Abstract
JAK inhibitors for myelofibrosis (MF) reduce spleen size, control constitutional symptoms, and may improve survival. We studied the clinical characteristics of 548 MF patients treated with JAK inhibitors from 2008–2016 to better understand predictors of splenic response. Response was defined as a 50% decrease in spleen size at early (3–4 months on therapy) and late (5–12 months) timepoints after therapy initiation. Early response positively correlated with higher doses of JAK inhibitor, baseline spleen size 5–10 cm, and HGB. Early response negatively correlated with baseline spleen size >20 cm and high WBC. The strongest predictor of late response was whether a patient had a response at the earlier timepoint (OR 8.88). Our response models suggest that clinical factors can be used to predict which patients are more likely to respond to JAK inhibitors, and those who do not achieve an early response, i.e. within 3–4 months, should consider alternative treatments.
Keywords: myelofibrosis, JAK inhibitors, predictive models, myeloproliferative neoplasms
Introduction
Myelofibrosis (MF) is a BCR-ABL1-negative myeloproliferative neoplasm characterized by megakaryocytic proliferation and atypia, and ineffective erythropoiesis [1]. Patients may present with constitutional symptoms, including fever, night sweats, pruritus, early satiety, and bone pain [2]. The median overall survival in MF ranges from 16 to 185 months, depending on the patient’s clinical characteristics [3,4]. Allogeneic hematopoietic stem cell transplantation remains the only curative therapy for MF; however, it has significant morbidity and risk of mortality [5,6].
Activation of the JAK-STAT pathway characterizes MF and results from mutations in JAK2, MPL, and CALR [7–13]. Newer therapies target JAK2 deregulation, although these agents are effective even in patients without one of the three mutations [14]. The JAK inhibitors pacritinib, ruxolitinib, fedratinib, and momelotinib have been developed to improve splenomegaly and constitutional symptoms [15,16]. In addition to improving signs and symptoms of the disease, ruxolitinib may also improve overall survival [17–20].
Despite the success of JAK inhibitors, it remains unclear which clinical factors predict response to therapy. The DIPSS and DIPSS-plus prognostic models can help make this determination, but these dynamic models were designed only to discriminate among patients with high and low rates of survival [3,21]. We describe a multivariate prediction model that identifies baseline and on-treatment clinical factors that predict response to therapy. Our approach aims to identify characteristics of MF patients who are more likely to respond to therapy, and further, define when a patient should be considered non-responsive to treatment.
Materials and Methods
Endpoints
We defined clinical response endpoints at two timepoints after initiation of JAK-inhibitor therapy, based on the literature and distribution of the retrospective data. The early endpoint was defined as having a follow-up splenic exam within 3–4 months of starting therapy, and the late endpoint was defined as having a follow-up splenic exam after 5–12 months on therapy. Response to therapy was based upon IWG-MRT criteria for splenic response by palpation below the left costal margin, with spleen measurement at each timepoint compared to spleen measurement at initiation of JAK-inhibitor therapy [22]. Response was defined as a ≥50% reduction in length of spleens measuring >5 cm at baseline. For patients with a spleen length of ≤ 5cm at baseline, we defined response as having a non-palpable spleen on exam. Among patients with valid endpoints, response was based upon the best splenic response out of all exams within each time period.
Training Cohorts
We constructed our predictive model for splenic response using a retrospective cohort of 383 patients with biopsy-proven MF receiving JAK-inhibitor therapy, before allogeneic stem cell transplant, between 2008–2016. This cohort included 203 patients enrolled in trials of ruxolitinib (INCB018424; Incyte), fedratinib (SAR302503; Sanofi-Aventis), momelotinib (CYT387; Gilead Sciences), or LY2784544 (Eli Lilly) at three sites (University of Michigan [MI]; Stanford University [ST]; Mayo Clinic--Scottsdale, AZ [MA]). We also included additional patients from MA who started on ruxolitinib following its FDA approval and 215 patients receiving pacritinib (SB1518) in the PERSIST-1 trial (PST1, CTI Biopharmaceuticals; ClinicalTrials.gov Identifier: NCT01773187). Among all 383 patients in the training cohort, 362 had a recorded early endpoint, 311 had a late timepoint, and 290 had endpoints at both times.
