Administering the anesthetic propofol after a brief reminder reduces retrieval of established emotional memory 24 hours later.
Abstract
The adjustment of maladaptive thoughts and behaviors associated with emotional memories is central to treating psychiatric disorders. Recent research, predominantly with laboratory animals, indicates that memories can become temporarily sensitive to modification following reactivation, before undergoing reconsolidation. A method to selectively impair reconsolidation of specific emotional or traumatic memories in humans could translate to an effective treatment for conditions such as posttraumatic stress disorder. We tested whether deep sedation could impair emotional memory reconsolidation in 50 human participants. Administering the intravenous anesthetic propofol following memory reactivation disrupted memory for the reactivated, but not for a non-reactivated, slideshow story. Propofol impaired memory for the reactivated story after 24 hours, but not immediately after propofol recovery. Critically, memory impairment occurred selectively for the emotionally negative phase of the reactivated story. One dose of propofol following memory reactivation selectively impaired subsequent emotional episodic memory retrieval in a time-dependent manner, consistent with reconsolidation impairment.
INTRODUCTION
Memory for traumatic experience can contribute to anxiety disorders (1–3), such as specific phobias or trauma and stressor-related disorders such as post traumatic stress disorder (PTSD) (4–6). An effective treatment for these disorders should selectively decrease these intrusive, pathological memories. A theoretical obstacle to developing these treatments has been a prevailing view that established memories are relatively fixed. That is, memories are initially labile and sensitive to interference by, e.g., electroconvulsive therapy (ECT) (7), general anesthesia (8), or protein synthesis inhibition (9), but stabilize over time during a period of consolidation, after which memories were considered to be established and no longer sensitive to disruption or modification (10). However, recent research using nonhuman animals challenges this classical view by showing that reactivating an old memory can temporarily return it to a labile state requiring restabilization processes to persist, referred to as reconsolidation, and rendering the memory restabilization susceptible to manipulation (11).
The development of protocols to selectively reduce unwanted memories via the disruption of reconsolidation could be of potential clinical benefit. However, evidence for reconsolidation in humans remains limited, primarily because the manipulations used to disrupt memory in animals, such as protein synthesis inhibitors, are toxic. Consequently, studies on reconsolidation in humans have largely used behavioral manipulations to target nondeclarative memory (12), including conditioned fear (13), which involves repeated pairings of a conditioned stimulus with an aversive unconditioned stimulus such as mild electric shock. Human psychopharmacological approaches using the β-adrenergic antagonist propranolol before or after the reactivation of conditioned fear for simple sensory stimuli show efficacy in blocking reconsolidation (14, 15). The rationale for trialing β-adrenergic antagonists in this context is based on previous reports that propranolol blocks emotional memory encoding (16, 17) and retrieval (18). In patient studies targeting reconsolidation to treat PTSD, β-adrenergic antagonists have shown promise (19–21). It should be noted, however, that some studies have failed to replicate these reconsolidation impairing effects in humans using behavioral manipulations (22, 23) or propranolol (22–25) to target conditioned fear or using propranolol in patients with PTSD (26).
Traumatic memories are more complex than simple associations formed by conditioning. These episodic memories are enriched with what-where-when contextual information (27), and their retrieval can be triggered by similar experiences (2, 28). Therefore, the modulation of reconsolidation of rare traumatic memories may need different manipulations than the ones used in previous studies using simple fear conditioning (12). Most of the extant studies on episodic memory reconsolidation have used noninvasive behavioral techniques, such as post-reactivation interference learning to update memory (29) instead of disrupting it. By contrast, we showed recently, in patients with unipolar depression, that ECT can selectively impair reconsolidation of a reactivated emotional episodic memory (30). This study design met criteria generated from nonhuman animal studies to provide compelling evidence for the reconsolidation phenomena in humans (31). These criteria include the requirement for (i) reactivation of consolidated memory by a reminder cue and (ii) that the memory effect be observed after sufficient time has elapsed for reconsolidation to take place (typically tested after 24 hours) and not immediately after reactivation (11, 30, 31). A third criterion, which is important for interpreting data as a disruption of reconsolidation (32), is that the manipulation targeting reconsolidation is delivered after reactivation and not before. This latter criterion was also met.
ECT comprises the application of short-acting general anesthesia, neuromuscular blockade, and cranial electrical stimulation to evoke generalized seizure activity. Although we attributed ECT-induced reconsolidation impairment in patients with depression to cranial electrical stimulation, whether the different components of ECT could individually impair reconsolidation remains an open question. A possibility that the anesthetic was, in part, responsible for ECT-induced reconsolidation impairment is particularly important, as the wide use of this pharmacological class in clinical practice suggests that it could be a relatively safe and accessible method to modify unwanted memories. The mechanism of action of most general anesthetics is the modulation of γ-aminobutyric acid (GABA) receptors, and data from animal models have shown that administration of GABA receptor type A (GABAA) agonists can disrupt reconsolidation (33–35). If anesthesia alone blocks reconsolidation, then targeted memory disruption in patients with psychiatric disorders could be achieved without the more invasive aspects of ECT. In support of this possibility, human neuroimaging data demonstrate that general anesthetics disrupt activity in the hippocampus and amygdala (36, 37), brain areas critically involved in emotional memory (38). We therefore tested the hypothesis that the intravenous anesthetic propofol impairs reconsolidation of emotional memories reactivated immediately before anesthetic administration in psychiatrically and neurologically healthy individuals who had been referred for endoscopy for clinical indications.
