Invasion of Yellowstone Lake by lake trout displaced bears and other cutthroat trout consumers in Yellowstone National Park.
Abstract
Predatory fish introduction can cause cascading changes within recipient freshwater ecosystems. Linkages to avian and terrestrial food webs may occur, but effects are thought to attenuate across ecosystem boundaries. Using data spanning more than four decades (1972–2017), we demonstrate that lake trout invasion of Yellowstone Lake added a novel, piscivorous trophic level resulting in a precipitous decline of prey fish, including Yellowstone cutthroat trout. Plankton assemblages within the lake were altered, and nutrient transport to tributary streams was reduced. Effects across the aquatic-terrestrial ecosystem boundary remained strong (log response ratio ≤ 1.07) as grizzly bears and black bears necessarily sought alternative foods. Nest density and success of ospreys greatly declined. Bald eagles shifted their diet to compensate for the cutthroat trout loss. These interactions across multiple trophic levels both within and outside of the invaded lake highlight the potential substantial influence of an introduced predatory fish on otherwise pristine ecosystems.
INTRODUCTION
Altered top-down effects following the loss of a native apex predator, or the addition of an exotic, may result in unanticipated changes to ecosystems (1). Introduced piscivorous fishes, in particular, have greatly altered freshwater ecosystems throughout the world (2). When a novel apex predator is introduced to a freshwater ecosystem, cascading changes can result where inverse patterns in abundance, productivity, or biomass of populations or communities emerge across links in the aquatic food web. The apex predator asserts top-down control, wherein planktivore biomass is reduced, zooplankton biomass and size are increased, and algal biomass declines (3). This trophic cascade concept arose from work in intertidal food webs (4) but has since been applied to predator-driven shifts in freshwater, marine, and terrestrial ecosystems (5). Movements of nutrients, prey, and predators among habitats are ubiquitous and are thought to strongly influence populations, communities, and food webs (6). In addition to causing within ecosystem effects (7, 8), there is also strong evidence that introduced apex predators can force across-ecosystem changes through food web linkages (9, 10). Because aquatic and terrestrial ecosystems are tightly linked through the fluxes of organisms, material, and energy (11), cross-ecosystem studies are valuable to improve the understanding of ecosystem responses to stressors (12) and for developing management strategies to conserve natural connectivity and function (13).
Here, we provide evidence of cascading interactions across the aquatic-terrestrial food web of the Yellowstone Lake watershed, a highly protected landscape within Yellowstone National Park and the Bridger-Teton wilderness of Wyoming (>3200 km2; fig. S1). The cascade was driven by the invasion of a previously nonexistent apex predator, lake trout (Salvelinus namaycush), which exhibited direct top-down effects on prey fish including native Yellowstone cutthroat trout (Oncorhynchus clarkii bouvieri). As lake trout complete their entire life history within the lake and preferentially use deep water, they are inaccessible to consumers and do not serve as an ecological substitute for cutthroat trout in the system (Fig. 1). Cutthroat trout evolved as the sole salmonid and dominant fish within the lake and are generally found in shallow waters where they were accessible to river otters (Lutra canadensis) and avian predators including osprey (Pandion haliaetus), bald eagles (Haliaeetus leucocephalus), and several colonial waterbirds. During spawning migrations, cutthroat trout became an important prey for grizzly bears (Ursus arctos) and American black bears (Ursus americanus). Following Euro-American settlement of the region in the late 1800s and early 1900s, other fishes not native to Yellowstone Lake were intentionally introduced, including the benthivorous longnose sucker (Catostomus catostomus). Longnose suckers became established, occupied localized areas of the lake, and used some tributary streams for spawning but never achieved the extensive spatial distribution and high abundance of the native cutthroat trout, estimated at 3.5 million (>350 mm in length) in the late 1970s.
Fig. 1. Topological placement of taxa in the Yellowstone Lake food web before (left) and after (right) invasion by nonnative lake trout.
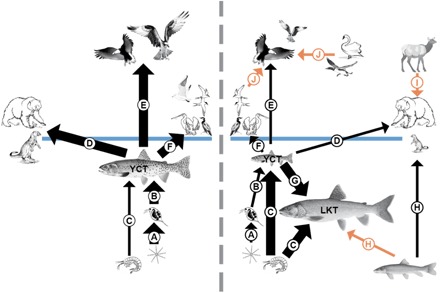
The conceptualization (nonmathematical) emphases are the cutthroat trout (YCT) and other components known (black arrows) or hypothesized (orange arrows) to be affected by the introduction of lake trout (LKT). Thick arrows indicate that the consumption of that food item is high by predator or herbivore, and thin arrows indicate that the consumption is low, within the aquatic (below the blue line) and across terrestrial (above the blue line) ecosystems. Letters represent consumption of (A) phytoplankton, (B) zooplankton, (C) amphipods, (D to G) cutthroat trout, (H) longnose suckers, (I) elk calves, and (J) common loon, trumpeter swan, American white pelican, double-crested cormorant, and Caspian tern. Organisms are not drawn to scale, although the size of the fish, osprey, and otter depicts observed shifts in abundance between periods. California gulls were present before lake trout invasion but no longer nest on Yellowstone Lake.
Using data spanning more than four decades (1972–2017), we present an observational study wherein the relationships among cutthroat trout and several closely linked components of the aquatic and terrestrial food webs are explored. We describe a multitude of pathways from introduced lake trout to both the Yellowstone Lake interaction web (within-system effects) and the surrounding terrestrial environment (across-system effects). We predicted that the cascading effects of the top predator would be strong within the aquatic ecosystem but would attenuate (weaken) across the aquatic-terrestrial ecosystem boundary as direct trophic interaction strengths decline (14). Because our study area is largely unaltered by humans and most of the natural functional linkages among native taxa remain intact, the lake trout invasion provided a unique opportunity to examine these predictions by comparing the magnitude of cascading effects among trophic levels both within the Yellowstone Lake aquatic ecosystem and across the aquatic-terrestrial ecosystem boundary in this pristine watershed.
RESULTS
Lake trout–induced cutthroat trout decline
The detection of nonnative lake trout in Yellowstone Lake in 1994 prompted the National Park Service to initiate a gillnetting program to suppress population growth and conserve the native cutthroat trout. Estimated abundance of age 2 and older lake trout increased from 125,000 fish in 1998 (15) to 953,000 fish in 2012, despite the gillnetting of 1.1 million fish during this period (Fig. 2A). The slope of average estimated abundance indicated that the lake trout population grew by 92,600 fish/year (three-piece segmented regression, R2 = 0.99, P < 0.01, df = 14) during 2005–2012 but was not significantly different from zero during 2013–2017 (β1 = −14,900, P = 0.09) due to a surge in suppression netting to an average of >71,000 effort units (effort unit = 100-m net per night) annually during 2012–2017 (fig. S2). Overall, >2.8 million lake trout were killed by gillnetting during 1995–2017. During 2012–2017 alone, the average total biomass of lake trout carcasses returned to deep (>65 m) areas of Yellowstone Lake exceeded 140,000 kg annually (fig. S2).
Fig. 2. Response of planktivorous and benthivorous fish to invasion of Yellowstone Lake by an apex predator.
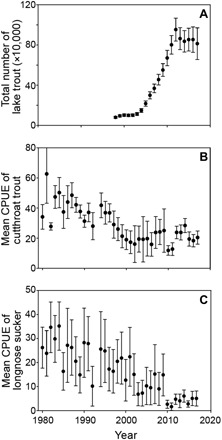
(A) Lake trout abundance estimated by a statistical catch-at-age model markedly increased between 1998 and 2012 when suppression gillnetting effort became great enough to curtail further population growth, (B) long-term decline in the average catch per unit effort (CPUE) of cutthroat trout, and (C) long-term decline in average CPUE of longnose suckers during annual fish population netting assessments on Yellowstone Lake, with 95% confidence intervals, from 1980 to 2017.
During the early stages of lake trout expansion, the lake trout consumption of cutthroat trout was substantial. The estimated 125,000 lake trout present in 1998 likely consumed 3 to 4 million cutthroat trout that year (15, 16). Predation by lake trout forced a precipitous, lake-wide decline in cutthroat trout during 1980–2003 (two-piece segmented regression, R2 = 0.69, β1 = −1.2, P < 0.01, df = 34; Fig. 2B) (17) and a shift in population size structure from dominance by small individuals (100 to 280 mm) to dominance by large individuals (400 to 600+ mm; fig. S3A). Concurrent with the decline in cutthroat trout was a steady, long-term decline in the introduced longnose suckers (single linear regression, R2 = 0.73, β1 = −0.73, P < 0.01, df = 36; Fig. 2C and fig. S3B), potentially also due to lake trout predation.
