Abstract
Hyperhomocysteinemia (Hhcy) is an independent risk factor for Alzheimer’s disease (AD). Visual dysfunction is commonly found and is positively correlated with the severity of cognitive defects in AD patients. Our previous study demonstrated that Hhcy induces memory deficits with AD-like tau and amyloid-β (Aβ) pathologies in the hippocampus, and supplementation with folate and vitamin B12 (FB) prevents the Hhcy-induced AD-like pathologies in the hippocampus. Here, we investigated whether Hhcy also induces AD-like pathologies in the retina and the effects of FB. An Hhcy rat model was produced by vena caudalis injection of homocysteine for 14 days, and the effects of FB were assessed by simultaneous supplementation with FB in drinking water. We found that Hhcy induced vessel damage with Aβ and tau pathologies in the retina, while simultaneous supplementation with FB remarkably attenuated the Hhcy-induced tau hyperphosphorylation at multiple AD-related sites and Aβ accumulation in the retina. The mechanisms involved downregulation of amyloid precursor protein (APP), presenilin-1, beta-site APP-cleaving enzyme 1, and protein phosphatase-2A. Our data suggest that the retina may serve as a window for evaluating the effects of FB on hyperhomocysteinemia-induced Alzheimer-like pathologies.
Electronic supplementary material
The online version of this article (10.1007/s12264-018-0293-8) contains supplementary material, which is available to authorized users.
Keywords: Hyperhomocysteinemia, Alzheimer’s disease, Retina, Folate, Vitamin B12
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by a progressive deterioration of cognitive functions and its initial symptoms are easily mistaken for stress or ageing [1]. In addition, AD is associated with high comorbidity burdens [2]. Pathologically, AD is characterized by the accumulation of extracellular plaques composed of β-amyloid (Aβ) and intracellular neurofibrillary tangles (NFTs) comprised of hyperphosphorylated tau in the brain. These pathological changes lead to synaptic dysfunction and neuronal loss [3]. Impaired visual function is also commonly reported in AD patients.
The development of effective medications to cure or delay the progressive brain degeneration in AD is a great challenge [4, 5]. Currently, the medications used to treat AD include rivastigmine, tacrine, donepezil, galantamine, and memantine. However, these drugs cannot stop or even delay the progression of the disease. Therefore, finding non-invasively detectable early indicators will help to assess the effectiveness of treatment strategies, and eventually help to treat or delay the progression of this type of neurodegenerative disease.
The retina, a developmental outgrowth of the brain, is considered to be a window through which to study the brain. The retina is marked by an array of pathological features in AD patients, including retinal ganglion cell (RGC) degeneration, nerve fiber layer thinning, and changes in vascular parameters. Moreover, recent studies have suggested that multiple sclerosis, Parkinson’s disease, and ischemic stroke also exhibit retinal abnormalities similar to the cerebral pathologies, and the retina is an appealing target in which to detect neurodegenerative disease [6–8]. The accumulation of hyperphosphorylated tau and Aβ has also been detected in the retina at early stages of AD [9, 10], indicating that AD is both a cerebral and an ocular disease [11]. However, the upstream factors inducing AD-like pathologies in the retina and whether they can be prevented and detected are still elusive.
Epidemiological and clinical investigations have shown that hyperhomocysteinemia (Hhcy) is an independent risk factor for AD [12–15]. In Hhcy mouse models, AD-like behavioral and neuropathological changes have been detected [16–19]. Our previous studies also demonstrated that elevation of plasma homocysteine (Hcy) induces AD-like Aβ accumulation and tau hyperphosphorylation in rats [20, 21]. Therefore, reducing the elevated plasma Hcy could be a promising strategy to arrest the AD process.
Hcy is a thiol-containing amino-acid formed by the demethylation of methionine. Hcy is catabolized through multiple pathways and one of these is dependent on folate and vitamin B12 (FB). Folate provides the methyl and vitamin B12 works as a co-enzyme for the methyltransferase [22]. FB can reduce the level of plasma Hcy [23]. Simultaneous supplementation with FB partially restores the plasma Hcy level and ameliorates the AD-like tau and Aβ pathologies in the hippocampus [20, 21]. Although it is commonly known that FB plays an important role in Hcy metabolism, the effects of FB treatment on Hhcy-induced AD-like lesions in the retina have not been reported to date.
In the present study, we produced an Hhcy model in adult rats by injecting Hcy through the vena caudalis as reported previously [21]. We found that in addition to vessel damage, Hhcy induced AD-like tau and Aβ pathologies in the retina, while simultaneous administration of FB remarkably attenuated these effects.
Materials and Methods
Antibodies and Chemicals
The primary antibodies used are listed in Table S1. Rhodamine Red-X-conjugated goat anti-mouse IgG (H+L) and Oregon Green 488-conjugated goat anti-rabbit IgG (H+L) were from Molecular Probes (Eugene, OR). DL-homocysteine was from Sigma Chemical Co. (St Louis, MO). Ketamine was from Hengrui Co. (Jiangsu, China) and xylazine was from Sigma Chemical Co. Folate was from Yabao Co. (Tianjin, China) and vitamin B12 was from Shiyao Co. (Shijiazhuang, China). The bicinchoninic acid BCA protein detection kit was from Pierce Chemical Co. (Rockford, IL).
Animals and Drug Administration
Male Sprague-Dawley rats (3–4 months old), supplied by the Experimental Animal Center of Tongji Medical College, Huazhong University of Science and Technology, were housed under a 12:12 light-dark cycle. The animal experiments were performed according to the “Policies on the Use of Animals and Humans in Neuroscience Research” revised by the Society for Neuroscience in 1995, and all animal experiments were approved by the Ethics Committee of Tongji Medical College. The rats were injected with Hcy (400 μg/kg per day) for 14 days with or without simultaneous supplementation with folate (4 mg/kg per day) and vitamin B12 (250 μg/kg per day) in their drinking water [21, 24] for 14 days. The injection was implemented from 09:00 to 11:30 each day. The animals were sacrificed 24 h after the final injection after recording the electroretinogram (ERG) and photographic examination of the fundus.
