Abstract
Key points
Mammalian cells are frequently exposed to stressors causing volume changes.
The transient receptor potential vanilloid 4 (TRPV4) channel translates osmotic stress into ion flux.
The molecular mechanism coupling osmolarity to TRPV4 activation remains elusive.
TRPV4 responds to isosmolar cell swelling and osmolarity translated via different aquaporins.
TRPV4 functions as a volume‐sensing ion channel irrespective of the origin of the cell swelling.
Abstract
Transient receptor potential channel 4 of the vanilloid subfamily (TRPV4) is activated by a diverse range of molecular cues, such as heat, lipid metabolites and synthetic agonists, in addition to hyposmotic challenges. As a non‐selective cation channel permeable to Ca2+, it transduces physical stress in the form of osmotic cell swelling into intracellular Ca2+‐dependent signalling events. Its contribution to cell volume regulation might include interactions with aquaporin (AQP) water channel isoforms, although the proposed requirement for a TRPV4–AQP4 macromolecular complex remains to be resolved. To characterize the elusive mechanics of TRPV4 volume‐sensing, we expressed the channel in Xenopus laevis oocytes together with AQP4. Co‐expression with AQP4 facilitated the cell swelling induced by osmotic challenges and thereby activated TRPV4‐mediated transmembrane currents. Similar TRPV4 activation was induced by co‐expression of a cognate channel, AQP1. The level of osmotically‐induced TRPV4 activation, although proportional to the degree of cell swelling, was dependent on the rate of volume changes. Importantly, isosmotic cell swelling obtained by parallel activation of the co‐expressed water‐translocating Na+/K+/2Cl− cotransporter promoted TRPV4 activation despite the absence of the substantial osmotic gradients frequently employed for activation. Upon simultaneous application of an osmotic gradient and the selective TRPV4 agonist GSK1016790A, enhanced TRPV4 activation was observed only with subsaturating stimuli, indicating that the agonist promotes channel opening similar to that of volume‐dependent activation. We propose that, contrary to the established paradigm, TRPV4 is activated by increased cell volume irrespective of the molecular mechanism underlying cell swelling. Thus, the channel functions as a volume‐sensor, rather than as an osmo‐sensor.
Keywords: aquaporin, ion channel, osmo‐sensor, osmotic swelling, transient receptor potential vanilloid 4 (TRPV4), TRP channels, volume regulation, volume‐sensor, water channel
Key points
Mammalian cells are frequently exposed to stressors causing volume changes.
The transient receptor potential vanilloid 4 (TRPV4) channel translates osmotic stress into ion flux.
The molecular mechanism coupling osmolarity to TRPV4 activation remains elusive.
TRPV4 responds to isosmolar cell swelling and osmolarity translated via different aquaporins.
TRPV4 functions as a volume‐sensing ion channel irrespective of the origin of the cell swelling.
Abbreviations
- AQP
aquaporin
- GSK101
GSK1016797A
- NKCC1
Na+/K+/2Cl− co‐transporter 1
- RR
ruthenium red
- TRPV4
transient receptor potential vanilloid 4
Introduction
Cellular structures in various organs and tissues may frequently be exposed to (patho)physiological factors or osmotic challenges that cause mechanical stress and/or volume changes of nearby cells (i.e. retinal neurons and glia in glaucoma) (Pinar‐Sueiro et al. 2011; Križaj et al. 2014), activity‐induced glial cell swelling (Haj‐Yasein et al. 2011; Larsen et al. 2014), changes in kidney/intestinal/ocular epithelia during food ingestion and urine filtration (Jo et al. 2016; Ryskamp et al. 2016); for review see Danziger & Zeidel (2015). Because maintenance of cell volume is a homeostatic imperative for cells of most origins (Okada & Maeno, 2001), cellular responses suitable for achieving an appropriate physiological reaction to the stressor in question are set in motion. A well‐established cellular response to mechanical stimuli, such as osmotically‐induced cell volume changes, involves regulation of ion channels (Hoffmann et al. 2009), which translate changes in membrane strain into altered ion permeation. In this manner, ion channel activation may affect membrane potential, signalling cascades, ion transporters and/or osmotic gradients, thereby initiating volume‐regulatory responses, albeit in an unresolved manner. Several volume‐sensitive ion channels have been identified (Hammami et al. 2009; Soe et al. 2009; Pedersen et al. 2015), one of which is the transient receptor potential vanilloid 4 (TRPV4) ion channel. TRPV4, also known as the vanilloid receptor related and osmotically activated channel (VR‐OAC) (Liedtke et al. 2000) or the osmo‐sensitive transient receptor potential vanilloid 4 (OTRPC4) (Liedtke et al. 2000; Strotmann et al. 2000), is a non‐selective cation channel expressed in many cell types (i.e. Müller cells, astrocytes, neurons of the circumventricular organs, epithelia of the trachea, oviduct, kidney, lung and cochlea, and in endothelium) (Liedtke et al. 2000; Strotmann et al. 2000; Wissenbach et al. 2000; Liedtke & Friedman, 2003; Jia et al. 2004; Tian et al. 2004; Andrade et al. 2005; Earley et al. 2005; Benfenati et al. 2011; Ryskamp et al. 2011). TRPV4 was initially described as an osmo‐sensor activated by hyposmolar stress (Liedtke et al. 2000; Strotmann et al. 2000; Nilius et al. 2001) and is still considered as such but, subsequently, other physiological stimuli were discovered, such as temperature (Vriens et al. 2004) and phospholipase A2‐mediated arachidonic acid production with subsequent conversion to epoxyeicosatrienoic acids (EETs) (Watanabe et al. 2003; Vriens et al. 2005; Fernandes et al. 2008; Ryskamp et al. 2014). TRPV4 thus appears to act as a polymodal sensory protein and is reported to be critical for regulation of intracellular Ca2+ signalling, osmo‐ and mechano‐transduction and temperature‐sensing, as well as cell volume regulation and the maintenance of Ca2+ homeostasis (Ye et al. 2012; Jo et al. 2015; White et al. 2016). Hereditary TRPV4 channelopathy mutations lead to a variety of phenotypic disorders (Dai et al. 2010; Nilius & Voets, 2013; Loukin et al. 2015) and the expression pattern and molecular characteristics of TRPV4 point to its putative involvement in pathophysiological conditions with increased pressure and edema formation, in skeletal dysplasias, inflammatory responses, and sensory/motor neuropathies (Ye et al. 2012; Jo et al. 2015; Ryskamp et al. 2015). Mice with genetic deletion of TRPV4 display impaired regulation of systemic tonicity (Liedtke & Friedman, 2003), although the molecular links between (i) altered osmolarity of the extracellular fluid and TRPV4 activation and (ii) TRPV4 activation and cell volume regulation remain elusive (Liedtke & Friedman, 2003; Nilius et al. 2004; Becker et al. 2005; Liedtke, 2005b; White et al. 2016). Synthetic agonists (i.e. GSK1016790A; hereafter referred to as GSK101) activate TRPV4 with high potency (Thorneloe et al. 2008; Jin et al. 2011). These are employed as the molecular stimuli in a range of experiments addressing a physiological role for TRPV4, although it is unresolved whether the synthetic agonists mimic the physiological stimuli or activates the channel in an alternative fashion.
Osmotically‐induced TRPV4 activation has been proposed to rely directly on a functional coupling with a specific aquaporin (AQP), such as AQP2 (Galizia et al. 2012), AQP4 (Benfenati et al. 2011), or AQP5 (Liu et al. 2006), rather than the swelling per se, although this was recently questioned (Jo et al. 2015; Mola et al. 2016). Thus, it remains unsettled whether TRPV4 responds directly to a change in osmolarity of the extracellular fluid, whether it relies on the osmotically‐induced cell volume change facilitated by the presence of a given AQP, and/or whether it simply responds to an increased cell volume even in the absence of AQPs and experimentally‐inflicted osmotic challenges: Is TRPV4 a bona fide osmo‐sensor or, rather, a volume‐sensor? We took advantage of the low intrinsic water permeability of the Xenopus laevis oocytes and our ability to introduce different AQP isoforms and cotransporters alongside TRPV4. In addition, our experimental protocols allowed us to determine the kinetics of the swelling‐induced activation of TRPV4 while concurrently monitoring the oocyte volume and TRPV4‐mediated membrane currents.
Methods
Ethical approval
The experiments were performed in accordance with the guidelines of the Danish Veterinary and Food Administration (Ministry of Environment and Food) and were approved by the animal facility at the Faculty of Health and Medical Sciences, University of Copenhagen. The experiments conform with the principles and regulations described in Grundy (2015). Experiments were performed on X. laevis oocytes. The surgical protocol by which the oocytes were retrieved was approved by The Danish National Committee for Animal Studies, Danish Veterinary and Food Administration (Ministry of Environment and Food).
RNA preparation and heterologous expression in X. laevis oocytes
cDNA encoding rat TRPV4, rat AQP4 and mouse Na+/K+/2Cl− co‐transporter (NKCC1), respectively, was subcloned into the oocyte expression vector pXOOM, and rat AQP1 was subcloned into a pOG1 vector, linearized downstream from the poly‐A segment, and in vitro transcribed using T7 mMessage machine in accordance with the manufacturer's instructions (Ambion, Austin, TX, USA). cRNA was extracted with MEGAclear (Ambion) and microinjected into defolliculated X. laevis oocytes: 4 ng of TRPV4 RNA/oocyte or in combination with 10 ng of AQP1, AQP4 RNA/oocyte or 50 ng of NKCC1 RNA/oocyte. Xenopus laevis frogs were obtained from Nasco (Fort Atkinson, WI, USA) or the National Center for Scientific Research (France) and housed under stringent care. The frogs were kept in tanks in a recirculating water facility, and fed twice weekly with Floating Frog Food 3/32 for adults and juveniles (Xenopus Express, Inc., Brooksville, FL, USA). Oocytes were collected from frogs under anaesthesia (2 g L−1 Tricain, 3‐aminobenzoic acid ethyl ester; A‐5040; Sigma‐Aldrich, St Louis, MO, USA). The preparation of defolliculated oocytes was carried out as described previously (Fenton et al. 2010) and the oocytes were maintained in Kulori medium (in mm): 90 NaCl, 1 KCl, 1 CaCl2, 1 MgCl2 and 5 Hepes (pH 7.4). Additionally, defolliculated oocytes were acquired from EcoCyte Bioscience (Castrop‐Rauxel, Germany). A TRP channel antagonist, ruthenium red (Vincent & Duncton, 2011) (RR) (1 μm; R‐2751; Sigma‐Aldrich) was added to the medium to prevent oocyte lysis and the oocytes were kept for 3–4 days at 19°C prior to the experiments.
