Supplemental digital content is available in the text.
Key Words: suPAR, serum, ascitic fluid, severe acute pancreatitis, prognosis
Abstract
Objectives
The aim of the study was to investigate the correlation of serum and ascitic fluid soluble form urokinase plasminogen activator receptor (suPAR) levels with patients' complications, disease severity, inflammatory markers, and prognosis in patients with severe acute pancreatitis (SAP).
Methods
Fifty patients with SAP, 47 patients with mild acute pancreatitis, and 50 healthy controls were enrolled. Serum samples were obtained from all participants after enrollment; meanwhile, ascitic fluid samples were collected from 20 patients with SAP who developed ascites. Serum and ascitic fluid suPAR levels were determined by enzyme-linked immunosorbent assay.
Results
Serum suPAR level was greatly elevated in patients with SAP than patients with mild acute pancreatitis and healthy controls. Receiver operating characteristic curve showed that serum suPAR presented with good value in predicting risk of pancreatic necrosis, pancreatic infection, and multiple organ dysfunction syndrome, whereas serum suPAR did not predict mortality. Serum suPAR level was also positively correlated with Acute Physiology and Chronic Health Evaluation II score, Balthazar index, and Sequential Organ Failure Assessment score. As to ascitic fluid suPAR, it was positively correlated with serum suPAR level, Acute Physiology and Chronic Health Evaluation II score, Sequential Organ Failure Assessment score, risk of pancreatic infection, and multiple organ dysfunction syndrome.
Conclusions
Serum and ascetic fluid suPAR levels could be served as markers for disease severity and risk of severe complications in patients with SAP.
Severe acute pancreatitis (SAP), as an inflammatory condition that is associated with multiple organ dysfunctions, presents with a high mortality rate ranging from 8% to 25%, which is a critical health issue worldwide.1,2 Early mortality of patients with SAP mainly owes to an overwhelming inflammatory reaction, whereas late mortality mainly results from sepsis-related complications such as septic shock and major bleeding that primarily arises from infected pancreatic necrosis.3,4 So as to decrease the mortality and improve the outcomes of patients with SAP, exploration of novel biomarkers for early diagnosis, disease monitoring, and prognosis is of great importance.
Urokinase plasminogen activator receptor (uPAR), which is extracellularly docked to the serum membrane by a glycosylphosphatidylinositol anchor, is expressed on various types of cells including neutrophils, lymphocytes, monocytes, macrophages, certain cancer cells, vascular endothelial cells, and so on.5–8 Accumulating research disclose that uPAR and its ligand urokinase plasminogen activator are involved in regulating numerous biological functions including cells proliferation, migration, adhesion, and angiogenesis and have been observed to promote tissue invasion in malignant diseases by converting plasminogen into plasmin, which leads to degradation of extracellular matrix.8–11 Through inflammatory stimulation, uPAR is cleaved from the cells surface by proteases to the soluble form of uPAR (suPAR), which has been detected in blood, urine, and cerebrospinal fluid.12–15 Blood suPAR level has been observed to be elevated in several infectious, inflammatory, and autoimmune diseases.14–18 Previous studies illuminate that plasma suPAR level presents a strong association with higher disease severity and increased mortality in critically ill patients,16,17 and high serum suPAR level has been discovered to be able to predict poor outcomes in patients with systemic inflammatory response syndrome (SIRS).18 However, the role of suPAR in monitoring disease severity and predicting outcomes in patients with SAP has not been explored.
Thus, this present study aimed to investigate the correlation of serum and ascitic fluid suPAR levels with patients' complications, disease severity, inflammatory markers, and prognosis in patients with SAP.
MATERIALS AND METHODS
Participants
Fifty patients with SAP who were admitted to the intensive care unit (ICU) at the Wuhan Central Hospital (Hubei, China) between June 2012 and December 2013 were consecutively enrolled in this study. The inclusion criteria were as follows: (1) diagnosed as SAP according to the Atlanta classification of acute pancreatitis; (2) older than 18 years; and (3) met at least 1 of the following items: (a) local complications such as necrosis, abscess, or pseudocyst; (b) organ failure (systolic blood pressure <90 mm Hg, Pao2 < 60 mm Hg or serucreatinine > 133 μmol/L); (c) an Acute Physiology and Chronic Health Evaluation (APACHE) II score of 8 or higher, or a Ranson score of 3 or higher; and (d) Balthazar index of 5 or higher. The exclusion criteria were as follows: (1) patients who had experienced previous attacks of acute pancreatitis; (2) patients who had received surgical intervention before admission; (3) patients who had pulmonary tuberculosis or human immunodeficiency virus; (4) patients with a known history of coagulative disorders; and (5) patients with a history of solid tumor or hematological malignances. Meanwhile, 50 patients with mild acute pancreatitis (MAP) as well as 50 age- and sex-matched healthy controls (HCs) were consecutively enrolled in this study.
Ethics Approval
This study was approved by the ethics committee of the Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology and was conducted in accordance with the Declaration of Helsinki. All participants or their statutory guardians provided written informed consents.
Data Collection and Assessments
Data from patients with SAP including age, sex, and etiology were collected and APACHE II score, Ranson score, Balthazar index, and Sequential Organ Failure Assessment (SOFA) score were assessed on admission. Pancreatic necrosis, pancreatic infection, and multiple organ dysfunction syndrome (MODS) occurrences during the admission were recorded. Pancreatic necrosis was detected according to the results of contrast-enhanced computed tomography performed at least 48 hours after the onset of the disease. Meanwhile, pancreatic infection was determined according to positive findings in bacterial culture of abdominal fluid and repeated temperature increments.
The use of vasoactive drugs, mechanical ventilation, continuous renal replacement therapy (CRRT), and surgical intervention during the hospitalization were also recorded. In addition, length of hospitalization, length of ICU stays, and the mortality during the hospitalization were documented. As for HCs, data of age and sex were collected.