Fifty participants were randomly assigned to one of two groups (A and B) matched for gender (Table 1). During emotional memory encoding, participants viewed slideshows of two distinct negative arousing stories. Both stories comprised three phases: Phases 1 and 3 were emotionally neutral, whereas phase 2 comprised the emotionally negative part of each story. The memory reactivation session took place 1 week after the encoding session (Fig. 1). To reactivate the memory and initiate a memory destabilization process, patients were presented with the first slide of one of the two stories and asked three questions about what had been visible behind a mask placed over a part of the slide. Immediately following memory reactivation, all participants received propofol and underwent endoscopy (gastroscopy, colonoscopy, or both) under deep sedation [depression of consciousness but spontaneous ventilation maintained (39)]. Memory for both stories was tested using a multiple-choice memory test after 24 hours (group A) or immediately after discharge from the recovery room (group B). All participants completed the digit symbol substitution test (DSST), a brief cognitive screening test of digit-symbol pairs, before emotional memory encoding and memory testing.
Table 1. Participant demographics and clinical details.
Twenty-five patients per group completed the study. One patient in group B was not included in analyses because of outlier-level performance on the DSST before recognition testing. Groups A and B did not differ on any demographical variables (age, gender, years of education, or type of endoscopy procedure) or in terms of dosage of other agents (midazolam or alfentanil) administered. However, there was a significant difference in the amount of propofol administered.
| Group A | Group B | Statistic (t, X2) | P | ||
| Gender* | Female | 10 | 9 | 0.86 | |
| Male | 15 | 15 | |||
| Age (years)† | Mean | 38.88 | 39.08 | t47 = −0.15 | 0.88 |
| SEM | 0.90 | 0.97 | |||
| Years of schooling† | Mean | 14.44 | 14.75 | t47 = −0.38 | 0.70 |
| SEM | 0.60 | 0.54 | |||
| Endoscopy procedure* | Colonoscopy | 13 | 10 | 0.23 | |
| Gastroscopy | 7 | 12 | |||
| Both | 5 | 2 | |||
| Endoscopy diagnosis* | Not pathological | 15 | 18 | 0.76 | |
| Inflammatory | 4 | 3 | |||
| Allergy | 1 | 1 | |||
| Vascular | 1 | 0 | |||
| Ulcer | 1 | 0 | |||
| Polyps | 3 | 2 | |||
| Propofol (mg/kg)† | Mean | 3.02 | 2.37 | t47 = 2.04 | 0.047‡ |
| SEM | 0.25 | 0.19 | |||
| Duration of deep sedation (min)† | Mean | 13.17 | 11.42 | t47 = 0.93 | 0.36 |
| SEM | 1.56 | 1.02 | |||
| Other pharmacological agents* | Yes | 13 | 13 | 0.88 | |
| No | 12 | 11 | |||
| Midazolam in mg† | n | 8 | 8 | ||
| Mean | 2.05 | 1.37 | t14 = 1.65 | 0.12 | |
| SEM | 0.35 | 0.21 | |||
| Alfentanil in mg† | n | 7 | 7 | ||
| Mean | 0.29 | 0.30 | t12 = −0.08 | 0.94 | |
| SEM | 0.06 | 0.06 |
*χ2.
†Independent t test.
‡Significant at P < 0.05.
Fig. 1. Study protocol.
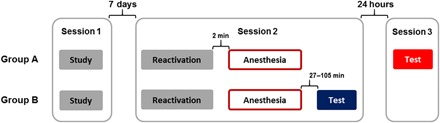
All patients, randomly assigned to one of two groups (A or B), underwent three sessions. Session 1 corresponded to day 1 and was the encoding session of the two emotional stories. Session 2 took place 7 days after day 1. All participants performed a memory reactivation task for one of the two emotional stories in the endoscopy unit. Immediately after, they received propofol, followed by the endoscopy procedure. For group B, session 3, the recognition memory test took place after the participants recovered from the procedure and were discharged from the recovery room. For group A, session 3 took place 24 hours after the endoscopy.
RESULTS
Cognitive status before memory encoding and retrieval
Groups A and B performed equally in the DSST task (F1,47 = 2.72, P = 0.11, η2p = 0.055) (fig. S1). Both groups improved with repetition of the task. That is, there was a main effect of experiment time point, encoding to recognition (F1,47 = 5.56, P = 0.023, η2p = 0.106), but no experiment time point by group interaction (F1,47 = 2.39, P = 0.13, η2p = 0.048), indicating a comparable cognitive performance across groups at the time of memory testing. Note that one participant from group B was discarded from further analyses because of a worsening of performance between the encoding and memory testing phases (3 SDs below the mean), which could indicate still being under the influence of propofol.
Propofol administration impairs emotional memory reconsolidation
To test our hypothesis that memory would be impaired in group A, but not group B, for the story reactivated before propofol administration, we performed a group (A and B) by reactivation (reactivated story and nonreactivated story) repeated-measures analysis of variance (ANOVA) on recognition memory scores. Variables that could have affected memory function were included as covariates of no interest. These comprised years of education, propofol dose (mg/kg), DSST performance immediately before the recognition test, which of the two stories was reactivated, which endoscopic procedure was performed (gastroscopy, colonoscopy, or both), and diagnostic outcome of the procedure (as this may have influenced levels of anxiety for a given patient). Furthermore, 27 of the 50 participants received adjuvant agents during deep sedation, which included midazolam or phenylpiperidine derivatives (Table 1); thus, adjuvant pharmacological agent administration during the endoscopy was also included as a covariate of no interest.