Interactions within the aquatic food web
The introduction of lake trout added a fourth trophic level resulting in cascading interactions within the aquatic food web of Yellowstone Lake, including a shift in cutthroat trout prey consumption and the biomass and individual lengths of zooplankton (Table 1). When cutthroat trout were abundant in 1989, they primarily consumed larger-bodied cladocerans, which comprised 80% of their diet (Fig. 1) (15). After cutthroat trout declined in 2011, cladocerans comprised only 11% of their diet, and the remaining fish more frequently consumed amphipods. The cutthroat trout diet was only 8% amphipods in 1989 but increased to 79% by 2011, likely due to increased amphipod availability after the cutthroat trout population declined (16, 17). After cutthroat trout declined and predation on large zooplankton was reduced, the biomass of smaller-bodied copepods was lower [analysis of variance (ANOVA), P < 0.001, df = 3; Fig. 3A], and the biomass of larger-bodied zooplankton was higher (P = 0.0008, df = 3; Fig. 3B) within the lake pelagic zone. As predicted by trophic cascade theory, the average individual length of large-bodied zooplankton also became longer (Hesperodiaptomus shoshone, P < 0.0001, df = 3; Daphnia pulicaria, P < 0.001, df = 3; fig. S4, A and B). Only a slight increase was noted during this period in the length of Leptodiaptomus ashlandi (P = 0.014, df = 3; fig. S4C), a small-bodied copepod that is generally too small to be effectively consumed by cutthroat trout (18).
Table 1. The effect size of predatory lake trout on aquatic and terrestrial ecosystems.
The response variables for the past (before lake trout population growth) and present (after) were compared using a log response ratio of means [log10(Xpresent/Xpast)]. Lake trout enhanced the variable when the ratio was positive and reduced the variable when the ratio was negative; ratios near zero indicate that the variable was similar between the two time periods. CI, confidence interval.
| Variable | Past* | Present | Log ratio | ||||
| Year(s) | Mean | 95% CI | Year(s) | Mean | 95% CI | ||
| Fish population responses | |||||||
| Lake trout abundance (total number × 1000) | 1998–2002 | 95.3 | 2.8 | 2013–2017 | 843.9 | 6.0 | 0.95 |
| Cutthroat trout abundance (CPUE) | 1980–1984 | 44.6 | 4.2 | 2013–2017 | 22.1 | 1.2 | −0.31 |
| Longnose sucker abundance (CPUE) | 1980–1984 | 30.0 | 1.5 | 2013–2017 | 4.6 | 0.4 | −0.82 |
| Cutthroat trout spawners (mean number observed) | 1989–1993 | 52.8 | 7.1 | 2013–2017 | 5.3 | 0.5 | −1.00 |
| Aquatic ecosystem effects | |||||||
| Small zooplankton biomass (mg/liter) | 1977–1980 | 67.8 | 27.3 | 2017 | 30.3 | 17.0 | −0.35 |
| Large zooplankton biomass (mg/liter) | 1977–1980 | 8.5 | 5.0 | 2017 | 102.9 | 24.0 | 1.08 |
| Chlorophyll a concentration (μg/liter) | 1972 | 2.2 | 0.3 | 2017 | 0.5 | 0.2 | −0.64 |
| Large zooplankton [H. shoshone length (mm)] | 1977–1980 | 2.4 | 0.2 | 2017 | 4.4 | 0.2 | 0.26 |
| Large zooplankton [D. pulicaria length (mm)] | 1977–1980 | 1.9 | 0.1 | 2017 | 2.7 | 0.0 | 0.16 |
| Small zooplankton [L. ashlandi length (mm)] | 1977–1980 | 0.7 | 0.0 | 2017 | 0.9 | 0.1 | 0.12 |
| Secchi disk depth (m)† | 1976 | 9.9 | 0.1 | 2005 | 11.4 | 0.1 | 0.06 |
| Terrestrial ecosystem effects | |||||||
| Bear occurrence on spawning streams (proportion of visits) | 1989–1993 | 0.5 | 0.0 | 2013–2017 | 0.2 | 0.0 | −0.30 |
| River otter use of cutthroat trout (prevalence in scat) | 2002–2003 | 0.73 | 0.06 | 2006–2008 | 0.53 | 0.14 | −0.14 |
| Osprey nest count (total number) | 1987–1991 | 37.6 | 2.2 | 2013–2017 | 3.2 | 0.1 | −1.07 |
| Osprey nest success (proportion that fledged) | 1987–1991 | 58.8 | 3.4 | 2013–2017 | 31.4 | 7.1 | −0.27 |
| Bald eagle nest count (total number) | 1985–1989 | 6.4 | 0.3 | 2013-2017 | 7.6 | 0.7 | 0.07 |
| Bald eagle nest success (proportion that fledged) | 1985–1989 | 56.0 | 6.1 | 2013–2017 | 70.4 | 3.6 | 0.10 |
*All data were collected before significant lake trout population growth with the exception of river otters, which were collected later during a period of lake trout population growth and cutthroat trout decline (24).
†Secchi depth was calculated using the model in fig. S6 based on 15 August (Julian day 227) 1976 and 2005.
Fig. 3. Changes in plankton due to decline of planktivorous cutthroat trout.
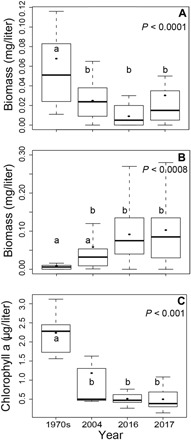
Between 1977–1980 (before lake trout introduction), 2004 (10 years after lake trout were found), and 2016–2017 (>20 years later), the biomass of (A) small zooplankton (L. ashlandi, Diacyclops, and nauplii) declined and (B) large zooplankton (D. pulicaria, D. schødleri, and H. shoshone) increased. (C) Chlorophyll a concentration, an indicator of phytoplankton biomass, was more than two times higher in 1972 (17) than in 2004 (16) or 2016–2017. Letters (a, b) indicate differences (P < 0.05) among means.
The shift in the zooplankton assemblage to dominance by larger-bodied cladocerans following the cutthroat trout decline resulted in lower phytoplankton biomass and increased water clarity. Chlorophyll a, a measure of phytoplankton biomass, was more than two times higher in 1972 (19) before lake trout introduction than it was in 2004 (20) or 2016–2017 (P < 0.001, df = 3; Fig. 3C and fig. S5). Secchi disk depths in the western region of Yellowstone Lake (West Thumb; fig. S1) during summer stratification were 1.6 m deeper in 2005 than in 1976 (t = 2.1, P = 0.05) and increased through the summer (t = 3.7, P = 0.001, R2 = 0.45). Secchi disk depths were shallower during 2016–2017 after lake trout removal efforts increased substantially (fig. S6). Recent Secchi depth variation may also be driven by the decomposition of >100,000 kg of lake trout carcasses annually placed in deep regions of the lake by the gillnetting program (fig. S2). Since water clarity is important in influencing heating of the water column, the thermal structure of Yellowstone Lake may have been altered by lake trout and, potentially, ongoing actions taken to suppress them. Although the thermal structure is typically unstable with a weak and variable thermocline (fig. S7), we have witnessed a 0.45°C per decade increase in surface water temperatures of Yellowstone Lake during the stratified period (15 July to 15 September), 1976–2018 (t = −2.4, P = 0.01, R2 = 0.17; fig. S8).
The decline of cutthroat trout in the lake extended into connected tributary streams. The lake trout–induced decline resulted in fewer spawning cutthroat trout returning to tributary streams (Fig. 4A) and, as a result, reduced transport of nutrients [e.g., ammonium (NH4+)] from Yellowstone Lake into the tributaries (18). We estimated that stream microbes removed 10 times more NH4+ excreted by cutthroat trout before the invasion of lake trout as compared to the mid-2000s. Therefore, transported NH4+ from spawning cutthroat trout was likely an integral part of nitrogen cycling in tributary streams in the past. We estimated that lake trout had a larger effect on nitrogen cycling within adjacent tributaries than within the lake itself because the spawning behavior of cutthroat trout concentrated them in tributaries, thus increasing the effect (18).
Fig. 4. Decline of spawning cutthroat trout and response by bears, eagles, and osprey.
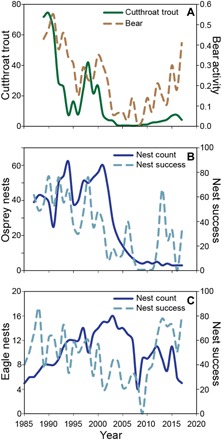
(A) Mean number of spawning adult cutthroat trout observed (solid line) and proportion of visits where activity by black and grizzly bears was found (dashed) during weekly spawning visual surveys of 9 to 11 tributaries located along the western side of Yellowstone Lake (1989–2017). (B) Number of nests (solid line) and nest success (dashed) during May to August 1987–2017 for osprey and (C) during April to June 1985–2017 for bald eagles, within approximately 1 km of the Yellowstone Lake shoreline, connected tributaries, and forested islands.
