Abstract
Anaplastic thyroid cancer (ATC) is a devastating disease with a dismal prognosis. Patients who have disease confined to the thyroid and who are able to undergo complete surgery and chemoradiation stand the best chance for survival. Unfortunately, nearly 50% of patients have distant metastases at diagnosis, and most present with locally advanced, unresectable tumors. Nevertheless, BRAF-mutated ATC patients represent a subset of cases who can benefit from a combination therapy with BRAF and MEK inhibitors. Here, a patient is presented with end-stage, locally advanced, unresectable ATC who was treated with this combination. Immunotherapy with pembrolizumab was added at the first sign of progression after which he achieved a partial response to therapy, enabling a complete surgical resection followed by postoperative chemoradiation to be undertaken. This novel neoadjuvant approach to BRAF-mutated ATC should be studied in further in clinical trials.
Keywords: : dabrafenib, trametinib, pembrolizumab, squamous, sarcomatoid, undifferentiated
Introduction
Anaplastic thyroid carcinoma (ATC) is one of the deadliest cancers in humans, with a median overall survival (OS) measured in months (1). Most patients present with rapidly growing locally advanced tumors that are not resectable (2), short of radical surgery (i.e., total laryngectomy and/or esophagectomy) with extremely undesirable functional consequences. Although surgery followed by chemoradiation has a beneficial effect upon survival, most patients develop distant metastasis, while others have locally progressive disease, quickly leading to their demise (3).
ATC is commonly derived from a differentiated tumor such as papillary or follicular thyroid cancer. Thus, ATCs often retain driver mutations present in the more differentiated cancer from which they are derived and accumulate more mutations that likely lead to the aggressiveness of the disease. Of the known driver mutations in ATC, only BRAFV600E has thus far been shown to be actionable. The combination of dabrafenib (a selective BRAF inhibitor) and trametinib (a selective MEK inhibitor) is now approved by the Food and Drug Administration for BRAFV600E-mutated ATC, based on the results of a basket trial that included BRAF-mutated ATC patients treated with these drugs (4). Of the 16 patients enrolled in this trial, one experienced a complete response, and 10 patients had partial responses (PR). The median OS was not reached but was 80% at one year. The authors' group has also published their experience with these drugs in six patients who would not have qualified for this particular basket trial due to inability to swallow or other disqualifying criteria (5). Of these six patients, three experienced a PR, and two had a minor response. As with other solid tumors, all patients developed drug resistance—a major limitation of kinase inhibitor therapy.
Herein, a case is described of an initially unresectable, end-stage, BRAF-mutated ATC patient treated with dabrafenib, trametinib, and pembrolizumab (“DTP”) prior to complete resection of the tumor, which was possible only after a meaningful response to these agents. This is the first reported case of the use of BRAF- and immune-directed drugs in the neoadjuvant setting in ATC.
Case Report
A 60-year-old male patient presented to his primary physician with a large, rapidly growing neck mass causing dysphagia. Baseline imaging showed an 8 cm left thyroid mass encasing the left common carotid artery, with tracheal deviation and significant lymphadenopathy but no distant metastases. Fine-needle aspiration (FNA) was consistent with ATC in the primary tumor (Fig. 1A) and papillary thyroid carcinoma (PTC) in a lymph node. He was started on weekly paclitaxel by his local oncologist, but following three doses, the tumor continued to increase in size. He then presented to the MD Anderson Cancer Center for a second opinion. The patient was experiencing hoarseness, inability to swallow solids, a weight loss of 50 lbs. over six weeks, and orthopnea. On exam, he had a massive neck mass spanning his entire neck, periorbital edema, and mild stridor at rest. Laryngoscopy showed significant supraglottic edema and pooling of secretions, interfering with visualization of the vocal cords. The patient declined tracheostomy, but a percutaneous gastrostomy tube had to be inserted.
