Abstract
Human networks of sexual contacts are dynamic by nature, with partnerships forming and breaking continuously over time. Sexual behaviours are also highly heterogeneous, so that the number of partners reported by individuals over a given period of time is typically distributed as a power-law. Both the dynamism and heterogeneity of sexual partnerships are likely to have an effect in the patterns of spread of sexually transmitted diseases. To represent these two fundamental properties of sexual networks, we developed a stochastic process of dynamic partnership formation and dissolution, which results in power-law numbers of partners over time. Model parameters can be set to produce realistic conditions in terms of the exponent of the power-law distribution, of the number of individuals without relationships and of the average duration of relationships. Using an outbreak of antibiotic resistant gonorrhoea amongst men have sex with men as a case study, we show that our realistic dynamic network exhibits different properties compared to the frequently used static networks or homogeneous mixing models. We also consider an approximation to our dynamic network model in terms of a much simpler branching process. We estimate the parameters of the generation time distribution and offspring distribution which can be used for example in the context of outbreak reconstruction based on genomic data. Finally, we investigate the impact of a range of interventions against gonorrhoea, including increased condom use, more frequent screening and immunisation, concluding that the latter shows great promise to reduce the burden of gonorrhoea, even if the vaccine was only partially effective or applied to only a random subset of the population.
Author summary
The formation and dissolution of sexual relationships in human populations constitute an ever-changing network of links between individuals through which sexually transmitted diseases spread. To study this phenomenon, we developed a dynamic simulation algorithm that can reproduce the same distribution of sexual contacts as observed in real populations. We applied our algorithm to the study of gonorrhoea outbreaks and showed that it results in significantly different patterns of transmission compared to models where the sexual network does not change or is ignored. We show how our model can be incorporated into existing algorithms of outbreak investigation based on genomic sequencing data. We also apply our model to the evaluation of a range of interventions frequently proposed to limit the spread of gonorrhoea transmission, and in particular we quantify the potential of vaccination strategies.
Introduction
In 2017 the WHO added Neisseria gonorrhoeae to its priority list of bacterial pathogens in response to the global spread of antibiotic resistance [1]. The bacteria have developed resistance to every therapy used against them, from penicillin through to third-generation cephalosporins [2, 3]. At a time when resistance to first line therapy (ceftriaxone 250-500mg in combination with azithromycin 1-2g) is increasingly observed [4], it is more important than ever to understand the transmission dynamics of the infection, and how interventions might be used to reduce the burden on antibiotic treatment [5].
It has been well documented that heterogeneity in sexual activity levels has an impact on disease transmission, with individuals who have many partners bearing much of the burden of disease [6–9]. However, the risk of acquiring and passing on a sexually transmitted infection (STI) depends not only on an individual’s sexual risk profile, but also on their position in the wider sexual network [10, 11]. Furthermore the structure of an underlying network affects the probability that an infection that is introduced to the network leads to an outbreak, as well as the size and longevity of any outbreaks that occur [12, 13]. As such, it is important to take into account the structure of the underlying sexual network when modelling STI outbreaks.
The distribution of the number of sexual contacts within a network is known as its degree distribution. Several studies have shown that real world sexual networks often have degree distributions that obey a power-law [14–16], where the probability of having k partners over a given period of time is proportional to k−γ. The constant γ is usually between 1 and 4, and different values have been observed in heterosexual and same-sex networks, as well as between genders [15]. Power-law networks exhibit high levels of heterogeneity, with the majority of individuals having a relatively small number of contacts, while a few have many. The standard method to simulate power-law networks is to use a system of preferential attachment, in which individuals are added one by one, connecting with a higher probability with existing individuals who already have a large number of partners [17]. Once all individuals have been added, the network thus created is guaranteed to have a static power-law distribution. It is important to note that even though the preferential attachment algorithm is dynamic in nature, the dynamic method used is purely a technique for generating a static network and does not in any way reflect the dynamics known to occur in real world sexual networks. Furthermore, transmission of infection occurs only once the network has been generated, with all partnerships being in place constantly from the beginning of the simulation of infection transmission until the end.
An alternative method of producing static networks with a power-law degree distribution has been proposed based on each network node having an intrinsic fitness parameter, and a function that determines the probability that a network connection exists between any two nodes depending on their fitness [18, 19]. In a sexual network, this can be thought of as each individual having an inherent propensity to seek new partnerships, relative to others in the network, with the probability of occurrence of each possible partnership depending on the mutual attraction of two individuals.
In a sexual network model, the rate of infection of an individual depends on whether their sexual partners are infectious, rather than on the prevalence of infection in the pool of potential partners, as in compartmental models. Compartmental models that do not explicitly represent partnerships have been shown to underestimate the importance of core groups of highly sexually active individuals in sustaining STI transmission, while overestimating the contribution of long-term partnerships and low-activity individuals [20]. Furthermore, several studies have shown that, in order to explain observed patterns of infection, it is important to take into account not only the network structure but also the duration of partnerships, and the gaps between them [21, 22]. It may therefore be necessary to use a dynamically evolving network to correctly simulate the spread of STI outbreaks. The power-law network methods described above [17–19] produce networks that are static and do not capture the dynamics of real networks. Conversely, several dynamic algorithms have been proposed where relationships are formed and dissolved over time [21, 23, 24], but they do not explicitly aim for the degree distribution over a year to be power-law distributed, as observed in real networks [14–16]. Since no algorithm has yet been designed to simulate a dynamic sexual network with the correct real-world properties of a power-law distribution of number of sexual partners over a year, the difference between such a realistic dynamic network and a more approximate static network has not been assessed.
Here we present a novel approach to dynamic network simulation using stochastic partnership formation and breakdown based on individuals’ intrinsic properties. We demonstrate that our method produces power-law networks, and that it can simulate a population reflecting the observed network characteristics in UK men who have sex with men (MSM). We then simulate an outbreak of gonorrhoea in three types of network: a fully-connected static network, a heterogeneous static network, and our novel heterogeneous dynamic network, showing important differences between all three models in terms of the resulting patterns of transmission. We estimate the resulting offspring distribution (the number of secondary cases caused by each primary case) and generation time distribution (time between infection of a primary case and infection of a secondary case) to assess the likelihood of super-spreading events predicted by each network structure, as well as the predicted duration of infection. We also compare the impact of the number of sexual partnerships on the probability of infection and transmission under each network structure, providing a basis for risk assessment. Finally, using the dynamic network model we investigate the impact of a range of interventions against gonorrhoea, including increased condom use, more frequent screening and a hypothetical vaccine.
Results
Analysis of survey data on number of partners
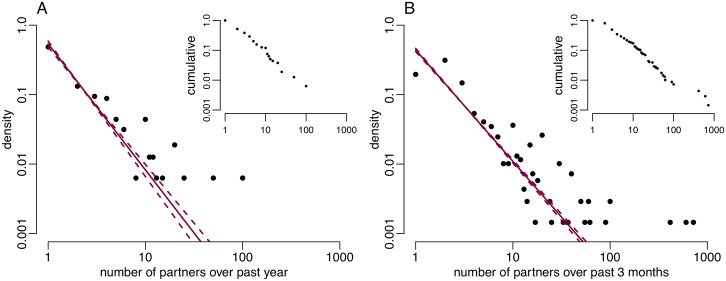
We first analysed the number of partners reported by MSM in the third National Survey of Sexual Attitudes and Lifestyles (Natsal-3), a population-based survey conducted in 2010-2012 [25–27]. 15.4% individuals reported zero partners, and amongst the remainder the distribution of number of partners approximately followed a power-law distribution (Fig 1A). We used Bayesian inference to estimate the exponent γ of this power-law distribution, and found a posterior mean of γ = 1.81 (95% credible interval: [1.69, 1.96]). This is comparable to estimates calculated based on the previous Natsal data, collected in 1990-1991 and 1999-2001, and the London Gay Men’s Sexual Health Survey [28], which were 1.57 (95% CI: [1.43, 1.72]), 1.75 (95% CI: [1.57, 1.95]) and 1.87 (95% CI: [1.80, 1.94]) respectively [15].