Effects on bears and otters
Lake trout invasion also caused substantial cascading effects that spun off the main aquatic interaction chain (knock-on effects) (21) and extended to terrestrial animals, such as grizzly and black bears, because spawning cutthroat trout were an important high-energy food for them (Fig. 1). Evidence of bear activity occurred at 46% of spawning stream surveys during 1989–1993 when spawning cutthroat trout were abundant at the beginning of the lake trout invasion (Fig. 4A and Table 1). However, concurrent with the subsequent decline in spawning cutthroat trout, evidence of bear activity also declined (Prais-Winsten time series, adjusted R2 = 0.86; table S1). No bear activity was found on surveyed spawning streams in 2008, 2009, or 2011. When compared to estimates obtained from 1997 to 2000, the number of grizzly bears visiting spawning streams a decade later (2007–2009) decreased by 63%, and the number of black bears decreased by 64 to 84% (22). The estimated number of spawning cutthroat trout consumed by grizzly bears declined from 20,910 in the late 1980s (23) to 2266 in the late 1990s (24) to only 302 in the late 2000s (25). Grizzly and black bears are opportunistic feeders with a flexible diet; consequently, they consumed other foods available in the Yellowstone Lake area when cutthroat trout abundance was low. Despite the cutthroat trout decline, grizzly bear abundance throughout Yellowstone National Park has remained stable.
River otters are a semi-aquatic predator that also relied on cutthroat trout as a primary source of food (Fig. 1). During 2002–2003, following a substantial decline in cutthroat trout, otters followed the movements of spawning cutthroat trout and were active on spawning streams. Cutthroat trout occurred in 73% of otter scat collected at 87 otter latrine sites during this period (26). Temporal changes in otter latrine activity occurred in response to further declines in spawning cutthroat trout. The visual counts of spawning cutthroat trout during 2006–2008 were the lowest ever recorded (table S2). By then, otter activity at latrine sites decreased and the prevalence of cutthroat trout in otter scat had declined to 53% (26). Otters supplemented their diet with alternative prey, including longnose suckers and amphibians, which are not likely comparable replacement foods. Abundance was estimated as 1 otter per 13.4-km shoreline in 2008, which is among the lowest reported for a river otter population. Estimates do not exist for periods before the cutthroat trout decline.
Displacement of avian fish predators
Yellowstone Lake supports a diversity of bird life including numerous species that preyed on fish. Densities of nesting ospreys declined concurrent with declines in prey fish (e.g., Prais-Winsten time series, longnose sucker, adjusted R2 = 0.83; table S1) from an average of 38 during 1987–1991 to 11 during 2004–2008 (27) and only 3 during 2013–2017 (Fig. 4B and Table 1). Nesting success during 1987–1991 averaged 59% but declined to zero during 2008–2011 when no young ospreys were fledged from Yellowstone Lake nests (spawning cutthroat trout, adjusted R2 = 0.84). An average of 31% of nests successfully fledged young during 2013–2017 (Fig. 4B). Although a few osprey nests remained on Yellowstone Lake, they were not observed foraging for cutthroat trout (28). Ospreys are obligate piscivores that do not switch to alternative food sources in the absence of fish. The few ospreys that remain have been leaving Yellowstone Lake to forage in large lakes of the upper Snake River (28), a distance of 8 to 10 km, where prey fish are more abundant.
During the 1960s–1970s, a period when pesticides affected bald eagles, there were typically four to six eagle nests on Yellowstone Lake. The nest count increased to an average of 11 during 2004–2008 (27) but then declined to 8 nests by 2013–2017 (Fig. 4C and Table 1). This variation in bald eagle nests was related to spawning cutthroat trout (Prais-Winsten time series, adjusted R2 = 0.91; table S1) and cutthroat trout and longnose sucker abundances within the lake (adjusted R2 = 0.90 and 0.84, respectively). There was also a steady long-term decline in eagle nest productivity over two decades concurrent with the lake-wide decline in prey fish. During 1985–1989, 56% of eagle nests on Yellowstone Lake successfully fledged young; however, nest success declined to zero in 2009 when prey fish abundance was low (Fig. 4C).
The number of American white pelicans (Pelecanus erythrorhynchos), double-crested cormorants (Phalacrocorax auritus), California gulls (Larus californicus), and Caspian terns (Hydroprogne caspia) fledged from colonies on Yellowstone Lake (Molly Islands; fig. S1) has been highly variable but has declined overall since the 1990s (29). Although the loss of prey fish is thought to be a factor influencing the colonial birds, because they are ground nesters on barren islands, the nesting success of these species was also strongly influenced by environmental factors such as lake surface levels (30). Flooding of the islands often results in total nesting failures.
DISCUSSION
We have documented changes to multiple aquatic and terrestrial trophic levels across a large landscape with relatively minimal confounding factors related to anthropogenic disturbance. Because the watershed of Yellowstone Lake is largely unaltered, we ascribe these trophic shifts to lake trout. Within the aquatic ecosystem, cascading effects of this introduced top predator remained strong and scaled predictably through four trophic levels following collapse of the planktivorous cutthroat trout. Large zooplankton biomass increased, small zooplankton biomass declined, and chlorophyll a concentrations declined (log10 ratios of 1.08, −0.35, and −0.64, respectively; Table 1). The predatory lake trout also indirectly altered nitrogen dynamics and transport to tributary streams, a nonconsumptive effect (31) through reduced NH4+ excretion by migratory cutthroat trout which had been concentrated in the streams (18).
Although trophic cascade theory predicted that effects would dissipate across ecosystem boundaries (14, 32), we instead found that effects remained strong across the aquatic-terrestrial ecosystem boundary, particularly for ospreys and bears (log10 ratios of −1.07 and −0.30, respectively; Table 1). Ospreys are obligate piscivores and, hence, were not able to switch prey in the absence of cutthroat trout, and their population declined in the riparian habitats around Yellowstone Lake. Grizzly and black bear frequency of occurrence on spawning tributaries and the use of cutthroat trout as a food resource were greatly reduced following the lake trout invasion. However, this was localized displacement, and their populations were not otherwise affected because only bears with home ranges neighboring Yellowstone Lake lost spawning cutthroat trout as a food resource. Since bears are omnivore generalists, they could make use of other foods.
Spatial and temporal variabilities in resources and consumers are considered to have a strong influence on the strength of cascading trophic interactions (33). Some resource subsidies are also reciprocal, generating feedbacks between coupled aquatic and terrestrial ecosystems (34, 35). Migratory fish, in particular, are considered important pulsed subsidies in riverine systems (36). The adult cutthroat trout spawning in tributaries during spring and juvenile cutthroat trout occurring in shallow waters within Yellowstone Lake during summer and autumn provided important pulsed subsidies for avian and terrestrial consumers during critical life history periods (e.g., weight gain following hibernation by bears and nesting and fledging of chicks by avian predators). Fish abundance, driven by a host of environmental factors, is naturally variable among years. We suggest that the strong behavioral response by some native terrestrial predators to seek alternative foods and/or be displaced from the ecosystem may have been heightened by the natural spatial and temporal variation in cutthroat trout availability. Similarly, anadromous fishes spawning in freshwater rivers and streams serve as prey for numerous wildlife species, affecting the biology of their populations and productivity of terrestrial ecosystems throughout the Pacific coast of North America (37). Highly mobile terrestrial predators respond and seek alternatives when fish prey resources diminish.
Trout introduced to mountain lakes elsewhere in the western United States have resulted in cascading interactions across aquatic-terrestrial ecosystem boundaries. The trophic levels involved, however, have typically included aquatic insects [e.g., mayflies (Ephemeroptera)] that we did not monitor or document changes for in Yellowstone Lake or tributary spawning streams. Introduced trout prey upon insect nymphs and reduce adult emergence from mountain lakes. The reduction of emergent aquatic insects affected an avian predator [rosy finch (Leucosticte tephrocotis dawsoni)] during a time when they were feeding their young (38). Experimentally, the introduction of cutthroat trout into small ponds has led to a trophic cascade by forcing a diet shift in a mesopredatory fish that increased the biomass and average size of insects emerging into the terrestrial system (39). In addition, fish presence in ponds has been shown to have effects that reverberate further into the terrestrial ecosystem by reducing predatory dragonfly abundances and increasing pollinating insects, resulting in an increase in pollination of plants (40). In small streams, introduced trout caused indirect effects that extended across the aquatic-terrestrial boundary by reducing emerging adult insects and depressing riparian spider abundance (35). Because cutthroat trout consume insects, it is possible that lake trout indirectly affected (increased) aquatic insect emergence through reduced predation by cutthroat trout when their abundance was low. The impact that this may have had on other aquatic and terrestrial trophic levels and their interactions across ecosystems at Yellowstone Lake is uncertain.