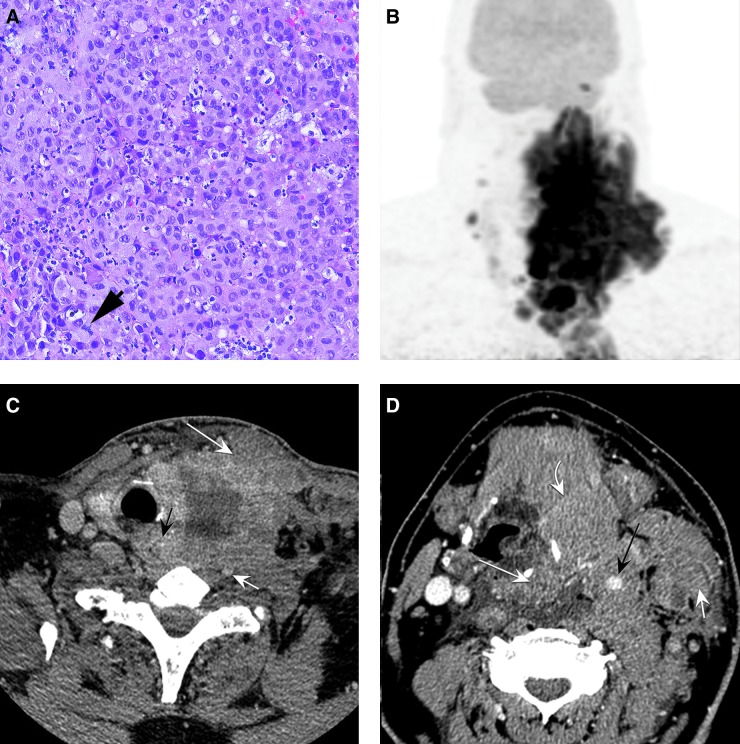
FIG. 1.
(A) Fine-needle aspiration (FNA) biopsy of the left supraclavicular lymph nodes shows sheets of neoplastic tumor cells, with an acute inflammatory infiltrate consistent with anaplastic thyroid carcinoma (ATC). The tumor cells lack conventional papillary thyroid carcinoma (PTC) nuclei and nested or papillary growth pattern and show mitotic activity (arrow; hematoxylin and eosin histology section at 200 × ). (B) Fluorodeoxyglucose (FDG)-positron emission tomography (PET)/computed tomography (CT) at presentation demonstrates a hypermetabolic mass engrossing the midline and left neck from the level of the skull base to the superior mediastinum, with additional hypermetabolic disease in the right lateral neck. (C) Pretreatment axial high-resolution contrast enhanced CT image at the level of the thyroid gland demonstrates a large heterogeneous hypodense mass arising from the left lobe, resulting in tracheal deviation toward the right. The tumor extends posteriorly to involve the tracheoesophageal groove, with direct invasion of the esophagus (black arrow), involvement of the prevertebral soft tissues, and encasement of the vertebral artery (small white arrow). Anteriorly, there is direct invasion of the sternocleidomastoid (large white arrow). (D) Axial CT image at the level of the supraglottis demonstrates 360° encasement of the carotid artery (black arrow). The tumor directly invades the supraglottic larynx and pyriform sinus (large white arrow), and there is anterior extension to involve the strap muscles (curved white arrow). The left internal jugular is occluded, and tumor directly invades the sternocleidomastoid (small white arrow).
Upon referral to the MD Anderson, staging with computed tomography (CT) and positron emission tomography (PET)/CT scans revealed a massive and infiltrative left thyroid mass extending to the mediastinum, with invasion of the paraspinal musculature, oropharynx, hypopharynx, and proximal esophagus, as well as extensive bilateral neck adenopathy and pre-vascular nodes in the mediastinum, with a 360° encasement of the left carotid artery (Fig. 1B–D). No fluorodeoxyglucose-avid distant metastases were identified. An FNA was again performed in order to obtain tissue for molecular testing and for BRAFV600E detection by immunohistochemistry, but insufficient cells were obtained due to extensive necrosis. However, blood for cell-free DNA (cfDNA) analysis was obtained. cfDNA was performed using Guardant 360 (6). The patient was given one dose of carboplatin plus nab-paclitaxel, but soon thereafter, the cfDNA result became available and revealed three mutations—BRAFV600E (variant allele frequency [VAF] 26.4%), TP53 R175H (VAF 22.3%), and EGFR G322S (VAF 1.6%)—as well as a BRAF amplification. Dabrafenib (150 mg p.o., b.i.d.) was then initiated soon after carboplatin plus nab-paclitaxel was administered. Due to his inability to swallow, dabrafenib was administered through his gastrostomy tube. Two days after initiating dabrafenib, the tumor and neck swelling subsided considerably, such that the patient was able to eat, drink, and take oral medications normally, and his stridor and orthopnea completely resolved. Trametinib (2 mg daily) was added to his regimen two weeks later, initially delayed due to insurance clearance.