Electroretinograms
Animals were dark-adapted overnight (> 12 h) and prepared for recordings under dim red illumination. Rats were intraperitoneally anesthetized with a mixture of 0.12 g/mL ketamine hydrochloride and 1.8 mg/mL xylazine hydrochloride. The injection volume was 8 mL/kg of body weight. Then, pupil dilation and topical corneal anesthesia were achieved using 0.5% tropicamide and 0.5% proparacaine, respectively (Alcon Laboratories, Fort Worth, TX). Custom-made silver chloride electrodes were placed on the right eye, with the reference electrode situated circumferentially around the corneoscleral limbus. A subdermal needle electrode (Technomed Europe, Maastricht, Netherlands) at the tail base served as the ground. Simultaneous ERGs were recorded using the RETI port (Roland Consult, Brandenburg an der Havel, Germany) Electrodiagnostic Testing System with brief, white light stimuli. Scotopic threshold responses were obtained for white flash stimuli (< 5 ms duration) and ERGs were recorded.
Fundus Photography
The central retina of rats was visualized under a microscope (Zeiss, Thornwood, NY) using hydroxypropyl methylcellulose ophthalmic (Goniosol; Novartis, Basel, Switzerland). The entire retina, including the peripheral retina up to the ora serrata in all directions, was examined and photographed using fundus camera (Micron IV; Toshiba, Irvine, CA). Briefly, the diameters of three of the most prominent vessels were estimated at 3 sites in its widest portion at equal distances from the center [25]. Before estimation of the diameter, the retinal photographs from all groups were randomized, and the estimations were made by three independent observers. The final vessel diameter was the average of the three estimates.
Hematoxylin-Eosin (HE) Staining
Rats were sacrificed by an overdose of chloral hydrate (1 g/kg) and perfused through the aorta with 100 mL 0.9% NaCl followed by 400 mL phosphate buffer containing 4% paraformaldehyde. The eyes were removed, embedded in paraffin, and then cut into sections (4 μm). The sections were collected consecutively in PBS for HE staining according to the instructions with the kit (Beyotime, China), and images were captured using an optical microscope (Olympus BX60, Tokyo, Japan).
Immunohistochemical Staining
Rats were sacrificed by an overdose of chloral hydrate (1 g/kg, i.p.) and perfused through the aorta with 100 mL 0.9% NaCl followed by 400 mL phosphate buffer containing 4% paraformaldehyde. The eyes were removed and paraffin-embedded and then cut into sections (4 μm). The sections of retina were collected consecutively in PBS for immunohistochemical staining. Free-floating sections were blocked with 0.3% H2O2 in absolute methanol for 30 min and nonspecific sites were blocked with bovine serum albumin for 30 min at room temperature. Then the sections were incubated overnight at 4 °C with primary antibodies (Table S1). After washing three times with PBS, the sections were incubated with biotin-labeled secondary antibodies for 1 h at 37 °C and washed again three times. The immunoreaction was measured using horseradish peroxidase-labeled antibodies for 1 h at 37 °C and visualized with the diaminobenzidine tetrachloride system (brown color). After three washes with PBS, positive staining of the tissues was detected with diaminobenzidine according to the manufacturer’s manual (ZSGB-BIO, Beijing, China). The sections were mounted and visualized by light microscopy (Olympus BX60, Tokyo, Japan), then the images were captured. For immunofluorescence staining, free-floating sections were incubated at 4 °C overnight with primary antibodies (Table S1). The secondary antibodies conjugated to Alexa-Fluor 488/546 were added to the coverslip and left for 1 h at room temperature, and then DAPI (1:1000) for 10 min. The coverslips were washed, mounted onto slides, and imaged under a Carl Zeiss LSM710 confocal microscope.
Sandwich Enzyme-Linked Immunosorbent Assay (ELISA)
ELISA was used to assess the plasma level of Hcy and the Aβ1-42 level in retinal extracts following the manufacturer’s instructions (Elabscience, Wuhan, China).
Western Blotting
Western blotting used the method established in our laboratory. Briefly, the dissected retinal tissues were homogenized or lysed in RIPA buffer and centrifuged for 10 min at 5000 ×g, then the supernatant was collected and the protein levels were analyzed. For Western blotting, protein extracts were separated by SDS/PAGE followed by transfer onto 0.2 µm nitrocellulose membranes. The membranes were blocked with 5% bovine serum albumin (Sigma) in Tris-buffered saline (TBS), then the membranes were washed with TBS containing 1% Tween-80 (Sigma) and incubated with primary antibodies in blocking buffer. The primary antibodies used in Western blotting were against APP (1:1000, Millipore, Billerica, MA), PS1 (1:500, Millipore), BACE1 (1:500, Santa Cruz, CA), Tau1 (1:1000, Chemicon, CA), pS262 (1:500, SAB, Zhejiang, China), pS356 (1:500, SAB), T-GSK-3β (1:1000, Cell Signaling, Danvers, MA), S9-GSK-3β (1:1000, Cell Signaling), PP2Ac (1:1000, Biosource, Camarillo, CA), p-PP2Ac (1:1000, Santa Cruz), M-PP2Ac (1:1000, Abcam, Cambridge, UK), and DM-PP2Ac (1:1000, Abcam) (Table S1). Blots were then incubated and visualized with the secondary antibodies (1:10,000; Odyssey, Li-Cor Biosciences, Nebraska, USA) at room temperature. The immunoreactive bands were visualized with the Odyssey Infrared Imaging System and quantitatively analyzed using ImageJ software (Version 1.5; National Institutes of Health, Bethesda, MD).
Statistical Analyses
Data were analyzed using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA) and SPSS version 21.0 for Windows (SPSS Inc., Chicago, IL). Student’s t-test for two-group comparisons, or one-way ANOVA followed by post hoc tests for multiple comparisons among more than two groups was used to determine the differences between the means among groups. The results are presented as mean ± SEM and P < 0.05 was accepted as statistically significant.