Hypothesized linkages to elk
The lake trout–induced decline in spawning cutthroat trout displaced bears from tributary streams, but indirect effects on alternative prey in the Yellowstone Lake area are less understood. Following the cutthroat trout decline within spawning tributaries, grizzly and black bears fed less upon them and shifted their diet to other foods, including elk (Cervus elaphus) calves (Fig. 1) (25). Each spring, thousands of elk that winter on lands at lower elevations in the Greater Yellowstone Ecosystem migrate to the interior of Yellowstone National Park. Elk calves born in the Yellowstone Lake area are vulnerable to predation, especially during the first few weeks after birth. Bear predation rates on elk calves may have increased after the cutthroat trout decline (41). By 2007–2009, grizzly bears had shifted to alternative prey, and the proportion of cutthroat trout in their diet had declined to 0% (42). Elk then accounted for 84% of all ungulates consumed by bears in the Yellowstone Lake area (25), suggesting that lake trout had some level of indirect negative impact on migratory elk using this area when spawning cutthroat trout were rare (41).
The reintroduction of gray wolves (Canis lupus) to Yellowstone National Park began in 1995, the year following the discovery of lake trout in Yellowstone Lake. The subsequent expansion of wolves across the park resulted in a terrestrial trophic cascade involving wolves, elk, cottonwood (Populus spp.), and willow (Salix spp.) due to wolves preying upon elk and altering their behavior (43). Although elk calves are preyed upon by wolves and other apex predators, including bears, coyotes (C. latrans), and cougars (Puma concolor), we do not attribute observed shifts in food resource use by bears and other consumers in the Yellowstone Lake area to interactions with wolves or other carnivores. Grizzly bears prey upon elk calves primarily during the first few days after birth, whereas wolves and other predators prey upon more ungulate calves later in the summer and winter, limiting their competition (44).
Bald eagle shift to alternative prey
At Glacier National Park, bald eagles were locally displaced after exotic crustaceans (Mysis relecta) invaded nearby Flathead Lake and indirectly forced a collapse of the introduced kokanee salmon (Oncorhynchus nerka) that the eagles preyed upon during autumn spawning migrations in McDonald Creek, a tributary of the Middle Fork Flathead River (8, 9). In contrast, we witnessed a slight increase in the number of bald eagle nests during the period of cutthroat trout decline in the 2000s, with a concurrent loss of nesting success to zero by 2009 (Fig. 4C). Bald eagles are opportunistic feeders and increased consumption of alternative prey, including scavenging carnivore-provided carcasses or winterkill. Bald eagles have been observed more frequently preying on common loons (Gavia immer) and trumpeter swan cygnets (Cygnus buccinators), which have declined recently in Yellowstone National Park (Fig. 1) (29). Reasons for the declines are unclear but may include the reduced availability of cutthroat trout as a food source for common loons and increased predation on loon chicks and trumpeter swan cygnets by bald eagles. We have also observed bald eagles preying on young white pelicans and double-crested cormorants on the Molly Islands, possibly contributing to their declines. The number of bald eagle nests has remained low and variable, but nesting success rebounded to an average of 70% during 2013–2017, likely due to switching prey in the absence of cutthroat trout. Magnitude of effects (log10 ratio) for bald eagle nest counts and success was only 0.07 and 0.10, respectively (Table 1), demonstrating that they were not greatly affected by the loss of cutthroat trout. This is similar to what occurred with bald eagles in the Aleutian archipelago that underwent a radical shift in diet in response to the collapse of sea otters (Enhydra lutris) during the 1990s, but with no associated influence on the abundance or reproductive success (45).
Other ecological drivers
Other landscape-level drivers of ecological change have emerged during the period of our study, which could have influenced the trophic interactions we have described. These include climate-driven shifts in nutrient deposition, stream flows and water temperatures, and, potentially, increased frequency of wildfires.
Plankton assemblages may have been influenced by altered nutrient cycling in Yellowstone Lake. Atmospheric deposition of nitrogen was ~9 μg N m−2 hour−1 and varied little during the study period (18), similar to lakes in the northeastern United States where total nitrogen concentrations have generally decreased since 1990 (46). The expansive wildfires of 1988 likely also moved terrestrial nutrients into Yellowstone Lake (47), fertilizing algae and microbes (bottom-up effects). However, Secchi disk depths, which are correlated with phytoplankton biomass (18), did not become shallower after the 1988 fires, indicating that any nutrient inputs were diluted by the large volume of the lake. In addition, the biomass of lake trout carcasses deposited by the gillnetting program returned existing nutrients to deep (>65 m) regions of the lake. In 2017 alone, 400,000 lake trout were deposited, returning ~8.5 mg N m−2. We would expect phytoplankton biomass to increase when nutrients are added to the lake; however, we observed a decrease concurrent with the lake trout invasion. Therefore, our results strongly suggest that trophic interactions were more important than nutrient dynamics in forcing shifts in plankton communities (Fig. 3 and fig. S4) and confirm earlier evidence that top-down control is strong in water (48, 49), especially in a low-diversity system such as Yellowstone Lake where great influence was exerted by a single species.
Climate change is predicted to alter stream flows and water temperatures in the western United States (50). Surface water temperatures of Yellowstone Lake have already increased at a rate of 0.45°C per decade during 1976–2018 (fig. S8). However, the thermal structure of Yellowstone Lake (e.g., isotherm depths), before lake trout invasion and currently, are typically unstable with a weak and variable thermocline that is established at 10 to 12 m (fig. S7). In spawning streams, climate shifts are suspected to influence cutthroat trout recruitment (51). During drought years, low water levels can cause mortality of juvenile cutthroat trout and/or restrict emigration to Yellowstone Lake (17). To estimate the degree to which drought may have caused the cutthroat trout decline, we have previously compared the discharge of the Yellowstone Lake outlet with indices of cutthroat trout abundance over time (20). We found that cutthroat trout abundance decreased during both wet and dry years. Although water temperature and flows may have strong influences in some years, we attribute the long-term (decadal-scale) loss of cutthroat trout and altered trophic structure of this system largely to lake trout predation.
Cutthroat trout and their terrestrial consumers have evolved over thousands of years with wildfire as a natural occurrence. Following the 1988 wildfires, Yellowstone’s ecosystems recovered rapidly with little human intervention (52). Climate has continued to warm since 1988, however, and the frequency of large wildfires has increased throughout the Rocky Mountain region. We do not attribute the significant loss of cutthroat trout to wildfires because recruitment of young cutthroat trout from spawning streams, the process most likely to be affected by wildfire, remained strong for more than a decade following 1988 (fig. S3). Cutthroat trout recruitment then severely declined concurrent with lake trout population growth. In addition, if wildfire were to have negatively affected bears, bald eagles, ospreys, or other cutthroat trout consumers, then those impacts would have also been documented elsewhere, outside of the Yellowstone Lake ecosystem, which has not been the case. These wildlife populations have remained stable or are increasing across Yellowstone National Park.
CONCLUSIONS
Our study illustrates the potential impact of a single invasive predatory species on otherwise pristine ecosystems. In addition to causing cascading interactions within Yellowstone Lake and tributary streams, lake trout indirectly forced declines, displacement, and/or prey shifting by bald eagles, ospreys, river otters, and bears. Because of the long-distance migrations of the spawning cutthroat trout (53), effects of lake trout on the ecology of these ecosystems likely extend far beyond the Yellowstone Lake shoreline and into the extremely remote, largely unmonitored reaches of the upper Yellowstone River and tributary drainages in the Thorofare region of the Bridger-Teton wilderness, Wyoming (fig. S1). During the late 2000s, cutthroat trout abundance within Yellowstone Lake was at its lowest, and impacts within and across ecosystems were most severe. To reverse these effects, concepts of cascading trophic interactions were adopted into the 2010 adaptive management plan for the restoration of Yellowstone Lake and connected terrestrial ecosystems (54). During 2012–2017, management actions were greatly increased, including a surge in suppression gillnetting effort (fig. S2) that resulted in a positive response by cutthroat trout [stabilized at catch-per-unit-effort (CPUE) of ~20; Fig. 2B]. Juveniles are again recruiting to the cutthroat trout population. After being absent for many years, spawning adult cutthroat trout are returning to some of the smaller tributaries, and bear use of these streams has increased as a result (Fig. 4A). Ospreys, however, have not yet responded to the recent increases in cutthroat trout prey (Fig. 4B). The outcome of restoration efforts to trophic levels within and across ecosystems in the Yellowstone Lake watershed remains uncertain. During the period of our study, both cutthroat trout and lake trout shifted their diets to a higher proportion of amphipods (Fig. 1). A better understanding of recruitment of both fish species in relation to amphipod and other prey abundance is required to inform future management actions on the lake. In addition, hypothesized linkages between bald eagles, grizzly bears, and their alternative prey species need to be more deeply explored. Lake trout suppression will be maintained in the interim, however, to further reduce their abundance, thereby allowing the potential for further cutthroat trout recovery to a level where they regain their ecological importance and once again support natural processes and biodiversity in Yellowstone National Park.