Supporting a hypothesis that anesthesia disrupts emotional memory reconsolidation, we observed a group by reactivation interaction (F1,40 = 4.84, P = 0.034, η2p = 0.108) (Fig. 2A). There was no main effect of reactivation (F1,40 = 0.31, P = 0.58, η2p = 0.008) or group (F1,40 = 1.46, P = 0.23, η2p = 0.035). Given that reconsolidation is a time-dependent process, we had predicted that reactivation-induced memory impairment should be present only in group A, but not in group B, as in group B, reconsolidation would not yet be complete. Confirming this prediction, planned paired t tests show reduced memory for the reactivated versus nonreactivated story in group A (t24 = −2.14, P = 0.043, d = 0.44), but no difference in memory for the reactivated versus nonreactivated story in group B (t23 = −0.87, P = 0.39, d = 0.17). The observed differences in recognition scores between groups A and B are not due to differences in memory reactivation, as there was no between-group difference in memory reactivation scores for the first slide of the reactivated story immediately before propofol administration (t47 = 0.70, P = 0.49, d = 0.20), i.e., both groups reactivated memory and performed above chance level (fig. S2).
Fig. 2. Propofol impaired memory for the reactivated story when tested after 24 hours (group A) but not when tested immediately after recovery from anesthesia (group B).
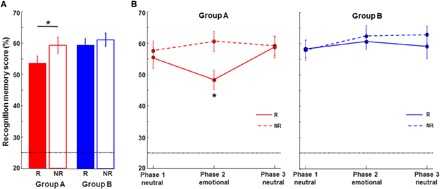
(A) Recognition memory scores for all slides of the reactivated and nonreactivated story (except the first slide) are plotted for each group. Scores (percentage) for each story per group: group A (n = 25 participants) reactivated mean (SEM) = 53.49 (2.29); nonreactivated mean = 59.20 (2.60); group B (n = 24 participants) reactivated mean = 59.52 (1.97); nonreactivated mean = 61.19 (2.11). (B) Percent correct recognition memory scores are plotted for the three story phases of the reactivated (R; solid line) and nonreactivated (NR; dashed line) story for each group. There is a significant impairment of memory for the emotional phase of the reactivated story (phase 2) in group A only. Chance recognition performance (25%) is indicated by the dotted horizontal line. *P < 0.05.
Both stories consisted of three phases: Phase 1 (slides 1 to 4) and phase 3 (slides 9 to 11) were of neutral content, whereas the middle part of the stories, phase 2 (slides 5 to 8), had emotionally negative content. Although we did not observe selectivity of reconsolidation disruption for any particular phase of the stories in our previous study involving ECT, recognition accuracy was generally low in those patients with severe depression, and we did not observe the typical enhancement of memory for the emotional phase of the stories (16). However, on examining recognition scores for reactivated versus nonreactivated stories as a function of phase in group A of the current study (Fig. 2B), selective reactivation–induced memory impairment for the emotional part of the story was evident. On post hoc testing, we observed memory differences for phase 2 between the reactivated versus nonreactivated stories [tphase2(24) = −3.05, P = 0.006, P = 0.033 Bonferroni corrected for six tests, d = −0.61] in group A, whereas reactivation did not affect memory for the neutral phases of the stories [tphase1(24) = −0.56, P = 0.58, d = −0.11; tphase3(24) = −1.20, P = 0.91, d = −0.024]. We observed no difference for any of the three phases in group B (P > 0.25 for all phases) (Fig. 2B).
Emotional memory reconsolidation impairment is not correlated with propofol dose
Propofol was administered entirely for clinical reasons, not for the purposes of the current study. To obtain deep sedation in all patients undergoing endoscopy, prescribed propofol doses are adjusted relative to the weight, age, and clinical condition of the patient. Critically, the degree of sedation was similar for all participants in our study. However, despite randomized group assignment, group A was prescribed, on average, a higher total propofol dose than group B (t47 = 2.04, P = 0.047, d = 0.59) (Table 1). To mitigate the likelihood that this dose difference contributed to the observed memory impairment in group A and not in group B, we included the dose of propofol as a covariate in the group by reactivation ANOVA described above. Furthermore, we found no linear association between the dose of propofol and phase 2 (emotional) recognition scores in group A (Pearson’s rreactivated = 0.22, P = 0.30; rnonreactivated = −0.007, P = 0.97; rreactivated minus nonreactivated = 0.17, P = 0.40) or in group B (Pearson’s rreactivated = 0.12, P = 0.56; rnonreactivated = −0.005, P = 0.98; rreactivated minus nonreactivated = 0.10, P = 0.63) (fig. S3). Last, we performed a median split on group A based on propofol dose and repeated the comparison of reactivated versus nonreactivated recognition scores for phase 2 (the emotional phase), taking only those 13 participants at, or below, the median propofol dose (2.97 mg/kg). The reactivation-induced reconsolidation impairment observed in group A (Fig. 2B) remains statistically significant in this subgroup [tphase2(12) = −2.61, P = 0.023, d = −0.73]. We therefore found no evidence that propofol dose accounts for the specific memory impairment of the emotional part of the reactivated story in group A. This suggests that deep sedation, and not propofol dose per se, is the important factor determining reconsolidation impairment.