Electrophysiology and volume recordings of oocytes
Conventional two‐electrode voltage clamp studies were performed using a DAGAN CA‐1B High Performance oocyte clamp (DAGAN, Minneapolis, MN, USA) with a Digidata 1440A interface controlled by pCLAMP software, version 10.5 (Molecular Devices, Burlingame, CA, USA). Electrodes were pulled (PIP5; HEKA Elektronik, Lambrecht, Germany) from borosilicate glass capillaries to a resistance of 2.5–4 MΩ when filled with 1 m KCl. The current traces were obtained by stepping the clamp potential from −20 mV to test potentials ranging from +50 mV to −130 mV (pulses of 200 ms) in increments of 15 mV. Recordings were low pass‐filtered at 500 Hz, sampled at 1 kHz and the steady‐state current activity was analysed at 140–180 ms after applying the test pulse. Oocytes were placed in an experimental recording chamber, perfused with various solutions and volume measurements were performed as described previously (Fenton et al. 2010). Briefly, the oocytes were placed in a small chamber with a glass bottom and perfused with solutions of interest. The volume of the oocytes was viewed from below via a long‐distance objective, and micrographs were captured continuously at a rate of 25 images s−1. In experiments where hyposmotic solutions were used to induce cell swelling, the perfusion solution consisted of (in mm): 50 NaCl, 2 KCl, 1 MgCl2, 1 CaCl2, 10 Hepes and 100 mm mannitol (Tris buffered pH 7.4, 220 mosmol l–1). Hyposmotic solutions were made subsequently by the removal of mannitol (∆20–100 mm mannitol) with resulting osmolarities of 120–200 mosmol l–1. Na+‐free (equiosmolar replacement with choline) and Ca2+‐free (equiosmolar replacement with Na+) solutions were used as perfusion solutions when investigating the contribution of endogenous Ca2+‐activated Cl− current to the whole‐cell current. For isosmotically‐induced cell swelling, the control perfusion solution consisted of (in mm): 100 NaCl, 2 KCl, 1 MgCl2, 1 CaCl2 and 10 Hepes (Tris buffered pH 7.4, 213 mosmol l–1), with KCl equiosmotically replacing Na+ to increase the K+ concentration to 15 mm. We activated NCKK1 by shrinking the oocytes in K+‐free solutions for 10–20 min, followed by an abrupt increase in the K+ concentration (15 mm) associated with a rapid onset of cotransporter‐mediated water influx, as described previously (Zeuthen & MacAulay, 2012). Only oocytes expressing NKCC1 at a level sufficient to display a 1.5% cell swelling within 3 min were employed for measurements. The osmotic water permeability was determined by L p = (J v)/(A × Δπ × V w), where Jv is the initial water flux during an osmotic challenge, A is the membrane surface ares (nine times the apparent area as a result of membrane folding; Zampighi et al. 1995), Δπ is the osmotic challenge and Vw is the partial molal volume of water (18 cm3 mol−1). Osmolarities of all solutions were confirmed to an accuracy of 1 mosmol l–1 with an osmometer Type 15 (Löser Messtechnik, Berlin, Germany). To stimulate TRPV4‐mediated currents, the experimental series was conducted in the presence of 100 nm GSK101 (G‐0798; Sigma‐Aldrich), whereas RR (100 μm) (R‐2751; Sigma‐Aldrich) was applied to suppress TRPV4 activity. Because variability in TRPV4‐mediated basal currents was observed, all experiments were set up as paired trials, and performed at room temperature (23°C). GSK101 was dissolved in DMSO (stock solution of 1 mm) and RR in water (stock solution of 1 mm). The stock solutions were kept as aliquots at −20°C.
Immunocytochemistry of oocytes, laser scanning microscopy and structured illumination microscopy
Uninjected oocytes and oocytes expressing TRPV4+AQP4 and TRPV4+AQP1 were fixed for 1 h at room temperature in 2% paraformaldehyde, washed and left in PBS overnight, before being incubated with primary antibodies (anti‐AQP1: AQP‐001, Alomone Lab, Jerusalem, Israel; anti‐AQP4: AQP‐004, Alomone Lab; anti‐TRPV4: ab63079, Abcam, Cambridge, UK) and conjugated secondary antibodies [Alexa Flour® 488 goat anti‐sheep: A‐11015, Invitrogen, Carlsbad, CA, USA; Alexa Flour® 546 goat anti‐rabbit: A‐11010, Invitrogen; Alexa Flour® 647 Phalloidin (F‐actin): A‐22287, Invitrogen]. Oocytes kept in 12‐well plates were mounted with ProlongGold (Invitrogen). The wells were sealed with a coverglas and kept from being exposed to light. Phalloidin was used as a morphological marker. To confirm that the proteins were expressed at the plasma membrane of the oocytes, micrographs were obtained at room temperature using a Zeiss LSM710 point laser (Argon Lasos RMC781272) scanning confocal microscope with a Zeiss EC Plan‐Neofluar 40×/ numerical aperture (NA) 1.3 oil objective (Carl Zeiss, Germany). All micrographs were sampled with scan mode as frame with one line step, a frame size of 512 × 512 and a bit depth of 16. No quantification was carried out from the micrographs as they only served to verify the expression of proteins of interest. Structured illumination microscopy was used to look more closely at the distribution of the membranous proteins and was performed using a Zeiss Plan‐Apochromat 63X/NA 1.4 oil immersion objective lens (DIC M27) (Carl Zeiss) and a sCMOS camera (PCO.edge, Kelheim, Germany) mounted on an ELyra PS.1 microscope (Carl Zeiss).
Statistical analysis
All data are presented as the mean ± SEM. Statistical significance was tested with a Student's paired t‐test or one‐way ANOVA with Dunnett's multiple comparison post hoc test, as indicated. P < 0.05 was considered statistically significant. The number of experiments (n) corresponds to independent measurements from at least three different preparations.
Results
To resolve whether TRPV4 activation is a direct cause of reduced osmolarity of the extracellular fluid or of the resulting AQP‐mediated cell volume change, we employed the X. laevis oocyte expression system. An inherent advantage of Xenopus oocytes is the low intrinsic water permeability of their cell membranes, which allows for a drastic increase in water permeability upon expression of an AQP (Fenton et al. 2010). Oocytes expressing TRPV4 alone will thus be expected to scarcely change volume upon brief exposure to even large osmotic gradients, whereas oocytes co‐expressing TRPV4 and AQP4 are predicted to respond with robust volume changes. Immunofluorescence micrographs revealed plasma membrane staining for TRPV4 and AQP4 upon microinjection of cRNA encoding TRPV4 and AQP4, whereas no staining was detected in uninjected control oocytes (Fig. 1 A). Structured illumination microscopy, which allows for images to be taken with a higher resolution than conventional confocal microscopy, demonstrated a close physical relationship of the two membrane proteins and confirmed their successful targeting to the plasma membrane (Fig. 1 A, right). Oocytes were abruptly challenged with a hyposmotic gradient of −100 mosmol l–1 (obtained by removal of 100 mm mannitol from the control solution to decrease the osmolarity at the same time as keeping the ionic strength constant) with continuous monitoring of their volume. The resultant cell volume response by TRPV4‐expressing oocytes was indistinguishable from that of the uninjected control oocytes with water permeabilities of 0.08 ± 0.01 × 10−3 cm s−1 for uninjected control oocytes and 0.07 ± 0.01 × 10−3 cm s−1 for TRPV4‐expressing oocytes, n = 20—21 (for representative volume responses, see Fig. 1 B). Expression of AQP4 increased the water permeability by ∼20‐fold compared to that of the uninjected oocytes (P < 0.001), regardless of whether or not TRPV4 was co‐expressed (compare 1.69 ± 0.09 × 10−3 cm s−1 for AQP4‐expressing oocytes and 1.40 ± 0.10 × 10−3 cm s−1 for TRPV4+AQP4‐expressing oocytes, n = 21–25) and these oocytes thus displayed prominent cell swelling upon exposure to the osmotic challenge (Fig. 1 B).
Figure 1. TRPV4 is activated by increased cell volume.