Results
FB Attenuates Hhcy Along with Amelioration of Impairments in the Retinal Artery and RGCs
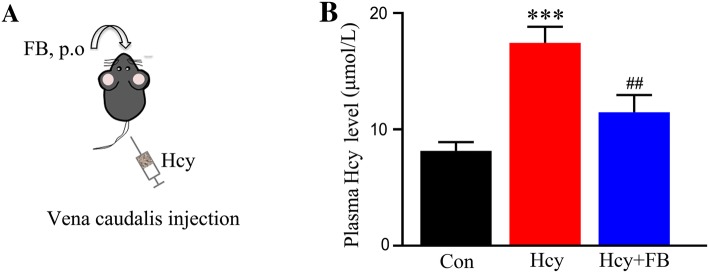
By vena caudalis injection of Hcy (400 μg/kg per day) for 14 days, we previously established an Hhcy rat model with AD-like tau and Aβ abnormalities in the hippocampus [18, 19]. To further investigate whether Hhcy also induces AD-like pathologies in the retina and the preventive effects of FB, we used the Hhcy rat model with simultaneous supplementation with FB (Fig. 1A). By measuring plasma Hcy using ELISA, we first confirmed that the injections increased the plasma Hcy level to ~ 17.5 µmol/L in rats, which is consistent with the Hhcy standard (> 15 µmol/L) for humans [10]. We also found that simultaneous supplementation with FB restored the plasma Hcy level in rats (Fig. 1B).
Fig. 1.
Simultaneous supplementation with FB reduces plasma Hcy level. A Schematic of procedures: rats were injected via the vena caudalis with Hcy (400 μg/kg per day) with or without folate and vitamin B12 (FB) supplementation. B FB reduced plasma Hcy levels in rats as measured by sandwich ELISA. ***P < 0.001 versus control; ##P < 0.01 versus Hcy at 14 days.
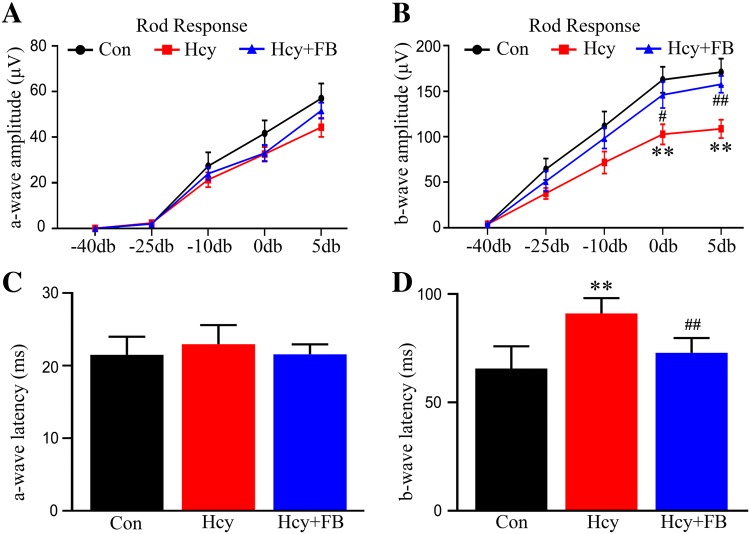
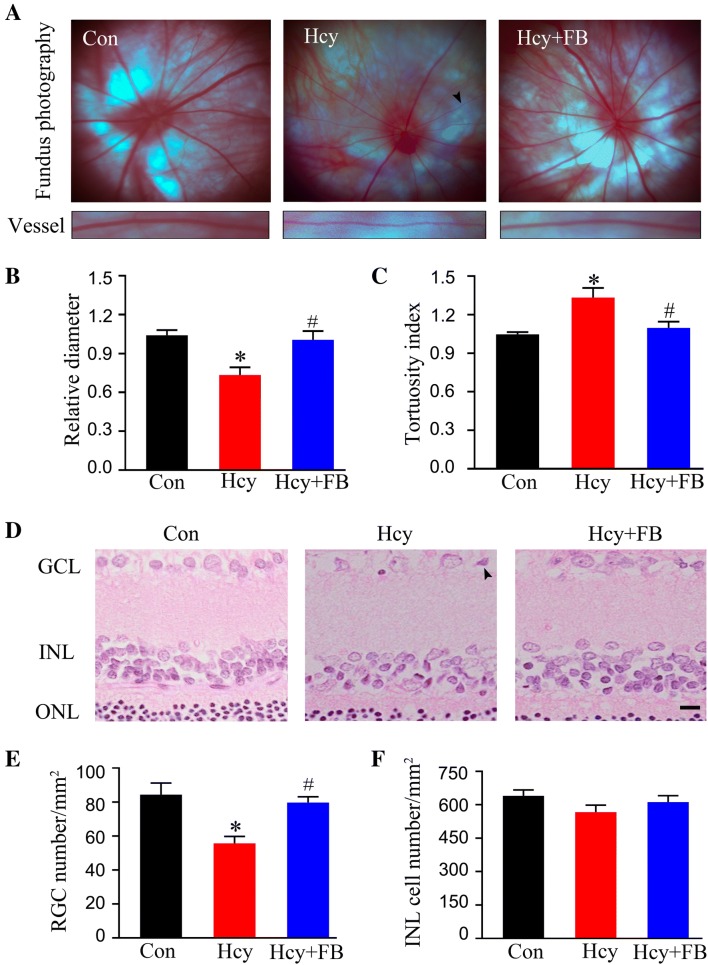
Next, we investigated how Hhcy impairs the retina and whether FB supplementation has beneficial effects on Hhcy. By ERG recording, we found that Hhcy significantly decreased the amplitude and increased the latency of the b-wave without changing the a-wave, while FB restored the b-wave to the normal level (Fig. 2A–D). Meanwhile, we conducted photographic fundus examination and observed that Hhcy induced arterial stenosis and distortion, while FB supplementation reversed these vessel abnormalities (Fig. 3A–C). By HE staining, we also observed that Hcy exposure induced nuclear shrinkage in the RGC layer and significantly decreased the number of RGCs (Fig. 3D, E), while FB supplementation attenuated these pathological changes. The cell number in the inner cell layer (INL) also showed a decreasing trend, but the difference was not statistically significant in Hhcy rats (Fig. 3F). These data together indicate that simultaneous FB supplementation attenuates Hhcy-induced retinal artery impairment and RGC loss in vivo.
Fig. 2.