Four weeks after initiating dabrafenib, the patient noticed a left supraclavicular mass. Restaging scans confirmed a significant partial response to therapy, but a slightly larger left supraclavicular lymph node, which was biopsied and confirmed to be ATC. Molecular testing performed on this lymph node showed BRAFV600E and TP53R175H mutations (identical to the initial cfDNA results prior to dabrafenib therapy), as well as a NRASQ61K mutation. The test was performed in the MD Anderson CLIA certified laboratory using next generation sequencing–based analysis, as previously described (7,8). The tumor PD-L1 (clone 22C3) score was >95% by immunohistochemistry. Pembrolizumab (200 mg administered intravenously; an anti-PD1 agent) was initiated five days after the development of the left supraclavicular lymph node. The patient noticed a decrease in the size of the lymph node approximately one week after pembrolizumab infusion. He was treated with infusions every three weeks and, three months after his initial visit to MD Anderson, underwent restaging scans. Imaging showed a remarkable improvement in the left hypermetabolic adenopathy and thyroid mass (Fig. 2A–C) so that the tumor was deemed resectable. Dabrafenib was withheld one day and trametinib three days prior to surgery. The third pembrolizumab infusion occurred 20 days prior to surgery, which consisted of a total thyroidectomy with en bloc resection of the esophageal muscularis layer, bilateral central compartment, and level II to Vb neck dissections, preserving the bilateral recurrent laryngeal nerves and two parathyroid glands (one additional parathyroid was auto-transplanted). The patient was discharged from the hospital on postoperative day 2.
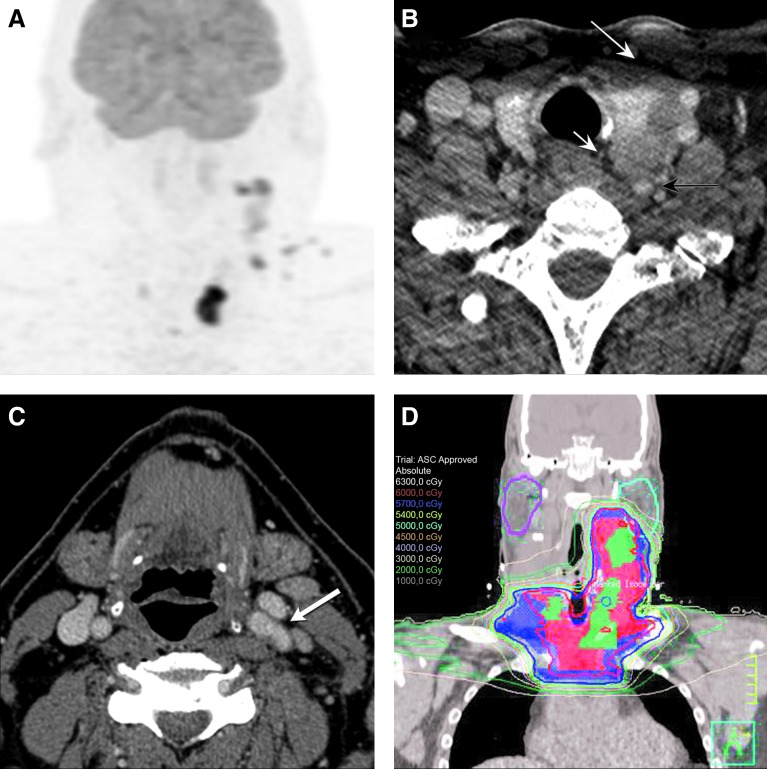
FIG. 2.