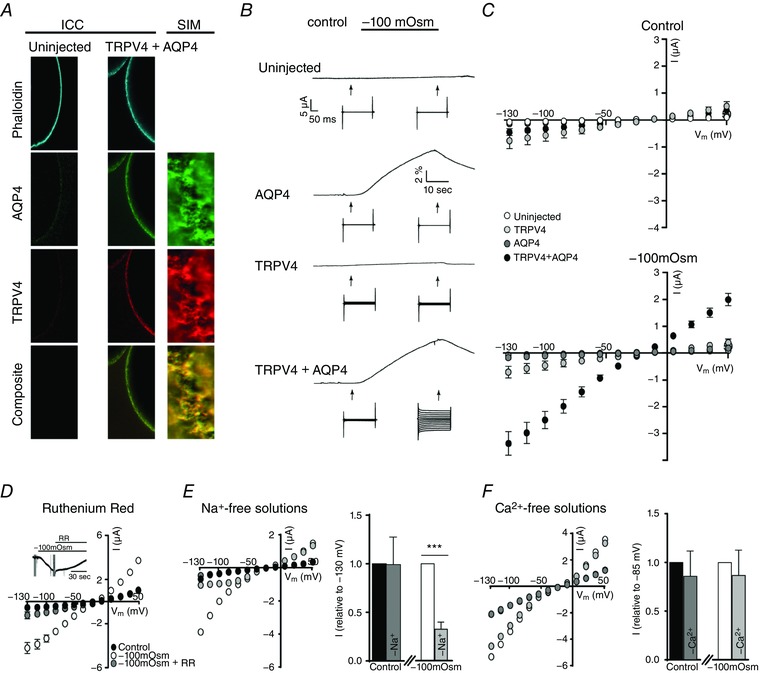
A, representative confocal laser scanning and structured illumination microscopy of an uninjected oocyte (left) and an oocyte expressing TRPV4 + AQP4 (right) after immunolabelling with phalloidin, anti‐AQP4 and anti‐TRPV4 antibodies confirmed the plasma membrane expression and close relationship of the proteins in oocytes. B, representative volume‐ and current traces obtained from oocytes voltage clamped at V m = −20 mV and challenged with a hyposmotic gradient (−100 mosmol l–1, indicated by a black bar). Current traces were recorded with a 200 ms step protocol (when indicated by an arrow) from an uninjected oocyte (top) and oocytes expressing either AQP4 (middle), TRPV4 (middle) or TRPV4 + AQP4 (bottom). C, summarized I/V curves from uninjected oocytes and oocytes expressing either AQP4, TRPV4, or TRPV4 + AQP4 in control solution (top) or during application of a hyposmotic solution (bottom; −100 mosmol l–1). D, application of ruthenium red (4 μm, 60 s) blocked the volume‐induced current activity of TRPV4 + AQP4‐expressing oocytes (summarized I/V curves) as seen in the representative current trace (D, insert). E, representative I/V curves obtained from a TRPV4 + AQP4 expressing oocyte exposed to regular and Na+‐free solutions (symbol colours correspond to the bar graph, right). The bar graph illustrates the current activity obtained in Na+‐free solutions (control and hyposmotic solutions) normalized to that recorded in regular solutions (control and hyposmotic solutions) at V m = −130 mV, n = 8–10) (E, bar graph). F, representative I/V curves obtained from a TRPV4 + AQP4 expressing oocyte exposed to regular and Ca2+‐free solutions (symbol colours correspond to the bar graph, right). The bar graph illustrates the current activity obtained in Ca2+‐free solutions (control and hyposmotic solutions) normalized to that recorded in regular solutions (control and hyposmotic solutions) at V m = −85 mV, n = 10) (F, bar graph). The magnitudes of TRPV4‐mediated currents (at V m = −85 mV) were compared using one‐way ANOVA with Dunnett's multiple comparison post hoc test or a Student's paired t‐test (for the normalized response). *** P < 0.001 (n = 8–25). [Color figure can be viewed at wileyonlinelibrary.com]
To determine whether TRPV4 sensed the imposed osmotic gradient or the resultant increase in cell volume, TRPV4‐mediated current activity during osmotic challenge was monitored with conventional two‐electrode voltage clamp (for representative current traces, see Fig. 1 B). Oocytes expressing only AQP4 failed to conduct detectable currents greater than those of uninjected oocytes both in control solution (compare −83 ± 27 nA for AQP4‐expressing oocytes and −41 ± 3 nA for uninjected oocytes at V m = −85 mV, n = 19) and upon application of the hyposmotic gradient (compare −85 ± 27 nA for AQP4‐expressing oocytes and −42 ± 4 nA for uninjected oocytes at V m = −85 mV, n = 19) (Fig. 1 C). TRPV4‐expressing oocytes displayed larger basal currents in control solution compared to uninjected and AQP4‐expressing oocytes (−402 ± 112 nA at V m = −85 mV, n = 18, P < 0.05) (Fig. 1 C), as observed previously (Strotmann et al. 2000). The hyposmotic gradient was insufficient to elicite TRPV4 activation (compare −402 ± 112 nA for control solution and −413 ± 90 nA for hyposmotic gradient application at V m = −85 mV, n = 18) (Fig. 1 C) unless the channel was co‐expressed with AQP4 (compare −267 ± 76 nA for control solution and −1983 ± 256 nA for hyposmotic solution at −85 mV, n = 25, P < 0.001) (Fig. 1 C). Application of 4 μm RR, a non‐selective TRP channel antagonist, resulted in an immediate reduction in current activity. When applied for 60 s, the antagonist blocked most of the sustained current, confirming its TRPV4 origin (Fig. 1 D, insert). Replacement of Na+ with equiosmolar choline in the recording solution reduced the swelling‐activated inward currents in TRPV4+AQP4‐expressing oocytes (compare −1314 ± 340 nA for control and −722 ± 230 nA for Na+‐free control, as well as −5076 ± 723 nA for hyposmotic gradient and −1639 ± 322 nA for Na+‐free hyposmotic gradient, V m = −130 mV, n = 8–10, P < 0.001) (for representative I/V curves, see Fig. 1 E). Normalized data display a significant difference between the swelling‐induced current activity obtained with Na+‐containing vs. Na+‐free solution (P < 0.001) (Fig. 1 E, bar graph), confirming the cationic nature of the TRPV4‐assigned membrane current. Removal of Ca2+ from the extracellular solution had only a minor effect on the obtained membrane currents (compare −818 ± 208 nA for control and −668 ± 163 nA for Ca2+‐free control, as well as −1625 ± 248 nA for hyposmotic gradient and −1354 ± 178 nA for Ca2+‐free hyposmotic gradient, V m = −85 mV, n = 10) (for representative I/V curves, see Fig. 1 F). Normalized data illustrate no significant difference between the current obtained with Ca2+‐containing vs. Ca2+‐free solution (Fig. 1 F, bar graph) excluding a major contribution from endogenous Ca2+‐activated Cl− channels to swelling‐evoked currents (Ackerman et al. 1994) under our experimental conditions. These data indicate that TRPV4 represents the main contributor to the observed current activity, and suggest that TRPV4 is sensitive to the resultant increase in cell volume rather than the hyposmotic gradient per se. Moreover, when expressed in oocytes, TRPV4 appears to require the presence of an AQP to swiftly translate osmotic challenges into abrupt cell swelling and subsequent channel activation.
TRPV4 has been suggested to require functional interaction exclusively with AQP4 (Benfenati et al. 2011). To further investigate isoform selectivity, we co‐expressed TRPV4 with the related AQP1 and observed successful plasma membrane targeting of both proteins by conventional confocal microscopy (Fig. 2 A). TRPV4+AQP1‐expressing oocytes exposed to a hyposmotic gradient (−100 mosmol l–1) responded with a robust volume change of a magnitude comparable to that of TRPV4 + AQP4‐expressing oocytes (compare 1.51 ± 0.2 × 10−3 cm s−1, n = 13, for TRPV4 + AQP1‐expressing oocytes and 1.40 ± 0.10 for TRPV4 + AQP4‐expressing oocytes, n = 25) (for representative volume trace, see Fig. 2 B). When co‐expressed with AQP1, TRPV4‐mediated current activity increased during application of the hyposmotic gradient (compare −240 ± 51 nA for control and −2723 ± 770 nA for hyposmotic treatment at V m = −85 mV, n = 13, P < 0.05) (Fig. 2 B and C). These data demonstrate that TRPV4 does not exhibit isoform selectivity with regard to functional interactions with AQP4, and that an osmotically‐induced increase in cell volume caused by co‐expression of AQP1 suffices for TRPV4 activation.
Figure 2. TRPV4 functionally interacts with AQP1 in oocytes.
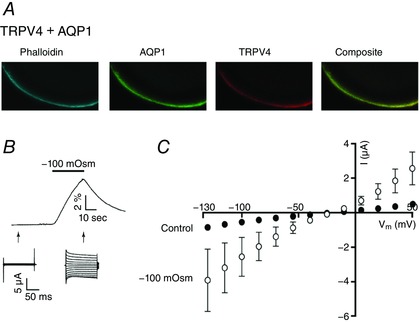
A, representative confocal laser scanning microscopy of an oocyte expressing TRPV4 + AQP1 after immunolabelling with phalloidin, anti‐AQP1 and anti‐TRPV4 illustrated plasma membrane targeting of both proteins. B, representative volume and current traces (arrows indicate when the current traces were obtained) from a TRPV4 + AQP1‐expressing oocyte challenged with a hyposmotic gradient (−100 mosmol l–1, indicated by a black bar). C, summarized I/V curves from oocytes expressing TRPV4 + AQP1 in control solution or in a hyposmotic solution (−100 mosmol l–1). TRPV4‐mediated activity (at V m = −85 mV) in different solutions was compared using one‐way ANOVA with Dunnett's multiple comparison post hoc test. n = 13. [Color figure can be viewed at wileyonlinelibrary.com]
To resolve the kinetics of swelling‐induced activation of TRPV4, oocytes expressing TRPV4 + AQP4 were exposed to progressively increased osmotic gradients (−20 to −100 mosmol l–1) and the current response was recorded during the initial volume increase (after 30 s of exposure to the test solution). TRPV4‐mediated current increased with the osmotic load; see representative volume‐ and current traces in Fig. 3 A, as well as summarized I/V relationships (n = 9) in Fig. 3 B. While no volume‐sensitive current response was recorded from oocytes exclusively expressing AQP4 (Fig. 3 Bi), TRPV4 activity in TRPV4 + AQP4‐expressing oocytes increased in a linear manner as a function of applied osmolarity (r 2 = 0.9493) and thus increased level of cell swelling (Fig. 3 C), with no signs of saturation of the response within the tested range of osmotic gradients. TRPV4 activity thus serves as a proxy read‐out of the magnitude of an abrupt volume change. To determine whether TRPV4, in addition to its ability to monitor an abrupt cell volume change, can also serve as an indirect measure of continuous cell volume changes, we exposed TRPV4 + AQP4‐expressing oocytes to a prolonged osmotic gradient (−100 mosmol l–1 for 180 s) with the TRPV4 activity continuously recorded every 30 s; see representative volume trace in Fig. 4 A, insert (arrows indicate recording times for TRPV4‐mediated current activity) and summarized I/V relationships (n = 9) in Fig. 4 A. The current (at V m = −85 mV) (left y‐axis) and volume changes in percentage (right y‐axis) were plotted as a function of time (Fig. 4 B) with the values recorded at the early time point aligned (given that TRPV4 activation occurs as a linear function of the initial volume response (30 s, Fig. 3)). The activity of the TRPV4‐mediated current reaches a plateau before the rate of volume change subsides. These data suggest that TRPV4 activity is not directly proportional to the changes in cell volume induced by sustained osmotic challenge.
Figure 3. Effects of osmotic load on TRPV4 activation.
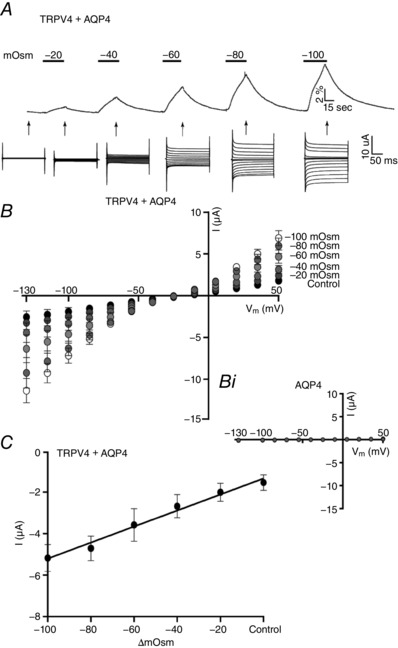
A, volume and current traces (recorded after 30 s of exposure to the given osmotic gradient, indicated by arrows) from a TRPV4 + AQP4‐expressing oocyte challenged with increasing osmotic gradients (−20 to −100 mosmol l–1, indicated by black bars). B, summarized I/V curves of TRPV4 + AQP4‐expressing oocytes exposed to increased osmotic loads, n = 9 (with the I/V curves of AQP4‐expressing oocytes given as (Bi)) (n = 9). C, summarized TRPV4‐mediated activity plotted as a function of the applied osmotic challenge (at V m = −85 mV, r 2 = 0.9493, n = 9).