Validation Cohort
To validate our model’s performance, we used an independent cohort of 165 patients receiving pacritinib in the PERSIST-2 trial (CTI Biopharmaceuticals; ClinicalTrials.gov Identifier: NCT02055781). Among these, 162 patients had a recorded early endpoint, 109 had a late endpoint, and 109 patients had endpoints at both times. Supplementary Figure S1 delineates the construction of our analysis cohorts.
Data Processing
Data from the training cohorts included 5 JAK inhibitors. To account for this heterogeneity, each patient’s initial dose level was scaled by the recommended phase 2 dose (RP2D) of the specific JAK inhibitor they received. The resulting dimensionless, normalized dose levels were then log2-transformed to calculate a relative initial dose for each patient; a relative dose of 0 corresponds to the RP2D. Equal relative initial dose levels of different JAK inhibitors were assumed to have equivalent associations with splenic response.
In addition to the normalized JAK inhibitor dose, we considered 13 additional covariates in our predictive model: seven measures of disease severity (1. spleen size by exam at baseline, 2. type of MF, 3. time from diagnosis to initiation of therapy, 4. bone marrow cellularity, 5. fibrosis grade by European Consensus criteria [23,24], 6. peripheral blood blasts >1%, and 7. blood transfusion requirement); three blood counts (platelet, white blood cell (WBC), hemoglobin (HGB)); and three other patient characteristics (sex, age at start of treatment, BMI). Jak2 mutation status was not consistently available for this retrospective cohort and was therefore excluded from analysis.
Model Building
Our initial analyses suggested that the association between baseline spleen size and the probability of splenic response was nonlinear. To account for this, we divided baseline spleen size into five categories, <= 5cm, (5cm, 10cm], (10cm, 15cm], (15cm, 20cm], and >20cm, and estimated associations separately for each category. Additionally, we examined statistical interactions between baseline spleen size, relative dose, and leukocytosis, and interactions between transfusion requirement and both HGB and thrombocytopenia.
We measured associations between response and candidate prognostic factors using Bayesian logistic regressions. After accounting for interactions and categorical variables, each multivariable model considered up to 33 odds-ratios (ORs), which quantify association. To measure the association between early and late response after adjusting for all other factors, we fit a third regression for predicting late response that included data on whether the patient had responded by the early timepoint. We used variable selection shrinkage priors to account for optimism and overfitting biases. We defined the ‘statistical significance’ of an association as having a greater than 66% probability of being positively or negatively associated with the outcome, referred to as relevance percentage. Note that standard measures of statistical significance, e.g. p-values, are not defined in the context of Bayesian regressions such as those here. Details of our modeling approach are given in Supplement S2.
Model Assessment
We thoroughly validated our models, applying cross-validation between the four training cohorts and external validation to the PST2 cohort. We assessed model performance using area under the curve (AUC) and goodness-of-fit methods.
Results
Descriptive Summaries
Supplementary Table S1 presents response rates in patients in model-building and validation data. In increasing order, the early response rates were 31% (PST2), 31% (ST), 35% (MI), 43% (PST1), and 64% (MA). The late response rates were 36% (PST2), 43% (ST), 47% (PST1), 50% (MI), and 60% (MA). Among the 290 patients in the model-building data with response at both timepoints, the early response rate was 41% (120/290); among these 120 patients, the late response rate was 78% (94/120). Among the 170 non-early responders, the late response rate was 26% (44/170).
Supplementary Tables S1 and S2 summarize the distributions of the evaluated prognostic factors. Because our primary aim was not to identify differences between patient populations but to use these differences to identify prognostic factors for splenic response, we present these differences without measures of statistical significance.
Splenic Response Models
Table 1 presents the ORs and relevance percentages for the selected predictors in the three regression models, after applying our model-building procedure. Evaluated predictors that were not selected in any model do not appear in Table 1. Each of the prognostic factors selected and discussed below is statistically significant as defined in the Methods (associations from categorical predictors were either all selected or all excluded). The selection of statistical interactions is clinically notable but complicates interpretation; we interpret results below but refer readers to Supplement S2 for details on calculating these associations.
Table 1.