Sample Collection
Blood samples were collected from patients with SAP and patients with MAP when they were admitted to the ICU (before therapeutic interventions) and from HCs after the enrollment. After centrifugation at 2000g at 4°C for 10 minutes, serum was subsequently isolated and frozen immediately at −80°C. Twenty of 50 patients with total SAP were detected with ascetic fluid by computerized tomography (CT) scanning. Abdominal fluid samples were then obtained from these patients by ultrasonically guided fine needle aspiration during admission and the samples were then stored at −80°C.
Soluble Form of uPAR, d-dimer, and PCT Measurements
Serum suPAR level and ascitic fluid suPAR level were detected by enzyme-linked immunosorbent assay (ELISA) using commercial ELISA kit (ViroGates, Birkerød, Denmark). Serum d-dimer and procalcitonin (PCT) levels were detected by electro chemiluminescence immunoassay (ECLIA) (Roche, Mannheim, Germany).
Statistics
Statistical analysis was performed using SPSS 22.0 software (SPSS Inc, Armonk, NY) and GraphPad Prism 6.0 (GraphPad Software, San Diego, Calif.). Data were mainly presented as median (range) and count (percentage). Comparison between the 2 groups was determined by Wilcoxon rank sum test; comparison among 3 groups was determined by Kruskal-Wallis H rank sum test, followed by Dunn's multiple comparison test; correlation between 2 continuous parameters was determined by Spearman test; the values of suPAR, d-dimer, and PCT levels in predicting pancreatic necrosis, pancreatic infection, and MODS risks were analyzed by receiver operating characteristic (ROC) curves, and factors predicting pancreatic necrosis, pancreatic infection, and MODS risk were analyzed by multivariate logistic regression model (Forward Stepwise - Wald). P < 0.05 was considered significant.
RESULTS
Patient Characteristics
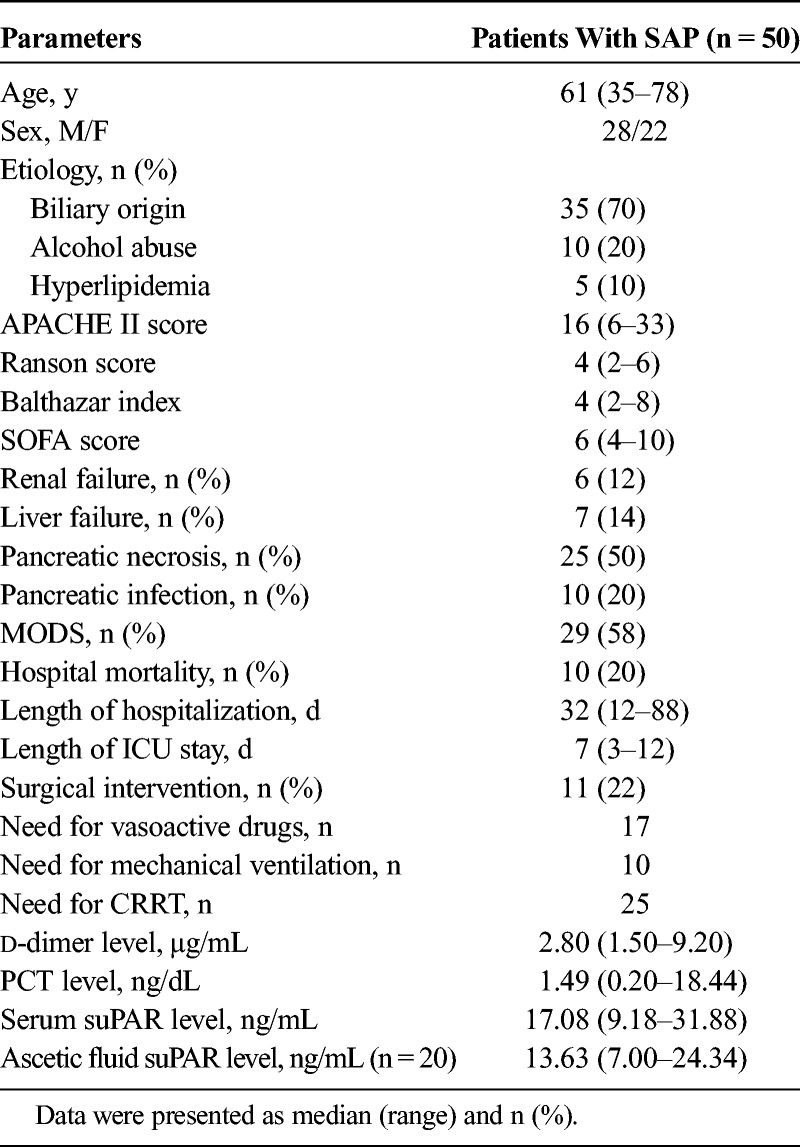
Fifty patients with SAP including 28 males and 22 females with a median age of 61 (range, 35–78) years were enrolled in this study. The median (range) APACHE II score, Ranson score, Balthazar index, and SOFA score were 16 (6–33), 4 (2–6), 4 (2–8), and 6 (4–10), respectively. Multiple organ dysfunction syndrome occurred in 29 (58%), pancreatic necrosis in 25 (50%), and pancreatic infection in 10 (20%); in addition, 10 patients (20%) died during the hospitalization. The median (range) values of serum suPAR level and ascetic fluid suPAR level were 17.08 (9.18–31.88) ng/mL and 13.63 (7.00–24.34) ng/mL, respectively. Other detailed information for patients with SAP are presented in Table 1.
TABLE 1.
