 4.
Comparison of Patients with Bacteremia due to antiseptic tolerant versus susceptible S. aureus
| Antiseptic Tolerant S. aureus, n=44 |
Antiseptic Susceptible S. aureus, n= 75 |
P value | Adjusted P value |
OR | 95% CI |
|
|---|---|---|---|---|---|---|
| Median Age, years (IQR) | 0.74 (0.13-8.3) | 6.3 (1.1-12.6) | 0.008 | 0.22** | 1.97 | 0.66-5.87 |
| Age ≤ 28 days | 8 (18.2) | 5 (6.7) | 0.07 | |||
| Median Duration of Bacteremia, days (IQR) | 1 (1-2) | 1 (1-2) | 0.67 | |||
| CLABSI | 15 (34.1) | 13 (17.3) | 0.046 | 0.88 | 0.9 | 0.24-3.34 |
| Line Removal | 10 (66.7) | 10 (76.9) | 0.69 | |||
| Musculoskeletal Infection | 1 (2.3) | 31 (41.3) | <0.001 | 0.03 | 0.15 | 0.03-0.84 |
| CHG Use in Prior 3 Months | 24 (54.5) | 25 (33.3) | 0.034 | 0.54 | 1.6 | 0.36-7.03 |
| One-Time CHG Use | 11 (24) | 11 (14.6) | 0.22 | |||
| Recurrent CHG Use | 13 (29.5) | 14 (18.6) | 0.18 | |||
| CVL in situ | 25 (56.8) | 21 (28) | 0.003 | 0.89 | 1.1 | 0.27-4.35 |
| Hospital Admission in Prior 3 Month | 37 (84.1) | 36 (48) | <0.001 | 0.62 | 0.56 | 0.06-5.53 |
| Surgery In Prior 3 Months | 23 (52.3) | 26 (34.6) | 0.08 | 0.22 | 0.37 | 0.08-1.78 |
| Underlying Conditions | 39 (88.6) | 38 (51.3) | <0.001 | 0.9 | 1.1 | 0.19-6.48 |
| Acquisition | <0.001 | 0.049† | 3.3 | 1.5-21.1 | ||
| Community-acquired | 7 (15.9) | 41 (54.6) | ||||
| Community-onset healthcare associated | 17 (38.6) | 16 (21.3) | ||||
| Nosocomial | 20 (45.5) | 18 (24) | ||||
| Antibiotics in Prior 3 Months | 39 (88.6) | 39 (52) | <0.001 | 0.25 | 2.94 | 0.47-18.24 |
| Infection Recurrence* | 8 (18.2) | 2 (2.6) | 0.005 | |||
| Mortality | 2 (4.5) | 2 (2.6) | 0.63 | |||
| Median Length of Stay, days (IQR) | 14 (6-84) | 10 (6-35) | 0.19 | |||
| Methicillin-resistance | 13 (29.5) | 12 (16.2) | 0.11 | |||
| Clindamycin-resistance | 13 (30.2) | 13 (17.6) | 0.17 |
3 patients with antiseptic tolerant S. aureus and infection recurrence had CLABSI initially treated with line in situ compared to 1 patient in the antiseptic susceptible group with infection recurrence. If these four patients are removed from the analyses, a statistically significant relationship between antiseptic tolerance and infection recurrence remains (5/41, 12.2%, vs. 1/74, 1.4%, p=0.03).
In multivariable analyses, age was dichotomized as > vs. ≤ 0.33 years (the bottom quartile of age for all patients with S. aureus bacteremia).
Acquisition of infection is dichotomized as healthcare associated (CO-HCA and nosocomial) vs. community-acquired.
Discussion
Despite advances in infection control and prevention, HAIs still cause substantial morbidity in the pediatric population with S. aureus, CoNS and enterococci being significant contributors.3 Antiseptics have been demonstrated to be an effective means to reduce the incidence of HAIs, however, concerns have been raised in recent years for the emergence of reduced susceptibility with widespread use of these agents.