Results from Early, Late, and Late-Given-Early splenic response models, given in terms of estimated odds ratios (ORs) for splenic response and relevance percentages. The latter is the larger of the probability that the OR exceeds 1 and the probability that the OR is less than 1. A dash (–) means that the predictor was not included in that model. A Relative Dose of 0 corresponds to the MTD of a JAK inhibitor. Supplement S2 describes how to calculate fitted probabilities using this table.
| Main Effects | Early | Late | Late-Given-Early |
|---|---|---|---|
| Intercept | 0.03 (96%) | 0.98 (51%) | 0.66 (79%) |
| Relative Dose (per doubling) | 1.90 (96) | 1.16 (70) | – |
| Spleen ≤ 5 | Reference | Reference | Reference |
| Spleen (5,10] vs. Ref. | 1.94 (94) | 1.63 (90) | 1.15 (78) |
| Spleen (10,15] vs. Ref. | 1.44 (86) | 1.08 (72) | 0.99 (58) |
| Spleen (15,20] vs. Ref. | 0.86 (71) | 0.99 (52) | 1.00 (50) |
| Spleen > 20 vs. Ref. | 0.27 (96) | 0.41 (97) | 0.92 (72) |
| WBC > 10 (per doubling) | 0.72 (95) | 0.96 (75) | – |
| Platelets > 450 (per doubling) | 1.61 (86) | – | – |
| HGB (per doubling) | 2.07 (94) | 1.22 (79) | – |
| Age (per 5 years) | 1.08 (91) | – | – |
| Weeks from DX | – | 0.97 (83) | 0.95 (86) |
| Transf. Required | 1.13 (75) | – | – |
| Cellularity Hypo. | 0.78 (84) | 0.87 (77) | – |
| Fibrosis MF-2 vs. MF-1 | 1.06 (68) | – | – |
| Fibrosis MF-3 vs. MF-1 | 0.80 (84) | 0.65 (95) | 0.83 (83) |
| Early Splenic Resp. = Yes | – | – | 8.88 (100) |
| Interaction Terms | |||
| Relative Dose*Spleen (5,10] | 0.99 (57) | 0.69 (82) | – |
| Relative Dose*Spleen (10,15] | 1.10 (72) | 1.19 (79) | – |
| Relative Dose*Spleen (15,20] | 1.04 (62) | 1.10 (73) | – |
| Relative Dose*Spleen >20 | 0.94 (62) | 1.00 (51) | – |
| WBC > 10 *Spleen (5,10] | 1.00 (51) | – | – |
| WBC > 10 *Spleen (10,15] | 1.00 (51) | – | – |
| WBC > 10 *Spleen (15,20] | 1.01 (55) | – | – |
| WBC > 10 *Spleen >20 | 0.87 (77) | – | – |
Early Response (First Column of Table 1)
The reference category for baseline spleen size was ‘<=5cm’. The OR for response to therapy per doubling of dose was 1.90 in this group. We summarize the interpretation for each spleen size group with respect to this reference:
5–10cm The OR at the standard dose was 1.94 times that of the reference group; the OR per doubling of dose was 1.88 (not significantly different from reference OR of 1.90). This group had a larger association with early response at the reference dose and a similar dose-response association.
10–15cm The OR at the standard dose was 1.44 times that of the reference group; the OR per doubling of dose was 2.09. This group had a larger association with early response at the reference dose and a larger dose-response association.
15–20cm The OR at the standard dose was 0.86 times that of the reference group; the OR per doubling of dose was 1.98 (not significantly different from reference). This group had a smaller association with early response at the reference dose and a similar dose-response association.
>20cm The OR at the standard dose was 0.27 times that of the reference group; the OR per doubling of dose was 1.79 (not significantly different from reference). This group had a much smaller base association with early response at the reference dose and a similar dose-response association.
High platelets, increased HGB, older age, transfusion requirement, and fibrosis grade of MF-2 (versus MF-1) were also associated with greater rates of early splenic response, whereas high WBC, hypocellularity and fibrosis grade of MF-3 were associated with lower rates of early response.
Late Response (Second Column of Table 1)
The OR per doubling of dose was 1.16 in the reference group. We summarize the interpretation for each spleen size group relative to this reference:
5–10cm The OR at the standard dose was 1.63 times that of the reference group; the OR per doubling of dose was 0.80. This group had a larger association with late response at the reference dose and a negative dose-response association.