Characteristics of Patients With SAP
Comparison of Serum suPAR Level Among Patients With SAP, MAP Patients, and HCs
The median (range) age of patients with MAP was 60 (41–73) years and there were 28 males and 19 females, whereas the median (range) age of HCs was 58 (40–70) years and there were 25 males and 25 females. No difference of age (P = 0.236) or sex (P = 0.327) among patients with SAP, patients with MAP, and HCs was discovered. The median (range) serum suPAR level was found to be greatly elevated in patients with SAP (17.08 [9.18–31.88]) compared with patients with MAP (9.739 [2.604–27.70]) (P = 0.006) and HCs (2.32 [0.20–13.28]) (P < 0.001) and was increased in patients with MAP than HCs (P < 0.001) (Fig. 1).
FIGURE 1.
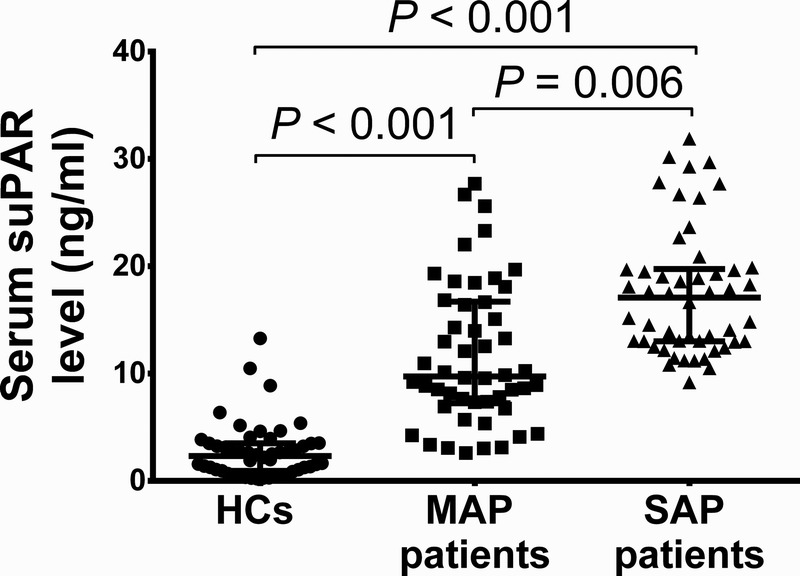
Serum suPAR level in patients with SAP, patients with MAP, and HCs. Serum suPAR level was dramatically increased in patients with SAP and patients with MAP than HCs and was enhanced in patients with SAP than patients with MAP. Comparison among 3 groups was determined by Kruskal-Wallis H rank sum test, followed by Dunn multiple comparison test, and P < 0.05 was considered as significant.
Correlation of Serum suPAR Level With Presence or Absence of Discontinuous Clinical Variables
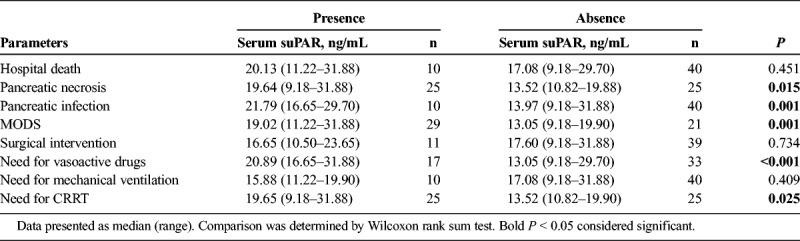
During the hospitalization, patients with presence of pancreatic necrosis (P = 0.015), pancreatic infection (P = 0.001), and MODS (P = 0.001) disclosed elevated serum suPAR compared with patients with absence of these conditions (Table 2). However, serum suPAR was only numerically increased in patients who died during the hospitalization than the survivors, but without statistical significance (P = 0.451). In addition, we also discovered that patients with presence of need for vasoactive drugs (P < 0.001) and CRRT (P = 0.025) illuminated raised serum suPAR compared with patients with absence of these conditions (Table 2).
TABLE 2.
Correlation of Serum SuPAR With Presence or Absence of Discontinuous Clinical Variables in Patients With SAP
Correlation of Serum suPAR Level With Disease Severity and Inflammation Markers
The APACHE II score, Ranson score, Balthazar index, and SOFA were assessed in patients with SAP, which disclosed that serum suPAR level was positively correlated with APACHE II score (R = 0.327, P = 0.020, Fig. 2A), Balthazar index (R = 0.298, P = 0.036, Fig. 2C), and SOFA score (R = 0.382, P = 0.006, Fig. 2D), although it did not associate with Ranson score (R = 0.182, P = 0.207, Fig. 2B). These indicated serum suPAR could serve as disease severity markers in patients with SAP. In addition, we also measured the expressions of inflammation markers (serum d-dimer and PCT), which revealed that serum suPAR presented with a tread of positive correlation with serum d-dimer level (R = 0.154, P = 0.285, Fig. 2E) and serum PCT level (R = 0.233, P = 0.104, Fig. 2F), but without statistical significance.
FIGURE 2.
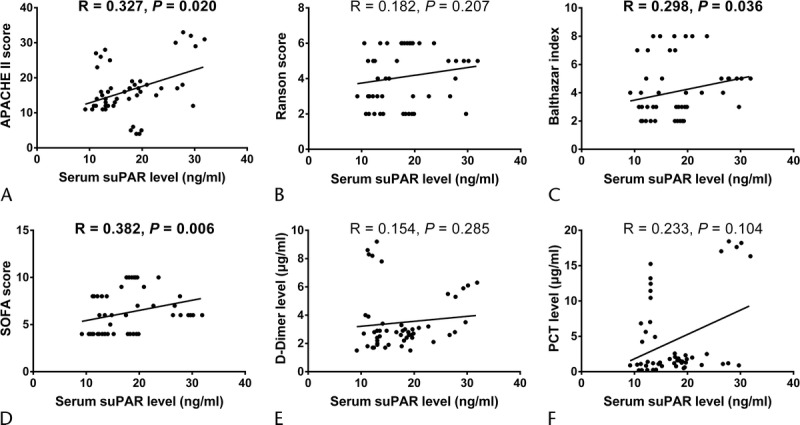
Correlation of serum suPAR level with disease severity and inflammation markers. Serum suPAR level was disclosed to be positively associated with APACHE II score (A), Balthazar index (C), and SOFA score (D). However, no correlation of serum suPAR level with Ranson score (B), d-dimer level (E), or PCT level (F) was observed. Correlation was determined by Spearman test, and P < 0.05 was considered as significant.