(A) FDG-PET/CT after treatment with dabrafenib, trametinib, and pembrolizumab demonstrates dramatic improvement in the neck disease, with residual foci of hypermetabolism in the left neck and superior mediastinum. (B) Posttreatment axial CT image now demonstrates a fat plane between the left thyroid lobe and the prevertebral soft tissues and the esophagus (small white arrow). Mass effect and tracheal deviation has resolved. Posteriorly, the left vertebral artery is no longer encased (black arrow), and anteriorly there is now a fat plane with the sternocleidomastoid (large white arrow). (C) Axial CT image at the level of the oropharynx shows that the left carotid artery is no longer encased (white arrow), and tumor involvement at the left pharyngeal wall and at the strap muscle has resolved. The patient underwent total thyroidectomy with en bloc resection of esophageal muscularis, bilateral central compartment dissection, bilateral level II–Vb neck dissection, preserving the bilateral recurrent laryngeal nerves and two parathyroid glands shortly after achieving a partial response to therapy. He then received radiation to the neck with radiosensitizing chemotherapy plus pembrolizumab. (D) A coronal view of the postoperative radiation planning CT scan. The red and blue lines represent the 60 Gy and 57 Gy isodose lines, respectively, while the color-washed areas within these lines demonstrate the clinical targets prescribed 60 and 57 Gy, respectively.
The surgical pathology showed residual multifocal, bilateral ATC, with the largest focus measuring 6.3 cm. There was 30% necrosis, lymphovascular invasion, extrathyroidal extension into skeletal muscle, and negative margins. The lymphadenopathy on the left was consistent with ATC, while the right lymphadenopathy was consistent with PTC.
Dabrafenib and pembrolizumab were resumed 10 and 13 days after surgery, respectively. Dabrafenib was withheld three days prior to external beam radiation to the neck. He received 60 Gy in 30 fractions to the sites of residual disease seen on the scans performed after systemic therapy (Fig. 2A), including the thyroid bed, left neck levels 2–5, and right neck level 4. A slightly lower dose (57 Gy) was given to the right neck up to the superior aspect of the mid-thyroid cartilage, lateral supraclavicular region, and upper mediastinum (Fig. 2D). The radiation was delivered over six weeks with chemotherapy (cisplatin + pembrolizumab). Pembrolizumab infusions were continued, and the patient returned for restaging scans eight weeks after completion of radiation. On exam, several nodules near the right-sided incision were noted (Fig. 3A). Imaging confirmed peripherally enhancing low-attenuation nodules in the right mid-neck in the sub-dermis measuring 11 and 14 mm (Fig. 3B and C) but no other sites of disease. An FNA could not be performed before the patient returned home, but he resumed dabrafenib plus trametinib, in addition to pembrolizumab. Soon after resumption of therapy, the nodules disappeared. He returned for restaging 12 weeks later (12 months after his initial diagnosis of ATC), and the dermal metastases had completely resolved on exam and imaging (Fig. 3D and E). Patient-reported outcome surveys, obtained at approximately 11 months after diagnosis, indicated an excellent quality of life, including continuation of normal leisure activities such as hunting and golf.