Figure 4. Effects of prolonged cell volume changes on TRPV4 activation.
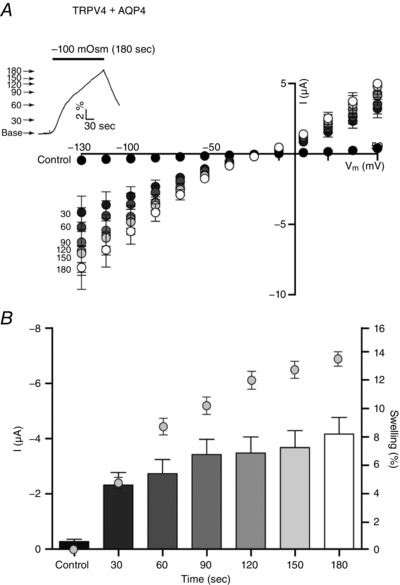
A, TRPV4 + AQP4‐expressing oocytes were challenged with an osmotic gradient (−100 mosmol l–1, indicated by a black bar) for 180 s (see insert for a representative volume trace) and a current trace was obtained every 30 s (indicated by arrows). Summarized I/V relationships are given at these time points (n = 9). B, At V m = −85 mV, TRPV4‐mediated current activity (bars) and resultant cell volume increase (symbols) (n = 9).
To determine whether TRPV4, in addition to the percentage of cell volume increase, senses the rate of cell swelling, we exposed TRPV4 + AQP4‐expressing oocytes to either a large osmotic challenge of −100 mosmol l–1 or a small osmotic challenge of −20 mosmol l–1. The oocytes were allowed to swell (at different rates) until they reached 3% volume increase (for representative volume traces, see Fig. 5 A and B), at which time the I/V relationship was recorded. Upon application of the large osmotic gradient, and thus with the fast rate of volume increase, the swelling‐induced TRPV4 response was robust (Fig. 5 A, compare −2936 ± 893 nA in the control condition and −7877 ± 743 nA after the 3% volume increase elicited by the large osmotic challenge, at V m = −85 mV, n = 9, P < 0.001). With the slow approach towards the 3% volume increase (−20 mosmol l–1), TRPV4 no longer responded to the applied osmotic gradient in terms of increased current activity (see representative I/V curve in Fig. 5 B and compare −2783 ± 983 nA in the control condition and −3152 ± 935 nA after the 3% volume increase, at V m = −85 mV, n = 8). To confirm that the variability within the recorded TRPV4 basal currents did not mask a putative TRPV4 activation under this paradigm, the membrane currents obtained after cell swelling were, in addition, normalized to their own control currents (at V m = −85 mV) (Fig. 5 B, insert). Although there was a tendency towards an increased current after prolonged cell swelling with a small osmotic gradient, no statistical significance was observed. We conclude that the rate of swelling is critical for osmotically‐induced swelling‐mediated TRPV4 activation.
Figure 5. The rate of cell swelling is a critical factor for TRPV4 activation.
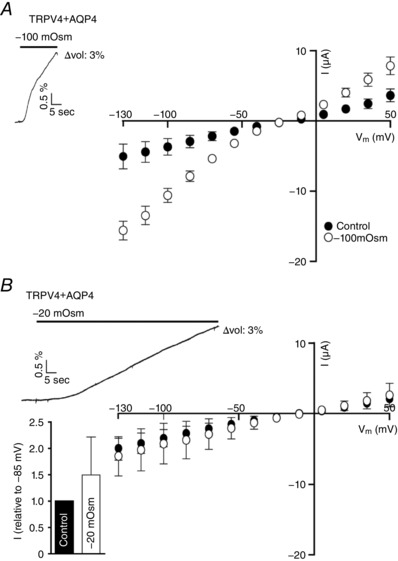
A, summarized I/V curves from TRPV4 + AQP4‐expressing oocytes in control solution and in hyposmotic solution (−100 mosmol l–1; recorded when a 3% increase in cell size was reached, n = 9), representative volume trace illustrated in insert. B, summarized I/V curves from a TRPV4 + AQP4‐expressing oocyte in control solution and in hyposmotic solution (−20 mosmol l–1; recorded when a 3% increase in cell size was reached, n = 8), representative volume trace illustrated in insert. TRPV4‐mediated current activity obtained after exposure to −20 mosmol l–1 was normalized to that obtained in control condition and displayed as an insert in (B). Statistical analysis of the TRPV4‐mediated activity (at V m = −85 mV) was carried out with one‐way ANOVA with Dunnett's multiple comparison post hoc test or with a Student's paired t‐test (for the normalized response).
Studies addressing the volume‐sensitivity of TRPV4 are usually conducted with large osmotic gradients (present study and e.g. (Loukin et al. 2010b; Benfenati et al. 2011)), which are unlikely to occur in a physiological setting. To determine whether swelling‐induced TRPV4 activation relies on such osmotically‐induced cell swelling or whether cell swelling of any origin leads to TRPV4 activation, we employed the concept of co‐transported water: co‐transporters, such as NKCC1, translocate water during their transport cycle in a manner independent of the osmotic gradient (MacAulay & Zeuthen, 2010; Zeuthen & MacAulay, 2012). Activation of NKCC1 will thus promote cell swelling in the absence of an osmotic gradient. Accordingly, isosmolar K+‐induced activation of the water‐transporting NKCC1 was employed to induce cell swelling, in the absence of an applied osmotic gradient, while monitoring TRPV4 activity. Oocytes were kept in a K+‐free solution (to activate NCKK1; Zeuthen & MacAulay, 2012), followed by an abrupt introduction of K+ (15 mm, equiosmolar replacement of Na+ to maintain ionic strength and osmolarity). Only in oocytes expressing NKCC1 (with or without co‐expression of TRPV4) did the introduction of high K+ initiate a rapid onset of water influx, as observed previously (Zeuthen & MacAulay, 2012), whereas the volume of uninjected and TRPV4‐expressing oocytes was unaffected by the applied change in the K+ concentration (for representative volume traces, see Fig. 6 A–D). Current traces recorded before and after the K+‐induced cell swelling demonstrated a robust TRPV4 activation in oocytes expressing both NKCC1 and TRPV4 (for representative current traces and I/V relationships, see Fig. 6 D), whereas no high K+‐mediated current was detected in uninjected, TRPV4‐ or NKCC1‐expressing oocytes (Fig. 6 A–C). Normalized and summarized data shown in Fig. 6 A–D thus illustrate that there was no significant difference between the current obtained before and after K+‐ exposure in uninjected oocytes (compare −56 ± 42 nA before and –67 ± 47 nA after K+‐exposure at V m = −85 mV, n = 9) and oocytes expressing either TRPV4 (compare −475 ± 283 nA for before and −500 ± 303 nA after K+‐exposure at V m = −85 mV, n = 9) or NKCC1 (compare −63 ± 56 nA for before and −59 ± 78 nA after K+‐exposure at V m = −85 mV, n = 9), whereas the current of TRPV4+NKCC1‐expressing oocytes increased by ∼3‐fold after the isosmotic cell swelling as a result of NKCC1‐mediated water transport (2183 ± 586 nA for before and −5770 ± 1911 nA after K+‐exposure at V m = −85 mV, n = 9, P < 0.05). These data highlight that TRPV4 acts as a genuine volume‐sensor, which can be activated directly by cell swelling, independently of AQPs and osmotic gradients.
Figure 6. Isosmolar cell swelling activates TRPV4 independently of AQPs and osmotic gradients.
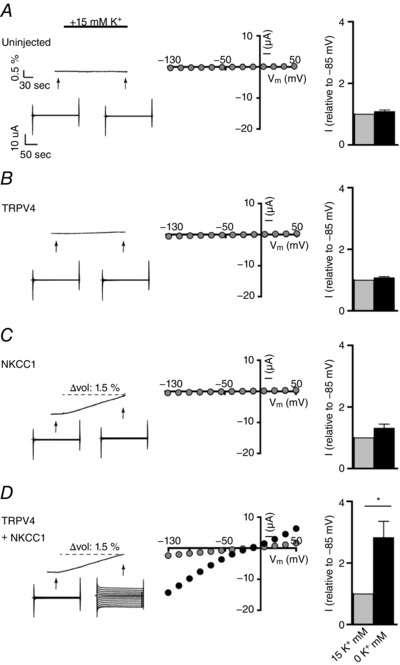
A–D, left: representative volume and current traces from an uninjected oocyte (A) and oocytes expressing TRPV4 (B) or NKCC1 (C) alone, or TRPV4 co‐expressing NKCC1 (D). Influx of water and thus cell swelling was initiated by an equiosmolar increase in external K+ concentration of 15 mm (indicated by a black bar) when NKCC1 was co‐expressed (C) and (D). Middle: representative I/V curves obtained before and after exposure to 15 mm K+. Right: normalized (to 0 mm K+ at V m = −85 mV) and summarized current activity (n = 9). The conditions (TRPV4‐mediated current activity in 0 mm K+ vs. 15 mm K+) were compared using a Student's paired t‐test. * P < 0.05.