FB ameliorates Hhcy-induced suppression of retinal activity in rats. A, B The electroretinogram (ERG) showed an unchanged a-wave (A) and a reduced b-wave amplitude (B) in the rod-specific response in rats injected with Hcy for 14 days, and that simultaneous supplementation with FB attenuated the changes. C, D The ERG showed an unchanged a-wave (C) and an increased b-wave latency (D) in the rod-specific response in rats injected with Hcy, while FB attenuated the changes. Data are expressed as mean ± SEM. **P < 0.01 versus control; #P < 0.05, ##P < 0.01 versus Hcy at 14 days.
Fig. 3.
FB attenuates Hhcy-induced vessel impairment and cell loss in the retina. A Representative images of the fundus; arrowheads indicate a tortuous and narrow artery. B, C Decreased vessel diameter with increased distorted vessels occurred in the Hcy group, and FB reversed the pathologies. D Representative images of HE staining of the retina; arrowhead indicates nuclear shrinkage. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer (scale bar, 20 μm). E, F Numbers of RGCs and INL cells in three groups analyzed using ImageJ. Data are expressed as mean ± SEM; n = 5/group; *P < 0.05 versus control; #P < 0.05 versus Hcy at 14 days.
FB Attenuates Hhcy-Induced Intracellular Aβ Accumulation in Rat Retina
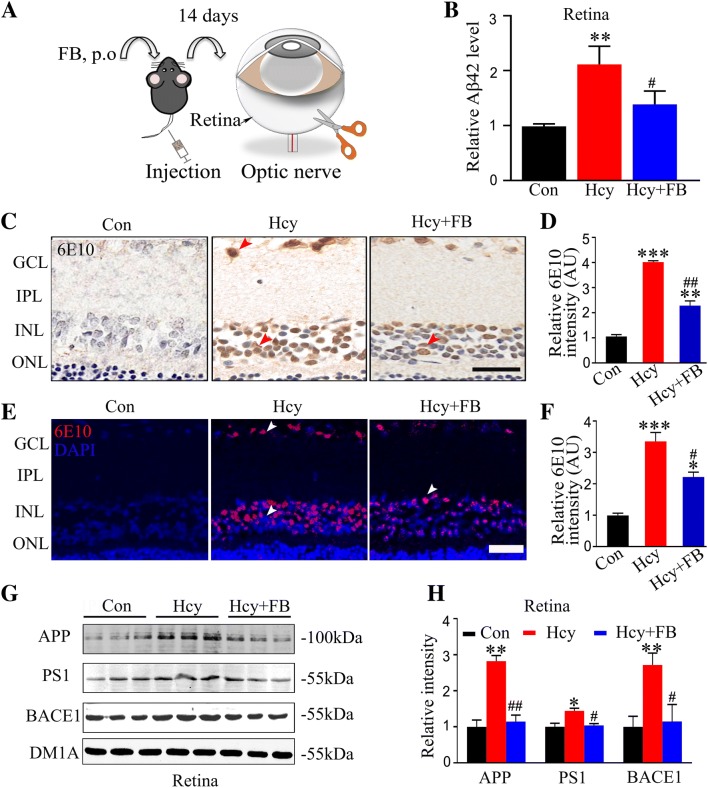
Aβ is the main component of amyloid plaques in the brain of AD patients and its accumulation is one of the main mechanisms that cause neurodegenerative diseases. To assess the effect of FB on retinal Aβ after Hcy injection, we measured the Aβ42 level in the retina by ELISA. We found that Hcy increased retinal Aβ after injection for 14 days, while FB supplementation almost restored it to the normal level (Fig. 4B). To further outline the Aβ pathology in the retina, we used immunohistochemistry and immunofluorescence staining for the antibody 6E10. We observed abundant intracellular Aβ accumulation in the ganglion cell layer (GCL) and INL and FB supplementation reduced this accumulation (Fig. 4C–F). We also found that FB attenuated the elevation of APP, PS1, and BACE 1 in the retina of the Hhcy group (Fig. 4G, H). These data suggested that Aβ and its synthesis are activated in the retina during Hhcy exposure, and FB attenuates these pathological changes.
Fig. 4.
FB attenuates Hhcy-induced Aβ accumulation in rat retina. A Schematic of procedures: Hcy was injected via the vena caudalis with or without FB supplementation, and the retinas were collected for measurements. B Aβ42 levels as measured by ELISA. C–F Representative retinal images and quantitation of Aβ accumulation measured by immunohistochemical and immunofluorescent staining for 6E10 (n = 5/group; scale bars, 50 μm; arrowheads indicate positive intracellular staining). G, H Western blots and quantitation showing increased levels of APP, PS1, and BACE1 in retinas with Hcy injection, and FB supplementation arrested the elevations (n = 3/group). Data are expressed as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001 versus control; #P < 0.05, ##P < 0.01 versus Hcy at 14 days.
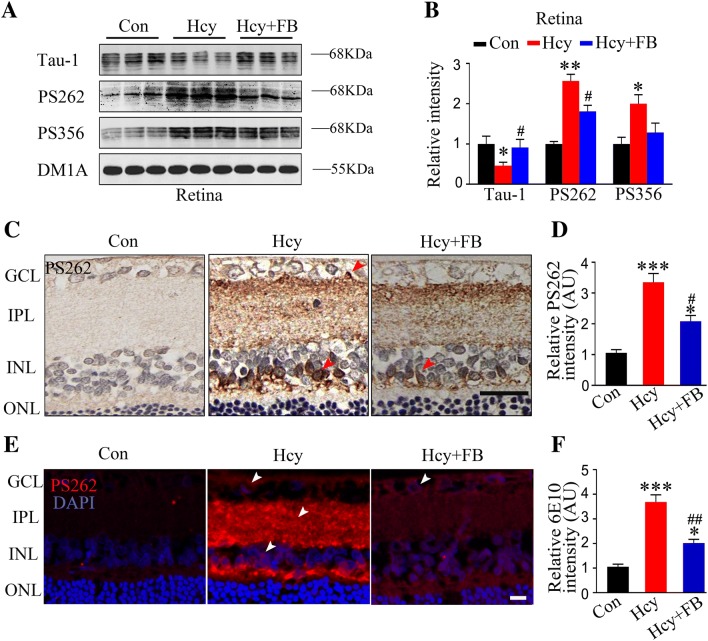
FB Reduces Hhcy-Induced Tau Hyperphosphorylation in Rat Retina
Hyperphosphorylated tau is the major protein component of NFTs in degenerating neurons of the AD brain [27–29]. Therefore, we analyzed tau phosphorylation in the retina using the phosphorylated tau-specific antibodies pS262 and pS356, which react with the AD-related epitopes [30–34]. By Western blotting, we found that Hhcy significantly increased the phosphorylation level of tau at PS262, PS356, and the tau-1 epitope, the latter reacting with the unphosphorylated sites at Ser198/199/202 (Fig. 5A, B). By immunohistochemistry and immunofluorescence staining, we observed the accumulation of pS262-tau in the INL, inner plexiform layer (IPL), and GCL in the Hcy group at 14 days, and FB rescued the tau hyperphosphorylation at Ser262 (Fig. 5C–F). These data together demonstrated that Hhcy induces tau hyperphosphorylation at multiple AD-related sites in the retina and simultaneous supplementation with FB attenuates this hyperphosphorylation.