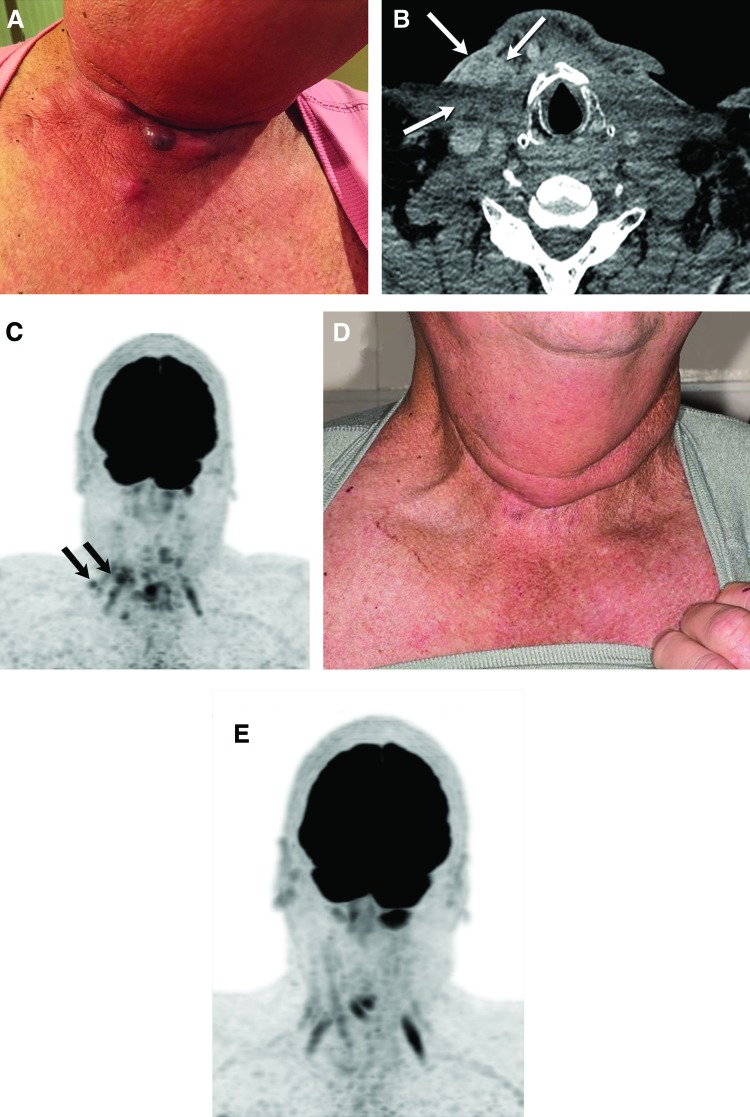
FIG. 3.
(A) Photograph of the patient's right neck demonstrating subdermal recurrences that occurred post surgery and chemoradiation, while on single-agent pembrolizumab. (B and C) Axial CT image at the level of the larynx demonstrates postoperative changes. Heterogeneously enhancing dermal and subdermal lesions in the right neck (white arrows) are hypermetabolic on the concurrent FDG-PET/CT (black arrows) and compatible with recurrent tumor. (D and E) The patient resumed the triple combination therapy (dabrafenib, trametinib, pembrolizumab) and achieved a complete resolution of metastatic lesions on exam and FDG-PET with excellent quality of life.
Discussion
To the authors' knowledge, this is the first report of the use of DTP, a BRAF- and immune-directed neoadjuvant therapy, for the management of BRAF-mutated ATC. Following induction therapy, surgery and chemoradiation followed by maintenance DTP were utilized. This case underscores several critical points that should be considered in patients who present with ATC. The first of these is the importance of identifying patients carrying a BRAFV600E mutation. This should be performed as soon as the ATC diagnosis is confirmed or suspected because dabrafenib plus trametinib have been shown to be effective in this patient population (4). BRAFV600E by immunohistochemistry is a rapid test that can be performed on biopsy material and has been validated in thyroid cancer (9–11). However, in this patient's case, due to extensive necrosis and lack of viable cells, this test could not be performed on DNA from the cancer tissue, but cfDNA for BRAF has been shown to be a reliable test in ATC patients (12,13) and was employed in this case to identify the mutation.
Second, this is the second report (14) to show that tracheostomy due to impending airway compromise may be averted with the use of targeted therapy in patients with BRAF-mutated ATC. This patient presented with dyspnea, dysphagia, and impending airway compromise and had rapid relief of stridor as well as severe dysphagia after only two days of dabrafenib, demonstrating that regression of tumor can occur very quickly. Although the contribution of the cytotoxic chemotherapy just prior to initiation of dabrafenib cannot be excluded, others (15,16) have described rapid symptomatic relief with BRAF inhibitors in ATC.