We have investigated the prevalence of AT genes among staphylococci and enterococci causing bacteremia at a large children’s hospital. Previous studies in CoNS have reported that 13–80% of isolates possessed AT genes.27,30–32 We found that 80.6% of CoNS isolates possessed either qacA/B or smr. The proportion of isolates with AT in our study is higher than that reported at most other institutions, particularly those centers caring for children. Soma reported that 65% of 18 CoNS isolates recovered from skin swabs studied at Seattle Children’s Hospital bore qacA/B;31 a similar proportion of CoNS were positive for qacA/B among a study of CLABSI isolates obtained from very low birth weight infants in France.30 The high frequency of qacA/B-positive CoNS in our population is comparable to a study from the United Kingdom in which 80% of bloodstream S. epidermidis isolates carried qacA/B.27 In our center we discovered that the frequency of detection of AT genes in CoNS was much higher than that detected among S. aureus (36.2%). Notably, however, the proportion of S. aureus isolates which were positive for AT genes was higher than that reported by other studies in pediatric populations (1–18.5%)22–24 but consistent with prior work at our institution.21,22
In previous studies in S. aureus conducted at our institution, the presence of AT genes was found to be independently associated with nosocomial infections and underlying medical conditions in the host.21 In the present study, antiseptic tolerant S. aureus were again associated with underlying medical conditions and nosocomial acquisition of infection as well as a number of other clinical factors in univariable analyses including recent hospitalization and the diagnosis of CLA-BSI. Importantly, we noted a statistically significant association with the presence of genotypic AT in CoNS and nosocomial acquisition of infection. These findings suggest that the healthcare environment and/or medical complexity select for AT in other organisms besides S. aureus. This is of particular importance given that previous studies examining the epidemiology and clinical impact of AT have largely focused on S. aureus with little attention given to other pathogens. CoNS was the predominant cause of all pediatric HAIs in a multinational study,37 highlighting the clinical significance of this pathogen. We found no other clinical variables to be associated with AT in CoNS; the small number of healthy patients with true CoNS infections likely limited the degree to which other clinical factors could be associated with AT in these organisms. In contrast to our findings in staphylococci, we observed no relationship between AT genes in enterococci and any examined clinical variable; this was likely a consequence of the small number of enterococci studied.
We have previously reported an association with AT in S. aureus and invasive infections as well as prolonged hospital stay.21 In the present study, we found an association with genotypic AT in S. aureus and recurrence of infection, however, the reasons for this observation are unclear. Work from ex vivo and in vivo models suggest that S. aureus expressing such multidrug-resistance efflux pumps may have an enhanced ability to colonize surfaces as well as cause disease.38,39 It is conceivable that if such organisms have an enhanced colonization capacity, they may be more likely to cause recurrent infection despite appropriate treatment. This finding is, in part, also likely related to the higher rate of CLA-BSI in the tolerant group and recurrences occurring as a result of not removing infected central lines in a minority of cases. One could also hypothesize that this phenomenon is a consequence of virulent strain types which happen to possess AT genes rather than a consequence of the genes themselves. Such findings further highlight the potential impact of antiseptic tolerant strains for public health. It is worth acknowledging, however, that the finding of higher recurrence in antiseptic tolerant S. aureus infections may have been a result of these patients being more ill and thus more likely to fail treatment.
There are additional limitations to this study that should be acknowledged. Foremost, these findings are from a single center with a previously described high prevalence of antiseptic tolerant S. aureus20,21 and thus our findings may not be generalizable to all regions. The retrospective nature of the study limits the degree to which clinical risk factors can be definitively associated with antiseptic tolerant organisms. Additionally, given that colonization cultures were not examined in this study, we are unable to assess the impact of antiseptic tolerant organism colonization on risk of subsequent bacteremia or the potential mitigating effects of antibiotic/antiseptic use.
In conclusion, genotypic AT is common among bloodstream staphylococci and E. faecalis isolates at our institution. The presence of AT genes was strongly associated with nosocomial acquisition of infection in staphylococci, implying a role for the hospital environment in selecting for these pathogens. Larger studies are needed to further explore and validate these findings.
Supplementary Material
Acknowledgements
Dr. Kaplan serves as PI on an investigator initiated clinical trial sponsored by Allergan as well as site PI of a clinical trial sponsored by Merck; Dr. McNeil serves as a co-investigator on these studies neither of which is directly related to the presented work. No authors have significant financial conflicts of interest.
Funding source
This study was funded by NIAID K23AI099159 and The Texas Children’s Hospital Pediatric Pilot Research Fund (both to JCM).