Fig 1.
Double logarithmic plot of degree distribution of number of partners for UK MSM A: reported over 1 year by Natsal-3 respondents. B: reported over 3 months in GRASP data from London. Inset: corresponding normalised cumulative degree distributions.
We performed the same analysis based on data collected between June and November 2004 as part of the national Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP) run by Public Health England (PHE) from individuals diagnosed with gonorrhoea in London [29, 30]. Fewer individuals had only one partner in the last three months than would be expected under a power-law distribution; however a power-law tail was observed for MSM having more than one partner. The inferred scale-parameter γ for gonorrhoea infected individuals was significantly lower than that in the Natsal-3 data at 1.60 (95% credible interval: [1.56, 1.65]) with non-overlapping credible intervals (Fig 1B).
Simulation of dynamic network model
We developed a new algorithm to simulate dynamic sexual networks in which relationships are being formed and broken down over time. To incorporate sexual behaviour heterogeneity, each individual in the network is characterised by a parameter λ that represents the propensity to make and break relationships. This λ parameter is analogous to the fitness property used in a previously published method to generate static power-law networks [18, 19]. We built upon this work to create a dynamically evolving network model, with mathematical properties such that individuals are involved over one year in a power-law distributed number of partnerships (see Materials and methods). In our model long-term partnerships form less frequently than short-term partnerships. High degree individuals have a higher turnover of partners than low degree individuals, rather than accumulating more long-term concurrent partners.
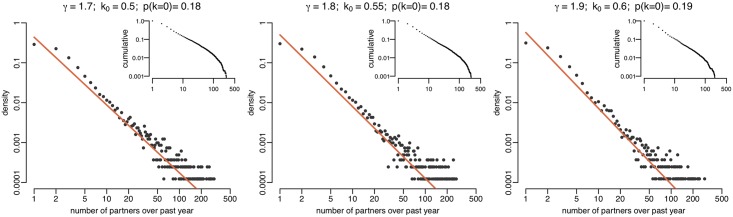
To demonstrate the ability of our algorithm to simulate realistic networks, we generated dynamic sexual networks of size N = 10,000 over one year using a power-law exponent γ equal to 1.7, 1.8 and 1.9 (Fig 2, S1 Fig). The network size was chosen to represent MSM aged between 15 and 65 in a UK city such as Brighton or central Manchester [31, 32]. Our algorithm also requires to set the parameter k0 which determines the proportion of individuals that do not have a sexual partnership during the year. Using values of k0 equal to 0.4, 0.5 and 0.6 respectively, we were able to produce networks exhibiting a power-law distribution of partnerships and proportion of individuals with zero partners over one year that were comparable to the 15.4% proportion in the Natsal-3 data (Fig 2). Finally, a third parameter ϕ in our method determines the rate of partnership breakdown, which in turn decreases the level of partnership concurrency in the network without affecting the distributions of partner numbers. The full mathematical description of parameters k0 and ϕ can be found in the Materials and methods section.
Fig 2. Double logarithmic plot of degree distributions of partners in last year generated with differing parameters γ and k0.
The orange lines represent the desired degree distribution p(k) = ck−γ. The proportion of individuals having no partners in the last year is shown in the title of each plot as p(k = 0). Inset: corresponding normalised cumulative degree distributions.
Effect of network model on simulated gonorrhoea outbreaks
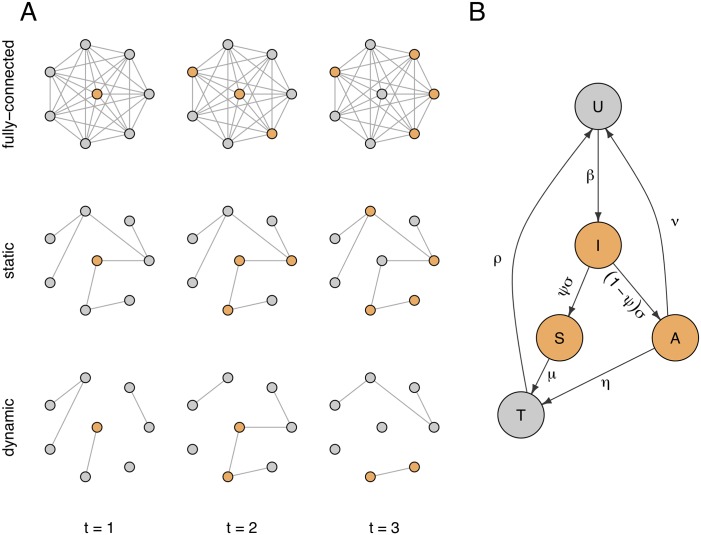
To assess the importance of the underlying sexual network structure and dynamism in the way gonorrhoea outbreaks spread, we performed a comparison of simulated gonorrhoea outbreaks on three types of networks (Fig 3A): a fully connected network, a static power-law network and our new dynamic power-law network. On each type of network we used the same model of gonorrhoea outbreak, adapted from a recent study [33] as illustrated in Fig 3B and described in the Materials and Methods section.
Fig 3.
A: Illustration of gonorrhoea transmission over time through the three network structures: fully connected, static and dynamic. B: Flow diagram of transmission model with rates of transition between infection states. Uninfected individuals (U) become infected after sexual contact with contagious individuals (highlighted in orange). Infections initially pass through an incubation period (I), before either developing symptoms (S) or remaining asymptomatic (A). Symptomatic individuals seek treatment, and asymptomatic infections identified by screening are also treated (T).
The flow parameters were calibrated for each of the three types of network in order to produce outbreaks of the same realistic size over a year (cf Materials and methods section). The resulting parameter values are summarised in Table 1 with no significant difference between the three models for any parameter except the rate of transmission per partnership β, which takes widely different values as expected. From the resulting simulations we analysed the offspring distribution, defined as the number of onward transmissions attributable to every case infected in the first year (Fig 4A) and we extracted the generation times, defined as the length of time from acquisition of infection to onward transmission to an uninfected partner (Fig 4B). The offspring distributions we derive are the average of 100 model realisations, based on all individuals that become infected in each simulation, and as such are conditional on the probability of infection. Both the offspring distribution and the generation time distribution exhibited important differences depending on the underlying type of sexual network considered (Fig 4).