Endoscopic procedure is unlikely to contribute to reconsolidation impairment in group A
Short-acting deep sedation is administered relatively routinely in the hospital setting. Thus, instead of exposing healthy participants to anesthesia that they would otherwise not need, we elected to perform the current study on psychiatrically and neurologically healthy individuals referred for endoscopy. The effects of propofol on memory reconsolidation were therefore studied in the context of an endoscopic procedure. This procedure may stimulate the vagus nerve either at esophageal intubation during gastroscopy or by stretching of the sigmoid mesentery during colonoscopy. Although the memory consequences of vagal stimulation in this context are unknown, continuous electrical stimulation of the vagus nerve in human patients with implanted stimulators has been shown to modulate cerebral blood flow in different brain areas, including the hippocampus and amygdala (40), raising a possibility that vagal stimulation contributed to the observed memory effects. However, it is reasonable to assume that those individuals undergoing both gastroscopy and colonoscopy would obtain more vagal stimulation than those having just one procedure. That is, whereas the effect of propofol does not depend on the dosage and would appear to reflect sedation level per se, any effect of vagal stimulation on reconsolidation could depend on total stimulation. We therefore tested the reconsolidation effects in group A and found these to be equivalent across the three subgroups undergoing either or both interventions when taking recognition scores for the entire story (F2,22 = 2.41, P = 0.11, η2p = 0.018) or just for the emotional phase 2 (F2,22 = 0.47, P = 0.63, η2p = 0.041). This makes it unlikely that vagal stimulation contributes to the memory effects we observe. In any case, vagal stimulation is thought to up-regulate memory (41), so if anything, this could have obscured reconsolidation disruption instead of contributing to it.
DISCUSSION
We demonstrate that a single dose of the GABAA agonist propofol following memory reactivation impaired reactivated, but not nonreactivated, episodic memory. In keeping with an explanation in terms of reconsolidation impairment, this reduction was only observed if tested 24 hours (group A), but not immediately (group B), after memory reactivation and propofol administration. Post hoc testing indicated that the memory impairment by propofol was selective for the negative phase of the reactivated story.
Evidence for the disruption of reconsolidation by anesthesia derives from animal studies investigating fear-conditioned memories (33–35). To date, the effects of general anesthesia have only been tested on emotional memory encoding in humans (36, 37). The anesthetic gas sevoflurane, a GABAA agonist, was shown to block the episodic memory enhancement associated with emotional arousal at subanesthetic doses, an effect associated with a reduction in connectivity between the amygdala and hippocampus during simultaneous glucose positron emission tomography scanning (36). This result in humans is supported by analogous data from rats showing that propofol (42) and sevoflurane (43) impair emotional learning, but not in animals that had undergone previous lesions to the amygdala. We therefore speculate that human amygdala activity may be highly sensitive to the inhibitory effects of anesthesia and that the emotional memory reconsolidation disruption by propofol observed here is possibly mediated by a down-regulation in amygdala and hippocampal activity and their coupling. A confirmation of this mechanism would be relevant to any application of propofol in the management of PTSD, as a disturbance in this circuit is considered a key pathophysiological feature of PTSD (4, 6).
An alternative potential mechanism underlying the effects of propofol on emotional memory reconsolidation we observe is via influencing the noradrenergic system. In vivo recordings in rodents have shown that propofol inhibits spontaneous firing in locus coeruleus (LC) (44). The noradrenergic antagonist propranolol has been shown to impair reconsolidation of conditioned fear but not episodic memory (14), making it less likely that the episodic memory reconsolidation impairment we observe reflects an effect of propofol on the noradrenergic system. We did not test for an effect of propofol on simple associative (conditioned) fear memory reconsolidation, but it is possible that propofol could impair reconsolidation of fear-conditioned memories via its action on LC. If this is not the case, the administration of both propofol and propranolol with a reminder cue may be required to inhibit conditioned fear and the episodic memory associated with the conditioning event. Reports that propranolol does disrupt reconsolidation of emotional episodic memory (45) would suggest that reconsolidation of both episodic emotional memory and conditioned fear could be disrupted by an effect of propofol on LC. However, in these studies, drug administration typically precedes memory reactivation, raising a possibility that any reduction in memory performance on subsequent testing reflects a sustained retrieval failure at reactivation (18, 26, 46) and not a disruption of reconsolidation, leaving the risk of the return of memory. The current design does not suffer from this limitation in interpreting the results as a disruption of reconsolidation, as administration of propofol is intravenous and therefore acts immediately following successful memory reactivation.
Considering recognition scores for each story in their entirety, our previous study (30) showed an ECT-evoked memory reduction in patients with depression of 8.48% down to chance levels, whereas in the current study, propofol-evoked reduction was 5.71%. Effect sizes for memory for the reactivated versus nonreactivated story in group A (Cohen’s d = 0.44) was lower than following ECT in the analogous group in our previous study (Cohen’s d = 0.90) (30). However, the effect of propofol is qualitatively different from that we reported for ECT, with propofol selectively reducing memory for the emotional phase of the reactivated story by 12.29% and not the neutral phases. Thus, whereas ECT may be a more potent method to disturb emotional episodic memory reconsolidation, propofol is less invasive and may be more selective to the emotional content of memories. Note that in our previous ECT study, the anesthetic administered as part of ECT was etomidate (30). Like propofol, etomidate potentiates the effects of GABA on GABAA receptors (47) and is known to impair learning and memory (48). However, etomidate also produces adrenal cortical inhibition. Given the role of cortisol in emotional memory (49), we eschewed this potential confound by performing this study in participants receiving propofol.
Determining the mechanism by which propofol reduces emotional memory (story phase 2) reconsolidation down to the same level as neutral memory (phases 1 and 3) will address a more fundamental question regarding the effects of emotion on memory. These observations suggest that either there is a degraded episodic memory that retains an emotional enhancement or reconsolidation of the emotional enhancement is impaired by propofol. Analogous effects of a reduction of emotional memory performance to the same level as neutral memory are observed following administration of propranolol (16–18, 50) or sevoflurane (36) at encoding. What is currently unknown is whether emotion simply strengthens the consolidation of an otherwise neutral memory trace or there is a qualitative difference between emotional and neutral memories. If the latter was the case, this would raise the additional question of whether the emotional and neutral components of an emotional memory are independent, but associated, or these two components are necessarily integrated and indivisible. Although the current results cannot discern between these possibilities, we do demonstrate that propofol evokes the same pattern of deficit at reconsolidation as is observed following pharmacological disruption of emotional memory consolidation.