The physiological impact of TRPV4 in various experimental settings is frequently based on data obtained with synthetic agonists in the absence of applied osmotic gradients (Thorneloe et al. 2008; Jin et al. 2011; Ryskamp et al. 2014). However, it remains unresolved whether this experimental manner of TRPV4 activation mimics that of cell swelling, or whether these two modes of action are mechanistically distinct and thus potentially additive. To resolve whether the modulatory effect of GSK101 on TRPV4 activity is independent of activation by cell swelling, we initially confirmed GSK101‐mediated TRPV4 activation in the oocyte expression system. Oocytes expressing either TRPV4 alone or co‐expressing AQP4 displayed robust membrane currents after 90 s of exposure to 100 nM GSK101 (compare −3667 ± 865 nA for TRPV4‐expressing oocytes in control solution to −7559 ± 663 nA upon application of GSK101 at V m of −85 mV, n = 9, P < 0.01 and −2936 ± 893 nA for TRPV4 + AQP4‐expressing oocytes in control solution to −8945 ± 913 nA upon application of GSK101 at V m of −85 mV, n = 9, P < 0.001), with no significant difference between the GSK101‐induced current whether or not AQP4 was co‐expressed; see Fig. 7 A–C for representative I/V relationships and Fig. 7 D for summarized data. No GSK101‐induced current activity was observed in oocytes expressing exclusively AQP4 (compare 90 ± 9 nA for AQP4 in control solution to 99 ± 14 nA upon application of GSK101 at V m of −85 mV, n = 9, Fig. 7 C and D). To determine whether GSK101 and cell swelling produced an additive activation of TRPV4, oocytes co‐expressing TRPV4 and AQP4 were initially exposed to a hyposmotic challenge (−100 mosmol l–1), with current traces illustrating robust TRPV4 activation, followed by an identical osmotic challenge with simultaneous application of GSK101 (for representative volume and current traces, see Fig. 8 A). Membrane currents obtained from oocytes expressing only AQP4 responded neither to cell swelling, nor to GSK101 (data not shown). Data summarized for TRPV4 + AQP4‐expressing oocytes demonstrated, at first, that coapplication of GSK101 failed to augment the cell swelling‐induced activation of TRPV4‐mediated currents (compare −7223 ± 1062 nA for hyposmolar gradient alone and −9078 ± 898 nA for GSK101+hyposmolar gradient at V m of −85 mV, n = 9). However, to confirm that the variability in the current level did not mask an additive TRPV4 activation by GSK101 following volume changes, the membrane currents obtained by cell swelling and GSK101 exposure in combination were normalized to the membrane currents obtained by cell swelling (at V m = −85 mV) (Fig. 8 B, insert). A slight increase was observed (P < 0.05), which illustrates that GSK101 exerts a small additive effect on the TRPV4 activity during osmotically‐induced cell swelling (with the tested osmotic challenge). To test the ability of an osmotic challenge to enhance GSK101‐mediated TRPV4‐activation, we conducted a slightly different experimental paradigm, where TRPV4 + AQP4‐expressing oocytes were exposed to a continuous GSK101 application, during which a hyposmotic challenge was introduced (−100 mosmol l–1, same concentration of GSK101) (for the experimental approach and representative volume and current traces, see Fig. 8 C). Representative I/V curves (Fig. 8 D) show that coapplication of the hyposmotic gradient failed to augment the GSK101‐triggered activation of TRPV4 (compare −1384 ± 92 nA for GSK101 alone and −1585 ± 84 nA for GSK101+hyposmolar gradient at V m of −85 mV, n = 9). Normalization of the currents obtained by GSK101 and cell swelling to those obtained with GSK101 alone (at V m = −85 mV) revealed no significant additive effect (Fig. 8 D, insert). Because the employed concentration of GSK101 is considered saturating for TRPV4 activation (Sullivan et al. 2012; Ryskamp et al. 2014) and the applied hyposmotic gradient close to (but most probably not) maximal, these data sets illustrate that following full activation of TRPV4 with one of these stimuli, an alternative stimulus evokes minimal additional facilitatory effect. To determine whether additive effects could be obtained with stimuli of reduced magnitude, we conducted an experimental paradigm identical to that of Fig. 8 A, albeit with a smaller osmotic challenge (−50 mosmol l–1) and a non‐saturating concentration of GSK101 (20 nm, near the EC50) (Sullivan et al. 2012; Ryskamp et al. 2014; Jo et al. 2015). Under these conditions, coapplication of GSK101 significantly augmented the effect of the cell swelling‐induced TRPV4 activation (compare −1005 ± 139 nA for hyposmolar gradient alone and −1369 ± 152 nA for GSK101+hyposmolar gradient at V m of −85 mV, n = 9, P < 0.05) (Fig. 8 E). With subsaturating stimuli, GSK101+hyposmotic gradient‐induced current activity normalized to that obtained by the osmotic gradient alone illustrates that GSK101 exerts an additive effect (P < 0.001, V m = −85 mV) (Fig. 8 E, insert). GSK101‐mediated activation of TRPV4 might therefore promote a channel conformation similar to that induced by cell swelling.
Figure 7. GSK1016790A‐mediated activation of TRPV4.

A–C, representative I/V curves from oocytes expressing TRPV4 (A), TRPV4 + AQP4 (B), or AQP4 (C), in control solution with or without application of GSK101 (100 nM, 90 s). D, TRPV4‐mediated activity recorded before and after activation of TRPV4 by GSK101 (at V m = −85 mV) (n = 9). The effect of GSK101 on TRPV4‐mediated current activity was analysed with one‐way ANOVA with Dunnett's multiple comparison post hoc test. ** P < 0.01, *** P < 0.001 (n = 9).
Figure 8. No additive effect of GSK1016790A and cell swelling‐ on TRPV4 activation.
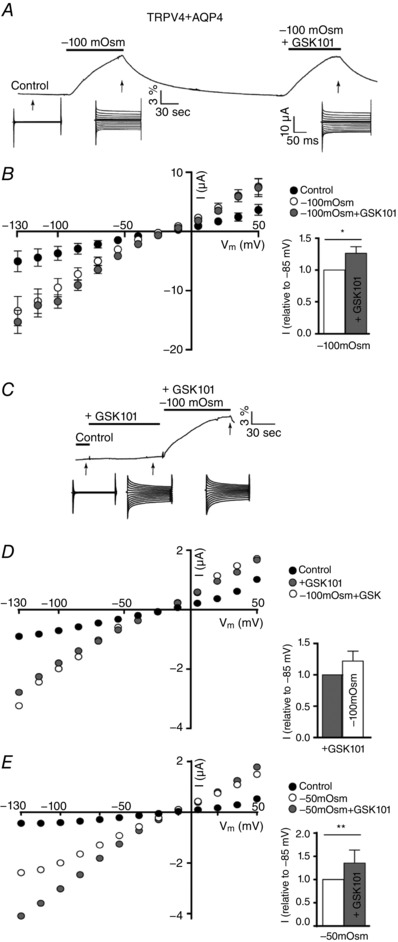
A, volume and current traces (arrows indicate when the current traces were recorded during the volume change) from a TRPV4 + AQP4‐expressing oocyte challenged with a hyposmotic gradient or a combination of a hyposmotic gradient and GSK101 (−100 mosmol l–1, 100 nM GSK101, 90 s of application, indicated by a black bar). B, summarized I/V relationships of TRPV4 + AQP4‐expressing oocytes obtained in control solution, in a hyposmolar solution (−100 mosmol l–1), or in a hyposmolar solution containing GSK101 (−100 mosmol l–1 + GSK101) (n = 9). TRPV4‐mediated current activity obtained after exposure to −100 mosmol l–1 + GSK101 was normalized to that obtained after exposure to −100 mosmol l–1 and displayed as an insert in (B). C, representative volume and current traces (arrows indicate when the current traces were recorded during stimulus application) from a TRPV4 + AQP4‐expressing oocyte challenged with GSK101 or a combination of GSK101 and a hyposmotic gradient (100 nM GSK101, −100 mosmol l–1, 90 s of application, indicated by a black bar). D, representative I/V curves from a TRPV4 + AQP4‐expressing oocyte challenged with GSK101 (100 nM) for 90 s at which point a hyposmotic gradient was introduced in addition to GSK101 (100 nM GSK101, −100 mosmol l–1, 90 s of application). TRPV4‐mediated current activity obtained after exposure to GSK101 + −100 mosmol l–1 was normalized to that obtained after exposure to GSK101 alone and displayed as an insert in (D) (n = 9). E, representative I/V curves from a TRPV4 + AQP4‐expressing oocyte challenged with a smaller hyposmotic gradient (−50 mosmol l–1) followed by a subsequent challenge of a combination of the gradient and GSK101 (−50 mosmol l–1, 20 nm GSK101, 90 s of application). TRPV4‐mediated current activity obtained after exposure to −50 mosmol l–1 + GSK101 was normalized to that obtained after exposure to −50 mosmol l–1 alone and displayed as an insert in (E) (n = 9). Statistical analysis of the TRPV4‐mediated activity (at V m = −85 mV) was carried out with one‐way ANOVA with Dunnett's multiple comparison post hoc test or with a Student's paired t‐test (for the normalized response). * P < 0.05, ** P < 0.01 (n = 9).
Discussion
The present study identifies TRPV4 as a volume‐sensing ion channel rather than the osmo‐sensor it is often referred to be, and demonstrates that TRPV4 responds to volume changes irrespective of the origin of the cell swelling. Genuine osmo‐sensing channels and transporters, such as the BetP, ProP and OpuA (Rubenhagen et al. 2000; van der Heide et al. 2001; Schiller et al. 2006), sense changes in the osmolarity of the surrounding fluid, whereas a volume‐sensor activates upon the ensuing change in cell volume. Most cells are osmo‐ and/or mechano‐sensitive (French, 1992). Such osmo‐sensitivity is considered to originate from the detection of membrane tension by ion channels (Kernan & Zuker, 1995; Sackin, 1995), which suggests that a change in cell volume, and not only an osmotic gradient, is essential for transduction. TRPV4 has been identified by its capacity to activate following exposure to hyposmotic gradients, possibly in response to membrane stretch achieved as a consequence of the osmotically‐induced cell swelling (Liedtke et al. 2000; Liedtke & Friedman, 2003; Liedtke, 2005a; Plant & Strotmann, 2007). Osmotically‐induced TRPV4 activation has been further suggested to require an obligatory formation of macromolecular complexes that include AQP4 or ‐5, whereby the given AQP isoform rather than cell swelling per se, activates the channel (Liu et al. 2006; Benfenati et al. 2011). To address this putative functional interaction directly, we expressed TRPV4, NKCC1 and AQP isoforms in the Xenopus oocyte membrane. We confirmed that the currents obtained in the TRPV4‐expressing oocytes originated from TRPV4 (by their cationic nature and RR‐sensitivity) and excluded a significant contribution from endogenous Xenopus Ca2+‐activated Cl− current (Ackerman et al. 1994; Morin et al. 1995). As described previously, basal current amplitudes varied from oocyte batch to oocyte batch (Strotmann et al. 2003), possibly indicating differences in temperature, batch‐specific levels of lipid metabolites, cell volume, channel trafficking and/or abundance of TRPV4 in the plasma membrane. Consistent with the earlier demonstration of distinct mechanisms involved in the generation of basal TRPV4‐mediated currents and in the channel response to hyposmotic gradients (Vriens et al. 2004), we found that a varying level of basal activity had no obvious effect on the response to activators such as cell swelling and agonist exposure. Activation of TRPV4 with cell swelling or agonist exposure yielded an almost linear current voltage relation, as previously observed in retinal Müller glia (Jo et al. 2015). Outward rectification of TRPV4‐mediated currents has been reported in studies employing TRPV4‐expressing HEK293 cells (Guler et al. 2002; Voets et al. 2002) and oocytes (Loukin et al. 2010a; Teng et al. 2015) and may have occurred partly as a consequence of the non‐physiological test solutions (i.e. following the removal of Na+/Ca2+ from the test solution).