Predictive Value of Serum suPAR for Occurrence of Pancreatic Necrosis, Pancreatic Infection, and MODS
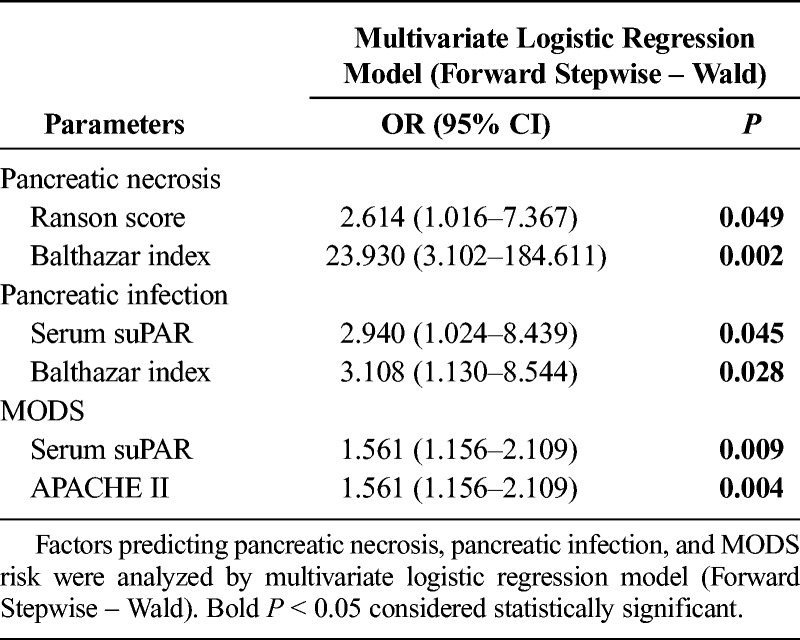
Receiver operating characteristic curves were subsequently performed to detect the predictive value of serum suPAR, serum d-dimer, and serum PCT levels for occurrence of pancreatic necrosis, pancreatic infection, and MODS in patients with SAP, which illuminated the following: (1) that serum suPAR presented with good value in predicting pancreatic necrosis risk with area under curve (AUC) of 0.70 (95% confidence interval [CI], 0.548–0.853), and sensitivity and specificity were 52.0% and 96.0%, respectively, at the best cutoff value (serum suPAR = 15.565 ng/mL), whereas serum d-dimer and serum PCT levels lacked predictive value for pancreatic necrosis risk (Fig. 3A); (2) that serum suPAR disclosed good value in predicting pancreatic infection risk with AUC of 0.833 (95% CI, 0.717–0.948), and sensitivity and specificity were 80.0% and 85.0%, respectively, at the best cutoff value (serum suPAR = 19.565 ng/mL), whereas serum d-dimer and serum PCT levels did not possess predictive value for pancreatic infection risk (Fig. 3B); (3) that serum suPAR had similar predictive value (AUC, 0.782; 95% CI, 0.656–0.907) as compared with serum d-dimer (AUC, 0.741; 95% CI, 0.605–0.877) and serum PCT (AUC, 0.722; 95% CI, 0.565–0.879) for MODS risk, and sensitivity and specificity were 72.4% and 76.2%, respectively, at the best cutoff value (serum suPAR = 15.914 ng/mL) (Fig. 3C). When being compared with common comprehensive assessing score, (1) serum suPAR disclosed better value compared with APACHE II and SOFA, similar value compared with Ranson, and worse value compared with Balthazar in predicting pancreatic necrosis (Supplementary Fig. 1A, http://links.lww.com/MPA/A704). (2) Serum suPAR disclosed better value compared with APACHE II, Ranson, and Balthazar, and similar value compared with SOFA in predicting pancreatic infection (Supplementary Fig. 1B, http://links.lww.com/MPA/A704). (3) Serum suPAR disclosed better value compared with Balthazar, similar value compared with Ranson, and worse value compared with APACHE II and SOFA in predicting MODS (Supplementary Fig. 1C, http://links.lww.com/MPA/A704). Furthermore, to attenuate the impact of compounding factors, multivariate logistic regression analysis was performed (Table 3), which disclosed that serum suPAR independently predicted risks of pancreatic infection and MODS but not pancreatic necrosis.
FIGURE 3.
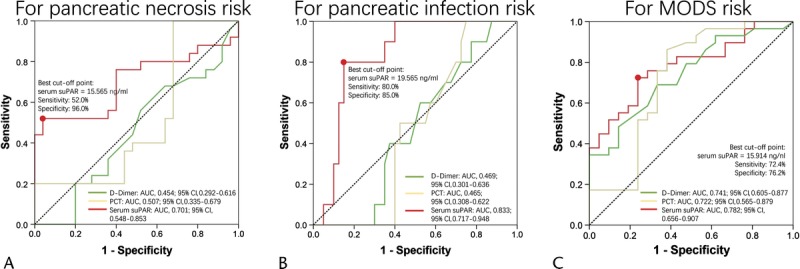
Receiver operating characteristic curve analysis of serum suPAR, d-dimer, and PCT level for risk of severe complications. Receiver operating characteristic curve revealed that serum suPAR disclosed good value in predicting risk of pancreatic necrosis (A, red line), pancreatic infection (B, red line), and MODS (C, red line). However, serum d-dimer (green line) and PCT could only predict risk of MODS (C), but not pancreatic necrosis (A) or pancreatic infection (B). Predicting value was determined by ROC curve analysis.