Table 1. Parameter notations, input range (cf S3 Appendix) and range of parameters that resulted in outbreaks persisting at least one year in at least 20% of simulations with at most 400 total diagnoses per year on average for each network structure.
| Parameter description | Input | Accepted parameter mean [95% range] | |||||
|---|---|---|---|---|---|---|---|
| Unit | range | Source | Fully connected | Static | Dynamic | ||
| β | within-partnership rate of transmission | year−1 | [0, 60] | fitting | 1.8 [1.1, 3.9] x10−3 | 0.11 [0.08, 0.15] | 24.0 [11.0, 53.9] |
| ψ | infections that become symptomatic | % | [40, 95] | [34–36] | 55 [40, 83] | 63 [41, 88] | 71 [41, 94] |
| 1/σ | duration of incubation period | days | [2, 10] | [37–41] | 4.8 [3.0, 8.0] | 4.7 [3.0, 7.8] | 4.6 [2.9, 7.8] |
| 1/ν | duration of carriage | days | [60, 550] | [42–46] | 223 [113, 459] | 167 [93, 392] | 166 [96, 385] |
| η | rate of screening when asymptomatic | year−1 | [0.5, 4] | [47–49] | 1.6 [0.8, 2.8] | 2.2 [1.0, 3.7] | 2.1 [1.0, 3.6] |
| 1/μ | duration of symptoms before treatment | days | [1, 30] | [50] | 2.8 [1.2, 21.1] | 2.7 [1.1, 15.4] | 2.7 [1.1, 13.2] |
| 1/ρ | time to recovery following treatment | days | [5, 10] | [51] | 6.9 [5.7, 8.5] | 6.9 [5.7, 8.4] | 7.0 [5.8, 8.5] |
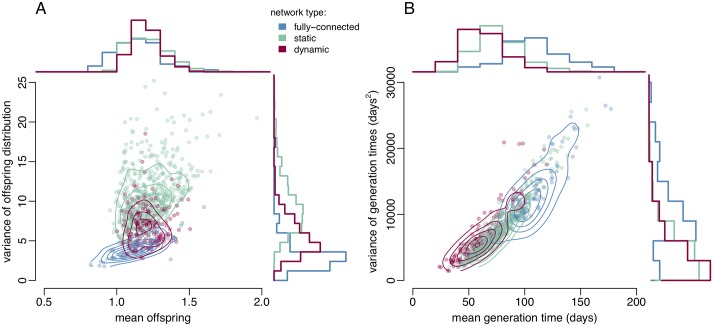
Fig 4. Scatter plots with overlaid density contours and marginal histograms for the mean and variance of A: the offspring distributions, and B: the distribution of generation times, for infections contracted in the first year of the outbreak.
Simulations under the fully connected, static, and dynamic network structures are shown in blue, green and red respectively.
For all three network structures (fully connected, static, and dynamic) mean numbers of offspring per infected individual were around 1.2 with overlapping 95% ranges (Fig 4A, X axis). This equality is due to the calibration of the models, which required outbreaks to be of similar sizes. However, we found that the variance in the simulated offspring distributions differed depending on network structure (Fig 4A, Y axis). Simulated outbreaks in both static and dynamic power-law networks had greater variance in the offspring distribution than the fully connected networks (4.2; 95% range: [1.9, 7.7]), due to the effect of heterogeneity in contact patterns. However, dynamic partnership formation and dissolution partially mitigated the impact of the network structure on the offspring distribution. The sample variance of the offspring distributions in the static power-law network (10.9; 95% range: [5.7, 20.3]) was on average greater than in the dynamic network (6.8; 95% range: [3.4, 12.0]), suggesting that adopting a static power-law network in disease models would overstate the importance of super-spreading events.
The distribution of mean generation times in the simulations is also affected by the underlying network structure (Fig 4B, X axis). Outbreaks in the dynamic network structure have a mean generation time of 63 days (95% range: [33, 101]). Generation times are overestimated when partnership dynamics are ignored, as in the case of the static power-law network structure (77 days; 95% range: [49, 124]), an effect which is exacerbated when heterogeneity in sexual activity levels is omitted, as in the fully connected network (109 days; 95% range: [54, 168]). In order to maintain persistence of the outbreak at realistically low prevalence, as is observed in gonorrhoea, outbreaks in the fully connected network overstate the proportion of asymptomatic infections compared to the dynamic network, 44.6% (95% range: [17.2%, 59.7%]) compared to 29.5% (95% range: [6.4%, 58.8%]) [6]. This larger untreated asymptomatic reservoir also serves to increase the variance of the simulated generation times from 6,960 (95% range: [2,100, 17,530]) in the dynamic networks to 13,500 (95% range: [3,850, 26,200]) in the fully connected networks (Fig 4B, Y axis).
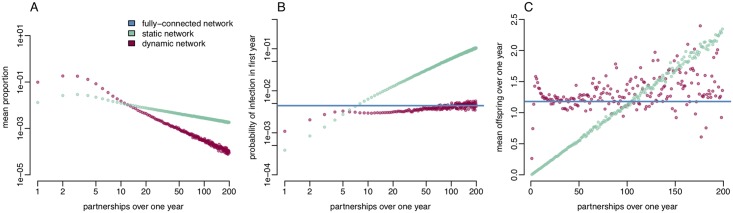
For both the static and dynamic power-law networks we investigated the relationship between the number of sexual partners that an individual has over one year, the probability of becoming infected, and the number of transmission events arising from those individuals who become infected. Fig 5 shows the proportion of infected individuals, the probability of an individual becoming infected in first year and the mean onward transmissions for infected individuals, split by the number of partners over one year. The proportion of infected individuals having fewer than three partners per year was lower than would be expected under a power-law distribution for both static and dynamic networks, however the distribution exhibited power-law behaviour for more highly active infectees (Fig 5A). This is similar to the pattern exhibited in the GRASP London data (Fig 1B).
Fig 5.
A: Proportion of infected individuals split by number of partners over one year B: Probability of an individual becoming infected in first year given their total number of partnerships. C: Mean transmission events for infected individuals with a given number of partnerships.
Compared to the dynamic network the static network structure appears to overestimate the burden of infection in individuals with more than 11 partners per year, while underestimating the burden in individuals with fewer partners.
Under the static network structure an individual’s probability of infection increases linearly with their annual number of partners (Fig 5B). When we allow for partnership dynamics, the probability of infection increases linearly at first, albeit at a slower rate than the static network, then levels off in individuals having more than five partners per year, eventually approximating the probability of infection observed in a fully connected network. This suggests that a static network structure may underestimate the risk of infection for individuals with few sexual partners.
There is a similar relationship between the expected number of onward transmission events from individuals and their number of partners over the year in the static and dynamic network structures (Fig 5C). An infected individual’s expected number of transmissions in the static network increases linearly with their total number of partnerships. For individuals with up to five partners per year, the dynamic network also shows a strong linear relationship between the expected offspring and number of partners. However, for individuals with more than five partners per year the relationship is less strong with a much greater variance in mean number of offspring. In the dynamic network the expected number of offspring is greater than one in individuals with at least three partners, whereas the mean offspring per person in the static network only becomes greater than one in individuals having more than 90 partners. The static network therefore likely overestimates the importance of very highly active individuals in maintaining transmission.