Third, progression occurred within one month of initiation of dabrafenib plus trametinib but could be salvaged with the addition of immunotherapy. The resistant tumor was confirmed to retain the BRAFV600E mutation present at baseline, but in addition, an NRAS mutation was identified, likely resulting in emergence of resistance. BRAF and RAS mutations are usually mutually exclusive in human malignancies, but can occur simultaneously in tumors resistant to BRAF inhibitors. Additionally, the emergence of a RAS mutation in vemurafenib-treated, BRAF-mutant PTC cell lines has also been described (17). Because RAS mutations are not actionable at this point in time, the addition of immunotherapy is a reasonable strategy, particularly in ATC, where the pathologic hallmark of the disease is infiltration of the tumor with white blood cells. ATC tumors have also been shown to express PD-L1 and other immune markers (18–20). Dadu et al. have shown that BRAF-mutated tumors have higher expression of PD-L1 (82%) when compared to BRAF wild-type tumors (13%; p = 0.06) (18). This patient's tumor highly expressed PD-L1 (>95%) at the time of progression, which could have made him more amenable to treatment with immunotherapy. It is unclear if this patient's PD-L1 expression was changed by the use of the BRAF/MEK inhibitor combination therapy, since tissue prior to initiating the targeted therapies was not available. Furthermore, the response to salvage immunotherapy in this patient could have also been attributed to the fact that the immunotherapy was started at the first sign of progression and when the progressive tumor was small. Smaller tumors have been shown to be more responsive to immunotherapy in a melanoma population (21).
Fourth, a tumor that was deemed previously unresectable because of 360° carotid artery encasement, prevertebral fascial, laryngotracheal, and superior mediastinal involvement, following treatment with DTP, became resectable after induction of meaningful tumor regression. The patient tolerated major surgery that included total thyroidectomy, bilateral central compartment dissection, and bilateral lateral neck dissection following neoadjuvant therapy, with preservation of critical functional organs (trachea, esophagus, larynx/recurrent laryngeal nerves, and parathyroid glands). Uruno et al. have reported their experience using neoadjuvant chemotherapy in ATC (22). They treated 40 patients with four to eight courses of weekly paclitaxel at presentation. Although 83% of patients responded, only 20 who did not have distant metastasis could undergo surgery. Five others with distant metastatic disease were later resected after a longer induction period. After surgery, paclitaxel was continued, and patients were later consolidated with external beam radiation. Remarkably, 16 patients received trimodal therapy and had a median survival of approximately two years.
Finally, despite adjuvant chemoradiation plus pembrolizumab, the patient experienced a recurrence of disease in the skin, on the side of the neck, with previously confirmed PTC. It is postulated that this could have been caused by the interruption of dabrafenib/trametinib to avoid radiation recall. However, following re-initiation of therapy with DTP, the patient had complete regression of these metastatic lesions, confirmed on PET/CT. The patient remains without evidence of disease 16 months after diagnosis, with excellent functional status and quality of life in what would have otherwise been a rapidly fatal outcome, based on symptom burden and advanced presentation.
It cannot be concluded, based on a single case report, that this approach is the ideal one for the management of advanced ATC. However, as part of the current approach, five other patients have recently been treated with surgically unresectable BRAF-mutated ATC using a similar approach, and a comparable ability to resect disease and initiate postoperative radiation therapy has been found in patients who would otherwise rapidly progress to functional impairment and death. It is unclear whether patients will require maintenance DTP after completion of surgery and chemoradiation, but patients who stop BRAF/MEK inhibitors postoperatively should be watched very closely. In case of local recurrence or distant metastasis, reinitiating systemic therapy is advisable.
The “quartet” of neoadjuvant BRAF/MEK inhibitors, immunotherapy, surgery, and adjuvant chemoradiation followed by maintenance BRAF/MEK inhibitors and immunotherapy is currently being studied in a prospective clinical trial (NCT03181100).
Author Disclosure Statement
M.E.C. has received research funding from Roche Genentech, Eisai, Kura, and Exelixis. M.E.C. has also received consultant fees from AstraZeneca, Exelixis, Eisai, LOXO, and Bayer. N.L.B. has received research funding from Novartis and consultant fees from Eisai. No competing financial interests exist for the remaining authors.