TABLE 3.
Multivariate Logistic Regression Analysis of Factors Predicting Pancreatic Necrosis, Pancreatic Infection, and MODS Risk
Correlation Between Ascetic Fluid suPAR Level and Serum suPAR Level
Twenty of 50 patients with total SAP were detected with ascetic fluid by CT scanning, and ascetic fluid suPAR level was determined in these patients. We found that ascetic fluid suPAR level was positively correlated with serum suPAR level (R = 0.851, P < 0.001, Fig. 4).
FIGURE 4.
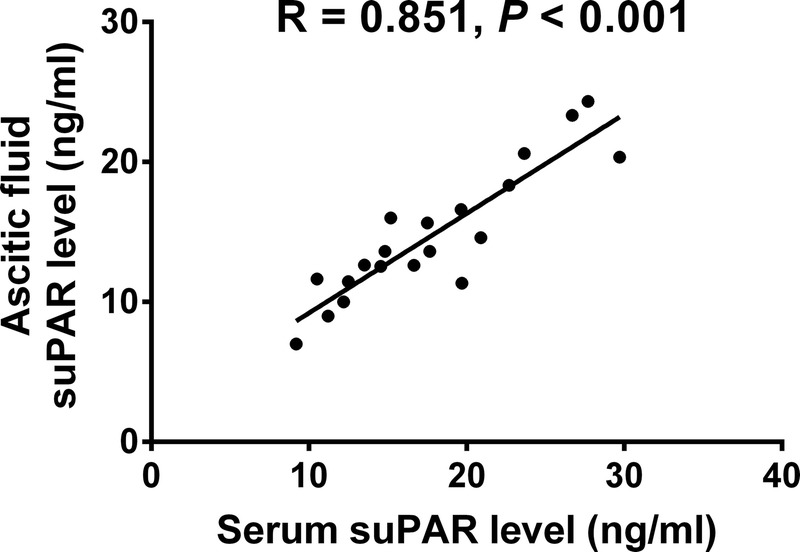
Correlation of ascetic fluid suPAR level with serum suPAR level. Ascetic fluid suPAR level was positively correlated with serum suPAR level. Correlation was determined by Spearman test, and P < 0.05 was considered as significant.
Association of Ascetic Fluid suPAR Level With Presence or Absence of Discontinuous Clinical Variables
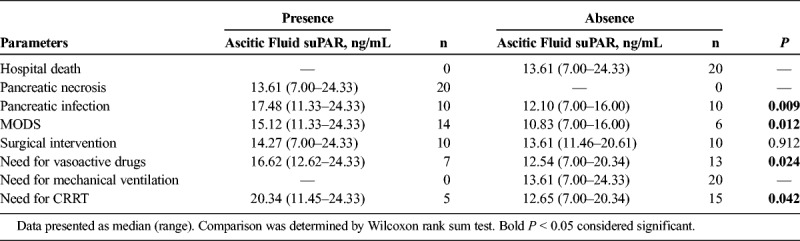
In the 20 patients with SAP with ascetic fluid, ascetic fluid suPAR level was observed to be elevated in patients with presence of pancreatic infection (P = 0.009), MODS (P = 0.012), need for vasoactive drugs (P < 0.001), and CRRT (P = 0.025) compared with patients with absence of these conditions (Table 4).
TABLE 4.
Correlation of Ascitic Fluid uPAR With Presence or Absence of Discontinuous Clinical Variables
Association of Ascetic Fluid suPAR Level With Disease Severity and Inflammation Markers
Ascetic fluid suPAR level was positively associated with APACHE II score (R = 0.563, P = 0.001, Fig. 5A) and SOFA score (R = 0.558, P = 0.011, Fig. 5D), although it did not associate with Ranson score (R = −0.033, P = 0.890, Fig. 5B) or Balthazar index (R = 0.145, P = 0.543, Fig. 5C). As to inflammation markers, ascetic fluid suPAR level was not correlated with serum d-dimer level (R = 0.365, P = 0.114, Fig. 5E) or serum PCT level (R = 0.173, P = 0.466, Fig. 5F).
FIGURE 5.
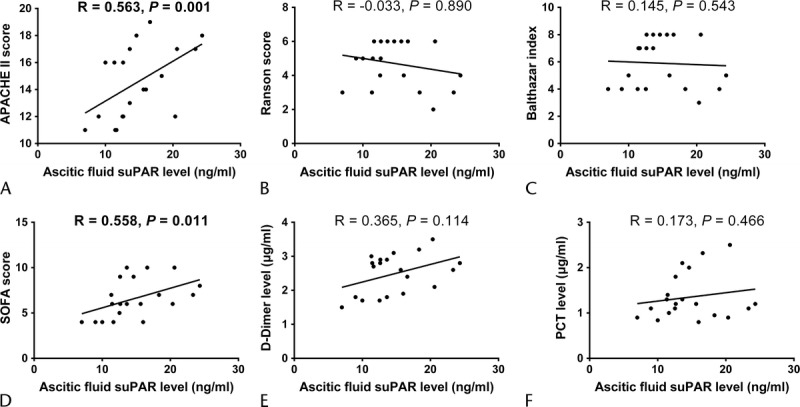
Correlation of ascetic fluid suPAR level with disease severity and inflammation markers. Ascetic fluid suPAR level was illuminated to be positively associated with APACHE II score (A) and SOFA score (D). However, no correlation of ascetic fluid suPAR level with Ranson score (B), Balthazar index (C), d-dimer level (E), or PCT level (F) was observed. Correlation was determined by Spearman test, and P < 0.05 was considered as significant.