Impact of interventions
Using the dynamic network model we investigated the impact of a range of interventions and preventative measures against gonorrhoea, including: 20% increased condom use (resulting in a reduction in the transmission rate β), 20% increased sexual health screening (an increase in parameter η), and the impact of a hypothetical gonorrhoea vaccine, deployed either at random to 20% of the network or targeted to a “core group” of 20% of individuals with the highest propensity to form partnerships (λ). In practice these people would be identified by clinics through repeat infections and/or co-infections, self-reported larger numbers of sexual partners, or though being named repeatedly as a contact by other patients with infection. It should be noted however that the original conception of the core group was in relation to a simple compartmental model with core and non-core parts of the population [52], whereas in a network model such as ours, the boundary of the core group is not clearly defined. Recent estimates suggest that a meningococcal B vaccine may be 31% [21%, 39%] effective against gonorrhoea [53]. The impact of vaccinating 20% of individuals at random with a vaccine that is 100% effective is comparable to vaccinating 65% [51%, 95%] of individuals with a vaccine of similar effectiveness. We assessed the one-year impact of these four measures on the probability of a outbreak stemming from a single introduction of gonorrhoea into a dynamic sexual network (Fig 6A), the total number of gonorrhoea diagnoses (Fig 6B), and the number of sexual health clinic visits from both screening and symptomatic treatment-seeking (Fig 6C).
Fig 6.
A: Proportion of outbreaks persisting for at least one year. B: Number of gonorrhoea diagnoses in first year. C: Number of clinic visits in first year.
The baseline proportion of simulated outbreaks persisting for at least one year from a single introduction of gonorrhoea was 31% (95% range: [15%, 49%]). All of the interventions we considered reduced the probability of an outbreak, with vaccination having the greatest impact; a fully effective vaccine administered to 20% of individuals in a randomised strategy reduced the probability of an outbreak by around a third to 21% (95% range: [7%, 40%]), a targeted strategy had a greater effect, reducing the probability to 19% (95% range: [5%, 36%]). The non-vaccine interventions had less of an impact. Increasing condom usage by 20% reduced the probability of an outbreak by around 10% to 28% (95% range: [13%, 47%]); A 20% increase in the rate of screening for asymptomatic cases had a similar effect, reducing the probability of an outbreak to 29% (95% range: [11%, 49%]).
The expected size of the visible outbreak in a population of 10,000, as measured by the total number of infected individuals diagnosed and receiving treatment, was reduced by a fifth from 98 (95% range: [25, 269]) to 79 (95% range: [19, 217]) with a 20% increase in condom-use, and could be halved using vaccination: down to 47 cases (95% range: [14, 114]) using the randomised strategy and 45 cases (95% range: [14, 105]) by targeting the most sexually-active individuals. However, a 20% increase in the screening rate resulted in a 5% increase in the visible outbreak size to 103 cases (95% range: [25, 295]), due to more asymptomatic cases receiving treatment.
There was a similar pattern in the burden of sexual health services, while increased condom use decreased the total number of clinic visits by 11% from 16,939 (95% range: [7,950, 28,810]) to 15,053 (95% range: [7,064, 25,602]). A 20% increase in the rate of screening, both of uninfected and asymptomatically infected individuals increased the total clinic visits by 20%, because the majority of testing is prompted by screening rather than symptomatic treatment seeking. The number of sexual health clinic visits remained stable in the vaccine scenarios, however the financial and administrative cost of initiating either a targeted or randomised vaccination programme must be considered once a vaccine candidate has been developed. It is important to note that while the targeted strategy is more effective, requires the ability to identify and vaccinate the 20% most sexually active individuals in a given population. It is unclear how effectively this could be done in practice, but could perhaps be offered at the GUM clinic at the same time as testing.
Discussion
Real-world sexual networks are dynamic by nature, and we have developed a method that can reproduce observed power-law distributions of numbers of sexual partners in the last year in a dynamic network, which accounts for heterogeneity of individual behaviour in the way relationships form and break down. Our model allows the user to specify the power-law distribution via the exponent γ, to vary the proportion of individuals having no partners via the parameter k0, and to set the average length of partnerships, via the parameter ϕ. Varying the length of partnerships for a given degree distribution impacts the pattern of partnership concurrency in the network. The longer the average partnership, the higher the degree of concurrency. While other models may be able to produce a power law distribution over time with the right choice of formation and breakdown partnership functions [24], we are the first to focus on this property and to demonstrate how it can be achieved. We implemented this dynamic simulation algorithm into a R package called simdynet which is freely available at https://github.com/lwhittles/simdynet.
Taking an outbreak of gonorrhoea as a case study, we found that failing to allow for sexual network structure (i.e. using the fully-connected network) resulted in an overestimation of the duration of carriage and asymptomatic reservoir. When network structure, but not dynamics of sexual partnership formation and breakage, was accounted for (i.e. using the static network) the model overstated the likelihood of super-spreading events and the burden of disease among individuals with high numbers of partners, compared to a dynamic model. While it is important to take heterogeneity into account, the traditional formulation of a core group [6, 21] might approximate the true transmission dynamics of gonorrhoea better than using a static power-law network. Our findings add support to previous modelling work that suggested that having more sexual partners does not greatly impact the rate at which antibiotic resistant gonorrhoea can spread [9].
We used our realistic dynamic power-law network model to investigate the impact of a range of interventions against gonorrhoea, including increased condom use, more frequent screening and immunisation. Our results confirm that vaccination shows great potential to reduce the burden of gonorrhoea [54]: if a random 20% of individuals were immune, then the probability of outbreaks persisting at least a year would be reduced by 16% with the outbreak size reduced on average by 31%. (Fig 6). Such a level of protection could be achieved either through vaccination of a small portion of the population with a highly effective vaccine, or by widespread use a less effective vaccine. For example, a recent retrospective case-control study has shown that the MeNZB vaccine against meningitis is cross-protective against gonorrhoea, with an estimated effectiveness between 20% and 40% [53, 55].
The dynamic sexual network we implemented is highly realistic, but also too computationally expensive to be used in many applications. For example if the population under study is very large, keeping track of every partnership formation and break down is clearly inefficient, especially for the study of the early stages of an outbreak of a new resistant strain in which only a small subset of individuals are being affected. However, we have computed from the full dynamic model the offspring distribution and distribution of generation time (Fig 4). These estimates allow for our model to be approximated as a stochastic branching process [56, 57], where infected individuals transmit to a number of secondary cases drawn from the offspring distribution, and the intervals of time between each primary and secondary cases are drawn from the generation time distribution. The advantage of such a model formulation is that it is much simpler than the full dynamic sexual network we described in this paper, but retains the same basic properties in terms of the transmission process. Furthermore, a branching model is at the basis of several recently developed methods to reconstruct transmission trees from genomic data, such as outbreaker [58], TransPhylo [59, 60] and phybreak [61]. Our estimates of the generation time distribution and offspring distribution therefore pave the way for these genomic epidemiology methods to be applied to the reconstruction of transmission in gonorrhoea outbreaks [42, 62–64].
We estimated that on average the mean and variance of the generation time distribution were equal to 63 days and 6980 days2, respectively (Fig 4B), which can be emulated using a Gamma distribution with shape and scale parameters equal to 0.57 and 110.48, respectively. The resulting 95% quantile range of the generation time stretches up to 298 days, which is in good agreement with an analysis of genomes from pairs of known sexual contact, in which the greatest observed time to most recent common ancestor was 8 months [42]. Since gonorrhoea can often remain asymptomatic, any outbreak reconstruction would need to account for the possibility of unsampled cases acting as intermediates in the transmission chains [42, 58, 60]. Accurate inference of unsampled cases requires in turn a good prior knowledge of the generation time distribution like the one we estimated here based on a dynamic power-law network. Both the mean and variance of the generation time distribution are overestimated when considering a fully-connected or static network (Fig 4B) which would likely result in an underestimation of the role played by unsampled cases.