MATERIALS AND METHODS
Lake trout suppression netting
Up to six large boats were used to capture lake trout with sinking gill nets during late-May to mid-October 1995–2017 (55). Suppression netting consisted of small-mesh (25 to 38 mm) and large-mesh (44 to 76 mm) bar measure gill nets targeting lake trout at depths typically greater than 20 m to reduce cutthroat trout bycatch. Nets were set shallower than 20 m at known spawning locations during peak spawning activity in autumn. Gill net soak time was typically three to four nights. Annual effort (effort unit = 100-m net per night) was 249 units in 1995 and increased to 90,349 units in 2017 (fig. S2). Trap nets were also used during 2010–2013 to target large lake trout (i.e., >450 mm) (55). Eight to 10 trap nets were deployed at fixed locations throughout Yellowstone Lake each year. The netted lake trout were cut to puncture air bladders and then returned to deep (>65 m) regions of Yellowstone Lake.
Lake trout estimated abundance
Statistical catch-at-age analysis (SCA) was used to estimate lake trout abundance and biomass through time (55, 56). The SCA model was estimated for lake trout age 2 and older (1998–2017). The model was fit to annual catch and age composition for suppression netting (1998–2017) and assessment netting (2010–2017; see below). Lake trout catch from trap nets and gill nets was pooled. Total effort in suppression netting was obtained for each year as the pooled catch among gill nets and trap nets divided by gill net CPUE. Instantaneous natural mortality was assumed to be 0.25 for age 2 and 0.16 for age 3 and older lake trout. Fishery selectivity was modeled as a logistic function of age for both suppression and assessment netting. Time-varying catchability was modeled with random deviations around a mean value for each netting type. For suppression netting, a different mean value was estimated for catchability in 1998–2000 and 2001–2017 to account for differences in fishery operation. The model was fit to catch and age composition from suppression netting and assessment netting datasets. Fits to observed catch in the suppression netting and CPUE in the assessment netting were modeled as lognormally distributed. Age compositions were assumed to follow multinomial distributions with a maximum effective sample size of 200 aged fish. Likelihood components for fits to suppression netting and assessment netting data sources were weighted by estimated SDs and effective sample sizes with additional weights not specified. Thus, suppression netting and assessment netting datasets influenced model results equally for years with both types of data. Wald approximations were used to compute approximate 95% confidence intervals (CIs) for abundance using asymptotic SDs produced by AD Model Builder (57).
Fish population netting assessments
Gill nets were used to assess the cutthroat trout and longnose sucker populations at 11 sites throughout the lake in mid-September 1980–2009 (fall netting assessment; fig. S1). At each site, five sinking experimental gill nets were set overnight perpendicular to shore. Nets were set 100 m apart with the near-shore end about 1.5 m deep. Nets were 1.5 m in height and 38 m length, consisting of 7.6-m panels of 19- to 51-mm bar measure. In 2010, a new protocol was developed and implemented through 2017 to encompass monitoring of lake trout. Twenty-four sites throughout the lake were sampled during early August with a total of six experimental gill nets per site (distribution netting; fig. S1). At each site, a small-mesh and large-mesh sinking gill net were set overnight at each of three depth strata [epilimnion (3 to 10 m), metalimnion (10 to 30 m), and hypolimnion (>40 m)]. Small-mesh gill nets were 2 m in height and 76 m length, consisting of 13.7-m panels of 19- to 51-mm bar measure. Large-mesh gillnets were 3.3 m in height and 68.6 m length, consisting of 13.7-m panels of 57- to 89-mm bar measure. Gill nets were set perpendicular to shore and nets within a stratum were set parallel 100 m apart. All fish caught in assessment netting were measured for total length, and otoliths were sampled from 10 fish per 1 cm length group for age determination.
Zooplankton monitoring
To measure zooplankton density, biomass, and size during 1977–1980, 2004, and 2016–2017, two samples were collected on each date during the ice-free season at four sites (Main Basin, West Thumb, South Arm, and Southeast Arm; fig. S1) with 20-m vertical hauls using nets with 80-μm mesh. Zooplankton samples before lake trout invasion (n = 20) were collected during 1977–1980 and were similar among years (ANOVA, P > 0.05); as a result, samples were combined for density, biomass, and size estimates (20). In 2004, 2016, and 2017, zooplankton samples were collected on 9 dates (n = 72), 10 dates (n = 80), and 9 dates (n = 72), respectively. Zooplankton samples were preserved in cold-sugared formalin in 2004 and prior. Samples were preserved with cold ethanol in 2016 and 2017. Zooplankton were enumerated and measured under a dissecting microscope using Sedgwick-Rafter cells. Zooplankton biomass was calculated using published length-mass regressions (58).
Phytoplankton monitoring
Phytoplankton biomass was estimated using chlorophyll a by collecting ~125 ml of lake water in West Thumb of Yellowstone Lake (fig. S1) from 5-m depth in 2004, 1.2 liters of water from 5-, 10-, and 15-m depths in 2005 (18), and 1.2 liters of water from 5- and 15-m depths during 2016–2017. Chlorophyll a was collected on 7 dates (n = 56), 3 dates (n = 36), 11 dates (n = 88), and 9 dates (n = 72) in 2004, 2005, 2016, and 2017, respectively. To concentrate phytoplankton, water was filtered through 25-mm PALL type A/E glass fiber filters. Chlorophyll a was extracted by incubating filters in 90% ethanol buffered with MgCO3 overnight, and concentration was measured using the acid method with a pheopigment correction (58) on a fluorometer (Turner Designs 700, Sunnyvale, CA). A secondary solid standard was calibrated using a primary chlorophyll a commercial standard of Anacystis nidulans (Sigma-Aldrich). Chlorophyll a before lake trout invasion was measured to 20-m depth in West Thumb during 1972 by acetone extraction and a spectrophotometer (19). Differences in extraction methods were corrected for (58).
Lake physical conditions
Light transmission was measured using a Secchi disk during ice-free seasons from 2005 (20) to 2017 in West Thumb of Yellowstone Lake (fig. S1). Light transmission before lake trout invasion (ice-free seasons during 1976–1991) was collected at the same site (47). The thermal structures of Yellowstone Lake (e.g., isotherm depths) were measured in the West Thumb in 1956 and 1959 using a bathythermograph and in 1996, 2006, 2009, 2015, and 2017 using a multiparameter sonde (Hydrolab Surveyor; fig. S7). Temperature was measured at the lake’s surface in four areas of Yellowstone Lake between 1976 and 2018 (fig. S8).
Spawning cutthroat trout and bear visual surveys
Visual surveys for spawning cutthroat trout and bear activity were conducted annually 1989–2017 on 9 to 11 tributaries located along the western side of Yellowstone Lake between Lake and Grant (fig. S1) (17). Spawning reaches were delineated on each tributary, and the standardized reaches were walked in an upstream direction once each week from May to July. The observed cutthroat trout were counted, and the activity by black bears and grizzly bears was estimated by noting the presence of scat, parts of consumed trout, fresh tracks, and/or bear sightings.
Bird surveys
All forested areas supporting mature trees up to 1 km from the Yellowstone Lake shoreline, approximately 1 km from the connected tributaries, and all forested islands were surveyed for evidence of nesting bald eagles and ospreys two to three times during the breeding season (April to June 1985–2017 for bald eagles and May to August 1987–2017 for ospreys) using a fixed-wing Super Cub airplane (27). In addition to aerial surveys, nests occurring along the roads were checked from the ground. All nests in which eggs were laid or an adult was observed in incubation or brooding posture were checked two to three times during each breeding season until the nesting attempt failed or nestlings fledged. For each breeding season, the number of breeding pairs and nesting success was determined for bald eagle and osprey populations. Breeding pairs refers to the number of paired bald eagles or ospreys that laid eggs per breeding season, and nesting success refers to the proportion of breeding pairs that raised at least one young to approximately 80% of fledging age per breeding season regardless of the number of breeding attempts made for that pair (27). Colonial waterbirds including American white pelicans, double-crested cormorants, California gulls, and Caspian terns nest on the Molly Islands in the Southeast Arm of Yellowstone Lake (fig. S1). Aerial photographs of the islands were taken throughout the breeding season each year to count nests and young and estimate nesting success for each species (29).