Predictive Value of Ascetic Fluid suPAR for Occurrence of Pancreatic Infection and MODS
Ascetic fluid suPAR disclosed good predictive value for risk of pancreatic infection (Fig. 6A) and MODS (Fig. 6B) with AUC of 0.840 (95% CI, 0.664–1.000) and AUC of 0.857 (95% CI, 0.667–1.000), respectively. The sensitivity and specificity were 60.0% and 100.0% at best cutoff point (suPAR = 16.310 ng/mL) for predicting pancreatic infection and were 85.7% and 83.3% at best cutoff point (suPAR = 12.580 ng/mL) for predicting MODS.
FIGURE 6.
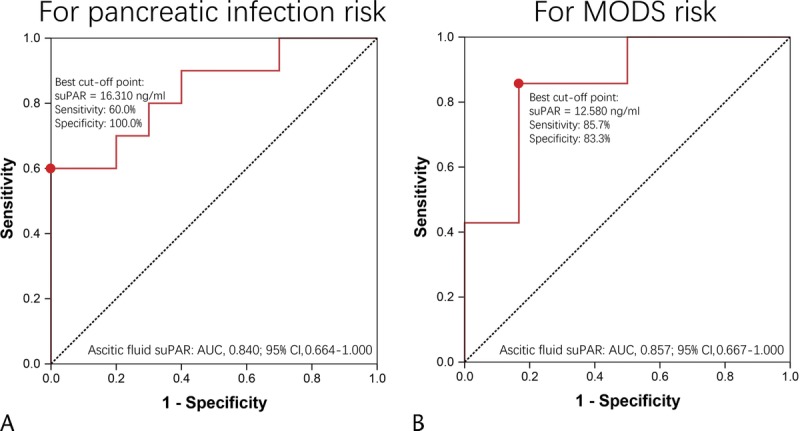
Receiver operating characteristic curve analysis of ascetic fluid suPAR for risk of severe complications. Receiver operating characteristic curve revealed that ascetic fluid suPAR disclosed good value in predicting risk of pancreatic infection (A) and MODS (B). Predicting value was determined by ROC curve analysis.
DISCUSSION
In this present study, we found that (1) serum suPAR level was greatly raised in patients with SAP than patients with MAP and HCs, and it correlated with elevated risk of pancreatic necrosis, pancreatic infection, and MODS as well as increased disease severity in patients with SAP, although it was not associated with levels of inflammatory markers or patient mortality; (2) ascetic fluid suPAR level was positively associated with serum suPAR level, correlated with elevated risk of pancreatic infection and MODS, and correlated with higher disease severity in patients with SAP, although it was not associated with levels of inflammatory markers.
Soluble form uPAR, as a receptor that is released from cell-membrane-bound uPAR, has been observed to not only participate in regulating various cells adhesion, proliferation, and migration, but also be involved in modulating coagulation, fibrinolysis, inflammation, and immune response.19,20 Accumulating studies reveal that blood suPAR is increased in various critical diseases including sepsis, SIRS, severe community-acquired pneumonia (CAP), acute liver failure, and so on.20–23 A case-control study reveals that plasma suPAR level is elevated in patients with sepsis compared with patients with SIRS and controls, which presents with a good value in distinguishing sepsis from SIRS and controls.21 Another case-control study shows that serum suPAR concentration is increased in patients with severe CAP compared with healthy individuals.22 Meanwhile, another cross-sectional cohort study reveals that serum suPAR is greatly elevated in patients with chronic liver diseases compared with controls.24 In addition, a recent case-control study illustrates that serum suPAR level is dramatically increased in acute liver failure patients, which is independent from the underlying etiology.23 Our previous study also disclosed that serum suPAR level was elevated in acute respiratory distress syndrome (ARDS) patients with sepsis than ARDS patients without sepsis.25 However, few studies have explored the dysregulation of blood suPAR level in patients with SAP. In this study, we found that serum suPAR level was greatly increased in patients with SAP compared with patients with MAP and HCs, which was in line with that blood suPAR level is upregulated in other critical diseases.20–23,25 The explanation of this result might be that patients with SAP presented with elevated inflammatory condition, enhanced immune response, and high possibility of multiple organ dysfunctions risk, whereas suPAR could promote plasminogen-activating pathways, increase inflammation, and modulate various cells adhesion, migration, as well as proliferation, which also leads to several organ injuries19,20; thus, patients with SAP illuminated greatly raised serum suPAR level.
As for the value of blood suPAR as marker for disease severity in monitoring critical diseases, a previous study illuminates that a high level of suPAR (defined as >11 ng/mL) is correlated with higher possibility of hypotension and elevated SOFA score in patients with bacteremia.26 Another study exhibits that serum suPAR concentration on admission is closely and independently associated with levels of inflammatory markers (tumor necrosis factor, C-reactive protein), as well as hepatic and renal dysfunction in sepsis patients.27 In a previous study from our research team, we observed that plasma suPAR level on the first therapeutical day was associated with higher risk of MODS in patients with SIRS.28 Another previous study from our research team found that plasma suPAR level was positively correlated with disease severity, serum PCT level, APACHE II score, and SOFA score in patients with ARDS.25 These indicated that suPAR could be served as marker for disease severity in critical diseases especially inflammation involved diseases. However, little is known about the role of suPAR in monitoring disease severity in patients with SAP. In this present study, we discovered that serum suPAR level was positively correlated with APACHE II score, Balthazar index, and SOFA score in patients with SAP, and suPAR level was raised in patients with presence of pancreatic necrosis, pancreatic infection, and MODS (these 3 conditions were severe complications related to SAP). The possible explanations were as follows: (1) that suPAR expression reflected coagulation, fibrinolysis, inflammation, and immune response in patients with SAP, which affected the disease severity and the occurrence of pancreatic necrosis and pancreatic infection a lot; (2) that suPAR promoted several organ injuries through regulating multiple pathways, which increased MODS risk. However, serum suPAR presented with only a tread of positive correlation with serum d-dimer level and serum PCT level, but without statistical significance in our study, these might result from that (1) the sample size was small that extreme value affected the results a lot and (2) suPAR reflected general activation of the immune system rather than exerting inflammatory actions in patients with SAP.