Literature Cited
- 1.Wisplinghoff H, Seifert H, Tallent SM, Bischoff T, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in pediatric patients in United States hospitals: epidemiology, clinical features and susceptibilities. Pediatr Infect Dis J 2003;22:686–691. [DOI] [PubMed] [Google Scholar]
- 2.Murray MT, Krishnamurthy G, Corda R, et al. Surgical site infections and bloodstream infections in infants after cardiac surgery. J Thorac Cardiovasc Surg 2014;148:259–265. [DOI] [PubMed] [Google Scholar]
- 3.Blackburn RM, Henderson KL, Minaji M, Meuller-Pedobdy B, Johnson AP, Sharland M. Exploring the epidemioogy of hospital-acquired bloodstream infections in children in England (January 2009- March 2010) by linkage of national hospital admissions and microbiological databases. J Pediatr Infect Dis Soc 2012;1:284–292. [DOI] [PubMed] [Google Scholar]
- 4.Bleasdale SC, Trick WE, Gonzalez IM, Lyles RD, Hayden MK, Weinstein RA. Effectiveness of chlorhexidine bathing to reduce catheter-associated bloodstream infections in medical intensive care unit patients. Arch Intern Med 2007;167:2073–2079. [DOI] [PubMed] [Google Scholar]
- 5.Climo MW, Yokoe DS, Warren DK, et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med 2013;368:533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee BY, Bartsch SM, Wong KF, et al. Beyond the Intensive Care Unit (ICU): Countywide Impact of Universal ICU Staphylococcus aureus Decolonization. Am J Epidemiol 2016;183:480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milstone AM, Elward A, Song X, et al. Daily chlorhexidine bathing to reduce bacteraemia in critically ill children: a multicentre, cluster-randomised, crossover trial. Lancet 2013;381:1099–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toltzis P, O’Riordan M, Cunningham DJ, et al. A statewide collaborative to reduce pediatric surgical site infections. Pediatrics 2014;134:e1174–1180. [DOI] [PubMed] [Google Scholar]
- 9.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol 1999;20:250–278; quiz 279–280. [DOI] [PubMed] [Google Scholar]
- 10.O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis 2011;52:e162–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yokoe DS, Anderson DJ, Berenholtz SM, et al. A compendium of strategies to prevent healthcare-associated infections in acute care hospitals: 2014 updates. Infect Control Hosp Epidemiol 2014;35 Suppl 2:S21–31. [DOI] [PubMed] [Google Scholar]
- 12.Rouch DA, Cram DS, DiBerardino D, Littlejohn TG, Skurray RA. Efflux-mediated antiseptic resistance gene qacA from Staphylococcus aureus: common ancestry with tetracycline- and sugar-transport proteins. Mol Microbiol 1990;4:2051–2062. [DOI] [PubMed] [Google Scholar]
- 13.Brown MH, Skurray RA. Staphylococcal multidrug efflux protein QacA. J Mol Microbiol Biotechnol 2001;3:163–170. [PubMed] [Google Scholar]
- 14.Paulsen IT, Brown MH, Littlejohn TG, Mitchell BA, Skurray RA. Multidrug resistance proteins QacA and QacB from Staphylococcus aureus: membrane topology and identification of residues involved in substrate specificity. Proc Natl Acad Sci U S A 1996;93:3630–3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Littlejohn TG, DiBerardino D, Messerotti LJ, Spiers SJ, Skurray RA. Structure and evolution of a family of genes encoding antiseptic and disinfectant resistance in Staphylococcus aureus. Gene 1991;101:59–66. [DOI] [PubMed] [Google Scholar]
- 16.Smith K, Gemmell CG, Hunter IS. The association between biocide tolerance and the presence or absence of qac genes among hospital-acquired and community-acquired MRSA isolates. J Antimicrob Chemother 2008;61:78–84. [DOI] [PubMed] [Google Scholar]
- 17.McNeil JC, Hulten KG, Kaplan SL, Mahoney DH, Mason EO. Staphylococcus aureus infections in pediatric oncology patients: high rates of antimicrobial resistance, antiseptic tolerance and complications. Pediatr Infect Dis J 2013;32:124–128. [DOI] [PubMed] [Google Scholar]
- 18.Suwantarat N, Carroll KC, Tekle T, et al. High prevalence of reduced chlorhexidine susceptibility in organisms causing central line-associated bloodstream infections. Infect Control Hosp Epidemiol 2014;35:1183–1186. [DOI] [PubMed] [Google Scholar]