Statistical analyses
Temporal trends for lake trout abundance estimates and annual mean CPUE for longnose suckers and cutthroat trout (CPUE and mean spawner counts) were assessed using linear regression to estimate increases or decreases in these fish populations over time. Annual mean values were used rather than individual observations for longnose suckers and cutthroat trout CPUE because of the implementation of a different sampling design in 2010 (see above). The visual examination of temporal patterns indicated that different slopes were evident for different time periods. Therefore, segmented regressions were fit using the R package segmented (59, 60) and compared to the fit from a single regression line using Akaike’s information criterion (AIC). Temporal autocorrelation in residuals was examined using a Durbin-Watson (DW) test in the lmtest package (61). A three-piece segmented regression (AIC = 195.7) fit the lake trout abundance time series (Fig. 2A) better than a single regression line (AIC = 248.4). The segmented regression indicated break points at year values equal to 2004.6 (95% CI = 2003.9 to 2005.2) and 2012.1 (95% CI = 2011.4 to 2012.6). Temporal autocorrelation was present in residuals for the lake trout regression (DW = 1.4, P < 0.01). However, we believed our slope estimate to be a reasonable indicator of the rate of change in abundance for 2005–2012, and the conclusion that abundance increased during this period would not change if we were able to correct for dependencies in the data. For cutthroat trout CPUE (Fig. 2B), a two-piece segmented regression (AIC = 260.6) described temporal variation better than a single regression line (AIC = 265.3), with a break point estimated at 2003.5 (95% CI = 1996.5 to 2010.5). For longnose sucker CPUE (Fig. 2C), a regression model with a single linear trend (β1 = −0.73, P < 0.01) described temporal variation (AIC = 235.5) better than a two-piece segmented regression model (AIC = 237.9). Temporal autocorrelation was not observed for regressions of longnose sucker (DW = 2.1, P = 0.59) or cutthroat trout (DW = 2.2, P = 0.53) CPUE through time. For the mean number of spawning adult cutthroat trout (Fig. 4A), there was a clear pattern in the log of the counts over time, and a model with two segments (AIC = 77.4) fits better than a single regression model (AIC = 104.2). The break point occurred at 2007.4 (95% CI = 2005.0 to 2009.6). The slope from 1989 to 2007 was −0.29 (P < 0.01) and from 2008 to 2017 was 0.30 (P < 0.01). Temporal autocorrelation was present in residuals (DW = 0.69, P < 0.01).
ANOVA was used to estimate differences in zooplankton biomass, zooplankton body length, and chlorophyll a among periods. If comparisons were significant, then Tukey’s honest significant difference was used to test for differences among periods. Data were analyzed using program R (62) and the plyr package (63).
The degree to which Secchi disk depths in West Thumb during summer stratification (15 July to 15 September) changed through time was analyzed using multiple regression by checking for first-order autocorrelations with a DW test using the lmtest package (61) and for higher-order correlations using program R. No temporal autocorrelation (i.e., DW test statistic of 2) was detected. Multiple regression analysis was conducted with Secchi depth as the dependent variable and both year and Julian day as independent variables to account for typical seasonal variation (increases) in water clarity as the summer progresses. We did not include data from 2016 to 2017 in our model as Secchi disk depths were shallower during these years and the models were not significant (P > 0.05). Similarly, we used multiple regression to analyze surface water temperatures recorded from four areas of Yellowstone Lake during the stratified period (15 July to 15 September) between 1976 and 2018. Year and Julian day were used as independent variables.
Prais-Winsten time series analysis was used to assess the degree to which cutthroat trout and longnose sucker abundances were related to the frequency of spawning stream use by bears and nest counts and success of ospreys and eagles. Prais-Winsten models describe annual time series data (autoregressive model with lag = 1) and can use an explanatory variable to explain additional variance. We chose this model because we had 29 to 33 years of temporal data, and this simpler model is sufficient to describe relationships with a dataset of this size. Visual surveys for spawning cutthroat trout and CPUE of cutthroat trout and longnose suckers in Yellowstone Lake (explanatory variables; table S2) were used to estimate the degree to which the declining abundance of these prey fish influenced bear activity and osprey and eagle nest counts and success (response variables; table S3) using
where Yt is the response variable in the form of annual time series data at time t, α is a constant, Xt is the explanatory variable in the form of time series data at time t, β is a vector of coefficients, and εt is the error term. We estimated which explanatory variable explained the most variation for each response variable. First-order autocorrelations were checked (and detected) with a DW test using the lmtest package (61), and higher-order correlations were calculated in program R (62). To normalize increasing variance, count data were square root–transformed, and proportional estimates (p) were transformed using log (p/1 − p) after zeros in proportional data were changed to 0.01. Prais-Winsten regression modeling that corrected for first-order autocorrelation was conducted with the package prais (64) in program R. Rho values indicated the time series fit, and t values specified the variance explained by the explanatory variable (table S1).
Magnitude of effect
To compare the lake trout–induced effect size across aquatic and terrestrial trophic levels, we estimated an effect size metric for each response variable using a log response ratio log10(Xpresent/Xpast), where Xpast is the mean of the variable before lake trout invasion and Xpresent is the mean of the same variable afterward (65). Means for fish, bears, ospreys, and bald eagles were for the initial 5 years (Xpast) and final 5 years (Xpresent) of the time series (Table 1). Means for plankton were for 1977–1980 (Xpast) and 2017 (Xpresent). Means for chlorophyll a were for 1972 (Xpast) and 2017 (Xpresent). Means for Secchi depth were for 1976 (Xpast) and 2005 (Xpresent). Data on river otter use of cutthroat trout (prevalence in scat) were collected only during the period of lake trout population growth and cutthroat trout decline (24), including 2002–2003 (Xpast) and 2006–2008 (Xpresent).
Supplementary Material
Acknowledgments
We thank R. Anderson-Sprecher (Department of Statistics, University of Wyoming) for providing guidance on time series analysis, T. Brenden (Michigan State University) for assisting with lake trout SCA modeling, A. Klein (Bozeman, Montana) for preparing all graphics, and C. Detjens (Gardiner, Montana) for editing and ensuring consistency of the text. We also thank R. Garrott, C. Guy, R. Hall, and J. Rotella for discussion and comments that improved this manuscript. Funding: This research was funded by Yellowstone Forever grant no. G022 and the U.S. National Park Service, Yellowstone National Park. Author contributions: T.M.K. compiled datasets and synthesis and led preparation of this report. L.M.T. contributed plankton and physical data and completed statistical analyses. J.L.A. (fish), K.A.G. (bears), and D.W.S. (birds) contributed and interpreted data. J.M.S. developed the lake trout statistical catch-at-age model, estimated annual abundances of lake trout, and completed trend analyses of fish populations. P.J.W. provided overall project coordination, contributed to manuscript preparation, and facilitated funding. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/3/eaav1139/DC1
Supplementary Text
Fig. S1. The watershed (>3200 km2) of Yellowstone Lake and tributary streams in Yellowstone National Park and the Bridger-Teton wilderness, Wyoming.
Fig. S2. Gillnetting effort expended and biomass of lake trout netted.
Fig. S3. Shift in size structure of prey fish populations during the period of lake trout invasion.
Fig. S4. Changes in plankton due to decline of planktivorous cutthroat trout.
Fig. S5. Phytoplankton biomass in the West Thumb of Yellowstone Lake.
Fig. S6. Secchi disk depths in the West Thumb of Yellowstone Lake.
Fig. S7. Depths of isotherms (°C) in the West Thumb of Yellowstone Lake.
Fig. S8. Surface water temperatures of Yellowstone Lake.
Table S1. Results of Prais-Winsten time series regressions.
Table S2. Fish explanatory variables used in time series and trend analyses.
Table S3. Bear and bird response variables used in time series analyses.