We found that co‐expression of an AQP in the Xenopus oocyte expression system simply accelerates osmotically‐induced cell swelling and is sufficient for TRPV4 activation, irrespective of the AQP isoform employed: both AQP4 and AQP1 co‐localize with TRPV4 in the membrane of cRNA microinjected oocytes and TRPV4‐expressing oocytes respond robustly to the introduction of a hyposmotic challenge whether co‐expressed with AQP1 or AQP4. TRPV4 activation is negligible upon the introduction of a similar osmotic gradient in a setting in which the oocyte is largely prevented from swelling on the tested time scale (in the absence of a co‐expressed AQP). Our data are consistent with recent reports in retinal and cortical glia, which concluded that rapid AQP‐dependent cell swelling optimizes osmotically‐induced TRPV4 activation (Jo et al. 2015; Mola et al. 2016). The advantages of our experimental setup allowed us to expand on these observations but also yielded fundamental new insights about the molecular mechanism of TRPV4 activation: (i) TRPV4 activity was assessed without contamination by glial volume‐sensitive ion channels; (ii) TRPV4 activation could be monitored in response to physiologically relevant cell swelling of only a few percent; and (iii) the ~ 20‐fold difference in osmotic water permeability of the oocyte membrane in the absence and presence of AQP‐expression allowed for a clear distinction in rate of cell swelling under these two conditions. Our data show that TRPV4 activation, induced by application of an osmotic gradient, occurs in response to AQP‐facilitated cell swelling. TRPV4, when co‐expressed with AQP4, responded in a linear manner to the magnitude of the abruptly introduced osmotic gradient applied within the tested range (−20 to −100 mosmol l–1). This finding is in agreement with previous reports of a relationship between osmotic load and the resulting TRPV4‐activation (Strotmann et al. 2000; Ryskamp et al. 2014). Nevertheless, TRPV4 current activity did not directly mirror the effects of cell volume changes in the presence of continuously applied osmotic challenges, as indicated by the saturation of TRPV4 activation at a time point at which cell swelling persisted. We therefore conclude that TRPV4 activity serves as a direct read‐out of cell volume on an immediate time scale (up to 30 s in the present study), at least when expressed in Xenopus oocytes. Interestingly, while TRPV4 responded robustly to a rapid cell volume increase (obtained by application of a large osmotic gradient of −100 mosmol l–1), a slow approach to the same percentage of volume increase (by prolonged application of a small osmotic gradient of −20 mosmol l–1) failed to activate TRPV4‐mediated currents to the same extent. These results may indicate that TRPV4 serves primarily as a cellular sensor for abrupt volume changes. Because membrane folding provides an uncommon excess surface area to Xenopus oocytes, we cannot exclude the possibility that the observed rate‐dependence might be an oocyte‐specific phenomenon. However, cells that lack AQPs display less TRPV4 activity as the rate of cell swelling is reduced (Jo et al. 2015; Mola et al. 2016), although these studies were not designed to obtain TRPV4 activity at a predefined percentage of cell swelling (obtained at the different rates of cell swelling).
The majority of experimental determinations of TRPV4 osmo‐/volume‐sensing have relied on application of large osmotic gradients of 100–150 mosmol l–1 (Benfenati et al. 2011; Loukin et al. 2011), however, such gradients are rarely, if ever, observed in physiology, or even pathophysiology. To determine whether volume‐activation of TRPV4 required osmotically‐induced cell swelling, we designed an experimental paradigm based on isosmotic cell swelling by co‐transporter‐mediated water transport (Zeuthen & MacAulay, 2012), which causes cell swelling in absence of any applied osmotic gradients and co‐expressed AQPs. Activation of NKCC1 leads to accumulation of osmolytes (Na+, K+, Cl−) concomitantly with the co‐transported water (near‐isotonic transportate; (Zeuthen & MacAulay, 2012)) and therefore a slight osmotic gradient may have been imposed across the oocyte membrane at any given time during the experimental procedure. We discovered that oocyte swelling obtained under such isosmotic conditions brings about robust TRPV4 activation. The independence from applied osmotic gradients cements the notion that TRPV4 functions as a sensor of cell volume rather than of the osmotic gradients facing a given cell.
The precise molecular mechanism that links cell swelling to TRPV4 channel activation remains unclear. Ion channels are most likely unable to directly sense cell swelling, and some form of molecular coupling must exist between the volume increase and the resultant cell swelling. The transmembrane helix 5 of TRPV4 is considered to be a required factor (Klausen et al. 2014) and cryo‐electron microscopy has revealed that TRPV4 resembles a ‘hanging basket’, with approximately 70% of its total structure located at the cytosolic side of the plasma membrane (Shigematsu et al. 2010). Such cytosolic bulk could suggest that intracellular sections of TRPV4 may be prone to regulatory factors and/or interaction with cytosolic proteins or the cellular cytoskeleton. In support of the latter, an intimate association between TRPV4, actin and microtubuli has been detected (Ramadass et al. 2007; Becker et al. 2009; Goswami et al. 2010) and a supra‐molecular cytoskeletal and regulatory kinase complex anchored at TRPV4 proposed (Goswami et al. 2010). In such a complex, TRPV4 could serve to integrate signals from various signalling cascades and cytoskeletal dynamics occurring during cell volume changes possibly in response to macromolecular crowding (Minton, 2006), post translational modification (Xu et al. 2003) (although later questioned; Vriens et al. 2004), or mechanical stimulation leading to increased membrane deformation (Suzuki et al. 2003; Matthews et al. 2010; Soya et al. 2014). Other chemical factors such as activation of phospholipase A2 with subsequent intracellular accumulation of arachidonic acid or its metabolites and derivatives, possibly released during cell volume changes (Thoroed et al. 1997; Basavappa et al. 1998; Pedersen et al. 2000), have been demonstrated as necessary for TRPV4 activation by hyposmotically‐induced cell swelling in HEK cells (Vriens et al. 2004) but not in Xenopus oocytes (Loukin et al. 2010a). Finally, it has been proposed that phosphatidylinositol‐4,5‐diphosphate binding to a motif in the cytosolic N‐terminus may cause rearrangements of the TRPV4 tail region causing channel opening during an osmotic challenge (Garcia‐Elias et al. 2013).
Another interesting, and still unresolved, question is how synthetic compounds, such as GSK101, employed as a potent, selective and routinely used TRPV4 agonist (Willette et al. 2008; Jin et al. 2011), activate the channel. We confirmed that the TRPV4 response to the agonist shows the typically delayed, yet almost linear, current–voltage response (Thorneloe et al. 2008; Ryskamp et al. 2014; Jo et al. 2015). With (near)saturating stimuli, the swelling‐induced TRPV4 activation was only slightly enhanced by concomitant application of GSK101 and the GSK101‐induced TRPV4 activation was unaffected by an additional osmotic challenge, suggesting that the GSK101‐elicited TRPV4 response, under these conditions, is barely additive to that of volume‐induced activation of TRPV4. A recent Ca2+ imaging study in chondrocytes suggested that GSK101 elicits more pronounced [Ca2+]i transients compared to hyposmotic loading (O'Conor et al. 2014). Consistent with this, we observed additivity between TRPV4 activation obtained by GSK101 and that obtained by an osmotic load, when the osmotic challenge and the GSK101 concentration were in the subsaturating range (Sullivan et al. 2012; Ryskamp et al. 2014; Jo et al. 2015). We therefore hypothesize that GSK101 activates TRPV4 in a manner that promotes conformational changes similar to those induced by osmotically‐induced cell swelling.
In summary, the results of the present study demonstrate that TRPV4 acts as a genuine volume‐sensor, rather than an osmo‐sensor. Using a rigorous, well‐defined and controlled cellular assay, we find that the channel responds to cell swelling irrespective of the presence of large osmotic gradients and co‐localization of AQPs. We propose that AQPs, during externally applied osmotic challenges, simply serve as a tool for efficiently achieving the fast volume changes that represent the optimal mode of TRPV4 activation. In such cases, TRPV4 activation serves as a functional read‐out of cell swelling. Our data indicate that GSK101‐mediated activation of TRPV4, to a large extent, mimics that of volume‐dependent activation, which highlights its usefulness in experimental settings. The molecular mechanisms that couple cell swelling to TRPV4 channel opening remain obscure, as do the downstream effectors connecting volume‐induced TRPV4 activation to cell volume regulation.
Additional information
Conflict of interest
The authors declare they have no competing interests.
Author contributions
TLTB, DK and NM conceived and designed the research. TLTB conducted the experiments. TLTB analysed the data. TLTB and NM interpretated the results. TLTB and NM prepared the figures. TLTB drafted the manuscript. TLTB, DK and NM edited and revised the manuscript. TLTB, DK and NM approved the final version of the manuscript submitted for publication.
Funding
The project was funded by the VELUX Foundation (107493) (TLTB), the National Institutes of Health (EY022076, EY027920, P30EY014800), the Willard L. Eccles Foundation, and an unrestricted support from Research to Prevent Blindness to the Moran Eye Institute (DK).
Acknowledgements
We thank technicians Charlotte Goos Iversen and Mette Assentoft, as well as the Core Facility for Integrated Microscopy, Faculty of Health and Medical Sciences, University of Copenhagen.
This is an Editor's Choice article from the 1 June 2017 issue.