- 19.Warren DK, Prager M, Munigala S, et al. Prevalence of qacA/B Genes and Mupirocin Resistance Among Methicillin-Resistant Staphylococcus aureus (MRSA) Isolates in the Setting of Chlorhexidine Bathing Without Mupirocin. Infect Control Hosp Epidemiol 2016;37:590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNeil JC, Kok EY, Vallejo JG, et al. Clinical and Molecular Features of Decreased Chlorhexidine Susceptibility among Nosocomial Staphylococcus aureus Isolates at Texas Children’s Hospital. Antimicrob Agents Chemother 2015;60:1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNeil JC, Hulten KG, Mason EO, Kaplan SL. Impact of Health Care Exposure on Genotypic Antiseptic Tolerance in Staphylococcus aureus Infections in a Pediatric Population. Antimicrob Agents Chemother 2017;61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fritz SA, Hogan PG, Camins BC, et al. Mupirocin and chlorhexidine resistance in Staphylococcus aureus in patients with community-onset skin and soft tissue infections. Antimicrob Agents Chemother 2013;57:559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson JG, Saye EJ, Jimenez-Truque N, et al. Frequency of disinfectant resistance genes in pediatric strains of methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol 2013;34:1326–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reich PJ, Boyle MG, Hogan PG, et al. Emergence of community-associated methicillin-resistant Staphylococcus aureus strains in the neonatal intensive care unit: an infection prevention and patient safety challenge. Clin Microbiol Infect 2016;22:645 e641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chapman AK, Aucott SW, Gilmore MM, Advani S, Clarke W, Milstone AM. Absorption and tolerability of aqueous chlorhexidine gluconate used for skin antisepsis prior to catheter insertion in preterm neonates. J Perinatol 2013;33:768–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson J, Bracken R, Tamma PD, Aucott SW, Bearer C, Milstone AM. Trends in Chlorhexidine Use in US Neonatal Intensive Care Units: Results From a Follow-Up National Survey. Infect Control Hosp Epidemiol 2016;37:1116–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hijazi K, Mukhopadhya I, Abbott F, et al. Susceptibility to chlorhexidine amongst multidrug-resistant clinical isolates of Staphylococcus epidermidis from bloodstream infections. Int J Antimicrob Agents 2016;48:86–90. [DOI] [PubMed] [Google Scholar]
- 28.Bischoff M, Bauer J, Preikschat P, Schwaiger K, Molle G, Holzel C. First detection of the antiseptic resistance gene qacA/B in Enterococcus faecalis. Microb Drug Resist 2012;18:7–12. [DOI] [PubMed] [Google Scholar]
- 29.Prag G, Falk-Brynhildsen K, Jacobsson S, Hellmark B, Unemo M, Soderquist B. Decreased susceptibility to chlorhexidine and prevalence of disinfectant resistance genes among clinical isolates of Staphylococcus epidermidis. APMIS 2014;122:961–967. [DOI] [PubMed] [Google Scholar]
- 30.Lepainteur M, Royer G, Bourrel AS, et al. Prevalence of resistance to antiseptics and mupirocin among invasive coagulase-negative staphylococci from very preterm neonates in NICU: the creeping threat? J Hosp Infect 2013;83:333–336. [DOI] [PubMed] [Google Scholar]
- 31.Soma VL, Qin X, Zhou C, Adler A, Berry JE, Zerr DM. The effects of daily chlorhexidine bathing on cutaneous bacterial isolates: a pilot study. Infect Drug Resist 2012;5:75–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang M, O’Donoghue MM, Ito T, Hiramatsu K, Boost MV. Prevalence of antiseptic-resistance genes in Staphylococcus aureus and coagulase-negative staphylococci colonising nurses and the general population in Hong Kong. J Hosp Infect 2011;78:113–117. [DOI] [PubMed] [Google Scholar]
- 33.Hulten KG, Kaplan SL, Gonzalez BE, et al. Three-year surveillance of community onset health care-associated staphylococcus aureus infections in children. Pediatr Infect Dis J 2006;25:349–353. [DOI] [PubMed] [Google Scholar]
- 34.Wang SH, Hines L, van Balen J, et al. Molecular and clinical characteristics of hospital and community onset methicillin-resistant Staphylococcus aureus strains associated with bloodstream infections. J Clin Microbiol 2015;53:1599–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hulten KG, Kaplan SL, Lamberth LB, et al. Hospital-acquired Staphylococcus aureus infections at Texas Children’s Hospital, 2001–2007. Infect Control Hosp Epidemiol 2010;31:183–190. [DOI] [PubMed] [Google Scholar]
- 36.Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 2009;49:1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zingg W, Hopkins S, Gayet-Ageron A, et al. Health-care-associated infections in neonates, children, and adolescents: an analysis of paediatric data from the European Centre for Disease Prevention and Control point-prevalence survey. Lancet Infect Dis 2017;17:381–389. [DOI] [PubMed] [Google Scholar]
- 38.Truong-Bolduc QC, Villet RA, Estabrooks ZA, Hooper DC. Native efflux pumps contribute resistance to antimicrobials of skin and the ability of Staphylococcus aureus to colonize skin. J Infect Dis 2014;209:1485–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ding Y, Onodera Y, Lee JC, Hooper DC. NorB, an efflux pump in Staphylococcus aureus strain MW2, contributes to bacterial fitness in abscesses. J Bacteriol 2008;190:7123–7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