REFERENCES AND NOTES
- 1.Estes J. A., Terborgh J., Brashares J. S., Power M. E., Berger J., Bond W. J., Carpenter S. R., Essington T. E., Holt R. D., Jackson J. B. C., Marquis R. J., Oksanen L., Oksanen T., Paine R. T., Pikitch E. K., Ripple W. J., Sandin S. A., Scheffer M., Schoener T. W., Shurin J. B., Sinclair A. R. E., Soulé M. E., Virtanen R., Wardle D. A., Trophic downgrading of planet Earth. Science 333, 301–306 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Cucherousset J., Olden J. D., Ecological impacts of non-native freshwater fishes. Fisheries 36, 215–230 (2011). [Google Scholar]
- 3.Carpenter S. R., Kitchell J. F., Hodgson J. R., Cascading trophic interactions and lake productivity. Bioscience 35, 634–639 (1985). [Google Scholar]
- 4.Paine R. T., Food webs: Linkage, interaction strength, and community infrastructure. J. Animal Ecol. 49, 666–685 (1980). [Google Scholar]
- 5.Pace M. L., Cole J. J., Carpenter S. R., Kitchell J. F., Trophic cascades revealed in diverse ecosystems. Trends Ecol. Evol. 14, 483–488 (1999). [DOI] [PubMed] [Google Scholar]
- 6.Polis G. A., Anderson W. B., Holt R. D., Toward an integration of landscape and food web ecology: The dynamics of spatially subsidized food webs. Annu. Rev. Ecol. Syst. 28, 289–316 (1997). [Google Scholar]
- 7.Croll D. A., Maron J. L., Estes J. A., Danner E. M., Byrd G. V., Introduced predators transform subarctic islands from grassland to tundra. Science 307, 1959–1961 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Ellis B. K., Stanford J. A., Goodman D., Stafford C. P., Gustafson D. L., Beauchamp D. A., Chess D. W., Craft J. A., Deleray M. A., Hansen B. S., Long-term effects of a trophic cascade in a large lake ecosystem. Proc. Natl. Acad. Sci. U.S.A. 108, 1070–1075 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spencer C. N., McClelland B. R., Stanford J. A., Shrimp stocking, salmon collapse, and eagle displacement. Bioscience 41, 14–21 (1991). [Google Scholar]
- 10.Eby L. A., Roach W. J., Crowder L. B., Stanford J. A., Effects of stocking-up freshwater food webs. Trends Ecol. Evol. 21, 576–584 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Bartels P., Cucherousset J., Steger K., Eklöv P., Tranvik L. J., Hillebrand H., Reciprocal subsidies between freshwater and terrestrial ecosystems structure consumer resource dynamics. Ecology 93, 1173–1182 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Soininen J., Bartels P., Heino J., Luoto M., Hillebrand H., Toward more integrated ecosystem research in aquatic and terrestrial environments. Bioscience 65, 174–182 (2015). [Google Scholar]
- 13.Massol F., Gravel D., Mouquet N., Cadotte M. W., Fukami T., Leibold M. A., Linking community and ecosystem dynamics through spatial ecology. Ecol. Lett. 14, 313–323 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Allen D. C., Wesner J. S., Synthesis: Comparing effects of resource and consumer fluxes into recipient food webs using meta-analysis. Ecology 97, 594–604 (2016). [PubMed] [Google Scholar]
- 15.Ruzycki J. R., Beauchamp D. A., Yule D. L., Effects of introduced lake trout on native cutthroat trout in Yellowstone Lake. Ecol. Appl. 13, 23–37 (2003). [Google Scholar]
- 16.Syslo J. M., Guy C. S., Koel T. M., Feeding ecology of native and nonnative salmonids during the expansion of a nonnative apex predator in Yellowstone Lake, Yellowstone National Park. Trans. Am. Fish. Soc. 145, 476–492 (2016). [Google Scholar]
- 17.Koel T. M., Bigelow P. E., Doepke P. D., Ertel B. D., Mahony D. L., Nonnative lake trout result in Yellowstone cutthroat trout decline and impacts to bears and anglers. Fisheries 30, 10–19 (2005). [Google Scholar]
- 18.Tronstad L. M., Hall R. O. Jr., Koel T. M., Introduced lake trout alter nitrogen cycling beyond Yellowstone Lake. Ecosphere 6, 1–24 (2015). [Google Scholar]
- 19.J. C. Knight, “The limnology of the West Thumb of Yellowstone Lake, Yellowstone National Park, Wyoming,” thesis, Montana State University, Bozeman, MT (1975). [Google Scholar]
- 20.Tronstad L. M., Hall R. O. Jr., Koel T. M., Gerow K. G., Introduced lake trout produced a four-level trophic cascade in Yellowstone Lake. Trans. Am. Fish. Soc. 139, 1536–1550 (2010). [Google Scholar]
- 21.Ripple W. J., Estes J. A., Schmitz O. J., Constant V., Kaylor M. J., Lenz A., Motley J. L., Self K. E., Taylor D. S., Wolf C., What is a trophic cascade? Trends Ecol. Evol. 31, 842–849 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Teisberg J. E., Haroldson M. A., Schwartz C. C., Gunther K. A., Fortin J. K., Robbins C. T., Contrasting past and current numbers of bears visiting Yellowstone cutthroat trout streams. J. Wildlife Manage. 78, 369–378 (2013). [Google Scholar]
- 23.Stapp P., Hayward G. D., Effects of an introduced piscivore on native trout: Insights from a demographic model. Biol. Invasions 4, 299–316 (2002). [Google Scholar]
- 24.Felicetti L. A., Schwartz C. C., Rye R. O., Gunther K. A., Crock J. G., Haroldson M. A., Waits L., Robbins C. T., Use of naturally occurring mercury to determine the importance of cutthroat trout to Yellowstone grizzly bears. Can. J. Zool. 82, 493–501 (2004). [Google Scholar]
- 25.Fortin J. K., Schwartz C. C., Gunther K. A., Teisberg J. E., Haroldson M. A., Evans M. A., Robbins C. T., Dietary adjustability of grizzly bears and American black bears in Yellowstone National Park. J. Wildlife Manage. 77, 270–281 (2013). [Google Scholar]
- 26.Crait J. R., Regehr E. V., Ben-David M., Indirect effects of bioinvasions in Yellowstone Lake: Response of river otters to declines in native cutthroat trout. Biol. Conserv. 191, 596–605 (2015). [Google Scholar]
- 27.Baril L. M., Smith D. W., Drummer T., Koel T. M., Implications of cutthroat trout declines for breeding ospreys and bald eagles at Yellowstone Lake. J. Raptor Res. 47, 234–246 (2013). [Google Scholar]
- 28.A. Soyland, “Effects of the introduced lake trout (Salvelinus namaycush) on the osprey (Pandion haliaetus) population in Yellowstone National Park,” thesis, Institute of Nature Management, Norwegian University of Life Science, Sørhellinga, Norway (2010). [Google Scholar]
- 29.L. E. Walker, D. W. Smith, B. J. Cassidy, E. M. Shields, M. D. Paulson, K. E. Duffy, “Yellowstone bird program 2017 annual report” (Technical Report YCR-2018-02, National Park Service, Yellowstone Center for Resources, Yellowstone National Park, WY, 2018).
- 30.Diem K. L., Pugesek B. H., American white pelicans at the Molly Islands, in Yellowstone National Park: Twenty-two years of boom and bust breeding, 1966–1987. Colon. Waterbird 17, 130–145 (1994). [Google Scholar]
- 31.Schmitz O. J., Hawlena D., Trussell G. C., Predator control of ecosystem nutrient dynamics. Ecol. Lett. 13, 1199–1209 (2010). [DOI] [PubMed] [Google Scholar]
- 32.Shurin J. B., Borer E. T., Seabloom E. W., Anderson K., Blanchette C. A., Broitman B., Cooper S. D., Halpern B. S., A cross-ecosystem comparison of the strength of trophic cascades. Ecol. Lett. 5, 785–791 (2002). [Google Scholar]
- 33.Leroux S. J., Loreau M., Dynamics of reciprocal pulsed subsidies in local and meta-ecosystems. Ecosystems 15, 48–59 (2012). [Google Scholar]
- 34.Nakano S., Murakami M., Reciprocal subsidies: Dynamic interdependence between terrestrial and aquatic food webs. Proc. Natl. Acad. Sci. U.S.A. 98, 166–170 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baxter C. V., Fausch K. D., Murakami M., Chapman P. L., Fish invasion restructures stream and forest food webs by interrupting reciprocal prey subsidies. Ecology 85, 2656–2663 (2004). [Google Scholar]
- 36.Flecker A. S., McIntyre P. B., Moore J. W., Anderson J. T., Taylor B. W., Hall R. O. Jr., Migratory fishes as material and process subsidies in riverine ecosystems. Am. Fish. Soc. Symp. 73, 559–592 (2010). [Google Scholar]
- 37.Schindler D. E., Scheuerell M. D., Moore J. W., Gende S. M., Francis T. B., Palen W. J., Pacific salmon and the ecology of coastal ecosystems. Front. Ecol. Environ. 1, 31–37 (2003). [Google Scholar]
- 38.Epanchin P. N., Knapp R. A., Lawler S. P., Nonnative trout impact an alpine-nesting bird by altering aquatic-insect subsidies. Ecology 91, 2406–2415 (2010). [DOI] [PubMed] [Google Scholar]
- 39.Rudman S. M., Heavyside J., Rennison D. J., Schluter D., Piscivore addition causes a trophic cascade within and across ecosystem boundaries. Oikos 125, 1782–1789 (2016). [Google Scholar]