References
- Ackerman MJ, Wickman KD & Clapham DE (1994). Hypotonicity activates a native chloride current in Xenopus oocytes. J Gen Physiol 103, 153–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade YN, Fernandes J, Vazquez E, Fernandez‐Fernandez JM, Arniges M, Sanchez TM, Villalon M & Valverde MA (2005). TRPV4 channel is involved in the coupling of fluid viscosity changes to epithelial ciliary activity. J Cell Biol 168, 869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavappa S, Pedersen SF, Jorgensen NK, Ellory JC & Hoffmann EK (1998). Swelling‐induced arachidonic acid release via the 85‐kDa cPLA2 in human neuroblastoma cells. J Neurophysiol 79, 1441–1449. [DOI] [PubMed] [Google Scholar]
- Becker D, Bereiter‐Hahn J & Jendrach M (2009). Functional interaction of the cation channel transient receptor potential vanilloid 4 (TRPV4) and actin in volume regulation. Eur J Cell Biol 88, 141–152. [DOI] [PubMed] [Google Scholar]
- Becker D, Blase C, Bereiter‐Hahn J & Jendrach M (2005). TRPV4 exhibits a functional role in cell‐volume regulation. J Cell Sci 118, 2435–2440. [DOI] [PubMed] [Google Scholar]
- Benfenati V, Caprini M, Dovizio M, Mylonakou MN, Ferroni S, Ottersen OP & Amiry‐Moghaddam M (2011). An aquaporin‐4/transient receptor potential vanilloid 4 (AQP4/TRPV4) complex is essential for cell‐volume control in astrocytes. Proc Natl Acad Sci USA 108, 2563–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Cho TJ, Unger S, Lausch E, Nishimura G, Kim OH, Superti‐Furga A & Ikegawa S (2010). TRPV4‐pathy, a novel channelopathy affecting diverse systems. J Hum Genet 55, 400–402. [DOI] [PubMed] [Google Scholar]
- Danziger J & Zeidel ML (2015). Osmotic homeostasis. Clin J Am Soc Nephrol 10, 852–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley S, Heppner TJ, Nelson MT & Brayden JE (2005). TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res 97, 1270–1279. [DOI] [PubMed] [Google Scholar]
- Fenton RA, Moeller HB, Zelenina M, Snaebjornsson MT, Holen T & MacAulay N (2010). Differential water permeability and regulation of three aquaporin 4 isoforms. Cell Mol Life Sci 67, 829–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes J, Lorenzo IM, Andrade YN, Garcia‐Elias A, Serra SA, Fernandez‐Fernandez JM & Valverde MA (2008). IP3 sensitizes TRPV4 channel to the mechano‐ and osmotransducing messenger 5‘‐6’‐epoxyeicosatrienoic acid. J Gen Physiol 131, i2. [DOI] [PubMed] [Google Scholar]
- French AS (1992). Mechanotransduction. Annu Rev Physiol 54, 135–152. [DOI] [PubMed] [Google Scholar]
- Galizia L, Pizzoni A, Fernandez J, Rivarola V, Capurro C & Ford P (2012). Functional interaction between AQP2 and TRPV4 in renal cells. J Cell Biochem 113, 580–589. [DOI] [PubMed] [Google Scholar]
- Garcia‐Elias A, Mrkonjic S, Pardo‐Pastor C, Inada H, Hellmich UA, Rubio‐Moscardo F, Plata C, Gaudet R, Vicente R & Valverde MA (2013). Phosphatidylinositol‐4,5‐biphosphate‐dependent rearrangement of TRPV4 cytosolic tails enables channel activation by physiological stimuli. Proc Natl Acad Sci USA 110, 9553–9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami C, Kuhn J, Heppenstall PA & Hucho T (2010). Importance of non‐selective cation channel TRPV4 interaction with cytoskeleton and their reciprocal regulations in cultured cells. PloS ONE 5, e11654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in the journal of physiology and experimental physiology. J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler AD, Lee H, Iida T, Shimizu I, Tominaga M & Caterina M (2002). Heat‐evoked activation of the ion channel, TRPV4. J Neurosci 22, 6408–6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haj‐Yasein NN, Jensen V, Vindedal GF, Gundersen GA, Klungland A, Ottersen OP, Hvalby O & Nagelhus EA (2011). Evidence that compromised K+ spatial buffering contributes to the epileptogenic effect of mutations in the human Kir4.1 gene (KCNJ10). Glia 59, 1635–1642. [DOI] [PubMed] [Google Scholar]
- Hammami S, Willumsen NJ, Olsen HL, Morera FJ, Latorre R & Klaerke DA (2009). Cell volume and membrane stretch independently control K+ channel activity. J Physiol 587, 2225–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann EK, Lambert IH & Pedersen SF (2009). Physiology of cell volume regulation in vertebrates. Physiol Rev 89, 193–277. [DOI] [PubMed] [Google Scholar]
- Jia Y, Wang X, Varty L, Rizzo CA, Yang R, Correll CC, Phelps PT, Egan RW & Hey JA (2004). Functional TRPV4 channels are expressed in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 287, L272–L278. [DOI] [PubMed] [Google Scholar]
- Jin M, Wu Z, Chen L, Jaimes J, Collins D, Walters ET & O'Neil RG (2011). Determinants of TRPV4 activity following selective activation by small molecule agonist GSK1016790A. PloS ONE 6, e16713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo AO, Lakk M, Frye AM, Phuong TT, Redmon SN, Roberts R, Berkowitz BA, Yarishkin O & Križaj D (2016). Differential volume regulation and calcium signaling in two ciliary body cell types is subserved by TRPV4 channels. Proc Natl Acad Sci USA 113, 3885–3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo AO, Ryskamp DA, Phuong TT, Verkman AS, Yarishkin O, MacAulay N & Križaj D (2015). TRPV4 and AQP4 channels synergistically regulate cell volume and calcium homeostasis in retinal muller glia. J Neurosci 35, 13525–13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernan M & Zuker C (1995). Genetic approaches to mechanosensory transduction. Curr Opin Neurol 5, 443–448. [DOI] [PubMed] [Google Scholar]
- Klausen TK, Janssens A, Prenen J, Owsianik G, Hoffmann EK, Pedersen SF & Nilius B (2014). Single point mutations of aromatic residues in transmembrane helices 5 and ‐6 differentially affect TRPV4 activation by 4alpha‐PDD and hypotonicity: implications for the role of the pore region in regulating TRPV4 activity. Cell Calcium 55, 38–47. [DOI] [PubMed] [Google Scholar]
- Križaj D, Ryskamp DA, Tian N, Tezel G, Mitchell CH, Slepak VZ & Shestopalov VI (2014). From mechanosensitivity to inflammatory responses: new players in the pathology of glaucoma. Curr Eye Res 39, 105–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen BR, Assentoft M, Cotrina ML, Hua SZ, Nedergaard M, Kaila K, Voipio J & MacAulay N (2014). Contributions of the Na(+)/K(+)‐ATPase, NKCC1, and Kir4.1 to hippocampal K(+) clearance and volume responses. Glia 62, 608–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W (2005a). TRPV4 as osmosensor: a transgenic approach. Pflugers Archiv 451, 176–180. [DOI] [PubMed] [Google Scholar]
- Liedtke W (2005b). TRPV4 plays an evolutionary conserved role in the transduction of osmotic and mechanical stimuli in live animals. J Physiol 567, 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W, Choe Y, Marti‐Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM & Heller S (2000). Vanilloid receptor‐related osmotically activated channel (VR‐OAC), a candidate vertebrate osmoreceptor. Cell 103, 525–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W & Friedman JM (2003). Abnormal osmotic regulation in trpv4–/– mice. Proc Natl Acad Sci USA 100, 13698–13703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Bandyopadhyay BC, Nakamoto T, Singh B, Liedtke W, Melvin JE & Ambudkar I (2006). A role for AQP5 in activation of TRPV4 by hypotonicity: concerted involvement of AQP5 and TRPV4 in regulation of cell volume recovery. J Biol Chem 281, 15485–15495. [DOI] [PubMed] [Google Scholar]
- Loukin S, Su Z & Kung C (2011). Increased basal activity is a key determinant in the severity of human skeletal dysplasia caused by TRPV4 mutations. PloS ONE 6, e19533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukin S, Su Z, Zhou X & Kung C (2010a). Forward genetic analysis reveals multiple gating mechanisms of TRPV4. J Biol Chem 285, 19884–19890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukin S, Zhou X, Su Z, Saimi Y & Kung C (2010b). Wild‐type and brachyolmia‐causing mutant TRPV4 channels respond directly to stretch force. J Biol Chem 285, 27176–27181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukin SH, Teng J & Kung C (2015). A channelopathy mechanism revealed by direct calmodulin activation of TrpV4. Proc Natl Acad Sci USA 112, 9400–9405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAulay N & Zeuthen T (2010). Water transport between CNS compartments: contributions of aquaporins and cotransporters. Neuroscience 168, 941–956. [DOI] [PubMed] [Google Scholar]
- Matthews BD, Thodeti CK, Tytell JD, Mammoto A, Overby DR & Ingber DE (2010). Ultra‐rapid activation of TRPV4 ion channels by mechanical forces applied to cell surface beta1 integrins. Integr Biol 2, 435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton AP (2006). Macromolecular crowding. Curr Biol 16, R269–271. [DOI] [PubMed] [Google Scholar]
- Mola MG, Sparaneo A, Gargano CD, Spray DC, Svelto M, Frigeri A, Scemes E & Nicchia GP (2016). The speed of swelling kinetics modulates cell volume regulation and calcium signaling in astrocytes: a different point of view on the role of aquaporins. Glia 64, 139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin XK, Bond TD, Loo TW, Clarke DM & Bear CE (1995). Failure of P‐glycoprotein (MDR1) expressed in Xenopus oocytes to produce swelling‐activated chloride channel activity. J Physiol 486, 707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Prenen J, Wissenbach U, Bodding M & Droogmans G (2001). Differential activation of the volume‐sensitive cation channel TRP12 (OTRPC4) and volume‐regulated anion currents in HEK‐293 cells. Pflugers Archiv 443, 227–233. [DOI] [PubMed] [Google Scholar]