10–15cm The OR at the standard dose was 1.08 times that of the reference group; the OR per doubling of dose was 1.38. This group had a larger association with late response at the reference dose and a larger dose-response association.
15–20cm The OR at the standard dose was 0.99 times that of the reference group (not significantly different from reference); the OR per doubling of dose was 1.31. This group had a similar association with late response at the reference dose and a larger dose-response association.
>20cm The OR at the standard dose was 0.41 times that of the reference group; the OR per doubling of dose was 1.16 (not significantly different from reference of 1.16). This group had a much smaller association with response at the reference dose and a similar dose-response association.
Increased HGB was also associated with greater rates of late splenic response, whereas high WBC, time from diagnosis, hypocellularity and fibrosis grade of MF-3 were associated with lower rates of late response.
Dynamic Prediction of Late Response (Late-given-Early)
The ORs in the third column of Table 1 represent predictions of late response given whether or not a patient achieved a response by the early timepoint. Apart from early response status, the model includes baseline spleen size, time from diagnosis, and fibrosis grade MF-3. Thus, no association with initial dose was apparent given these predictors. A patient’s early response status is a highly meaningful predictor for late response (OR 8.88). Translating this quantity, if a patient who did not achieve a response by the early timepoint has a 25% probability of achieving a late response, an identical patient who did achieve a response by the early timepoint would have a 75% probability of having a late response. This comports with our early observation that 26% of early non-responders were late responders, compared to 78% of early responders. Across all patients, the average late response rate was 49%.
We can visualize how the probabilities of an early response (left panel) and late response (right panel) shift with WBC count and spleen size prior to the start of therapy for a hypothetical patient (Figure 1). When the patient has a normal WBC count (7*109/L), their probability of response depends on baseline spleen size. With a baseline spleen size of 5–10 cm, this patient would have a better probability of an early response (0.70) than with a spleen size <5 cm (0.50) or >20 cm (0.20) at baseline. If the WBC were elevated (35*109/L), the probability of an early response would decrease by 0.10–0.15 for each spleen size category. This difference in response based on baseline spleen size and WBC count is no longer seen when the late response model accounts for whether the patient achieved a response at the earlier timepoint (Figure 2). This suggests that achievement of an early response is a much stronger predictor of achieving a late response than baseline WBC count and spleen size.
Figure 1.
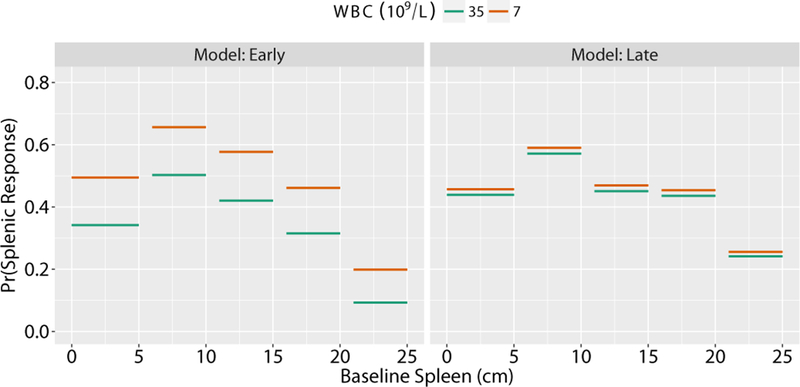
Estimated probabilities of splenic response using the Early and Late models. To demonstrate how the early (left panel) and late (right panel) models can be used to predict splenic response, plots based on increasing spleen size are shown for a hypothetical patient treated with JAK-inhibitor therapy. Two possible WBC counts were inputted (blue circles=7*109/L WBC; yellow triangles=35*109/L WBC). All other patient characteristics are fixed: 70 year old male diagnosed 140 weeks ago treated at 100% of the recommended phase 2 dose, platelets 210*109/L, Hgb 10.7 g/dL, requiring transfusions at baseline, and European Consensus Criteria MF-3.
Figure 2.