In contrast to the current study, our previous ECT experiment (30) included a third group (group C), for which patients with severe depression underwent the same emotional memory protocol but were not given ECT following memory reactivation. Here, we elected not to include an analogous nontreatment control group for two reasons. First, group C patients in our previous study showed memory enhancement for the reactivated material in the absence of ECT. Although this enhancement was observed in patients with severe depression, it is likely that a similar enhancement would occur in the current study, given cross-species evidence from rodents (51) and healthy human participants (52) that reactivation can strengthen episodic memory via reconsolidation in the absence of post-reactivation treatment designed to impair it. Second, as propofol was administered as part of a clinical intervention, a nontreatment control group (i.e., a group not undergoing deep sedation plus endoscopy following memory reactivation) would have differed on key aspects besides anesthesia that would have likely influenced memory function. These include the absence of anxiety associated with the endoscopic procedure and diagnostic outcome, as well as the anxiety associated with undergoing anesthesia.
We demonstrate propofol-induced reconsolidation impairment at an interval of 24 hours after reactivation and deep sedation. Testing memory after a longer period (e.g., 1 week) would have allowed us to determine whether memory recovers over time. Memory recovery at 1 week would indicate that the reduced recognition memory for the reactivated story observed in group A at 24 hours reflects a temporary retrieval impairment or temporary inhibition of the memory trace and not a reconsolidation blockade. However, the current paradigm is not suited to repeated memory testing (i.e., immediately after recovery from propofol, at 24 hours and at 1 week) within the same individuals. The multiple-choice questionnaires are highly structured, providing substantial information for new learning of the stories that would render performance on a repeat memory test a poor indication of what is remembered from the initial learning experience. This is why we elected to have two groups (immediate testing and at 24-hour delay), as opposed to one group being tested both immediately and at 24 hours, to prevent confounding effects of new learning during recognition.
The aim of this study was to test for a method to reduce aversive episodic memories in a relatively noninvasive way by reactivating these memories before a dose of intravenous anesthetic is administered. The results presented here pertain to impairing the reconsolidation of aversive episodic memories learned in an experimental context by healthy individuals. Thus, these emotional memories remain quite distant from those formed during truly stressful life experiences. While here we provide a proof of concept that a routine anesthetic procedure impairs reconsolidation and could potentially be used to treat psychiatric disorders in which abnormal emotional memory plays a role, clinical trials are required to apply these findings to patients with pathological, traumatic memories. Reactivation of these memories before propofol administration could be achieved by simple script-driven recall through to immersive virtual reality, tailored to the patients’ traumatic event. However, we note that disorders such as PTSD are multifaceted disorders. PTSD involves recurrent, intrusive recollection of the trauma memory and peritraumatic memory disturbances (4, 5, 53), and these different facets may vary in sensitivity to alteration following reactivation. One limiting factor may be the age of the memory; in some animal models, older memories seem to be more resistant to reconsolidation blockade (54). However, there is also evidence that altering parameters of the reactivation session, such as increasing duration (55), can destabilize remote memories (56). The administration of propofol with simultaneous recording of the electroencephalogram may provide useful markers of the depth of sedation and loss of consciousness (57) potentially predictive of efficacy of reconsolidation impairment across patients.
MATERIALS AND METHODS
Study population
Fifty participants, without history of neurological and psychiatric illnesses, were recruited from the gastroenterology clinic of the Hospital Clínico San Carlos, Madrid, with an age range of 30 to 45 years (Table 1). Participants were invited to join the study because they had been referred for a routine endoscopy procedure (gastroscopy and/or colonoscopy) involving brief, deep sedation. Participants were randomly assigned to one of two groups (A and B) matched for gender (15 men per group). All participants were free of psychoactive medication and were only under stable pharmacological treatment related to gastrointestinal conditions (22 of 50 participants). The two groups did not differ in terms of age, educational level, and endoscopic procedure or ensuing diagnosis (Table 1). The ethical committee of the Hospital Clínico San Carlos approved the study, and all participants provided written informed consent after the nature and possible consequences of the studies were explained. All methods were carried out in accordance with the Declaration of Helsinki.
Emotional stories
Participants viewed slideshows of two distinct negative arousing stories on a 16″ laptop computer screen. Each story consisted of 11 slides, each accompanied by an auditory narrative, presented via computer speakers through the integrated device high-definition audio (mean: 62 dB, in a range between 42 and 80 dB at a distance of 15 cm). Each slide was shown for 20 s, with a total presentation time of 3.6 min per story. Story 1 contained scanned analog images, whereas story 2 consisted of modern digital photographs. To minimize interference due to learning two stories in a short period of time, the narrator for story 1 was male, and the narrator for story 2 was female (30). Both stories were adapted to the Spanish language and were identical in structure, grammar, and details. Both stories comprised three phases: Phase 1 (slides 1 to 4) was emotionally neutral, phase 2 (slides 5 to 8) comprised the emotionally negative part of each story, and phase 3 (slides 9 to 11) was again of neutral content.
Emotional memory encoding
Session 1 took place in the gastroenterology consulting room 1 week before the patient’s endoscopy (Fig. 1). The order in which the two narrated slideshow stories were presented was randomized across participants. The encoding session for both stories lasted for approximately 15 min.