Fig. 5.
FB attenuates Hhcy-induced tau hyperphosphorylation in rat retina. A, B Western blots of phosphorylated tau at Ser262, Ser356, and the Tau-1 epitope in the retina (n = 3/group). Note that Tau-1 reacts with the dephosphorylated tau at the Ser198/199/202 epitope. C–F Retinal images and quantitation of tau hyperphosphorylation as measured by immunohistochemical and immunofluorescent staining using PS262 (n = 5/group; scale bars, 50 μm; arrowheads indicate representative intracellular positive staining). Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 versus control; #P < 0.05, ##P < 0.01 versus Hcy at 14 days.
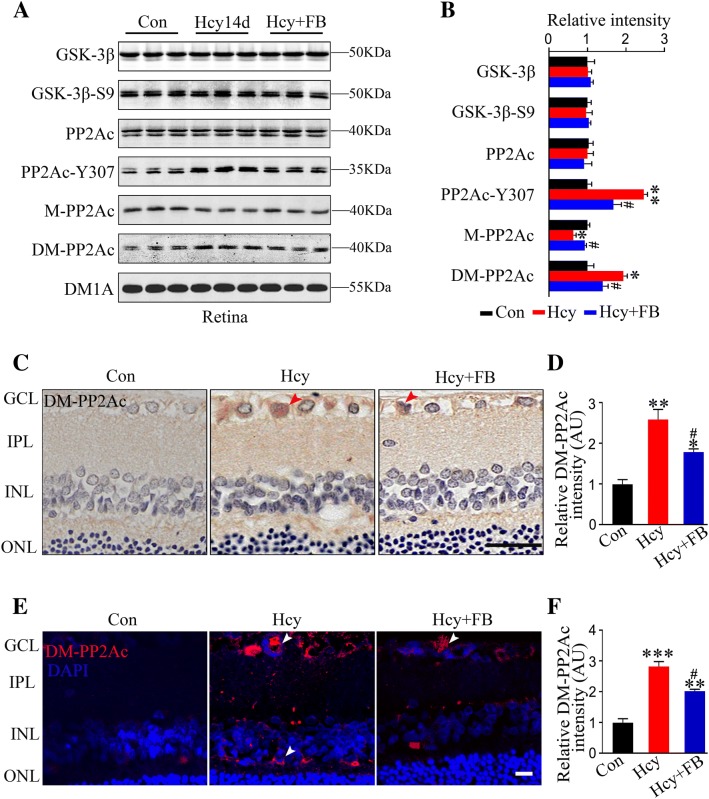
FB Ameliorates Hhcy-Induced Protein Kinase and Protein Phosphatase Imbalance in Rat Retina
The activation of protein kinases and/or inhibition of protein phosphatases (PPs) are the direct cause of tau hyperphosphorylation, and since GSK-3β and PP2A are the most implicated, we measured their levels. We found that Hhcy with or without FB did not change the total GSK-3β or Ser9-phosphorylated GSK-3β in the retina (Fig. 6A, B). Hcy exposure for 14 days significantly decreased methylated PP2Ac (M-PP2Ac, the active form) along with increased demethylation (DM-PP2Ac, the inactive form) and no change in the total PP2Ac level; FB restored the levels (Fig. 6A, B). The beneficial effects of FB on DM-PP2Ac in the GCL, IPL, and INL were also demonstrated by immunofluorescent staining (Fig. 6C–F). These data suggested that FB supplementation modulates the Hhcy-induced imbalance of PP2A.
Fig. 6.
FB attenuates Hhcy-induced PP2Ac inhibition in rat retina. A, B Western blots and quantitation of the levels of GSK-3β, GSK-3β-S9, PP2Ac, PP2Ac-Y307, M-PP2Ac, and DM-PP2Ac in the retina (n = 3/group). Hcy injection decreased the methylated PP2Ac (M-PP2Ac) along with increased demethylated PP2Ac (DM-PP2Ac). Increased tyrosine-307 phosphorylation of PP2Ac (PP2Ac-Y307) was detected with no changes of GSK-3β and GSK-3β-S9 in the Hcy group and FB restored the PP2A activity. C–F Representative images and quantitation of DM-PP2Ac in the retina measured by immunohistochemistry (C, D) and immunofluorescence (E, F). Increased DM-PP2Ac immunoreactivity occurred in the GCL and INL of the Hcy group, and FB ameliorated this increase (n = 5/group; scale bars, 10 μm; arrowheads indicate representative intracellular positive staining). Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 versus control; #P < 0.05 versus Hcy at 14 days.
Discussion
AD is characterized pathologically by the formation of numerous senile plaques and NFTs in specific brain regions, with a progressive decline in several cognitive fields [35, 36]. The major components of the tangles and plaques are hyperphosphorylated tau protein and Aβ, respectively. During the last decades, the therapeutic strategies developed to target misfolded proteins in the AD brain have been disappointing, because these strategies are unable to stop the cognitive decline [37–40]. Since the main lesion is the accumulation of hyperphosphorylated tau and Aβ in the hippocampus, the therapeutic effect of a drug cannot be visualized at an early stage. Therefore, a non-invasive and accurate visualization window for AD studies is urgently needed.