- Nilius B & Voets T (2013). The puzzle of TRPV4 channelopathies. EMBO Rep 14, 152–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Vriens J, Prenen J, Droogmans G & Voets T (2004). TRPV4 calcium entry channel: a paradigm for gating diversity. Am J Physiol Cell Physiol 286, C195–C205. [DOI] [PubMed] [Google Scholar]
- O'Conor CJ, Leddy HA, Benefield HC, Liedtke WB & Guilak F (2014). TRPV4‐mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc Natl Acad Sci USA 111, 1316–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y & Maeno E (2001). Apoptosis, cell volume regulation and volume‐regulatory chloride channels. Comp Biochem Physiol A Mol Integr Physiol 130, 377–383. [DOI] [PubMed] [Google Scholar]
- Pedersen S, Lambert IH, Thoroed SM & Hoffmann EK (2000). Hypotonic cell swelling induces translocation of the alpha isoform of cytosolic phospholipase A2 but not the gamma isoform in Ehrlich ascites tumor cells. Eur J Biochem 267, 5531–5539. [DOI] [PubMed] [Google Scholar]
- Pedersen SF, Klausen TK & Nilius B (2015). The identification of a volume‐regulated anion channel: an amazing Odyssey. Acta Physiol 213, 868–881. [DOI] [PubMed] [Google Scholar]
- Pinar‐Sueiro S, Urcola H, Rivas MA & Vecino E (2011). Prevention of retinal ganglion cell swelling by systemic brimonidine in a rat experimental glaucoma model. Clin Exp Ophthalmol 39, 799–807. [DOI] [PubMed] [Google Scholar]
- Plant TD & Strotmann R (2007). TRPV4: a multifunctional nonselective cation channel with complex regulation In TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades, ed. Liedtke WB. & Heller S. Boca Raton, FL, CRC Press/Taylor & Francis. [PubMed] [Google Scholar]
- Ramadass R, Becker D, Jendrach M & Bereiter‐Hahn J (2007). Spectrally and spatially resolved fluorescence lifetime imaging in living cells: TRPV4‐microfilament interactions. Arch Biochem Biophys 463, 27–36. [DOI] [PubMed] [Google Scholar]
- Rubenhagen R, Ronsch H, Jung H, Kramer R & Morbach S (2000). Osmosensor and osmoregulator properties of the betaine carrier BetP from Corynebacterium glutamicum in proteoliposomes. J Biol Chem 275, 735–741. [DOI] [PubMed] [Google Scholar]
- Ryskamp DA, Frye AM, Phuong TT, Yarishkin O, Jo AO, Xu Y, Lakk M, Iuso A, Redmon SN, Ambati B, Hageman G, Prestwich GD, Torrejon KY & Križaj D (2016). TRPV4 regulates calcium homeostasis, cytoskeletal remodeling, conventional outflow and intraocular pressure in the mammalian eye. Sci Rep 6, 30583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryskamp DA, Iuso A & Križaj D (2015). TRPV4 links inflammatory signaling and neuroglial swelling. Channels 9, 70–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryskamp DA, Jo AO, Frye AM, Vazquez‐Chona F, MacAulay N, Thoreson WB & Križaj D (2014). Swelling and eicosanoid metabolites differentially gate TRPV4 channels in retinal neurons and glia. J Neurosci 34, 15689–15700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryskamp DA, Witkovsky P, Barabas P, Huang W, Koehler C, Akimov NP, Lee SH, Chauhan S, Xing W, Renteria RC, Liedtke W & Križaj D (2011). The polymodal ion channel transient receptor potential vanilloid 4 modulates calcium flux, spiking rate, and apoptosis of mouse retinal ganglion cells. J Neurosci 31, 7089–7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackin H (1995). Mechanosensitive channels. Annu Rev Physiol 57, 333–353. [DOI] [PubMed] [Google Scholar]
- Schiller D, Ott V, Kramer R & Morbach S (2006). Influence of membrane composition on osmosensing by the betaine carrier BetP from Corynebacterium glutamicum . J Biol Chem 281, 7737–7746. [DOI] [PubMed] [Google Scholar]
- Shigematsu H, Sokabe T, Danev R, Tominaga M & Nagayama K (2010). A 3.5‐nm structure of rat TRPV4 cation channel revealed by Zernike phase‐contrast cryoelectron microscopy. J Biol Chem 285, 11210–11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soe R, MacAulay N & Klaerke DA (2009). Modulation of Kir4.1 and Kir4.1‐Kir5.1 channels by small changes in cell volume. Neurosci Lett 457, 80–84. [DOI] [PubMed] [Google Scholar]
- Soya M, Sato M, Sobhan U, Tsumura M, Ichinohe T, Tazaki M & Shibukawa Y (2014). Plasma membrane stretch activates transient receptor potential vanilloid and ankyrin channels in Merkel cells from hamster buccal mucosa. Cell Calcium 55, 208–218. [DOI] [PubMed] [Google Scholar]
- Strotmann R, Harteneck C, Nunnenmacher K, Schultz G & Plant TD (2000). OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol 2, 695–702. [DOI] [PubMed] [Google Scholar]
- Strotmann R, Schultz G & Plant TD (2003). Ca2+‐dependent potentiation of the nonselective cation channel TRPV4 is mediated by a C‐terminal calmodulin binding site. J Biol Chem 278, 26541–26549. [DOI] [PubMed] [Google Scholar]
- Sullivan MN, Francis M, Pitts NL, Taylor MS & Earley S (2012). Optical recording reveals novel properties of GSK1016790A‐induced vanilloid transient receptor potential channel TRPV4 activity in primary human endothelial cells. Mol Pharmacol 82, 464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Mizuno A, Kodaira K & Imai M (2003). Impaired pressure sensation in mice lacking TRPV4. J Biol Chem 278, 22664–22668. [DOI] [PubMed] [Google Scholar]
- Teng J, Loukin SH, Anishkin A & Kung C (2015). L596‐W733 bond between the start of the S4‐S5 linker and the TRP box stabilizes the closed state of TRPV4 channel. Proc Natl Acad Sci USA 112, 3386–3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneloe KS, Sulpizio AC, Lin Z, Figueroa DJ, Clouse AK, McCafferty GP, Chendrimada TP, Lashinger ES, Gordon E, Evans L, Misajet BA, Demarini DJ, Nation JH, Casillas LN, Marquis RW, Votta BJ, Sheardown SA, Xu X, Brooks DP, Laping NJ & Westfall TD (2008). N‐((1S)‐1‐{[4‐((2S)‐2‐{[(2,4‐dichlorophenyl)sulfonyl]amino}‐3‐hydroxypropanoyl)‐1‐piperazinyl]carbonyl}‐3‐methylbutyl)‐1‐benzothiophene‐2‐carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity: Part I. J Pharmacol Exp Ther 326, 432–442. [DOI] [PubMed] [Google Scholar]
- Thoroed SM, Lauritzen L, Lambert IH, Hansen HS & Hoffmann EK (1997). Cell swelling activates phospholipase A2 in Ehrlich ascites tumor cells. J Membr Biol 160, 47–58. [DOI] [PubMed] [Google Scholar]
- Tian W, Salanova M, Xu H, Lindsley JN, Oyama TT, Anderson S, Bachmann S & Cohen DM (2004). Renal expression of osmotically responsive cation channel TRPV4 is restricted to water‐impermeant nephron segments. Am J Physiol Renal Physiol 287, F17–F24. [DOI] [PubMed] [Google Scholar]
- van der Heide T, Stuart MC & Poolman B (2001). On the osmotic signal and osmosensing mechanism of an ABC transport system for glycine betaine. EMBO J 20, 7022–7032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent F & Duncton MA (2011). TRPV4 agonists and antagonists. Curr Top Med Chem 11, 2216–2226. [DOI] [PubMed] [Google Scholar]
- Voets T, Prenen J, Vriens J, Watanabe H, Janssens A, Wissenbach U, Bodding M, Droogmans G & Nilius B (2002). Molecular determinants of permeation through the cation channel TRPV4. J Biol Chem 277, 33704–33710. [DOI] [PubMed] [Google Scholar]
- Vriens J, Owsianik G, Fisslthaler B, Suzuki M, Janssens A, Voets T, Morisseau C, Hammock BD, Fleming I, Busse R & Nilius B (2005). Modulation of the Ca2 permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res 97, 908–915. [DOI] [PubMed] [Google Scholar]
- Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T & Nilius B (2004). Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci USA 101, 396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T & Nilius B (2003). Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 424, 434–438. [DOI] [PubMed] [Google Scholar]
- White JP, Cibelli M, Urban L, Nilius B, McGeown JG & Nagy I (2016). TRPV4: molecular conductor of a diverse orchestra. Physiol Rev 96, 911–973. [DOI] [PubMed] [Google Scholar]
- Willette RN, Bao W, Nerurkar S, Yue TL, Doe CP, Stankus G, Turner GH, Ju H, Thomas H, Fishman CE, Sulpizio A, Behm DJ, Hoffman S, Lin Z, Lozinskaya I, Casillas LN, Lin M, Trout RE, Votta BJ, Thorneloe K, Lashinger ES, Figueroa DJ, Marquis R & Xu X (2008). Systemic activation of the transient receptor potential vanilloid subtype 4 channel causes endothelial failure and circulatory collapse: part 2. J Pharmacol Exp Ther 326, 443–452. [DOI] [PubMed] [Google Scholar]
- Wissenbach U, Bodding M, Freichel M & Flockerzi V (2000). Trp12, a novel Trp related protein from kidney. FEBS Lett 485, 127–134. [DOI] [PubMed] [Google Scholar]
- Xu H, Zhao H, Tian W, Yoshida K, Roullet JB & Cohen DM (2003). Regulation of a transient receptor potential (TRP) channel by tyrosine phosphorylation. SRC family kinase‐dependent tyrosine phosphorylation of TRPV4 on TYR‐253 mediates its response to hypotonic stress. J Biol Chem 278, 11520–11527. [DOI] [PubMed] [Google Scholar]
- Ye L, Kleiner S, Wu J, Sah R, Gupta RK, Banks AS, Cohen P, Khandekar MJ, Bostrom P, Mepani RJ, Laznik D, Kamenecka TM, Song X, Liedtke W, Mootha VK, Puigserver P, Griffin PR, Clapham DE & Spiegelman BM (2012). TRPV4 is a regulator of adipose oxidative metabolism, inflammation, and energy homeostasis. Cell 151, 96–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampighi GA, Kreman M, Boorer KJ, Loo DD, Bezanilla F, Chandy G, Hall JE & Wright EM (1995). A method for determining the unitary functional capacity of cloned channels and transporters expressed in Xenopus laevis oocytes. J Membr Biol 148, 65–78. [DOI] [PubMed] [Google Scholar]
- Zeuthen T & MacAulay N (2012). Cotransport of water by Na(+)‐K(+)‐2Cl(‐) cotransporters expressed in Xenopus oocytes: NKCC1 versus NKCC2. J Physiol 590, 1139–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]