Memory reactivation
This session took place 1 week after the encoding session. Once the participant was supine in the hospital endoscopy room and the intravenous cannula was placed, memory for one of the two stories was reactivated. Which of the two stories was reactivated was counterbalanced across participants within each group (for 12 participants in each group, story 1 was reactivated). To reactivate the memory and initiate a destabilization process, patients were presented with the first slide of one of the two stories and asked three questions about what had been visible behind a mask placed over a part of the slide. After the patient answered the question, the related part of the mask was removed. The entire slide was visible after all three questions were answered. Answers were provided by free recall and recorded using a tape recorder by the investigator. If the patient was unable to answer freely, a two-alternative forced choice question was posed. Reactivation score was calculated as the number of questions answered correctly by free recall (multiplied by 2) plus the number of correctly answered questions by multiple choice (yielding a maximum score of 6). Immediately following memory reactivation, all participants received propofol and underwent endoscopy. The reactivation session lasted for approximately 1 to 2 min.
Deep sedation
All participants were administered propofol (2,6-diisopropylphenol) by an anesthetist; initial dose: 100 to 200 mg; additional doses: intravenous (IV) dose of 25 to 75 mcg/kg per minute or incremental IV bolus doses of 30 to 50 mg. Furthermore, 27 of the 50 participants received adjuvant agents (Table 1), which included midazolam or phenylpiperidine derivatives (alfentanil or remifentanil). That is, although all participants received propofol, the memory study did not interfere with the individual anesthetist’s prescribing preferences for adjuvant agents. Two participants in each group were administered both midazolam and alfentanil as adjuvant agents. Only one participant in group B received remifentanil (2 mcg). Participants maintained spontaneous ventilation and were monitored with a peripheral pulse oximeter for SpO2. Across all participants, the mean (SD) duration under deep sedation was 12.3 (6.6) min with no significant between-group difference in duration (Table 1).
Memory testing
Memory for both stories was tested after 24 hours (group A) or after 27 to 105 min (group B), which corresponded to the time taken for group B participants to recover from the procedure and be discharged from the recovery room (mean: 60.4 min; SD: 21.3 min). Memory was assessed using a multiple-choice test (30) conducted in the same consultation room where the stories were initially encoded. Following the order of the 11 presented slides, three to five multiple-choice questions were posed per slide with four answer options. Memory performance on the first slide for both stories was excluded from the memory score, as this slide was used for memory reactivation. Testing memory for both stories required approximately 1 hour.
Digit Symbol Substitution Test
The DSST was completed by all participants before emotional memory encoding and memory testing. For nine digit-symbol pairs, the task was to write down the associated digits underneath 115 symbols as fast and with as few errors as possible.
Statistical analysis
Recognition memory scores were entered into ANOVA, with effect sizes reported as partial η2 (η2p). t test effect sizes were reported as Cohen’s d. For paired t tests, the values provided pertain to drm (Cohen’s effect size for repeated measures). All t tests are two-tailed. For ANOVAs, the assumption of sphericity was assessed by Mauchly’s test, and Greenhouse-Geisser correction was used if applicable.
Supplementary Material
Acknowledgments
We thank members of the endoscopy unit of the Hospital Clinical San Carlos, Madrid, Spain. Funding: This work was supported by grants from the Spanish Ministry of Economy and Competition (SAF2014-62116-EXP and SAF2015-65982-R) to B.A.S. and a Marie Curie Career Integration Fellowship to B.A.S. (FP7-PEOPLE-2011-CIG 304248). M.C.W.K. was supported by an H2020 Marie Sklodowska-Curie Fellowship and a Society in Science–Branco Weiss fellowship. B.A.S. was supported by a NARSAD Independent Investigator Grant from the Brain and Behavior Research Foundation. Author contributions: B.A.S, M.C.W.K., and G.F. designed the study. A.G.V., E.R., and M.V.A. organized the patient recruitment and acquired data. A.G.V., M.C.W.K., S.M., and B.A.S. analyzed the data. A.G.V., M.C.W.K., and B.A.S. wrote the paper with input from all authors. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/3/eaav3801/DC1
Fig. S1. DSST performance for groups A and B.
Fig. S2. Memory reactivation scores.
Fig. S3. Reconsolidation impairment is not correlated with propofol dose.