Blood suPAR level also reveals good value in predicting prognosis in several critical diseases including severe CAP, bacteremia, SIRS, sepsis, ARDS, multiple myeloma, and so on.22,23,25,29–32 For instance, serum suPAR level presents with good value in predicting mortality in severe CAP patients with AUC of 0.772,22 and plasma suPAR level is markedly upregulated in nonsurvivors compared with survivors in patients with bacteremia, and the AUC of suPAR in the prediction of case fatality is 0.8421; moreover, plasma suPAR level correlates with increased 30-day mortality in patients with SIRS.29 In addition, a previous cohort study in the Netherlands showed that plasma suPAR expression is increased in nonsurvivors compared with survivors in ARDS patients, which also presents with a good value in distinguishing nonsurvivors from survivors by ROC curve.30 Another study in Italy shows that baseline suPAR level correlates with increased 7- and 30-day mortality in patients with sepsis.31 These results suggest that high blood suPAR level could be regarded as a biomarker for predicting worse prognosis in critical diseases. However, the prognostic role of blood suPAR level in patients with SAP is still unknown. In our study, we observed no difference of serum suPAR level between nonsurvivors and survivors in patients with SAP, whereas it was numerically elevated in nonsurvivors than survivors, which was inconsistent with its prognostic value in other critical diseases. The possible explanations were as follows: (1) the total sample size was small, and thus, extreme value affected the results a lot; (2) number of death events was only 10 (20%), which affected the statistical power. Thus, further study enrolling more patients with SAP to investigate the prognostic role of serum suPAR level is of great importance.
During the hospitalization of patients with SAP in this present study, 20 patients developed ascites; thus, we also detected the level of ascetic fluid suPAR in these patients. We found that ascetic fluid suPAR level was positively correlated with serum suPAR level, and it was positively associated with APACHE II score and SOFA score as well as the occurrence of pancreatic infection and MODS. These indicated that ascetic fluid suPAR level could also serve as a marker for disease severity and risk of severe complications in patients with SAP with ascetic fluid, and this result was in line with a previous study that ascetic fluid suPAR was associated with a severe course of spontaneous bacterial peritonitis and worse outcome in patients with decompensated cirrhosis.33 However, ascetic fluid sample was less feasible to obtain than serum sample, and the detection of ascetic fluid suPAR was later than serum suPAR, which delayed the prognostic estimation in patients with SAP.
There were some limitations in this study. Firstly, the sample size was small, which might lead to greater bias due to patient selection and data collection and result in lack of statistical power. However, SAP presented with low occurrence and more time is needed to recruit more patients. Secondly, the age of the patients included in this study were all older than 35 years; thus, the correlation of suPAR level with patients' complications, disease severity, inflammatory markers, and prognosis in younger patients with SAP was not explored. Thirdly, the patients of this study mainly came from Middle China, which might cause patient selection bias.
In conclusion, serum and ascetic fluid suPAR levels could serve as markers for disease severity and risk of severe complications in patients with SAP.
Supplementary Material
Footnotes
This study was supported by Research Fund Project of Wuhan Central Hospital (YQ13B03).
The authors declare no conflict of interest.
D.L. and Y.W. contributed equally to this work.
Supplemental digital contents are available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.pancreasjournal.com).
REFERENCES
- 1.Portelli M, Jones CD. Severe acute pancreatitis: pathogenesis, diagnosis and surgical management. Hepatobiliary Pancreat Dis Int. 2017;16:155–159. [DOI] [PubMed] [Google Scholar]
- 2.Appelros S, Lindgren S, Borgström A. Short and long term outcome of severe acute pancreatitis. Eur J Surg. 2001;167:281–286. [DOI] [PubMed] [Google Scholar]
- 3.Zerem E. Treatment of severe acute pancreatitis and its complications. World J Gastroenterol. 2014;20:13879–13892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brisinda G, Vanella S, Crocco A, et al. Severe acute pancreatitis: advances and insights in assessment of severity and management. Eur J Gastroenterol Hepatol. 2011;23:541–551. [DOI] [PubMed] [Google Scholar]
- 5.Eugen-Olsen J, Giamarellos-Bourboulis EJ. suPAR: rhe unspecific marker for disease presence, severity and prognosis. Int J Antimicrob Agents. 2015;46(suppl 1):S33–S34. [DOI] [PubMed] [Google Scholar]
- 6.Vassalli JD, Baccino D, Belin D. A cellular binding site for the Mr 55,000 form of the human plasminogen activator, urokinase. J Cell Biol. 1985;100:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stoppelli MP, Corti A, Soffientini A, et al. Differentiation-enhanced binding of the amino-terminal fragment of human urokinase plasminogen activator to a specific receptor on U937 monocytes. Proc Natl Acad Sci U S A. 1985;82:4939–4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol. 2002;3:932–943. [DOI] [PubMed] [Google Scholar]
- 9.Madsen CD, Ferraris GM, Andolfo A, et al. uPAR-induced cell adhesion and migration: vitronectin provides the key. J Cell Biol. 2007;177:927–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madsen CD, Sidenius N. The interaction between urokinase receptor and vitronectin in cell adhesion and signalling. Eur J Cell Biol. 2008;87:617–629. [DOI] [PubMed] [Google Scholar]