References
- 1.Smallridge RC, Ain KB, Asa SL, Bible KC, Brierley JD, Burman KD, Kebebew E, Lee NY, Nikiforov YE, Rosenthal MS, Shah MH, Shaha AR, Tuttle RM; American Thyroid Association Anaplastic Thyroid Cancer Guidelines Taskforce 2012. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid 22:1104–1139 [DOI] [PubMed] [Google Scholar]
- 2.Ahmed S, Ghazarian MP, Cabanillas ME, Zafereo M, Williams MD, Vu T, Schomer DF, Debnam JM. 2017. Imaging of anaplastic thyroid carcinoma. Am J Neuroradiol 2017. December 14 [Epub ahead of print]; DOI: 10.3174/ajnr.A5487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rao SN, Zafereo M, Dadu R, Busaidy NL, Hess K, Cote GJ, Williams MD, William WN, Sandulache V, Gross N, Gunn GB, Lu C, Ferrarotto R, Lai SY, Cabanillas ME. 2017. Patterns of treatment failure in anaplastic thyroid carcinoma. Thyroid 27:672–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Subbiah V, Kreitman RJ, Wainberg ZA, Cho JY, Schellens JHM, Soria JC, Wen PY, Zielinski C, Cabanillas ME, Urbanowitz G, Mookerjee B, Wang D, Rangwala F, Keam B. 2018. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J Clin Oncol 36:7–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iyer P, Dadu R, Ferrarotto R, Busaidy N, Habra MA, Zafereo M, Gross ND, Hess K, Gule-Monroe M, Williams MD, Cabanillas M. 2018. Real world experience with targeted therapy for the treatment of anaplastic thyroid carcinoma. Thyroid 28:79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lanman RB, Mortimer SA, Zill OA, Sebisanovic D, Lopez R, Blau S, Collisson EA, Divers SG, Hoon DS, Kopetz ES, Lee J, Nikolinakos PG, Baca AM, Kermani BG, Eltoukhy H, Talasaz A. 2015. Analytical and clinical validation of a digital sequencing panel for quantitative, highly accurate evaluation of cell-free circulating tumor DNA. PLoS One 10:e0140712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh RR, Patel KP, Routbort MJ, Reddy NG, Barkoh BA, Handal B, Kanagal-Shamanna R, Greaves WO, Medeiros LJ, Aldape KD, Luthra R. 2013. Clinical validation of a next-generation sequencing screen for mutational hotspots in 46 cancer-related genes. J Mol Diagn 15:607–622 [DOI] [PubMed] [Google Scholar]
- 8.Kanagal-Shamanna R, Portier BP, Singh RR, Routbort MJ, Aldape KD, Handal BA, Rahimi H, Reddy NG, Barkoh BA, Mishra BM, Paladugu AV, Manekia JH, Kalhor N, Chowdhuri SR, Staerkel GA, Medeiros LJ, Luthra R, Patel KP. 2014. Next-generation sequencing-based multi-gene mutation profiling of solid tumors using fine needle aspiration samples: promises and challenges for routine clinical diagnostics. Mod Pathol 27:314–327 [DOI] [PubMed] [Google Scholar]
- 9.Smith AL, Williams MD, Stewart J, Wang WL, Krishnamurthy S, Cabanillas ME, Roy-Chowdhuri S. 2018. Utility of the BRAF p.V600E immunoperoxidase stain in FNA direct smears and cell block preparations from patients with thyroid carcinoma. Cancer Cytopathol 2018. March 26 [Epub ahead of print]; DOI: 10.1002/cncy.21992 [DOI] [PubMed] [Google Scholar]
- 10.Wobker SE, Kim LT, Hackman TG, Dodd LG. 2015. Use of BRAF v600e immunocytochemistry on FNA direct smears of papillary thyroid carcinoma. Cancer Cytopathol 123:531–539 [DOI] [PubMed] [Google Scholar]
- 11.Zimmermann AK, Camenisch U, Rechsteiner MP, Bode-Lesniewska B, Rossle M. 2014. Value of immunohistochemistry in the detection of BRAF(V600E) mutations in fine-needle aspiration biopsies of papillary thyroid carcinoma. Cancer Cytopathol 122:48–58 [DOI] [PubMed] [Google Scholar]