The offspring distribution was estimated to have a mean and variance equal to 1.2 and 6.8, respectively (Fig 4A). Since the variance is greater than the mean, the offspring distribution is over-dispersed compared to a Poisson process, which indicates the presence of super-spreaders [65–67], although this transmission heterogeneity is not as pronounced as would be implied by an unstructured or static network model (Fig 4A). In a branching model, over-dispersion can be implemented using a Negative-Binomial distribution for the number of offspring, which in this context is often parametrised in terms of its mean and dispersion parameter k, with lower values of k indicating more over-dispersion [60, 65, 68, 69]. Here we estimated that k = 0.257, which with a mean offspring number of 1.2 gives a 95% quantile ranging up to nine secondary cases, compared to only four cases for a Poisson distribution with the same mean. We ensured that our estimates were appropriate for use in simple branching process models by confirming that there is no correlation between successive offspring distributions (S2 Fig). These estimates of the generation time distribution and offspring dispersion parameter pave the way for future studies of genomic epidemiology in gonorrhoea outbreaks.
Materials and methods
Estimation of power-law exponents from real data
The third National Survey of Sexual Attitudes and lifestyles in the UK (Natsal-3), was conducted between September 2010 and August 2012 in 15,000 adults aged between 16 and 74 [25–27]. We extracted the number of same-sex partners over one year for the 188 men who reported sexual contact with another man within the past five years. 15.4% of MSM reported having no same sex partners over the past year.
In addition to the Natsal data we examined the number of partners reported by 691 MSM diagnosed with gonorrhoea in a collection of 2,045 isolates sampled between June and November 2004 from 13 major sexual health clinics throughout London as part of the national Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP), run by Public Health England (PHE) [29]. PHE has produced a GRASP report annually since 2000 to monitor trends in resistance and susceptibility to the drugs used to treat gonorrhoea in England and Wales, which is used to inform national treatment guidelines and strategy. The GRASP data from London represent 54% of the 3,754 cases reported across the city at that time [30, 42, 70].
We fitted the power-law distribution using Bayesian inference, implemented via a Monte Carlo Markov Chain, to obtain obtained posterior estimates of γ based on the Natsal-3 and GRASP London datasets, using an uninformative prior. Five chains with over-dispersed starting points were run for 100,000 iterations after a 10,000 iterations burn-in period and thinned by a factor of 100. The convergence of the MCMC was assessed by visual inspection of the trace plots, and confirmed to have a Gelman-Rubin criterion of < 1.1 [71, 72].
Simulating a dynamic power-law network using vertex intrinsic fitness
Network dynamics
We consider a population of N sexually active men who have sex with men (MSM). Each individual j = 1, …, N has a propensity λj to form new partnerships which is randomly drawn from a probability distribution with density f(λ), and cumulative density function . The events that can occur with regard to the network composition are: a new partnership forming (event a) or a partnership breakup (event d). These events occur as Poisson processes: the (time invariant) rate of individual j forming a partnership with individual l (given they are not already in a partnership together) is given by the function a(λj, λl). The rate of individuals j and l breaking up, given they are in a partnership, is denoted d(λj, λl).
The state of each possible partnership is independent from all others, with no limit on the number of concurrent partners. Each possible partnership {j, l} can be thought of as being ‘on’ or ‘off’ (i.e. is in existence at a particular point in time or not). We can then derive a stationary distribution of the network, and use this to derive the probability at time t that each possible partnership exists. By considering the average time spent in each state (on or off) we find that the probability of pair of individuals {j, l} being in a partnership at time t is:
| (1) |
Conversely the probability they are not in a partnership at t is simply:
| (2) |
Given that two individuals are not in a partnership at time t the time to forming a partnership, is distributed Exp(a(λj, λl)), so the probability of them forming a partnership in the next year is . Combining these two cases, we can calculate the probability that two individuals have been in a partnership over the last year:
| (3) |
Analytical expression of the degree distribution
Let kj denote the degree distribution of individual j, that is the number of partnerships involving j that have been active at some point over the past year. The probability that individual j was in partnership at some point with an individual l of unknown λl is equal to:
| (4) |
The distribution of kj given λj is therefore , and its expectation is:
| (5) |
Since κ(.) is a continuous, monotonically increasing function of λ, we can use the method of transformations to find the degree distribution p(κ):
| (6) |
Power-law distribution of partners over a year
We require the network of partnerships over the last year to have a power-law degree distribution, so:
| (7) |
The constant c is determined by the fact that , where k0 = limλ→0 κ(λ) and k∞ = limλ→∞ κ(λ). For γ ≠ 1 (in accordance with observations from real-world networks) we have:
So
| (8) |
While k0 is an input of the model, k∞ is chosen to be as large as possible, subject to the condition that limλj,λl→∞ q(λj, λl) ≤ 1. We equate Eqs 6 and 7 and rearrange to obtain an expression for the density of λ in terms of the degree distribution:
| (9) |
Integrating both sides between 0 and λ we obtain:
And after rearrangement:
| (10) |
Following [19], we consider the case where:
| (11) |
Chosen arbitrarily to enable analytical solution.
Substituting Eq 11 into 5 we obtain:
| (12) |
where
Equating Eqs 10 and 12, then multiplying by f(λ) and integrating between 0 and ∞ we obtain:
So
| (13) |
Rearranging 12 we obtain:
| (14) |
Substituting Eqs 10 and 13 into 14 we obtain:
| (15) |
Combining Eqs 15 and 11 we deduce the function q(λj, λl) required to the network to follow a power-law distribution. The functions governing partnership formation and dissolution can then be inferred by considering the simple case where d(λj, λl) = ϕa(λj, λl) where ϕ is a constant. This behaviour is in line with evidence from Natsal-3 that the mean partnership duration is negatively correlated with the number of partners reported by MSM over one year (S3 Fig). so Eq 3 becomes
| (16) |
We set the minimum rate of partnership breakup , so that the longest partnerships in the network are Exponentially distributed with a mean of 50 years. Applying this constraint and rearranging Eq 16 we obtain:
| (17) |
We deduce an algorithm for the simulation of a static snapshot of the network over a year (S1 Appendix) and an algorithm for the simulation of the dynamic network (S2 Appendix). Both algorithms were implemented in a R package called simdynet which is freely available at https://github.com/lwhittles/simdynet.
Simulation of gonorrhoea outbreaks
In order to investigate the patterns of transmission under different network structures, we consider a stochastic individual-based model of gonorrhoea, adapted from [33]. Individuals are initially uninfected (U) and become infected probabilistically at rate β due to sexual contact with an contagious individual as dictated by the underlying sexual network structure. Infected individuals initially pass through an incubation period (I) which they leave at rate σ. A proportion ψ of those infected then develop symptoms (S), whereas the remainder enters an asymptomatic stage (A). In men, gonococcal infection can occur in the rectum, pharynx and/or urethra, resulting in different rates of onward transmission and probabilities of developing symptoms [73]. We do not explicitly model separate anatomical sites of infection, therefore the rate of transmission, β, and the likelihood of developing symptoms, ψ, should be seen as an average for any infection site. Asymptomatic individuals (A) undergo screening and receive treatment at rate η, otherwise recovery from asymptomatic infection happens (either naturally or following unrelated antibiotic treatment) at rate ν. The symptomatic individuals (S) seek treatment at rate μ. Individuals who have been treated recover from the infection and become uninfected again at rate ρ. The contagious population is denoted C = I + S + A, since individuals in treatment are assumed either to no longer be contagious or to abstain from sexual activity in accordance with treatment guidelines [51].