- 40.Knight T. M., McCoy M. W., Chase J. M., McCoy K. A., Holt R. D., Trophic cascades across ecosystems. Nature 437, 880–883 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Middleton A. D., Morrison T. A., Fortin J. K., Robbins C. T., Proffitt K. M., White P. J., McWhirter D. E., Koel T. M., Brimeyer D. G., Fairbanks W. S., Kauffman M. J., Grizzly bear predation links the loss of native trout to the demography of migratory elk in Yellowstone. Proc. R. Soc. B 280, 20130870 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.J. K. Fortin, “Niche separation of grizzly (Ursus arctos) and American black bears (Ursus americanus) in Yellowstone National Park,” thesis, Washington State University, Pullman, WA (2011). [Google Scholar]
- 43.Boyce M. S., Wolves for Yellowstone: Dynamics in time and space. J. Mammal. 99, 1021–1031 (2018). [Google Scholar]
- 44.F. T. van Manen, M. A. Haroldson, K. A. Gunther, Ecological niche, in Yellowstone Grizzly Bears: Ecology and Conservation of an Icon of Wilderness, P. J. White, K. A. Gunther, F. T. van Manen, Eds. (Yellowstone Forever, Yellowstone National Park, 2017). [Google Scholar]
- 45.Anthony R. G., Estes J. A., Ricca M. A., Keith Miles A., Forsman E. D., Bald eagles and sea otters in the Aleutian Archipeligo: Indirect effects of trophic cascades. Ecology 89, 2725–2735 (2008). [DOI] [PubMed] [Google Scholar]
- 46.Oliver S. K., Collins S. M., Soranno P. A., Wagner T., Stanley E. H., Jones J. R., Stow C. A., Lottig N. R., Unexpected stasis in a changing world: Lake nutrient and chlorophyll trends since 1990. Glob. Change Biol. 23, 5455–5467 (2017). [DOI] [PubMed] [Google Scholar]
- 47.Theriot E. C., Fritz S. C., Gresswell R. E., Longterm limnological data from the larger lakes of Yellowstone National Park, Wyoming, USA. Arctic Alpine Res. 29, 304–314 (1997). [Google Scholar]
- 48.Strong D. R., Are trophic cascades all wet? Differentiation and donor-control in speciose ecosystems. Ecology 73, 747–754 (1992). [Google Scholar]
- 49.Halaj J., Wise D. H., Terrestrial trophic cascades: How much do they trickle? Am. Nat. 157, 262–281 (2001). [DOI] [PubMed] [Google Scholar]
- 50.B. B. Shepard, R. Al-Chokhachy, T. M. Koel, M. A. Kulp, N. Hitt, Likely responses of native and invasive salmonid fishes to climate change in the Rocky Mountains and Appalachian Mountains, in Climate Change in Wildlands: Pioneering Approaches to Science and Management, A. J. Hansen, W. B. Monahan, D. M. Theobald, S. T. Olliff, Eds. (Island Press, 2016). [Google Scholar]
- 51.L. R. Kaeding, “Relative contributions of climate variation, lake trout predation, and other factors to the decline of Yellowstone Lake cutthroat trout during the three recent decades,” thesis, Montana State University, Bozeman, MT (2010). [Google Scholar]
- 52.Romme W. H., Boyce M. S., Gresswell R., Merrill E. H., Minshall G. W., Whitlock C., Turner M. G., Twenty years after the 1988 Yellowstone fires: Lessons about disturbance and ecosystems. Ecosystems 14, 1196–1215 (2011). [Google Scholar]
- 53.Ertel B. D., McMahon T. E., Koel T. M., Gresswell R. E., Burckhardt J. C., Life history migrations of adult Yellowstone cutthroat trout in the upper Yellowstone River. N. Am. J. Fish. Manage. 37, 743–755 (2017). [Google Scholar]
- 54.Koel T. M., White P. J., Ruhl M. E., Arnold J. L., Bigelow P. E., Detjens C. R., Doepke P. D., Ertel B. D., An approach to conservation of native fish in Yellowstone. Yellowstone Science 25, 4–11 (2017). [Google Scholar]
- 55.J. M. Syslo, “Dynamics of Yellowstone cutthroat trout and lake trout in the Yellowstone Lake Ecosystem: A case study for the ecology and management of non-native fishes,” thesis, Montana State University, Bozeman, MT (2015). [Google Scholar]
- 56.Syslo J. M., Guy C. S., Bigelow P. E., Doepke P. D., Ertel B. D., Koel T. M., Response of non-native lake trout (Salvelinus namaycush) to 15 years of harvest in Yellowstone Lake, Yellowstone National Park. Can. J. Fish. Aquat. Sci. 68, 2132–2145 (2011). [Google Scholar]
- 57.Fournier D. A., Skaug H. J., Ancheta J., Ianelli J., Magnusson A., Maunder M. N., Nielsen A., Sibert J., AD Model Builder: Using automatic differentiation for statistical inference of highly parameterized complex nonlinear models. Optim. Method Software 27, 233–249 (2012). [Google Scholar]
- 58.L. M. Tronstad, “Ecosystem consequences of declining Yellowstone cutthroat trout in Yellowstone Lake and spawning streams,” thesis, University of Wyoming, Laramie, WY (2008). [Google Scholar]
- 59.Muggeo V. M. R., Estimating regression models with unknown break-points. Stat. Med. 22, 3055–3071 (2003). [DOI] [PubMed] [Google Scholar]
- 60.Muggeo V. M. R., Segmented: An R package to fit regression models with broken-line relationships. R News 8, 20–25 (2008). [Google Scholar]
- 61.Zeileis A., Hothorn T., Diagnostic checking in regression relationships. R News 2, 7–10 (2002). [Google Scholar]
- 62.R Core Development Team, R: A language and environment for statistical computing (R Foundation for Statistical Computing, 2013).
- 63.Wickham H., The split-apply-combine strategy for data analysis. J. Stat. Software 40, 1–29 (2011). [Google Scholar]
- 64.F. Mohr, Prais: Prais-Winsten estimation procedure for AR(1) serial correlation (R package version 0.1.1, 2015); https://github.com/FranzMohr/Prais.
- 65.Hedges L. V., Gurevhitch J., Curtis P. S., The meta-analysis of response ratios in experimental ecology. Ecology 80, 1150–1156 (1999). [Google Scholar]
- 66.Morgan L. A., Shanks P., Lovalvo D., Pierce K., Lee G., Webring M., Stephenson W., Johnson S., Finn C., Schulze B., Harlan S., The floor of Yellowstone Lake is anything but quiet! New discoveries in lake mapping. Yellowstone Science 11, 15–30 (2003). [Google Scholar]
- 67.Kilham S. S., Theriot E. C., Fritz S. C., Linking planktonic diatoms and climate change in large lakes of the Yellowstone ecosystem using resource theory. Limnol. Oceanogr. 41, 1052–1062 (1996). [Google Scholar]
- 68.M. A. Kaplinksi, “Geomorphology and geology of Yellowstone Lake, Yellowstone National Park, Wyoming,” thesis, Northern Arizona University, Flagstaff, AZ (1991). [Google Scholar]
- 69.Finn C. A., Morgan L. A., High-resolution aeromagnetic mapping of volcanic terrain, Yellowstone National Park. J. Volcanol. Geotherm. Res. 115, 207–231 (2002). [Google Scholar]
- 70.J. D. Varley, P. D. Schullery, Yellowstone Fishes: Ecology, History, and Angling in the Park (Stackpole Books, 1998). [Google Scholar]
- 71.Gresswell R. E., Varley J. D., Effects of a century of human influence on the cutthroat trout of Yellowstone Lake. Am. Fish. Soc. Symp. 4, 45–52 (1988). [Google Scholar]
- 72.Kaeding L. R., Boltz G. D., Spatial and temporal relations between fluvial and allacustrine Yellowstone cutthroat trout, Oncorhynchus clarkii bouvieri, spawning in the Yellowstone River, outlet stream of Yellowstone Lake. Environ. Biol. Fishes 61, 395–406 (2001). [Google Scholar]
- 73.Koel T. M., Mahony D. L., Kinnan K. L., Rasmussen C., Hudson C. J., Murcia S., Kerans B. L., Myxobolus cerebralis in native cutthroat trout of the Yellowstone Lake ecosystem. J. Aquat. Anim. Health 18, 157–175 (2006). [Google Scholar]
- 74.Murcia S., Kerans B. L., MacConnell E., Koel T. M., Myxobolus cerebralis infection patterns in Yellowstone cutthroat trout after natural exposure. Dis. Aquat. Org. 71, 191–199 (2006). [DOI] [PubMed] [Google Scholar]
- 75.Interlandi S. J., Kilham S. S., Theriot E. C., Responses of phytoplankton to varied resource availability in large lakes of the Greater Yellowstone Ecosystem. Limnol. Oceanogr. 44, 668–682 (1999). [Google Scholar]
- 76.Bergum D. J., Gunther K. A., Baril L. M., Birds and mammals that consume Yellowstone cutthroat trout in Yellowstone Lake and its tributaries. Yellowstone Science 25, 86–89 (2017). [Google Scholar]
- 77.N. G. Benson, “Limnology of Yellowstone Lake in relation to the cutthroat trout” (Research report 56, U. S. Department of the Interior, Fish and Wildlife Service, Bureau of Sport Fisheries and Wildlife, Washington, DC, 1961).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/3/eaav1139/DC1
Supplementary Text
Fig. S1. The watershed (>3200 km2) of Yellowstone Lake and tributary streams in Yellowstone National Park and the Bridger-Teton wilderness, Wyoming.
Fig. S2. Gillnetting effort expended and biomass of lake trout netted.
Fig. S3. Shift in size structure of prey fish populations during the period of lake trout invasion.
Fig. S4. Changes in plankton due to decline of planktivorous cutthroat trout.
Fig. S5. Phytoplankton biomass in the West Thumb of Yellowstone Lake.
Fig. S6. Secchi disk depths in the West Thumb of Yellowstone Lake.
Fig. S7. Depths of isotherms (°C) in the West Thumb of Yellowstone Lake.
Fig. S8. Surface water temperatures of Yellowstone Lake.
Table S1. Results of Prais-Winsten time series regressions.
Table S2. Fish explanatory variables used in time series and trend analyses.
Table S3. Bear and bird response variables used in time series analyses.