REFERENCES AND NOTES
- 1.Ressler K. J., Mayberg H. S., Targeting abnormal neural circuits in mood and anxiety disorders: From the laboratory to the clinic. Nat. Neurosci. 10, 1116–1124 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams J. M. G., Barnhofer T., Crane C., Herman D., Raes F., Watkins E., Dalgleish T., Autobiographical memory specificity and emotional disorder. Psychol. Bull. 133, 122–148 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garakani A., Mathew S. J., Charney D. S., Neurobiology of anxiety disorders and implications for treatment. Mt. Sinai J. Med. 73, 941–949 (2006). [PubMed] [Google Scholar]
- 4.Layton B., Krikorian R., Memory mechanisms in posttraumatic stress disorder. J. Neuropsychiatry Clin. Neurosci. 14, 254–261 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Rubin D. C., Berntsen D., Bohni M. K., A memory-based model of posttraumatic stress disorder: Evaluating basic assumptions underlying the PTSD diagnosis. Psychol. Rev. 115, 985–1011 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pitman R. K., Rasmusson A. M., Koenen K. C., Shin L. M., Orr S. P., Gilbertson M. W., Milad M. R., Liberzon I., Biological studies of post-traumatic stress disorder. Nat. Rev. Neurosci. 13, 769–787 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duncan C. P., The retroactive effect of electroshock on learning. J. Comp. Physiol. Psychol. 42, 32–44 (1949). [DOI] [PubMed] [Google Scholar]
- 8.Glass P. S., Bloom M., Kearse L., Rosow C., Sebel P., Manberg P., Bispectral analysis measures sedation and memory effects of propofol, midazolam, isoflurane, and alfentanil in healthy volunteers. Anesthesiology 86, 836–847 (1997). [DOI] [PubMed] [Google Scholar]
- 9.Davis H. P., Squire L. R., Protein synthesis and memory: A review. Psychol. Bull. 96, 518–559 (1984). [PubMed] [Google Scholar]
- 10.McGaugh J. L., Memory--a century of consolidation. Science 287, 248–251 (2000). [DOI] [PubMed] [Google Scholar]
- 11.Nader K., Schafe G. E., Le Doux J. E., Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406, 722–726 (2000). [DOI] [PubMed] [Google Scholar]
- 12.M. C. W. Kroes, D. Schiller, J. E. LeDoux, E. A. Phelps, Translational approaches targeting reconsolidation, in Translational Neuropsychopharmacology (Springer, 2015), pp. 197–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schiller D., Monfils M.-H., Raio C. M., Johnson D. C., LeDoux J. E., Phelps E. A., Preventing the return of fear in humans using reconsolidation update mechanisms. Nature 463, 49–53 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kindt M., Soeter M., Vervliet B., Beyond extinction: Erasing human fear responses and preventing the return of fear. Nat. Neurosci. 12, 256–258 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Kindt M., Soeter M., Pharmacologically induced amnesia for learned fear is time and sleep dependent. Nat. Commun. 9, 1316 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cahill L., Prins B., Weber M., McGaugh J. L., β-adrenergic activation and memory for emotional events. Nature 371, 702–704 (1994). [DOI] [PubMed] [Google Scholar]
- 17.Strange B. A., Hurlemann R., Dolan R. J., An emotion-induced retrograde amnesia in humans is amygdala- and β-adrenergic-dependent. Proc. Natl. Acad. Sci. U.S.A. 100, 13626–13631 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kroes M. C. W., Strange B. A., Dolan R. J., β-adrenergic blockade during memory retrieval in humans evokes a sustained reduction of declarative emotional memory enhancement. J. Neurosci. 30, 3959–3963 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaiva G., Ducrocq F., Jezequel K., Averland B., Lestavel P., Brunet A., Marmar C. R., Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biol. Psychiatry 54, 947–949 (2003). [DOI] [PubMed] [Google Scholar]
- 20.Brunet A., Orr S. P., Tremblay J., Robertson K., Nader K., Pitman R. K., Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J. Psychiatr. Res. 42, 503–506 (2008). [DOI] [PubMed] [Google Scholar]
- 21.Kindt M., van Emmerik A., New avenues for treating emotional memory disorders: Towards a reconsolidation intervention for posttraumatic stress disorder. Ther. Adv. Psychopharmacol. 6, 283–295 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soeter M., Kindt M., Disrupting reconsolidation: Pharmacological and behavioral manipulations. Learn. Mem. 18, 357–366 (2011). [DOI] [PubMed] [Google Scholar]
- 23.Kredlow M. A., Unger L. D., Otto M. W., Harnessing reconsolidation to weaken fear and appetitive memories: A meta-analysis of post-retrieval extinction effects. Psychol. Bull. 142, 314–336 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bos M. G. N., Beckers T., Kindt M., Noradrenergic blockade of memory reconsolidation: A failure to reduce conditioned fear responding. Front. Behav. Neurosci. 8, 412 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schroyens N., Beckers T., Kindt M., In search for boundary conditions of reconsolidation: A failure of fear memory interference. Front. Behav. Neurosci. 11, 65 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wood N. E., Rosasco M. L., Suris A. M., Spring J. D., Marin M.-F., Lasko N. B., Goetz J. M., Fischer A. M., Orr S. P., Pitman R. K., Pharmacological blockade of memory reconsolidation in posttraumatic stress disorder: Three negative psychophysiological studies. Psychiatry Res. 225, 31–39 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Baddeley A., Conway M., Aggleton J., Tulving E., Episodic memory and common sense: How far apart? Philos. Trans. R. Soc. B Biol. Sci. 356, 1505–1515 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee J. L. C., Reconsolidation: Maintaining memory relevance. Trends Neurosci. 32, 413–420 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forcato C., Burgos V. L., Argibay P. F., Molina V. A., Pedreira M. E., Maldonado H., Reconsolidation of declarative memory in humans. Learn. Mem. 14, 295–303 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kroes M. C. W., Tendolkar I., van Wingen G. A., van Waarde J. A., Strange B. A., Fernández G., An electroconvulsive therapy procedure impairs reconsolidation of episodic memories in humans. Nat. Neurosci. 17, 204–206 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Strange B. A., Kroes M. C. W., Fan J. E., Dolan R. J., Emotion causes targeted forgetting of established memories. Front. Behav. Neurosci. 4, 175 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finnie P. S. B., Nader K., The role of metaplasticity mechanisms in regulating memory destabilization and reconsolidation. Neurosci. Biobehav. Rev. 36, 1667–1707 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Makkar S. R., Zhang S. Q., Cranney J., Behavioral and neural analysis of GABA in the acquisition, consolidation, reconsolidation, and extinction of fear memory. Neuropsychopharmacology 35, 1625–1652 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang S., Cranney J., The role of GABA and anxiety in the reconsolidation of conditioned fear. Behav. Neurosci. 122, 1295–1305 (2008). [DOI] [PubMed] [Google Scholar]
- 35.Bustos S. G., Maldonado H., Molina V. A., Midazolam disrupts fear memory reconsolidation. Neuroscience 139, 831–842 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Alkire M. T., Gruver R., Miller J., McReynolds J. R., Hahn E. L., Cahill L., Neuroimaging analysis of an anesthetic gas that blocks human emotional memory. Proc. Natl. Acad. Sci. U.S.A. 105, 1722–1727 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pryor K. O., Root J. C., Mehta M., Stern E., Pan H., Veselis R. A., Silbersweig D. A., Effect of propofol on the medial temporal lobe emotional memory system: A functional magnetic resonance imaging study in human subjects. Br. J. Anaesth. 115, i104–i113 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richardson M. P., Strange B. A., Dolan R. J., Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nat. Neurosci. 7, 278–285 (2004). [DOI] [PubMed] [Google Scholar]
- 39.American Society of Anesthesiologists, Continuum of depth of sedation: Definition of general anesthesia and levels of sedation/analgesia (2004).