Model calibration
Using the Gillespie algorithm described in S2 Appendix we generated dynamic sexual networks exhibiting a power-law distribution of partnerships and proportion of individuals with zero partners over one year that were comparable to the Natsal-3 data. A thousand sets of parameters were sampled by Latin Hypercube Sampling from input ranges selected based on published sources (cf Table 1 and S3 Appendix). For each underlying network structure (fully connected, static and dynamic) we seeded infection 100 times and simulated over three years for each parameter set. Parameter sets that produced outbreaks persisting at least one year in fewer than 20% of simulations were discarded, as were parameter sets that resulted in total diagnoses exceeding 400 cases per year on average. Thus only parameter sets that resulted in realistic gonorrhoea outbreaks were retained.
We considered a suite of interventions and preventative measures against gonorrhoea, including: increased condom use (resulting in a reduction in the transmission rate β), increased sexual health screening (an increase in η), and the impact of a hypothetical gonorrhoea vaccine, deployed either at random or targeted to the individuals with the highest propensity to form new partnerships (λ). We assessed the one-year impact on the probability of a outbreak stemming from the introduction of gonorrhoea into a dynamic sexual network, the number of sexual health clinic visits from both screening and symptomatic treatment-seeking, and the total number of gonorrhoea diagnoses.
Supporting information
The orange lines represent the desired degree distribution p(k) = ck−γ. The proportion of individuals having no partners in the last year is shown in the title of each plot as p(k = 0).
(PDF)
(PDF)
The number of partners reported by MSM over one year is negatively correlated with the average length of the relationships. This suggests that in a realistic model the expected duration of partnerships formed by high degree individuals should be shorter than for low-degree individuals.
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability
All relevant data are within the paper, its Supporting Information files, and the freely accessible repository https://github.com/lwhittles/simdynet.
Funding Statement
This work was funded by the UK National Institute for Health Research (NIHR) Health Protection Research Unit (HPRU) in Modelling Methodology at Imperial College London in partnership with Public Health England (PHE) (grant HPRU-2012-10080), the MRC Centre for Global Infectious Disease Analysis (grant MR/R015600/1), and the Medical Research Council (grant MR/N010760/1). The views expressed are those of the authors and not necessarily those of the Department of Health and Social Care, MRC, NIHR, NHS, or Public Health England. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Tacconelli E, Magrini N, Kahlmeter G, Singh N. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. World Health Organization; 2017; p. 1–7. [Google Scholar]
- 2. Unemo M. Current and future antimicrobial treatment of gonorrhoea—the rapidly evolving Neisseria gonorrhoeae continues to challenge. BMC Infectious Diseases. 2015;15(364):364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Whittles LK, White PJ, Paul J, Didelot X. Epidemiological Trends of Antibiotic Resistant Gonorrhea in the United Kingdom. Antibiotics. 2018;7(3):60 10.3390/antibiotics7030060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Papp JR, Abrams AJ, Nash E, Katz AR, Kirkcaldy RD, Connor NPO, et al. Azithromycin resistance and decreased ceftriaxone susceptibility in Neisseria gonorrhoeae, Hawaii, USA. Emerging Infectious Diseases. 2017;23(5):830–832. 10.3201/eid2305.170088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grad YH, Goldstein E, Lipsitch M, White PJ. Improving control of antibiotic-resistant gonorrhea by integrating research agendas across disciplines: key questions arising from mathematical modeling. The Journal of Infectious Diseases. 2016;213:883–890. 10.1093/infdis/jiv517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garnett GP, Mertz KJ, Finelli L, Levine WC, St Louis ME. The transmission dynamics of gonorrhoea: modelling the reported behaviour of infected patients from Newark, New Jersey. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 1999;354:787–797. 10.1098/rstb.1999.0431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Turner KME, Garnett GP, Ghani AC, Sterne JAC, Low N. Investigating ethnic inequalities in the incidence of sexually transmitted infections: mathematical modelling study. Sexually Transmitted Infections. 2004;80:379–385. 10.1136/sti.2003.007575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. White PJ, Ward H, Cassell JA, Mercer CH, Garnett GP. Vicious and virtuous circles in the dynamics of infectious disease and the provision of health care: gonorrhea in Britain as an example. The Journal of Infectious Diseases. 2005;192:824–836. 10.1086/432004 [DOI] [PubMed] [Google Scholar]
- 9. Fingerhuth SM, Bonhoeffer S, Low N, Althaus CL. Antibiotic-resistant Neisseria gonorrhoeae spread faster with more treatment, not more sexual partners. PLoS Pathogens. 2016;12(5):e1005611 10.1371/journal.ppat.1005611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ghani A, Ison C, Ward H, Garnett G, Bell G, Kinghorn G, et al. Sexual partner networks in the transmission of sexually transmitted diseases. Sexually Transmitted Diseases. 1996;23(6):498–503. [DOI] [PubMed] [Google Scholar]
- 11. Ghani A, Garnett G. Risks of acquiring and transmitting sexually transmitted diseases in sexual partner networks. Sexually Transmitted Diseases. 2000;27:579–587. [DOI] [PubMed] [Google Scholar]
- 12. Bansal S, Grenfell BT, Meyers LA. When individual behaviour matters: homogeneous and network models in epidemiology. Journal of the Royal Society Interface. 2007;4(16):879–91. 10.1098/rsif.2007.1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stack JC, Bansal S, Kumar VSA, Grenfell B. Inferring population-level contact heterogeneity from common epidemic data. Journal of the Royal Society Interface. 2012;10(78):20120578 10.1098/rsif.2012.0578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liljeros F, Edling CR, Amaral LA, Stanley HE, Aberg Y. The web of human sexual contacts. Nature. 2001;411(6840):907–908. 10.1038/35082140 [DOI] [PubMed] [Google Scholar]
- 15. Schneeberger A, Mercer C, Gregson S, Ferguson N, Nyamukapa C, Anderson R, et al. Scale-free networks and sexually transmitted diseases. Sexually Transmitted Diseases. 2004;31(6):380–387. 10.1097/00007435-200406000-00012 [DOI] [PubMed] [Google Scholar]
- 16. Keeling MJ, Danon L, Ford AP, House T, Jewell CP, Roberts GO, et al. Networks and the epidemiology of infectious disease. Interdisciplinary Perspectives on Infectious Diseases. 2011;284909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Albert R, Barabási AL. Statistical mechanics of complex networks. Reviews of Modern Physics. 2002;74(1):47–97. 10.1103/RevModPhys.74.47 [DOI] [Google Scholar]
- 18. Caldarelli G, Capocci A, De Los Rios P, Muñoz MA. Scale-free networks from varying vertex intrinsic fitness. Physical Review Letters. 2002;89(25):258702 10.1103/PhysRevLett.89.258702 [DOI] [PubMed] [Google Scholar]
- 19. Servedio VDP, Caldarelli G, Buttà P. Vertex intrinsic fitness: how to produce arbitrary scale-free networks. Physical Review E. 2004;70:2–5. 10.1103/PhysRevE.70.056126 [DOI] [PubMed] [Google Scholar]
- 20. Johnson LF, Geffen N. A comparison of two mathematical modeling frameworks for evaluating sexually transmitted infection epidemiology. Sexually Transmitted Diseases. 2016;43(3):139–46. [DOI] [PubMed] [Google Scholar]
- 21. Chen MI, Ghani AC, Edmunds J. Mind the gap: the role of time between sex with two consecutive partners on the transmission dynamics of gonorrhea. Sexually Transmitted Diseases. 2008;35(5):435–444. 10.1097/OLQ.0b013e3181612d33 [DOI] [PubMed] [Google Scholar]
- 22. Ong JBS, Fu X, Lee GKK, Chen MIC. Comparability of results from pair and classical model formulations for different sexually transmitted infections. PLoS ONE. 2012;7(6):e39575 10.1371/journal.pone.0039575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Robinson K, Cohen T, Colijn C. The dynamics of sexual contact networks: effects on disease spread and control. Theoretical Population Biology. 2012;81(2):89–96. 10.1016/j.tpb.2011.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miller JC, Slim AC, Volz EM. Edge-based compartmental modelling for infectious disease spread. Journal of the Royal Society Interface. 2012;9:890–906. 10.1098/rsif.2011.0403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erens B, Phelps A, Clifton S, Hussey D, Mercer CH, Tanton C, et al. National Survey of Sexual Attitudes and Lifestyles 3 Technical Report Volume 1: Methodology. London: National Centre for Social Research; 2013. November.