Hcy concentration increases with age in normal human plasma [12, 13, 41, 42]. Epidemiological studies have shown that patients with high plasma Hcy levels display more rapid neurodegenerative changes, and > 40% of AD patients have high levels of plasma Hcy. Therefore, Hhcy has been proposed to be an independent risk factor for AD [12]. An alternative route of Hcy metabolism is degradation to cysteine by the transsulfuration pathway through two sequential vitamin B6-dependent reactions [43]. Folate deficiency results in decreased leucine carboxyl methyltransferase-1 and PP2A subunit protein levels which, combined with tau hyperphosphorylation [44] and vitamin B deficiency, induce Hhcy and selectively impair the performance of apolipoprotein-E-deficient mice in the Morris water maze [45]. Previous studies have demonstrated that administration of Hcy through the vena caudalis for 14 days induces a spatial memory deficit with AD-like tau and β-amyloid pathologies in the hippocampus [20, 21]. Simultaneous supplementation with folate and vitamin B12 partially restores the plasma Hcy level and ameliorates the tau and β-amyloid pathologies in the hippocampus [20, 21]. In the current study, we found that Hcy injected for 14 days induced the accumulation of Aβ and phosphorylated tau protein in the retina in addition to vessel impairment, and FB attenuated the AD-like pathological changes. With regard to retinal biomarkers in AD, reports have been controversial [46]. Some support the idea that retinal pathology can be used as a biomarker for AD [47, 48], while others suggest that Aβ, phosphorylated tau, and α-synuclein are not deposited in the eye in a manner analogous to the brain, or that these molecules are present at lower levels or in different forms in the eye [49]. Our data revealed that FB not only ameliorates the Hhcy-induced lesions in the hippocampus, but also rescues the AD-like pathologies in the retina.
The direct cause of tau hyperphosphorylation is an imbalance of protein kinase and protein phosphatase. To explore the mechanism underlying the changes of hyperphosphorylated tau with Hhcy, we examined the activities of kinases and phosphatase. Previous studies have reported that Hhcy results in increased phosphorylation of PP2Ac-Y307 and DM-PP2Ac, thus it inhibits PP2Ac activity and increases the hyperphosphorylation of tau protein [50]. We showed that simultaneous administration of FB reduced the phosphorylation of tau and DM-PP2Ac in the retina. Meanwhile, FB increased the level of M-PP2Ac, thereby restoring the activity of PP2Ac and reducing the tau phosphorylation. The mechanism underlying the attenuation of PP2A by FB is currently not fully understood. As simultaneous supplementation with FB significantly decreased the plasma Hcy level, which has also been reported [13], the beneficial effects of FB on PP2A may be through reducing plasma Hcy level or directly acting on the phosphatase.
We used folate (4 mg/kg per day) and vitamin B12 (250 μg/kg per day) in rats by referring to previous reports [20, 21]. In one report, 6.7 mg/kg per day folate in mouse was used and no adverse reaction was shown [44]. In a more recent report, 500 μg/day vitamin B12 was used [51]. In humans, 8–80 µg/kg per day folate and 3–30 µg/kg per day vitamin B12 have been recommended for supplementary treatment of Hhcy [52]; these are much lower than the doses used in rats or mice. However, 0.4 mg–1 g/day folate and 1 mg/day vitamin B12 for 1 month have been prescribed for treating human macrocytic anemia [50]; by conversion, 7 µg–17 mg/kg per day for folate and 17 µg/kg per day for vitamin B12 may be used for a person weighing 60 kg. It seems that the doses of vitamin B12 and folate used in humans and mice are different, which may be considered in future studies.
In summary, we demonstrate in the present study that Hhcy induces AD-like tau hyperphosphorylation and Aβ accumulation in the retina in addition to vessel impairment, while simultaneous supplementation with FB efficiently reduces plasma Hcy levels with attenuation of the AD-like pathologies in the retina. Our data suggest that the retina may serve as a window for the early non-invasive detection of AD-like pathologies and for evaluating the intervention effects of therapeutic compounds in some disorders with a high risk for AD, such Hhcy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Prof. Xiangtian Zhou of the School of Optometry and Ophthalmology and Eye Hospital, Wenzhou Medical College, China, for technical support. This work was supported in part by the Natural Science Foundation of China (91632305, 91632111, 31730035, and 81721005), and by the Ministry of Science and Technology of China (2016YFC1305800).
Compliance with Ethical Standards
Conflict of interest
The authors declare no competing interests.
Contributor Information
Jian-Zhi Wang, Email: wangjz@mail.hust.edu.cn.
Ying Yang, Email: yingyang@hust.edu.cn.