- 12.Sandulache VC, Williams MD, Lai SY, Lu C, William WN, Busaidy NL, Cote GJ, Singh RR, Luthra R, Cabanillas ME. 2017. Real-time genomic characterization utilizing circulating cell-free DNA in patients with anaplastic thyroid carcinoma. Thyroid 27:81–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iyer P, Cote G, Dadu R, Ferrarotto R, Busaidy N, Hofmann M, Zafereo M, Williams MD, Subbiah V, Cabanillas ME. 2017. Circulating BRAFV600E cell-free DNA detected by droplet digital PCR (ddPCR) as a biomarker in the management of anaplastic thyroid carcinoma (ATC) patients. Thyroid 27:A-115 [Google Scholar]
- 14.Cabanillas ME, Busaidy N, Khan SA, Gunn GB, Dadu R, Rao SN, Waguespack SG. 2016. Molecular diagnostics and anaplastic thyroid carcinoma: the time has come to harvest the high hanging fruit. Int J Endocr Oncol 3:221–233 [Google Scholar]
- 15.Lim AM, Taylor GR, Fellowes A, Cameron L, Lee B, Hicks RJ, McArthur GA, Angel C, Solomon B, Rischin D. 2016. BRAF inhibition in BRAFV600E-positive anaplastic thyroid carcinoma. J Natl Compr Canc Netw 14:249–254 [DOI] [PubMed] [Google Scholar]
- 16.Agarwal R, Wang J, Wilson K, Barrett W, Morris JC. 2016. Response to targeted therapy in BRAF mutant anaplastic thyroid cancer. J Natl Compr Canc Netw 14:1203–1207 [DOI] [PubMed] [Google Scholar]
- 17.Danysh BP, Rieger EY, Sinha DK, Evers CV, Cote GJ, Cabanillas ME, Hofmann MC. 2016. Long-term vemurafenib treatment drives inhibitor resistance through a spontaneous KRAS G12D mutation in a BRAF V600E papillary thyroid carcinoma model. Oncotarget 7:30907–30923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dadu R, Vilalobos PA, Para Cuentas ER, ROdriguez Canales J, Wistuba I, Zhou S, Williams MD, Cabanillas ME. 2016. Anaplastic thyroid cancer (ATC) is a hot immunogenic environment: immunoprofiling of a large cohort of ATC tumors. Thyroid 26:abstr 12 [Google Scholar]
- 19.Bastman JJ, Serracino HS, Zhu Y, Koenig MR, Mateescu V, Sams SB, Davies KD, Raeburn CD, McIntyre RC, Jr, Haugen BR, French JD. 2016. Tumor-infiltrating T cells and the PD-1 checkpoint pathway in advanced differentiated and anaplastic thyroid cancer. J Clin Endocrinol Metab 101:2863–2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chintakuntlawar AV, Rumilla KM, Smith CY, Jenkins SM, Foote RL, Kasperbauer JL, Morris JC, Ryder M, Alsidawi S, Hilger C, Bible KC. 2017. Expression of PD-1 and PD-L1 in anaplastic thyroid cancer patients treated with multimodal therapy: results from a retrospective study. J Clin Endocrinol Metab 102:1943–1950 [DOI] [PubMed] [Google Scholar]
- 21.Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, Xu W, Harmon S, Giles JR, Wenz B, Adamow M, Kuk D, Panageas KS, Carrera C, Wong P, Quagliarello F, Wubbenhorst B, D'Andrea K, Pauken KE, Herati RS, Staupe RP, Schenkel JM, McGettigan S, Kothari S, George SM, Vonderheide RH, Amaravadi RK, Karakousis GC, Schuchter LM, Xu X, Nathanson KL, Wolchok JD, Gangadhar TC, Wherry EJ. 2017. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 545:60–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uruno T, Ogimi Y, Saito F, Masaki C, Akaishi J, Tomoda C, Matsuzu K, Suzuki A, Ohkuwa K, Shibuya H, Kitagawa W, Nagahama N, Sugino K, Ito K. 2015. Proposal of a new staging system and treatment algorithm for anaplastic thyroid cancer by induction weekly paclitaxel. Thyroid 25:abstr 645 [Google Scholar]