- 26. Erens B, Phelps A, Clifton S, Mercer CH, Tanton C, Hussey D, et al. Methodology of the third British National Survey of Sexual Attitudes and Lifestyles (Natsal-3). Sexually Transmitted Infections. 2014;90(2):84–9. 10.1136/sextrans-2013-051359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson A. National Survey of Sexual Attitudes and Lifestyles, 2010-2012. [data collection]. UK Data Service. 2018;2nd Ed.
- 28. Dodds JP, Nardone A, Mercey DE, Johnson AM. Increase in high risk sexual behaviour among homosexual men, London 1996-8: cross sectional, questionnaire study. British Medical Journal. 2000;320(7248):1510–1511. 10.1136/bmj.320.7248.1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.GRASP Steering Group. The Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP) Year 2004 report. London: Health Protection Agency; 2005.
- 30. Choudhury B, Risley CL, Ghani AC, Bishop CJ, Ward H, Fenton KA, et al. Identification of individuals with gonorrhoea within sexual networks: a population-based study. The Lancet. 2006;368(9530):139–146. 10.1016/S0140-6736(06)69003-X [DOI] [PubMed] [Google Scholar]
- 31.Park N. Revised population estimates for England and Wales: mid-2012 to mid-2016. ONS; 2018.
- 32. Van Kampen S, Fornasiero M, Lee W. Producing modelled estimates of the size of the lesbian, gay and bisexual (LGB) population of England. London: Public Health England; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Whittles LK, White PJ, Didelot X. Estimating the fitness cost and benefit of cefixime resistance in Neisseria gonorrhoeae to inform prescription policy: a modelling study. PLoS Medicine. 2017;14(10):e1002416 10.1371/journal.pmed.1002416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bissessor M, Tabrizi SN, Fairley CK, Danielewski J, Whitton B, Bird S, et al. Differing Neisseria gonorrhoeae bacterial loads in the pharynx and rectum in men who have sex with men: Implications for gonococcal detection, transmission, and control. Journal of Clinical Microbiology. 2011;49(12):4304–4306. 10.1128/JCM.05341-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kent CK, Chaw JK, Wonq W, Liska S, Gibson S, Hubbard G, et al. Prevalence of rectal, urethral, and pharyngeal chlamydia and gonorrhea detected in 2 clinical settings among men who have sex with men: San Francisco, California, 2003. Clinical Infectious Diseases. 2005;41(1):67–74. 10.1086/430704 [DOI] [PubMed] [Google Scholar]
- 36. Norris Turner A, Carr Reese P, Ervin M, Davis JA, Fields KS, Bazan JA. HIV, rectal chlamydia and rectal gonorrhea in men who have sex with men attending an STD clinic in a midwestern US city. Sexually Transmitted Diseases. 2013;40(6):433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Harrison WO, Hooper RR, Wiesner PJ, Campbell AF, Karney WW, Reynolds GH, et al. A trial of minocycline given after exposure to prevent gonorrhea. New England Journal of Medicine. 1979;300(19):1074–1078. 10.1056/NEJM197905103001903 [DOI] [PubMed] [Google Scholar]
- 38. McCutchan JA. Epidemiology of venereal urethritis: comparison of gonorrhea and nongonococcal urethritis. Reviews of Infectious Diseases. 1984;6(5):669–688. 10.1093/clinids/6.5.669 [DOI] [PubMed] [Google Scholar]
- 39. Lodin A. Has the incubation period of gonorrhoea undergone a change? Acta dermato-venereologica. 1955;35(6):457–462. [PubMed] [Google Scholar]
- 40. Sherrard J, Barlow D. Gonorrhoea in men: clinical and diagnostic aspects. Genitourinary Medicine. 1996;72(6):422–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Korenromp EL, Sudaryo MK, de Vlas SJ, Gray RH, Sewankambo NK, Serwadda D, et al. What proportion of episodes of gonorrhoea and chlamydia becomes symptomatic? International Journal of STD and AIDS. 2002;13(2):91–101. 10.1258/0956462021924712 [DOI] [PubMed] [Google Scholar]
- 42. Didelot X, Dordel J, Whittles LK, Collins C, Bilek N, Bishop CJ, et al. Genomic analysis and comparison of two gonorrhoea outbreaks. mBio. 2016;7(3):e00525–16. 10.1128/mBio.00525-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hazel A, Marino S, Simon C. An anthropologically based model of the impact of asymptomatic cases on the spread of Neisseria gonorrhoeae. Journal Of The Royal Society Interface. 2015;12(106):20150067 10.1098/rsif.2015.0067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Handsfield HH, Lipman TO, Harnisch JP, Tronca E, Holmes KK. Asymptomatic gonorrhea in men. New England Journal of Medicine. 1974;290(3):117–123. 10.1056/NEJM197401172900301 [DOI] [PubMed] [Google Scholar]
- 45. Fairley CK, Chen MY, Bradshaw CS, Tabrizi SN. Is it time to move to nucleic acid amplification tests screening for pharyngeal and rectal gonorrhoea in men who have sex with men to improve gonorrhoea control? Sexual Health. 2011;8(1):9–11. 10.1071/SH10134 [DOI] [PubMed] [Google Scholar]
- 46. Jin F, Prestage GP, Mao L, Kippax SC, Pell CM, Donovan B, et al. Incidence and risk factors for urethral and anal gonorrhoea and chlamydia in a cohort of HIV-negative homosexual men: the Health in Men Study. Sexually Transmitted Infections. 2007;83(2):113–119. 10.1136/sti.2006.021915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clutterbuck D, Flowers P, Barber T, Wilson H, Nelson M, Hedge B, et al. The 2010 UK national guidelines on safer sex advice. The Clinical Effectiveness Group of the British Association for Sexual Health and HIV (BASHH) and the British HIV Association (BHIVA); International Journal of STD & AIDS. 2012; 23(6): 381–388. [DOI] [PubMed] [Google Scholar]
- 48.Ross J, Brady M, Clutterbuck D, Doyle T, Hart G, Hughes G, et al. BASHH recommendations for testing for sexually transmitted infections in men who have sex with men. British Association of Sexual Health and HIV; 2014.