References
- 1.Waldemar G, Dubois B, Emre M, Georges J, McKeith IG, Rossor M, et al. Recommendations for the diagnosis and management of Alzheimer’s disease and other disorders associated with dementia: EFNS guideline. Eur J Neurol. 2007;14:e1–26. doi: 10.1111/j.1468-1331.2006.01605.x. [DOI] [PubMed] [Google Scholar]
- 2.Wang QH, Wang X, Bu XL, Lian Y, Xiang Y, Luo HB, et al. Comorbidity burden of dementia: a hospital-based retrospective study from 2003 to 2012 in seven cities in china. Neurosci Bull. 2017;33:703–710. doi: 10.1007/s12264-017-0193-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardy J. A hundred years of Alzheimer’s disease research. Neuron. 2006;52:3–13. doi: 10.1016/j.neuron.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 4.Mendes D, Oliveira MM, Moreira PI, Coutinho J, Nunes FM, Pereira DM, et al. Beneficial effects of white wine polyphenols-enriched diet on Alzheimer’s disease-like pathology. J Nutr Biochem. 2018;55:165–177. doi: 10.1016/j.jnutbio.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Sun BL, Li WW, Zhu C, Jin WS, Zeng F, Liu YH, et al. Clinical research on Alzheimer’s disease: progress and perspectives. Neurosci Bull. 2018 doi: 10.1007/s12264-018-0249-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Archibald NK, Clarke MP, Mosimann UP, Burn DJ. The retina in Parkinson’s disease. Brain. 2009;132:1128–1145. doi: 10.1093/brain/awp068. [DOI] [PubMed] [Google Scholar]
- 7.Calabresi PA, Balcer LJ, Frohman EM. Retinal pathology in multiple sclerosis: insight into the mechanisms of neuronal pathology. Brain. 2010;133:1575–1577. doi: 10.1093/brain/awq133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ong YT, De Silva DA, Cheung CY, Chang HM, Chen CP, Wong MC, et al. Microvascular structure and network in the retina of patients with ischemic stroke. Stroke. 2013;44:2121–2127. doi: 10.1161/STROKEAHA.113.001741. [DOI] [PubMed] [Google Scholar]
- 9.Koronyo-Hamaoui M, Koronyo Y, Ljubimov AV, Miller CA, Ko MK, Black KL, et al. Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. Neuroimage. 2011;54(Suppl 1):S204–217. doi: 10.1016/j.neuroimage.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.La Morgia C, Ross-Cisneros FN, Koronyo Y, Hannibal J, Gallassi R, Cantalupo G, et al. Melanopsin retinal ganglion cell loss in Alzheimer disease. Ann Neurol. 2016;79:90–109. doi: 10.1002/ana.24548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hart NJ, Koronyo Y, Black KL, Koronyo-Hamaoui M. Ocular indicators of Alzheimer’s: exploring disease in the retina. Acta Neuropathol. 2016;132:767–787. doi: 10.1007/s00401-016-1613-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 13.Clarke R, Smith AD, Jobst KA, Refsum H, Sutton L, Ueland PM. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch Neurol. 1998;55:1449–1455. doi: 10.1001/archneur.55.11.1449. [DOI] [PubMed] [Google Scholar]
- 14.Hooshmand B, Solomon A, Kareholt I, Leiviska J, Rusanen M, Ahtiluoto S, et al. Homocysteine and holotranscobalamin and the risk of Alzheimer disease: a longitudinal study. Neurology. 2010;75:1408–1414. doi: 10.1212/WNL.0b013e3181f88162. [DOI] [PubMed] [Google Scholar]
- 15.Ravaglia G, Forti P, Maioli F, Martelli M, Servadei L, Brunetti N, et al. Homocysteine and folate as risk factors for dementia and Alzheimer disease. Am J Clin Nutr. 2005;82:636–643. doi: 10.1093/ajcn/82.3.636. [DOI] [PubMed] [Google Scholar]
- 16.Miller AL. The methionine-homocysteine cycle and its effects on cognitive diseases. Altern Med Rev. 2003;8:7–19. [PubMed] [Google Scholar]
- 17.Ho PI, Ortiz D, Rogers E, Shea TB. Multiple aspects of homocysteine neurotoxicity: glutamate excitotoxicity, kinase hyperactivation and DNA damage. J Neurosci Res. 2002;70:694–702. doi: 10.1002/jnr.10416. [DOI] [PubMed] [Google Scholar]
- 18.Kamath AF, Chauhan AK, Kisucka J, Dole VS, Loscalzo J, Handy DE, et al. Elevated levels of homocysteine compromise blood-brain barrier integrity in mice. Blood. 2006;107:591–593. doi: 10.1182/blood-2005-06-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pacheco-Quinto J, Rodriguez de Turco EB, DeRosa S, Howard A, Cruz-Sanchez F, Sambamurti K, et al. Hyperhomocysteinemic Alzheimer’s mouse model of amyloidosis shows increased brain amyloid beta peptide levels. Neurobiol Dis. 2006;22:651–656. doi: 10.1016/j.nbd.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Zhang CE, Wei W, Liu YH, Peng JH, Tian Q, Liu GP, et al. Hyperhomocysteinemia increases beta-amyloid by enhancing expression of gamma-secretase and phosphorylation of amyloid precursor protein in rat brain. Am J Pathol. 2009;174:1481–1491. doi: 10.2353/ajpath.2009.081036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang CE, Tian Q, Wei W, Peng JH, Liu GP, Zhou XW, et al. Homocysteine induces tau phosphorylation by inactivating protein phosphatase 2A in rat hippocampus. Neurobiol Aging. 2008;29:1654–1665. doi: 10.1016/j.neurobiolaging.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 22.Schnyder G, Roffi M, Pin R, Flammer Y, Lange H, Eberli FR, et al. Decreased rate of coronary restenosis after lowering of plasma homocysteine levels. N Engl J Med. 2001;345:1593–1600. doi: 10.1056/NEJMoa011364. [DOI] [PubMed] [Google Scholar]
- 23.Mattson MP, Shea TB. Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci. 2003;26:137–146. doi: 10.1016/S0166-2236(03)00032-8. [DOI] [PubMed] [Google Scholar]
- 24.Sasaki K, Duan J, Murohara T, Ikeda H, Shintani S, Shimada T, et al. Rescue of hypercholesterolemia-related impairment of angiogenesis by oral folate supplementation. J Am Coll Cardiol. 2003;42:364–372. doi: 10.1016/S0735-1097(03)00629-6. [DOI] [PubMed] [Google Scholar]
- 25.Gupta SK, Kumar B, Nag TC, Agrawal SS, Agrawal R, Agrawal P, et al. Curcumin prevents experimental diabetic retinopathy in rats through its hypoglycemic, antioxidant, and anti-inflammatory mechanisms. J Ocul Pharmacol Ther. 2011;27:123–130. doi: 10.1089/jop.2010.0123. [DOI] [PubMed] [Google Scholar]
- 26.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brion JP, Couck AM, Conreur JL. Calcineurin (phosphatase 2B) is present in neurons containing neurofibrillary tangles and in a subset of senile plaques in Alzheimer’s disease. Neurodegeneration. 1995;4:13–21. doi: 10.1006/neur.1995.0002. [DOI] [PubMed] [Google Scholar]