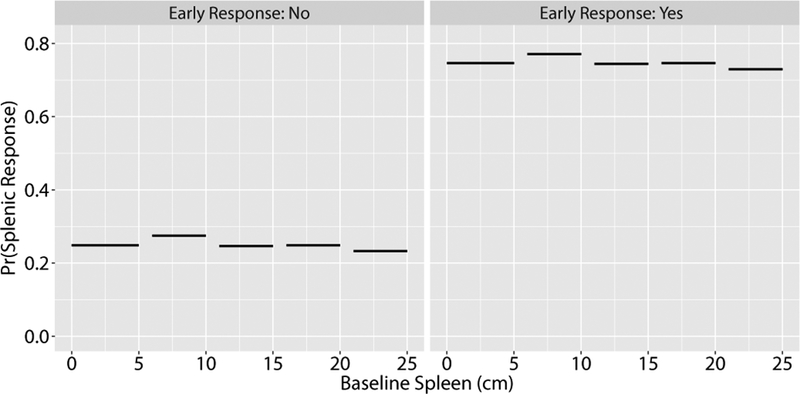
Estimated probabilities of splenic response for the Late-Given-Early model. This plot shows the application of the late-given-early model for predicting splenic response based on initial spleen sizes of a hypothetical patient treated with JAK-inhibitor therapy. Probabilities are stratified by no early response (left panel) or early response (right panel), with the fixed patient characteristics described in Figure 1.
The discrimination and calibration of these models is presented in Supplementary Table S3 and Supplementary Figures S2 and S3. The cross-validated and validated AUCs are, respectively, 0.679 and 0.616 (Early), 0.641 and 0.523 (Late), and 0.765 and 0.792 (Late-given-Early). Lack of model fit was most evident among patients with small baseline spleen sizes (Supplementary Figure S2) or low baseline platelet levels (Supplementary Figure S3).
Discussion
We developed prediction models for splenic response to JAK-inhibitor therapy using retrospective data from 548 patients across three academic centers and two industry-based cohorts. We evaluated 14 clinical prognostic factors, with a specific hypothesis that spleen size by palpation at initiation of JAK-inhibitor therapy would be associated with splenic response. For timepoints approximately 3–4 months (‘early’) and 5–12 months (‘late’) after therapy initiation, splenic response correlated with spleen size prior to therapy initiation, WBC, HGB, transfusion requirement, and fibrosis grade, as well as other factors. The finding that both higher levels of HGB and a transfusion requirement predict for early response further suggests that response can occur along the spectrum of disease phenotypes, with a loss of response once the patient’s marrow becomes too fibrotic and hypocellular. Our two most important findings are that (i) patients with intermediate spleen sizes (between approximately 5–15 cm) were more likely to be responders than patients with smaller (non-palpable) or larger spleens and (ii) early-responding patients had high probabilities of maintaining response.
JAK-inhibitor therapy is commonly started when a patient has an IPSS score of intermediate-2 or higher, or for the treatment of constitutional symptoms and splenomegaly [25,26]. Patients with symptomatic splenomegaly are considered for treatment, and no strict cutoff in size is used for therapy initiation. We found that the probability of response was highest for patients with spleen sizes between 5 and 15 cm at therapy initiation. These patients were more likely to respond to JAK-inhibitor therapy than those started on therapy when their spleens were non-palpable or less than 5 cm in size. Patients with spleen sizes greater than 20 cm were least likely to respond, even less so when they also had a high WBC.
The underlying biology of this disease could explain this varying pattern of response to therapy depending on baseline spleen size. We hypothesize that a more proliferative phenotype in myelofibrosis could give patients a better chance of responding to JAK inhibitor therapy. Patients with spleen sizes less than 5 cm may have disease progressing too slowly for treatment to be effective. Those with intermediate spleen sizes may be in a more proliferative phase of the disease, in which interruption of signaling with JAK inhibitors can temper disease progression. Patients with large spleen sizes and high WBCs may have progressed too far, with marrow too fibrotic and hypocellular, for these agents to modify disease course. Future translational studies could elucidate whether the biology of this disease follows these patterns.