- 40.Barnes A., Duncan R., Chisholm J. A., Lindsay K., Patterson J., Wyper D., Investigation into the mechanisms of vagus nerve stimulation for the treatment of intractable epilepsy, using 99m Tc-HMPAO SPET brain images. Eur. J. Nucl. Med. Mol. Imaging 30, 301–305 (2003). [DOI] [PubMed] [Google Scholar]
- 41.Clark K. B., Naritoku D. K., Smith D. C., Browning R. A., Jensen R. A., Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat. Neurosci. 2, 94–98 (1999). [DOI] [PubMed] [Google Scholar]
- 42.Alkire M. T., Vazdarjanova A., Dickinson-Anson H., White N. S., Cahill L., Lesions of the basolateral amygdala complex block propofol-induced amnesia for inhibitory avoidance learning in rats. Anesthesiology 95, 708–715 (2001). [DOI] [PubMed] [Google Scholar]
- 43.Alkire M. T., Nathan S. V., Does the amygdala mediate anesthetic-induced amnesia? Basolateral amygdala lesions block sevoflurane-induced amnesia. Anesthesiology 102, 754–760 (2005). [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y., Yu T., Yuan J., Yu B.-W., The ventrolateral preoptic nucleus is required for propofol-induced inhibition of locus coeruleus neuronal activity. Neurol. Sci. 36, 2177–2184 (2015). [DOI] [PubMed] [Google Scholar]
- 45.Schwabe L., Nader K., Wolf O. T., Beaudry T., Pruessner J. C., Neural signature of reconsolidation impairments by propranolol in humans. Biol. Psychiatry 71, 380–386 (2012). [DOI] [PubMed] [Google Scholar]
- 46.Kroes M. C. W., Tona K.-D., den Ouden H. E. M., Vogel S., van Wingen G. A., Fernández G., How administration of the beta-blocker propranolol before extinction can prevent the return of fear. Neuropsychopharmacology 41, 1569–1578 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Forman S. A., Clinical and molecular pharmacology of etomidate. Anesthesiology 114, 695–707 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin L. J., Oh G. H. T., Orser B. A., Etomidate targets α5γ-aminobutyric acid subtype A receptors to regulate synaptic plasticity and memory blockade. Anesthesiology 111, 1025–1035 (2009). [DOI] [PubMed] [Google Scholar]
- 49.Buchanan T. W., Lovallo W. R., Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology 26, 307–317 (2001). [DOI] [PubMed] [Google Scholar]
- 50.Strange B. A., Dolan R. J., β-adrenergic modulation of emotional memory-evoked human amygdala and hippocampal responses. Proc. Natl. Acad. Sci. U.S.A. 101, 11454–11458 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inda M. C., Muravieva E. V., Alberini C. M., Memory retrieval and the passage of time: From reconsolidation and strengthening to extinction. J. Neurosci. 31, 1635–1643 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kroes M. C. W., Dunsmoor J. E., Lin Q., Evans M., Phelps E. A., A reminder before extinction strengthens episodic memory via reconsolidation but fails to disrupt generalized threat responses. Sci. Rep. 7, 10858 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.A. P. Association, Diagnostic and Statistical Manual of Mental Disorders (DSM-5®) (American Psychiatric Pub, ed. 5, 2013). [Google Scholar]
- 54.Milekic M. H., Alberini C. M., Temporally graded requirement for protein synthesis following memory reactivation. Neuron 36, 521–525 (2002). [DOI] [PubMed] [Google Scholar]
- 55.Suzuki A., Josselyn S. A., Frankland P. W., Masushige S., Silva A. J., Kida S., Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J. Neurosci. 24, 4787–4795 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elsey J. W. B., Kindt M., Breaking boundaries: Optimizing reconsolidation-based interventions for strong and old memories. Learn. Mem. 24, 472–479 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Purdon P. L., Pierce E. T., Mukamel E. A., Prerau M. J., Walsh J. L., Wong K. F. K., Salazar-Gomez A. F., Harrell P. G., Sampson A. L., Cimenser A., Ching S., Kopell N. J., Tavares-Stoeckel C., Habeeb K., Merhar R., Brown E. N., Electroencephalogram signatures of loss and recovery of consciousness from propofol. Proc. Natl. Acad. Sci. U.S.A. 110, E1142–E1151 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/3/eaav3801/DC1
Fig. S1. DSST performance for groups A and B.
Fig. S2. Memory reactivation scores.
Fig. S3. Reconsolidation impairment is not correlated with propofol dose.