- 49. Frankis J, Goodall L, Clutterbuck D, Abubakari R, Flowers P. Regular STI testing amongst men who have sex with men and use social media is suboptimal—a cross-sectional study. International Journal of STD & AIDS. 2017;28(6):573–583. 10.1177/0956462416636780 [DOI] [PubMed] [Google Scholar]
- 50. Mercer CH, Aicken CRH, Estcourt CS, Keane F, Brook G, Rait G, et al. Building the bypass–implications of improved access to sexual healthcare: evidence from surveys of patients attending contrasting genitourinary medicine clinics across England in 2004/2005 and 2009. Sexually Transmitted Infections. 2012;88(1):9–15. 10.1136/sextrans-2011-050257 [DOI] [PubMed] [Google Scholar]
- 51. Bignell C, FitzGerald M. UK national guideline for the management of gonorrhoea in adults, 2011. International Journal of STD & AIDS. 2011;22(10):541–547. 10.1258/ijsa.2011.011267 [DOI] [PubMed] [Google Scholar]
- 52. Hethcote HW, Yorke JA. Modeling gonorrhea in a population with a core group In: Levin S, editor. Gonorrhoea Transmission Dynamics and Control. Berlin, Heidelberg: Springer-Verlag; 1984. p. 32–48. [Google Scholar]
- 53. Petousis-Harris H, Paynter J, Morgan J, Saxton P, McArdle B, Goodyear-Smith F, et al. Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: a retrospective case-control study. The Lancet. 2017;390(10102):1603–1610. 10.1016/S0140-6736(17)31449-6 [DOI] [PubMed] [Google Scholar]
- 54. Craig AP, Gray RT, Edwards JL, Apicella MA, Jennings MP, Wilson DP, et al. The potential impact of vaccination on the prevalence of gonorrhea. Vaccine. 2015;33(36):4520–4525. 10.1016/j.vaccine.2015.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Seib KL. Gonorrhoea vaccines: a step in the right direction. The Lancet. 2017;390(10102):1567–1569. 10.1016/S0140-6736(17)31605-7 [DOI] [PubMed] [Google Scholar]
- 56. Becker N. Estimation for discrete time branching processes with application to epidemics. Biometrics. 1977;33(3):515–22. 10.2307/2529366 [DOI] [PubMed] [Google Scholar]
- 57. Farrington CP, Kanaan MN, Gay NJ. Branching process models for surveillance of infectious diseases controlled by mass vaccination. Biostatistics (Oxford, England). 2003;4(2):279–295. 10.1093/biostatistics/4.2.279 [DOI] [PubMed] [Google Scholar]
- 58. Jombart T, Cori A, Didelot X, Cauchemez S, Fraser C, Ferguson N. Bayesian reconstruction of disease outbreaks by combining epidemiologic and genomic data. PLoS Computational Biology. 2014;10(1):e1003457 10.1371/journal.pcbi.1003457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Didelot X, Gardy J, Colijn C. Bayesian inference of infectious disease transmission from whole-genome sequence data. Molecular Biology and Evolution. 2014;31(7):1869–1879. 10.1093/molbev/msu121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Didelot X, Fraser C, Gardy J, Colijn C. Genomic infectious disease epidemiology in partially sampled and ongoing outbreaks. Molecular Biology and Evolution. 2017;34(4):997–1007. 10.1093/molbev/msw275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Klinkenberg D, Backer JA, Didelot X, Colijn C, Wallinga J. Simultaneous inference of phylogenetic and transmission trees in infectious disease outbreaks. PLoS Computational Biology. 2017;13(5):1–32. 10.1371/journal.pcbi.1005495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. De Silva D, Peters J, Cole K, Cole MJ, Cresswell F, Dean G, et al. Whole-genome sequencing to determine Neisseria gonorrhoeae transmission: an observational study. The Lancet Infectious Diseases. 2016;16(11):1295–1303. 10.1016/S1473-3099(16)30157-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fifer H, Cole M, Hughes G, Padfield S, Smolarchuk C, Woodford N, et al. Sustained transmission of high-level azithromycin-resistant Neisseria gonorrhoeae in England: an observational study. The Lancet Infectious Diseases. 2018;18(5):573–581. [DOI] [PubMed] [Google Scholar]
- 64. Peters J, Cresswell F, Amor L, Cole K, Dean G, Didelot X, et al. Whole genome sequencing of Neisseria gonorrhoeae reveals transmission clusters involving patients of mixed HIV serostatus. Sexually Transmitted Infections. 2018;94(2):138–143. 10.1136/sextrans-2017-053198 [DOI] [PubMed] [Google Scholar]
- 65. Lloyd-Smith JO, Schreiber SJ, Kopp PE, Getz WM. Superspreading and the effect of individual variation on disease emergence. Nature. 2005;438(7066):355–359. 10.1038/nature04153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Galvani AP, May RM. Epidemiology: dimensions of superspreading. Nature. 2005;438(7066):293–295. 10.1038/438293a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Garske T, Rhodes CJ. The effect of superspreading on epidemic outbreak size distributions. Journal of Theoretical Biology. 2008;253(2):228–237. 10.1016/j.jtbi.2008.02.038 [DOI] [PubMed] [Google Scholar]
- 68. Grassly NC, Fraser C. Mathematical models of infectious disease transmission. Nature Reviews Microbiology. 2008;6(6):477–487. 10.1038/nrmicro1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Li LM, Grassly NC, Fraser C. Quantifying transmission heterogeneity using both pathogen phylogenies and incidence time series. Molecular Biology and Evolution. 2017;34(11):2982–2995. 10.1093/molbev/msx195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Risley CL, Ward H, Choudhury B, Bishop CJ, Fenton KA, Spratt BG, et al. Geographical and demographic clustering of gonorrhoea in London. Sexually Transmitted Infections. 2007;83(6):481–487. 10.1136/sti.2007.026021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gelman A, Rubin DB. Inference from iterative simulation using multiple sequences. Statistical Science. 1992;7(4):457–472. 10.1214/ss/1177011136 [DOI] [Google Scholar]
- 72. Brooks SPB, Gelman AG. General methods for monitoring convergence of iterative simulations. Journal of Computational and Graphical Statistics. 1998;7(4):434–455. 10.2307/1390675 [DOI] [Google Scholar]
- 73. Platt R, Rice P, McCormack W. Risk of acquiring gonorrhea and prevalence of abnormal adnexal findings among women recently exposed to gonorrhea. JAMA. 1983;250(23):3205–3209. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The orange lines represent the desired degree distribution p(k) = ck−γ. The proportion of individuals having no partners in the last year is shown in the title of each plot as p(k = 0).
(PDF)
(PDF)
The number of partners reported by MSM over one year is negatively correlated with the average length of the relationships. This suggests that in a realistic model the expected duration of partnerships formed by high degree individuals should be shorter than for low-degree individuals.
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper, its Supporting Information files, and the freely accessible repository https://github.com/lwhittles/simdynet.