- 11.Danø K, Rømer J, Nielsen BS, et al. Cancer invasion and tissue remodeling—cooperation of protease systems and cell types. APMIS. 1999;107:120–127. [DOI] [PubMed] [Google Scholar]
- 12.Stephens RW, Pedersen AN, Nielsen HJ, et al. ELISA determination of soluble urokinase receptor in blood from healthy donors and cancer patients. Clin Chem. 1997;43:1868–1876. [PubMed] [Google Scholar]
- 13.Ostergaard C, Benfield T, Lundgren JD, et al. Soluble urokinase receptor is elevated in cerebrospinal fluid from patients with purulent meningitis and is associated with fatal outcome. Scand J Infect Dis. 2004;36:14–19. [DOI] [PubMed] [Google Scholar]
- 14.Ostrowski SR, Haastrup E, Langkilde A, et al. The uPA/uPAR system and suPAR in HIV infection. In: Alfano M, ed. Soluble Factors Mediating Innate Immune Responses to HIV Infection. Sharjah, UAE: Bentham E-Books; 2009:85–110. [Google Scholar]
- 15.Thunø M, Macho B, Eugen-Olsen J. suPAR: the molecular crystal ball. Dis Markers. 2009;27:157–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koch A, Tacke F. Why high suPAR is not super—diagnostic, prognostic and potential pathogenic properties of a novel biomarker in the ICU. Crit Care. 2011;15:1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jalkanen V, Yang R, Linko R, et al. SuPAR and PAI-1 in critically ill, mechanically ventilated patients. Intensive Care Med. 2013;39:489–496. [DOI] [PubMed] [Google Scholar]
- 18.Kofoed K, Eugen-Olsen J, Petersen J, et al. Predicting mortality in patients with systemic inflammatory response syndrome: an evaluation of two prognostic models, two soluble receptors, and a macrophage migration inhibitory factor. Eur J Clin Microbiol Infect Dis. 2008;27:375–383. [DOI] [PubMed] [Google Scholar]
- 19.Parsons PE, Eisner MD, Thompson BT, et al. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005;33:1–6; discussion 230–232. [DOI] [PubMed] [Google Scholar]
- 20.Backes Y, van der Sluijs KF, Mackie DP, et al. Usefulness of suPAR as a biological marker in patients with systemic inflammation or infection: a systematic review. Intensive Care Med. 2012;38:1418–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng M, Chang M, Zheng H, et al. Clinical value of soluble urokinase-type plasminogen activator receptor in the diagnosis, prognosis, and therapeutic guidance of sepsis. Am J Emerg Med. 2016;34:375–380. [DOI] [PubMed] [Google Scholar]
- 22.Luo Q, Ning P, Zheng Y, et al. Serum suPAR and syndecan-4 levels predict severity of community-acquired pneumonia: a prospective, multi-centre study. Crit Care. 2018;22:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koch A, Zimmermann HW, Gassler N, et al. Clinical relevance and cellular source of elevated soluble urokinase plasminogen activator receptor (suPAR) in acute liver failure. Liver Int. 2014;34:1330–1339. [DOI] [PubMed] [Google Scholar]
- 24.Zimmermann HW, Koch A, Seidler S, et al. Circulating soluble urokinase plasminogen activator is elevated in patients with chronic liver disease, discriminates stage and aetiology of cirrhosis and predicts prognosis. Liver Int. 2012;32:500–509. [DOI] [PubMed] [Google Scholar]
- 25.Wu X, Hu K, Yu L, et al. Correlation of plasma suPAR expression with disease risk and severity as well as prognosis of sepsis-induced acute respiratory distress syndrome. Int J Clin Exp Pathol. 2017;10:11378–11383. [PMC free article] [PubMed] [Google Scholar]
- 26.Huttunen R, Syrjänen J, Vuento R, et al. Plasma level of soluble urokinase-type plasminogen activator receptor as a predictor of disease severity and case fatality in patients with bacteraemia: a prospective cohort study. J Intern Med. 2011;270:32–40. [DOI] [PubMed] [Google Scholar]
- 27.Koch A, Voigt S, Kruschinski C, et al. Circulating soluble urokinase plasminogen activator receptor is stably elevated during the first week of treatment in the intensive care unit and predicts mortality in critically ill patients. Crit Care. 2011;15:R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu XL, Yu L, Long D, et al. [Dynamic changes in plasma levels of urokinase type plasminogen activator and urokinase type plasminogen activator receptor in patients with systemic inflammatory response syndrome]. [Article in Chinese]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2011;23:478–481. [PubMed] [Google Scholar]
- 29.Reichsoellner M, Raggam RB, Wagner J, et al. Clinical evaluation of multiple inflammation biomarkers for diagnosis and prognosis for patients with systemic inflammatory response syndrome. J Clin Microbiol. 2014;52:4063–4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geboers DG, de Beer FM, Tuip-de Boer AM, et al. Plasma suPAR as a prognostic biological marker for ICU mortality in ARDS patients. Intensive Care Med. 2015;41:1281–1290. [DOI] [PubMed] [Google Scholar]
- 31.Casagranda I, Vendramin C, Callegari T, et al. Usefulness of suPAR in the risk stratification of patients with sepsis admitted to the emergency department. Intern Emerg Med. 2015;10:725–730. [DOI] [PubMed] [Google Scholar]
- 32.Shen J, Wang Q, Wang J, et al. Analysis of soluble urokinase plasminogen activator receptor in multiple myeloma for predicting prognosis. Oncol Lett. 2015;10:2403–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zimmermann HW, Reuken PA, Koch A, et al. Soluble urokinase plasminogen activator receptor is compartmentally regulated in decompensated cirrhosis and indicates immune activation and short-term mortality. J Intern Med. 2013;274:86–100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