Regarding prediction of response duration, we found that early response status was highly prognostic for late response status: the odds of late splenic response were nearly 9 times larger among early responders than early non-responders. After adjusting for early response status, other prognostic factors, including spleen size at therapy initiation and initial dose of JAK inhibitor, had significantly less association with late response. Data beyond 6 months support our findings: among patients who were not responders at 3 months from initiation but did eventually respond at 6 months, 45% continued to respond at their final recorded splenic exam (median 92 weeks from initiation), and among patients who were responders at both 3 and 6 months, 68% were still responding at their final recorded splenic exam (median 82 weeks from initiation; results not shown). Thus, early response status was also associated with longer-term (>6 month) splenic response.
Our study has some limitations mainly because of its retrospective nature. Rates of response varied between cohorts – even after adjusting for predictors. Additionally, we included patients with non-palpable or small (5–10 cm) spleens at baseline because they are commonly seen in clinical practice and increase the efficiency of our analysis, even though they are excluded from the IWG-MRT criteria. To control for potential differences between treatment centers, we estimated separate institution-specific means in the regression model.
When the study was initiated, JAK2 inhibitors were not widely in use, so data from multiple trials of JAK2 inhibitors were pooled. While we acknowledge variability between agents, all inhibit JAK2 and will similarly affect JAK/STAT signaling, with or without additional effects mediated by JAK1 inhibition. Our measure of dose of the JAK inhibitor does not account for intra-patient dose adjustments made in response to a patient’s tolerance. However, dose reductions and adjustments take place in all clinical studies with JAK inhibitors, and responses are typically analyzed based on intended dose, not actual dose. We have also made the simplifying assumption that each JAK inhibitor has the same association with response (as a percentage of the RP2D), all other things being equal. Although the response rates to these agents may differ in subpopulations, no data suggest that the differences in response would be large enough to confound this analysis [27].
The intention of our models was not to assess the overall utility of JAK-inhibitor therapy in MF, nor to compare the efficacy of different JAK inhibitors. Rather, our study identified patient characteristics that help to explain why only some patients respond. Established scoring systems, such as IPSS, DIPSS, or DIPSS-plus are designed to predict survival in a treatment-agnostic context. Unlike those models, our models include spleen size at therapy initiation, time from diagnosis to start of therapy, and grade of MF in the marrow, and were developed with different endpoints and target populations. In all, this study shows that clinical factors, particularly spleen size, can be used to predict which patients are more likely to respond to JAK-inhibitor therapy. Those who respond after about three months of treatment are more likely to continue to respond, whereas those who do not should be considered for alternative therapies.
Supplementary Material
Acknowledgments
This study was supported by research funding from CTI Biopharmaceuticals (PSB, MT), the National Institutes of Health grant P30-CA046592 (PSB), and the Michigan Institute for Clinical and Health Research (MICHR) and REDCap Database Support CTSA grant UL1TR000433 (KM).
Footnotes
Declaration of interest
KM, JM, CP, KG, AW report no conflict of interest
PSB: Research support – CTI Biopharma
RM: Consultant – Novartis, Ariad, Galena; Research Support – Incyte, Gilead, CTI, Promedior, Celgene
JRG: Consultant – Gilead, CTI BIOPharma, Incyte; Funding Support – Gilead, CTI BIOPharma
LW: Full-time employee of CTI BIOPharma
JWS: Full-time employee of CTI BIOPharma
MT: Past advisory board – CTI Biopharma; Research support – CTI Biopharma, Incyte, Gilead and NS Pharma
References
- [1].Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. [DOI] [PubMed] [Google Scholar]
- [2].Mesa RA, Schwager S, Radia D, et al. The Myelofibrosis Symptom Assessment Form (MFSAF): an evidence-based brief inventory to measure quality of life and symptomatic response to treatment in myelofibrosis. Leuk. Res 2009;33:1199–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gangat N, Caramazza D, Vaidya R, et al. DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol 2011;29:392–397. [DOI] [PubMed] [Google Scholar]