- 28.Minthon L, Hesse C, Sjogren M, Englund E, Gustafson L, Blennow K. The apolipoprotein E epsilon4 allele frequency is normal in fronto-temporal dementia, but correlates with age at onset of disease. Neurosci Lett. 1997;226:65–67. doi: 10.1016/S0304-3940(97)00230-9. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi M, Tsujioka Y, Yamada T, Tsuboi Y, Okada H, Yamamoto T, et al. Glycosylation of microtubule-associated protein tau in Alzheimer’s disease brain. Acta Neuropathol. 1999;97:635–641. doi: 10.1007/s004010051040. [DOI] [PubMed] [Google Scholar]
- 30.Biernat J, Gustke N, Drewes G, Mandelkow EM, Mandelkow E. Phosphorylation of Ser262 strongly reduces binding of tau to microtubules: distinction between PHF-like immunoreactivity and microtubule binding. Neuron. 1993;11:153–163. doi: 10.1016/0896-6273(93)90279-Z. [DOI] [PubMed] [Google Scholar]
- 31.Vincent IJ, Davies P. Phosphorylation characteristics of the A68 protein in Alzheimer’s disease. Brain Res. 1990;531:127–135. doi: 10.1016/0006-8993(90)90765-4. [DOI] [PubMed] [Google Scholar]
- 32.Wang JZ, Liu F. Microtubule-associated protein tau in development, degeneration and protection of neurons. Prog Neurobiol. 2008;85:148–175. doi: 10.1016/j.pneurobio.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Su Y, Wang J, Sun S, Wang T, Qiao X, et al. Rapamycin decreases tau phosphorylation at Ser214 through regulation of cAMP-dependent kinase. Neurochem Int. 2013;62:458–467. doi: 10.1016/j.neuint.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 34.Collin L, Bohrmann B, Gopfert U, Oroszlan-Szovik K, Ozmen L, Gruninger F. Neuronal uptake of tau/pS422 antibody and reduced progression of tau pathology in a mouse model of Alzheimer’s disease. Brain. 2014;137:2834–2846. doi: 10.1093/brain/awu213. [DOI] [PubMed] [Google Scholar]
- 35.Karran E, Mercken M, De Strooper B. The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nat Rev Drug Discov. 2011;10:698–712. doi: 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- 36.Reitz C, Mayeux R. Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol. 2014;88:640–651. doi: 10.1016/j.bcp.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aisen PS, Gauthier S, Ferris SH, Saumier D, Haine D, Garceau D, et al. Tramiprosate in mild-to-moderate Alzheimer’s disease - a randomized, double-blind, placebo-controlled, multi-centre study (the Alphase Study) Arch Med Sci. 2011;7:102–111. doi: 10.5114/aoms.2011.20612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370:311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 39.Golde TE, Schneider LS, Koo EH. Anti-abeta therapeutics in Alzheimer’s disease: the need for a paradigm shift. Neuron. 2011;69:203–213. doi: 10.1016/j.neuron.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Green RC, Schneider LS, Amato DA, Beelen AP, Wilcock G, Swabb EA, et al. Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial. JAMA. 2009;302:2557–2564. doi: 10.1001/jama.2009.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCaddon A, Davies G, Hudson P, Tandy S, Cattell H. Total serum homocysteine in senile dementia of Alzheimer type. Int J Geriatr Psychiatry. 1998;13:235–239. doi: 10.1002/(SICI)1099-1166(199804)13:4<235::AID-GPS761>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 42.Obeid R, Herrmann W. Mechanisms of homocysteine neurotoxicity in neurodegenerative diseases with special reference to dementia. FEBS Lett. 2006;580:2994–3005. doi: 10.1016/j.febslet.2006.04.088. [DOI] [PubMed] [Google Scholar]
- 43.Finkelstein JD. Methionine metabolism in mammals. J Nutr Biochem. 1990;1:228–237. doi: 10.1016/0955-2863(90)90070-2. [DOI] [PubMed] [Google Scholar]
- 44.Sontag JM, Nunbhakdi-Craig V, Montgomery L, Arning E, Bottiglieri T, Sontag E. Folate deficiency induces in vitro and mouse brain region-specific downregulation of leucine carboxyl methyltransferase-1 and protein phosphatase 2A B(alpha) subunit expression that correlate with enhanced tau phosphorylation. J Neurosci. 2008;28:11477–11487. doi: 10.1523/JNEUROSCI.2816-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Troen AM, Shukitt-Hale B, Chao W-H, Albuquerque B, Smith DE, Selhub J, et al. The cognitive impact of nutritional homocysteinemia in Apolipoprotein-E deficient mice. J Alzheimers Dis. 2006;9:381–392. doi: 10.3233/JAD-2006-9403. [DOI] [PubMed] [Google Scholar]
- 46.Ong SS, Doraiswamy PM, Lad EM. Controversies and future directions of ocular biomarkers in Alzheimer disease. JAMA Neurol. 2018;75:650–651. doi: 10.1001/jamaneurol.2018.0602. [DOI] [PubMed] [Google Scholar]
- 47.Ascaso FJ, Cruz N, Modrego PJ, Lopez-Anton R, Santabarbara J, Pascual LF, et al. Retinal alterations in mild cognitive impairment and Alzheimer’s disease: an optical coherence tomography study. J Neurol. 2014;261:1522–1530. doi: 10.1007/s00415-014-7374-z. [DOI] [PubMed] [Google Scholar]
- 48.Koronyo Y, Biggs D, Barron E, Boyer DS, Pearlman JA, Au WJ, et al. Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer’s disease. JCI Insight 2017, 2. [DOI] [PMC free article] [PubMed]
- 49.Ho CY, Troncoso JC, Knox D, Stark W, Eberhart CG. Beta-amyloid, phospho-tau and alpha-synuclein deposits similar to those in the brain are not identified in the eyes of Alzheimer’s and Parkinson’s disease patients. Brain Pathol. 2014;24:25–32. doi: 10.1111/bpa.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Regland B, Abrahamsson L, Gottfries CG, Magnus E. Vitamin B12 analogues, homocysteine, methylmalonic acid, and transcobalamins in the study of vitamin B12 deficiency in primary degenerative dementia. Dement Geriatr Cogn Disord. 1990;1:272–277. doi: 10.1159/000107152. [DOI] [Google Scholar]
- 51.Shu XJ, Li ZF, Chang YW, Liu SY, Wang WH, Li X. Different doses of folic acid and vitamin B12 to treat rabbits with deep venous thrombosis and hyperhomocysteinemia. Exp Ther Med. 2018;15:2874–2878. doi: 10.3892/etm.2018.5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guilliams TG. Homocysteine - a risk factor for vascular diseases: guidelines for the clinical practice. JANA. 2004;7:11–24. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.