- [4].Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113:2895–2901. [DOI] [PubMed] [Google Scholar]
- [5].Kröger N, Giorgino T, Scott BL, et al. Impact of allogeneic stem cell transplantation on survival of patients less than 65 years of age with primary myelofibrosis. Blood. 2015;125:3347–3350; quiz 3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kröger NM, Deeg JH, Olavarria E, et al. Indication and management of allogeneic stem cell transplantation in primary myelofibrosis: a consensus process by an EBMT/ELN international working group. Leukemia. 2015;29:2126–2133. [DOI] [PubMed] [Google Scholar]
- [7].Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. The Lancet. 2005;365:1054–1061. [DOI] [PubMed] [Google Scholar]
- [8].Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. [DOI] [PubMed] [Google Scholar]
- [9].Kralovics R, Passamonti F, Buser AS, et al. A Gain-of-Function Mutation of JAK2 in Myeloproliferative Disorders. N. Engl. J. Med 2005;352:1779–1790. [DOI] [PubMed] [Google Scholar]
- [10].Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic Mutations of Calreticulin in Myeloproliferative Neoplasms. N. Engl. J. Med 2013;369:2379–2390. [DOI] [PubMed] [Google Scholar]
- [11].Nangalia J, Massie CE, Baxter EJ, et al. Somatic CALR Mutations in Myeloproliferative Neoplasms with Nonmutated JAK2. N. Engl. J. Med 2013;369:2391–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pikman Y, Lee BH, Mercher T, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3:e270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Scott LM, Tong W, Levine RL, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N. Engl. J. Med 2007;356:459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Santos FPS, Verstovsek S. JAK2 inhibitors for myelofibrosis: why are they effective in patients with and without JAK2V617F mutation? Anticancer Agents Med. Chem 2012;12:1098–1109. [DOI] [PubMed] [Google Scholar]
- [15].Gupta V, Mesa RA, Deininger MWN, et al. A phase 1/2, open-label study evaluating twice-daily administration of momelotinib in myelofibrosis. Haematologica. 2017;102:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mesa RA, Vannucchi AM, Mead A, et al. Pacritinib versus best available therapy for the treatment of myelofibrosis irrespective of baseline cytopenias (PERSIST-1): an international, randomised, phase 3 trial. Lancet Haematol. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Verstovsek S, Mesa RA, Gotlib J, et al. Efficacy, safety and survival with ruxolitinib in patients with myelofibrosis: results of a median 2-year follow-up of COMFORT-I. Haematologica. 2013;98:1865–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Vannucchi AM, Kantarjian HM, Kiladjian J-J, et al. A pooled analysis of overall survival in COMFORT-I and COMFORT-II, 2 randomized phase III trials of ruxolitinib for the treatment of myelofibrosis. Haematologica. 2015;100:1139–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Verstovsek, et al. A Pooled Overall Survival (OS) Analysis of 5-Year Data from the COMFORT-I and COMFORT-II Trials of Ruxolitinib for the Treatment of Myelofibrosis (MF). ASH 2016 Present. 3110 2016; [Google Scholar]
- [20].Cervantes F, Vannucchi AM, Kiladjian J-J, et al. Three-year efficacy, safety, and survival findings from COMFORT-II, a phase 3 study comparing ruxolitinib with best available therapy for myelofibrosis. Blood. 2013;122:4047–4053. [DOI] [PubMed] [Google Scholar]
- [21].Cervantes F How I treat myelofibrosis. Blood. 2014;124:2635–2642. [DOI] [PubMed] [Google Scholar]
- [22].Tefferi A, Cervantes F, Mesa R, et al. Revised response criteria for myelofibrosis: International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) and European LeukemiaNet (ELN) consensus report. Blood. 2013;122:1395–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gianelli U, Vener C, Bossi A, et al. The European Consensus on grading of bone marrow fibrosis allows a better prognostication of patients with primary myelofibrosis. Mod. Pathol 2012;25:1193–1202. [DOI] [PubMed] [Google Scholar]
- [24].Thiele J, Kvasnicka HM, Facchetti F, et al. European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica. 2005;90:1128–1132. [PubMed] [Google Scholar]
- [25].Verstovsek S, Mesa RA, Gotlib J, et al. A Double-Blind, Placebo-Controlled Trial of Ruxolitinib for Myelofibrosis. N. Engl. J. Med 2012;366:799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Harrison C, Kiladjian J-J, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N. Engl. J. Med 2012;366:787–798. [DOI] [PubMed] [Google Scholar]
- [27].Hobbs GS, Rozelle S, Mullally A. The Development and Use of Janus Kinase 2 Inhibitors for the Treatment of Myeloproliferative Neoplasms. Hematol. Oncol. Clin. North Am 2017;31:613–626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


